NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Evidence reviews for clinical and cost effectiveness of non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA alone or in combination for people with a probability of mediastinal malignancy
Review questions
RQ 1.1: What is the clinical and cost effectiveness of using non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA as the first invasive test for people with a probability of mediastinal malignancy?
RQ 1.2: What is the clinical and cost-effectiveness of EBUS-TBNA alone, EUS-FNA alone or EBUS-TBNA and EUS-FNA in combination compared with surgical staging to diagnose and/or stage lung cancer?
Introduction
Since publication of the existing guideline CG121, a randomised controlled trial (RCT) suggested that the use of endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) and occasional use of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in the diagnosis of lung cancer enabled:
- faster treatment decisions compared to conventional diagnosis and staging;
- fewer invasive investigations per person compared to conventional diagnosis and staging;
- improved survival (all-cause hazard ratio) compared to conventional diagnosis and staging in a post-hoc analysis (Navani 2015).
Conventional diagnosis and staging included CT-guided biopsy and non-ultrasound-guided TBNA. Another RCT suggested that EBUS-TBNA in combination with EUS-FNA is more effective and less expensive than standard surgical staging alone (Annema 2010, Sharples 2012). Therefore, the purposes of this review are to:
- Determine the effectiveness of using non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA as the first invasive test for people with a probability of mediastinal malignancy.
- Determine the effectiveness of EBUS-TBNA alone, EUS-FNA alone or EBUS-TBNA and EUS-FNA in combination compared with surgical staging to diagnose and/or stage lung cancer.
Table 1PICO table
| Population | Patients with suspected/ confirmed lung cancer (Pre-diagnosis and CT std. clinical evaluation) |
|---|---|
| Interventions |
|
| Comparator | The gold standard investigation (histological/ cytological confirmation and pathological TNM - Or follow up period adequate to confirm outcome - Normally pathology from surgical resection but could be another technique in specified circumstances. |
| Outcomes |
|
Table 2PICO table
| Population | Patients with suspected/ confirmed lung cancer (Pre-diagnosis and CT std. clinical evaluation) |
|---|---|
| Interventions |
|
| Comparator |
|
| Outcomes |
|
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in appendix A, and the methods section in appendix B. In particular, the minimally important differences (MIDs) used in this review are summarised in appendix B.
Declarations of interest were recorded according to NICE’s 2018 conflicts of interest policy.
Clinical evidence
Included studies
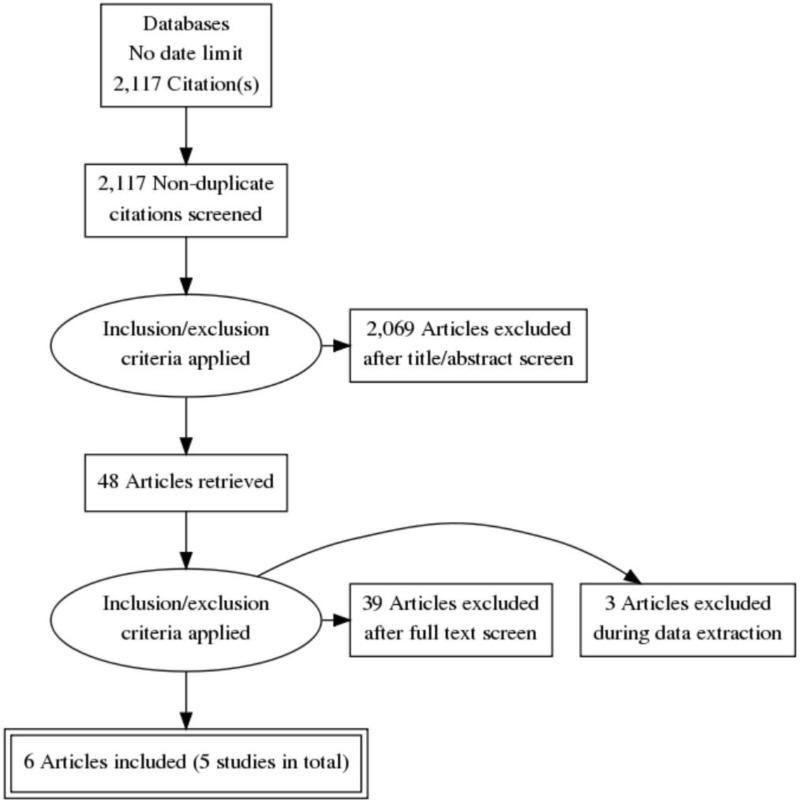
This review was conducted as part of a larger update of the NICE Lung cancer: diagnosis and management guideline (CG121). A systematic literature search for RCTs and systematic reviews with no date limit yielded 2,117 references.
Papers returned by the literature search were screened on title and abstract, with 48 full-text papers ordered as potentially relevant systematic reviews or RCTs. RCTs were excluded if they did not meet the criteria of enrolling patients with suspected or confirmed lung cancer.
Six papers representing 5 unique RCTs were included after full text screening. Three of these were cross-sectional diagnostic RCTs: Annema 2010 (n=241, follow-up period 1 year), Kang 2014 (n=160, follow-up period 3–5 days), Tournoy 2008 (n=40 days, median follow-up period 2 nights). Two studies were interventional RCTs: Larsen 2005 (n=104, median follow-up period 1.3 and 1.4 years for each arm respectively) and Navani 2015 (n=132, median follow-up period 503 days and 312 days for each arm respectively). Multiple papers reporting results of the same study were identified and collated, so that each study rather than individual reports was the unit of interest in the review, therefore there were 5 unique studies. The following reference standards were used - for benign results: surgical confirmation and for malignant results: pathology.
For the search strategy, please see appendix C. For the clinical evidence study selection flowchart, see appendix D. For the full evidence tables and full GRADE profiles for included studies, please see appendix E and appendix F.
Excluded studies
Details of the studies excluded at full-text review are given in appendix G along with a reason for their exclusion.
Summary of clinical studies included in the evidence review
Five randomised controlled studies were included in this review. The following studies met the inclusion criteria for RQ 1.1: Larsen 2005 and Tournoy 2008. The following study met the inclusion criteria for RQ 1.2: Annema 2010. The following studies met the inclusion criteria for both RQ 1.1 and 1.2: Kang 2014 and Navani 2015.
Study locations
One randomised controlled study was from the UK (Navani 2015), 1 was from the Netherlands, Belgium and the UK (Annema 2010), 1 was from South Korea (Kang 2014), 1 was from Denmark (Larsen 2005) and 1 was from Belgium (Tournoy 2008).
Outcomes and sample sizes
The reported outcomes with extractable data were diagnostic performance (preferably sensitivity, diagnostic negative predictive value, staging sensitivity), mortality, in-patient admission, pneumothorax, other complications, patient acceptability, anxiety and psychological problems, time to treatment decision, time to diagnosis and staging, number of investigations per person, number of outpatient attendances per person and quality of life. Additional non-protocol outcome measures were recorded. Rather than exclude them, the committee decided that they were worthy of consideration. The non-protocol outcome measures were: number of avoidable thoracotomies and recurrence during a specified follow-up time. The committee wanted to know the number of avoidable thoracotomies because unnecessary thoracotomies can be distressing for patients. Recurrence during a specified follow-up time was useful for the economic modelling. The sample sizes ranged from 40 participants to 257 across studies.
See full evidence tables and GRADE profiles Appendix E and Appendix F.
Quality assessment of clinical studies included in the evidence review
See appendix F for full GRADE tables.
Economic evidence
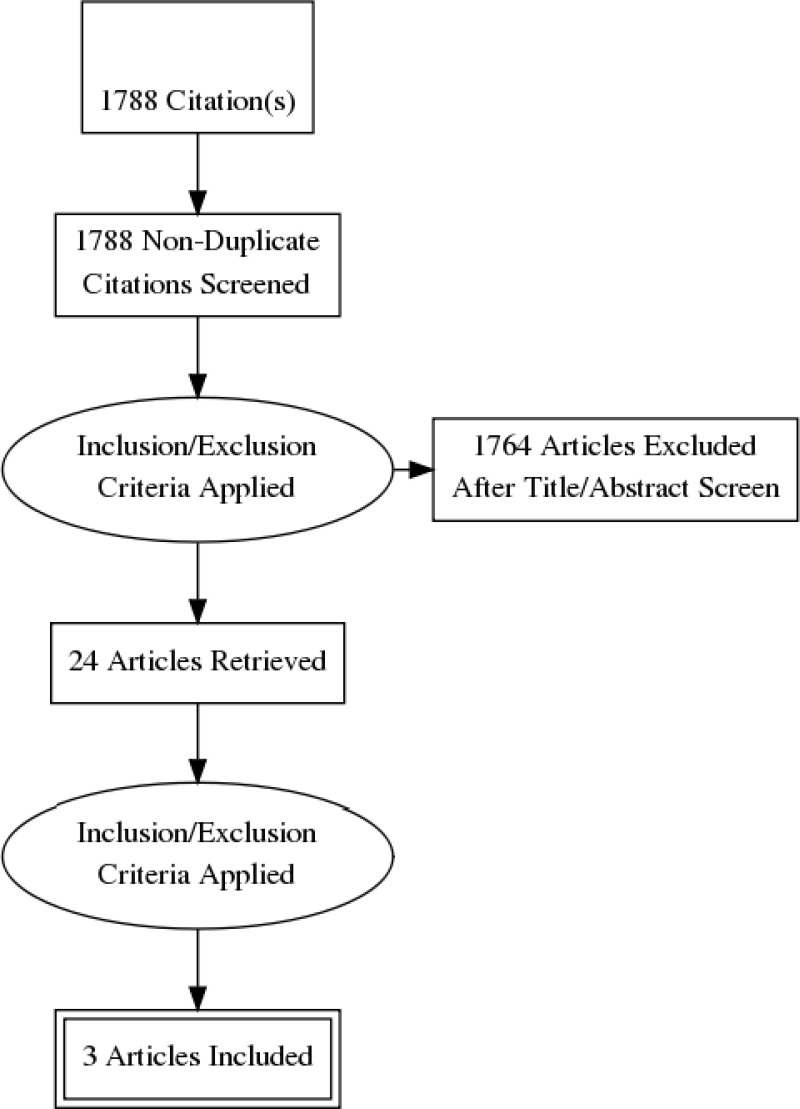
Standard health economic filters were applied to the clinical search for this question, and a total of 1,788 citations was returned. Details of the literature search are provided in Appendix C. Following review of titles and abstracts, 24 full-text studies were retrieved for detailed consideration. One relevant cost–utility analysis, 1 health economics paper with a survival model and one health economics paper with an influence diagram were identified. Therefore 3 studies were included in this review.
EBUS-FNA plus EBUS-TBNA vs surgical staging
Sharples et al. (2012) conducted a cost-utility analysis alongside a 6-month RCT (ASTER) in the UK, Belgium and the Netherlands (n=247). Patients were eligible for the trial if they had known/suspected non-small cell lung cancer (NSCLC), with suspected mediastinal lymph node involvement; otherwise eligible for surgery with curative intent; clinically fit for endosonography and surgery; and had no evidence of metastatic disease. Patients were excluded from the trial if they had previous lung cancer treatment; concurrent malignancy; uncorrected coagulopathy; or were not suitable for surgical staging. One hundred and twenty three patients were randomised to endosonography followed by surgical staging if no nodal metastases were found at endosonography, whilst 118 patients were randomised to surgical staging alone. The primary research objective of the study was to determine whether endosonography is better than standard surgical staging techniques in terms of sensitivity, diagnostic accuracy and negative predictive value for diagnosing and staging the mediastinum in lung cancer. A secondary research objective was to conduct a comparative cost analysis of the diagnostic strategies of the two trial arms.
Endosonography in this study was EBUS-TBNA combined with EUS-FNA. Surgical staging was performed by (video) mediastinoscopy, left anterior mediastinoscopy or video-assisted thoracoscopy or combination.
The authors’ base case adopted a UK NHS perspective. Resource use was collected in terms of numbers of procedures done, (surgical, radiotherapy, chemotherapy) treatments administered, hospital and hospice stays. Costs were taken from the Department of Health (DoH) NHS reference costs 2008–2009. Cost estimates for endosonography were estimated by Papworth Hospital finance department. The price year was 2008–2009.
Utility was measured using the EQ-5D at baseline, end of staging, 2 months and 6 months, using a UK tariff.
Bayesian parametric modelling was used to estimate final expected costs and quality-adjusted life-years (QALYs) while simultaneously estimating missing data based on randomisation group, centre and stage.
Base-case results for patients for whom complete information on trial costs and QALYs were available (endosonography n=58, surgical staging n=56) are shown in Table 3.
Table 3
Costs and effects from Sharples et al. (2012).
Endosonography followed by surgical staging compared to surgical staging alone was £972 cheaper and produced 0.00652 more QALYs, rending endosonography followed by surgical staging as a dominant strategy. (Strategies that are dominant cost less and are more effective than their comparator.)
Because of the very small QALY difference, the authors concluded that an ICER could not be reliably estimated but in the probabilistic sensitivity analysis, 63% of bootstrapped samples showed endosonography dominated (which means it was less expensive and produced more benefit compared to) surgical staging and endosonography was cost-effective at a threshold of £30,000/QALY in 99.9% of samples.
EBUS-TBNA vs conventional approaches
Navani et al. (2015) conducted a cost-effectiveness analysis alongside LUNG-BOOST, an open-label, multicentre, pragmatic, randomised controlled trial. Patients were recruited from 6 centres in the UK, who were suspected to have stage I to IIIA lung cancer on the basis of CT scans of the neck, thorax, and upper abdomen were eligible for trial entry. For inclusion into the trial, patients had to be aged at least 18 years and fit enough to undergo thoracotomy and lung resection. Exclusion criteria were significant concurrent malignant disease or any condition or concurrent medicine that contraindicated EBUS-TBNA or mediastinoscopy. Patients with known extrathoracic malignant disease, supraclavicular lymphadenopathy, or pleural effusion were also excluded. Of the 133 RCT participants, 66 participants were randomised to endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), whilst 67 patients were randomised to conventional diagnosis and staging (CDS).
