NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients. London: National Institute for Health and Clinical Excellence (NICE); 2012 Sep. (NICE Clinical Guidelines, No. 151.)

Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients.
Show detailsA1. Introduction
Neutropenic sepsis causes significant morbidity and mortality in patients receiving chemotherapy and can lead to reduced chemotherapy dose intensity and increased overall treatment costs (Cullen 2009). There are two approaches to preventing neutropenic sepsis: destroying potentially dangerous bacteria or enhancing immunity. Because there is great uncertainty over the cost-effectiveness of the different prophylactic medicines and whether primary or secondary prophylaxis is more cost effective, the guideline Development Group (GDG) prioritised this topic for health economic analysis (See Economic Plan in the full Evidence Review).
A1.1. Prophylactic medicines
There are two commonly used prophylactic medicines for preventing neutropenic sepsis, namely antibiotics and G-CSF. Evidence was reported for two types of antibitioics: quinolones and cotrimoxazole. However the GDG chose to focus on the evidence related to quinolones because of concerns that changing anti-microbial resistance patterns meant the cotrimoxazole trials may no longer be applicable (Gafter-Gvili, et al., 2005). The quinolones are a family of synthetic broad-spectrum antibiotics which can be used to kill or slow down the growth of bacteria. The most commonly used subset of quinolones is fluoroquinolone. Pre-emptive use of oral quinolones can reduce the likelihood of neutropenic sepsis (Gafter-Gvili, 2005), but may incur patient-related risks of gut disturbance, allergy, etc and more general risks related to the development of antibiotic resistance within the population.
Recently, the use of Granulocyte colony-stimulating factor (G-CSF) to prevent neutropenic sepsis has increased substantially (Aapro, et al., 2006). G-CSF is a colony-stimulating factor hormone which can be used to raise neutrophil counts, and shorten the duration of neutropenia, by stimulating the bone marrow to produce neutrophils. However, adverse effects include bone pain, headache and nausea, and rarely more serious complications such as anaphylaxis, respiratory failure and splenic rupture. G-CSF must be given by injection, and this may lead to local reactions at the site of administration, and repeated injections may not be desired by patients. Pegylated G-CSF only needs to be given once with each cycle of chemotherapy, but the cost-effectiveness is unknown, comparing to quinolones.
A1.2. Eligibility criteria for prophylaxis
Patients who have had a prior episode of neutropenic sepsis are more likely to become neutropenic with repeated doses of chemotherapy than patients who have never experienced this complication, thus putting them at greater risk of neutropenic sepsis (Cullen 2007). There is uncertainty over the eligibility criteria for prophylaxis. Should it be provided to all cancer patients receiving chemotherapy which is likely to cause neutropenia (primary prophylaxis) or should it only be provided to patients with a previous episode of neutropenic sepsis (secondary prophylaxis)? Compared to primary prophylaxis, secondary prophylaxis prevents less episodes of neutropenic sepsis, and thus is associated with a higher cost. However, secondary prophylaxis may reduce the overall use of prophylactic medicine and thus avoid potential side effects such as antibiotic resistance.
Because of the large patient group covered by this topic and the potentially significant difference in cost of different treatment options, this topic was identified as a high economic priority by the GDG.
A systematic review of the economic evidence for this topic was carried out (Chapter 5). No cost-effectiveness analysis was found which directly addressed the clinical question. As a result, de novo models have been built to inform recommendations.
A2. De novo economic model (overview)
A2.1. Aim
The aim of this economic analysis was to examine which of the following prophylactic strategies is the most cost-effective for cancer patients who are receiving outpatient chemotherapy (defined as patients with planned inpatient treatment of less than 10-days post- chemotherapy):
- Nothing/placebo
- Primary prophylaxis with quinolones
- Primary prophylaxis with G-CSF
- Primary prophylaxis with G-CSF and quinolones
- Primary prophylaxis with PEG-G-CSF
- Secondary prophylaxis with quinolones
- Secondary prophylaxis with G-CSF
- Secondary prophylaxis with G-CSF and quinolones
- Secondary prophylaxis with PEG-G-CSF
A subgroup analysis was conducted for the following three patient groups:
- Patients with a solid tumour (aged 18 years and older)
- Patients with non-Hodgkin lymphoma (aged 18 years and older)
- Patients with Hodgkin lymphoma (aged 18 years and older)
This economic analysis does not cover:
- Cancer patients whose chemotherapy regimen includes G-CSF for dose intensity reasons.
- Cancer patients with planned inpatient treatment of greater than 10-days post-chemotherapy. It is acknowledged that the costs of prophylaxis and treatment of neutropenic sepsis for inpatient-only management are lower than outpatient management.
- Paediatric cancer patients (aged less than 18 years). Due to considerable clinical heterogeneity in the treatment regimens for this patient group, and a paucity of direct evidence, a representative model for economic analysis could not be built.
- The impact of different prophylactic strategies on subsequent courses of chemotherapy. The consequence of this bias is discussed in detail in section A9.2.3.
- Antibiotic resistance. The best available evidence identified to address the issue of antibiotic resistance caused by use of quinolones was derived from two systematic reviews: one was a review conducted for this guideline (see Section 5.1) and the other was a Cochrane review undertaken by Gafter-Gvili, et al., (2005). The conclusions of these two reviews were very similar. After use of quinolones, although there is an increase in colonisation with bacteria resistant to quinolones, there was no statistically significant increase in the number of infections caused by pathogens resistant to quinolones. The GDG were aware of the potential limitations of these two reviews but could not find any better evidence to answer the clinical question. Therefore the GDG decided to qualitatively consider the potential increase in antibiotic resistance and its impact on cancer patients when agreeing their recommendations, instead of quantitatively model it in the economic analysis.
A2.2. Key model assumptions
- None of the prophylaxis strategies included in the model could improve patient's short-term mortality.
- The sensitivity and specificity of diagnosing neutropenic sepsis is 100%.
- Patients could only develop one episode of neutropenic sepsis during one cycle of chemotherapy.
- If a patient stops receiving chemotherapy, he or she would not be at risk of developing neutropenic sepsis.
- The effectiveness of each prophylactic strategy (relative reduction of neutropenic sepsis) would be the same for patients at different levels of risk of developing neutropenic sepsis.
- The effectiveness of each prophylactic strategy (relative reduction of neutropenic sepsis) would be the same for patients who are receiving primary or secondary prophylaxis.
A2.3. Model structure
Decision trees are used to reflect key events in the clinical pathway in order to compare costs and health effects for the interventions of interest. In this economic analysis, two decision trees were constructed to cover two different populations:
- model A for adult patients with Hodgkin lymphoma, and
- model B for adult patients with a solid tumour or non-Hodgkin lymphoma.
The details of both models can be found below. A Markov process was embedded in both decision trees to model the recurrence of neutropenic sepsis within one course of chemotherapy.
- Model A: ‘Continue to receive full dose-chemotherapy
- This model assumes patients will continue to receive full-dose chemotherapy regardless of previous episodes of neutropenic sepsis. Figure A1 illustrates the key health states in the model and possible transitions between them.
- Model B: ‘Dose-reduction chemotherapy’
- This model assumes that if patients develop one episode of neutropenic sepsis, they will then receive dose-reduction chemotherapy. If they develop two episodes of neutropenic sepsis chemotherapy will be discontinued. Figure A2 illustrates the key health states in the model and possible transitions between them.
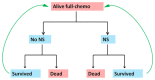
Figure A1
Model A – Simplified transition state diagram. Alive full-chemo = Alive caner patients who are receiving full-dose chemotherapy NS = neutropenic sepsis.
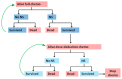
Figure A2
Model B – Simplified transition state diagram. Alive full-chemo = Alive cancer patients who are receiving full-dose chemotherapy Alive dose-reduction chemo = Alive cancer patients who are receiving dose-reduction chemotherapy
The volume of clinical data to inform the relative risk of overall mortality (each prophylactic strategy versus nothing/placebo) was very sparse for the three patient subgroups included in the model. What's more, of the studies that report this outcome their quality was assessed by GRADE as low since none were designed to investigate the effect of GCSF on short-term mortality and the death rate between different arms was low. Therefore the GDG decided to assume that the overall mortality would be the same for each prophylactic strategy, and only looked at the efficacy of each strategy in terms of preventing neutropenic sepsis. Since the baseline short-term overall mortality rate for our target population group is normally very low; unless there were any prophylactic strategies that could significantly reduce short-term overall mortality, this bias is unlikely to change our conclusion.
A2.4. Time horizon
The time horizon of both models (A and B) was one course of chemotherapy, as the GDG were only interested in short-term outcomes. The number of cycles within one course of chemotherapy, and length of each cycle were estimated for each patient subgroup by the GDG (Table A1).
Table A1
Number and length of chemotherapy cycle for each patient subgroup.
A2.5. Software
The cost-effectiveness analyses were conducted using TreeAge pro 2010.
A3. Cost-effectiveness model - inputs
The cost-effectiveness analysis required clinical evidence, health-related preferences (utilities), healthcare resource use and costs. High quality evidence on all relevant parameters was essential; however, these data were not always available. Where published evidence was sparse, the expert opinion of the GDG was used to estimate relevant parameters.
A3.1. Clinical data
A3.1.1. Risk of neutropenic sepsis
Risk of neutropenic sepsis – baseline risk
The baseline risk of neutropenic sepsis for each patient subgroup was obtained from the clinical evidence review of this topic (Appendix 4 of full evidence review) and is presented in Table A2. A range of different risk levels (5-100% per cycle of chemotherapy) were tested in a one-way sensitivity analysis.
Table A2
Baseline risk of neutropenic sepsis (one course of chemotherapy).
The relative risk of a neutropenic sepsis event in the first cycle of chemotherapy compared with cycle two onwards was calculated as 3.69 (Cullen, 2007) (Table A3). The relative risk of further febrile neutropenia episodes in a patient who had experienced previous episodes was calculated as 5.96 (Cullen, 2007) (Table A3). This means that once patients have experienced one episode of neutropenic sepsis, their baseline risk of neutropenic sepsis will be increased with any subsequent chemotherapy.
Table A3
Relative risk of neutropenic sepsis (different cycles, with or without previous neutropenic sepsis).
Model B (‘Dose-reduction chemotherapy’) assumes that once a patient develops one episode of neutropenic sepsis they will start to receive dose-reduction chemotherapy. It is generally considered that a reduction in chemotherapy dose is likely to reduce the patient's risk of neutropenic sepsis, and thus decrease short-term mortality. However, very little clinical evidence comparing chemotherapy dose and the risk of neutropenic sepsis was identified. Therefore in our economic model, it is assumed that chemotherapy dose has no impact on the risk of neutropenic sepsis or short-term mortality. This bias favours all prophylactic strategies except nothing/placebo.
Risk of neutropenic sepsis - relative effects
The relative risk of neutropenic sepsis for each prophylactic strategy was obtained from the clinical evidence review of this topic (Appendix 4 of full evidence review) and is presented in Table A4.
Table A4
Relative risk of neutropenic sepsis (each prophylaxis strategy versus nothing/placebo).
Only a very small volume of clinical evidence for secondary prophylaxis was identified. Therefore it was assumed that the effectiveness of each prophylactic strategy (relative reduction of neutropenic sepsis, and relative reduction of short-term overall mortality) would be the same for patients who are receiving primary or secondary prophylaxis.
A3.1.2. Overall mortality
Overall mortality - baseline risk
The baseline overall mortality for a patient with neutropenic sepsis was obtained from the systematic reviews of the clinical evidence conducted for this topic (Appendix 4 of full evidence review) and is presented in Table A5.
Table A5
Overall mortality for patients with neutropenic sepsis who received no prophylaxis (baseline risk within our course of chemotherapy).
Overall mortality - relative effects
The volume of clinical data to inform the relative risk of overall mortality (each prophylactic strategy versus nothing/placebo) was very sparse for the three patient subgroups included in the model. What's more, of the studies that report this outcome their quality was assessed by GRADE as low since none were designed to investigate the effect of GCSF on short-term mortality and the death rate between different arms was low. The GDG decided to assume that the overall mortality would be the same for each prophylactic strategy, and only looked at the efficacy of each strategy in terms of preventing neutropenic sepsis. Since the baseline short-term overall mortality rate for our target population group is very low; unless there were any prophylactic strategies that could significantly reduce short-term overall mortality, this bias is unlikely to change our conclusion.
For those patients who died during chemotherapy, the probability of dying from infection (infection-related mortality divided by all cause mortality) was obtained from the clinical evidence reviews conducted for this topic (Appendix 4 of full evidence review) and is presented in Table A6.
Table A6
Probability of dying from infection (infection-related mortality/all cause mortality).
A3.2. Utility scores
Utility weights were required to estimate quality adjusted life years (QALYs).
In this analysis the utility decrement due to incidence and treatment of neutropenic sepsis (base-case model) and death were all considered. Utility decrement due to neutropenia was not considered in the economic model for two reasons. Firstly, neutropenia often coincides with other side-effects of chemotherapy, so it is difficult to judge whether the utility decrement is caused by neutropenia alone or other side-effects of chemotherapy. Secondly little evidence was identified which reported utility decrement of neutropenia using EQ-5D, which is the tool recommended by NICE.
A3.2.1. Utility decrement due to neutropenic sepsis and its treatment
Wherever possible, utility data was taken from studies conducted in the UK and using EQ-5D.
Many studies reported utility decrement due to neutropenic sepsis. However, none of those studies were considered to be entirely applicable to the UK settings except Brown, (2001). The most common reasons for inapplicability were:
- Studies were conducted in countries other than the U.K.
- Studies didn't specify the treatment settings for neutropenic sepsis patients: entire inpatient, entire outpatient or inpatient followed by outpatient.
It is generally considered that patients receiving outpatient treatment have better quality of life, comparing with patients receiving inpatient treatment.
Only one paper reported separate utility data for neutropenic sepsis patients receiving treatment in both inpatient and outpatient settings (Brown, 2001). The utility data reported by Brown, (2001) is presented in Table A7.
Table A7
Utility decrement of neutropenic sepsis in different settings.
A3.3. Resource use and cost
The costs considered in this economic analysis were those relevant to the UK NHS, and included the cost of each prophylactic strategy, the costs of each diagnostic investigation and the costs of inpatient and outpatient treatment. Unit costs were based on the British National Formulary (BNF 62), NHS reference costs (2009-10) or the Unit Costs of Health and Social Care (Curtis, 2010).
The cost of chemotherapy was not included as the economic model was only looking at the prevention and treatment of neutropenic sepsis.
Due to the short time horizon of this economic analysis(less than one year), costs and health outcomes were not discounted.
A3.3.1. Prophylactic medicine cost
The costs of each prophylactic medicine included in the model are provided in Table A9.
Table A9
Prophylactic medicine cost per person per cycle.
A3.3.2. Single ambulance journey
Patients with suspected neutropenic sepsis need to see a healthcare professional as soon as possible. However, there is a scarcity of evidence for the use of an ambulance for the target population. It is reported that the use of an ambulance is positively associated with age (Health and Social Care Information Centre, 2009-10). Therefore the use of an ambulance for each patient subgroup was estimated based on their age distribution. The age distribution of each patient subgroup was obtained from the Cancer Research UK website (http://info.cancerresearchuk.org/cancerstats/).
Table A10 shows the estimated ambulance use and associated cost for each patient subgroup. The detailed calculation process can be found in section A11: Cost of ambulance.
Table A10
Estimated ambulance use and cost for each patient subgroup.
A3.3.3. Cost of treating neutropenic sepsis
There is a HRG code for ‘Febrile neutropenia associated with malignancy’: £5,373 (Department of Health, 2011). However, this HRG cost was considered to be inappropriate to our model for two reasons:
- Different target population. This economic analysis only looks at adult patients who are receiving outpatient chemotherapy (defined as patients with inpatient treatment of less than 10-day post-chemotherapy). In contrast to patients who are receiving inpatient chemotherapy (defined as patients with inpatient treatment of greater than 10-day post-chemotherapy), our target population rarely use Intensive Care/Therapy Unit (ICU/ITU) or antifungal drugs; both of which are very expensive. This means the treatment cost for our target population (outpatient group) will be much lower than it for the inpatient group. The HRG code, however, didn't report separate results for patients who are receiving chemotherapy in different settings.
Therefore, the cost of treating neutropenic sepsis was estimated based on the clinical pathway desgined by this guideline (Algorithm: Summary of recommendations).
Unit cost of hospital bed day
According to the NHS reference cost (2009-10), the average cost of an excess bed day is £255, which includes the cost of staff, medication, routine examination and treatment. Therefore the cost of any diagnostic tests and intravenous antibiotic were not double counted. The average cost of an excess bed day is provided in Table A11.
Table A11
Cost of an excess hospital bed day.
Length of hospital stay
Several recent large-scale studies (Schilling, 2011, Lingaratnam, 2011, Lathia, 2009) reported the average length of hospital stay for patients with febrile neutropenia. However, none of these studies were considered to be applicable to our model for three reasons:
- None of the studies were conducted in the UK
- It is generally considered that the length of hospital stay is different for patients who are at different risk of serious adverse outcomes: low-risk patients can receive outpatient management from the outset or for early discharge after a period of inpatient observation and investigation (Section 4.4); while high-risk patients need to stay in hospital until they are afebrile. However, none of the studies reported separate outcomes (length of stay) for patients who are receiving outpatient chemotherapy (defined as patients with inpatient treatment of less than 10-day post-chemotherapy) at different risk of serious adverse outcomes.
Therefore, an estimate of the baseline hospital stay for the economic model was made by the GDG (Table A12), based upon the recommendation in this guideline. The GDG also estimated the percentage of high-risk patients for all three patient subgroups (Table A13).
Table A12
Baseline length of hospital stay for neutropenic sepsis patients who did not receive any prophylaxis.
Table A13
Percentage of neutropenic sepsis patients at high risk of serious adverse outcome.
A recent systematic review by Sung, et al., (2007) reported that the use of prophylactic CSF is associated with a reduction of hospital stay of 2.41 days (95% CI: 1.70-3.13 days) (see Table A14). However this paper did not report baseline hospital days used in the included studies; therefore, an estimate of baseline hospital day was made by the GDG. If it is assumed the average length of hospital stay is 8-day, then the relative reduction of hospital days due to use of G-CSF would be 2.41/8=30.13%. In this model, the average hospitalisation duration for high-risk patients was assumed to be 7 days. So the reduction in hospital days due to use of G-CSF was calculated as 2.11 days (=7*30.13%). It is assumed that the use of prophylactic CSF won't reduce the length of hospital stay for neutropenic sepsis patients at low risk of serious adverse outcomes.
Table A14
Reduced hospital bed days due to use of prophylactic G-CSF (for neutropenic sepsis patients at high risk of serious adverse outcomes only).
As the Sung, et al., review (2007) did not report separate data for patients with different types of cancer it was assumed that the reduction of hospital days would be the same for all three patient subgroups.
It was noted that whilst the Sung, et al., review (2007) included 148 papers comparing G-CSF with placebo/nothing, only 43 reported the reduction of hospital days due to prophylactic G-CSF. So the pooled data might be affected by publication bias. This bias favours G-CSF.
Outpatient treatment and daily telephone contact after discharge (for neutropenic sepsis patients at low risk of serious adverse outcomes only)
In the economic model, it is assumed that neutropenic sepsis patients at a low-risk of serious adverse outcomes can step down to outpatient treatment with oral antibiotics, after the first 48-hour inpatient observation and investigation. For this group of patients, it is assumed that telephone follow-up will last for two days after the patient is discharged from hospital.
Oral antibiotics
Patients who are allergic to penicillin will receive different oral antibiotics to patients who are not allergic. It is estimated that about 10% of neutropenic sepsis patients are allergic to penicillin. The weighted cost of oral antibiotics is presented in Table A15.
Table A15
Weighted cost of oral antibiotic for patients with neutropenic sepsis who are at low risk of serious adverse outcomes.
Daily telephone contact
For patients with neutropenic sepsis and a low-risk of serious adverse outcomes, it is assumed that telephone follow-up will last for two days after the patient is discharged from hospital. It is assumed that each phone call will take a nurse about 10 minutes to complete. The estimated cost of this telephone follow-up is presented in Table A16.
Table A16
Cost of daily telephone contact.
A4. Sensitivity analysis
Three different kinds of sensitivity analysis were conducted to test the robustness of the results of each economic model.
A4.1. Structural sensitivity analysis
A structural sensitivity analysis was conducted to test the robustness of results in each model structure. In model B patients could only develop a maximum of two episodes of neutropenic sepsis and then their chemotherapy would be discontinued, so these patients would no longer be at risk of neutropenic sepsis. However, in Model A, patients who have developed two episodes of neutropenic sepsis will keep on receiving full-dose chemotherapy, and will continue to be at high risk of neutropenic sepsis. Therefore model A (‘carry on regardless’) is a high-risk model when compared to model B (‘dose-reduction model’), even when their baseline risks are the same. This is because the baseline risk can be increased after the patient has developed one episode of neutropenic sepsis.
This means if one prophylactic strategy is not cost-effective in model B, it could potentially become cost-effective in model A (as the risk of neutropenic sepsis has been increased). However if one prophylactic strategy is not cost-effective in model A, then using model B will only make this intervention even less cost-effective. Therefore structural sensitivity analysis has only been conducted for model B (i.e. patients with solid tumour and non-hodgkin lymphoma).
A4.2. One-way sensitivity analysis
For each model, over sixteen scenarios (including the data ranges) were considered and are detailed below:
- Number of cycles of chemotherapy (varies for each patient subgroup)
- Baseline risk of neutropenic sepsis per chemotherapy cycle: Cycle 2 onwards21: (5 - 100%)
- Relative risk of a neutropenic sepsis episode: Cycle 1 versus Cycle 2 onwards (1-10)
- Relative risk of a neutropenic sepsis episode: previous neutropenic sepsis versus no previous neutropenic sepsis (1-10)
- Relative risk of a neutropenic sepsis episode: each prophylactic strategy versus nothing/placebo (0.1 – 0.95)
- Probability of self administrating PEG-G-CSF or G(M)-CSF (0-100%)
- Probability of using an ambulance for patients with neutropenic sepsis (0-100%)
- Probability of patients with neutropenic sepsis who are at high risk of serious adverse events (varies for each patient subgroup)
- Days of using G(M)-CSF for each cycle of chemotherapy (5-11 days)
- Days of inpatient treatment for neutropenic sepsis patients at low-risk of serious adverse events (1-6 days)
- Days of inpatient treatment for neutropenic sepsis patients at high-risk of serious adverse events (6-14 days)
- Cost per hospital bed day (£100 - £1000)
- Drug discounts of PEG-G-CSF and G(M)-CSF (0% - 90%)
- Utility decrement due to inpatient treatment of neutropenic sepsis (0.14-0.38)
- Utility decrement due to outpatient treatment of neutropenic sepsis (0-0.15).
A4.3. Probabilistic sensitivity analysis (PSA)
Probabilistic sensitivity analysis was performed to assess the robustness of the model results against plausible variations in the model parameters. For each patient subgroup, the main results were re-calculated 5000 times.
A summary of all parameters used in the probabilistic sensitivity analysis for each patient subgroup is provided in Table A17 to A19.
Table A17
Summary of parameters used in probabilistic sensitivity analysis (Solid tumour adult).
Table A18
Summary of parameters used in probabilistic sensitivity analysis (non-Hodgkin lymphoma adult).
Table A19
Summary of parameters used in probabilistic sensitivity analysis (Hodgkin lymphoma adult).
A5. Interpreting results
The results of cost-effectiveness analyses are usually presented as incremental cost-effectiveness ratios (ICERs). This is calculated by dividing the difference in cost associated with two alternatives by the difference in QALYS (formula below).
By calculating the difference in benefits, a cost per QALY can be calculated for each comparison.
NICE'S report ‘Social value judgments: principles for the development of NICE guidance’ sets out the principles that GDGs should consider when judging whether an intervention offers good value for money.
In general, an intervention is considered to be cost effective by NICE if either of the following criteria applied:
- The intervention is less costly and more clinically effective compared with all the other relevant alternative strategies. In this case, an ICER is not calculated, or
- Compared with the next best strategy, the intervention has an ICER of less than £20,000 per quality adjusted life-year (QALY).
A6. Results – Solid tumour sub group
The results for adult patients with a solid tumour are presented below in the following order:
- base case analysis (Section A6.1)
- structural sensitivity analysis (Section A6.2)
- one-way sensitivity analysis (Section A6.3)
- probabilistic sensitivity analysis (Section A6.4)
For all sections, separate results are presented for patients who can or cannot take quinolones.
A6.1. Base case analysis
A6.1.1. For patients who can take quinolone
For adult patients with a solid tumour and who can take quinolone, clinical evidence was available for all nine strategies of interest (Section A2.1). Compared to quinolone alone, G(M)-CSF and G(M)-CSF + quinolone are more expensive and less effective in terms of preventing neutropenic sepsis (Table A4 and A11). Therefore all primary and secondary prophylactic strategies involving, G(M)-CSF and G(M)-CSF + quinolone were excluded from the analysis. As a result cost-effectiveness was only formally examined for the following five strategies:
- Nothing/placebo
- Primary prophylaxis with quinolone
- Secondary prophylaxis with quinolone
- Primary prophylaxis with PEG-G-CSF
- Secondary prophylaxis with PEG-G-CSF
The incremental costs and incremental QALYs in the base case analysis for each of the five strategies are summarised in Table A20, and shown graphically in Figure A3. Taking primary prophylaxis with quinolone as the reference (least expensive) strategy, all other strategies were shown to be less effective and also more costly except primary prophylaxis with PEG-G-CSF. Compared to the reference strategy, use of primary PEG-G-CSF produces 3.3×10-4 more QALYs and incurs £1,899.8 in additional costs. This yields an incremental cost-effectiveness ratio (ICER) of £5.7 million/QALY, which exceeds the NICE willingness to pay (WTP) threshold of £20,000/QALY. Therefore primary prophylaxis with PEG-G-CSF was considered not to be cost effective. At a willingness to pay (WTP) threshold of £20,000/QALY, primary prophylaxis with quinolone is the most cost-effective strategy.
Table A20
Incremental costs and effectiveness by treatment strategy for solid tumour patients who can take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
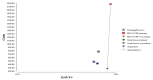
Figure A3
Incremental costs and effectiveness by treatment strategy for solid tumour patients who can take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
A6.1.2. For patients who cannot take quinolone
For adult patients with a solid tumour who cannot take quinolone, cost-effectiveness was only formally examined for the following strategies (all strategies containing quinolone were excluded):
- Nothing/placebo
- Primary prophylaxis with G(M)-CSF
- Secondary prophylaxis with G(M)-CSF
- Primary prophylaxis with PEG-G-CSF
- Secondary prophylaxis with PEG-G-CSF.
The incremental costs and incremental QALYs in the base case analysis for each of the five strategies are summarised in Table A21, and shown graphically in Figure A4. Taking nothing/placebo as the reference (least expensive) strategy, the other four strategies were shown to be more effective but were each associated with a very high ICER (all > £0.6 million/QALY) and were not considered to be cost effective. Therefore at a willingness to pay (WTP) threshold of £20,000/QALY, nothing/placebo is the most cost-effective strategy.
Table A21
Incremental costs and effectiveness by treatment strategy for solid tumour patients who can not take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
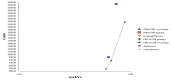
Figure A4
Incremental costs and effectiveness by treatment strategy for solid tumour patients who can not take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
A6.2. Structural sensitivity analysis
A6.2.1. For patients who can take quinolone
For patients with a solid tumour who can take quinolone, the results of the structural sensitivity analysis are summarised in Table A22, and shown graphically in Figure A5. When using the high-risk model (Model A, ‘carry on regardless’), primary prophylaxis with quinolone remains the most cost-effective strategy at a WTP threshold of £20,000/QALY.
Table A22
Incremental costs and effectiveness by treatment strategy for solid tumour patients who can take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
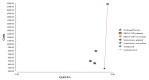
Figure A5
Incremental costs and effectiveness by treatment strategy for solid tumour patients who can take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
A6.2.2. For patients who can not take quinolone
For adult patients with a solid tumour who cannot take quinolone, the results of the structural sensitivity analysis are summarised in Table A23, and shown graphically in Figure A6. When using the high-risk model (Model A, ‘carry on regardless’), nothing/placebo remains the most cost-effective strategy at a WTP threshold of £20,000/QALY.
Table A23
Incremental costs and effectiveness by treatment strategy for solid tumour patients who cannot take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
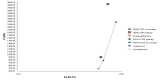
Figure A6
Incremental costs and effectiveness by treatment strategy for solid tumour patients who can not take quinolone (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 34.41%).
A6.3. One-way sensitivity analysis
A6.3.1. For patients who can take quinolone
Over sixteen scenarios were considered and tested using one-way sensitivity analysis (Section A4.2). The results of one-way sensitivity analyses for adult patients with a solid tumour who can take quinolones are presented below in the following order:
- Primary prophylaxis with quinolone v.s Secondary prophylaxis with quinolone (Table A24)
- Primary prophylaxis with quinolone v.s Nothing/Placebo (Table A25)
- Primary prophylaxis with quinolone v.s Secondary prophylaxis with PEG-G-CSF (Table A26)
- Primary prophylaxis with quinolone v.s Primary prophylaxis with PEG-G-CSF (Table A27)
Table A24
One-way sensitivity analyses results for solid tumour patients who can take quinolone: Primary prophylaxis with quinolones v.s Secondary prophylaxis with quinolone.
Table A25
One-way sensitivity analyses results for solid tumour patients who can take quinolone: Primary prophylaxis with quinolones v.s Nothing/Placebo.
Table A26
One-way sensitivity analyses results for solid tumour patients who can take quinolone: Primary prophylaxis with quinolones v.s Secondary prophylaxis with PEG-G-CSF.
Table A27
One-way sensitivity analyses results for solid tumour patients who can take quinolone: Primary prophylaxis with quinolones v.s Primary prophylaxis with PEG-G-CSF.
For adult patients with a solid tumour who can take quinolones, the conclusion of the base case analysis (primary prophylaxis with quinolone being the most cost-effective prophylactic strategy) was robust to all scenarios tested, except for relative risk of a neutropenic sepsis episode (quinolones versus nothing/placebo). When the relative risk of a neutropenic sepsis episode (quinolones versus nothing/placebo) was above 0.787, nothing/placebo became the most cost-effective strategy, at a WTP threshold of £20,000 per QALY.
Table A26 and A27 show that primary or secondary prophylaxis with PEG-G-CSF is never the most cost-effective strategy, even when extreme scenarios were considered, for example: 100% risk of neutropenic sepsis per cycle of chemotherapy, 90% drug discount of PEG-G-CSF, extended length of hospital stay for neutropenic sepsis patients (6-day for low-risk patients and 14-day for high-risk patients) etc.
A6.3.2. For patients who can not take quinolone
Over sixteen scenarios were considered and tested using one-way sensitivity analysis (Section A4.2). The results of one-way sensitivity analyses for adult patients with a solid tumour who can take quinolones are presented below in the following order:
- Nothing/Placebo v.s Secondary prophylaxis with PEG-G-CSF (Table A28)
- Nothing/Placebo v.s Secondary prophylaxis with G(M)-CSF (Table A29)
- Nothing/Placebo v.s Primary prophylaxis with PEG-G-CSF (Table A30)
- Nothing/Placebo with quinolone v.s Primary prophylaxis with G(M)-CSF (Table A31)
Table A28
One-way sensitivity analyses results for solid tumour patients who cannot take quinolone: Nothing/placebo v.s Secondary prophylaxis with PEG-G-CSF.
Table A29
One-way sensitivity analyses results for solid tumour patients who can not take quinolone: Nothing/placebo v.s Secondary prophylaxis with G(M)-CSF.
Table A30
One-way sensitivity analyses results for solid tumour patients who cannot take quinolone: Nothing/placebo v.s Primary prophylaxis with PEG-G-CSF.
Table A31
One-way sensitivity analyses results for solid tumour patients who can take quinolone: Nothing/ placebo v.s Primary prophylaxis with G(M)-CSF.
For adult patients with a solid tumour who cannot take quinolones, the conclusion of the base case analysis (nothing/placebo being the most cost-effective prophylaxis strategy) was robust to all scenarios tested (Section A4.2), except for discounting the cost of PEG-G-CSF. At a WTP threshold of £20,000/QALY:
- When the discount to the cost of PEG-G-CSF was over 73.85% (corresponding price: £179.5 per single subcutaneous injection (6mg)), secondary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
- When the discount to the cost of PEG-G-CSF was over 84.13% (corresponding price: £108.9 per single subcutaneous injection (6mg)), primary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
Table A29 and A31 show that primary or secondary prophylaxis with G(M)-CSF is never the most cost-effective strategy, even when extreme scenarios were considered, for example: 100% risk of neutropenic sepsis per cycle of chemotherapy, 90% drug discount of G(M)-CSF, reduced days of using G(M)-CSF (5-day per cycle of chemotherapy), reduced daily dose (one vial of G(M)-CSF for all adult patients regardless of patient weight) etc.
A6.4. Probabilistic sensitivity analysis
A6.4.1. For patients who can take quinolones
For patients with a solid tumour who can take quinolones, the probability of primary prophylaxis with quinolone becoming cost-effective is always 100%, at a willingness to pay between £10,000 to £40,000 per QALY.
A6.4.2. For patients who cannot take quinolones
For patients with a solid tumour who cannot take quinolones, the probability of nothing/placebo becoming cost-effective is always 100%, at a willingness to pay between £10,000 to £40,000 per QALY.
A7. Results – Non-Hodgkin lymphoma sub group
The results for patients with adult non-Hodgkin lymphoma are presented below in the following order:
- base case analysis (section A7.1)
- structural sensitivity analysis (section A7.2)
- one-way sensitivity analysis (section A7.3)
- probabilistic sensitivity analysis (section A7.4)
Both strategies including quinolone are excluded from formal cost-effectiveness analysis, either because of no clinical evidence (quinolone alone) or prior dominated (more expensive and less effective) by other strategies (quinolone plus G(M)-CSF). The reasons for exclusion are detailed in section A7.1. As a result, no separate analyses were conducted for adult patients with non-Hodgkin lymphoma who can or cannot take quinolones.
A7.1. Base case analysis
For adult/elderly patients with non-Hodgkin lymphoma, no clinical evidence was identified for the use of quinolone alone for either primary or secondary prophylaxis therefore neither strategy was included in this analysis.
Compared to G(M)-CSF alone, G(M)-CSF + quinolone is more expensive and less effective in terms of preventing neutropenic sepsis (Table A4 and A9) so both primary and secondary prophylactic G(M)-CSF + quinolone strategies were excluded. As a result cost-effectiveness was only formally examined for the following five strategies:
- Nothing/placebo
- Primary prophylaxis with G(M)-CSF
- Secondary prophylaxis with G(M)-CSF
- Primary prophylaxis with PEG-G-CSF
- Secondary prophylaxis with PEG-G-CSF
The incremental costs and incremental QALYs in the base case analysis for each of the five strategies are summarised in Table A32, and shown graphically in Figure A7. Taking nothing/placebo as the reference (least expensive) strategy, the other four strategies were shown to be more effective, but were each associated with a very high ICER (all > £1.2 million/QALY) and were not considered to be cost effective. Therefore at a WTP threshold of £20,000/QALY, nothing/placebo is the most cost-effective strategy.
Table A32
Incremental costs and effectiveness by treatment strategy for non-Hodgkin lymphoma patients (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 44.22%).

Figure A7
Incremental costs and effectiveness by treatment strategy for patients with non-Hodgkin lymphoma (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 44.22%).
A7.2. Structural sensitivity analysis
For adult patients with non-Hodgkin lymphoma, the results of the structural sensitivity analysis are summarised in Table A33, and shown graphically in Figure A8. When using the high-risk model (Model A, ‘carry on regardless’), nothing/placebo remains the most cost-effective strategy, at a WTP threshold of £20,000/QALY.
Table A33
Incremental costs and effectiveness by treatment strategy for non-Hodgkin lymphoma patients; Model A (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 44.22%).

Figure A8
Incremental costs and effectiveness by treatment strategy for non-Hodgkin lymphoma patients, model A (baseline risk of neutropenic sepsis of one course of chemotherapy: 44.22%).
A7.3. One-way sensitivity analysis
Over sixteen scenarios were considered and tested using one-way sensitivity analysis (Section A4.2). The results of one-way sensitivity analyses for adult patients with a solid tumour who can take quinolones are presented below in the following order:
- Nothing/Placebo v.s Secondary prophylaxis with PEG-G-CSF (Table A34)
- Nothing/Placebo v.s Secondary prophylaxis with G(M)-CSF (Table A35)
- Nothing/Placebo v.s Primary prophylaxis with PEG-G-CSF (Table A36)
- Nothing/Placebo with quinolone v.s Primary prophylaxis with G(M)-CSF (Table A37)
Table A34
One-way sensitivity analyses results for non-Hodgkin lymphoma patients: Nothing/placebo v.s Secondary prophylaxis with PEG-G-CSF.
Table 35
One-way sensitivity analyses results for non-Hodgkin lymphoma patients: Nothing/placebo v.s Secondary prophylaxis with G(M)-CSF.
Table A36
One-way sensitivity analyses results for non-Hodgkin lymphoma patients: Nothing/placebo v.s Primary prophylaxis with PEG-G-CSF.
Table A37
One-way sensitivity analyses results for non-Hodgkin lymphoma patients: Nothing/placebo v.s Primary prophylaxis with G(M)-CSF.
For adult patients with non-Hodgkin lymphoma, the conclusion of the base case analysis (i.e. nothing/placebo being the most cost-effective prophylactic strategy) was robust to all of scenarios tested (Section A4.2), except for discounting the cost of PEG-G-CSF. At a WTP threshold of £20,000/QALY:
- When the discount to the cost of PEG-G-CSF was over 83.49% (corresponding price: £113.3 per single subcutaneous injection (6mg)), secondary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
- When the discount to the cost of PEG-G-CSF was over 89.12% (corresponding price: £74.7 per single subcutaneous injection (6mg)), primary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
Table A35 and A37 show that primary or secondary prophylaxis with G(M)-CSF is never the most cost-effective strategy, even when extreme scenarios were considered, for example: 100% risk of neutropenic sepsis per cycle of chemotherapy, 90% drug discount of G(M)-CSF, reduced days of using G(M)-CSF (5-day per cycle of chemotherapy), reduced daily dose (one vial of G(M)-CSF for all adult patients regardless of weight) etc.
A7.4. Probabilistic sensitivity analysis
For patients with non-Hodgkin lymphoma, the probability for nothing/placebo becoming cost-effective is always 100%, at a willingness to pay between £10,000 to £40,000 per QALY.
A8. Results – Hodgkin lymphoma sub group
The results for adult patients with Hodgkin lymphoma are presented below in the following order:
- base case analysis (Section A8.1)
- one-way sensitivity analysis (Section A8.2)
- probabilistic sensitivity analysis (Section A8.3)
Structural sensitivity analysis was not conducted for this patient group. The reason for which is detailed in section A4.1. Both strategies including quinolone (quinolone alone and quinolone plus G(M)-CSF) were excluded from formal cost-effectiveness analysis, because of no clinical evidence. As a result, no separate analyses were conducted for adult patients with Hodgkin lymphoma who can or cannot take quinolones.
A8.1. Base case analysis
For adult patients with Hodgkin lymphoma, clinical evidence was only available for the use of G(M)-CSF for either primary or secondary prophylaxis. Therefore cost-effectiveness was only formally examined for the following three strategies:
- Nothing/placebo
- Primary prophylaxis with G(M)-CSF
- Secondary prophylaxis with G(M)-CSF
The incremental costs and incremental QALYs in the base case analysis for each of the three strategies are summarised in Table A38, and shown graphically in Figure A9. Taking nothing/placebo as the reference (least expensive) strategy, the other two strategies were shown to be more effective, but were each associated with a very high ICER (both > £18.2 million/QALY) and were therefore not considered to be cost effective. Therefore at a WTP threshold of £20,000/QALY, nothing/placebo is the most cost-effective strategy.
Table A38
Incremental costs and effectiveness by treatment strategy for patients with Hodgkin lymphoma (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 20.27%).
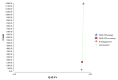
Figure A9
Incremental costs and effectiveness by treatment strategy for patients with Hodgkin lymphoma (using a baseline risk of neutropenic sepsis for one course of chemotherapy of 20.27%).
A8.1.2. One-way sensitivity analysis
Over sixteen scenarios were considered and tested using one-way sensitivity analysis (Section A4.2). The results of one-way sensitivity analyses for adult patients with Hodgkin lymphoma are presented below in the following order:
- Nothing/Placebo v.s Secondary prophylaxis with G(M)-CSF (Table A39)
- Nothing/Placebo with quinolone v.s Primary prophylaxis with G(M)-CSF (Table A40)
Table A39
One-way sensitivity analyses results for Hodgkin lymphoma patients: Nothing/placebo v.s Secondary prophylaxis with G(M)-CSF.
Table A40
One-way sensitivity analyses results for Hodgkin lymphoma patients: Nothing/placebo v.s Primary prophylaxis with G(M)-CSF.
For adult patients with Hodgkin lymphoma, the conclusion of the base case analysis (nothing/placebo being the most cost-effective prophylactic strategy) was robust to all scenarios tested (Section A4.2).
Table A39 and A40 show that primary or secondary prophylaxis with G(M)-CSF is never the most cost-effective strategy, even when extreme scenarios were considered, for example: 100% risk of neutropenic sepsis per cycle of chemotherapy, 90% drug discount, reduced days of using G(M)-CSF (5-day per cycle of chemotherapy), reduced daily dose (one vial of G(M)-CSF for all adult patients regardless of weight) etc.
A8.1.3. Probabilistic sensitivity analysis
For patients with Hodgkin lymphoma, the probability of nothing/placebo becoming cost-effective is always 100%, at a willingness to pay between £10,000 to £40,000 per QALY.
A9. Discussion
A9.1. Summary of results
The aim of this economic analysis was to determine which prophylactic strategy is the most cost-effective for cancer patients who are receiving chemotherapy.
The findings of the base-case analysis for all three patient sub–groups are summarised below.
At the NICE WTP threshold of £20,000 per QALY,
- For patients with a solid tumour and who can take quinolone, primary prophylaxis with quinolone is the most cost-effective prophylactic strategy.
- For patients with a solid tumour and who cannot take quinolone, no prophylaxis is the most cost-effective strategy.
- For patients with non-Hodgkin lymphoma or Hodgkin lymphoma, no prophylaxis is the most cost-effective strategy.
All the results in the analysis were robust to both structural sensitivity analysis and probabilistic sensitivity analysis,
The one-way sensitivity analysis that was conducted showed that the model was robust to all scenarios tested (Section A4.2), except for relative risk of neutropenic sepsis (quinolone versus nothing/placebo) and discounting the cost of PEG-G-CSF.
For patients with a solid tumour and who can take quinolone:
- When the relative risk of a neutropenic sepsis episode (quinolones versus nothing/placebo) was above 0.787, nothing/placebo became the most cost-effective strategy, at a WTP threshold of £20,000 per QALY.
For patients with a solid tumour and who cannot take quinolone:
- When the discount to the cost of PEG-G-CSF was over 73.85% (corresponding price: £179.5 per single subcutaneous injection (6mg)), secondary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
- When the discount to the cost of PEG-G-CSF was over 84.13% (corresponding price: £108.9 per single subcutaneous injection (6mg)), primary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
For patients with non-Hodgkin lymphoma:
- When the discount to the cost of PEG-G-CSF was over 83.49% (corresponding price: £113.3 per single subcutaneous injection (6mg)), secondary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
- When the discount to the cost of PEG-G-CSF was over 89.12% (corresponding price: £74.7 per single subcutaneous injection (6mg)), primary prophylaxis with PEG-G-CSF became the most cost-effective strategy.
Primary or secondary prophylaxis with G(M)-CSF is never the most cost-effective strategy for any of the three patient groups of interest, even when extreme scenarios were considered, for example: 100% risk of neutropenic sepsis per cycle of chemotherapy, 90% drug discount of G(M)-CSF, reduced days of using G(M)-CSF (5-day per cycle of chemotherapy), reduced daily dose (one vial of G(M)-CSF for all adult patients regardless of weight) etc.
A9.2. Potential limitations within the model
A9.2.1. Relative risk of overall mortality
The volume of evidence that reported relative risk data for overall mortality obtained from the clinical evidence review was very sparse. What's more, of the studies that report this outcome their quality was assessed by GRADE as low since none were designed to investigate the effect of GCSF on short-term mortality and the death rate between different arms was low. Based on these two reaons, as well as the low short-term overall mortality rate for patients receiving chemotherapy (less than 1%) the GDG decided not to consider the survival difference of different prophylactic strategies in the base-case model.
The likely impact of this assumption is that for those prophylactic strategies that can improve short-term overall mortality, their effectiveness was underestimated in our analysis. However, since the baseline short-term overall mortality for the target population is assumed to be very low, the effect of this bias is likely to be small.
A9.2.2. Relative risk of neutropenic sepsis
A total of 202 RCTs were included for this topic. However only one of these studies directly compared the effectiveness of G(M)-CSF or PEG-G-CSF with quinolone (Herbst. et al., 2009). Therefore in our economic analysis, each prophylactic strategy was only compared with nothing/placebo and not with each other. The direction of this bias is unknown.
As there was only one head-to-head trial directly comparing G-CSF with quinolone, a network meta-analysis was considered unfeasible for this economic model.
A9.2.3. Impact of prophylactic strategy on subsequent chemotherapy
Although our systematic review of cost-effectiveness studies did identify several studies trying to model the impact of using G-CSF on patients long-term survival for patients with stage II breast cancer by maintaining chemotherapy dose (Borget, et al., (2009); Danova, et al., (2008); Liu, et al., (2009); Lyman, (2009 (a)); Lyman, (2009 (b)); Ramsey, (2009); Whyte, et al., (2011)), none of these studies used any direct clinical data. Instead, these studies were trying to build an indirect relationship between use of G-CSF and patient long-term survival. They stated that G-CSF could prevent neutropenic sepsis; neutropenic sepsis is a risk factor of receiving dose-reduction chemotherapy and dose-reduction chemotherapy is a risk factor for patient long-term survival. Then based on this hypothesis, the authors claimed that G-CSF could improve patient long-term survival. However, these assumptions are in contrast with more direct evidence:
- Papaldo et al (2005) shows that the addition of varying intensity schedules of open-label G-CSF to high-dose epirubicin/cyclophosphamide chemotherapy in patients with stage I and II breast cancer had no significant impact on the delivered dose-intensity compared with the non-G-CSF arms.
- Results from the Impact of Neutropenia in Chemotherapy Euroopean study group (INC-EU) prospective obervational study shows that the impact of primary prophylaxis with G-CSF on relative dose intensity is not significant (Pettengell 2008).
- A recent meta-analysis (Shitara, et al., 2011) shows that neutropenia experienced during chemotherapy is actually associated with improved survival in patients with advanced cancer or haematological malignancies undergoing chemotherapy. This implies that experiencing side effects of chemotherapy might not be associated with impaired long term survival.
In order to investigate the impact of prophylactic strategy on subsequent chemotherapy we would need to conduct a systematic review to identify which specific patient group(s) were likely to benefit from dose-intense/dense chemotherapy. Having identified these patient group(s) we would then need to search for and appraise RCTs comparing dose-intense chemotherapy + GCSF with normal chemotherapy + no GCSF. Data would be needed on overall survival/relapse free survival, the cost of chemotherapy regimes and patients future quality of life. Given that the guideline covers all cancer patients, from paediatric to adult, and the multitude of different chemotherapy regimens used in these different groups, it would be extremely complicated to model and a vast amount of data would be required.
This bias works against any prophylactic strategies that could potentially improve patient long-term survival or relapse free survival by maintaining chemotherapy dose.
A9.3. Compared with published studies
A total of 10 studies were identified in the systematic review of economic evidence for this topic (Full evidence review). However, none of these studies include all of the interventions that the GDG considered relevant for the topic (Section A2.1).
