All rights reserved. This material may be freely reproduced for educational and not-for-profit purposes. No reproduction by or for commercial organisations, or for commercial purposes, is allowed without the express written permission of NICE.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Introduction
This Evidence Update identifies new evidence that might reinforce or generate future change to the practice laid out in the following reference guidance:
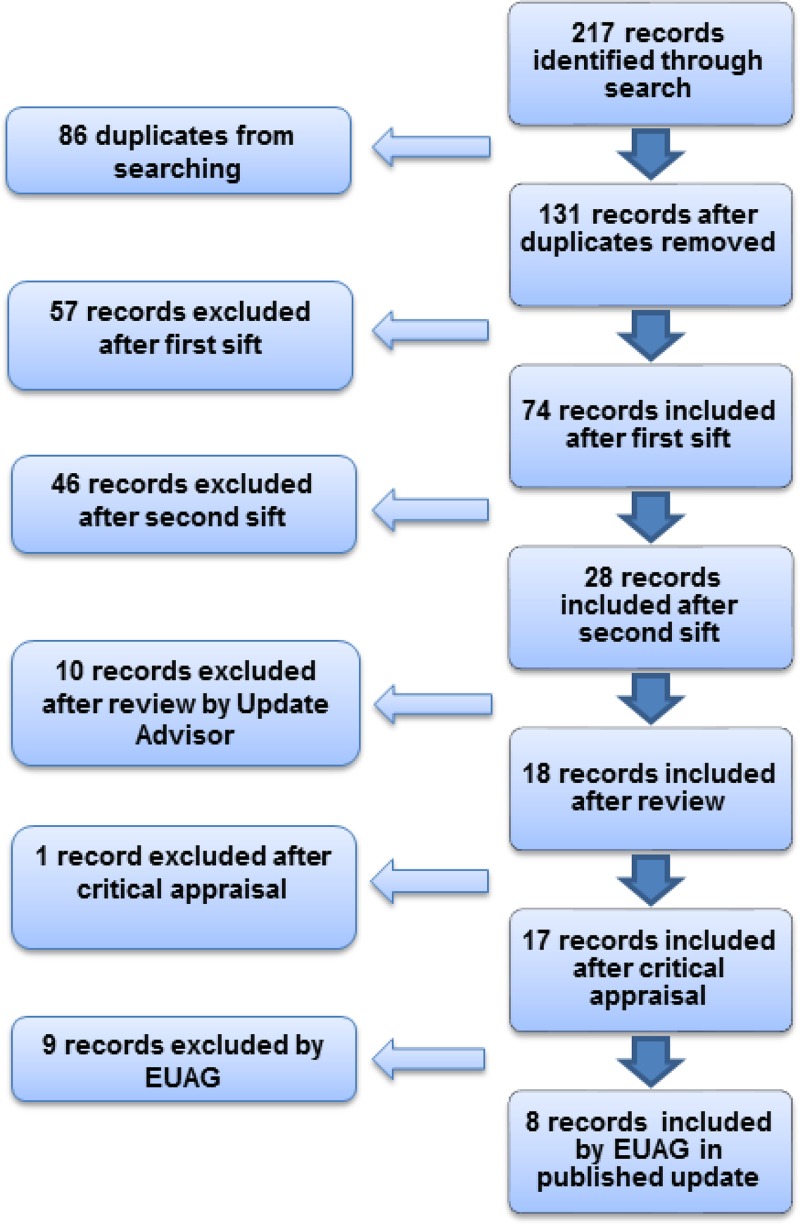
Just over 200 pieces of evidence were identified and assessed, of which 9 were selected for the Evidence Update (see Appendix A for details of the evidence search and selection process). An Evidence Update Advisory Group, comprised of subject experts, reviewed the prioritised evidence and provided a commentary.
Feedback
If you have any comments you would like to make on this Evidence Update, please email ku.shn.ecnedive@sutcatnoc
Key messages
The following table summarises what the Evidence Update Advisory Group (EUAG) decided were the key messages for this Evidence Update. It also indicates the EUAG’s opinion on whether new evidence identified by the Evidence Update reinforces or has the potential to generate future change to the current guidance listed in the introduction.