The primary endpoint was the time from first outpatient appointment with the respiratory specialist to treatment decision by the multidisciplinary team, after completion of the diagnosis and staging procedures. Analysis took a UK NHS perspective.
Effectiveness in this study was measured using mean time to treatment decision from the first outpatient appointment with the respiratory specialist, using hazard ratios. This is in contrast to the NICE reference case, where effects are measured in QALYs. Unit costs were obtained from NHS reference costs, NICE 2011 lung cancer guideline, and a published study; these were multiplied by the resource use and summed across all resource items. The price year was 2010–2011.
Lung cancer was diagnosed in 57 (86%) patients in the CDS group and 50 (76%) in the EBUS group (p=0·196), and clinical staging did not differ significantly between the groups in patients with non-small-cell lung cancer.
The median time-to-treatment decision was longer after CDS (29 days [95% CI 23–35]), than after EBUS (14 days [14–15]; HR 1·98, 95% CI 1·39–2·82, p<0·0001) in the intention-to-diagnose population. Therefore, patients in the EBUS group of the trial were likely to receive a treatment decision twice as fast as patients in the CDS group. A greater proportion of patients had diagnosis and staging completed by 14 days in the EBUS group than in the CDS group (35 [53%] vs 8 [12%], p<0·0001). In the subset of patients with non-small-cell lung cancer, initial EBUS-TBNA resulted in a shorter time-to-treatment decision of 15 days (95% CI 14–16), compared with 30 days (95% CI 23–34) in the CDS group (HR 2·09, 95% CI 1·38–3·15, p=0·0002).
In a post-hoc analysis, the median survival of patients with non-small-cell lung cancer in the EBUS group of 503 days (95% CI 312–715) was longer than the median survival in the CDS group of 312 days (95% CI 231–488; HR 0·60, 0·37–0·98, p=0·0382;). An exploratory analysis of lung cancer patients who underwent surgery suggested that postoperative survival was better in the EBUS group than in the CDS group.
For diagnosis and staging, EBUS-TBNA was found to cost £2,407 (SD £180.50) whilst CDS was found to cost £2,348 (SD £192.20). This represents an incremental cost for EBUS-TBNA of £59 (95% CI −£463 to £581). Mean initial treatment costs per patient in those diagnosed with lung cancer were £4452 (£180·00) and £4261 (£257·90), respectively (difference £191, 95% CI −447 to 829).
The results from the trial suggest that routine use of EBUS-TBNA as an initial investigation after a staging CT for suspected lung cancer scan results in a faster treatment decision, with fewer investigations at no significant difference in cost, and, in post-hoc analysis, seems to improve survival, compared with conventional diagnosis and staging methods.
Influence Diagram model to determine optimal sequence of tests for mediastinal staging of lung cancer
Luque et al. (2016) created an influence diagram (ID) model for a Spanish public healthcare system to determine the optimal sequence of tests for the mediastinal staging of non-small cell lung cancer (NSCLC) by considering sensitivity, specificity, and the economic cost of each test. This was stated to be important, as correct staging of the disease as early as possible helps to determine which patients may benefit from surgery and, in turn, to avoid dangerous, painful, and unnecessary surgery when metastasis has already occurred.
The model assumed that all patients first had a computed tomography (CT) scan, and then could have a transbronchial needle aspiration (TBNA), positron emission tomography (PET), endobronchial ultrasound (EBUS), endoscopic ultrasound (EUS), or a mediastinoscopy (MED) in various sequences.
IDs are a new modelling method that makes use of advanced statistical and computer science techniques to handle problems where the numbers of sequential decisions and probabilities are too large to be easily evaluated by a conventional decision tree. An auxiliary Bayesian network was built that could handle every possible sequence of tests as well as patients’ decisions and outcomes.
The ID model was evaluated twice, first without considering economic costs, and then considering cost effectiveness using a willingness-to-pay of €30,000 per QALY, the shadow threshold estimated for the Spanish health system. The authors performed several types of sensitivity analysis to study the effect of the uncertainty in the numerical parameters of the model.
The authors reported the optimal strategies using the two different criteria. When considering only effectiveness, a positive computed tomography (CT) scan should be followed by a transbronchial needle aspiration (TBNA) and an endobronchial ultrasound (EBUS). Endoscopic ultrasound (EUS) and mediastinoscopy are then used to either confirm negative findings or when the results of two tests are contradictory. When the CT scan is negative, a positron emission tomography (PET) and EBUS are performed. EUS and mediastinoscopy are used in the case of negative or contradictory results.
Economic model conducted for the 2011 NICE lung cancer guideline
The economic model built for the 2011 NICE lung cancer guideline included a range of diagnosis and staging strategies for people with an intermediate probability of mediastinal malignancy.
The model was a decision tree comprising 27 possible strategies which included one or several of neck ultrasound, PET-CT, conventional TBNA, EBUS TBNA and mediastinoscopy in various orders. Patients at each final end point entered a two state Markov model comprising survival and death states.
Disease prevalence, distribution of treatment options and survival estimates were drawn from registry data and expert opinion. Costs were drawn from standard NHS sources and resource use was drawn from expert opinion. The test accuracy data was drawn from expert opinion. Utility data were drawn from published literature and expert opinion.
The model concluded that PET-CT followed by conventional TBNA was the optimal strategy. This was due to the combination of high sensitivity and low cost parameters used within the model for these tests. The model was reasonably robust with regards to deterministic sensitivity analysis but no probabilistic sensitivity analysis was conducted. The guideline committee concluded that while the model had a number of limitations, the results provided them with useful information when developing a diagnostic testing algorithm.
Evidence statements
EUS-FNA followed by EBUS-TBNA vs straight to surgical staging
Effectiveness data
Low to moderate-quality evidence from 1 RCT reporting data on 241 people with suspected N2 or N3 mediastinal lymph node involvement found that there was a greater number of avoidable thoracotomies in people offered EUS-FNA followed by EBUS-TBNA compared to people who went straight to surgical staging. However, there was no difference in the number of people experiencing a pneumothorax, the total number of complications, quality of life at 6 months, or the number of people who died between staging and 6 months later.
Diagnostic accuracy data
Moderate-quality evidence from 1 RCT reporting data on 241 people with suspected N2 or N3 mediastinal lymph node involvement found the sensitivity of EUS-FNA followed by EBUS-TBNA was 93.3% and the negative predictive value was 92.7% (with a prevalence of 53.7%). The sensitivity of the straight to surgical staging arm was 78.3% and the negative predictive value was 85.3% (with a prevalence of 44.1%).
Bronchoscopy, EBUS-TBNA then EUS(B)-FNA if necessary vs bronchoscopy, EUS(B)-FNA then EBUS-TBNA if necessary
Effectiveness data
Very low-quality evidence from 1 RCT reporting data from 160 people with histologically confirmed or strongly suspected, potentially operable non-small cell lung cancer found that the data could not differentiate the number of people experiencing a pneumothorax or patient tolerance 3–5 days after the interventions.
Diagnostic accuracy data
Low-quality evidence from 1 RCT reporting data from 148 people with histologically confirmed or strongly suspected, potentially operable non-small cell lung cancer found that the sensitivity of bronchoscopy, EBUS-TBNA, then EUS(B)-FNA was 85.3% and the negative predictive value was 88.0% (with a prevalence of 45.9%). The sensitivity of bronchoscopy, EUS (B)-FNA, then EBUS-TBNA was 90.4% and the negative predictive value was 95.2% (with a prevalence of 33.8%). For the bronchoscopy, EBUS-TBNA, then EUS (B)-FNA arm, the sensitivity of EBUS-TBNA was 81.4% and its negative predictive value was 86.2% (with a prevalence 45.9%). In the bronchoscopy, EUS (B)-FNA, then EBUS-TBNA arm, the sensitivity of EUS(B)-FNA was 59.6% and its negative predictive value was 82.5% (with a prevalence of 33.8%).
Mediastinoscopy + EUS-FNA vs mediastinoscopy + EUS-FNA only if CT shows invasion adjacent to the oesophagus
Effectiveness data
High-quality evidence from 1 RCT reporting data from 104 people with suspected or diagnosed lung cancer after CT/PET, bronchoscopy, TBNA/TTNA, lung function tests and general examination found that there was a greater number of avoidable thoracotomies in the mediastinoscopy + EUS-FNA arm compared to the mediastinoscopy + EUS-FNA only if CT shows invasion adjacent to the oesophagus arm. However, moderate-quality data could not differentiate between complications, recurrence or death.
EBUS-TBNA (or EUS-FNA) vs conventional diagnosis and staging (bronchoscopy or CT-guided biopsy etc.)
Effectiveness data
High to moderate-quality evidence from 1 RCT reporting data from 132 people with suspected stage I to IIIA lung cancer on CT neck, thorax and upper abdomen showed that there was a reduction in time to treatment decision, a reduction in the number of investigations per person, an increase in the duration of survival (hazard ratio), an increase in the number of people who had diagnosis and staging competed by 14 days and an increase in the number of people diagnosed and staged with one investigation for EBUS-TBNA (or EUS-FNA) compared to conventional diagnosis and staging (bronchoscopy or CT-guided biopsy etc.) However, the data could not differentiate between the number of avoidable thoracotomies and the number of people experiencing a pneumothorax or in-patient admissions.
Diagnostic accuracy data
High to moderate-quality evidence from 1 RCT reporting data from 132 people with suspected stage I to IIIA lung cancer on CT neck, thorax and upper abdomen showed that for EBUS-TBNA (or EUS-FNA) the sensitivity was 92.0% and the negative predictive value was 90.0% (with a prevalence of 75.8%).
EUS-FNA vs straight to surgical staging
Effectiveness data
Moderate to low-quality evidence from 1 RCT reporting data from 40 people who had proven or suspected NSCLC or suspected mediastinal lymph node invasion on CT/PET found that the date could not differentiate the numbers of people experiencing perforation or bleeding.
Diagnostic accuracy data
Low-quality evidence from 1 RCT reporting data from 40 people who had proven or suspected NSCLC or suspected mediastinal lymph node invasion on CT/PET found that the sensitivity for EUS-FNA for all was 93.0% and the negative predictive value was 83.0% (with a prevalence of 73.7%). For people who went straight to surgical staging, the sensitivity was 73.0% and the negative predictive value was 73.0% (with a prevalence of 52.3%).
Reference standards: For benign results, surgical confirmation. For malignant results, pathology.
Health economics evidence statements
One directly applicable UK, Belgian and Dutch based cost-utility analysis with potentially serious limitations compared endosonography followed by surgical staging with surgical staging alone for the staging of potentially resectable lung cancer. Endosonography followed by surgical staging compared to surgical staging alone was found to be a dominant strategy. A cost-effectiveness acceptability curve (CEAC) for endosonography followed by surgery if negative showed that 92% of the scenarios involved cost savings.
One partially applicable UK cost-effectiveness analysis with potentially serious limitations compared endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), to conventional diagnosis and staging (CDS) for diagnosis and staging in patients who were suspected to have stage I to IIIA lung cancer on the basis of CT scans of the neck, thorax, and upper abdomen. EBUS-TBNA for investigation was found to be slightly more expensive than CDS, but resulted in a shortened median time to treatment decision of nearly 50%. A post-hoc analysis revealed that the median survival time was greater for those in the EBUS-TBNA arm of the trial compared to those in the CDS arm.
One directly applicable economic model with very serious limitations found that PET-CT followed by conventional TBNA was the most cost effective strategy for people with an intermediate probability of mediastinal malignancy.
One partially applicable influence diagram model with very serious limitations found that when considering only effectiveness, the optimal strategy following a positive computed tomography (CT) scan was transbronchial needle aspiration (TBNA), followed by an endobronchial ultrasound (EBUS), and an endoscopic ultrasound (EUS). When the CT scan is negative, the optimal strategy was positron emission tomography (PET) followed by EBUS, and EUS. When taking into account costs, the optimal strategy following a positive CT scan was TBNA only; with an EBUS being done only when the CT scan or the TBNA is negative.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The committee highlighted that the outcomes that matter most are time to treatment decision, number of investigations per patient, patient acceptability, reduction of avoidable thoracic surgery and diagnostic sensitivity and negative predictive value This is because the committee agreed that these two diagnostic accuracy measurements are the ones that that matter most to clinicians and people with suspected / confirmed lung cancer.
The committee agreed that the outcomes in Kang 2014 (adverse events, patient satisfaction, sensitivity and negative predictive value) are less relevant because both arms of the trial involve giving patients 3 endoscopic interventions. This is less relevant because in the UK, healthcare professionals aim to use fewer endoscopic interventions.
The quality of the evidence
The committee agreed that the quality of evidence for using EBUS-TBNA as a first invasive test was good particularly with regard to the study by Navani et al. (2015). The committee also confirmed that the evidence for when EUS-FNA should be used as a first invasive test or as a second invasive test following EBUS-TBNA was of a lower quality: The methods section of Navani 2015 says the following: “If a target node was inaccessible with EBUS-TBNA then EUS-FNA as an alternative procedure was allowed.” The word “inaccessible” is an inexact term. For example, this term does not specify which lung stations are inaccessible by EBUS-TBNA. In Navani 2015, EUS-FNA was conducted for 2 people who met the inclusion criteria out of 66 (the others had EBUS-TBNA because they had suspicious lesions in lung stations accessible by EBUS-TBNA). To specify a more exact treatment protocol that includes EUS-FNA, there is an issue of collecting enough data. Therefore, the committee agreed that it might never be possible to have a study that specifies the exact usage of EUS-FNA. This is because the outcomes depend on too many variables such as the study population. In addition, Kang 2014 had vague inclusion criteria, non-significant results and had indirect evidence because the in the UK clinicians aim to give patients fewer than 3 endoscopic interventions. The committee also noted that EUS-FNA is particularly good at reaching lung stations 8, 9 and 4L.
Benefits and harms
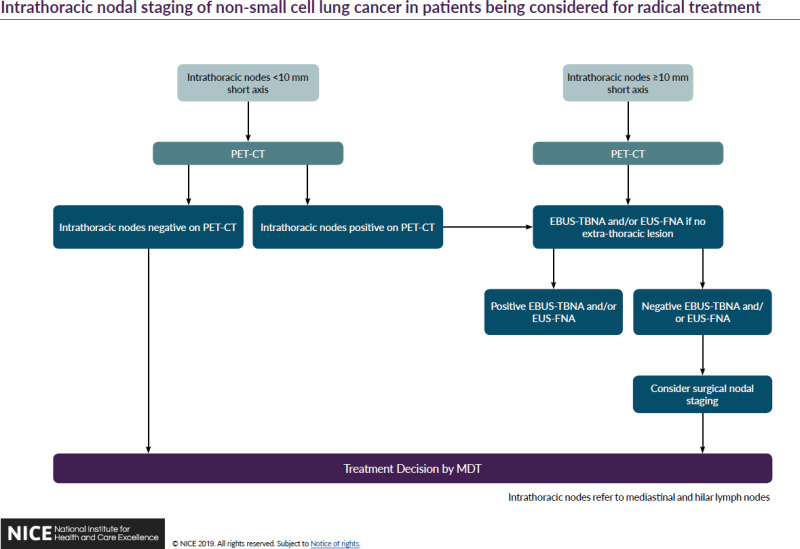
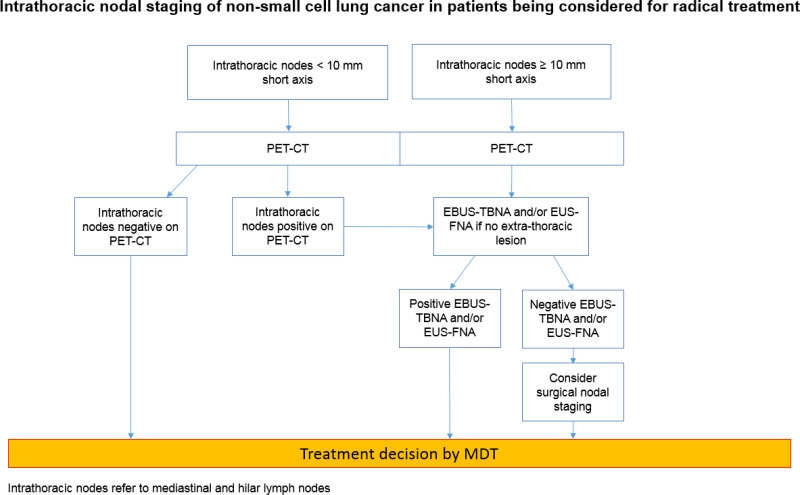
The committee agreed that EBUS-TBNA and/or EUS-FNA should be offered as a first invasive test for diagnosis and staging lung cancer with a probability of having mediastinal malignancy. This is because the committee decided that the findings of Navani 2015 showed that for EBUS-TBNA (or EUS-FNA) there was a reduction in time to treatment decision, a reduction in the number of investigations per patient and an increase in the number of people diagnosed and staged with one investigation compared to conventional diagnosis and staging (bronchoscopy or CT-guided biopsy etc.). The committee also found it plausible that the higher rates of survival in the EBUS-TBNA arm of the trial might be related to the faster treatment decisions those patients received. In addition, the committee noted that the findings in Annema 2010 and Larsen 2005 show that EBUS-TBNA and/or EUS-FNA as a first invasive test for people with a probability of having mediastinal malignancy, reduces the number of avoidable thoracic surgeries compared to people who go straight to surgical staging. Finally, EBUS-TBNA and EUS-FNA are generally performed as day case procedures under sedation and are safer, faster, cheaper and repeatable if necessary compared to surgical staging. The committee decided to recommend that EBUS-TBNA and EUS-FNA be offered together where indicated as this would be better for patients and consume less resources than if the two procedures were performed on separate occasions.
Cost effectiveness and resource use
The committee examined cost data on the various procedures and acknowledged that although it was recognised to be less sensitive than EBUS-TBNA, conventional TBNA would be the cheaper option for accessing lymph nodes via the trachea. They noted, however, that the large apparent cost differences between conventional TBNA and EBUS-TBNA are an artefact of certain pricing codes used in published sources (Luque et al. 2016 and the 2011 version of this guideline) and are likely to be far smaller in reality, as the only difference between the procedures are the marginal costs associated with the EBUS equipment and the difference between the costs of the needles. This was calculated at a little over £300 per procedure (see Appendix J). In addition many NHS trusts already have the EBUS equipment.
The committee considered whether they should recommend a cost saving strategy that put conventional TBNA first in a sequenced diagnostic pathway, followed by EBUS-TBNA for patients testing negative. The committee rejected this for several reasons. Firstly, they recognised the direction of travel in NHS policy is for time-to-diagnosis to be significantly reduced (a 28 day wait is to be trialled from 2018 and is intended to become national policy by 2020). Secondly, they noted that the National Optimal Lung Cancer Pathway recently published by the Lung Clinical Expert Group recommends that biopsy results should be available to the MDT within 21 days of initial suspicion of lung cancer on a CT scan. Thirdly, they recognised the practical difficulty of scheduling multiple tests for patients within this short time window and also took into account the views of lay members, who highlighted the importance of reducing the distressing wait for a diagnosis. Fourthly, the committee took into account patient representatives’ unease about undergoing multiple uncomfortable tests, which often require recovery time in a hospital bed. As noted above, the committee had experience of some patients being reluctant to return for further tests if the initial test was negative. Also as noted above, the committee found it plausible that extending time to diagnosis, even by a short time, may adversely affect treatment outcomes.
The committee also considered the cost-utility analysis in the Sharples et al. 2012 study and agreed that due to similar QALY estimates for EBUS/EUS and surgical staging, the analysis would reduce to a cost-comparison as concluded by the paper authors. However, they did not have confidence in the costing of endosonography in the Sharples et al. 2012 study as presented because the combined cost of EBUS-TBNA and EUS-FNA was less than the cost of EBUS-TBNA alone that had been provided in other sources produced at a similar time (the NICE 2011 Lung Cancer guideline update and in Navani et al. 2012).The committee also considered the influence diagram model by Luque et al. (2016), which suggested using cheaper tests before EBUS-TBNA but disregarded the evidence due to lack of face validity in the model’s diagnostic accuracy and cost data, particularly for conventional TBNA, which was costed at €80 rather than the ~£1,200 estimated for this update (see Appendix J).
Other factors the committee took into account
The committee gave special consideration to people living in deprived areas. This is because socioeconomic status was identified as a potential equality issue in the equity impact assessment. However, the committee agreed that no additional recommendations were necessary. The committee did not have any reason to believe that the interventions work better or worse in different groups. In addition, there was no data available specific to this population.
Appendix A. Review protocols
Review protocol for the clinical and cost effectiveness of using non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA as the first invasive test for people with a probability of mediastinal malignancy
| Field (based on PRISMA-P) | Content |
|---|---|
| Review question | What is the clinical and cost effectiveness of using non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA as the first invasive test for people with a probability of mediastinal malignancy? |
| Type of review question | Diagnostic and intervention |
| Objective of the review | This area was identified as requiring updating during the 2016 surveillance review. It is anticipated that recommendation on the use of non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA will be affected. |
| Eligibility criteria – population | Patients with suspected/ confirmed lung cancer (Pre-diagnosis and CT std. clinical evaluation) or in other words, people with a probability of mediastinal malignancy |
| Eligibility criteria – interventions |
|
| Eligibility criteria – gold standard | The gold standard investigation (histological/ cytological confirmation and pathological TNM - Or follow up period adequate to confirm outcome - Normally pathology from surgical resection but could be another technique in specified circumstances. |
| Outcomes and prioritisation |
|
| Eligibility criteria – study design |
|
| Other inclusion exclusion criteria |
|
| Proposed sensitivity/sub-group analysis, or meta-regression | No subgroup analysis identified |
| Selection process – duplicate screening/selection/analysis |
10% of the abstracts were reviewed by two reviewers, with any disagreements resolved by discussion or, if necessary, a third independent reviewer. If meaningful disagreements were found between the different reviewers, a further 10% of the abstracts were reviewed by two reviewers, with this process continued until agreement is achieved between the two reviewers. From this point, the remaining abstracts will be screened by a single reviewer. This review made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. See Appendix B for more details. |
| Data management (software) | See Methods Appendix B |
| Information sources – databases and dates |
See Appendix C Main Searches:
Citation searching will be carried out in addition on analyst/committee selected papers. The search will not be date limited because this is a new review question. Economics:
The search will not be date limited because this is a new review question. |
| Identify if an update | This is not an update, this is a new review question. |
| Author contacts | Guideline update |
| Highlight if amendment to previous protocol | For details please see section 4.5 of Developing NICE guidelines: the manual |
| Search strategy – for one database | For details please see appendix C |
| Data collection process – forms/duplicate | A standardised evidence table format will be used, and published as appendix F (clinical evidence tables) or I (economic evidence tables). |
| Data items – define all variables to be collected | For details please see evidence tables in appendix F (clinical evidence tables) or I (economic evidence tables). |
| Methods for assessing bias at outcome/study level | See Appendix B |
| Criteria for quantitative synthesis | See Appendix B |
| Methods for quantitative analysis – combining studies and exploring (in)consistency | See Appendix B |
| Meta-bias assessment – publication bias, selective reporting bias | See Appendix B |
| Confidence in cumulative evidence | See Appendix B |
| Rationale/context – what is known | For details please see the introduction to the evidence review in the main file. |
| Describe contributions of authors and guarantor |
A multidisciplinary committee developed the evidence review. The committee was convened by the NICE Guideline Updates Team and chaired by Gary McVeigh in line with section 3 of Developing NICE guidelines: the manual. Staff from the NICE Guideline Updates Team undertook systematic literature searches, appraised the evidence, conducted meta-analysis and cost-effectiveness analysis where appropriate, and drafted the evidence review in collaboration with the committee. For details please see Developing NICE guidelines: the manual. |
| Sources of funding/support | The NICE Guideline Updates Team is an internal team within NICE. |
| Name of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| Roles of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| PROSPERO registration number | N/A |
Review protocol for the clinical and cost-effectiveness of EBUS-TBNA alone, EUS-FNA alone or EBUS-TBNA and EUS-FNA in combination compared with surgical staging to diagnose and/or stage lung cancer
What is the clinical and cost-effectiveness of EBUS-TBNA alone, EUS-FNA alone or EBUS-TBNA and EUS-FNA in combination compared with surgical staging to diagnose and/or stage lung cancer?
| Field (based on PRISMA-P) | Content |
|---|---|
| Review question | What is the clinical and cost-effectiveness of EBUS-TBNA alone, EUS-FNA alone or EBUS-TBNA and EUS-FNA in combination compared with surgical staging to diagnose and/or stage lung cancer? |
| Type of review question | Diagnostic and intervention |
| Objective of the review | This area was identified as requiring updating during the 2016 surveillance review. Anticipated recommendations may cover which test is most appropriate for diagnosing or staging of lung cancer. |
| Eligibility criteria – population | Patients with suspected/ confirmed lung cancer (Pre-diagnosis and CT std. clinical evaluation) |
| Eligibility criteria – interventions |
|
| Eligibility criteria – gold standard |
|
| Outcomes and prioritisation |
|
| Eligibility criteria – study design |
|
| Other inclusion exclusion criteria |
|
| Proposed sensitivity/sub-group analysis, or meta-regression | No subgroup analysis identified |
| Selection process – duplicate screening/selection/analysis |
10% of the abstracts were reviewed by two reviewers, with any disagreements resolved by discussion or, if necessary, a third independent reviewer. If meaningful disagreements were found between the different reviewers, a further 10% of the abstracts were reviewed by two reviewers, with this process continued until agreement is achieved between the two reviewers. From this point, the remaining abstracts will be screened by a single reviewer. This review made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. See Appendix B for more details. |
| Data management (software) | See Methods Appendix B |
| Information sources – databases and dates |
See Appendix C Main Searches:
Citation searching will be carried out in addition on analyst/committee selected papers. The search will not be date limited because this is a new review question. Economics:
The search will not be date limited because this is a new review question. |
| Identify if an update | This is not an update, this is a new review question. |
| Author contacts | Guideline update |
| Highlight if amendment to previous protocol | For details please see section 4.5 of Developing NICE guidelines: the manual |
| Search strategy – for one database | For details please see appendix C |
| Data collection process – forms/duplicate | A standardised evidence table format will be used, and published as appendix E (clinical evidence tables) or I (economic evidence tables). |
| Data items – define all variables to be collected | For details please see evidence tables in appendix E (clinical evidence tables) or I (economic evidence tables). |
| Methods for assessing bias at outcome/study level | See Appendix B |
| Criteria for quantitative synthesis | See Appendix B |
| Methods for quantitative analysis – combining studies and exploring (in)consistency | See Appendix B |
| Meta-bias assessment – publication bias, selective reporting bias | See Appendix B |
| Confidence in cumulative evidence | See Appendix B |
| Rationale/context – what is known | For details please see the introduction to the evidence review in the main file. |
| Describe contributions of authors and guarantor |
A multidisciplinary committee developed the evidence review. The committee was convened by the NICE Guideline Updates Team and chaired by Gary McVeigh in line with section 3 of Developing NICE guidelines: the manual. Staff from the NICE Guideline Updates Team undertook systematic literature searches, appraised the evidence, conducted meta-analysis and cost-effectiveness analysis where appropriate, and drafted the evidence review in collaboration with the committee. For details please see Developing NICE guidelines: the manual. |
| Sources of funding/support | The NICE Guideline Updates Team is an internal team within NICE. |
| Name of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| Roles of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| PROSPERO registration number | N/A |
Appendix B. Methods
Priority screening
The reviews undertaken for this guideline all made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. This uses a machine learning algorithm (specifically, an SGD classifier) to take information on features (1, 2 and 3 word blocks) in the titles and abstract of papers marked as being ‘includes’ or ‘excludes’ during the title and abstract screening process, and re-orders the remaining records from most likely to least likely to be an include, based on that algorithm. This re-ordering of the remaining records occurs every time 25 additional records have been screened.
