NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | Gd-DTPA l-Cystine bisisopropyl amide copolymers |
 |
| Abbreviated name: | GCIC | |
| Synonym: | ||
| Agent Category: | Compound (polymers) | |
| Target: | A non-targeted probe with confinement in the vasculature, and activity accumulation in the liver and kidneys. | |
| Target Category: | Nonspecific confinement to the vascular space | |
| Method of detection: | Magnetic resonance imaging (MRI) | |
| Source of contrast /signal: | Gadolinium (Gd) | |
| Activation: | No | |
| Studies: |
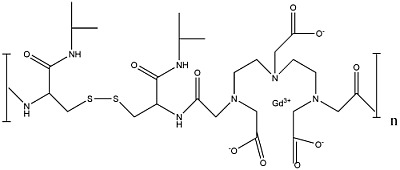
| Gd-DTPA l-cystine bisisopropyl amide copolymers structure. Click on PubChem (SID 47193369) for more information. |
Background
[PubMed]
The Gd-DTPA l-cystine bisisopropyl amide copolymer (GCIC) is a biodegradable, macromolecular contrast agent designed for contrast enhancement of the blood pool, liver, and kidneys for magnetic resonance imaging (MRI) (1). The gadolinium(III) ion (Gd3+) is a paramagnetic lanthanide metal ion with seven unpaired electrons.
MRI signals depend on a wide range of parameters. The key factor of conventional MRI contrast is the interaction of the total water signal (proton density) and the magnetic properties of the tissues (2, 3). Various paramagnetic and superparamagnetic contrast agents can increase the sensitivity and specificity of MRI. Current clinical agents are predominately Gd-based contrast agents (GBCA) and are largely nonspecific, low molecular weight compounds. These agents have transient tissue retention, a wide distribution into the extracellular space, and rapid excretion from the body (3-5). There is a need to develop intravascular MRI contrast agents that have a sufficiently long intravascular half-life (t½) to allow imaging of the vasculature and aid in the detection of cancer and cardiovascular diseases (6, 7).
Current strategies to prolong the intravascular t½ include the chelation of paramagnetic ions to macromolecules and the use of superparamagnetic nanoparticles (1, 6, 7). Macromolecular contrast agents are generally large enough (>20 kDa) so that they do not readily diffuse across the healthy vascular endothelium and are not rapidly excreted. These agents are retained in the vasculature for a sufficiently prolonged period of time to allow for imaging, and they also preferentially accumulate in disease tissues with leaky vasculature, such as cancers and vascular disease. Most macromolecular GBCAs are prepared by the conjugation of Gd3+ chelates to biomedical polymers including poly(amino) acids (8, 9), polysaccharides (10, 11), dendrimers (12, 13), and proteins (14), or by the copolymerization of diethylenetriamine pentaacetic acid (DTPA) dianhydride with diamines and the complexation with Gd3+ (15, 16). However, the development of these macromolecular GBCAs has been hampered by potential Gd toxicity associated with the slow degradation of chemically modified biomedical polymers (6, 17). Smaller macromolecules (<20 kDa) are cleared more rapidly by the kidneys but their effectiveness may also be compromised. One approach to improve the safety of macromolecular GBCAs is the development of small molecules (<1.2 kDa) with a hydrophilic Gd3+ complex and a hydrophobic region for reversible noncovalent binding to serum albumin (6, 18). Lu et al. (17, 19) proposed another approach by designing biodegradable macromolecular polydisulfide GBCAs. These agents have disulfide bonds incorporated into a polymeric backbone, and these bonds can be readily reduced by the thiol-disulfide exchange reaction with endogenous or exogenous thiols, such as glutathione and cysteine. As a result, these macromolecules are broken down into smaller complexes that are readily excreted by the kidneys. The Gd-DTPA-cystamine copolymer was the first such agent synthesized by the copolymerization of cystamine and DTPA dianhydride (17). A series of polydisulfide-based macromolecular GBCAs with different structural modifications around the disulfide bonds have been synthesized and evaluated by the same research team of Lu et al. (17, 20-23). Kaneshiro et al. (1) reported the synthesis and evaluation of GCIC and two other derivatives (Gd-DTPA l-cystine bisamide copolymers (GCAC) and Gd-l-cystine bispropyl amide copolymer (GCPC)) with different amide substituents at the cystine carboxylic groups. All three neutral agents were cleaved in vivo into low molecular weight Gd3+ chelates and were cleared rapidly in rats.
Both renal and extrarenal toxicities have been reported after the clinical use of GBCAs in patients with underlying kidney disease (24-26). In 2007, the US FDA requested manufacturers of all GBCAs to add new warnings that exposure to GBCAs increases the risk for nephrogenic systemic fibrosis in patients with advanced kidney disease.
Synthesis
[PubMed]
Kaneshiro et al. (1) described the synthesis of GCIC from (t-butoxycarbonyl-l-cystine)2 (t-BOC-l-Cys)2. Cystine bisisopropyl amide was first prepared. Briefly, N-hydroxysuccinimide and (t-BOC-l-Cys)2 were dissolved and mixed in tetrahydrofuran (THF). N, N’-Dicyclohexylcarbodiimide was added to the mixture, and it was stirred overnight at room temperature. N,N’-dicyclohexylurea (DCU) was precipitated out of the THF solution, and removed by filtration. isopropyl amine was then added to the filtrate while stirring at –5ºC. Additional DCU was precipitated out and removed. The solvent was also removed to produce the crude product. The dry residue was dissolved in chloroform and washed three times with deionized water at pH 10. The organic layer was dried, filtered, and concentrated to dryness. The dry product was washed with ethyl acetate, filtered, and then washed with diethyl ether to yield (t-BOC)2-cystine bisisopropyl amide. t-Boc protection was then removed by trifluoroacetic acid at 30 min at room temperature. The product was precipitated out, separated and concentrated to yield l-cystine bisisopropyl amide. The final yield was 71%.
The DTPA l-cystine bisisopropyl amide copolymer (DCIC) was synthesized by condensation copolymerization of equimolar amounts of DTPA dianhydride and l-cystine bisisopropyl amide (1). Briefly, l-cystine bisisopropyl amide was dissolved in triethylamine (TEA) and anhydrous dimethyl sulfoxide (DMSO) while stirred in an ice-water bath at 5ºC. TEA acted as a base to neutralize the salts of monomers and as a solvent to increase the solubility of the copolymers. DTPA dianhydride was then added over a 25-min period. After 40 min, the solidified mixture was removed and allowed to come to room temperature. Additional DMSO was added and the mixture was stirred overnight at room temperature. DTPA l-cystine bisisopropyl amide copolymers were precipitated in acetone and dissolved in double ionized water (DI H2O) at pH 7. The copolymer was dialyzed and concentrated to dryness. The yield was 60.0%. The resulting product was mixed with a large excess of Gd triacetate in DI H2O (pH 5.5) and stirred at room temperature for 1 h. Free Gd3+ ions were removed by size-exclusion chromatography. The yield was 71.0%. The DTPA l-cystine bisamide copolymers were anionic and had a large hydrodynamic volume. The complexation of the copolymers with Gd3+ ions produced a neutral GCIC with a significant reduction in hydrodynamic volume. Using size-exclusion chromatography, the number average molecular weight (Mn, the total weight of all the polymer molecules divided by the total number of polymer molecules) and the weight average molecular weight (Mw based on the concept of weight fractions) of GCIC were determined to be 16.6 kDa and 25.0 kDa, respectively. The Gd content was determined to be 162 mg Gd/g or 16.2% by the inductively coupled argon plasma optical emission spectrometer. This was lower than the calculated value of 18.4%; Kaneshiro et al. (1) suggested that this difference might be attributed to the association of water molecules to the hydrophilic polymers.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The in vitro longitudinal relaxivity (R1) of GCIC based on T1 relaxation time measurement at room temperature by a 3T scanner (inversion recovery prepared turbo spin-echo pulse sequence) was 5.56 mM−1s−1 (1). The in vitro transverse relaxivity (R2) was 6.53 mM−1s−1. In comparison, the R1 of Gd-DTPA-BMEA was 4.62 mM−1s−1.
GCIC was stable in the solid state for at least 6 months of cold storage as its molecular weight distribution did not change.
In vitro degradation of DCIC (copolymers without Gd) and GCIC with and without the presence of l-cysteine (15 μM) at 37ºC was studied (1). In the presence of cysteine, DCIC completely degraded into low molecular weight species within 24 h. DCIC with bulky isopropyl groups appeared to degrade slower than the other two copolymers, DTPA l-cystine bisamide copolymers and DTPA l-cystine bisproplyl amide copolymers. GCIC completely degraded into low molecular weight oligomers after 75 min. Measurement by matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry indicated that the mass of the degradation product with one, two, and three repeat units was ~835.2, 1668.4, and ~2502.6 (m/z), respectively. Kaneshiro et al. (1) suggested that the steric effect around the disulfide bonds significantly affected the degradation rate of the copolymers in the presence of cysteine. The degradation rate appeared to decrease in the order of GCAC, GCPC, and GCIC as the steric hindrance around the disulfide bonds increased.
Animal Studies
Rodents
[PubMed]
In vivo metabolic studies of GCIC were conducted in rats with a dose of 0.1 mmol Gd/kg by i.v. injection (1). The major metabolites for GCIC had a mass (m/z) of 718.25, 875.15, 913.11, and 988.02, 1589.36, and 1707.29 were identified in urine samples measured by positively-charged labeled MALDI-TOF mass spectrometry (α-cyano-4-hydroxycinnamic acid matrix) at 8 h. Three unknown peaks were found with masses (m/z) of 895.09, 1215.02, and 1397.51. The 718 peak corresponded to the ligand of the repeat units. The 875.15 and 913,11 metabolites represented the monomeric repeat units with a K+ ion and two K+ ions, respectively. The 1589.36 peak corresponded to two GCIC monomeric peat units with two of its isopropyl amide groups hydrolyzed. The 1707.29 peak corresponded to two GCIC monomeric peat units plus a K+ ion. There were no major metabolites identified in urine samples at 24 h. With negatively-charged labeled MALDI-TOF mass spectrometry, the masses (m/z) identified in urine samples at 8 h were 589.04, 734.08, and 911.04. Two unknown metabolite with 531.97 and 631.03 were found. The structures of these metabolites were not known (20). No metabolites were observed at 24 h. On the basis of these observations, Kaneshiro et al. (1) suggested that the degradation processes of GCIC were more complicated than the in vitro disulfide-thiol exchange reactions. Other degradative processes such as enzymatic reactions and oxidation might be involved. Since all metabolites had higher masses than Gd-DTPA (584.04 Da), this might indicate that the Gd-DTPA-complex was intact and stable in plasma. The absence of major metabolites at 24 h indicated that the majority of GCIC was excreted and cleared from the body within a short time period.
In vivo MRI imaging was performed with a 3T MRI scanner in three rats (1). Each rat received an i.v. dose of 0.1 mmol Gd/kg. Images were obtained with a wrist coil using a 3D FLASH pulse sequence. Strong contrast enhancement was observed within the heart, blood vessels, liver, and kidneys at 2 min. The contrast enhancement gradually decreased but was still visible at 30 min. Contrast enhancement was also observed and gradually increased over time in the urinary bladder. Kaneshiro et al. (1) observed that GCIC had more significant contrast enhancement in the blood pool of rats than that of Gd-DPTA-BMEA.
NIH Support
R01 EB00489, R33 CA095873,
References
- 1.
- Kaneshiro T.L. , Ke T. , Jeong E.K. , Parker D.L. , Lu Z.R. Gd-DTPA L-cystine bisamide copolymers as novel biodegradable macromolecular contrast agents for MR blood pool imaging. Pharm Res. 2006; 23 (6):1285–94. [PubMed: 16729223]
- 2.
- Morawski A.M. , Lanza G.A. , Wickline S.A. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr Opin Biotechnol. 2005; 16 (1):89–92. [PubMed: 15722020]
- This MICAD chapter is not included in the Open Access Subset, because it was authored / co-authored by one or more investigators who was not a member of the MICAD staff.
- Review Gd-DTPA l-Cystine bispropyl amide copolymers.[Molecular Imaging and Contrast...]Review Gd-DTPA l-Cystine bispropyl amide copolymers.Cheng KT, Lu ZR, Kaneshiro T. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Gd-DTPA l-Cystine bisamide copolymers.[Molecular Imaging and Contrast...]Review Gd-DTPA l-Cystine bisamide copolymers.Cheng KT, Lu ZR, Kaneshiro T. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Gd-DTPA L-cystine bisamide copolymers as novel biodegradable macromolecular contrast agents for MR blood pool imaging.[Pharm Res. 2006]Gd-DTPA L-cystine bisamide copolymers as novel biodegradable macromolecular contrast agents for MR blood pool imaging.Kaneshiro TL, Ke T, Jeong EK, Parker DL, Lu ZR. Pharm Res. 2006 Jun; 23(6):1285-94. Epub 2006 Jun 1.
- Gadolinium Magnetic Resonance Imaging.[StatPearls. 2024]Gadolinium Magnetic Resonance Imaging.Ibrahim MA, Hazhirkarzar B, Dublin AB. StatPearls. 2024 Jan
- Review Polyion complex micelles of poly(ethylene glycol)-b-poly(L-lysine)-gadolinium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-dextran sulfate.[Molecular Imaging and Contrast...]Review Polyion complex micelles of poly(ethylene glycol)-b-poly(L-lysine)-gadolinium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-dextran sulfate.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Gd-DTPA l-Cystine bisisopropyl amide copolymers - Molecular Imaging and Contrast...Gd-DTPA l-Cystine bisisopropyl amide copolymers - Molecular Imaging and Contrast Agent Database (MICAD)
- Giraffa camelopardalis antiquorum haplotype Rafiki/ZA4124 cytochrome b (cytb) ge...Giraffa camelopardalis antiquorum haplotype Rafiki/ZA4124 cytochrome b (cytb) gene, complete cds; tRNA-Thr and tRNA-Pro genes, complete sequence; and control region, partial sequence; mitochondrialgi|146262877|gb|EF442265.1|Nucleotide
- Penaeus vannamei obstructor A1 mRNA, partial cdsPenaeus vannamei obstructor A1 mRNA, partial cdsgi|1226142480|gb|MF415537.1|Nucleotide
- Penaeus vannamei obstructor F1 mRNA, complete cdsPenaeus vannamei obstructor F1 mRNA, complete cdsgi|1226142486|gb|MF415540.1|Nucleotide
- Homo sapiens NIMA related kinase 6 (NEK6), transcript variant 1, mRNAHomo sapiens NIMA related kinase 6 (NEK6), transcript variant 1, mRNAgi|1890250860|ref|NM_001145001.3|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro