NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Evidence reviews for the clinical and cost-effectiveness of first use of thoracic radiotherapy for people with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies
Review questions
RQ 3.5: In people with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies, when is first use of thoracic radiotherapy clinically and cost effective?
Introduction
New evidence has become available since the previous guideline was published that may have an impact on existing recommendations. A randomised controlled trial (RCT) suggests that some people with extensive-stage SCLC with a partial response to first-line treatment have improved survival if they have thoracic radiotherapy and prophylactic cranial irradiation (PCI) compared to those who have PCI alone (Slotman 2015). Experts advise us that oncologists are already adopting the approach in their practice. Therefore, this review aims to compare thoracic radiotherapy to no thoracic radiotherapy for people with extensive-stage SCLC who have had had first-line treatment with systemic anti-cancer therapies who have had a partial response.
Table 1PICO table
| Population | People with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies who have had a partial response |
|---|---|
| Intervention | Thoracic radiotherapy |
| Comparator | No thoracic radiotherapy |
| Outcomes |
|
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual (2014). Methods specific to this review question are described in the review protocol in appendix A, and the methods section in appendix B. In particular, the minimally important differences (MIDs) used in this review are summarised in appendix B.
Declarations of interest were recorded according to NICE’s 2018 conflicts of interest policy.
Clinical evidence
Included studies
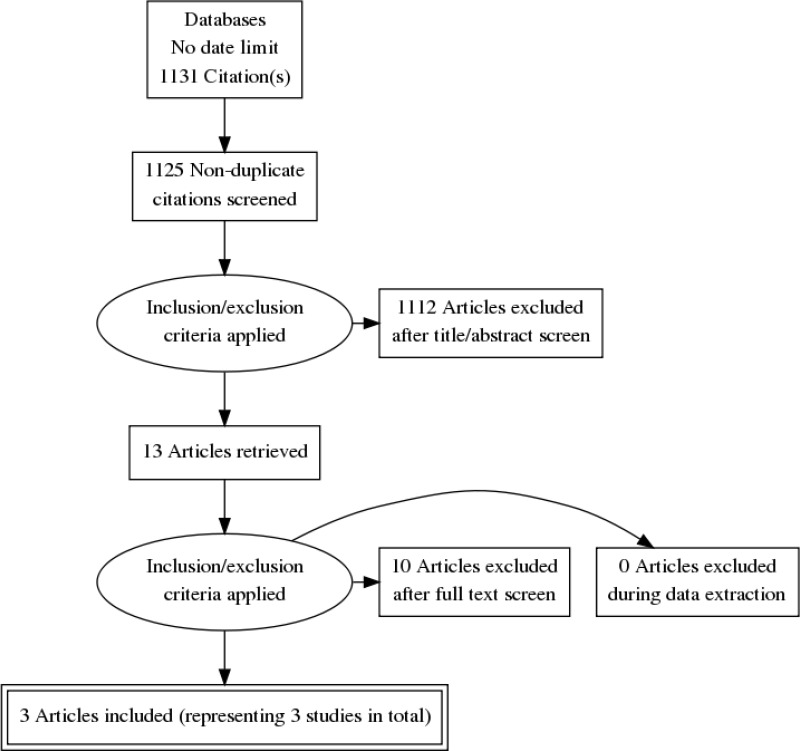
This review was conducted as part of a larger update of the NICE Lung cancer: diagnosis and management guideline (CG121). A systematic literature search for RCTs and systematic reviews of RCTs with no date limit yielded 1,131 references.
Papers returned by the literature search were screened on title and abstract, with 13 full-text papers ordered as potentially relevant RCTs, systematic reviews of RCTs or if no RCT data available, quasi-randomised controlled trials or prospective data. Studies were excluded if they did not meet the criteria of enrolling participants with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies who have had a partial response.
Three papers representing 3 unique RCTs, were included after full text screening: Gore 2017 (RCT, n=86, indefinite follow-up but with a median of 9 months), Slotman 2015 (RCT, n=495, indefinite follow-up but with a median of 24 months), Jeremic 1999 (RCT, n=109, indefinite follow-up but with a median of 9 months),
For the search strategy, please see appendix C. For the clinical evidence study selection flowchart, see appendix D. For the full evidence tables and full GRADE profiles for included studies, please see appendix E and appendix F.
Excluded studies
Details of the studies excluded at full-text review are given in appendix H along with a reason for their exclusion.
Summary of clinical studies included in the evidence review
Three randomised controlled studies were included in this review.
Study locations
One RCT was from the Netherlands, UK, Norway and Belgium, 1 RCT was from the USA, and 1 RCT was from Yugoslavia.
Outcomes and sample sizes
The reported outcomes with extractable data were mortality (hazard ratio, survival rates at various intervals and median survival), response to treatment (median disease-free survival, hazard ratio for time to progression, risk ratio whose cancer had progressed at various intervals, median time to first relapse and duration of response) and the risk ratio of participants who experienced a grade 3 or higher adverse event. The sample sizes for the 3 RCTs were n=690 altogether.
See full evidence tables and Grade profiles Appendix E and Appendix F.
Quality assessment of clinical studies included in the evidence review
See appendix F for full GRADE tables.
Economic evidence
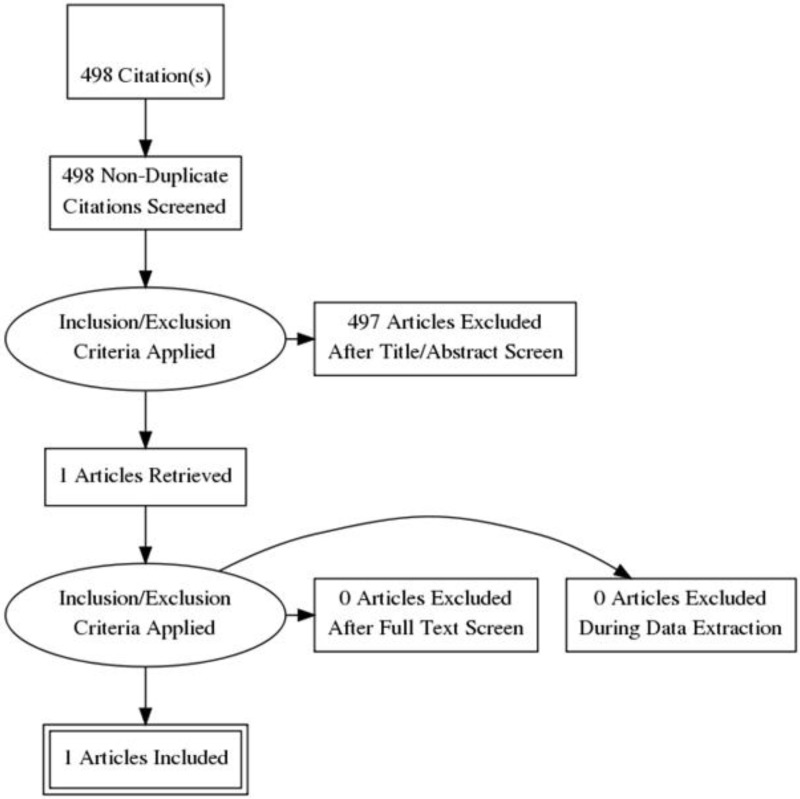
Standard health economic filters were applied to the clinical search for this question, and a total of 498 citations was returned. Details of the literature search are provided in Appendix C. Following review of titles and abstracts, 1 full-text study was retrieved for detailed consideration. One relevant cost–utility analysis with a partitioned survival model was identified. Therefore 1 study was included in this review.
