All rights reserved. NICE copyright material can be downloaded for private research and study, and may be reproduced for educational and not-for-profit purposes. No reproduction by or for commercial organisations, or for commercial purposes, is allowed without the written permission of NICE.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Introduction
Evidence Updates are intended to increase awareness of new evidence – they do not replace current NICE guidance and do not provide formal practice recommendations.
Evidence Updates reduce the need for individuals, managers and commissioners to search for new evidence. For contextual information, this Evidence Update should be read in conjunction with the relevant clinical guideline, available from the NICE Evidence Services topic page for sickle cell disease.
This Evidence Update provides a summary of selected new evidence published since the literature search was last conducted for the following NICE guidance:
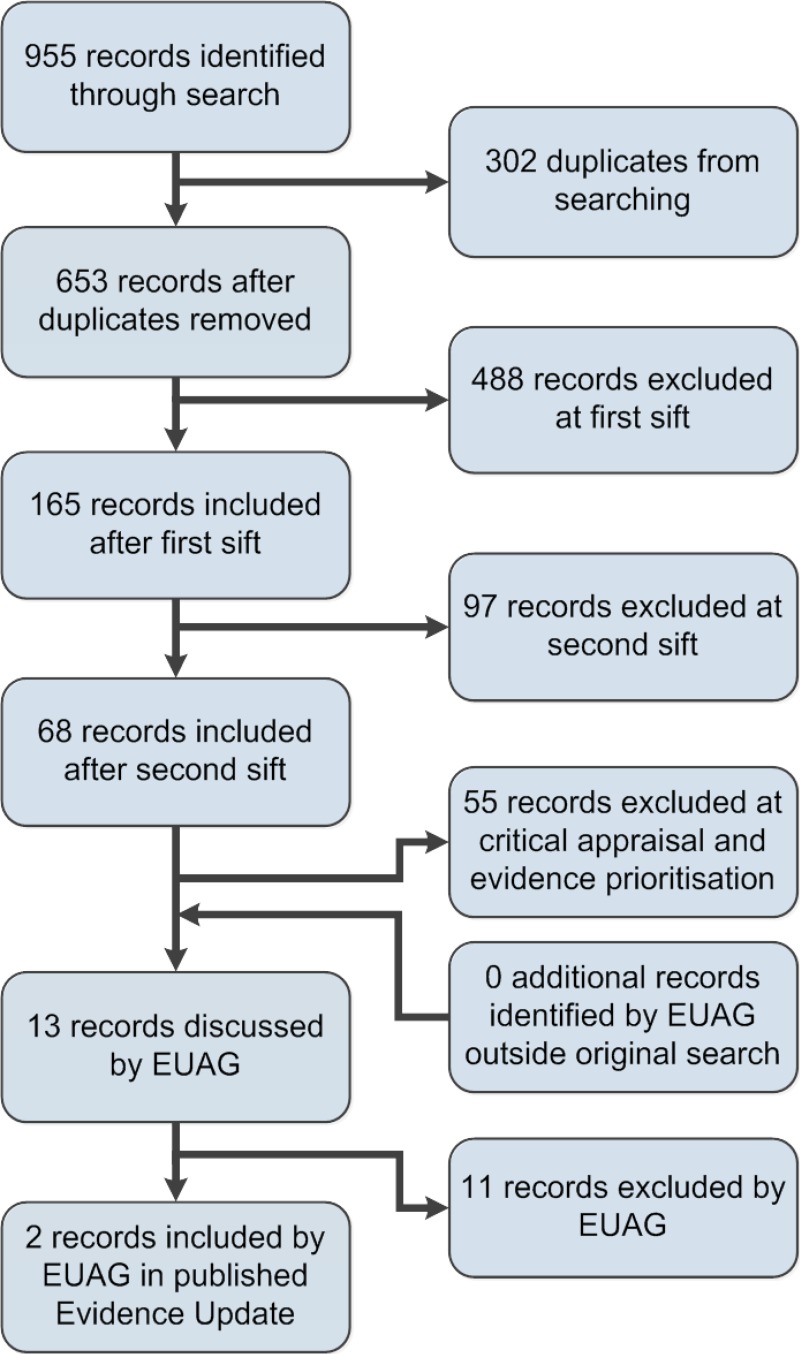
A search was conducted for new evidence from 1 May 2011 to 10 Jan 2014. A total of 955 pieces of evidence were initially identified. After removal of duplicates, a series of automated and manual sifts were conducted to produce a list of the most relevant references. The remaining 13 references underwent a rapid critical appraisal process and then were reviewed by an Evidence Update Advisory Group, which advised on the final list of 2 items selected for the Evidence Update. See Appendix A for details of the evidence search and selection process.
Evidence selected for inclusion in this Evidence Update may highlight a potential impact on guidance: that is, a high-quality study, systematic review or meta-analysis with results that suggest a change in practice. Evidence that has no impact on guidance may be a key read, or may substantially strengthen the evidence base underpinning a recommendation in the NICE guidance.
The Evidence Update gives a preliminary assessment of changes in the evidence base and a final decision on whether the guidance should be updated will be made by NICE according to its published processes and methods.
This Evidence Update was developed to help inform the review proposal on whether or not to update NICE clinical guideline 143 (NICE CG143). The process of updating NICE guidance is separate from both the process of an Evidence Update and the review proposal.
See the NICE clinical guideline development methods guides for further information about updating clinical guidelines.
NICE Pathways
NICE pathways bring together all related NICE guidance and associated products on the condition in a set of interactive topic-based diagrams. The following NICE Pathways cover advice and recommendations related to this Evidence Update:
- Sickle cell acute painful episode. NICE Pathway
Feedback
If you would like to comment on this Evidence Update, please email ku.shn.ecnedive@sutcatnoc
Key points
The following table summarises the key points for this Evidence Update and indicates whether the new evidence may have a potential impact on NICE CG143. Please see the full commentaries for details of the evidence informing these key points.
The section headings used in the table below are taken from NICE CG143.
Evidence Updates do not replace current NICE guidance and do not provide formal practice recommendations.
| Potential impact on guidance | ||
|---|---|---|
| Key point | Yes | No |
| Management of underlying pathology | ||
| ✓ | |
| ✓ | |
1. Commentary on new evidence
These commentaries focus on the ‘key references’ identified through the search process and prioritised by the EUAG for inclusion in the Evidence Update, which are shown in bold text. Supporting references provide context or additional information to the commentary. Section headings are taken from NICE CG143.
1.1. Individualised assessment at presentation
No new key evidence for this section was selected for inclusion in this Evidence Update.
1.2. Primary analgesia
No new key evidence for this section was selected for inclusion in this Evidence Update.
1.3. Reassessment and ongoing management
No new key evidence for this section was selected for inclusion in this Evidence Update.
1.4. Possible acute complications
No new key evidence for this section was selected for inclusion in this Evidence Update.
1.5. Management of underlying pathology
Intravenous magnesium sulfate in children
NICE CG143 does not contain recommendations about intravenous magnesium sulfate in acute painful sickle cell episodes.
Goldman et al. (2013) conducted a double-blind, placebo-controlled randomised trial of magnesium sulfate3 in children with an acute painful sickle cell episode to assess the effect on length of stay in hospital. Secondary outcomes were pain score measured by the Faces Pain Scale-Revised and visual analogue scales, total dose of analgesics and adverse events. Participants received intravenous magnesium sulfate (100 mg/kg, maximum 2 g every 8 hours or placebo (saline identical to the active substance in appearance and administration). De Franceschi et al. (2000) showed that oral magnesium pidolate reduced the number of days patients had painful episodes, so the present study was designed to assess whether magnesium can treat painful episodes in addition to its preventive effects.
The sample size calculation determined that 63 participants were needed in each arm to have 80% power to detect a reduction in length of stay in hospital from 5 days to 4 days. The study recruited 98 children with an overall mean age of 12.4 years (range 4–18 years) who had 104 acute painful episodes: 51 episodes in the magnesium sulfate group and 53 episodes in the placebo group.
The mean length of stay in hospital for the magnesium sulfate group was 132.6 (standard deviation [SD] 106.6) hours and for the placebo group was 117.9 (SD 72.8) hours. Magnesium sulfate was therefore associated with a slightly higher mean length of stay in hospital (mean of 14.7 hours longer), but this difference between groups was not significant (p=0.41).
Median pain scores throughout admission did not differ significantly between groups (magnesium sulfate=5.4 [SD 2.5]; placebo=5.3 [SD 2.3], p=0.80). Morphine was administered as a continuous infusion in 98 of 104 episodes, at a mean cumulative dose of 28.2 (SD 10.9) micrograms/kg per hour in the magnesium sulfate group and 28.4 (SD 8.8) micrograms/kg per hour in the placebo group. Children additionally received morphine bolus in 88 episodes, paracetamol in 83 episodes and ibuprofen in 77 episodes. Other drugs used less frequently included intravenous hydromorphone, patient-controlled morphine, oral morphine, oral hydromorphone, and oral codeine. Generally, the mean cumulative dose did not differ significantly between groups.
Pain at the infusion site was more common in the magnesium sulfate group (7 patients) than in the control group (2 patients). Other adverse reactions were rare: transient hypotension was seen in 1 patient in each group and nausea or vomiting occurred in 2 patients in each group.
Limitations of the study included that it was conducted at a single centre, and it did not recruit the full sample of patients to allow statistical power. Recruitment ceased because the principal investigator moved to another hospital during the study.
This randomised controlled trial found that intravenous magnesium sulfate did not reduce length of stay in hospital, pain scores, or cumulative dose of analgesics compared with placebo in children with acute painful sickle cell episodes. This evidence is unlikely to impact on recommendations in NICE CG143, which does not cover this intervention.
Key reference
- Goldman RD, Mounstephen W, Kirby-Allen M et al (2013) Intravenous magnesium sulfate for vaso occlusive episodes in sickle cell disease. Pediatrics 132: e1634–41 [PubMed: 24276838]
Supporting reference
- De Franceschi L, Bachir D, Galacteros F et al (2000) Oral magnesium pidolate: effects of long-term administration in patients with sickle cell disease. British Journal of Haematology 108: 284–9 [PubMed: 10691856]
Arginine in children
NICE CG143 does not contain recommendations about arginine in acute painful sickle cell episodes.
Morris et al. (2013) did a randomised double-blind, placebo-controlled trial to investigate the use of L-arginine4 in children with an acute painful sickle cell episode. L-arginine is a precursor to the synthesis of the vasodilator nitric oxide. Arginine metabolism and the vasodilatory effects of nitric oxide may be impaired in people with sickle cell disease, thus this study aimed to assess the effects of L-arginine supplementation during an acute painful sickle cell episode. Participants were recruited from the emergency department, haematology clinic, day hospital and wards; however recruitment occurred only when study staff, the research pharmacist and parents were present on-site.
Patients were randomly assigned to receive L-arginine 100 mg/kg 3 times daily for 5 days (maximum cumulative dose 10 g) or until discharge from hospital (whichever occurred first), or placebo. Patients could receive L-arginine by oral or intravenous administration, with a matched tablet or saline used in the placebo group. Outcomes were length of stay in hospital, total dose of analgesia as standard opioid equivalents, and pain scores measured by visual analogue scale and Faces Pain Scale.
Overall, 38 patients (mean age 13.9 years, range 3.6–19 years) had 56 acute pain episodes, however in 2 episodes, patients received no opioid analgesia, which was an eligibility requirement, so data for 54 episodes were analysed.
Length of stay in hospital was 4.1 (SD 1.8) days in the L-arginine group and 4.8 (SD 2.5) days in the placebo group; this difference was not significant (p=0.34). Most episodes (50 of 54) were treated with intravenous morphine, 3 were treated with intravenous hydromorphone, and 1 was treated with intravenous pethidine. Mean cumulative opioid dose was significantly lower in the arginine group (1.9 [SD 2.0] mg/kg) than in the placebo group (4.1 [SD 4.1] mg/kg, p=0.02). Pain scores at discharge were also significantly lower in the arginine group (mean=1.9 [SD 2.4]) than in the placebo group (mean=3.9 [SD 2.9], p=0.01).
Acute chest syndrome developed in 3 patients in the L-arginine group and 1 in the placebo group. Acute clinical deterioration as a consequence of acute chest syndrome led to 1 withdrawal from the study in the placebo group. Other adverse events were 1 case of urticaria in the L-arginine group and 1 case of raised liver enzymes in the placebo group.
Limitations of the study include that it was conducted in a single centre and had convenience recruitment of participants (that is, only when specified staff were present). The authors stated that the study was not sufficiently powered to detect a change in length of hospital stay, which may account for the lack of significance in the difference between groups for this outcome.
This study suggests that L-arginine may be associated with lower use of opioid analgesia and lower pain scores compared with placebo in children with acute painful sickle cell episodes; however length of stay in hospital may not be affected. These findings need to be confirmed in a large randomised controlled trial, so no impact on NICE CG143 is expected.
Key reference
- Morris CR, Kuypers FA, Lavrisha L et al (2013) A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 98 1375–82 [PMC free article: PMC3762093] [PubMed: 23645695]
1.6. Non-pharmacological interventions
No new key evidence for this section was selected for inclusion in this Evidence Update.
1.7. Settings and training
No new key evidence for this section was selected for inclusion in this Evidence Update.
1.8. Discharge information
No new key evidence for this section was selected for inclusion in this Evidence Update.
2. New evidence uncertainties
During the development of the Evidence Update, the following evidence uncertainties were identified for the UK Database of Uncertainties about the Effects of Treatments (UK DUETs).
Management of underlying pathology
Further evidence uncertainties for sickle cell disease can be found in the UK DUETs database and in the NICE research recommendations database. UK DUETs was established to publish uncertainties about the effects of treatments that cannot currently be answered by referring to reliable up-to-date systematic reviews of existing research evidence.
Appendix A. Methodology
Scope
The scope of this Evidence Update is taken from the scope of the reference guidance:
- Sickle cell acute painful episode. NICE clinical guideline 143 (2012)
Searches
The literature was searched to identify studies and reviews relevant to the scope. Searches were conducted of the following databases, covering the dates 1 May 2011 (the end of the search period of NICE clinical guideline 143) to 10 January 2014:
- CDSR (Cochrane Database of Systematic Reviews)
- CENTRAL (Cochrane Central Register of Controlled Trials)
- CINAHL (Cumulative Index to Nursing and Allied Health Literature)
- DARE (Database of Abstracts of Reviews of Effects)
- EMBASE (Excerpta Medica database)
- HTA (Health Technology Assessment) database
- MEDLINE (Medical Literature Analysis and Retrieval System Online)
- MEDLINE In-Process
- NHS EED (Economic Evaluation Database)
- PsycINFO
The Evidence Update search strategy replicates the strategy used by NICE CG143 (for key words, index terms and combining concepts) as far as possible. Where necessary, the strategy is adapted to take account of changes in search platforms and updated indexing language.
Table 1 provides details of the MEDLINE search strategy used, which was adapted to search the other databases listed above. The search strategy was used in conjunction with validated Scottish Intercollegiate Guidelines Network search filters for RCTs and systematic reviews.
Figure 1 provides details of the evidence selection process. The list of evidence excluded after review by the Chair of the EUAG, and the full search strategies, are available on request from ku.shn.ecnedive@sutcatnoc
See the NICE Evidence Services website for more information about how NICE Evidence Updates are developed.
Table 1MEDLINE search strategy (adapted for individual databases)
| 1 | exp Anemia, Sickle Cell/ |
| 2 | exp Pain/ |
| 3 | Acute Disease/ |
| 4 | (pain$ or acute$ or cris$ or episode$).ti,ab. |
| 5 | or/2-4 |
| 6 | 1 and 5 |
| 7 | (sickl$ adj10 (pain$ or acute$ or cris$ or episode$)).ti,ab. |
| 8 | 6 or 7 |
Appendix B. The Evidence Update Advisory Group and Evidence Update project team
Evidence Update Advisory Group
The Evidence Update Advisory Group is a group of topic experts who reviewed the prioritised evidence from the literature search and advised on the development of the Evidence Update.
- Dr Michele Afif – ChairConsultant Paediatrician, North West London Hospitals NHS Trust
- Dr Brigitta BrandnerConsultant Anaesthetist, University College London Hospitals
- Dr Jo HowardConsultant Haematologist, Guy’s and St Thomas’ Hospital, London
- Dr Kate RyanConsultant Haematologist, Manchester Royal Infirmary
- Ms Sekayi TangayiService Manager and Specialist Nurse, Sickle Cell and Thalassaemia Centre, East London NHS Foundation Trust
Evidence Update project team
- Marion SpringAssociate Director
- Dr Chris AlcockClinical Lead – NICE Evidence Services
- Chris WeinerConsultant Clinical and Public Health Adviser
- Cath WhiteProgramme Manager
- Swapna MistryProject Manager
- Mike RaynorInformation Specialist
- Fran WilkieCritical Appraiser
- Lynne KincaidMedical Writer
Footnotes
- 1
- 2
At the time of publication of this Evidence Update, magnesium sulfate or L-arginine did not have UK marketing authorisation for acute painful sickle cell episodes and are not recommended by NICE CG143 for this indication.
- 3
At the time of publication of this Evidence Update, magnesium sulfate did not have UK marketing authorisation for acute painful sickle cell episodes and is not recommended by NICE CG143 for this indication.
- 4
At the time of publication of this Evidence Update, L-arginine did not have UK marketing authorisation for acute painful sickle cell episodes and is not recommended by NICE CG143 for this indication.
- Sickle cell acute painful episodeSickle cell acute painful episode
- Glucosamine 6-phosphate N-acetyltransferase [Caenorhabditis elegans]Glucosamine 6-phosphate N-acetyltransferase [Caenorhabditis elegans]gi|17557234|ref|NP_505654.1|Protein
- PREDICTED: Lemur catta axin 1 (AXIN1), transcript variant X1, mRNAPREDICTED: Lemur catta axin 1 (AXIN1), transcript variant X1, mRNAgi|2168868863|ref|XM_045540948.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...