NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Effectiveness of cannabis-based medicinal products for the treatment of severe treatment-resistant epilepsy
Introduction
Severe treatment-resistant epilepsy, or drug-resistant epilepsy, is defined by the International League Against Epilepsy as epilepsy that has not responded to trials of 2 tolerated and appropriately chosen and used anti-epileptic drug regimens (as monotherapies or in combination) to achieve sustained freedom from seizures.
There are about 600,000 people in the UK with a diagnosis of epilepsy taking antiepileptic drug treatment; the prevalence of drug-resistant epilepsy is about 30% of all people with epilepsy on treatment (NICE Clinical Knowledge summary on epilepsy; The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis).
The NICE guideline on diagnosing and managing epilepsies covers diagnosing, treating and managing epilepsy and seizures in children, young people and adults in primary and secondary care. It offers best practice advice on managing epilepsy to improve health outcomes so that people with epilepsy can fully participate in daily life. The NICE guideline is currently being updated as two guidelines: Epilepsies in adults: diagnosis and management update and Epilepsies in children: diagnosis and management.
The aim of this review is to examine the effectiveness of cannabis-based medicinal products (CBMPs) for people with severe treatment-resistant epilepsy This review also aims to identify adverse events, complications and contraindications associated with the use of CBMPs. Additionally, this review will examine individual patient requirements, treatments durations, reviewing and stopping criteria for the use of CBMPs.
Review question
What is the clinical and cost effectiveness of cannabis-based medicinal products for people with severe treatment-resistant epilepsy?
What are the adverse effects or complications of cannabis-based medicinal products for people with severe treatment-resistant epilepsy?
What are the contraindications, potential interactions and risks and cautions for use of cannabis-based medicinal products for people with severe treatment-resistant epilepsy?
What are the individual patient monitoring requirements, treatment durations, reviewing and stopping criteria, including how should treatment be withdrawn or stopped, for use of cannabis-based medicinal products for people with severe treatment-resistant epilepsy?
PICO table
| Population |
Adults, young people, children and babies with severe treatment-resistant epilepsy. Specific considerations will be given to:
|
|---|---|
| Interventions | Cannabis-based medicinal product |
| Comparator |
|
| Outcomes |
|
Evidence review
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual (2018). A review protocol was developed to encompass the four review questions around effectiveness, adverse events, contraindications and monitoring requirements. This review protocol can be found in Appendix A. Methods specific to the review questions are described in the review protocol in Appendix B.
Declarations of interest were recorded according to NICE’s 2018 conflicts of interest policy.
A broad search strategy was used to identify all studies that examined the effectiveness of cannabis-based medicinal products (CBMPs) in the treatment of intractable nausea and vomiting, chronic pain, spasticity and severe treatment-resistant epilepsy. The review protocol highlighted in Table 1 and Appendix A was used to identify studies associated with severe treatment-resistant epilepsy.
For the adult population, randomised controlled trials (RCTs) and systematic review of RCTs were considered. The committee noted that a minimum of 5 RCTs were required to provide adequate evidence. If fewer than 5 RCTs were identified, prospective observational studies would also be considered for inclusion.
For children, RCTs and systematic reviews of RCTs were considered. The review protocol also specified that in the event of fewer than 5 RCTs being identified, observational cohort studies would be considered for inclusion. The committee expected that there would be fewer studies for children than adults and so both prospective and retrospective observational studies would be considered.
Additional information on safety concerns and contraindications will be obtained from the Summary of Product Characteristics and other relevant sources, such as the U.S Food and Drugs Administration.
Studies were also excluded if they examined the use of:
- Synthetic cannabinoids in schedule 1 of the 2001 regulations,
- Smoked cannabis-based products
The review protocol also specifies that where possible, subgroup analyses would be conducted to explore the effectiveness of cannabis-based medicinal products in young people, children and babies, pregnant women and women who are breastfeeding, people with existing substance abuse and people with hepatic and renal failure. However, no evidence was available to carry out these subgroup analyses.
Protocol deviations
The review protocol stated that if fewer than 5 RCTs were identified then prospective cohort studies would be included. However, full-text screening of observational studies found no prospective cohort studies that met the inclusion criteria. It was therefore agreed to deviate from the protocol and include single-arm study designs as part of the review. This resulted in the inclusion of 11 single-arm observational studies.
Clinical evidence
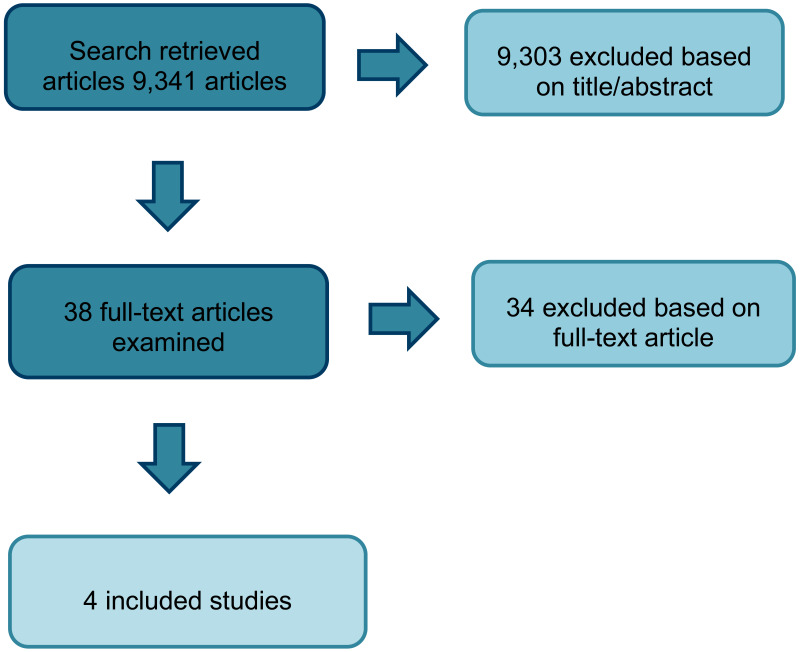
A total of 19,491 RCTs and systematic reviews were identified from the search. After removing duplicates, 9,341 references were screened on their titles and abstracts. 38 studies were obtained and reviewed against the inclusion criteria as described in the review protocol for severe treatment-resistant epilepsy (Appendix A). Overall, 4 parallel RCTs were included (2 for Dravet syndrome and 2 for Lennox-Gastaut syndrome - see Table 2). The use of cannabidiol (CBD) for Dravet and Lennox-Gastaut syndromes were listed as part of the exclusion criteria because this is currently being considered by technology appraisals. However, given the limited number of RCTs available for the use of cannabis for epilepsy, these studies were included in the evidence review to provide the committee with an overview of the current available evidence. This also gave the committee an opportunity to discuss whether the results of these studies could be applied to other types of epilepsy in the absence of any RCT evidence for other epilepsy syndromes. No studies were identified for any of the subgroup analyses.
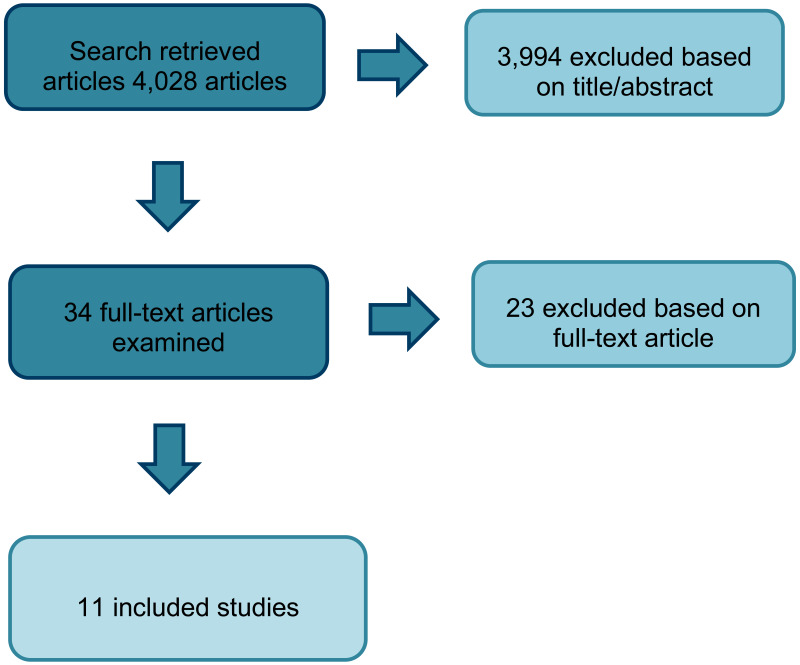
As fewer than 5 RCTs were identified, observational studies were also incorporated into the literature search. From a database of 4,028 observational studies, 34 studies were identified as potentially relevant. Following full text review of the 34 studies, 11 observational studies were included in the review. All 11 studies were single-arm observational studies; 8 were prospective analyses, 2 were retrospective and 1 was unclear. Whereas the RCT evidence only examined the use of CBD products, the observational studies included CBD products and those containing both THC and CBD. Data for the single-arm trials are presented in Appendix K.
See Appendix E for evidence tables and Appendix I for excluded studies. See Appendix K for a summary of the included single-arm observational studies, including the constituents and doses used in each study.
Quality assessment of clinical studies included in the evidence review
The 2 RCTs identified for Lennox-Gastaut syndrome were assessed as low risk of bias. The 2 RCTs identified for Dravet syndrome were downgraded for providing limited information on random sequence allocation, allocation concealment or whether assessors were aware of the intervention. All 4 studies were downgraded for indirectness as they assessed patients with Lennox-Gastaut or Dravet syndrome rather than other types of epilepsy that were within the inclusion criteria. See Appendix G for full GRADE tables and Appendix F for forest plots in situations where data have been meta-analysed.
The 11 single-arm observational studies identified were very low quality. All of these studies were downgraded for indirectness as the inclusion of single-arm studies was a deviation from the protocol.
Interventions
Each of the 4 included RCTs examined the use of CBD oil for treating different forms of epilepsy: 2 studies looked at Dravet syndrome and 2 looked at Lennox-Gastaut syndrome.
Most of the single-arm studies also used CBD oil as the active treatment although 2 used capsules containing both delta-9-tetrahydrocannabinol (THC) and CBD. Of the 11 single-arm observational studies included, 1 examined the use of CBD for the treatment of Dravet syndrome, 8 examined cannabis-based medicinal products for intractable epilepsy (6 using CBD oil, 2 using THC:CBD oil), 1 examined the use of CBD for febrile infection-related epilepsy syndrome and 1 used CBD for drug-resistant epilepsy in tuberous sclerosis complex.
At the time of writing this evidence review, no CBMP had a UK marketing authorisation for the management of treatment-resistant epilepsy.
Summary of clinical studies included in the evidence review
Table 2
summary of included RCT studies.
See Appendix E for evidence tables and Appendix H for further information on adverse events.
As part of this evidence review, in addition to reviewing efficacy and safety data, studies were reviewed for information about patient monitoring and reviewing and stopping criteria when cannabis-based medicinal products were prescribed.
The interventions, doses, monitoring and stopping criteria are summarised in tables 4 and 5 below:
Table 4
summary of interventions and doses in the included studies.
See Appendix E for evidence tables.
Economic evidence
Included studies
A systematic review of the economic literature was conducted. 1,863 studies were retrieved by the search. No economic studies were identified which were applicable to this review question and no full-text copies of articles were requested.
Excluded studies
No full-text copies of articles were requested for this review and so there is no excluded studies list.
Economic model
No economic modelling was undertaken for this review because of a lack of economic evidence and because the results from the clinical evidence could not be directly applied to all treatment-resistant epilepsies.
Summary of evidence
The summary of evidence in this section reflects the evidence on effectiveness of CBMPs. Evidence statements are stratified by population and reflect evidence that was statistically significant. Further information on adverse events is also provided. Evidence statements are only provided for outcomes for the RCT studies because the single-arm trials did not have a control group against which to make comparisons. The format of the evidence summary table is explained in the methods in Appendix B. Further information on adverse events is provided in Appendix H.