Thoracic Radiation Therapy in Extensive-Stage Small Cell Lung Cancer
Patrice et al. (2017) conducted a cost-utility study comparing standard therapy with thoracic radiation therapy versus stand therapy alone for extensive-stage small cell lung cancer (ES-SLCL). Treatment effects were from the Chest Radiotherapy Extensive-Stage Small Cell Lung Cancer Trial (CREST, RCT (Nederlands Trial Register, number NTR1527, n=498). This study is Slotman 2015, which is included in this review. People who participated in CREST had demonstrated a response to induction chemotherapy. Participants were randomised to receive PCI with Thoracic Radiation Therapy (TRT) (n=247) or PCI alone (n=248).
A partitioned survival model was created to estimate the direct medical costs and QALYs from a US health care payers’ perspective. The base case time horizon was 24 months (consistent with the maximum progression free survival) whilst an additional analysis had a time horizon of the participants’ lifetime. Parametric probability distributions were independently fitted to the estimated individual patient time-to-event for OS and PFS for each treatment group to address uncertainty associated with small patient numbers at the tails of the Kaplan-Meier survival curves. Curve fitting was performed in the R program (R Foundation for Statistical Computing, Vienna, Austria).
Participants entered the model in the progression free survival health state after completing the induction chemotherapy.
TRT costs were obtained from the 2016 Centers for Medicare & Medicaid Services Physician Fee Schedule (CMSPFS) national payment amount. Post-treatmentt surveillance costs associated with the PFS health state were obtained from the 2016 CMSPFS and included a level 3 established patient office visit, chest and/or abdominal computed tomography scans, and laboratory work every 3 months during years 1 and 2, every 6 months during years 3 through 5, and annually thereafter. At the time of progression, an additional 1-time cost was incurred for workup and restaging of disease that was derived from the relapse patterns reported in the CREST and calculated using the 2016 CMSPFS.
The model assumed that PPS costs were incurred through the second to last month of life, and the terminal cost was assigned in the last month of life. Costs were inflated to 2016 US dollars using the medical care component of the US Chained Consumer Price Index. A discount rate of 3% was used for costs and outcomes beyond one year.
Patient preferences for the PFS and PPS health states associated with metastatic lung cancer were obtained from the literature and were elicited from members of the general public using standard gamble techniques (Nafees, 2008). Utility values for metastatic non-SCLC were used as a proxy for the comparable ES-SCLC health states based on available data.
Results of the study are shown in Table 2 and Table 3.
Table 2
Results from Patrice (2017) for Thoracic Radiation Therapy with Standard Therapy compared to Standard Therapy Alone (24 month horizon).
Table 3
Results from Patrice (2017) for Thoracic Radiation Therapy with Standard Therapy compared to Standard Therapy Alone (Patient lifetime horizon).
In the base case 24 month scenario analysis, the TRT strategy produced 0.049 QALYs whilst resulting in a saving of $538, rending TRT as dominant as compared to Standard Therapy alone. In the patient life time horizon analysis, the TRT strategy resulted in an ICER of $194,726/QALY. The authors explained this relatively high ICER by highlighting that post-treatment participants who had survived experienced high costs of salvage therapy.
In the 24 months one-way deterministic sensitivity analysis, the TRT ICER was found to be most sensitive to changes in the parameters of the TRT and ST PFS and OS distributions. In the patient lifetime one-way deterministic sensitivity analysis, the use of alternative PFS distributions resulted in the TRT ICERs ranging from $79,291 to $381,264. For the 24-month time horizon probabilistic sensitivity analysis, TRT was expected to be cost-effective and preferred over the ST strategy in 68%, 81%, and 96% of the simulations at willingness-to-pay thresholds of $50,000/QALY, $100,000/QALY, and $200,000/QALY, respectively. In contrast, when a lifetime horizon was assumed, ST was expected to be cost-effective and preferred over the TRT strategy in 89%, 82%, and 55% of the simulations at willingness-to-pay thresholds of $50,000/QALY, $100,000/QALY, and $200,000/QALY, respectively.
The authors concluded that by use of the actual follow-up interval reported in the CREST, adding TRT to chemotherapy and PCI strongly dominates a strategy of chemotherapy and PCI alone in participants with ES SCLC. Since the long-term incremental survival benefit of TRT is small relative to ongoing incremental costs to manage progressive metastatic disease, the ICER of TRT is less favourable and situated near the upper boundary of contemporary thresholds for cost-effectiveness when evaluating a lifetime scenario.
Evidence statements
For all participants who had at least a partial response to chemotherapy: thoracic radiotherapy + prophylactic cranial irradiation (PCI) vs PCI only
Very low to low-quality evidence from 2 RCTs reporting data on 581 people who had at least a partial response found that the data favoured those who had thoracic radiotherapy + PCI for the risk ratio of people still alive at 1.5 and 2 years, hazard ratio for progression and the risk ratio of cancer progression at 3 months compared to people who had PCI. However, the data could not differentiate mortality (all-cause hazard ratio), progression-free survival at 6 months, risk ratio for cancer progression at 1 year and adverse events.
For participants who had a complete extra-thoracic response (and who had either a complete or partial thoracic response to chemotherapy: Accelerated hyperfractionated radiation therapy + carboplatin/etoposide + PCI + 2x cisplatin/etoposide vs 2x cisplatin/etoposide + PCI + 2x cisplatin/etoposide
Very low to low-quality evidence from 1 RCT reporting data on 109 people who had a complete extra-thoracic response (and who had either a complete or partial thoracic response) found that the data favoured accelerated hyperfractionated radiation therapy for mortality (risk ratio of people alive at 1, 2, 3, 4 and 5 years), the risk ratio of people experiencing nausea and vomiting grade 3 and above, the risk ratio of people experiencing alopecia grade 3 or above and the risk ratio of people experiencing kidney toxicity grade 3 or above compared to people who had no radiation therapy. The data favoured people who had no radiation therapy for the risk ratio of people experiencing oesophageal toxicity grade 3 or above compared to accelerated hyperfractionated radiation therapy. However, the data could not differentiate thoracic recurrence-free survival at 5 years, extra-thoracic metastases-free survival at 5 years, the risk ratio of people experiencing leukopenia grade 3 or above, the risk ratio of people experiencing thrombocytopenia grade 3 or above, the risk ratio of people experiencing anaemia grade 3 or above, the risk ratio of people experiencing infection grade 3 or above, the risk ratio of people experiencing bronchopulmonary toxicity grade 3 or above or the risk ratio of people requiring hospital admission for an adverse event.
Health economics evidence statement
One partially applicable partitioned survival model with minor limitations compared thoracic radiation therapy and standard therapy with standard therapy alone for participants with extensive-small cell lung cancer in the US. In the base case 24 month analysis, the thoracic radiation therapy was found to be less expensive and more effective than standard therapy alone, and therefore a dominant treatment strategy. In the life time analysis, the ICER was found to be $194,726 per QALY. The lifetime analysis showed that the difference in the effectiveness of the treatments was 0.09 QALYs (0.16 life years).
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The committee agreed that the outcome that matters most is mortality. This is because in the opinion of the committee, the life expectancy for someone with SCLC is generally so short that just a few months of extra life makes a lot of difference.