A9.3.1. Different types of G(M)-CSF versus each other
Six out of 10 studies compared different types of G-CSF with each other. All six studies considered two efficacies of G-CSF (i) preventing neutropenic sepsis and (ii) improving patient long-term survival by facilitating chemotherapy. The conclusions of these six studies are as follows:
For patients with at least 20% risk of febrile neutropenia:
- Primary prophylaxis with PEG-G-CSF is more effective and less expensive than primary prophylaxis with 11-day G-CSF (Borget, 2009; Liu, 2009; Lyman, 2009(b))
- Primary prophylaxis with PEG-G-CSF is more effective and more expensive than primary prophylaxis with 6-day G-CSF; and the ICER of PEG-G-CSF is less than the NICE WTP threshold of £20,000 per QALY (Borget, 2009; Danova, 2008; Liu, 2009; Lyman, 2009(a); Lyman, 2009(b))
- Primary prophylaxis with PEG-G-CSF is more effective and more expensive than secondary prophylaxis with PEG-G-CSF; and the ICER of primary prophylaxis with PEG-G-CSF is 3.3 times higher than the NICE WTP threshold of £20,000 per QALY (Ramsey, 2009).
Our analysis only considered the efficacy of G(M)-CSF in preventing neutropenic sepsis (Section A9.1.3); and didn't differentiate between 6 or 11-day G(M)-CSF. Despite these differences, the conclusions of our analysis (Section A6) are consistent with the conclusions of the six included papers above:
At the NICE WTP threshold of £20,000 per QALY
- Primary prophylaxis with PEG-G-CSF is more cost-effective than primary prophylaxis with G(M)-CSF.
- Secondary prophylaxis with PEG-G-CSF is more cost effective than primary prophylaxis with PEG-G-CSF.
A9.3.2. G(M)-CSF versus nothing/placebo
Two of the 10 studies (Lathia, 2009; Whyte, et al., (2011)) compared G-CSF with placebo. Lathia, (2009) considered G-CSF's efficacy in preventing neutropenic sepsis only (same as our analysis), and reported that compared to nothing, the ICER for primary prophylaxis with G(M)-CSF and primary prophylaxis with PEG-G-CSF are £0.94 million/QALY and £2.39 million/QALY respectively (converted to 2011 UK pounds), for patients with at least 20% risk of febrile neutropenia. This conclusion is consistent with our results.
Whyte (2011) considered primary and secondary prophylaxis with all different types of G-CSF and compared them with nothing/placebo. Their study concluded that at NICE willingness-to-pay threshold of £20,000 per QALY:
- for patients with a febrile neutropenia risk level of 11% -37%, secondary PEG-G-CSF is the most cost-effective strategy.
- for patients with a febrile neutropenia risk greater than 38%, primary PEG-G-CSF is the the most cost-effective strategy.
However Whyte (2011) considered three efficacies of G-CSF (i) preventing neutropenic sepsis; (ii) improving patient short-term survival (by preventing febrile neutropenia); and (iii) improving patient long-term survival by facilitating chemotherapy, whilst our analysis only considered the efficacy of G(M)-CSF in preventing neutropenic sepsis (Section A9.1.3). What's more, it is acknowledged that the efficacy data of G-CSF in terms of improving short-term and long-term survival estimated by Whyte et al., (2011) differ substantively from the data reported by best available evidence (Note 28, Table 5.10)
A9.3.3. G(M)-CSF plus quinolone versus quinolone alone
Two of 10 studies compared G(M)-CSF plus quinolone with quinolone alone, for patients with at least 20% risk of febrile neutropenia. Timmer-Bonte, (2006) compared primary prophylaxis with G(M)-CSF plus quinolone to primary prophylaxis with quinolone alone and Timmer-Bonte, (2008) compared secondary prophylaxis with G(M)-CSF plus quinolone to secondary prophylaxis with quinolone alone. Both papers considered G-CSF's efficacy in preventing neutropenic sepsis only (same as our analysis), and found out that G-CSF plus quinolone is more clinically effective than quinolone alone but is associated with a very high ICER (£0.27 million per febrile neutropenia-free cycle of chemotherapy (Timmer-Bonte, 2008) and £4149 per one percent decrease of the probability of febrile neutropenia (Timmer-Bonte, 2006). Neither study reported an ICER in terms of incremental cost per QALY, so it was very difficult to compare their results with ours.
A9.4. Implications for future research
Further research that could improve the model for this topic would include collecting the following additional data/information:
- A head-to-head RCT which directly compares G-CSF with quinolone
- The impact of the prophylactic strategy of neutropenic sepsis on patients' long-term survival
- The impact of prophylactic quinolone on antibiotic resistance
A10. Cost of different types of G(M)-CSF
Two types of G(M)-CSF are currently used in the U.K practice: filgrastim and lenograstim. The daily drug cost of filgrastim and lenograstim are presented in Table A41 and A42 separately. In our economic anlayisis, the daily cost of G(M)-CSF is the average cost of filgrastim and lenograstim (Table 43).
Table A41
Daily cost of filgrastim.
Table A42
Daily cost of Lenograstim.
Table A43
Daily cost of G(M)-CSF used in economic analysis.
The unit cost of G(M)-CSF per day was calculated based on the average cost of all G(M)-CSF brands listed by British National Formulary 62.
A11. Cost of ambulance for each patient subgroup
According to the recent report ‘Accident and Emergency Attendances in England (Experimental Statistics) 2009-10’ (Health and Social Care Information Centre, 2011), the use of an ambulance is positively associated with age (Figure A10). Therefore the ambulance use for each patient subgroup was calculated based on their age distribution (Table A44).
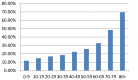
Figure A10
Use of ambulance by all A & E attendances by age (2009-10).
Table A44
Age distribution and estimated ambulance use for each patient subgroup.
A12. Average cost of oral antibiotics
The cost of oral antibiotics was calculated based on cost data obtained from the British National Formulary assuming no wastage (Table A45).
Table A45
Average cost of oral antibiotics for patients with neutropenic sepsis.
A13 References
- Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M, Kearney N, Lyman GH, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinskil C., European Organisation for Research and Treatment of Cancer (EORTC). Granulocyte Colony-Stimulating Factor (G-CSF). Guidelines Working Party. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. [PubMed: 16750358]
- Bertaccini A, Ceccarelli R, Urbinati M, et al. BSP-PC (Bononian Satisfaction Profile-Prostate Cancer): development and validation of a ‘disease-specific’ questionnaire for the evaluation of health-related quality of life in patients with prostate cancer. Arch Ital Urol Androl. 2003;75(4):187–94. [PubMed: 15005491]
- Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Quality of Life Research. 2010;19:391–400. [PubMed: 20084462]
- Bonadonna G, et al. 30 years' follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005 Jan 29;330(7485):217. Epub 2005 Jan 13. [PMC free article: PMC546063] [PubMed: 15649903]
- Briggs A, Sculpher M, Cloaxton K. Decision Modelling for Economic Evaluation. Oxford: Oxford University Press; 2006.
- Conner-Spady BL, Cumming C, Nabholtz JM, et al. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant. 2005;36(3):251–9. [PubMed: 15937502]
- Cullen MH, et al. Rational selection of patients for antibacterial prophylaxis after chemotherapy. Journal of Clinical Oncology. 2007;25(30):4821–28. [PubMed: 17947731]
- Department of Health. NHS Trusts and PCTs combined reference cost schedules 2010-11. [25th June 2012]. http://www
.dh.gov.uk /en/Publicationsandstatistics /Publications /PublicationsPolicyAndGuidance /DH_131140 . - Doorduijn J, Buijt I, Holt B, et al. Self-reported quality of life in elderly patients with aggressive non-Hodgkin lymphoma treated with CHOP chemotherapy. Eur J Haematol. 2005;75(2):116–23. [PubMed: 16000127]
- Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35. [PubMed: 12488289]
- Grigg A, Solal-Celigny P, Hoskin P, et al. Open-label, randomized study of pegfilgrastim vs. daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin's lymphoma. Leuk Lymphoma. 2003;44:1503–1508. [PubMed: 14565651]
- Health and Social Care Information Centre, Hospital Episode Statistics. Accident and Emergency Attendances in England (Experimental Statistics). 2009-10.
