NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.

TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades.
Show detailsAbstract
The expression of the TRPC2 channel in rodents appears largely restricted to neurons of the vomeronasal organ (VNO) and is detected at lower levels in few other tissues. The characteristics of TRPC2 expression, as well as the availability of the TRPC2 gene as a tool for genetic manipulation, has led to significant advances in understanding the biology of the vomeronasal organ and pheromone detection in rodents and across evolution.
INTRODUCTION
In order to ensure reproductive success, animals have evolved sensory and behavioral strategies to identify, respond to, and attract suitable mating partners. In many vertebrate and invertebrate species, chemical cues called pheromones carry the species- and gender-specific information required for mating. Detection of pheromones triggers the activation of likely hard-wired brain circuits, which in turn lead to stereotyped changes in the behavior and endocrine state of the animal. Molecular and physiological approaches have recently explored how the information provided by pheromones is detected in the nose and how the activation of defined sets of pheromone receptors is translated into specific behavioral and physiological outputs [9]. Molecular studies of chemosensory systems have made tremendous progress and opened new avenues of research with the discovery of receptor gene families and essential components of the signal transduction cascade in the main olfactory epithelium (MOE) and the vomeronasal organ (VNO).
THE MOLECULAR BIOLOGY OF PHEROMONE SENSING IN MAMMALS
Surgical removal of olfactory and vomeronasal structures has traditionally assigned the role of the main olfactory system to the sense of smell, resulting in the detection of a large variety of volatile odorants [1, 5], while the vomeronasal system is thought to carry most of the detection of gender- and species-specific cues involved in the control of mating and aggressive behavior [11]. In the nasal cavity, volatile chemical cues interact with olfactory receptors (ORs) expressed in the MOE, while nonvolatile signals carried in the nasal mucus are actively pumped into the lumen of the VNO, where they can activate vomeronasal receptors (VRs) (Figure 3.1A and B). VNO neurons send their axonal projections to a distinct area of the dorsal telecephalon, the accessory olfactory bulb (AOB). Unlike mitral cells of the main olfactory bulb (MOB) that mainly innervate cortical areas, AOB mitral cells bypass the cerebral cortex and project directly to the medial amygdala, which, in turn, innervates nuclei of the hypothalamus involved in aggression and reproduction. Sensory neurons located in the apical layer of the VNO neuroepithelium each express a single member of the V1R family of VNO receptors and project to the anterior AOB, while neurons of the basal half of the neuroepithelium express receptors of the V2R gene family and send axons to the posterior AOB (Figure 3.1B) (reviewed in reference [9]). The two murine VR gene families include 150–200 functional genes each and although both belong to the G-protein coupled receptor (GPCR) gene superfamily, they are phylogenetically unrelated to each other and to the ORs. Recent studies indicate thatV1R-expressing neurons respond to low molecular weight organic molecules [11, 18 44], while V2R-expressing cells respond to peptides [15 17]. The V2Rs require the specific interaction with MHC Class Ib molecules M10s in order to be expressed in vitro and in vivo [24].
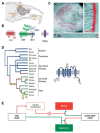
FIGURE 3.1
(A) The vomeronasal system: pheromone signals are detected by neurons of the vomeronasal organ (VNO) in the ventral nasal septum. VNO axons project to the accessory olfactory bulb (AOB), which in turn connects to nuclei of the vomeronasal amygdala in (more...)
Neurons in the main olfactory system and vomeronasal system utilize distinct signaling components to transduce chemical stimuli. The main components of the olfactory transduction cascade—Golf, ACIII, and OCNC1—are not expressed in vomeronasal sensory neurons [2], suggesting the existence of a different pathway for sensory transduction in the VNO. Because of similarity with phototransduction in the Drosophila eye [30] and chemosensation in C. elegans [8], it was suggested that a TRP homologue might be involved in vertebrate pheromone signal transduction [20]. Indeed, TRP2/TRPC2, a cation channel belonging to the transient receptor potential (TRP) family of ion channels, appears highly expressed in VNO neurons and specifically localized to the microvilli, the proposed site of pheromone transduction (Figure 3.1C), suggesting a direct role in the VNO signaling cascade.