The relevant NICE guidance development centres have been made aware of this evidence, which will be considered when guidance is reviewed. For further details of the evidence behind these key messages and the specific guidance that may be affected, please see the full commentaries.
| Effect on guidance | ||
|---|---|---|
| Key message | Potential change | No change |
| Care for all babies | ||
| ✓ | |
| Measuring and monitoring bilirubin thresholds during phototherapy | ||
| Phototherapy | ||
| ✓ | |
| ✓ | |
| ✓ | |
| ✓ | |
| ✓ | |
| Stopping phototherapy | ||
| ✓ | |
| Exchange transfusion | ||
| ✓ | |
1. Commentary on new evidence
These commentaries analyse the key references identified specifically for the Evidence Update, which are identified in bold text.
1.1. Information for parents or carers
No new key evidence was found for this section.
1.2. Care for all babies
A randomised controlled trial (RCT) by Mishra et al. (2009) investigated whether transcutaneous bilirubinometry reduced the need to test for total serum bilirubin (TSB) compared with visual evaluation of neonatal jaundice. Infants of gestational age of 35 weeks or more were enrolled; the exclusion criteria were Rhesus haemolytic disease, neonatal intensive care needs for more than 24 hours, major congenital malformation, or previous phototherapy. Each infant (n = 617) underwent a standardised visual assessment (all performed by the same doctor), and then had transcutaneous bilirubinometry. A random method was then used for each infant to decide which result from the two assessments would be used to determine whether to test TSB.
The sample size needed to show a 40% reduction in blood sampling was stated as 492 in each group; however, only 303 infants were allocated to visual inspection, and only 314 to transcutaneous bilirubinometry. No reason was given for the under-recruitment. The number of blood samples to estimate TSB was lower in the transcutaneous bilirubinometry group compared with the visual inspection group (55 of 314 [17.5%] vs 80 of 303 [26.4%], relative risk = 0.66, 95% confidence interval [CI] 0.49 to 0.90, p = 0.008). Limitations of the study include that only one doctor conducted the visual assessment, which could have resulted in bias, and the visual assessment was not independently validated.
The results of this study support current clinical practice to not rely only on visual assessment of jaundice, as recommended in NICE CG98.
Key reference
- Mishra S, Chawla D, Agarwal R et al. (2009) Transcutaneous bilirubinometry reduces the need for blood sampling in neonates with visible jaundice. Acta Paediatrica 98: 1916–9. Abstract: www
.onlinelibrary.wiley .com/doi/10.1111/j .1651-2227.2009.01505 .x/abstract;jsessionid =B08B0B96CCF32B0C3FB73E15A59F9CA6 .d01t04 [PubMed: 19811459]
1.3. Management and treatment of hyperbilirubinaemia
No new key evidence was found for this section.
1.4. Measuring and monitoring bilirubin thresholds during phototherapy
Phototherapy
Donneborg et al. (2010) performed an RCT of 112 infants (gestational age ≥ 33 weeks) with non-haemolytic hyperbilirubinaemia, no other medical conditions, and receiving phototherapy, to compare supine positioning (53 infants) with alternation between supine and prone positioning every 3 hours (59 infants). The authors stated that this study was powered to detect a 5% difference in TSB in 24 hours. No significant difference was seen in reduction in TSB from baseline at 12 hours (supine = 32%, 94 micromol/l; alternating = 32%, 92 micromol/l; p = 0.86) or at 24 hours (supine = 50%, 145 micromol/l; alternating = 39%, 141 micromol/l; p = 0.66). Loose stool was the only side effect of phototherapy noted, with no difference between groups.
The authors also sent a questionnaire to all 41 neonatal departments in Denmark and Norway and found that two-thirds of neonatal departments routinely alternated the infant’s position during phototherapy.
By contrast with this practice, in the UK supine positioning only is advised to help to prevent sudden infant death syndrome, which is in line with recommendations in NICE CG98. The results of this study suggest no clinical effect of alternating position in phototherapy, so this evidence is unlikely to affect NICE CG98.