Clinical evidence
Cannabidiol for Dravet syndrome
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Reduction in frequency of total seizures from baseline | |||||
| 20 mg/kg/day | |||||
| 1 (Devinsky 2017) | Parallel RCT | 120 | Median percentage point difference (IQR) −19.20 (−39.25, −1.17) | Low | Favours CBD |
| Reduction in total seizures from baseline | |||||
| 20 mg/kg/day | |||||
| 1 (Devinsky 2017) | Parallel RCT | 120 | Median percentage point difference (IQR) −22.8 (−41.1, −5.4) | Low | Favours CBD |
| Total adverse events | |||||
| 20 mg/kg/day | |||||
| 1 (Devinsky 2017) | Parallel RCT | 120 | RR 1.25 (1.06, 1.48) | Low | Favours placebo |
Commonly reported adverse events
- At a dose of 5 mg/kg.day, commonly reported adverse events included pyrexia, somnolence, sedation, abnormal behaviour and ataxiaAt a dose of 10 mg/kg/day, commonly reported adverse events included pyrexia, somnolence, vomiting, decreased appetite, vomiting, nasopharyngitis, convulsion, pneumonia and rash
- At a dose of 20 mg/kg/day, commonly reported adverse events included decreased appetite, somnolence, diarrhoea, fatigue and vomiting
Cannabidiol for Lennox-Gastaut syndrome
| No. of studies | Study design | Sample size | Effect size (95% CI) | Quality | Interpretation of effect |
|---|---|---|---|---|---|
| Number of people achieving 50% seizure reduction | |||||
| 10 mg/kg/day | |||||
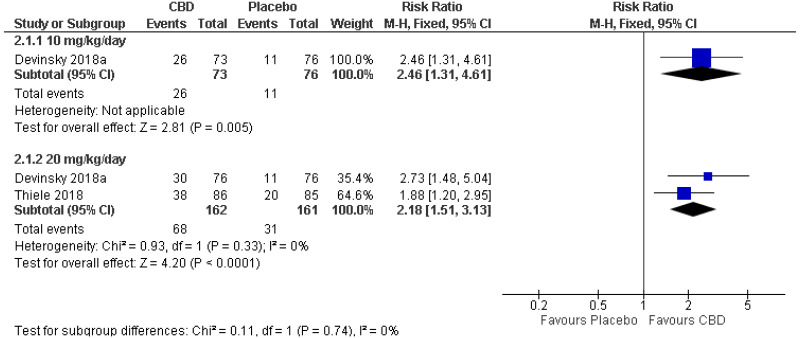
| 1 (Devinsky 2018) | Parallel RCT | 149 |
RR 2.46 (1.31, 4.61) | Moderate | Favours CBD |
| 20 mg/kg/day | |||||
| 2 (Devinsky 2018, Thiele 2018) | Parallel RCTs | 323 |
RR 2.18 (1.51, 3.13) | Moderate | Favours CBD |
| Reduction in total seizures from baseline | |||||
| 10 mg/kg/day | |||||
| 1 (Devinsky 2018) | Parallel RCT | 149 |
Median percentage point difference (IQR) −19.5 (−330.4, −7.5) | Moderate | Favours CBD |
| 20 mg/kg/day | |||||
| 1 (Devinsky 2018) | Parallel RCT | 152 |
Median percentage point difference (IQR) −18.8 (−31.8, −4.4) | Moderate | Favours CBD |
| 1 (Thiele 2018) | Parallel RCT | 171 |
Median percentage point difference (IQR) −21.1 (−33.3, −9.4) | Moderate | Favours CBD |
| Reduction in drop seizures from baseline | |||||
| 10 mg/kg/day | |||||
| 1 (Devinsky 2018) | Parallel RCT | 149 |
Median percentage point difference (IQR) −19.2 (−31.2, −7.7) | Moderate | Favours CBD |
| 20 mg/kg/day | |||||
| 1 (Devinsky 2018) | Parallel RCT | 152 |
Median percentage point difference (IQR) −21.6 (−34.8, −6.7) | Moderate | Favours CBD |
| 1 (Thiele 2018) | Parallel RCT | 171 |
Median percentage point difference (IQR) −17.21 (−30.32, −4.09) | Moderate | Favours CBD |
Commonly reported adverse events
- At a dose of 10 mg/kg/day, commonly reported adverse events included somnolence, decreased appetite, upper respiratory tract infection, diarrhoea and status epilepticus
- At a dose of 20 mg/kg/day, commonly reported adverse events included somnolence, diarrhoea, decreased appetite, pyrexia and upper respiratory tract infection
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The committee decided that outcomes including the proportion of patients achieving 50% or greater reduction in seizures and percentage reduction in seizures from baseline were key outcomes for assessing effectiveness. The number of adverse events was also considered important to evaluate the safety of CBMPs. Other outcomes considered by the committee included the dose, treatment duration, contraindications, monitoring requirements and stopping criteria.
The quality of the evidence
There were only 4 RCTs which evaluated the use of CBMPs in severe treatment-resistant epilepsy. RCT evidence for Dravet syndrome ranged from very low to low quality and evidence for Lennox-Gastaut syndrome ranged from low to moderate quality. Each RCT was rated as partially applicable as they examined the effectiveness of Epidiolex for the treatment of Dravet or Lennox Gastaut syndromes, which did not meet the inclusion criteria for this review. Although different types of epilepsy may have some common mechanisms, the committee agreed that there are differences in underlying pathologies that mean the results of these studies could not inform recommendations on other epilepsy syndromes.
Given the low number of RCTs, evidence from 11 observational studies were also considered. Each of these studies were single-arm studies, 2 of which were retrospective. Whereas the RCT evidence examined only CBD products, the observational studies included both CBD and THC: CBD products: 8 examined the use of pure CBD and 3 used THC: CBD plant-extract. There was a wide range of doses used and most studies included people with a diagnosis of severe treatment-resistant epilepsy, rather than a specific epilepsy syndrome. Other studies looked specifically at either Dravet syndrome, febrile infection-related epilepsy syndrome or tuberous sclerosis complex but these were informed by a single study for each condition. Although most studies included both adults and children only 1 of these separated the results by age, making it difficult to determine whether this is a factor in the effectiveness of CBMPs.
Each of the observational studies were downgraded for being at high risk of bias as a result of the single-arm study design. This design does not provide an estimate of the effect of an intervention and by not including a comparison group there was also no way to determine how outcomes would have changed either without CBMPs or with a different treatment. Some of the studies also had very low participant numbers and little information about the methods used. The committee agreed that the very low quality of evidence and absence of a control arm for comparisons meant that these results could not be used to make any recommendations.
The committee agreed that the very low quality of evidence and lack of RCTs meant it was not currently possible to make any recommendations for the use of CBMPs for severe treatment-resistant epilepsy. The only RCT evidence available was for the use of Epidiolex for Lennox Gastaut or Dravet syndromes, both of which will form part of a technology appraisal update and so were excluded from this review. Instead they agreed that it was important that people with severe treatment-resistant epilepsy and their patients and carers were made aware of the current limited understanding of the effectiveness of these products. Existing research was used to help form research recommendations to help improve the quality of evidence in the future.
Benefits and harms
There are a number of anti-epileptic treatments which may reduce the frequency and severity of seizures in people with epilepsy. However, not all patients respond to these treatments and some may experience adverse events. Cannabidiol (Epidyolex) has been licensed by the MHRA as an add-on treatment for seizures associated with Lennox-Gastaut syndrome or Dravet syndrome, in conjunction with clobazam, for people aged 2 years and over and is listed under Schedule 2 of the 2001 Regulations. All other CBMPs are currently unlicensed for the treatment of epilepsy but There are some reports of individual patients benefitting from their use as adjuvant therapy for reducing seizure frequency when other treatments have failed. However, current research is limited and of low quality making it difficult to quantify how effective CBMPs are for this population.
A potential harm associated with CBMPs is the high number of adverse events. However, current RCT research focuses on people with Dravet and Lennox-Gastaut syndrome, both of which are populations who often experience adverse events. Without further research it is unclear whether a similar number of adverse events would be experienced by people with other epilepsy syndromes following the use of CBMPs. The observational studies also reported high adverse events, with up to 98% of people experiencing an adverse event. However, the low-quality single-arm design of these studies means it is not possible to determine how many of these events were likely to be a result of CBMPs. The committee were concerned about the current lack of high-quality evidence including the potential for adverse events, particularly because most of the research for severe treatment-resistant epilepsy is in children and young people where adverse events could have long-term effects. People with severe treatment-resistant epilepsy also tend to have more severe illness than those with other conditions that may benefit from CBMPs, and the effects of an adverse events may therefore be more severe. Current research has also investigated a range of different CBMPs and it is currently unclear how adverse events may vary between these different products.
Given the limited amount of research currently available for the use of CBMPs for treatment-resistant epilepsy, the committee decided that making no recommendation was preferable to making a recommendation against the use of CBMPs. Not making a recommendation against their use means that people who are currently benefitting from the use of CBMPs can continue with treatment, and specialists, people with epilepsy and their carers will not be prevented from making individualised treatment decisions. A recommendation against the use of CBMPs would also prevent any future research into their effectiveness. The committee agreed that this would not be helpful as further research is necessary to provide a greater understanding of the potential benefits and harms of these products. There was also concern that a recommendation against the prescribing of CBMPs could lead to an increase in patients and carers using unprescribed (over the counter/internet) CBMPs. This could potentially be harmful given the unmonitored nature of these products and limited understanding about their effects and how they may react with concomitant medications.
Cost effectiveness and resource use
Since no recommendations were made for clinical practice, the issue of cost-effectiveness was not considered explicitly, and no resource impact is expected. Broadly, the committee were aware that CBMPs are expensive but had the potential to generate significant gains in quality of life and reduction in resource use in those patients who respond very well to treatment. Importation costs currently account for a significant proportion of the costs of some CBMPs but these are expected to drop over time following the recent regulatory changes.
Other factors the committee took into account
Throughout the committee discussion, a key concern was the lack of high-quality evidence for severe treatment-resistant epilepsy. Currently, anyone using CBMPs for severe treatment-resistant epilepsy must be granted an individual funding request. However, it was noted that some applications are currently being denied because of a lack of evidence for the efficacy of CBMPs. This supports the need for further research into the effectiveness of CBMPs so that treatment decisions can be made based on a stronger and more extensive evidence base.
A key discussion point for the committee was the constituents that make up CBMPs. There are a range of CBMPs, some of which contain either purified CBD alone or purified CBD combined with THC. Others contain CBD and THC from whole-plant extracts. The committee agreed that although most of the current evidence for severe treatment-resistant epilepsy has evaluated the use of pure CBD products, it is also important to know whether the addition of THC to CBD has further benefits or a different adverse event profile. There were also questions over whether CBD-rich plant extract might be effective. Some of the observational studies used CBD-rich extract rather than pure CBD but the different effects were not considered by the committee given the low quality of these studies.
The committee also had concerns over the doses and monitoring of CBMPs. Although the RCTs and some of the observational studies used pharmaceutical grade cannabidiol, others used non-pharmaceutical grade products. These are unlikely to have the same standards of production and so there was concern that the concentration of CBD and THC in these products could be variable. This may be a particular issue for CBMPs that are from whole-plant extracts as the concentration of THC and CBD in these plants can vary widely making it more difficult to standardise the dose of medication.
The committee were aware of ongoing research in this area including trials of cannabidiol in tuberous sclerosis complex and infantile spasms and felt that this evidence, when published, could be an important consideration in the discussions of future committees looking at this topic.
This evidence review supports the research recommendations on CBD for severe treatment-resistant epilepsy and THC in combination with CBD for severe treatment-resistant epilepsy.
Glossary
Cannabis-based medicinal products
In this guideline cannabis-based medicinal products include:
- cannabis-based products for medicinal use as set out by the UK Government in the 2018 Regulations
- the licensed products delta-9-tetrahydrocannibinol and cannabidiol (Sativex) and nabilone
- plant-derived cannabinoids such as pure cannabidiol (CBD)
- synthetic compounds which are identical in structure to naturally occurring cannabinoids such as delta-tetrahydrocannabinol (THC), for example, dronabinol.
Appendix A. Review protocols
Review protocol for clinical effectiveness, cost effectiveness, contraindications, potential interactions, individual patient monitoring requirements, treatment durations, reviewing and stopping criteria for cannabis based medicinal products
| Field (based on PRISMA-P | Content |
|---|---|
| Review question |
What is the clinical and cost effectiveness of cannabis-based medicinal products for people with severe treatment-resistant epilepsy? What are the adverse effects or complications of cannabis-based medicinal products for people with severe treatment-resistant epilepsy? What are the contraindications, potential interactions and risks and cautions for use of cannabis-based medicinal products for people with severe treatment-resistant epilepsy? What are the individual patient monitoring requirements, treatment durations, reviewing and stopping criteria, including how should treatment be withdrawn or stopped, for use of cannabis-based medicinal products for people with severe treatment-resistant epilepsy? |
| Type of review question | Intervention |
| Objective of the review | To determine the effectiveness, harms and cost-effectiveness of cannabis-based medicinal products in reducing severe treatment-resistant epilepsy |
| Eligibility criteria – population/disease/c ondition/issue/domain |
Adults, young people, children and babies with severe treatment-resistant epilepsy. Specific considerations will be given to:
Studies where epilepsy is being managed by cannabis in one arm will be included. Cannabis cannot be used as a first-line or second-line treatment because the population of interest is severe treatment-resistant epilepsy. |
| Eligibility criteria – intervention |
Cannabis-based products for medicinal use (as per government definition): A cannabis-based product for medicinal use that is a preparation or other product, other than one to which paragraph 5 of part 1 of schedule 4 applies, which: is or contains cannabis, cannabis resin, cannabinol or a cannabinol derivative (not being dronabinol or its stereoisomers) is produced for medicinal use in humans; and is a medicinal product, or a substance or preparation for use as an ingredient of, or in the production of an ingredient of, a medicinal product (MDR 2018 regulations) Synthetic compounds which are identical in structure to naturally occurring cannabinoids such as delta-9-tetrahydrocannabinol (THC) for example dronabinol Licensed products Sativex and nabilone Plant-derived cannabinoids such as pure cannabidiol For the purpose of this guideline, all the interventions above will be classed as cannabis-based medicinal products. |
| Eligibility criteria – comparator |
Placebo Any relevant treatment Combination of treatments Usual or standard care. |
| Outcomes |
Proportion of patients achieving seizure freedom 50% or greater reduction in seizures Reduction of seizures from baseline Quality of life scores Serious adverse events Adverse events including but not limited to: sleep problems, fatigue, road traffic accidents, psychological distress, dizziness, headache, confusion state, paranoia, psychosis, substance dependence, diarrhoea at the start of treatment Withdrawals due to adverse events Complications due to adverse events Change in cognition Substance abuse due to the use of cannabis-based medicinal product. Misuse/diversion Hepatic and renal failure Outcomes requiring a narrative synthesis: Contraindications as listed in exclusion criteria Monitoring requirements, treatment durations, reviewing and stopping criteria, including how should treatment be withdrawn stopped as discussed in the methods of included studies. |
| Eligibility criteria – study design |
For adults: RCTs Systematic reviews of RCTs The committee noted that a minimum of 5 RCTs were required to provide adequate evidence. If less than five RCTs identified, prospective cohort studies will be used. For children: RCTs Systematic reviews of RCTs If less than five RCTs identified, prospective and retrospective cohort studies will be used. Additional information on safety concerns and contraindications will be obtained from the Summary of Product Characteristics and other relevant sources, such as the U.S Food and Drugs Administration. |
| Other inclusion/exclusion criteria |
Inclusion Cannabis-based products for medicinal use when other treatments haven’t helped or have been discounted. Exclusion Synthetic cannabinoids in schedule 1 of the 2001 regulations, Smoked cannabis-based products Studies which do not report the doses or the concentration of cannabinoid constituents. For randomised crossover studies, washout periods of less than 1 week. |
| sub-group analysis |
Subgroups, where possible, will include: Young people, children and babies Pregnant women and women who are breastfeeding People with existing substance abuse Spasticity in relation to multiple sclerosis (MS) People with hepatic and renal failure |
| Selection process – duplicate screening/selection/a nalysis | 10% of the abstracts will be reviewed by two reviewers, with any disagreements will be resolved by discussion or, if necessary, a third independent reviewer. If meaningful disagreements are found between the different reviewers, a further 10% of the abstracts will be reviewed by two reviewers, with this process continuing until agreement is achieved between the two reviewers. From this point, the remaining abstracts will be screened by a single reviewer. |
| Data management (software) | See Appendix B. |
| Information sources – databases and dates |
Sources to be searched Clinical searches - Medline, Medline in Process, Medline EPub Ahead of Print, Embase, Cochrane CDSR, CENTRAL, DARE (legacy records), HTA, MHRA. Economic searches - Medline, Medline in Process, Medline EPub Ahead of Print, Embase, Econlit, NHS EED (legacy records) and HTA, with economic evaluations and quality of life filters applied. Supplementary search techniques None identified Limits Studies reported in English Study design RCT, SR and Observational filter will be applied (as agreed) Animal studies will be excluded from the search results Conference abstracts will be excluded from the search results No date limit will be set. |
| Identify if an update | N/A |
| Author contacts | Guideline updates team |
| Highlight if amendment to previous protocol | This is a new protocol. |
| Search strategy – for one database | For details please see Appendix C of relevant chapter. |
| Data collection process – forms/duplicate | A standardised evidence table format will be used, and published as Appendix D (clinical evidence tables) or H (economic evidence tables). |
| Data items – define all variables to be collected | For details please see evidence tables in Appendix D (clinical evidence tables) or H (economic evidence tables). |
| Methods for assessing bias at outcome/study level |
Study checklists were used to critically appraise individual studies. For details please see Appendix H of Developing NICE guidelines: the manual The following checklists will be used: Risk of bias of intervention studies - systematic reviews and meta-analyses will be assessed using the Risk of Bias in Systematic Reviews (ROBIS) checklist Risk of bias of intervention studies – randomised controlled trials (individual or cluster) will be assessed using the Cochrane risk of bias (RoB) 2.0 tool Risk of bias of cohort studies will be assessed using Cochrane ROBINS-I The risk of bias across all available evidence was evaluated for each outcome using an adaptation of the ‘Grading of Recommendations Assessment, Development and Evaluation (GRADE) toolbox’ developed by the international GRADE working group http://www |
| Criteria for quantitative synthesis | For details please see section 6 of Developing NICE guidelines: the manual |
| Methods for quantitative analysis – combining studies and exploring (in)consistency | For details please see the methods and process section of the main file. |
| Meta-bias assessment – publication bias, selective reporting bias | For details please see section 6 of Developing NICE guidelines: the manual. |
| Confidence in cumulative evidence | For details please see sections 6 of Developing NICE guidelines: the manual |
| Rationale/context – what is known | For details please see the introduction to the evidence review in the main file. |
| Describe contributions of authors and guarantor |
A multidisciplinary committee [add link to history page of the guideline] developed the evidence review. The committee was convened by NICE Guideline Updates Team and chaired by Steve Pilling in line with section 3 of Developing NICE guidelines: the manual. Staff from NICE undertook systematic literature searches, appraised the evidence, conducted meta-analysis and cost-effectiveness analysis where appropriate, and drafted the evidence review in collaboration with the committee. For details please see Developing NICE guidelines: the manual. |
| Sources of funding/support | The NICE Guideline Updates Team is an internal team within NICE. |
| Name of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
| Roles of sponsor | The NICE Guideline Updates Team is an internal team within NICE. |
Appendix B. Methods
1.1. Priority screening
The reviews undertaken for this guideline all made use of the priority screening functionality with the EPPI-reviewer systematic reviewing software. This uses a machine learning algorithm (specifically, an SGD classifier) to take information on features (1, 2 and 3 word blocks) in the titles and abstract of papers marked as being ‘includes’ or ‘excludes’ during the title and abstract screening process, and re-orders the remaining records from most likely to least likely to be an include, based on that algorithm. This re-ordering of the remaining records occurs every time 25 additional records have been screened.
As an additional check to ensure this approach did not miss relevant studies, the included studies list of included systematic reviews were searched to identify any papers not identified through the primary search.
1.2. Evidence synthesis and meta-analyses
Where possible, meta-analyses were conducted to combine the results of quantitative studies for each outcome. For continuous outcomes analysed as mean differences, where change from baseline data were reported in the trials and were accompanied by a measure of spread (for example standard deviation), these were extracted and used in the meta-analysis. Where measures of spread for change from baseline values were not reported, the corresponding values at study end were used and were combined with change from baseline values to produce summary estimates of effect. These studies were assessed to ensure that baseline values were balanced across the treatment groups; if there were significant differences at baseline these studies were not included in any meta-analysis and were reported separately. For continuous outcomes analysed as standardised mean differences, where only baseline and final time point values were available, change from baseline standard deviations were estimated, assuming a correlation coefficient of 0.5.
1.3. Evidence of effectiveness of interventions
Quality assessment
Parallel RCTs were quality assessed using the Cochrane Risk of Bias Tool 2.0.
Each individual study was classified into one of the following three groups:
- Low risk of bias – The true effect size for the study is likely to be close to the estimated effect size.
- Some concern around risk of bias – There is a possibility the true effect size for the study is substantially different to the estimated effect size.
- High risk of bias – It is likely the true effect size for the study is substantially different to the estimated effect size.
Single-arm observational studies were quality assessed using the Institute of Health Economics (IHE) Quality Appraisal Checklist for Case Series Studies. Each of these studies were classified into one of the following three groups:
- Low risk of bias – The true result for the study is likely to be close to the estimated result
- Moderate risk of bias – There is a possibility the true result for the study is substantially different to the estimated result.
- High risk of bias – It is likely the true result for the study is substantially different to the estimated result.
Each individual study was also classified into one of three groups for directness, based on if there were concerns about the population, intervention, comparator and/or outcomes in the study and how directly these variables could address the specified review question. Studies were rated as follows:
- Direct – No important deviations from the protocol in population, intervention, comparator and/or outcomes.
- Partially indirect – Important deviations from the protocol in one of the population, intervention, comparator and/or outcomes.
- Indirect – Important deviations from the protocol in at least two of the following areas: population, intervention, comparator and/or outcomes.
All RCTs in this review examined the effect of CBMP, specifically cannabidiol, in relation to either Dravet or Lennox-Gastaut syndrome. Cannabidiol for both conditions fell within the exclusion criteria of the protocol, but the studies were included because of the lack of other RCTs for epilepsy. Given that both Dravet and Lennox-Gastaut syndromes make up a small proportion of epilepsy-related conditions and the results could not be directly applied to other forms of epilepsy, it was decided that all RCTs should be rated as partially indirect and downgraded accordingly in the quality assessment.
All observational studies were single-arm studies, the inclusion of which was a deviation from the protocol. As single-arm studies were not within the included study designs initially stated in the protocol it was decided that each of these studies should also be rated as partially indirect.
Methods for combining intervention evidence
Meta-analyses of interventional data were conducted with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al. 2011).
A pooled relative risk was calculated for dichotomous outcomes (using the Mantel–Haenszel method) reporting numbers of people having an event. Both relative and absolute risks were presented, with absolute risks calculated by applying the relative risk to the pooled risk in the comparator arm of the meta-analysis (all pooled trials).
Fixed- and random-effects models (der Simonian and Laird) were fitted for all syntheses, with the presented analysis dependent on the degree of heterogeneity in the assembled evidence. Fixed-effects models were the preferred choice to report, but in situations where the assumption of a shared mean for fixed-effects model were clearly not met, even after appropriate pre-specified subgroup analyses were conducted, random-effects results are presented. Fixed-effects models were deemed to be inappropriate if one or both of the following conditions was met:
- Significant between study heterogeneity in methodology, population, intervention or comparator was identified by the reviewer in advance of data analysis. This decision was made and recorded before any data analysis was undertaken.
- The presence of significant statistical heterogeneity in the meta-analysis, defined as I2≥50%.
Meta-analyses were performed in Cochrane Review Manager V5.3.
Minimal clinically important differences (MIDs)
The Core Outcome Measures in Effectiveness Trials (COMET) database was searched to identify published minimal clinically important difference thresholds relevant to this guideline. In addition, the Guideline Committee were asked to prospectively specify any outcomes where they felt a consensus MID could be defined from their experience.
No MIDs were identified. Therefore, line of no effect was used to assess imprecision.
When decisions were made in situations where MIDs were not available, the ‘Evidence to Recommendations’ section of that review should make explicit the committee’s view of the expected clinical importance and relevance of the findings. In particular, this includes consideration of whether the whole effect of a treatment (which may be felt across multiple independent outcome domains) would be likely to be clinically meaningful, rather than simply whether each individual sub outcome might be meaningful in isolation.
GRADE for pairwise meta-analyses of interventional evidence
GRADE was used to assess the quality of evidence for the selected outcomes as specified in ‘Developing NICE guidelines: the manual (2018)’. Data from all study designs was initially rated as high quality and the quality of the evidence for each outcome was downgraded or not from this initial point, based on the criteria given in Table 1
Table 1. Rationale for downgrading quality of evidence for intervention studies
The quality of evidence for each outcome was upgraded if any of the following three conditions were met:
- Data from non-randomised studies showing an effect size sufficiently large that it cannot be explained by confounding alone.
- Data showing a dose-response gradient.
- Data where all plausible residual confounding is likely to increase our confidence in the effect estimate.
Summary of the evidence
The evidence is presented in the form of a table because the committee agreed in advance that effect sizes would be an important consideration. Summary of evidence is stratified by population and reflects evidence that was statistically significant.
Where the data are only consistent, at a 95% confidence level, with an effect in one direction (i.e. one that is ‘statistically significant’), and the magnitude of that effect is most likely to meet or exceed the MID (i.e. the point estimate is not in the zone of equivalence). In such cases, we state that the evidence showed that there is an effect. In all other cases, we state that the evidence could not differentiate between the comparators.
Appendix C. Literature search strategies
A single systematic search was conducted for all of the questions within this evidence review between 19th December 2018 and 21st January 2019. The following databases were searched MEDLINE, MEDLINE in Process, MEDLINE e pub Ahead of print, Embase, (all via the Ovid platform), Cochrane Database of Systematic Reviews CENTRAL (all via the Wiley platform), and the HTA and DARE databases (both via the CRD platform). NICE inhouse RCT, systematic review, and observational filters were attached where appropriate.
The MEDLINE strategy is presented below. This was translated for other databases
- Medical Marijuana/
- cannabinoids/ or cannabidiol/ or cannabinol/ or cannabis/
- ((cannabi* or hemp or marijuana or marihuana) adj4 (medicine* or medicinal or medical or oil or oils or product* or extract* or therap* or CBD or vap* or spray* or inhal* or compound* or resin* or derivative*)).tw.
- (epidiolex* or cannabidiol* or cannabinoid*).tw.
- (sativex or nabiximols or tetrabinex or nabidiolex).tw.
- (nabilone or cesamet).tw.
- (tilray* or bedrocan* or bedrobinol* or bedica* or bediol* or bedrolite*).tw.
- Dronabinol/
- (dronabinol* or marinol* or syndros*).tw.
- (9-ene-tetrahydrocannabinol* or 9enetetrahydrocannabinol*).tw.
- (THC or tetrahydrocannabinol*).tw.
- ("delta(1)-thc*" or “delta(1)-tetrahydrocannabinol*” or “delta(9)-thc*” or “delta(9)-tetrahydrocannabinol*”).tw.
- (9-delta-tetra-hydrocannabinol* or “9-delta-THC*” or “9 delta tetra hydrocannabinol*” or “9 delta THC*”).tw.
- (1-delta-tetra-hydrocannabinol* or “1-delta-THC*” or “1 delta tetra hydrocannabinol” or “1 delta thc*”).tw.
- THCa.tw.
- CBDa.tw.
- cannabinol*.tw.
- cannabigerol*.tw.
- cannabichromene*.tw.
- (tetrahydrocannabivarin* or THCV).tw.
- (cannabidivarin* or CBDV).tw.
- or/1-21
- animals/ not humans/
- 22 not 23
- limit 24 to english language
- Randomized Controlled Trial.pt.
- Controlled Clinical Trial.pt.
- Clinical Trial.pt.
- exp Clinical Trials as Topic/
- Placebos/
- Random Allocation/
- Double-Blind Method/
- Single-Blind Method/
- Cross-Over Studies/
- ((random$ or control$ or clinical$) adj3 (trial$ or stud$)).tw.
- (random$ adj3 allocat$).tw.
- placebo$.tw.
- ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw.
- (crossover$ or (cross adj over$)).tw.
- or/20-33
- Meta-Analysis.pt.
- Network Meta-Analysis/
- Meta-Analysis as Topic/
- Review.pt.
- exp Review Literature as Topic/
- (metaanaly$ or metanaly$ or (meta adj3 analy$)).tw.
- (review$ or overview$).ti.
- (systematic$ adj5 (review$ or overview$)).tw.
- ((quantitative$ or qualitative$) adj5 (review$ or overview$)).tw.
- ((studies or trial$) adj2 (review$ or overview$)).tw.
- (integrat$ adj3 (research or review$ or literature)).tw.
- (pool$ adj2 (analy$ or data)).tw.
- (handsearch$ or (hand adj3 search$)).tw.
- (manual$ adj3 search$).tw.
- or/35-48
- 34 or 49
- 19 and 50
- Observational Studies as Topic/
- Observational Study/
- Epidemiologic Studies/
- exp Case-Control Studies/
- exp Cohort Studies/
- Cross-Sectional Studies/
- Controlled Before-After Studies/
- Historically Controlled Study/
- Interrupted Time Series Analysis/
- Comparative Study.pt.
- case control$.tw.
- case series.tw.
- (cohort adj (study or studies)).tw.
- cohort analy$.tw.
- (follow up adj (study or studies)).tw.
- (observational adj (study or studies)).tw.
- longitudinal.tw.
- prospective.tw.
- retrospective.tw.
- cross sectional.tw.
- or/26-45
- 25 and 46
- 57 or 79
Searches to identify economic evidence were run on 20th December 2018 in MEDLINE, MEDLINE in Process, MEDLINE e pub Ahead of print, Econlit and Embase (all va the Ovid platform), NHS EED and the Health Technology Assessment Database (via the CRD platform). NICE inhouse economic evaluation and Quality of Life filters were attached to lines 1 to 25 of the core strategy (lines 1 to 25 of the MEDLINE version shown above) in the MEDLINE and Embase databases. The MEDLINE version of the filters is displayed below.
Economic evaluations
Economics/
- exp “Costs and Cost Analysis”/
- Economics, Dental/
- exp Economics, Hospital/
- exp Economics, Medical/
- Economics, Nursing/
- Economics, Pharmaceutical/
- Budgets/
- exp Models, Economic/
- Markov Chains/
- Monte Carlo Method/
- Decision Trees/
- econom$.tw.
- cba.tw.
- cea.tw.
- cua.tw.
- markov$.tw.
- (monte adj carlo).tw.
- (decision adj3 (tree$ or analys$)).tw.
- (cost or costs or costing$ or costly or costed).tw.
- (price$ or pricing$).tw.
- budget$.tw.
expenditure$.tw.
(value adj3 (money or monetary)).tw.
(pharmacoeconomic$ or (pharmaco adj economic$)).tw.
or/1-25
- Quality of Life
- "Quality of Life"/
- quality of life.tw.
- "Value of Life"/
- Quality-Adjusted Life Years/
- quality adjusted life.tw.
- (qaly$ or qald$ or qale$ or qtime$).tw.
- disability adjusted life.tw.
- daly$.tw.
- Health Status Indicators/
- (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
- (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
- (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
- (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw.
- (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
- (euroqol or euro qol or eq5d or eq 5d).tw.
- (qol or hql or hqol or hrqol).tw.
- (hye or hyes).tw.
- health$ year$ equivalent$.tw.
- utilit$.tw.
- (hui or hui1 or hui2 or hui3).tw.
- disutili$.tw.
- rosser.tw.
- quality of wellbeing.tw.
- quality of well-being.tw.
- qwb.tw.
- willingness to pay.tw.
- standard gamble$.tw.
- time trade off.tw.
- time tradeoff.tw.
- tto.tw.
- or/1-30
A search of the MHRA was undertaken on the 24th January 2019 to look for safety updates, alerts and recalls. The search terms are displayed below.
- Sativex
- Dronabinol
- Epidiolex
- Nabiximols
- Abalone
- Tetrabinex
- Nabidiolex
- Cesamet
- Tilray
- Bedrocan
- Bedrobinol
- Bedica
- Bediol
- Bedrolite
- Marinol
- Syndros
- THC
- Tetrahydrocannabinol
- Cannabinol
- Cannibigerol
- Cannabichromene
- Tetrahydrocannabivarin
- Cannabidivarin
Appendix D. Clinical evidence study selection
Appendix E. Clinical evidence table
E.1. Parallel RCTs
Appendix F. Forest plots and median tables
Dravet syndrome
Median change in seizure frequency from baseline: Total seizures (20 mg/kg/day)
| Study | CBD (median, IQR) | Placebo (median, IQR) | Median difference (percentage points, 95% CI) |
|---|---|---|---|
| 20 mg/kg/day | |||
| Devinsky 2017 | −28.6% | −9.0% |
−19.2 (−39.25, −1.17) |
Median change in seizure frequency from baseline: Convulsive seizures (20 mg/kg/day)
| Study | CBD (median, IQR) | Placebo (median, IQR) | Median difference (percentage points, 95% CI) |
|---|---|---|---|
| 20 mg/kg/day | |||
| Devinsky 2017 |
−38.9% (−69.5, −4.8) |
−13.3% (−52.5, 20.2) |
−22.8 (−41.1, −5.4) |
Lennox-Gastaut syndrome
Median change in seizure frequency from baseline: Total seizures
| Study | CBD (median, IQR) | Placebo (median, IQR) | Median difference (percentage points, 95% CI) |
|---|---|---|---|
| 10 mg/kg/day | |||
| Devinsky 2018 | −36.4% | −18.4% |
−19.5 (−30.4, −7.5) |
| 20 mg/kg/day | |||
| Devinsky 2018 | −38.4% | −18.4% |
−18.8 (−31.8, −4.4) |
| Thiele 2018 |
−41.2% (−62.9, −13.0) |
−13.7% (−45.0, 7.3) |
−21.1 (−33.3, −9.4) |
Median change in seizure frequency from baseline: Drop seizures
| Study | CBD (median, IQR) | Placebo (median, IQR) | Median difference (percentage points, 95% CI) |
|---|---|---|---|
| 10 mg/kg/day | |||
| Devinsky 2018 | −37.2% | −17.2% |
−19.2 (−31.2, −7.7) |
| 20 mg/kg/day | |||
| Devinsky 2018 | −41.9% | −17.2% |
−21.6 (−34.8, −6.7) |
| Thiele 2018 |
−43.9% (−69.6, −1.9) |
−21.8% (−45.7, 1.7) |
−17.21 (−30.32, −4.09) |
Appendix G. GRADE tables
Dravet syndrome
| No. of studies | Study design | Sample size | Effect size (95% CI) | Absolute risk (control) | Absolute risk (interventi on) | Risk of bias | Inconsiste ncy | Indirect ness | Imprecisi on | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of people achieving 50% seizure reduction (RR>1 favours CBD) | ||||||||||
| 1 (Devinsky 2017) | Parallel RCT | 120 | RR 1.57 (0.94, 2.62) | 27 per 100 | 43 per 100 (25, 71) | Serious1 | N/A2 | Serious3 | Serious4 | Very low |
| Median change in seizure frequency from baseline: Total seizures (Median difference <0 favours CBD) | ||||||||||
| 1 (Devinsky 2017) | Parallel RCT | 120 | Median percentage point difference (IQR) −19.20 (−39.25, −1.17) | - | - | Serious1 | N/A2 | Serious3 | Not serious | Low |
| Median change in seizure frequency from baseline: Convulsive seizures (Median difference <0 favours CBD) | ||||||||||
| 1 (Devinsky 2017) | Parallel RCT | 120 | Median percentage point difference (IQR) −22.8 (−41.1, −5.4) | - | - | Serious1 | N/A2 | Serious3 | Not serious | Low |
| Total adverse events (RR<1 favours CBD) | ||||||||||
| 1 (Devinsky 2017) | Parallel RCT | 120 | RR 1.25 (1.06, 1.48) | 75 per 100 | 93 per 100 (79, 100) | Serious1 | N/A2 | Serious3 | Not serious | Low |
| Treatment-emergent adverse events: CBD 5 mg/kg/day (RR<1 favours CBD) | ||||||||||
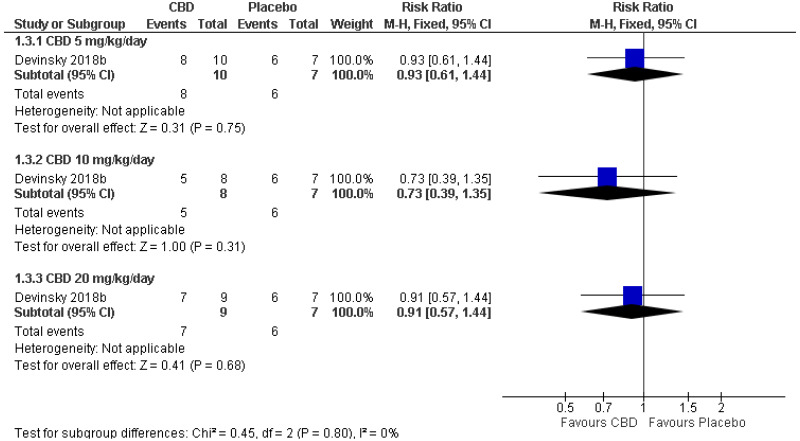
| 1 (Devinsky 2018) | Parallel RCT | 17 | RR 0.93 (0.61, 1.44) | 86 per 100 | 80 per 100 (52, 100) | Serious1 | N/A2 | Serious3 | Serious4 | Very low |
| Treatment-emergent adverse events: CBD 10 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 15 | RR 0.73 (0.39, 1.35) | 86 per 100 | 63 per 100 (33, 100) | Serious1 | N/A2 | Serious3 | Serious4 | Very low |
| Treatment-emergent adverse events: CBD 20 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 16 | RR 0.91 (0.57, 1.44) | 86 per 100 | 78 per 100 (49, 100) | Serious1 | N/A2 | Serious3 | Serious4 | Very low |
| Serious adverse events: CBD 5 mg/kg/day (RR<1 favours CBD) | ||||||||||
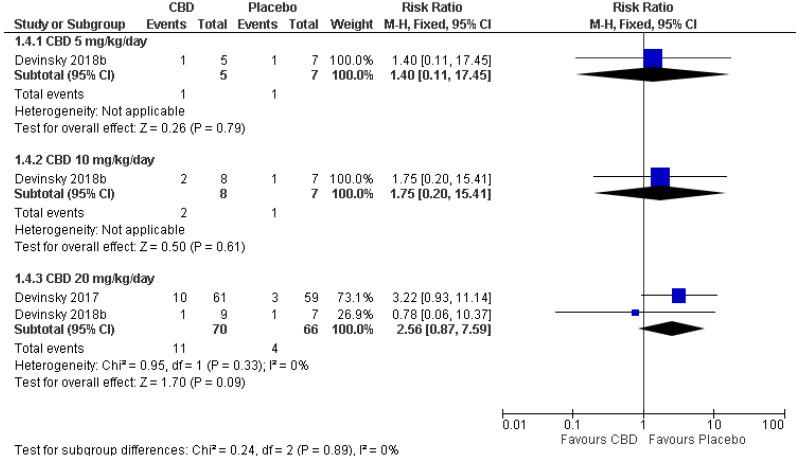
| 1 (Devinsky 2018) | Parallel RCT | 12 | RR 1.40 (0.11, 17.45) | 14 per 100 | 20 per 100 (2, 100) | Serious1 | N/A2 | Serious3 | Serious4 | Very low |
| Serious adverse events: CBD 10 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 15 | RR 1.75 (0.20, 15.41) | 14 per 100 | 25 per 100 (3, 100) | Serious1 | N/A2 | Serious3 | Serious4 | Very low |
| Serious adverse events: CBD 20 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 2 | Parallel RCTs | 136 | RR 2.56 (0.87, 7.59) | 6 per 100 | 16 per 100 (5, 46) | Serious6 | Not serious | Serious5 | Serious4 | Very low |
| Withdrawals due to adverse events: CBD 10 mg/kg/day (RR<1 favours CBD) | ||||||||||
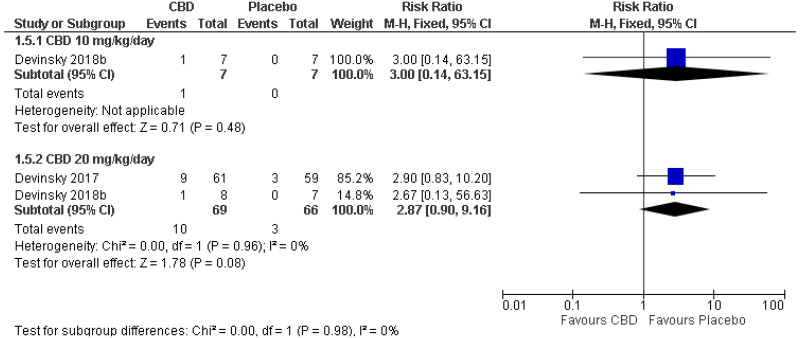
| 1 (Devinsky 2018) | Parallel RCT | 14 |
RR 3.00 (0.14, 63.15) | 7 per 100 | 21 per 100 (1, 100) | Serious1 | N/A2 | Serious3 | Serious4 | Very low |
| Withdrawals due to adverse events: CBD 20 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 2 | Parallel RCTs | 135 | RR 2.87 (0.90, 9.16) | 5 per 100 | 13 per 100 (4, 42) | Serious6 | Not serious | Serious5 | Serious4 | Very low |
- 1
Single study at moderate or high risk of bias. Downgraded 1 level
- 2
Inconsistency N/A as only 1 study
- 3
Single study rated as partially direct. Downgraded 1 level
- 4
95% confidence interval crosses line of no effect. Downgraded 1 level
- 5
> 33.3% of the weight in a meta-analysis came from partially direct studies. Downgraded 1 level
- 6
> 33.3% of the weight in a meta-analysis came from studies at moderate or high risk of bias. Downgraded 1 level
Lennox-Gastaut syndrome
| No. of studies | Study design | Sample size | Effect size (95% CI) | Absolute risk (control) | Absolute risk (intervent ion) | Risk of bias | Inconsist ency | Indirectn ess | Imprecisi on | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of people achieving 50% seizure reduction: 10 mg/kg/day (RR>1 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 149 | RR 2.46 (1.31, 4.61) | 14 per 100 | 36 per 100 (19, 67) | Not serious | N/A1 | Serious2 | Not serious | Moderate |
| Number of people achieving 50% seizure reduction: 20 mg/kg/day (RR>1 favours CBD) | ||||||||||
| 2 | Parallel RCTs | 323 | RR 2.18 (1.51, 3.13) | 14 per 100 | 32 per 100 (22, 45) | Not serious | Not serious | Serious3 | Not serious | Moderate |
| Median change in seizure frequency from baseline: Total seizures 10 mg/kg/day (Median percentage point difference <0 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 149 | Median percentage point difference (IQR) −19.5 (−30.4, −7.5) | - | - | Not serious | N/A1 | Serious2 | Not serious | Moderate |
| Median change in seizure frequency from baseline: Total seizures 20 mg/kg/day (Median percentage point difference <0 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 152 | Median percentage point difference (IQR) −18.8 (−31.8, −4.4) | - | - | Not serious | N/A1 | Serious2 | Not serious | Moderate |
| Median change in seizure frequency from baseline: Total seizures 20 mg/kg/day (Median percentage point difference <0 favours CBD) | ||||||||||
| 1 (Thiele 2018) | Parallel RCT | 171 | Median percentage point difference (IQR) −21.1 (−33.3, −9.4) | - | - | Not serious | N/A1 | Serious2 | Not serious | Moderate |
| Median change in seizure frequency from baseline: Drop seizures 10 mg/kg/day (Median percentage point difference <0 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 149 | Median percentage point difference (IQR) −19.2 (−31.2, −7.7) | - | - | Not serious | N/A1 | Serious3 | Not serious | Moderate |
| Median change in seizure frequency from baseline: Drop seizures 20 mg/kg/day (Median difference <0 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 152 | Median percentage point difference (IQR) −21.6 (−34.8, −6.7) | - | - | Not serious | N/A1 | Serious2 | Not serious | Moderate |
| Median change in seizure frequency from baseline: Drop seizures 20 mg/kg/day (Median difference <0 favours CBD) | ||||||||||
| 1 (Thiele 2018) | Parallel RCT | 171 | Median percentage point difference (IQR) −17.21 (−30.32, −4.09) | - | - | Not serious | N/A1 | Serious2 | Not serious | Moderate |
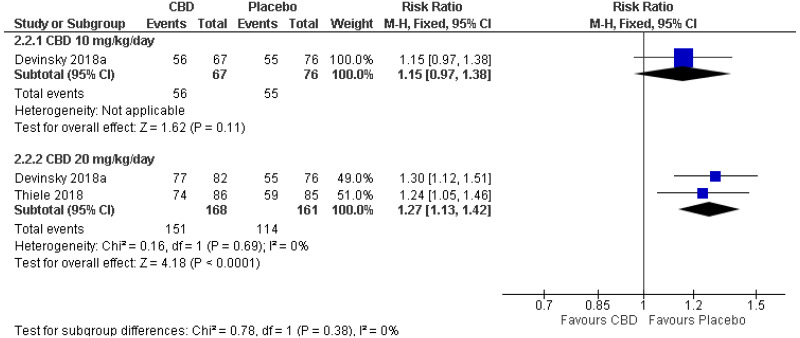
| All-cause adverse events: 10 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 143 | RR 1.15 (0.97, 1.38) | 72 per 100 | 83 per 100 (70, 100) | Not serious | N/A1 | Serious2 | Serious4 | Low |
| All-cause adverse events: 20 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 2 | Parallel RCTs | 329 | RR 1.27 (1.13, 1.42) | 71 per 100 | 90 per 100 (80, 100) | Not serious | Not serious | Serious3 | Not serious | Moderate |
| Treatment-related adverse events: 20 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 1 (Thiele 2018) | Parallel RCT | 171 | RR 1.81 (1.29, 2.54) | 34 per 100 | 62 per 100 (44, 87) | Not serious | N/A1 | Serious2 | Not serious | Moderate |
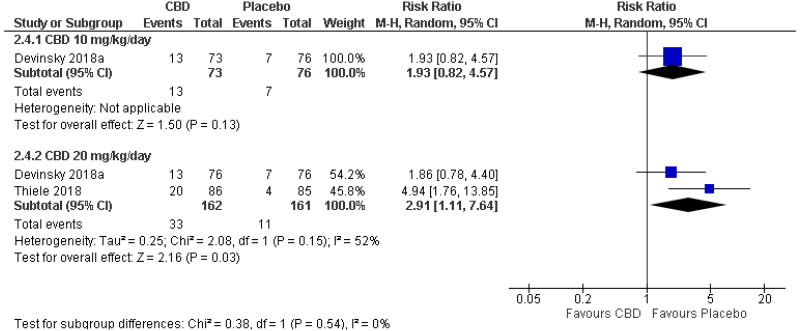
| Serious adverse events: 10 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 149 | RR 1.93 (0.82, 4.57) | 9 per 100 |
18 per 100 (8, 42) | Not serious | N/A1 | Serious2 | Serious4 | Low |
| Serious adverse events: 20 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 2 | Parallel RCTs | 323 | RR 2.91 (1.11, 7.64) | 7 per 100 |
20 per 100 (8, 52) | Not serious | Serious5 | Serious3 | Not serious | Low |
| Withdrawals due to adverse events: 10 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 1 (Devinsky 2018) | Parallel RCT | 149 | RR 1.04 (0.07, 16.34) | 1 per 100 |
1 per 100 (0, 22) | Not serious | N/A1 | Serious1 | Serious4 | Low |
| Withdrawals due to adverse events: 20 mg/kg/day (RR<1 favours CBD) | ||||||||||
| 2 | Parallel RCTs | 323 | RR 8.94 (2.11, 37.93) | 1 per 100 |
11 per 100 (3, 47) | Not serious | Not serious | Serious3 | Not serious | Moderate |
- 1
Inconsistency N/A as only 1 study
- 2
Single study rated as partially direct. Downgraded 1 level
- 3
> 33.3% of the weight in a meta-analysis came from partially direct studies. Downgraded 1 level
- 4
95% confidence interval crosses line of no effect. Downgraded 1 level
- 5
I2 between 33.3% and 66.7%. Downgraded one level
Appendix H. Adverse events
Dravet syndrome
| Study | Adverse events reported |
|---|---|
| Devinsky 2017 |
Adverse events experience by ≥10% of participants (CBD 20 mg/kg/day) CBD (n=61): Gastrointestinal (Diarrhoea 31%; Vomiting 15%); General (Fatigue 20%; Pyrexia 15%); Upper respiratory tract infection 11%; Decreased appetite 28%; Nervous system (Convulsion 11%; Lethargy 13%; Somnolence* 36%) Placebo (n=59): Gastrointestinal (Diarrhoea 10%; Vomiting 5%); General (Fatigue 3%; Pyrexia 8%); Upper respiratory tract infection 8%; Decreased appetite 5%; Nervous system (Convulsion 5%; Lethargy 5%; Somnolence* 10%) *Of the patients with somnolence, 82% in CBD group and 83% in placebo group were taking clobazam concomitantly Serious adverse events CBD: Status epilepticus (5%), Elevated aminotransferase levels (20%)** Placebo: Status epilepticus (5%), Elevated aminotransferase levels (2%)** ** All patients with elevated aminotransferase levels were taking a form of valproate |
| Devinsky 2018 |
Adverse events experienced by ≥1 participant CBD 5 mg/kg/day (n=10): Pyrexia 30%; Somnolence 20%; Sedation 20%; Vomiting 10%; Ataxia 20%; Gastroenteritis viral 10%; Abnormal behaviour 30%; Gastroenteritis 10%; Pharyngitis streptococcal 10%; Psychomotor hyperactivity 10% CBD 10 mg/kg/day (n=8): Pyrexia 38%; Somnolence 38%; Decreased appetite 13%; Vomiting 13%; Nasopharyngitis 13%; Convulsion 13%; Pneumonia 13%; Rash 13% CBD 20 mg/kg/day (n=9): Decreased appetite 44%; Sedation 22%; Vomiting 11%; Nasopharyngitis 11%; Ataxia 11%; Gastroenteritis viral 11%; Fatigue 11%; Upper abdominal pain 22%; Pneumonia 11%; Rash 11%; Viral infection 11% Placebo (n=7): Somnolence 14%; Nasopharyngitis 14%; Gastroenteritis viral 14%; Fatigue 29%; Convulsion 2%; Gastroenteritis 29%; Viral infection 14%; Pharyngitis streptococcal 14%; Psychomotor hyperactivity 14% |
Lennox-Gastaut syndrome
| Study | Adverse events reported |
|---|---|
| Devinsky 2018 |
Adverse events experienced by ≥10% participants CBD 10 mg/kg/day (n=73): Somnolence* 21% (mild 13%; moderate 6%; severe 1%); Decreased appetite 16% (mild 12%; moderate 4%); Diarrhoea 10% (mild 9%; moderate 1%); Upper respiratory tract infection 16% (mild 15%; moderate 1%); Pyrexia 9% (mild 7%; moderate 1%); Vomiting 6% (mild 3%; moderate 3%); Mild nasopharyngitis 4%; Status epilepticus 10% (mild 1%; moderate 6%; severe 3%) CBD 20 mg/kg/day (n=76): Somnolence* 30% (mild 22%; moderate 7%; severe 1%); Decreased appetite 26% (mild 18%; moderate 6%; severe 1%); Diarrhoea 15% (mild 12%; moderate 2%); Upper respiratory tract infection 13% (mild 10%; moderate 4%); Pyrexia 10% (mild 10%); Vomiting 10% (mild 10%); Mild nasopharyngitis 11%; Status epilepticus 5% (mild 1%; moderate 4%) Placebo (n=76): Somnolence* 5% (mild 4%; moderate 1%); Decreased appetite 8% (mild 7%; moderate 1%); Diarrhoea 8% (mild 8%); Upper respiratory tract infection 14% (mild 14%); Pyrexia 16% (mild 14%); Vomiting 12% (mild 12%); Mild nasopharyngitis 7%; Status epilepticus 4% (mild 3%; moderate 1%) *Of the patients with somnolence, 79% in 10 mg/kg/day group, 60% in 20 mg/kg/day group and 25% in placebo group were taking clobazam concomitantly Serious treatment-related adverse events (reported for both CBD groups combined): Elevated aspartate aminotransferase concentration (1%); Elevated alanine aminotransferase concentration (1%), Elevated γ-glutamyltransferase concentration (1%), Somnolence (1%), Increased seizures during weaning (1%), Nonconvulsive status epilepticus (1%); Lethargy (1%); Constipation (1%), Worsening chronic cholecystitis (1%) |