The quality of the evidence
The committee agreed that the quality of the evidence was low or very low. The committee agreed that the methods used in Slotman 2015 reflect UK practice whereas the methods used in Jeremic 1999 and Gore 2017 do not. For example, Slotman 2015 had a total radiation dose of 30 Gy. By contrast, Jeremic 1999 used a total radiation dose of 54 Gy and Gore 2017 used a total radiation dose of 45 Gy. Slotman 2015 used both 2D and 3D radiotherapy planning techniques but Jeremic 1999 did not. The committee agreed that Slotman 2015 was better quality than Gore 2017. This is because in Gore 2017, those randomised to the thoracic radiotherapy plus PCI arm were on average 5 years older compared to the PCI only arm (comparing median ages of the two groups). A potential risk of bias in Slotman 2015 is that measuring mortality beyond 1 year was not included in the study protocol. However, measuring mortality beyond 1 year is usually normal for cancer studies that include mortality as an outcome.
Benefits and harms
The committee agreed that the recommendation should be a “consider” because there was inconsistency across studies and the benefits of thoracic radiotherapy, such as survival, are experienced by a minority of people who undergo the intervention. For example in Slotman 2015, there is no difference in mortality at 1 year for people who have thoracic radiotherapy and those who do not. However, the data favours thoracic radiotherapy compared to no radiotherapy at 1.5 years and 2 years. This might suggest there is a subgroup of participants who respond to treatment better than others do. However, there is insufficient data to investigate this possibility further.
The committee agreed that the disadvantage to people receiving thoracic radiotherapy would be the journeys that they would have to make to hospital in order to receive it. However, the committee agreed that this would be outweighed by the advantage of improved survival.
In Slotman 2015 and Gore 2017, the data could not differentiate for adverse events grade 3 or above. However, the investigators did not state that they powered these studies to detect adverse events. In the committee’s experience, some people do experience adverse events but the potential benefit of increased survival is more important to patients.
In Jeremic 1999, more people receiving thoracic radiotherapy experienced oesophageal toxicity grade 3 or above compared to people who did not have thoracic radiotherapy. However, the total dose of radiation was relatively high at 54 Gy compared to 30 Gy in Slotman 2015, which is more representative of current practice.
The committee specified that thoracic radiotherapy should be given alongside prophylactic cranial irradiation. This is to match recommendation 1.4.92 and how thoracic radiotherapy was used in all 3 RCTs they reviewed. There was no evidence on the effectiveness of thoracic radiotherapy alone in the 3 RCTs. With regards to recommendation 1.4.92, people who have prophylactic cranial irradiation have improved survival. This was the finding of the study most relevant to UK practice (Slotman 2007).
Slotman 2015 was the RCT that most closely resembled current practice. This study involved administering thoracic radiotherapy and PCI to participants who had a partial response at distant sites and within the thorax. Therefore, the committee agreed that the recommendation should reflect this.
Cost effectiveness and resource use
The Patrice et al 2017 cost-effectiveness analysis that was included in this review was based on the clinical evidence from the Slotman 2015 trial. This is a US based analysis so the costs and ICERs are not relevant to the UK context, but, as the underpinning clinical data were based on the Slotman 2015 trial and the methods used to calculate QALYs were high quality and not health system specific, the committee considered the estimates of differential QALY gain to be relevant. The committee considered this evidence along with the QALYs only analysis of PCI (Evidence Review H) and noted that it was highly likely that offering both interventions together would be cost effective, particularly as much, if not all, of the costs of the intervention can be shared. This means that in many situations, the addition of thoracic radiotherapy to prophylactic cranial irradiation will gain QALYs with a negligible up front resource use. A full discussion of this evidence can be found in Appendix J of Evidence Review H.
According to advice from experts, oncologists in the UK are already adopting the thoracic radiotherapy approach in Slotman 2015. While this recommendation applies to people with a greater range of thoracic response than the previous guideline recommendation, it also specifies that thoracic radiotherapy should only be considered alongside prophylactic cranial irradiation. The committee changed the ‘offer’ recommendation for prophylactic cranial irradiation made in the previous guideline to a ‘consider’, which might lead to a small reduction in its use and therefore the number of situations in which thoracic radiotherapy is considered. Therefore, this recommendation is expected to lead to a negligible change in resource use.
Other factors the committee took into account
The committee noted that in the studies it was a requirement for the participants to have a good performance status before thoracic radiotherapy was undertaken.
Appendix A. Review protocols
Review protocol for when first use of thoracic radiotherapy is clinically and cost effective for people with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies
In people with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies, when is first use of thoracic radiotherapy clinically and cost effective?
| Field (based on PRISMA-P) | Content |
|---|---|
| Review question | In people with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies, when is first use of thoracic radiotherapy clinically and cost effective? |
| Type of review question | Intervention |
| Objective of the review | This area was identified as requiring updating in the 2016 surveillance review. The aim of the evidence review is to establish at what point during systemic anti-cancer therapy thoracic radiotherapy should be offered. |
| Eligibility criteria – population | People with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies who have had a partial response |
| Eligibility criteria – interventions | Thoracic radiotherapy |
| Eligibility criteria – comparator | No thoracic radiotherapy |
| Outcomes and prioritisation |
|
| Eligibility criteria – study design |
|
| Other inclusion exclusion criteria |
|
| Proposed sensitivity/sub-group analysis, or meta-regression | Partial or complete vs stable response to thoracic radiotherapy |
| Selection process – duplicate screening/selection/analysis |
10% of the abstracts were reviewed by two reviewers, with any disagreements resolved by discussion or, if necessary, a third independent reviewer. If meaningful disagreements were found between the different reviewers, a further 10% of the abstracts were reviewed by two reviewers, with this process continued until agreement is achieved between the two reviewers. From this point, the remaining abstracts will be screened by a single reviewer. This review made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. See Appendix B for more details. |
| Data management (software) | See Methods Appendix B |
| Information sources – databases and dates | See Appendix C Main Searches:
Citation searching will be carried out in addition on analyst/committee selected papers. The search will not be date limited because this is a new review question. Economics:
The search will not be date limited because this is a new review question. |
| Identify if an update |
This is to update the following recommendation: 1.4.54 Offer prophylactic cranial irradiation to patients with extensive-stage disease SCLC and WHO performance status 2 or less, if their disease has not progressed on first-line treatment. [new 2011] |
| Author contacts | Guideline update |
| Highlight if amendment to previous protocol | For details please see section 4.5 of Developing NICE guidelines: the manual |
| Search strategy – for one database | For details please see appendix C |
| Data collection process – forms/duplicate | A standardised evidence table format will be used, and published as appendix F (clinical evidence tables) or I (economic evidence tables). |
| Data items – define all variables to be collected | For details please see evidence tables in appendix F (clinical evidence tables) or I (economic evidence tables). |
| Methods for assessing bias at outcome/study level | See Appendix B |
| Criteria for quantitative synthesis | See Appendix B |
| Methods for quantitative analysis – combining studies and exploring (in)consistency | See Appendix B |
| Meta-bias assessment – publication bias, selective reporting bias | See Appendix B |
| Confidence in cumulative evidence | See Appendix B |
| Rationale/context – what is known | For details please see the introduction to the evidence review in the main file. |
| Describe contributions of authors and guarantor |
A multidisciplinary committee developed the evidence review. The committee was convened by the NICE Guideline Updates Team and chaired by Gary McVeigh in line with section 3 of Developing NICE guidelines: the manual. Staff from the NICE Guideline Updates Team undertook systematic literature searches, appraised the evidence, conducted meta-analysis and cost-effectiveness analysis where appropriate, and drafted the evidence review in collaboration with the committee. For details please see Developing NICE guidelines: the manual. |
| Sources of funding/support | The NICE Guideline Updates Team is an internal team within NICE. |
| Name of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| Roles of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| PROSPERO registration number | N/A |
Appendix B. Methods
1.1. Priority screening
The reviews undertaken for this guideline all made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. This uses a machine learning algorithm (specifically, an SGD classifier) to take information on features (1, 2 and 3 word blocks) in the titles and abstract of papers marked as being ‘includes’ or ‘excludes’ during the title and abstract screening process, and re-orders the remaining records from most likely to least likely to be an include, based on that algorithm. This re-ordering of the remaining records occurs every time 25 additional records have been screened.