TRPC2 PROTEIN STRUCTURE AND BINDING PARTNERS
TRPC2 was first identified as a pseudogene in the database of human-expressed sequence tags (ESTs), and its presence in other genomes was confirmed through sequencing of partial clones from mice and cows [38, 39, 43]. A full-length sequence of rodent TRPC2 was subsequently identified simultaneously by two groups of researchers [20, 36]. The TRPC2 cDNA isolated from the rat vomeronasal organ (rTRPC2) by Liman et al. encodes a protein of 885 amino acids [20], and its mouse orthologue (mTRPC2β) encodes a protein of 890 amino acids that is 96 percent similar [12]. Two long forms of mouse TRPC2 were identified by Vannier and colleagues (clones 14 and 17 or mTRPC2a and mTRPC2b) [36]; the predicted proteins of 1,172 and 1,072 amino acids, respectively, differ from the protein expressed in the VNO in that they contain extended N-termini. Subsequently a shorter variant (886 amino acids) of mTRPC2 (clone α) [12] (see also reference [40]) and three variants of mTRPC2 that encode proteins consisting of only portions of the extended N-terminal region from clones 14 and 17 were reported [7, 40]. The variant expressed in the VNO has been confirmed by isolating the full cDNA from multiple cDNA libraries and through comparison of the size of the encoded protein with that of the native protein [20]; moreover, the functionality of TRPC2 in the VNO has been clearly demonstrated (see below). For these reasons, unless otherwise noted, “TRPC2” will refer to the VNO splice variant. The possibility that other splice variants exist and have distinct functional roles is intriguing and merits additional study.
The highest expression of TRPC2 is in sensory neurons of the vomeronasal organ, where it represents as much as 1/10,000 of the mRNA (Figure 3.1C) [20]. In these cells, the TRPC2 protein is remarkably localized to the sensory microvilli [20, 28], actin-based structures that are specialized for the detection of chemical signals. Outside the vomeronasal organ, TRPC2 expression is low [12, 20]; it has been detected in the main olfactory epithelium [20], testes, heart, brain, liver, spleen, and erythrocytes [12, 39]. Notably, these tissues do not express the VNO form of TRPC2 (mTRPC2β), suggesting that they represent priming from alternate promoters. In sperm, TRPC2 protein has been detected in the acrosome region, suggesting a possible role in fertilization [14].
The TRPC2 protein is structurally related to other TRP family members and is predicted to have, like these proteins and more distantly related voltage-gated K+ channels, six transmembrane domains and the ability to tetramerize. TRPC2 does not heteromultimerize with other TRPC channel subunits [13] (but see reference [6]), and thus native channels may either form homotetramers or may multimerize with more distantly related channel subunits. The predicted cytoplasmic N-terminus contains ankryn repeat domains, and the cytoplasmic C-terminus contains a coiled coil domain, both of which might mediate protein–protein interactions [20, 36]. Interaction between TRPC2 and the IP3 receptor has been demonstrated [4, 35], and a conserved motif in the C-terminus of TRPC2 has been identified that binds the IP3 receptor and calmodulin [34]. Calmodulin has been shown to interact with the N-terminus of the long form of TRPC2 [40]. A novel protein named enkurin was identified from a yeast-two hybrid screen with the N-terminus of TRPC2, and the function of this protein is as yet unknown [33]. Given the difficulty in studying the functional properties of TRPC2 (see below), the significance of these protein interactions and of the domain structure of TRPC2 is not known.
TRPC2 Mechanism of Activation
Understanding the mechanism of activation of TRPC2 is critical to understanding its role in pheromone detection and other physiological processes. Despite the importance of this problem, it has remained refractory to study and there is presently no single agreed-upon mechanism for its activation. In heterologous cells, mTRPC2 (splice variants A and B) was reported to be activated by depletion of Ca2+ stores by thapsigargin and to function as a capacitative Ca2+ entry channel [10, 35, 36]. Other experiments, however, showed that in heterologous cells TRPC2 (splice variants A, α, and β) is largely trapped in the endoplasmic reticulum, impeding study of its functional properties [12]. (ER Liman, D Liu and J Appler, unpublished) An alternative is to study native TRPC2 channels. In sperm cells, thapsigargin induces a rise in Ca2+ that can be partially blocked by an antibody against an extracellular domain of TRPC2, suggesting that in these cells TRPC2 may be store operated [14]. In sensory neurons from the VNO, TRPC2 is unlikely to be activated by depletion of Ca2+ stores, because the channel is localized in sensory microvilli at a considerable distance from Ca2+ stores [20, 28]. In these cells, TRPC2 may be gated by diacylglycerol, a conclusion based on the observation that VNO sensory neurons from TRPC2 knockout animals are missing a DAG-gated conductance found in wild-type cells [25]. It is possible that there are different modes of activation of TRPC2, depending on the splice variant or the cell type in which it is expressed. Resolution of this controversy will require the successful expression of TRPC2 in heterologous cell types or its reintroduction into native cells.
TRPC2 and the Evolution of Pheromone Sensing
The essential function and nearly exclusive expression of TRPC2 in the vomeronasal organ have made it an excellent marker to study changes in VNO function during evolution. In fish, which do not have a structurally distinct VNO, TRPC2 is expressed in the olfactory epithelium in a population of apical microvillar cells that also express VRs, and it is not expressed in the basal ciliated cells that express ORs [31]. The microvillar cells appear specialized for detecting amino acids [23] and send segregated projections to the lateral portion of the olfactory bulb [31]. It is thus likely that the VNO arose by segregation of the microvillar cells from the ciliated cells, possibly as a response to terrestrial life. The main olfactory epithelium is well suited for detecting airborne chemicals that enter the nasal cavity during the respiratory cycle, whereas the VNO is better suited for detecting nonvolatile chemicals whose delivery is based on the presence of coinciding sensory and neuroendocrine signals [26].
Whether humans have a functional VNO has been difficult to determine using histological or functional techniques, and therefore it has been the subject of intense debate [29]. The observation that the TRPC2 gene is a pseudogene in humans [21, 38], as are most of the vomeronasal receptors of the V1R family [16], is strong evidence that the human VNO is vestigial. When did humans lose a functional VNO? A comprehensive analysis of the TRPC2 gene in extant primates has revealed that TRPC2 is a pseudogene in all Old World monkeys and apes, but not in New World monkeys (Figure 3.1D). [21, 42]. Analysis of nucleotide substitutions during the predicted evolution of the TRPC2 gene shows that it changed through relaxed selective pressure in the ancestors of New World monkeys and apes, coincident with the development of trichromatic vision through duplication of the green opsin gene [21]. It is likely that, at that time in evolution, visual signaling replaced the use of pheromones in communicating social and reproductive status. This conclusion is consistent with strong sexual dimorphism seen in Old World monkeys and primates.
From VNO Activation to Behavioral Changes
The surgical ablation of the VNO or the AOB in rodents has been shown to impair mating and territorial defense of the animal, thus suggesting a critical role in gender and social recognition [11]. However, clear differences in the degree of behavioral defects were observed in different species and, to some extent, in various reports of the same experiment. This can be easily explained by the behavioral variability existing between different species and strains and by the inherent difficulty of the surgical procedure performed on a sensory organ prone to regeneration. Genetic ablation of TRPC2 in mice provided a new experimental system to assess the requirement of TRPC2 function in VNO signaling and to directly investigate the repertoire of VNO-mediated sensory responses and behaviors [19, 32]. First, it appears that the TRPC2 deficiency dramatically impairs the sensory activation of VNO neurons by urine pheromones, thus confirming the critical role of TRPC2 in the VNO signal transduction cascade. In addition, the absence of pheromone detection mediated by VNO signaling has striking behavioral consequences. TRPC2−/− male mice appear unable to recognize the sexual identity of their conspecifics: they fail to display the pheromone-evoked aggression toward male intruders that is normally seen in wild-type males and, remarkably, they display courtship and mounting behavior indiscriminately toward both males and females. These data contradict the established notion that VNO activity is required for the initiation of male-female mating behavior in mice and suggest instead a critical role in ensuring sex discrimination (Figure 3.1E). Defects in maternal aggression, male territory marking, and recognition of social dominance also appear impaired in the TRPC2−/− mouse line. The ability of TRPC2−/− males to mate, although indiscriminately with conspecifics of both sexes, emphasizes the essential role played by other sensory modalities in the control of reproductive behavior. Indeed, the VNO does not appear to be the sole detector of pheromonal cues. Recent evidence demonstrated that areas of the hypothalamus controlling reproduction and fertility receive inputs from the MOE [3, 41]. Likewise, odorant detection processed in the MOB is involved in the attraction of females toward males [22], and olfactory cues recently emerged as essential stimuli for reproductive behavior [27, 41].
CONCLUSION
The discovery of TRPC2 expression in the vomeronasal organ has led to major breakthroughs in understanding the molecular and cellular processes of pheromone signal transduction. Moreover, it has provided an essential tool to investigate the role of pheromone and vomeronasal signaling in organisms and across evolution. However, the most mechanistic aspects of TRPC2 function remain to be uncovered. Progress in this direction should provide novel insights into the translation of chemosensory detection into electrical signals and lead to a deeper understanding of the sensory coding of pheromones.
REFERENCES
- 1.
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. [PubMed: 9459443]
- 2.
- Berghard A, Buck LB, Liman ER. Evidence for distinct signaling mechanisms in two mammalian olfactory sense organs. Proc Natl Acad Sci USA. 1996;93:2365–2369. [PMC free article: PMC39802] [PubMed: 8637879]
- 3.
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. [PubMed: 16290036]
- 4.
- Brann JH, Dennis JC, Morrison EE, Fadool DA. Type-specific inositol 1,4,5-trisphosphate receptor localization in the vomeronasal organ and its interaction with a transient receptor potential channel, TRPC2. J Neurochem. 2002;83:1452–1460. [PMC free article: PMC3082845] [PubMed: 12472899]
- 5.
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. [PubMed: 8893025]
- 6.
- Chu X, Tong Q, Cheung JY, Wozney J, Conrad K, Mazack V, Zhang W, Stahl R, Barber DL, Miller BA. Interaction of TRPC2 and TRPC6 in erythropoietin modulation of calcium influx. J Biol Chem. 2004;279:10514–10522. [PubMed: 14699131]
- 7.
- Chu X, Tong Q, Wozney J, Zhang W, Cheung JY, Conrad K, Mazack V, Stahl R, Barber DL, Miller BA. Identification of an N-terminal TRPC2 splice variant which inhibits calcium influx. Cell Calcium. 2005;37:173–182. [PubMed: 15589997]
- 8.
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. [PMC free article: PMC6573730] [PubMed: 9334401]
- 9.
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. [PubMed: 12838330]
- 10.
- Gailly P, Colson-Van Schoor M. Involvement of trp-2 protein in store-operated influx of calcium in fibroblasts. Cell Calcium. 2001;30:157–165. [PubMed: 11508995]
- 11.
- Halpern M. The organization and function of the vomeronasal system, Annual review of. Neuroscience. 1987;10:325–362. [PubMed: 3032065]
- 12.
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Cloning, expression and subcellular localization of two novel splice variants of mouse transient receptor potential channel 2. Biochem J. 2000;351:115–122. [PMC free article: PMC1221341] [PubMed: 10998353]
- 13.
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA. 2002;99:7461–7466. [PMC free article: PMC124253] [PubMed: 12032305]
- 14.
- Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. [PubMed: 11331878]
- 15.
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. [PubMed: 16208374]
- 16.
- Kouros-Mehr H, Pintchovski S, Melnyk J, Chen YJ, Friedman C, Trask B, Shizuya H. Identification of nonfunctional human VNO receptor genes provides evidence for vestigiality of the human VNO. Chem Senses. 2001;26:1167–1174. [PubMed: 11705802]
- 17.
- Leinders-Zufall T, Brennan P, Widmayer P, Chandramani SP, Maul-Pavicic A, Jager M, Li XH, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. [PubMed: 15528444]
- 18.
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. [PubMed: 10866200]
- 19.
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–6381. [PMC free article: PMC122956] [PubMed: 11972034]
- 20.
- Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA. 1999;96:5791–5796. [PMC free article: PMC21939] [PubMed: 10318963]
- 21.
- Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci USA. 2003;100:3328–3332. [PMC free article: PMC152292] [PubMed: 12631698]
- 22.
- Lin da Y, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. [PubMed: 15724148]
- 23.
- Lipschitz DL, Michel WC. Amino acid odorants stimulate microvillar sensory neurons. Chem Senses. 2002;27:277–286. [PubMed: 11923189]
- 24.
- Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, Kumanovics A, Fischer Lindahl K, Dulac C. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. [PubMed: 12628182]
- 25.
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. [PubMed: 14642279]
- 26.
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. [PubMed: 12595684]
- 27.
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. [PubMed: 16261133]
- 28.
- Menco BP, Carr VM, Ezeh PI, Liman ER, Yankova MP. Ultrastructural localization of G-proteins and the channel protein TRP2 to microvilli of rat vomeronasal receptor cells. J Comp Neurol. 2001;438:468–489. [PubMed: 11559902]
- 29.
- Meredith M. Human vomeronasal organ function: a critical review of best and worst cases. Chem Senses. 2001;26:433–445. [PubMed: 11369678]
- 30.
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. [PubMed: 8646774]
- 31.
- Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25:4889–4897. [PMC free article: PMC6724860] [PubMed: 15901770]
- 32.
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. [PubMed: 11823606]
- 33.
- Sutton KA, Jungnickel MK, Wang Y, Cullen K, Lambert S, Florman HM. Enkurin is a novel calmodulin and TRPC channel-binding protein in sperm. Dev Biol. 2004;274:426–435. [PubMed: 15385169]
- 34.
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem. 2001;276:21303–21310. [PMC free article: PMC1847329] [PubMed: 11290752]
- 35.
- Tong Q, Chu X, Cheung JY, Conrad K, Stahl R, Barber DL, Mignery G, Miller BA. Erythropoietin-modulated calcium influx through TRPC2 is mediated by phospholipase Cgamma and IP3R. Am J Physiol Cell Physiol. 2004;287:C1667–1678. [PubMed: 15329338]
- 36.
- Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion–activated capacitative Ca2+ entry channel. Proc Natl Acad Sci USA. 1999;96:2060–2064. [PMC free article: PMC26736] [PubMed: 10051594]
- 37.
- Webb DM, Cortes-Ortiz L, Zhang J. Genetic evidence for the coexistence of pheromone perception and full trichromatic vision in howler monkeys. Mol Biol Evol. 2004;21:697–704. [PubMed: 14963105]
- 38.
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–9656. [PMC free article: PMC40860] [PubMed: 7568191]
- 39.
- Wissenbach U, Schroth G, Philipp S, Flockerzi V. Structure and mRNA expression of a bovine trp homologue related to mammalian trp2 transcripts. FEBS Lett. 1998;429:61–66. [PubMed: 9657384]
- 40.
- Yildirim E, Dietrich A, Birnbaumer L. The mouse C-type transient receptor potential 2 (TRPC2) channel: alternative splicing and calmodulin binding to its N-terminus. Proc Natl Acad Sci USA. 2003;100:2220–2225. [PMC free article: PMC151321] [PubMed: 12601176]
- 41.
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. [PubMed: 16290037]
- 42.
- Zhang J, Webb DM. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc Natl Acad Sci USA. 2003;100:8337–8341. [PMC free article: PMC166230] [PubMed: 12826614]
- 43.
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. [PubMed: 8646775]
- 44.
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. [PubMed: 12214233]
- Review The TRPC2 ion channel and pheromone sensing in the accessory olfactory system.[Naunyn Schmiedebergs Arch Phar...]Review The TRPC2 ion channel and pheromone sensing in the accessory olfactory system.Zufall F. Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr; 371(4):245-50.
- Review TRPC channels in pheromone sensing.[Vitam Horm. 2010]Review TRPC channels in pheromone sensing.Kiselyov K, van Rossum DB, Patterson RL. Vitam Horm. 2010; 83:197-213.
- Characterization of TRPC2, an essential genetic component of VNS chemoreception, provides insights into the evolution of pheromonal olfaction in secondary-adapted marine mammals.[Mol Biol Evol. 2010]Characterization of TRPC2, an essential genetic component of VNS chemoreception, provides insights into the evolution of pheromonal olfaction in secondary-adapted marine mammals.Yu L, Jin W, Wang JX, Zhang X, Chen MM, Zhu ZH, Lee H, Lee M, Zhang YP. Mol Biol Evol. 2010 Jul; 27(7):1467-77. Epub 2010 Feb 8.
- Vomeronasal signal deficiency enhances parental behavior in socially isolated male mice.[Physiol Behav. 2017]Vomeronasal signal deficiency enhances parental behavior in socially isolated male mice.Orikasa C, Kondo Y, Katsumata H, Terada M, Akimoto T, Sakuma Y, Minami S. Physiol Behav. 2017 Jan 1; 168:98-102. Epub 2016 Nov 10.
- TRICK or TRP? What Trpc2(-/-) mice tell us about vomeronasal organ mediated innate behaviors.[Front Neurosci. 2015]TRICK or TRP? What Trpc2(-/-) mice tell us about vomeronasal organ mediated innate behaviors.Yu CR. Front Neurosci. 2015; 9:221. Epub 2015 Jun 23.
- TRPC2 and the Molecular Biology of Pheromone Detection in Mammals - TRP Ion Chan...TRPC2 and the Molecular Biology of Pheromone Detection in Mammals - TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades
Your browsing activity is empty.
Activity recording is turned off.
See more...