| Thiele 2018 |
Treatment-related adverse events experienced by ≥10% participants (20 mg/kg/day) CBD (n=86): Diarrhoea 13% (mild 10%; moderate 2%); Somnolence 14% (mild 6%; moderate 8%); Pyrexia 1% (moderate 1%); Decreased appetite 9% (mild 6%; moderate 2%; severe 1%); Vomiting 7% (mild 3%; moderate 2%; severe 1%) Placebo (n=85): Diarrhoea 4% (mild 4%); Somnolence 8% (mild 5%; moderate 4%); Pyrexia 1% (mild 1%); Decreased appetite 1% (moderate 1%); Vomiting 5% (mild 4%; moderate 1%) All-cause adverse events experienced by ≥10% participants CBD (n=86): Diarrhoea 19% (mild 14%; moderate 3%; severe 1%); Somnolence* 15% (mild 6%; moderate 9%); Pyrexia 13% (mild 8%; moderate 5%); Decreased appetite 13% (mild 8%; moderate 3%; severe 1%); Vomiting 10% (mild 3%; moderate 6%; severe 1%) Placebo (n=85): Diarrhoea 8% (mild 7%; moderate 1%); Somnolence* 9% (mild 6%; moderate 4%); Pyrexia 8% (mild 6%; moderate 2%); Decreased appetite 2% (mild 1%; moderate 1%); Vomiting 16% (mild 11%; moderate 6%) * Of the patients with somnolence, 69% in the CBD group and 88% in the placebo group were taking clobazam concomitantly Serious treatment-related adverse events experienced by >3% patients (only reported for CBD): Increased alanine aminotransferase concentration (5%); Increased aspartate aminotransferase concentration (5%); Increased γ-glutamyltransferase concentration (3%); Pneumonia (6%); Acute respiratory failure (3%) |
Appendix I. Excluded studies
Clinical studies
| Study | Reason for exclusion |
|---|---|
| Cunha, J. M., Carlini, E. A., Pereira, A. E. et al. (1980) Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21(3): 175-85 | Results not presented in an extractable format |
| Mechoulam, R. and Carlini, E. A. (1978) Toward drugs derived from cannabis. Die Naturwissenschaften 65(4): 174-9 | Non-English language article |
| (2018) Cannabidiol (CBD) treatment effect and adverse events (AES) by time in patients with lennox-gastaut syndrome (LGS): pooled results from 2 trials. Neurology conference70thannualmeetingoftheamericanacademyofneurologyaan2018unitedstates90(15sup plement1) | Conference abstract |
| Ali, Shayma; Scheffer, Ingrid E.; Sadleir, Lynette G. Efficacy of cannabinoids in paediatric epilepsy. Developmental medicine and child neurology 61(1): 13-18 | Narrative review |
| Cross, J. H., Devinsky, O., Laux, L. et al. (2017) Cannabidiol (CBD) reduces convulsive seizure frequency in dravet syndrome: results of a multi-centre, randomised, double-blind, placebo-controlled trial (GWPCARE1). Epilepsia. Conference: 32nd international epilepsy congress. Spain 58(supplement5): 12 | Conference abstract |
| Devinsky, O., Cross, J. H., Laux, L. et al. (2017) Cannabidiol (CBD) reduces convulsive seizure frequency in Dravet syndrome: results of a multi-center, randomized, double-blind, placebo-controlled trial (GWPCARE1). Neurotherapeutics. Conference: 19th annual meeting of the american society for experimental neurotherapeutics, ASENT 2017. United states 14(3): 824 | Conference abstract |
| Elliott, J., DeJean, D., Clifford, T. et al. (2018) Cannabis-based products for pediatric epilepsy: A systematic review. Epilepsia | Review article. The bibliography was reviewed for possible includes |
| Gloss, David and Vickrey, Barbara (2014) Cannabinoids for epilepsy. The Cochrane database of systematic reviews: cd009270 | Review article. The bibliography was reviewed for possible includes |
| Gloss, David and Vickrey, Barbara (2012) Cannabinoids for epilepsy. The Cochrane database of systematic reviews: cd009270 | Review article. The bibliography was reviewed for possible includes |
| Halford, J., Marsh, E., Mazurkiewicz-Beldzinska, M. et al. (2018) Long-term Safety and Efficacy of Cannabidiol (CBD) in Patients with Lennox-Gastaut Syndrome (LGS): results from Open-label Extension Trial (GWPCARE5). Neurology. Conference: 70th annual meeting of the american academy of neurology, AAN 2018. United states 90(15supplement1nopagination) | Conference abstract |
| Joshi, C., Thiele, E., Marsh, E. et al. (2017) Treatment with Cannabidiol (CBD) Significantly Reduces Drop and Total Seizure Frequency in Lennox-Gastaut Syndrome (LGS): results of a Multicenter, Randomized, Double-blind, Placebo Controlled Trial (GWPCARE4). Annals of neurology 82(s21): 293abstractno42 | Conference abstract |
| Koo, Chung Mo and Kang, Hoon-Chul (2017) Could Cannabidiol be a Treatment Option for Intractable Childhood and Adolescent Epilepsy?. Journal of epilepsy research 7(1): 16-20 | Review article. The bibliography was reviewed for possible includes |
| Lattanzi, Simona, Brigo, Francesco, Cagnetti, Claudia et al. (2018) Efficacy and Safety of Adjunctive Cannabidiol in Patients with Lennox-Gastaut Syndrome: A Systematic Review and Meta-Analysis. CNS drugs 32(10): 905-916 | No outcomes of interest |
| Lattanzi, Simona, Brigo, Francesco, Trinka, Eugen et al. (2018) Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs 78(17): 1791-1804 | Review article. The bibliography was reviewed for possible includes |
| Lippiello, Pellegrino, Balestrini, Simona, Leo, Antonio et al. (2016) From Cannabis to Cannabidiol to Treat Epilepsy, Where Are We?. Current pharmaceutical design 22(42): 6426-6433 | Review article. The bibliography was reviewed for possible includes |
| Mazurkiewicz-Beldzinska, M., Thiele, E. A., Benbadis, S. et al. (2017) Treatment with cannabidiol (CBD) significantly reduces drop seizure frequency in lennox-gastaut syndrome (LGS): results of a multi-centre, randomised, double-blind, placebocontrolled trial (GWPCARE4). Epilepsia. Conference: 32nd international epilepsy congress. Spain 58(supplement5): 55 | Conference abstract |
| Messenheimer, J. A., O’Brien, T., Berkovic, S. et al. (2018) Transdermal cannabidiol (CBD) gel for the treatment of focal epilepsy in adults. Neurology. Conference: 70th annual meeting of the american academy of neurology, AAN 2018. United states 90(24): e2188 | Conference poster |
| Miller, I., Devinsky, O., Nabbout, R. et al. (2018) Maintenance of long-term safety and efficacy of cannabidiol (CBD) treatment in dravet syndrome (DS): results of the open-label extension (OLE) trial (GWPCARE5). Neurology. Conference: 70th annual meeting of the american academy of neurology, AAN 2018. United states 90(15supplement1nopagination) | Conference abstract |
| Moore, Y. and Robinson, R. (2018) Cannabidiol reduced frequency of convulsive seizures in drug resistant Dravet syndrome. Archives of Disease in Childhood: Education and Practice Edition 103(5): 278-279 | Letter (non-peer-reviewed information) |
| Neale, Michelle (2017) Efficacy and safety of cannabis for treating children with refractory epilepsy. Nursing children and young people 29(7): 32-37 | Review article. The bibliography was reviewed for possible includes |
| Nickels, K. (2017) Cannabidiol in patients with intractable epilepsy due to TSC: A possible medication but not a miracle. Epilepsy Currents 17(2): 91-92 | Letter (non-peer-reviewed information) |
| Pamplona, Fabricio A.; da Silva, Lorenzo Rolim; Coan, Ana Carolina (2018) Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis. Frontiers in neurology 9: 759 | Review article. The bibliography was reviewed for possible includes |
| Patel, A., Devinsky, O., Cross, J. H. et al. (2017) Cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox-Gastaut syndrome (LGS): results of a dose-ranging, multi-center, randomized, double-blind, placebo-controlled trial (GWPCARE3). Neurology. Conference: 69th american academy of neurology annual meeting, AAN 2017. United states 89(8): e100 | Conference poster |
| Reithmeier, Darren, Tang-Wai, Richard, Seifert, Blair et al. (2018) The protocol for the Cannabidiol in children with refractory epileptic encephalopathy (CARE-E) study: a phase 1 dosage escalation study. BMC Pediatrics 18(1): 221 | Observational study. No control group |
| Ridler, C. (2017) Epilepsy: Cannabidiol reduces seizure frequency in Dravet syndrome. Nature Reviews Neurology 13(7): 383 | Letter (non-peer-reviewed information) |
| Schoedel, K., Etges, T., Levy-Cooperman, N. et al. (2018) A randomized, double-blind, placebo-controlled, crossover study to evaluate the abuse potential of purified cannabidiol (CBD) in subjects with a history of recreational polydrug use. Neurology. Conference: 70th annual meeting of the American academy of neurology, AAN 2018. United states 90(15supplement1nopagination) | Conference abstract |
| Stockings, Emily, Zagic, Dino, Campbell, Gabrielle et al. (2018) Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. Journal of neurology, neurosurgery, and psychiatry 89(7): 741-753 | Review article. The bibliography was reviewed for possible includes |
| Thiele, E. A., Mazurkiewicz-Beldzinska, M., Benbadis, S. et al. (2017) Treatment with cannabidiol (CBD) significantly reduces drop seizure frequency in Lennox Gastaut Syndrome (LGS): results of a multi - Center, randomized, double-blind, Placebo-controlled trial (GWPCARE4). Neurotherapeutics. Conference: 19th annual meeting of the american society for experimental neurotherapeutics, ASENT 2017. United states 14(3): 824-825 | Conference abstract |
| Wong, Shane Shucheng and Wilens, Timothy E. (2017) Medical Cannabinoids in Children and Adolescents: A Systematic Review. Pediatrics 140(5) | Review article. The bibliography was reviewed for possible includes |
| Wright, S., Devinsky, O., Thiele, E. A. et al. (2017) Cannabidiol (CBD) in Dravet syndrome: a randomised, dose-ranging pharmacokinetics and safety trial (GWPCARE1). Epilepsia. Conference: 32nd international epilepsy congress. Spain 58(supplement5): 56 | Conference abstract |
| Yap, Megan, Easterbrook, Laura, Connors, Jan et al. (2015) Use of cannabis in severe childhood epilepsy and child protection considerations. Journal of paediatrics and child health 51(5): 491-496 | Review article. The bibliography was reviewed for possible includes |
| Zuberi, S., Devinsky, O., Patel, A. et al. (2017) Cannabidiol (CBD) significantly reduces drop and total seizure frequency in Lennox-Gastaut syndrome (LGS): results of a dose-ranging, multicentre, randomised, double-blind, placebo-controlled trial (GWPCARE3). Epilepsia. Conference: 32nd international epilepsy congress. Spain 58(supplement5): S13-S14 | Conference abstract |
Economic studies
Appendix J. Research recommendations
1. What is the clinical and cost effectiveness of CBD in epileptic disorders in children, young people and adults?
4 RCTs were identified for the use of CBD for severe treatment-resistant epilepsy. These studies showed some effectiveness in relation to Lennox-Gastaut and Dravet syndromes but there is currently no RCT evidence for the effectiveness and safety of CBD for other epilepsy syndromes.
Further research is needed using a robust study design such as a parallel RCT to explore the clinical and cost effectiveness of CBD treatment for people with severe treatment-resistant epilepsy. Studies should be UK based and consider the effects on both adults and children. Research in this area is essential to determine whether recommendations for the use of cannabis-based medicinal products can be made in the future to help improve patient outcomes.
| PICO |
Population: Adults and children with genetic (idiopathic) generalised epilepsies, genetic epilepsies, structural epilepsies, metabolic epilepsies and developmental and epileptic encephalopathies Specific subgroups:
Cannabis based product, containing CBD only, defined as:
Outcomes:
|
|---|---|
| Current evidence base | 4 RCTS and 11 observational studies |
| Study design | Randomised controlled trial |
| Other comments | Study should be adequately powered and include an adequate follow-up period. |
2. Does the addition of THC to CBD have an effect on seizure frequency, brain structure and neurophysiological performance when compared with both CBD alone and placebo in epileptic disorders in children, young people and adults?
4 RCTs were identified for the use of CBD for severe treatment-resistant epilepsy. These studies evaluated the use of CBD but none included the addition of THC. There is currently no RCT evidence for the effectiveness and safety of using THC added to CBD for people with severe treatment-resistant epilepsy.
Further research is needed using a robust study design such as a parallel RCT to establish whether THC added to CBD can have benefits for the treatment of people with severe treatment-resistant epilepsy compared to the use of CBD alone. Studies should be UK based and consider the effects on both adults and children. Research in this area is essential to determine whether recommendations for the use of cannabis-based medicinal products can be made in the future to help improve patient outcomes.
| PICO |
Population: Adults and children with genetic (idiopathic) generalised epilepsies, genetic epilepsies, structural epilepsies, metabolic epilepsies and developmental and epileptic encephalopathies Specific subgroups:
Cannabis based product, including both THC and CBD, defined as:
|
|---|---|
| Current evidence base | 4 RCTS and 11 observational studies |
| Study design | Randomised controlled trial |
| Other comments | Study should be adequately powered and include an adequate follow-up period |
Appendix K. Single-arm observational studies
Constituents and doses for single-arm observational studies
Cannabis-based medicinal products for Dravet syndrome
| Intervention | Maintenance dose | |
|---|---|---|
| McCoy 2018 | Oil-based cannabidiol extract (CBD:THC ratio 50:1) | Maximum 16 mg/kg/day CBD |
Cannabis-based medicinal products for intractable epilepsy
| Intervention | Maintenance dose | |
|---|---|---|
| Devinsky 2016 | 99% pure oil-based cannabidiol extract (Epidiolex) | Maximum 25 mg/kg/day |
| Rosenberg 2017 | 99% pure oil-based cannabidiol extract (Epidiolex) | Maximum 50 mg/kg/day |
| Sands 2019 | 99% pure oil-based cannabidiol extract (Epidiolex) | Target 25 mg/kg/day |
| Szaflarski 2018 | 99% pure oil-based cannabidiol extract (Epidiolex) | Maximum 50 mg/kg/day |
| Tzadok 2016 | CBD-enriched cannabis oil (CBD:THC ratio 20:1) | Range 1-20 mg/kg/day |
| Neubauer 2018 | 98% pure oil-based cannabidiol | Maximum 16 mg/kg/day |
| Hausman-Kedem 2018 | CBD-enriched cannabis oil (CBD:THC ratio 20:1) (Cheesepie and Avidekel) | Maximum 50 mg/kg/day |
| Chen 2018 | 99% pure oil-based cannabidiol extract (Epidiolex) | Target 25 mg/kg/day |
Cannabis-based medicinal products for febrile infection-related epilepsy syndrome
| Intervention | Maintenance dose | |
|---|---|---|
| Gofshteyn 2017 | 99% pure oil-based cannabidiol extract (Epidiolex) | Maximum 25 mg/kg/day |
Cannabis-based medicinal products for drug-resistant epilepsy in tuberous sclerosis complex
| Intervention | Maintenance dose | |
|---|---|---|
| Hess 2016 | 99% pure oil-based cannabidiol extract (Epidiolex) |
Maximum 25 mg/kg/day (for some who tolerated CBD, maximum was increased to 50 mg/kg/day) |
Cannabis-based medicinal products for Dravet syndrome
Number of people achieving 50% seizure reduction (all seizure types)
| n | % responders | Quality | Indirectness | |
|---|---|---|---|---|
| 5 months follow-up | ||||
| McCoy 2018 | 20 | 63% | Very low | Partially indirect |
All-cause adverse events
| n | % with adverse events | Quality | Indirectness | |
|---|---|---|---|---|
| 5 months follow-up | ||||
| McCoy 2018 | 20 | 95% | Very low | Partially indirect |
Withdrawals due to adverse events
| n | % withdrawals | Quality | Indirectness | |
|---|---|---|---|---|
| 5 months follow-up | ||||
| McCoy 2018 | 20 | 0% | Very low | Partially indirect |
Improvements in quality of life from baseline (QOLCE score)
| n | Change in quality of life – mean (SD) | Quality | Indirectness | |
|---|---|---|---|---|
| 5 months follow-up | ||||
| McCoy 2018 | 20 | 6.4 | Very low | Partially indirect |
Cannabis-based medicinal products for intractable epilepsy
Number of people achieving 50% seizure reduction (all seizure types)
| n | % responders | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Devinsky 2016 | 162 | 37% | Very low | Partially indirect |
| Rosenberg 2017 | 48 | 42% | Very low | Partially indirect |
| Sands 2019 | 26 | 38% | Very low | Partially indirect |
| Szaflarski 2018 |
Children: 70 Adults: 62 |
Children: 61% Adults: 49% | Very low | Partially indirect |
| 6 months follow-up | ||||
| Sands 2019 | 26 | 57% | Very low | Partially indirect |
| 9-12 months follow-up | ||||
| Sands 2019 (9 months) | 26 | 42% | Very low | Partially indirect |
|
Tzadok 2016 (3-12 months: median 10 months) | 74 | 51% | Very low | Partially indirect |
| Szaflarski 2018 (11 months) |
Children: 70 Adults: 62 |
Children: 63% Adults: 65% | Very low | Partially indirect |
| Sands 2019 (12 months) | 26 | 38% | Very low | Partially indirect |
| 12-18 months follow-up | ||||
|
Neubauer 2018 (6-29 months: median 14 months) | 66 | 49% | Very low | Partially indirect |
| Sands 2019 (18 months) | 26 | 42% | Very low | Partially indirect |
|
Hausman-Kedem 2018 (3-33 months: median 18 months) | 57 | 46% | Very low | Partially indirect |
| 24 months follow-up | ||||
| Sands 2019 | 26 | 35% | Very low | Partially indirect |
| 36 months follow-up | ||||
| Sands 2019 | 26 | 27% | Very low | Partially indirect |
Number of people achieving 50% seizure reduction (by seizure type)
| n | % responders | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Motor seizures | ||||
| Devinsky 2016 | 162 | 39% | Very low | Partially indirect |
| Atonic seizures | ||||
| Devinsky 2016 | 32 | 56% | Very low | Partially indirect |
| Tonic seizures | ||||
| Devinsky 2016 | 65 | 40% | Very low | Partially indirect |
| Tonic-clonic seizures | ||||
| Devinsky 2016 | 89 | 34% | Very low | Partially indirect |
All-cause adverse events
| n | % with adverse events | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Chen 2018 | 40 | 98% | Very low | Partially indirect |
| Devinsky 2016 | 162 | 79% | Very low | Partially indirect |
| Hausman-Kedem 2018 | 57 | 46% | Very low | Partially indirect |
| Sands 2019 | 26 | 81% | Very low | Partially indirect |
| 10 months follow-up | ||||
|
Tzadok 2016 (3-12 months: median 10 months) | 74 | 46% | Very low | Partially indirect |
| 14 months follow-up | ||||
|
Neubauer 2018 (6-29 months: median 14 months) | 66 | 8% | Very low | Partially indirect |
All-cause serious adverse events
| n | % with serious adverse events | % with serious treatment-related adverse events | Quality | Indirectness | |
|---|---|---|---|---|---|
| 3 months follow-up | |||||
| Chen 2018 | 40 | 38% | 15% | Very low | Partially indirect |
| Devinsky 2016 | 162 | 30% | 12% | Very low | Partially indirect |
Withdrawals due to adverse events
| n | % withdrawals due to adverse events | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Chen 2018 | 40 | 10% | Very low | Partially indirect |
| Devinsky 2016 | 162 | 3% | Very low | Partially indirect |
| Hausman-Kedem 2018 | 57 | 18% | Very low | Partially indirect |
| Sands 2019 | 26 | 8% | Very low | Partially indirect |
| Szaflarski 2018 |
Children: 70 Adults: 62 |
Children: 3% Adults 3% | Very low | Partially indirect |
| 10 months follow-up | ||||
|
Tzadok 2016 (3-12 months: median 10 months) | 74 | 7% | Very low | Partially indirect |
Improvements in quality of life from baseline (QOLCE score)
| n | Change in quality of life – mean (SD) | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Rosenberg 2017 | 48 | 8.1 (9.9) | Very low | Partially indirect |
Improvements in cognition from baseline
| n | % people with improvements in quality of life from baseline | Quality | Indirectness | |
|---|---|---|---|---|
| 14 months follow-up | ||||
|
Neubauer 2018 (6-29 months: median 14 months) | 66 | 5% | Very low | Partially indirect |
Cannabis-based medicinal products for febrile infection-related epilepsy syndrome
Reduction in seizures from baseline (all seizure types)
| n | % reduction in seizures | Quality | Indirectness | |
|---|---|---|---|---|
| 1 month follow-up | ||||
| Gofshteyn 2017 | 6 | 90.9% (±18.9) | Very low | Partially indirect |
| 11 months follow-up | ||||
| Gofshteyn 2017 | 6 | 65.3% (±29.3) | Very low | Partially indirect |
Reduction in seizures from baseline (by seizure type)
| n | % reduction in seizures | Quality | Indirectness | |
|---|---|---|---|---|
| Non convulsive: 1 month follow-up | ||||
| Gofshteyn 2017 | 6 | 99.6% (±0.5) | Very low | Partially indirect |
| Convulsive: 1 month follow-up | ||||
| Gofshteyn 2017 | 6 | 75.0% (±35.4) | Very low | Partially indirect |
| Focal motor: 1 month follow-up | ||||
| Gofshteyn 2017 | 6 | 99.6% (±0.5) | Very low | Partially indirect |
| Focal motor: 11 months follow-up | ||||
| Gofshteyn 2017 | 6 | 62.3% (±44.7) | Very low | Partially indirect |
| Focal with impaired consciousness, dyscognitive: 1 month follow-up | ||||
| Gofshteyn 2017 | 6 | 99.6% (±0.5) | Very low | Partially indirect |
| Focal with impaired consciousness, dyscognitive: 11 months follow-up | ||||
| Gofshteyn 2017 | 6 | 62.4% (±44.9) | Very low | Partially indirect |
Cannabis-based medicinal products for drug-resistant epilepsy in tuberous sclerosis complex
Number of people achieving 50% seizure reduction (all seizure types)
| n | % responders | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Hess 2016 | 18 | 50% | Very low | Partially indirect |
Number of people achieving 50% seizure reduction (by seizure type)
| n | % responders | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Atonic seizures | ||||
| Hess 2016 | 4 | 75% | Very low | Partially indirect |
| Tonic seizures | ||||
| Hess 2016 | 7 | 46% | Very low | Partially indirect |
| Tonic-clonic seizures | ||||
| Hess 2016 | 6 | 67% | Very low | Partially indirect |
| Epileptic spasms | ||||
| Hess 2016 | 4 | 75% | Very low | Partially indirect |
| Complex partial seizures | ||||
| Hess 2016 | 13 | 54% | Very low | Partially indirect |
Treatment-related adverse events
| n | % with treatment-related adverse events | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Hess 2016 | 18 | 67% | Very low | Partially indirect |
Withdrawals due to adverse events
| n | % withdrawals due to adverse events | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Hess 2016 | 18 | 11% | Very low | Partially indirect |
Improvements in cognition from baseline
| n | % people with improvements in quality of life from baseline | Quality | Indirectness | |
|---|---|---|---|---|
| 3 months follow-up | ||||
| Hess 2016 | 14 | 86% | Very low | Partially indirect |
Narrative outcomes – dose, patient monitoring and stopping criteria
Cannabis-based medicinal products for Dravet syndrome
Dose
One study used a maximum CBD dose of 16 mg/kg/day, with patients ending on a final dose ranging between 7 and 16 mg/kg/day. There was an 8 week titration phase beginning with a dose of 2 mg/kg/day taken twice daily. This was increased by 2 mg/kg/day every week until the maximum dose was reached. No information was provided on when the 2 doses were taken during the day.
Patient monitoring
Adverse events were monitored, and the dose was no longer increased if there was evidence of excessive somnolence, anorexia, diarrhoea and weight loss. No information was provided on the timing of monitoring visits.
Stopping criteria
No information was provided for stopping criteria.
Cannabis-based medicinal products for intractable epilepsy
Dose
Seven studies used a variety of doses, ranging from 16 – 50 mg/kg/day. Although 2 studies reported a maximum dose of 50 mg/kg/day, 1 study reported that no patients exceeded 30 mg/kg/day. The other study, which included both children and adults, reported an average dose of 17.5 mg/kg/day at 12 weeks for children and 20.2 mg/kg/day for adults. No studies reported the length of titration phases but most reported that the initial dose was increased each week until either the maximum dose or tolerance was reached. No studies provided information on the timing of doses. One study reported that if at least 50% seizure reduction had been achieved by after 6 months then they attempted to reduce the doses of other AEDs.
Patient monitoring
Four studies reported the timing of clinic follow-up visits which ranged from every 2 weeks to 2 within the first 6 months of beginning treatment. Some had different follow-up times for different outcomes, with 1 reporting that adverse events were assessed every 2 weeks whilst reviews of seizure diaries, use of rescue medication and laboratory tests took place every 4 weeks. Most studies reviewed seizure frequency and adverse events. Other common assessments included blood count and liver function tests. One study reported that doses could be decreased between clinic visits over the phone if there was evidence of worsening seizures or side-effects. However, increases in dose could only be made in person at a clinic visit.
Stopping criteria
Four studies reported stopping criteria, 3 of which were related to adverse events. Adverse events that resulted in stopping treatment included allergy, somnolence, worsening seizures, gastrointestinal intolerance, severe weight loss and hyperammonaemia. One study stopped treatment if they thought that patients or carers were inadequately reporting seizures.
Cannabis-based medicinal products for febrile infection-related epilepsy syndrome
Dose
One study used a maximum dose of 25 mg/kg/day, with patients taking a range of doses from 15 – 25 mg/kg/day. The study states that the initial dose was slowly titrated to the maximum dose, but no information was provided on the length of the titration phase or how the dose was titrated.
Patient monitoring
Monitoring included a review of seizure frequency and adverse events. Prolonged video EEG and clinical assessments were also used to measure a person’s response to treatment. No information was provided on the timing of clinic visits.
Stopping criteria
Limited information was provided for stopping criteria although up-titration of the dose was stopped for 1 patient who had a significant reduction in seizures, reporting less than one per week.
Cannabis-based medicinal products for drug-resistant epilepsy in tuberous sclerosis complex
Dose
One study used an initial maximum dose of 25 mg/kg/day although people who continued to have seizures and tolerated CBD were permitted to continue increasing the dose to a maximum of 50 mg/kg/day. During the titration phase the initial dose was increased by 5 mg/kg once a week but no information was provided on the length of the titration phase. During the first 3 months of treatment other concomitant AEDs, with the exception of clobazam, were kept stable. After this the doses of CBD and other AEDs could be changed monthly to optimise seizure control.
Patient monitoring
Monitoring included a review of frequency and type of seizures as reported by patients or carers, adverse events, concomitant AEDs, changes in cognition and behaviour and epilepsy-related hospital admissions. Medication was reviewed if people experienced an increase in seizure frequency. For most patients who experienced an increase in seizure frequency this was only during the first 6 months. Doses of CBD and AEDs were reduced at 9 months which resulted in a reduction in seizure frequency. If patients who were taking clobazam experienced either adverse events or elevated plasma levels of clobazam and N-desmethylclobazam then the dose of clobazam was reduced. No information was provided on the timing of clinic visits.
Stopping criteria
No information was provided for stopping criteria.
Appendix L. Included studies
Parallel RCTs
| Study |
|---|
| Devinsky O, Marsh E, Friedman D et al. (2016) Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. The Lancet. Neurology 15(3): 270-278 |
| Devinsky, Orrin, Cross, J. Helen, Laux, Linda et al. (2017) Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. The New England journal of medicine 376(21): 2011-2020 |
| Devinsky, Orrin, Patel, Anup D., Cross, J. Helen et al. (2018) Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. The New England journal of medicine 378(20): 1888-1897 |
| Thiele, Elizabeth A., Marsh, Eric D., French, Jacqueline A. et al. (2018) Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London, England) 391(10125): 1085-1096 |
Single-arm observational studies
| Study |
|---|
| Chen, Kerrie-Anne, Farrar, Michelle, Cardamone, Michael et al. (2018) Cannabidiol for treating drug-resistant epilepsy in children: the New South Wales experience. The Medical journal of Australia 209(5): 217-221 |
| Devinsky O, Marsh E, Friedman D et al. (2016) Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. The Lancet. Neurology 15(3): 270-278 |
| Gofshteyn, Jacqueline S., Wilfong, Angus, Devinsky, Orrin et al. (2017) Cannabidiol as a Potential Treatment for Febrile Infection-Related Epilepsy Syndrome (FIRES) in the Acute and Chronic Phases. Journal of child neurology 32(1): 35-40 |
| Hausman-Kedem, Moran; Menascu, Shay; Kramer, Uri (2018) Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents - An observational, longitudinal study. Brain & development 40(7): 544-551 |
| Hess, Evan J., Moody, Kirsten A., Geffrey, Alexandra L. et al. (2016) Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 57(10): 1617-1624 |
| McCoy, Blathnaid, Wang, Laura, Zak, Maria et al. (2018) A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Annals of clinical and translational neurology 5(9): 1077-1088 |
| Neubauer, D.; Perkovic Benedik, M.; Osredkar, D. (2018) Cannabidiol for treatment of refractory childhood epilepsies: Experience from a single tertiary epilepsy center in Slovenia. Epilepsy and Behavior 81: 79-85 |
| Rosenberg, Evan C., Louik, Jay, Conway, Erin et al. (2017) Quality of Life in Childhood Epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia 58(8): e96-e100 |
| Sands, Tristan T., Rahdari, Shahryar, Oldham, Michael S. et al. (2018) Long-Term Safety, Tolerability, and Efficacy of Cannabidiol in Children with Refractory Epilepsy: Results from an Expanded Access Program in the US. CNS drugs |
| Szaflarski, J. P., Bebin, E. M., Cutter, G. et al. (2018) Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy and Behavior 87: 131-136 |
| Tzadok, Michal, Uliel-Siboni, Shimrit, Linder, Ilan et al. (2016) CBD-enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure 35: 41-4 |
Final
Evidence review underpinning the research recommendations on epilepsy
These evidence reviews were developed by NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Effectiveness of cannabis-based medicinal products for the treatment of severe treatment-resistant epilepsy
- Glossary
- Review protocols
- Methods
- Literature search strategies
- Clinical evidence study selection
- Clinical evidence table
- Forest plots and median tables
- GRADE tables
- Adverse events
- Excluded studies
- Research recommendations
- Single-arm observational studies
- Included studies
- Review Epilepsies: diagnosis and management[ 2021]Review Epilepsies: diagnosis and management. 2021 May 12
- Review Evidence review for spasticity: Cannabis-based medicinal products: Evidence review C[ 2019]Review Evidence review for spasticity: Cannabis-based medicinal products: Evidence review C. 2019 Nov
- Rufinamide add-on therapy for drug-resistant epilepsy.[Cochrane Database Syst Rev. 2020]Rufinamide add-on therapy for drug-resistant epilepsy.Panebianco M, Prabhakar H, Marson AG. Cochrane Database Syst Rev. 2020 Nov 8; 11(11):CD011772. Epub 2020 Nov 8.
- Gabapentin add-on treatment for drug-resistant focal epilepsy.[Cochrane Database Syst Rev. 2021]Gabapentin add-on treatment for drug-resistant focal epilepsy.Panebianco M, Al-Bachari S, Hutton JL, Marson AG. Cochrane Database Syst Rev. 2021 Jan 12; 1(1):CD001415. Epub 2021 Jan 12.
- Review Lamotrigine add-on for drug-resistant partial epilepsy.[Cochrane Database Syst Rev. 2016]Review Lamotrigine add-on for drug-resistant partial epilepsy.Ramaratnam S, Panebianco M, Marson AG. Cochrane Database Syst Rev. 2016 Jun 22; 2016(6):CD001909. Epub 2016 Jun 22.
- Evidence review for epilepsyEvidence review for epilepsy
- LOC100132787 [Homo sapiens]LOC100132787 [Homo sapiens]Gene ID:100132787Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...