NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
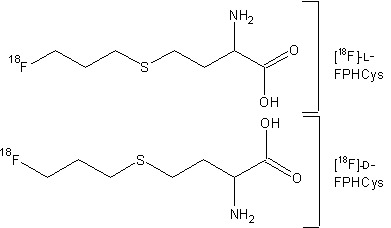
| Chemical name: | l- and d-S-(3-[18F]fluoropropyl)homocysteine |
|
| Abbreviated name: | l- and d-[18F]FPHCys | |
| Synonym: | ||
| Agent Category: | Compound | |
| Target: | LAT1 amino acid transporter | |
| Target Category: | Transporter | |
| Method of detection: | Positron emission tomography (PET) | |
| Source of signal / contrast: | 18F | |
| Activation: | No | |
| Studies: |
| Structures of L- and D-[18F]FPHCys |
Background
[PubMed]
High energy consumption and constant proliferation are the hallmarks of all cells with a malignant phenotype. To maintain a high pace of protein and DNA synthesis, such cells have an increased demand for various nutrients, glucose, and amino acids (aa). Therefore, radiotracers such as 18F-labeled fluorodeoxyglucose, which is taken up by the cell through the glucose transporter, are often used with positron emission tomography (PET) to detect cancerous tumors. This agent, however, is not able to distinguish between malignant tissue, inflammation, and tissues that normally have high glucose consumption, such as the brain (1). As an alternative, radiolabeled aa and their derivatives, such as those of phenylalanine and tyrosine, have been used to detect neoplastic tumors because these lesions show increased utilization of aa for the synthesis of proteins and other cellular components (2, 3). To accommodate the increased demand for aa, the malignant cells overexpress the aa transporters (phenylalanine and tyrosine use the l-type transporter), and this phenomenon promotes the rapid uptake and accumulation of the radiolabeled aa in the tumors. Therefore, noninvasive imaging with a radiolabeled aa can be used to detect cancerous lesions within a short time after administration of an aa tracer. Among the various radiolabeled aa tracers, [18F]-α-methyl-tyrosine is often used in the clinic, but the low yield of the final labeled product prohibits the use of this labeled compound in most oncology centers (1).
S-(2-[11C]methyl)-l-methionine ([11C]MET; half-life of 11C = ~20 min) is another aa derivative that is widely used for the PET imaging of tumors, but because MET contributes to a diverse array of biosynthetic reactions such as DNA synthesis, protein synthesis, etc., the radiolabeled macromolecules derived from [11C]MET along with the biodegradation products of the labeled molecules generate a high background signal in the tissues (4). As a consequence, the imaging of tumors with [11C]MET has limited utility in the clinic and this tracer has been used primarily for the noninvasive visualization of gliomas in the brain, and very little information is available to indicate that labeled MET is suitable for the detection of tumors in other parts of the body (3, 4). Investigators developed S-(2-[18F]fluoroethyl)-L-homocysteine, an analog of MET, as a possible agent for the imaging of cancerous tumors, but this agent was unstable in aqueous media and could be used for the detection of these lesions (5). In a continued effort to develop an aa derivative that can be used for the imaging of non-glioma tumors, the d and l enantiomers of S-(3-[18F]fluoropropyl)homocysteine ([18F]-d-FPHCys and [18F]-l-FPHCys) were synthesized, and the biodistribution and tumor imaging properties of these compounds in nude mice bearing xenograft tumors derived from different non-glioma human cancer cell lines was investigated (4, 6).
Related Resource Links
Amino acid transporter related chapters in MICAD
Clinical trials related to amino acid transporters
Clinical trials using amino acids as imaging agents
Amino acid transporters in Online Mendelian Inheritance in Man (OMIM)
Gene information regarding l- type amino acid transporters
Cystinuria – an inherited defect of amino acid transport
Human amino acid transport disorders [PubMed]
Human amino acid metabolism disorders [PubMed]
Synthesis
[PubMed]
The synthesis of d-FPHCys and l-FPHCys and their labeling with 18F have been described by Bourdier et al. (4). The enantiomeric purity, radiochemical purity, and radiochemical yield of both enantiomers of [18F]FPHCys were typically >98%, >98%, and 20 ± 5%, respectively. The total time to prepare both tracers, from purification with high-performance liquid chromatography (HPLC), formulation, and sterile filtration through a 0.22-μm filter (for biological studies), was ~65 min. Both 18F-labeled compounds had a specific activity of >185 GBq/μmol (~5 Ci/μmol). The stability of the two enantiomers was not reported.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The uptake of [18F]-d-FPHCys and [18F]-l-FPHCys was studied in A375 (human malignant melanoma cell line) and HT29 (human colorectal adenocarcinoma cell line) cells (4). Radioactivity from [18F]-l-FPHCys was rapidly taken up by the A375 cells (with 50% of total accumulation within the initial 2 min after exposure); accumulation peaked at 60 min (9% of total dose/105 cells) and gradually decreased to ~4.5% of total dose by 240 min. A similar uptake profile was observed with [18F]-d-FPHCys in the A375 cells; however, the initial uptake was more gradual compared to that of [18F]-L-FPHCys, and the maximum accumulation of label was 6.8% of total dose/105 cells at 60 min after exposure, which was followed by a gradual decrease in uptake up to 240 min (~3% of total dose). The maximum uptake of [18F]-l-FPHCys and [18F]-d-FPHCys by the HT29 cells was 2% and 1.6% of total dose, respectively, at 15 min, and the accumulation decreased to ~1% of total dose for both tracers at 240 min.
From competitive inhibition experiments it was determined that the D and L labeled enantiomers of FPHCys were transported in the two cells types by the l aa transporter (LAT) system (4). In another study, [18F]-d-FPHCys was shown to be transported into A431 (human epidermoid carcinoma cell line) and Colo 205 (human colorectal adenocarcinoma cell line) cells by the LAT1 subtype of the LAT (6). In this study, HT29 and PC3 (a human prostate adenocarcinoma cell line) cells showed a low uptake of the tracer, and both cell types were determined to have a low expression of LAT1 (as assessed with quantitative polymerase chain reaction of LAT1 mRNA). The uptake of [18F]-d-FPHCys by the four cell lines was shown to correlate well (R2 = 0.85) with the expression of LAT1 in these cells.
Animal Studies
Rodents
[PubMed]
The biodistribution of [18F]-d-FPHCys and [18F]-l-FPHCys was studied in nude mice bearing A375 cell xenograft tumors (n = 3 animals/group) as described by Bourdier et al. (4). Results obtained from this study were presented as percent of injected dose per gram tissue (% ID/g). The amounts of tracer in the blood from [18F]-l-FPHCys and [18F]-d-FPHCys were 5.17 ± 0.18% ID/g and 3.51 ± 0.68% ID/g, 4.44 ± 0.54% ID/g and 2.51 ± 0.35% ID/g, 2.32 ± 0.33% ID/g and 0.89 ± 0.10% ID/g, and 1.38 ± 0.37% ID/g at 15, 30, 60, and 120 min postinjection (p.i.), respectively. The accumulation of radioactivity from [18F]-l-FPHCys in the tumor was 9.41 ± 1.21% ID/g, 8.28 ± 0.81% ID/g, 5.64 ± 0.95% ID/g, and 4.40 ± 0.67% ID/g at 15, 30, 60, and 120 min p.i., respectively. With [18F]-l-FPHCys, the uptake of label in the tumor was 6.01 ± 0.77% ID/g, 5.87 ± 1.14% ID/g, 3.91 ± 0.32% ID/g, and 1.68 ± 0.27% ID/g at 15, 30, 60, and 120 min p.i., respectively. The tumor/blood ratios for [18F]-d-FPHCys and [18F]-l-FPHCys at 60–120 min p.i. were ~4–5 and ~2–3, respectively. HPLC analysis showed that 90% and 80% of [18F]-d-FPHCys remained intact in the pancreas and urine at 60 min, respectively; however, only 30% (pancreas) and 10% (urine) of [18F]-l-FPHCys remained intact at the same time point. At 120 min p.i., a very low percentage (<3%) of both labeled enantiomers was incorporated into proteins of the brain, pancreas, plasma, and the tumors, indicating that the tracers had low incorporation in the macromolecules. No blocking studies were reported.
Whole-body PET images were acquired over a period of 120 min after intravenous injection of either [18F]-d-FPHCys or [18F]-l-FPHCys in nude mice bearing A375 tumors (n = 3 animals) (4). Images acquired at 60 min p.i. showed that the kidneys, followed by the tumors, had the maximum accumulation of radioactivity with both labeled compounds. Bladders of mice injected with [18F]-d-FPHCys showed a higher retention of radioactivity than those injected with [18F]-l-FPHCys. In addition, compared with [18F]-l-FPHCys, a lower background uptake of label from [18F]-d-FPHCys was observed in the liver and the muscles. No blocking studies were reported.
In another study, whole-body PET imaging was performed on nude mice bearing A431, Colo 205, PC3, or HT29 cell tumors (n = 6–10 animals/cell type) at 90 min p.i. of [18F]-d-FPHCys (6). From the images it was determined that the amount of radioactivity in the A431and Colo 205 cell tumors was ~3-fold higher than background, and accumulated radioactivity in the PC3 and HT29 cell tumors was ~2-fold higher than background, indicating that uptake of the label in these lesions had a high correlation (R2 = 0.99) with expression of the LAT1 transporter in these cells (see In Vitro Studies section for details).
From these studies, the investigators concluded that [18F]-d-FPHCys can be a suitable imaging agent for the detection of tumors in mice, although more work is necessary before it can be used in the clinic (4, 6).
References
- 1.
- Kong F.L., Ali M.S., Zhang Y., Oh C.S., Yu D.F., Chanda M., Yang D.J. Synthesis and evaluation of amino acid-based radiotracer 99mTc-N4-AMT for breast cancer imaging. J Biomed Biotechnol. 2011;2011:276907. [PMC free article: PMC3085329] [PubMed: 21541217]
- 2.
- Ganapathy V., Thangaraju M., Prasad P.D. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121(1):29–40. [PubMed: 18992769]
- 3.
- la Fougere C., Suchorska B., Bartenstein P., Kreth F.W., Tonn J.C. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13(8):806–19. [PMC free article: PMC3145468] [PubMed: 21757446]
- 4.
- Bourdier T., Shepherd R., Berghofer P., Jackson T., Fookes C.J., Denoyer D., Dorow D.S., Greguric I., Gregoire M.C., Hicks R.J., Katsifis A. Radiosynthesis and biological evaluation of L- and D-S-(3-[18F]fluoropropyl)homocysteine for tumor imaging using positron emission tomography. J Med Chem. 2011;54(6):1860–70. [PubMed: 21351733]
- 5.
- Bourdier T., Fookes C.J.R., Pham T.Q., Greguric I., Katsifis A. Synthesis and stability of S-(2-[18F]fluoroethyl)-L-homocysteine for potential tumour imaging. Journal of Labelled Compounds and Radiopharmaceuticals. 2008;51(11):369–373.
- 6.
- Denoyer, D., L. Kirby, K. Waldeck, P. Roselt, O.C. Neels, T. Bourdier, R. Shepherd, A. Katsifis, and R.J. Hicks, Preclinical characterization of (18)F-D-FPHCys, a new amino acid-based PET tracer. Eur J Nucl Med Mol Imaging, 2011. [PubMed: 22160176]
- Review (99m)Tc-Labeled O-[3-(1,4,8,11-tetraazabicyclohexadecane)-propyl]-α-methyl tyrosine.[Molecular Imaging and Contrast...]Review (99m)Tc-Labeled O-[3-(1,4,8,11-tetraazabicyclohexadecane)-propyl]-α-methyl tyrosine.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(18)F]Fluoromethyl-d-tyrosine.[Molecular Imaging and Contrast...]Review [(18)F]Fluoromethyl-d-tyrosine.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (4S)-4-(3-[(18)F]Fluoropropyl)-l-glutamate.[Molecular Imaging and Contrast...]Review (4S)-4-(3-[(18)F]Fluoropropyl)-l-glutamate.Chopra A. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review p-(2-[(18)F]Fluoroethyl)-l-phenylalanine.[Molecular Imaging and Contrast...]Review p-(2-[(18)F]Fluoroethyl)-l-phenylalanine.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Preclinical characterization of 18F-D-FPHCys, a new amino acid-based PET tracer.[Eur J Nucl Med Mol Imaging. 2012]Preclinical characterization of 18F-D-FPHCys, a new amino acid-based PET tracer.Denoyer D, Kirby L, Waldeck K, Roselt P, Neels OC, Bourdier T, Shepherd R, Katsifis A, Hicks RJ. Eur J Nucl Med Mol Imaging. 2012 Apr; 39(4):703-12. Epub 2011 Dec 13.
- L- and D-S-(3-[18F]fluoropropyl)homocysteine - Molecular Imaging and Contrast Ag...L- and D-S-(3-[18F]fluoropropyl)homocysteine - Molecular Imaging and Contrast Agent Database (MICAD)
- Cy5-Glu-Pro-Asp-acyloxymethyl ketone - Molecular Imaging and Contrast Agent Data...Cy5-Glu-Pro-Asp-acyloxymethyl ketone - Molecular Imaging and Contrast Agent Database (MICAD)
- DY682 - Molecular Imaging and Contrast Agent Database (MICAD)DY682 - Molecular Imaging and Contrast Agent Database (MICAD)
- N,N´-Bis(2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl-3-methoxypropyl)-acetamidol]-...N,N´-Bis(2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl-3-methoxypropyl)-acetamidol]-2,4,6-triiodoisophthalamide - Molecular Imaging and Contrast Agent Database (MICAD)
- TOTO-3 - Molecular Imaging and Contrast Agent Database (MICAD)TOTO-3 - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro