I.2.1. Model overview
I.2.1.1. Comparators
The model compares the cost effectiveness of four strategies for the management of incontinence due to neurogenic lower urinary tract dysfunction (NLUTD):
Augmentation Cystoplasty (AC) is a well established, major, open surgical technique where the bladder is made larger or ‘augmented’ by incorporating a bowel segment into the bladder. Most commonly an ileal segment is used but alternatives include a section of the large intestine. The incorporation of intestine into the bladder prevents effective bladder contractions from occurring and patients usually cannot void completely following the surgery and therefore need to perform clean intermittent self catheterisation.
The second intervention is the injection of botulinum toxin type A (BTX) into the bladder wall. BTX is currently not licensed for this indication but various trials have shown it to be effective in reducing the frequency of incontinence episodes1–3 in patients with incontinence due to NLUTD. The protocol for administration of BTX varies but the method used in this model is 30 endoscopic injections of 300u or 200u into the bladder wall. Patients with neurogenic LUT dysfunction will mostly need to use intermittent catheterisation to empty the bladder effectively following the treatment.
The third strategy is whereBTX is administered for two cycles and then AC is conducted in 100% of those that do not respond to BTX (BTX100AC) BTX continues to be administered in those that do respond.
The final comparator is no treatment or “best supportive care” (No-Rx). This comparator is included as an arm where patients opt to manage their incontinence with a mixture of incontinence appliances: pads, indwelling catheters, sheaths and suprapubic catheters.
I.2.1.2. Population
The population in this model is made up of patients with NLUTD (Myelomeningocele, Spinal Cord Injury, Multiple Sclerosis etc.) and bladder over-activity who are unresponsive or intolerant to anticholinergic medication. The patients in the base case are considered to be adults as the paucity of data on children prevents an adequate analysis for the paediatric age group. However the cost effectiveness in children will be tested in a sensitivity analysis.
The trial that the data for BTX3 utilises measures effectiveness in patients with an average age of 49. The AC study used patients with an average age of 34. The AC study defined its population with a range, 17–66, as the BTX study falls fairly centrally within this range; the base case age was selected as 49. The distribution of men and women across the studies were also defined. In the Cruz study, there were around 40% men and in the AC study, there were 76% men. If a pooled average is taken this comes to a sex distribution of 53% female and 47% male.
Using this base case patient, it is possible to find standard mortality data for the UK 4 and determine life expectancy, thus allowing a lifetime horizon to be considered in the model. The model uses a standardised mortality ratio from a group of patients with spinal cord injury5. Standard mortally for the UK will be considered in a sensitivity analysis. Subgroup analysis will be carried out on different patients to determine cost effectiveness in a paediatric population.
However, not all of the comparators are relevant in every situation. For some patients, such as multiple sclerosis patients, the AC comparator is not relevant as they are not suitable for this surgical option. There are therefore two base case comparisons. Base case 1 is all the comparators compared together. The second base case analysis is simply BTX compared with No-Rx.
I.2.1.3. Time horizon, perspective, discount rates used
The time horizon is defined as a lifetime using a 3.5% discount rate per year on both outcomes and costs but this was varied between 0 and 6% for outcomes and costs in a sensitivity analysis as per the NICE reference case6. A specific analysis will be done on a discount factor of 1.5% for Quality Adjusted Life Years (QALYs) and 3.5% for costs. The analysis is conducted from the National Health Service and Personal Social Service perspective.
I.2.2. Approach to modelling
A decision tree was constructed in Windows Excel® to model the comparison of cost and effectiveness of the interventions. Life tables were then attached to each of the final health states in the tree and a hypothetical cohort of a thousand patients was run through the model. The trials that were used to inform the model used frequency of incontinence episodes as the main outcome. Quality of Life weights were attached to being either incontinent, continent or having mild incontinence on the basis of the frequency of episodes. As adverse events and the presence or absence of urinary tract infections have important quality of life and cost implications, these were also included. The cost components included costs of the treatment itself, the ongoing costs associated with adverse events and any monitoring or follow up treatments.
I.2.2.1. Model structure
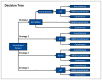
The decision tree compared the three management strategies (AC, BTX and BTX with AC in non-responders) and one no treatment strategy emanating from the initial choice node. Then at each of the chance nodes, a probability is attached that is determined by the effectiveness of the given treatments. At the end of each branch there is a Markov model for each of the outcome states that enable calculation of costs and QALYs over the time horizon. This allows the consideration of mortality data and life expectancy. The structure of the decision tree can be seen in .
Decision Tree. BTX100AC : BTX for two attempts then transfer to Augmentation if BTX is unsuccessful. BTX: Botulinum Toxin. NoRx: No Treatment. AC: Augmentation Cystoplasty. Note: At every health state, a patient may progress to the “absorptive” (more...)
With each of the four options, an incontinent patient upon receiving treatment will either become continent meaning that the treatment was effective, they will have improved continence but will not be fully continent, mild incontinence, or they will remain incontinent. Each of these options is determined by the effectiveness of each treatment. This is true of all the arms but there is a slight difference in the BTX100AC arm. In this arm, the patient will receive two cycles of BTX treatment, however those patients who remain incontinent, rather than remaining in the incontinent state for the rest of the model, they will opt to undergo an AC, incurring all the benefits and harms of that treatment arm. It is assumed that the AC that a patient receives following attempted BTX is as effective as an AC received without attempted BTX. In the BTX only arm, the patients that do not respond will receive BTX for two cycles then will no longer receive BTX and will manage their incontinence using appliances such as pads and catheters.
The frequency of incontinence episodes is used as the main outcome. Due to the inconsistent reporting of the effectiveness of treatments between studies, assumptions had to be made about the frequency of incontinence episodes that constituted each outcome. This was done so that costs and effects could be calculated. It was assumed that in the continent group a patient would suffer from one incontinence episode per week, in the mild incontinent group, they would suffer from two episodes per day and in the incontinent group, they would suffer from five episodes per day. All these options were given an assumed standard error 20% of the mean and normally distributed for the probabilistic analysis.
Table 1Frequency of incontinence episodes as defined by continence status
View in own window
| Incontinent | Mild Incontinent | Continent |
|---|
| Number of episodes per day | 5 | 2 | 0.14 |
Once a patient is in one of the outcome groups; continent, mild incontinent or incontinent, it is assumed that they will remain within this group for the duration of the lifetime horizon. In order to model this, life tables are attached to each of the outcome groups. Life tables are mortality rates for a given population, in this case England and Wales4. In the base case analysis a standardised mortality ratio (SMR) is used that fits the mortality to a more appropriate population. The SMR used was from a retrospective 50 year study in spinal cord injury patients5. This SMR increased the mortality rate in patients by a factor of between two and four, depending on age.
The use of a life table allows costs and outcomes to be calculated over a lifetime. It allows the cycle length, the period over which these costs and outcomes are borne, to be varied in a sensitivity analysis. The model uses the mean time that patients requested retreatment with BTX as the cycle length, enabling the costs and outcomes of each intervention to be calculated over the same period. The trial3 used as the basis of the BTX data reported 8 months mean time to request retreatment and 10 months as the median. Therefore the mean is used as the base case but the median is tested in a sensitivity analysis. This cycle length determined the frequency of reinjection with BTX. Changing the cycle length will not impact on the effectiveness of BTX. BTX is assumed to retain the same effectiveness no matter how long or short the reinjection time is. This is a substantial simplification that reduces the precision of the result, due to the uncertainty about how long the patient will remain continent for. The effectiveness data is only for 12 weeks, however the 8 month reinjection period is based on the same study so there is internal consistency
I.2.2.2. Uncertainty
The model is built probabilistically to take account of the uncertainty around parameter point estimates. In order to do this a probability distribution is defined for each model input. So that when the model is run, a value for each input gets randomly selected from its respective probability distribution simultaneously. This is done repeatedly – 1000 times – and results are summarised. Probability distributions are based on error estimates from data sources, for example: the standard error around a point estimate. The number of simulations used was chosen considering the Monte Carlo error of the incremental costs, QALYs and net monetary benefit using the methods as described by Koehler and colleagues7. It is set to ensure that the Monte Carlo error is not more than 5% of the standard error for these parameters.
In addition, various deterministic (one-way) sensitivity analyses were undertaken to test the robustness of model assumptions and data sources. In these, one or more inputs are changed and the analysis is rerun to see the impact on results. This was done using the deterministic (non-probabilistic) data.
I.2.3. Model inputs
Model inputs were based on clinical evidence identified in the systematic review undertaken for the guideline, supplemented by additional data from standard national sources. Model inputs were agreed by clinical members of the GDG.
I.2.3.1. Initial cohort settings
The Model is based on a hypothetical cohort of patients. Baseline patients are defined as patients suffering from incontinence from NLUTD. The patient has undergone treatment with antimuscarinics and is either intolerant or is unresponsive to them. Therefore the baseline patient currently manages their incontinence with catheters in order to void and absorptive pads or incontinence sheaths in order to counteract incontinence episodes. The base case patient is 49 years old, 53% female and 47% male. There was no acceptable data in children for either BTX or AC, therefore the same data is used in the paediatric sensitivity analysis although there are differences in the rate of adverse events.
I.2.3.2. Treatment effects
No studies were identified in the clinical review that compared botulinum toxin (BTX) with augmentation cystoplasty (AC) directly. Therefore studies that compared BTX and AC to “usual care” were used. However considering BTX and AC are both interventions in those where first line treatment had failed, usual care consisted of no treatment (No-RX).
The data for the effectiveness of BTX comes from a randomised controlled trial of 275 patients3. This study compared the use of 30 intradetrusor injections of 200U and 300U of “botulinum toxin type A” (BTX) with placebo over a period of 12 weeks. In the base case, this analysis will be looking at 200U of BTX. To measure the effectiveness of the treatment, the frequency of incontinence episodes is used. The study showed a decrease from baseline in the frequency of episodes at 6 and 12 weeks compared to placebo. In the study, the mean frequency of incontinence episodes was reported. However in order to be able to put the data into the model in a comparable form with the AC data, it was necessary to convert it to a categorical variable. The categorical variable was: those patients who did not respond to treatment (incontinent), those who responded but were not completely dry (mild incontinence) and those who were completely dry after treatment (continent). A request was therefore submitted to the authors of the paper for additional dataa. The data was supplied in the form of a responder analysis. The data was consistent with that presented in the original paper but was in a more applicable form for this analysis. The study also showed the time to request retreatment with BTX: mean 8 months median 10 months. A Dirichlet distribution was applied to take into account the uncertainty around the probability point estimates (The Dirichlet distribution is the multivariate generalisation of the beta distribution that confines all the parameters between 0 and 1 and allows order to be maintained between linked probabilities). However there was no long-term, follow-up, data of BTX for the treatment of neurological incontinence. An assumption therefore had to be made on the basis of several studies8–10 that the bladder wall does not lose responsiveness and that the therapeutic effect of BTX is maintained after long term usage.
There were no randomised controlled trials carried out on Augmentation Cystoplasty (AC) identified in the clinical review. However there were several observational studies conducted that inform the model on the effectiveness of AC. These studies where not meta-analysed due to heterogeneity. All of these studies had relatively low sample sizes and most were from inappropriate settings. One study by Reyblat et al. 200911 was from a US setting, was of an acceptable size and provided enough data on outcomes to incorporate into the model. This study was therefore selected to form the basis of the analysis of the AC arm of the model. The outcomes of this study were measured using a categorical variable: incontinence, mild incontinence or continence. The probabilistic parameters or the AC outcomes were given using a Dirichlet distribution, in order to maintain the order of probabilities.
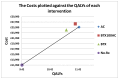
It was assumed that in the No-Rx arm, patients remained incontinent throughout. A summary of the treatment effects used in model is provided in .
Overview of treatment effects used in the model.
I.2.3.3. Adverse Events
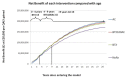
The other impact that these interventions are evaluated for is adverse events (AEs) and urinary tract infections (UTIs). One of the suggested benefits of BTX over AC is the fact that it produces fewer side effects. This is captured in the analysis. The probability of UTIs and AEs can be seen in . The data used to inform the adverse event and UTI probabilities were from various different study lengths meaning they all had to be standardised to the same cycle length. In order to do this the following equations are used:
Adverse Event Probabilities.
Probability to annual rate:
Annual rate to cycle length probability:
The GDG considered that the two most important side effects associated with BTX are haematuria and urinary retention. The probability of a patient experiencing haematuria following BTX treatment is given in a study by Schurch et al. 20051, this probability was given over one year and was incorporated using the method described above. It was recognised that urinary retention is an adverse event associated with BTX however the costs and effects associated with it are not modelled. This was due to the fact that the entire population is likely to undertake intermittent catheterisation and therefore urinary retention adds no extra burden.