Research is currently ongoing as to what are the appropriate thresholds where reviewing of abstract can be stopped, assuming a defined threshold for the proportion of relevant papers it is acceptable to miss on primary screening. As a conservative approach until that research has been completed, the following rules were adopted during the production of this guideline:
- In every review, at least 50% of the identified abstract (or 1,000 records, if that is a greater number) were always screened.
- After this point, screening was only terminated when the threshold was reached for a number of abstracts being screened without a single new include being identified. This threshold was set according to the expected proportion of includes in the review (with reviews with a lower proportion of includes needing a higher number of papers without an identified study to justify termination), and was always a minimum of 250.
- A random 10% sample of the studies remaining in the database when the threshold were additionally screened, to check if a substantial number of relevant studies were not being correctly classified by the algorithm, with the full database being screened if concerns were identified.
As an additional check to ensure this approach did not miss relevant studies, the included studies lists of included systematic reviews were searched to identify any papers not identified through the primary search.
1.2. Incorporating published systematic reviews
For all review questions where a literature search was undertaken looking for a particular study design, systematic reviews containing studies of that design were also included. All included studies from those systematic reviews were screened to identify any additional relevant primary studies not found as part of the initial search.
1.2.1. Quality assessment
Individual systematic reviews were quality assessed using the ROBIS tool, with each classified into one of the following three groups:
- High quality – It is unlikely that additional relevant and important data would be identified from primary studies compared to that reported in the review, and unlikely that any relevant and important studies have been missed by the review.
- Moderate quality – It is possible that additional relevant and important data would be identified from primary studies compared to that reported in the review, but unlikely that any relevant and important studies have been missed by the review.
- Low quality – It is possible that relevant and important studies have been missed by the review.
Each individual systematic review was also classified into one of three groups for its applicability as a source of data, based on how closely the review matches the specified review protocol in the guideline. Studies were rated as follows:
- Fully applicable – The identified review fully covers the review protocol in the guideline.
- Partially applicable – The identified review fully covers a discrete subsection of the review protocol in the guideline (for example, some of the factors in the protocol only).
- Not applicable – The identified review, despite including studies relevant to the review question, does not fully cover any discrete subsection of the review protocol in the guideline.
1.2.2. Using systematic reviews as a source of data
If systematic reviews were identified as being sufficiently applicable and high quality, and were identified sufficiently early in the review process (for example, from the surveillance review or early in the database search), they were used as the primary source of data, rather than extracting information from primary studies. The extent to which this was done depended on the quality and applicability of the review, as defined in Table 4. When systematic reviews were used as a source of primary data, and unpublished or additional data included in the review which is not in the primary studies was also included. Data from these systematic reviews was then quality assessed and presented in GRADE/CERQual tables as described below, in the same way as if data had been extracted from primary studies. In questions where data was extracted from both systematic reviews and primary studies, these were cross-referenced to ensure none of the data had been double counted through this process.
Table 4. Criteria for using systematic reviews as a source of data
1.3. Evidence synthesis and meta-analyses
Where possible, meta-analyses were conducted to combine the results of quantitative studies for each outcome. For continuous outcomes analysed as mean differences, where change from baseline data were reported in the trials and were accompanied by a measure of spread (for example standard deviation), these were extracted and used in the meta-analysis. Where measures of spread for change from baseline values were not reported, the corresponding values at study end were used and were combined with change from baseline values to produce summary estimates of effect. These studies were assessed to ensure that baseline values were balanced across the treatment groups; if there were significant differences at baseline these studies were not included in any meta-analysis and were reported separately. For continuous outcomes analysed as standardised mean differences, where only baseline and final time point values were available, change from baseline standard deviations were estimated, assuming a correlation coefficient of 0.5.
1.4. Evidence of effectiveness of interventions
1.4.1. Quality assessment
Individual RCTs and quasi-randomised controlled trials were quality assessed using the Cochrane Risk of Bias Tool. Other study were quality assessed using the ROBINS-I tool. Each individual study was classified into one of the following three groups:
- Low risk of bias – The true effect size for the study is likely to be close to the estimated effect size.
- Moderate risk of bias – There is a possibility the true effect size for the study is substantially different to the estimated effect size.
- High risk of bias – It is likely the true effect size for the study is substantially different to the estimated effect size.
Each individual study was also classified into one of three groups for directness, based on if there were concerns about the population, intervention, comparator and/or outcomes in the study and how directly these variables could address the specified review question. Studies were rated as follows:
- Direct – No important deviations from the protocol in population, intervention, comparator and/or outcomes.
- Partially indirect – Important deviations from the protocol in one of the population, intervention, comparator and/or outcomes.
- Indirect – Important deviations from the protocol in at least two of the following areas: population, intervention, comparator and/or outcomes.
1.4.2. Methods for combining intervention evidence
Meta-analyses of interventional data were conducted with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al. 2011).
Where different studies presented continuous data measuring the same outcome but using different numerical scales (e.g. a 0–10 and a 0–100 visual analogue scale), these outcomes were all converted to the same scale before meta-analysis was conducted on the mean differences. Where outcomes measured the same underlying construct but used different instruments/metrics, data were analysed using standardised mean differences (Hedges’ g).
A pooled relative risk was calculated for dichotomous outcomes (using the Mantel–Haenszel method) reporting numbers of people having an event, and a pooled incidence rate ratio was calculated for dichotomous outcomes reporting total numbers of events. Both relative and absolute risks were presented, with absolute risks calculated by applying the relative risk to the pooled risk in the comparator arm of the meta-analysis (all pooled trials).
Fixed- and random-effects models (der Simonian and Laird) were fitted for all syntheses, with the presented analysis dependent on the degree of heterogeneity in the assembled evidence. Fixed-effects models were the preferred choice to report, but in situations where the assumption of a shared mean for fixed-effects model were clearly not met, even after appropriate pre-specified subgroup analyses were conducted, random-effects results are presented. Fixed-effects models were deemed to be inappropriate if one or both of the following conditions was met:
- Significant between study heterogeneity in methodology, population, intervention or comparator was identified by the reviewer in advance of data analysis. This decision was made and recorded before any data analysis was undertaken.
- The presence of significant statistical heterogeneity in the meta-analysis, defined as I2≥50%.
In any meta-analyses where some (but not all) of the data came from studies at high risk of bias, a sensitivity analysis was conducted, excluding those studies from the analysis. Results from both the full and restricted meta-analyses are reported. Similarly, in any meta-analyses where some (but not all) of the data came from indirect studies, a sensitivity analysis was conducted, excluding those studies from the analysis.
Meta-analyses were performed in Cochrane Review Manager V5.3, with the exception of incidence rate ratio analyses which were carried out in R version 3.3.4.
