NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Review question: How effective are spot protein/creatinine ratio or albumin/creatinine ratio measurements as compared with a 24 hour urine collection for the identification of proteinuria in women with hypertensive disorders of pregnancy?
Introduction
The reliable detection of significant proteinuria is important in women with new-onset hypertension during pregnancy because it helps distinguish between those pregnancies with pre-eclampsia and those with gestational hypertension and this determines the pathways for future monitoring and management.
Traditionally proteinuria has been assessed initially by urine dipstick (which can be read visually or by an automated device) and confirmed by various methods of laboratory quantification either using spot samples of urine, or 24 hour urine collection. A 24 hour urine collection is a time-consuming procedure for the woman, and in recent years spot urinary protein:creatinine ratio (PCR) and spot urinary albumin:creatinine ratio (ACR) (which are widely used outside maternity services) have been increasingly used in pregnant women. International definitions have recommended certain thresholds of PCR and ACR for diagnosis of ‘significant proteinuria’, and which are included in definitions of pre-eclampsia.
The aim of this review is to determine the best method for assessing proteinuria and to determine if currently used thresholds of PCR and ACR are correct to diagnose significant proteinuria.
Summary of the protocol
See Table 1 for a summary of the Population, Index test, Reference test, and Outcome (PIRO) characteristics of this review.
Table 1
Summary of the protocol (PIRO table).
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual 2014. Methods specific to this review question are described in the review protocol in appendix A.
Declaration of interests were recorded according to NICE’s 2018 conflicts of interest policy (see Register of interests).
Included studies reported data for ACR in mg/mmol only. Study data for PCR was reported as mg/mmol, mg/mg, mg/g, mg/dL, mg, and presented without units. We made the pragmatic decision to transform the data for direct comparison using the approximate conversion factor, for example, PCR 0.30 (ratio without units) = PCR 0.30 mg/mg = PCR 30mg/mmol = PCR of 300mg/g. Data are presented here to 2 decimal places only (as a ratio), and in whole numbers when converted back into mg/mmol.
Following conversion to a ratio, meta-analysis was performed when at least 4 different studies reported data at the same cut-off threshold. This was possible at PCR cut-off points 0.15, 0.19, 0.20, 0.30, 0.40, and 0.45 only.
Sub-group analyses were only possible at PCR 0.30, where 4 studies (Bhatti 2018, Kyle 2008, Leanos-Miranda 2007, Mohseni 2013) excluded spot urine samples taken at the first morning void. The remaining 6 studies reporting at PCR 0.30 included samples taken at the first morning void (though not exclusively first void), or did not report this (second subgroup analysis: Amin 2015, Durnwald 2003, Lamontagne 2014, Saudan 1997, Waugh 2017, Wilkinson 2013).
Imprecision was assessed according to pre-specified thresholds for sensitivity (a critical outcome measure), which were identified by the guideline committee as representing clinically meaningful results. Sensitivity of ≥90% was regarded as high, and ≥75% was regarded as moderate.
Clinical evidence
Included studies
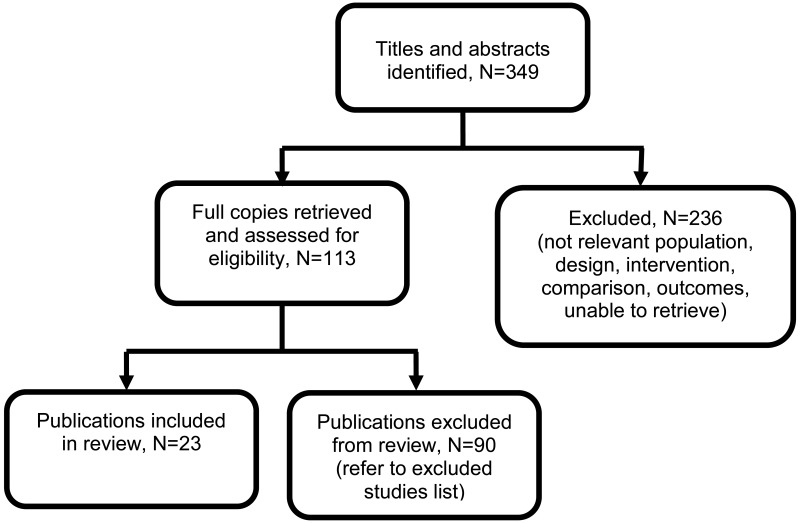
Twenty-three studies were included in this review.
Four studies were retrospective cohort studies (Al 2004, Park 2013, Rodriguez-Thompson 2001, Stout 2013), 17 were prospective cohort studies (Amin 2015, Bhatti 2018, Durnwald 2003, Dwyer 2008, Kucukgoz Gulec 2017, Kyle 2008, Lamontagne 2014, Leanos-Miranda 2007, Mohseni 2013, Rizk 2007, Saudan 1997, Tun 2012, Valdes 2016, Waugh 2005, Waugh 2017, Wheeler 2007, Wilkinson 2013), 1 descriptive cohort study (Nisar 2017) and 1 case-series (Eslamian 2011).
Four studies reported on the diagnostic accuracy of ACR (Kyle 2008, Waugh 2005, Waugh 2017, Wilkinson 2013), and 22 studies reported on the diagnostic accuracy of PCR (Al 2004, Amin 2015, Bhatti 2018, Durnwald 2003, Dwyer 2008, Eslamian 2011, Kucokgoz-Gulec 2017, Lamontagne 2014, Leanos-Miranda 2007, Mohseni 2013, Nisar 2017, Park 2013, Rizk 2007, Rodriguez-Thompson 2001, Saudan 1997, Stout 2013, Tun 2012, Valdes 2016, Waugh 2017, Wheeler 2007, Wilkinson 2013),
One study (Mohseni 2013) presented data for spot/random samples collected at two time points (10am and 4pm) related to the same 24 hour collection. To avoid double counting, we took the decision to use only the data presented for the 10am sample as these reported more conservative estimates for diagnostic accuracy (consistently lower sensitivity at each cut-off).
