NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.

TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades.
Show detailsTRPP
Polycystin Ciliary Localization and ADPKD in C. Elegans
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common monogenic diseases, affecting 1 in 400 to 1 in 1,000 individuals. In ADPKD patients, the kidney accumulates multiple cysts, which ultimately cause end-stage renal disease. Mutation in the PKD1 or PKD2 gene accounts for 95 percent of ADPKD cases [1, 2]. PKD1 encodes polycystin-1 (PC-1), a 4,302 amino acid protein with a large extracellular domain, a G-protein-coupled receptor proteolytic site (GPS), eleven transmembrane (TM) domains, and an intracellular C-terminus (Figure 19.1) [1, 3]. The polycystin/lipoxygenase/alpha-toxin (PLAT) domain is located in the first cytoplasmic loop between TM1 and TM2 and has been postulated to be involved in membrane–protein or protein–protein interactions [4]. The PLAT domain is conserved in all PC-1 family members and is also found in a variety of membrane-or lipid-associated proteins. Polycystin-2 (PC-2, encoded by PKD2) is a transient receptor protein polycystin (TRPP) family member [2] and acts as a nonselective cation channel (reviewed in reference [5]). Mammalian PC-1 and PC-2 have been demonstrated to localize to primary cilia of kidney epithelial cells [6, 7] where they function as a mechanosensitive channel [8]. ADPKD is one of a number of human genetic diseases that are rooted in defects in cilia formation, maintenance, or function [9, 10, 11].
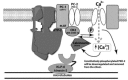
FIGURE 19.1
Polycystin localization on the ciliary membrane and interacting proteins. The KLP-6 kinesin-3 is required for polycystin-mediated sensory behaviors and TRPP localization. KLP-6 may act as a molecular motor transporting the TRPP complex on microtubules (more...)
C. elegans provides a powerful model system to understand ADPKD and other diseases of ciliary basis [12]. In C. elegans, LOV-1 and PKD-2 are homologous to polycystin-1 and polycystin-2, respectively [13, 14, 15]. Similar to PC-1, LOV-1 has eleven predicted TM domains, an intracellular PLAT domain between TM1 and TM2, and a large extracellular domain. The extracellular domains of LOV-1 and PC-1 are divergent, suggesting that a species-specific ligand may activate the putative receptor LOV-1. LOV-1 does possess a GPS site, suggesting that proteolytic processing may occur as demonstrated for human PC-1 and sea urchin REJ3, a polycystin-like protein [16, 17]. Like PC-2, PKD-2 has six TM domains, an extracellular polycystin loop domain between TM1 and TM2, and a C-tail possessing coiled-coil and potential Ca2+-binding EF hand domains [14, 18]. LOV-1 and PKD-2 localize to ciliated endings on dendrites and in neuronal cell bodies of male-specific sensory neurons [13, 14]. lov-1 and pkd-2 are required for two mating behaviors (response to mate contact and location of the mate’s vulva) and are postulated to sense cues from the mate [13, 14]. Hence, the connection between the polycystins, cilia, and sensory function seems to be an ancient one.
TRP vanilloid (TRPV) channels also localize to cilia in mammals, Drosophila, and the nematode [19, 20, 21]. In C. elegans, the TRPV channels OSM-9 and OCR-2 depend on each other for ciliary localization and sensory function [22]. In Drosophila, the ciliary localization of TRPV hearing channels Nanchung (NAN) and Inactive (IAV) is also codependent [23]. Human PC-1 has been implicated in transporting PC-2 from the endoplasmic reticulum (ER) to the plasma membrane [24]. In C. elegans sensory neurons, PKD-2 ciliary stabilization requires LOV-1, and vice versa [25]. In a lov-1 mutant background, PKD-2 levels are greatly reduced in cilia. Hence, TRPP partnering is required for optimal ciliary targeting. Interestingly, PKD-2 forms abnormal aggregates in neuronal cell bodies of lov-1 mutants [25]. Protein aggregation has not been examined or described in ADPKD cysts.
