NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
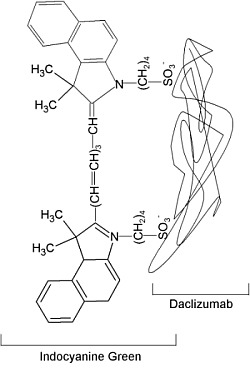
| Chemical name: | Daclizumab complexed to near-infrared fluorophore indocyanine green |
 |
| Abbreviated name: | Dac-ICG | |
| Synonym: | ||
| Agent Category: | Monoclonal antibody | |
| Target: | Interleukin 2 receptor α chain (CD25) | |
| Target Category: | Receptor | |
| Method of detection: | Optical: Near-infrared (NIR) fluorescence | |
| Source of signal / contrast: | Indocyanine green | |
| Activation: | Yes | |
| Studies: |
| Proposed structure of Daclizumab-indocyanine green conjugate. |
Background
[PubMed]
The technique of non-invasive optical imaging is based on the principle that when a dye is exposed to light it absorbs energy and is elevated from the steady state to a high-energy state. To return to its normal, low-energy state, the molecule releases energy in the form of photons with wavelengths that are either in the visible (400–700 nm) or the near infrared (NIR; 700–900 nm) range (1). Although both types of dyes can be used for imaging, in general NIR agents are favored over those functional in the visible range because fluorescence from NIR probes can be detected in deep tissue, have low autofluorescence, and generate low background signals because tissues do not absorb in the NIR wavelength range (2). Among the various NIR agents, only one, indocyanine green (ICG; also known as IR-125 or Cardiogreen), with absorption at ~780 nm and emission at ~820 nm, is approved by the United States Food and Drug Administration (US FDA) for clinical applications (3) and is under evaluation in several clinical trials for other applications. ICG is a tricarbocyanine dye that is fluorescent only in the free state and easily binds to proteins through non-covalent linkages (such as ionic, hydrophobic, electrostatic, or hydrogen bonds). When bound to proteins ICG is non-fluorescent, but on dissociation from the protein complex it reverts to the fluorescent state and the signal has been shown to be suitable for in vivo imaging (4).
The interleukin-2 (IL-2) receptor (IL2R) consists of three subunits (α, β, and γ) that are non-covalently linked. Among these subunits, the γ-chain is a constitutive component of the cell membrane, but the α- (also known as CD25) and β-chains have to be induced by IL-2 and other related ligands to bring about functionality to the receptor (5). The functions of the various subunits of IL2R and the signaling pathways used to mediate its activity are described elsewhere (5). Induction of the α-chain is believed to impart specificity to the receptor and indicates activation of the T cells and dendritic cells that participate in the development of an immune response and play a role in the progression of various malignancies and autoimmune diseases (6, 7). Once activated, the receptor-ligand complex is internalized by the cell for inactivation through enzymatic digestion in the lysosomes (8, 9). Because of its possible role in the development of allograft rejection, cancer, autoimmune diseases, etc., daclizumab (Dac), a commercially available humanized monoclonal antibody (MAb) directed toward the IL2R α-chain, is approved by the US FDA to prevent or treat renal allograft rejection (10). It is also under investigation in several clinical trials for the treatment of a variety of ailments, including cancer.
The characteristic properties of ICG (fluorescent only in an unbound state) and the IL2R α-chain (internalization by the cell) prompted Ogawa et al. to develop an imaging probe for cancer by complexing ICG to Dac (Dac-ICG) (4). The investigators used the Dac-ICG complex to image cells that express the IL2R α-chain under in vitro conditions and showed that the complex could also be used for the imaging of IL2R α-chain–expressing xenograft tumors in mice.
Synthesis
[PubMed]
The synthesis of Dac-ICG was described by Ogawa et al. (4). ICG-N-hydroxysulfosuccinimide ester (ICG-sulfo-OSu) and Dac were obtained from commercial sources. Dac was incubated with ICG-sulfo-OSu (Dac:ICG ratio was either 1:1 or 1:5) in phosphate buffer (pH 8.5) for 30 min at room temperature. The Dac-ICG complex was purified on a PD-10 Sephadex G50 column with phosphate-buffered saline (PBS; pH not reported) as the elution buffer. With the reaction conditions used to generate the MAb-ICG complex, either oneor five ICG molecules (Dac-ICG(1:1) or Dac-ICG(1:5)) were reported to be bound to each Dac molecule, respectively.
For use as a control, a human polyclonal IgG-Cy5.5 complex was also synthesized in the manner described above. The storage conditions and stability of the various MAb-ICG complexes were not reported.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
Ogawa et al. performed fluorescence imaging with Dac-ICG(1:1) and Dac-ICG(1:5) solutions in PBS under in vitro conditions (4). Fluorescence of the two complexes was measured before and after exposure to a mixture of sodium dodecyl sulfate (SDS) and 2-mercaptoethanol (2-ME). Fluorescence was detected only in solutions exposed to the denaturing (SDS) and reducing (2-ME) agents. The Dac-ICG(1:5) solution exposed to SDS and 2-ME was reported to have a higher fluorescence compared to the Dac-ICG(1:1) solution under the same conditions, suggesting that the degree of fluorescence obtained from a dissociated MAb-ICG complex was proportionate to the number of ICG molecules bound to the MAb molecule. This indicated that dissociation of ICG from the MAb-ICG complex resulted in reversion of the dye from a non-fluorescent state to a fluorescent state.
Animal Studies
Rodents
[PubMed]
Xenograft tumors were generated after subcutaneous (s.c.) injection of A431cells (of human skin carcinoma origin ) transfected either with the red fluorescent protein (A431/DsRed, used as control) or the IL2R α-chain (ATAC4 cells) in the right and left dorsum, respectively, of mice (n = 10 animals) for imaging studies (4). The Dac-ICG (1:1 or 1:5) complexes were respectively administered intravenously to the animals (n = 5 mice/Dac-ICG complex) through the tail vein 14–18 days after s.c. injection of the cells. Fluorescent imaging was performed on the mice 0–4 days after injection of the Dac-ICG complexes. During this period, fluorescence was observed only in the ATAC4 cell tumors, and the signal was higher with the Dac-ICG(1:5) complex versus the Dac-ICG(1:1) complex. Coinjection of the Dac-ICG(1:5) complex with the control IgG-Cy5.5 complex showed that all tumors were fluorescent with the non-specific Cy5.5 complex, but only the ATAC4 tumors were fluorescent with the Dac-ICG complex (fluorescence from the two dyes in the images was separated using appropriate software), indicating that the latter complex was specific for tumors expressing the IL2R α-chain. After treatment of the mice with the two Dac-ICG complexes, a steady increase in fluorescence with Dac-ICG(1:5) was reported in the ATAC4 tumors for 1 day (~150 autofluorescence units (au)), and it gradually decreased to ~100 au by day 4. During the same period, tumors of mice injected with the Dac-ICG(1:1) complex showed a steady fluorescence of ~20 au. A histological examination of the A431 and ATAC4 cell tumors using the hematoxylin and eosin stain showed that both tumor types had the same histology. No competition studies using animals pretreated with Dac were reported.
With observations from this study, the investigators concluded that the Dac-ICG complex could probably be adapted for tumor imaging in humans during surgery. However, they cautioned that the Dac-ICG complex could have a different toxicity compared with individual components of the complex, and extensive clinical testing of Dac-ICG would have to be performed before its application in humans.
References
- 1.
- Sharma R. , Wendt J.A. , Rasmussen J.C. , Adams K.E. , Marshall M.V. , Sevick-Muraca E.M. New horizons for imaging lymphatic function. Ann N Y Acad Sci. 2008; 1131 :13–36. [PMC free article: PMC3094766] [PubMed: 18519956]
- 2.
- Luker G.D. , Luker K.E. Optical imaging: current applications and future directions. J Nucl Med. 2008; 49 (1):1–4. [PubMed: 18077528]
- 3.
- Sakka S.G. Assessing liver function. Curr Opin Crit Care. 2007; 13 (2):207–14. [PubMed: 17327744]
- 4.
- Ogawa, M., N. Kosaka, P.L. Choyke, and H. Kobayashi, In vivo Molecular Imaging of Cancer with a Quenching Near-Infrared Fluorescent Probe Using Conjugates of Monoclonal Antibodies and Indocyanine Green. Cancer Res, 2009. [PMC free article: PMC2788996] [PubMed: 19176373]
- 5.
- Gaffen S.L. Signaling domains of the interleukin 2 receptor. Cytokine. 2001; 14 (2):63–77. [PubMed: 11356007]
- 6.
- Driesen J. , Popov A. , Schultze J.L. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology. 2008; 213 (9-10):849–58. [PubMed: 18926299]
- 7.
- Ali E. , Healy B. , Stazzone L. , Brown B. , Weiner H. , Khoury S. Daclizumab in treatment of multiple sclerosis patients. Mult Scler. 2009; 15 (2):272–274. [PubMed: 19136546]
- 8.
- Woodman P. ESCRT proteins, endosome organization and mitogenic receptor down-regulation. Biochem Soc Trans. 2009; 37 (Pt 1):146–50. [PubMed: 19143620]
- 9.
- Nabholz M. , Combe M.C. , Corthesy P. , Lowenthal J. , Gabathuler R. Interleukin 2 receptor traffic in a murine cytolytic T cell line. Eur J Immunol. 1987; 17 (6):783–90. [PubMed: 3109923]
- 10.
- Kim S.E. Daclizumab treatment for multiple sclerosis. Pharmacotherapy. 2009; 29 (2):227–35. [PubMed: 19170591]
- Daclizumab complexed to near-infrared fluorophore indocyanine green - Molecular ...Daclizumab complexed to near-infrared fluorophore indocyanine green - Molecular Imaging and Contrast Agent Database (MICAD)
- 177Lu-DOTA-Gly-4-aminobenzoyl-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 - Molecular Im...177Lu-DOTA-Gly-4-aminobenzoyl-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 - Molecular Imaging and Contrast Agent Database (MICAD)
- 18F-Labeled neogalactosylalbumin - Molecular Imaging and Contrast Agent Database...18F-Labeled neogalactosylalbumin - Molecular Imaging and Contrast Agent Database (MICAD)
- Mus musculus alkB homolog 3, alpha-ketoglutarate-dependent dioxygenase (Alkbh3),...Mus musculus alkB homolog 3, alpha-ketoglutarate-dependent dioxygenase (Alkbh3), transcript variant 15, non-coding RNAgi|2463695008|ref|NR_184795.1|Nucleotide
- Gadolinium-diethylenetriamine tetraacetic acid-icosahedral closo-borane12 scaffo...Gadolinium-diethylenetriamine tetraacetic acid-icosahedral closo-borane12 scaffold - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro