Clinical Description
Familial cerebral cavernous malformations (FCCM) is a disorder characterized by multiple cerebral cavernous malformations (CCMs) without a developmental venous anomaly. Individuals with FCCM may present with seizure, headaches, or focal neurologic deficits with or without associated cerebral hemorrhage. In some instances, individuals present for screening or evaluation of unrelated concerns and the cavernous malformation(s) are asymptomatic [Riant et al 2010].
To date, it is estimated that more than 1,000 families have been reported with FCCM and a pathogenic variant in one of the FCCM-associated genes. The following is a description of the phenotypic features associated with this disorder.
Table 2.
Familial Cerebral Cavernous Malformations: Frequency of Select Features
View in own window
| Feature 1 | % of Persons w/Feature 2 | Comment |
|---|
|
Cavernous malformation of the central nervous system presenting with:
|
| Seizures | 20%-40% | The cumulative incidence of childhood seizures is ~20% (~60% by age 80 yrs). 3 |
| Focal neurologic deficits | 25%-30% | Focal neurologic deficits most commonly occur w/intracranial hemorrhage but may occur w/o hemorrhage as well. Focal neurologic deficits should localize to the CCM to be considered symptomatic. |
| Nonspecific headaches | 10%-30% | Primary headache disorders are common in persons w/CCMs; however, headaches may also occur due to hemorrhage &/or hydrocephalus. |
| Symptomatic intracerebral hemorrhage | 30%-40% | 30%-40% of persons initially present w/symptomatic hemorrhage. 4 |
| Spinal cord cavernous malformations | ~16%-70% | Up to 70% of persons w/pathogenic variants in KRIT1 may have at least 1 spinal cavernous malformation, although many persons remain asymptomatic. |
|
Extraneuronal manifestations
|
| Cutaneous vascular malformations (CVMs) | 9%-20% | Three types of CVMs have been reported:
Hyperkeratotic cutaneous capillary-venous malformations (HCCVMs) Punctate capillary malformations (PCMs) Deep blue nodules (DBNs) 5
|
| Retinal vascular lesions | ~5% | May incl retinal cavernomas & choroidal hemangiomas 6 |
CCM = cerebral cavernous malformation; CVM = cutaneous vascular malformation; FCCM = familial cerebral cavernous malformations
- 1.
Up to 40%-50% of individuals with FCMM are asymptomatic.
- 2.
Data reflect percentage of individuals with the feature at the time of initial presentation.
- 3.
- 4.
- 5.
- 6.
Neurologic findings. Up to 50% of individuals with a heterozygous germline pathogenic variant in either KRIT1, CCM2, or PDCD10 may be initially asymptomatic, although at least half of these individuals have identifiable CCM lesions on brain imaging [Battistini et al 2007, Fischer et al 2013]. CCMs have been reported in infants and children, but most individuals with FCCM present with symptoms between the second and fifth decades. In a study of individuals who have undergone genetic testing, 20% of symptomatic individuals presented under the age of 15 years [Denier et al 2006].
Clinically affected individuals may present with seizures (20%-40%), focal neurologic deficits (35%-50%), and nonspecific headaches (10%-30%). These symptoms may or may not be associated with symptomatic hemorrhage in the brain or the spinal cord [Zafar et al 2019]. Brain or spinal cord hemorrhage are common and occur in up to 30%-40% of individuals initially presenting with cavernous malformation(s) [Zafar et al 2019, Alalfi et al 2023, Weinsheimer et al 2023].
In large natural history studies, the risk of prospective, symptomatic hemorrhage does not seem to differ between familial and sporadic CCMs [Horne et al 2016, Taslimi et al 2016, Gross & Du 2017]. However, these studies had limited follow-up data and did not discern the effect of the number or type of lesions on hemorrhage risk. Prior studies had estimated the risk of prospective, symptomatic hemorrhage in FCCM to be between 2.8% and 16.5% per year depending on prior history of hemorrhage [Zabramski et al 1994, Labauge et al 2000, Carrión-Penagos et al 2020, Santos et al 2022, Weinsheimer et al 2023]. Most recently, the largest study of individuals with FCCM (n=321) estimated an overall hemorrhage risk of 2.8 per 100 person years [Weinsheimer et al 2023]. However, the risk was higher in individuals with prior hemorrhage, at 4.5 per 100 person years compared to 2.0 per 100 person years in those without prior hemorrhage. Furthermore, this study noted a higher risk of future hemorrhage based on total lesion count. Another study of children with FCCM (n=41) reported a five-year risk of hemorrhage of 5% per person year, with higher rates in those with pathogenic variants in CCM2, positive family history, and prior symptomatic hemorrhage [Geraldo et al 2023].
Seizures are common in FCCM during an individual's lifetime. One large study of individuals with FCCM (n=479) reported that by age 80 years, 60.4% of individuals had at least one seizure [Fox et al 2021]. The prospective risk of seizures has been estimated at approximately 1.2%-1.5% per person year in those not initially presenting with seizures [Taslimi et al 2019, Alalfi et al 2023]. Overall, seizure risk is estimated to be increased with higher lesion counts, larger CCMs, and possibly a pathogenic variant in PDCD10 [Fox et al 2021].
Brain imaging. Recommended standard imaging in individuals with cavernous malformations includes standard MRI sequences with either susceptibility-weighted imaging (SWI) on a 3 Tesla or higher-magnet machine [Akers et al 2017]. SWI allows the visualization of microscopic lesions, which are common in FCCM. Gradient echo (GRE) is acceptable but is less sensitive than SWI at assessing lesion count.
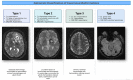
Four characteristic types of lesions have been described [Zabramski et al 1994] by MRI and histology (see ). Dividing CCMs into these radiologic and histologic types can be clinically useful in predicting hemorrhage risk [Nikoubashman et al 2015].
The clinical significance of small lesions (classified as Zabramski type 4) seen on MRI (sometimes referred to as cerebral dot-like cavernous malformations) is unclear. For these lesions, a mean hemorrhage rate of 1.3% per year was found over a period of 5.5 years in 18 children with either an inherited or a de novo heterozygous pathogenic variant in KRIT1 or PDCD10. However, these individuals were all asymptomatic [Nikoubashman et al 2015].
FCCM is a dynamic disease based on neuroimaging. Several studies suggest that new lesions appear at a rate between 0.2 to one lesion per person year [Brunereau et al 2000, Labauge et al 2001, Taslimi et al 2019, Santos et al 2022]. Studies reporting on de novo lesions can be limited if sequences (SWI vs GRE), slice thickness, and magnet strength differ. In both FCCM and sporadic CCM, lesions may change in size and signal characteristics over time.
In FCCM, 70%-86% of lesions are supratentorial and 16%-24% infratentorial in location [Brunereau et al 2000, Labauge et al 2001]. Of the infratentorial lesions, almost half occur in the brain stem. In addition, one study of individuals with FCCM found a higher prevalence of white matter intensities in younger individuals with FCCM compared to those with sporadic CCM [Golden et al 2015a], the clinical consequences of which are not yet known.
Spinal cord cavernous malformations (SCCMs). SCCMs are common in FCCM. In one study of individuals with known KRIT1 pathogenic variants, at least one lesion was found in up to 70% of individuals using standard and GRE imaging of the cervical and thoracic spine [Mabray et al 2020]. While spinal lesions may be common, symptomatic spinal cord lesions in FCCM are rare. In one small study (n=75) of individuals with FCCM, seven (9.3%) initially presented with symptomatic hemorrhage in the spinal cord and only one had a prospective spinal cord hemorrhage [Alalfi et al 2023].
Systemic vascular abnormalities. Vascular lesions outside the central nervous system have been reported in association with multiple intracranial cavernous malformations.
Cutaneous vascular malformations were found in 38 out of 417 individuals with FCCM [
Sirvente et al 2009]. Of the 38, 13 had skin lesions classified as capillary malformations; 15 individuals had hyperkeratotic cutaneous capillary-venous malformations (HCCVMs); eight had venous malformations; and two had unclassified lesions.
Bluish nodules and other subcutaneous nodules have been described as subtypes of venous malformations.
Some affected individuals have skin lesions removed secondary to bleeding, pain, protrusion, cosmetic concerns, or concern for malignancy.
Atypical vertebral hemangioma. Osseous vascular malformations of the vertebral bodies of the spine may also be present in individuals with FCCM. Rarely these lesions can be associated with pathologic fractures. Histopathology demonstrated capillary-venous malformation. These lesions are visible on spine MRI and are more atypical appearing than most vertebral hemangiomas, as they are hyperintense on T
2-weighted images but hypointense on T
1-weighted images [
Tandberg et al 2020].
Other systemic manifestations
Scoliosis. In a series of 18 affected individuals with pathogenic variants in
PDCD10, scoliosis was identified in 39% of individuals [
Shenkar et al 2015].
Brain tumors. In a series of 18 affected individuals with pathogenic variants in
PDCD10, 5 of 18 individuals had a brain tumor, including meningioma (n=2), acoustic neuroma (n=2), and cerebellar astrocytoma (n=1) [
Shenkar et al 2015].
Adrenal gland calcifications have been rarely reported and are suspected to represent small vascular lesions, with limited histologic confirmation [
Strickland et al 2017].
Histopathology. Histopathologic findings on resected CCMs show:
Closely clustered enlarged capillary channels (caverns) ranging from two to 55 mm (mean: 8 mm) with a single layer of endothelium without normal mature vessel wall elements or intervening brain parenchyma;
Thrombosis and intra- and extralesional hemorrhage. Edema may surround lesions with recent hemorrhage.