1. Clinical Characteristics of Holoprosencephaly
Holoprosencephaly (HPE), the most common malformation of the forebrain in humans, is a structural anomaly of the brain resulting from failed or incomplete forebrain division in the third to fourth weeks of gestation; the forebrain (prosencephalon) incompletely cleaves into right and left hemispheres, deep brain structures, and the olfactory and optic bulbs and tracts [Gropman & Muenke 2005, Dubourg et al 2007, Grinblat & Lipinski 2019].
While HPE is often first identified on prenatal ultrasound examination [Kousa et al 2018], it is most frequently diagnosed during the newborn period when abnormal facial findings and/or neurologic presentation prompt further evaluation. Infants with normal facies or only mildly abnormal facies and either mild or intermediate brain anomalies may not be diagnosed until the first year of life when neuroimaging studies obtained during evaluation for developmental delay and/or failure to thrive reveal HPE [Weiss et al 2018b].
Imaging of the brain by CT scan or MRI defines the type of HPE and identifies associated CNS anomalies [Hahn & Barnes 2010, Griffiths & Jarvis 2016, Kousa et al 2018]. The study of choice is cranial MRI examination, preferably obtained with adequate sedation at a pediatric center experienced in evaluating children for structural brain anomalies. Review of the study by a radiologist and/or other clinician familiar with the clinical subtypes of HPE is essential, as subtle midline anomalies may be missed, and non-HPE-related malformation findings may be mistaken for findings of HPE [Solomon et al 2009b].
Types of HPE
HPE, a continuum of brain malformations, is traditionally divided into the following types (in decreasing order of severity) (reviewed in Hahn & Barnes [2010]):
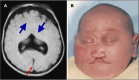
Alobar HPE, in which there is a single "monoventricle" and no separation of the cerebral hemispheres (), is the most severe form. The range of findings includes:
Alobar HPE A. MRI of alobar holoprosencephaly (HPE), the most severe form of HPE, characterized by an enlarged midline monoventricle (holoventricle, red/thin arrow) with fusion of the frontal lobes and the midline gray matter structures (thalami and basal (more...)
Cyclopia: single eye or partially divided eye in single orbit with a proboscis above the eye
Cyclopia without proboscis
Ethmocephaly: extremely closely spaced eyes but separate orbits with proboscis between the eyes
Cebocephaly: closely spaced eyes with single-nostril nose
Closely spaced eyes
Anophthalmia or microophthalmia
Premaxillary agenesis with median cleft lip, closely spaced eyes, depressed nasal ridge
Bilateral cleft lip
Relatively normal facial appearance (especially in persons with pathogenic variants in ZIC2)
Semilobar HPE. The left and right frontal and parietal lobes are fused and the interhemispheric fissure is only present posteriorly (). The range of findings includes:
Semilobar HPE A. MRI showing semilobar HPE. Note fusion of the frontal lobes, but presence of some septation posteriorly with presence of a falx and interhemispheric fissure (red/thin arrow). The splenium of the corpus callosum is present but more anterior (more...)
Closely spaced eyes
Anophthalmia/microophthalmia
Depressed nasal ridge
Absent nasal septum
Flat nasal tip
Bilateral cleft lip with median process representing the philtrum-premaxilla anlage
Midline cleft (lip and/or palate)
Relatively normal facial appearance
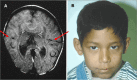
Lobar HPE. Most of the right and left cerebral hemispheres and lateral ventricles are separated but the frontal lobes, most rostral aspect of the telencephalon, are fused, especially ventrally (). The range of findings includes:
Lobar HPE A. MRI in axial plane depicting lobar HPE, the least severe of the major types of HPE. The cerebral hemispheres are separated (blue/thick arrows); the ventricles are misshapen as a result of absence of the septum pellucidum. The posterior portion (more...)
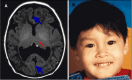
Middle interhemispheric fusion variant (MIHF/MIHV or syntelencephaly). The posterior frontal and parietal lobes fail to separate, with varying lack of cleavage of the basal ganglia and thalami and absence of the body of the corpus callosum but presence of the genu and splenium of the corpus callosum ().