1.4.3. Minimal clinically important differences (MIDs)
The Core Outcome Measures in Effectiveness Trials (COMET) database was searched to identify published minimal clinically important difference thresholds relevant to this guideline. However, no relevant MIDs were found. In addition, the Guideline Committee were asked to specify any outcomes where they felt a consensus MID could be defined from their experience. In particular, any questions looking to evaluate non-inferiority (that one intervention is not meaningfully worse than another) required an MID to be defined to act as a non-inferiority margin. However, the committee agreed that in their experience, they could not define any MIDs. This is because the committee agreed that the protocol outcomes were objective rather than subjective measures and the committee were not aware of evidence supporting the use of MIDs for the protocol’s outcomes. Therefore, the line of no effect was used as the MID for risk ratios, hazard ratios and mean differences.
1.4.4. GRADE for pairwise meta-analyses of interventional evidence
GRADE was used to assess the quality of evidence for the selected outcomes as specified in ‘Developing NICE guidelines: the manual (2014)’. Data from all study designs was initially rated as high quality and the quality of the evidence for each outcome was downgraded or not from this initial point, based on the criteria given in Table 5
Table 5. Rationale for downgrading quality of evidence for intervention studies
The quality of evidence for each outcome was upgraded if any of the following three conditions were met:
- Data from non-randomised studies showing an effect size sufficiently large that it cannot be explained by confounding alone.
- Data showing a dose-response gradient.
- Data where all plausible residual confounding is likely to increase our confidence in the effect estimate.
1.4.5. Publication bias
Publication bias was assessed in two ways. First, if evidence of conducted but unpublished studies was identified during the review (e.g. conference abstracts, trial protocols or trial records without accompanying published data), available information on these unpublished studies was reported as part of the review. Secondly, where 10 or more studies were included as part of a single meta-analysis, a funnel plot was produced to graphically assess the potential for publication bias.
1.4.6. Evidence statements
Evidence statements for pairwise intervention data are classified in to one of four categories:
- Situations where the data are only consistent, at a 95% confidence level, with an effect in one direction (i.e. one that is ‘statistically significant’), and the magnitude of that effect is most likely to meet or exceed the MID (i.e. the point estimate is not in the zone of equivalence). In such cases, we state that the evidence showed that there is an effect.
- Situations where the data are only consistent, at a 95% confidence level, with an effect in one direction (i.e. one that is ‘statistically significant’), but the magnitude of that effect is most likely to be less than the MID (i.e. the point estimate is in the zone of equivalence). In such cases, we state that the evidence could not demonstrate a meaningful difference.
- Situations where the confidence limits are smaller than the MIDs in both directions. In such cases, we state that the evidence demonstrates that there is no meaningful difference.
- In all other cases, we state that the evidence could not differentiate between the comparators.
For outcomes without a defined MID or where the MID is set as the line of no effect (for example, in the case of mortality), evidence statements are divided into 2 groups as follows:
- We state that the evidence showed that there is an effect if the 95% CI does not cross the line of no effect.
- The evidence could not differentiate between comparators if the 95% CI crosses the line of no effect.
1.5. Health economics
Literature reviews seeking to identify published cost–utility analyses of relevance to the issues under consideration were conducted for all questions. In each case, the search undertaken for the clinical review was modified, retaining population and intervention descriptors, but removing any study-design filter and adding a filter designed to identify relevant health economic analyses. In assessing studies for inclusion, population, intervention and comparator, criteria were always identical to those used in the parallel clinical search; only cost–utility analyses were included. Economic evidence profiles, including critical appraisal according to the Guidelines manual, were completed for included studies.
Economic studies identified through a systematic search of the literature are appraised using a methodology checklist designed for economic evaluations (NICE guidelines manual; 2014). This checklist is not intended to judge the quality of a study per se, but to determine whether an existing economic evaluation is useful to inform the decision-making of the committee for a specific topic within the guideline.
There are 2 parts of the appraisal process. The first step is to assess applicability (that is, the relevance of the study to the specific guideline topic and the NICE reference case); evaluations are categorised according to the criteria in Table 6.
Table 6. Applicability criteria
In the second step, only those studies deemed directly or partially applicable are further assessed for limitations (that is, methodological quality); see categorisation criteria in Table 7.
Table 7. Methodological criteria
Where relevant, a summary of the main findings from the systematic search, review and appraisal of economic evidence is presented in an economic evidence profile alongside the clinical evidence.
Appendix C. Literature search strategies
Scoping search strategies
Scoping searches Scoping searches were undertaken on the following websites and databases (listed in alphabetical order) in April 2017 to provide information for scope development and project planning. Browsing or simple search strategies were employed.
| Guidelines/website |
|---|
| American Cancer Society |
| American College of Chest Physicians |
| American Society for Radiation Oncology |
| American Thoracic Society |
| Association for Molecular Pathology |
| British Lung Foundation |
| British Thoracic Society |
| Canadian Medical Association Infobase |
| Canadian Task Force on Preventive Health Care |
| Cancer Australia |
| Cancer Care Ontario |
| Cancer Control Alberta |
| Cancer Research UK |
| Care Quality Commission |
| College of American Pathologists |
| Core Outcome Measures in Effectiveness Trials (COMET) |
| Department of Health & Social Care |
| European Respiratory Society |
| European Society for Medical Oncology |
| European Society of Gastrointestinal Endoscopy |
| European Society of Thoracic Surgery |
| General Medical Council |
| Guidelines & Audit Implementation Network (GAIN) |
| Guidelines International Network (GIN) |
| Healthtalk Online |
| International Association for the Study of Lung Cancer |
| MacMillan Cancer Support |
| Medicines and Products Regulatory Agency (MHRA) |
| National Audit Office |
| National Cancer Intelligence Network |
| National Clinical Audit and Patient Outcomes Programme |
| National Health and Medical Research Council - Australia |
| National Institute for Health and Care Excellence (NICE) - published & in development guidelines |
| National Institute for Health and Care Excellence (NICE) - Topic Selection |
| NHS Choices |
| NHS Digital |
| NHS England |
| NICE Clinical Knowledge Summaries (CKS) |
| NICE Evidence Search |
| Office for National Statistics |
| Patient UK |
| PatientVoices |
| Public Health England |
| Quality Health |
| Royal College of Anaesthetists |
| Royal College of General Practitioners |
| Royal College of Midwives |
| Royal College of Nursing |
| Royal College of Pathologists |
| Royal College of Physicians |
| Royal College of Radiologists |
| Royal College of Surgeons |
| Scottish Government |
| Scottish Intercollegiate Guidelines Network (SIGN) |
| UK Data Service |
| US National Guideline Clearinghouse |
| Walsall community Health NHS Trust |
| Welsh Government |
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between October 2017 and April 2018 for 9 review questions (RQ).
Searches were re-run in May 2018.
Where appropriate, in-house study design filters were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 3.
Search strategy
|
Medline Strategy, searched 12th February 2018 Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Strategy: |
|---|
| 1 Small Cell Lung Carcinoma/ |
| 2 Carcinoma, Small Cell/ |
| 3 SCLC.tw. |
| 4 ((pancoast* or superior sulcus or pulmonary sulcus) adj4 (tumo?r* or syndrome*)).tw. |
| 5 or/1–4 |
| 6 ((small or oat or reserve or round) adj1 cell adj1 (lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 7 (non adj1 small adj1 cell adj1 (lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 8 6 not 7 |
| 9 5 or 8 |
| 10 exp Radiography, Thoracic/ |
| 11 ((chest* or thorac* or thorax) adj4 (radiotherap* or radiotreat* or roentgentherap* or radiosurg* or radiograph*)).tw. |
| 12 ((chest* or thorac* or thorax) adj4 (radiat* or radio* or irradiat* or roentgen or x-ray or xray) adj4 (therap* or treat* or repair* or oncolog* or surg*)).tw. |
| 13 ((chest* or thorac* or thorax) adj4 (RT or RTx or XRT)).tw. |
| 14 (TRT or TCRT).tw. |
| 15 or/10–14 |
| 16 exp Radiotherapy/ |
| 17 Radiation Oncology/ |
| 18 radiotherapy.fs. |
| 19 or/16–18 |
| 20 exp Thorax/ |
| 21 (chest* or thorac* or thorax).tw. |
| 22 20 or 21 |
| 23 19 and 22 |
| 24 15 or 23 |
| 25 9 and 24 |
| 26 limit 25 to english language |
| 27 Animals/ not Humans/ |
| 28 26 not 27 |
Note: In-house RCT, observational studies and systematic review filters were appended. No date limit as this is a new question.