One study (Waugh 2017) performed multiple analyses for PCR based on the different assays performed at the local laboratory, or central study laboratory using two different assays (BZC assay and PGR assay). To reflect clinical practice, we have used results from the local laboratory PCR analysis for inclusion in this review. Assays for ACR were conducted at the central laboratory only, therefore these data were included in the review.
See the literature search strategy in appendix B and clinical evidence study selection flow chart in appendix C.
Excluded studies
Studies not included in this review with reasons for their exclusions are provided in appendix K.
Summary of clinical studies included in the evidence review
A summary of the studies that were included in this review are presented in Table 2.
Table 2
Summary of included studies.
See appendix D for the clinical evidence tables, appendix E for the Forest plots, and appendix M for a graphical representation of the data (scatter plots showing results for sensitivity and specificity by cut-off threshold).
Quality assessment of clinical outcomes included in the evidence review
See appendix F for full GRADE tables.
Economic evidence
A systematic review of the economic literature was conducted but no relevant studies were identified which were applicable to this review question. Economic modelling was not undertaken for this question because other topics were agreed as higher priorities for economic evaluation
The committee were aware of an economic analysis conducted as part of a large, UK-based study (Waugh 2017). However this study was not included in the economic evidence review because it assessed the cost-effectiveness of strategies to diagnose severe pre-eclampsia rather than the diagnosis of proteinuria.
Evidence statements
Spot albumin:creatinine ratio (ACR) for the identification of significant proteinuria (≥300mg/24 hours)
Cut-off threshold: 1.0 mg/mmol
- One cohort study (N=132 samples from 89 women) provided moderate quality evidence to show very high sensitivity and low specificity when using an ACR cut-off point of 1.0 mg/mmol to identify significant proteinuria. Likelihood ratios show this is not a useful test when the test is positive, but is a very useful test when negative.
Cut-off threshold: 1.5 mg/mmol
- One cohort study (N=132 samples from 89 women) provided low quality evidence to show very high sensitivity and low specificity when using an ACR cut-off point of 1.5 mg/mmol to identify significant proteinuria. Likelihood ratios show this is not a useful test when the test is positive, but is a very useful test when negative.
Cut-off threshold: 2.0 mg/mmol
- Meta-analysis of 4 cohort studies (N=1412) provided very low quality evidence to show very high sensitivity and low specificity when using an ACR cut-off point of 2.0 mg/mmol to identify significant proteinuria. Likelihood ratios show this is not a useful test when the test is positive, but is a very useful test when negative.
Cut-off threshold: 2.5 mg/mmol
- One cohort study (N=132 samples from 89 women) provided low quality evidence to show very high sensitivity and moderate specificity when using an ACR cut-off point of 2.5 mg/mmol to identify significant proteinuria. Likelihood ratios show this is not a useful test when the test is positive, but is a very useful test when negative.
Cut-off threshold: 3.0 mg/mmol
- One cohort study (N=132 samples from 89 women) provided low quality evidence to show high sensitivity and moderate specificity when using an ACR cut-off point of 3.0 mg/mmol to identify significant proteinuria. Likelihood ratios show this is not a useful test when the test is positive, and a moderately useful test when negative.
Cut-off threshold: 3.5 mg/mmol
- One cohort study (N=150) provided low quality evidence to show very high sensitivity and moderate specificity when using an ACR cut-off point of 3.5 mg/mmol to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when the test is positive, and a very useful test when negative.
- A second cohort study (N=132 samples from 89 women) provided low quality evidence to show high sensitivity and moderate specificity when using an ACR cut-off point of 3.5 mg/mmol to identify significant proteinuria. Likelihood ratios show this is not a useful test when the test is positive, and a moderately useful test when negative.
Cut-off threshold: 8.0 mg/mmol
- One cohort study (N=150) provided low quality evidence to show very high sensitivity and very high specificity when using an ACR cut-off point of 8.0 mg/mmol to identify significant proteinuria. Likelihood ratios show this is a very useful test when the test is positive, and a very useful test when negative.
Spot protein:creatinine ratio (PCR) for the diagnosis of significant proteinuria (≥300mg/24 hours)
Cut-off threshold: 0.08 (~8mg/mmol)
- One cohort study (N=356) provided high quality evidence to show very high sensitivity and very low specificity when using a PCR cut-off point of 0.08 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive or negative.
Cut-off threshold: 0.10 (~10mg/mmol)
- One cohort study (N=132) provided moderate quality evidence to show very high sensitivity and very low specificity when using a PCR cut-off point of 0.10 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, but is very useful when negative (LR- not calculable due to sensitivity=1.00).
Cut-off threshold: 0.12 (~12mg/mmol)
- One cohort study (N=356) provided moderate quality evidence to show high sensitivity and very low specificity when using a PCR cut-off point of 0.12 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive or negative.
Cut-off threshold: 0.13 (~13mg/mmol)
- One cohort study (N=185) provided moderate quality evidence to show high sensitivity and low specificity when using a PCR cut-off point of 0.13 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, and is moderately useful when the result is negative.
Cut-off threshold: 0.14 (~14mg/mmol)
- One cohort study (N=138) provided high quality evidence to show very high sensitivity and low specificity when using a PCR cut-off point of 0.14 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, but is very useful when negative (LR- not calculable due to sensitivity=1.00).
Cut-off threshold: 0.15 (~15mg/mmol)
- Meta-analysis of 5 cohort studies (N=696) provided low quality evidence to show very high sensitivity and low specificity when using a PCR cut-off point of 0.15 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, but is very useful when negative.
Cut-off threshold: 0.16 (~16mg/mmol)
- One cohort study (N=138) provided high quality evidence to show very high sensitivity and low specificity when using a PCR cut-off point of 0.16 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, but is very useful when negative.