- Herbst C, Naumann F, Kruse EB, Monsef I, Bohlius J, Schulz H, et al. Prophylactic antibiotics or G-CSF for the prevention of infections and improvement of survival in cancer patients undergoing chemotherapy. Cochrane Database of Systematic Reviews. 2009;(1) [Review] [91 refs] CD007107, 2009., CD007107. [PubMed: 19160320]
- Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. British Journal of Cancer. 2006;95:683–90. [PMC free article: PMC2360509] [PubMed: 16967055]
- Nafees B, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008 Oct 21;6:84. [PMC free article: PMC2579282] [PubMed: 18939982]
- Norum J, Vonen B, Olsen JA, et al. Adjuvant chemotherapy (5- fluorouracil and levamisole) in Dukes' B and C colorectal carcinoma: a cost-effectiveness analysis. Ann Oncol. 1997;8(1):65–70. [PubMed: 9093709]
- Norum J, Angelsen V, Wist E, et al. Treatment costs in Hodgkin disease: a cost-utility analysis. Eur J Cancer. 1996;32A(9):1510–7. [PubMed: 8911110]
- Pickard AS, et al. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Quality Life Outcomes. 2010;8:4. [PMC free article: PMC2248572] [PubMed: 18154669]
- Jansen SJ, Otten W, van de Velde CJ, et al. The impact of the perception of treatment choice on satisfaction with treatment, experienced chemotherapy burden and current quality of life. Br J Cancer. 2004;91(1):56–61. [PMC free article: PMC2364741] [PubMed: 15162143]
- Pettengell R, Donatti C, Hoskin P, et al. The impact of follicularlymphoma on health-related quality of life. Ann Oncol. 2008;19:570–6. [PubMed: 18056649]
- Romieu G, Clemens M, Mahlberg R, et al. Pegfilgrastim supports delivery of FEC-100 chemotherapy in elderly patients with high risk breast cancer: a randomized phase 2 trial. Crit Rev Oncol Hematol. 2007;64:64–72. [PubMed: 17317205]
- Sandblom G, Carlsson P, Sennfalt K, et al. A population-based study of pain and quality of life during the year before death in men with prostate cancer. Br J Cancer. 2004;90(6):1163–8. [PMC free article: PMC2409660] [PubMed: 15026796]
- Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43(7):736–49. [PubMed: 15970790]
- Slovacek L, Slovackova B, Jebavy L. Global quality of life in patients who have undergone the hematopoietic stem cell transplantation: finding from transversal and retrospective study. Exp Oncol. 2005;27(3):238–42B. [PubMed: 16244589]
- Boran P, et al. Translation and cultural adaptation of health utilities index with application to paediatric oncology patients during neutropenia and recovery in Turkey. Paediatric Blood & Cancer. 2011;56(5):812–17. [PubMed: 21370416]
- Blane Schilling M, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: A retrospective study. Experimental and Therapeutic Medicine. 2011;2(5):859–866. Date of Publication: September 2011.5 (2000): September. [PMC free article: PMC3440789] [PubMed: 22977589]
- Cheng S, et al. Health-related quality of life anticipated with different management strategies for paediatric febrile neutropaenia. British Journal of Cancer. 2011;105(5):606–611. Date of Publication: 23 Aug 2011.5 (2011): 23. [PMC free article: PMC3188924] [PubMed: 21694729]
- Health and Social Care Information Centre, Hospital Episode Statistics. Accident and Emergency Attendances in England (Experimental Statistics). 2009-10.
- Lathia N. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116:742–748. [PubMed: 20029970]
- Lingaratnam S, et al. The disease and economic burden of neutropenic fever in adult patients in Australian cancer treatment centres 2008: analysis of the Victorian Admitted Episodes Dataset. Internal Medicine Journal. 2011;41(1b):121–29. [PubMed: 21272176]
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [PubMed: 3657876]
- Sacco JJ, et al. The Average Body Surface Area of Adult Cancer Patients in the UK: A Multicentre Retrospective Study. PLoS One. 2010 Jan 28;5(1):e8933. [PMC free article: PMC2812484] [PubMed: 20126669]
- Sung L, et al. Identification of paediatric cancer patients with poor quality of life. British Journal of Cancer. 2009;100:82–88. [PMC free article: PMC2634672] [PubMed: 19066605]
- Whyte S, et al. Cost-effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health. 2011 Jun;14(4):465–74. [PubMed: 21669371]
- Borget I, Di Palma M, Leonard R. PEG-G-CSF - A health economic model to assess overall cost-effectiveness. EJHP Practice. 2009;15(5):58–61.
- Danova M, et al. Cost-effectiveness of PEG-G-CSF versus six days of filgrastim for preventing febrile neutropenia in breast cancer patients. Tumori. 2009;95(2):219–26. [PubMed: 19579869]
- Lathia N, et al. Cost-Effectiveness of Filgrastim and PEG-G-CSF as Primary Prophylaxis against Febrile Neutropenia in Lymphoma Patients Receiving R-CHOP Chemotherapy. Blood Conference.var.pagings. 2009:22.
- Liu Z, et al. The economic value of primary prophylaxis using PEG-G-CSF compared with filgrastim in patients with breast cancer in the UK. Applied Health Economics and Health Policy. 2009;7(3):193–205. [PubMed: 19799473]
- Lyman G. Cost-effectiveness of PEG-G-CSF versus 6-day filgrastim primary prophylaxis in patients with non-Hodgkin's lymphoma receiving CHOP-21 in United States. Current Medical Research And Opinion. 2009;25(2):401–411. (a) [PubMed: 19192985]
- Lyman GH. Cost-effectiveness of PEG-G-CSF versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clinical Therapeutics. 2009;31(5):1092–1104. [PubMed: 19539110]
- NHS reference costs 2009-2010. Department of Health; Jan 13, 2011.
- Ramsey SD. Cost-effectiveness of primary versus secondary prophylaxis with PEG-G-CSF in women with early-stage breast cancer receiving chemotherapy. Value in Health. 2009;12(2):217–225. [PubMed: 18673353]
- Timmer-Bonte JNH, et al. Cost-effectiveness of adding granulocyte colony-stimulating factor to primary prophylaxis with antibiotics in small-cell lung cancer. Journal of Clinical Oncology. 2006;24(19):2991–97. [PubMed: 16682725]
- Timmer-Bonte JNH, et al. Modeling the cost effectiveness of secondary febrile neutropenia prophylaxis during standard-dose chemotherapy. Journal of Clinical Oncology. 2008;26(2):290–96. [PubMed: 18182670]
- Whyte S, et al. Cost-effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value in Health. 2011 Jun;14(4):465–74. [PubMed: 21669371]
References of included economic evidence
Footnotes
- 21
In the economic analysis, it is assumed that the relative risk of a neutropenic sepsis event in the first cycle of chemotherapy compared with cycle two onwards is 3.69 (Cullen, 2007). Therefore by testing a range of 5-100% risk of neutropenic sepsis (per cycle) for Cycle 2 onwards; we tested a range of 1.4-100% risk of neutropenic sepsis (per cycle) for the first cycle of chemotherapy.
- 22
Average cost of filgrastim and lenograstim assuming that the daily dose of G(M)-CSF for all adult patients is one vial (one single 30 million-unit syringe of filgrastim or one single 33.6 million-unit syringe of lenograstim) regardless of patient weight.
- 23
Average cost of filgrastim and lenograstim, based on BNF recommended dose (500,000 units/kg daily) and patient weight distribution reported by: Green 2003, Romieu 2007, and Gigg 2003. The detailed calculation process is reported in appendix A10.
- Introduction
- De novo economic model (overview)
- Cost-effectiveness model - inputs
- Sensitivity analysis
- Interpreting results
- Results – Solid tumour sub group
- Results – Non-Hodgkin lymphoma sub group
- Results – Hodgkin lymphoma sub group
- Discussion
- Cost of different types of G(M)-CSF
- Cost of ambulance for each patient subgroup
- Average cost of oral antibiotics
- A13 References
- A cost-utility analysis of primary and secondary prophylaxis with G(M)-CSF and/o...A cost-utility analysis of primary and secondary prophylaxis with G(M)-CSF and/or quinolones for the prevention of neutropenic sepsis - Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients
Your browsing activity is empty.
Activity recording is turned off.
See more...