Study Design Filters
| The MEDLINE SR, RCT, and observational studies filters are presented below. |
|---|
| Systematic Review |
| 1. Meta-Analysis.pt. |
| 2. Meta-Analysis as Topic/ |
| 3. Review.pt. |
| 4. exp Review Literature as Topic/ |
| 5. (metaanaly$ or metanaly$ or (meta adj3 analy$)).tw. |
| 6. (review$ or overview$).ti. |
| 7. (systematic$ adj5 (review$ or overview$)).tw. |
| 8. ((quantitative$ or qualitative$) adj5 (review$ or overview$)).tw. |
| 9. ((studies or trial$) adj2 (review$ or overview$)).tw. |
| 10. (integrat$ adj3 (research or review$ or literature)).tw. |
| 11. (pool$ adj2 (analy$ or data)).tw. |
| 12. (handsearch$ or (hand adj3 search$)).tw. |
| 13. (manual$ adj3 search$).tw. |
| 14. or/1–13 |
| 15. animals/ not humans/ |
| 16. 14 not 15 |
| RCT |
| 1 Randomized Controlled Trial.pt. |
| 2 Controlled Clinical Trial.pt. |
| 3 Clinical Trial.pt. |
| 4 exp Clinical Trials as Topic/ |
| 5 Placebos/ |
| 6 Random Allocation/ |
| 7 Double-Blind Method/ |
| 8 Single-Blind Method/ |
| 9 Cross-Over Studies/ |
| 10 ((random$ or control$ or clinical$) adj3 (trial$ or stud$)).tw. |
| 11 (random$ adj3 allocat$).tw. |
| 12 placebo$.tw. |
| 13 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw. |
| 14 (crossover$ or (cross adj over$)).tw. |
| 15 or/1–14 |
| 16 animals/ not humans/ |
| 17 15 not 16 |
| Observational |
| 1 Observational Studies as Topic/ |
| 2 Observational Study/ |
| 3 Epidemiologic Studies/ |
| 4 exp Case-Control Studies/ |
| 5 exp Cohort Studies/ |
| 6 Cross-Sectional Studies/ |
| 7 Controlled Before-After Studies/ |
| 8 Historically Controlled Study/ |
| 9 Interrupted Time Series Analysis/ |
| 10 Comparative Study.pt. |
| 11 case control$.tw. |
| 12 case series.tw. |
| 13 (cohort adj (study or studies)).tw. |
| 14 cohort analy$.tw. |
| 15 (follow up adj (study or studies)).tw. |
| 16 (observational adj (study or studies)).tw. |
| 17 longitudinal.tw. |
| 18 prospective.tw. |
| 19 retrospective.tw. |
| 20 cross sectional.tw. |
| 21 or/1–20 |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Apr 2015
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the review question search strategies. For some health economics strategies additional terms were added to the original review question search strategies (see sections 4.2, 4.3 and 4.4) The searches were conducted between October 2017 and April 2018 for 9 review questions (RQ).
Searches were re-run in May 2018.
Searches were limited to those in the English language. Animal studies were removed from results.
Economic evaluation and quality of life filters
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom$.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov$.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree$ or analys$)).tw. |
| 20 (cost or costs or costing$ or costly or costed).tw. |
| 21 (price$ or pricing$).tw. |
| 22 budget$.tw. |
| 23 expenditure$.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic$ or (pharmaco adj economic$)).tw. |
| 26 or/1–25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly$ or qald$ or qale$ or qtime$).tw. |
| 7 disability adjusted life.tw. |
| 8 daly$.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health$ year$ equivalent$.tw. |
| 19 utilit$.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili$.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble$.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1–30 |
Health economics search strategy
|
Medline Strategy, searched 13th February 2018 Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Strategy: |
|---|
| 1 Small Cell Lung Carcinoma/ |
| 2 Carcinoma, Small Cell/ |
| 3 SCLC.tw. |
| 4 ((pancoast* or superior sulcus or pulmonary sulcus) adj4 (tumo?r* or syndrome*)).tw. |
| 5 or/1–4 |
| 6 ((small or oat or reserve or round) adj1 cell adj1 (lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 7 (non adj1 small adj1 cell adj1 (lung* or pulmonary or bronch*) adj3 (cancer* or neoplasm* or carcinoma* or tumo?r* or lymphoma* or metast* or malignan* or blastoma* or carcinogen* or adenocarcinoma* or angiosarcoma* or chrondosarcoma* or sarcoma* or teratoma* or microcytic*)).tw. |
| 8 6 not 7 |
| 9 5 or 8 |
| 10 exp Radiotherapy/ |
| 11 Radiation Oncology/ |
| 12 exp Radiography, Thoracic/ |
| 13 radiotherapy.fs. |
| 14 (radiotherap* or radiotreat* or roentgentherap* or radiosurg*).tw. |
| 15 ((radiat* or radio* or irradiat* or roentgen or x-ray or xray) adj4 (therap* or treat* or repair* or oncolog* or surg*)).tw. |
| 16 (RT or RTx or XRT or TRT or TCRT).tw. |
| 17 or/10–16 |
| 18 9 and 17 |
| 19 limit 18 to english language |
| 20 Animals/ not Humans/ |
| 21 19 not 20 |
Appendix D. Evidence Study Selection
Appendix E. Clinical evidence tables
Download PDF (283K)
Appendix F. GRADE tables
For all participants who had at least a partial response to chemotherapy: thoracic radiation + PCI vs PCI only
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | RT + PCI | PCI | Summary of results (95% CI) | |
| Mortality: all-cause hazard ratio (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 2 (Gore 2017, Slotman 2015) | RCT | Very serious1 | Not serious | Very serious2 | Serious3 | 291 | 290 | HR 1.03 (0.62, 1.71)3 | Very low |
| Mortality: risk ratio of participants still alive at 1.5 years (values over 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Not serious | 247 | 248 | RR 1.83 (1.12, 2.98) | Low |
| Mortality: risk ratio of participants still alive at 2 years (values over 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Not serious | 247 | 248 | RR 4.59 (2.07, 10.20) | Low |
| Response to treatment: hazard ratio for progression (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 2 (Gore 2017, Slotman 2015) | RCT | Very serious1 | Not serious | Not serious | Not serious | 291 | 290 | HR 0.68 (0.52, 0.88) | Low |
| Response to treatment: risk ratio whose cancer had progressed at 3 months (values under 1 favour radiotherapy + PCI) | |||||||||
| 1 (Gore 2015) | RCT | Very serious1 | Not serious | N/A | Not serious | 44 | 42 | RR 0.26 (0.12, 0.58) | Low |
| Response to treatment: progression-free survival at 6 months (values over 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 1.18 (0.85, 1.65) | Very low |
| Response to treatment: risk ratio whose cancer had progressed at 1 year (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Gore 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 44 | 42 | RR 0.95 (0.76, 1.20) | Very low |
| Adverse events: risk ratio of people who experienced a grade 3 or higher adverse event (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Gore 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 44 | 42 | RR 1.53 (0.78, 2.98) | Very low |
| Adverse events: risk ratio of people experiencing cough grade 3 or above (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 0.33 (0.01, 8.18) | Very low |
| Adverse events: risk ratio of people experiencing dysphagia grade 3 or above (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 3.01 (0.12, 73.58) | Very low |
| Adverse events: risk ratio of people experiencing dyspnoea grade 3 or above (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 0.75 (0.17, 3.33) | Very low |
| Adverse events: risk ratio of people experiencing oesophagitis grade 3 or above (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 9.04 (0.49, 166.95) | Very low |
| Adverse events: risk ratio of people experiencing fatigue grade 3 or above (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 1.23 (0.52, 2.91) | Very low |
| Adverse events: risk ratio of people experiencing insomnia grade 3 or above (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 1.51 (0.25, 8.94) | Very low |
| Adverse events: risk ratio of people experiencing nausea or vomiting grade 3 or above (values below 1 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 3.01 (0.12, 73.58) | Very low |
| Adverse events: risk ratio of people experiencing headache grade 3 or above (values below 0 favour thoracic radiotherapy + PCI) | |||||||||
| 1 (Slotman 2015) | RCT | Very serious1 | Not serious | N/A | Serious3 | 247 | 248 | RR 1.51 (0.25, 8.94) | Very low |
- 1
For Gore 2017: Those randomised to the thoracic radiotherapy arm were a median of 5 years older. There was no allocation concealment and no blinding of outcome assessment. For Slotman 2015: No blinding of outcome assessment. Treatment of disease progression was left to the discretion of each of the participating centres and there were 4 different countries involved. The authors’ protocol was to look at outcomes at 1 year. However, other time intervals were also looked at so there is the possibility of cherry-picking data.