Cut-off threshold: 0.17 (~17mg/mmol)
- One cohort study (N=138) provided moderate quality evidence to show high sensitivity and low specificity when using a PCR cut-off point of 0.16 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, but is very useful when negative.
Cut-off threshold: 0.18 (~18mg/mmol)
- One cohort study (N=185) provided low quality evidence to show moderate sensitivity and low specificity when using a PCR cut-off point of 0.18 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive or negative.
- A second cohort study (N=138) provided moderate quality evidence to show high sensitivity and low specificity when using a PCR cut-off point of 0.18 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, but is moderately useful when negative.
Cut-off threshold: 0.19 (~19mg/mmol)
- Meta-analysis of 5 cohort studies (N=878) provided moderate quality evidence to show moderate sensitivity and low specificity when using a PCR cut-off point of 0.19 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive or negative.
Cut-off threshold: 0.20 (~20mg/mmol)
- Meta-analysis of 6 cohort studies (N=1179) provided very low quality evidence to show high sensitivity and low specificity when using a PCR cut-off point of 0.20 to identify significant proteinuria. Likelihood ratios show this is not a useful test when the result is positive, but is moderately useful when negative.
Cut-off threshold: 0.21 (~21mg/mmol)
- One cohort study (N=476) provided moderate quality evidence to show moderate sensitivity and moderate specificity when using a PCR cut-off point of 0.21 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when the result is positive or negative.
- Two cohort studies (not meta-analysed: N=138, N=126) provided moderate quality evidence to show moderate sensitivity and moderate specificity when using a PCR cut-off point of 0.21 to identify significant proteinuria. Likelihood ratios show this is not a useful test when positive, but is moderately useful when negative.
Cut-off threshold: 0.22 (~22mg/mmol)
- One cohort study (N=100) provided low quality evidence to show moderate sensitivity and high specificity when using a PCR cut-off point of 0.22 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive, and moderately useful when negative.
Cut-off threshold: 0.25 (~25mg/mmol)
- One cohort study (N=100) provided low quality evidence to show very high sensitivity and moderate specificity when using a PCR cut-off point of 0.25 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive, and very useful when negative.
- One cohort study (N=132) provided low quality evidence to show moderate sensitivity and high specificity when using a PCR cut-off point of 0.25 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive, and moderately useful when negative.
Cut-off threshold: 0.28 (~28mg/mmol)
- One cohort study (N=116) provided moderate quality evidence to show low sensitivity and very high specificity when using a PCR cut-off point of 0.28 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive, but not a useful test when negative.
- One cohort study (N=205) provided high quality evidence to show moderate sensitivity and low specificity when using a PCR cut-off point of 0.28 to identify significant proteinuria. Likelihood ratios show this is not a useful test when positive or negative.
Cut-off threshold: 0.30 (~30mg/mmol)
- Meta-analysis of 10 cohort studies (N=3224) provided very low quality evidence to show high sensitivity and high specificity when using a PCR cut-off point of 0.30 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive and negative.
- Sub-group analysis for 4 cohort studies which excluded the 1st morning urine void (N=1620) provided very low quality evidence to show high sensitivity and very high specificity when using a PCR cut-off point of 0.30 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive and negative
- Sub-group analysis for 6 cohort studies which included first morning urine samples, or did not specify that these samples were excluded, (N=1604) provided very low quality evidence to show moderate sensitivity and moderate specificity when using a PCR cut-off point of 0.30 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive and negative.
Cut-off threshold: 0.35 (~35mg/mmol)
- One cohort study (N=67) provided moderate quality evidence to show high sensitivity and low specificity when using a PCR cut-off point of 0.35 to identify significant proteinuria. Likelihood ratios show this is not a useful test when positive, but is very useful when negative.
- A second cohort study (N=100) provided low quality evidence to show moderate sensitivity and very high specificity when using a PCR cut-off point of 0.35 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive and moderately useful when negative.
Cut-off threshold: 0.36 (~36mg/mmol)
- One cohort study (N=83) provided moderate quality evidence to show low sensitivity and moderate specificity when using a PCR cut-off point of 0.36 to identify significant proteinuria. Likelihood ratios show this is not a useful test when positive or negative.
- A second cohort study (N=72) provided moderate quality evidence to show low sensitivity and high specificity when using a PCR cut-off point of 0.36 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive, but not useful when negative.
Cut-off threshold: 0.39 (~39mg/mmol)
- One cohort study (N=220) provided moderate quality evidence to show low sensitivity and low specificity when using a PCR cut-off point of 0.39 to identify significant proteinuria. Likelihood ratios show this is not a useful test when positive or negative.
Cut-off threshold: 0.40 (~40mg/mmol)
- Meta-analysis of 4 cohort studies (N=743) provided very low quality evidence to show low sensitivity and moderate specificity when using a PCR cut-off point of 0.40 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive, but not a useful test when negative.
Cut-off threshold: 0.45 (~45mg/mmol)
- Meta-analysis of 4 cohort studies (N=625) provided very low quality evidence to show low sensitivity and very high specificity when using a PCR cut-off point of 0.45 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive, but not a useful test when negative.
Cut-off threshold: 0.49 (~49mg/mmol)
- One cohort study (N=185) provided moderate quality evidence to show low sensitivity and moderate specificity when using a PCR cut-off point of 0.49 to identify significant proteinuria. Likelihood ratios show this is not a useful test when positive or negative.
Cut-off threshold: 0.50 (~50mg/mmol)
- One cohort study (N=67) provided low quality evidence to show moderate sensitivity and moderate specificity when using a PCR cut-off point of 0.50 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive and negative.
- A second cohort study (N=220) provided high quality evidence to show low sensitivity and moderate specificity when using a PCR cut-off point of 0.50 to identify significant proteinuria. Likelihood ratios show this is not a useful test when positive or negative.
Cut-off threshold: 0.53 (~53mg/mmol)
- One cohort study (N=205) provided moderate quality evidence to show moderate sensitivity and high specificity when using a PCR cut-off point of 0.53 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive, and moderately useful when negative.
