NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Addendum to Clinical Guideline 164, Familial breast cancer. London: National Institute for Health and Care Excellence (NICE); 2017 Mar. (Clinical Guideline Addendum, No. 164.1.)
N.1. Introduction
Since the update to this guideline in 2013, new evidence has been published on chemoprevention using aromatase inhibitors. The evidence suggests that these agents are effective in preventing breast cancer in postmenopausal women, although there remain concerns regarding their adverse event profile, specifically reduction in bone mineral density and an increased occurrence of fractures. The original economic analysis for the 2013 update investigated the cost consequences of chemoprevention as an overall strategy (with patients receiving either tamoxifen or raloxifene) compared to no chemoprevention. This evaluation updates the original economic analysis by considering the cost consequences of three chemopreventive agents (tamoxifen, raloxifene, and anastrozole) compared to no chemoprevention in postmenopausal women at high-risk and moderate-risk of breast cancer.
N.2. Methods
N.2.1. Type of evaluation
Although this topic would be a suitable candidate for cost utility modelling, it was determined that a simple cost consequences model adapted from the analysis developed for the 2013 guideline update would be sufficient to provide estimates of incremental costs and outcomes associated with each of the comparators. This type of evaluation was chosen as the previous analysis demonstrated that chemoprevention as an overall strategy is likely to be cost effective at a threshold of £20,000 per QALY. Therefore the objective of this analysis was to compare the relative costs, benefits, and side effects of different chemoprevention in their natural units.
N.2.2. Target population
The population for this analysis is high- and moderate-risk postmenopausal women with no personal history of breast cancer, who have no history or increased risk of thromboembolic disease or endometrial cancer, and who are eligible for chemoprevention with any of the chemopreventive agents included in the analysis. Risk levels are defined according to the following lifetime risks of breast cancer from age 20:
- High risk population: 30% or greater
- Moderate risk population: Greater than 17% but less than 30%
N.2.3. Interventions
The interventions included in the analysis are the following chemopreventive agents, administered once-daily over a five year period:
- Tamoxifen 20mg
- Raloxifene 60mg
- Anastrozole 1mg
N.2.4. Comparator
The comparator in the analysis is no chemoprevention.
N.2.5. Time horizon
Since chemoprevention has the potential to reduce the long-term incidence of breast cancer, a lifetime time horizon was used in this analysis.
N.2.6. Health outcomes
The primary measure of health outcomes in the analysis is cases of breast cancer prevented as a result of chemoprevention. Secondary measures are incremental endometrial cancer cases, thromboembolic events, and fractures, compared to no chemoprevention.
N.2.7. Perspective
The analysis was conducted from the perspective of the NHS and personal and social services (PSS).
N.2.8. Discounting
A discount rate of 3.5% per annum was applied to all costs incurred after the first year.
N.2.9. Model structure
A Markov structure with a cycle length of one year was used to simulate the progression of patients over a lifetime time horizon. The model used three health states: healthy, breast cancer, and dead, as shown in Figure 2.
For the no chemoprevention arm of the model, age-specific incidence rates of breast cancer and baseline mortality were used to inform progression probabilities. Each year, living patients (either with or without breast cancer) could also experience adverse events: endometrial cancer, venous thromboembolism, or fracture. Patients could experience four categories of fracture: hip fracture, wrist fracture, vertebral fracture, or other fracture. The number of adverse events of each category per year was calculated by multiplying the number of living patients by the annual baseline probability of experiencing each event.
For the treatment arms of the model (tamoxifen, raloxifene, and anastrozole) relative risks were applied to the baseline incidence rates for breast cancer incidence, endometrial cancer, venous thromboembolism, and fracture in order to inform transition probabilities and adverse event probabilities.
N.2.10. Patient cohort characteristics
The assumption was made that patients of the age 50 and above are postmenopausal. The model simulated the progression of four patient age groups of patients: 50-59 years old, 60-69 years old, 70-79 years, and 80 years old plus, in order to capture the heterogeneity of the patient population. The distribution of patients among these age groups was derived from Office for National Statistics mid-2015 population data for females in the UK [accessed 23rd September 2016], and is shown in Table 17.
Table 17Age distribution among women aged at least 50 in the UK
| Age (years) | 50-59 | 60-69 | 70-79 | ≥80 |
|---|---|---|---|---|
| Proportion of women | 34.6% | 29.3% | 20.9% | 15.3% |
N.2.11. Baseline breast cancer risk
Due to the scarcity of data on age-dependent baseline risk of breast cancer, five-year risks of breast cancer were generated using the BOADICEA assessment tool [http://ccge.medschl.cam.ac.uk/boadicea/] for representative high- and moderate-risk patients. Baseline risk data are displayed in Table 18. These values were converted to one year risks in order to inform transition probabilities for the model.
Patient profiles in the BOADICEA tool were selected to produce lifetime breast cancer risks which were consistent with definitions of high- and moderate-risk patients in this guideline: high-risk patients are associated with a 30% or greater risk of breast cancer from age 20 and moderate-risk patients are associated with a risk of between 17% and 30%.
The high-risk BOADICEA profile consisted of a 20 year old female patient who had not been tested for genetic mutations, but whose mother had a confirmed BRCA1 mutation. The moderate-risk profile consisted of a 20 year old female patient who had not been tested for genetic mutations, and whose mother had also not been tested, but had been diagnosed with breast cancer at age 20.