- Research is currently ongoing as to what are the appropriate thresholds where reviewing of abstract can be stopped, assuming a defined threshold for the proportion of relevant papers it is acceptable to miss on primary screening. As a conservative approach until that research has been completed, the following rules were adopted during the production of this guideline:
- In every review, at least 50% of the identified abstract (or 1,000 records, if that is a greater number) were always screened.
- After this point, screening was only terminated when the threshold was reached for a number of abstracts being screened without a single new include being identified. This threshold was set according to the expected proportion of includes in the review (with reviews with a lower proportion of includes needing a higher number of papers without an identified study to justify termination), and was always a minimum of 250.
- A random 10% sample of the studies remaining in the database when the threshold were additionally screened, to check if a substantial number of relevant studies were not being correctly classified by the algorithm, with the full database being screened if concerns were identified.
- As an additional check to ensure this approach did not miss relevant studies, the included studies lists of included systematic reviews were searched to identify any papers not identified through the primary search.
Evidence synthesis and meta-analyses
Where possible, meta-analyses were conducted to combine the results of studies for each outcome. For mean differences, where change from baseline data were reported in the studies and were accompanied by a measure of spread (for example standard deviation), these were extracted and used in the meta-analysis. Where measures of spread for change from baseline values were not reported, the corresponding values at study end were used and were combined with change from baseline values to produce summary estimates of effect. All studies were assessed to ensure that baseline values were balanced across the treatment/comparison groups; if there were significant differences in important confounding variables at baseline these studies were not included in any meta-analysis and were reported separately.
When averages were given as medians, no meta-analysis of the data were performed.
Evidence of effectiveness of interventions
Quality assessment
Individual RCTs and quasi-randomised controlled trials were quality assessed using the Cochrane Risk of Bias Tool. Cohort studies were quality assessed using the CASP cohort study checklist. Each individual study was classified into one of the following three groups:
- Low risk of bias – The true effect size for the study is likely to be close to the estimated effect size.
- Moderate risk of bias – There is a possibility the true effect size for the study is substantially different to the estimated effect size.
- High risk of bias – It is likely the true effect size for the study is substantially different to the estimated effect size.
Each individual study was also classified into one of three groups for directness, based on if there were concerns about the population, intervention, comparator and/or outcomes in the study and how directly these variables could address the specified review question. Studies were rated as follows:
- Direct – No important deviations from the protocol in population, intervention, comparator and/or outcomes.
- Partially indirect – Important deviations from the protocol in one of the population, intervention, comparator and/or outcomes.
- Indirect – Important deviations from the protocol in at least two of the following areas: population, intervention, comparator and/or outcomes.
Methods for combining intervention evidence
Meta-analyses of interventional data were conducted with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al. 2011).
Where different studies presented continuous data measuring the same outcome but using different numerical scales (e.g. a 0–10 and a 0–100 visual analogue scale), these outcomes were all converted to the same scale before meta-analysis was conducted on the mean differences. Where outcomes measured the same underlying construct but used different instruments/metrics, data were analysed using standardised mean differences (Hedges’ g).
A pooled relative risk was calculated for dichotomous outcomes (using the Mantel–Haenszel method). Both relative and absolute risks were presented, with absolute risks calculated by applying the relative risk to the pooled risk in the comparator arm of the meta-analysis.
Fixed- and random-effects models (der Simonian and Laird) were fitted for all syntheses, with the presented analysis dependent on the degree of heterogeneity in the assembled evidence. Fixed-effects models were the preferred choice to report, but in situations where the assumption of a shared mean for fixed-effects model were clearly not met, even after appropriate pre-specified subgroup analyses were conducted, random-effects results are presented. Fixed-effects models were deemed to be inappropriate if one or both of the following conditions was met:
- Significant between study heterogeneity in methodology, population, intervention or comparator was identified by the reviewer in advance of data analysis. This decision was made and recorded before any data analysis was undertaken.
- The presence of significant statistical heterogeneity in the meta-analysis, defined as I2≥50%.
In any meta-analyses where some (but not all) of the data came from studies at high risk of bias, a sensitivity analysis was conducted, excluding those studies from the analysis. Results from both the full and restricted meta-analyses are reported. Similarly, in any meta-analyses where some (but not all) of the data came from indirect studies, a sensitivity analysis was conducted, excluding those studies from the analysis.
Meta-analyses were performed in Cochrane Review Manager v 5.3.
Minimal clinically important differences (MIDs)
The Core Outcome Measures in Effectiveness Trials (COMET) database was searched to identify published minimal clinically important difference thresholds relevant to this guideline. However, no relevant MIDs were found. In addition, the Guideline Committee were asked to specify any outcomes where they felt a consensus MID could be defined from their experience. In particular, any questions looking to evaluate non-inferiority (that one intervention is not meaningfully worse than another) required an MID to be defined to act as a non-inferiority margin. However, the committee agreed that in their experience, they could not define any MIDs. This is because the committee agreed that the protocol outcomes were objective rather than subjective measures and the committee were not aware of evidence supporting the use of MIDs for the protocol’s outcomes. This was particularly the case for sensitivity and negative predictive value. The line of no effect was used as a MID for risk ratios and hazard ratios. Diagnostic accuracy outcomes do not have a line of no effect. Therefore, imprecision for diagnostic accuracy was graded using participant numbers only.
GRADE for pairwise meta-analyses of interventional evidence
GRADE was used to assess the quality of evidence for the selected outcomes as specified in ‘Developing NICE guidelines: the manual (2014)’. Data from RCTs was initially rated as high quality and the quality of the evidence for each outcome was downgraded or not from this initial point. If non-RCT evidence was included for intervention-type systematic reviews then these were initially rated as either moderate quality (quasi-randomised studies) or low quality (cohort studies) and the quality of the evidence for each outcome was further downgraded or not from this point, based on the criteria given in Table 4.
Table 4. Rationale for downgrading quality of evidence for intervention studies
The quality of evidence for each outcome was upgraded if any of the following five conditions were met:
- Data from non-randomised studies showing an effect size sufficiently large that it cannot be explained by confounding alone.
- Data showing a dose-response gradient.
- Data where all plausible residual confounding is likely to increase our confidence in the effect estimate.
Publication bias
Publication bias was assessed in two ways. First, if evidence of conducted but unpublished studies was identified during the review (e.g. conference abstracts, trial protocols or trial records without accompanying published data), available information on these unpublished studies was reported as part of the review. Secondly, where 10 or more studies were included as part of a single meta-analysis, a funnel plot was produced to graphically assess the potential for publication bias.
Evidence statements
Evidence statements for pairwise intervention data are classified in to one of four categories:
- Situations where the data are only consistent, at a 95% confidence level, with an effect in one direction (i.e. one that is ‘statistically significant’), and the magnitude of that effect is most likely to meet or exceed the MID (i.e. the point estimate is not in the zone of equivalence). In such cases, we state that the evidence showed that there is an effect.
- Situations where the data are only consistent, at a 95% confidence level, with an effect in one direction (i.e. one that is ‘statistically significant’), but the magnitude of that effect is most likely to be less than the MID (i.e. the point estimate is in the zone of equivalence). In such cases, we state that the evidence could not demonstrate a meaningful difference.
- Situations where the data are consistent, at a 95% confidence level, with an effect in either direction (i.e. one that is not ‘statistically significant’) but the confidence limits are smaller than the MIDs in both directions. In such cases, we state that the evidence demonstrates that there is no difference.
- In all other cases, we state that the evidence could not differentiate between the comparators.
Diagnostic test accuracy evidence
In this guideline, diagnostic test accuracy (DTA) data are classified as any data in which a test result or the output of an algorithm – is observed in some people who have the condition of interest at the time of the test and some people who do not. Such data either explicitly provide, or can be manipulated to generate, a 2x2 classification of true positives and false negatives (in people who, according to the reference standard, truly have the condition) and false positives and true negatives (in people who, according to the reference standard, do not).
The ‘raw’ 2×2 data can be summarised in a variety of ways. Those that were used for decision making in this guideline are as follows:
- Sensitivity is the probability that the feature will be positive in a person with the condition.
- sensitivity = TP/(TP+FN)
- Negative predictive value is the probability that people for whom the feature is negative truly do not have the condition.
- negative predictive value = TN/(TN+FN)
Negative predictive value was used rather than specificity. This is because all studies assumed that the pathologist made no false positives. Therefore, sensitivity and negative predictive value (with prevalence information) are more meaningful measurements of performance because they do not involve false positives.
Quality assessment
Individual studies were quality assessed using the QUADAS-2 tool, which contains four domains: patient selection, index test, reference standard, and flow and timing. Each individual study was classified into one of the following two groups:
- Low risk of bias – Evidence of non-serious bias in zero or one domain.
- Moderate risk of bias – Evidence of non-serious bias in two domains only, or serious bias in one domain only.
- High risk of bias – Evidence of bias in at least three domains, or of serious bias in at least two domains.
Each individual study was also classified into one of three groups for directness, based on if there were concerns about the population, index features and/or reference standard in the study and how directly these variables could address the specified review question. Studies were rated as follows:
- Direct – No important deviations from the protocol in population, index feature and/or reference standard.
- Partially indirect – Important deviations from the protocol in one of the population, index feature and/or reference standard.
- Indirect – Important deviations from the protocol in at least two of the population, index feature and/or reference standard.
Modified GRADE for diagnostic test accuracy evidence
GRADE has not been developed for use with diagnostic studies; therefore a modified approach was applied using the GRADE framework. GRADE assessments were only undertaken for sensitivity and negative predictive values (that are provided in the context of the prevalences of lung cancer). The committee thought that it was very unlikely that pathologists would identify non-cancerous cells as cancerous. Therefore, the committee agreed that the false positive rate for all techniques was likely to be 0. Therefore, all calculated outcomes that involve a false positive value are not meaningful. For example, specificity and likelihood ratios. GRADE quality ratings were calculated using the same criteria as for randomised controlled trials, given in Table 4. For example, the committee agreed that if the sample size was 26 to 40, the outcome was downgraded once. If the sample size was 25 or less, the outcome was downgraded twice. This is because neither sensitivity nor negative predictive value have a line of no effect with which to rate imprecision.
Appendix C. Literature search strategies
Scoping search strategies
Scoping searches Scoping searches were undertaken on the following websites and databases (listed in alphabetical order) in April 2017 to provide information for scope development and project planning. Browsing or simple search strategies were employed.
| Guidelines/website |
|---|
| American Cancer Society |
| American College of Chest Physicians |
| American Society for Radiation Oncology |
| American Thoracic Society |
| Association for Molecular Pathology |
| British Lung Foundation |
| British Thoracic Society |
| Canadian Medical Association Infobase |
| Canadian Task Force on Preventive Health Care |
| Cancer Australia |
| Cancer Care Ontario |
| Cancer Control Alberta |
| Cancer Research UK |
| Care Quality Commission |
| College of American Pathologists |
| Core Outcome Measures in Effectiveness Trials (COMET) |
| Department of Health & Social Care |
| European Respiratory Society |
| European Society for Medical Oncology |
| European Society of Gastrointestinal Endoscopy |
| European Society of Thoracic Surgery |
| General Medical Council |
| Guidelines & Audit Implementation Network (GAIN) |
| Guidelines International Network (GIN) |
| Healthtalk Online |
| International Association for the Study of Lung Cancer |
| MacMillan Cancer Support |
| Medicines and Products Regulatory Agency (MHRA) |
| National Audit Office |
| National Cancer Intelligence Network |
| National Clinical Audit and Patient Outcomes Programme |
| National Health and Medical Research Council - Australia |
| National Institute for Health and Care Excellence (NICE) - published & in development guidelines |
| National Institute for Health and Care Excellence (NICE) - Topic Selection NHS Choices |
| NHS Digital |
| NHS England |
| NICE Clinical Knowledge Summaries (CKS) |
| NICE Evidence Search |
| Office for National Statistics |
| Patient UK |
| PatientVoices |
| Public Health England |
| Quality Health |
| Royal College of Anaesthetists |
| Royal College of General Practitioners |
| Royal College of Midwives |
| Royal College of Nursing |
| Royal College of Pathologists |
| Royal College of Physicians |
| Royal College of Radiologists |
| Royal College of Surgeons |
| Scottish Government |
| Scottish Intercollegiate Guidelines Network (SIGN) |
| UK Data Service |
| US National Guideline Clearinghouse |
| Walsall community Health NHS Trust |
| Welsh Government |
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between October 2017 and April 2018 for 9 review questions (RQ).
Searches were re-run in May 2018.
Where appropriate, in-house study design filters were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 3.
Search strategy
|
Medline Strategy, searched 3rd November 2017 Database: Ovid MEDLINE(R) 1946 to October Week 4 2017 Search Strategy: |
|---|
| 1 exp Lung Neoplasms/ |
| 2 ((lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 3 ((pancoast* or superior sulcus or pulmonary sulcus) adj4 (tumo?r* or syndrome*)).tw. |
| 4 ((lung* or pulmonary or bronch*) adj4 (oat or small or non-small) adj4 cell*).tw. |
| 5 (SCLC or NSCLC).tw. |
| 6 or/1–5 |
| 7 exp Biopsy, Fine-Needle/ |
| 8 Biopsy, Needle/mt [Methods] |
| 9 (TBNA* or EBUSTBNA* or TBNB* or EUS-FNA* or EUSFNA* or EUS-FNB* or EUSFNB*).tw. |
| 10 (EUS* adj2 (FNA* or FNB*)).tw. |
| 11 ((transbronch* or trans-bronch*) adj4 needle* adj4 (aspirat* or biops* or prick* or perforat* or ruptur*)).tw. |
| 12 ((endoscop* or endobronch*) adj4 (ultras* or echo* or sonogra* or tomograph* or doptone*) adj4 (needle* or fine or hollow*) adj4 (aspirat* or biops* or prick* or perforat* or ruptur*)).tw. |
| 13 (EUS* adj4 (needle* or fine or hollow*) adj4 (aspirat* or biops* or prick* or perforat* or ruptur*)).tw. |
| 14 or/7–13 |
| 15 6 and 14 |
| 16 Animals/ not Humans/ |
| 17 15 not 16 |
| 18 limit 17 to english language |
Note: In-house RCT, observational studies and systematic review filters were appended. No date limit as these were new questions.
Study Design Filters
| The MEDLINE SR, RCT, and observational studies filters are presented below. |
|---|
| Systematic Review |
| 1. Meta-Analysis.pt. |
| 2. Meta-Analysis as Topic/ |
| 3. Review.pt. |
| 4. exp Review Literature as Topic/ |
| 5. (metaanaly$ or metanaly$ or (meta adj3 analy$)).tw. |
| 6. (review$ or overview$).ti. |
| 7. (systematic$ adj5 (review$ or overview$)).tw. |
| 8. ((quantitative$ or qualitative$) adj5 (review$ or overview$)).tw. |
| 9. ((studies or trial$) adj2 (review$ or overview$)).tw. |
| 10. (integrat$ adj3 (research or review$ or literature)).tw. |
| 11. (pool$ adj2 (analy$ or data)).tw. |
| 12. (handsearch$ or (hand adj3 search$)).tw. |
| 13. (manual$ adj3 search$).tw. |
| 14. or/1–13 |
| 15. animals/ not humans/ |
| 16. 14 not 15 |
| RCT |
| 1 Randomized Controlled Trial.pt. |
| 2 Controlled Clinical Trial.pt. |
| 3 Clinical Trial.pt. |
| 4 exp Clinical Trials as Topic/ |
| 5 Placebos/ |
| 6 Random Allocation/ |
| 7 Double-Blind Method/ |
| 8 Single-Blind Method/ |
| 9 Cross-Over Studies/ |
| 10 ((random$ or control$ or clinical$) adj3 (trial$ or stud$)).tw. |
| 11 (random$ adj3 allocat$).tw. |
| 12 placebo$.tw. |
| 13 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw. |
| 14 (crossover$ or (cross adj over$)).tw. |
| 15 or/1–14 |
| 16 animals/ not humans/ |
| 17 15 not 16 |
| Observational |
| 1 Observational Studies as Topic/ |
| 2 Observational Study/ |
| 3 Epidemiologic Studies/ |
| 4 exp Case-Control Studies/ |
| 5 exp Cohort Studies/ |
| 6 Cross-Sectional Studies/ |
| 7 Controlled Before-After Studies/ |
| 8 Historically Controlled Study/ |
| 9 Interrupted Time Series Analysis/ |
| 10 Comparative Study.pt. |
| 11 case control$.tw. |
| 12 case series.tw. |
| 13 (cohort adj (study or studies)).tw. |
| 14 cohort analy$.tw. |
| 15 (follow up adj (study or studies)).tw. |
| 16 (observational adj (study or studies)).tw. |
| 17 longitudinal.tw. |
| 18 prospective.tw. |
| 19 retrospective.tw. |
| 20 cross sectional.tw. |
| 21 or/1–20 |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Apr 2015
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the review question search strategies. For some health economics strategies additional terms were added to the original review question search strategies (see sections 4.2, 4.3 and 4.4) The searches were conducted between October 2017 and April 2018 for 9 review questions (RQ).
Searches were re-run in May 2018.
Searches were limited to those in the English language. Animal studies were removed from results.
Economic evaluation and quality of life filters
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom$.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov$.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree$ or analys$)).tw. |
| 20 (cost or costs or costing$ or costly or costed).tw. |
| 21 (price$ or pricing$).tw. |
| 22 budget$.tw. |
| 23 expenditure$.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. |
| 26 or/1–25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly$ or qald$ or qale$ or qtime$).tw. |
| 7 disability adjusted life.tw. |
| 8 daly$.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health$ year$ equivalent$.tw. |
| 19 utilit$.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili$.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble$.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. 30 tto.tw. |
| 31 or/1–30 |
Health economics search strategy
|
Medline Strategy, searched 6th November 2017 Database: Ovid MEDLINE(R) 1946 to October Week 4 2017 Search Strategy: |
|---|
| 1 exp Lung Neoplasms/ |
| 2 ((lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 3 ((pancoast* or superior sulcus or pulmonary sulcus) adj4 (tumo?r* or syndrome*)).tw. (756) |
| 4 ((lung* or pulmonary or bronch*) adj4 (oat or small or non-small) adj4 cell*).tw. |
| 5 (SCLC or NSCLC).tw. |
| 6 or/1–5 |
| 7 exp Biopsy, Fine-Needle/ |
| 8 Biopsy, Needle/mt [Methods] |
| 9 (TBNA* or EBUSTBNA* or TBNB* or EUS-FNA* or EUSFNA* or EUS-FNB* or EUSFNB*).tw. |
| 10 (EUS* adj2 (FNA* or FNB*)).tw. |
| 11 ((transbronch* or trans-bronch*) adj4 needle* adj4 (aspirat* or biops* or prick* or perforat* or ruptur*)).tw. |
| 12 ((endoscop* or endobronch*) adj4 (ultras* or echo* or sonogra* or tomograph* or doptone*) adj4 (needle* or fine or hollow*) adj4 (aspirat* or biops* or prick* or perforat* or ruptur*)).tw. |
| 13 (EUS* adj4 (needle* or fine or hollow*) adj4 (aspirat* or biops* or prick* or perforat* or ruptur*)).tw. |
| 14 exp Positron-Emission Tomography/ |
| 15 (positron emission adj2 compute* adj2 (tomograph* or assist*)).tw. |
| 16 (PET* adj2 CT).tw. |
| 17 Mediastinoscopy/ |
| 18 Mediastinoscopes/ |
| 19 Mediastinum/dg [Diagnostic Imaging] |
| 20 (mediastinoscop* or mediastinotom*).tw. |
| 21 ((neck* or collum or collar) adj4 US).tw. |
| 22 or/7–21 |
| 23 exp Neck/ |
| 24 Neck Muscles/ |
| 25 exp Cervical Vertebrae/ |
| 26 (neck* or collum or collar).tw. |
| 27 ((cervical or C) adj4 vertebra*).tw. |
| 28 or/23–27 |
| 29 exp Ultrasonography/ |
| 30 (ultras* or echo* or sonogra* or tomograph* or doptone*).tw. |
| 31 29 or 30 |
| 32 28 and 31 |
| 33 22 or 32 |
| 34 6 and 33 (10309) |
| 35 Animals/ not Humans/ |
| 36 34 not 35 |
| 37 limit 36 to english language |
Appendix D. Evidence study selection for RQ 1.1 and RQ 1.2
Appendix E. Clinical evidence tables
Download PDF (375K)
Appendix F. GRADE tables
RQ 1.1: Mediastinoscopy + EUS-FNA vs mediastinoscopy + EUS-FNA only if CT shows invasion adjacent to the oesophagus: intervention evidence
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EUS-FNA | EUS-FNA if CT shows invasion | Summary of results (95% CI) | |
| Safety: complications (RR >1 favours EUS-FNA if CT shows invasion adjacent to the oesophagus) | |||||||||
| 1 (Larsen 2005) | RCT | Not serious | Not serious | N/A | Serious1 | 53 | 51 | N/A2 | Moderate |
| Safety: number of avoidable thoracotomies (RR >1 favours EUS-FNA if CT shows invasion adjacent to the oesophagus) | |||||||||
| 1 (Larsen 2005) | RCT | Not serious | Not serious | N/A | Not serious | 53 | 51 | RR 0.37 (0.14, 0.96) | High |
| Recurrence or death during a median follow-up time of 1.3 years (range 0.2–2.4 years) for routine EUS-FNA and 1.4 years (range 0.2–2.4 years) for EUS-FNA if local invasion (RR >1 favours EUS-FNA if CT shows invasion adjacent to the oesophagus) | |||||||||
| 1 (Larsen 2005) | RCT | Not serious | Not serious | N/A | Serious1 | 53 | 51 | RR 0.48 (0.15, 1.50) | Moderate |
- 1
Non-significant result
- 2
Not applicable - no events in either arm
RQ 1.1: EUS-FNA vs straight to surgical staging: intervention evidence
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EUS-FNA | Straight to surgical staging | Summary of results (95% CI) | |
| Safety: in-patient admission for staging only, median number of nights | |||||||||
| 1 (Tournoy 2008) | RCT | Not serious | Not serious | N/A | Serious1 | 19 | 21 | EUS-FNA: median = 0 nights; straight to surgical staging: median = 2 nights (range: 1–22)2 | Moderate |
| Safety: perforation / bleeding (RR >1 favours surgical staging) | |||||||||
| 1 (Tournoy 2008) | RCT | Not serious | Not serious | N/A | Very serious1,3 | 19 | 21 | RR 0.37 (0.02, 8.50) | Low |
- 1
Small number of participants. Downgraded once because the sample size is 26 to 40
- 2
These results are presented as they are because they are expressed as medians
- 3
Non-significant result
RQ 1.1: EUS-FNA vs straight to surgical staging: diagnostic accuracy evidence. Reference standards: For benign results, surgical confirmation. For malignant results, pathology
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Negative predictive value (95%CI) | Prevalence | Risk of bias | Indirectness | Inconsistency | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| EUS-FNA for all | ||||||||||
| 1 (Tournoy 2008) | RCT | 19 | 93.0% (66.0, 99.0) | 83.0% (35.0%, 99.0) | 73.7% | Not serious | Not serious | N/A | Very serious1 | Low |
| Straight to surgical staging | ||||||||||
| 1 (Tournoy 2008) | RCT | 21 | 73.0% (39.0, 93.0) | 73.0% (39.0, 93.0) | 52.3% | Not serious | Not serious | N/A | Very serious1 | Low |
- 1
Very small number of participants. Downgraded twice because the sample size is below 25
RQ 1.1 and RQ 1.2: Bronchoscopy, EBUS-TBNA then EUS (B)-FNA if necessary on mediastinal nodes inaccessible or difficult to access by EBUS-TBNA vs bronchoscopy, EUS-FNA then EBUS-TBNA if necessary on mediastinal nodes inaccessible or difficult to access by EUS-FNA: intervention evidence
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EBUS-TBNA then EUS-FNA | EUS-FNA then EBUS-TBNA | Summary of results (95% CI) | |
| Safety: pneumothorax (RR >1 favours EUS-FNA then EBUS-TBNA) | |||||||||
| 1 (Kang 2014) | RCT | Serious1 | Serious2 | N/A | Serious3 | 80 | 80 | RR 0.33 (0.01, 8.20) | Very low |
| Patient satisfaction: overall tolerance at 3–5 days after the interventions. Visual analogue scale from 1–10 (values >0 EUS-FNA then EBUS-TBNA) | |||||||||
| 1 (Kang 2014) | RCT | Serious1 | Serious2 | N/A | Serious3 | 80 | 80 | MD −0.54 (−1.28, 0.20) | Very low |
- 1
Vague inclusion criteria
- 2
Both arms of the trial involve giving patients 3 endoscopic interventions. Therefore, this is indirect evidence because in the UK, healthcare professionals aim to use fewer endoscopic interventions
- 3
Non-significant result
RQ 1.1 and RQ 1.2: Bronchoscopy, EBUS-TBNA then EUS (B)-FNA if necessary vs bronchoscopy, EUS-FNA then EBUS-TBNA if necessary: diagnostic accuracy evidence. Reference standards: For benign results, surgical confirmation. For malignant results, pathology
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Negative predictive value (95%CI) | Prevalence | Risk of bias | Indirectness | Inconsistency | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Bronchoscopy, EBUS-TBNA, then EUS-FNA arm | ||||||||||
| 1 (Kang 2014) | RCT | 74 | 85.3% (68.3, 93.0) | 88.0% (75.1, 94.7) | 45.9% | Serious1 | Serious2 | N/A | Not serious | Low |
| Bronchoscopy, EUS-FNA, then EBUS-TBNA arm | ||||||||||
| 1 (Kang 2014) | RCT | 74 | 90.4% (71.8, 97.2) | 95.2% (84.8, 98.6) | 33.8% | Serious1 | Serious2 | N/A | Not serious | Low |
| Bronchoscopy, EBUS-TBNA, then EUS-FNA arm: EBUS-TBNA only | ||||||||||
| 1 (Kang 2014) | RCT | 74 | 81.4% (65.2, 91.1) | 86.2% (73.1, 93.4) | 45.9% | Serious1 | Serious2 | N/A | Not serious | Low |
| Bronchoscopy, EUS-FNA, then EBUS-TBNA arm: EUS-FNA only | ||||||||||
| 1 (Kang 2014) | RCT | 74 | 59.6% (40.3, 76.4) | 82.5% (70.8, 90.2) | 33.8% | Serious1 | Serious2 | N/A | Not serious | Low |
- 1
Vague inclusion criteria
- 2
Both arms of the trial involve giving patients 3 endoscopic interventions. Therefore, this is indirect evidence because in the UK, healthcare professionals aim to use fewer endoscopic interventions
RQ 1.1 and RQ 1.2: EBUS-TBNA (or EUS-FNA) vs conventional (bronchoscopy or CT-guided biopsy etc): intervention evidence
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EBUS-TBNA (or EUS-FNA) | Conventional | Summary of results (95% CI) | |
| Safety: pneumothorax (RR >1 favours conventional (bronchoscopy or CT-guided biopsy etc)) | |||||||||
| 1 (Navani 2015) | RCT | Not serious | Not serious | N/A | Serious1 | 66 | 66 | RR 1.00 (0.06, 15.65) | Moderate |
| Safety: in-patient admissions (RR >1 favours conventional (bronchoscopy or CT-guided biopsy etc)) | |||||||||
| 1 (Navani 2015) | RCT | Not serious | Not serious | N/A | Serious1 | 66 | 66 | RR 0.33 (0.01, 8.04) | Moderate |
| Timing: time to treatment decision | |||||||||
| 1 (Navani 2015) | RCT | Not serious | Not serious | N/A | Not serious | 66 | 66 | EBUS-TBNA/EUS-FNA: median = 14 days (1415); bronchoscopy = 29 days (23–35)2 | High |
| Timing: number of people who had diagnosis and staging completed by 14 days (RR >1 favours EBUS-TBNA (or EUS-FNA)) | |||||||||
| 1 (Navani 2015) | RCT | Not serious | Not serious | N/A | Not serious | 66 | 66 | RR 4.38 (2.20, 8.71) | High |
| Number of investigations per person (values >0 favour conventional (bronchoscopy or CT-guided biopsy etc)) | |||||||||
| 1 (Navani 2015) | RCT | Not serious | Not serious | N/A | Not serious | 66 | 66 | MD −0.69 (−0.95, −0.43) | High |
| Number of people diagnosed and staged with one investigation (RR >1 favours EBUS-TBNA (or EUS-FNA)) | |||||||||
| 1 (Navani 2015) | RCT | Not serious | Not serious | N/A | Not serious | 66 | 66 | RR 3.75 (1.86, 7.56) | High |
| Number of avoidable thoracotomies at 1 year (RR >1 favours EBUS-TBNA (or EUS-FNA)) | |||||||||
| 1 (Navani 2015) | RCT | Not serious | Not serious | N/A | Serious1 | 66 | 66 | RR 2.60 (0.98, 6.88) | Moderate |
| Duration of survival: median number of days | |||||||||
| 1 (Navani 2015) | RCT | Serious3 | Not serious | N/A | Serious1 | 66 | 66 | EBUS-TBNA/EUS-FNA: median = 503 days (312–715); bronchoscopy = 312 days (231–488)2 | Low |
| Duration of survival: hazard ratio (HR >1 favours conventional (bronchoscopy / CT guided biopsy etc)) | |||||||||
| 1 (Navani 2015) | RCT | Not serious Serious3 | Not serious | N/A | Not serious | 66 | 66 | HR 0.60 (0.37, 0.98) | Moderate |
- 1
Non-significant result
- 2
These results are presented as they are because they are expressed as medians
- 3
Post-hoc analysis
RQ 1.1 and RQ 1.2: EBUS-TBNA (or EUS-FNA) vs conventional (bronchoscopy or CT-guided biopsy etc): diagnostic accuracy evidence. Reference standards: For benign results, surgical confirmation. For malignant results, pathology
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Negative predictive value (95%CI) | Prevalence | Risk of bias | Indirectness | Inconsistency | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| EBUS-TBNA. If node cannot be accessed, then EUS-FNA | ||||||||||
| 1 (Navani 2015) | RCT | 66 | 92.0% (78.0, 98.0) | 90.0% (72.0, 97.0) | 75.8% | Not serous | Not serious | N/A | Not serious | High |
RQ 1.2: EUS-FNA followed by EBUS-TBNA vs straight to surgical staging: intervention evidence
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EUS-FNA followed by EBUS-TBNA | Straight to surgical staging | Summary of results (95% CI) | |
| Safety: pneumothorax (RR >1 favours surgical staging) | |||||||||
| 1 (Annema 2010) | RCT | Serious1 | Not serious | N/A | Serious2 | 123 | 118 | RR 0.96 (0.06, 15.16) | Low |
| Safety: total number of complications (RR >1 favours surgical staging) | |||||||||
| 1 (Annema 2010) | RCT | Serious1 | Not serious | N/A | Serious2 | 123 | 118 | RR 0.82 (0.28, 2.38) | Low |
| Quality of life change at 6 months from randomisation, EQ-5D (values >0 favour EUS-FNA + EBUS-TBNA) | |||||||||
| 1 (Annema 2010) | RCT | Serious1 | Not serious | N/A | Serious2 | 123 | 118 | MD 0.01 (−0.07, 0.09) | Low |
| Number of avoidable thoracotomies (RR >1 favours surgical staging) | |||||||||
| 1 (Annema 2010) | RCT | Serious1 | Not serious | N/A | Not serious | 123 | 118 | RR 0.41 (0.20, 0.86) | Moderate |
| Number of people who died between staging and 6 months later (RR >1 favours surgical staging) | |||||||||
| 1 (Annema 2010) | RCT | Serious1 | Not serious | N/A | Serious2 | 123 | 118 | RR 0.78 (0.34, 1.83) | Low |
- 1
Details of randomisation not given
- 2
Non-significant result
RQ 1.2: EUS-FNA followed by EBUS-TBNA vs straight to surgical staging: diagnostic accuracy evidence. Reference standards: For benign results, surgical confirmation. For malignant results, pathology
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Negative predictive value (95%CI) | Prevalence | Risk of bias | Indirectness | Inconsistency | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| EUS-FNA followed by EBUS-TBNA | ||||||||||
| 1 (Annema 2010) | RCT | 123 | 93.3% (84.2, 97.3) | 92.7% (83.0, 97.1) | 53.7% | Serious1 | Not serious | N/A | Not serious | Moderate |
| Straight to surgical staging (mediastinoscopy) | ||||||||||
| 1 (Annema 2010) | RCT | 117 | 78.3% (65.3, 87.4) | 85.3% (75.6, 91.5%) | 44.1% | Serious1 | Not serious | N/A | Not serious | Moderate |
- 1
Details of randomisation not given
Appendix G. Excluded Studies
Excluded clinical studies
| Short title | Title | Reason for exclusion |
|---|---|---|
| Adams (2009) | Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis | Systematic review of non-randomised controlled trials |
| Akulian (2014) | Molecular profiling of adenocarcinoma of the lung | Review article but not a systematic review |
| Almeida (2012) | Bronchoscopy and endobronchial ultrasound for diagnosis and staging of lung cancer | Review article but not a systematic review |
| Anantham (2010) | Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis and staging of lung cancer | Review article but not a systematic review |
| Boonsarngsuk (2015) | Comparison of diagnostic performances among bronchoscopic sampling techniques in the diagnosis of peripheral pulmonary lesions | Non-randomised study |
| Casal (2012) | Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration | No relevant outcomes. The randomisation is not between two different arms of a trial. Lung cancer is mentioned as a coincidence, it is not the main focus |
| Chao 2009 | Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: a randomized trial | This study is on radial EBUS, which is not in the protocol |
| Dango (2010) | Endobronchial ultrasound-guided transbronchial needle aspiration and its role in non-small cell lung cancer: Diagnostic impact and limitations | Review article but not a systematic review |
| Darwiche (2013) | Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging | Non-randomised study |
| Ernst (2008) | Diagnosis of mediastinal adenopathy-realtime endobronchial ultrasound guided needle aspiration versus mediastinoscopy | Non-randomised study |
| Fritscher-Ravens (2003) | Mediastinal lymph node involvement in potentially resectable lung cancer: comparison of CT, positron emission tomography, and endoscopic ultrasonography with and without fine-needle aspiration | Non-randomised study |
| Fritscher-Ravens (2003) | Endoscopic ultrasound evaluation in the diagnosis and staging of lung cancer | Review article but not a systematic review |
| Godbout (2016) | Evaluation of pulmonary nodules using the spyglass direct visualization system combined with radial endobronchial ultrasound: A clinical feasibility study | Non-randomised study |
| Gompelmann (2014) | Role of endobronchial and endoscopic ultrasound in pulmonary medicine | Review article but not a systematic review |
| Govert (1999) | A prospective comparison of fiberoptic transbronchial needle aspiration and bronchial biopsy for bronchoscopically visible lung carcinoma | Non-randomised study |
| Grah (2011) | Comparison of 21 gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration: a randomised trial | Conference abstract |
| Gu (2009) | Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis | Systematic review of non-randomised controlled trials |
| Hassan (2010) | Comparative study of efficacy of brush cytology and transthoracic fine needle aspiration cytology in the diagnosis of bronchogenic carcinoma | Non-randomised study |
| Herth (2004) | Conventional vs Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Randomized Trial | No relevant outcomes. The outcome of interest is diagnostic yield. Diagnostic yield is the likelihood that a test or procedure will provide the information needed to establish a diagnosis. It is not a measurement of diagnostic accuracy. |
| Herth (2005) | Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes | Non-randomised study |
| Hwangbo (2010) | Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer | Non-randomised study |
| Jiang (2014) | TBNA with and without EBUS: A comparative efficacy study for the diagnosis and staging of lung cancer | Non-randomised study |
| Kramer (2003) | Current Concepts in the Mediastinal Lymph Node Staging of Nonsmall Cell Lung Cancer | Systematic review of non-randomised controlled trials |
| Lardinois (2011) | Pre- and intra-operative mediastinal staging in non-small-cell lung cancer | Review article but not a systematic review |
| Micames (2007) | Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis | Systematic review of non-randomised controlled trials |
| Mullan (2004) | CT-guided fine-needle aspiration of lung nodules: effect on outcome of using coaxial technique and immediate cytological evaluation | Non-randomised study |
| Oezkan (2017) | Feasibility study of using 19G needle for EBUS-TBNA: a prospective-randomized comparison of 19G and 22G EBUSneedles | Conference abstract |
| Ost (2016) | Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry | Non-randomised study |
| Paone (2005) | Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions | Study is on EBUS-TBB, not EBUS-TBNA |
| Puri (2009) | Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis | No relevant outcomes. Lung cancer is mentioned as a coincidence, it is not the main focus |
| Roth 2011 | A randomised trial of endobronchial ultrasound guided sampling in peripheral lung lesions | This study is on radial EBUS, not EBUS-TBNA |
| Saji (2011) | Comparison of 21-gauge and 22-gauge Needles for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration of Mediastinal and Hilar Lymph Nodes | Non-randomised study |
| Schreiber (2003) | Performance characteristics of different modalities for diagnosis of suspected lung cancer: Summary of published evidence | Systematic review of non-randomised controlled trials |
| Soja (2010) | Usefulness of transbronchial needle aspiration for initial lung cancer staging | Non-randomised study |
| Szlubowski (2012) | A comparison of the combined ultrasound of the mediastinum by use of a single ultrasound bronchoscope versus ultrasound bronchoscope plus ultrasound gastroscope in lung cancer staging: a prospective trial | Non-randomised study |
| Trisolini 2015 | Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping | The comparison of EBUS-TBNA vs EBUS-TBNA with Rapid On-Site Evaluation (ROSE) is not in the protocol |
| Wagner (1989) | Transbronchial fine-needle aspiration. Reliability and limitations | Non-randomised study |
| Xi (2017) | Distant metastasis and survival outcomes after computed tomography-guided needle biopsy in resected stage I-III non-small cell lung cancer | Non-randomised study |
| Yarmus (2011) | A randomized prospective trial of the utility of rapid on-site evaluation of transbronchial needle aspirate specimens | Study on bronchoscopy |
| Yarmus (2015) | A randomized controlled trial evaluating airway inspection effectiveness during endobronchial ultrasound bronchoscopy | No relevant outcomes |
| Yasuda (2009) | Mediastinal lymph node staging in potentially resectable non-small cell lung cancer: a prospective comparison of CT and EUS/EUS-FNA | Non-randomised study |
| Zhang (2013) | Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis | Systematic review of non-randomised controlled trials. There was one RCT included, which we are already including. |
Excluded economic studies
| Paper | Primary reason for exclusion |
|---|---|
| Bongers, M.L., Coupé, V.M., De Ruysscher, D., Oberije, C., Lambin, P. and Uyl-de Groot, C.A., 2015. Individualized Positron Emission Tomography–Based Isotoxic Accelerated Radiation Therapy Is Cost-Effective Compared With Conventional Radiation Therapy: A Model-Based Evaluation. International Journal of Radiation Oncology* Biology* Physics, 91(4), pp.857–865. | Not conducted in a health care system similar to the UK. |
| Czarnecka-Kujawa, K., Rochau, U., Siebert, U., Atenafu, E., Darling, G., Waddell, T.K., Pierre, A., De Perrot, M., Cypel, M., Keshavjee, S. and Yasufuku, K., 2017. Cost-effectiveness of mediastinal lymph node staging in non–small cell lung cancer. The Journal of thoracic and cardiovascular surgery, 153(6), pp.1567–1578. | Not conducted in a health care system similar to the UK. |
| Deppen, S.A., Davis, W.T., Green, E.A., Rickman, O., Aldrich, M.C., Fletcher, S., Putnam Jr, J.B. and Grogan, E.L., 2014. Cost-effectiveness of initial diagnostic strategies for pulmonary nodules presenting to thoracic surgeons. The Annals of thoracic surgery, 98(4), pp.1214–1222. | Not conducted in a health care system similar to the UK. |
| Dietlein, M., Weber, K., Gandjour, A., Moka, D., Theissen, P., Lauterbach, K.W. and Schicha, H., 2000. Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: priority for a PET-based strategy after nodal-negative CT results. European journal of nuclear medicine, 27(11), pp.1598–1609. | Not conducted in a health care system similar to the UK. |
| Dietlein, M., Weber, K., Gandjour, A., Moka, D., Theissen, P., Lauterbach, K.W. and Schicha, H., 2000. Cost-effectiveness of FDG-PET for the management of solitary pulmonary nodules: a decision analysis based on cost reimbursement in Germany. European journal of nuclear medicine, 27(10), pp.1441–1456. | Not conducted in a health care system similar to the UK. |
| Esnaola, N.F., Lazarides, S.N., Mentzer, S.J. and Kuntz, K.M., 2002. Outcomes and Cost-Effectiveness of Alternative Staging Strategies for Non–Small-Cell Lung Cancer. Journal of clinical oncology, 20(1), pp.263–273. | Not conducted in a health care system similar to the UK. |
| Han, Y., Xiao, H., Zhou, Z., Yuan, M., Zeng, Y., Wu, H. and Fang, Y., 2015. Cost-effectiveness analysis of strategies introducing integrated 18F-FDG PET/CT into the mediastinal lymph node staging of non-small-cell lung cancer. Nuclear medicine communications, 36(3), pp.234–241. | Not conducted in a health care system similar to the UK. |
| Hayashi, K., Abe, K., Yano, F., Watanabe, S., Iwasaki, Y. and Kosuda, S., 2005. Should mediastinoscopy actually be incorporated into the FDG PET strategy for patients with non-small cell lung carcinoma?. Annals of nuclear medicine, 19(5), pp.393–398. | Not conducted in a health care system similar to the UK. |
| Lejeune, C., Al Zahouri, K., Woronoff-Lemsi, M.C., Arveux, P., Bernard, A., Binquet, C. and Guillemin, F., 2005. Use of a decision analysis model to assess the medicoeconomic implications of FDG PET imaging in diagnosing a solitary pulmonary nodule. The European Journal of Health Economics, 6(3), pp.203–214. | Not conducted in a health care system similar to the UK. |
| León, N.G., Escalona, S., Bandrés, B., Belda, C., Callejo, D. and Blasco, J.A., 2014. 18f-fluorodeoxyglucose positron emission tomography/computed tomography accuracy in the staging of non-small cell lung cancer: Review and cost-effectiveness. Radiology research and practice, 2014. | Not conducted in a health care system similar to the UK. |
| Meyers, B.F., Haddad, F., Siegel, B.A., Zoole, J.B., Battafarano, R.J., Veeramachaneni, N., Cooper, J.D. and Patterson, G.A., 2006. Cost-effectiveness of routine mediastinoscopy in computed tomography–and positron emission tomography–screened patients with stage I lung cancer. The Journal of thoracic and cardiovascular surgery, 131(4), pp.822–829. | Not conducted in a health care system similar to the UK. |
| Navani, N. and Janes, S.M., 2013. Endobronchial Ultrasound–guided Transbronchial Needle Aspiration for Lymphoma: The Final Frontier. | Not conducted in a health care system similar to the UK. |
| Navani, N., Nankivell, M., Woolhouse, I., Harrison, R.N., Munavvar, M., Oltmanns, U., Falzon, M., Kocjan, G., Rintoul, R.C. and Janes, S.M., 2011. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. Journal of Thoracic Oncology, 6(9), pp.1505–1509. | Not conducted in a health care system similar to the UK. |
| Navani, N., Lawrence, D.R., Kolvekar, S., Hayward, M., McAsey, D., Kocjan, G., Falzon, M., Capitanio, A., Shaw, P., Morris, S. and Omar, R.Z., 2012. Endobronchial ultrasound–guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. American journal of respiratory and critical care medicine, 186(3), pp.255–260. | Not conducted in a health care system similar to the UK. |
| Rintoul, R.C., Glover, M.J., Jackson, C., Hughes, V., Tournoy, K.G., Dooms, C., Annema, J.T. and Sharples, L.D., 2014. Cost effectiveness of endosonography versus surgical staging in potentially resectable lung cancer: a health economics analysis of the ASTER trial from a European perspective. Thorax, 69(7), pp.679–681. | Not conducted in a health care system similar to the UK. |
| Sari, A.A., Ravaghi, H., Mobinizadeh, M. and Sarvari, S., 2013. The cost-utility analysis of PET-scan in diagnosis and treatment of non-small cell lung carcinoma in Iran. Iranian Journal of Radiology, 10(2), p.61. | Not conducted in a health care system similar to the UK. |
| Schreyögg, J., Weller, J., Stargardt, T., Herrmann, K., Bluemel, C., Dechow, T., Glatting, G., Krause, B.J., Mottaghy, F., Reske, S.N. and Buck, A.K., 2010. Cost-effectiveness of hybrid PET/CT for staging of non-small cell lung cancer. J Nucl Med, 51(11), pp.1668–75. | Not conducted in a health care system similar to the UK. |
| Scott, W.J., Shepherd, J. and Gambhir, S.S., 1998. Cost-effectiveness of FDG-PET for staging non–small cell lung cancer: a decision analysis. The Annals of thoracic surgery, 66(6), pp.1876–1884. | Not conducted in a health care system similar to the UK. |
| Søgaard, R., Fischer, B.M.B., Mortensen, J., Rasmussen, T.R. and Lassen, U., 2013. The Optimality of Different Strategies for Supplemental Staging of Non–Small-Cell Lung Cancer: A Health Economic Decision Analysis. Value in health, 16(1), pp.57–65. | Not conducted in a health care system similar to the UK. |
| van Loon, J., Grutters, J.P., Wanders, R., Boersma, L., Dingemans, A.M.C., Bootsma, G., Geraedts, W., Pitz, C., Simons, J., Brans, B. and Snoep, G., 2010. 18FDG-PET-CT in the follow-up of non-small cell lung cancer patients after radical radiotherapy with or without chemotherapy: an economic evaluation. European Journal of Cancer, 46(1), pp.110–119. | Not conducted in a health care system similar to the UK. |
| Wang, Y.T. and Huang, G., 2012. Is FDG PET/CT cost-effective for pre-operation staging of potentially operative non-small cell lung cancer?–from Chinese healthcare system perspective. European journal of radiology, 81(8), pp.e903–e909. | Not conducted in a health care system similar to the UK. |
Appendix H. References
Clinical Studies - Included
- Annema J T, van Meerbeeck, J P, Rintoul R C, Dooms C, Deschepper E, Dekkers O M, De Leyn, P, Braun J, Carroll N R, Praet M, de Ryck, F, Vansteenkiste J, Vermassen F, Versteegh M I, Veselic M, Nicholson A G, Rabe K F, and Tournoy K G (2010) Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 304(20), 2245–52 [PubMed: 21098770]
- Kang H J, Hwangbo B, Lee G K, Nam B H, Lee H S, Kim M S, Lee J M, Zo J I, Lee H S, and Han J Y (2014) EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 69(3), 261–8 [PubMed: 24172712]
- Larsen S S, Vilmann P, Krasnik M, Dirksen A, Clementsen P, Maltbaek N, Lassen U, Skov B G, and Jacobsen G K (2005) Endoscopic ultrasound guided biopsy performed routinely in lung cancer staging spares futile thoracotomies: preliminary results from a randomised clinical trial. Lung Cancer 49(3), 377–85 [PubMed: 16102606]
- Navani N, Nankivell M, Lawrence D R, Lock S, Makker H, Baldwin D R, Stephens R J, Parmar M K, Spiro S G, Morris S, Janes S M, and Lung Boost trial investigators (2015) Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. The Lancet Respiratory Medicine 3(4), 282–9 [PMC free article: PMC4648022] [PubMed: 25660225]
- Sharples L D, Jackson C, Wheaton E, Griffith G, Annema J T, Dooms C, Tournoy K G, Deschepper E, Hughes V, Magee L, Buxton M, Rintoul R C (2012) Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technology Assessment 16(18), 1–100 [PubMed: 22472180]
- Tournoy K G, De Ryck, F, Vanwalleghem L R, Vermassen F, Praet M, Aerts J G, Van Maele, G, van Meerbeeck, and J P (2008) Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. American Journal of Respiratory & Critical Care Medicine 177(5), 531–5 [PubMed: 17962631]
Clinical studies – Excluded
- Adams K, Shah P L, Edmonds L, and Lim E (2009) Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 64(9), 757–62 [PubMed: 19454408]
- Akulian J A, Belanger A R, and Yarmus L (2014) Molecular profiling of adenocarcinoma of the lung. Minerva Pneumologica 53(1), 1–8
- Almeida F A (2012) Bronchoscopy and endobronchial ultrasound for diagnosis and staging of lung cancer. Cleveland Clinic Journal of Medicine 79 Electronic Suppl 1, eS11–6 [PubMed: 22614959]
- Anantham D, and Koh M S (2010) Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis and staging of lung cancer. Thoracic Cancer 1(1), 9–16 [PubMed: 27755787]
- Boonsarngsuk V, Kanoksil W, and Laungdamerongchai S (2015) Comparison of diagnostic performances among bronchoscopic sampling techniques in the diagnosis of peripheral pulmonary lesions. Journal of Thoracic Disease 7(4), 697–703 [PMC free article: PMC4419296] [PubMed: 25973236]
- Casal R F, Staerkel G A, Ost D, Almeida F A, Uzbeck M H, Eapen G A, Jimenez C A, Nogueras-Gonzalez G M, Sarkiss M, and Morice R C (2012) Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest 142(3), 568–573 [PMC free article: PMC3610596] [PubMed: 22156610]
- Chao T Y, Chien M T, Lie C H, Chung Y H, Wang J L, and Lin M C (2009) Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: a randomized trial. Chest 136(1), 229–236 [PubMed: 18812446]
- Dango S, Guenter J, and Passlick B (2010) Endobronchial ultrasound-guided transbronchial needle aspiration and its role in non-small cell lung cancer: Diagnostic impact and limitations. Thoracic Cancer 1(2), 70–76 [PubMed: 27755780]
- Darwiche K, Zarogoulidis P, Baehner K, Welter S, Tetzner R, Wohlschlaeger J, Theegarten D, Nakajima T, and Freitag L (2013) Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Annals of Oncology 24(11), 2866–70 [PubMed: 24026539]
- Ernst A, Anantham D, Eberhardt R, Krasnik M, and Herth F J (2008) Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer 3(6), 577–82 [PubMed: 18520794]
- Fritscher-Ravens A (2003) Endoscopic ultrasound evaluation in the diagnosis and staging of lung cancer. Lung Cancer 41(3), 259–67 [PubMed: 12928117]
- Fritscher-Ravens A, Bohuslavizki K H, Brandt L, Bobrowski C, Lund C, Knofel W T, and Pforte A (2003) Mediastinal lymph node involvement in potentially resectable lung cancer: comparison of CT, positron emission tomography, and endoscopic ultrasonography with and without fine-needle aspiration. Chest 123(2), 442–51 [PubMed: 12576364]
- Godbout K, Martel S, Simon M, Lampron N, and Delage A (2016) Evaluation of pulmonary nodules using the spyglass direct visualization system combined with radial endobronchial ultrasound: A clinical feasibility study. Open Respiratory Medicine Journal 10, 79–85 [PMC free article: PMC5220169] [PubMed: 28144366]
- Gompelmann D, and Herth F J (2014) Role of endobronchial and endoscopic ultrasound in pulmonary medicine. Respiration 87(1), 3–8 [PubMed: 24296947]
- Govert J A, Dodd L G, Kussin P S, and Samuelson W M (1999) A prospective comparison of fiberoptic transbronchial needle aspiration and bronchial biopsy for bronchoscopically visible lung carcinoma. Cancer 87(3), 129–34 [PubMed: 10385443]
- Grah C, Griff S, Mairinger T, Bollmann L, Temme H, Seiberth R, and Vogelbusch M (2011) Comparison of 21 gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration: a randomised trial. Journal of thoracic oncology. 6(6 suppl. 2), S1148
- Gu P, Zhao Y Z, Jiang L Y, Zhang W, Xin Y, and Han B H (2009) Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. European Journal of Cancer 45(8), 1389–96 [PubMed: 19124238]
- Hassan M Q, Ahamad M S, Ahmed S, and Chowdhury M A (2010) Comparative study of efficacy of brush cytology and transthoracic fine needle aspiration cytology in the diagnosis of bronchogenic carcinoma. Bangladesh Medical Research Council Bulletin 36(1), 37–9 [PubMed: 21280559]
- Herth F, Becker H D, and Ernst A (2004) Conventional vs Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Randomized Trial. Chest 125(1), 322–325 [PubMed: 14718460]
- Herth F J. F, Lunn W, Eberhardt R, Becker H D, and Ernst A (2005) Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. American Journal of Respiratory and Critical Care Medicine 171(10), 1164–1167 [PubMed: 15665318]
- Hwangbo B, Lee G K, Lee H S, Lim K Y, Lee S H, Kim H Y, Lee H S, Kim M S, Lee J M, Nam B H, and Zo J I (2010) Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 138(4), 795–802 [PubMed: 20348194]
- Jiang J, Browning R, Lechtzin N, Huang J, Terry P, and Wang K P (2014) TBNA with and without EBUS: A comparative efficacy study for the diagnosis and staging of lung cancer. Journal of Thoracic Disease 6(5), 416–420 [PMC free article: PMC4015015] [PubMed: 24822097]
- Kramer H, and Groen H J. M (2003) Current Concepts in the Mediastinal Lymph Node Staging of Nonsmall Cell Lung Cancer. Annals of Surgery 238(2), 180–188 [PMC free article: PMC1422673] [PubMed: 12894010]
- Lardinois D (2011) Pre- and intra-operative mediastinal staging in non-small-cell lung cancer. Swiss Medical Weekly 141(MARCH), [PubMed: 21384283]
- Micames C G, McCrory D C, Pavey D A, Jowell P S, and Gress F G (2007) Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 131(2), 539–48 [PubMed: 17296659]
- Mullan C P, Kelly B E, Ellis P K, Hughes S, Anderson N, and McCluggage W G (2004) CT-guided fine-needle aspiration of lung nodules: effect on outcome of using coaxial technique and immediate cytological evaluation. Ulster Medical Journal 73(1), 32–6 [PMC free article: PMC2475445] [PubMed: 15244123]
- Oezkan F, Wolters C, Franzen D, Hager T, Eisenmann S, Freitag L, He K, Weinreich G, and Darwiche K (2017) Feasibility study of using 19G needle for EBUS-TBNA: a prospective-randomized comparison of 19G and 22G EBUS-needles. American journal of respiratory and critical care medicine. Conference: american thoracic society international conference, and ATS 2017. United states 195(no pagination),
- Ost D E, Ernst A, Lei X, Kovitz K L, Benzaquen S, Diaz-Mendoza J, Greenhill S, Toth J, Feller-Kopman D, Puchalski J, Baram D, Karunakara R, Jimenez C A, Filner J J, Morice R C, Eapen G A, Michaud G C, Estrada Y Martin R. M, Rafeq S, Grosu H B, Ray C, Gilbert C R, Yarmus L B, Simoff M, and Registry A QuIRE Bronchoscopy (2016) Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. American Journal of Respiratory & Critical Care Medicine 193(1), 68–77 [PMC free article: PMC4731617] [PubMed: 26367186]
- Paone G, Nicastri E, Lucantoni G, Della Iacono, R, Battistoni P, D’Angeli A L, and Galluccio G (2005) Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 128(5), 3551–3557 [PubMed: 16304312]
- Puri R, Vilmann P, Saftoiu A, Skov B G, Linnemann D, Hassan H, Garcia E S. G, and Gorunescu F (2009) Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scandinavian Journal of Gastroenterology 44(4), 499–504 [PubMed: 19117242]
- Roth K, Eagan T M, Andreassen A H, Leh F, and Hardie J A (2011) A randomised trial of endobronchial ultrasound guided sampling in peripheral lung lesions. Lung Cancer 74(2), 219–25 [PubMed: 21481486]
- Saji J, Kurimoto N, Morita K, Nakamura M, Inoue T, Nakamura H, and Miyazawa T (2011) Comparison of 21-gauge and 22-gauge Needles for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration of Mediastinal and Hilar Lymph Nodes. Journal of Bronchology and Interventional Pulmonology 18(3), 239–246 [PubMed: 23208567]
- Schreiber G, and McCrory D C (2003) Performance characteristics of different modalities for diagnosis of suspected lung cancer: Summary of published evidence. Chest 123(1 SUPPL.), 115S–128S [PubMed: 12527571]
- Soja J, Szlubowski A, Kocon P, Czajkowski W, Grzanka P, Tomaszewska R, Cmiel A, and Kuzdzal J (2010) Usefulness of transbronchial needle aspiration for initial lung cancer staging. Polskie Archiwum Medycyny Wewnetrznej 120(7–8), 264–269 [PubMed: 20693956]
- Szlubowski A, Soja J, Kocon P, Talar P, Czajkowski W, Rudnicka-Sosin L, Cmiel A, and Kuzdzal J (2012) A comparison of the combined ultrasound of the mediastinum by use of a single ultrasound bronchoscope versus ultrasound bronchoscope plus ultrasound gastroscope in lung cancer staging: a prospective trial. Interactive Cardiovascular & Thoracic Surgery 15(3), 442–6; discussion 446 [PMC free article: PMC3422922] [PubMed: 22623626]
- Trisolini R, Cancellieri A, Tinelli C, de Biase, D, Valentini I, Casadei G, Paioli D, Ferrari F, Gordini G, Patelli M, and Tallini G (2015) Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 148(6), 1430–7 [PubMed: 26158441]
- Wagner E D, Ramzy I, Greenberg S D, and Gonzalez J M (1989) Transbronchial fine-needle aspiration. Reliability and limitations. American Journal of Clinical Pathology 92(1), 36–41 [PubMed: 2750706]
- Xi Y, Fan J, Che D, Zhai K, Ren T, Feng X, Shang L, Hu J, Yu Y, and Meng Q (2017) Distant metastasis and survival outcomes after computed tomography-guided needle biopsy in resected stage I-III non-small cell lung cancer. Journal of Cancer 8(16), 3356–3361 [PMC free article: PMC5665052] [PubMed: 29158808]
- Yarmus L, Kloot T, Lechtzin N, Napier M, Dressel D, and Feller-Kopman D (2011) A randomized prospective trial of the utility of rapid on-site evaluation of transbronchial needle aspirate specimens. Journal of bronchology & interventional pulmonology 18(2), 121–127 [PubMed: 23169079]
- Yarmus L, Akulian J, Ortiz R, Thompson R, Oakjones-Burgess K, Arias S, Semaan R, Feller-Kopman D, Lee H, and Wang Kp (2015) A randomized controlled trial evaluating airway inspection effectiveness during endobronchial ultrasound bronchoscopy. Journal of thoracic disease 7(10), 1825–1832 [PMC free article: PMC4635275] [PubMed: 26623106]
- Yasuda I, Kato T, Asano F, Okubo K, Omar S, Kako N, Yasuda S, Sano K, Soehendra N, and Moriwaki H (2009) Mediastinal lymph node staging in potentially resectable non-small cell lung cancer: a prospective comparison of CT and EUS/EUS-FNA. Respiration 78(4), 423–31 [PubMed: 19672051]
- Zhang R, Ying K, Shi L, Zhang L, and Zhou L (2013) Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. European Journal of Cancer 49(8), 1860–7 [PubMed: 23481511]
Health Economic studies – Included
- Luque, M., Díez, F. J., & Disdier, C. (2016). Optimal sequence of tests for the mediastinal staging of non-small cell lung cancer. BMC medical informatics and decision making, 16(1), 9. [PMC free article: PMC4727341] [PubMed: 26813400]
- Navani, N., Nankivell, M., Lawrence, D. R., Lock, S., Makker, H., Baldwin, D. R., … & Janes, S. M. (2015). Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. The Lancet Respiratory Medicine, 3(4), 282–289. [PMC free article: PMC4648022] [PubMed: 25660225]
- Sharples, L. D., Jackson, C., Wheaton, E., Griffith, G., Annema, J. T., Dooms, C., … & Buxton, M. (2012). Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. [PubMed: 22472180]
- NICE (2011) Clinical Guideline 121 Lung cancer: diagnosis and management. Appendix 4: Economic model to compare different testing strategies to stage the mediastinum in patients with NSCLC
Health Economic studies – Excluded
- Bongers, M. L., Coupé, V. M., De Ruysscher, D., Oberije, C., Lambin, P., & Uyl-de Groot, C. A. (2015). Individualized Positron Emission Tomography–Based Isotoxic Accelerated Radiation Therapy Is Cost-Effective Compared With Conventional Radiation Therapy: A Model-Based Evaluation. International Journal of Radiation Oncology* Biology* Physics, 91(4), 857–865. [PubMed: 25752401]
- Czarnecka-Kujawa, K., Rochau, U., Siebert, U., Atenafu, E., Darling, G., Waddell, T. K., … & Yasufuku, K. (2017). Cost-effectiveness of mediastinal lymph node staging in non–small cell lung cancer. The Journal of Thoracic and Cardiovascular Surgery, 153(6), 1567–1578. [PubMed: 28283236]
- Deppen, S. A., Davis, W. T., Green, E. A., Rickman, O., Aldrich, M. C., Fletcher, S., … & Grogan, E. L. (2014). Cost-effectiveness of initial diagnostic strategies for pulmonary nodules presenting to thoracic surgeons. The Annals of thoracic surgery, 98(4), 1214–1222. [PMC free article: PMC4186897] [PubMed: 25087933]
- Dietlein, M., Weber, K., Gandjour, A., Moka, D., Theissen, P., Lauterbach, K. W., & Schicha, H. (2000). Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: priority for a PET-based strategy after nodal-negative CT results. European journal of nuclear medicine, 27(11), 1598–1609. [PubMed: 11105815]
- Dietlein, M., Weber, K., Gandjour, A., Moka, D., Theissen, P., Lauterbach, K. W., & Schicha, H. (2000). Cost-effectiveness of FDG-PET for the management of solitary pulmonary nodules: a decision analysis based on cost reimbursement in Germany. European journal of nuclear medicine, 27(10), 1441–1456. [PubMed: 11083532]
- Esnaola, N. F., Lazarides, S. N., Mentzer, S. J., & Kuntz, K. M. (2002). Outcomes and cost-effectiveness of alternative staging strategies for non–small-cell lung cancer. Journal of clinical oncology, 20(1), 263–273. [PubMed: 11773178]
- Han, Y., Xiao, H., Zhou, Z., Yuan, M., Zeng, Y., Wu, H., & Fang, Y. (2015). Cost-effectiveness analysis of strategies introducing integrated 18F-FDG PET/CT into the mediastinal lymph node staging of non-small-cell lung cancer. Nuclear medicine communications, 36(3), 234–241. [PubMed: 25460305]
- Lejeune, C., Al Zahouri, K., Woronoff-Lemsi, M. C., Arveux, P., Bernard, A., Binquet, C., & Guillemin, F. (2005). Use of a decision analysis model to assess the medicoeconomic implications of FDG PET imaging in diagnosing a solitary pulmonary nodule. The European Journal of Health Economics, 6(3), 203–214. [PubMed: 15834623]
- Gómez León, N., Escalona, S., Bandrés, B., Belda, C., Callejo, D., & Blasco, J. A. (2014). 18f-fluorodeoxyglucose positron emission tomography/computed tomography accuracy in the staging of non-small cell lung cancer: Review and cost-effectiveness. Radiology Research and Practice, 2014. [PMC free article: PMC4238277] [PubMed: 25431665]
- Medford, A. R. L., Agrawal, S., Free, C. M., & Bennett, J. A. (2010). A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration, 79(6), 482–489. [PubMed: 20110643]
- Meyers, B. F., Haddad, F., Siegel, B. A., Zoole, J. B., Battafarano, R. J., Veeramachaneni, Patterson, G. A. (2006). Cost-effectiveness of routine mediastinoscopy in computed tomography–and positron emission tomography–screened patients with stage I lung cancer. The Journal of thoracic and cardiovascular surgery, 131(4), 822–829. [PubMed: 16580440]
- Navani, N., & Janes, S. M. (2013). Endobronchial Ultrasound–guided Transbronchial Needle Aspiration for Lymphoma: The Final Frontier. [PMC free article: PMC3863735] [PubMed: 24236584]
- Navani, N., Nankivell, M., Woolhouse, I., Harrison, R. N., Munavvar, M., Oltmanns, Janes, S. M. (2011). Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. Journal of Thoracic Oncology, 6(9), 1505–1509. [PMC free article: PMC3361007] [PubMed: 21792077]
- Navani, Neal, et al. “Endobronchial ultrasound–guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial.” American journal of respiratory and critical care medicine 186.3 (2012): 255–260. [PMC free article: PMC3423452] [PubMed: 22652031]
- Rintoul, Robert C., et al. “Cost effectiveness of endosonography versus surgical staging in potentially resectable lung cancer: a health economics analysis of the ASTER trial from a European perspective.” Thorax (2013): thoraxjnl-2013. [PubMed: 24064440]
- Sari, A. A., Ravaghi, H., Mobinizadeh, M., & Sarvari, S. (2013). The cost-utility analysis of PET-scan in diagnosis and treatment of non-small cell lung carcinoma in Iran. Iranian Journal of Radiology, 10(2), 61. [PMC free article: PMC3767016] [PubMed: 24046780]
- Schreyögg, J., Weller, J., Stargardt, T., Herrmann, K., Bluemel, C., Dechow, T., … & Buck, A. K. (2010). Cost-effectiveness of hybrid PET/CT for staging of non–small cell lung cancer. Journal of Nuclear Medicine, 51(11), 1668–1675. [PubMed: 21051648]
- Scott, W. J., Shepherd, J., & Gambhir, S. S. (1998). Cost-effectiveness of FDG-PET for staging non–small cell lung cancer: a decision analysis. The Annals of thoracic surgery, 66(6), 1876–1884. [PubMed: 9930463]
- Søgaard, R., Fischer, B. M. B., Mortensen, J., Rasmussen, T. R., & Lassen, U. (2013). The Optimality of Different Strategies for Supplemental Staging of Non–Small-Cell Lung Cancer: A Health Economic Decision Analysis. Value in health, 16(1), 57–65. [PubMed: 23337216]
- van Loon, J., Grutters, J. P., Wanders, R., Boersma, L., Dingemans, A. M. C., Bootsma, G., … & Snoep, G. (2010). 18 FDG-PET-CT in the follow-up of non-small cell lung cancer patients after radical radiotherapy with or without chemotherapy: an economic evaluation. European Journal of Cancer, 46(1), 110–119. [PubMed: 19944595]
- Wang, Y. T., & Huang, G. (2012). Is FDG PET/CT cost-effective for pre-operation staging of potentially operative non-small cell lung cancer?–from Chinese healthcare system perspective. European journal of radiology, 81(8), e903–e909. [PubMed: 22698711]
Appendix I. Health Economics Evidence Tables
Download PDF (201K)
Appendix J. Unit Costs of TBNA, EBUS-TBNA and EUS-FNA
Table 5. Test Costs drawn from published sources
Table 6. Micro costing of EBUS-TBNA from Navani 2012 (supplementary data)
Appendix K. Intrathoracic nodal staging of non-small cell lung cancer in patients being considered for radical treatment
Final
Evidence reviews
These evidence reviews were developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Evidence reviews for clinical and cost effectiveness of non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA alone or in combination for people with a probability of mediastinal malignancy
- Review protocols
- Methods
- Literature search strategies
- Evidence study selection for RQ 1.1 and RQ 1.2
- Clinical evidence tables
- GRADE tables
- Excluded Studies
- References
- Health Economics Evidence Tables
- Unit Costs of TBNA, EBUS-TBNA and EUS-FNA
- Intrathoracic nodal staging of non-small cell lung cancer in patients being considered for radical treatment
- Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS).[Endoscopy. 2015]Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS).Vilmann P, Clementsen PF, Colella S, Siemsen M, De Leyn P, Dumonceau JM, Herth FJ, Larghi A, Vazquez-Sequeiros E, Hassan C, et al. Endoscopy. 2015 Jun; 47(6):545-59. Epub 2015 Jun 1.
- Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions.[Endoscopy. 2005]Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions.Vilmann P, Krasnik M, Larsen SS, Jacobsen GK, Clementsen P. Endoscopy. 2005 Sep; 37(9):833-9.
- Review Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis.[Respir Care. 2015]Review Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis.Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. Respir Care. 2015 Jul; 60(7):1040-50. Epub 2015 Mar 10.
- Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: Are two better than one in mediastinal staging of non-small cell lung cancer?[J Thorac Cardiovasc Surg. 2014]Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: Are two better than one in mediastinal staging of non-small cell lung cancer?Oki M, Saka H, Ando M, Kitagawa C, Kogure Y, Seki Y. J Thorac Cardiovasc Surg. 2014 Oct; 148(4):1169-77. Epub 2014 May 15.
- Review Transesophageal endoscopic ultrasound with bronchoscope-guided fine-needle aspiration for diagnostic and staging purposes: a narrative review.[J Thorac Dis. 2023]Review Transesophageal endoscopic ultrasound with bronchoscope-guided fine-needle aspiration for diagnostic and staging purposes: a narrative review.Hong G, Oki M. J Thorac Dis. 2023 Sep 28; 15(9):5088-5098. Epub 2023 Aug 21.
- Evidence reviews for effectiveness of non-ultrasound-guided TBNA, EBUS-TBNA or E...Evidence reviews for effectiveness of non-ultrasound-guided TBNA, EBUS-TBNA or EUS-FNA for people with a probability of mediastinal malignancy
- Arabidopsis thaliana Core-2/I-branching beta-1,6-N-acetylglucosaminyltransferase...Arabidopsis thaliana Core-2/I-branching beta-1,6-N-acetylglucosaminyltransferase family protein (AT3G21310), mRNAgi|1063712919|ref|NM_001338526.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...