NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent
This evidence review supports recommendations 1.5.3 to 1.5.4.
Review question
What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
Introduction
Surgical resection is the standard mode of treatment for colorectal liver metastases. However, questions have been raised on whether it is better to do a simultaneous or staged resection of the primary tumour and the liver metastases, if chemotherapy is beneficial in addition to liver resection, and whether treatment methods other than surgical resection could be used to treat colorectal liver metastasis. The aim of this review is to find out what is the optimal combination and sequence of treatments in patients with metastatic colorectal cancer in the liver amenable to treatment with curative intent.
Summary of the protocol
Please see Table 1 for a summary of the population, intervention, comparison and outcomes (PICO) characteristics of this review.
Table 1
Summary of the protocol (PICO table).
For further details see the review protocol in appendix A.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual 2014. Methods specific to this review question are described in the review protocol in appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy until 31 March 2018. From 1 April 2018, declarations of interest were recorded according to NICE’s 2018 conflicts of interest policy. Those interests declared until April 2018 were reclassified according to NICE’s 2018 conflicts of interest policy (see Register of Interests)
Clinical evidence
Included studies
Three randomised controlled trials (RCTs; reported in 4 publications) and 18 retrospective cohort studies were included in this review (Abbott 2012; Abelson 2017; Bartolini 2018; De Haas 2010; Eltawil 2014; EORTC 40983 trial [Nordlinger 2013; Nordlinger 2008]; FFCD trial and ENG trial [Mitry 2008]; FFCD trial only [Portier 2006]; Gleisner 2008; Hof 2018; Imai 2017; Karibori 2010; Masuda 2018; Mayo 2013; Moug 2010; Patrono 2014; Vallance 2018; van Amerongen 2016; van der Poel 2018; Wang 2018; Yoshidome 2008).
The included studies are summarised in Table 2.
Twelve retrospective cohort studies compared simultaneous resection of the colorectal tumour and liver metastases to staged resection (mainly colorectal resection first) (Abbott 2012; Abelson 2017; Bartolini 2018; De Haas 2010; Hof 2018; Karibori 2010; Mayo 2013; Moug 2010; Patrono 2014; Vallance 2018; van der Poel 2018; Yoshidome 2008). Three RCTs compared chemotherapy in addition to surgery to surgery alone (EORTC 40983 trial [Nordlinger 2013; Nordlinger 2008]; FFCD trial and ENG trial [Mitry 2008]; FFCD trial only [Portier 2006]). Five retrospective cohort studies compared ablation with resection to resection alone (Eltawil 2014; Gleisner 2008; Imai 2017; Masuda 2018; van Amerongen 2016) and one retrospective cohort study compared ablation alone to resection alone (Wang 2018).
See the literature search strategy in appendix B and study selection flow chart in appendix C.
Excluded studies
Studies not included in this review with reasons for their exclusions are provided in appendix K.
Summary of clinical studies included in the evidence review
Summaries of the studies that were included in this review are presented in Table 2.
Table 2
Summary of included studies.
See the full evidence tables in appendix D and the forest plots in appendix E.
Quality assessment of clinical outcomes included in the evidence review
See the clinical evidence profiles in appendix D.
Economic evidence
Included studies
A systematic review of the economic literature was conducted but no economic studies were identified which were applicable to this review question.
Excluded studies
A global search of economic evidence was undertaken for all review questions in this guideline. See Supplement 2 for further information.
Economic model
The cost effectiveness of simultaneous versus staged resection in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
See appendix J for the full report of the economic analysis.
This economic analysis aims to estimate the outcomes, patient quality of life and costs of a simultaneous approach to resection compared to a staged approach in patients with colorectal cancer presenting with liver metastases.
Methods
Population
The model considers patients with colorectal cancer presenting with liver metastases where surgical resection of both the primary cancer and the metastases is considered the most appropriate treatment option.
Intervention and comparator
Two approaches to the surgical resection of the cancer were considered in the economic model:
- Staged Approach: This approach consists of 2 surgical operations, 1 for the resection of the primary colorectal cancer and 1 for the resection of the liver metastases.
- Simultaneous Approach: This approach involves 1 surgical operation to resect both the primary colorectal cancer and the liver metastases.
Model Structure
A partitioned survival analysis was developed to estimate the expected life expectancy, quality adjusted life years (QALYs) and costs associated with the 2 approaches considered by this economic analysis. A partitioned survival analysis divides the model cohort between different health states based on survival curves derived for overall survival (OS) and disease-free survival (DFS) derived from the accompanying clinical evidence review. The expected OS and DFS are then calculated from the area under the respective curves
Model parameters
Clinical inputs
Socioeconomic and demographics variables
The model assumed a uniform age of the cohort of 60 years of age based and 60% male.
Overall and disease-free survival
Survival curves for the economic model were estimated entirely from the accompanying clinical evidence report. Two models were reported one which adjusted for biases in the survival estimates identified in the clinical evidence review and one which used the reported values.
Adverse events
The proportion of adverse events for either approach was taken from the accompanying clinical evidence review.
Perioperative period
Perioperative period in the study was estimated from one US costing study a retrospective study at 1 US hospital. The studied reported a median length of stay of 7 days for the simultaneous approach and 13 days (total across both resections) for the staged approach.
Resource use and costs
Cost of resection and resection related complications
All resection costs were taken from NHS Reference Costs 2016/17.
To estimate the cost of the resections in the simultaneous approach it was not appropriate to combine the 2 costs as undertaken for the staged approach. This is because both sets of costs would include costs for pre-assessment, surgical preparation, anaesthesia, time in surgical theatre and many other items that can be ‘shared’ between the 2 procedures by combining them To estimate the cost for the simultaneous approach the cost of the staged approach was adjusted using data on total operative time and length of stay in hospital. Abbott 2012, identified in the accompanying clinical evidence review, estimated that total operating time in the simultaneous approach was 144 minutes shorter compared to the staged approach. This was converted into a cost using estimates of the cost of operating theatre time
Length of stay in hospital also differed between the 2 arms of the model with simultaneous approach resulting in 6 less days in hospital as discussed above. This mean cost was then multiplied by the reduction in days and subtracted from the procedure cost for the staged approach. This was in addition to the adjustment for operative time.
Resource use and cost of further treatment
All patients who have disease recurrence will go on to receive further treatment or if not appropriate palliative care. Of those patients with recurrent disease who go on to receive further treatment three broad types of treatment were identified by the committee-hepatic resection, extrahepatic resection and chemotherapy.
The proportion of patients going on to receive further treatment was taken from a UK cost utility study comparing operable to non-operable treatments for liver metastases. Costs for both hepatic and extrahepatic resection were again taken from NHS Reference Costs.
For disease recurrence, which was considered not amenable to resection, patients received systemic chemotherapy treatment. Two chemotherapy regimens were used by the economic model which were considered to cover the majority of chemotherapy received in the NHS for this patient group following inoperable disease recurrence-FOLFOX and FOLFIRI. Costs were taken from the ‘Drug and Pharmaceutical Electronic Market Information Tool’ (eMit) for all drug components. Administration costs were taken from NHS Reference Costs 2016.
Cost of palliative care
Given the relatively short life expectancy of the model cohort and that the majority of patients would die as a result of their disease a one off cost of palliative care was applied to the entirety of the cohort during their final year of life. This is to represent the increase in resource use experienced during the final months of a patient’s life.
Quality of life
Quality of life weights for the model were taken from previous cost-effectiveness study of patients (identical in age to our cohort) with rectal cancer.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was also conducted to assess the combined parameter uncertainty in the model.
Results
Base-case results
The base case results of the analysis are shown in Table 3. The results show an increase in life expectancy of half a year with the simultaneous approach corresponding to a 0.28 QALY increase compared to the staged approach. The simultaneous approach also led to reduction in costs just below £2,500. In the base-case analysis the simultaneous approach dominated (was both cost saving and health increasing).
Table 3
Base-case results.
When the unadjusted results of the accompanying clinical evidence review are used to inform the economic model and adjustments for survival have not been made to account for individuals who only received the first resection life expectancy is now higher in the staged group (potentially for reasons discussed above) with an associated higher QALY as well (Table 4). The simultaneous approach remains cost saving (even more so given the larger number of liver resections in the staged approach). If a £20,000 per QALY threshold is considered, in line with Developing NICE guidelines: the manual, the reduction in costs would not justify the decrease in QALYs, that is, only £16,506 is saved for every QALY forgone.
Table 4
Results of the economic model using values reported in the accompanying clinical evidence review.
Probabilistic Sensitivity Analysis
Base-case assumptions
The results of 10,000 runs of the PSA are shown using ICER scatterplots and cost-effectiveness acceptability curves (CEAC). The ICER scatter plots show the incremental costs and QALYs associated with each of the 10,000 runs of the PSA along with the mean result. The CEAC graphs show the probability of each strategy being considered cost-effective at the various cost-effectiveness thresholds on the x axis.
Figure 1 presents the probabilistic results of the base case analysis. Of these 10,000 iterations over 75% of them are health improving (to the right of the Y-axis) and over 80% are cost decreasing (below the X-axis) with the majority of iterations being both cost saving and health increasing.
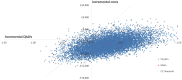
Figure 1
ICER scatterplot base case results. CE: cost effectiveness; ICER: incremental cost-effectiveness ratio; QALYs: quality-adjusted life years
Figure 2 presents the CEAC for the base case results. The probability that the simultaneous approach is the preferred option is 80% at a cost effectiveness ratio of £0 i.e. where the cheapest option is preferred. At £20,000 threshold there is a 86% probability of the simultaneous approach being the preferred option. This remains above 80% beyond values above the £100,000 threshold. For no threshold does a staged approach become the preferred option.

Figure 2
Cost effectiveness acceptability curve base case results.
Clinical evidence review values results
When results from the clinical evidence review are used to inform the economic model there is a greater degree of uncertainty around the results (Figure 3). When these inputs are considered a greater number of iterations show the simultaneous approach as cost saving (94%) but with less than 20% of iterations being health improving.

Figure 3
ICER scatterplot clinical evidence review inputs. CE: cost effectiveness; ICER: incremental cost-effectiveness ratio; QALYs: quality-adjusted life years
The CEAC for this analysis shows uncertainty around the preferred option (Figure 4). At the £20,000 threshold 42% showed the simultaneous approach to be cost effective although as shown by the cost effectiveness plane the majority of these would also be health decreasing. As the threshold increases the probability of the staged approach being the most cost effective option also increases. The threshold at which the CEACs cross and we are indifferent between the two options is £17,000 per QALY.

Figure 4
Cost effectiveness acceptability curve clinical evidence review inputs.
Conclusions
Both versions of the model gave differing results. The base-case results, where survival in the staged approach had been adjusted, showed the simultaneous approach as both health improving and cost saving. This conclusion was robust to both probabilistic and deterministic sensitivity analysis. The secondary analysis using survival estimates from the accompanying clinical evidence review presented the simultaneous approach as health decreasing and cost decreasing but not cost effective although the PSA highlighted considerable uncertainty around this conclusion.
Evidence statements
Clinical evidence statements
Comparison 1: Simultaneous resection versus staged resection
Critical outcomes
Liver-progression free survival
- Very low quality evidence from 3 retrospective cohort studies (N=250) showed no clinically important difference in liver-progression free survival between people who underwent simultaneous resection or staged resection for metastatic colorectal cancer in the liver.
Overall survival
- Low to very low quality evidence from 8 retrospective cohort studies (N=2031) showed no clinically important difference in overall survival between people who underwent simultaneous resection or staged resection for metastatic colorectal cancer in the liver.
Quality of life
No evidence was identified to inform this outcome.
Important outcomes
Disease-free survival
- Very low quality evidence from 2 retrospective cohort studies (N=196) showed mixed results for disease-free survival. Evidence from 1 study showed no clinically important difference in disease-free survival between people who underwent simultaneous resection or staged resection for metastatic colorectal cancer in the liver. Evidence from another study showed a clinically important worse disease-free survival for people who underwent simultaneous resection compared to those who underwent staged resection for metastatic colorectal cancer in the liver.
Treatment-related mortality
- Very low quality evidence from 2 retrospective cohort studies (N=238) showed that there were no clinically important differences in treatment-related mortality (postoperative mortality, 30-day or 60-day) between people who underwent simultaneous resection or staged resection for metastatic colorectal cancer in the liver.
Any grade 3 or 4 adverse event
- Very low quality evidence from 1 retrospective cohort study (N=64) showed no clinically important difference in grade 3 or 4 adverse events between people who underwent simultaneous resection or staged resection for metastatic colorectal cancer in the liver.
- Very low quality evidence from 2 retrospective cohort studies (N=1,552) showed no clinically important difference in major events within 30 days (myocardial infarction, stroke, pulmonary embolism, shock, in-hospital death), return to operating theatre, anastomotic leak, acute liver failure, or liver abscess between people who underwent simultaneous resection or staged resection for metastatic colorectal cancer in the liver.
- Very low quality evidence from 2 retrospective cohort studies (N=1,552) showed no clinically important difference in likelihood of readmission within 30 days in people who underwent simultaneous resection compared to those who underwent staged resection for metastatic colorectal cancer in the liver.
- Very low quality evidence from 1 retrospective cohort study (N=52) showed no clinically important difference in postoperative complications between people who underwent simultaneous resection or staged resection for metastatic colorectal cancer in the liver.
Comparison 2: Surgery and SACT versus surgery alone
Critical outcomes
Liver-progression free survival
No evidence for a specific outcome
Overall survival
- Moderate quality evidence from 2 RCTs (N=666) pooled together showed that there may be a clinically important better overall survival for people who received chemotherapy in addition to surgery compared to those who underwent surgery alone for metastatic colorectal cancer in the liver but there is uncertainty around the estimate. However, pooling the results might not be appropriate because the chemotherapy regimens in these two RCTs were different in terms of 1) the timing of the chemotherapy and 2) the chemotherapy drugs used. In one RCT (N=302) the patients received fluorouracil (5-FU) and leucovorin (folinic acid) postoperatively and in the other RCT (N=364) the patients received FOLFOX (leucovorin, fluorouracil and oxaliplatin) pre- and post-operatively. If the RCTs are considered individually, there was no clinically important difference in overall survival in people who received chemotherapy in addition to surgery compared to those who underwent surgery alone for metastatic colorectal cancer in the liver.
Quality of life
No evidence was identified to inform this outcome.
Important outcomes
Disease-free survival
- Moderate quality evidence from 2 RCTs (N=666) pooled together showed that there is a clinically important better disease-free survival for people who received chemotherapy in addition to surgery compared to those who underwent surgery alone for metastatic colorectal cancer in the liver. However, pooling the results might not be appropriate because the chemotherapy regimens in these two RCTs were different in terms of 1) the timing of the chemotherapy and 2) the chemotherapy drugs used. In one RCT (N=302) the patients received fluorouracil (5-FU) and leucovorin (folinic acid) postoperatively and in the other RCT (N=364) the patients received FOLFOX (leucovorin, fluorouracil and oxaliplatin) pre- and post-operatively. If the RCTs are considered individually, the evidence showed that there may be a clinically important better disease-free survival in people who received chemotherapy in addition to surgery compared to those who underwent surgery alone for metastatic colorectal cancer in the liver but there is uncertainty around the estimate.
Treatment-related mortality
- Moderate quality evidence from 1 RCT (N=364) showed no clinically important difference in treatment-related mortality between people who received chemotherapy in addition to surgery and those who underwent surgery alone for metastatic colorectal cancer in the liver.
Any grade 3 or 4 adverse event
- Moderate quality evidence from 1 RCT (N=166) showed that around 25% of the people who received chemotherapy in addition to surgery had grade 3 or 4 chemotherapy-related adverse events compared to 0% in those who had surgery alone for metastatic colorectal cancer in the liver.
Comparison 3: Ablation ± resection versus resection alone
Critical outcomes
Liver-progression free survival
- Very low quality evidence from 1 retrospective cohort study (N=124) showed no clinically important difference in liver disease-free survival between people who underwent liver resection and RFA and those who underwent resection alone for metastatic colorectal cancer in the liver.
Overall survival
- Very low quality evidence from 4 retrospective cohort studies (N=1,177) showed mixed results. Evidence from 2 studies (N=298) showed no clinically important difference in overall survival between people who underwent liver resection and RFA and those who underwent resection alone for metastatic colorectal cancer in the liver. However, evidence from 2 other studies (N=879) showed a clinically important worse overall survival in people who underwent liver resection and RFA compared to those who underwent resection alone for metastatic colorectal cancer in the liver.
- Very low quality evidence from 1 retrospective cohort study (N=149) showed no clinically important benefit in overall survival for high risk patients who underwent liver resection alone compared to those who underwent liver resection + RFA for metastatic colorectal cancer in the liver.
- Very low quality evidence from 1 retrospective cohort study (N=568) showed no clinically important benefit in overall survival for low risk patients who underwent liver resection alone compared to those who underwent liver resection + RFA for metastatic colorectal cancer in the liver.
- Very low quality evidence from 1 retrospective cohort study (N=138) showed no clinically important difference in overall survival between people who received RFA alone and those who underwent resection alone for metastatic colorectal cancer in the liver.
Quality of life
No evidence was identified to inform this outcome.
Important outcomes
Disease-free survival
- Very low quality evidence from 4 retrospective cohort studies (N=1,177) showed mixed results. Evidence from 3 studies (N=930) showed no clinically important difference in disease-free survival between people who underwent liver resection and RFA and those who underwent resection alone for metastatic colorectal cancer in the liver. However, evidence from 1 study (N=247) showed a clinically important worse disease-free survival in people who underwent liver resection and RFA compared to those who underwent resection alone for metastatic colorectal cancer in the liver.
Treatment-related mortality
- Very low quality evidence from 1 retrospective cohort study (N=124) showed no clinically important difference in 90-day mortality between people who received RFA in addition to resection and those who underwent resection alone for metastatic colorectal cancer in the liver.
Any grade 3 or 4 adverse event
- Very low quality evidence from 1 retrospective cohort study (N=124) showed no clinically important difference in grade 3 or 4 adverse events between people who received RFA in addition to resection and those who underwent resection alone for metastatic colorectal cancer in the liver.
Comparison 4: SABR versus resection or ablation
No evidence was identified to inform this comparison.
Economic evidence statements
A bespoke economic model was created for this topic to investigate the cost effectiveness of a simultaneous compared to a staged approach to resection. The study took a UK NHS+PSS perspective and was informed by evidence identified in the accompanying clinical evidence review. Two models were created one using the survival outcomes from the clinical evidence review and another where the inputs had been adjusted to account for biases in these estimates. The adjusted analysis showed a simultaneous approach to be both cost saving and health improving. This result was robust to sensitivity analysis with a greater than 85% probability of being cost effective at a £20,000 per QALY threshold. The analysis using the results from the clinical evidence review showed a simultaneous approach to be cost saving but also health decreasing with a staged approach being the preferred option at a £20,000 per QALY threshold. There was however large uncertainty around this conclusion. For both analyses a simultaneous approach was cost saving under the majority of iterations during the probabilistic sensitivity analysis.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
Liver progression-free survival and overall survival were considered critical outcomes for decision making because progression of the liver metastases suggests ineffective treatment, potentially requiring further treatment and affecting overall survival. Quality of life was a critical outcome because of the impact that different treatment options can have on patients’ functioning and the potential long term adverse effects.
Disease-free survival, meaning survival without disease recurrence or progression anywhere in the body, was an important outcome because it reflects effectiveness of treatment, and can mean additional treatments and affect overall survival. Additionally, treatment-related mortality and adverse events were also important outcomes, as they are indicative of the short-term side effects of treatments.
The quality of the evidence
Evidence was available for the comparison of simultaneous versus staged resection, for which evidence was available on all outcomes except quality of life; surgery and SACT versus surgery alone, for which evidence was available for all outcomes except liver-progression free survival and quality of life; and ablation (with or without resection) versus resection alone, for which evidence was available on all other outcomes except quality of life.
No evidence was identified on stereotactic body radiation therapy or stereotactic ablative radiotherapy.
The quality of the evidence was assessed using GRADE and varied from very low to moderate quality.
Only observational evidence was available for comparing simultaneous resection to staged resection and comparing ablation with or without resection to resection alone. The evidence was mostly of very low quality, varying from very low to low. The main reason for downgrading the quality of the evidence was imprecision of the effect estimate due to small sample sizes or risk of bias due to lack of adequate controlling for confounding factors. Even when baseline characteristics between the groups were controlled for (usually through multivariate regression or propensity score matching), there were serious concerns about the comparability of the groups included in these retrospective studies as the committee thought that there were underlying clinical reasons why one patient received treatment X and another received treatment Y. For example, in the included studies local ablation in addition to resection was largely done because some metastases were unresectable.
Because of the serious concerns about the comparability of the groups included in these retrospective studies, and the differences between studies in their criteria for resectability their results were not pooled in meta-analysis.
For comparing liver resection with SACT to liver resection alone RCT evidence was available and it was of moderate quality. The main reason for downgrading the quality of the evidence was imprecision of the effect estimate due to small sample sizes.
Benefits and harms
There was some evidence that simultaneous resection worsened liver progression-free survival, however, evidence from more studies showed no difference in overall survival or disease-free survival. No difference was seen in treatment-related mortality and morbidity. Because there was not enough evidence to show that one approach was better than the other, the patient’s perspective on the effect of each approach on quality of life is particularly important to guide clinicians. No quality of life evidence was available, however, it can be assumed that if simultaneous resection is feasible it would also be preferred by patients in order to avoid having to go through two major surgeries with waiting time in between.
The committee agreed that there are problems with comparability of the patient groups in the included studies. In some cases a staged resection was done because a simultaneous resection was not possible due to clinical reasons, therefore, the groups were not similar enough to be truly comparable.
Due to the low quality of the evidence paired with the inconclusive evidence on which approach is better, the committee agreed that it cannot recommend one approach over the other. Instead, the decision should be based on a careful consideration by a multidisciplinary team consisting of experts in both colorectal and liver surgeries.
Moderate quality evidence from three RCTs suggested that SACT in addition to liver resection was beneficial in terms of disease-free survival and possibly overall survival compared to liver resection alone. Chemotherapy increased the rate of treatment-related adverse events with around a quarter of patients in one trial having grade 3 or 4 adverse events due to chemotherapy. There was no evidence on quality of life, however, considering the adverse effects that chemotherapy might cause, it could be assumed that quality of life might be impaired as a result of chemotherapy, at least in the short-term. The potential gain in survival should therefore be balanced with the potential effect on quality of life and morbidity.
The RCTs were different in terms of chemotherapy regimen used (oxaliplatin-based FOLFOX versus 5-FU and leucovorin without oxaliplatin) and the timing of administering the chemotherapy (before and after resection versus after resection only). This makes it more difficult to draw definitive conclusions from the evidence when the RCTs are pooled together while individually they lack statistical power.
Additional caution is needed when interpreting the evidence because the included RCTs are relatively old. For example, patient population has since changed because more synchronous disease is detected currently due to improvements in detection. Having a synchronous versus metachronous disease can make a big difference in terms of treatment effectiveness and survival but unfortunately the available data did not allow that type of analysis. In addition, the regimens used in the RCTs are somewhat outdated and more options for chemotherapy are available in current practice. If the RCTs would be conducted at current time, the committee would expect to see a bigger difference in survival.
Taking into consideration these different aspects of the evidence, the committee agreed that chemotherapy should be considered for people who are suitable for liver resection.
Local ablative techniques have been suggested as an alternative to surgical resection for people not fit for surgery and with potentially less mortality and morbidity associated with major surgery. The LAVA randomised trial was established to compare these two approaches, however, the trial was discontinued due to poor recruitment of patients. With no RCT evidence available on the effectiveness and safety of local ablative technique as an alternative to resection, observational studies were sought. Very low quality observational evidence from retrospective cohort studies was available comparing local ablation in combination with liver resection versus liver resection alone, or comparing local ablation alone to liver resection alone. The committee had major concerns about the usefulness of this data because the comparability of the groups in these studies. For most patients in the ablation group, ablation was only used because resection was not possible due to patient fitness or clinical reasons. Therefore, the ablation group would likely have more advanced disease or be less likely to achieve good outcome.
Cost effectiveness and resource use
The committee considered 2 versions of a bespoke economic model to investigate the cost effectiveness of a staged versus simultaneous approach to resection, 1 using the results of the clinical evidence review and another where the inputs had been adjusted to account for biases in the clinical evidence around survival. The adjusted analysis showed a simultaneous approach to be both cost saving and health improving. This result was robust to sensitivity analysis with a greater than 85% probability of being cost effective at a £20,000 per QALY threshold. The analysis using the results from the clinical evidence review showed a simultaneous approach to be cost saving but also health decreasing with a staged approach being the preferred option at a £20,000 per QALY threshold. There was uncertainty around this conclusion. For both analyses a simultaneous approach was cost saving under the majority of iterations during the probabilistic sensitivity analysis.
The committee was of the opinion that a simultaneous approach was unlikely to be health decreasing and the survival difference (although not statistically significant) identified in the clinical evidence review was most likely a result of selection bias. Given this the committee concluded that a simultaneous approach was likely a cost effective use of resources. However, with uncertainty around the clinical inputs and the potential for harm, either through a less effective approach or through inefficient use of resources, the committee did not feel it appropriate to recommend one approach over the other.
The committee acknowledge the majority of the clinical evidence was retrospective observational studies with the previously discussed weaknesses. Given the importance of this parameter for informing economic decisions the confidence with which they could make this conclusion was reduced. The committee considered that it was unlikely there would be future high quality evidence for this clinical topic as they consider it would be difficult to recruit to a staged arm of any RCT with patients’ preferences being towards one operation with a shorter total hospital stay.
References
Abbott 2012
Abbott D, Cantor S, Hu C, et al. (2012) Optimizing clinical and economic outcomes of surgical therapy for patients with colorectal cancer and synchronous liver metastases. Journal of the American College of Surgeons 215(2): 262–70 [PMC free article: PMC3688254] [PubMed: 22560316]Abelson 2017
Abelson J, Michelassi F, Sun T, et al. (2017) Simultaneous Resection for Synchronous Colorectal Liver Metastasis: the New Standard of Care? Journal of Gastrointestinal Surgery 21(6): 975–82 [PubMed: 28411351]Bartolini 2018
Bartolini I, Ringressi M, Melli F, et al. (2018) Analysis of prognostic factors for resected synchro-nous and metachro-nous liver metastases from colorectal cancer. Gastroenterology Research and Practice [PMC free article: PMC6079496] [PubMed: 30116264]Cicero 2018
Cicero G, De Luca R and Dieli F (2018) Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic colorectal cancer. Onco Targets and Therapy 11: 3059–3063 [PMC free article: PMC5975605] [PubMed: 29872317]Curtis 2018
Curtis L and Burns A (2018) Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of KentDe Haas 2010
De Haas R, Adam R, Wicherts D, et al. (2010) Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. British Journal of Surgery 97(8): 1279–89 [PubMed: 20578183]Department of Health 2018