Table 18Baseline five-year breast cancer incidence in high- and moderate-risk women
| Age (years) | 50-54 | 55-59 | 60-64 | 65-69 | 70-74 | ≥75 |
|---|---|---|---|---|---|---|
| High-risk women | 5.4% | 4.7% | 4.3% | 3.8% | 2.9% | 2.6% |
| Moderate-risk women | 2.8% | 2.7% | 3.0% | 2.8% | 2.4% | 2.1% |
N.2.12. Mortality rate
Age-specific mortality data for females were taken from Office for National Statistics National Life Tables: England and Wales for 2013-15 [accessed 23rd September 2016]
N.2.13. Baseline adverse event risks
Baseline risks for the incidence of endometrial cancer, thromboembolic events, and fractures were taken from the placebo arm of Cuzick et al. (2015). This study was selected as it is the analysis of tamoxifen with the largest patient numbers identified by the clinical review. Pooling of studies from the clinical literature review to derive baseline data was not possible, as analyses used varying follow-up lengths. Table 19 displays baseline adverse event incidence rates used in the model.
Table 19Baseline incidence of adverse events
| Adverse event | Endometrial cancer (median follow-up 16 years) | Thromboembolic event (median follow-up 95.6 months) | Fracture (median follow-up 95.6 months) |
|---|---|---|---|
| Incidence | 0.56% | 1.9% | 6.57% |
The distribution of fracture categories was derived from Scholes et al (2014) – an epidemiological study of the prevalence of fractures in adults aged 55 and above. Table 20 shows the relative incidence of fracture categories.
Table 20Relative incidence of fracture categories
| Fracture Category | Hip fracture | Wrist fracture | Vertebral fracture | Other fractures |
|---|---|---|---|---|
| Relative incidence | 3.2% | 22.1% | 2.1% | 72.6% |
N.2.14. Relative risks for chemoprevention
Relative risks of breast cancer incidence and adverse event incidence were taken from the results of the clinical literature review and are shown in Table 21.
Table 21Relative risks of breast cancer and adverse event incidence for each chemoprevention treatment
| Treatment | Tamoxifen versus placebo | Tamoxifen versus Raloxifene | Anastrozole versus placebo |
|---|---|---|---|
| Breast cancer incidence | 0.70 | 0.81 | 0.51 |
| Endometrial cancer incidence | 2.12 | 1.76 | 0.61 |
| Thromboembolic event incidence | 1.49 | 1.31 | 1.21 |
| Fracture incidence | 0.91 | 1.09 | 1.11 |
N.2.15. Adherence to chemoprevention
For the base case, the assumption was made that 50% of patients discontinued chemoprevention after one year of treatment, with the remaining 50% continuing treatment for the full 5 years. This estimate was elicited via expert opinion. It was assumed that patients discontinuing after one year had the same risk of breast cancer and adverse events as patients with receiving no chemoprevention.
N.2.16. Duration of treatment effect
The assumption was made that, in the base case, reduction in the incidence of breast cancer resulting from chemoprevention persisted over patients’ lifetime. It was assumed that change in risk level of endometrial cancer and thromboembolic events caused by chemoprevention lasted for the duration of treatment (i.e. the first five years of the model), and the change in risk level of fractures lasted for five years after the end of treatment (i.e. the first ten years of the model), after which incidence rates of adverse events returned to baseline levels.
N.2.17. Costs
Annual costs of tamoxifen, raloxifene, and anastrozole were taken from the September 2016 Electronic Drug Tariff [accessed 23rd September 2016], and are shown in Table 22.
Table 22Annual costs of chemoprevention
| Treatment | Tamoxifen | Raloxifene | Anastrozole |
|---|---|---|---|
| Annual cost | £37.23 | £48.62 | £15.90 |
The assumption was made that all patients receiving chemoprevention would also incur the cost of two GP consultations per year while treatment was ongoing. The cost of a GP consultation was estimated to be £38 (including direct care staff costs and excluding qualification costs), taken from the PSSRU Unit Costs of Health and Social Care 2015. It was also assumed that patients treated with anastrozole incurred the cost of a DEXA scan at the outset of treatment, to screen patients for osteoporosis, due to concerns of reduction in bone mineral density associated with aromatase inhibitors. The cost of a DEXA scan was estimated to be £62, taken from the NHS National Tariff Payment System 2016/17 [accessed 23rd September, 2016].
Costs associated with a case of breast cancer were taken from the model for the 2013 guideline update, which were originally derived from the NICE costing report published with the full guideline. These costs were adjusted to 2015 prices using consumer price index rates from the Office for National Statistics. Costs associated with a case of breast cancer are shown in Table 23.
Table 23Cost components of breast cancer treatment
| Category | Surgery | Radiotherapy | Chemotherapy | Other drug costs | Total |
|---|---|---|---|---|---|
| Cost | £2,824.75 | £1,872.68 | £3,880.35 | £6,310.82 | £14,888.59 |
Costs of endometrial cancer and thromboembolic events were also taken from the model produced for the 2013 update, adjusted to 2015 prices. The cost of endometrial cancer was originally sourced from Hind et al (2007). The cost of a thromboembolic event was assumed to be that of deep vein thrombosis, derived from NHS Reference Costs 2011/12. Costs of hip fracture, wrist fracture, vertebral fracture, and other fractures were taken from Dolan et al (1998), and adjusted to 2015 prices. Costs of all adverse events used in the model are shown in Table 24.
Table 24Adverse event costs
| Adverse event | Cost |
|---|---|
| Endometrial cancer | £4,684.62 |
| Thromboembolic event | £878.92 |
| Hip fracture | £17,139.30 |
| Wrist fracture | £668.43 |
| Vertebral fracture | £684.14 |
| Other fractures | £1,911.03 |
N.2.18. Sensitivity analysis
Both deterministic and probabilistic sensitivity analyses were used to characterise the uncertainty surrounding the base case results of the model.
For the deterministic sensitivity analysis, cost per breast cancer case prevented was calculated compared to no chemoprevention for each intervention, under each of the following scenarios:
- Relative risks for breast cancer incidence and adverse events replaced by hazard ratios where available from the clinical literature
- Baseline incidence of breast cancer increased by 100% and reduced by 50%
- Relative risks of treatments for breast cancer incidence changed to lower and upper 95% confidence intervals
- Adherence changed to 100% and 25%
- Baseline incidence of adverse events (endometrial cancer, thromboembolic events, and fractures) increased by 100% and reduced by 50%
- Relative risks of adverse events set to lower and upper 95% confidence intervals
- Cost of treatment increased by 100% and reduced by 50%
- Costs of adverse events increased by 100% and reduced by 50%
- Cost of breast cancer increased by 100% and reduced by 50%
- Relative risks of adverse events persist for 20 years after the end of treatment
- Breast cancer treatment is associated with no chemotherapy costs (this scenario was included to reflect the fact that chemoprevention is only effective in preventing ER-positive breast cancers, which are less susceptible to treatment with chemotherapy)
- Relative risks for breast cancer, endometrial cancer and thromboembolic event incidence for raloxifene changed to those from the RUTH trial comparing raloxifene to placebo in postmenopasal women (Barrett-Connor et al, 2006). This sensitivity analysis was conducted as the committee felt that the evidence comparing raloxifene to tamoxifen may provide an unfavourable representation of the effectiveness of raloxifene in preventing breast cancer. The RUTH trial was selected as a representative study comparing raloxifene to placebo in a relevant patient population.Table 25 shows the relative risks used in this sensitivity analysis.
Table 25Relative risk values used for deterministic sensitivity analysis of raloxifine using results from the RUTH trial
| Parameter | Breast cancer incidence | Endometrial cancer incidence | Thromboembolic event incidence | Fracture incidence |
|---|---|---|---|---|
| Relative risk (raloxifene versus placebo) | 0.57 | 1.24 | 1.45 | 0.92 |
For the probabilistic sensitivity analysis, all model input parameters were assigned probability distributions (rather than being expressed as point estimates) to reflect the uncertainty surrounding the available clinical and cost data. 1,000 iterations of the model were run, each drawing random values from parameter distributions.
Probability parameters were assigned beta distributions in order to account for the fact that probability values must lie between 0 and 1. Cost parameters were assigned gamma distributions, to ensure that costs could not be negative. Relative risks were assigned a distribution by raising the exponential constant to the power of a normal distribution of the natural logarithm of the parameter.
Where available, standard errors or 95% confidence intervals were used to inform the shape of distributions. For parameters for which these values were not available, it was assumed that standard error was 10% of the parameter mean.
N.3. Results – high risk patients
N.3.1. Deterministic results
Base case cost results for a cohort of 1,000 high risk patients are displayed in Table 26. Results show that tamoxifen and raloxifene incur an additional cost of £97,346 and £237,865 per 1,000 patients, respectively, compared to no treatment. This additional cost is incurred primarily from costs of chemoprevention and monitoring consultations with GPs, although it partially offset by a reduction in the cost of breast cancer treatment. Conversely, anastrozole produces a cost saving of £34,539 per 1,000 patients, mostly achieved through a reduction in the cost of breast cancer treatment.
Table 27 displays health outcomes for a cohort of 1,000 patients. All chemoprevention strategies demonstrate a reduction in breast cancer cases, with anastrozole achieving the highest reduction (36 cases prevented). The incidence of adverse events is relatively uniform across all comparators, although the incidence of thromboembolic events is slightly elevated for all chemopreventive agents (in particular for tamoxifen), and the incidence of fractures is slightly decreased by tamoxifen and raloxifene, and slightly increased by anastrozole.
Table 28 displays incremental cost effectiveness results for each chemopreventive agent compared to no treatment, reported in terms of incremental cost per breast cancer case prevented. Raloxifene is associated with a substantially higher cost per case prevented than other chemopreventive agents, due to a high drug acquisition cost and a relatively low efficacy. Anastrozole is associated with a negative ICER (i.e. it dominates no chemoprevention), as it results in lower costs and fewer breast cancer cases than no treatment.
Results are also reported in terms of the QALY gain required per prevented breast cancer case for each treatment to be cost effective at a threshold of £20,000/QALY. Treatment with tamoxifen and raloxifene requires a gain of 0.23 and 1.27 QALYs per breast cancer case prevented, respectively, while the value for anastrozole is negative, as this treatment dominates no chemoprevention.
Table 26Cost results per 1,000 high-risk patients
| Cost category | No chemoprevention | Tamoxifen | Raloxifene | Anastrozole |
|---|---|---|---|---|
| Chemoprevention drugs | £0 | £102,947 | £134,442 | £43,966 |
| Chemoprevention monitoring | £0 | £210,152 | £210,152 | £210,152 |
| Breast cancer | £1,525,164 | £1,309,356 | £1,428,772 | £1,167,124 |
| Endometrial cancer and thromboembolic events | £58,294 | £64,749 | £59,700 | £57,854 |
| Fractures | £276,539 | £270,138 | £264,795 | £284,362 |
| DEXA scans | £0 | £0 | £0 | £62,000 |
| Total costs | £1,859,997 | £1,957,343 | £2,097,862 | £1,825,458 |
Table 27Health outcomes per 1,000 high-risk patients
| Outcome category | No chemoprevention | Tamoxifen | Raloxifene | Anastrozole |
|---|---|---|---|---|
| Breast cancer cases | 152 | 131 | 142 | 116 |
| Endometrial cancer cases | 9 | 10 | 9 | 8 |
| Thromboembolic events | 60 | 63 | 61 | 61 |
| Fractures | 211 | 208 | 205 | 216 |
Table 28Incremental cost effectiveness results compared to no treatment for high-risk patients
| Tamoxifen | Raloxifene | Anastrozole | |
|---|---|---|---|
| Cost per breast cancer case prevented | £4,621 | £25,387 | -£984 |
| QALY gain required per breast cancer case prevented to be cost effective at £20,000 threshold | 0.23 | 1.27 | dominant |
In order to verify that chemoprevention with these agents is cost effective at a theshold of £20,000, a simple Markov chain was constructed to estimate the QALY difference between a 50 year old woman with breast cancer and a healthy 50 year old woman over the course of five years. Using an annual cycle length, with the mortality and utility inputs listed in Table 29 and a discount rate of 3.5% per year, an estimate of 1.33 incremental QALYs per breast cancer case prevented was produced. Based on this value, it is likely that, in high risk patients, chemprevention with all three agents is cost effective compared to no treatment.
Table 29Parameters used to estimate the incremental QALYs associated with preventing a case of breast cancer
| Parameter | Value | Source |
|---|---|---|
| Annual breast cancer-related mortality: BRCA+ women with breast cancer – 50-59 years old | 5.67% | Brekelmans et al, 2007 |
| Annual background mortality rate: 50 years old | 0.21% | Office for National Satitistics – National life Tables: England and Wales |
| Baseline utility for individual affected with breast cancer | 0.68 | Peasgood et al, 2010 |
| Baseline utility for individual without cancer but with positive BRCA test result | 0.895 | Grann et al, 2011 |
N.3.2. Sensitivity analysis
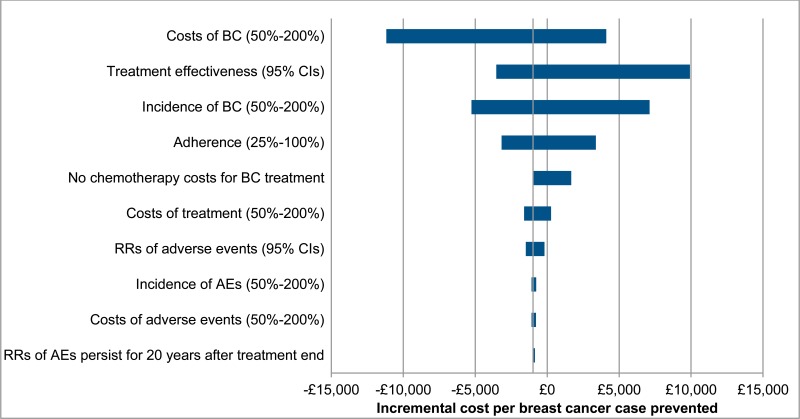
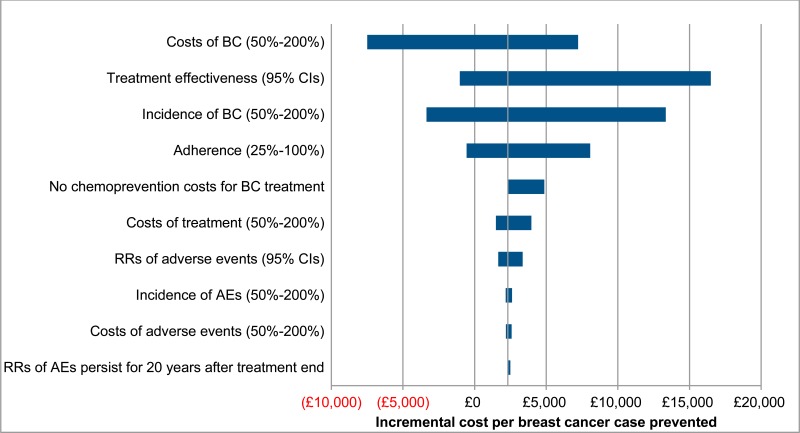
Results of one-way sensitivity analysis are displayed in Table 30. Results for anastrozole are displayed as a tornado diagram in Figure 3, to illustrate the magnitude of sensitivity across parameters. These results show that the cost effectiveness of treatment is particularly sensitive to changes in the baseline incidence and relative risks of breast cancer. This is because these parameters have a considerable impact on both the number of breast cancer cases prevented and the magnitude of costs, as breast cancer treatment constitutes a large proportion of total costs. For this reason, results are also sensitive to changes in the cost per case of breast cancer.
Comparatively, cost effectiveness results are insensitive to changes in the baseline incidence, relative risks, and costs of adverse events. This is because the majority of adverse events have a low baseline incidence rate, low cost of treatment, or relative risks for chemoprevention that do not deviate far from 1. This also explains why extending the persistence of relative risks associated with adverse events to 20 years after the end of treatment does not considerably change cost effectiveness.
Removing all chemotherapy costs for breast cancer treatment results a moderate increase in incremental costs per breast cancer case prevented. However, due to chemotherapy costs compising a relatively small proportion of total breast cancer treatment costs, these changes are unlikely to be sufficiently large to affect decisions. This demonstrates that, even in the extreme scenario that no patients with ER-postive breast cancer receive chemoprevention, results are robust.
Cost effectiveness results are also relatively sensitive to changes in estimated patient adherence, as a reduction in the number of adherent patients increases drug costs, but infers no benefits in breast cancer cases prevented.
Finally, using relative risks for breast cancer and adverse event rates derived from the RUTH trial results in a considerably lower incremental cost per breast cancer case prevented, indicating that there is significant uncertainty regarding both the effectiveness and the cost effectiveness of raloxifene.
Table 30One-way sensitivity analysis results – incremental cost per breast cancer case prevented for high-risk patients
| Scenario | Tamoxifen | Raloxifene | Anastrozole |
|---|---|---|---|
| Incidence of BC reduced by 50% | £17,316 | £54,990 | £7,116 |
| Incidence of BC increased by 100% | -£1,916 | £10,676 | -£5,266 |
| Treatment relative risks for breast cancer incidence set to lower 95% CI | £1,104 | £54,056 | -£3,542 |
| Treatment relative risks for breast cancer incidence set to upper 95% CI | £12,268 | £14,820 | £9,919 |
| Adherence set to 100% | £1,948 | £18,773 | -£3,168 |
| Adherence set to 25% | £9,967 | £38,615 | £3,385 |
| Incidence of adverse events reduced by 50% | £4,622 | £25,948 | -£1,090 |
| Incidence of adverse events increased by 100% | £4,607 | £24,206 | -£759 |
| Treatment relative risks of adverse events set to lower 95% CI | £4,048 | £26,395 | -£1,490 |
| Treatment relative risks of adverse events set to upper 95% CI | £5,359 | £24,642 | -£192 |
| Costs of treatment reduced by 50% | £2,178 | £18,213 | -£1,609 |
| Costs of treatment increased by 100% | £9,508 | £39,736 | £268 |
| Costs of adverse events reduced by 50% | £4,620 | £25,939 | -£1,089 |
| Costs of adverse events increased by 100% | £4,624 | £24,284 | -£773 |
| Costs of breast cancer reduced by 50% | £9,744 | £30,531 | £4,114 |
| Costs of breast cancer increased by 100% | -£5,624 | £15,099 | -£11,179 |
| Relative risks of adverse events persist for 20 years after end of treatment | £5,034 | £24,788 | -£848 |
| Breast cancer treatment is associated with no chemotherapy costs | £7,291 | £28,068 | £1,674 |
| Relative risks for raloxifene taken from the RUTH trial | - | £1040 | - |
Figure 3Tornado diagram of one way sensitivity analysis results for anastrozole (high-risk patients)
Mean probabilistic sensitivity analysis results are displayed in Table 31. These values show that mean probabilistic results are generally comparable to deterministic results.
Table 31Mean PSA results for high-risk post-menopausal patients
| Tamoxifen | Raloxifene | Anastrozole | |
|---|---|---|---|
| Incremental cost (versus no chemoprevention) | £100,699.78 | £249,453.45 | -£32,489.16 |
| Breast cancer cases prevented | 21 | 9 | 35 |
| Cost/BC case prevented | £4,758.28 | £28,367.96 | -£919.36 |
| QALYs required per BC case averted to be CE | 0.24 | 1.42 | -0.05 |
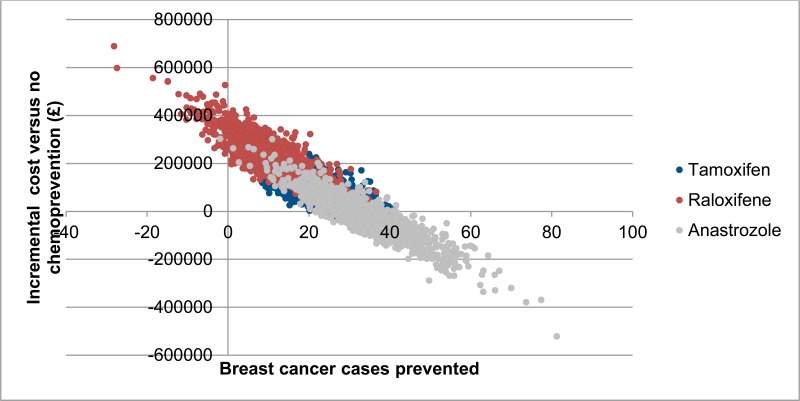
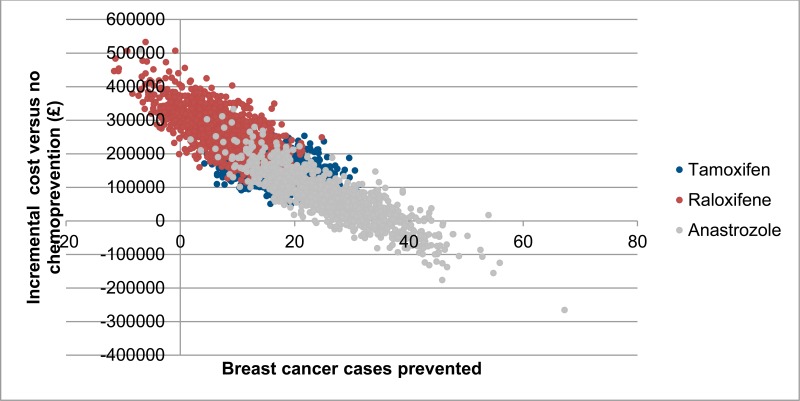
Costs and breast cancer cases prevented for each of the 1,000 probabilistic iterations are shown in Figure 4. There is considerable overlap in the results for all chemopreventive agents, but there is a trend towards lower incremental costs and higher number of breast cancer cases for anastrozole. Moreover, anastrozole is associated with a relatively high probability of being cost saving compared to no chemoprevention (59%). Probabilistic results also show that there is an observable negative correlation between number of breast cancer cases prevented and incremental cost. This is because treatment of breast cancer comprises a large proportion of costs in the model, meaning that iterations in which a higher number of breast cancer cases are prevented are also likely to result in higher cost savings.
Figure 4Probabilistic sensitivity analysis results – incremental cost and breast cancer cases prevented per 1,000 high-risk patients
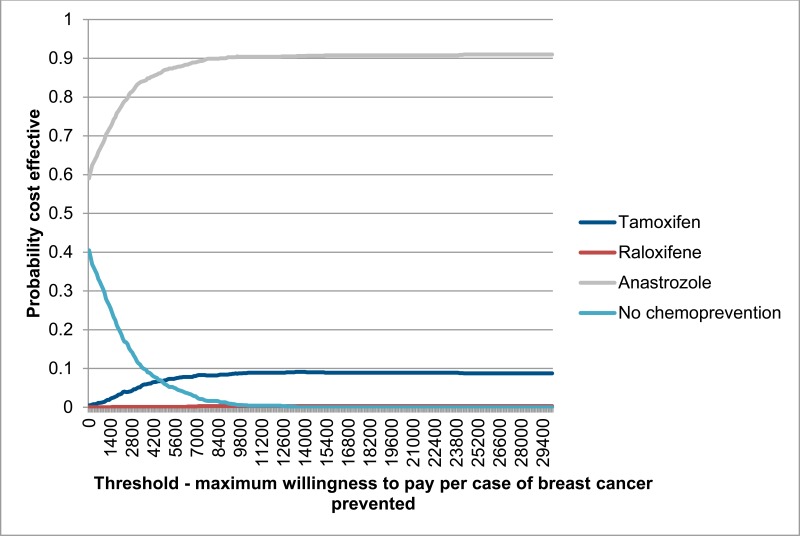
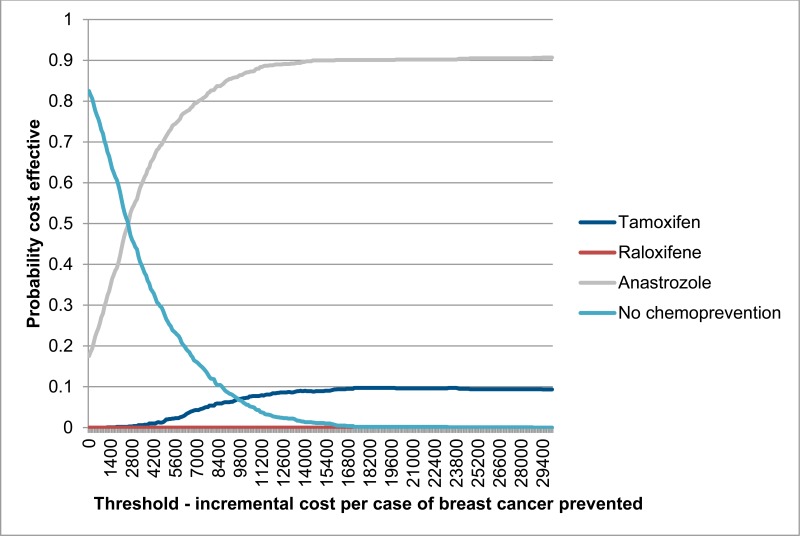
Figure 5 shows probabilistic results displayed as a cost effectiveness acceptability curve, plotting the probability of each comparator being the most cost effective option at a range of willingness to pay thresholds for the prevention of one case of breast cancer. Results show that at all thresholds anastrozole has the highest probability of being the most cost effective treatment. At a threshold of £20,000 per breast cancer case prevented, the probability of anastrozole being the most cost effective treatment is probabilities of anastrozole being the most cost effective treatment is 89%.
N.4. Results – moderate risk patients
N.4.1. Deterministic results
Base case cost and health outcome results for a cohort of 1,000 moderate risk patients are presented in Table 32. Numbers of adverse events were identical to those of the high-risk cohort, so are not tabulated again. Results show that, due to the lower baseline rate of breast cancer incidence, all chemoprevention treatments are associated with a lower number of prevented breast cancer cases, and higher incremental costs than in the high-risk cohort. Anastrozole is also associated with a positive incremental costs compared to no treatment, rather than being cost saving.
However, treatment with anastrozole still results in lower total costs and a higher number of breast cancer cases prevented than tamoxifen and raloxifene. This is reflected in Table 33, which for each chemopreventive agent shows the cost per breast cancer prevented and QALYs required per breast cancer case prevented to be cost effective at a £20,000 threshold. These results indicate that, although chemoprevention is not as cost effective for moderate risk patients, anastrozole is still expected to be cost effective, both compared to no chemoprevention and compared to other chemopreventive agents.
Table 32Cost and health outcome results per 1,000 moderate-risk patients
| No chemoprevention | Tamoxifen | Raloxifene | Anastrozole | |
|---|---|---|---|---|
| Cost outcomes | ||||
| Cost of breast cancer treatment | £1,100,825 | £942,318 | £1,029,747 | £839,066 |
| Total costs | £1,435,657 | £1,590,305 | £1,698,837 | £1,497,400 |
| Health outcomes | ||||
| Breast cancer cases | 113 | 97 | 106 | 87 |
Table 33Incremental cost effectiveness results compared to no treatment for moderate-risk patients
| Tamoxifen | Raloxifene | Anastrozole | |
|---|---|---|---|
| Cost/BC case prevented | £9,606 | £36,566 | £2,314 |
| QALYs required per BC case averted to be CE | 0.48 | 1.83 | 0.12 |
N.4.2. Sensitivity analysis
One-way sensitivity analysis results are displayed in Table 34. Results for anastrozole are displayed as a tornado diagram in Figure 6, to illustrate the magnitude of sensitivity across parameters. As with the high-risk cohort of patients, cost effectiveness results for moderate-risk patients are particularly sensitive to changes in parameters affecting the incidence or cost of breast cancer, and relatively insensitive to changes in parameters affecting incidence or cost of adverse events.
Table 34One-way sensitivity analysis results – cost per breast cancer case prevented per 1,000 moderate-risk patients
| Tamoxifen | Raloxifene | Anastrozole | |
|---|---|---|---|
| Incidence of BC reduced by 50% | £26,959 | £77,256 | £13,338 |
| Incidence of BC increased by 100% | £797 | £16,271 | -£3,358 |
| Treatment relative risks for breast cancer incidence set to lower 95% CI | £5,031 | £73,744 | -£1,032 |
| Treatment relative risks for breast cancer incidence set to upper 95% CI | £19,536 | £22,858 | £16,492 |
| Adherence set to 100% | £6,109 | £27,956 | -£561 |
| Adherence set to 25% | £16,601 | £53,787 | £8,064 |
| Incidence of adverse events reduced by 50% | £9,607 | £37,296 | £2,174 |
| Incidence of adverse events increased by 100% | £9,588 | £35,028 | £2,610 |
| Treatment relative risks of adverse events set to lower 95% CI | £8,856 | £37,878 | £1,648 |
| Treatment relative risks of adverse events set to upper 95% CI | £10,572 | £35,597 | £3,356 |
| Costs of treatment reduced by 50% | £6,409 | £27,227 | £1,490 |
| Costs of treatment increased by 100% | £16,001 | £55,246 | £3,962 |
| Costs of adverse events reduced by 50% | £9,604 | £37,284 | £2,176 |
| Costs of adverse events increased by 100% | £9,609 | £35,130 | £2,591 |
| Costs of breast cancer reduced by 50% | £14,529 | £41,504 | £7,220 |
| Costs of breast cancer increased by 100% | -£240 | £26,691 | -£7,497 |
| Relative risks of adverse events persist for 20 years after end of treatment | £10,146 | £35,786 | £2,493 |
| Breast cancer treatment is associated with no chemotherapy costs | £10,146 | £35,786 | £2,493 |
| Relative risks for raloxifene taken from the RUTH trial | - | £4,959 | - |
Figure 6Tornado diagram of one-way sensitivity analysis results for anastrozole (moderate-risk patients)
Mean results of the probabilistic sensitivity analysis are shown in Table 35. As with the results of the high-risk patient population, these values show that deterministic and mean PSA results are largely consistent.
Table 35Mean PSA results for moderate-risk post-menopausal patients
| Tamoxifen | Raloxifene | Anastrozole | |
|---|---|---|---|
| Incremental cost (versus no chemoprevention) | £155,098 | £266,093 | £67,762 |
| Breast cancer cases prevented | 16 | 7 | 26 |
| Cost/BC case prevented | £9,488 | £36,912 | £2,569 |
| QALYs required per BC case averted to be CE | 0.47 | 1.85 | 0.13 |
Figure 7 shows the results of the 1,000 probabilistic iterations for moderate risk patients plotted on a cost effectiveness plane. These results show a similar overall pattern to those of the high-risk cohort – anastrozole shows a trend towards lower costs and more cases of breast cancer prevented, though these is considerable overlap between results of different treatments. The results differ from those of the high-risk patients in that all treatments are, overall, less cost effective compared to no treatment. For this group of patients, anastrozole has an 18% probability of being cost saving compared to no treatment.
The cost effectiveness acceptability curve for moderate-risk patients (shown in Figure 8) reinforces these findings – at low thresholds no chemoprevention is the most likely option to be cost effective. However, at a relatively low threshold of £2,600 per breast cancer case prevented, anastrozole becomes the treatment with the highest probability of being cost effective.
Figure 7Probabilistic sensitivity analysis results – incremental cost and breast cancer cases prevented per 1,000 moderate-risk patients
N.5. Discussion
The results of this cost consequences analysis show that anastrozole is likely to be cost effective in preventing the incidence of breast cancer in high- and moderate-risk postmenopausal women. In the base case results, anastrozole shows improvements in both the number of breast cancer cases and total costs compared to tamoxifen and particularly raloxifene. Although results indicate that chemopreventive agents are more cost effective compared to no treatment in high-risk patients (both in terms of costs and number of breast cancer cases prevented), anastrozole is still associated with relatively a low ICER for the moderate-risk population (£2,314 per breast cancer case prevented).
Results indicate that, although associated with higher ICERs than anastrozole, tamoxifen is also likely to be cost effective (£4,621 and £9,606 per breast cancer case prevented for high- and moderate- risk patients, respectively). The cost effectiveness of raloxifene is less clear, with base case case results showing incremental costs per breast cancer case prevented of £25,387 and £36,566 for high- and moderate-risk patients. This would indicate that a gain of at least 1.27 and 1.83 QALYs, respectively, would have to be achieved per breast cancer case prevented for raloxifene to be cost effective at a threshold of £20,000. However, results of the sensitivity analysis in which data from the RUTH trial are used to inform the model indicate that raloxifene is associated with considerably lower ICERs (£668 and £4,473 for high- and moderate- risk patients). This implies that there is a high degree of uncertainty surrounding the cost effectiveness of raloxifene.
Results are robust in the sensitivity analysis, with anastrozole consistently showing the highest probability of being cost effective at any threshold in high risk patients, and the highest probability in moderate-risk patients at thresholds over £2,600 per breast cancer prevented and above.
While anastrozole results in a higher number of fractures (4 more fractures than no chemoprevention per 1,000 patients), this number is relatively low in absolute terms. Moreover, it is likely that the harm of these adverse events would be more than offset by the benefit gained in breast cancer cases prevented.
This analysis was associated with a number of limitations. First, the model assumes the same rate of mortality for healthy women and women with breast cancer, and that there is no risk of death associated with adverse events. As the primary health outcome of the analysis is breast cancer cases prevented, rather than QALYs, this assumption is unlikely to affect results considerably, although it may result in a small overestimation of the number of adverse events in treatment arms. Second, the proportion of patients discontinuing treatment after one year was based on expert assumption, as the true value is unknown. Third, the assumption was made that the benefits of chemoprevention persist over a patients’ lifetime, while relative risks of adverse events either return to baseline after the end of treatment or persist for five years after the end of treatment. While these assumptions are plausible, the true timeframe over which treatment effects persist is not precisely known (although sensitivity analysis has shown that results are generally robust to changes in these assumptions). Fourth, the assumption is made that chemoprevention affects the risks of all types of fractures equally whereas, in reality, it is likely that the incidence of fractures relating to osteoporosis are disproportionately affected. Finally, the analysis does not distinguish between different subsets of breast cancer. As chemoprevention only prevents ER-positive cancers, the incremental costs of prevented breast cancer cases are likely to differ from the average costs of breast cancer as a whole. While this assumption has been explored in sensitivity analysis by removing the cost of chemotherapy treatment (as ER-positive breast cancers are less receptive to chemotherapy), it is likely that the costs of treating breast cancer cases prevented by chemoprevention also differ in other ways.
In conclusion, although this analysis is limited to assessing the cost consequences, rather than the cost utility, of chemoprevention, it seems very likely from the results that anastrozole represens a cost effective means of preventing breast cancer in postmenopausal women, both compared to no treatment, and compared to currently recommended chemopreventive agents.
N.6. References
- Barrett-Connor, E., Mosca, L., Collins, P., Geiger, M.J., Grady, D., Kornitzer, M., McNabb, M.A. and Wenger, N.K., 2006. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. New England Journal of Medicine, 355(2), pp.125–137. [PubMed: 16837676]
- Brekelmans, C.T.M., Tilanus-Linthorst, M.M.A., Seynaeve, C., vd Ouweland, A., Menke-Pluymers, M.B.E., Bartels, C.C.M., Kriege, M., van Geel, A.N., Burger, C.W., Eggermont, A.M.M. and Meijers-Heijboer, H., 2007. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1-and non-BRCA1/2 families as compared to sporadic breast cancer cases. European Journal of Cancer, 43(5), pp.867–876. [PubMed: 17307353]
- Curtis, L., and Burns, A. 2015. Unit Costs of Health and Social Care 2015. Personal Social Services Research Unit. University of Kent.
- Cuzick, J., Sestak, I., Cawthorn, S., Hamed, H., Holli, K., Howell, A., Forbes, J.F. and IBIS-I Investigators, 2015. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. The Lancet Oncology, 16(1), pp.67–75. [PMC free article: PMC4772450] [PubMed: 25497694]
- Dolan, P. and Torgerson, D.J., 1998. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporosis International, 8(6), pp.611–617. [PubMed: 10326069]
- Electronic Drug Tarriff. Available online from: http://www
.nhsbsa.nhs .uk/PrescriptionServices/4940.aspx - Grann, V.R., Patel, P.R., Jacobson, J.S., Warner, E., Heitjan, D.F., Ashby-Thompson, M., Hershman, D.L. and Neugut, A.I., 2011. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast cancer research and treatment, 125(3), pp.837–847. [PMC free article: PMC3615889] [PubMed: 20644999]
- Hind, D., Ward, S., De Nigris, E., Simpson, E., Carroll, C. and Wyld, L., 2007. Hormonal therapies for early breast cancer: systematic review and economic evaluation. [PubMed: 17610808]
- NHS National Tariff Payment System 2016/17. Available online from: https://www
.gov.uk/government /publications /nhs-national-tariff-payment-system-201617 - Office for National Statistics – Mid-2015 Population Estimates for UK, England and Wales, Scotland and Northern Ireland. Available online from: http://www
.ons.gov.uk /peoplepopulationandcommunity /populationandmigration /populationestimates /datasets /populationestimatesforukenglandandwalesscotlandandnorthernireland - Office for National Satitistics – National life Tables: England and Wales. Available online from: http://www
.ons.gov.uk /peoplepopulationandcommunity /birthsdeathsandmarriages /lifeexpectancies /datasets /nationallifetablesenglandandwalesreferencetables - Peasgood, T., Ward, S.E. and Brazier, J., 2010. Health-state utility values in breast cancer. Expert review of pharmacoeconomics & outcomes research, 10(5), pp.553–566. [PubMed: 20950071]
- Scholes, S., Panesar, S., Shelton, N.J., Francis, R.M., Mirza, S., Mindell, J.S. and Donaldson, L.J., 2014. Epidemiology of lifetime fracture prevalence in England: a population study of adults aged 55 years and over. Age and ageing, 43(2), pp.234–240. [PubMed: 24231585]
- NICE Clinical Guideline 164: Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer *[ Surveillance report 2018 - Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer (2013) NICE guideline CG164] (ro:nicecg164surv1) *[ 2019 exceptional surveillance of familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer (NICE guideline CG164)] (ro:nicecg164surv2)
- Economic modelling report - Addendum to Clinical Guideline 164, Familial breast ...Economic modelling report - Addendum to Clinical Guideline 164, Familial breast cancer
- txid56723[orgn] AND "specimen-voucher RO24"[All Fields] (2)Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...