Treatment of Manifestations
Practice parameters, including information on surgery, have been outlined by the following professional groups:
Colonic polyps. For individuals with FAP, endoscopic surveillance with colonoscopy is generally recommended to start between ages ten and 15 years with an interval of every one to two years [Weiss et al 2016, van Leerdam et al 2019, Yang et al 2020]. All polyps >5 mm should be resected. If the polyp burden is manageable and no definitive indications for surgery are noted, it is reasonable to delay colectomy and monitor with endoscopic surveillance [Ishikawa et al 2016]. Absolute indications for colectomy include documented or suspected colorectal cancer (CRC) or significant symptoms (e.g., obstruction, bleeding), although these are uncommon in the absence of cancer. Relative indications for colectomy include presence of multiple large adenomas (>10 mm) that cannot be reasonably managed by endoscopy, a significant increase in adenoma number between surveillance examinations, presence of adenomas with high-grade dysplasia, or inability to adequately survey the colon (e.g., due to innumerable diminutive adenomas or limited access or compliance with colonoscopy). In individuals age ten to 20 years in whom adenomas are <6 mm and without villous component or high-grade dysplasia, delay in colectomy may be considered to allow for physical and emotional maturity.
For individuals with an attenuated FAP phenotype, delaying the initiation of colonoscopy until late adolescence can be considered [Weiss et al 2016, van Leerdam et al 2019, Yang et al 2020]. Colonoscopic surveillance and polypectomy every one to two years can often be effective and delay or even prevent the need for colectomy [Knudsen et al 2010]. Approximately one third of individuals have colonic polyps that are limited enough in number that surveillance with periodic colonoscopic polypectomy is sufficient (see Surveillance) [Patel et al 2016].
Surgical options for colectomy include the following:
Total proctocolectomy with ileal pouch anal anastomosis (IPAA). This can be performed laparoscopically, laparoscopically-assisted, robotically, or open. The IPAA can be stapled, leaving 1-2 cm of anal transition epithelium and low rectal mucosa; or it can be hand-sewn after a complete anal mucosectomy. This is a multistage surgery.
Total colectomy with ileorectal anastomosis (IRA). This can also be performed with minimally invasive surgical techniques and is a single-stage surgery.
Total proctocolectomy with permanent ileostomy. This can also be performed with minimally invasive surgical techniques and is a single-stage surgery.
The choice of procedure depends on the clinical circumstances.
An IPAA is generally performed in individuals with FAP with a high rectal polyp burden (generally considered as >20 adenomas in the rectum or presence of advanced rectal neoplasia) or as a second procedure after IRA when rectal disease burden cannot be managed endoscopically [
Warrier & Kalady 2012]. The advantage of this procedure is near-elimination of the risk for rectal cancer and relatively good preservation of bowel function. However, there may be an increased risk of bladder/sexual dysfunction compared to colectomy with IRA and functional results can be variable.
A study of individuals with FAP and ileal pouches found that 57% had adenomatous polyps in the ileal pouch [
Groves et al 2005].
An IRA is generally considered when the rectal polyp burden is minimal and deemed to be endoscopically manageable (usually in the setting of attenuated FAP). It is a technically straightforward procedure with low complication rates. It is usually associated with good functional outcome and minimizes risk of sexual or urinary dysfunction. When performed in appropriate individuals, the risk for rectal cancer or need for proctectomy after IRA is low [
Church et al 2001]. This is not an optimal surgical choice if there is severe rectal disease or the individual cannot reliably undergo endoscopic surveillance of the remaining rectum postoperatively.
A total proctocolectomy with end ileostomy is rarely required unless a contraindication to IPAA is present (e.g., a mesenteric desmoid preventing a pouch from reaching pelvic floor, low rectal cancer invading pelvic floor, or individual preference due to poor sphincter control) and a proctocolectomy is necessary (due to rectal polyp/cancer burden).
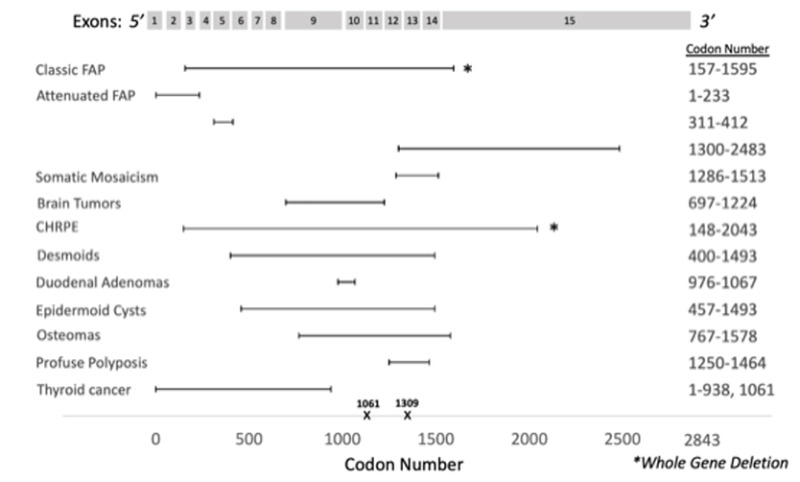
Duodenal adenomas. Current guidelines recommend duodenal screening at age 20-25 years or earlier if colectomy is planned [van Leerdam et al 2019, Yang et al 2020, Weiss et al 2021]. This recommendation applies to all colon phenotypes and to both classic and attenuated FAP. Surveillance intervals are determined based on the Spigelman scoring system [Spigelman et al 1989], which incorporates duodenal polyp number, size, histology, and dysplasia grade to categorize affected individuals into five stages (see Table 5). Guidelines generally agree that for low-risk individuals (Stage 0 and I) a five-year interval for duodenal screening is appropriate, and for moderate-risk individuals (Stage II) a three-year interval is recommended. For higher-risk individuals (Stage III and IV) at least annual endoscopy is needed; for individuals with Stage IV there should be a consideration of surgical referral.
Table 5.
Spigelman Scoring System for Duodenal Adenomas in Familial Adenomatous Polyposis
View in own window
| Spigelman Scoring System |
|---|
| Criteria | 1 point | 2 points | 3 points |
|---|
| Polyp number | 1-4 | 5-20 | >20 |
| Polyp size (mm) | 1-4 | 5-10 | >10 |
| Histology | Tubular | Tubulovillous | Villous |
| Dysplasia | Mild | Moderate | Severe |
Endoscopic or surgical removal of duodenal and/or ampullary adenomas is recommended by standard polypectomy techniques such as snaring and endoscopic mucosal resection. If there are too many polyps to remove, the focus should be on removing polyps >1 cm in size or those with concerning features. European Society of Gastrointestinal Endoscopy guidelines [van Leerdam et al 2019] caution against removing small polyps due to concern for fibrosis limiting future resection, but this has not been reported as a limiting factor in clinical practice.
Indications for surgery for advanced duodenal polyposis are Stage III with high-grade dysplasia, Stage IV disease, and malignancy. Surgical options for advanced duodenal polyposis include pancreaticoduodenectomy (Whipple procedure) and pancreas-sparing duodenectomy, which is a good option when the papilla is not involved and there is no suspicion for cancer. These surgeries have high associated morbidities and should be performed at high-volume centers and preferably by those with expertise in FAP.
Ampullary adenomas. Specific endoscopic techniques are needed to ensure adequate examination. This can be accomplished with a side-viewing duodenoscope or a clear-cap distal attachment to a forward-viewing gastroscope – approaches that have been found to be equivalent [Abdelhafez et al 2019]. Ampullary biopsy has been shown to be safe with a low risk for pancreatitis and there should be a low threshold to biopsy if there is a suspicion for an adenoma [Mehta et al 2021]. Guidelines vary on whether to include ampullary adenomas with duodenal polyposis in determining a surveillance interval; if a small ampullary adenoma is identified, most experts recommend repeat surveillance within three years. Although small ampullary adenomas can be monitored without resection, any adenomas >1 cm in size or with advanced histology should be resected. Ampullectomy carries a high rate of complications and should be performed by experienced providers [Roos et al 2021]. Recurrence after ampullectomy is common and close endoscopic surveillance is needed. Surgical considerations for ampullary adenomas are similar to those for advanced duodenal polyposis.
Gastric polyps. Gastric surveillance should be performed at the time of duodenal surveillance. Recommended guidelines for gastric surveillance are not as developed as those for duodenal polyposis, but with increasing incidence of gastric cancer in individuals with FAP these guidelines will likely evolve. Removal of all polyps that are concerning for adenomas / pyloric gland adenomas or advanced changes (dysplasia) is recommended along with random sampling of fundic gland polyps (FGPs). Some experts recommend using polyp number, size, histology, dysplasia, and other features to guide surveillance [Mankaney et al 2017]; endoscopic criteria (Surveillance for Pathology Associated with Cancer on Endoscopy; see Mankaney et al [2020]) can aid in optical diagnosis of high-risk pathology. If advanced neoplasia is noted on sampling, surgical gastrectomy should be considered.
Thyroid nodules and cancer. Treatment of thyroid nodules and papillary thyroid carcinoma, including the cribriform variant, is similar to that of sporadic disease [Abdullah Suhaimi et al 2015].
Osteomas may be removed for cosmetic reasons.
Desmoid tumors. Available treatments include surgical excision (associated with high rates of recurrence), nonsteroidal anti-inflammatory drugs (NSAIDs), anti-estrogens, cytotoxic chemotherapy, and radiation [Smith et al 2000a, Tonelli et al 2003, Gega et al 2006]. A review of desmoid treatments can be found in Guillem et al [2006] and the Desmoid Tumor Working Group [2020].
Adrenal tumors. Standard treatment is indicated as needed for adrenal masses.
Chemoprevention. There are currently no FDA-approved chemopreventive agents for FAP. Individuals interested in chemoprevention should be encouraged to enroll in an ongoing clinical trial (see Therapies Under Investigation). Note: The FDA has stated that changes in adenoma number and size are insufficient for approval and that clear evidence of clinical benefit is required. Cited examples of clinical benefit include decreased risk for CRC or reduced need for surgery; current trials are designed to address these endpoints.
NSAIDs. Non-placebo-controlled trials and observational studies on sulindac were initially promising, showing remarkable reduction in polyp size and number. However, these preliminary studies were limited in their design (non-placebo controlled; limited number of affected individuals; some individuals with only surveyable rectum). Several controlled trials subsequently confirmed a decrease in polyp burden during sulindac therapy [Labayle et al 1991, Giardiello et al 1993a, Nugent et al 1993]. However, rapid reappearance or increase in polyp number was observed after sulindac was discontinued [Labayle et al 1991, Giardiello et al 1993a]. A subsequent study designed to evaluate primary prevention of polyps in individuals with APC pathogenic variants showed a statistically nonsignificant trend toward benefit compared to placebo [Giardiello et al 2002].
The FDA initially approved celecoxib for FAP based on evidence of decreased colon polyp burden and size (as well as modest decrease in the duodenum) [Steinbach et al 2000, Phillips et al 2002]. However, due to cardiovascular and cerebrovascular safety concerns, FDA approval for celecoxib for FAP was withdrawn and rofecoxib was also taken off the market.
Aspirin has traditionally been shown to be of little or no benefit in FAP [Burn et al 2001, Ishikawa et al 2013], but a recent randomized trial showed a potential benefit in suppressing large polyps [Ishikawa et al 2021].
Interest in combination of NSAIDs with other drugs was raised when reports of sulindac plus difluoromethylornithine (DFMO) showed marked reduction in sporadic metachronous adenomas [Meyskens et al 2008]. In a randomized placebo-controlled study of 92 participants with FAP, sulindac plus erlotinib (an EGF receptor inhibitor) resulted in decreased duodenal polyp burden compared to placebo after six months of use [Samadder et al 2016]. Adverse events were common in the treatment group (87% experienced an acne-like rash), although serious adverse events were rare (2 participants) [Samadder et al 2016]. A secondary analysis of this trial also showed a decrease in colorectal polyp burden [Samadder et al 2018]. A criticism of this trial is that the endpoints are not clearly clinically meaningful. When celecoxib was compared to celecoxib plus DFMO, there was no significant difference in polyp burden within a defined endoscopic field (however, when more comprehensive video assessment was used, there was a decrease in polyp burden in the combination therapy group) [Lynch et al 2016]. More recently, a large trial did not show that disease progression was different with the combination of sulindac and eflornithine than with either drug separately [Burke et al 2020].
Note: NSAID use before colectomy remains experimental (see Therapies Under Investigation).
GAPPS. There are no current guidelines for management of GAPPS, but some experts have recommended gastroscopy starting at age 15 years with removal of all polyps that are concerning for adenomas / pyloric gland adenomas or advanced changes (dysplasia) along with random sampling of FGPs. If advanced neoplasia is noted on sampling, standard surgical gastrectomy should be considered [Author, personal communication].
Pregnancy Management
Pregnancy / fertility / hormone use. Limited information is available on the effect of pregnancy on women with FAP. A study of 162 women with FAP compared fertility rates before and after two types of colorectal surgery with a control population. Women with FAP who had not yet undergone surgery had the same fertility as a control population of normal women. Additionally, those women with FAP who had had a colectomy with IRA had the same fertility as the control population. Fertility was significantly reduced in women with FAP who had had a proctocolectomy with IPAA compared to the control population [Olsen et al 2003].
In another study, the prevalence of self-reported fertility problems was similar among individuals with FAP who had undergone IRA, IPAA, or proctocolectomy with ileostomy. However, those who had had their first surgical procedure at a younger age had more postoperative fertility problems [Nieuwenhuis et al 2010].
Limited evidence supports an association between desmoid tumor development or growth and pregnancy [Sinha et al 2011], and there has been an association with a more benign course of desmoids after pregnancy [Church & McGannon 2000].
Women who have undergone colectomy are considered to be at the same risk for obstetric complications as any other woman who has had major abdominal surgery and are more likely to be delivered by C-section than those without such surgery.
In a study of women with FAP at the time of their colectomy, no association was found between pregnancy history and colonic polyp severity; however, the proportion of parous women with severe duodenal disease was significantly higher than the proportion of nulliparous women [Suraweera et al 2007].
Some studies have suggested that female hormones protect against CRC development in the general population. In one woman, reduction in polyps after use of oral contraceptives was observed [Giardiello et al 2005].