Department of Health (2018) NHS reference costs 2016 to 2017. London: The Stationery OfficeEjaz 2010
Ejaz A, Semenov E, Spolverato G, et al. (2010) Synchronous primary colorectal and liver metastasis: impact of operative approach on clinical outcomes and hospital charges. HPB 16(12): 1117–26 [PMC free article: PMC4253336] [PubMed: 24965845]Eltawil 2014
Eltawil K, Boame N, Mimeault R, et al. (2014) Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. Journal of Surgical Oncology 110(6): 734–8 [PubMed: 24965163]EORTC 40983 trial (Nordlinger 2013; Nordlinger 2008)
Nordlinger B, Sorbye H, Glimelius B, et al. (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncology 14(12): 1208–15 [PubMed: 24120480]
Nordlinger B, Sorbye H, Glimelius B et al. (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371(9617): 1007–16 [PMC free article: PMC2277487] [PubMed: 18358928]FFCD trial and ENG trial (Mitry 2008)
Mitry E, Fields A, Bleiberg H, et al. (2008) Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. Journal of Clinical Oncology 26(30): 4906–11 [PubMed: 18794541]FFCD trial (Portier 2006)
Portier G, Elias D, Bouche O, et al. (2006) Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. Journal of Clinical Oncology 24(31): 4976–82 [PubMed: 17075115]Georghiou 2014
Georghiou T and Bardsley M. (2014) Exploring the cost of care at the end of life. Nuffield Trust. London: Nuffield TrustGleisner 2008
Gleisner A, Choti M, Assumpcao L, et al. (2008) Colorectal liver metastases: Recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Archives of Surgery 143(12): 1204–12 [PubMed: 19075173]Hof 2018
Hof J, Joosten H, Havenga K, et al. (2018) Radiofrequency ablation is beneficial in simultaneous treatment of synchronous liver metastases and primary colorectal cancer. PLoS ONE 13(3):e0193385 [PMC free article: PMC5854258] [PubMed: 29543821]Imai 2017
Imai K, Allard M, Castro Benitez C, et al. (2017) Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. British Journal of Surgery 104(5): 570–9 [PubMed: 28112813]Kaibori 2010
Kaibori M, Iwamoto S, Ishizaki M, et al. (2010) Timing of resection for synchronous liver metastases from colorectal cancer. Digestive Diseases and Sciences 55(11): 3262–70 [PubMed: 20112062]Masuda 2018
Masuda T, Margonis G, Andreatos N, et al. (2018) Combined hepatic resection and radio-frequency ablation for patients with colo-rectal cancer liver metastasis: A viable option for patients with a large number of tumors. Anticancer Research 38(11): 6353–6360 [PubMed: 30396957]Mayo 2013
Mayo S, Pulitano C, Marques H, et al. (2013) Surgical management of patients with synchronous colorectal liver metastasis: A multicenter international analysis. Journal of the American College of Surgeons 216(4): 707–18 [PMC free article: PMC3994665] [PubMed: 23433970]Miller 2000
Miller A, Cantor S, Peoples G, et al (2000) Quality of life and cost effectiveness analysis of therapy for locally recurrent rectal cancer. Diseases of the Colon and Rectum 43(12): 1695–1701 [PubMed: 11156453]Moug 2010
Moug S, Smith D, Leen E, et al. (2010) Evidence for a synchronous operative approach in the treatment of colorectal cancer with hepatic metastases: a case matched study. European Journal of Surgical Oncology 36(4): 365–70 [PubMed: 20034757]ONS 2018
Office for National Statistics National life tables, UK [online: accessed 25 November 2018]Pattamatta 2019
Pattamatta M, Evers S, Smeets B, et al (2019) An economic evaluation of perioperative enteral nutrition in patients undergoing colorectal surgery (SANICS II study). Journal of Medical Economics 22(3): 238–244 [PubMed: 30523724]Patrono 2014
Patrono D, Paraluppi G, Perino M, et al. (2014) Posthepatectomy liver failure after simultaneous versus staged resection of colorectal cancer and synchronous hepatic metastases. Il Giornale di Chirurgia 35(3-4): 86–93 [PMC free article: PMC4321594] [PubMed: 24841686]Ramsay 2012
Ramsay C, Pickard R, Robertson C, et al. (2012) Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technology Assessment 16 (41) [PMC free article: PMC4780976] [PubMed: 23127367]Rao 2017
Rao C, Sun Myint A, Athanasiou T (2017) Avoiding Radical Surgery in Elderly Patients With Rectal Cancer Is Cost-Effective. Diseases of the Colon and Rectum 60(1): 30–42 [PubMed: 27926555]Roberts 2015
Roberts K, Sutton A, Prasad K, et al (2015). Cost-utility analysis of operative versus non-operative treatment for colorectal liver metastases. British Journal of Surgery 102(4): 388–98 [PubMed: 25624168]Ruers 2017
Ruers T, Van Coevorden F, Punt C, et al. (2017) Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. Journal of the National Cancer Institute 109(9) [PMC free article: PMC5408999] [PubMed: 28376151]Seymour 2007
Seymour M, Maughan T, Ledermann J, et al. (2007) Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 14; 370(9582): 143–152 [PubMed: 17630037]Vallance 2018
Vallance A, van der Meulen J, Kuryba A, et al. (2018) The timing of liver resection in patients with colorectal cancer and synchronous liver metastases: a population-based study of current practice and survival. Colorectal Disease 16 [PubMed: 29338108]Van Amerongen 2016
Van Amerongen M, Van Der Stok E, Futterer J, et al. (2016) Short term and long term results of patients with colorectal liver metastases undergoing surgery with or without radiofrequency ablation. European Journal of Surgical Oncology 42(4): 523–30 [PubMed: 26856957]van der Poel 2018
van der Poel M, Tanis, P, Marsman H, et al. (2018) Laparoscopic combined resection of liver metastases and colorectal cancer: a multicenter, case-matched study using propensity scores. Surgical Endoscopy 33(4): 1124–1130 [PMC free article: PMC6430752] [PubMed: 30069639]Wang 2018
Wang L, Zhang Z, Yan X, et al. (2018) Radiofrequency ablation versus resection for technically resectable colorectal liver metastasis: A propensity score analysis. World Journal of Surgical Oncology 16(1): 207 [PMC free article: PMC6190664] [PubMed: 30322402]Woods 2017
Woods B, Sideris E, Palmer S, et al. (2017) NICE Decision Support Unit Technical Support Document 19: Partitioned Survival Analysis for Decision Modelling in Health Care: A Critical Review. Sheffield: Decision Support Unit [Available from http://www.nicedsu.org.uk] Yoshidome 2008
Yoshidome H, Kimura F, Shimizu H, et al. (2008) Interval period tumor progression: Does delayed hepatectomy detect occult metastases in synchronous colorectal liver metastases? Journal of Gastrointestinal Surgery 12(8): 1391–8 [PubMed: 18491195]
Appendices
Appendix A. Review protocol
Review protocol for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
Appendix B. Literature search strategies
Literature search strategies for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
A combined search was conducted for the following two review questions:
- What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
- What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver not amenable to treatment with curative intent?
Databases: Embase/Medline
Last searched on: 12/02/2019
| # | Search |
|---|---|
| 1 | (exp colorectal cancer/ or exp colon tumor/ or exp rectum tumor/) use emez |
| 2 | exp colorectal neoplasms/ use ppez |
| 3 | ((colorect* or colo rect* or colon or colonic or rectal or rectum) adj3 (adenocarcinoma* or cancer* or carcinoma* or malignan* or neoplas* or oncolog* or tumo?r*)).tw. |
| 4 | or/1-3 |
| 5 | liver metastasis/ use emez |
| 6 | liver/ use ppez |
| 7 | exp neoplasm metastasis/ use ppez |
| 8 | 6 and 7 |
| 9 | ((Liver or hepatic*) adj3 (disseminat* or metasta* or migrat*)).tw. |
| 10 | ((colorect* or colo rect* or colon or colonic or rectal or rectum) adj3 (liver metasta* or hepatic* metasta*)).tw. |
| 11 | 5 or 8 or 9 |
| 12 | 4 and 11 |
| 13 | 10 or 12 |
| 14 | hepatectomy/ use ppez or segmentectomy/ use emez |
| 15 | (Hepatectom* or segmentectom*).tw. |
| 16 | (exp liver resection/ or metastasis resection/) use emez |
| 17 | Metastasectomy/ use ppez |
| 18 | metastasectom*.tw. |
| 19 | ((liver or hepatic*) adj3 (excis* or metastasectom* or resect* or surg*)).tw. |
| 20 | or/14-19 |
| 21 | exp *antineoplastic agent/ use emez |
| 22 | exp antineoplastic agents/ use ppez |
| 23 | exp *Antineoplastic Protocols/ use ppez |
| 24 | multimodality cancer therapy/ use emez |
| 25 | cancer therapy/ use emez |
| 26 | exp *chemotherapy/ use emez |
| 27 | *cancer combination chemotherapy/ use emez |
| 28 | Cancer Vaccines/ use ppez |
| 29 | cancer vaccine/ use emez |
| 30 | cancer immunotherapy/ use emez |
| 31 | exp antibodies, monoclonal/ use ppez or monoclonal antibody/ use emez |
| 32 | chemosaturat*.tw. |
| 33 | ((anti canc* or anticanc* or anticancerogen* or anticarcinogen* or anti neoplas* or antineoplas* or anti tumo?r* or antitumo?r* or cytotoxic*) adj3 (agent* or drug* or protocol* or regimen* or treatment* or therap*)).ti. |
| 34 | (SACT or chemotherap* or immunotherap* or biological agent* or biological therap*).ti. |
| 35 | or/21-34 |
| 36 | 20 and 35 |
| 37 | ((combin* or delay* or simultaneous* or stage*) adj3 (resect* or surg*)).tw. |
| 38 | (liver-first or liverfirst).tw. |
| 39 | bowel first.tw. |
| 40 | or/37-39 |
| 41 | radiofrequency ablation/ use emez or ablation techniques/ use ppez |
| 42 | microwave thermotherapy/ use emez or irreversible electroporation/ use emez or electroporation/ use ppez |
| 43 | ((percutaneous* or radiofrequen* or radio-frequen* or RF or microwave*) adj3 ablat*).tw. |
| 44 | electroporat*.tw. |
| 45 | (RFA or MWA or IRE).tw. |
| 46 | or/41-45 |
| 47 | (radiosurgery/ or stereotactic body radiation therapy/ or stereotactic radiosurgery/ or cyberknife/) use emez |
| 48 | radiosurgery/ use ppez |
| 49 | (Stereotactic* adj2 (irradiation* or RT or radiation* or radioablation* or radiosurg* or radiotherap* or therap* or treat*)).tw. |
| 50 | (SBRT or SABRT or SABR or cyberknife or cyber knife).tw. |
| 51 | or/47-50 |
| 52 | chemoembolization/ use emez |
| 53 | exp embolization, therapeutic/ use ppez |
| 54 | ((transarterial or trans-arterial or transcatheter or trans-catheter) adj2 chemoemboli?ation).tw. |
| 55 | (irinotecan adj4 beads).tw. |
| 56 | (DEBIRI or TACE).tw. |
| 57 | or/52-56 |
| 58 | radioembolization/ use emez |
| 59 | radioemboli?ation.tw. |
| 60 | ((intraarterial or intra-arterial) adj3 brachytherapy).tw. |
| 61 | (SIRT or “selective internal radiation therapy”).tw. |
| 62 | or/58-61 |
| 63 | limit 35 to yr=“2000 - current” |
| 64 | limit 57 to yr=“2000 - current” |
| 65 | limit 62 to yr=“2000 - current” |
| 66 | 36 or 40 or 46 or 51 or 63 or 64 or 65 |
| 67 | 13 and 66 |
| 68 | limit 67 to (yr=“1995 - current” and english language) |
| 69 | Letter/ use ppez |
| 70 | letter.pt. or letter/ use emez |
| 71 | note.pt. |
| 72 | editorial.pt. |
| 73 | Editorial/ use ppez |
| 74 | News/ use ppez |
| 75 | exp Historical Article/ use ppez |
| 76 | Anecdotes as Topic/ use ppez |
| 77 | Comment/ use ppez |
| 78 | Case Report/ use ppez |
| 79 | case report/ or case study/ use emez |
| 80 | (letter or comment*).ti. |
| 81 | or/69-80 |
| 82 | randomized controlled trial/ use ppez |
| 83 | randomized controlled trial/ use emez |
| 84 | random*.ti,ab. |
| 85 | or/82-84 |
| 86 | 81 not 85 |
| 87 | animals/ not humans/ use ppez |
| 88 | animal/ not human/ use emez |
| 89 | nonhuman/ use emez |
| 90 | exp Animals, Laboratory/ use ppez |
| 91 | exp Animal Experimentation/ use ppez |
| 92 | exp Animal Experiment/ use emez |
| 93 | exp Experimental Animal/ use emez |
| 94 | exp Models, Animal/ use ppez |
| 95 | animal model/ use emez |
| 96 | exp Rodentia/ use ppez |
| 97 | exp Rodent/ use emez |
| 98 | (rat or rats or mouse or mice).ti. |
| 99 | or/86-98 |
| 100 | 67 not 99 |
| 101 | limit 100 to (yr=“1995 - current” and english language) |
| 102 | limit 101 to yr=“1995 - 2012” |
| 103 | limit 101 to yr=“2013-current” |
| 104 | remove duplicates from 102 |
| 105 | remove duplicates from 103 |
| 106 | 104 or 105 |
Database: Cochrane Library
Last searched on: 12/02/2019
| # | Search |
|---|---|
| 1 | MeSH descriptor: [Colorectal Neoplasms] explode all trees |
| 2 | ((colorect* or colo rect* or colon or colonic or rectal or rectum) near/3 (adenocarcinoma* or cancer* or carcinoma* or malignan* or neoplas* or oncolog* or tumo?r*)):ti,ab,kw |
| 3 | #1 or #2 |
| 4 | MeSH descriptor: [Neoplasm Metastasis] explode all trees |
| 5 | MeSH descriptor: [Liver] explode all trees |
| 6 | #4 and #5 |
| 7 | ((Liver or hepatic*) near/3 (disseminat* or metasta* or migrat*)):ti,ab,kw |
| 8 | ((colorect* or colo rect* or colon or colonic or rectal or rectum) near/3 (liver metasta* or hepatic* metasta*)):ti,ab,kw |
| 9 | #6 or #7 |
| 10 | #3 and #9 |
| 11 | #8 or #10 |
| 12 | MeSH descriptor: [Hepatectomy] this term only |
| 13 | (Hepatectom* or segmentectom*):ti,ab,kw |
| 14 | MeSH descriptor: [Metastasectomy] this term only |
| 15 | metastasectom*:ti,ab,kw |
| 16 | ((liver or hepatic*) near/3 (excis* or metastasectom* or resect* or surg*)):ti,ab,kw |
| 17 | MeSH descriptor: [Antineoplastic Agents] explode all trees |
| 18 | MeSH descriptor: [Antineoplastic Protocols] explode all trees |
| 19 | MeSH descriptor: [Cancer Vaccines] explode all trees |
| 20 | MeSH descriptor: [Antibodies, Monoclonal] explode all trees |
| 21 | chemosaturat*:ti,ab,kw |
| 22 | ((anti canc* or anticanc* or anticancerogen* or anticarcinogen* or anti neoplas* or antineoplas* or anti tumo?r* or antitumo?r* or cytotoxic*) near/3 (agent* or drug* or protocol* or regimen* or treatment* or therap*)):ti,ab,kw |
| 23 | (SACT or chemotherap* or chemosaturat* or immunotherap* or biological agent* or biological therap*):ti,ab,kw |
| 24 | ((combin* or delay* or simultaneous* or stage*) near/3 (resect* or surg*)):ti,ab,kw |
| 25 | (liver-first or liverfirst):ti,ab,kw |
| 26 | “bowel first”:ti,ab,kw |
| 27 | MeSH descriptor: [Ablation Techniques] explode all trees |
| 28 | ((percutaneous* or radiofrequen* or radio-frequen* or RF or microwave*) near/3 ablat*):ti,ab,kw |
| 29 | electroporat*:ti,ab,kw |
| 30 | (RFA or MWA or IRE):ti,ab,kw |
| 31 | MeSH descriptor: [Radiosurgery] this term only |
| 32 | (Stereotactic* near/2 (irradiation* or RT or radiation* or radioablation* or radiosurg* or radiotherap* or therap* or treat*)):ti,ab,kw |
| 33 | (SBRT or SABRT or SABR or cyberknife or cyber knife):ti,ab,kw |
| 34 | MeSH descriptor: [Chemoembolization, Therapeutic] this term only |
| 35 | ((transarterial or trans-arterial or transcatheter or trans-catheter) near/2 chemoemboli?ation):ti,ab,kw |
| 36 | (irinotecan near/4 beads):ti,ab,kw |
| 37 | (DEBIRI or TACE):ti,ab,kw |
| 38 | radioemboli?ation:ti,ab,kw |
| 39 | ((intraarterial or intra-arterial) near/3 brachytherapy):ti,ab,kw |
| 40 | (SIRT or “selective internal radiation therapy”):ti,ab,kw |
| 41 | {or #12-#40} |
| 42 | #11 and #41 Publication Year from 1995 to 2018 |
Appendix C. Clinical evidence study selection
Clinical study selection for: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
Appendix D. Clinical evidence tables
Clinical evidence tables for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
Table 6. Clinical evidence tables (PDF, 843K)
Appendix E. Forest plots
Forest plots for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
Figure 7. Comparison 1: Simultaneous resection versus staged resection – Overall survival
Figure 8. Comparison 1: Simultaneous resection versus staged resection – Disease-free survival
Figure 9. Comparison 1: Simultaneous resection versus staged resection – Treatment-related mortality
Figure 13. Comparison 2: Adjuvant chemotherapy versus surgery alone – Overall survival
Figure 14. Comparison 2: Adjuvant chemotherapy versus surgery alone – Disease-free survival
Figure 15. Comparison 2: Adjuvant chemotherapy versus surgery alone – Treatment-related mortality
Figure 18. Comparison 3: Liver resection plus RFA versus liver resection alone – Overall survival
Figure 19. Comparison 3: RFA alone versus liver resection alone – Overall survival
Figure 21. Comparison 3: Liver resection plus RFA versus liver resection alone – 90-day mortality
Appendix F. GRADE tables
GRADE tables for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
Appendix G. Economic evidence study selection
Economic evidence study selection for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
A global search of economic evidence was undertaken for all review questions in this guideline. See Supplement 2 for further information.
Appendix H. Economic evidence tables
Economic evidence tables for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
No economic evidence was identified which was applicable to this review question.
Appendix I. Economic evidence profiles
Economic evidence profiles for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
No economic evidence was identified which was applicable to this review question.
Appendix J. Economic analysis
Economic evidence analysis for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
An economic analysis was undertaken to estimate the cost-effectiveness of simultaneous versus staged resection in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent.
Introduction
Surgical resection is the standard mode of treatment for colorectal cancer presenting with liver metastases when the cancer is amenable to treatment with curative intent. Traditionally, the resection of the primary colorectal cancer and the liver metastasis has taken a staged approach with the resections performed over two surgical operations. In recent times some centres have taken a simultaneous approach performing both resections during the same surgical procedure. A simultaneous approach has potential to both decrease costs and increase patient quality of life and satisfaction through reduced hospitalisation, number of operations and total operating time and reduced perioperative recovery periods. This economic analysis aims to estimate the outcomes, patient quality of life and costs of a simultaneous approach to resection compared to a staged approach in patients with colorectal cancer presenting with liver metastases.
Methods
Population
The model considers patients with colorectal cancer presenting with liver metastases where surgical resection of both the primary cancer and the metastases is considered the most appropriate treatment option. Only patients where the procedure is intended to remove all malignant tissue (R0 margins) are considered by this analysis. Patients were not excluded 26 from analysis based on previous treatment or future planned treatment including systemic anti-cancer therapies (SACTs).
Intervention and comparator
Two approaches to the surgical resection of the cancer were considered in the economic 30 model:
Staged Approach: This approach consists of 2 surgical operations, 1 for the resection of the primary colorectal cancer and 1 for the resection of the liver metastases. The surgical operations are performed during unique hospitalisations and both will consist of standalone pre-operative assessment and preparation, surgical procedure, perioperative recovery and surgical follow-up. Both resections are usually performed within a few months of each other but the model allowed for the second resection to occur within a maximum of 6 months of the first. Whilst the colorectal and liver resection can be performed in either order the majority of staged procedures in England remove the primary colorectal cancer first. This also accounts for the majority of operations used by the studies identified in the accompanying clinical evidence review. The economic model therefore assumes that the colorectal cancer resection is performed prior to the resection of the liver metastases.
Simultaneous Approach: This approach involves 1 surgical operation to resect both the primary colorectal cancer and the liver metastases. Only 1 hospitalisation, pre-operative assessment and preparation, surgical procedure, perioperative recovery period and surgical follow-up is need with this approach.
Other methods of treatment for this patient group were considered by the review protocol including stereotactic ablative radiotherapy (SABR) and adjuvant chemotherapy alongside resection. These were not considered by the economic model as in the case of SABR it was considered unlikely that clinical evidence would be identified to inform the model and there is no clarity on the most appropriate patient group for these techniques or its exact position in any treatment pathway. It was therefore not feasible to meaningfully model such interventions. Adjuvant chemotherapy was not considered by the economic model. It was not considered that having adjuvant radiotherapy could change the preferred option between staged and simultaneous resection. Whilst the use of adjuvant radiotherapy, including whether to use it and what regimen to use, may be of economic interest this question was considered likely to have a greater impact upon quality of life and resource use and was prioritised for bespoke economic modelling.
Model Structure
A partitioned survival analysis was developed to estimate the expected life expectancy, quality adjusted life years (QALYs) and costs associated with the 2 approaches considered by this economic analysis. A partitioned survival analysis divides the model cohort between different health states based on survival curves derived for overall survival (OS) and disease-free survival (DFS) derived from the accompanying clinical evidence review. The expected OS and DFS are then calculated from the area under the respective curves. For our model, 3 mutually exclusive health states were derived for the cohort to be partitioned into:
- alive without progressed disease (equal to the area under the DFS curve)
- alive with progressed disease (equal to the area between the DFS curve and the OS curve)
- death (area above the OS curve).
An illustrative example of the structure of the partitioned survival analysis is shown in Figure 23.
Figure 23. Illustrative example of partitioned survival analysis
A partitioned survival analysis approach was chosen over other modelling approaches, for example, a state transition model as only absolute survival estimates at limited set time points were reported by the identified studies and these were the only survival estimates synthesised and reported by the meta-analyses in the accompanying clinical evidence review. Consequently all OS and DFS estimates in the model were derived from these outcomes. Given the scarcity of the time points at which these estimated it was difficult to estimate plausible transition probabilities for use in a state transition model. It was also possible to extrapolate survival beyond that reported by the studies in the accompanying clinical evidence review. How this evidence was used to inform the OS and DFS curves for the economic model is discussed in detail below. This approach is widely used in models of the cost effectiveness of oncology interventions. A review of recent NICE Technology Appraisals in oncology found that this approach was used in 73% of submissions (Woods 2017).
While not a consideration in choosing the most appropriate modelling approach, a partitioned survival analysis is a more intuitive modelling approach for metastases in cancer than state transition models. Evidence from trials and observational studies where survival is a key outcome are almost exclusively reported as median overall and disease or progression-free survival with accompanying hazard ratio and Kaplan Meier survival curves. As these are the primary inputs for partitioned survival analysis the inputs can be easily compared with those observed in the included trials and other external sources. The model can also be more 2 easily compared, for validity, with any potential future study which consider the relevant 3 interventions.
A partitioned survival analysis was performed for both interventions considered in the economic evaluation and total time spent in each health state for the model cohort was 6 calculated. Each health state was assigned a quality of life weighting so that survival could 7 be adjusted to QALYs.
The economic component of the model was built and run in Microsoft Excel 2013. The model had a cycle length of 1 year. The model had a time horizon of 36 years for which, based on Office of National Statistics (ONS) life tables, over 99.9% of a general population sample would have died (ONS 2018). This percentage would be even higher for a population with metastatic cancer. The model would therefore comfortably cover a sufficient time horizon to capture all outcomes, QALYs and costs. The model took a NHS and Personal Social Services perspective (PSS) and only outcomes relevant to either organisation were considered.
For the reporting of outcomes in the model staged resection was considered the comparator and simultaneous the intervention. The model was run with a hypothetical cohort of 1000 people although costs and outcomes are reported on a per person basis.
Model parameters
Clinical inputs
Socioeconomic and demographics variables
The model assumed a uniform age of the cohort of 60 years of age based and 60% male identical to those reported in the only study (Mayo 2013), identified in the accompanying clinical evidence review, that reported overall survival. The uniform age of the model cohort was not varied during either deterministic or probabilistic sensitivity analysis as evidence was not available, given the 1 study identified, to do this in a meaningful way. The proportion of males was varied using a beta distribution based on the absolute numbers reported (Table 16).
Overall and disease-free survival
Survival curves for the economic model were estimated entirely from the accompanying clinical evidence report. The accompanying clinical evidence review identified one study which reported overall survival for this patient group. (Mayo 2013) The study estimated an absolute overall survival from a staged approach at 44.0% after 5 years. From the same study the hazard ratio for a simultaneous compared to a staged approach was 1.08 (0.89-1.32) suggesting decreased overall survival in the simultaneous approach although the 95% confidence interval crossed the point of no difference. The study was a retrospective cohort study involving 1004 patients. Full details of the study are available in the accompanying clinical evidence review.
It was considered by the committee that retrospective cohort studies would always overestimate the overall survival of the staged approach. This is because there will be a subset of patients in the staged approach who would receive the first stage of the resection, with intention for the second stage, but did not receive both either because of disease progression, change in the frailty of the patient making further surgery inappropriate or through death. This sub patient group is likely to be older, have more aggressive disease or greater comorbidities. Their life expectancy is less than that of the rest of the population. The 3 retrospective studies did not or were unable to identify these patients and they were excluded from the analysis increasing the mean survival for the cohort. A prospective cohort study or randomised controlled trial would have eliminated this selection bias. Based on single centre cohort studies it is estimated that between 16% and 35% of patients intended to receive both resections in a staged approach will not receive the second operation. (Mayo 2013) The majority of these patients, if simultaneous approach was favoured, would have received both parts of the resection. There is possibly a small number of patients for which performing the first resection, without full intention of performing the second resection (i.e. a planned reevaluation of the disease and patient condition) and that a simultaneous approach maybe inappropriate. Whilst this group is not considered by the economic model they are will be included in the retrospective studies. However, as their number will be small it is unlikely that they will alter the results of the economic model.
Overall survival curves for a staged approach were estimated using the 5 year overall survival and assuming an exponential function in the absence of an estimated Kaplan Meier survival curve. Given the biases in the estimates of survival discussed above it was assumed in the base case that 25.5% of patients intended to receive both resections would only receive the initial one, the median of the two proportions discussed above. It was considered by the committee that such patients would instead receive treatment with non-curative intent given their poor prognosis, advanced cancer or frailty. It was considered by the committee that individuals in this group would follow the prognosis of those enrolled in the FOCUS trial (Seymour 2007). This was an RCT involving 2135 previously untreated patients with advanced colorectal cancer receiving treatment with non-curative intent comparing a number of different treatment strategies. All treatment strategies considered by the RCT reported a median overall survival between 13.9 months and 16.7 months. To approximately account for this omitted group in the base-case overall survival for the staged approach was adjusted to assume that 25.5% of patients had been excluded from the analysis and that these would have a mean survival of 16.7 months the largest median value reported in the FOCUS trial. Mean values were not reported in the trial which would be a more appropriate value to adjust by. The median survival is likely to be an underestimate of the true survival. The overall survival of this subgroup did not vary during the PSA although the proportion of the group was varied on a uniform distribution across the range of reported estimates (16%-35%) from the single centre cohort studies.
The population of the FOCUS trial are about 5 years older, more male and have a worse performance scores compared to the population in Mayo 2013 and assumed for this model. As above though it is assumed tha this subsection of the population would be older and frailer than the rest of the population. The focus study is also reasonably old and survival would now likely be greater. This will be somewhat accounted for in the unadjusted population estimates.
Up to 5 years survival curves for the simultaneous approach were calculated from the hazard ratio reported in the accompanying clinical evidence review and the unadjusted overall survival curve for the staged approach following the usual proportional hazard assumptions. After 5 years in the model equal hazards are assumed. This hazard ratio was varied during probabilistic sensitivity analysis following the 95% confidence interval (0.89-1.32) reported in the accompanying clinical evidence review with a log normal distribution.
Disease-free survival was estimated using similar methods. Again an exponential function was assumed for the 52.0% disease-free survival at 2 years estimated in the accompanying clinical evidence review for the staged approach. Both the baseline rate and relative effect were varied for both overall and disease free survival during PSA. To account for the omitted patients who did not receive both resections it was assumed that all patients in this group would not be disease-free after the first year and the disease-free survival curve was adjusted to account for this. It is unlikely that in a patient in which cancer has not progressed significantly but is fit for surgery that the second resection would not be performed. Disease-free survival for patients in the simultaneous approach arm was again estimated using the same assumptions as for overall survival using the estimated hazard ratio for disease-free survival (1.30 95% CI 0.77-2.18). Again the hazard ratio was used against the unadjusted disease-free survival curve for the staged approach. The hazard ratio was varied using a log normal distribution. It was assumed there was no correlation between overall and disease-free survival during the PSA although there is some evidence for a weak to moderate relationship between the two outcomes in metastatic colorectal cancer (Cicero 2018). Where during the PSA disease-free survival was estimated to be greater than overall survival it was adjusted to be equal to that of the overall survival estimate. This was to avoid any logical anomalies in the economic model.
A deterministic sensitivity analysis was also performed where no adjustments were made to the overall or disease-free survival estimates from the accompanying clinical evidence review.
All patients who had disease recurrence would receive either further treatment or palliative care. The treatments received did not alter the survival in the model as these were already accounted for in the survival estimates from the observational studies survival estimates are based on. These further treatments were added solely as costs and are discussed in detail below.
Mortality
The model split death into disease specific mortality and other cause mortality. Whilst this made no difference to the overall survival in the economic model it was useful for assigning palliative care costs (discussed below). Other cause mortality was assumed to be identical to that of the general population which was estimated using Office of National Statistics Life Tables for England and Wales 2014-2016. Estimated mortality was weighted by the age and percentage male in the model cohort (ONS 2018). All other death was assumed to be as a result of the metastasised colorectal cancer or related complications.
The accompanying clinical evidence review identified 1 study reporting on treatment-related mortality which did not report on any events. The model therefore assumed that there would be no death as a direct result of the treatment approach received. This assumption was held for all sensitivity analyses.
Adverse events
The proportion of adverse events for either approach was taken from the accompanying clinical evidence review. Only grade 3 and grade 4 adverse events, those deemed either severe or potentially life threatening, were included in the economic model as these were considered to be the only adverse events which would add additional costs, relative to the base resection costs, and reduce quality of life over the usual detriment from significant surgery.
The accompanying clinical evidence review only identified 1 study (Moug 2010) which reported grade 3 and grade 4 adverse events. This study reported 1 adverse event, in the simultaneous group, for the 64 patients divided equally across the arms. This difference was not statistically significant. Adverse events for both arms in the economic model were inputted, in absolute terms, identically to the values reported in Moug 2010 (3% staged, 0% simultaneous). The committee highlighted that they thought such adverse events were likely to be very rare in these patient groups and, if there was to be any difference between the two groups, it was most clinically plausible for it to be higher in the staged group where a greater number of operations are needed.
Perioperative period
For all resections there will be some perioperative period given that this is a relatively major operation. Patients will have to spend some period of time in hospital following resection regardless of whether a staged or simultaneous approach is taken. For the purposes of the model the perioperative period was assumed to be equal to the length of stay in hospital although it is acknowledged that recovery after surgery is likely to continue after discharge from hospital. However, the significant health detriments and costs are most likely to occur during this hospitalised period.
Length of stay in the study was estimated from one US costing study a retrospective study at 1 US hospital of 224 patients undergoing resection either as a staged or simultaneous procedure between 1990 and 2012 (Ejaz 2014). The studied reported a median length of stay of 7 days for the simultaneous approach and 13 days (total across both resections) for the staged approach. These perioperative periods were applied to all patients in the model.
Resource use and costs
Cost of resection and resection related complications
All resection costs were taken from NHS Reference Costs 2016/17 (Department of Health 2018). For the staged approach resection was best matched by the currency description ‘Complex Large Intestine Procedure’ for the colorectal resection and ‘Complex, Hepatobiliary or Pancreatic Procedure’ performed on an inpatient basis. Both procedures reported differing reference costs based on the number of clinical complications of the patient. As the model structure modelled complications from surgery separately the cost of either was based on the no complications (CC score 0-2) reference cost. The total costs of a staged approach was equal to the two weighted mean costs combined.
As the model cohort did not exclude any patient based on comorbidities a weighted mean of all costs reported were used for all currency descriptions with complications reported. This weighted cost then had the no complications cost subtracted to estimate a cost of complications. To avoid double counting of complications between the 2 resections a mean cost of the estimated complications cost for both stages was applied in the model. Costs were weighted based on the total number of full consultant episodes (FCE) reported in the reference costs. ‘Complex Large Intestine Procedure’ reported reference costs for both patients under and over 19 years of age. Given the 60 year average age used for the cohort of the model only the over 19 year of age costs were included (Table 10).
In the staged approach arm of the model for patients who receive the first stage (colorectal cancer resection) but do not proceed to the second stage their total cost of procedure will be equal just the primary colorectal cancer resection cost.
Table 10. NHS reference costs used to estimate the cost of staged resection
To estimate the cost of the resections in the simultaneous approach it was not appropriate to combine the 2 costs as undertaken for the staged approach. This is because both sets of costs would include costs for pre-assessment, surgical preparation, anaesthesia, time in surgical theatre and many other items that can be ‘shared’ between the 2 procedures by combining them. This will lead to both reduced overall operating time and resource use.
No currency description was identified in the NHS Reference Costs which was applicable to the simultaneous approach. To estimate the cost for the simultaneous approach the cost of the staged approach was adjusted using data on total operative time and length of stay in hospital. Abbott 2012, identified in the accompanying clinical evidence review, estimated that total operating time in the simultaneous approach was 144 minutes shorter compared to the staged approach. This was converted into a cost using estimates from Ramsay 2012. Ramsey 2012 was a systematic review and economic model of laparoscopic and robotic surgery for the removal of the prostate in individuals with prostate cancer. The economic model estimated a cost per hour of operating time of £1,266. This was converted into a cost for the 144 minutes difference and subtracted from the staged approach cost. Whilst removal of a prostate is fundamentally a different type of surgical operation, the pay grade and number of personnel in attendance and size of operating theatre would be almost identical between the 2 and it was the committee’s opinion that this was a reasonable estimate of operating costs.
Length of stay in hospital also differed between the 2 arms of the model with simultaneous approach resulting in 6 less days in hospital as discussed above. This reduced time in hospital was again converted into a cost. This was done by using the excess bed day costs for ‘Complex Large Intestine Procedures, 19 years and over’ again weighted against the FCEs in the NHS Reference Costs. This mean cost was then multiplied by the reduction in days and subtracted from the procedure cost for the staged approach. This was in addition to the adjustment for operative time. The cost of complications was assumed to be identical to that of the staged approach. Full details of how costs for the simultaneous approach were calculated are presented in Table 11.
Resource use and cost of further treatment
All patients who have disease recurrence will go on to receive further treatment or if not appropriate palliative care. Of those patients with recurrent disease who go on to receive further treatment three broad types of treatment were identified by the committee-hepatic resection, extrahepatic resection and chemotherapy. Hepatic resection is an identical surgical operation to the liver resection stage of the staged approach. It was assumed that given the post-surgical surveillance these patients would be under that any recurrence would be identified reasonably quickly and that complications from any operation in this group would be minimal. Extrahepatic resection is defined as where disease has recurred near to but outside of the liver most commonly in the bile ducts. Chemotherapy was the treatment choice for patients with recurrence in either the hepatic or extra-hepatic regions where this is not amenable to treatment or to other parts of the body where surgery would not be considered appropriate.
The proportion of patients going on to receive further treatment was taken from a UK cost utility study comparing operable to non-operable treatments for liver metastases (Roberts 2015). The study was excluded from the economic evidence review as comparisons of operable to non-operable treatments were outside of the scope of the protocol. The proportion for each type of treatment was taken from one prospectively maintained database of individuals undergoing surgery or chemotherapy for liver metastases at 1 UK hospital between 1992 and 2001. Estimates of the proportion of patients undergoing each type of further treatment are presented in Table 12.
Table 12. Estimate of proportions undergoing each type of treatment from Roberts 2015
Costs for both hepatic and extrahepatic resection were again taken from NHS Reference Costs. Hepatic resection was costed identically to the resection part of the staged approach although the assumption was made, given the follow-up of these patients, that the recurrence would be identified rapidly and the ‘Complex, Hepatobiliary or Pancreatic Procedure - without complications-with CC score 0-2’ description and costs were assumed. Extrahepatic resection was costed as ‘Complex Thoracic Procedures, 19 years and over’ and as for other procedures in the model a weighted average of all CC scores was calculated based on FCEs in an inpatient setting. The estimated costs for both approaches are presented in Table 13.
Table 13. Estimation of hepatic and extrahepatic resection costs
For disease recurrence, which was considered not amenable to resection, patients received systemic chemotherapy treatment. Two chemotherapy regimens were used by the economic model which were considered to cover the majority of chemotherapy received in the NHS for this patient group following inoperable disease recurrence-FOLFOX and FOLFIRI. An annual cost for each regimen was estimated based on committee estimates of the quantity of each component and the number of cycles. Patients who had recurred disease and received chemotherapy were assumed to continue receiving it annually until death and the cost was applied for every 1 year model cycle. The resource use and quantity for the chemotherapy regimens used in the study are presented in Table 14. Prices were taken from the ‘Drug and Pharmaceutical Electronic Market Information Tool’ (eMit) [Accessed March 2019] for all drug components. Administration costs were taken from NHS Reference Costs 2016/17 using the currency description ‘Deliver Complex Chemotherapy, including Prolonged Infusional Treatment, at First Attendance’ on a day case and regular day/night basis. Given the costs for both chemotherapy regimens were almost identical (less than £1 difference) no differentiation was made between them in the model and a mean cost of the two regimens was applied to all patients receiving chemotherapy following recurrence.
Table 14. Cost of chemotherapy treatment following recurrence
The committee also considered that cetuximab or panitumumab may be used for patients whose disease recurs but is not amenable to further surgery. Cetuximab or panitumumab is used in the NHS in conjunction with Cetuximab and panitumumab for previously untreated metastatic colorectal cancer (TA439) alongside either the FOLFOX or FOLFIRI chemotherapy regimens. This may either be given to shrink cancer tumours to allow surgery or to palliate. Resource impact tools for TA439 estimated that 65% of patients receiving either FOLFIRI or FOLFOX for metastatic cancer would receive this alongside cetuximab or panitumumab.
The list prices for cetuximab is £890.50 for a 100ml vial and panitumumab £1,517.16 per 20ml vial Both drugs are currently provided to the NHS, by the manufacturers, under the terms of a confidential patient access scheme (for panitumumab) and commercial access agreement (for cetuximab) and the cost to the NHS is likely to be significantly less than the list price. It has not been possible to transparently include these costs in the economic model. Therefore, costs for cetuximab and panitumumab were not included in the base-case analysis. This does not imply that these costs will not be significant but that the access agreements are priced so that the net benefit, assuming NICE’s threshold of £20,000 per QALY gained is identical to society’s willingness to pay per QALY gained, then the addition of either drug to FOLFIRI or FOLFOX will have no impact upon the incremental cost effectiveness ratios or ultimately the decision around the preferred approach to resection. A deterministic sensitivity analysis was also undertaken which used the list price for both drugs to investigate if this would alter the preferred approach to resection. These 2 values were considered to cover all plausible costs.
Cost of palliative care
Given the relatively short life expectancy of the model cohort and that the majority of patients would die as a result of their disease a one off cost of palliative care was applied to the entirety of the cohort during their final year of life. This is to represent the increase in resource use experienced during the final months of a patient’s life. This one off cost was taken from Georghiou 2014. The study used medical records of over 1,836 patients with cancer at multiple UK hospitals and hospices to estimate resource use and publically available UK costs to estimate a total cost for the final 90 days of life. An average cost for patients with cancer was used from the report. These costs are presented in Table 15.
Table 15. Costs of palliative care for patients with cancer from Georghiou 2014
The above costs includes ‘local authority-funded care’. The methods of calculation from the original report may include costs, such as personal contributions to care, which are not strictly covered by the NHS & PSS perspective used for this economic model. A deterministic sensitivity analysis was therefore undertaken which removed this cost from the total palliative care cost estimate.
Quality of life
Quality of life weights for the model were taken from previous cost-effectiveness study of patients (identical in age to our cohort) with rectal cancer (Rao 2017). The Markov simulation model compared radical surgery to a ‘watch and wait’ and wait approach. The model estimated utility weights both following surgery and radiation (0.86) and following disease recurrence (0.78) based on the authors clinical opinion, a previous Dutch study of resection and a previous cost effectiveness study of treatment for recurrent rectal cancer (Miller 2000). These represented the disease-free and disease recurrence groups in our model. These utility weights were used to adjust overall survival and calculate QALYs. The committee cancer in the liver amenable to treatment with curative intent considered that these utilities were probably an overestimate of the true utility of this cohort given the morbidity from major surgery. The difference between disease-free and recurrent disease was also considered too small with recurrent disease likely to lead to greater anxiety, morbidity from further surgery or adverse events from chemotherapy or other treatment. These values were therefore given a wide range during the PSA.
Utility values for the peri-operative period was taken from an economic evaluation conducted alongside the SANICS II RCT investigating the use of perioperative enteral nutrition in patients undergoing colorectal surgery (Pattamatta 2019). This was an RCT of 265 patients at 3 large Dutch hospitals and 2 Danish hospitals. Participants in the study completed the EuroQol 5 dimension 5 level (EQ-5D-5L) questionnaire and scored using the ‘Dutch tariff’ preferences elicited from the general population of the Netherlands. It was the committee’s opinion that patients in this trial would have identical quality of life during the perioperative period as for the patient group in the model cohort. As the study reported higher quality of life for the perioperative stage then the disease-free state used in our model (0.89 versus 0.86) it would not have been logical to include this value in our model and imply that quality of life is greater during a patients stay in hospital then when they are disease-free. A QALY detriment was therefore calculated for the perioperative period which was equal to the difference of the highest and lowest reported utilities values, in the patient group, during the RCT. This was the difference between presurgery and at 3 months post surgery where quality of life had decreased by 0.06 points. The utility weight for the peri-operative period was therefore the disease-free utility state (0.86) minus this difference. The utility weight used for peri-operative period in the model was therefore 0.80.
Death was given a utility value of 0 in the model as is standard in economic evaluations.
Inflation
All costs in the model were converted and inflated to UK sterling 2017 prices, to match the cost year from the NHS Reference Costs, using the IMF Purchasing Power Parities for Healthcare and inflation indices reported by Curtis 2018 where necessary.
Discounting
All health and cost outcomes were discounted at a rate of 3.5% per annum in line with developing NICE guidelines: the manual. This was not varied during sensitivity analyses.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was also conducted to assess the combined parameter uncertainty in the model. In this analysis, the values that are utilised in the base-case are replaced with values drawn randomly from the distributions assigned to them. This was done for 10,000 iterations and the different outcomes of these iterations presented both diagrammatically (in the forms of a cost effectiveness plane and cost effectiveness acceptability curve) and in terms of mean from these iterations to reflect the uncertainty around the inputs and consequently the outcomes of the model. The distributions for all parameters used during the probabilistic sensitivity analysis are presented in Table 16.
Table 16. Distributions used during the probabilistic sensitivity analysis
Results
Base-case results
The base case results of the analysis are shown in Table 17. The results show an increase in life expectancy of half a year with the simultaneous approach corresponding to a 0.28 QALY increase compared to the staged approach. The simultaneous approach also led to reduction in costs just below £2,500. In the base-case analysis the simultaneous approach dominated (was both cost saving and health increasing).
When the unadjusted results of the accompanying clinical evidence review are used to inform the economic model and adjustments for survival have not been made to account for individuals who only received the first resection life expectancy is now higher in the staged group with an associated higher QALY as well (Table 18). The simultaneous approach remains cost saving (even more so given the larger number of liver resections in the staged approach). If a £20,000 per QALY threshold is considered, in line with Developing NICE guidelines: the manual, the reduction in costs would not justify the decrease in QALYs, that is only £16,506 is saved for every QALY forgone.
The conclusions of both the base-case and alternative assumption remain when the list prices of cetuximab and panitumumab are included and when local authority costs are removed from the estimate of palliative care. As both of these assumptions are considered the upper bound alternative to the base-case assumptions, and they did not alter the conclusions of the model, intermediate estimate of these value are not presented. The difference between both approaches for the percentage of the cohort receiving cetuximab or panitumumab or palliative care was never greater than 3% and therefore even more extreme assumptions would not alter these results unless unfeasibly large or small.
Probabilistic Sensitivity Analysis
Base-case assumptions
The results of 10,000 runs of the PSA are shown using ICER scatterplots and cost-effectiveness acceptability curves (CEAC). The ICER scatter plots show the incremental costs and QALYs associated with each of the 10,000 runs of the PSA along with the mean result. The CEAC graphs show the probability of each strategy being considered cost-effective at the various cost-effectiveness thresholds on the x axis.
Figure 24 presents the probabilistic results of the base case analysis. Of these 10,000 iterations over 75% of them are health improving (to the right of the Y-axis) and over 80% are cost decreasing (below the X-axis) with the majority of iterations being both cost saving and health increasing.
Figure 24. ICER scatterplot base case results
Figure 24 presents the CEAC for the base case results. The probability that the simultaneous approach is the preferred option is 80% at a cost effectiveness ratio of £0 i.e. where the cheapest option is preferred. At £20,000 threshold there is a 86% probability of the simultaneous approach being the preferred option. This remains above 80% beyond values above the £100,000 threshold. For no threshold does a staged approach become the preferred option.
Figure 25. Cost effectiveness acceptability curve base case results
Clinical evidence review values results
When results from the clinical evidence review are used to inform the economic model there is a greater degree of uncertainty around the results. (Figure 26) When these inputs are considered a greater number of iterations show the simultaneous approach as cost saving (94%) but with only 20% of iterations being health improving.
Figure 26. ICER scatterplot clinical evidence review inputs
The CEAC for this analysis shows uncertainty around the preferred option (Figure 27). At the £20,000 threshold 42% showed the simultaneous approach to be cost effective although as shown by the scatterplot the majority of these would also be health decreasing. As the threshold increases the probability of the staged approach being the most cost effective option also increases. The threshold at which the CEACs cross and we are indifferent between the two options is £17,000 per QALY.
Figure 27. Cost effectiveness acceptability curve clinical evidence review inputs
Conclusions
Both versions of the model gave differing results. The base-case results, where survival in the staged approach had been adjusted, showed the simultaneous approach as both health improving and cost saving. This conclusions was robust to both probabilistic and deterministic sensitivity analysis. The secondary analysis using survival estimates from the accompanying clinical evidence review presented the simultaneous approach as health decreasing and cost decreasing but not cost effective although the PSA highlighted considerable uncertainty around this conclusion. Given the sensitivity of survival estimates to the conclusions of the economic model and the biases with the observational data used to inform the economic model it was difficult to form strong conclusions from either model.
Appendix K. Excluded studies
Excluded clinical studies for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
Appendix L. Research recommendations
Research recommendations for review question: What is the optimal combination and sequence of treatments in patients presenting with metastatic colorectal cancer in the liver amenable to treatment with curative intent?
No research recommendations were made for this review question.
Final
Evidence reviews
Developed by the National Guideline Alliance part of the Royal College of Obstetricians and Gynaecologists
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Curative liver resection for metastatic breast cancer.[Eur J Surg Oncol. 2000]Curative liver resection for metastatic breast cancer.Maksan SM, Lehnert T, Bastert G, Herfarth C. Eur J Surg Oncol. 2000 Apr; 26(3):209-12.
- Review Multimodal treatment strategies for colorectal liver metastases.[Swiss Med Wkly. 2021]Review Multimodal treatment strategies for colorectal liver metastases.Birrer DL, Tschuor C, Reiner C, Fritsch R, Pfammatter T, Garcia Schüler H, Pavic M, De Oliveira M, Petrowsky H, Dutkowski P, et al. Swiss Med Wkly. 2021 Feb 15; 151:w20390. Epub 2021 Feb 15.
- Liver resection for colorectal cancer metastases.[Curr Oncol. 2013]Liver resection for colorectal cancer metastases.Gallinger S, Biagi JJ, Fletcher GG, Nhan C, Ruo L, McLeod RS. Curr Oncol. 2013 Jun; 20(3):e255-65.
- Simultaneous versus staged liver resection of synchronous liver metastases from colorectal cancer.[Int J Colorectal Dis. 2007]Simultaneous versus staged liver resection of synchronous liver metastases from colorectal cancer.Thelen A, Jonas S, Benckert C, Spinelli A, Lopez-Hänninen E, Rudolph B, Neumann U, Neuhaus P. Int J Colorectal Dis. 2007 Oct; 22(10):1269-76. Epub 2007 Feb 21.
- Review Simultaneous Resection of Primary Colorectal Cancer and Synchronous Liver Metastases: Contemporary Practice, Evidence and Knowledge Gaps.[Oncol Ther. 2021]Review Simultaneous Resection of Primary Colorectal Cancer and Synchronous Liver Metastases: Contemporary Practice, Evidence and Knowledge Gaps.Kleive D, Aas E, Angelsen JH, Bringeland EA, Nesbakken A, Nymo LS, Schultz JK, Søreide K, Yaqub S. Oncol Ther. 2021 Jun; 9(1):111-120. Epub 2021 Mar 23.
- Treatment for metastatic colorectal cancer in the liver amenable to treatment wi...Treatment for metastatic colorectal cancer in the liver amenable to treatment with curative intent
Your browsing activity is empty.
Activity recording is turned off.
See more...
