NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
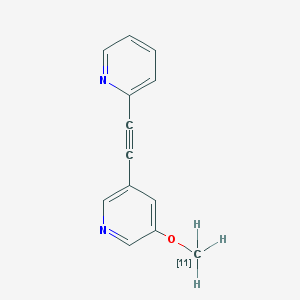
| Chemical name: | 2-(2-(5-[11C]Methoxypyridin-3-yl)ethynyl)pyridine |
|
| Abbreviated name: | [11C]M-PEPy | |
| Synonym: | ||
| Agent Category: | Compound | |
| Target: | Metabotropic glutamate subtype 5 (mGlu5) receptor (mGluR5 or mGluR5) | |
| Target Category: | Receptor binding | |
| Method of detection: | Positron emission tomography (PET) | |
| Source of signal: | 11C | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
2-(2-(5-[11C]Methoxypyridin-3-yl)ethynyl)pyridine ([11C]M-PEPy) is a radioligand developed for positron emission tomography (PET) imaging of metabotropic glutamate (mGlu) receptor subtype 5 (mGluR5 or mGluR5) in the central nervous system (CNS) (1, 2). 11C is a positron emitter with a physical half-life of 20.3 min.
Glutamate is a major excitatory neurotransmitter at CNS synapses. Many neuroanatomical CNS projection pathways contain glutamatergic neurons (3). Glutamate produces its excitatory effects by acting on cell-surface ionotropic glutamate or mGluRs (4). The mGluRs are G-protein–coupled receptors, and the eight mGluR subtypes are further subdivided into groups I, II, and III. The group I receptors include mGluR1 and mGluR5, and they are found predominantly in postsynaptic locations. The mGluR5s are found with high to moderate density in the frontal cortex, caudate, putamen, nucleus accumbens, olfactory tubercle, hippocampus, and dorsal horn of the spinal cord, whereas the density in the cerebellum is low. These receptors couple with phospholipase C and up- or downregulate neuronal excitability. They have been implicated in a variety of diseases in the CNS, including anxiety, depression, schizophrenia, Parkinson’s disease, and drug addiction or withdrawal. These receptors are also involved in the modulation of various pain states, which makes them attractive targets for therapeutic drug development.
PET and single-photon emission computed tomography imaging of radioligands that target mGluR5s can be used to visualize and study the CNS mGluR5s in normal and pathological states. Some mGluR5 antagonists have been successfully labeled, but their in vivo visualization has been hampered by high lipophilicity, unfavorable brain uptake kinetics, or high metabolism (5, 6). 2-Methyl-6-(2-phenylethynylpyridine (MPEP) and its analogs, M-MPEP and M-PEPy, have been identified as potent and highly selective noncompetitive antagonists for mGluR5 (1, 7-9). M-MPEP was reported to have approximately six-fold higher in vitro affinity (50% inhibition concentration = 3.4 nM) compared to 5-[(2-methyl-1,3-thizaol-4-yl)-ethynyl]pyridine (MTEP), an mGluR5 antagonist with moderately high affinity (2, 9). Yu et al. (1) synthesized [11C]M-PEPy and demonstrated the feasibility of using it as a PET ligand for in vivo imaging.
Synthesis
[PubMed]
Cosford et al. (9) reported the synthesis of the phenolic precursor 5-(2-(pyridin-2-yl)ethynyl)pyridin-3-ol of M-PEPy. Briefly, sodium methoxide was reacted with 3,5-dibromopyridine in dimethylformamide at 60ºC for 18 h to produce 3-bromo-5-methoxypyridine. M-PEPy was prepared by Sonogoshira cross-coupling of 2-ethynylpyridine with 3-bromo-5-methoxypyridine. M-PEPy was converted to the phenolic precursor by reaction with methylene bromide in the presence of aluminum bromide at 0–25ºC for 1 h. The yield was 76%. Yu et al. (1) reported the radiosynthesis of [11C]M-PEPy by radiolabeling this phenolic precursor with [11C]methyl iodide ([11C]CH3I) under basic conditions. [11C]CH3I was prepared from [11C]carbon dioxide with the use of the 14N(p, α)11C reaction. The best yield condition was determined to be with the use of potassium hydroxide (KOH) as the base. In this radiolabeling reaction, 4–10 mg of solid KOH powder was added to 0.5–1.0 mg of the precursor dissolved in acetonitrile before [11C]CH3I was added to the reaction mixture. The mixture was heated at 90ºC for 6 min. The radioligand was purified with high-performance liquid chromatography (HPLC). The radiochemical purity was >98.1 ± 1.6% (n = 9), and the specific activity was 31 ± 4.4 GBq/μmol (840 ± 120 mCi/μmol). The radiochemical yield was 1.47 ± 0.70 GBq (39.8 ± 18.8 mCi) at the end of synthesis (EOS). The time of synthesis was ~45 min.
Severance et al. (2) reported the radiosynthesis of [11C]M-PEPy with the precursor desmethyl-M-PEPy with the use of [11C]methyl triflate, which was synthesized from [11C]methyl iodide. Desmethyl-M-PEPy was dissolved in acetone and mixed with aqueous sodium hydroxide. [11C]Methyl triflate was transported into the mixture at room temperature over a period of 5 min, and then mixture was heated at 60ºC for 2 min. The final product was purified with semipreparative HPLC; the radiochemical purity was >99%. The total time of synthesis was 30 min from the end of bombardment with an average yield of 30 ± 5.5% (n = 10) based on [11C]CO2 at EOS. The average specific activity of [11C]M-PEPy was 70.3 ± 33.3 GBq/μmol (1.9 ± 0.9 Ci/μmol) (n = 9).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
Severance et al. (2) studied the in vitro binding of [11C]M-MPEPy with the use of 20-μm sections of human prefrontal cortex, striatum, hippocampus, occipital cortex, and cerebellum, as well as rat brain sections. The sections were incubated with [11C]M-MPEP for 40 min. The sections were then washed and studied with a phosphor imager. Nonspecific binding was evaluated by coincubation with unlabeled M-MPEPy. High specific binding was observed in human brain sections of hippocampus, striatum, ventral striatum, frontal cortex, and occipital cortex. Similar high specific binding was also observed in the hippocampus and cortex of the rat brain sections. Minimal specific binding was found in the both human and rat cerebellum. The hippocampus/cerebellum ratio was ~8 in the human sections and ~37 in the rat sections.
Animal Studies
Rodents
[PubMed]
Dynamic PET imaging of [11C]M-PEPy was performed in male rats (1). Each halothane-anesthetized rat received 111 ± 72.89 MBq (3.00 ± 1.97 mCi) [11C]M-PEPy in 0.43 ± 0.16 μg (2–3 nM in 0.1 ml or ~3.57 nmol calculated from the mean specific activity of 0.84 mCi/nmol) by i.v. administration. Dynamic imaging for 1 h after administration showed that the radioligand was primarily eliminated from the body through gastrointestinal pathways with accumulation of radioactivity in the liver, pancreas, and intestine. The radioactivity appeared to be in the bile and was excreted through the common bile duct. No accumulation of radioactivity was observed in the bladder during the 1 h of imaging. Imaging of the brain showed that the maximum radioactivity accumulation was in the olfactory area. The maximum value of the olfactory bulb/cerebellum radioactivity ratio was 8.1 at 10 min. At 10 min, the highest radioactivity level was in the olfactory bulb, followed by the striatum, hippocampus, and cortex. The authors suggested that this distribution pattern appeared to be similar to that of the brain mGluR5s in rats (10). The radioactivity levels (n = 13) of the whole brain in percentage of the injected dose per cubic centimeter (% ID/cc) as obtained from volumetric region-of-interest image analysis were 0.136 ± 0.097 (5 min), 0.099 ± 0.063 (10 min), 0.082 ± 0.051 (20 min), 0.074 ± 0.047 (30 min), 0.068 ± 0.044 (40 min), 0.061 ± 0.041 (50 min), and 0.050 ± 0.045 (60 min). The radioactivity levels (% ID/cc) of the olfactory bulb were 0.83 ± 0.79 (5 min), 0.73 ± 0.62 (10 min), 0.56 ± 0.42 (20 min), 0.45 ± 0.32 (30 min), 0.38 ± 0.26 (40 min), 0.36 ± 0.23 (50 min), and 0.30 ± 0.22 (60 min). The [11C]M-PEPy radioactivity levels were reversible in all areas of the body. When a blocking dose of 10 mg/kg unlabeled MPEP was administered intravenously 5 min before the radioligand injection (79.18 ± 56.98 MBq (2.96 ± 1.51 mCi) in 0.74 ± 0.38 μg (~3.52 nmol calculated from the mean specific activity of 0.84 mCi/nmol)), the radioactivity levels (n = 8) of the olfactory lobe were decreased by 84.6% at 5 min and by 60.5% at 40 min. In the other parts of the brain, MPEP decreased radioactivity levels of [11C]M-PEPy in the early time points: at 5 min the decreases were 44.9% in the whole brain, 47.3% in the striatum, 46.9% in the cortex, and 28.7% in the hippocampus; at 40 min, the corresponding decreases were 19.1% in the whole brain, 14.9% in the striatum, 28.1% in the cortex, and 31.4% in the hippocampus. No ex vivo verification was presented.
In the in vivo metabolism study, HPLC analysis showed [11C]M-PEPy to be rapidly metabolized after administration (1). The percentage of the radioligand that remained intact in the plasma after administration (n = 3) was 20.1 ± 4.9% at 10 min and 6.9 ± 3.1% at 30 min. No lipophilic metabolite from [11C]M-PEPy was found in the plasma.
Severance et al. (2) performed micro-PET imaging of [11C]M-PEPy in anesthetized (urethane) rats. Each rat received a dose of 12.53 ± 9.0 MBq (0.34 ± 0.24 mCi) [11C]M-PEPy with a specific activity of 61.94 ± 5.4 GBq/μmol (1.67 ± 0.15 Ci/μmol). Radioactivity was observed in the olfactory epithelium and the brain. The radioactivity reached a maximum at 2–3 min and then rapidly decreased. The average hippocampus/cerebellum ratio at 20 min was 1.6 ± 0.4. Ex vivo studies were conducted in rats without anesthesia (dose = 6.92 ± 2.66 MBq (0.19 ± 0.72 mCi); specific activity = 52.17 GBq/μmol (1.41 Ci/μmol)). The rats were euthanized after a 15-min brain imaging. The brains were removed, and different brain sections were dissected for counting. The average hippocampus/cerebellum radioactivity ratio was 1.8 ± 0.3.
Non-Human Primates
[PubMed]
Yu et al. (1) briefly reported PET imaging studies of [11C]M-PEPy in primates. The authors observed that the basic radioactivity distribution was the same as that observed in rats; no data were presented. Severance et al. (2) performed two [11C]M-PEPy scans in one male baboon. A dose of 170.2 ± 16.3 MBq (4.6 ± 0.24 mCi) [11C]M-PEPy (specific activity = 119.88 ± 18.13 GBq/μmol (3.24 ± 0.49 Ci/μmol)) was administered to the baboon. Accumulation of radioactivity was observed throughout the brain. The highest radioactivity was found in the hippocampus and frontal cortex. The lowest radioactivity area was the cerebellum. The hippocampus/cerebellum and frontal cortex/cerebellum ratios of radioactivity at 20 min averaged 1.4 ± 0.1 and 1.2 ± 0.1, respectively; these ratios peaked between 10 and 20 min. The hippocampus/cerebellum ratio decreased to 1 by 40 min.
NIH Support
NIH/NIBIB grant EB01850, PHS MH062185.
References
- 1.
- Yu M., Tueckmantel W., Wang X., Zhu A., Kozikowski A.P., Brownell A.L. Methoxyphenylethynyl, methoxypyridylethynyl and phenylethynyl derivatives of pyridine: synthesis, radiolabeling and evaluation of new PET ligands for metabotropic glutamate subtype 5 receptors. Nucl Med Biol. 2005;32(6):631–40. [PubMed: 16026710]
- 2.
- Severance A.J., Parsey R.V., Kumar J.S., Underwood M.D., Arango V., Majo V.J., Prabhakaran J., Simpson N.R., Van Heertum R.L., Mann J.J. In vitro and in vivo evaluation of [11C]MPEPy as a potential PET ligand for mGlu5 receptors. Nucl Med Biol. 2006;33(8):1021–7. [PubMed: 17127176]
- 3.
- Pin J.P., Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34(1):1–26. [PubMed: 7623957]
- 4.
- Slassi A., Isaac M., Edwards L., Minidis A., Wensbo D., Mattsson J., Nilsson K., Raboisson P., McLeod D., Stormann T.M., Hammerland L.G., Johnson E. Recent advances in non-competitive mGlu5 receptor antagonists and their potential therapeutic applications. Curr Top Med Chem. 2005;5(9):897–911. [PubMed: 16178734]
- 5.
- Ametamey S.M., Kessler L.J., Honer M., Wyss M.T., Buck A., Hintermann S., Auberson Y.P., Gasparini F., Schubiger P.A. Radiosynthesis and Preclinical Evaluation of 11C-ABP688 as a Probe for Imaging the Metabotropic Glutamate Receptor Subtype 5. J Nucl Med. 2006;47(4):698–705. [PubMed: 16595505]
- 6.
- Patel S., Ndubizu O., Hamill T., Chaudhary A., Burns H.D., Hargreaves R., Gibson R.E. Screening cascade and development of potential Positron Emission Tomography radiotracers for mGluR5: in vitro and in vivo characterization. Mol Imaging Biol. 2005;7(4):314–23. [PubMed: 16080024]
- 7.
- Gasparini F., Andres H., Flor P.J., Heinrich M., Inderbitzin W., Lingenhohl K., Muller H., Munk V.C., Omilusik K., Stierlin C., Stoehr N., Vranesic I., Kuhn R. [(3)H]-M-MPEP, a potent, subtype-selective radioligand for the metabotropic glutamate receptor subtype 5. Bioorg Med Chem Lett. 2002;12(3):407–9. [PubMed: 11814808]
- 8.
- Patel S., Krause S.M., Hamill T., Chaudhary A., Burns D.H., Gibson R.A. In vitro characterization of [3H]MethoxyPyEP, an mGluR5 selective radioligand. Life Sci. 2003;73(3):371–9. [PubMed: 12757844]
- 9.
- Cosford N.D., Roppe J., Tehrani L., Schweiger E.J., Seiders T.J., Chaudary A., Rao S., Varney M.A. [3H]-methoxymethyl-MTEP and [3H]-methoxy-PEPy: potent and selective radioligands for the metabotropic glutamate subtype 5 (mGlu5) receptor. Bioorg Med Chem Lett. 2003;13(3):351–4. [PubMed: 12565928]
- 10.
- Shigemoto R., Nomura S., Ohishi H., Sugihara H., Nakanishi S., Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163(1):53–7. [PubMed: 8295733]
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review 2-(2-(3-[(11)C]Methoxyphenyl)ethynyl)pyridine.[Molecular Imaging and Contrast...]Review 2-(2-(3-[(11)C]Methoxyphenyl)ethynyl)pyridine.Cheng KT. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 2-[(11)C]Methyl-6-(2-phenylethynyl)pyridine.[Molecular Imaging and Contrast...]Review 2-[(11)C]Methyl-6-(2-phenylethynyl)pyridine.Cheng KT. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(18)F]3-Fluoro-5-[(2-methyl-1,3-thiazol-4-yl)ethynyl]benzonitrile.[Molecular Imaging and Contrast...]Review [(18)F]3-Fluoro-5-[(2-methyl-1,3-thiazol-4-yl)ethynyl]benzonitrile.The MICAD Research Team. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(18)F]3-Fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile.[Molecular Imaging and Contrast...]Review [(18)F]3-Fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile.Cheng KT. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 3-(6-Methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-(11)C-methyl-oxime.[Molecular Imaging and Contrast...]Review 3-(6-Methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-(11)C-methyl-oxime.Cheng KT. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- 2-(2-(5-[11C]Methoxypyridin-3-yl)ethynyl)pyridine - Molecular Imaging and Contra...2-(2-(5-[11C]Methoxypyridin-3-yl)ethynyl)pyridine - Molecular Imaging and Contrast Agent Database (MICAD)
- 99mTc-Anti-ED-B fibronectin L19-(Gy)3-Cys-Ala scFv antibody fragment - Molecular...99mTc-Anti-ED-B fibronectin L19-(Gy)3-Cys-Ala scFv antibody fragment - Molecular Imaging and Contrast Agent Database (MICAD)
- (2S,αS)-2-(α-(2-[125I]Iodophenoxy)benzyl)morpholine - Molecular Imaging and Cont...(2S,αS)-2-(α-(2-[125I]Iodophenoxy)benzyl)morpholine - Molecular Imaging and Contrast Agent Database (MICAD)
- [99mTc]-Pentavalent dimercaptosuccinic acid - Molecular Imaging and Contrast Age...[99mTc]-Pentavalent dimercaptosuccinic acid - Molecular Imaging and Contrast Agent Database (MICAD)
- N,N-Diethyl-2-2(2-(4-[11C]methoxyphenyl)-5,7-dimethyl-pyrazolo(1,5-a)pyrimidin-3...N,N-Diethyl-2-2(2-(4-[11C]methoxyphenyl)-5,7-dimethyl-pyrazolo(1,5-a)pyrimidin-3-yl)-acetamide - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro