NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.

TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades.
Show detailsINTRODUCTION
The TRPV subfamily has had increasing attention since some channels in this group have been shown to be sensitive to a broad range of environmental stimuli, including heat, osmosensitivity, and mechanical stress. In addition, TRPV proteins are widely expressed in a range of cell types in lower and higher organisms. Although some TRPVs were originally found in the sensory system, ubiquitous expression in the whole body suggests that they play important roles in both sensory and nonsensory transduction functions. All mammalian homologues of TRPVs are calcium-permeable channels, with TRPV1–4 characterized as moderately calcium-selective cationic channels (Nilius, Voets et al. 2005; O'Neil and Brown 2003; Benham, Davis et al. 2002). This calcium permeability is physiologically important because Ca2+ has an obligatory role in regulating diverse cellular functions (e.g., fertilization, muscle contraction, exocytosis, and so on). There is increasing evidence that TRPV1–4 are sensitive to physical stimuli such as osmolarity, stretching, and shear stress (Liedtke and Kim 2005; O'Neil and Heller 2005). Whereas TRPV4 appears to be crucial for some relevant forms of cellular mechanosensitivity, the activation of TRPVs by mechanostress has not been fully elucidated for all channels of this group (O'Neil and Heller 2005).
Cellular responses to stretch or shear stimuli by blood flow are one of the key elements in muscle tone regulation (Figure 28.1A). In cell-attached and inside-out patch-recording modes, membrane stretch applied through the recording pipette activates nonselective cationic channels in vascular smooth muscles (Kirber et al. 1988; Davis et al. 1992; Ohya et al. 1998; see review: Beech et al. 2004). The unitary conductances range from 8 to 64 pS for monovalent cations, and a cationic channel blocker, Gd3+, is effective to block the channel. In whole-cell recordings, application of longitudinal cell stretch or cell swelling by pressure on the patch pipette or hypotonic bath solution also evokes Ca2+-permeable cationic currents in vascular myocytes (Davis et al. 1992). Additionally, in cardiac atrial and ventricular myocytes, nonselective cationic channels are activated by cell swelling as well as membrane stretch (Clemo and Baumgarten 1997; Zhang et al. 2000; Kamkin et al. 2003). Similar nonselective cationic channels sensitive to mechanical stimuli including shear stress are also identified in vascular endothelial cells (Lansman et al. 1987; Oike et al. 1994; see review: Nilius and Droogmans 2001). Although extensive studies to identify a molecular candidate of these mechanosensitive channels have been performed, information is still limited (Kanzaki, Nagasawa et al. 1999; Gillespie and Walker 2001). Nevertheless, the mechanosensitive nature of the channels seems to be conserved in higher organisms for some TRP channels, and it is likely that TRPC1, TRPC6, TRPV2, TRPM4, TRPA1, TRPP1, and possibly TRPV4 are potential candidates for the mechanosensitive channels in various native organs (Maroto et al. 2005; Welsh et al. 2002; Muraki et al. 2003; Iwata et al. 2003; Earley, Waldron et al. 2004; Corey et al. 2004; Nauli et al. 2003; Liedtke 2005). In this section, we focus on expression, function, and mechanosensitivity of TRPV2 (a member of the vanilloid receptor TRP subfamily) in circulatory organs such as vascular smooth muscles, cardiac muscles, and the endothelium. Heat activation and trafficking mechanisms of TRPV2 and expression of TRPV2 in neuronal organs will be discussed in another section (Caterina et al. 1999; Kanzaki, Zhang et al. 1999; Boels et al. 2001; Barnhill et al. 2004; Benham, Gunthorpe et al. 2003).
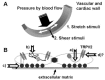
FIGURE 28.1
(A) Schematic diagram showing mechanical stimuli to which vascular and cardiac walls are exposed. (B) Potential converging pathways involved in activation of TRPV2 by mechanical stimuli: (a) involvement of ankyrin repeats in mechanoactivation of TRPV2; (more...)
Expression of TRPV2 in Circulatory Organs
TRPV2 is mainly expressed in a subpopulation of medium to large sensory neurons and is also distributed in the brain and spinal cord (Caterina et al. 1999; Bender et al. 2005). In nonsensory organs including vascular and cardiac myocytes, TRPV2 is distributed as mRNA and protein (Kanzaki, Zhang et al. 1999; Muraki et al. 2003; (Iwata et al. 2003; review: O'Neil and Brown 2003). The mRNA expression of TRPV2 is also detected in human pulmonary (Fantozzi et al. 2003) and umbilical vein endothelial cells (Figure 28.2E). Based on mRNA expression of TRPV2 in mice, it is speculated that TRPV2 is widely expressed in arterial myocytes, which can be influenced by a broad range of blood pressures (Muraki et al. 2003). Although intracellular localization of the protein was evident, some growth factors localized TRPV2 to the plasma membrane (Kanzaki, Zhang et al. 1999; Boels et al. 2001; Iwata et al. 2003). The translocation of TRPV2 was also observed when myotubes were subjected to a cyclic stretch (Iwata et al. 2003). The mechanisms of this translocation of TRPV2 are not, however, clear because PI3 kinase inhibitors are effective in some cells and not in others. Nevertheless, localization of the TRPV2 protein to the plasma membrane without treatment with growth factors is found in rat adult dorsal root ganglions, cerebral cortex (Liapi and Wood 2005), and mouse arterial myocytes (Muraki et al. 2003). Glycosylation of TRPV2 has been suggested (~95 KDa) (Kanzaki, Zhang et al. 1999), but the apparent molecular mass of TRPV2 in cardiac and aortic smooth muscles is 85–90 KDa (Iwata et al. 2003; Muraki and Imaizumi, unpublished), close to the predicted mass of full-length TRPV2 (~86 KDa).
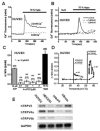
FIGURE 28.2
Activation of human TRPV2-like channel currents in human umbilical vein endothelial cells (HUVEC). Activation of the channel was monitored as an increase in Ca2+ fluorescence signal in the cell. (A) In the perfusion of a bathing solution with Ca2+ (a (more...)
Mechanosensation of TRPV2 in Circulatory Organs
In vascular smooth muscles and cardiac myocytes, striking new evidence has emerged for TRPV2’s role (Muraki et al. 2003; Iwata et al. 2003) In mouse aortic mycoytes, cell swelling caused by hypotonic solution activated a nonselective cationic channel current (NSCC) and elevated [Ca2+]i. These responses were not affected by diltiazem, a Ca2+ antagonist, and caffeine, a Ca2+ releaser from the sarcoplasmic reticulum, whereas responses were effectively inhibited by ruthenium red, a TRPV blocker. Elevation of [Ca2+]i by the hypotonic stimulation but not activation of NSCC was abolished by the removal of external Ca2+, suggesting that Ca2+ entry through NSCC has an obligatory role in elevation of [Ca2+]i. Although TRPV2 and TRPV4, but not TRPV3, were present in mouse aortas as mRNA, 4αPDD, a potent activator of TRPV4, was not effective to aortic myocytes. Significant immunoreactivity to mouse TRPV2 protein was detected in mouse aortic, mesenteric, and basilar arterial myocytes. Treatment of mouse aortas with TRPV2 antisense oligonucleotides suppressed hypotonic stimulation–induced activation of NSCC and elevation of [Ca2+]i as well as expression of TRPV2 protein. Although further evidence should be gathered, these results suggest that TRPV2 in vascular myocytes is an essential component of NSCC activated by membrane stretch and cell swelling.
On the other hand, it was evident that TRPV2 expression was increased in cultured myotubes prepared from δ-sarcoglycan-deficient BIO14.6 hamster and mdx mice (a mouse model of dystrophin-deficient Duchenne muscular dystrophy). Moreover, a cyclic stretch as well as growth factors translocated TRPV2 to the plasma membrane. In BIO14.6 myotubes, 45Ca2+ uptake was greater compared with the controls and significantly inhibited by RuR. Myocyte damage measured by releasing creatine phosphokinase (CK) from myotube cytoplasm was increased in BIO14.6 myotubes to which a cyclic cell stretch was applied. In the presence of RuR, stretch-induced myocyte damage was significantly suppressed. Treatment of BIO14.6 myotubes with TRPV2 antisense oligonucleotides reduced the TRPV2 protein expression and elevation of [Ca2+]i and CK release by cyclic cell stretch, strongly indicating that TRPV2 has an obligatory role in cell abnormalities. In transgenic mice overexpressing TRPV2 in cardiac myocytes, prominent anasarca was observed at the age of ~180 days. Furthermore, the mice that died peripartum developed globally enlarged hearts. The morphological changes in the transgenic mice were similar to those observed in damaged cardiac muscles with Ca2+ overload. These results demonstrate a close link between TRPV2 expression levels and myocyte damage/loss of in vivo heart function.
In Chinese hamster ovary K1 (CHO-K1) cells transfected with mouse TRPV2 (mTRPV2-CHO), membrane stretch through the recording pipette and cell swelling caused by hypotonic solution activated NSCC, while not in control of CHO-K1. Moreover, stretch of mTRPV2–CHO cultured on an elastic silicone membrane elevated their [Ca2+]i (Muraki et al. 2003). Consistently, 45Ca2+ uptake in mTRPV2–CHO was significantly greater than in nontransfected cells, and the uptake was further enhanced by cyclic stretch (Iwata et al. 2003). Taken together, these results provide us with new insight that TRPV2 is a mechanosensitive channel.
TRPV4 also appears to display mechnosensitive properties, at least to hypotonic stress and possibly to fluid flow or shear stress. Because TRPV4 has been highly expressed in mouse aortic endothelial cells, TRPV4 is a potential candidate as a mechanosensor to shear stress/fluid flow in vascular endothelial cells (Watanabe, Vriens et al. 2002; Vriens, Watanabe et al. 2004). However, it has been reported that TRPV2 is distributed in vascular endothelial cells (Fantozzi et al. 2003; see also Figure 28.2E), suggesting that TRPV2 also has a functional role in sensing mechanical stimuli in the endothelium. As shown in Figure 28.2A, application of 70 percent hypotonic solution to HUVECS elevated [Ca2+]i in 2.2 mM Ca2+-containing bathing solution, but not in the solution without Ca2+. Hypoosmotic solution–induced elevation of [Ca2+]i was effectively inhibited by 1 and 3 μM RuR, strongly suggesting that TRPV-like channels are involved in the response. Exposure of HUVECs to 4αPDD, a TRPV4 activator, in a concentration range between 0.1 and 3 μM caused a slight increase in [Ca2+]i. In response to a mild heat stimulus (20–46°C) HUVECS exhibited a modest rise in [Ca2+]i (0.13 ± 0.01, n = 21, Figure 28.2D, open squares). In contrast, a higher heat stimulus (20–60°C, Figure 28.2D, closed circles) caused a significantly larger rise once the temperature exceeded ~55°C. Although further extensive studies are required, TRPV2 as well as TRPV4, both of which are expressed in the vascular endothelium, are potential mechanosensors in the vascular endothelium.
Potential Activation Mechanisms of TRPV2 by Mechanical Stimuli
Our studies and other additional data strongly suggest that TRPV2 may be a mechanosensor in circulatory organs. However, the mechanisms involved in opening TRPV2 by membrane stretch or hypoosmotic cell swelling have not been determined. Moreover, at present, the mechanisms in opening TRPV4 by mechanostress are not elucidated. Deletion of ankyrin repeats, which are present in the N-terminal region of TRPVs, abolished heat activation of TRPV4 (Watanabe, Vriens et al. 2002). Because the ankyrin repeats interact with certain cytoskeletal proteins, this region of TRPV2 might also be important for acceptance of applied mechanical stimuli (Figure 28.1B[a]). In addition, it is possible that cell swelling or membrane stretch produces a certain endogenous ligand that activates TRPV2 (Figure 28.1B[b]). Some fatty acids released by cell stretching modify channel activity. Metabolites of arachidonic acid or diacylglycerol might affect TRPV2 as they do TRPV4 and TRPC6 (Watanabe, Vriens et al. 2003; Slish et al. 2002). A micro-domain composed with channels, lipids, receptors, and/or enzymes could also be an effector of local change of membrane tension by membrane stretch, cell swelling, or shear stress (Figure 28.1B[c]). It has been proposed that adhesion molecules and integrins play important roles in endothelial mechanosensing in addition to their involvement in cell attachment and migration (Shyy and Chien 2002). Also TRPV4 has a tight interaction with ryanodine receptors as well as K+ channels, all of which are involved in regulating vascular tones (Earley, Heppner et al. 2005). Further studies may provide a new line of evidence of a tight interaction of TRPV2 with adhesion molecules, cytoskeletal proteins, or signal transduction units. This is important when compared with the delay of opening of the transduction channels after mechanical stimuli in fly mechanoreceptors (submillisecond response time of mechanogated channels in the fly; Earley, Heppner et al. 2005; review: Gillespie and Walker 2001): those of TRPV2 and TRPV4 after physical stimuli seem to be relatively slow (not determined precisely, at least ~1 second), indicating that these channels are mechanosensitive but not mechanogating (Figure 28.1B[d]).
CONCLUSIONS
TRPVs including TRPV2 display multimodal activation by diverse chemical and physical stimuli and function as molecular integrators. The available evidence demonstrates that TRPV2 appears to function as a molecular sensor of heat and mechanical stimuli. In circulatory organs, TRPV2 may regulate blood pressure and cell degeneration under some pathophysiological conditions. Other roles of TRPV2 continue to be explored in an attempt to define the role of translocation of TRPV2 by growth factors. Furthermore, mechanisms of activation of TRPV2 by mechanical stimuli and interactions of TRPV2 with functional molecules should be defined in the future.
REFERENCES
- Barnhill JC, Stokes AJ, et al. RGA protein associates with a TRPV ion channel during biosynthesis and trafficking. J Cell Biochem. 2004;91(4):808–20. [PubMed: 14991772]
- Beech DJ, Muraki K, et al. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559(Pt. 3):685–706. [PMC free article: PMC1665181] [PubMed: 15272031]
- Bender FL, Mederos YSM, et al. The temperature-sensitive ion channel TRPV2 is endogenously expressed and functional in the primary sensory cell line F-11. Cell Physiol Biochem. 2005;15:1–4. 183–94. [PubMed: 15665528]
- Benham CD, Davis JB, et al. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002;42(7):873–88. [PubMed: 12069898]
- Benham CD, Gunthorpe MJ, et al. TRPV channels as temperature sensors. Cell Calcium. 2003;33:5–6. 479–87. [PubMed: 12765693]
- Boels K, Glassmeier G, et al. The neuropeptide head activator induces activation and translocation of the growth-factor-regulated Ca(2+)-permeable channel GRC. J Cell Sci. 2001;114(Pt. 20):3599–606. [PubMed: 11707512]
- Caterina MJ, Rosen TA, et al. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–41. [PubMed: 10201375]
- Clemo HF, Baumgarten CM. Swelling-activated Gd3+-sensitive cation current and cell volume regulation in rabbit ventricular myocytes. J Gen Physiol. 1997;110(3):297–312. [PMC free article: PMC2229368] [PubMed: 9276755]
- Corey DP, García-Anoveros J, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432(7018):723–30. [PubMed: 15483558]
- Davis MJ, Donovitz JA, et al. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992;262(4 Pt. 1):C1083–88. [PubMed: 1373561]
- Earley S, Heppner TJ, et al. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005 [PubMed: 16269659]
- Earley S, Waldron BJ, et al. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95(9):922–29. [PubMed: 15472118]
- Fantozzi I, Zhang S, et al. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(6):L1233–45. [PubMed: 12909593]
- Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413(6852):194–202. [PubMed: 11557988]
- Iwata Y, Katanosaka Y, et al. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161(5):957–67. [PMC free article: PMC2172975] [PubMed: 12796481]
- Kamkin A, Kiseleva I, et al. Characterization of stretch-activated ion currents in isolated atrial myocytes from human hearts. Pflügers Arch. 2003;446(3):339–46. [PubMed: 12799902]
- Kanzaki M, Nagasawa M, et al. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science. 1999;285(5429):882–86. [PubMed: 10436155]
- Kanzaki M, Zhang YQ, et al. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1(3):165–70. [PubMed: 10559903]
- Kirber MT, Walsh JV Jr, et al. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflügers Arch. 1988;412(4):339–45. [PubMed: 2459658]
- Lansman JB, Hallam TJ, et al. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers. Nature. 1987;325(6107):811–13. [PubMed: 2434860]
- Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci. 2005;22(4):825–34. [PubMed: 16115206]
- Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol. 2005;567(Pt 1):53–58. [PMC free article: PMC1474158] [PubMed: 15961428]
- Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci. 2005;62(24):2985–3001. [PubMed: 16314934]
- Maroto R, Raso A, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7(2):179–85. [PubMed: 15665854]
- Muraki K, Iwata Y, et al. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93(9):829–38. [PubMed: 14512441]
- Nauli SM, Alenghat FJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–37. [PubMed: 12514735]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81(4):1415–59. [PubMed: 11581493]
- Nilius B, Voets T, et al. TRP channels in disease. Sci STKE. 2005;2005:295–re8. [PubMed: 16077087]
- Ohya Y, Adachi N, et al. Stretch-activated channels in arterial smooth muscle of genetic hypertensive rats. Hypertension. 1998;31(1 Pt. 2):254–58. [PubMed: 9453312]
- Oike M, Droogmans G, et al. Mechanosensitive Ca2+ transients in endothelial cells from human umbilical vein. Proc Natl Acad Sci USA. 1994;91(8):2940–44. [PMC free article: PMC43490] [PubMed: 8159684]
- O'Neil RG, Brown RC. The vanilloid receptor family of calcium-permeable channels: molecular integrators of microenvironmental stimuli. News Physiol Sci. 2003;18:226–31. [PubMed: 14614154]
- O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflügers Arch. 2005;451(1):193–203. [PubMed: 15909178]
- Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91(9):769–75. [PubMed: 12411390]
- Slish DF, Welsh DG, et al. Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283(6):H2196–201. [PubMed: 12388226]
- Vriens J, Watanabe H, et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101(1):396–401. [PMC free article: PMC314196] [PubMed: 14691263]
- Watanabe H, Vriens J, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–38. [PubMed: 12879072]
- Watanabe H, Vriens J, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–51. [PubMed: 12354759]
- Welsh DG, Morielli AD, et al. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90(3):248–50. [PubMed: 11861411]
- Zhang YH, Youm JB, et al. Stretch-activated and background non-selective cation channels in rat atrial myocytes. J Physiol. 2000;523(Pt. 3):607–19. [PMC free article: PMC2269835] [PubMed: 10718741]
- Review Osmomechanical-Sensitive TRPV Channels in Mammals.[Neurobiology of TRP Channels. ...]Review Osmomechanical-Sensitive TRPV Channels in Mammals.Moore C, Liedtke WB. Neurobiology of TRP Channels. 2017
- TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes.[Circ Res. 2003]TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. Circ Res. 2003 Oct 31; 93(9):829-38. Epub 2003 Sep 25.
- Review The mechanosensitive nature of TRPV channels.[Pflugers Arch. 2005]Review The mechanosensitive nature of TRPV channels.O'Neil RG, Heller S. Pflugers Arch. 2005 Oct; 451(1):193-203. Epub 2005 May 21.
- Hypotonic-induced stretching of plasma membrane activates transient receptor potential vanilloid channels and sodium-calcium exchangers in mouse odontoblasts.[J Endod. 2013]Hypotonic-induced stretching of plasma membrane activates transient receptor potential vanilloid channels and sodium-calcium exchangers in mouse odontoblasts.Sato M, Sobhan U, Tsumura M, Kuroda H, Soya M, Masamura A, Nishiyama A, Katakura A, Ichinohe T, Tazaki M, et al. J Endod. 2013 Jun; 39(6):779-87. Epub 2013 Mar 15.
- Review Determining the Crystal Structure of TRPV6.[Calcium Entry Channels in Non-...]Review Determining the Crystal Structure of TRPV6.Saotome K, Singh AK, Sobolevsky AI. Calcium Entry Channels in Non-Excitable Cells. 2018
- A New Insight into the Function of TRPV2 in Circulatory Organs - TRP Ion Channel...A New Insight into the Function of TRPV2 in Circulatory Organs - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
- Role of TRPV4 in the Mechanotransduction of Shear Stress in Endothelial Cells - ...Role of TRPV4 in the Mechanotransduction of Shear Stress in Endothelial Cells - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
- The TRPC Family of Ion Channels: Relation to the TRP Superfamily and Role in Rec...The TRPC Family of Ion Channels: Relation to the TRP Superfamily and Role in Receptor- and Store-Operated Calcium Entry - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
Your browsing activity is empty.
Activity recording is turned off.
See more...