The Kinesin KLP-6 Regulates TRPP Function and Localization
The klp-6 mutant was identified based on its Rsp (response) and Lov (location of vulva) defects in the same genetic screen that yielded the lov-1/PC-1 mutant [26]. In klp-6 mutants, PKD-2 abnormally accumulates at the base of cilia as opposed to the cilium proper and also accumulates along dendrites. klp-6 encodes a kinesin-like protein of 928 amino acids that belongs to the kinesin-3 (previously known as UNC-104/Kif1A) family [27, 28, 29]. Kinesin-3 family members are composed of an N-terminal, motor head region containing ATP- and microtubule-binding domains, followed by a coiled-coil and fork-head associated (FHA) domain that may facilitate multimer-ization [30], and a C-terminal tail of variable length and domain composition. Single homologues of KLP-6 are found in multiple vertebrate genomes. Alignments of C. elegans, zebrafish, mouse, and rat KLP-6 revealed 34 percent identity and 52 percent similarity overall, a highly conserved motor domain (53 percent identity), and middle and tail domains conserved to a lesser extent. The C-terminal tail of kinesin enables many regulatory properties relating to cargo recognition, motor activity, and subcellular trafficking [31–34]. If the klp-6 mutant produces a protein, the truncated protein is predicted to be deficient in cargo-binding but to retain microtubule-binding ability. KLP-6 is coexpressed and colocalized with LOV-1/PC-1 and PKD-2/PC-2 in male-specific sensory neurons.
KLP-6 and the polycystins may function as an evolutionarily conserved ciliary unit (Figure 19.1). KLP-6 may act as a motor protein to directly transport multiple signaling molecules and distribute membrane receptors to specific ciliary subzones. Additionally, or possibly alternatively to the motor model, KLP-6 may function as a novel anchor-like protein and tether the polycystins and other unidentified proteins to ciliary microtubules. Mammalian polycystins function as mechanosensors in primary cilia of renal epithelial tubules, and mechanosensory complexes typically interact with microtubule or actin filaments via cytoskeletal-associated proteins. KLP-6 promises new routes to understanding cilia function, sensory behaviors, and potentially ADPKD.
Intraflagellar Transport Moves TRPV but Not TRPP In Cilia
The development of all cilia and flagella requires intraflagellar transport (IFT) [35]. IFT is an evolutionarily conserved, microtubule-based motility first observed as microscopic particles moving up and down the length of the flagella of the green alga Chlamydomonas [36]. The cellular machinery driving IFT was determined using primarily cellular and biochemical approaches [35]. The IFT machinery contains heterotrimeric kinesin-2 and retrograde cytoplasmic dynein motors that move IFT particles and cargo to and from the distal tips of cilia. The IFT particle is composed of two complexes (A or B) containing 16 to 18 polypeptides. A simple model involves kinesin-2 and complex B polypeptides regulating anterograde transport, and dynein and complex A polypeptides regulating retrograde transport. In mammals, disruption of the kinesin-2 IFT motor or Polaris IFT complex B polypeptide results in embryos lacking cilia and exhibiting abnormal left-right development and polycystic kidney disease [37, 38, 39]. Polycystin-2 is also required to establish left-right asymmetry in mice [40]. PC-2 is found on nodal cilia and required for the generation of an asymmetric calcium signal, suggesting that PC-2 functions as a mechanosensor in nodal cilia [41].
A role for IFT in transporting ciliary membrane proteins has recently been described. Using time-lapse fluorescence microscopy and genetics, we demonstrated IFT-dependent vectorial transport of select GFP-tagged sensory receptors within the ciliary membrane of C. elegans sensory neurons in vivo [42]. TRPV channels OSM-9 and OCR-2 [21, 22] move in cilia at rates comparable to the IFT machinery, and this motility is disrupted in IFT mutant backgrounds. Surprisingly, motility of TRPP channel PKD-2 is not detected, suggesting that PKD-2 may diffuse into the ciliary membrane, that PKD-2 may be physically restrained at the base of the cilium, or that at least two mechanisms regulate ciliary protein localization.
TRPP1 PLAT Binding Partners
The evolutionarily conserved PLAT domain has been proposed to mediate protein–protein or protein–lipid interactions [4]. In wild-type C. elegans males, overexpression of the PLAT domain alone or a LOV-1/PC-1 TM segment followed by the PLAT domain (TM-PLAT) interferes with male sensory behaviors [43]. PLAT and TM-PLAT do not localize to cilia, indicating that the PLAT domain is not sufficient for LOV-1/PC-1 ciliary targeting [43]. To identify targets of the C. elegans PLAT domain, a yeast two hybrid screen was performed [43]. ATP-2, an ATP synthase subunit, and KIN-10, the regulatory beta subunit of the protein kinase CK2 (casein kinase 2), were isolated and validated [43, 44]. ATP-2 and KIN-10 also associate with the human PLAT domain.