Middle interhemispheric fusion (MIHF) A. MRI in axial plane depicting middle interhemispheric variant of HPE in which the anterior portions of the frontal lobes and the occipital lobes are well separated. The sylvian fissures are oriented nearly vertically (more...)
The range of findings includes:
Septopreoptic type. Nonseparation is restricted to the septal and/or preoptic regions; described in small case series [Hahn et al 2010].
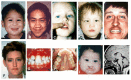
Microforms of HPE (also termed "microform HPE") are clinical subtypes of HPE defined by the presence of HPE-related craniofacial anomalies without structural brain defects on imaging. They may occur in simplex HPE (i.e., a single occurrence of HPE in a family) or in relatives of probands with classic forms of HPE (). Their clinical spectrum includes the following:
Microforms of holoprosencephaly (HPE) spectrum with milder craniofacial anomalies in the absence of neurologic findings A. Premaxillary agenesis with repaired bilateral clefts of the lip
Other Structural CNS Findings
Other structural CNS findings that may occur with but are not specific to HPE:
Anomalies of midline structures: undivided thalami, agenesis of the corpus callosum (OMIM
217990), callosal dysgenesis [
Kidron et al 2016], absent septum pellucidum, and absent or hypoplastic olfactory bulbs and tracts (arrhinencephaly) and optic bulbs and tracts
Macrocephaly secondary to hydrocephalus
Dandy-Walker malformation
Neuronal migration anomalies
Abnormal circle of Willis
Caudal dysgenesis
Craniofacial Anomalies
The continuum of craniofacial anomalies, present in approximately 80% of individuals with HPE, includes cyclopia, synophthalmia, or a proboscis at the severe end and normal facies in individuals who have, but are not expressing, an HPE pathogenic variant inherited in an autosomal dominant manner. Common subtle facial features in individuals without obvious craniofacial findings include microcephaly (although hydrocephalus can result in macrocephaly), closely spaced eyes (also known as hypotelorism; potentially severe), depressed nasal ridge, and cleft lip and/or palate. A single maxillary central incisor may be present; although a nonspecific finding, it is a distinctive microform in autosomal dominant HPE [Lacbawan et al 2009, Richieri-Costa & Ribeiro 2010].
Of note, subtle facial anomalies in mildly affected family members can be easily overlooked [Lacbawan et al 2009, Solomon et al 2009a].
Malformations of the nose include complete absence, agenesis of the nasal cartridge, and proboscis (flat nose with a single central nostril without nasal bones).
Palatal anomalies include various midline and lateral clefts, midline palatal ridge, bifid uvula, high-arched palate, and absence of the superior labial frenulum [Solomon et al 2010a].
The extremely variable phenotypic expression occurs both in simplex HPE (i.e., a single occurrence in a family) and among members of the same family with an inherited form of HPE.
Clinical Manifestations of HPE
Clinical manifestations (reviewed in Levey et al [2010] and Solomon et al [2010a]) commonly observed in children with HPE include the following:
Developmental delay is present in all individuals with the HPE spectrum of CNS anomalies. The degree of delay is variable, correlating with the severity of the brain malformation, but tends to be severe.
Seizures are common, and may be difficult to control.
Hydrocephalus can occur, and may result in macrocephaly, rather than the more commonly observed microcephaly.
Neural tube defects occur in a small proportion of individuals.
Hypothalamic and brain stem dysfunction may lead to swallowing difficulties and instability of temperature, heart rate, and respiration.
Pituitary dysfunction is manifest by partial or complete panhypopituitarism with abnormal function of any or all of the anterior and/or posterior pituitary hormones, though central diabetes insipidus is by far the most common finding in persons with nonchromosomal, nonsyndromic HPE [
Lacbawan et al 2009,
Solomon et al 2010a].
Short stature and
failure to thrive are common, especially in more severely affected children. Growth hormone deficiency and/or
chromosome anomalies may in part be responsible for poor growth in some individuals.
Feeding difficulties may be a major problem in children with HPE. At least part of the difficulty may derive from axial hypotonia, poor suck as a result of neurologic complications, lethargy, seizures and their effects, side effects of medications, and lack of interest. Often gastroesophageal reflux, choking, and gagging occur with feeds. Additional common problems include slowness in eating, frequent pauses, and frank vomiting with risk of aspiration. Oral-sensory dysfunction may affect feeding especially when associated with textural aversion and labial and lingual weakness. Children with cleft lip and/or palate often have additional difficulties with oral feeding.
Excessive intestinal gas/colic, irritability, and constipation frequently occur [
Levey et al 2010].
Aspiration pneumonia can be a complication of poor coordination of swallowing.
Erratic sleep patterns can occur.
Life expectancy. A common misperception is that children with HPE do not survive beyond early infancy. While this is the case for the most severely affected children, a significant proportion of more mildly affected children (as well as some severely affected children) survive past age 12 months. In fact, a proportion of individuals with HPE of various subtypes, including severe subtypes, survive until adulthood.
The longer survival may be on account of recent advances in diagnostic methods, including brain imaging methods that allow for early detection of both severe and mild malformations. Improvement in the management of HPE over time may have also contributed to longer survival [Levey et al 2010, Pineda-Alvarez et al 2010, Weiss et al 2018b].
The distribution of HPE subtypes appears to be similar among children, adolescents, and adults, with semilobar HPE representing approximately 50% of HPE in both children and adults. The exception is alobar HPE, which appears to be less frequent in adults than children [Weiss et al 2018a].
Among affected individuals with abnormal chromosome complement, an inverse relationship exists between the severity of the facial phenotype and length of survival.
Among affected adolescents and adults, sensorineural hearing loss is found in approximately 30% and cortical vision impairment in approximately 20% [Weiss et al 2018a].
Of note, 60% of adolescent and adult survivors have severe involvement: they are nonambulatory and nonverbal with minimal hand function, and full dependence on caregivers [Weiss et al 2018a]. There is a correlation between the degree of developmental delay and the severity of the brain malformation or HPE subtype [Levey et al 2010, Weiss et al 2018a].
2. Genetic Causes of Holoprosencephaly
Chromosome Abnormalities with Holoprosencephaly
Approximately 25%-50% of individuals with HPE have a chromosome abnormality. Chromosome abnormalities are nonspecific and either numeric or structural, and can involve any chromosome [Dubourg et al 2018].
Individuals with HPE and a normal chromosome complement cannot be distinguished from those with an abnormal chromosome complement based on craniofacial abnormality or HPE subtype; however, individuals with HPE caused by a chromosome abnormality are more likely to have other organ system involvement, resulting in a more severe clinical course in most.
Numeric chromosome abnormalities. Trisomy 13, the most common cause of HPE, is observed in 40%-60% of HPE of all causes and about 75% of HPE caused by chromosome abnormalities. Birth prevalence of trisomy 13 is 1:5000. Arrhinencephaly is seen in about 70% of individuals with trisomy 13.
The other common aneuploidies associated with HPE include trisomy 18 and triploidy. Various other aneuploidies have been reported [Kagan et al 2010, Solomon et al 2010c, Petracchi et al 2011, Toufaily et al 2016, Rosa et al 2017].
Structural chromosome abnormalities associated with HPE have been reported in virtually all chromosomes. The most frequent are deletions or duplications involving various regions of 13q, and del(18p), del(7)(q36), dup(3)(p24-pter), del(2)(p21), and del(21)(q22.3) [Hu et al 2018]. Many of these regions contain genes known to be associated with autosomal dominant nonsyndromic HPE (, ).
Pathogenic copy number variations (CNVs). Chromosomal microarray (CMA) includes array-based comparative genomic hybridization (array CGH) and SNP array. CMA has identified pathogenic CNVs (including loci already known to be associated with HPE) in 10% of all individuals with HPE. Note that CNV detection rates may vary by testing laboratory and methodology [Dubourg et al 2018, Hu et al 2018].
Monogenic Syndromes with Holoprosencephaly as an Occasional Finding
Approximately 18%-25% of individuals with HPE have a pathogenic variant in a single gene causing syndromic HPE. At least 25 different conditions in which HPE is an occasional finding have been described; the majority of these disorders are rare. Some of the more common are summarized in [Kruszka & Muenke 2018].
Table 1.
Syndromes with Holoprosencephaly as an Occasional Finding: Monogenic Causes
View in own window
Single-Gene Disorders with Isolated (Nonsyndromic) Holoprosencephaly
The nonsyndromic forms of HPE best understood at a molecular genetic level are inherited in an autosomal dominant manner (see , ).
The phenotype of individuals with pathogenic variants in genes associated with nonsyndromic HPE is extremely variable even within the same family, ranging from alobar HPE with cyclopia to clinically normal [Solomon et al 2010a, Solomon et al 2010b, Dubourg et al 2018].
In the majority of individuals with HPE, a correlation exists between the facial anomalies and the gene involved and/or type of pathogenic variant (see and Types of HPE). However, it is important to note that in many cases this correlation cannot be made.
Facial findings in holoprosencephaly (HPE) A. Alobar HPE with cyclopia and proboscis above the single eye
Table 2a.
Nonsyndromic Holoprosencephaly: Most Common Monogenic Causes
View in own window
| Gene 1 | % of All
Nonsyndromic
HPE | Craniofacial
Findings | Other Key Features / Comment | Selected
OMIM Entries |
|---|
|
SHH
| 5.4%-5.9% 2 | Spectrum of HPE-related craniofacial features |
|
142945
|
|
ZIC2
| 4.8%-5.2% 2 | Bitemporal narrowing, upslanted palpebral fissures, large ears, short nose w/anteverted nares, & broad & deep philtrum 3 |
|
609637
|
|
SIX3
| ~3% 6 | Spectrum of HPE-related craniofacial features |
|
157170
|
|
TGIF1
| <1% 2 | Spectrum of HPE-related craniofacial features | May demonstrate the entire spectrum of severity 7 |
142946
|
- 1.
Genes are listed in order of most commonly involved.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
Table 2b.
Nonsyndromic Holoprosencephaly: Less Common Monogenic Causes
View in own window
| Gene 1 | % of All
Nonsyndromic
HPE | Craniofacial
Findings | Other Key Features / Comment | Selected
OMIM
Entries |
|---|
|
CDON
| Rare | Rare | Range of classic HPE-spectrum features described in several unrelated persons 2 |
614226
|
|
CNOT1
| <1.5% | Microtia, microcephaly, epicanthal folds, long philtrum | Semilobar HPE described in 2 unrelated persons Neonatal diabetes mellitus requiring insulin may be a feature. Pancreatic exocrine insufficiency may be present. May be assoc w/sensorineural & conductive hearing loss w/ossicle anomalies 3
| |
|
DISP1
| <1.2% | Bilateral cleft lip/palate, hypotelorism, single central maxillary incisor | Facial features may be consistent w/HPE-spectrum anomalies w/o corresponding brain anomalies. 4 |
607502
|
|
DLL1
| <1% | Rare | Facial features may be consistent w/HPE-spectrum anomalies. 5 |
606582
|
|
FGF8
| <2.2% | Spectrum of HPE-related craniofacial features | Range of classic HPE-spectrum features described in several unrelated persons 6 |
600483
|
|
FGFR1
| ~ 1.2% | Spectrum of HPE-related craniofacial features | Isolated HPE 7 | |
|
KMT2D
| Rare | Spectrum of HPE-related craniofacial features | Range of classic HPE-spectrum features described in 2 unrelated persons 8 | |
|
PPP1R12A
| Rare | Wide spectrum of craniofacial features | HPE, urogenital malformations, & DD 9 | |
|
RAD21
| Rare | Spectrum of HPE-related craniofacial features |
| |
|
SMC1A
| Rare | Spectrum of HPE-related craniofacial features | Range of classic HPE-spectrum features described in 5 unrelated females 10 | |
|
SMC3
| Rare | HPE-related craniofacial features | HPE-spectrum features described in 1 person w/semilobar HPE 10 | |
|
STAG2
| Rare | Spectrum of HPE-related craniofacial features | Range of classic HPE-spectrum features described in 6 unrelated females 10 | |
|
STIL
| Rare | Rare |
| |
- 1.
Genes are listed in alphabetic order.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
Variants in other candidate genes including FOXH1, GAS1, NODAL, PTCH1, SUFU, and CRIPTO (formerly TDGF1) have seldom been reported in individuals with nonsyndromic HPE. However, because investigations of several large HPE cohorts have failed to reproduce these findings, more data may be needed to confirm the possible role of these genes in HPE pathogenesis.
Although GLI2 pathogenic variants were described as a cause of HPE, it has become clear that GLI2 variants do not cause HPE, but rather a distinct phenotype characterized by pituitary anomalies, polydactyly, and subtle facial features (sometimes similar to HPE facial features) consistent with Culler-Jones syndrome (OMIM 615849) [Bear et al 2014, Bear & Solomon 2015].
3. Evaluation Strategies to Identify the Genetic Cause of Holoprosencephaly in a Proband
Establishing a specific genetic cause of holoprosencephaly (HPE) can aid in discussions of prognosis (which are beyond the scope of this GeneReview) and genetic counseling.
Evaluations to determine a specific genetic cause of holoprosencephaly usually involve the following.
Prenatal history to identify possible environmental causes. The most common teratogen in humans known to cause HPE is diabetes mellitus. While both gestational and pre-gestational diabetes are risk factors for HPE, pre-gestational diabetes requiring insulin confers the highest (>10-fold increased) HPE risk [Johnson & Rasmussen 2010, Tinker et al 2019].
Physical examination. A detailed physical examination should be conducted with special emphasis on extracranial features, especially those associated with syndromic forms of HPE (see ) [Kruszka & Muenke 2018]. Of note, it is not uncommon to find extracranial anomalies in individuals with nonsyndromic HPE (see , ) [Martinez et al 2018].
Family history. A three-generation family history should be taken with attention to pregnancy loss, neonatal deaths, and relatives with manifestations of holoprosencephaly and/or developmental delay with documentation of relevant findings through direct examination or review of medical records, including results of molecular genetic testing.
Focused examination of the parents and apparently normal sibs (whenever possible) to identify milder manifestations of HPE.
Molecular genetic testing. Approaches can include a combination of gene-targeted testing (multigene panel or single-gene testing) and comprehensive genomic testing (chromosomal microarray analysis, genome sequencing, exome sequencing, or exome array). Gene-targeted testing requires the clinician to hypothesize which gene(s) are likely involved, whereas genomic testing does not.
Chromosomal microarray analysis (CMA) using oligonucleotide or SNP arrays detects genome-wide large deletions/duplications in all forms of HPE, including apparently nonsyndromic HPE and HPE with associated anomalies. CMA has been successful in identifying pathogenic variants in up to 14% of individuals with HPE who have a normal
karyotype and no causative
gene identified on
multigene panel testing.
Chromosome analysis. Diagnostic methods for detecting
chromosome abnormalities in individuals with HPE are not different from those routinely used in the investigation of numeric and structural
cytogenetic abnormalities in those with other genetic conditions or birth defects. Recommended methods include: G-banding
karyotype and CMA [
Kruszka et al 2018]. If there is clinical suspicion for trisomy 13, a karyotype should be done first; otherwise, a CMA should be done first [
Pineda-Alvarez et al 2010,
Solomon et al 2010c], including trisomy 13.
Single-gene testing. When a specific
syndromic cause of HPE is considered (),
sequence analysis of the gene of interest is performed first to detect
missense,
nonsense, and
splice site variants and small intragenic deletions/insertions. Note: Depending on the sequencing method used and the gene involved, single-
exon, multiexon, or whole-gene deletions/duplications may not be detected. If no variant is detected by the sequencing method used, the next step is to consider gene-targeted
deletion/duplication analysis to detect exon and whole-gene deletions or duplications.
A multigene panel that includes some or all of the genes listed in , , and is most likely to identify the genetic cause of HPE while limiting identification of variants of
uncertain significance and pathogenic variants in genes that do not explain the underlying
phenotype. Note: (1) The genes included in the panel and the diagnostic
sensitivity of the testing used for each
gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this
GeneReview. Of note, given the rarity of some of the genes associated with HPE, some panels may not include all the genes mentioned in this overview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused
exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include
sequence analysis,
deletion/duplication analysis, and/or other non-sequencing-based tests. For this disorder a multigene panel that also includes deletion/duplication analysis is recommended.
For an introduction to multigene panels click
here. More detailed information for clinicians ordering genetic tests can be found
here.
Comprehensive
genomic testing does not require the clinician to determine which
gene(s) are likely involved.
Exome sequencing is most commonly used;
genome sequencing is also possible. If
exome sequencing is not diagnostic – and particularly when evidence supports
autosomal dominant or mendelian inheritance –
exome array (when clinically available) may be considered to detect multiexon deletions or duplications that cannot be detected by
sequence analysis.
For an introduction to comprehensive
genomic testing click
here. More detailed information for clinicians ordering genomic testing can be found
here.
4. Genetic Counseling
Genetic counseling is the process of providing individuals and families with
information on the nature, mode(s) of inheritance, and implications of genetic disorders to help them
make informed medical and personal decisions. The following section deals with genetic
risk assessment and the use of family history and genetic testing to clarify genetic
status for family members; it is not meant to address all personal, cultural, or
ethical issues that may arise or to substitute for consultation with a genetics
professional. —ED.
If a genetic etiology (chromosome abnormality, monogenic condition, or pathogenic variant in a HPE-associated gene) is established in a proband with HPE, specific counseling for recurrence risk is indicated [Hadley et al 2018].
Chromosome Abnormality – Risk to Family Members
Parents of a proband
Parents of a child with a numeric
chromosome abnormality (e.g., trisomy or triploidy) are expected to be chromosomally and phenotypically normal.
Parents of a child with a structural unbalanced
chromosome rearrangement (e.g.,
deletion,
duplication) are at risk of having a balanced chromosome rearrangement and should be offered chromosome analysis.
Sibs of a proband
Sibs of a child with a numeric
chromosome abnormality are at a slightly increased risk of having a similar chromosome abnormality (depending on the specific abnormality and the age of the mother) with a similar or different
phenotype.
The risk to the sibs of a child with a structural unbalanced
chromosome rearrangement depends on the chromosome status of the parents:
If neither parent has a structural rearrangement, the risk to sibs is negligible.
If a parent has a balanced structural rearrangement, the risk is increased and depends on the specific rearrangement and possibly other variables.
Offspring of a proband. Individuals with HPE and a chromosome rearrangement are unlikely to reproduce.
Other family members. The risk to other family members depends on the status of the proband's parents: if a parent has a chromosome rearrangement, the parent's family members are at risk and can be offered chromosome analysis.
Autosomal Dominant Inheritance – Risk to Family Members
Parents of a proband
Molecular genetic testing is recommended for the parents of a
proband with an apparent
de novo pathogenic variant. Recommendations may also include evaluation of the parents for mild manifestations of HPE.
The family history of some individuals diagnosed with HPE may appear to be negative because of reduced
penetrance and failure to recognize the disorder in family members; this is particularly true for families with HPE caused by pathogenic variants in
SIX3 [
Stokes et al 2018]. In some cases, a single mild manifestation is the only clue that a given individual has
autosomal dominant nonsyndromic HPE and thus is at increased risk of having affected offspring. Note, however, that none of the mild manifestations is pathognomonic for HPE and each can occur as an
isolated finding apart from the HPE spectrum. Therefore, an apparently negative family history cannot be confirmed unless appropriate
molecular genetic testing has been performed on the parents of the
proband.
Sibs of a proband. The risk to the sibs of the proband depends on the genetic status of the parents:
If a parent is affected or has an HPE
pathogenic variant (with or without clinical manifestations), the risk to sibs of inheriting the variant is 50%. Empiric studies indicate that sibs who inherit a pathogenic variant have a 20% risk for HPE, 15% risk for an HPE microform, and a 15% likelihood of a normal
phenotype.
If the parents have not been tested for the HPE
pathogenic variant in the
proband but are clinically unaffected and the family history is negative, the risk to the sibs of the proband appears to be low. However, sibs of a proband with clinically unaffected parents are still presumed to be at increased risk for HPE because of the possibility of reduced
penetrance in a parent or parental
germline mosaicism.
Offspring of a proband
Although severely affected individuals do not reproduce, individuals with mild forms and microforms of
autosomal dominant HPE may do so. The clinical manifestations and severity in offspring may range from mild to severe.
Other family members. The risk to other family members depends on the status of the proband's parents: if a parent is affected or has a pathogenic variant, the parent's family members may be at risk.
Autosomal Recessive Inheritance – Risk to Family Members
Parents of a proband
Sibs of a proband
Offspring of a proband. To date, individuals with STIL-HPE are not known to reproduce.
Other family members. Each sib of the proband's parents is at a 50% risk of being a carrier of a STIL pathogenic variant.
Carrier detection. Carrier testing for at-risk relatives requires prior identification of the STIL pathogenic variants in the family.
X-Linked Inheritance – Risk to Family Members
Parents of a female proband
A female
proband with
X-linked HPE caused by a
pathogenic variant in
STAG2 or
SMC1A may have inherited a pathogenic variant from either her mother or her father, or the pathogenic variant may be
de novo.
To date, the majority of individuals with
X-linked HPE are female, represent
simplex cases (i.e., a single occurrence in the family), and have the disorder as the result of a
de novo pathogenic variant.
Molecular genetic testing is recommended for both parents of a female
proband.
If the
STAG2 or
SMC1A pathogenic variant found in the
proband cannot be detected in the leukocyte DNA of either parent, the proband most likely has a
de novo pathogenic variant. Another possible explanation is that the proband inherited a pathogenic variant from a parent with
germline mosaicism.
Note: The mother of a
proband who is found to be
heterozygous for a
STAG2 or
SMC1A pathogenic variant may have favorable skewed X inactivation that results in her being mildly affected or unaffected.
Parents of a male proband
If a male is the only affected family member (i.e., a
simplex case), the mother may be
heterozygous or the affected male may have a
de novo pathogenic variant, in which case the mother is not heterozygous. To date,
X-linked HPE has been reported in only one male; the affected male had the disorder as the result of a
de novo
STAG2 truncating variant [
Aoi et al 2019];
SMC1A-HPE has not been reported in a male
proband.
Molecular genetic testing is recommended for the mother of a male
proband.
The father of an affected male will not have the disorder nor will he be
hemizygous for an
X-linked HPE-causing
pathogenic variant; therefore, he does not require further evaluation/testing.
Sibs of a female proband. The risk to sibs depends on the genetic status of the parents:
If the mother of the
proband has a
STAG2 or
SMC1A pathogenic variant, the chance of transmitting it in each pregnancy is 50%.
Females who inherit the
pathogenic variant are at high risk of developing HPE, although skewed X inactivation may result in variable phenotypic expression.
Because the large majority of individuals affected with
X-linked HPE identified to date are females, it is very likely that males who inherit
STAG2 or
SMC1A variants either do not survive or present a severe HPE
phenotype [
Aoi et al 2019,
Kruszka et al 2019b].
If the father of the
proband has a
STAG2 or
SMC1A pathogenic variant, he will transmit it to all his daughters and none of his sons.
If the
proband represents a
simplex case and if the
STAG2 or
SMC1A pathogenic variant cannot be detected in the leukocyte DNA of either parent, the risk to sibs is low but greater than that of the general population because of the possibility of parental
germline mosaicism.
Sibs of a male proband. The risk to sibs depends on the genetic status of the mother:
If the mother of the
proband has a
STAG2 or
SMC1A pathogenic variant, the chance of transmitting it in each pregnancy is 50% (see
Sibs of a female proband).
If the
proband represents a
simplex case (i.e., a single occurrence in a family) and if the
STAG2 or
SMC1A pathogenic variant cannot be detected in the leukocyte DNA of the mother, the risk to sibs is greater than that of the general population because of the possibility of maternal
germline mosaicism.
Offspring of a proband
Each child of a female
proband with
X-linked HPE has a 50% chance of inheriting the
STAG2 or
SMC1A pathogenic variant. Female probands with frank HPE are very unlikely to reproduce.
Each daughter of a male
proband with
X-linked HPE has a 50% chance of inheriting the
STAG2 or
SMC1A pathogenic variant. Surviving male probands with frank HPE are very unlikely to reproduce.
Other family members. The risk to other family members depends on the genetic status of the proband's parents: if a parent is affected or has a STAG2 or SMC1A pathogenic variant, the parent's family members may be at risk.
Prenatal Testing and Preimplantation Genetic Testing
High-Risk Pregnancies
Molecular genetic testing. Once the HPE-causing pathogenic variant(s) have been identified in an affected family member, prenatal testing for a pregnancy at increased risk for HPE and preimplantation genetic testing are possible.
Fetal ultrasound examination. For families with nonsyndromic HPE and no identifiable etiology, alobar HPE can be diagnosed by prenatal ultrasound examination between ten to 14 weeks of gestation based on abnormal facial morphology (as seen in many cases) and absence of the normal configuration of the choroid plexuses within the lateral ventricles, called "butterfly sign" [Kousa et al 2018, Calloni et al 2019]. Milder degrees of HPE including semilobar or lobar HPE cannot reliably be detected by prenatal ultrasound examination.
Lobar HPE can be recognized in utero with ultrasound. However, a specific diagnosis is often difficult and relies on qualitative evaluation of the morphology of the ventricles. Though not specific, antenatal demonstration of an echogenic linear structure running anterior-posterior within the third ventricle is highly suggestive of lobar HPE, and can assist this difficult diagnosis.
Fetal MRI is routinely used as a second-line investigation in several centers to evaluate CNS structure when ultrasound studies have suggested the presence of an anomaly [Edwards & Hui 2018]. MRI is useful for the evaluation of the posterior fossa and the median telencephalon as well as for etiologic clarification of hydrocephalus. Fetal MRI is particularly valuable for clarifying the anatomic subtypes of HPE, and thus informing on its severity [Edwards & Hui 2018, Kousa et al 2018, Calloni et al 2019]. Ultrafast MRI minimizes artifacts caused by fetal motion. Because MRI involves no exposure to radiation, it appears to be safe.
Low-Risk Pregnancies
When HPE is found on routine prenatal ultrasound examination in a fetus not known to be at increased risk for HPE, an extensive fetal examination, preferably using high-resolution ultrasound examination (e.g., examination with 3D ultrasound) to determine the presence of additional structural anomalies is indicated [Edwards & Hui 2018]. Additional testing on amniotic fluid may be done to both establish the cause of HPE and assist in the management of the pregnancy and the recurrence risk counseling of the parents. Such testing can include the following [Kruszka et al 2018]:
It is essential to bear in mind that if the fetus has HPE identified by ultrasound examination, medical and parental decision making about the pregnancy may occur independent of a specific genetic diagnosis.
Chapter Notes
Author History
Andrea L Gropman, MD, FAAP, FACMG; Children's National Health System (2000-2020)
Paul Kruszka, MD, MPH (2020-present)
Maximilian Muenke, MD, FACMG (2000-present)
Benjamin D Solomon, MD; National Human Genome Research Institute (2010-2020)
Cedrik Tekendo-Ngongang, MD (2020-present)
Revision History
5 March 2020 (bp) Comprehensive update posted live
29 August 2013 (me) Comprehensive update posted live
3 November 2011 (cd) Revision: clinical testing available for FOXH1 and NODAL
30 March 2010 (me) Comprehensive update posted live
5 March 2008 (me) Comprehensive update posted live
11 March 2005 (me) Comprehensive update posted live
27 January 2003 (me) Comprehensive update posted live
27 December 2000 (pb) Overview posted live
August 2000 (mm) Original submission