- 2
There is significant statistical heterogeneity in the meta-analysis (I2≥66.7%).
- 3
The effect size crosses the line of no effect.
- 4
Random effects model used because the total dose of thoracic radiation was: Slotman 2015, 30 Gy; Gore 2017, 45 Gy. In addition, in Gore 2017, those randomised to the thoracic radiation + PCI arm were on average 5 years older compared to the PCI arm.
For people who had a complete extra-thoracic response (and who had either a complete or partial thoracic response) to chemotherapy: Accelerated hyperfractionated radiation therapy + carboplatin/etoposide + PCI + 2x cisplatin/etoposide vs 2x cisplatin/etoposide + PCI + 2x cisplatin/etoposide
| Quality assessment | No of people | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | RT + 2x chemo + PCI + 2x chemo | 2x chemo + PCI + 2x chemo | Summary of results (95% CI) | |
| Mortality: risk ratio of participants still alive at 1 year (values over 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 1.41 (1.00, 2.00) | Very low |
| Mortality: risk ratio of participants still alive at 2 years (values over 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 1.37 (0.80, 2.37) | Very low |
| Mortality: risk ratio of participants still alive at 3 years (values over 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 1.47 (0.65, 3.32) | Very low |
| Mortality: risk ratio of participants still alive at 4 years (values over 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 2.29 (0.62, 8.40) | Very low |
| Mortality: risk ratio of participants still alive at 5 years (values over 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 2.45 (0.50, 12.11) | Very low |
| Response to treatment: thoracic recurrence-free survival at 5 years (values over 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 2.70 (0.92, 7.96) | Very low |
| Response to treatment: extra-thoracic metastases-free survival at 5 years (values over 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 1.84 (0.85, 3.98) | Very low |
| Adverse events: risk ratio of people experiencing leukopenia grade 3 or above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 0.74 (0.51, 1.07) | Very low |
| Adverse events: risk ratio of people experiencing thrombocytopenia grade 3 or above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 0.67 (0.39, 1.15 | Very low |
| Adverse events: risk ratio of people experiencing anaemia grade 3 and above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 0.54 (0.21, 1.35) | Very low |
| Adverse events: risk ratio of people experiencing infection grade 3 and above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 0.75 (0.41, 1.39) | Very low |
| Adverse events: risk ratio of people experiencing nausea and vomiting grade 3 and above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Not serious | 55 | 54 | RR 0.27 (0.11, 0.68) | Low |
| Adverse events: risk ratio of people experiencing alopecia grade 3 or above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Not serious | 55 | 54 | RR 0.22 (0.11, 0.46) | Low |
| Adverse events: risk ratio of people experiencing kidney toxicity grade 3 or above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Not serious | 55 | 54 | RR 0.04 (0.00, 0.65) | Low |
| Adverse events: risk ratio of people experiencing oesophageal toxicity grade 3 or above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Not serious | 55 | 54 | RR 30.45 (1.85, 496.43) | Low |
| Adverse events: risk ratio of people experiencing bronchopulmonary toxicity grade 3 or above (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 6.88 (0.36, 130.01) | Very low |
| Adverse events: risk ratio of people requiring hospital admission for an adverse event (values below 1 favour RT + 2x chemo + PCI + 2x chemo) | |||||||||
| 1 (Jeremic 1999) | RCT | Very serious1 | Not serious | N/A | Serious2 | 55 | 54 | RR 0.54 (0.21, 1.35) | Very low |
- 1
The chemotherapy interventions are different for each arm of the trial. In addition, participants were selected for the RCT because they were expected to have a better outcome: they had a complete extra-thoracic response to the chemotherapy before they were randomised. People who were thought to have a worse prognosis (partial extra-thoracic response) were placed into cohort study arms where comparison was not possible.
- 2
The effect size crosses or touches the line of no effect.
