NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Development of a systemic anti-cancer therapy algorithm
Introduction
The following review questions were identified and included in the scope for the update of Lung cancer: diagnosis and management guideline (CG 121):
What is the clinical and cost effectiveness of the following systemic anti-cancer therapy regimens for treating NSCLC?
- platinum combinations compared with non-platinum combinations in people with advanced NSCLC (stage III or IV)
- non-platinum monotherapy compared with non-platinum combinations in people with advanced NSCLC (stage III or IV) who cannot tolerate platinum combinations
New evidence was identified during the 4-year surveillance process that may change current recommendations. This included systematic review evidence on gemcitabine plus paclitaxel and docetaxel-based doublet therapy and new RCT evidence on third-generation drugs plus platinum drugs.
The guideline committee discussed the review questions and the need for clinical guidance in this area and agreed that instead of updating the chemotherapy for NSCLC recommendations (2005 recommendations 1.4.40 – 1.4.43) the guideline update should develop an algorithm outlining the treatment pathway for systemic anti-cancer therapy treatments. This algorithm would provide a clear overview and contextualisation of systemic anti-cancer therapy treatments.
Methods and Process
The algorithms were drafted based on effectiveness and cost effectiveness recommendations from all relevant Technology Appraisals (TAs) for non-small cell lung cancer alongside expert clinical knowledge provided by the guideline committee. Additionally systematic anti-cancer therapy recommendations to support the algorithms were agreed based on recommendations from the relevant TAs.
Targeted Expert Engagement
A pre-consultation engagement exercise was agreed to support the development of the algorithms by providing selected expert groups the opportunity to review the draft algorithms ahead of the formal public stakeholder consultation.
The targeted engagement exercise is summarised below:
- The guideline committee identified the British Thoracic Oncology Group and the British Thoracic Society as professional bodies whose membership has expert knowledge of systemic anti-cancer therapy.
- Ten survey questions were drafted including seven closed and three open questions.
- The survey was administered online with a web link sent to participating organisations requesting that the survey be shared with their membership and completed online. The survey was open from 11th May to 3rd June 2018, with reminder emails, to promote a greater response.
- Additionally the Lung Cancer Clinical Expert Group (CEG) were contacted. Rather than completing the survey this stakeholder provided a series of individual comments which contributed towards the development of the algorithm.
- A second round of engagement with the Lung Cancer CEG was conducted following revision of the draft algorithm.
Results
British Thoracic Oncology Group and the British Thoracic Society (online survey)
A total of 3 responses were received from the online survey.
| Lung cancer - targetted consultation - 3 survey results received | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q2 | Do you think the algorithm is organised in a helpful and understandable way? | ||||||||
| RESPONSES | |||||||||
| Yes 2 | No 1 | ||||||||
| Q3 | Do you think the algorithm is organised in a helpful and understandable way? Please explain your response | ||||||||
| RESPONSES | |||||||||
| its quite confusing in the top algorithm with the arrows switching sides. Would it be better for 1 algorithm with targetable mutations and one without? Each individual side is easy to follow | No | ||||||||
| There are a few omissions and inaccuracies which have been corrected. | Yes | ||||||||
| Q4 |
The current Lung Cancer diagnosis and management update (CG121) recommends the below: Chemotherapy for advanced NSCLC should be a combination of a single third generation drug (docetaxel, gemcitabine, paclitaxel or vinorelbine) plus a platinum drug. Either carboplatin or cisplatin may be administered, taking account of their toxicities, efficacy and convenience. [2005 CGC 121 recommendation] The update committee would like to remove docetaxel from this recommendation as they are of the view that docetaxel should not be used with a platinum drug. Do you agree? | ||||||||
| RESPONSES | |||||||||
| Yes 1 | No 1 | ||||||||
| Q4 | If you disagree, please explain why? | ||||||||
| Docetaxel and carboplatin is a valid combination licensed and appraised. Not used very much but should be included as an option | |||||||||
| Q5 | Please review the statements below and rate them on your level of agreement, 1 being strongly disagree, 5 being strongly agree. | ||||||||
| Q5a | The algorithm and recommendations reflect best clinical practice | ||||||||
| RESPONSES | |||||||||
| Strongly disagree | Disagree | Neither agree/disagree | Agree | Strongly agree | |||||
| 1 | 1 | 1 | |||||||
| Q5b | The algorithm and recommendations reflect the wording and intentions of NICE’s guidance in this area to date | ||||||||
| RESPONSES | |||||||||
| Strongly disagree | Disagree | Neither agree/disagree | Agree | Strongly agree | |||||
| 1 | 2 | ||||||||
| Q6 | Please explain your ratings for the above statements | ||||||||
| RESPONSES | |||||||||
| Consider carboplatin as well as cisplatin in recommending platinum based chemotherapy | |||||||||
| Generally fit for purpose and reflect NICE guidance but some inaccuracies which have been corrected on behalf of BTOG and returned to NICE | |||||||||
| Q7 | Are you aware of any regional variation in the implementation of systematic anti-cancer therapy guideline recommendations? | ||||||||
| RESPONSES | |||||||||
| Yes 2 | No 1 | ||||||||
| Q7a | If yes, please explain | ||||||||
| RESPONSES | |||||||||
| Access to better diagnostics and pathology. Knowledge of access of drugs through cdf as very frequent changes | |||||||||
| Q8 | Finally, how can the algorithm be improved (if at all)? Please use the box to suggest any changes you think should be made? | ||||||||
| RESPONSES | |||||||||
| Algorithm amended on behalf of BTOG Main division should be squamous vs nonsquamous PDL1 >50% and >1-49% and unknown Nivolumab TA484 omitted please see amendments | |||||||||
Lung Cancer Clinical Expert Group (CEG)
Comments from the first round of engagement with the Lung Cancer (CEG) are summarised below:
- There is a clear need for the algorithms to reflect current clinical practice alongside the recommendations from relevant technology appraisal (TA) guidance.
- The algorithms will require updating as new evidence emerges either through research or the publication of further TA guidance.
- Identification of a number of factual errors in the initial draft algorithms particularly in relation to genetic markers for disease, the possible variations in 1st, 2nd and 3rd line treatment based on clinical practice and the overall presentation of the algorithms e.g. by histology or squamous/ non-squamous sub-type.
Comments from the second round of engagement with the Lung Cancer (CEG) are summarised below:
- The systematic anti-cancer therapy recommendations were considered repetitive for the reader and would be better paraphrased.
- The need to keep the algorithm updated was again emphasised.
- The recommendations need to include the caveats imposed on the Cancer Drug Fund.
- The need to take into account the further restrictions in practice imposed by accessing therapies through the Blueteq system.
- The algorithm does not reflect current practice as this is driven by the NHS England’s permissions stated by each CDF drug indication which is usually a more limited remit of the NICE approved indication.
Committee Discussion
The committee discussed the outcome of the online survey and the feedback received from the CEG. It was agreed that the results from the online survey were limited and mixed. Therefore they provided no clear direction for further development of the algorithms.
The Lung Cancer CEG comments were discussed by the guideline committee and further revisions made. The committee had initially proposed removing recommendation 1.4.41 which recommends docetaxel combined with a platinum drug for advanced NSCLC as they considered this combination to be toxic and poorly tolerated by patients. However comments from the lung cancer CEG indicated that experts were content to keep docetaxel combined with a platinum drug for advanced NSCLC as an option for systemic therapy. This treatment option has therefore remained.
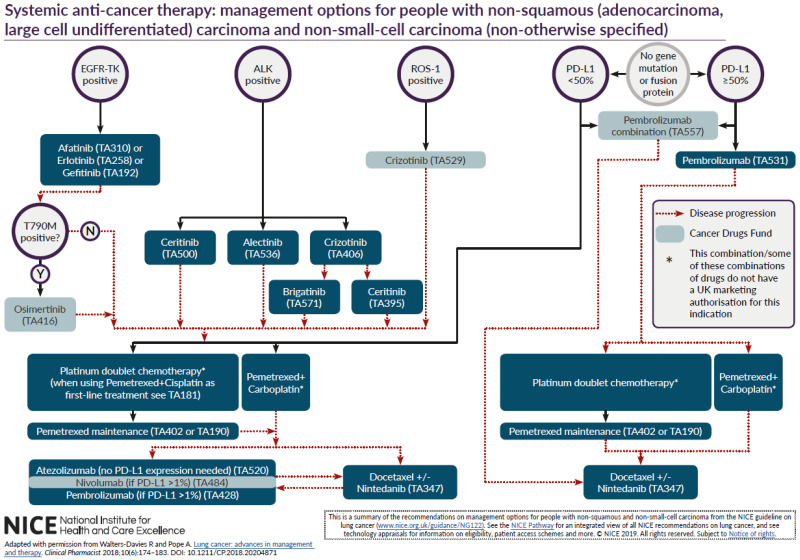
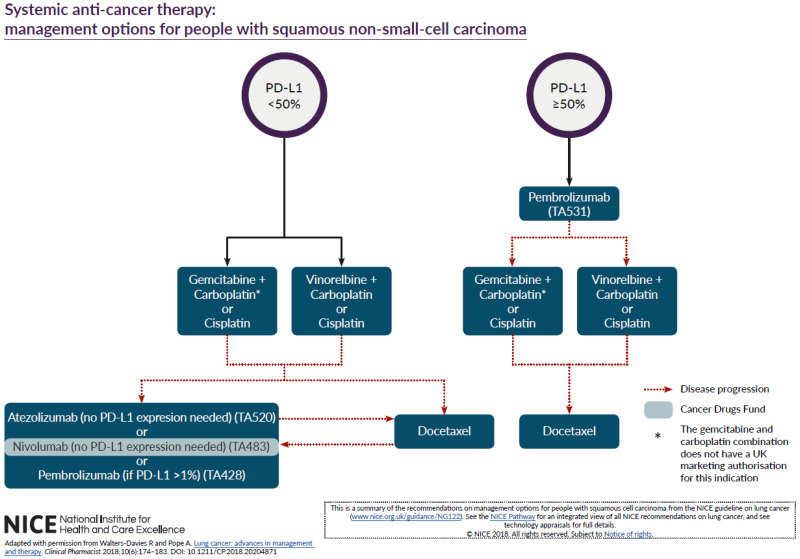
The committee discussed the difficulties in developing a set of algorithms which are able to reflect NICE’s TAs in the context of clinical practice and recommended that the algorithm be adapted from a published algorithm developed by Rhiannon Walters-Davies and Anthony Pope (Clinical Pharmacist, June 2018, Volume 10 (6)). Copyright permission was obtained by NICE to use this published algorithm although some modifications were made in line with comments received and using the wider experience and expertise of the committee.
A set of written recommendations were also discussed and agreed by the committee to support the algorithms. Because this exercise did not have an accompanying evidence review, the committee were unable to stratify their recommendations by the performance status of patients. It is recognised, however, that most patients in clinical trials of systemic therapy have a good performance status and a reasonable prospect of treatment improving their survival and that this should reflect the population being offered systemic therapy in clinical practice.
Amendments following the targeted engagement exercise
Between consultation and publication of this guideline, technology appraisal guidance on first line pembrolizumab combination therapy and brigatinib after crizotinib was published. The non-squamous algorithm and associated guideline recommendations were updated to reflect their place in the treatment pathway. The committee decided not to include negative technology appraisal guidance in the algorithms or recommendations for presentational clarity. They also decided to omit positive technology appraisal guidance on 2nd line crizotinib (TA422) because the evidence for this TA related to people who are ALK positive but had been treated with chemotherapy first line and they felt that patients would now optimally be treated with targeted therapy first line. This is because ALK testing is now routine in clinical practice. They also decided to omit TA374, which includes positive guidance on erlotinib after delayed confirmation of EGFR positive status, again because they felt that EGFR testing is routine practice and people would be treated with targeted agents first line. However, it should be noted that both of these technology appraisals remain extant guidance.
The algorithm indicates that immunotherapy may be considered after docetaxel+/- nintedanib in some patients and vice versa. It should be noted that the intent of this area of the algorithm is not to suggest that people go “back and forth” between the two but that one may be tried on progression in certain circumstances if the other has been given first. The committee felt that this only applied to very few patients and chose not to make specific recommendations about this area of the algorithm, feeling that it was an area of specialist care best left to clinical judgement and patient preference.
Final
Evidence reviews
These evidence reviews were developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Review [Gemcitabine in the first line therapy of advanced and metastatic non-small-cell lung carcinoma (NSCLC): review of the results of phase III studies].[Onkologie. 2005]Review [Gemcitabine in the first line therapy of advanced and metastatic non-small-cell lung carcinoma (NSCLC): review of the results of phase III studies].Langer F, Helsberg K, Schütte WH, Leschinger MI. Onkologie. 2005 Mar; 28 Suppl 1:1-28. Epub 2005 Mar 30.
- Review Paclitaxel: a pharmacoeconomic review of its use in non-small cell lung cancer.[Pharmacoeconomics. 2001]Review Paclitaxel: a pharmacoeconomic review of its use in non-small cell lung cancer.Plosker GL, Hurst M. Pharmacoeconomics. 2001; 19(11):1111-34.
- Review First-line chemotherapy for advanced-stage non-small-cell lung cancer: focus on docetaxel.[Clin Lung Cancer. 2005]Review First-line chemotherapy for advanced-stage non-small-cell lung cancer: focus on docetaxel.Ramalingam S. Clin Lung Cancer. 2005 Dec; 7 Suppl 3:S77-82.
- Review Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline.[Lung Cancer. 2005]Review Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline.Chu Q, Vincent M, Logan D, Mackay JA, Evans WK, Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care. Lung Cancer. 2005 Dec; 50(3):355-74. Epub 2005 Aug 31.
- Review Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.[Health Technol Assess. 2006]Review Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, Stark D, Palmer S, Riemsma R. Health Technol Assess. 2006 Mar; 10(9):1-132. iii-iv.
- Development of a systemic anti-cancer therapy algorithmDevelopment of a systemic anti-cancer therapy algorithm
- PREDICTED: Homo sapiens histone deacetylase 4 (HDAC4), transcript variant X21, m...PREDICTED: Homo sapiens histone deacetylase 4 (HDAC4), transcript variant X21, mRNAgi|2462578914|ref|XM_054344741.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...