Key reference
Donneborg ML, Knudsen KB, Ebbesen F (2010) Effect of infants’ position on serum bilirubin level during conventional phototherapy. Acta Paediatrica 99: 1131–4 Abstract: www.onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2010.01885.x/abstract [PubMed: 20528799]
In an open-label multicentre RCT, Kumar at el (2010) enrolled 272 infants who developed hyperbilirubinaemia in the first 7 days after birth (gestational age ≥ 35 weeks; baseline exclusion of infants with haemolytic disease, sepsis, congenital malformation, and those needing exchange transfusion) to phototherapy with either light-emitting diode (LED; 142 babies) or compact fluorescent tube (CFT; 130 babies). This non-inferiority study was powered to show that the two light types resulted in similar duration of phototherapy (defined as up to 6 hours difference).
The median duration of phototherapy was similar between the groups (CFT = 26 hours, interquartile range 22–36 hours, LED = 25 hours, interquartile range 22–36 hours; p = 0.44). No statistically significant differences were seen in: rate of fall of TSB; ‘failure of phototherapy’ (defined as rising TSB, or more than 20 mg/dl [342 micromol/l]); exchange transfusions; or rebound in TSB needing phototherapy. Side effects were stated as rare and comparable between groups. However, seven babies in the CFT group underwent phototherapy for more than 60 hours, which was unexplained in the paper. Other limitations of the study include differences in the amount of body-surface area covered by the light sources, and possible differences in the calibration of bilirubinometers.
NICE CG98 currently recommends ‘blue-light’ phototherapy without specifying a particular light source such as LED or CFT. This study did not find a difference in effect between light sources, which supports the current recommendation.
Key reference
Kumar P, Murki S, Malik GK et al. (2010) Light-emitting diodes versus compact fluorescent tubes for phototherapy in neonatal jaundice: a multi-centre randomized controlled trial. Indian Pediatrics 47: 131–7. Full text: www.indianpediatrics.net/feb2010/131.pdf [PubMed: 19578227]
An RCT by Naderi et al. (2009) compared double with triple light sources for phototherapy in 40 otherwise healthy newborn babies with hyperbilirubinaemia (gestational age > 37 weeks). In the double phototherapy group, 20 infants had two fluorescent lamps placed 25 cm away; one above and one in line with the baby. In triple phototherapy, 40 infants had two lamps placed identically to the double phototherapy group, and a third lamp, also alongside the infant, at a distance of 35 cm. TSB did not differ between groups at admission (p= 0.170), or after 8 hours (p= 0.590), 16 hours (p= 0.760), and 24 hours (p= 0.370), although the report contained no information on how the study was powered. Additionally, no difference in mean length of hospital stay (p = 0.211) or in rate of decline in TSB was seen (p = 0.5). Side-effects were few and did not differ between groups.
In NICE CG98, multiple phototherapy is recommended for infants who do not respond to single phototherapy, and in those with very high or rapidly rising TSB. However, the number of light sources to use is not specified. This study provides limited evidence that three light sources are not better than two, which is unlikely to affect NICE CG98.
Key reference
Naderi S, Safdarian F, Mazloomi D et al. (2009) Efficacy of double and triple phototherapy in term newborns with hyperbilirubinaemia: the first clinical trial. Paediatric Neonatology 50: 266–9. Full text: www.download.journals.elsevierhealth.com/pdfs/journals/1875-9572/PIIS1875957209600759.pdf [PubMed: 20025139]
Silva et al. (2009) conducted an RCT of single or double phototherapy for non-haemolytic hyperbilirubinaemia in newborn babies who had no signs of sepsis or congenital malformation admitted to a single neonatal unit. A total of 37 infants underwent single phototherapy and 40 underwent double phototherapy (planned enrolment of 37 infants in each group with power to detect a 20% increase in the rate of bilirubin reduction). The mean decrease in bilirubin level after 24 hours was greatest in the double phototherapy group (mean ± standard deviation [SD] = 5.1 ± 2.2 mg/dl [87.2 ± 37.6 micromol/l]) compared with the single phototherapy group (4.3 ± 2.1 mg/dl [73.5 ± 35.9 micromol/l]), but this was not statistically significant (p = 0.18). No adverse events related to phototherapy were noted.
This study suggests no general advantage of double over single phototherapy. However, the authors note that the higher efficacy of double phototherapy might have reached significance if more patients had been included in the study.
NICE CG98 recommends multiple phototherapy (number of light sources not defined) for selected subgroups of babies who have not responded to single phototherapy or have rapidly rising or very high TSB. This study provides no new evidence for these special populations and is unlikely to affect NICE CG98.
Key reference
Silva I, Luco M, Tapia JL et al. (2009) Single vs. double phototherapy in the treatment of full-term newborns with nonhemolytic hyperbilirubinemia. Jornal de Pediatrica 85: 455–8. Full text: www.scielo.br/scielo.php?pid=S0021-75572009000500015&script=sci_arttext&tlng=en [PubMed: 19830352]
In a single-centre study, Sivanandan et al. (2009) conducted an RCT of phototherapy with or without white slings (curtains) hung from the sides of CFT equipment in 84 healthy infants (gestational age ≥ 37 weeks). Each infant was assigned to one of the two treatment groups (both n = 42); however the authors stated that detecting a difference in duration of phototherapy of 20% would require 75 participants in each group. No reason was given for the under-recruitment in this study. The duration of phototherapy did not differ between groups (slings: mean ± SD = 23.3 ± 12.9 hours; no-slings = 24.9 ± 15.4; p = 0.6, mean difference −1.67, 95% CI −8.00 to 4.66). Additionally, no differences in TSB levels were seen between groups (8 hour TSB, TSB at end of phototherapy, rate of TSB fall, absolute TSB fall, or percentage TSB fall).
NICE CG98 states ‘do not use white curtains routinely’. The conclusions of this under-powered study are aligned with that recommendation and no change would be anticipated.
Key reference
- Sivanandan S, Chawla D, Misra S et al. (2009) Effect of sling application on efficacy of phototherapy in healthy term neonates with non-hemolytic jaundice: a randomized controlled trial. Indian Pediatrics 46: 23–8. Full text: www
.indianpediatrics.net/jan2009/23.pdf [PubMed: 19179714]
Stopping phototherapy
A pilot study by Barak et al. (2009) (intended to determine the sample size needed for a larger trial) investigated whether phototherapy could safely be stopped earlier by using a higher TSB limit to indicate when to end treatment. Infants (n = 53, gestational age > 36 weeks) meeting criteria for phototherapy were randomly assigned to either the high threshold group (n = 26, phototherapy stopped when TSB was 17 micromol/l less than the threshold for starting phototherapy) or to the low threshold group (n = 27, stopping at 51 micromol/l below phototherapy threshold). One infant did not complete follow-up and three did not have a second rebound bilirubin check 24 hours after stopping phototherapy.
The duration of phototherapy was significantly shorter in the high-threshold group than in the low-threshold group (mean ± SD = 22 ± 13 hours vs 27 ± 12 hours respectively, p = 0.031). Length of hospital stay was also significantly reduced (84 ± 29 hours vs 94 ± 24 hours respectively, p = 0.05). Additionally, no difference in the need for further phototherapy was seen between groups (5 infants in each group, p = 0.58).
Within the limitations of this small, non-blinded, single-centre, single-doctor study, the results suggest that a higher limit of TSB for ceasing phototherapy may be safe and effective. If replicated in a larger study, such evidence may be a consideration for future reviews of NICE CG98. Further data from a definitive study are needed.
Key reference
- Barak M, Berger I, Dollberg S et al. (2009) When should phototherapy be stopped? A pilot study comparing two targets of serum bilirubin concentration. Acta Paediatrica 98; 277–81. Abstract: www
.onlinelibrary.wiley .com/doi/10.1111/j .1651-2227.2008.01015.x/abstract [PubMed: 19143666]
1.5. Factors that influence the risk of kernicterus
No new key evidence was found for this section.
1.6. Formal assessment for underlying disease
No new key evidence was found for this section.
1.7. Care of babies with prolonged jaundice
No new key evidence was found for this section.
1.8. Intravenous immunoglobulin
No new key evidence was found for this section.
1.9. Exchange transfusion
An RCT by Shahian et al. (2010) was conducted to evaluate an albumin infusion given before exchange transfusion in 50 babies (gestational age > 37 weeks) with non-haemolytic hyperbilirubinaemia (TSB ≥ 25 mg/dl [427.5 micromol/l]) who had not responded to ‘intensive’ phototherapy. The study was powered to detect a difference in TSB of at least 6 mg/dl (102.6 micromol/l) 12 hours after the exchange transfusion. In the albumin group, 25 infants received an albumin infusion of 1 mg/kg, 1 hour before exchange transfusion, and in the control group, exchange transfusion was done without prior albumin infusion.
TSB was significantly lower in the albumin group compared with the control group at both 6 hours (mean ± SD = 14.4 ± 1.7 mg/dl [246.2 ± 29.1 micromol/l] vs 21.7 ± 3.2 mg/dl [371.1 ± 54.7 micromol/l] respectively, p < 0.001), and at 12 hours (8 ± 1.5 mg/dl [136.8 ± 25.7 micromol/l] vs 16.1 ± 2.1 mg/dl [275.3 ± 35.9 micromol/l] respectively, p < 0.001). Albumin levels were similar between groups at baseline and at 24 hours. Duration of phototherapy was also significantly shorter in the albumin group (8.6 ± 2.4 hours vs 25 ± 8.2 hours, p < 0.001). No infant in the albumin group needed a second exchange transfusion but four infants in the control group did.
This study of 50 infants is small, but exchange transfusions for severe hyperbilirubinaemia in otherwise healthy near-term or term babies are rare (around 25 cases per year in the UK (Manning et al. 2007). This single-centre study provides some evidence to support albumin infusion and because the potential population for a UK study is so small, conducting a larger study would be difficult. However, the definition of ‘intensive’ phototherapy used in this trial may not represent the high irradiance of multiple phototherapy used in the UK, so fewer such babies in the UK might progress to needing exchange transfusion.
NICE CG98 lists albumin priming before exchange transfusion as a ‘do not use’ intervention. However, the full version of NICE CG98 lists only one small study from 1976 addressing the use of albumin before exchange transfusion and the Guideline Development Group therefore stated that albumin priming could not be recommended because of an ‘absence of evidence’. The evidence from Shahain et al. (2010) may be a consideration in future reviews of NICE guidance.
Key reference
- Shahian M, Moslehi MA et al. (2010) Effect of albumin administration prior to exchange transfusion in term neonates with hyperbilirubinaemia – a randomized controlled trial. Indian Pediatrics 47: 241–4. Full text: www
.indianpediatrics.net/mar2010/241.pdf [PubMed: 19578230]
Supporting reference
- Manning D, Todd P, Maxwell M et al. (2007) Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Archives of Disease in Childhood Fetal and Neonatal Edition 92: F342–6. Full text: www
.fn.bmj.com/content/92/5/F342 [PMC free article: PMC2675352] [PubMed: 17074786]
1.10. Other therapies
No new key evidence was found for this section.
2. New evidence uncertainties
In developing this Evidence Update EUAG acknowledged that evidence around the topic of neonatal jaundice may be limited and that measuring outcomes in small trials can be complex. Accordingly, the need for further research was outlined in the document.
No new evidence uncertainties were identified during the Evidence Update process, however current uncertainties for neonatal jaundice can be found in the NHS Evidence UK Database of Uncertainties about the Effects of Treatments (DUETs) at www.library.nhs.uk/duets/ and in the NICE research recommendations database at www.nice.org.uk/research/index.jsp?action=rr
Appendix A. Methodology
Scope
The scope of this Evidence Update is taken from the scope of the reference guidance:
- Neonatal jaundice. NICE clinical guideline 98 (2010). Available from www.nice.org.uk/guidance/CG98
Searches
The literature was searched to identify studies and reviews relevant to the scope. Searches were conducted of the following databases, covering the dates 1 June 2009 (the end of the search period of the most recent NICE clinical guideline) to 1 November 2011:
- CINAHL
- Cochrane Database of Systematic Reviews – Cochrane Library
- Cochrane Central Register of Controlled Trials
- Embase
- MEDLINE
- Database of Abstracts of Effects
Table 1 provides details of the search strategy used, which was adapted to search the other databases listed above. Several search strategies were used in the original guideline to answer specific clinical questions (see Appendix I of the full guideline). The search for this Evidence Update focused on management so no searches were done for diagnosis and assessment studies (for example diagnostic test accuracy studies). A highly specific search strategy was developed to provide a focused set of results, which was thoroughly tested to ensure that the comprehensiveness of the results was not compromised. The search strategy was used in conjunction with validated Scottish Intercollegiate Guidelines Network search filters for RCTs and systematic reviews (www.sign.ac.uk/methodology/filters.html).
Figure 1 provides details of the evidence selection process. The long list of evidence excluded after review by the Update Adviser (the chair of the EUAG), and the full search strategies, are available on request from ku.shn.ecnedive@sutcatnoc
Table 1MEDLINE search strategy (adapted for individual databases)
| 1 | exp Jaundice, Neonatal/ |
| 2 | Hyperbilirubinemia, Neonatal/ |
| 3 | or/1-2 |
| 4 | exp Infant, Newborn/ |
| 5 | newborn?.ti,ab. |
| 6 | neonate?.ti,ab. |
| 7 | neonatal$.ti,ab. |
| 8 | preterm.ti,ab. |
| 9 | or/4-8 |
| 10 | Hyperbilirubinemia/ |
| 11 | exp Jaundice/ |
| 12 | hyperbilirubin$.ti,ab. |
| 13 | bilirubin?emia?.ti,ab. |
| 14 | jaundice?.ti,ab. |
| 15 | Kernicterus/ |
| 16 | kernicterus.ti,ab. |
| 17 | (bilirubin adj2 encephalopath$).ti,ab. |
| 18 | (icterus adj2 neonatorum).ti,ab. |
| 19 | or/10-18 |
| 20 | 9 and 19 |
| 21 | or/3,20 |
Appendix B. The Evidence Update Advisory Group and NHS Evidence project team
Evidence Update Advisory Group
The Evidence Update Advisory Group is a group of subject experts who review the prioritised evidence obtained from the literature search and provide the commentary for the Evidence Update.
- Professor Neil Marlow – ChairProfessor of Neonatal Medicine, University College London
- Dr Kevin IvesConsultant Neonatologist, Oxford John Radcliffe Hospital NHS Trust
- Dr Donal ManningConsultant Paediatrician, Wirral Hospital University Foundation NHS Trust
- Dr Janet M RennieConsultant in Neonatal Medicine, University College London Hospitals
- Dr Ryan WatkinsConsultant Neonatologist, Brighton and Sussex University Hospitals NHS Trust
NHS Evidence project team
- Alan LovellEvidence Hub Manager
- Danielle WorsterInformation Specialist
- Lynne KincaidEditor
Footnotes
- 1
NICE-accredited guidance is denoted by the accreditation symbol

Evidence Updates provide a regular, often annual, summary of selected new evidence published since the literature search was last conducted for the accredited guidance they update. They reduce the need for individuals, managers and commissioners to search for new evidence and inform guidance developers of new evidence in their field. In particular, Evidence Updates highlight any new evidence that might reinforce or generate future change to the practice described in the most recent, accredited guidance, and provide a commentary on the potential impact. Any new evidence that may impact current guidance will be notified to the appropriate NICE guidance development centres. For contextual information, Evidence Updates should be read in conjunction with the relevant clinical guideline, available from the NHS Evidence topic page (www.evidence.nhs.uk/topic/jaundice-newborn). NHS Evidence is a service provided by NICE to improve use of, and access to, evidence-based information about health and social care.
Evidence Updates do not replace current accredited guidance and do not provide formal practice recommendations.
National Institute for Health and Clinical Excellence
Level 1A
City Tower
Piccadilly Plaza
Manchester M1 4BT
- Neonatal jaundiceNeonatal jaundice
- SRP161717 (1)SRA
- T-cell receptor gamma, partial [Danio rerio]T-cell receptor gamma, partial [Danio rerio]gi|65333957|gb|AAY42318.1|Protein
- Saprolegnia delica strain ABDN_72 18S ribosomal RNA gene, partial sequence; inte...Saprolegnia delica strain ABDN_72 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequencegi|536725642|gb|KF420265.1|Nucleotide
- ceroid-lipofuscinosis neuronal protein 5 precursor [Rattus norvegicus]ceroid-lipofuscinosis neuronal protein 5 precursor [Rattus norvegicus]gi|300794450|ref|NP_001178618.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...