The side effects associated with AC are more extensive. They include: ileus, bowel obstruction, perforation of the augmented bladder, bladder stones and re-augmentation. Some of these AEs represent serious events while the incidence of the various complications varies from the relatively common (bladder stones) to the rare (perforation). Where possible, the probability of experiencing an adverse event was taken from the same study as the clinical effectiveness, i.e. Reyblat et al. 200911. This was possible for ileus, bladder stones and bladder obstruction as these were all measured outcomes with an average follow-up of 2.5 years. Ileus was considered in a different way from the other adverse events because it is a one off event. Whereas the probability of stones, obstruction or perforation continued throughout the length of time that a patient was in the model; ileus is a complication arising specifically around the time of the surgery and therefore the associated probability and costs are simply attached to the cost of the AC. The probability of having a perforated augmentation bladder is taken from Metcalfe et al. 200612, this study, in a young-adult and paediatric population, had an average follow-up of 3.8 years enabling conversion to a one year probability. The probability was therefore considered to be 2.4% per year. However, this figure was thought to be too high for adults and the GDG assumed that this figure was 4 times less in adults: 0.6% per annum. The final adverse event is the probability of redoing the surgery due to an issue with the augmentation bladder. This was assumed by the GDG to be at a probability of 30–40% over 10 years. This was converted to a standardised probability using the midpoint of this estimate, 35% and the range for the sensitivity analyses.
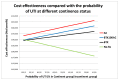
Urinary tract infections (UTIs) are defined as the symptomatic UTIs that require treatment. This definition was used as asymptomatic bacteriuria is universally present in this population of patients. A clear definition of what is classified as a UTI, which UTIs are treated and which UTIs are reported is not available. The baseline rate of UTIs in the population was taken from the study by Gamé et al. 200713, this study was used because the studies in the clinical review did not report the UTIs associated with continence status which was required so that the UTIs could be standardised for all the interventions. This gave a rate of 1.77 UTIs per patient in the six months running up to BTX injection. As the pre-BTX population is in the same as the pre-AC population, this baseline rate of UTI could apply to both. If this rate is converted to a probability it comes to 93% every 8 months. The Gamé study gives a reduction for patients who are continent to a 28% probability of a UTI after 8 months. The GDG considered that for patients who are continent post AC, a similar reduction is seen. Due to the paucity of data in this area, an assumption was made that the mild incontinent and continent groups experienced the same level of UTIs. This means that the rate of UTIs are associated with the level of incontinence and not with the treatment used. The probability of having UTIs is also tested in a sensitivity analysis.
I.2.3.4. Utilities
The Utility for the main outcome, incontinence, were taken from a study by Hollingworth 201014 this study evaluated the quality of life associated with incontinence, using the SF-6D utility measure. The baseline utility of a patient with neurological incontinence is 0.66 according to the Hollingsworth study, see . The quality of life weights for “successful” treatment are 0.78 for continence and 0.75 for mild incontinence. The Utility loss from UTI is taken from the Infection Prevention guideline model on catheterisation. This gives a 0.05 reduction in quality of life and is based on UTI in catheterised patients with spinal cord injury. There is a potential limitation in that by counting UTI utility loss, we are double counting. The Utility data associated with incontinence potentially includes UTI quality of life loss. In order to measure the impact that this has on cost effectiveness, a sensitivity analysis was done, setting the utility loss from UTIs to zero.
The utility for haematuria resulting from BTX was not included because it is not considered to be painful nor does it not cause long term negative impacts. Haematuria is normally be followed up due to cancer risk but there is an obvious cause here, so this is considered unlikely. There is some potential for patient anxiety, but with explanation of the situation from the physician this should be relieved.
The AE utility loss comes from a combination of studies. The availability of utility data to populate this part of the model was very poor. Sullivan et al. 201115 provide a catalogue of EQ-5D disutilities and was used to input the disutility of a bladder stones which came to −0.02. Another AE was perforation or rupture of the augmented bladder. For this the Jansen 2007 study was used, although this study was in a different population and the event was not exactly the same, it was considered by the GDG to be a close approximation which came to −0.48. The same study was also used to provide data on bowel obstruction and diarrhoea which when combined came to a utility loss of −0.18. When the utility losses are combined with the probabilities of given events, the utility loss from AC comes to −0.004 per augmentation.
The method used to combine the, per cycle, probabilities with utilities for AEs in AC are given below to demonstrate this type of calculation:
View in own window
| Disutility for bladder stones | = −0.02 |
| Probability of bladder stones per cycle | = 0.019 |
| = 0.019*−0.02 |
| = −0.000376 |
| Disutility for bladder perforation | = 0.488 |
| Probability of bladder perforation per cycle (assumed 4x less in adult augments) | = 0.00393 |
| = 0.00393*−0.488 |
| = −0.00192 |
| Disutility for general bowel disruption (Diarrhoea, blockage, Ileus) | = − 0.184 |
| Probability for bowel disruption per cycle | = 0.0074 |
| = 0.0074*−0.184 |
| = −0.0014 |
| Combined disutility | = −0.000376 + −0.00192 +−0.0014 |
| = − 0.0037 |
This same method is used to generate the other disutilities occurring in the model.
The utilities in that need to be are converted into disutilities using the simple conversion of 1-utility. The reason this is done is to limit the utilities by 0 and 1 when the probabilistic analysis is done. The utilities are then combined with the life years to provide a weighting to produce Quality Adjusted Life Years (QALYs). The QALYs are calculated for each cycle and the sum total over a lifetime is divided by the cohort size to provide the number of QALYs per person. Patients who move into the death state accrue zero QALYs.
I.2.3.5. Resource use and cost
It was possible to cost the resource use using official UK sources: NHS reference costs 2009/10, NHS supply Chain Catalogue 2011, the NHS drug tariff and the British National Formulary (BNF) 60. Where an appropriate cost could be found that fully covered the aspect of resource use required, this was attached. However, assumptions had to be made when the cost was not so clear. All the costs that were incorporated into the model can be found in .
The cost of BTX was constructed using a combination of the NHS reference cost for “injection of substance into bladder wall” and the price of either 200U or 300U of BTX from the BNF-60. However because BTX is not yet licensed for this use, this cost is fairly speculative and was tested in a sensitivity analysis. The adverse events associated with the injection of BTX were haematuria and urinary retention. The cost of haematuria is assumed as the cost of a consultation with a GP.
The cost of AC was simply the cost of a “major open procedure/reconstruction” in the NHS Reference costs 2009/10. This cost could also form the basis of re-augmentation and the cost of repairing a bladder perforation. The cost of bladder stone removal is a combination of endoscopic and open removal. Costing bowel obstruction required the assumption that 70% would simply require an extra week in hospital whereas 30% would require a major surgical procedure to remove the obstruction. The treatment for ileus simply consisted of an extra period in hospital; the Reyblat11 study put the mean at 4.9 days.
The costs outlined above were the costs of treatment. The long term costs besides continued BTX treatment and the AEs associated with BTX and AC were the costs of each continence state. These included the costs of UTIs and the costs of incontinence appliances. The Cost of a UTI was calculated as the cost of a Healthcare consultation (£32), a dipstick analysis (£0.07), first-line antibiotic treatment (£2) and the dispensing fee (£1.96). In total this came to £36.
The costs of incontinence appliances were costed based on GDG assumptions about the average usage by patients. Pads were costed at £0.25, intermittent catheters costed £0.75, Indwelling catheters costed £5.31 with 30 minutes of district nurse time coming to £32 every 6 weeks and sheaths cost £0.79. All these appliances were used at different rates depending on the health state that the patient was in.
In the Incontinent group it was assumed:
- -
25% of men and 50% of women would manage on pads and intermittent catheters.
- -
40% of men and 50% of women would manage on indwelling catheters.
- -
35% of men would manage with sheaths.
In the Mild Incontinent and Continent groups it was assumed:
- -
100% of men and women would use intermittent catheters.
- -
All of these would wear a pad and manage episodes with pads based on the frequency defined by the treatment effectiveness.
It was also recognised that children use different levels of incontinence appliances. It was assumed, for simplicity, that patients under the age of 10 use pads only if incontinent to manage episodes. The costs of all appliances combined and multiplied by cycle length can be found in .
These costs are then added to the number of patients in each health state while they remain within the model at each cycle. For AC, the main cost is that of the operation at the beginning, the follow on costs are then the cost multiplied by the probability of any adverse events they may incur at any given time. For BTX, the cost of the injection of BTX is incurred at every cycle as is the cost of any side effects.
I.2.4. Computations
Some methods of eliciting distributional parameters simply require the number of events and the total number in the study. The beta distribution for, example, is defined by α and β, α being the number of events and β being the total study size minus α. However often this data is not available and more complex computations have to be made in order to make the data probabilistic. This usually entails using the mean of a sample as the point estimate and some an error estimate such as a confidence interval or a standard error, is used to determine the shape of a distribution: the slope of the line and the intercept.
To elicit distribution parameters for the beta distribution (α,β) the method of moments was used (μ = mean, s = variance):
In order to elicit the distribution parameters for the gamma distribution, the method of moments was also used:
The distributional parameters for the lognormal distribution were elicited from the mean and standard error using the method of moments: