NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | [11C]Norepinephrine |
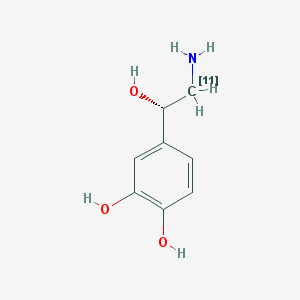
|
| Abbreviated name: | [11C]NE | |
| Synonym: | [11C]Norepinephrine hydrochloride, racemic [11C]norepinephrine | |
| Agent Category: | Compound | |
| Target: | Norepinephrine transporter (NET), vesicular monoamine transporter (VMAT), neuronal storage vesicle, monoamine oxidase (MAO), catechol-O-methyl transferase (COMT) | |
| Target Category: | Transporter binding, storage and metabolism in the sympathetic nervous system (SNS) | |
| Method of detection: | Positron Emission Tomography (PET) | |
| Source of signal/contrast: | 11C | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
[11C]Norepinephrine ([11C]NE) is a radioligand developed for positron emission tomography (PET) imaging of the sympathetic nervous system (SNS). It is a catecholamine analog labeled with 11C, a positron emitter with a physical half-life (t½) of 20.4 min (1).
Many diseases affect the SNS, and imaging of pathologic changes of adrenergic transmission has been an important area of PET research (2, 3). Most postganglionic sympathetic neurons in the autonomic nervous system release the neurotransmitter norepinephrine (NE), which stimulates adrenergic receptors in various effector organs (4). There are different types of adrenergic receptors, and NE stimulates α1, β1, and certain β2 receptors. The NE transporter (NET) is a transmembrane protein located in the adrenergic nerve terminals that is responsible for active reuptake (uptake-1) of NE released from neurons (5). NE is stored in the neuronal vesicles and is released on stimulation. Significant expression of NET is found in major organs of the SNS such as the heart and brain. There is substantial evidence that aberrations in cardiac SNS function contribute to the morbidity and mortality associated with cardiac diseases (6).
Molecular probes with structures closely related to NE can be used to assess the integrity of presynaptic sympathetic nerve terminals in various diseases. [123I]meta-Iodobenzylguanidine ([123I]MIBG), a single-photon emission tomography (SPECT) agent, has been developed and used for neuronal imaging (7). Efforts have been made to develop a positron-emitting tracer because of the inadequate quantitative information and lower spatial resolution obtained by SPECT imaging with [123I]MIBG (7). Studies with [14C]NE and [3H]NE have shown that when radiolabeled NE was injected intravenously into animals, NE rapidly disappeared from the circulation and accumulated in organs rich in SNS (7, 8). NE is taken up by NET and transported by vesicular monoamine transporter into storage vesicles. However, radiolabeled NE is subject to metabolism by both monoamine oxidase and catechol-O-methyl transferase. Langer and Haldin (7) reviewed and compared various PET and SPECT trances for mapping the cardiac nervous system.
Synthesis
[PubMed]
Racemic 11C-labeled NE ((±)-[11C]NE) was first synthesized by Fowler et al. (9). The synthetic approach was similar to the synthesis of [11C]dopamine. The synthesis of radiolabeled. NE is complicated by the presence of the stereogenic center generated by a β-hydroxyl group. In this synthesis (9, 10), [11C]NE was prepared by reduction of cyanohydrins with borane. Briefly, a mixture of sodium cyanide, 3,4-dihydroxybenzaldehyde, and sodium bisulfite was heated at 70 ºC for 30 min and then extracted with ether. After the ether was evaporated, a 1 M borane was added to the residue after the ether was evaporated and allowed to stand 5 min at room temperature. The NE hydrochloride was separated by Dowex 50 W-Xs chromatography with a yield of 14.2%. The radiosynthesis prepared Na[11C]CN from H[11C]CN and used Na[11C]CN in the same procedure to give a radiochemical yield of approximately 10% based on the initial activity of H[11C]CN at the end of bombardment (EOB).
Nagren et al. (11) used no-carrier-added [11C]nitromethane as the starting chemical to react with 3,4-(methylenedioxy)-benzaldehyde to produce [β-11C]3,4-(methylenedioxy)-β-nitrophenethyl alchol. This intermediate product was then reduced with Raney nickel in 30% formic acid to produce [11C]NE. The final product was purified by high-performance liquid chromatography (HPLC). [11C]Nitromethane was prepared from [11C]carbon dioxide by the 14N(p,α)11C reaction. The radiochemical yield was 20-25% (decay-corrected activity from [11C]CO2) with a total synthesis time of 65-75 min (from EOB to the final filtered product). The reported specific activity was 26-56 GBq(700-1500 Ci)/µmol. In another study, Nagren et al. (12) described the use of a preparative HPLC method for the separation of (−)- and (+)-[11C]NE.
Animal Studies
Other Non-Primate Mammals
[PubMed]
Fowler et al. (9) evaluated the biodistribution of carrier-free, racemic [11C]NE in 4 dogs. The radioactivities in percentage of injected dose/gram of tissue, in the heart at 15, 30, 60, and 90 min after injection were 0.017, 0.023, 0.025, and 0.023, respectively. The heart/blood, heart/lung, heart/liver, heart/kidney, and heart/adrenal medulla ratios at 1 h were 11.04, 4.89, 2.28, 0.908, and 0.28, respectively. The biological t½ in the blood was less than 2 min.
Non-Human Primates
[PubMed]
Farde et al. (13, 14) studied the heart uptake of racemic [11C]NE with PET imaging in 2 cynomolgus monkeys. Racemic [11C]NE was prepared with a specific activity >37,000 GBq (1000 Ci)/mmol. A dose of 20-40 MBq (0.54-1.08 mCi at the time of administration) was injected intravenously into the monkey. After the injection, there was a rapid, high uptake of radioactivity by the heart. The radioactivity in the myocardium was more than 10 times higher than that in the adjacent lung tissue and 5 times more than that in the plasma. Following a 1-h pretreatment with desipramine (3 mg/kg i.v.; a reuptake inhibitor), the radioactivity of [11C]NE was reduced by 80-85%. This uptake inhibition probably indicated that a major proportion of the radioactivity represented binding and transport of this radioactive tracer by NET into the neuronal vesicles. More than 90% of plasma radioactivity represented unchanged ligand 30 min after injection (15).
NIH Support
NIMH Grant 42105-7.
References
- 1.
- Rosenspire K.C. , Haka M.S. , Van Dort M.E. , Jewett D.M. , Gildersleeve D.L. , Schwaiger M. , Wieland D.M. Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: a false transmitter agent for heart neuronal imaging. J Nucl Med. 1990; 31 (8):1328–34. [PubMed: 2384800]
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review R-(−)-[(11)C]Epinephrine.[Molecular Imaging and Contrast...]Review R-(−)-[(11)C]Epinephrine.Cheng KT. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(11)C]meta-Hydroxyephedrine.[Molecular Imaging and Contrast...]Review [(11)C]meta-Hydroxyephedrine.Cheng KT. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(11)C]-p-Hydroxyphenethylguanidine.[Molecular Imaging and Contrast...]Review [(11)C]-p-Hydroxyphenethylguanidine.Cheng KT, Raffel DM, Jung YW. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 1R-[(11)C]Phenylephrine.[Molecular Imaging and Contrast...]Review 1R-[(11)C]Phenylephrine.Cheng KT. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (S,S)-[(11)C]Methylreboxetine.[Molecular Imaging and Contrast...]Review (S,S)-[(11)C]Methylreboxetine.The MICAD Research Team. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- [11C]Norepinephrine - Molecular Imaging and Contrast Agent Database (MICAD)[11C]Norepinephrine - Molecular Imaging and Contrast Agent Database (MICAD)
- PREDICTED: Mus musculus zinc finger protein (C2H2 type) 276 (Zfp276), transcript...PREDICTED: Mus musculus zinc finger protein (C2H2 type) 276 (Zfp276), transcript variant X4, mRNAgi|1907190523|ref|XM_006531229.5|Nucleotide
- cytochrome b, partial (mitochondrion) [Criniger barbatus]cytochrome b, partial (mitochondrion) [Criniger barbatus]gi|1389162463|gb|AWK02532.1|Protein
- mitogen-activated protein kinase 10 isoform 12 [Mus musculus]mitogen-activated protein kinase 10 isoform 12 [Mus musculus]gi|2249007066|ref|NP_001394528.1|Protein
- [123I]Vascular endothelial growth factor - Molecular Imaging and Contrast Agent ...[123I]Vascular endothelial growth factor - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 Non–primate non-rodent mammals
Non–primate non-rodent mammals