NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
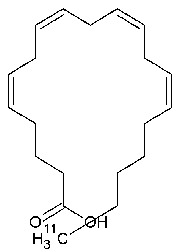
| Chemical name: | 1-[11C]Arachidonic acid |
 |
| Abbreviated name: | 1-[11C]AA | |
| Synonym: | ||
| Agent Category: | Small molecule | |
| Target: | Phospholipids | |
| Target Category: | Lipids | |
| Method of detection: | Positron emission tomography (PET) | |
| Source of signal / contrast: | 11C | |
| Activation: | No | |
| Studies: |
| Structure of 1-[11C]arachidonic acid. |
Background
[PubMed]
Arachidonic acid (AA), also known as all-cis-5,8,11,14-eicosatetraenoic acid (20:4n-6, a ω-6 fatty acid), is an essential fatty acid that is found primarily at the sn-2 position of most membrane phospholipids (PLs). Through the AA cascade it is a precursor for the synthesis of prostaglandins and leukotrienes, which have been implicated in the development of a variety of neurological disorders in mammals (1). The AA is released from the PLs by phospholipase A2 (PLA2) enzymes (both cytosolic and secretory forms) that are calcium dependent, and they are usually receptor activated. The released AA and its metabolites are believed to modulate a variety of processes in mammals including aging, ion channel functioning, neuropsychiatric disorders, inflammation, and diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (2-7). Because cytokines, nitric oxide, and glutamate influence calcium mobilization in the tissue, they are known to affect PLA2 activation, the release of AA from PLs, and neuroinflammation observed with different neurological diseases (8-13).
The AD brain has been shown to have higher than normal PLA2 enzyme activity and increased levels of cytokines, β-amyloid proteins and peptides, glutamatergic markers, and AA metabolites (14-18). Esposito et al. envisioned that elevated AA metabolism in the AD brain could perhaps be used to image the neuroinflammation during the course and therapy of the disease because this fatty acid is either synthesized de novo or from linoleic acid (18:2n-6), which can be considered as its precursor in mammals (19). Using AA labeled with radioactive carbon (1-11C-AA), investigators have determined the regional brain AA incorporation coefficient (K*), which is the ratio of radioactivity in the brain and the integral of AA in plasma, developed using quantitative autoradiography in rodents with 14C-labeled AA (20) and positron emission tomography (PET) in non-human primates (21) and humans with 11C-labeled AA (19, 22). In addition, labeled AA has been shown to be an ideal brain imaging agent, and K* was not influenced by regional cerebral blood flow (CBF) changes measured with water containing radioactive oxygen (15O-water) (19).
Synthesis
[PubMed]
The methods described by Chang et al. (21) and Channing et al. (23) were used for the synthesis of 1-11C-AA (19, 22). The radiochemical was routinely obtained at the end of synthesis in ~35 min and was formulated in 0.9% saline containing 8% serum (source of the serum used was not specified) (21). Stability of the formulated tracer was not reported. Radiochemical purity of 1-11C-AA was reported to be >95% as determined with high-performance liquid chromatography with a specific activity of ~37 GBq/μmol (~1 Ci/μmol; n = 10) at the end of bombardment. In another publication the specific activity of 1-11C-AA was reported to be 3,700 MBq/μmol (100 mCi/μmol) with a radiochemical purity of >97.6% (19).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
Much literature is available regarding AA activity under in vitro conditions (PubMed), however only some studies are presented in this section. Readers interested to learn more about the in vitro investigations with AA are encouraged to check references available by clicking on the above link. It is also pertinent to mention that all studies reviewed in this section were performed with unlabeled AA.
Using a mouse epidermal JB6 cell line model the effects of omega 3 fatty acids such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and AA (an omega 6 fatty acid) on phorbol 12-tetradecanoate 13-acetate (TPA)- induced or epidermal growth factor (EGF) induced transcription activator protein 1 (AP-1) transactivation and the subsequent transformation of the cells was investigated (24). Among the two omega 3 fatty acids DHA was more effective at inhibiting cell transformation, but AA inhibited the beneficial effects of DHA. The investigators concluded the dietary ratio of omega 3 and omega 6 fatty acids may be an important factor in tumorigenesis under in vivo conditions.
In another study it was demonstrated that an AA metabolite (hydroxyeicosatetraenoic acid (15(S)-HETE) stimulated the adhesion of human metastatic human breast carcinoma MDA-MB-435 cells to the extracellular matrix (type IV collagen) by activating the p38 mitogen-activated protein kinase (25). This indicated that 15(S)-HETE initiated the signal transduction pathway responsible for adhesion of the cells to the extracellular matrix. AA was also shown to induce MDA-MB-231 cell migration by Navarro-Tito et. al. (26). Using tumor derived endothelial cells from human breast carcinomas it was shown that AA promotes Ca2+ entry into the cells during early phases of angiogenesis and this AA activity is inhibited by carboxyamidotriazole (27). This indicated that AA may have a role in angiogenesis during tumor development.
Animal Studies
Rodents
[PubMed]
Basselin et al. used autoradiography to investigate the effect of a lithium chloride (LiCl) or control diet on the K* of 1-14C-AA in brains of rats after an intracerebroventricular infusion of either lipopolysaccharide (LPS), which induces neuroinflammation and elevates AA metabolism in the brain, or artificial cerebrospinal fluid (aCSF) (28). Compared with animals on the aCSF diet, LPS was reported to significantly increase the K* in 28 regions of the brain in rats on the control diet. Under the same conditions, LiCl prevented an increase in K* in 18 of the same regions of the brain in rats on the control diet. LiCl was reported to increase K* in 14 regions (primarily in the visual and auditory systems) of the brain in rats fed aCSF. Markers of AA metabolism such as PLA2 activity as well as prostaglandin E2 and thromboxane B2 levels were increased by LPS infusion in the animals on the control diet, but no such increase was observed in rats treated with LPS and fed the LiCl diet.
Non-Human Primates
[PubMed]
The incorporation of 1-11C-AA was investigated using PET in the brains of normocapnic (normal carbon dioxide levels in arterial blood) and hypercapnic (increased carbon dioxide blood levels) monkeys (n = 4) (21). CBF was measured in the animals with 15O-water before the intravenous infusion of 1-11C-AA. Dynamic PET scans were performed on the animals at various time points starting at 0 min for up to 60 min after the infusion of labeled AA. During the same time, arterial blood samples were taken from the animals. The half-life of 1-11C-AA in the blood was reported to be 1.1 min, and radioactivity in the brain reached a steady-state within 10 min. The K* of 1-11C-AA was determined to be 1.1–1.2 × 10-4 ml/sec per gram of gray matter and remained consistent at all time points even after a 2.6-fold increase in CBF caused by hypercapnia in the animals. The investigators concluded that the incorporation of 11C-AA could be quantified in monkeys with PET and that the accumulation of this radiochemical in the brain was independent of blood flow.
Human Studies
[PubMed]
Giovacchini et al. used PET to measure the incorporation of 1-11C-AA in the brains of eight rested, young, and healthy adult humans (22). The K* and cerebral blood volume (Vb) were determined for the gray and white matter in the subjects. The K* values were 5.6 ± 1.2 and 2.6 ± 0.5 μL/min per mL in the gray and white matter, respectively. The Vb values were reported to remain unchanged for the data analysis periods from 20 to 60 min.
In another study, the effect of healthy aging on 1-11C-AA incorporation, blood volume, and blood flow was investigated in eight young (27 ± 5 y) and seven old (65 ± 9 y) healthy individuals after applying the partial-volume correction (PVC) (29). No differences between the K* and Vb values of the young and old individuals were reported before or after the application of PVC. However, a significant reduction of CBF was apparent in the frontal cortex of individuals in the older group. After normalization to the global gray area of the brain, the K*, Vb, and CBF values were significantly reduced in frontal lobe of the older individuals, but this difference disappeared after application of the PVC. The investigators concluded that brain function as determined with PET imaging is affected minimally by healthy aging in humans.
PET imaging was also used to study signal transduction by 1-11C-AA in the human brain during visual stimulation (30). 1-11C-AA was administered to the individuals through the intravenous route, and K* of the labeled fatty acid was determined in different parts of the brain. Sixteen healthy individuals were enrolled for the study; they were split into two groups each having eight subjects. One group was subjected to visual stimulation by flash frequencies of either 2.9 Hz or 7.8 Hz and compared with the second group, which was not visually stimulated and was kept under dark conditions (0 Hz). CBF was measured after an intravenous injection of 15O-water in the individuals kept under the same conditions for visual stimulation. Compared with the dark conditions, a significant increase in K* (2.3–8.9%) was observed in the Brodmann areas 17, 18, and 19 as well as in some frontal, parietal, and temporal cortical regions of the brain in individuals who were flash-stimulated at either frequency. The CBF was reported to increase significantly (3.1–22%) in these subjects, usually in areas of the brain comparable to those mentioned above. Reduced K* and CBF levels were also reported in some frontal brain areas of these individuals. From this study it was concluded AA plays a role in the signaling process provoked by visual stimulation, since visual stimulation at flash frequencies of 2.9 and 7.8 Hz compared to 0 Hz modifies both K* for AA and rCBF in visual and related areas of the human brain.
Because neuroinflammation is believed to play a role in the pathogenesis of AD, Esposito et al. used PET with 1-11C-AA to determine the K* for AA in patients with AD (19). Eight patients with AD (mean age, 71.7 ± 11.2 y) and nine normal control subjects (mean age, 68.7 ± 5.6 y) were enrolled in the study. CBF in the individuals was determined after an intravenous injection of 15O-water, and 1-11C-AA was administered to the individuals 15 min later to determine the K* for AA, with or without the application of PVC. Compared with the control group, the K* of global gray matter, with or without the application of PVC, was significantly elevated in subjects belonging to the AD group, but the global CBF (without the application of PVC) was reduced significantly (P < 0.05) in these individuals. The K* for AA of individuals in the AD group, after the application of PVC, was elevated in 78 of the 90 identified hemispheric gray matter regions of the brain, particularly in the neocortex, but this was not apparent in the caudate, palladium, or the thalamic regions. From these observations the investigators concluded that an upregulated AA metabolism is associated with neuroinflammation, and PET with 1-11C-AA can be used to detect neuroinflammation in patients with brain diseases, including AD.
References
- 1.
- Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007; 102 (3):577–86. [PMC free article: PMC2084377] [PubMed: 17403135]
- 2.
- Calder P.C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008; 52 (8):885–97. [PubMed: 18504706]
- 3.
- Hudson C. , Gotowiec A. , Seeman M. , Warsh J. , Ross B.M. Clinical subtyping reveals significant differences in calcium-dependent phospholipase A2 activity in schizophrenia. Biol Psychiatry. 1999; 46 (3):401–5. [PubMed: 10435206]
- 4.
- McGahon B. , Clements M.P. , Lynch M.A. The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience. 1997; 81 (1):9–16. [PubMed: 9300396]
- 5.
- Meves H. Arachidonic acid and ion channels: an update. Br J Pharmacol. 2008; 155 (1):4–16. [PMC free article: PMC2527843] [PubMed: 18552881]
- 6.
- Patrick C.B. , Krzywkowski P. , Ramassamy C. , Poirier J. , Rapoport S.I. , Murphy E.J. Phospholipase A2 activity is decreased selectively in the hippocampus of aged apolipoprotein E deficient mice. Neurosci Lett. 2000; 288 (3):211–4. [PubMed: 10889345]
- 7.
- Smalheiser N.R. , Swanson D.R. Calcium-independent phospholipase A2 and schizophrenia. Arch Gen Psychiatry. 1998; 55 (8):752–3. [PubMed: 9707387]
- 8.
- Lehtonen J.Y. , Holopainen J.M. , Kinnunen P.K. Activation of phospholipase A2 by amyloid beta-peptides in vitro. Biochemistry. 1996; 35 (29):9407–14. [PubMed: 8755719]
- 9.
- Mattson M.P. , Chan S.L. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium. 2003; 34 (4-5):385–97. [PubMed: 12909083]
- 10.
- Orr S.K. , Bazinet R.P. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs. 2008; 9 (7):735–43. [PubMed: 18600579]
- 11.
- Sun G.Y. , Horrocks L.A. , Farooqui A.A. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007; 103 (1):1–16. [PubMed: 17561938]
- 12.
- Rao J.S. , Ertley R.N. , DeMar J.C. Jr, Rapoport S.I. , Bazinet R.P. , Lee H.J. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007; 12 (2):151–7. [PubMed: 16983392]
- 13.
- Rao J.S. , Ertley R.N. , Lee H.J. , DeMar J.C. Jr, Arnold J.T. , Rapoport S.I. , Bazinet R.P. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007; 12 (1):36–46. [PubMed: 16983391]
- 14.
- Farooqui A.A. , Horrocks L.A. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006; 12 (3):245–60. [PubMed: 16684969]
- 15.
- Jana M. , Palencia C.A. , Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol. 2008; 181 (10):7254–62. [PMC free article: PMC2701549] [PubMed: 18981147]
- 16.
- Kashani A. , Lepicard E. , Poirel O. , Videau C. , David J.P. , Fallet-Bianco C. , Simon A. , Delacourte A. , Giros B. , Epelbaum J. , Betancur C. , El Mestikawy S. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol Aging. 2008; 29 (11):1619–30. [PubMed: 17531353]
- 17.
- Sanchez-Mejia R.O. , Newman J.W. , Toh S. , Yu G.Q. , Zhou Y. , Halabisky B. , Cisse M. , Scearce-Levie K. , Cheng I.H. , Gan L. , Palop J.J. , Bonventre J.V. , Mucke L. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat Neurosci. 2008; 11 (11):1311–8. [PMC free article: PMC2597064] [PubMed: 18931664]
- 18.
- Steinman L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008; 118 (11):3557–63. [PMC free article: PMC2575716] [PubMed: 18982162]
- 19.
- Esposito G. , Giovacchini G. , Liow J.S. , Bhattacharjee A.K. , Greenstein D. , Schapiro M. , Hallett M. , Herscovitch P. , Eckelman W.C. , Carson R.E. , Rapoport S.I. Imaging neuroinflammation in Alzheimer's disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008; 49 (9):1414–21. [PMC free article: PMC2587283] [PubMed: 18703605]
- 20.
- Robinson P.J. , Noronha J. , DeGeorge J.J. , Freed L.M. , Nariai T. , Rapoport S.I. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992; 17 (3):187–214. [PubMed: 1467810]
- 21.
- Chang M.C. , Arai T. , Freed L.M. , Wakabayashi S. , Channing M.A. , Dunn B.B. , Der M.G. , Bell J.M. , Sasaki T. , Herscovitch P. , Eckelman W.C. , Rapoport S.I. Brain incorporation of [1-11C]arachidonate in normocapnic and hypercapnic monkeys, measured with positron emission tomography. Brain Res. 1997; 755 (1):74–83. [PubMed: 9163542]
- 22.
- Giovacchini G. , Chang M.C. , Channing M.A. , Toczek M. , Mason A. , Bokde A.L. , Connolly C. , Vuong B.K. , Ma Y. , Der M.G. , Doudet D.J. , Herscovitch P. , Eckelman W.C. , Rapoport S.I. , Carson R.E. Brain incorporation of [11C]arachidonic acid in young healthy humans measured with positron emission tomography. J Cereb Blood Flow Metab. 2002; 22 (12):1453–62. [PubMed: 12468890]
- 23.
- Channing M.A. , Simpson N. Radiosynthesis of 1-[11C]polyhomoallylic acids. Journal of Labelled Compounds and Radiopharmaceuticals. 1993; 33 (6):541–546.
- 24.
- Liu G. , Bibus D.M. , Bode A.M. , Ma W.Y. , Holman R.T. , Dong Z. Omega 3 but not omega 6 fatty acids inhibit AP-1 activity and cell transformation in JB6 cells. Proc Natl Acad Sci U S A. 2001; 98 (13):7510–5. [PMC free article: PMC34699] [PubMed: 11416221]
- 25.
- Nony P.A. , Kennett S.B. , Glasgow W.C. , Olden K. , Roberts J.D. 15S-Lipoxygenase-2 mediates arachidonic acid-stimulated adhesion of human breast carcinoma cells through the activation of TAK1, MKK6, and p38 MAPK. J Biol Chem. 2005; 280 (36):31413–9. [PubMed: 16000313]
- 26.
- Navarro-Tito N. , Robledo T. , Salazar E.P. Arachidonic acid promotes FAK activation and migration in MDA-MB-231 breast cancer cells. Exp Cell Res. 2008; 314 (18):3340–55. [PubMed: 18804105]
- 27.
- Fiorio Pla A. , Grange C. , Antoniotti S. , Tomatis C. , Merlino A. , Bussolati B. , Munaron L. Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol Cancer Res. 2008; 6 (4):535–45. [PubMed: 18403634]
- 28.
- Basselin M. , Villacreses N.E. , Lee H.J. , Bell J.M. , Rapoport S.I. Chronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammation. J Neurochem. 2007; 102 (3):761–72. [PubMed: 17488274]
- 29.
- Giovacchini G. , Lerner A. , Toczek M.T. , Fraser C. , Ma K. , DeMar J.C. , Herscovitch P. , Eckelman W.C. , Rapoport S.I. , Carson R.E. Brain incorporation of 11C-arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med. 2004; 45 (9):1471–9. [PubMed: 15347713]
- 30.
- Esposito G. , Giovacchini G. , Der M. , Liow J.S. , Bhattacharjee A.K. , Ma K. , Herscovitch P. , Channing M. , Eckelman W.C. , Hallett M. , Carson R.E. , Rapoport S.I. Imaging signal transduction via arachidonic acid in the human brain during visual stimulation, by means of positron emission tomography. Neuroimage. 2007; 34 (4):1342–51. [PMC free article: PMC2040045] [PubMed: 17196833]
- Functional consequences of phospholipase A2 activation in human monocytes.[Adv Exp Med Biol. 1990]Functional consequences of phospholipase A2 activation in human monocytes.Hoffman T, Brando C, Lizzio EF, Lee C, Hanson M, Ting K, Kim YJ, Abrahamsen T, Puri J, Bonvini E. Adv Exp Med Biol. 1990; 279:125-36.
- Fatty acid and phospholipid selectivity of different phospholipase A2 enzymes studied by using a mammalian membrane as substrate.[Biochem J. 1994]Fatty acid and phospholipid selectivity of different phospholipase A2 enzymes studied by using a mammalian membrane as substrate.Diez E, Chilton FH, Stroup G, Mayer RJ, Winkler JD, Fonteh AN. Biochem J. 1994 Aug 1; 301 ( Pt 3)(Pt 3):721-6.
- Imaging neuroinflammation in Alzheimer's disease with radiolabeled arachidonic acid and PET.[J Nucl Med. 2008]Imaging neuroinflammation in Alzheimer's disease with radiolabeled arachidonic acid and PET.Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, Hallett M, Herscovitch P, Eckelman WC, Carson RE, et al. J Nucl Med. 2008 Sep; 49(9):1414-21. Epub 2008 Aug 14.
- Review [Phospholipase A2: characteristics and function].[Cesk Fysiol. 1998]Review [Phospholipase A2: characteristics and function].Sedláková A, Kohút A. Cesk Fysiol. 1998 Sep; 47(3):95-103.
- Review Phospholipase A2.[J Biochem. 2002]Review Phospholipase A2.Murakami M, Kudo I. J Biochem. 2002 Mar; 131(3):285-92.
- 1-[11C]Arachidonic acid - Molecular Imaging and Contrast Agent Database (MICAD)1-[11C]Arachidonic acid - Molecular Imaging and Contrast Agent Database (MICAD)
- Gd-DTPA l-Cystine bisisopropyl amide copolymers - Molecular Imaging and Contrast...Gd-DTPA l-Cystine bisisopropyl amide copolymers - Molecular Imaging and Contrast Agent Database (MICAD)
- Giraffa camelopardalis antiquorum haplotype Rafiki/ZA4124 cytochrome b (cytb) ge...Giraffa camelopardalis antiquorum haplotype Rafiki/ZA4124 cytochrome b (cytb) gene, complete cds; tRNA-Thr and tRNA-Pro genes, complete sequence; and control region, partial sequence; mitochondrialgi|146262877|gb|EF442265.1|Nucleotide
- Penaeus vannamei obstructor A1 mRNA, partial cdsPenaeus vannamei obstructor A1 mRNA, partial cdsgi|1226142480|gb|MF415537.1|Nucleotide
- Penaeus vannamei obstructor F1 mRNA, complete cdsPenaeus vannamei obstructor F1 mRNA, complete cdsgi|1226142486|gb|MF415540.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...

 Rodents
Rodents