NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients. London: National Institute for Health and Clinical Excellence (NICE); 2012 Sep. (NICE Clinical Guidelines, No. 151.)

Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients.
Show details4. Signs and symptoms of neutropenic sepsis (Topic A)
Guideline subgroup members
Helen Clayson (lead), Anne Davidson, Nicola Perry and Janie Thomas.
Review question
Which symptoms and/or signs experienced by patients in the community predict neutropenic sepsis?
Rationale
Neutropenic sepsis is a potentially fatal complication following anti-cancer treatments. Most people receive anti-cancer treatments as outpatients and symptoms and/or signs that might predict the development of neutropenic sepsis often occur in patients in the community. Delay in diagnosis is associated with poor outcomes, sometimes resulting in avoidable deaths. There is great variation in those symptoms and/or signs that may predict the development of neutropenic sepsis; this leads to variations in practice such as in the information given to patients and the criteria for urgent admission to hospital. Overdiagnosis results in inappropriate admissions to hospital and this may delay anti-cancer treatments; underdiagnosis or delay in diagnosis puts patients at risk of serious infections or, at worst, avoidable death due to neutropenia-related infections. Topic A addresses this variation in practice and aims to examine the evidence around several key symptoms and signs to assess their utility in the community as predictors of neutropenic sepsis.
Question in PICO format
| Patients/population | Symptoms/signs | Reference test | Target condition |
|---|---|---|---|
| Patients in the community, who have received anti-cancer treatment | “Gold standard” definition of neutropenic sepsis (see topic D1) or accept whatever reference standard was used in the original studies |
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Cochrane Library, BNI, Cinah, Psychinfo, Web of Science (SCI & SSCI) and ISI Proceedings.
There were no publication date limits set. The searches were conducted between the 20th April and the 3rd May 2011, and updated on 7th November 2011.
The information specialist (SB) did the first screen of the literature search results. Two reviewers (NB & KF) selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question. The full articles were then ordered and appraised.
Data synthesis
One reviewer (KF) extracted data and assessed study quality using items from the QUADAS checklist for diagnostic studies. Where possible, the sensitivity and specificity of the particular test was extracted into 2×2 tables. The heterogeneity of outcome measures precluded data pooling.
RESULTS
Results of literature searches
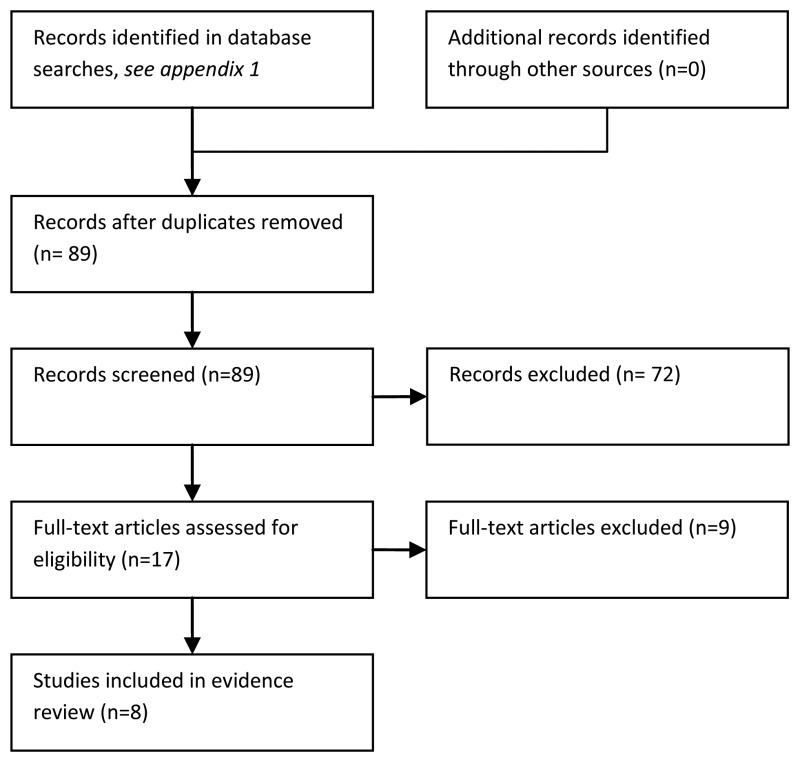
After de-duplication, 112 records were identified. After a preliminary sift, eighty-nine papers were reviewed. Seventeen papers were ordered and eight were included in this summary (Ammann et al., 2003, Ammann et al., 2004, Ammann et al., 2010, Chayakulkeeree et al., 2003, Hakim et al., 2010, Klaassen et al., 2000, Klastersky et al., 2000 and West et al., 2004).
Description of included studies
There was no evidence about signs and symptoms in the community that might predict severe sepsis, mortality or sepsis within a time period. Instead, the included papers reported largely retrospective data on patients who had presented at hospital with treatment induced neutropenia and fever. As part of the initial clinical assessment, some variables of interest were noted i.e. high temperature, appearance, mucositis, altered mental status, gastrointestinal upset or signs of infection.
The limited spectrum of patients in these studies means we do not have evidence about patients in the community setting. This is an important shortcoming as the sensitivity and specificity of symptoms or signs in the community might differ greatly from their sensitivity and specificity in secondary care.
The target condition in all studies was the clinical outcome for patients where an unfavourable outcome could be considered as one or more of the following: sepsis, bacteremia, serious medical complications, microbiologically documented infection, critical care, fever relapse, positive urine/blood cultures or death due to infection. Unfortunately, these variables were frequently considered in groups and hence results for each outcome could not be extracted.
Six studies recruited paediatric patients, one study recruited adults only and one had a mixed population of children and adults. The absolute proportion of haematological malignancies varied across studies, or was not documented, but was often half or more than half of the patients. Tests were typically, but not exclusively, performed on patients admitted with fever and neutropenia, before the initiation of empiric antimicrobial therapy.
Two studies presented data on a single episode of neutropenic fever per patient whilst the majority included multiple episodes in their analyses. Multiple episodes may not be independent and could introduce bias. Investigators performed univariate analyses using Mann Whitney, X2 or Student's t-tests. Significant variables were applied to multivariate analyses using backwards or forwards stepwise logistic regression. Only univariate results are presented here since covariates were irrelevant to the question.
Blinding was rarely used. Blinding is where reference tests are interpreted without knowledge of the index test results and vice-versa. The reference test (or gold standard) is the definitive test whereas the index test is the factor under investigation (e.g. oral mucositis). For index tests in prospective studies, clearly lack of blinding is not an issue since the outcome of interest could not have been known at the time of presentation.
Table 4.1 is a summary of study quality, according to the QUADAS check list. Only three studies were prospective.
Table 4.1
Study quality according to QUADAS criteria.
In all cases, two by two tables, sensitivity and specificity were calculated as far as possible from the data presented in each study although odds ratios and P values were as reported by the authors.
Study quality and results
There was no direct evidence about signs and symptoms of cancer patients in the community that might predict neutropenic sepsis. The available evidence came from retrospective studies of patients who had presented at hospital with treatment induced neutropenia and fever. This evidence is summarised in Table 4.2 and in Figures 4.2 to 4.8. By including only patients with confirmed neutropenia and fever these studies are not a representative spectrum of patients in the community. The sensitivity and specificity of symptoms or signs for neutropenic sepsis in the community might differ from that in secondary care. Studies typically reported composite outcomes encompassing severe bacterial infection, death and critical care. For these reasons the evidence is of very low quality.
Table 4.2
Signs and symptoms as predictors of adverse outcome in patients with fever and neutropenia.

Figure 4.2
Temperature. This plot suggests that about half of the patients that develop neutropenic sepsis have a temperature of > 39°C whereas about a fifth of patients that don't develop sepsis also have a similarly high temperature. Since patients (more...)
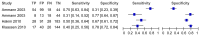
Figure 4.3
General appearance. This outcome was measured in paediatric patients who, in the opinion of the clinician, looked unwell. The results are similar to those of temperature in that about half of the children that developed an adverse event looked unwell (more...)
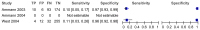
Figure 4.4
Chills. The two sets of data available for this variable both showed very high specificity and very low sensitivity, that is to say that the majority of patients who experienced an adverse event (‘severe bacterial infection’ in the case (more...)

Figure 4.5
Mucositis. The two sets of data for Ammann et al., 2010 relate respectively to ‘oral mucositis to any degree’ and ‘other mucositis to any degree’. The observed relationship between sensitivity and specificity across the (more...)
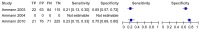
Figure 4.6
Clinical signs of an infection. Approximately 20% of patients, regardless of outcome, had clinical signs of infection. The ROC curve (see Figure vii) suggests clinical signs of infection were slightly more common in patients with good outcomes. In these (more...)

Figure 4.7
Confused mental state. This is a similar result to those for the variable chills. It suggests that confused mental state might be useful in indentifying patients at risk of adverse events. The absence of confused mental state, however, is not a good indicator (more...)
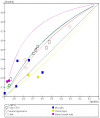
Figure 4.8
Summary ROC curve for all variables.
Evidence statements
There was uncertainty about which signs and symptoms predict neutropenic sepsis and its complications in cancer patients in the community due to a lack of published evidence.
Chills and altered mental status were associated with adverse outcome in two secondary care studies, but most patients with neutropenic sepsis did not experience either of these symptoms.
REFERENCES
- Ammann RA, Hirt A, Luthy AR, Aebi C. Identification of children presenting with fever in chemotherapy-induced neutropenia at low risk for severe bacterial infection. Medical & Pediatric Oncology. 2003;41:436–443. [PubMed: 14515382]
- Ammann RA, Hirt A, thy AR, Aebi C. Predicting bacteremia in children with fever and chemotherapy-induced neutropenia. Pediatr.Infect.Dis.J. 2004;23:61–67. [PubMed: 14743049]
- Ammann RA, Bodmer N, Hirt A, Niggli FK, Nadal D, Simon A, Ozsahin H, Kontny U, Kuhne T, Popovic MB, Luthy AR, Aebi C. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. J.Clin.Oncol. 2010;28:2008–2014. [PubMed: 20231680]
- Chayakulkeeree M, Thamlikitkul V. Risk index for predicting complications and prognosis in Thai patients with neutropenia and fever. J.Med.Assoc.Thai. 2003;86:212–223. [PubMed: 12757060]
- Hakim H, Flynn PM, Srivastava DK, Knapp KM, Li C, Okuma J, Gaur AH. Risk prediction in pediatric cancer patients with fever and neutropenia. Pediatr.Infect.Dis.J. 2010;29:53–59. [PubMed: 19996816]
- Klaassen RJ, Goodman TR, Pham B, Doyle JJ. “Low-risk” prediction rule for pediatric oncology patients presenting with fever and neutropenia. J.Clin.Oncol. 2000;18:1012–1019. [PubMed: 10694551]
- Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, Gallagher J, Herrstedt J, Rapoport B, Rolston K, Talcott J. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J.Clin.Oncol. 2000;18:3038–3051. [PubMed: 10944139]
- West DC, Marcin JP, Mawis R, He J, Nagle A, Dimand R. Children with cancer, fever, and treatment-induced neutropenia: risk factors associated with illness requiring the administration of critical care therapies. Pediatr.Emerg.Care. 2004;20:79–84. [PubMed: 14758303]
EVIDENCE TABLES
Download PDF (627K)
5. Investigations appropriate for risk stratification and management. (Topic D2)
Guideline group members
Anne Davidson (lead), Jeanette Hawkins, Paul Wallman, Mark Holland, Wendy King and Barry Hancock
Review question
Which tests predict outcome and response to treatment in patients with suspected neutropenic sepsis?
Rationale
The majority of protocols for the management of febrile neutropenia or suspected neutropenic sepsis will recommend a number of routine laboratory investigations. Some which are an essential component of routine patient management, for example renal function tests, may also predict a more complicated course in terms of neutropenic sepsis. Other tests such as CRP and ESR are used as more specific markers of infection and may influence decisions regarding length of stay. Recent studies have suggested that investigations such as procalcitonin, IL6 and IL8 may be useful in outcome prediction, although these are not widely available in all hospitals in the United Kingdom. Lactate is routinely used in the management of patients with septic shock, but is not necessarily measured at the outset of febrile neutropenic episodes. It has been suggested that early measurement of lactate may predict the development of septic shock in patients with febrile neutropenia.
Although the absolute neutrophil count is generally used in management protocols for febrile neutropenia, monocyte count and lymphocyte count may also be useful independent prognostic factors.
It would be extremely useful to develop an evidence based guideline based on an understanding of which tests most accurately predict patients at high risk of an adverse outcome. An early prediction of patients at higher risk of an adverse outcome may prompt more aggressive management and intensive monitoring with a potential reduction in mortality rates. Tests which accurately predict patients at low, or no, risk of serious clinical infection could incorporated into risk stratification management protocols.
Question in PICO format
| Patients/population | Tests | Outcomes |
|---|---|---|
| Patients with suspected neutropenic sepsis |
|
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Psychinfo and BMI. The full strategy will be available in the full guideline. There were no publication date limits set. The date of the search was January 5th 2011, and it was updated on November 7th 2011.
Papers ordered for other topics (D1 and E1) were also checked for eligibility for this topic.
Selection of studies
The information specialist (SB) did the first screen of the literature search results. One reviewer (NB) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question. The full articles were then obtained for and checked against the inclusion criteria.
Data synthesis
One reviewer (NB) extracted data and assessed study quality was assessed using ten items from the QUADAS checklist for diagnostic studies. Where possible the sensitivity and specificity of the particular test was extracted into 2×2 tables.
When there were sufficient studies reporting the diagnostic accuracy of a given test for a particular outcome meta-analysis was attempted. If there was no evidence of heterogeneity of both sensitivity and specificity then separate univariate meta-analyses of sensitivity and specificity were done using random effects models. If there was heterogeneity then the DiagMeta package within the R statistical computing program was used to fit a bivariate ROC model (Chappell, Raab and Wardlaw, 2009).
In some cases studies did not use a cut-off threshold but reported the mean and standard deviation of a biomarker according to outcome group. In studies where only the median and range were reported for a given biomarker, methods described by Hozo, Djulbegovic and Hozo (2005) were used to estimate the mean and standard deviation. Meta-analysis of the mean difference between outcome groups was done using RevMan 5.0.
RESULTS
Results of the literature searches
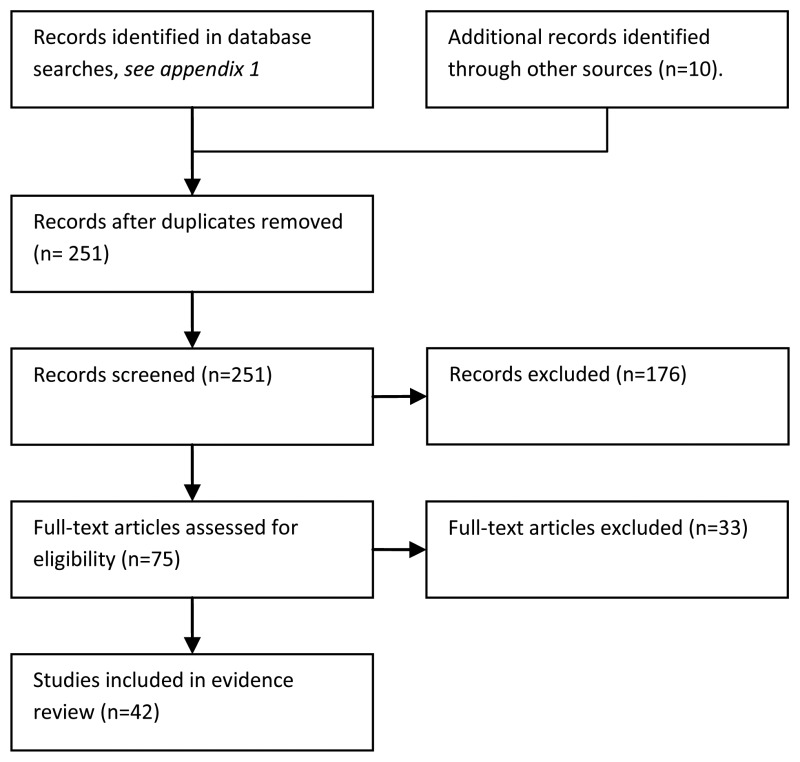
21/42 studies were done in children, 18/42 were in adults and 3 were in adults and children. Most of the febrile neutropenic episodes were experienced by patients with haematological malignancy. Twelve studies included only patients with haematological malignancy. In 25 of the 30 remaining studies more than 50% of the included patients had haematological malignancy.
Tests were typically done on admission for fever and neutropenia, before the initiation of antimicrobial therapy. Some studies repeated tests over the first few days of fever, to compare how serum levels of biomarkers changed over time in patients with and without severe infection.
Figure 5.2 is a summary of study quality, according to the QUADAS check list. 25/42 studies were prospective. It was unclear in 16/42 studies how patients were selected for inclusion (for example whether it was a consecutive or random sample of eligible patients) this is a potential source of bias.
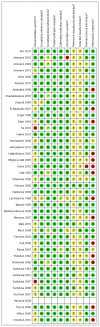
Figure 5.2
Summary of study quality using QUADAS criteria.
Blinding was rarely used. Blinding is where reference tests were interpreted without knowledge of the index test results and vice-versa. The reference test (sometimes called the gold standard test) is the definitive test, whereas the index test is the one under investigation (e.g. serum CRP level). For index tests in prospective studies, lack of blinding should not be a problem as the eventual outcome of the patient would be unknown at the time of admission.
Study quality and results
There were relatively few studies of tests to predict mortality in patients admitted for fever and neutropenia. There was very limited evidence about CRP, lactate, full blood count, liver function tests or kidney function tests for the prediction of length of hospital stay. This evidence is summarised in Table 5.1. Our searches identified no studies of tests to predict the requirement for critical care; however there was some evidence about tests to predict severe sepsis and documented infection.
Table 5.1
Diagnostic Accuracy for Investigations appropriate for risk stratification and management.
Tests were typically done on admission for fever and neutropenia, before the initiation of antimicrobial therapy. Some studies repeated tests over the first few days of fever, to compare how serum levels of biomarkers changed over time in patients with and without severe infection.
25 of the 42 studies were prospective. It was unclear in 16/42 studies how patients were selected for inclusion (for example whether it was a consecutive or random sample of eligible patients) this is a potential source of bias. Blinding was explicitly used in 6/42 studies. For index tests in prospective studies, lack of blinding to the reference standard result should not be a problem as the eventual outcome of the patient would be unknown at the time of admission. Similarly lack of blinding to the index test result should not influence objective outcomes like mortality.
Evidence statements
Mortality
Lactate, albumin and creatinine levels had reasonable specificity (93%, 88% and 89% respectively) but low sensitivity (53% or less) to predict short term mortality in patients with fever and neutropenia, with only data from a single study for each of these tests. Santolaya, et al., (2007) and Wilbur, et al., (2000) reported blood urea nitrogen (at thresholds of 180 and 260 mg/L respectively) had good specificity (86% to 94%) but moderate to low sensitivity (43% to 69%) to predict short term mortality.
Santolaya, et al., (2007) only reported the sensitivity and specificity of laboratory tests whose results differed significantly between patients who died and survived. In their study ANC, AMC, CRP, BUN and CRP differed significantly between those the two groups, whereas there was no significant difference between the groups in terms of platelets, creatinine, glycemia or lactate dehydrogenase (LDH).
Length of hospital stay
Pastura, et al., (2004) carried out a prospective study to derive a predictive model for length of hospital stay in children with haematological malignancy, neutropenia and presumed infection. Granulocyte count < 0.1 × 109/L was considered as a predictive factor in this study, but was excluded from the final multivariate model due to lack of statistical significance. Pastura, et al., final predictive model included ill appearance, age ≥6 years, presence of CVC and disease status as relapse.
Critical care and severe sepsis
Ammann, et al., (2010) reported a prospective study of predictive factors for serious medical complications in children with fever and chemotherapy induced neutropenia. Serious medical complications were defined as death, complication requiring intensive care treatment or complication judged as potentially life threatening by the treating doctor. Ammann, et al., (2010) constructed a multivariate risk score for serious complications, by selecting factors (from a list of 31 candidates) significantly associated with serious complications on univariate analysis. Their final model included four predictive factors: chemotherapy more intensive than ALL maintenance, haemoglobin level ≥90 g/L at presentation, leukocyte count <0.3 g/L at presentation and platelet count <50 g/L at presentation.
Five studies (Ahn, et al., 2011; Erten, et al., 2004; Hamalainen, et al., 2008, 2010 and Santolaya, 2008) compared the mean levels of serum CRP at admission in patients who did and did not develop severe sepsis. Although mean serum CRP level was higher in patients who went on to develop severe sepsis (mean difference 45 mg/L higher, 95% C.I. 32 to 58 mg/L higher) there was considerable overlap between the two groups. Hamalainen, et al., (2008, 2010) recorded CRP levels in the days following admission for fever and neutropenia. They observed a widening difference between the serum CRP levels of patients with severe sepsis and others over the first days of fever – from 53 mg/L on admission to 135 mg/L after four days.
Documented infection
Meta-analysis according to cut-off threshold was done for CRP (Table 5.1). In theory sensitivity should decrease and specificity should increase as the CRP threshold is raised, but this was not the case perhaps due to heterogeneity. AMC and ANC were poor predictors of documented infection.
Some studies (Arber, et al., 2000, El-Maghraby, et al., 2007, Engel, et al., Hitoglou-Hatzi 2005, Katz, et al., 1993, Massaro, et al., 2007, Martinez-Albarran, et al., 2009, Santolaya, et al., 1994, Tezcan, et al., 2006 and Yonemori, et al., 2001) compared the mean levels of serum CRP at admission for fever and neutropenia in those patients who went on to have a documented infection and patients with fever of unknown or viral origin. Mean CRP level was invariably higher in the patients who went on to have a documented infection: mean difference 35 mg/L higher (95%C.I. 26 to 44 mg/L higher). The greatest differences were seen in studies involving children, however there was significant heterogeneity in the results from paediatric studies.
There was a large range of serum CRP levels recorded in those with documented infections and in those with fever of unknown origin with considerable overlap in the distribution of CRP levels in the two groups. Thus it is unlikely that a single CRP threshold could achieve acceptable sensitivity and specificity for the prediction of documented infection.

Figure 5.3
Sensitivity and specificity of tests to predict mortality.

Figure 5.4
Sensitivity and specificity of tests to predict severe sepsis.

Figure 5.5
Sensitivity and specificity of tests to predict documented infection.
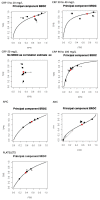
Figure 5.6
Summary ROC curves for CRP, AMC and ANC for the prediction of documented infection.

Figure 5.7
Mean difference in serum CRP between patients with severe infection and others: on the day of admission and on the following four days.

Figure 5.8
Mean difference in serum CRP level at admission between patients with documented infection and others.
EVIDENCE TABLES
Ahn 2011 (PDF, 214K)
Ammann 2003 (PDF, 218K)
Ammann 2004 (PDF, 215K)
Ammann 2010 (PDF, 218K)
Arber 2000 (PDF, 213K)
Asturias 2010 (PDF, 216K)
Avabratha 2009 (PDF, 215K)
Chayakulkeeree 2003 (PDF, 218K)
Diepold 2008 (PDF, 213K)
El-Maghraby 2007 (PDF, 213K)
Engel 1998 (PDF, 213K)
Erten 2004 (PDF, 214K)
Ha 2010 (PDF, 215K)
Hakim 2010 (PDF, 217K)
Hamalainen 2008 (PDF, 213K)
Hamalainen 2010 (PDF, 217K)
Hatzistilianou 2007 (PDF, 213K)
Hitoglou-Hatzi 2005 (PDF, 213K)
Karan 2002 (PDF, 319K)
Katz 1992 (PDF, 318K)
Kitanovski 2006 (PDF, 319K)
Klassen 2000 (PDF, 317K)
Klastersky 2000 (PDF, 321K)
Lehrnbecher 1999 (PDF, 316K)
Manian 1995 (PDF, 319K)
Martinez-Albarran 2009 (PDF, 213K)
Massaro 2007 (PDF, 316K)
Mato 2010 (PDF, 316K)
Moon 2009 (PDF, 321K)
Persson 2004 (PDF, 325K)
Prat 2008 (PDF, 317K)
Ramzi 2007 (PDF, 317K)
Riikonen 1993 (PDF, 316K)
Rondinelli 2006 (PDF, 324K)
Santolaya 1994 (PDF, 316K)
Santolaya 2001 (PDF, 318K)
Santolaya 2007 (PDF, 317K)
Santolaya 2008 (PDF, 318K)
Secmeer 2007 (PDF, 317K)
Spasova 2009 (PDF, 315K)
Tezcan 2006 (PDF, 317K)
Wilbur 2000 (PDF, 425K)
Yonemori 2001 (PDF, 422K)
REFERENCES
- Ahn S, Lee YS, Chun YH, Kwon IH, Kim W, Lim KS, et al. Predictive factors of poor prognosis in cancer patients with chemotherapy-induced febrile neutropenia. Supportive Care in Cancer. 2011;19:1151–1158. [PubMed: 20552376]
- Ammann RA, Hirt A, Luthy AR, Aebi C. Identification of children presenting with fever in chemotherapy-induced neutropenia at low risk for severe bacterial infection. Medical & Pediatric Oncology. 2003;41:436–443. [PubMed: 14515382]
- Ammann RA, Hirt A, thy AR, Aebi C. Predicting bacteremia in children with fever and chemotherapy-induced neutropenia. Pediatric Infectious Disease Journal. 2004;23:61–67. [PubMed: 14743049]
- Ammann RA, Bodmer N, Hirt A, Niggli FK, Nadal D, Simon A, et al. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2008–2014. [PubMed: 20231680]
- Arber C, Passweg JR, Fluckiger U, Pless M, Gregor M, Tichelli A, et al. C-reactive protein and fever in neutropenic patients. Scandinavian Journal of Infectious Diseases. 2000;32:515–520. [PubMed: 11055657]
- Asturias EJ, Corral JE, Quezada J. Evaluation of six risk factors for the development of bacteremia in children with cancer and febrile neutropenia. Current Oncology. 2010;17:59–63. [PMC free article: PMC2854640] [PubMed: 20404980]
- Avabratha KS, Rau ATK, Venkataravanamma P, Rau A. Significance of C-reactive protein during febrile neutropenia in pediatric malignancies. Indian Pediatrics. 2009;46:797–799. [PubMed: 19430077]
- Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat Med. 2009;28(21):2653–68. [PubMed: 19591118]
- Chayakulkeeree M, Thamlikitkul V. Risk index for predicting complications and prognosis in Thai patients with neutropenia and fever. Journal of the Medical Association of Thailand. 2003;86:212–223. [PubMed: 12757060]
- Diepold M, Noellke P, Duffner U, Kontny U, Berner R. Performance of Interleukin-6 and Interleukin-8 serum levels in pediatric oncology patients with neutropenia and fever for the assessment of low-risk. BMC infectious diseases. 2008;8 [PMC free article: PMC2292194] [PubMed: 18321393]
- El-Maghraby SM, Moneer MM, Ismail MM, Shalaby LM, El-Mahallawy HA. The diagnostic value of C-reactive protein, interleukin-8, and monocyte chemotactic protein in risk stratification of febrile neutropenic children with hematologic malignancies. Journal of Pediatric Hematology/Oncology. 2007;29:131–136. [PubMed: 17356388]
- Engel A, Mack E, Kern P, Kern WV. An analysis of interleukin-8, interleukin-6 and C-reactive protein serum concentrations to predict fever, gram-negative bacteremia and complicated infection in neutropenic cancer patients. Infection. 1998;26:213–221. [PubMed: 9717678]
- Erten N, Genc S, Besisik SK, Saka B, Karan MA, Tascioglu C. The predictive and diagnostic values of procalcitonin and C-reactive protein for clinical outcome in febrile neutropenic patients. Journal of the Chinese Medical Association. 2004;67:217–221. [PubMed: 15357107]
- Ha YE, Song JH, Kang WK, Peck KR, Chung DR, Kang CI, et al. Clinical factors predicting bacteremia in low-risk febrile neutropenia after anti-cancer chemotherapy. Support Care Cancer. 2010 [PubMed: 20931237]
- Hakim H, Flynn PM, Srivastava DK, Knapp KM, Li C, Okuma J, et al. Risk prediction in pediatric cancer patients with fever and neutropenia. Pediatric Infectious Disease Journal. 2010;29:53–59. [PubMed: 19996816]
- Hamalainen S, Kuittinen T, Matinlauri I, Nousiainen T, Koivula I, Jantunen E. Neutropenic fever and severe sepsis in adult acute myeloid leukemia (AML) patients receiving intensive chemotherapy: Causes and consequences. Leukemia and Lymphoma. 2008;49:495–501. [PubMed: 18297526]
- Hamalainen S, Juutilainen A, Kuittinen T, Nousiainen T, Matinlauri I, Pulkki K, et al. Serum amino-terminal pro-brain natriuretic peptide in hematological patients with neutropenic fever: A prospective comparison with C-reactive protein. Leukemia and Lymphoma. 2010;51:1040–1046. [PubMed: 20470220]
- Hatzistilianou M, Rekleity A, Athanassiadou F, DeLutiis MA, Conti P, Catriu D. Serial procalcitonin responses in infection of children with secondary immunodeficiency. Clinical and Investigative Medicine. 2007;30:E75–E85. [PubMed: 17716545]
- Hitoglou-Hatzi S, H M, G D, et al. Serum adenosine deaminase and procalcitonin concentrations in neutropenic febrile children with acute lymphoblastic leukaemia. Clinical & Experimental Medicine. 2005;5:60–65. [PubMed: 16096855]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res.Methodol. 2005;5:13. [PMC free article: PMC1097734] [PubMed: 15840177]
- Karan MA. Predictive value of higher plasma interleukin-6 levels in patients with febrile neutropenia. Archives of Medical Research. 2002;33:557–561. [PubMed: 12505102]
- Katz JA, Mustafa MM, Bash RO, Cash JV, Buchanan GR. Value of C-reactive protein determination in the initial diagnostic evaluation of the febrile, neutropenic child with cancer. Pediatric Infectious Disease Journal. 1992;11:708–712. [PubMed: 1448309]
- Kitanovski L, Jazbec J, Hojker S, Gubina M, Derganc M. Diagnostic accuracy of procalcitonin and interleukin-6 values for predicting bacteremia and clinical sepsis in febrile neutropenic children with cancer. European Journal of Clinical Microbiology and Infectious Diseases. 2006;25:413–415. [PubMed: 16767494]
- Klaassen RJ, Goodman TR, Pham B, Doyle JJ. ‘Low-risk’ prediction rule for pediatric oncology patients presenting with fever and neutropenia. Journal of Clinical Oncology. 2000;18:1012–1019. [PubMed: 10694551]
- Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. Journal of Clinical Oncology. 2000;18:3038–3051. [PubMed: 10944139]
- Lehrnbecher T, Venzon D, De HM, Chanock SJ, Kuhl J. Assessment of measuring circulating levels of interleukin-6, interleukin-8, C-reactive protein, soluble Fc receptor type III, and Mannose-binding protein in febrile children with cancer and neutropenia. Clinical Infectious Diseases. 1999;29:414–419. [PubMed: 10476751]
- Manian FA. A prospective study of daily measurement of C-reactive protein in serum of adults with neutropenia. Clinical Infectious Diseases. 1995;21:114–121. [PubMed: 7578719]
- Martinez-Albarran M, Perez-Molina JDJ, Gallegos-Castorena S, Sanchez-Zubieta F, Del Toro-Arreola S, Troyo-Sanroman R, et al. Procalcitonin and C-reactive protein serum levels as markers of infection in a pediatric population with febrile neutropenia and cancer. Pediatric Hematology and Oncology. 2009;26:414–425. [PubMed: 19657991]
- Massaro KSR, Costa SF, Leone C, Chamone DAF. Procalcitonin (PCT) and C-reactive Protein (CRP) as severe systemic infection markers in febrile neutropenic adults. BMC infectious diseases. 2007;7:2007. Article Number. [PMC free article: PMC2217552] [PubMed: 18034890]
- Mato AR, Luger SM, Heitjan DF, Mikkelsen ME, Olson E, Ujjani C, et al. Elevation in serum lactate at the time of febrile neutropenia (FN) in hemodynamically-stable patients with hematologic malignancies (HM) is associated with the development of septic shock within 48 hours. Cancer Biology and Therapy. 2010;9:585–589. [PubMed: 20160493]
- Moon JM, Chun BJ. Predicting the complicated neutropenic fever in the emergency department. Emergency Medicine Journal. 2009;26:802–806. [PMC free article: PMC2773519] [PubMed: 19850806]
- Pastura PS, Land MG, Santoro-Lopes G, Pastura P, Land MGP, Santoro-Lopes G. Predictive model for the length of hospital stay of children with hematologic malignancies, neutropenia, and presumed infection. Journal of Pediatric Hematology and Oncology. 2004;12:813–816. [PubMed: 15591901]
- Persson L, Engervall P, Magnuson A, Vikerfors T, Soderquist B, Hansson L-O, et al. Use of inflammatory markers for early detection of bacteraemia in patients with febrile neutropenia. Scandinavian Journal of Infectious Diseases. 2004;36:365–371. [PubMed: 15287382]
- Prat C, Sancho JM, Dominguez J, Xicoy B, Gimenez M, Ferra C, et al. Evaluation of procalcitonin, neopterin, C-reactive protein, IL-6 and IL-8 as a diagnostic marker of infection in patients with febrile neutropenia. Leukemia and Lymphoma. 2008;49:1752–1761. [PubMed: 18661397]
- Ramzi J, Mohamed Z, Yosr B, Karima K, Raihane B, Lamia A, et al. Predictive factors of septic shock and mortality in neutropenic patients. Hematology. 2007;12:543–548. [PubMed: 17852435]
- Riikonen P, Jalanko H, Hovi L, Saarinen UM. Fever and neutropenia in children with cancer: diagnostic parameters at presentation. Acta Paediatrica. 1993;82:271–275. [PubMed: 8495083]
- Rondinelli PIP, Ribeiro KDCB, De CB. A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. Journal of Pediatric Hematology/Oncology. 2006;28:665–670. [PubMed: 17023827]
- Santolaya ME, Cofre J, Beresi V. C-reactive protein: a valuable aid for the management of febrile children with cancer and neutropenia. Clinical Infectious Diseases. 1994;18:589–595. [PubMed: 8038314]
- Santolaya ME, Alvarez AM, Becker A, Cofre J, Enriquez N, O'Ryan M, et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. Journal of Clinical Oncology. 2001;19:3415–3421. [PubMed: 11454890]
- Santolaya ME, Alvarez AM, CL, Becker A, Mosso C, et al. Admission clinical and laboratory factors associated with death in children with cancer during a febrile neutropenic episode. Pediatric Infectious Disease Journal. 2007;26:794–798. [PubMed: 17721373]
- Santolaya ME, Alvarez AM, Aviles CL, Becker A, King A, Mosso C, et al. Predictors of severe sepsis not clinically apparent during the first twenty-four hours of hospitalization in children with cancer, neutropenia, and fever: a prospective, multicenter trial. The Pediatric infectious disease journal. 2008;27:538–543. [PubMed: 18458649]
- Secmeer G, Devrim I, Kara A, Ceyhan M, Cengiz B, Kutluk T, et al. Role of procalcitonin and CRP in differentiating a stable from a deteriorating clinical course in pediatric febrile neutropenia. Journal of Pediatric Hematology/Oncology. 2007;29:107–111. [PubMed: 17279007]
- Spasova MI, Terzieva DD, Tzvetkova TZ, Stoyanova AA, Mumdzhiev IN, Yanev IB, et al. Interleukin-6, interleukin-8, interleukin-10, and C-reactive protein in febrile neutropenia in children with malignant diseases. Folia Med (Plovdiv). 2005;47:46–52. [PubMed: 16761394]
- Tezcan G, Kupesiz A, Ozturk F, Ogunc D, Gultekin M, Yesilipek A, et al. Episodes of fever and neutropenia in children with cancer in a tertiary care medical center in Turkey. Pediatric Hematology and Oncology. 2006;23:217–229. [PubMed: 16517538]
- Wilbur DW, Rentschler RE, Couperus JJ, Camacho ES, Godfrey TE, Hilliard DA. Identifying neutropenic febrile cancer patients at risk for early death. Infections in Medicine. 2000;17:347. -+
- Yonemori K, Kanda Y, Yamamoto R, Hamaki T, Suguro M, Chizuka A, et al. Clinical value of serial measurement of serum C-reactive protein level in neutropenic patients. Leukemia and Lymphoma. 2001;41:607–614. [PubMed: 11378578]
6. Emergency assessment in secondary/tertiary care of a person with suspected neutropenic sepsis. (Topic C)
Guideline subgroup members for this topic
Paul Wallman (lead), Anne Davidson, Janie Thomas and Barbara Crosse
Review question
Should additional peripheral blood culture in patients with a central line, CRP (C-reactive protein), urinalysis, chest x-ray, lactate, blood gases be used in the emergency empirical assessment of a person with suspected neutropenic sepsis?
Rationale
Patients with acute cancer often present to hospital, by self presentation or referral by a GP or a community nurse or health worker. This may be to a specialist hospital or a local / district general hospital Emergency Department, with symptoms complicating their underlying disease or treatment thereof. Some of these symptoms may suggest the complication of neutropenic sepsis.
In such patients in the Emergency Department / Room, do any ‘standard’ tests that we currently perform add weight, or conversely, assist in refuting a diagnosis of neutropaenic sepsis or its source? Such a standard test would include the full blood count (FBC) to take a look at the number of white cells in the sample; neutropenic would be denoted by a low number of neutrophils in this sample. However, doing tests are not necessarily instantaneous and so there may be a delay in getting such blood results back to the ‘bedside’. As clinicians, should we be waiting for the results of tests prior to the initiation of treatment of a patient with suspected neutropenic sepsis?
What are the risks and the benefits of initiating treatments prior to the results of the accepted standard tests, and conversely what are the risks or benefits of delaying the treatment of neutropenic sepsis until receipt of the test results? What does the evidence suggest and do these standard tests actually guide treatment decisions or in fact delay treatments that reduce mortality and morbidity?
Question in PICO format
| Patients/population | Tests | Reference standard test | Target Condition | Outcomes |
|---|---|---|---|---|
| Patients in secondary or tertiary care with suspected neutropenic sepsis |
| Use whatever reference standards are reported in the individual studies. | Sepsis |
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Psychinfo and BMI. There were no publication date limits set. The date of the search was 27th of June 2011, and it was updated on 7th November 2011.
Papers ordered for other topics (A, D1 and D2) were also checked for eligibility for this topic.
Selection of studies
The information specialist (SB) did the first screen of the literature search results. Two reviewers (NB and CL) then independently selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question. The full articles were then obtained and checked against the inclusion criteria.
Data synthesis
One reviewer extracted information about diagnostic accuracy into 2 × 2 tables of true/false positives and true/false negatives for each test/outcome combination in each study. A proportion of studies were appraised by a second reviewer (CL). One reviewer (NB) extracted data and assessed study quality was assessed using eight items from the QUADAS checklist for diagnostic studies.
The evidence for CRP as an initial test in patients with neutropenic fever had already been reviewed for topic D2, so this analysis was updated with any extra studies identified in the search. We also aimed to record the rate at which management decisions were influenced by each test and any influence of tests on the timing of treatment or diagnosis.
RESULTS
Study quality and results
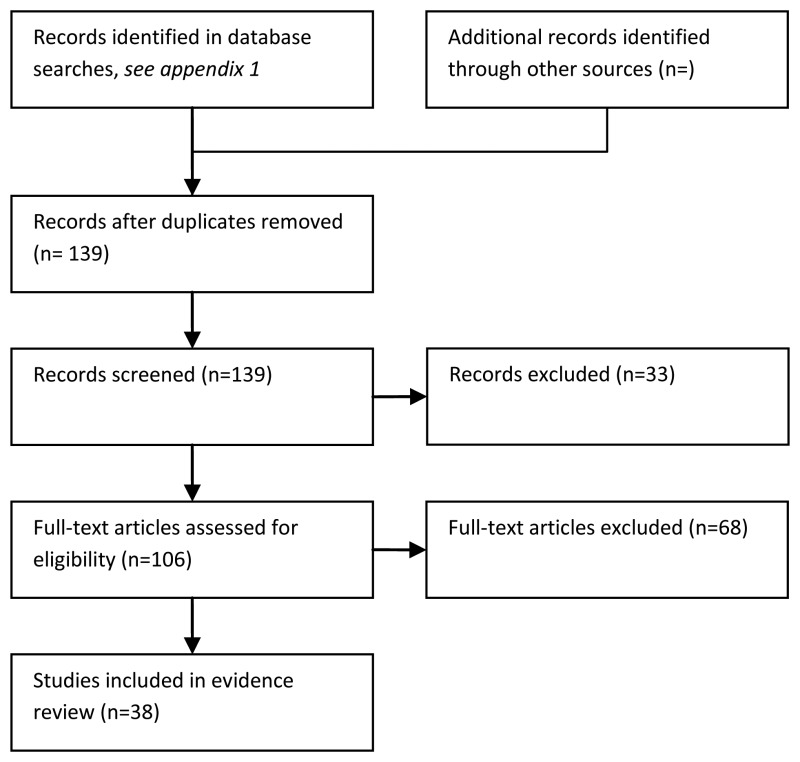
The overall quality of the studies was low (Figure 6.2), because most did not include a representative spectrum of patients. 32/38 of the studies included only patients with confirmed neutropenia and fever, a subset of the relevant population of patients presenting with fever where neutropenia is suspected but not yet confirmed. The accuracy of tests in the emergency department setting could be different from that reported in the included studies.

Figure 6.2
Study quality.
Only 2/38 studies were carried out in emergency departments: Ha, et al., 2010 (but including only low risk patients – MASCC ≥21) and Moon, et al., (2009). The evidence is summarised in table 6.1 below.
Table 6.1
Chest X-ray and additional peripheral blood cultures in the emergency assessment of patients with suspected neutropenic sepsis.
Evidence statements
Chest x-ray
Diagnosis of sepsis
Chest X-ray had a high sensitivity for bacterial pneumonia in two studies (Oude Nihuis, et al., 2003 and Renoult, et al., 2004), all cases of bacterial pneumonia were evident on the chest X-ray. A systematic review of the clinical features of radiographic pneumonia in children with fever and neutropenia (Phillips, et al,, 2011), identified 4 studies with 278 patients. The prevalence of pneumonia was 5% and Philips, et al., (2011) estimated that symptoms of respiratory distress had a negative predictive value of 98% (95% C.I. 96% to 99%). The probability of pneumonia in a child without respiratory symptoms was 1.9%.
In five studies, chest X-ray had widely varying sensitivity and specificity for severe sepsis or its complications (Badiei, et al., 2011, Chayakulkeeree, et al., 2003, Klastersky, et al., 2000, Moon, et al., 2009, and Wilbur, et al., 2000). Moon, et al., (2009) considered the use of chest X-ray in the emergency department to predict complicated fever in patients presenting with fever and neutropenia. In this study chest X-ray had a high positive likelihood ratio of 20.26 for complicated fever – a positive chest X-ray increased the odds of complicated fever by a factor of 20.
Clinical value of Test
Two studies considered the influence of chest X-ray on clinical management (Oude Nihuis, et al., 2003 and Renoult, et al., 2004). Both concluded that the results of chest X-ray did not influence the choice of antibiotic treatment.
Time to diagnosis or initiation of treatment
None of the included studies reported this outcome.
Peripheral blood culture (in patients with a central line)
Diagnosis of sepsis
Scheienmann, et al., (2010) found that peripheral blood cultures were positive in some cases where central cultures were not. In their series of 228 episodes of bacteraemia the peripheral blood culture was the only positive culture in 28 cases. Thus doing both peripheral blood cultures and central cultures could improve sensitivity for the detection of bacteraemia.
Blot, et al., (1998) reported that in patients where both central venous and peripheral blood cultures were positive the differential time to positivity (DPT) could help indicate catheter related sepsis. Earlier positivity of the central venous culture of two or more hours, when compared to the peripheral culture, increased the odds of catheter-related sepsis by three times.
Clinical value of Test
There was no direct evidence about the influence of peripheral blood cultures on clinical management decisions. However, Scheienmann, et al., (2010) surveyed Canadian healthcare professionals about their attitudes to obtaining peripheral blood cultures. The main reason given by the healthcare professionals for not obtaining peripheral blood cultures was that they do not provide any additional information and that phlebotomy is associated with a risk of complications
Time to diagnosis or initiation of treatment
None of the included studies reported this outcome.
CRP, Lactate and Blood gases
Evidence for these tests is reviewed in chapter 5: Investigations appropriate for risk stratification and management.
Urinalysis
Diagnostic accuracy
Moon, et al., (2009) reported a positive test for urine nitrates had sensitivity of 5% and specificity of 90% for complications of neutropenic sepsis. Thus a positive test was unlikely both in those with and without complications. Other studies mentioned using urinalysis in their initial assessment of patients with suspected neutropenic sepsis (for example Katz, et al., 1992) but did not report its results.
Clinical value of Test, Time to diagnosis or initiation of treatment
The influence of urinalysis on treatment decisions, time to diagnosis or initiation of treatment was not reported.
REFERENCES
- Asturias EJ, Corral JE, Quezada J. Evaluation of six risk factors for the development of bacteremia in children with cancer and febrile neutropenia. Current Oncology. 2010;17:59–63. [PMC free article: PMC2854640] [PubMed: 20404980]
- Avabratha KS, Rau ATK, Venkataravanamma P, Rau A. Significance of C-reactive protein during febrile neutropenia in pediatric malignancies. Indian Pediatrics. 2009;46(9) [PubMed: 19430077]
- Badiei Z, Khalesi M, Alami MH, Kianifar HR, Banihashem A, Farhangi H, Razavi AR. Risk factors associated with life-threatening infections in children with febrile neutropenia: a data mining approach. Journal of Pediatric Hematology/Oncology. 2011;33(1):e9–e12. [PubMed: 21102352]
- Blot F, Schmidt E, Nitenberg G, Tancrède C, Leclercq B, Laplanche A, Andremont A. Earlier positivity of central-venous- versus peripheral-blood cultures is highly predictive of catheter-related sepsis. J Clin Microbiol. 1998 Jan;36(1):105–9. [PMC free article: PMC124817] [PubMed: 9431930]
- Chayakulkeeree M, T V. Risk index for predicting complications and prognosis in Thai patients with neutropenia and fever. Journal of the Medical Association of Thailand. 2003;86(3):212–23. [PubMed: 12757060]
- Diepold M, Noellke P, Duffner U, Kontny U, Berner R. Performance of Interleukin-6 and Interleukin-8 serum levels in pediatric oncology patients with neutropenia and fever for the assessment of low-risk. BMC infectious diseases. 2008;8 [PMC free article: PMC2292194] [PubMed: 18321393]
- El-Maghraby SM, Moneer MM, Ismail MM, Shalaby LM, El-Mahallawy HA. The diagnostic value of C-reactive protein, interleukin-8, and monocyte chemotactic protein in risk stratification of febrile neutropenic children with hematologic malignancies. Journal of Pediatric Hematology/Oncology. 2007 Mar;29(3):131–136. 2007.Date of Publication: Mar 2007. [PubMed: 17356388]
- Erten N, G S, B SK, S B, K MA, T C. The predictive and diagnostic values of procalcitonin and C-reactive protein for clinical outcome in febrile neutropenic patients. Journal of the Chinese Medical Association: JCMA. 2004;67(5):217–21. [PubMed: 15357107]
- Ha YE, Song JH, Kang WK, Peck KR, Chung DR, Kang CI, et al. Clinical factors predicting bacteremia in low-risk febrile neutropenia after anti-cancer chemotherapy. Support Care Cancer. 2010 [PubMed: 20931237]
- Hatzistilianou M, Rekleity A, Athanassiadou F, DeLutiis MA, Conti P, Catriu D. Serial procalcitonin responses in infection of children with secondary immunodeficiency. Clinical and Investigative Medicine. 2007;30:E75–E85. [PubMed: 17716545]
- Heney D, Lewis IJ, Evans SW, Banks R, Bailey CC, Whicher JT. Interleukin-6 and its relationship to C-reactive protein and fever in children with febrile neutropenia. Journal of Infectious Diseases. 1992;165:886–890. [PubMed: 1569338]
- Hitoglou-Hatzi S, H M, G D, et al. Serum adenosine deaminase and procalcitonin concentrations in neutropenic febrile children with acute lymphoblastic leukaemia. Clinical & Experimental Medicine. 2005;5:60–65. [PubMed: 16096855]
- Karan MA. Predictive value of higher plasma interleukin-6 levels in patients with febrile neutropenia. Archives of Medical Research. 2002;33:557–561. [PubMed: 12505102]
- Katz JA, Mustafa MM, Bash RO, Cash JV, Buchanan GR. Value of C-reactive protein determination in the initial diagnostic evaluation of the febrile, neutropenic child with cancer. Pediatric Infectious Disease Journal. 1992;11:708–712. [PubMed: 1448309]
- Kitanovski L, Jazbec J, Hojker S, Gubina M, Derganc M. Diagnostic accuracy of procalcitonin and interleukin-6 values for predicting bacteremia and clinical sepsis in febrile neutropenic children with cancer. European Journal of Clinical Microbiology and Infectious Diseases. 2006;25:413–415. [PubMed: 16767494]
- Klaassen RJ, Goodman TR, Pham B, Doyle JJ. ‘Low-risk’ prediction rule for pediatric oncology patients presenting with fever and neutropenia. Journal of Clinical Oncology. 2000;18:1012–1019. [PubMed: 10694551]
- Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. Journal of Clinical Oncology. 2000;18:3038–3051. [PubMed: 10944139]
- Lodahl D, Schroder H. Procalcitonin adds to diagnosis, but does not reduce initial antibiotics in febrile neutropenic children. Danish Medical Bulletin. 2011;58:A4233. [PubMed: 21371399]
- Manian FA. A prospective study of daily measurement of C-reactive protein in serum of adults with neutropenia. Clinical Infectious Diseases. 1995;21:114–121. [PubMed: 7578719]
- Martinez-Albarran M, Perez-Molina JDJ, Gallegos-Castorena S, Sanchez-Zubieta F, Del Toro-Arreola S, Troyo-Sanroman R, et al. Procalcitonin and C-reactive protein serum levels as markers of infection in a pediatric population with febrile neutropenia and cancer. Pediatric Hematology and Oncology. 2009;26:414–425. [PubMed: 19657991]
- Massaro K, Costa S, Leone C, Chamone D. Procalcitonin (PCT) and C-reactive Protein (CRP) as severe systemic infection markers in febrile neutropenic adults. BMC Infectious Diseases. 2007;7:137. [PMC free article: PMC2217552] [PubMed: 18034890]
- Mato AR, Luger SM, Heitjan DF, Mikkelsen ME, Olson E, Ujjani C, et al. Elevation in serum lactate at the time of febrile neutropenia (FN) in hemodynamically-stable patients with hematologic malignancies (HM) is associated with the development of septic shock within 48 hours. Cancer Biology and Therapy. 2010;9:585–589. [PubMed: 20160493]
- Moon JM, Chun BJ. Predicting the complicated neutropenic fever in the emergency department. Emergency Medicine Journal. 2009;26:802–806. [PMC free article: PMC2773519] [PubMed: 19850806]
- Oude Nijhuis CS, Vellenga E, Daenen SM, van der Graaf WT, Gietema JA, Groen HJ, et al. Lipopolysaccharide-binding protein: a possible diagnostic marker for Gram-negative bacteremia in neutropenic cancer patients. Intensive Care Medicine. 2003;29:2157–2161. [PubMed: 14569424]
- Oude Nijhuis CS, Gietema JA, Vellenga E, Daenen SM, de Bont ES, Kamps WA, et al. Routine radiography does not have a role in the diagnostic evaluation of ambulatory adult febrile neutropenic cancer patients. European Journal of Cancer. 2003;39:2495–2498. [PubMed: 14602135]
- Park Y, Kim DS, Park SJ, Seo HY, Lee SR, Sung HJ, et al. The suggestion of a risk stratification system for febrile neutropenia in patients with hematologic disease. Leukemia Research. 2010;34:294–300. [PubMed: 19762083]
- Persson L, Engervall P, Magnuson A, Vikerfors T, Soderquist B, Hansson LO, et al. Use of inflammatory markers for early detection of bacteraemia in patients with febrile neutropenia. Scandinavian Journal of Infectious Diseases. 2004;36:365–371. [PubMed: 15287382]
- Phillips B, Wade R, Westwood M, Riley R, Sutton AJ. Systematic review and meta-analysis of the value of clinical features to exclude radiographic pneumonia in febrile neutropenic episodes in children and young people. Journal of Paediatrics and Child Health. 2011 [PubMed: 22050289] [CrossRef]
- Renoult E, Buteau C, Turgeon N, Moghrabi A, Duval M, Tapiero B. Is routine chest radiography necessary for the initial evaluation of fever in neutropenic children with cancer? Pediatric Blood and Cancer. 2004 Sep;43(3):224–228. 2004.Date of Publication: Sep 2004. [PubMed: 15266405]
- Riikonen P, Jalanko H, Hovi L, Saarinen UM. Fever and neutropenia in children with cancer: diagnostic parameters at presentation. Acta Paediatrica. 1993;82:271–275. [PubMed: 8495083]
- Rondinelli PIP, Ribeiro KDCB, De CB. A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. Journal of Pediatric Hematology/Oncology. 2006;28:665–670. [PubMed: 17023827]
- Santolaya ME, Cofre J, Beresi V. C-reactive protein: a valuable aid for the management of febrile children with cancer and neutropenia. Clinical Infectious Diseases. 1994;18:589–595. [PubMed: 8038314]
- Santolaya ME, Alvarez AM, Becker A, Cofre J, Enriquez N, O'Ryan M, et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. Journal of Clinical Oncology. 2001;19:3415–3421. [PubMed: 11454890]
- Scheinemann K, Ethier MC, Dupuis LL, Richardson SE, Doyle J, Allen U, et al. Utility of peripheral blood cultures in bacteremic pediatric cancer patients with a central line. Supportive Care in Cancer. 2010;18:913–919. [PubMed: 19727845]
- Secmeer G, Devrim I, Kara A, Ceyhan M, Cengiz B, Kutluk T, et al. Role of procalcitonin and CRP in differentiating a stable from a deteriorating clinical course in pediatric febrile neutropenia. Journal of Pediatric Hematology/Oncology. 2007;29:107–111. [PubMed: 17279007]
- Wilbur DW, Rentschler RE, Couperus JJ, Camacho ES, Godfrey TE, Hilliard DA. Identifying neutropenic febrile cancer patients at risk for early death. Infections in Medicine. 2000;17:347. -+
- Yonemori K, Kanda Y, Yamamoto R, Hamaki T, Suguro M, Chizuka A, et al. Clinical value of serial measurement of serum C-reactive protein level in neutropenic patients. Leukemia & Lymphoma. 2001;41:607–614. [PubMed: 11378578]
EVIDENCE TABLES
Download PDF (655K)
7. Risk stratification scores or algorithms. (Topic E1)
Review question
Which is the most valid published risk stratification score or algorithm for influencing management and predicting outcome in patients with neutropenic sepsis?