Appendix G. Forest plots
For all participants who had at least a partial response to chemotherapy: thoracic radiotherapy + PCI vs PCI
Appendix H. Excluded Studies
| Short Title | Title | Reason for exclusion |
|---|---|---|
| Giuliani (2011) | Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy | Study outcome data included participants whose extensive SCLC did not respond to initial chemotherapy |
| Li-Ming (2017) | Receipt of thoracic radiation therapy and radiotherapy dose are correlated with outcomes in a retrospective study of three hundred and six patients with extensive stage small-cell lung cancer | Study outcome data included participants whose extensive SCLC did not respond to initial chemotherapy |
| Luan (2015) | Efficacy of 3D conformal thoracic radiotherapy for extensive-stage small-cell lung cancer: A retrospective study | Study outcome data included participants whose extensive SCLC did not respond to initial chemotherapy |
| Luo (2017) | Timing of thoracic radiotherapy in the treatment of extensive-stage small-cell lung cancer: important or not? | Study outcome data included participants whose extensive SCLC did not respond to initial chemotherapy This study included an unknown number of participants who had a stable response after chemotherapy. Our protocol inclusion criteria specify a partial response. This is an important distinction because there might not be much difference between an effect of radiotherapy that is statistically significant and one that is not for people who have had a partial response. |
| Mahmoud (2016) | Intrathoracic extensive-stage small cell lung cancer: assessment of the benefit of thoracic and brain radiotherapy using the SEER database | Study outcome data included people whose extensive SCLC did not respond to initial chemotherapy |
| Palma (2016) | Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Meta-Analysis | This systematic review was searched for relevant studies. This systematic review was considered for inclusion. However, it meta-analyses studies that we believe are not comparable. |
| Slotman (2015) | [Letter regarding the study:: Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial (Slotman 2015)] | This is a non-peer reviewed letter |
| Xu (2017) | Thoracic radiotherapy (TRT) improved survival in both oligo- and polymetastatic extensive stage small cell lung cancer | Study outcome data included people whose extensive SCLC did not respond to initial chemotherapy |
| Yee (2012) | Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer | Single-arm study |
| Zhu (2011) | Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis | Study outcome data included people whose extensive SCLC did not respond to initial chemotherapy Although only 9/60 participants had either stable disease or progressive disease after chemotherapy, this small number of participants might make a difference to the outcomes that we are trying to assess. This is because for the people with extensive SCLC who respond to chemotherapy, the effect of radiotherapy might be borderline between statistically significant and not statistically significant. |
Appendix H. References
Clinical Studies - Included
- Gore E M (2017) Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. Journal of Thoracic Oncology 12(10), 1561–70 [PMC free article: PMC5610652] [PubMed: 28648948]
- Jeremic B (1999) Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol 17(7), 2092–9 [PubMed: 10561263]
- Slotman B J (2015) Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 385(9962), 36–42 [PubMed: 25230595]
Clinical studies – Excluded
- Giuliani M E, Atallah S, Sun A, Bezjak A, Le L W, Brade A, Cho J, Leighl N B, Shepherd F A, and Hope A J (2011) Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy. Clinical Lung Cancer 12(6), 375–9 [PubMed: 21729647]
- Li-Ming X, Zhao L J, Simone C B, 2nd, Cheng C, Kang M, Wang X, Gong L L, Pang Q S, Wang J, Yuan Z Y, and Wang P (2017) Receipt of thoracic radiation therapy and radiotherapy dose are correlated with outcomes in a retrospective study of three hundred and six patients with extensive stage small-cell lung cancer. Radiotherapy & Oncology 125(2), 331–337 [PubMed: 29079309]
- Luan Z, Huang W, Zhang J, Dong W, Zhang W, Li B, Zhou T, Li H, Zhang Z, Wang Z, Sun H, and Yi Y (2015) Efficacy of 3D conformal thoracic radiotherapy for extensive-stage small-cell lung cancer: A retrospective study. Experimental and Therapeutic Medicine 10(2), 671–678 [PMC free article: PMC4509057] [PubMed: 26622373]
- Luo J, Xu L, Zhao L, Cao Y, Pang Q, Wang J, Yuan Z, and Wang P (2017) Timing of thoracic radiotherapy in the treatment of extensive-stage small-cell lung cancer: important or not?. Radiation Oncology 12(1), 42 [PMC free article: PMC5331725] [PubMed: 28245874]
- Mahmoud O, Kwon D, Greenfield B, Wright J L, and Samuels M A (2016) Intrathoracic extensive-stage small cell lung cancer: assessment of the benefit of thoracic and brain radiotherapy using the SEER database. International Journal of Clinical Oncology 21(6), 1062–1070 [PubMed: 27380168]
- Palma D A, Warner A, Louie A V, Senan S, Slotman B, and Rodrigues G B (2016) Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Meta-Analysis. Clinical Lung Cancer 17(4), 239–44 [PubMed: 26498503]
- Slotman B J (2015) Radiotherapy for extensive stage small-cell lung cancer - Authors’ reply. Lancet 385(9975), 1292 [PubMed: 25890910]
- Yee D (2012) Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiation and Oncology 102(2), 234–8 [PubMed: 21930323]
- Xu L M, Cheng C, Kang M, Luo J, Gong L L, Pang Q S, Wang J, Yuan Z Y, Zhao L J, and Wang P (2017) Thoracic radiotherapy (TRT) improved survival in both oligo- and polymetastatic extensive stage small cell lung cancer. Scientific Reports 7(1), 9255 [PMC free article: PMC5569074] [PubMed: 28835666]
- Zhu H (2011) Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer 117(23), 5423–31 [PubMed: 21563176]
Health Economic studies – Included
- Patrice, G.I., Lester-Coll, N.H., James, B.Y., Amdahl, J., Delea, T.E. and Patrice, S.J., 2018. Cost-Effectiveness of Thoracic Radiation Therapy for Extensive-Stage Small Cell Lung Cancer Using Evidence From the Chest Radiotherapy Extensive-Stage Small Cell Lung Cancer Trial (CREST). International Journal of Radiation Oncology* Biology* Physics, 100(1), pp.97–106. [PubMed: 29029885]
Health Economic studies – Excluded
None.
Appendix I. Health Economics Evidence Tables
Download PDF (141K)
Final
Evidence reviews
These evidence reviews were developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Evidence reviews for the clinical and cost-effectiveness of first use of thoracic radiotherapy for people with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies
- Review protocols
- Methods
- Literature search strategies
- Evidence Study Selection
- Clinical evidence tables
- GRADE tables
- Forest plots
- Excluded Studies
- References
- Health Economics Evidence Tables
- Current Patterns of Care for Patients with Extensive-Stage SCLC: Survey of U.S. Radiation Oncologists on Their Recommendations Regarding Prophylactic Cranial Irradiation.[J Thorac Oncol. 2016]Current Patterns of Care for Patients with Extensive-Stage SCLC: Survey of U.S. Radiation Oncologists on Their Recommendations Regarding Prophylactic Cranial Irradiation.Jain A, Luo J, Chen Y, Henderson MA, Thomas CR Jr, Mitin T. J Thorac Oncol. 2016 Aug; 11(8):1305-1310. Epub 2016 May 26.
- The role of postoperative thoracic radiotherapy and prophylactic cranial irradiation in early stage small cell lung cancer: Patient selection among ESTRO experts.[Radiother Oncol. 2020]The role of postoperative thoracic radiotherapy and prophylactic cranial irradiation in early stage small cell lung cancer: Patient selection among ESTRO experts.Putora PM, De Ruysscher D, Glatzer M, Widder J, Van Houtte P, Troost EGC, Slotman BJ, Ramella S, Pöttgen C, Peeters S, et al. Radiother Oncol. 2020 Apr; 145:45-48. Epub 2019 Dec 27.
- Prophylactic Cranial Irradiation for Limited-Stage Small-Cell Lung Cancer: Survey of US Radiation Oncologists on Current Practice Patterns.[Clin Lung Cancer. 2018]Prophylactic Cranial Irradiation for Limited-Stage Small-Cell Lung Cancer: Survey of US Radiation Oncologists on Current Practice Patterns.Farrell MJ, Yahya JB, Degnin C, Chen Y, Holland JM, Henderson MA, Jaboin JJ, Harkenrider MM, Thomas CR Jr, Mitin T. Clin Lung Cancer. 2018 Jul; 19(4):371-376. Epub 2018 Feb 23.
- Review What is the role of radiotherapy for extensive-stage small cell lung cancer in the immunotherapy era?[Transl Lung Cancer Res. 2019]Review What is the role of radiotherapy for extensive-stage small cell lung cancer in the immunotherapy era?Nesbit EG, Leal TA, Kruser TJ. Transl Lung Cancer Res. 2019 Sep; 8(Suppl 2):S153-S162.
- Review Prophylactic Cranial Irradiation for Extensive Small-Cell Lung Cancer.[J Oncol Pract. 2017]Review Prophylactic Cranial Irradiation for Extensive Small-Cell Lung Cancer.Schild SE, Sio TT, Daniels TB, Chun SG, Rades D. J Oncol Pract. 2017 Nov; 13(11):732-738.
- Evidence reviews for the clinical and cost-effectiveness of first use of thoraci...Evidence reviews for the clinical and cost-effectiveness of first use of thoracic radiotherapy for people with extensive-stage SCLC who have had first-line treatment with systemic anti-cancer therapies
Your browsing activity is empty.
Activity recording is turned off.
See more...