NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | Radioiodinated (Z)-2-(4-(2-hydroxyethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one |
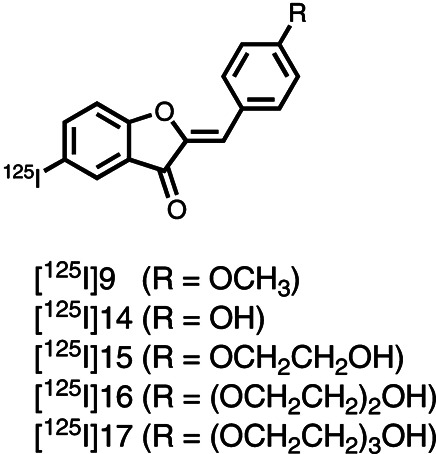
|
| Abbreviated name: | [125I]15 | |
| Synonym: | ||
| Agent Category: | Compounds | |
| Target: | β-amyloid (Aβ) | |
| Target Category: | Accepters | |
| Method of detection: | Single-photon emission computed tomography (SPECT) | |
| Source of signal / contrast: | 125I | |
| Activation: | No | |
| Studies: |
| Structures of aurone derivatives by Maya et al. (1). |
Background
[PubMed]
Radioiodinated (Z)-2-(4-(2-hydroxyethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one (compound 15), abbreviated as [125I]15, is an aurone derivative synthesized by Maya et al. for single-photon emission computed tomography(SPECT) of Alzheimer’s disease (AD) by targeting β-amyloid (Aβ) (1). The other four aurone derivatives include radioiodinated (Z)-2-(4-methoxybenzylidene)-5-iodobenzofuran-3(2H)-one (compound 9), (Z)-2-(4-hydroxybenzylidene)-5-iodobenzofuran-3(2H)-one (compound 14), (Z)-2-(4-(2-(2-hydroxyethoxy)ethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one (compound 16), and (Z)-2-(4-(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one (compound 17), which are abbreviated as [125I]9, [125I]14, [125I]16, and [125I]17, respectively.
AD is characterized in pathology by the presence of extracellular Aβ plaques, intraneuronal neurofibrillary tangles, and neuronal loss in the cerebral cortex (2, 3). Of them, Aβ deposit is the earliest neuropathological marker and is relatively specific to AD and closely related disorders. Aβ plaques are composed of abnormal paired helical filaments 5–10 nm in size. These filaments are largely made of insoluble Aβ peptides that are 40 or 42 amino acids in length (4).
In recent years, molecular imaging by targeting the extracellular Aβ has been intensively investigated in attempts to detect early AD, assess Aβ content in vivo, determine the timing of anti-plaque therapy, and evaluate the therapeutic efficacy (4). Radiolabeled Aβ40 peptides were tested first, but they showed poor penetration ability to cross the blood–brain barrier (BBB) (4). Based on the fact that Aβ can be specifically stained in vitro with dyes of Congo red, chrysamine G, and thioflavin-T, an effort was made to develop imaging agents with these dyes. This effort, however, was in general unsuccessful because the bulky ionic groups of heteroatoms in these dyes prevent them from crossing the BBB (2). Importantly, a large class of derivatives (e.g., aminonaphthalenes, benzothiazoles, stilbenes, and imidazopyridines) was synthesized with these dyes as templates (4). Clinical and preclinical studies have shown that these derivatives not only possess a high binding affinity with Aβ plaques as their parent compounds, but also exhibit good penetration ability through the BBB and rapid washout from brain with low to no plaque deposits.
Ono et al. first synthesized a class of radioiodinated flavone derivatives that present a high binding affinity with Aβ plaques and good penetration ability through the BBB (5). However, these flavone derivatives display poor clearance from the brain, which leads to a high brain background. The investigators then explored another class of flavonoids with aurone as the core structure (6, 7). Aurone is a heterocyclic chemical compound that contains a benzofuran element associated with a benzylidene linked in position 2 and a chalcone-like group being closed into a five-member ring. The aurone derivatives possess a nucleophilic group (NH2, NHMe, or NMe2) at the 4' position and a radioiodine at the 5 position. Although these aurone derivatives exhibit a strong binding affinity with Aβ (inhibition constant (Ki) = 1.2–6.8 nM), high penetration ability through the BBB (1.9%−4.6% injected dose per gram tissue (ID/g) at 2 min), and a fast washout from the brain (0.3%−0.5% ID/g at 30 min), the pharmacokinetics of these compounds are less favorable for brain imaging than the pharmacokinetics of the agent [123I]IMPY (6-iodo-2-(4'-dimethylamino)phenyl-imidazo[1,2]pyridine), which is the only SPECT agent to be tested in humans to date (1, 8, 9). The investigators also modified the flavone and aurone derivatives by pegylating them with 1–3 units of ethylene glycol at the 4' position or by conjugating them with the chelating agent bis-amino-bis-thiol (BAT) (7). Favorable pharmacokinetics for brain imaging was observed for the pegylated derivatives ([18F]8(a–c)) but not for the BAT-chelated derivatives ([99mTc]BAT-FL and [99mTc]BAT-AR) (6, 7).
This series of chapters summarizes the data obtained with flavone and aurone derivatives, including [125I]15, [125I]9, [125I]14, [125I]16, [125I]17, [99mTc]BAT-FL, [99mTc]BAT-AR, [18F]8(a–c), [125I]3, and [18F]3 (1, 6-8). This chapter presents the data obtained with [125I]15, [125I]9, [125I]14, [125I]16, and [125I]17 (1).
Synthesis
[PubMed]
The aurone backbone was synthesized by an Aldol reaction of benzofuranones with benzaldehydes using Al2O3 in chloroform at room temperature (1). The tributyltin derivatives were prepared by using a halogen-to-tributyltin exchange reaction catalyzed by Pd(0). Radioiodination of the tributyltin derivatives resulted in the agents [125I]9, [125I]14, [125I]15, [125I]16, and [125I]17. The radiochemical identities of the radioiodinated compounds were verified by coinjection with nonradioactive compounds with high-performance liquid chromatography (HPLC). The radiochemical yields were 25%−57%, and the radiochemical purities were >95% after purification with HPLC. It was anticipated that the no-carrier-added preparation would result in a final product bearing a theoretical specific activity similar to that of 125I (81.4 TBq/mmol (2,200 Ci/mmol)).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The binding affinities of the unradiolabeled compounds 9, 14, 15, 16, and 17 were measured with Aβ(1−42) aggregates using (Z)-2-(4-aminobenzylidene)-5-iodobenzofuran-3(2H)-one ([125I]AAU, Kd = 4.2 nM) as the competing radioligand (5). The Ki values estimated for compounds 9, 14, 15, 16, and 17 were 2.9, 1.3, 1.1, 3.4, and 2.6 nM, respectively, similar to the values of known Aβ imaging agents such as SB-13 (1.2 nM), PIB (2.8 nM), and IMPY (1.4 nM), indicating that these aurone derivatives have binding affinity strong enough to test clinically (1).
The binding of the aurone derivatives with mouse Aβ plaques was confirmed in brain sections of double transgenic AD mice (Tg2576) (1). Fluorescent staining showed that many amyloid plaques were clearly stained with the derivatives, and the labeling patterns were consistent with that observed with thioflavin S staining. These results suggest that these aurone derivatives can specifically bind with Aβ plaques in the mouse brain.
Animal Studies
Rodents
[PubMed]
Biodistribution studies were performed in normal ddY mice (n = 4–5 mice/time point for each agent) after tail vein injection of the agents (4.2−6.3 kBq (113.5–170.3 nCi)) (1). The brain uptake values ranged from 1.7% to 4.5% ID/g (4.51% ID/g for [125I]15) at 2 min after injection, a level sufficient for imaging Aβ plaques in the brain. The five agents also displayed good clearance from the normal brain (0.1%−0.4% ID/g (0.24% ID/g for [125I]15) at 30 min after injection). The brain2min/brain30min ratios (an index of the washout rate) for [125I]9, [125I]14, [125I]15, [125I]16, and [125I]17 were 15.4, 8.3, 18.8, 9.7, and 15.6, respectively. [125I]15 had the best washout index and was superior to [123I]IMPY (11.1). The kidney, liver, and intestine were the normal organs with the highest radioactivity.
Human Studies
[PubMed]
Postmortem brain tissues from an autopsy-confirmed case of AD and a control subject were used to analyze the binding of [125I]15 with human Aβ plaques in brain sections with autoradiography (1). High radioactivity was observed in the brain sections, and the hot spots were consistent with those observed with in vitro immunohistochemical staining in the same brain sections. Normal human brain displayed no evident accumulation of [125I]15. These results demonstrate the feasibility of using [125I]15 as a SPECT probe for detecting Aβ plaques in the brains of AD patients.
References
- 1.
- Maya Y., Ono M., Watanabe H., Haratake M., Saji H., Nakayama M. Novel radioiodinated aurones as probes for SPECT imaging of beta-amyloid plaques in the brain. Bioconjug Chem. 2009;20(1):95–101. [PubMed: 19072219]
- 2.
- Ono M. Development of positron-emission tomography/single-photon emission computed tomography imaging probes for in vivo detection of beta-amyloid plaques in Alzheimer's brains. Chem Pharm Bull (Tokyo). 2009;57(10):1029–39. [PubMed: 19801854]
- 3.
- Mathis C.A., Wang Y., Klunk W.E. Imaging beta-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr Pharm Des. 2004;10(13):1469–92. [PubMed: 15134570]
- 4.
- Vallabhajosula S. Positron emission tomography radiopharmaceuticals for imaging brain Beta-amyloid. Semin Nucl Med. 2011;41(4):283–99. [PubMed: 21624562]
- 5.
- Ono M., Maya Y., Haratake M., Ito K., Mori H., Nakayama M. Aurones serve as probes of beta-amyloid plaques in Alzheimer's disease. Biochem Biophys Res Commun. 2007;361(1):116–21. [PubMed: 17644062]
- 6.
- Ono M., Watanabe R., Kawashima H., Kawai T., Watanabe H., Haratake M., Saji H., Nakayama M. 18F-labeled flavones for in vivo imaging of beta-amyloid plaques in Alzheimer's brains. Bioorg Med Chem. 2009;17(5):2069–76. [PubMed: 19201614]
- 7.
- Ono M., Ikeoka R., Watanabe H., Kimura H., Fuchigami T., Haratake M., Saji H., Nakayama M. 99mTc/Re complexes based on flavone and aurone as SPECT probes for imaging cerebral beta-amyloid plaques. Bioorg Med Chem Lett. 2010;20(19):5743–8. [PubMed: 20797860]
- 8.
- Watanabe H., Ono M., Kimura H., Kagawa S., Nishii R., Fuchigami T., Haratake M., Nakayama M., Saji H. A dual fluorinated and iodinated radiotracer for PET and SPECT imaging of beta-amyloid plaques in the brain. Bioorg Med Chem Lett. 2011;21(21):6519–22. [PubMed: 21920750]
- 9.
- Newberg A.B., Wintering N.A., Plossl K., Hochold J., Stabin M.G., Watson M., Skovronsky D., Clark C.M., Kung M.P., Kung H.F. Safety, biodistribution, and dosimetry of 123I-IMPY: a novel amyloid plaque-imaging agent for the diagnosis of Alzheimer's disease. J Nucl Med. 2006;47(5):748–54. [PubMed: 16644743]
- PubMedLinks to PubMed
- Review (99m)Tc-Bis-amino-bis-thiol-conjugated 6-(3-bromopropoxy)-2-(4-(dimethylamino)phenyl)-4H-chromen-4-one and (Z)-5-(3-bromopropoxy)-2-(4-(dimethylamino)benzylidene)benzofuran-3(2H)-one.[Molecular Imaging and Contrast...]Review (99m)Tc-Bis-amino-bis-thiol-conjugated 6-(3-bromopropoxy)-2-(4-(dimethylamino)phenyl)-4H-chromen-4-one and (Z)-5-(3-bromopropoxy)-2-(4-(dimethylamino)benzylidene)benzofuran-3(2H)-one.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Fluorinated and iodinated (Z)-2-(4-(2-fluoroethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one.[Molecular Imaging and Contrast...]Review Fluorinated and iodinated (Z)-2-(4-(2-fluoroethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (18)F-Labeled fluoropegylated 6-fluoroethoxy-4'-dimethylaminoflavone, 6-(2-(2-fluoro-ethoxy)-ethoxy)-4'-dimethylaminoflavone, and 6-(2-(2-(2-fluoro-ethoxy)-ethoxy)ethoxy)-4'-dimethylaminoflavone.[Molecular Imaging and Contrast...]Review (18)F-Labeled fluoropegylated 6-fluoroethoxy-4'-dimethylaminoflavone, 6-(2-(2-fluoro-ethoxy)-ethoxy)-4'-dimethylaminoflavone, and 6-(2-(2-(2-fluoro-ethoxy)-ethoxy)ethoxy)-4'-dimethylaminoflavone.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Fluoro-pegylated (1E,4E)-1-(4-(dimethylamino)phenyl)-5-(4-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)phenyl)penta-1,4-dien-3-one and (1E,4E)-1-(4-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)phenyl)-5-(4-(methylamino)phenyl)penta-1,4-dien-3-one.[Molecular Imaging and Contrast...]Review Fluoro-pegylated (1E,4E)-1-(4-(dimethylamino)phenyl)-5-(4-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)phenyl)penta-1,4-dien-3-one and (1E,4E)-1-(4-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)phenyl)-5-(4-(methylamino)phenyl)penta-1,4-dien-3-one.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Radioiodinated (1E,4E)-1-(4-aminophenyl)-5-(4-iodophenyl)penta-1,4-dien-3-one and (1E,4E)-1-(4-iodophenyl)-5-(4-(methylamino)phenyl)penta-1,4-dien-3-one.[Molecular Imaging and Contrast...]Review Radioiodinated (1E,4E)-1-(4-aminophenyl)-5-(4-iodophenyl)penta-1,4-dien-3-one and (1E,4E)-1-(4-iodophenyl)-5-(4-(methylamino)phenyl)penta-1,4-dien-3-one.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Radioiodinated (Z)-2-(4-(2-hydroxyethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one...Radioiodinated (Z)-2-(4-(2-hydroxyethoxy)benzylidene)-5-iodobenzofuran-3(2H)-one - Molecular Imaging and Contrast Agent Database (MICAD)
- Self-quenching Alexa fluor 680 conjugated to trastuzumab - Molecular Imaging and...Self-quenching Alexa fluor 680 conjugated to trastuzumab - Molecular Imaging and Contrast Agent Database (MICAD)
- 2-(2-Nitroimidazol-1H-yl)-(3-[18F]fluoropropyl)acetamide - Molecular Imaging and...2-(2-Nitroimidazol-1H-yl)-(3-[18F]fluoropropyl)acetamide - Molecular Imaging and Contrast Agent Database (MICAD)
- Endocrinology of Male Reproduction - EndotextEndocrinology of Male Reproduction - Endotext
- Staff training - DementiaStaff training - Dementia
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro