Clinical Description
The natural history of cytochrome P450 oxidoreductase deficiency (PORD) varies because it encompasses a wide phenotypic spectrum. However, steroid abnormalities, which occur in all individuals with PORD, can be associated with a number of characteristics.
The summary of clinical characteristics is based on 26 studies on 140 individuals with molecularly confirmed PORD published to date (June 2017).
Cortisol deficiency found in PORD varies, but is present in the majority of individuals.
Based on ACTH stimulation tests, Krone et al [2012] reported severe cortisol deficiency (requiring permanent hydrocortisone replacement) in 43% of individuals, and partial cortisol deficiency (requiring glucocorticoid replacement during stress only) in 40%; no replacement was required in 10% of the cohort.
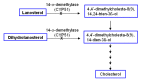
Mineralocorticoid excess due to inhibition of 17α-hydroxylase activity can result in hypertension, which typically manifests in young adulthood.
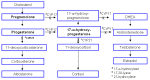
Disorders of sex development (DSD) occur in approximately 75% of individuals with molecularly confirmed PORD. DSD can occur in both sexes: 46,XY DSD (e.g., small penis, undescended testes) and 46,XX DSD (e.g., enlarged clitoris, fused and hypoplastic labia). The unusual finding that both sexes can present with DSD (e.g., virilized genitalia in 46,XX and undermasculinized genitalia in 46,XY) was suggested to be caused by the presence of an alternative pathway to dihydrotestosterone [Arlt et al 2004] (see Molecular Genetics). The manifestation of DSD is related to genotype, with some pathogenic variants leading to normal male virilization and 46,XX DSD in girls while other pathogenic variants result in normal female genital appearance and 46,XY DSD in boys (see Genotype-Phenotype Correlations).
Primary amenorrhea was the presenting feature in at least one woman with PORD (milder phenotype) [Scott et al 2007].
Large ovarian cysts with a tendency to spontaneous rupture are present in a number of adolescent and adult females with PORD [Scott et al 2007, Fukami et al 2009, Idkowiak et al 2011].
Poor masculinization and delayed puberty have been reported in some males, but spontaneous progression during puberty has also been observed [Fukami et al 2005, Hershkovitz et al 2008, Idkowiak et al 2011]. Hypospermatogenesis was documented on testicular biopsy in a male with PORD [Fukami et al 2005].
Fertility may be a concern. No reports describe reproduction in individuals with PORD; thus, the prevalence of infertility among individuals with PORD remains uncertain.
Signs of maternal virilization during pregnancy with an affected fetus, including hirsutism, enlargement of the nose and lips, deepening of the voice, and acne, have been reported in women during pregnancies in which fetuses were later found to have PORD [Fukami et al 2009, Krone et al 2012, Reisch et al 2013].
Skeletal abnormalities of the Antley-Bixler syndrome (ABS) phenotype are frequently observed in individuals with PORD. The severity of malformations varies from mild to moderate and severe. Functional studies are currently lacking but individuals with milder skeletal features similar to those in classic ABS probably have moderate PORD. Those with mild PORD tend to have few if any notable physical characteristics. Krone et al have introduced a clinical scoring system rating the severity of the skeletal and craniofacial malformations in PORD (Table 2) [Krone et al 2012], which is useful for systematic assessment of malformations in PORD.
Skeletal malformations occur in approximately 85% of individuals with molecularly confirmed PORD. Elbow ankylosis, often from radiohumeral synostosis, causes fixation of the elbow in a flexed position. Elbow extension may be restricted in the absence of radiohumeral synostosis. Neonatal fractures and
congenital bowing of the long bones (especially the femurs) are common. Other common malformations of the limbs include long palms, camptodactyly, other joint contractures, arachnodactyly, clubfeet, irregularly positioned toes, and rocker-bottom feet. Vertebral and rib anomalies, hypoplasia of the scapula, scoliosis, and narrow chest and/or pelvis have been reported. Other skeletal anomalies reported in individuals with PORD include diastases of the radioulnar joint, ulnar deviation of the wrists, marfanoid habitus, flattened metacarpal epiphyses, cubitus valgus, brachymetacarpia, and brachytelephalangy.
Craniofacial anomalies. Craniosynostosis is usually severe and most commonly involves the coronal and lambdoid sutures, resulting in turricephaly; synostosis of other cranial sutures has also been reported. Other craniofacial anomalies include frontal bossing, enlarged anterior fontanelle, severe midface retrusion, choanal stenosis or atresia, short bulbous nose, depressed nasal bridge, narrow mouth, high arched narrow palate, and dysplastic ears that may be low-set with stenotic external auditory canals. In milder forms, the craniofacial features – if present – may not be as easily identified at birth and/or tend to be less severe than those in individuals with severe disease. Although craniosynostosis and/or brachycephaly may be observed, surgical treatment may not require as many procedures. Individuals may have conductive hearing loss.
Hydrocephalus requiring ventriculoperitoneal shunt insertion has been reported in a number of affected individuals [
Krone et al 2012].
Associated anomalies, presumably not related to the disruption of sterol or steroid synthesis, are rare in individuals with PORD. These include urinary tract anomalies including several individuals with renal pelvic dilatation and vesicoureteral reflux [Krone et al 2012, Bonamichi et al 2016] and one individual with unilateral renal agenesis [Krone et al 2012]. Gastrointestinal conditions are reported in one individual with PORD who developed severe gastroesophageal reflux and constipation [Williamson et al 2006] and another individual with an anteriorly placed anus [Krone et al 2012]. Other observations include a two vessel umbilical cord in two individuals with PORD; Arnold-Chiari malformation and a frontal capillary hemangioma have each been reported in one individual [Krone et al 2012].
Cognitive function and development. There is a paucity of data on cognitive function and developmental outcomes in individuals with PORD. Developmental delays have been reported in a number of children with PORD, mainly delayed speech and language development and fine motor skills, presumably secondary to conductive hearing loss, skeletal abnormalities, multiple surgical procedures with anesthesia, and prolonged hospitalization with immobility [Williamson et al 2006, Sahakitrungruang et al 2009]. Early and effective management of upper airway obstruction, craniosynostosis, hydrocephalus, and hearing loss appear to be a prerequisite for good cognitive development.
Prognosis is primarily determined by the severity of the skeletal and craniofacial malformations. Stillbirth has been reported in infants with very severe skeletal malformations [Krone et al 2012, Reisch et al 2013]. In individuals with mild to moderate clinical features, the prognosis is guarded in infancy and improves with age. Early death caused by respiratory complications is a concern. However, with careful airway management, many children with ABS survive and the prognosis may be reasonably good. Prospective follow-up studies describing the natural course of PORD are currently lacking.