ATP-2 is a component of the ATP synthase, which is composed of two functional domains: a catalytic F1 portion and a membrane-embedded F0 portion. C. elegans ATP-2 is the beta subunit, or active site, of the F1 portion [45]. The mitochondrial respiratory chain (MRC) generates the majority of cellular ATP. ATP-2 and other ATP synthase components but not MRC I–IV colocalize with PKD-2 in male-specific sensory cilia [43]. Moreover, knockdown of the ATP synthase but not MRC I–IV produces polycystin-like male sensory defects [43]. While the ATP synthase localizes primarily to the inner mitochondria membrane [46], its presence at the cell membrane has been reported [47–52]. The ATP synthase has also been found in plasma membrane lipid rafts along with beta-tubulin [53, 54]. Our results suggest that the ciliary-localized ATP synthase may play a previously unsuspected role in polycystin signaling.
C. elegans LOV-1 and human PC-1 also bind the regulatory subunit of CK2 [44]. Protein phosphorylation by the coordinated activities of protein kinases and phosphatases is central to many signal transduction pathways. CK2 and calcineurin/protein phosphatase 2B (PP2B) modulate PKD-2 function and ciliary localization. CK2 and the Ca2+-activated phosphatase calcineurin act antagonistically to regulate PKD-2. A “phospho-defective” PKD-2 mutant protein trafficks normally to cilia but exhibits attenuated function, while a “phospho-mimetic” PKD-2 is defective in both function and ciliary localization. tax-6 regulates PKD-2 ciliary localization but not ciliogenesis or gene expression. A dynamic phosphorylation cycle modulates normal polycystin function and ciliary distribution.
Interestingly, CK2 has been implicated in the regulation of mammalian PC-2 activity and trafficking to the plasma membrane [55, 56], and PC-1 has been shown to activate a calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway [57]. Mammalian PC-2S812 is constitutively phosphorylated in vivo. [55] This CK2 site (S812) is not conserved in C. elegans PKD-2. Walz and colleagues have shown that trafficking of PC-2 from the ER to the plasma membrane involves CK2 phosphorylation at S812 in an acidic cluster region and PACS proteins [56]. There is no data to suggest that S812 is critical to PC-2 ciliary localization, and there is no acidic cluster region found in C. elegans PKD-2. Distinct mechanisms are likely required for localizing polycystin-2 to the plasma membrane and cilium.
The PLAT domain of C. elegans LOV-1 and human PC-1 may coordinate CK2 localization and activity to a key PKD-2/PC-2 CK2 site (Figure 19.1). Upon stimulation, the polycystin mechanosensitive complex is activated [8] and phosphorylated by CK2, causing an increase in intracellular Ca2+. Elevation in intracellular Ca2+ concentration activates TAX-6/calcineurin, resulting in dephosphorylation of PKD-2/PC-2 and a return to an inactive state. Phosphorylation of PKD-2/PC-2 may serve as a mechanism for modulating channel properties; for attenuation of sensory signals; for clustering of channels, receptors, and signaling molecules; or for receptor internalization. In C. elegans, an imbalance between inactive and active PKD-2 states culminates in functional defects represented by a reduction in male mating behavior. CK2 and calcineurin have been individually implicated in other behaviors such as Drosophila circadian rhythms and mammalian learning and memory, respectively [58, 59], as well as the regulation of ion channel activity or localization [55, 56, 60–64].
Future Directions
Recent genomic and proteomic approaches have identified components required for formation and function of cilia and flagella [65–71]. In contrast to ciliogenesis, very little is known regarding “sensorigenesis,” the process by which a cilium is specialized for a particular function. Cilia often possess unique morphologies and express a distinct repertoire of sensory receptors and signaling molecules. The polycystins are required for the flow-induced mechanosensory properties of kidney cilia [8, 72, 73, 74], with defects resulting in ADPKD. How is this essential polycystin sensory complex regulated? How does the renal primary cilium sense urine flow? A genome-wide expression profiling of ray-enriched genes identified novel cwp (coexpressed with polycystins) genes whose expression patterns are identical to pkd-2 and lov-1. [75] These cell-type specific factors would regulate not only PKD-2 localization, but also contribute to the generation of sex-specific sensory behaviors. Future investigations of the genetic, cellular, molecular, and biochemical roles of these candidates and others in polycystin localization and function are warranted.