Cut-off threshold: 0.55 (~55mg/mmol)
- One cohort study (N=67) provided low quality evidence to show moderate sensitivity and moderate specificity when using a PCR cut-off point of 0.55 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive and negative.
- A second cohort study (N=83) provided high quality evidence to show low sensitivity and moderate specificity when using a PCR cut-off point of 0.55 to identify significant proteinuria. Likelihood ratios show this not a useful test when positive or negative.
Cut-off threshold: 0.60 (~60mg/mmol)
- One cohort study (N=66) provided moderate quality evidence to show high sensitivity and very high specificity when using a PCR cut-off point of 0.595 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive and negative.
- A second cohort study (N=67) provided low quality evidence to show moderate sensitivity and moderate specificity when using a PCR cut-off point of 0.599 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive and when negative.
- A third cohort study (N=102) provided moderate quality evidence to show moderate sensitivity and moderate specificity when using a PCR cut-off point of 0.60 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive, but not a useful test when negative.
Cut-off threshold: 0.63 (~63mg/mmol)
- One cohort study (N=46) provided low quality evidence to show moderate sensitivity and very high specificity when using a PCR cut-off point of 0.63 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive (LR+ not calculable due to specificity=1.00) and moderately useful when negative.
Cut-off threshold: 0.75 (~75mg/mmol)
- One cohort study (N=102) provided moderate quality evidence to show low sensitivity and very high specificity when using a PCR cut-off point of 0.75 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive (LR+ not calculable due to specificity=1.00), but not a useful test when negative.
Cut-off threshold: 0.86 (~86mg/mmol)
- One cohort study (N=83) provided high quality evidence to show very low sensitivity and high specificity when using a PCR cut-off point of 0.86 to identify significant proteinuria. Likelihood ratios show this is a moderately useful test when positive, but is not a useful test when negative.
Cut-off threshold: 0.90 (~90mg/mmol)
- One cohort study (N=102) provided high quality evidence to show low sensitivity and very high specificity when using a PCR cut-off point of 0.90 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive (LR+ not calculable due to specificity=1.00), but not a useful test when negative.
Cut-off threshold: 1.19 (~119mg/mmol)
- One cohort study (N=356) provided high quality evidence to show very low sensitivity and very high specificity when using a PCR cut-off point of 1.19 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive, but is not a useful test when negative.
Cut-off threshold: 1.40 (~140mg/mmol)
- One cohort study (N=83) provided high quality evidence to show very low sensitivity and very high specificity when using a PCR cut-off point of 1.40 to identify significant proteinuria. Likelihood ratios show this is a very useful test when positive, but is not a useful test when negative.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
Sensitivity and negative likelihood ratio were prioritised over specificity and positive likelihood ratio in this review. The main priority in testing for proteinuria is to ensure that women who may have pre-eclampsia are identified, to allow for appropriate monitoring and/or management. Therefore the priority is to ensure that a test detects these women (sensitivity). Whilst false positives may mean that women undergo unnecessary follow up, this is less of a concern than missing women who may need altered surveillance or intervention.
The quality of the evidence
Albumin:creatinine ratio (ACR)
Limited evidence from 4 cohort studies was classed as very low to moderate quality evidence. There was no serious risk of bias across any of the included studies: often not all women recruited/enrolled in the study were included in the analysis, but reasons for exclusion were well documented and valid (incomplete 24 hour urine collection, gave birth during 24 hour collection period, documented urine infection, refused consent/willingness to participate), and judged to have no to low impact on the risk of bias.
Individual studies were downgraded due to imprecision with wide confidence intervals (based on the critical outcome of sensitivity). Where studies could be pooled, the evidence was downgraded due to very high heterogeneity (assessed using the I2 statistic). However, it was noted that heterogeneity is often extremely high with diagnostic accuracy studies, and therefore this downgrading of the evidence should be interpreted with caution. Only one cut-off threshold had sufficient data for meta-analysis (2.0 mg/mmol). The remaining cut-off points reported were from individual studies that each reported at multiple thresholds.
Protein:creatinine ratio (PCR)
The quality of the evidence ranged from very low to high. There was no serious risk of bias across any of the included studies: often not all women recruited/enrolled in the study were included in the analysis, but reasons for exclusion were well documented and valid (incomplete 24 hour urine collection, gave birth during 24 hour collection period, documented urine infection, refused consent/willingness to participate), and judged to have no to low impact on the risk of bias.
Individual studies were downgraded for imprecision with wide confidence intervals (based on the critical outcome of sensitivity). Where studies could be pooled, evidence was often downgraded due to very high heterogeneity (assessed using the I2 statistic). However, it was noted that heterogeneity is often extremely high with diagnostic accuracy studies, and therefore this downgrading of the evidence should be interpreted with caution. When subgrouping was possible (at cut-off threshold PCR 30mg/mmol), heterogeneity remained very high within each subgroup.
Multiple cut-off thresholds were reported, with individual studies often reporting more than one threshold each. Studies reported cut-offs that were pre-defined (prior to study commencement), or selected based on the data (exploratory testing using the AUC/ROC). Studies utilising the AUC reported the optimal cut-off (where sensitivity and specificity were optimised), and/or the cut-offs that produced maximum sensitivity or maximum specificity. Other included studies reported a range of cut-offs where the reasoning for selection was unclear (arbitrary selection).
Due to the extensive range of thresholds reported by the included studies to identify proteinuria ≥300mg/24hours, the committee decided to review a graphical representation/overview (appendix M) of sensitivity and specificity for all thresholds available from the evidence, and in particular a PCR threshold of 30 mg/mmol (ratio 0.30) as it is the most commonly used in clinical practice (CG107 NICE guideline 2010), before focussing on other thresholds of interest (based on the graphical representation).
Benefits and harms
The main priority in testing for significant proteinuria is to ensure that women who have/may have pre-eclampsia are identified and offered appropriate follow up and monitoring. The gold standard for assessment/diagnosis of significant proteinuria is currently by 24 hour urine collection and analysis. This can cause delays in commencement of treatment, and the process itself can be awkward and cumbersome. Furthermore, the committee noted that, although this is regarded as the “gold standard”, the results still may be misleading. Samples may be incomplete, leading to an under-estimation of the quantity of protein. The quantity of protein excreted may also fluctuate slightly from day to day, therefore an individual woman may have a “positive” result on one day, and a “negative” result on the next. Studies have also previously identified a lack of repeatability in laboratory based testing of proteinuria – the specific assay used to identify protein varies between individual laboratories, and may lead to an under/over-estimation of the degree of proteinuria. The committee discussed the reliability of this “gold standard” in itself and agreed that, though it was not perfectly reliable, as it stands it is the only appropriate reference standard for significant proteinuria (to compare to spot PCR and ACR).
From the woman’s perspective, the committee discussed the negative connotations associated with being labelled as having pre-eclampsia, often based on dipstick screening alone, before the results of a 24 hour urine collection were available. The committee discussed common situations, where women are hospitalised for suspected pre-eclampsia and undergo unnecessary further testing and monitoring, when ultimately significant proteinuria is never identified. The anxiety caused by such admissions, the disruption to the woman and her family, and the health economic issues associated with lengthy admission were discussed. There was a strong feeling that a quicker, easier, simpler, and accurate test for significant proteinuria in pregnancy should be favoured.
The sensitivity of both ACR and PCR tests at the thresholds recommended was high, giving confidence to women and health care professionals that those with a negative test do not have significant proteinuria. Therefore it was considered safe and appropriate to recommend the use of these tests in preference to a 24 hour urine collection. The committee noted that ACR and PCR may not be sufficient in pregnant women with additional comorbidities (such as renal disease in pregnancy). Therefore a 24 hour urine collection may still be appropriate and useful in a specialist setting.
The evidence presented showed that, by excluding the first morning urine void, diagnostic accuracy appeared to improve (both sensitivity and specificity). Evidence for this was only available for PCR analysis at a threshold of 30 mg/mmol, but the committee considered it was probably of relevance to other thresholds for PCR, and for ACR. The committee could only speculate on the reasons for the first morning urine void decreasing diagnostic accuracy. Possible factors could be the effects of posture overnight on kidney function, the concentration of the first urine void in the morning, and increased proteinuria associated with exercise. The committee therefore concluded it would be wise to recommend not using the first morning urine void, to maximise the diagnostic accuracy for both PCR and ACR.
The committee discussed the widespread use of urine dipstick analysis in both primary and secondary care settings. As per the previous version of this guideline, the committee agreed that automated dipstick analysis should be used as a screening test to establish whether a woman requires further testing using PCR or ACR, but it should not be used for a definitive diagnosis. The committee agreed that the use of visual analysis of a dipstick test was highly subjective, therefore should be minimised and halted where possible, to be replaced by automated dipstick analysis, at least in secondary care (for example, it would not be practicable to expect all community midwives to carry an automated reader with them). This should ensure that women who need further assessment of proteinuria, and those in whom proteinuria is not present, can be safely identified and followed up as appropriate.
The evidence for diagnostic accuracy for PCR clearly showed sensitivity as very high at lower thresholds - at such thresholds the false negative rate is very low (a negative result can be taken with high confidence), whereas specificity was very low at the lowest thresholds (very low confidence in a positive result). As the threshold increased, sensitivity began to drop, and specificity rose. The majority of the evidence for PCR was at the threshold of 30mg/mmol, which is already commonly used in clinical practice for the identification of significant proteinuria. At this threshold, meta-analysis of 10 studies (including over 3000 women) confirmed high sensitivity and high specificity, with comparatively narrow confidence intervals, therefore the committee supported the use of this threshold.
In discussing the evidence for ACR use in the identification of significant proteinuria, the committee discussed the reasoning and scientific rationale behind the use of albumin compared to total protein (as in PCR). The scientific rationale suggests that, as albumin is a small molecule, it can pass from the kidneys into the urine sooner than other proteins. Therefore albumin may appear in the urine and be detected by an ACR test in the early stages of pre-eclampsia, before proteinuria or clinical symptoms and signs of pre-eclampsia may be present. Detecting these low levels of albuminuria may be useful in early detection of proteinuria, to monitor women for the development of pre-eclampsia.
The evidence for ACR was not as clear as with PCR, due to the limited number of studies, with small sample sizes, that could be included within the review. Sensitivity was noted to be high across all studies, at every threshold. In assessing the available evidence, both sensitivity and specificity appeared to be maximised at a threshold of 8.0 mg/mmol. However, there were no data for thresholds between 3.5 mg/mmol and 8.0 mg/mmol. The single study which reported data at a threshold of 8.0 mg/mmol included only 150 women, and showed very wide confidence intervals for sensitivity. In addition to the evidence presented within the review, the committee were aware of, and discussed, additional data reported in a recent, large, UK-based study (Waugh 2017). This study assessed the identification of proteinuria (with a reference standard of 24 hour urinary protein ≥300mg), and also the prediction of severe pre-eclampsia (with either the NICE definition of severe pre-eclampsia, or a clinician diagnosis of severe pre-eclampsia as the reference standard). For the purposes of this review, only the data relating to identification of proteinuria were relevant to the protocol.
The committee noted that Waugh 2017 presented additional data regarding the prediction of severe pre-eclampsia (as defined by NICE), which included further analyses of different ACR thresholds. In this analysis, it was noted that an ACR of 8.0 mg/mmol had comparable performance to that of a PCR of 30 mg/mmol. The ACR threshold of 8.0 mg/mmol was also used in a health economic model which was conducted as part of the Waugh study - which considered a clinical diagnosis of severe pre-eclampsia - and supported this ACR threshold as the most suitable and cost effective assessment.
Based on this additional information, and the limited evidence at 8.0 mg/mmol within this review, the committee supported the use of a threshold of 8mg/mmol for ACR when using this in the diagnosis of pre-eclampsia. The committee were aware that this threshold is different to that used for detection of microalbuminura in the non-pregnant population. However, they agreed that, on the basis of the evidence reviewed, it was appropriate to use a threshold of 8 mg/mmol for pregnant women.
Some ACR tests are designed as point-of-care or bedside assessments, and may be useful due to speed of obtaining the results. However, the data presented to the committee and used to aid decision making was based on ACR analysis performed within laboratories, and not at point-of-care. Consequently, the committee could not make a recommendation to use point-of-care ACR tests, and the recommendations regarding ACR results are based upon the diagnostic accuracy of laboratory tested spot/random urine samples. The committee discussed the potential for ACR point-of-care tests in the future, with improved technology allowing accurate and efficient testing to be undertaken, with results in minutes instead of the hours normally required for laboratory testing.
The difference in diagnostic accuracy for the identification of significant proteinuria was marginal between the two tests (PCR and ACR), therefore the committee did not recommend one test over the other. Local availability of the two tests could be used to determine which method is utilised. They noted that ACR testing was found to be more cost effective in the study by Waugh 2017, but again this was for the prediction of severe (clinician diagnosed) pre-eclampsia, rather than identification of significant proteinuria. The committee agreed that there was no benefit to performing both tests, as it provides no additional information.
The committee discussed when re-testing of ACR or PCR should be performed, if appropriate. No evidence addressing this issue was assessed. The committee noted that there is wide variation in the time taken for PCR and ACR results to be reported, and that this may impact on when a result should be repeated. Some laboratories are able to report a result within hours, while others take several days. It is unclear whether a false positive PCR or ACR result may resolve rapidly (over the course of the day), or whether it would be better to wait for several days before re-testing. Therefore this was left to the discretion of the health care professional, in discussion with the woman, taking into account other features of the pregnancy, clinical signs and symptoms of pre-eclampsia, and the local availability of testing, and the committee recommended that a re-testing schedule is developed according to the laboratory time available at local/regional level.
The committee noted that, whilst the evidence included in this review relates to the identification of proteinuria, a sequelae to that is often the diagnosis of pre-eclampsia. Typically, the urine may be checked because of an episode of hypertension. Therefore the identification of proteinuria in that urine sample would consequently lead to a firm diagnosis of pre-eclampsia being made, and a woman being offered intensive monitoring, follow up, and possible admission to hospital. The committee were aware that proteinuria is occasionally found to resolve on a subsequent urine sample (particularly when the initial sample showed a relatively low level of protein). Therefore they recommended consideration of repeating the PCR or ACR measurement in the absence of any other clinical symptoms or signs of pre-eclampsia. Clearly, if other defining features of pre-eclampsia were present, then the proteinuria result may need no confirmation.
The committee reiterated throughout the discussion that the results of either ACR or PCR in the assessment of proteinuria should be interpreted alongside the presence of hypertension and the other clinical signs and symptoms of pre-eclampsia. As emphasised at the start of this evidence report, and in keeping with international guidelines, the absence of proteinuria does not exclude the possibility of pre-eclampsia. Some women may develop other clinical features of the condition before developing significant proteinuria. Furthermore, although the sensitivity of ACR and PCR tests is high, false negative results may still occur. Therefore clinicians and women need to be vigilant to the other symptoms and signs of the disease, and not rely on the presence or absence of significant proteinuria alone as a defining feature.
Cost effectiveness and resource use
A systematic review of the economic literature was conducted but no relevant studies were identified which were applicable to this review question.
Use of spot urine tests for PCR or ACR should reduce the delay in identifying significant proteinuria, as compared with using a 24 hour urine test. This should reduce unnecessary hospital admissions for women in whom proteinuria can be confidently excluded. Furthermore, it will allow targeted follow up for women who are found to have a positive result.
Women who have a positive result for significant proteinuria are currently offered intensive follow up and monitoring, due to the suspicion/diagnosis of pre-eclampsia. Repeating the PCR/ACR tests for those with marginally elevated results is likely to increase the number of tests requested. However, this should also improve the diagnostic accuracy, by detecting those women in whom the first result was falsely positive. This will allow a step-down in follow up and monitoring for these women, reducing unnecessary resource use.
Other factors the committee took into account
The committee reviewed a graphical representation of the data regarding sensitivity and specificity of the PCR and ACR tests at different thresholds (Appendix M). This highlighted the high sensitivity and specificity of ACR at a threshold of 8mg/mmol, although the wide confidence interval for sensitivity was noted. Similarly, the committee noted the high sensitivity and specificity at a PCR threshold of 30mg/mmol, with comparatively narrow confidence intervals around the point estimate from the meta-analysis.
As discussed above, the committee were aware of the large diagnostic accuracy study (Waugh 2017) that was commissioned as a result of the previous guideline. Only the data which considered identification of significant proteinuria (reference standard ≥300mg in 24 hours) were directly relevant to this systematic review. However, the committee were aware of, and discussed, the other findings of the study – including reference standards of a diagnosis of severe pre-eclampsia (either as defined in this guideline, or a clinician diagnosis). The committee agreed that these were also important and relevant outcomes, which should be taken into account when appraising the evidence.
References
Al 2004
Al, R. A., Baykal, C., Karacay, O., Geyik, P. O., Altun, S., Dolen, I., Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy, Obstetrics & Gynecology, 104, 367–71, 2004 [PubMed: 15292013]Amin 2015
Amin, S. V., Illipilla, S., Hebbar, S., Rai, L., Kumar, P., Pai, M. V., Quantifying Proteinuria in Hypertensive Disorders of Pregnancy, International Journal of Hypertension, 2014, 941408, 2015 [PMC free article: PMC4181784] [PubMed: 25302114]Bhatti 2018
Bhatti, S., Cordina, M., Penna, L., Sherwood, R., Dew, T., Kametas, N. A., The effect of ethnicity on the performance of protein-creatinine ratio in the prediction of significant proteinuria in pregnancies at risk of or with established hypertension: an implementation audit and cost implications, Acta Obstetricia et Gynecologica Scandinavica, 97, 598–607, 2018 [PubMed: 29355892]Durnwald 2003
Durnwald, C., Mercer, B., A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia, American Journal of Obstetrics & Gynecology, 189, 848–52, 2003 [PubMed: 14526328]Dwyer 2008
Dwyer, B. K., Gorman, M., Carroll, I. R., Druzin, M., Urinalysis vs urine protein - Creatinine ratio to predict significant proteinuria in pregnancy, Journal of Perinatology, 28, 461–467, 2008 [PMC free article: PMC2743480] [PubMed: 18288120]Eslamian 2011
Eslamian, L., Behnam, F., Tehrani, Z. F., Jamal, A., Marsoosi, V., Random urine protein creatinine ratio as a preadmission test in hypertensive pregnancies with urinary protein creatinine ratio, Acta Medica Iranica, 49, 81–4, 2011 [PubMed: 21598214]Kucukgoz Gulec 2017
Kucukgoz Gulec, U., Sucu, M., Ozgunen, F. T., Buyukkurt, S., Guzel, A. B., Paydas, S., Spot Urine Protein-to-Creatinine Ratio to Predict the Magnitude of 24-Hour Total Proteinuria in Preeclampsia of Varying Severity, Journal of Obstetrics & Gynaecology Canada: JOGC, 21, 21, 2017 [PubMed: 28647444]Kyle 2008
Kyle, P. M., Fielder, J. N., Pullar, B., Horwood, L. J., Moore, M. P., Comparison of methods to identify significant proteinuria in pregnancy in the outpatient setting, BJOG: An International Journal of Obstetrics and Gynaecology, 115, 523–527, 2008 [PubMed: 18201282]Lamontagne 2014
Lamontagne, A., Cote, A. M., Rey, E., The urinary protein-to-creatinine ratio in Canadian women at risk of preeclampsia: does the time of day of testing matter?, Journal of Obstetrics & Gynaecology Canada: JOGC, 36, 303–8, 2014 [PubMed: 24798667]Leanos-Miranda 2007
Leanos-Miranda, A., Marquez-Acosta, J., Romero-Arauz, F., Cardenas-Mondragon, G. M., Rivera-Leanos, R., Isordia-Salas, I., Ulloa-Aguirre, A., Protein:creatinine ratio in random urine samples is a reliable marker of increased 24-hour protein excretion in hospitalized women with hypertensive disorders of pregnancy, Clinical Chemistry, 53, 1623–8, 2007 [PubMed: 17660273]Mohseni 2013
Mohseni, S. M., Moez, N., Naghizadeh, M. M., Abbasi, M., Khodashenas, Z., Correlation of random urinary protein to creatinine ratio in 24-hour urine samples of pregnant women with preeclampsia, Journal of Family & Reproductive Health, 7, 95–101, 2013 [PMC free article: PMC4064777] [PubMed: 24971109]Nisar 2017
Nisar, N., Akhtar, N., Dars, S., Diagnostic accuracy of spot urine protein-creatinine ratio in women with pre-eclapmsia, Medical Forum Monthly, 28, 6–10, 2017Park 2013
Park, Jung-Hwa, Chung, Dawn, Cho, Hee-Young, Kim, Young-Han, Son, Ga-Hyun, Park, Yong-Won, Kwon, Ja-Young, Random urine protein/creatinine ratio readily predicts proteinuria in preeclampsia, Obstetrics & gynecology science, 56, 8–14, 2013 [PMC free article: PMC3784101] [PubMed: 24327974]Rizk 2007
Rizk, D. E. E., Agarwal, M. M., Pathan, J. Y., Obineche, E. N., Predicting proteinuria in hypertensive pregnancies with urinary protein-creatinine or calcium-creatinine ratio, Journal of Perinatology, 27, 272–277, 2007 [PubMed: 17453039]Rodriguez-Thompson 2001
Rodriguez-Thompson, D., Lieberman, E. S., Use of a random urinary protein-to-creatinine ratio for the diagnosis of significant proteinuria during pregnancy, American Journal of Obstetrics & Gynecology, 185, 808–11, 2001 [PubMed: 11641656]Saudan 1997
Saudan, P. J., Brown, M. A., Farrell, T., Shaw, L., Improved methods of assessing proteinuria in hypertensive pregnancy, British Journal of Obstetrics & Gynaecology, 104, 1159–64, 1997 [PubMed: 9332994]Stout 2013
Stout, M. J., Scifres, C. M., Stamilio, D. M., Diagnostic utility of urine protein-to-creatinine ratio for identifying proteinuria in pregnancy, Journal of Maternal-Fetal & Neonatal Medicine, 26, 66–70, 2013 [PMC free article: PMC4118637] [PubMed: 23020712]Tun 2012
Tun, C., Quinones, J. N., Kurt, A., Smulian, J. C., Rochon, M., Comparison of 12-hour urine protein and protein:creatinine ratio with 24-hour urine protein for the diagnosis of preeclampsia, American Journal of Obstetrics & Gynecology, 207, 233.e1–8, 2012 [PubMed: 22939731]Valdes 2016
Valdes, E., Sepulveda-Martinez, A., Tong, A., Castro, M., Castro, D., Assessment of Protein: Creatinine Ratio versus 24-Hour Urine Protein in the Diagnosis of Preeclampsia, Gynecologic and Obstetric Investigation, 81, 78–83, 2016 [PubMed: 26045043]Waugh 2005
Waugh, J. J. S., Bell, S. C., Kilby, M. D., Blackwell, C. N., Seed, P., Shennan, A. H., Halligan, A. W. F., Optimal bedside urinalysis for the detection of proteinuria in hypertensive pregnancy: A study of diagnostic accuracy, BJOG: An International Journal of Obstetrics and Gynaecology, 112, 412–417, 2005 [PubMed: 15777437]Waugh 2017
Waugh, J., Hooper, R., Lamb, E., Robson, S., Shennan, A., Milne, F., Price, C., Thangaratinam, S., Berdunov, V., Bingham, J., Spot protein-creatinine ratio and spot albumin-creatinine ratio in the assessment of pre-eclampsia: A diagnostic accuracy study with decision-analytic model-based economic evaluation and acceptability analysis, Health Technology Assessment, 21, 1–90, 2017 [PMC free article: PMC5672500] [PubMed: 29064366]Wheeler 2007
Wheeler, Thomas L., 2nd, Blackhurst, Dawn W., Dellinger, Eric H., Ramsey, Patrick S., Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia, American Journal of Obstetrics and Gynecology, 196, 465.e1–4, 2007 [PubMed: 17466704]Wilkinson 2013
Wilkinson, C., Lappin, D., Vellinga, A., Heneghan, H.M., O’Hara, R., Monaghan, J., Spot urinary protein analysis for excluding significant proteinuria in pregnancy, Journal of Obstetrics and Gynaecology, 33, 24–27, 2013 [PubMed: 23259873]
Appendices
Appendix A. Review protocol
Appendix B. Literature search strategies
Review question search strategies
Databases: Medline; Medline EPub Ahead of Print; and Medline In-Process & Other Non-Indexed Citations
Databases: Embase; and Embase Classic
Databases: Cochrane Central Register of Controlled Trials; Cochrane Database of Systematic Reviews; Database of Abstracts of Reviews of Effects; and Health Technology Assessment
Health economics search strategies
Databases: Medline; Medline EPub Ahead of Print; and Medline In-Process & Other Non-Indexed Citations
Databases: Embase; and Embase Classic
Databases: Cochrane Central Register of Controlled Trials; Health Technology Assessment; and NHS Economic Evaluation Database
Appendix D. Clinical evidence tables
Table 4. Clinical evidence tables (PDF, 1.9M)
Appendix E. Forest plots
Figure 1. Forest plot for ACR cut-off 2.0 mg/mmol
Figure 2. Forest plot for PCR cut-off 0.15 (15 mg/mmol
Figure 3. Forest plot for PCR cut-off 0.19 (19 mg/mmol)
Figure 4. Forest plot for PCR cut-off 0.20 (20 mg/mmol)
Figure 5. Forest plot for PCR cut-off 0.30 (30 mg/mmol): overall
Appendix F. GRADE tables
Appendix H. Economic evidence tables
No economic evidence was identified for this review question.
Appendix I. Health economic evidence profiles
No economic evidence was identified for this review question
Appendix J. Health economic analysis
No health economic analysis was conducted for this review question
Appendix K. Excluded studies
Clinical studies
Table 7. Clinical excluded studies with reasons for exclusion
Economic studies
Table 8. Economic excluded studies with reasons for exclusion
Appendix L. Research recommendations
No research recommendations were made for this review question.
Appendix M. Additional Graphs
FINAL
Evidence reviews
These evidence reviews were developed by The National Guideline Alliance hosted by the Royal College of Obstetricians and Gynaecologists
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Review Spot protein-creatinine ratio and spot albumin-creatinine ratio in the assessment of pre-eclampsia: a diagnostic accuracy study with decision-analytic model-based economic evaluation and acceptability analysis.[Health Technol Assess. 2017]Review Spot protein-creatinine ratio and spot albumin-creatinine ratio in the assessment of pre-eclampsia: a diagnostic accuracy study with decision-analytic model-based economic evaluation and acceptability analysis.Waugh J, Hooper R, Lamb E, Robson S, Shennan A, Milne F, Price C, Thangaratinam S, Berdunov V, Bingham J. Health Technol Assess. 2017 Oct; 21(61):1-90.
- Review Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice.[Crit Rev Clin Lab Sci. 2020]Review Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice.Kamińska J, Dymicka-Piekarska V, Tomaszewska J, Matowicka-Karna J, Koper-Lenkiewicz OM. Crit Rev Clin Lab Sci. 2020 Aug; 57(5):345-364. Epub 2020 Feb 14.
- A prospective study of the impact of automated dipstick urinalysis on the diagnosis of preeclampsia.[Hypertens Pregnancy. 2004]A prospective study of the impact of automated dipstick urinalysis on the diagnosis of preeclampsia.Phelan LK, Brown MA, Davis GK, Mangos G. Hypertens Pregnancy. 2004; 23(2):135-42.
- The accuracy of spot urinary protein-to-creatinine ratio in confirming proteinuria in pre-eclampsia.[Aust N Z J Obstet Gynaecol. 2012]The accuracy of spot urinary protein-to-creatinine ratio in confirming proteinuria in pre-eclampsia.Cade TJ, Gilbert SA, Polyakov A, Hotchin A. Aust N Z J Obstet Gynaecol. 2012 Apr; 52(2):179-82. Epub 2012 Feb 15.
- Protein-creatinine ratio and albumin-creatinine ratio for the diagnosis of significant proteinuria in pregnant women with hypertension: Systematic review and meta-analysis of diagnostic test accuracy.[Pregnancy Hypertens. 2021]Protein-creatinine ratio and albumin-creatinine ratio for the diagnosis of significant proteinuria in pregnant women with hypertension: Systematic review and meta-analysis of diagnostic test accuracy.Geneen LJ, Webster KE, Reeves T, Eadon H, Maresh M, Fishburn S, Chappell LC. Pregnancy Hypertens. 2021 Aug; 25:196-203. Epub 2021 Jul 1.
- Evidence review for assessment of proteinuriaEvidence review for assessment of proteinuria
Your browsing activity is empty.
Activity recording is turned off.
See more...