Rationale
Patients receiving cancer treatment are at risk of potentially life threatening sepsis caused by neutropenia and early empiric broad spectrum antibiotic therapy significantly reduces mortality. Standard therapy requires hospitalisation until both the fever and neutropenia have resolved with average inpatient stays of around 5 days.
However, around 40% of patients treated for febrile neutropenia are not found to have either clinical or microbiologically proven infection. These patients may be termed at “low risk” from serious infection and various risk stratification approaches have been used to help identify low risk patients suitable for either outpatient management from the outset or for early discharge after a period of inpatient observation and investigation (a “step-down” approach).
The ideal stratification system would accurately identify a group of low risk patients with no risk of mortality from sepsis, would be simple to use by medical and healthcare professionals with little or no specific oncology or haematology experience, and use either clinical parameters or laboratory parameters which are widely available and inexpensive. In addition there are a number of “early warning” scoring systems used in both general paediatric and adult practice which have not been widely tested or validated in this population which may be useful in supporting a step-down approach.
There is no single risk stratification system is in widespread use in either adult or paediatric practice and there are considerable variations in practice. A simple, reliable and safe risk stratification system has the potential to significantly reduce hospitalisation rates without increasing overall mortality.
Question in PICO format
| Patients/population | Risk score or algorithm | Outcomes |
|---|---|---|
| Patients with suspected neutropenic sepsis. |
| Accuracy for prediction of
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Psychinfo and BMI. The search was limited to papers published from 1999 onwards. The date of the search was 13th December 2010, and it was updated on 2nd November 2011.
Selection of studies
The information specialist (SB) did the first screen of the literature search results. One reviewers (KF) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question. The full articles were then obtained and checked against the inclusion criteria.
Data synthesis
One reviewer (KF) extracted information about diagnostic accuracy into 2 × 2 tables of true/false positives and true/false negatives for each test/outcome combination in each study.
RESULTS
Study quality and results
Eight prospective or retrospective observational studies were identified that validated the Multinational Association of Supportive Care in Cancer (MASCC) risk index (Baskaran, et al., 2008; De Souza Viana, et al., 2008; Innes, et al., 2008; Ahn, et al., 2010; Uys, et al., 2007; Klastersky, et al., 2006; Hui, et al., 2010 and Cherif, et al., 2006. These papers provided data on the sensitivity and specificity of this risk score in determining which adult patients presenting with neutropenia and fever, were at low risk of developing ‘serious medical complications’. There was no specific evidence on ‘early warning signs’ in neutropenic sepsis.
Phillips, et al., (2010) presented a systematic review of the discriminatory performance of risk prediction rules in febrile neutropenic episodes in children and young people. Six of the twenty studies included studies were prospective, but the studies were at low risk of verification procedure bias and unclear risk of interpretation bias (according to the QUADAS criteria). Three other papers about paediatric clinical decision rules were identified (Dommett, et al., 2009; Ammann, et al., 2010 and Marcher, et al., 2010).
The evidence is summarised in Table 7.1.
Table 7.1
Studies of clinical decision rules to identify patients at low risk of adverse outcome in patients with fever and neutropenia.
Evidence Statements
Paediatric patients
Six studies evaluated the Klaassen rule which uses a single feature: an absolute monocyte count of greater than 100/mm3 to predict paediatric patients with significant infection. Sensitivity ranged from 37% to 100% and specificity from 23% to 58%.
Evidence from three studies suggests the Amman rule (Ammann, et al., 2003) to predict paediatric patients at low risk of significant bacterial has high sensitivity (95% to 100%) but low specificity (9% to 22%). This means that most patients at low risk of adverse outcome would be labelled as high risk.
The Alexander rule to predict adverse clinical consequences was evaluated by three studies (Alexander, et al. 2002; Ammann, et al., 2010 and Dommet, et al., 2009; see Phillips et al., 2010 ). Results were heterogeneous with sensitivity ranging from 59% to 94% and specificity 9% to 65%.
Four studies evaluated the PINDA rule for identification of patients at low risk of significant bacterial infection. Two South American studies from the rules' authors (Santoloya, et al., 2002 and 2003; see Phillips et al., 2010) showed high sensitivity and specificity, however these findings were not replicated by two European validation studies (Ammann, et al., 2010 and Macher, et al., 2009).
Other paediatric clinical decision rules have been proposed (Phillips, et al., 2010) but are validated by less than three studies.
Adult patients
Eight studies reported the sensitivity and specificity of the MASCC risk score to identify adult patients with neutropenia and fever at low risk of serious medical complications. There was considerable heterogeneity in study results which precluded statistical meta-analysis, but no obvious explanatory factor was identified (see Figures 7.2 and 7.3). The sensitivity of MASCC score < 21 (for the prediction of serious medical complications) ranged between 40% and 80% whilst the specificity ranged between 59% and 95%.
Figure 7.2
Summary ROC curve, sensitivity and specificity for MASCC studies.
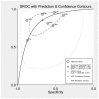
Figure 7.3
Summary ROC curve for MASCC studies with the added extra information of the % solid tumour patients in study. Additional bivariate diagnostic meta-analysis by clinical lead Dr. Bob Phillips, studies are numbered in alphabetical order.
EVIDENCE TABLES
Download PDF (722K)
- Identification and Assessment: guideline chapter four - Neutropenic Sepsis: Prev...Identification and Assessment: guideline chapter four - Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients
- phospholipase D2 isoform 1 [Mus musculus]phospholipase D2 isoform 1 [Mus musculus]gi|699045644|ref|NP_001289404.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...