THE TRPC CHANNEL SUBFAMILY IN C. ELEGANS
The C. elegans genome encodes three TRPC subfamily members: TRP-1, TRP-2, and TRP-3 [15, 76]. TRP-1 and TRP-2 share 35–45 percent sequence identity with human TRPCs, while the homology between TRP-3 and human TRPCs is 25–30 percent. All three worm TRPCs possess the same domain structure as their mammalian counterparts. This includes three to four ankyrin repeats and a coiled-coil domain in the N-terminus, followed by six putative transmembrane domains and a TRP homology domain in the C-terminus. Although TRP-3 has been characterized in vivo (see below), functional analyses of TRP-1 and TRP-2 have not been reported [76].
TRP-3 Is Required for Sperm–Egg Interactions during Fertilization
TRP-3 protein is enriched in sperm as evidenced by antibody staining [76]. A microarray study also reveals that TRP-3 mRNA is enriched in sperm [77]. Consistent with the expression pattern, trp-3 deletion mutants are nearly sterile with an average fertility of ~5 percent of wild-type hermaphrodites [76]. This sterile phenotype is due to a defect in sperm, but not in oocytes or ovulation. Likewise, trp-3 males are also nearly sterile. Further analysis indicates that trp-3 mutant sperm are developmentally normal and motile and can make contact with oocytes in the spermatheca where sperm are stored and fertilization takes place. These results indicate that the sterile phenotype in trp-3 results from a defect in fertilization [76].
Fertilization is triggered by a series of specialized sperm–egg interactions including gamete recognition, binding, and fusion; however, the molecular mechanisms underlying sperm–egg interactions are not well understood [78]. Because of its facile genetics, C. elegans has recently emerged as a genetic model for the study of fertilization [79]. Despite their morphological differences from mammalian sperm and lack of acrosome, nematode sperm have basic functions common to all sperm, including spermatogenesis, sperm activation (spermiogenesis), motility, gamete recognition/adhesion, and gamete fusion [79]. In particular, the absence of egg coats in C. elegans oocytes greatly facilitates the analysis of fertilization [79]. Thus, during nematode fertilization, gamete binding and fusion likely follow gamete contact/recognition. Interestingly, trp-3 mutant sperm can bind to the oocyte, suggesting that the mutant sperm may be defective in sperm–egg fusion [76]. However, the lack of a robust in vitro sperm–egg binding assay in C. elegans precludes the measurement of sperm–egg binding affinity. Thus, whether trp-3 mutations impair sperm–egg binding remains an open question. Notwithstanding, these analyses demonstrate that the sterile phenotype of trp-3 mutants results from a defect of trp-3 sperm in mediating sperm–egg interactions during fertilization.
Are the functions of TRPC proteins conserved in fertilization? All seven mammalian TRPCs are found to be expressed in sperm and have been suggested to play roles in regulating sperm acrosome reaction and motility, but their roles in sperm–egg binding/fusion have not been examined [80, 81]. Specifically, mouse TRPC3 and TRPC6 are localized to the posterior part of the sperm head (the region that mediates sperm–egg binding and fusion), raising the possibility that these TRPCs might play roles in these processes [80, 81]. Nevertheless, genetic ablation of individual TRPCs in mice, such as TRPC2, TRPC4, and TRPC6, has not revealed a defect in fertility [82, 83, 84]. It is possible that the functions of mouse TRPCs in fertilization are redundant.
TRP-3 Translocates from Intracellular Vesicles to the Plasma Membrane during Sperm Activation
In spermatids, TRP-3 is localized to the membranous organelles (MOs), a class of intracellular vesicles derived from the ER/Golgi during spermatogenesis [76]. By contrast, in mature sperm (spermatozoa), TRP-3 is localized to the plasma membrane [76]. This suggests that TRP-3 undergoes protein translocation during sperm activation, a process by which spermatids develop into mature sperm. The MOs in spermatids fuse with the plasma membrane during sperm activation and become permanently attached to the plasma membranes of mature sperm. This process is also accompanied by the development of a pseudopod, resulting in the transformation of round immotile spermatids into polarized motile mature sperm (Figure 19.2). In the sterile fer-1 mutants, the MOs fail to fuse with the plasma membrane, while sperm activation proceeds [85]. In support of the translocation model, TRP-3 no longer undergoes protein translocation and is instead restricted to the MOs in fer-1 mutant sperm [76]. Translocation of TRP-3 thus provides an in vivo mechanism for the regulation of TRP-3 function. A similar phenomenon has been observed with the Drosophila TRPC member TRPL and mammalian TRPC5 in response to light stimulation and epidermal growth factor treatment, respectively [86, 87].
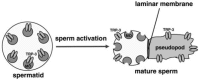
FIGURE 19.2
A schematic model showing TRP-3 translocation during sperm activation (spermiogenesis). The circles depict the MO vesicles, while the ovals represent TRP-3. Adapted from reference [76].
Questions and Future Directions
Questions remain as to how TRP-3 is activated in vivo and how TRP-3 activation leads to gamete fusion. Identifying the genes that genetically interact with trp-3 might provide insights into these questions. Several genes such as spe-9, spe-38, and spe-42, when mutated, lead to similar sperm defects to those of trp-3 mutants [88, 89, 90]. These genes all encode membrane proteins, but their potential interactions with trp-3 have not been evaluated. As with many mammalian TRPCs, expression of TRP-3 in HEK293 cells promotes both receptor- and store-operated calcium entry [76]. Because fertilization in nematodes occurs very rapidly after gamete contact [79], relatively slow kinetics of the store depletion makes it unlikely to be the physiological signal leading to TRP-3 activation in vivo. Thus, TRP-3 might function as a receptor-operated channel in sperm. If so, the binding of sperm receptors and oocyte ligands in the plasma membrane might signal the opening of TRP-3 in sperm, and the ensuing calcium influx would then trigger a series of signaling events culminating in gamete fusion.
ACKNOWLEDGMENTS
We thank Paul W. Sternberg (California Institute of Technology) for the intellectually challenging and rich scientific environment. Research in our laboratories is supported by the NIH (M.M.B. and X.Z.S.X.), PKD Foundation (M.M.B.), and University of Michigan BSSP Program (X.Z.S.X.).
REFERENCES
- 1.
- Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–60. [PubMed: 7663510]
- 2.
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–42. [PubMed: 8650545]
- 3.
- Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–98. [PubMed: 12191984]
- 4.
- Bateman A, Sandford R. The PLAT domain: a new piece in the PKD1 puzzle. Curr Biol. 1999;9:R588–90. [PubMed: 10469604]
- 5.
- Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39:585–88. [PubMed: 12925273]
- 6.
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–80. [PubMed: 12062067]
- 7.
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–16. [PubMed: 12239239]
- 8.
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. [PubMed: 12514735]
- 9.
- Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–55. [PubMed: 12495842]
- 10.
- Watnick T, Germino G. From cilia to cyst. Nat Genet. 2003;34:355–56. [PubMed: 12923538]
- 11.
- Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–36. [PubMed: 15466257]
- 12.
- Barr MM. Caenorhabditis elegans as a model to study renal development and disease: sexy cilia. J Am Soc Nephrol. 2005;16:305–12. [PubMed: 15647338]
- 13.
- Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–89. [PubMed: 10517638]
- 14.
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–46. [PubMed: 11553327]
- 15.
- Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol. 2006;68:719–36. [PubMed: 16460289]
- 16.
- Qian F, Boletta A, Bhunia AK, Xu H, Liu L, Ahrabi AK, Watnick TJ, Zhou F, Germino GG. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1–associated mutations. Proc Natl Acad Sci USA. 2002;99:16981–86. [PMC free article: PMC139255] [PubMed: 12482949]
- 17.
- Mengerink KJ, Moy GW, Vacquier VD. suREJ3, a polycystin-1 protein, is cleaved at the GPS domain and localizes to the acrosomal region of sea urchin sperm. J Biol Chem. 2002;277:943–48. [PubMed: 11696547]
- 18.
- Koulen P, Duncan RS, Liu J, Cohen NE, Yannazzo JA, McClung N, Lock-hart CL, Branden M, Buechner M. Polycystin-2 accelerates Ca2+ release from intracellular stores in Caenorhabditis elegans. Cell Calcium. 2005;37:593–601. [PubMed: 15862350]
- 19.
- Andrade YN, Fernandes J, Vazquez E, Fernandez-Fernandez JM, Arniges M, Sanchez TM, Villalon M, Valverde MA. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168:869–74. [PMC free article: PMC2171792] [PubMed: 15753126]
- 20.
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, Kernan MJ, Kim C. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. [PubMed: 12819662]
- 21.
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–69. [PMC free article: PMC6573730] [PubMed: 9334401]
- 22.
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–18. [PubMed: 12160748]
- 23.
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–66. [PMC free article: PMC6730075] [PubMed: 15483124]
- 24.
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–94. [PubMed: 11140688]
- 25.
- Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2 to cilia PKD-2. Development. 2006 [PubMed: 16943275]
- 26.
- Peden EM, Barr MM. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr Biol. 2005;15:394–404. [PubMed: 15753033]
- 27.
- Siddiqui SS. Metazoan motor models: kinesin superfamily in C. elegans. Traffic. 2002;3:20–28. [PubMed: 11872139]
- 28.
- Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–80. [PubMed: 12600311]
- 29.
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L. A standardized kinesin nomenclature. J Cell Biol. 2004;167:19–22. [PMC free article: PMC2041940] [PubMed: 15479732]
- 30.
- Lee JR, Shin H, Choi J, Ko J, Kim S, Lee HW, Kim K, Rho SH, Lee JH, Song HE, Eom SH, Kim E. An intramolecular interaction between the FHA domain and a coiled coil negatively regulates the kinesin motor KIF1A. Embo J. 2004;23:1506–15. [PMC free article: PMC391070] [PubMed: 15014437]
- 31.
- Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell. 2000;103:569–81. [PubMed: 11106728]
- 32.
- Coy DL, Hancock WO, Wagenbach M, Howard J. Kinesin’s tail domain is an inhibitory regulator of the motor domain. Nat Cell Biol. 1999;1:288–92. [PubMed: 10559941]
- 33.
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. [PubMed: 11986669]
- 34.
- Seiler S, Kirchner J, Horn C, Kallipolitou A, Woehlke G, Schliwa M. Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nat Cell Biol. 2000;2:333–38. [PubMed: 10854323]
- 35.
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. [PubMed: 12415299]
- 36.
- Kozminski KG, Forscher P, Rosenbaum JL. Three flagellar motilities in Chlamydomonas unrelated to flagellar beating. Video supplement. Cell Motil Cytoskeleton. 1998;39:347–48. [PubMed: 9556339]
- 37.
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003 [PMC free article: PMC154337] [PubMed: 12672950]
- 38.
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge polycystic kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–55. [PubMed: 10804177]
- 39.
- Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–99. [PMC free article: PMC30966] [PubMed: 11251073]
- 40.
- Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet. 2000;24:75–78. [PubMed: 10615132]
- 41.
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. [PubMed: 12859898]
- 42.
- Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–99. [PubMed: 16169494]
- 43.
- Hu J, Barr MM. ATP-2 interacts with the PLAT domain of LOV-1 and is involved in Caenorhabditis elegans polycystin signaling. Mol Biol Cell. 2005;16:458–69. [PMC free article: PMC545878] [PubMed: 15563610]
- 44.
- Hu J, Bae Y-K, Knobel KM, Barr MM. Casein kinase II and calcineurin modulate TRPP function and ciliary localization. Mol Biol Cell. 2006;17:2200–11. [PMC free article: PMC1446073] [PubMed: 16481400]
- 45.
- Tsang WY, Sayles LC, Grad LI, Pilgrim DB, Lemire BD. Mitochondrial respiratory chain deficiency in Caenorhabditis elegans results in developmental arrest and increased life span. J Biol Chem. 2001;276:32240–46. [PubMed: 11410594]
- 46.
- Boyer PD. The ATP synthase—a splendid molecular machine. Annu Rev Biochem. 1997;66:717–49. [PubMed: 9242922]
- 47.
- Das B, Mondragon MO, Sadeghian M, Hatcher VB, Norin AJ. A novel ligand in lymphocyte-mediated cytotoxicity: expression of the beta subunit of H+ transporting ATP synthase on the surface of tumor cell lines. J Exp Med. 1994;180:273–81. [PMC free article: PMC2191542] [PubMed: 8006588]
- 48.
- Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA. 2001;98:6656–61. [PMC free article: PMC34409] [PubMed: 11381144]
- 49.
- Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA. 1999;96:2811–16. [PMC free article: PMC15851] [PubMed: 10077593]
- 50.
- Chang SY, Park SG, Kim S, Kang CY. Interaction of the C-terminal domain of p43 and the alpha subunit of ATP synthase. Its functional implication in endothelial cell proliferation. J Biol Chem. 2002;277:8388–94. [PubMed: 11741979]
- 51.
- Arakaki N, Nagao T, Niki R, Toyofuku A, Tanaka H, Kuramoto Y, Emoto Y, Shibata H, Magota K, Higuti T. Possible role of cell surface H(+)-ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res. 2003;1:931–39. [PubMed: 14638865]
- 52.
- Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezon E, Champagne E, Pineau T, Georgeaud V, Walker JE, Terce F, Collet X, Perret B, Barbaras R. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. [PubMed: 12511957]
- 53.
- Bae TJ, Kim MS, Kim JW, Kim BW, Choo HJ, Lee JW, Kim KB, Lee CS, Kim JH, Chang SY, Kang CY, Lee SW, Ko YG. Lipid raft proteome reveals ATP synthase complex in the cell surface. Proteomics. 2004;4:3536–48. [PubMed: 15378739]
- 54.
- Li N, Shaw AR, Zhang N, Mak A, Li L. Lipid raft proteomics: Analysis of in-solution digest of sodium dodecyl sulfate-solubilized lipid raft proteins by liquid chromatography-matrix-assisted laser desorption/ionization tandem mass spectrometry. Proteomics. 2004;4:3156–66. [PubMed: 15378691]
- 55.
- Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem. 2004;279:19987–95. [PubMed: 14742446]
- 56.
- Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. Embo J. 2005;24:705–16. [PMC free article: PMC549624] [PubMed: 15692563]
- 57.
- Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, Wallace DP, Hempson SJ, Calvet JP. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem. 2004;279:55455–64. [PubMed: 15466861]
- 58.
- Blau J. A new role for an old kinase: CK2 and the circadian clock. Nat Neurosci. 2003;6:208–10. [PubMed: 12601377]
- 59.
- Lee JI, Ahnn J. Calcineurin in animal behavior. Mol Cells. 2004;17:390–96. [PubMed: 15232211]
- 60.
- Kuhara A, Inada H, Katsura I, Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;33:751–63. [PubMed: 11879652]
- 61.
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–18. [PubMed: 15195093]
- 62.
- Wu ZZ, Chen SR, Pan HL. TRPV1 activation downregulates voltage-gated calcium channels through calcium-dependant calcineurin in sensory neurons. J Biol Chem. 2005;280:18142–51. [PubMed: 15746091]
- 63.
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–32. [PubMed: 15691846]
- 64.
- Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–58. [PubMed: 15363395]
- 65.
- Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002;1:451–65. [PubMed: 12169685]
- 66.
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–39. [PubMed: 15137945]
- 67.
- Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, McKay SJ, Huang P, Swoboda P, Jones SJ, Marra MA, Baillie DL, Moerman DG, Shaham S, Leroux MR. Functional genomics of the cilium, a sensory organelle. Curr Biol. 2005;15:935–41. [PubMed: 15916950]
- 68.
- Efimenko E, Bubb K, Mak HY, Holzman T, Leroux MR, Ruvkun G, Thomas JH, Swoboda P. Analysis of xbx genes in C. elegans. Development. 2005;132:1923–34. [PubMed: 15790967]
- 69.
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–52. [PubMed: 15137946]
- 70.
- Keller LC, Romijn EP, Zamora I, Yates JR, 3rd &, Marshall WF. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr Biol. 2005;15:1090–98. [PubMed: 15964273]
- 71.
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–13. [PMC free article: PMC2171396] [PubMed: 15998802]
- 72.
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. [PubMed: 11687880]
- 73.
- Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. [PubMed: 12532278]
- 74.
- Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–20. [PubMed: 12920399]
- 75.
- Portman DS, Emmons SW. Identification of C. elegans sensory ray genes using whole-genome expression profiling. Dev Biol. 2004;270:499–512. [PubMed: 15183729]
- 76.
- Xu XZ, Sternberg PW. A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell. 2003;114:285–97. [PubMed: 12914694]
- 77.
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJ, Davis EB, Scherer S, Ward S, Kim SK. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6:605–16. [PubMed: 11030340]
- 78.
- Jungnickel MK, Sutton KA, Florman HM. In the beginning: lessons from fertilization in mice and worms. Cell. 2003;114:401–14. [PubMed: 12941269]
- 79.
- Geldziler B, Kadandale P, Singson A. Molecular genetic approaches to studying fertilization in model systems. Reproduction. 2004;127:409–16. [PubMed: 15047931]
- 80.
- Sutton KA, Jungnickel MK, Wang Y, Cullen K, Lambert S, Florman HM. Enkurin is a novel calmodulin and TRPC channel binding protein in sperm. Dev Biol. 2004;274:426–35. [PubMed: 15385169]
- 81.
- Castellano LE, Trevino CL, Rodriguez D, Serrano CJ, Pacheco J, Tsutsumi V, Felix R, Darszon A. Transient receptor potential (TRPC) channels in human sperm: expression, cellular localization and involvement in the regulation of flagellar motility. FEBS Lett. 2003;541:69–74. [PubMed: 12706821]
- 82.
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasore-laxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–27. [PubMed: 11175743]
- 83.
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–81. [PMC free article: PMC122956] [PubMed: 11972034]
- 84.
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–500. [PubMed: 11823606]
- 85.
- Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci. 1997;110(Pt 9):1073–81. [PubMed: 9175703]
- 86.
- Bahner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. [PubMed: 11931743]
- 87.
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–20. [PubMed: 15258588]
- 88.
- Singson A, Mercer KB, L’Hernault SW. The C. elegans spe-9 gene encodes a sperm transmembrane protein that contains EGF-like repeats and is required for fertilization. Cell. 1998;93:71–79. [PubMed: 9546393]
- 89.
- Chatterjee I, Richmond A, Putiri E, Shakes DC, Singson A. The Caenorhabditis elegans spe-38 gene encodes a novel four-pass integral membrane protein required for sperm function at fertilization. Development. 2005;132:2795–808. [PubMed: 15930110]
- 90.
- Kroft TL, Gleason EJ, L’Hernault SW. The spe-42 gene is required for sperm-egg interactions during C. elegans fertilization and encodes a sperm-specific transmembrane protein. Dev Biol. 2005;286:169–81. [PubMed: 16120437]
- Review Polycystins: polymodal receptor/ion-channel cellular sensors.[Pflugers Arch. 2005]Review Polycystins: polymodal receptor/ion-channel cellular sensors.Delmas P. Pflugers Arch. 2005 Oct; 451(1):264-76. Epub 2005 May 12.
- Review Polycystic Kidney Disease.[Compr Physiol. 2017]Review Polycystic Kidney Disease.Ghata J, Cowley BD Jr. Compr Physiol. 2017 Jun 18; 7(3):945-975. Epub 2017 Jun 18.
- Review [Pathophysiology, epidemiology, clinical presentation, diagnosis and treatment options for autosomal dominant polycystic kidney disease].[Nephrol Ther. 2015]Review [Pathophysiology, epidemiology, clinical presentation, diagnosis and treatment options for autosomal dominant polycystic kidney disease].Noël N, Rieu P. Nephrol Ther. 2015 Jul; 11(4):213-25. Epub 2015 Jun 22.
- Genes homologous to the autosomal dominant polycystic kidney disease genes (PKD1 and PKD2).[Eur J Hum Genet. 1999]Genes homologous to the autosomal dominant polycystic kidney disease genes (PKD1 and PKD2).Veldhuisen B, Spruit L, Dauwerse HG, Breuning MH, Peters DJ. Eur J Hum Genet. 1999 Dec; 7(8):860-72.
- ATP-2 interacts with the PLAT domain of LOV-1 and is involved in Caenorhabditis elegans polycystin signaling.[Mol Biol Cell. 2005]ATP-2 interacts with the PLAT domain of LOV-1 and is involved in Caenorhabditis elegans polycystin signaling.Hu J, Barr MM. Mol Biol Cell. 2005 Feb; 16(2):458-69. Epub 2004 Nov 24.
- TRP Channel Functioning in Mating and Fertilization - TRP Ion Channel Function i...TRP Channel Functioning in Mating and Fertilization - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
- Voltage and Temperature Gating of ThermoTRP Channels - TRP Ion Channel Function ...Voltage and Temperature Gating of ThermoTRP Channels - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
Your browsing activity is empty.
Activity recording is turned off.
See more...