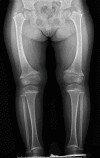
Clinical Description
Schmid metaphyseal chondrodysplasia (SMCD) is typically diagnosed in early childhood and is the most common and least severe metaphyseal chondrodysplasia [Al Kaissi et al 2018]. It results from disrupted calcification of metaphyseal cartilage and consequent restricted longitudinal growth of bones with preservation of the epiphyses. The clinical and radiographic features are usually not present at birth, but manifest in early childhood with limb shortening, genu varum, and a waddling gait [Bateman et al 2005]. The pattern of radiographic features is generally similar across individuals with SMCD, but clinical severity varies considerably [Mäkitie et al 2005]. There are no extraskeletal manifestations.
A comprehensive review of the published reports identified at least 130 unrelated individuals with SMCD and a confirmed pathogenic variant in COL10A1. The following description of the phenotypic features associated with this condition is based on these reports and author observations in a multidisciplinary skeletal dysplasia clinic.
Table 2.
Clinical and Radiographic Features of Schmid Metaphyseal Chondrodysplasia
View in own window
| Features | % of Persons
w/Feature | Comment |
|---|
|
Clinical
| Waddling gait | >80% | |
| Short stature | >60% | Typically apparent by age 2 yrs |
| Genu varum | >60% | Genu valgum less commonly reported |
|
Radiographic
| Metaphyseal dysplasia of proximal/distal femora, proximal tibiae | ~100% | Splaying, flaring, widening, cupping |
| Cupped &/or sclerotic anterior rib ends | >90% | |
| Coxa vara | >80% | Angle head & shaft of femur <120° |
| Short long bones | >60% | Typically apparent by age 2 yrs |
| Metaphyseal dysplasia of hands | 47% 1 | Metaphyseal cupping, short proximal phalanges/metacarpals |
| Vertebral dysplasia | 9% 2 | Usually mild; platyspondyly, anterior rounding, indentations, & posterior wedging |
Growth. Most neonates with SMCD have normal growth parameters. Progressive growth failure typically begins in the second year of life with individuals coming to medical attention after age two years with short-limbed short stature and bowed legs [Mäkitie et al 2005]. Adult height is typically more than 3.5 SD below the mean, although a wide spectrum that overlaps normal height has been reported [Mäkitie et al 2005]. No standard growth curves for SMCD are available.
Musculoskeletal. Most individuals with SMCD have genu varum (outward bowing at the knees), although valgus deformity has been reported. Waddling gait due to coxa vara is common by age two years and may require surgical correction. Joint pain in the knees and hips is common in adults and children with the condition and may limit physical activity. Chronic joint pain may develop and limit mobility for some individuals with SMCD. Lumbar lordosis may be present with typical onset by age three to five years.
Radiographic findings. The metaphyses of the long bones become flared or widened. The proximal and distal femoral and proximal tibial metaphyses are consistently affected (ragged, cupped, sclerotic, or splayed) with widening of the growth plates. Coxa vara (reduced angle to <120° between the head and the shaft of the femur) is seen in a majority of individuals and distinguishes SMCD from other forms of metaphyseal chondrodysplasia. Tibial and femoral bowing is typical. Metaphyseal irregularities of the distal ulnae and radii and enlarged capital femoral epiphyses are less commonly reported [Lachman et al 1988, Al Kaissi et al 2018]. The anterior rib ends are often cupped or sclerotic [Bateman et al 2005].
Hand involvement is present in fewer than half of individuals and is usually mild. Metaphyseal cupping of the distal metacarpals and proximal phalanges and shortening of the phalanges may be seen and are more pronounced in the fifth ray. Hand features may become less apparent with age [Elliott et al 2005].
Vertebral involvement is less common and, when present, is usually mild. Reported findings include platyspondyly and vertebral endplate anomalies (e.g., rounding of the anterior aspects of the vertebral bodies, superior and inferior indentations of the vertebral bodies, posterior wedging of the vertebrae) [Hasegawa et al 1994, Savarirayan et al 2000]. Vertebral changes may become less apparent with age.
Obesity. Limited mobility due to chronic joint pain may contribute to the development of obesity and associated comorbidities.
Craniofacial. The craniofacial bones and facial appearance are normal [Bateman et al 2005].
Neurodevelopment. Intelligence is normal. Attainment of early motor milestones is usually preserved in infancy; however, mild gross and fine motor delays may accompany orthopedic complications in early childhood.
Homozygotes. Biallelic pathogenic variants in COL10A1 have been associated with a more severe phenotype [Ain et al 2018] and in some cases embryonic lethality [Zhang et al 2018].