NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Effectiveness of endovascular aneurysm repair, open surgical repair and non-surgical management of unruptured abdominal aortic aneurysms
Review question
What are the relative benefits and harms of EVAR, open surgical repair and non-surgical management in people with unruptured abdominal aortic aneurysms?
Introduction
This review question aims to assess the advantages and disadvantages of elective endovascular aneurysm repair (EVAR) in comparison with conventional open surgical repair for the treatment of unruptured abdominal aortic aneurysms (AAAs). Furthermore, this question aims to explore advantages and disadvantages of elective EVAR in comparison with non-surgical management when open surgical repair is not possible.
PICO table
Table 1
Inclusion criteria.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual (2014). Methods specific to this review question are described in the review protocol in Appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A Cochrane systematic review (Paravastu et al. 2014) comparing EVAR and open surgical repair of unruptured AAAs was identified as a reliable source of randomised controlled trials (RCTs) relevant to this review question. Since the systematic review was published in 2014, the Cochrane Vascular Group worked in collaboration with the NICE Guideline Updates Team and performed update literature searches to facilitate identification of any RCTs published after publication of the systematic review by Paravastu et al. (2014). Data were extracted from the systematic review, individual RCTs within it, and RCTs identified from update literature searches to compare the efficacy of elective EVAR with that of open surgical repair of ‘simple’ unruptured infrarenal aneurysms. Since the Cochrane systematic review did not explicitly consider complex aneurysm anatomies (such as juxtarenal and suprarenal type IV aneurysms) a supplementary literature search was performed by NICE.
In 2019, this evidence review was updated because long-term data was published from the OVER trial.
Studies were excluded if they:
- were not in English
- were not full reports of the study (for example, published only as an abstract)
- were not peer-reviewed.
The original protocol for this review (Appendix A) indicated that, for complex AAAs only, non-randomised comparative studies and prospective cohort studies could also be included. However, this identified only 1 study (Donas et al., 2012). In consultation on the draft version of this guideline, stakeholders argued that this protocol was too restrictive and, as a result, the review had failed to identify relevant nonrandomised evidence in both infrarenal and complex AAAs. Stakeholders also agreed that the 1 study of complex AAA that had been included under the original protocol was of limited relevance (it was the only available cohort study with a prospective design, but it was small and took no steps to address the selection biases inherent in observational designs, whereas there are some retrospective studies that are superior in both respects).
In response to this feedback, an additional review of casemix-adjusted observational evidence for both infrarenal and complex AAAs was undertaken. The methods and results of this review are detailed in Evidence review K2.
Because all stakeholders agreed that the prospective cohort study identified in the original review (Donas et al., 2012) added little to the evidence-base, and because it did not meet the criteria for the new review (as it did not perform any form of casemix adjustment), we consider it is superseded. Accordingly, we have removed details from this review. The consultation draft of this document remains available on NICE’s website for any reader who wishes to see what was said about Donas et al. (2012) in our original review.
Clinical evidence
Included studies
Standard EVAR
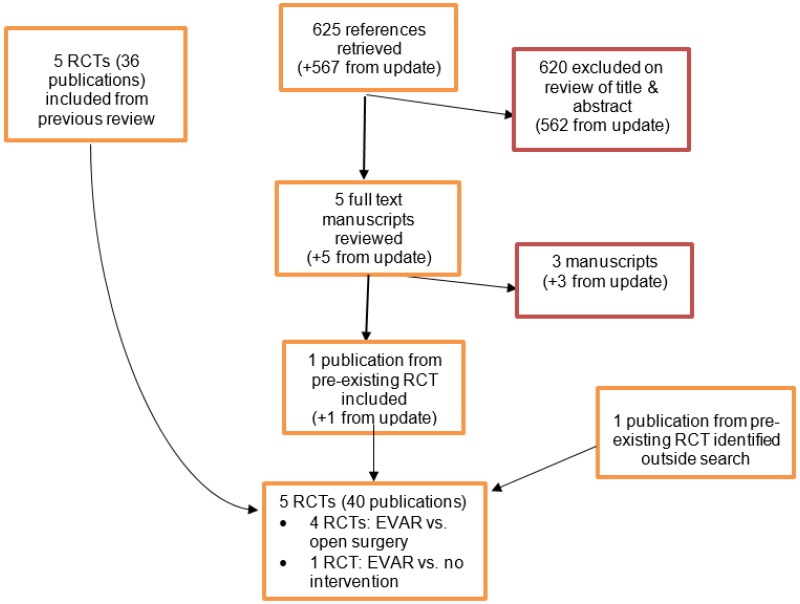
The 2014 Cochrane systematic review (Paravastu et al, 2014) included 4 RCTs (reported across multiple publications) comparing EVAR with open surgical repair of infrarenal AAA. The update literature search performed by Cochrane Vascular Group yielded 354 abstracts, of which 4 full manuscripts were ordered. Of the 4 articles reviewed, an additional publication reporting an RCT (EVAR-1 trial) that was already included in the Cochrane review was identified. Thus, a total of 4 RCTs, published across multiple publications, was considered relevant for comparisons between standard EVAR and open surgical repair of unruptured AAAs. The 2014 Cochrane systematic review included 1 RCT (EVAR-2 trial) comparing EVAR with non-surgical management, in patients for whom open surgical repair was considered unsuitable. The update literature search performed by Cochrane Vascular Group yielded 1 publication reporting long-term follow-up of the EVAR-2 trial.
In December 2017, Cochrane performed another literature search to identify studies which were published during guideline development. The search yielded a total of 296 abstracts; of which, 4 full manuscripts were ordered. Upon review of these 4 articles, a publication of another RCT (DREAM trial) already included in the Cochrane review was identified.
Excluded studies
The list of papers excluded at full-text review, with reasons, is given in Appendix J.
Summary of clinical studies included in the evidence review
A summary of the included studies is provided in the tables below.
Standard EVAR compared with open surgical repair of unruptured infrarenal AAA
| Study | Details |
|---|---|
| Paravastu SC, Jayarajasingam R, Cottam R et al. (2014) Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev;(1): CD004178. doi: 10.1002/14651858.CD004178.pub2. [PubMed: 24453068] [CrossRef] |
Study design: systematic review Location: UK Population: patients with unruptured AAA Sample size: 4 RCTs including 2,745 participants Follow-up: 30 days, up to 4 years, up to 8 years Intervention: standard EVAR using any type of endovascular device Comparators: open surgical repair Outcomes: All-cause mortality, aneurysm-related mortality, endograft-related complications, major complications, minor complications, and quality of life. Assessed at the following time points: 30 days, up to 4 years up to 8 years. Note: details about included studies can be found in Appendix D |
| ACE trial (results reported in multiple publications outlined in the Cochrane systematic review) |
Study design: multicentre, non-blinded, randomised controlled trial Location: France Population: patients with asymptomatic unruptured abdominal aortic or aorto-iliac aneurysm Sample size: 299; 99% male Follow-up: up to 4 years Intervention: standard EVAR Comparators: Open surgical repair Outcomes: All-cause mortality, major adverse events (myocardial infarction, permanent stroke, permanent haemodialysis, major amputation, paraplegia and bowel infarction), vascular reinterventions and minor complications |
|
DREAM trial (results reported in multiple publications outlined in the Cochrane systematic review) NB: a new publication was identified from update searches van Schaik T G, Yeung KK, Verhagen HJ et al. (2017) Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery 54 (5), 671 [PubMed: 29061270] |
Study design: multicentre, non-blinded, randomised controlled trial Location: Netherlands Population: patients with unruptured AAA Sample size: 351; 91% male Follow-up: up to 15 years 3 Intervention: standard EVAR Comparators: Open surgical repair Outcomes: All-cause mortality, aneurysm-related mortality, complications and reintervention rates |
|
EVAR1 trial (results reported in multiple publications outlined in the Cochrane systematic review) NB: new publications were identified from update searches Patel R, Sweeting MJ, Powell JT et al. (2016) Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repairtrial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 388(10058):2366–2374. [PubMed: 27743617] Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technology Assessment (Winchester, England). 2018 Jan;22(5):1. [PMC free article: PMC5817412] [PubMed: 29384470] |
Study design: multicentre, non-blinded, randomised controlled trial Location: UK Population: patients with unruptured AAA Sample size: 1,252; 91% male Follow-up: up to 15 years Intervention: standard EVAR Comparators: Open surgical repair Outcomes: All-cause mortality, aneurysm-related mortality, complications and reintervention rates |
|
OVER trial (results reported in multiple publications outlined in the Cochrane systematic review) NB: a new publication was identified from update searches Lederle FA, Kyriakides TC, Stroupe KT, Freischlag JA, Padberg Jr FT, Matsumura JS, Huo Z, Johnson GR. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. New England Journal of Medicine. 2019 May 30;380(22):2126–35 [PubMed: 31141634]. |
Study design: multicentre, non-blinded, randomised controlled trial Location: USA Population: patients with unruptured AAA Sample size: 881; 99% male Follow-up: 14 years Intervention: standard EVAR Comparators: Open surgical repair Outcomes: All-cause mortality, aneurysm-related mortality, complications and reintervention rates |
EVAR vs no intervention for patients in whom open surgery is not considered appropriate
| Study | Details |
|---|---|
|
Sweeting M J, Patel R, Powell J T, and Greenhalgh R M (2017) Endovascular Repair of Abdominal Aortic Aneurysm in Patients Physically Ineligible for Open Repair: Very Long-term Follow-up in the EVAR-2 Randomized Controlled Trial. Annals of Surgery. 24 [PubMed: 28742684] Note: Other publications relating to this trial that reported data at different follow-up periods were considered |
Study design: multicentre, non-blinded, randomised controlled trial Location: UK Population: patients with large aneurysms in whom open surgical repair was considered inappropriate Sample size: 404; sex-specific proportions were not reported Follow-up: 12 years Intervention: EVAR Comparators: open surgical repair Outcomes: All-cause mortality, aneurysm-related mortality, graft-related complications and graft-related re-interventions. |
See Appendix D for full evidence tables.
Quality assessment of clinical studies included in the evidence review
See Appendix F for full GRADE tables.
Economic evidence
Included studies
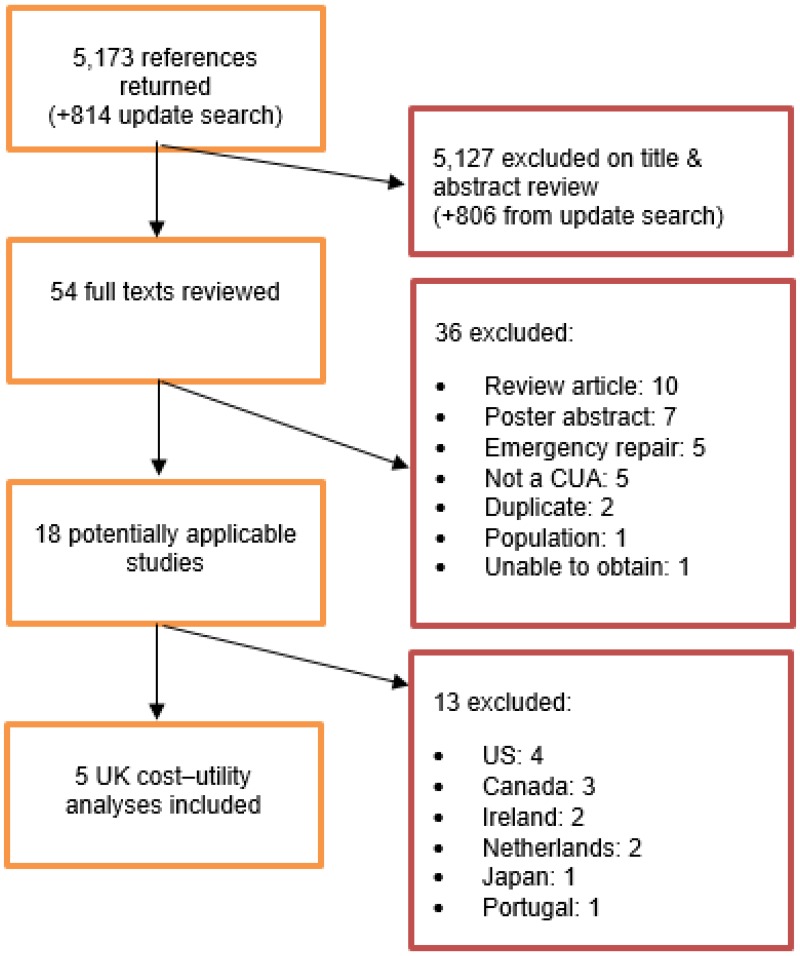
A systematic review of economic literature was conducted jointly for all review questions in this guideline by applying standard health economic filters to a clinical search for AAA (see Appendix B). A total of 5,173 studies was identified. The studies were reviewed to identify cost–utility analyses exploring the costs and effects of elective surgical procedures to repair unruptured AAAs. Studies that met the eligibility criteria were assessed using the quality appraisal criteria as outlined in the Developing NICE guidelines: the manual (2014).
Following an initial review of titles and abstracts, the full texts of 46 studies were retrieved for detailed consideration. Following full-text review, 15 of the 46 studies were judged to be potentially applicable cost–utility analyses for elective repair. Of these, 5 were UK studies. It was decided to exclude the non-UK studies because of their lower applicability to UK practice.
An update search was conducted in December 2017, to identify any relevant cost–utility analyses that had been published during guideline development. This search returned 814 studies. Following review of titles and abstracts, the full texts of 8 studies were retrieved for detailed consideration. Three were determined to be potentially applicable for elective repair; however they were non-UK studies, and were selectively excluded. A total of 5 studies was therefore included as economic evidence for the elective repair of unruptured AAA.
Excluded studies
Studies that were excluded after full-text review, and reasons for exclusion, are provided in Appendix J – Excluded studies.
Summary of studies included in the economic evidence review
Michaels et al. (2005)
Michaels et al. (2005) published the first UK cost–utility analysis comparing EVAR with open surgical repair for the elective repair of infrarenal AAA, based on early (perioperative; 30-day) results of the EVAR-1 and DREAM trials. The analysis modelled a cohort of 70-year old men with an initial AAA diameter of 5.5cm. A decision tree was developed to model the surgical procedure followed by general population survival for 10 years. Other inputs, such as EVAR complications, were derived from a 2005 NICE review of non-RCT data. Costs and QALYs were both discounted by 3.5% per year. Model results (Table 3) suggest that EVAR is associated with a high ICER of over £100,000/QALY compared with open surgical repair, with a near 0% likelihood of the ICER falling under £20,000 per QALY gained.
Table 3
NICE cost–utility model results, population for whom open surgical repair is an option.
A secondary analysis was also reported comparing EVAR with providing no intervention; however it was based on non-randomised evidence only, therefore these results have been excluded due to possessing very serious limitations.
Epstein et al. (2008)
Epstein et al. (2008) developed a lifetime Markov model comparing EVAR with open surgical repair in the UK, based on 4-year data from the EVAR-1 randomised study. Perioperative outcomes included mortality and conversion from EVAR to open surgical repair, followed by symptom-free survival subject to risks of major cardiovascular events, AAA-related readmission and death. All-cause mortality rates were assumed to converge after 2 years. Health-related quality of life effects (EQ-5D), resource use and costs were informed by data collected during EVAR-1. All outcomes were discounted by 3.5% per year.
The model found EVAR to incur higher total costs and accrue fewer QALYs per patient than open surgical repair (Table 3), and the difference in costs was statistically significant. EVAR had a 1% probability of having an ICER of £20,000 or less per QALY gained, which remained less than 10% in all but extreme scenario analyses.
Chambers et al. (2009)
Chambers et al. (2009) developed an NIHR-funded cost–utility model as part of their EVAR health technology assessment to support NICE Technology Appraisal 167. It evaluated EVAR in 2 populations: people who are fit enough to undergo open surgical repair and people who are not. For the primary analysis comparing EVAR with open surgical repair, a Markov model was developed using patient-level data from the EUROSTAR registry dataset, with a similar structure to the Epstein et al. (2008) model. The EUROSTAR data informed multivariable models predicting baseline risks of perioperative mortality, postoperative AAA-related mortality and other cause mortality, with relative risks informed by the DREAM and EVAR-1 studies or expert advice. The aneurysm-related mortality benefit associated with EVAR was assumed to persist for the lifetime horizon. Quality of life (EQ-5D) and resource use inputs were informed by the EVAR-1 trial. Outcomes were discounted by 3.5% per year.
EVAR was found to be associated with a QALY gain, and to incur a higher cost per patient, compared with open surgical repair, resulting in an ICER was £48,990 per QALY gained (Table 3). The probability of EVAR possessing an ICER below £20,000 was 26%.
The secondary analysis evaluated EVAR compared with continued monitoring or discharge without intervention. This analysis included the option of repairing AAA at diameters below 5.5 cm, such that the study is relevant to the question of early intervention for this guideline. Its methods and details are described fully in Evidence review F. Briefly, the authors concluded that EVAR may have an ICER below £20,000 compared with providing no intervention in somebody with a 5.5 cm aneurysm aged 73 or younger. In people with larger aneurysms, EVAR became increasingly cost effective, compared with no intervention (e.g. it was cost effective in people aged up to 79 years old if the AAA is 8.0 cm).
Brown et al. (2012)
Brown et al. (2012) conducted an economic evaluation with a Markov model broadly similar to the Epstein et al. (2008) and Chambers et al. (2009) models, with the inclusion of a waiting period via an ‘intention to treat’ analysis, with outcomes divided into more granular time periods: randomisation to 6 months, 6 months for 4 years, 4–8 years, and after 8 years. Data up to 8 years were informed by mid-term outcomes of EVAR-1. Quality of life (EQ-5D) and resource use inputs were obtained from the EVAR-1 data. Outcomes were discounted by 3.5% per year. Results (Table 3) suggest that EVAR is dominated by open surgical repair, with higher overall costs and fewer total QALYs per patient, with the EVAR ICER being £20,000 per QALY gained or better in 1% of model runs.
The authors also conducted a within-trial economic analysis based on the EVAR-2 trial, comparing EVAR with ‘no intervention’ for infrarenal AAA in people deemed unfit for open surgical repair. Quality of life (EQ-5D) and resource use were from the trial, captured in the same manner as the EVAR-1 study. The within-trial intention-to-treat analysis (8-year duration) found EVAR to have a mean ICER of £264,900 per QALY gained over ‘no intervention’, with 0% probability of the ICER being under £20,000. Results of a lifetime analysis, with survival extrapolated using parametric survival curves fitted to the EVAR-2 data, reduced the EVAR ICER to £30,274 per QALY gained. However, costs were not extrapolated beyond the trial.
Epstein et al. (2014)
Epstein et al. (2014) presented a further iteration of the Epstein et al. (2008) model, using outcomes data from the ACE, DREAM, EVAR-1 (8-year data) and OVER studies. Clinical and resource use inputs were obtained from each trial. The trial data were not synthesised. Instead, 4 sets of results were presented. The reintervention rate following open surgical repair was estimated using EVAR-1 trial data, with relative effects from each study used to estimate EVAR reintervention rates. Quality of life was informed by the EVAR-1 EQ-5D data. To normalise country-specific follow-up protocols, the authors applied a single postoperative outpatient CT scan for open surgical repair patients and continued annual monitoring following EVAR. Outcomes were discounted by 3.5% per year.
EVAR was dominated by open surgical repair in the EVAR-1 and ACE analyses, with an ICER of almost £3,000,000 per QALY gained in the DREAM analysis (Table 3). Each analysis predicted a 0% probability of EVAR having an ICER below £20,000 per QALY gained compared with open surgical repair. Conversely, the OVER analysis found a cost saving and QALY gain per patient for EVAR, with a 91% probability that its ICER is under £20,000. The authors attribute this to higher hospital costs in the US setting of the OVER trial, and the fact that the OVER trial predicts more favourable long-term survival for EVAR compared with the other trials.
Table 2
Cost–utility results of included economic studies – all infrarenal AAA repair.
Further details of the included economic studies are available in Appendix H – Economic evidence tables and the separate economic analysis appendix.
Economic model
The effectiveness of EVAR compared with open surgical repair for the repair of unruptured AAAs was identified as a priority for new economic analysis. Clinical evidence has become available since the existing technology appraisal (TA 167) was published, including the ACE and OVER trials, as has longer-term data from the DREAM and EVAR trials. Furthermore, the TA guidance is focused on infrarenal AAA, whereas the scope of this guideline has a wider population containing other types of AAA. A new economic model was therefore developed to support decision-making in this area.
Methods
The model began at the point when the decision is made to conduct, or not to conduct, the elective repair of an AAA. Two distinct populations were modelled: (1) those for whom open surgical repair is a suitable intervention, comparing EVAR with open surgical repair; and (2) those for whom open surgical repair is not a suitable intervention, because of raised intraoperative risk, comparing EVAR with no intervention. Much of the input data for these 2 models was informed by anonymised patient-level survival data from the EVAR-1 and EVAR-2 trials, respectively, which the EVAR trial investigators provided to NICE. Within each population, the model also evaluated infrarenal AAAs and complex AAAs as separate groups. The perspective on costs was those incurred by the NHS and Personal Social Services (PSS), and the perspective on outcomes was the direct health effects for people using AAA services. The main outcomes were incremental costs and QALYs, and the resulting ICER. The model time horizon was the lifetime of the patient (to a maximum age of 100), composed of 1-month cycles, with all outcomes discounted by 3.5% per year (Developing NICE guidelines, 2014).
In the population for whom open surgical repair is a suitable intervention, modelled patients were first at risk of death while waiting for their elective intervention: 2 months for infrarenal EVAR and any open surgical repair; 3 months for complex EVAR. The extended waiting time for complex EVAR is due to the need for most EVAR devices to be custom-made to suit the patient’s aortic anatomy, whereas standard EVAR devices suitable for infrarenal AAAs are readily available. This was followed by 1 perioperative cycle, in which the intervention occurs, with a risk of perioperative mortality. In the base-case model, this was informed by the UK National Vascular Registry (2017) data on EVAR (0.4%), representing a current snapshot of UK practice outcomes. To estimate the OSR perioperative mortality rate relative to EVAR, the model used the results of a Cochrane systematic review of elective AAA repair trials (odds ratio for EVAR versus open surgical repair: 0.33; Paravastu et al., 2014). This approach combined using an estimate of current UK practice outcomes (the registry) for baseline data and the best available randomised evidence for the relative effectiveness between EVAR and OSR from (the Cochrane review).
Surviving patients move into the post-perioperative survival (long-term) phase of the model, informed by general population mortality rates, calibrated to post-perioperative survival data from the EVAR-1 open surgical repair arm (though the EVAR arm would have been equally appropriate for this). The long-term relative effectiveness of EVAR was informed by hazard ratio from a meta-analysis of long-term elective repair data (EVAR-1, DREAM and OVER). Throughout the model, patients are at risk of complications requiring reintervention; initial rates are informed by the EVAR-1 trial but, for EVAR only, these are modified using observational data (Verzini et al., 2014) with the aim of reflecting a presumed reduction in rates of reintervention with EVAR since the RCTs were conducted. Laparotomy-related reinterventions, such as bowel resection, are also captured based on US Medicare data.
In the population for whom open surgical repair is not a suitable intervention, EVAR waiting time, perioperative and long-term mortality data were informed by the only relevant RCT: the EVAR-2 trial. For this population, survival on the comparator strategy of ‘no intervention’ was modelled from the point of randomisation, with no waiting time or perioperative periods. The ‘no intervention’ survival data were adjusted for the effect of crossover, using the rank preserving structure failure-time (RPSFT) technique, as one-third of participants randomised to this arm instead received EVAR. The RPSFT method is a well established method for accounting for trial crossover, estimating what the survival of trial participants who switched arm would have looked like had they not switched (the counterfactual), and adjusting the observed treatment effect accordingly. The same technique to calibrate general population survival data as described above was then used. Postoperative EVAR complications were included using event rates reported in the EVAR-2 study. On the ‘no intervention’ arm, the model includes the complication of the unrepaired AAA rupturing. In the EVAR-2 trial, the rate of rupture was reported to be 12.4% per year. This rate is used to determine the proportion of patients in each cycle who require emergency repair (noting that 89% of EVAR-2 ruptures were fatal before emergency intervention could be commenced).
In order to explore subgroup effects, the models for both populations were configured so that perioperative and long-term survival estimates could be influenced by effect modifiers. For perioperative mortality, the effects of age, AAA diameter and sex were captured based on data from the European ‘Vascunet’ registry (Mani et al., 2015; Budtz-Lily et al., 2017). AAA diameter was a significant predictor of death, more prominently for EVAR, and age was a predictor of perioperative death for open surgical repair. For post-perioperative mortality, multivariable Cox regressions using the EVAR-1 data found AAA size to be a significant determinant of long-term survival. Using the EVAR-2 data, being treated with EVAR was associated with improved survival for up to 4.5 years. The effect of age was implicitly captured in this by our use of calibrated general population survival data. Effect modifiers were used in specific subgroup analyses and in probabilistic sensitivity analysis, to fully explore the effect of uncertain patient characteristics on outcomes. Our base-case deterministic results are evaluated for the trial mean cohorts.
Base case overall survival curves are presented in Figure 1 and Figure 2.
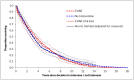
Figure 2
Base case overall survival profile – infrarenal AAAs – population for whom open surgical repair is not an option, versus EVAR-2 trial data.
People with more complex aneurysms – that is, cases in which a standard EVAR graft cannot be used within the terms of its instructions for use – were also simulated in the model, as a separate subpopulation in elective cases (the analyses do not distinguish between infrarenal and complex aneurysms in the emergency population for whom open surgical repair is a potentially suitable option). There are no randomised data comparing EVAR and open surgical repair for the repair of complex AAAs. The model therefore used the UK National Vascular Registry (2017), which reports perioperative mortality rates in UK practice for complex repair. Taking the registry’s EVAR mortality rate (3.5%) as the starting point, the model applies the relative effect from the Cochrane meta-analysis of elective infrarenal AAA repairs to this value to obtain an estimated complex repair perioperative mortality rate for open surgical repair (9.9%). The relevant effect modifiers may then be applied to the resulting baseline estimates. In the population for whom open surgical repair is not a suitable option, the Registry data were used to estimate a ‘relative effect of complexity’ on perioperative mortality following EVAR, relative to infrarenal EVAR (odds ratio = 8.7). This relative effect is used to increase the perioperative mortality rate from the EVAR-2 trial, to estimate the equivalent EVAR perioperative mortality rate in people with complex aneurysms. Owing to the absence of long-term evidence, post-perioperative survival and reintervention rates for people with repaired complex aneurysms were assumed to be equal to those for people with repaired infrarenal aneurysms; the guideline committee advised that this is a plausible assumption. The overall survival of people on the ‘no intervention’ strategy, based on EVAR-2 trial data, was assumed to be independent of aneurysm complexity, due to the absence of long-term survival data in people with untreated complex AAA. Again, the guideline committee advised that this was a reasonable approach to take.
Resource use was obtained from the detailed published EVAR-1 data (Brown et al. 2012), to which up-to-date national unit costs were applied, and from unadjusted National Vascular Registry data (2017). The cost of standard and complex EVAR devices were obtained from NHS Trusts by members of the guideline development committee. Following advice from the committee, a strategy of ‘no intervention’ is assumed to incur non-zero costs, associated with a further outpatient attendance and CT scan. Quality of life was primarily informed by the published 1-year EVAR-1 EQ-5D data, supplemented by decrements for complications identified by informal searches.
For complete details of model methods and parameters, please see the separate economic analysis appendix.
Results
In the base-case model, in a cohort for whom open surgical repair is a suitable option, elective EVAR was found to be dominated by open surgical repair, producing fewer QALYs at a higher total NHS and personal social service (PSS) cost (Table 3). Probabilistic sensitivity analysis showed that its ICER had 9.1% likelihood of being £20,000 per QALY gained or better. The only parameter that causes the cost-effectiveness conclusion to change when deterministically varied between its plausible bounds is the post-perioperative mortality hazard ratio. When it is set to its lower 95% confidence interval (0.95) to favour EVAR instead of the base-case estimate in favour of OSR (1.05), the incremental net monetary benefit (INMB) becomes positive if a QALY is valued at £20,000, and the ICER is £13,753 per QALY gained. For the repair of complex AAAs in this population, the base-case ICER was £34,288 per QALY gained. Here, EVAR was associated with a nontrivial QALY gain of 0.284 per patient, due to the wider gap between EVAR and open surgical repair in estimated perioperative mortality – that is, fewer individuals are predicted to survive open surgical repair to experience any improved long-term survival prospects. However, this benefit is offset by the substantially higher device cost associated with complex EVAR, such that it remains unlikely (16.4%) to have an ICER of £20,000 per QALY gained or better. This finding is sensitive to variations in the complex EVAR device cost, the 30-day mortality odds ratio and the post-perioperative mortality hazard ratio. Subgroup analyses mostly suggest that there are no groups in which EVAR represented an effective use of NHS resources, when compared with open surgical repair; however, if one perioperative risk-modification model is used, the probability that complex EVAR might be optimal exceeds 50% for nonagenarian men and women aged 80–95 who have smaller AAAs.
In the population for whom open surgical repair is not a suitable option, an EVAR strategy was compared with offering no AAA repair. On the comparator arm, the individual does not undergo any surgical procedure, and therefore faces no waiting time or perioperative mortality risk. However, they continue living with an unrepaired AAA that is at risk of rupturing. The ICER for EVAR compared with this strategy was found to be £430,602 per QALY gained (Table 4), with a modest gain in QALYs (0.030) coming at a high additional cost (£13,012) per patient. No parameter had the capacity to change the conclusion about this ICER in one-way sensitivity analysis, and probabilistic sensitivity analysis showed a 0.02% probability that the ICER is £20,000/QALY or better. For the repair of complex AAAs in this population, the base-case cost–utility results showed that EVAR was clearly dominated by the ‘no intervention’ strategy. The relatively high perioperative mortality rate associated with complex EVAR, which is never offset by differences in long-term survival, causes a net loss of QALYs, while the high cost of the custom-built device leads to a high incremental cost. Here, EVAR has a 0% probability of having an ICER of £20,000 per QALY gained or better. No subgroup could be identified in which standard or complex EVAR represented an effective use of NHS resources, when compared with no intervention in people for whom open surgical repair is not a suitable option.
Table 4
NICE cost–utility model results, population for whom open surgical repair is not an option.
For detailed results, sensitivity analyses and discussion, including limitations and comparison with published analyses, please see the separate health economics appendix.
Evidence statements
EVAR compared with open repair in people for whom open surgery is considered a suitable option
Clinical evidence
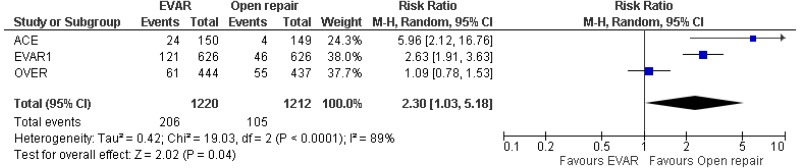
- Four RCTs provided moderate to high-quality evidence on all-cause mortality in people with unruptured AAAs in whom surgery was considered appropriate. The studies reported that:
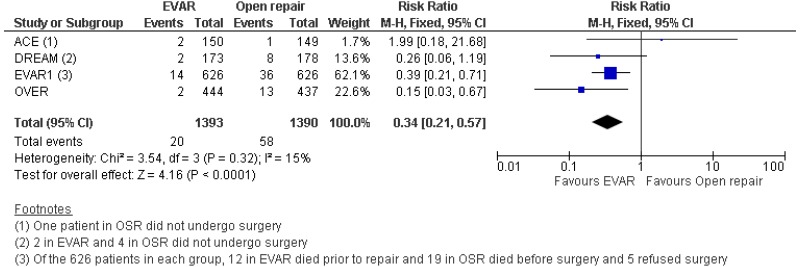
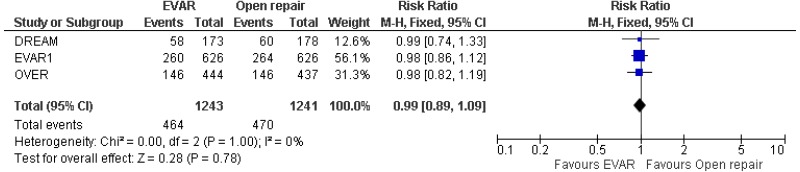
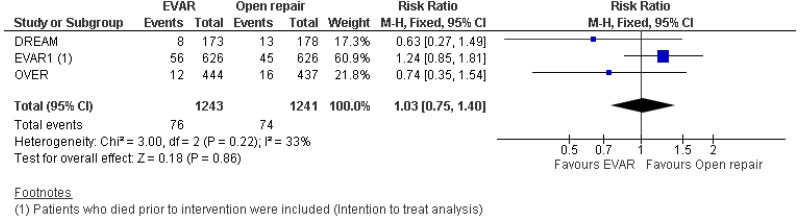
- Perioperative mortality (30-day or in-hospital) was lower with EVAR than with open surgical repair (high-quality evidence; from 4 RCTs, including 2,783 people).
- 0–6-month mortality was higher with open surgical repair than with EVAR (high-quality evidence from 4 RCTs, including 2,783 people).
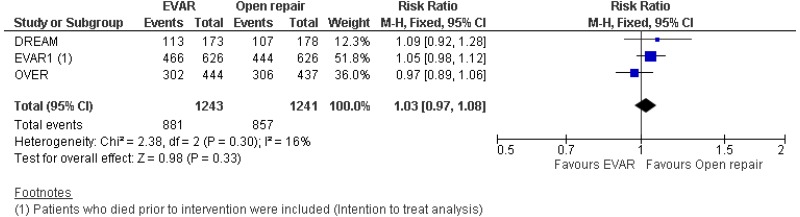
- 6-month–4-year mortality could not be differentiated between EVAR and open surgical repair (moderate-quality evidence from 4 RCTs, including 2,664 people).
- 4–8-year mortality could not be differentiated between EVAR and open surgical repair (moderate-quality evidence from 2 RCTs, including 1,665 people).
- Above 8-year mortality could not be differentiated between EVAR and open surgical repair (moderate-quality evidence from 2 RCTs, including 1,230 people).
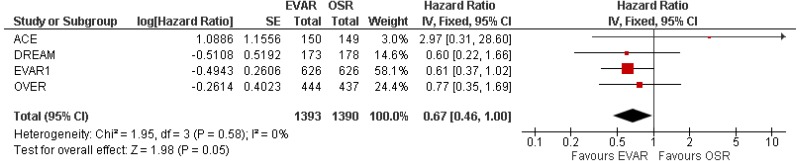
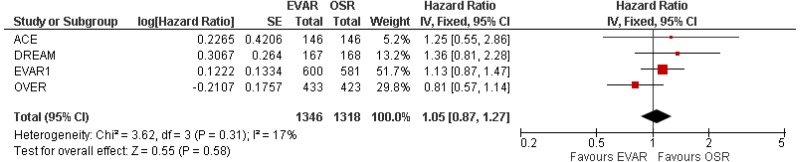
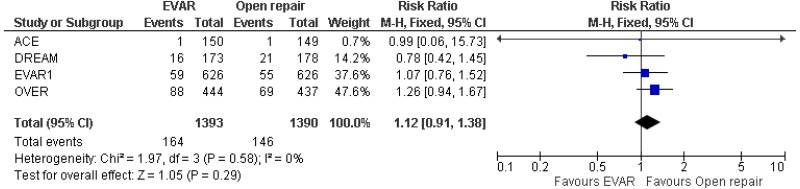
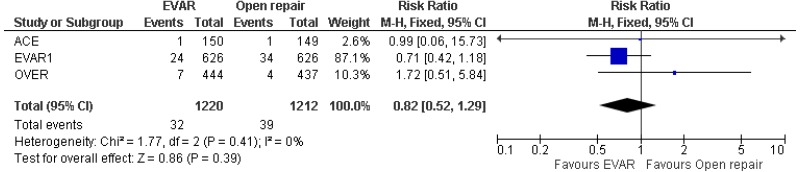
- There was no difference in 0–15-year mortality between EVAR and open surgical repair (high-quality evidence from 3 RCTs, including 2,484 people).
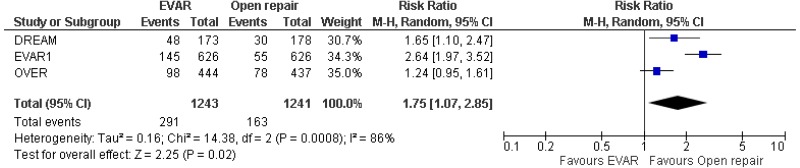
- Four RCTs provided very low- to high-quality evidence on AAA-specific mortality in people with unruptured AAAs in whom surgery was considered appropriate. The studies reported that:
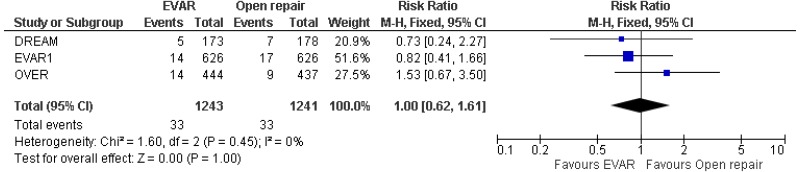
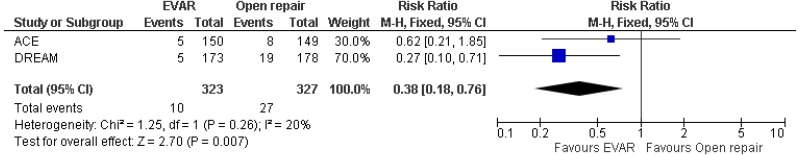
- 0–4-year AAA-specific mortality could not be differentiated between EVAR and open surgical repair (very low-quality evidence from 4 RCTs, including 2,783 people).
- 0–8- year AAA-specific mortality could not be differentiated between EVAR and open surgical repair (moderate-quality evidence from 3 RCTs, including 2,484 people).
- 0–15-year AAA-specific mortality could not be differentiated between EVAR and open surgical repair (moderate-quality evidence from 3 RCTs, including 2,484 people).
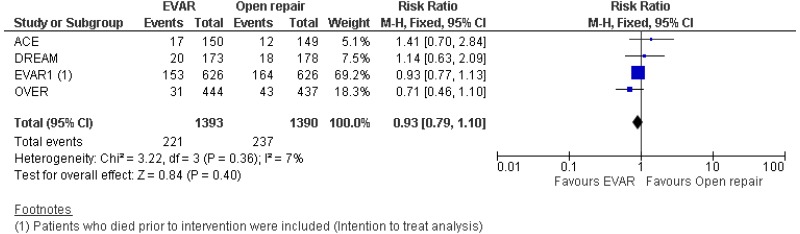
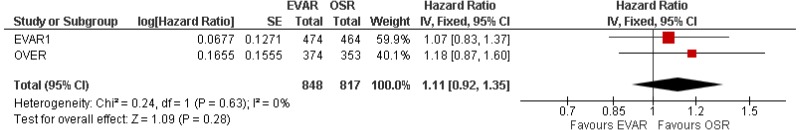
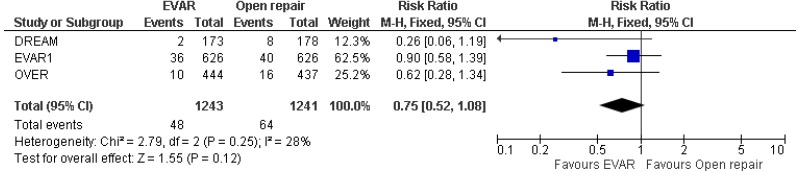
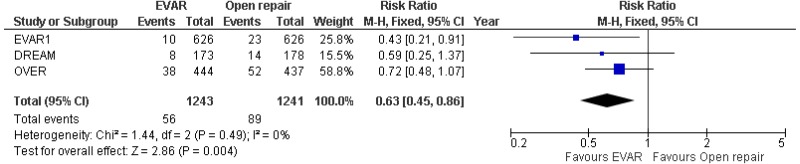
- 8–15-year AAA-specific mortality was higher with EVAR than with open surgical repair (high-quality evidence from 1 RCT including 1,252 people).
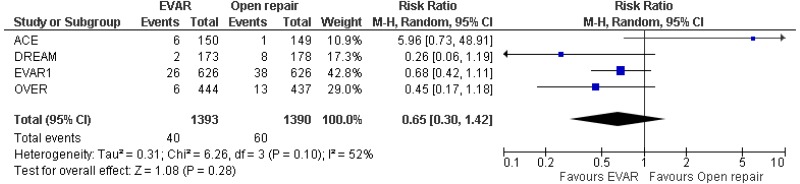
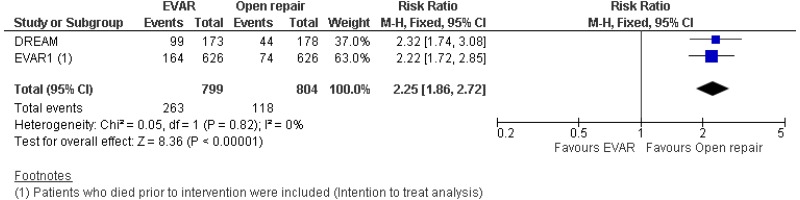
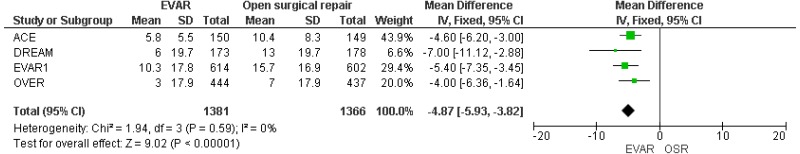
- High-quality evidence from 4 RCTs, including 2,747 people with unruptured AAAs, reported shorter length of hospital stay in patients treated by EVAR compared with those treated by open repair.
- Moderate-quality evidence from 4 RCTs, including 2,783 people, reported lower rates of pulmonary-related mortality in patients treated by EVAR than those treated by open surgery. Low- to moderate-quality evidence from 4 RCTs, including 2,783 people with unruptured AAAs, could not differentiate cardiac- and stroke-related mortality rates between patients treated by EVAR and those treated by open repair (follow-up not reported).
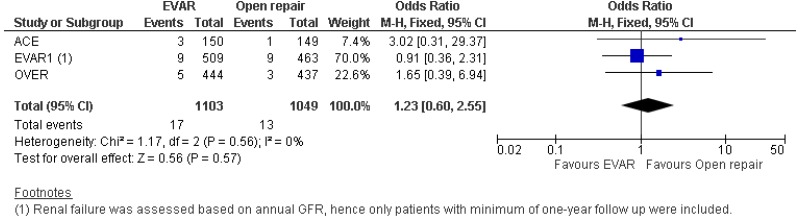
- High-quality evidence from 2 RCTs, including 2,432 people with unruptured AAAs, reported lower pulmonary complication rates in patients treated by EVAR compared with those treated by open repair (follow-up not reported). Low-quality evidence from 3 RCTs, including up to 2,432 people with unruptured AAAs, could not differentiate non-fatal stroke, sexual dysfunction and renal complication rates between patients treated by EVAR and those treated by open repair (follow-up not reported).
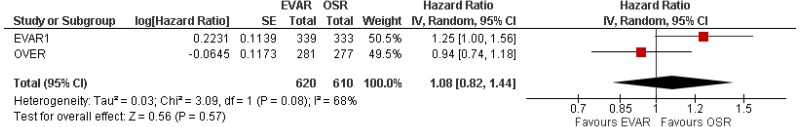
- Very low-quality evidence from 3 RCTs, including 2,484 people with unruptured AAAs, reported higher rates of any type of reintervention in patients treated by EVAR compared with those treated by open repair at 4-year and 8-year follow-up. Moderate-quality evidence from 1 RCT, including 546 people with unruptured AAA, could not differentiate rates of any type of reintervention between patients treated by EVAR and those treated by open repair between 8- and 15-year follow-up. When considering total follow-up periods, high-quality evidence from 2 RCTs including 1,603 people reported higher rates of any type of reintervention in patients treated by EVAR than those treated by open repair at follow-up of up to 15 years.
- High-quality evidence from 1 RCT, including 351 people with unruptured AAA reported higher rates of AAA-related reintervention in patients treated by EVAR compared with those treated by open repair at follow-up of up to 15 years. High-quality evidence from another RCT including up to 1,252 people with unruptured AAAs, reported higher rates of life-threatening reintervention in patients treated by EVAR compared with those treated by open repair at follow-up of up to 15 years.
- Moderate-quality evidence from 1 RCT, including 1,341 people with unruptured AAAs, could not differentiate quality of life measures (SF-36, and EQ-5D scores) between patients treated by EVAR and those treated by open repair at 2-year follow-up.
The results of a review of casemix-adjusted observational studies comparing EVAR and open repair are presented in Evidence review K2.
Economic evidence
Published evidence
- Five partially applicable cost–utility analyses with potentially serious limitations, based on data from the ACE, DREAM and EVAR-1 trials, found that EVAR was either dominated by open surgical repair, or associated with an ICER of £48,990 to £2.8 million per QALY gained. The EVAR ICER was associated with a 0% to 26% probability of being £20,000 per QALY gained or better. One of these studies, when using data from the OVER trial, found EVAR to have higher incremental QALYs and lower incremental costs than open surgical repair, with a 91% probability of its ICER being £20,000 per QALY gained or better.
NICE model
- One directly applicable cost–utility analysis with minor limitations found EVAR to produce fewer QALYs per patient at a higher cost per patient than open repair, for the elective repair of infrarenal AAAs in people for whom open repair may be an appropriate intervention. This result was robust to one-way sensitivity analyses. The ICER had 9% probability of being £20,000 or better.
Complex EVAR compared with open repair for people with complex AAAs
Clinical evidence
No randomised trials of complex EVAR compared with open repair for people with complex AAAs were identified. The results of a review of casemix-adjusted observational studies are presented in Evidence review K2.
Economic evidence
Published evidence
- No cost–utility analyses were identified in this population.
NICE model
- One directly applicable cost–utility analysis with potentially serious limitations found EVAR to have an ICER of £34,288 per QALY gained compared with open repair, for the elective repair of complex AAAs in people for whom open repair may be an appropriate intervention. The finding that EVAR is unlikely to be associated with an ICER of £20,000 per QALY or better was robust to most one-way sensitivity analyses. The ICER had a 16% probability of being £20,000 or better.
EVAR vs no intervention in people for whom open surgery is not considered a suitable option
Clinical evidence
- Low- to moderate-quality evidence from 1 RCT, including 404 people with unruptured AAAs that were considered unsuitable for open repair, could not differentiate all-cause mortality rates between patients treated by EVAR and those who received no intervention at 6-month, 4-year, 8-year and 12-year follow-up.
- Low-quality evidence from 1 RCT, including 404 people with unruptured AAAs that were considered unsuitable for open repair, could not differentiate AAA-related mortality rates between patients treated by EVAR and those who received no intervention at 6-month follow-up. Conversely, moderate-quality evidence from the same study reported lower AAA-related mortality rates in patients treated by EVAR compared with those who received no intervention at 4- and 8-year follow-up.
- Very low-quality evidence from 1 RCT, including 404 people with unruptured AAAs that were considered unsuitable for open repair, could not differentiate rates of fatal myocardial infarction and stroke-related mortality between patients treated by EVAR and those who received no intervention at 4-year follow-up.
- Low-quality evidence from 1 RCT, including 404 people with unruptured AAAs that were considered unsuitable for open repair, reported higher rates of non-fatal myocardial infarction in patients treated by EVAR than those who received no intervention at 4-year follow-up. Low-quality-evidence from the same trial could not differentiate rates of non-fatal stroke in patients treated by EVAR compared with those who received no intervention at 4-year follow-up.
- Very low-quality evidence from 1 RCT, including 404 people with unruptured AAAs that were considered unsuitable for open repair, could not differentiate quality of life measures (SF-36, and EQ-5D scores) between patients treated by EVAR and those who received no intervention at 2-year follow-up.
Economic evidence
Published evidence
- One partially applicable cost–utility analysis with potentially serious limitations, based on the EVAR-2 trial, found that EVAR had an ICER of £264,900 per QALY compared with no treatment over 8 years, with 0% probability of this being less than £20,000. A lifetime analysis with very serious limitations had an equivalent ICER of £30,274 and probability of 23%.
NICE model
- One directly applicable cost–utility analysis with minor limitations found EVAR to be associated with an ICER of £430,602 compared with no intervention, for the elective repair of infrarenal AAAs in people for whom open repair is not considered to be a suitable intervention. This result was robust to one-way sensitivity analyses. The ICER had 0.02% probability of being £20,000 or better.
- The equivalent result for the repair of complex AAAs, in an analysis with minor limitations, showed EVAR to be dominated by no intervention, with a 0% probability of its ICER being £20,000 or better.
Research recommendations
RR6. What is the effectiveness and cost-effectiveness of complex EVAR versus open surgical repair in people with an unruptured AAA for whom open surgical repair is a suitable option?
Other evidence sources
The key randomised controlled trials (RCTs) in this area are relatively old. The committee looked at more recent observational evidence, to see if changes in surgical techniques and technology have led to different outcomes. The observational studies are at high risk of bias, but their findings are broadly in line with the RCTs. They show that, while outcomes from EVAR have improved over the last 15 years, outcomes from open surgical repair have also improved by roughly the same amount. This means the difference in outcomes between the two has remained fairly constant. See evidence review K2.
Registries like the National Vascular Registry can provide a useful snapshot of current practice, and the analyses that informed the committee’s decision-making made use of data from them. However, they are not designed to evaluate the comparative benefits and harms of different surgical approaches, such as EVAR and open surgical repair. Therefore, they cannot be considered a reasonable alternative to RCT data. In addition, an analysis using the registry data showed that EVAR still did not provide greater long-term benefits than open surgical repair, and that it still has higher net costs.
The committee’s discussion of the evidence
Please note that NICE amended recommendations 1.5.1 to 1.5.6 on repairing unruptured aneurysms, after the committee’s proposed recommendations were reviewed by NICE’s Board.
Interpreting the evidence
The outcomes that matter most
The committee agreed that decision-making needs to balance the short- and long-term impacts of AAA repair. Naturally, the risk of perioperative mortality is a critical consideration that weighs heavily in the minds of people undergoing repair. However, long-term survival and the need for reintervention are also vital determinants of the overall value provided by the different approaches. This is because committee members believed that the fundamental goal of AAA repair is to ensure that people live as long as possible and have the best quality of life possible following intervention.
The quality of the evidence
Unruptured infrarenal AAA in people for whom open repair would be suitable
The committee agreed that, in cases of infrarenal AAA, the high-quality randomised evidence relating to all-cause mortality and perioperative resource-use was sufficient to demonstrate that EVAR is superior to open surgery during the first 30 days after repair.
The committee also reviewed casemix-adjusted observational evidence, to explore the commonly asserted view that these short-term benefits for EVAR, relative to open repair, will have increased in magnitude in the 15-or-so years since the RCTs recruited their participants. This evidence was judged to be of very low quality. Observational evidence is at greater risk of bias than randomised trials, because the people who receive each treatment will be systematically different in characteristics that have an independent effect on the outcomes of interest (‘selection bias’). Some included studies adopt recommended methods of adjusting for potential confounding factors; however, none of them have done this in a very rigorous way. Common issues include a failure to account for AAA anatomy among adjustment variables, limited consideration of missing data, and a failure to examine the overlap (or ‘common support’) of matched cohorts, a step that is critical to assess the validity of matching methods (see Faria et al., 2015). The review also included studies that use naive multivariable regression in an attempt to isolate the independent effect of treatment when controlling for other covariates of outcome. This is a less reliable method and, again, the studies have not been well performed for the purpose of estimating risk-adjusted differences between EVAR and OSR. Although they acknowledged the flaws of the observational evidence-base, the committee stated that it was valuable to have some data that bridged the gap between the relatively old RCTs and the current context of its decision-making.
The randomised evidence comparing the long-term effects of EVAR and OSR is also of high quality. Three of the 4 RCTs report survival and reintervention data for periods ranging between 8 and 15 years after randomisation, with few patients lost to follow-up. The committee understood that, when looking at post-perioperative survival (that is, long-term survival conditional on surviving the first 30 postoperative days), there is good evidence – not only from the appearance of relevant plots, but also from formal hypothesis testing – that a simple assumption of proportional hazards between EVAR and OSR is well supported by the data.
The committee were aware that the largest, most directly applicable RCT, EVAR-1, has been criticised in the past for focusing only on graft-related reinterventions, and not collecting data on other secondary procedures. This may introduce bias into the estimation of benefits and harms because, while EVAR reinterventions will be well covered by this approach, reinterventions that are required following OSR are much more likely to be complications of the laparotomy (for example, incisional hernia). However, the committee were also aware that the EVAR-1 investigators retrospectively obtained data on hernia interventions required following EVAR and OSR for all trial participants, using HES data and medical record review. These data were reported in the long-term follow-up reports (Patel et al., 2016; Patel et al., 2018). The committee understood that these data had been incorporated in the base-case HE model (see below).
The casemix-adjusted observational data also included several studies that report long-term survival and/or reintervention rates. The committee understood that data for analyses of these outcomes were mostly derived from published Kaplan–Meier curves, using a well validated method (Guyot et al., 2012), that had produced accurate results (as proved by the close replication of published data). Again, the limitations of the observational studies render them of very low quality; however, the committee found it helpful to have additional data to validate the findings of the RCTs, with most of it coming from a more recent time.
Unruptured complex AAA in people for whom open repair would be suitable
The committee noted that the evidence comparing complex EVAR with open surgical repair was limited in quantity and quality. No RCTs were identified and the 9 casemix-adjusted observational studies that met the eligibility criteria had nontrivial limitations. As in the infrarenal studies, there was a failure to account for AAA anatomy among adjustment variables and a failure to examine the common support of matched cohorts. In 2 of the included studies (Raux et al., 2014; Tinelli et al., 2018), the EVAR and OSR cohorts being compared derived from different institutions in different countries. The committee noted that, while the authors of these studies had carefully tried to match the patients according to their characteristics, differences between the health systems in which they received their repairs could not be controlled for. The committee thought this was particularly likely to introduce bias in measurement of resource-use, which is heavily dependent on structural and cultural factors that have relatively little to do with the results of each operation. Only 1 study reported long-term outcomes, and it was subject to this limitation; therefore, the committee were especially cautious about these results. Six of the 9 included studies are based on the same dataset (the American College of Surgeons’ National Surgical Quality Improvement Program), so care had to be taken to ensure that participants were not double-counted in analyses. One of the included studies (Michel et al., 2015) was judged partially applicable because it includes a proportion of participants (around 6%) with supradiaphragmatic thoracoabdominal aneurysms, which are outside the scope of this guideline.
Unruptured infrarenal AAA in people for whom open repair is unsuitable because of medical comorbidities
The committee considered that the single RCT (EVAR-2 trial) comparing EVAR with no intervention highlighted no differences in most outcome measures between groups; however, the study had some limitations. The committee noted that a considerable proportion (34%) of the no intervention group ultimately received EVAR. This was not taken into account by investigators in earlier publications of this study. However, the most recent publication (Sweeting et al., 2017) presented an analysis using an established statistical technique (rank-preserving structural failure time; RPSFT) to correct for any bias introduced in this way, and the committee were also aware that the original modelling undertaken for this guideline had used the same technique. Nevertheless, the committee recognised that, while plausible, the assumptions underpinning the RPSFT cannot be empirically validated.
Benefits and harms
Unruptured infrarenal AAA in people for whom open repair would be suitable
The committee agreed that, for unruptured infrarenal AAAs in people for whom open surgical repair is a suitable option, the benefits of EVAR (and the harms of open surgical repair) are concentrated in the perioperative period. Both the randomised evidence and the adjusted observational data demonstrate that people who undergo EVAR have approximately 3 times lower odds of perioperative death than people who have open repair, they have better short-term quality of life, and they also recover more quickly (which means they consume fewer healthcare resources). The magnitude of perioperative mortality benefit does not appear to have increased over time. In consultation, the committee heard from many stakeholders who assert that perioperative mortality with infrarenal EVAR has generally fallen since the RCTs were undertaken. The casemix-adjusted observational evidence shows that this is correct, but the same is also true of mortality for OSR, when it is measured in cohorts that are matched for prognostic factors with EVAR candidates. The net result is that the relative effect has remained stable over time. The committee agreed that this finding provides strong validation that the RCT evidence is a good estimate of relative effect in current practice.
Conversely, the harms of EVAR (and the benefits of open surgical repair) are found in the medium-to-long-term outcomes of this group. In the RCTs, there is no significant difference in survival between the treatments until 8 years post-surgery. After this point, open surgery yields significantly better survival than EVAR. The casemix-adjusted observational evidence suggests that the RCTs may somewhat underestimate the excess mortality that is associated with EVAR in the medium and long term. The committee speculated that this finding may come about because the RCTs all ensured relatively rigorous follow-up of people who had EVAR (with annual CTs), whereas the real-world evidence is likely to reflect a less intensive surveillance protocol. As such, it is plausible that late endograft dysfunction would be more likely to be noticed in the RCTs, giving investigators a chance to intervene before critical failure occurred.
The committee also noted clear evidence that reintervention rates are higher – approximately double – with EVAR than with open repair. This is true in both the RCTs and the observational evidence. Following consultation, the committee accepted stakeholders’ contention that reintervention rates are likely to have reduced for EVAR in the time since the RCTs were undertaken (this led them to revise the reintervention parameter of their economic model; see below). However, they remained certain that there are more secondary procedures after EVAR than there are after open surgical repair (even when laparotomy-related reinterventions are carefully considered).
Unruptured complex AAA in people for whom open repair would be suitable
On reviewing the evidence relating to complex AAA, the committee noted that there is no evidence that EVAR is associated with benefits in terms of perioperative mortality, as in infrarenal cases (although the benefits in reduced perioperative resource-use are similar). Only 1 included study reports long-term results; this suggests that, when people survive the perioperative period, those who have undergone EVAR face double the hazard of death of those whose AAA was repaired in an open operation. The committee agreed that this would be an extremely important finding, if true; however, they concluded that, owing to uncertainties in the quality of the evidence (discussed above), it is difficult to be sure whether there is any negative effect of EVAR, let alone one that big.
As the committee were unconvinced by the data relating to complex EVAR, they discussed the potential for harm if patients who could receive open repair are offered complex EVAR. Committee members agreed that it would be inappropriate to recommend the use of complex EVAR as standard practice. However, the committee noted that the data relating to open surgical repair for complex AAA are also uncertain, and so the balance of benefits, harms and costs in this population is also uncertain. To reduce this uncertainty, the committee agreed that complex EVAR should only be performed in the well-controlled environment of an RCT. As a result, a research recommendation was made to ensure that data would be collected to inform future updates of the guideline.
Unruptured infrarenal AAA in people for whom open repair is unsuitable because of medical comorbidities
Regarding infrarenal AAA in patients with medical contraindications to open surgical repair, the committee agreed that it is difficult to ignore the RCT evidence showing that intervention confers no net survival benefit for people in this group. As would be expected, managing people for whom open repair is unsuitable conservatively leads to a higher rate of rupture and AAA-related death. However, the short- and long-term risks associated with EVAR in people with this degree of comorbidity are enough to counterbalance this benefit.
Nevertheless, the committee recognised that there are challenges to the generalisability of EVAR-2 to contemporary practice, in large measure because of its deliberately non-prescriptive eligibility criteria. Therefore, the committee agreed that it would be valuable to generate new high-quality research in this area and made a research recommendation noting that such a study would be helpful.
Unruptured complex AAA in people for whom open repair is unsuitable because of medical comorbidities
In the absence of evidence relating to complex EVAR in patients with medical contraindications to open surgical repair, the committee considered evidence from other AAA patient populations (alongside original health economic modelling; see below). Having seen convincing evidence that, when compared with no intervention, standard EVAR does not represent a reasonable balance of benefits, harms and costs for people with infrarenal AAA, the committee agreed that the most optimistic expectation possible is that EVAR outcomes would be no worse in people with complex AAAs. The more likely outcome is that they will be substantially worse, owing to higher perioperative mortality. Moreover, while it is inconceivable that there would be additional benefits for this population, compared with the infrarenal group, it is certain that complex EVAR grafts cost more than standard EVAR grafts (see below). Therefore, while the committee discussed whether research was warranted in this area, they decided that it would be unethical to randomise people to an expensive intervention that is known to have a high risk of perioperative mortality, when there is no realistic prospect of long-term benefits that would justify the costs.
Cost effectiveness and resource use
Unruptured infrarenal AAA in people for whom open repair would be suitable
The committee discussed the cost-effectiveness evidence for the repair of unruptured infrarenal AAA. The committee were aware that this population, for whom open surgical repair is a suitable option, comprised the majority of both clinical and published economic evidence for this review question. The committee agreed that the published UK economic evidence could only reasonably be interpreted as evidence that EVAR was not likely to be an effective use of NHS resources, though it was noted that none of the studies included the longest-term follow-up that is currently available, namely 15 years of data from the EVAR-1 trial. The committee therefore considered evidence from the new economic model developed for this guideline.
The committee were satisfied with the modelling approach of: (1) using National Vascular Registry data to inform baseline perioperative mortality, and the results of a Cochrane meta-analysis to inform relative effects; (2) estimating long-term survival by calibrating general population mortality to the EVAR-1 open surgical repair data conditional on surviving for 30 days after the intervention; and (3) estimating relative long-term survival using a hazard ratio from a meta-analysis of long-term data from 3 RCTs (DREAM, EVAR-1 and OVER). The committee agreed that the new economic model provides compelling evidence that EVAR is not a cost-effective option for infrarenal AAA compared with open surgical repair. The base-case model results indicate that EVAR produces fewer QALYs than open surgery at a higher total cost to the NHS and PSS, and this is reflected in the probabilistic results, with a low probability of its ICER being £20,000 per QALY gained or better. Results were also robust to scenario and one-way sensitivity analyses, including using only EVAR-1 study data.
The committee discussed the cost results from the new model, and agreed that the high acquisition cost of EVAR was likely to be the key cost difference between EVAR and open surgery in practice. It advised that the modelled cost of complications following EVAR appeared low compared with clinical experience. However, it was agreed that any increase in EVAR complication costs would strengthen the cost-effectiveness results in favour of open surgical repair, and would therefore not affect interpretation of the evidence. The committee also discussed the cost of routine monitoring following EVAR and advised that, in practice, adherence to scheduled monitoring following EVAR is less than 100%. The committee discussed the implications of this on the cost-effectiveness evidence. It agreed that, although the expected cost of ongoing monitoring per patient may be lower than the model predicts, this would be counteracted to some degree because people who fail to attend scheduled scans may be more likely to experience complications that require reintervention. The committee also saw that the model conclusion did not change when assumptions were applied that were favourable to EVAR, but highly implausible, such as assuming monitoring appointments following EVAR incur no cost, or that no post-EVAR complications occur. It was therefore agreed that, while the effect of non-adherence to follow-up appointments on EVAR cost-effectiveness results is unclear, it cannot plausibly be sufficient to change conclusions drawn from the new economic model.
The committee discussed the use of the RCTs to inform much of the new model, noting that a potential criticism of the model is its use of the relatively old evidence. They noted that more recent casemix-adjusted observational evidence has closely comparable results, with no evidence that the relative difference between the approaches has changed over time (though both have got better). They agreed that this strongly validates the model’s base case approach.
When feedback was sought from stakeholders during consultation on draft guidance, a common suggestion was that, instead of using RCT data for perioperative mortality, the model should make use of current data from the National Vascular Registry (NVR). The committee discussed and firmly rejected this idea. They agreed that the NVR is probably a relatively faithful snapshot of prevailing practice; however, this means that it faithfully reflects deeply ingrained selection habits, and no NVR data available to the committee make any attempt to adjust for these. The committee also expressed concern that there is a very high risk of reporting bias in the data that get submitted to the registry (for example, which AAAs get classified as ‘infrarenal’ is very likely to vary depending on the type of repair attempted). They also noted that their concerns were validated by the review of casemix-adjusted observational evidence undertaken for this guideline. Among the 38 studies reporting perioperative mortality that attempt to provide balanced cohorts (either by randomisation or by risk-adjustment), only 1 small study has ever found that EVAR is associated with a perioperative mortality benefit of the magnitude implied by unadjusted NVR data. Therefore, the committee were convinced that it would be inappropriate to use these data for their base-case health economic model. Despite these misgivings, the committee were interested to see a sensitivity analysis in which the NVR mortality data were used. This showed that EVAR would be associated with an ICER of over £55,000/QALY.
However, there were some areas in which the committee received feedback from stakeholders during consultation that they found more persuasive, and they agreed that it was appropriate to revise the model to take advantage of more recent data. In particular, they accepted that the rate of reintervention following EVAR procedures has fallen over time. This may be, as many people claim, because endograft technology has become more durable over time. However, the committee also considered it important to note that, over this period, knowledge has developed regarding which graft complications demand revision and which can be left alone. In order to reflect this change, the model was revised to adjust the RCT data using evidence from an Italian before–after study cited by multiple stakeholders, which compares results with older-generation stent-grafts (as used in the RCTs) with more recent technology (Verzini et al., 2014).
The committee also acknowledged a common stakeholder argument that, compared with when the RCTs were undertaken, people undergoing EVAR now spend much less time in hospital in general and in critical care in particular. The committee agreed that this corresponds with their experience, too. They acknowledged that the HE modelling supporting their decision-making should ideally reflect the resource use that would be expected if the decision being simulated were adopted in present-day NHS practice. Therefore, the committee accepted a suggestion from several stakeholders that, instead of the RCTs, the model should rely on the most recent resource-use data from the NVR. The committee expressed significant misgivings about this: there is no reason to suspect that the selection biases that made them unwilling to rely on the NVR for perioperative mortality data would pose any less of a risk to resource-use data, even though the attraction of a current data source with good coverage of UK NHS activity is obvious.
In the event, this discussion was moot for infrarenal AAAs, as the NVR data are very closely comparable with the results from the EVAR-1 RCT on which the consultation draft placed reliance. The NVR data suggest that the average person undergoing EVAR spends 2.95 fewer days in critical care than the typical OSR candidate, and 6.19 fewer days in hospital overall. The equivalent differences in the EVAR-1 RCT were 2.93 days and 6.00 days. From this, the committee understood that, although perioperative length of stay has certainly decreased for people undergoing EVAR, it has decreased an almost identical amount for OSR.
The committee discussed the QALY outcomes of the model, recognising that incremental QALYs were fairly small in absolute terms (equivalent to around 3 weeks of perfect health), and the point estimate was more uncertain than for incremental costs. However, the unequivocal high incremental cost associated with EVAR led the committee to agree that the ‘true’ QALY gain for EVAR would need to be implausibly high for EVAR to be cost effective compared with open surgery (via, for example, superior long-term survival in EVAR patients, counter to the available long-term evidence). To achieve an ICER of £20,000 per QALY gained, EVAR would need to generate 0.146 QALYs more than open surgery per patient, compared with a base-case estimate of 0.056 QALYs less than open surgery. The committee were aware that modelled and empirical survival curves crossed over, with a longer-term survival benefit associated with open surgical repair offsetting its worse perioperative outcomes. The committee saw that the model suggests open surgical repair is increasingly cost-effective in younger patients, and agreed that this was consistent with its expectations, as younger people will typically be more likely to survive the open surgery procedure and experience the long-term survival benefit.
The committee discussed whether the cost-effectiveness evidence suggested that there may be differences in the balance of benefits and harms in people with different characteristics. They reviewed a series of subgroup analyses in which cohorts with different age, sex and AAA diameters were simulated. None of the preferred ICERs were qualitatively sensitive to these factors. The committee therefore determined that there was no identifiable subgroup for whom EVAR represents a reasonable use of NHS resources, so its recommendations were appropriate to the relevant population as a whole.
The committee also discussed whether the cost-effectiveness results for EVAR might be influenced by a person’s underlying life expectancy. In particular, if it were possible to identify individuals who are less likely to live to experience the long-term survival benefit associated with open surgical repair, might EVAR be a cost-effective intervention for those people? A threshold analysis was conducted in which the hazard ratio used to calibrate general population survival to ‘match’ the EVAR-1 population was varied between 1 and 15. These values indicated a relatively healthy population with a mortality hazard equal to the general population of the same age, and an extremely unhealthy population with mortality hazard of 15-times the general population, respectively. Across this range of underlying life expectancies, EVAR remained associated with ICERs substantially worse than £20,000 per QALY gained, when compared with open surgical repair.
Unruptured infrarenal AAA in people for whom open repair is unsuitable because of medical comorbidities
The committee then considered the cost-effectiveness evidence for infrarenal AAA repair in people for whom open surgical repair is not a suitable option due to medical comorbidity. This evidence comprised 1 published, UK cost–utility analysis, and modelling conducted for this guideline. The committee were aware of the extensive trial crossover that occurred in EVAR-2, from the ‘no intervention’ control arm to EVAR, and that its per-protocol analysis breaks trial randomisation in a way that is likely to bias in favour of EVAR (as it can be expected that participants who ‘crossed over’ to receive EVAR were the fittest members of the cohort, with the longest life expectancy). The committee therefore placed greater emphasis on the economic model, which adjusted for crossover using a well established statistical method (RPSFT). These data did not show any long-term survival benefit for EVAR compared with no intervention. The committee explained that many people with AAAs die with – rather than from – their aneurysms, and this would be particularly true in a population which is defined by the presence of comorbidities that are invariably life-limiting.
The committee advised that, since the population for which open surgical repair is unsuitable is defined by substantial medical comorbidity, the most appropriate analysis uses calibrated general population life tables at 1999–2001 levels; not inflating the analysis to 2015–16 lifetables, which would reflect a general increase in the health of the UK population. The committee then discussed its preferred base-case ICER for EVAR, which exceeded £430,000 per QALY gained compared with ‘no intervention’, and agreed that this indicates EVAR for this population is not an effective use of NHS resources. The committee also understood that variation of parameters to extreme values – for example, assuming no survival differences beyond 5 years, and assuming there are no EVAR graft complications at any time – do not cause the ICER to fall to a level that would be considered to represent good value for money. To achieve an ICER of £20,000 per QALY gained in this population, compared with providing no intervention, EVAR would need to generate 0.651 incremental QALYs per patient, compared with a base case estimate of 0.030 QALYs.
In consultation feedback, some stakeholders expressed uneasiness about the possible effects of crossover in EVAR-2, and raised reasonable objections about the use of the RPSFT method to adjust for it. In response to this, an extreme-case sensitivity analysis was performed, assuming that everyone who crossed over from the no intervention arm to the treatment arm of EVAR-2 would have died immediately had they not done so. This found that EVAR would be associated with a QALY gain of 0.691 and an ICER of £18,314/QALY under this extreme assumption. The committee agreed that the fact that this totally implausible scenario produces an ICER that is only just below £20,000/QALY is a very strong indication that the ‘true’ ICER must be very much higher, with no realistic prospect of representing an effective use of NHS resources.
The committee discussed whether the cost-effectiveness evidence suggests that there may be differences in the balance of benefits and harms between men and women, and older and younger people. None of the preferred ICERs were sensitive to the sex of the cohort; nor were they sensitive to differences in age or AAA size. The committee therefore determined that there was no identifiable subgroup for whom EVAR represents a reasonable use of NHS resources, so its recommendations were appropriate to the relevant population as a whole.
The committee discussed whether living with an unrepaired AAA may cause psychological morbidity for people who are not offered repair. They noted that there is no evidence of this: the EVAR-2 RCT found no significant differences in EQ-5D between people receiving EVAR and those randomised to no intervention; nor was there a detectable effect on the SF-36 mental component summary score.
Committee members reported that some patients are relieved to learn that they do not have to undergo intervention. They agreed that the information patients are given is critical. Some clinicians inform patients that they have a ‘ticking time-bomb’ inside them. However, as the people for whom surgical repair is inappropriate are subject to comorbidities that are inevitably life-limiting, it is important to provide a realistic appraisal of the competing hazards they face. The committee agreed that it would be good practice to advise people in this situation that the EVAR-2 trial showed no overall survival benefit for people receiving EVAR.
For all these reasons, a negative impact of living with an untreated AAA was not included in the base-case economic model. However, the impact of a large lifetime utility decrement of 0.1 was explored in sensitivity analysis. The ICER for EVAR compared with no intervention remained worse than £30,000/QALY. The committee agreed that this demonstrates that no plausible level of disutility could be enough to counterbalance the harms and costs associated with EVAR.
Unruptured complex AAA in people for whom open repair would be suitable
The committee discussed the cost-effectiveness evidence for the repair of unruptured complex AAA. The committee agreed that here the term ‘complex’ has a broad meaning, generally referring to non-standard AAA repairs. Typically, a complex AAA is one for which a standard EVAR device cannot be used within the terms of its instructions for use (IFU), and a complex device is one that is custom made, requiring bespoke adaptations, such as fenestrations and branches. The committee agreed that optimal decision-making for this population would be based on detailed analysis of reliable data subdividing people according to types of complex aneurysm and repair. However, with the possible exception of fenestrated EVAR (fEVAR; see below), there is a critical dearth of specific evidence in this area. Therefore, in the absence of data enabling focused analysis on different types of complex AAA, the committee agreed that it would be of value to explore more general evidence which combines experience with various types of complex AAA repair.
The committee were aware that there is no randomised comparative evidence evaluating EVAR and open repair for complex AAA. They understood that there are 2 broad approaches that can be used to estimate cost–utility results in the economic model. The first approach, which constitutes the base-case model, relies on a degree of assumption regarding the transferability of data on infrarenal AAA. The committee advised that, once a person has survived to 30 days after their intervention, survival thereafter is expected to be relatively similar to people with repaired infrarenal AAA. On this basis, the use of data for infrarenal AAA to model long-term survival was agreed to be a reasonable approach. The second possible approach would be to rely on lower-quality data from the directly applicable population, as identified in the review of casemix-adjusted observational data. This approach was pursued as a sensitivity analysis.
The committee were aware that the bespoke nature of many complex EVAR devices has implications for obtaining reliable unit costs. However, they were satisfied that an average cost obtained from 3 NHS Trusts was likely to adequately reflect a typical UK cost, significantly in excess of the cost of a standard EVAR device. Only one ‘off-the-shelf’ complex endograft appears in the NHS Supply Chain Catalogue – a fenestrated anaconda device manufactured by Vascutek. The costs of this graft are similar to those estimated from the committee data (although cases with more than 2 fenestrations cost somewhat more).
The committee reviewed the ICERs predicted by the base-case economic model for the repair of unruptured, complex AAA. The committee noted that EVAR was associated with more net QALYs than open surgery in this population, as it is predicted – using a relative effect generalised from the infrarenal setting – to have a larger absolute perioperative survival benefit than in the infrarenal population, owing to the higher underlying risk of surgery, in these patients. This means fewer patients are expected to survive to experience any long-term survival benefits of open surgery. The committee agreed that these results were plausible, though less certain than in the unruptured infrarenal population, because of the lack of directly applicable clinical evidence. However, they agreed that the magnitude of these uncertain benefits were unlikely to be sufficient to outweigh the unambiguous additional costs associated with complex EVAR compared with open surgical repair, as reflected in a base-case ICER of over £34,000 per QALY gained and a very small probability of the true figure being £20,000 or better. To achieve an ICER of £20,000 per QALY gained, complex EVAR would need to generate 0.485 QALYs more than open surgery per patient, compared with a base case estimate of 0.284 QALYs.
The committee advised that the 30-day mortality rate reported in the NVR for open repair in this population (18.4%) is very high compared with what would be expected for the open repair of an ‘average’ complex AAA in practice. They thought that the estimate for EVAR (3.5%) is more representative of current practice. They agreed that this discrepancy reflects substantial selection bias: they suggested that, in many units, the only cases that are offered open repair are those that are so anatomically complex that an endovascular approach is impossible, and it is unsurprising that those cases would be subject to a very high risk of perioperative death. The committee noted that this view is shared by the authors of the NVR report, who comment that ‘direct comparison of [EVAR versus open repair] figures is difficult and the open procedures may represent a more complex anatomical AAA to repair.’
Furthermore, the committee agreed that the weaknesses of the NVR data are likely to be exacerbated by substantial reporting biases. They suggested that the kinds of anatomical features that constitute ‘complexity’ vary between the approaches and are very likely to have been reported differently in returns to the NVR. For example, there are cases that, in an open operation, can be accomplished with an infrarenal cross-clamp, whereas the same anatomy would require a fenestrated EVAR graft, rendering the case ‘complex’ for EVAR. Biases like this would lead to the infrarenal and complex NVR results looking worse for open repair than for EVAR.
In this way, the committee concluded with confidence that, due to the selection and reporting biases underlying the NVR data, a cost-effectiveness analysis using the reported complex repair perioperative mortality rates directly would not provide a meaningful comparison of EVAR and open surgical repair. Rather, the preferred approach was to take the EVAR NVR data as the baseline mortality rate – as it more closely reflects clinical experience than the open surgical repair value – and then apply a measure of relative effect to this, derived from (infrarenal) RCT evidence, to estimate the mortality rate for open surgical repair.
The committee discussed other assumptions applied in the model. They were mindful that the use of NVR data to estimate perioperative resource-use almost certainly biased the model in favour of EVAR (for the reasons referred to above: there is no reason to suspect that the selection biases that made the committee unwilling to rely on the NVR for perioperative mortality data would pose any less of a risk to resource-use data).
The committee also discussed complication rates used in the model. They agreed that complex AAA repairs are likely to be more susceptible to subsequent complications and reintervention than infrarenal aneurysm repairs. The committee noted that a scenario analysis had been included in the model that applied a complication rate double that of infrarenal repair, and that this led to a notable increase in the ICER for EVAR versus open repair.
The committee saw that, when the new model is configured to use best-available data that are specific to complex AAAs from casemix-adjusted observational evidence, EVAR becomes massively dominated by open repair (with a lifetime health loss of more than 1.6 QALYs).
The committee also reviewed the results of an analysis that had attempted to estimate cost–utility results for fEVAR, as a specific subtype of complex AAA. This suggested that fEVAR is dominated by OSR, with somewhat worse net health effects (0.095 QALYs worse than OSR), but substantially higher costs (slightly more than £10,000), even though fEVAR benefits from the likely biased estimate of perioperative resource use for complex AAA from the NVR. They understood that the model’s strongly negative conclusion has been shared by other authors who have attempted to analyse the cost effectiveness of fEVAR compared with open repair (Michel et al., 2015, 2018; Ciani et al., 2018).
The committee were satisfied that the new model provides a reasonable prediction of the likely cost-effectiveness of EVAR in people with a complex unruptured AAA for whom surgical repair is a suitable option. However, they were cautious about the lack of directly applicable, randomised comparative evidence underlying the model, as this increases uncertainty regarding the true ICER for EVAR in this population. The committee were also mindful that the model had plausibly demonstrated that the benefits of complex EVAR may outweigh its harms, albeit at a cost that was very unlikely to be justified by any gains. The committee therefore made a recommendation that the use of EVAR in this population should be limited to the context of an RCT (that should include resource-use in its data collection), to ensure that any use of EVAR in this population provides direct, comparative clinical effectiveness and cost-effectiveness evidence. Mindful of the heterogeneity of the ‘complex’ AAA category, they added the stipulation that this research should be stratified in a way that will help to reveal any differences in the balance of benefits, harms and costs between EVAR and OSR according to AAA anatomy (at least distinguishing between juxtarenal, pararenal and suprarenal AAAs).
Unruptured complex AAA in people for whom open repair is unsuitable because of medical comorbidities
The committee then discussed complex AAA repair in people for whom open surgical repair is not a suitable option, because of concerns regarding medical comorbidity. The committee agreed that outcomes associated with complex EVAR would certainly be no better than infrarenal EVAR, and would probably be worse, whereas outcomes in complex AAA patients who receive no intervention are not likely to be different to infrarenal AAA patients who receive no intervention. The committee were also aware that bespoke EVAR devices for complex repair are more expensive than standard EVAR devices for infrarenal repair, and that the ICER for infrarenal AAA repair in this population was £430,602 per QALY gained. The committee therefore agreed that complex EVAR will be more expensive than standard EVAR and will provide health outcomes that are at best equivalent and at worst substantially less favourable, meaning there is no possibility that EVAR could be cost effective in this population compared with a strategy of ‘no intervention’. This result is clearer than in people with complex AAA for whom open surgery is a suitable option, where the base-case ICER for EVAR compared with open surgery was £34,288/QALY (described above). In this population, it is feasible that EVAR may be more likely to be cost-effective than in infrarenal cases, because AAA complexity also worsens the expected outcomes from open surgery.
The committee were aware that there is no published cost-effectiveness evidence in this population, and so the only evidence was from the economic model developed by NICE. The base-case model found EVAR to be dominated by a strategy of ‘no intervention’, though the committee recognised that the analysis had necessarily been informed by some assumptions, such as generalising long-term survival data from the EVAR-2 population, and low-quality data, namely estimating a ‘complexity effect’ from the National Vascular Registry. The estimated EVAR perioperative mortality rate of 42% was felt to be much higher than observed in clinical practice; therefore this analysis was deemed to be more speculative than the infrarenal AAA repair analyses conducted for this guideline. However, the unequivocal result of EVAR being dominated was seen to be supportive of the committee’s view that complex EVAR cannot be cost effective in this population. The committee therefore made a strong recommendation against the use of EVAR in people with a complex unruptured AAA for whom surgical repair is not a suitable option.
The committee considered whether the cost-effectiveness evidence suggests there may be differences in the balance of benefits and harms between men and women, for the elective repair of unruptured complex AAA. None of the preferred ICERs from the modelling were sensitive to the sex of the cohort; nor were they sensitive to differences in age or AAA size. The committee therefore determined that there was no identifiable subgroup for whom complex EVAR represents a reasonable use of NHS resources, so its recommendations were appropriate to the relevant population as a whole.
Other factors the committee took into account
Equality considerations
The committee agreed that the effectiveness and cost-effectiveness evidence discussed above was compelling. However, they were also mindful of their responsibility to consider the broader context of their decision-making. NICE’s Social Value Judgements stipulate that ‘Decisions about whether to recommend interventions should not be based on evidence of their relative costs and benefits alone. NICE must consider other factors when developing its guidance, including the need to distribute health resources in the fairest way within society as a whole’ (Principle 3). Accordingly, the committee gave careful consideration to factors that might lead them to depart from decision-making that simply seeks to maximise population-level QALYs.
In particular, the committee explored whether limiting access to EVAR would result in any unfairness to identifiable groups of people with AAA.
For the comparison of EVAR and OSR, it is commonly asserted that access to EVAR is most vital for people with higher baseline risk of perioperative mortality (that is, the oldest and most comorbid). However, the committee were aware that some evidence suggests the opposite. In a subgroup analysis of the EVAR-1 cohort, Brown et al. (2007) found that EVAR only confers a perioperative survival benefit, compared with OSR, in people judged to benefit from ‘good’ fitness. In OVER, Lederle et al. (2012) found that younger participants had a significant benefit from EVAR whereas older people did not (indeed, the results were very nearly reversed).
The committee also discussed the comparison of EVAR and no intervention, in people for whom OSR is not a suitable option, in this context. It might be argued that, because the population in question is – by definition – sicker and likely to be older than the average person with AAA, it is unfair to deny such people repair, when younger, fitter people have access to OSR. The committee did not agree with this argument. They noted that their primary reason for not recommending EVAR, in this populationwas that there is no evidence that it results in better outcomes. Therefore, it cannot be said that anyone is being denied a meaningful benefit. On the contrary, the committee agreed that the many people with AAA and life-limiting comorbidities who are currently receiving EVAR are being inappropriately exposed to risk (and clinicians are imposing unnecessary opportunity costs on the NHS by doing so).
The committee discussed any potential differences between postoperative outcomes of EVAR between men and women. They agreed that, although the majority of the evidence presented was in men, the issue of whether a different balance of benefits, harms and costs could be expected in women was explored in the original economic model. These analyses found no evidence of any subgroup effects of a sufficient magnitude to overturn the results in the wider cohort. Similarly, regression analyses based on the Vascunet dataset suggest that female sex is a greater risk factor for people undergoing EVAR than it is for people having OSR (Mani et al., 2015; Budtz-Lilly et al., 2017).
Therefore, the committee were content that their recommendations did not induce any particular inequality with respect to sex, so no recommendations were made that were specific to women.
Patient choice
The committee discussed their responsibility to provide guidance that acknowledges individual patients’ differing preferences at length. The committee advised that patients often express a preference for EVAR compared with open surgical repair, typically due to the increased short-term risks associated with open surgery. In response to the consultation draft of this guideline, stakeholders made the committee aware of a small amount of research formally eliciting patient preferences regarding EVAR and open surgery (Winterborn et al., 2009; Reise et al., 2010; Faggioli et al., 2011). This evidence suggests that, when offered a choice, people tend to express a preference for EVAR over OSR. However, the committee noted that – as would be expected, given the state of available evidence at the time they were undertaken – none of these studies provided participants with information about long-term outcomes with EVAR and OSR, and certainly none referred to an excess hazard of mortality being associated with EVAR, for people who survive the initial operation. Since the committee found the evidence that there are differences in long-term survival expectation convincing, they felt this had to be a critical consideration in weighing up the benefits and harms of EVAR and OSR. Accordingly, they agreed that the results of these studies were of limited relevance to the present-day decision problem. The committee heard that, to the extent that a stated preference for EVAR reflects the priority people place on short-term benefits over long-term risks, this had been captured in the model by making use of evidence showing a larger quality of life decrement following open surgery, compared with EVAR, and by discounting health outcomes over time. The committee were also aware of evidence showing that AAA patients put much more weight on future outcomes than surgeons (Dion et al., 2017). The committee noted that, while individual choice is important in all care provided by the NHS, this did not compel them to recommend care that is not cost effective, as per Principle 5 of NICE’s Social Value Judgements. Given this, and based on its assessment of the evidence from the new economic model (and other published economic evaluations), the committee made strong recommendations that people with an unruptured infrarenal AAA for whom open surgical repair is a suitable option should be offered open surgical repair, and that EVAR should not be offered in such cases. They agreed that it would be to the benefit of the average candidate for elective AAA repair if the vascular community adopted a broader focus that puts appropriate weight on medium-to-long-term outcomes, rather than concentrating on the perioperative period.
Symptomatic AAA
The committee discussed whether it was necessary to specify AAA symptomatology in their recommendations. Although none of the RCTs included participants with symptomatic AAAs, it was noted that several of the studies identified in the review of casemix-adjusted non-randomised evidence include symptomatic (or ‘emergent’) cases. Among these, only 1 reports results for symptomatic cases though, helpfully, that is one of the few UK studies in the dataset. In univariable analysis across EVAR and OSR, Choke et al. (2012) found that symptomatic AAAs may be associated with a higher risk of perioperative death; however, at a 95% confidence level, the data are comfortably consistent with no difference (OR=1.94 [0.64 to 5.95]). The committee were not aware of any data exploring the possibility of interaction between symptomatic status and repair approach, which would be necessary to inform any specific recommendations regarding the relative benefit of EVAR and OSR, in these patients. However, as noted above, many of the studies included in the review of observational data included emergent cases, and the fact that pooled results from these studies are closely comparable to results from RCTs provides some validation for the committee’s view that the balance of benefits and harms is unlikely to be very different in such cases.
Feasibility of randomised research
As the committee had concluded that the best way to resolve uncertainty regarding the benefits, harms and costs of complex EVAR would be to limit such activity to a randomised trial, they were obliged to discuss the feasibility of such research in detail. In consultation, multiple stakeholders expressed the view that such an RCT would not be considered ethical by the vascular community, mostly because of a perceived lack of equipoise (with complex EVAR thought to be the superior option). This perception is predominantly based on unadjusted figures from the NVR, which the committee rejected as being completely unrepresentative of the results that would be expected if cases were selected at random (see above). Moreover the committee agreed that the uncertainty about the long-term effects of complex EVAR is substantial enough that, even if it could be shown that complex EVAR is associated with a large reduction in perioperative mortality, there should be real equipoise about whether any such effect translates into net health gain over a patient’s lifetime. The 1 study in the review of casemix-adjusted observational evidence that reported post-perioperative data reflected a substantial benefit for open repair over EVAR for complex AAAs. If estimates such as these were to prove accurate, open repair would be the superior approach even if the unadjusted, almost certainly biased NVR perioperative numbers were true. In this context, it is extremely difficult to see how a RCT could be considered unethical on the grounds that complex EVAR is unimpeachably superior.
The committee also noted that, if the community will tolerate a learning curve when introducing new technologies, it would be perverse not to see the benefit in empowering the workforce to provide a higher long-term standard of care when the ‘innovation’ that is required is for surgeons to refamiliarise themselves with older techniques.
In a related way, consultation elicited stakeholder feedback suggesting that a well designed registry might provide an adequate alternative to an RCT. In several cases, the NIHR-funded UK-COMPASS study, which is currently collecting observational data on juxtarenal aneurysms, was highlighted. The committee’s view was that, as current practice is subject to strong prior beliefs about the relative benefits and harms of EVAR and OSR for complex AAA, randomisation is critical to provide an unbiased estimate of comparative effectiveness. Stakeholders assert that a randomised trial is unfeasible, because the vascular community has a strong consensus that EVAR is superior to OSR, which is reflected in a strong preference to offer EVAR wherever possible. However, if this is true across the vascular community, there can be no expectation that observational evidence of current practice will provide a valid basis on which to compare EVAR and OSR for complex AAAs, no matter how carefully it is collected or how rigorously it is analysed. It is only where there is a good degree of overlap between the types of people who receive different treatments that it is even theoretically possible to isolate the independent effect of treatment on outcomes (see Faria et al., 2015). If prevalent attitudes lead to OSR being offered to a small, highly selected group of people with complex AAAs, who have little in common with the people who receive complex EVAR, it will not be possible to estimate a counterfactual, and the beliefs that led to the selection cannot be validated or disproven.
Some stakeholders also suggested in consultation that the numbers of people requiring complex repair are too low to make recruitment to a trial possible. The committee noted that the NVR reports over 2,000 complex procedures in the last 3 years, and agreed that this suggests that any such concerns are overstated.
Implementation challenges
The committee recognised that the recommendations represent a substantial change to practice and some resistance to change may be encountered. The committee were under no illusion regarding the perioperative mortality risks associated with EVAR and OSR: it is inarguable that the latter has significantly greater odds of death, probably around a threefold increase. However, the committee felt certain that it should be possible to optimise systems so that OSR, as well as EVAR, is associated with a low absolute risk of mortality. They firmly disagreed with stakeholders who suggest that returning to an OSR-led approach to AAA repair will inevitably lead to perioperative mortality levels that were seen before EVAR became the predominant mode of repair. They noted the impact of the Vascular Society’s AAA Quality Improvement Programme, the provisions of which raised standards in EVAR and OSR alike. The introduction of the National AAA Screening Programme, starting in 2008, has also led to many AAAs being diagnosed at a smaller diameter and at a younger age than would be the case if they had been left to present symptomatically or incidentally; this will also have contributed to lower perioperative risk for both procedures. The committee also argued that many general improvements in patient care have had beneficial impacts for the perioperative survival of people undergoing both EVAR and OSR. Factors such as improvements in imaging technology, better cardiovascular risk management (including increasingly widespread use of statins), improved prevention and treatment of nosocomial infections would all contribute to reducing perioperative mortality across the board. The committee noted that the 2 most recent datapoints in the supplementary review of casemix-adjusted observational evidence report perioperative mortality rates of 0.6% and 0.5% for OSR (Sugimoto et al., 2017 and Symonides et al., 2018). They considered the latter figure an especially attractive target, as it comes from a recent, countrywide database of publicly funded practice in Europe (Poland). In view of these features, the committee saw no reason why the NHS should not aspire to a similarly low level of perioperative mortality.
The committee also thought about a number of practical implementation challenges that may be encountered.
First, the committee acknowledged that the predominance of endovascular techniques for most unruptured AAAs, in recent years, could mean that the skills-mix of the current vascular workforce is ill-equipped to deal with a rebalancing of activity in favour of open repair. The committee were unconvinced by this argument. They expressed the view that modern vascular units should be competent to provide the necessary volume of open repair of AAA, and cited a small amount of evidence that supports this view (Beiles et al., 2016; Modrall et al. 2011). They also noted that the Intercollegiate Surgical Curriculum Programme’s Vascular Surgery Curriculum places greater emphasis on open repair than endovascular techniques. In consultation, stakeholders express related concerns about the implications of the guidance for recruitment and training of vascular surgeons. However the Royal College of Surgeons report a ‘high competition ratio of 14:1’ for vascular surgical trainee positions. Relatedly, the committee noted that, while service models addressing volume–outcome dynamics were explicitly beyond the scope of this guideline, it remains possible for the NHS to give consideration to an appropriate level of centralisation, if that is deemed necessary to optimise results.
Second, the committee considered the argument that, regardless of the funds that might be made available from cost savings elsewhere, there is currently a lack of capacity in the NHS – in critical care beds in particular – to handle the additional demand that the committee’s recommendations would create. The committee considered this argument to be unduly nihilistic: given their confident interpretation of the evidence that OSR is associated with better net outcomes and lower total costs than EVAR, it should not be acceptable to continue to rely on the inferior approach because it forms the basis of current capacity planning. However, the committee accepted that – at least in the short term – one possible knock-on effect of increased use of OSR (and the critical care capacity it necessitates) would be a lengthier waiting list for AAA repair. To explore this possibility, a scenario analysis was undertaken in the infrarenal economic model. One extra month was added to the OSR waiting time, making it 3 months compared with 2 for EVAR. OSR remained the dominant option. In fact, further exploration shows that the waiting list for OSR would have to be over 3 months longer than that for EVAR before EVAR would generate more lifetime discounted QALYs than OSR and over 8 months longer than that for EVAR before OSR would be associated with an ICER worse than £20,000/QALY. The committee were reassured by this analysis, as they did not believe that there is any risk of waiting lists reaching extreme lengths such as these.
Third, the committee discussed whether it will be possible to retain skilled EVAR capacity (which may be necessary for the repair of ruptured AAAs), if the elective EVAR workload is likely to reduce to near-zero. Detailed considerations are discussed in Evidence review T.
Appendices
Appendix A. Review protocols
Review protocol for assessing the effectiveness of endovascular aneurysm repair compared with open surgical repair of unruptured abdominal aortic aneurysms
| Review question 12 |
The original question was: What is the effectiveness of EVAR compared to open repair surgery in reducing morbidity and mortality in people with unruptured abdominal aortic aneurysms? The committee agreed to retrospectively change the question to: What are the relative benefits and harms of EVAR, open surgical repair and non-surgical management in people with unruptured abdominal aortic aneurysms? | |||||
|---|---|---|---|---|---|---|
| Objectives |
To assess the advantages and disadvantages of elective endovascular aneurysm repair in comparison with conventional open surgical repair for the treatment of unruptured abdominal aortic aneurysms To explore the subgroup effects of various patient characteristics, leading to more tailored recommendations | |||||
| Type of review | Intervention | |||||
| Language | English only | |||||
| Study design |
| |||||
| Interventions | ||||||
| Standard (on-IFU) EVAR | Complex EVAR | |||||
| Off-IFU use of standard EVAR | Other complex EVAR | |||||
| Infrarenal |
Systematic reviews RCTs Quasi-RCTs |
Systematic reviews RCTs Quasi-RCTs
UK registry data (National Vascular Registry) |
Systematic reviews RCTs Quasi-RCTs
UK registry data (National Vascular Registry) | |||
| Juxtarenal |
Systematic reviews RCTs Quasi-RCTs |
Systematic reviews RCTs Quasi-RCTs
UK registry data (National Vascular Registry) |
Systematic reviews RCTs Quasi-RCTs
UK registry data (National Vascular Registry) | |||
| Suprarenal / ‘type IV’ | - | - |
Systematic reviews RCTs Quasi-RCTs Non-randomised controlled trials Prospective cohort studies UK registry data (National Vascular Registry) | |||
| Status |
Published papers only (full text) No date restrictions | |||||
| Population |
People undergoing surgery for a confirmed unruptured abdominal aortic aneurysm Subgroups: fitness for surgery, age, sex, comorbidities (including cardiovascular disease, renal disease, COPD, obesity), ethnicity | |||||
| Intervention |
Elective standard (on-IFU) EVAR for infrarenal and juxtarenal abdominal aortic aneurysms Elective complex EVAR for infrarenal, juxtarenal and suprarenal abdominal aortic aneurysms, including: fenestrated EVAR EVAR with chimneys EVAR with snorkels branched grafts ‘CHIMPS’ (CHIMneys, Periscopes, Snorkels) infrarenal devices used for juxtarenal AAA – that is, off-IFU use of standard devices Open repair Non-surgical intervention Summary: | |||||
| No surgery | Open repair | Standard (on-IFU) EVAR | Off-IFU use of standard EVAR | Other complex EVAR | ||
| Infrarenal | ✓ | ✓ | ✓ | ✓ | Iliac-branched only | |
| Juxtarenal | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Suprarenal / ‘type IV’ | ✓ | ✓ | - | - | ✓ | |
| Comparator | Each other | |||||
| Outcomes |
Mortality/survival Peri- and post-operative complications Successful exclusion of the aneurysm, aneurysm rupture, or further aneurysm growth Need for reintervention Quality of life Resource use, including length of hospital or intensive care stay, and costs | |||||
| Other criteria for inclusion / exclusion of studies |
Exclusion: Non-English language Abstract/non-published | |||||
| Baseline characteristics to be extracted in evidence tables |
Age Sex Size of aneurysm Comorbidities | |||||
| Search strategies | See Appendix B | |||||
| Review strategies |
| |||||
| Key papers | Sharath Chandra Paravastu, V, Rubaraj
Jayarajasingam, Rachel
Cottam, Simon
J. Palfreyman, Jonathan
A. Michaels, and Steven
M. Thomas. Endovascular repair of abdominal aortic aneurysm. Cochrane Database of Systematic Reviews (1), 2014 – SYSTEMATIC REVIEW [PubMed: 24453068]; included papers:
| |||||
- 1
The original protocol for this question specified that a limited selection of observational evidence could be considered for complex AAA only. However, only 1 study met these criteria and, in consultation on the draft guideline, stakeholders agreed that it was of limited relevance. Therefore, a much wider range of observational evidence covering all types of AAA was conducted; full details of methods and results are reported in Evidence review K2.
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review question 12
Paravastu SC, Jayarajasingam R, Cottam R et al. (2014) Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev;(1): CD004178. doi: 10.1002/14651858.CD004178.pub2 [PubMed: 24453068] [CrossRef].
|
Medline Strategy, searched 15th August 2017 Database: Ovid MEDLINE(R) 1946 to August Week 1 2017 Search Strategy: |
|---|
| 1 Aortic Aneurysm, Abdominal/ |
| 2 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco-abdom* or aort* or spontan* or juxtarenal* or juxta-renal* or juxta renal* or paraerenal* or para-renal* or para renal* or suprarenal* or supra renal* or supra-renal* or short neck* or short-neck* or shortneck* or visceral aortic segment*)).tw. |
| 3 (AAA or cAAA).tw. |
| 4 or/1-3 |
| 5 exp Stents/ |
| 6 Vascular Surgical Procedures/ or Blood Vessel Prosthesis/ or Blood Vessel Prosthesis Implantation/ |
| 7 (blood adj4 vessel* adj4 (transplant* or graft* or implant*)).tw. |
| 8 (endovasc* or endostent* or endograft* or EVAR* or Palmaz or stent* or graft*).tw. |
| 9 (endovascular* adj4 aneurysm* adj4 repair*).tw. |
| 10 (endovascular* adj4 aort* adj4 repair*).tw. |
| 11 or/5-10 |
| 12 4 and 11 |
| 13 Aortic Aneurysm, Abdominal/su [Surgery] |
| 14 12 or 13 |
| 15 (complex or fenestrat* or branched or chimney* or snorkel* or periscope* or sandwich* or CHIMPS).tw. |
| 16 14 and 15 |
| 17 (FEVAR or F-EVAR or BEVAR or B-EVAR or BREVAR or BR-EVAR or CHEVAR or CH-EVAR or Co-EVAR or CoEVAR or Co-FEVAR or CoFEVAR).tw. |
| 18 (complex adj4 EVAR*).tw. |
| 19 17 or 18 |
| 20 16 or 19 |
| 21 animals/ not humans/ |
| 22 20 not 21 |
| 23 limit 22 to english language |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom*.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov*.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree* or analys*)).tw. |
| 20 (cost or costs or costing* or costly or costed).tw. |
| 21 (price* or pricing*).tw. |
| 22 budget*.tw. |
| 23 expenditure*.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic* or (pharmaco adj economic*)).tw. |
| 26 or/1-25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly* or qald* or qale* or qtime*).tw. |
| 7 disability adjusted life.tw. |
| 8 daly*.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health* year* equivalent*.tw. |
| 19 utilit*.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili*.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble*.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1-30 |
Appendix C. Clinical evidence study selection
Cochrane systematic review update search
Complex EVAR versus open surgery study selection
Appendix D. Clinical evidence tables
Standard EVAR compared with open surgical repair of simple AAA
Download PDF (121K)
Studies included in the systematic review by Paravastu et al.
Download PDF (175K)
EVAR vs no intervention for patients in whom open surgery is not considered appropriate
Download PDF (151K)
Appendix E. Forest plots
EVAR compared with open surgery for patients in whom open surgery is considered appropriate
Appendix F. GRADE tables
EVAR compared with open surgery for patients in whom open surgery is considered appropriate
Mortality
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| All-cause mortality at 30 days or within hospital; effect sizes below 1 favour EVAR | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Not serious | 1,362 | 1,361 | RR 0.34 (0.21, 0.57) | High |
| All-cause mortality up to 4 years; effect sizes below 1 favour EVAR | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 1,393 | 1,390 | RR 0.93 (0.79, 1.10) | Moderate |
| All-cause mortality up to 8 years; effect sizes below 1 favour EVAR | |||||||||
| 3 (DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Not serious | 1,243 | 1,241 | RR 0.99 (0.89, 1.09) | High |
| All-cause mortality up to 15 years; effect sizes below 1 favour EVAR | |||||||||
| 3 (EVAR1, DREAM & OVER trials) | RCTs | Not serious | Not serious | Not serious | Not serious | 1,243 | 1,241 | RR 1.03 (0.97, 1.08) | High |
| All-cause mortality between 0 to 6 months; effect sizes below 1 favour EVAR | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Not serious | 1,393 | 1,390 | HR 0.67 (0.46, 1.00) | High |
| All-cause mortality between >6 months to 4 years; effect sizes below 1 favour EVAR | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 1,346 | 1,318 | HR 1.05 (0.87, 1.27) | Moderate |
| All-cause mortality between >4 years to 8 years; effect sizes below 1 favour EVAR | |||||||||
| 2 (EVAR1 & OVER) | RCTs | Not serious | Not serious | Not serious | Serious1 | 848 | 817 | HR 1.11 (0.92, 1.35) | Moderate |
| All-cause mortality >8 years; effect sizes below 1 favour EVAR | |||||||||
| 2 (EVAR1 & OVER) | RCTs | Not serious | Not serious | Not serious | Serious1 | 620 | 610 | HR 1.08 (0.82, 1.44) | Moderate |
| AAA-related mortality up to 4 years; effect sizes below 1 favour EVAR | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Serious2 | Very serious3 | 1,393 | 1,390 | RR 0.65 (0.30, 1.42) | Very low |
| AAA-related mortality up to 8 years; effect sizes below 1 favour EVAR | |||||||||
| 4 (DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 1,243 | 1,241 | RR 0.75 (0.52, 1.08) | Moderate |
| AAA-related mortality between 8 and 15 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR1 | RCT | Not serious | Not serious | Not serious | Not serious | 626 | 626 | HRa 5.82 (1.64, 20.65) | High |
| AAA-related mortality up to 15 years; effect sizes below 1 favour EVAR | |||||||||
| 3 (EVAR 1, DREAM & OVER trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 1,243 | 1,241 | RR 1.03 (0.75, 1.40) | Moderate |
| Cardiac-related mortality (follow-up not specified); effect sizes below 1 favour EVAR | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 1,393 | 1,390 | RR 1.12 (0.91, 1.38) | Moderate |
| Stroke-related mortality (follow-up not specified); effect sizes below 1 favour EVAR | |||||||||
| 3 (DREAM, EVAR1 and OVER trials) | RCTs | Not serious | Not serious | Not serious | Very serious3 | 1,243 | 1,241 | RR 1.00 (0.62, 1.61) | Low |
| Pulmonary-related mortality (follow-up not specified); effect sizes below 1 favour EVAR | |||||||||
| 3 (DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 1,243 | 1,241 | RR 0.63 (0.45, 0.86) | Moderate |
- a
Hazard ratios were reported adjusting for age, sex, maximum aneurysm diameter, FEV1, log creatinine, statin use, BMI, smoking status, systolic blood pressure, and total cholesterol
- 1
Confidence interval crosses one line of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 1 level.
- 2
I2 value between 33.3% and 66.7%, downgrade 1 level.
- 3
Confidence interval crosses two lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Endograft-related complications
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Any endograft complication (not specified) | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Very serious1 | Not serious | 1,393 | N/A |
ACE: 27.3% (41/150) DREAM: 27.7% (48/173) EVAR1: 45.0% (282/626) OVER: 24.8% (110/444) Overall rate: 34.5% | Low |
| Endoleaks | |||||||||
| 4 (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Very serious1 | Not serious | 1,296 | N/A |
ACE: 27.3% (41/150) DREAM: 11.7% (20/173) EVAR1: 22.3% (118/529) OVER: 24.8% (110/444) Overall rate: 22.3% | Low |
| Graft migration | |||||||||
| 2 (DREAM & EVAR1 trials) | Systematic review (2 RCTs) | Not serious | Not serious | Not serious | Not serious | 799 | N/A |
DREAM: 4.0% (7/173) EVAR1: 1.9% (12/444) Overall: 3.1% (15/617) | Low |
- 1
Unexplained variation in complication rates reported across included studies, downgrade 2 levels.
Other complications
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Non-fatal stroke (follow-up not reported); effect sizes below 1 favour EVAR | |||||||||
| 3 (ACE, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Very serious1 | 1,220 | 1,212 | RR 0.82 (0.52, 1.29) | Low |
| Pulmonary complications (follow-up not reported); effect sizes below 1 favour EVAR | |||||||||
| 2 (ACE & DREAM trials) | RCTs | Not serious | Not serious | Not serious | Not serious | 323 | 327 | RR 0.38 (0.18, 0.76) | High |
| Renal complications (follow-up not reported); effect sizes below 1 favour EVAR | |||||||||
| 3(ACE, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Very serious1 | 1,103 | 1,049 | RR 1.23 (0.60, 2.55) | Low |
| Sexual dysfunction (follow-up not reported); effect sizes below 1 favour EVAR | |||||||||
| ACE trial | RCT | Not serious | Not serious | Not serious | Very serious1 | 150 | 148 | RR 0.63 (0.25, 1.58) | Low |
- 1
Confidence interval crosses two lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Need for reintervention
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Any reintervention up to 4 years; effect sizes below 1 favour EVAR | |||||||||
| 3 (ACE, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Very serious1 | Serious2 | 1,220 | 1,212 | RR 2.30 (1.03, 5.18) | Very low |
| Any reintervention up to 8 years; effect sizes below 1 favour EVAR | |||||||||
| 3v(DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Very serious1 | Serious2 | 1,243 | 1,241 | RR 1.75 (1.07, 2.85) | Very low |
| Any reintervention between 8 and 15 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR1 | RCT | Not serious | Not serious | Not serious | Serious3 | 264 | 282 | HRa 1.51 (0.71, 3.19) | Moderate |
| Any reintervention up to 15 years; effect sizes below 1 favour EVAR | |||||||||
| 3 (EVAR1, DREAM & OVER trials) | RCT | Not serious | Not serious | Not serious | Not serious | 1,243 | 1,241 | RR 1.87 (1.61, 2.18) | High |
| AAA-related reintervention up to 15 years; effect sizes below 1 favour EVAR | |||||||||
| 1 DREAM trial | RCT | Not serious | Not serious | N/A | Not serious | 178 | 173 | RR 6.66 (3.70, 12.5,) | High |
| Life threatening reintervention up to 15 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR1 | RCT | Not serious | Not serious | N/A | Not serious | 302 | 300 | HRa 2.09 (1.42, 3.08) | High |
- a
Hazard ratios were reported adjusting for age, sex, maximum aneurysm diameter, FEV1, log creatinine, statin use, BMI, smoking status, systolic blood pressure, and total cholesterol
- 1
I2 value >66.7%, downgrade 2 levels.
- 2
Confidence interval crosses one line of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 1 level.
- 3
Non-significant result (95% CI crosses the line of no effect), downgrade 1 level.
Quality of life
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Mean changes in SF-36 Mental component scores at 2 years; effect sizes below 0 favour EVAR | |||||||||
| 1 EVAR1 trial | RCT | Not serious | Not serious | N/A | Serious1 | 1,220 | 1,212 | MD 0.92 (−0.39, 2.23) | Moderate |
| Mean changes in SF-36 physical component scores at 2 years; effect sizes below 0 favour EVAR | |||||||||
| 1 EVAR1 trial | RCT | Not serious | Not serious | Not serious | Serious1 | 1,220 | 1,212 | MD −0.20 (−1.59, 1.19) | Moderate |
| Mean changes in EQ-5D scores at 2 years; effect sizes below 0 favour EVAR | |||||||||
| 1 EVAR1 trial | RCT | Not serious | Not serious | Not serious | Serious1 | 1,103 | 1,049 | MD 0.01 (−0.01, 0.03) | Moderate |
- 1
Non-significant result (95% CI crosses the line of no effect), downgrade 1 level.
Length of stay
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Length of hospital stay; effect sizes below 0 favour EVAR | |||||||||
| 4, (ACE, DREAM, EVAR1 & OVER trials) | RCTs | Not serious | Not serious | Not serious | Not serious | 1,381 | 1,366 | MD −4.87(−5.93, −3.82) | High |
EVAR vs no intervention for patients in whom open surgery is not considered appropriate
Mortality
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | No intervention | Summary of results | |
| All-cause mortality at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Serious2 | 197 | 207 | HRa 1.32 (0.68, 2.54) | Moderate |
| All-cause mortality at 4 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Serious2 | 197 | 207 | HRa 1.02 (0.75, 1.37) | Low |
| All-cause mortality at 8 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Serious2 | 197 | 207 | HRa 0.96 (0.61, 1.51) | Low |
| All-cause mortality at 12 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Not serious | Not serious | Not serious | Serious2 | 197 | 207 | HRa 0.83 (0.65, 1.07) | Moderate |
| AAA-related mortality at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Serious2 | 197 | 207 | HRa 1.78 (0.75, 4.21) | Low |
| AAA-related mortality at 4 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Not serious | 197 | 207 | HRa 0.34 (0.16, 0.72) | Moderate |
| AAA-related mortality at 8 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Not serious | 197 | 207 | HRa 0.17 (0.04, 0.84) | Moderate |
| Fatal myocardial infarction at 4 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Very serious3 | 197 | 207 | RR 0.74 (0.38, 1.42) | Very low |
| Stroke-related mortality at 4 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Very serious3 | 197 | 207 | RR 1.75 (0.42, 7. 23) | Very low |
- a
Hazard ratios were reported adjusting for age, sex, maximum aneurysm diameter, FEV1, log creatinine, statin use, BMI, smoking status, systolic blood pressure, and total cholesterol
- 1
Investigators analyses did not take into account a considerably high rate of crossover (34%) from the no intervention group to the EVAR group, downgrade 1 level.
- 2
Non-significant result (95% CI crosses the line of no effect), downgrade 1 level.
- 3
Confidence interval crosses two lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Endograft-related complications and reintervention
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Any graft-related complication (including endoleak, infection, stenosis, migration, thrombosis rupture, and kinking) | |||||||||
| 1 EVAR2 trial | RCT | Not serious | Not serious | Not serious | Not serious | 197 | N/A | 49.2% (97/197) | High |
| Graft-related reinterventions | |||||||||
| 1 EVAR2 trial | RCT | Not serious | Not serious | Not serious | Not serious | 197 | N/A | 27.9% (55/197) | High |
Major complications
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Cardiovascular events (not specified) at 4 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Serious2 | 197 | 207 | HRa 1.07 (0.60, 1.91) | Low |
| Non-fatal myocardial infarction at 4 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Serious2 | 197 | 207 | RR 5.25 (1.17, 23.68) | Low |
| Non-fatal stroke at 4 years; effect sizes below 1 favour EVAR | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Very serious3 | 197 | 207 | RR 1.84 (0.55, 6.18) | Very low |
- a
Hazard ratios were reported adjusting for age, sex, maximum aneurysm diameter, FEV1, log creatinine, statin use, BMI, smoking status, systolic blood pressure, and total cholesterol
- 1
Investigators’ analyses did not take into account a considerably high rate of crossover (34%) from the no intervention group to the EVAR group, downgrade 1 level.
- 2
Non-significant result (95% CI crosses the line of no effect), downgrade 1 level.
- 3
Confidence interval crosses two lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Quality of life
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| SF-36 scores at 2 years | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Very serious2 | 197 | 207 | No difference between groups. | Very low |
| EQ-5D scores at 2 years | |||||||||
| 1 EVAR2 trial | RCT | Serious1 | Not serious | Not serious | Very serious2 | 197 | 207 | No difference between groups. | Very low |
- 1
Investigators’ analyses did not take into account a considerably high rate of crossover (34%) from the no intervention group to the EVAR group, downgrade 1 level.
- 2
Effect sizes and measures of dispersion were not reported, downgrade 2 levels.
Appendix H. Economic evidence tables
Download PDF (241K)
Appendix J. Excluded studies
Clinical studies
| No. | Study | Reason for exclusion |
|---|---|---|
| 1 | Belczak Sergio Quilici, Lanziotti Luiz, Botelho Yuri et al. (2014) Open and endovascular repair of juxtarenal abdominal aortic aneurysms: a systematic review. Clinics (Sao Paulo, and Brazil) 69, 641–6 [PMC free article: PMC4192422] [PubMed: 25318097] | Systematic review including studies that prospective and retrospective cohort studies. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 2 | Brooks M J, Brown L C, and Greenhalgh R M (2006) Defining the Role of Endovascular Therapy in the Treatment of Abdominal Aortic Aneurysm: results of a Prospective Randomized Trial. Advances in surgery 40, 191–204 [PubMed: 17163102] | Narrative review |
| 3 | Brown L C, Epstein D, Manca A, Beard J D, Powell J T, and Greenhalgh R M (2004) The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. European journal of vascular and endovascular surgery 27(4), 372–381 [PubMed: 15015186] | Protocol for the EVAR trial that we have already included |
| 4 | Bruen Kevin J, Feezor Robert J, Daniels et al. (2011) Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. Journal of vascular surgery 53, 895–5 [PubMed: 21211934] | Authors collected data from patients who underwent EVAR and compared their results with retrospectively collected data from historical controls. |
| 5 | Bulder R M. A, Bastiaannet E, Hamming J F, and Lindeman J H. N (2019) Meta-analysis of long-term survival after elective endovascular or open repair of abdominal aortic aneurysm. British Journal of Surgery 106(5), 523–533 [PubMed: 30883709] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 6 | Chen Z G, Tan S P, Diao Y P, Wu Z Y, Miao Y Q, and Li Y J (2019) The long-term outcomes of open and endovascular repair for abdominal aortic aneurysm: A meta-analysis. Asian Journal of Surgery 23, 23 [PubMed: 30914154] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 7 | de Bruin , J L, Vervloet M G, Buimer M et al. (2013) Renal function 5 years after open and endovascular aortic aneurysm repair from a randomized trial. : John Wiley and Sons Ltd (Southern Gate, Chichester, West Sussex PO19 8SQ, United Kingdom) | Conference abstract. |
| 8 | Deery SE, Lancaster RT, Gubala AM et al. (2017) Early experience with fenestrated endovascular compared to open repair of complex abdominal aortic aneurysms in a high-volume open aortic center. Annals of vascular surgery [PubMed: 29217447] | Retrospective cohort study design. |
| 9 | Di Xiao, Ye Wei, Liu Chang-Wei et al. (2013) Fenestrated endovascular repair for pararenal abdominal aortic aneurysms: a systematic review and meta-analysis. Annals of vascular surgery 27, 1190–200 [PubMed: 23973095] | Systematic review that assessed data from retrospective case series (single arm, non-comparative studies). Case series are not listed for inclusion in the review protocol. |
| 10 | Donas Konstantinos P, Torsello Giovanni, Pitoulias Georgios A et al. (2011) Surgical versus endovascular repair by iliac branch device of aneurysms involving the iliac bifurcation. Journal of vascular surgery 53, 1223–9 [PubMed: 21276683] | Retrospective cohort study design. |
| 11 | Donas Konstantinos P, Torsello Giovanni et al. (2012) Early outcomes for fenestrated and chimney endografts in the treatment of pararenal aortic pathologies are not significantly different: a systematic review with pooled data analysis. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists 19, 723–8 [PubMed: 23210868] | Systematic review that assessed data from retrospective and prospective case series (single arm, non-comparative studies). Case series are not listed for inclusion in the review protocol. |
| 12 | Fanelli F (2017) Do the long-term outcomes of EVAR justify its generalised use? Cardiovascular and interventional radiology. Conference: cardiovascular and interventional radiological society of europe, and CIRSE 2017. Denmark 40(2 Supplement 1), S58–s59 | Conference abstract |
| 13 | Gallitto E, Gargiulo M, Freyrie A et al. (2015) The endovascular treatment of juxta-renal abdominal aortic aneurysm using fenestrated endograft: early and mid-term results. The Journal of cardiovascular surgery , [PubMed: 26417936] | Case series |
| Gok E, Onalan M A, Beyaz M O, Karatepe C, Cinar B, Alpagut I U, Goksel O S, and Dayioglu E (2016) Quality of life after endovascular repair versus open surgery for abdominal aortic aneurysms. American journal of cardiology. Conference: 12th international congress of update in cardiology and cardiovascular surgery. Antalya turkey. Conference start: 20160310. Conference end: 20160313. Conference publication: (var.pagings) 117, S17–S18 | Conference abstract | |
| 14 | Gupta P K, Brahmbhatt R, Kempe K et al. (2017) Thirty-day outcomes after fenestrated endovascular repair are superior to open repair of abdominal aortic aneurysms involving visceral vessels. Journal of Vascular Surgery , [PubMed: 28711400] | Retrospective cohort study involving retrospective analysis of data from an American surgical registry. |
| 15 | Han Y, Zhang S, Zhang J et al. (2017) Outcomes of Endovascular Abdominal Aortic Aneurysm Repair in Octogenarians: Meta-analysis and Systemic Review. European Journal of Vascular and Endovascular Surgery. [PubMed: 28822680] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 16 | Health Quality, and Ontario (2009) Fenestrated endovascular grafts for the repair of juxtarenal aortic aneurysms: an evidence-based analysis. Ontario health technology assessment series 9, 1–51 [PMC free article: PMC3377528] [PubMed: 23074534] | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 17 | Katsargyris Athanasios, Oikonomou Kyriakos, Klonaris Chris et al. (2013) Comparison of outcomes with open, fenestrated, and chimney graft repair of juxtarenal aneurysms: are we ready for a paradigm shift? Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists 20, 159–69 [PubMed: 23581756] | Systematic review that assessed data from retrospective and prospective case series (single arm, non-comparative studies). Case series are not listed for inclusion in the review protocol. |
| 18 | Lederle F A, Stroupe K T, Kyriakides T C, Ge L, and Freischlag J A (2016) Long-term Cost-effectiveness in the Veterans Affairs Open vs Endovascular Repair Study of Aortic Abdominal Aneurysm: a Randomized Clinical Trial. [PubMed: 27627802] | Investigators performed secondary data analysis using data from a study (OVER trial) that is included in a systematic review identified as relevant to this review question. No additional relevant data was reported in this new publication. |
| 19 | Li B, Khan S, Salata K, Hussain M A, de Mestral , C , Greco E, Aljabri B A, Forbes T L, Verma S, and Al-Omran M (2019) A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. Journal of Vascular Surgery 27, 27 [PubMed: 31147117] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 20 | Li Yue, Zhang Tao, Guo Wei et al. (2015) Endovascular chimney technique for juxtarenal abdominal aortic aneurysm: a systematic review using pooled analysis and meta-analysis. Annals of vascular surgery 29, 1141–50 [PubMed: 26004962] | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 21 | Locham S S, Nejim B, Aridi H et al. (2017) Perioperative outcomes of patients undergoing fenestrated endovascular repair vs open repair of intact abdominal aortic aneurysms involving the visceral vessels: 10-year national study. Journal of the American College of Surgeons 225 (4 Supplement 1), S220 | Conference abstract |
| 22 | Nordon I M, Hinchliffe R J, Holt P J et al. (2009) Modern treatment of juxtarenal abdominal aortic aneurysms with fenestrated endografting and open repair-a systematic review. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 38, 35–41 [PubMed: 19346140] | Systematic review that assessed data from prospective and retrospective case series (single arm, non-comparative studies). Case series are not listed for inclusion in the review protocol. |
| 23 | Orr Nathan T, Davenport Daniel L, Minion David J, and Xenos Eleftherios S (2017) Comparison of perioperative outcomes in endovascular versus open repair for juxtarenal and pararenal aortic aneurysms: A propensity-matched analysis. Vascular 25, 339–345 [PubMed: 27903931] | Retrospective cohort study involving retrospective analysis of data from an American surgical registry. |
| 24 | Patel R, Powell J T, Sweeting M J, Epstein D M, Barrett J K, and Greenhalgh R M (2018) The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technology Assessment (Winchester, and England) 22(5), 1–132 [PMC free article: PMC5817412] [PubMed: 29384470] | Economic analysis. This report was forwarded to our economists for consideration. |
| 25 | Raux Maxime, Patel Virendra I, Cochennec Frederic et al. (2014) A propensity-matched comparison of outcomes for fenestrated endovascular aneurysm repair and open surgical repair of complex abdominal aortic aneurysms. Journal of vascular surgery 60, 858–4 [PubMed: 24835042] | Retrospective cohort study. |
| 26 | Sala-Almonacil VA, Zaragoza-Garcia JM, Ramirez-Montoya M et al. (2017) Fenestrated and chimney endovascular aneurysm repair versus open surgery for complex abdominal aortic aneurysms. The Journal of cardiovascular surgery 58(6), 801–813 [PubMed: 28128541] | Study employed a mixture of study designs: prospectively collected data of patients who underwent EVAR was compared against data from a historical cohort |
| 27 | Spanos K, Karathanos C, Athanasoulas A, Saleptsis V, Vasilopoulos I, Xhepa S, Matsagkas M, and Giannoukas A D (2017) Renal Function Impairment in Patients Undergoing Elective EVAR vs. Elective Open Repair During Follow up Period: A Systematic Review of the Literature. Current Vascular Pharmacology 15(2), 103–111 [PubMed: 27697066] | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 28 | Spanos K, Karathanos C, Athanasoulas A, Saleptsis V, Vasilopoulos I, Xhepa S, Matsagkas M, and Giannoukas A D (2017) Re: ‘Re. Renal Function Impairment in Patients Undergoing Elective EVAR vs Elective Open Repair During Follow up Period: A Systematic Review of the Literature’. Current Vascular Pharmacology 15(2), 113–114 [PubMed: 28472918] | Non-peer-reviewed letter |
| 29 | Stather P W, Sidloff D, Dattani N et al. (2013) Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. [PubMed: 23475697] | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 30 | Stroupe K T, Lederle F A, Matsumura J S, Kyriakides T C, Jonk Y C, Ge L, and Freischlag J A (2012) Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. Journal of Vascular Surgery 56(4), 901–909.e2 [PubMed: 22640466] | Economic analysis. This report was forwarded to our economists for consideration. |
| 31 | Tsilimparis Nikolaos, Perez Sebastian, Dayama Anand et al. (2013) Endovascular repair with fenestrated-branched stent grafts improves 30-day outcomes for complex aortic aneurysms compared with open repair. Annals of vascular surgery 27, 267–73 [PubMed: 23403330] | Retrospective cohort study involving retrospective analysis of data from an American surgical registry. |
| 32 | Ultee Klaas H. J, Zettervall Sara L, Soden Peter A et al. (2017) Perioperative outcome of endovascular repair for complex abdominal aortic aneurysms. Journal of vascular surgery 65, 1567–1575 [PMC free article: PMC5438879] [PubMed: 28216344] | Retrospective cohort study involving retrospective analysis of data from an American surgical registry. |
| 33 | van Lammeren GW, Unlu C, Verschoor S et al. (2017) Results of open pararenal abdominal aortic aneurysm repair: single centre series and pooled analysis of literature. Vascular 25(3), 234–241 [PubMed: 27565511] | Case series |
| 34 | Van Schaik , T G, Yeung K K, Verhagen H J, De Bruin , J L, Van Sambeek , Mrhm , Balm R, Zeebregts C J, Van Herwaarden , J A, and Blankensteijn J D (2017) Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. European journal of vascular and endovascular surgery 54(5), 671– [PubMed: 29061270] | Conference abstract |
| 35 | Vierhout B P, Pol R A, Ott M A, Pierie M E. N, van Andringa de Kempenaer, T M G, Hissink R J, Wikkeling O R. M, Bottema J T, Moumni M E, and Zeebregts C J (2019) Randomized multicenter trial on percutaneous versus open access in endovascular aneurysm repair (PiERO). Journal of Vascular Surgery 69(5), 1429–1436 [PubMed: 30292613] | This study is EVAR vs EVAR |
| 36 | Williamson J S, Ambler G K, Twine C P, Williams I M, and Williams G L (2018) Elective Repair of Abdominal Aortic Aneurysm and the Risk of Colonic Ischaemia: Systematic Review and Meta-Analysis. European Journal of Vascular & Endovascular Surgery 56(1), 31–39 [PubMed: 29636250] | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 37 | Yaoguo Yang, Zhong Chen, Lei Kou, and Yaowen Xiao (2017) Treatment of complex aortic aneurysms with fenestrated endografts and chimney stent repair: Systematic review and meta-analysis. Vascular 25, 92–100 [PubMed: 26846442] | Systematic review comparing 2 approaches of performing complex EVAR (fenestrated versus chimney endografts). The aim of this review question is to compare complex EVAR with open surgical repair or no intervention. Thus, comparisons between different types of complex EVAR are out of scope of this review question. |
Economic studies
| Study | Primary reason for exclusion |
|---|---|
| Selectively excluded | |
| Blackhouse et al. (2009). A cost-effectiveness model comparing endovascular repair to open surgical repair of abdominal aortic aneurysm in Canada. Value in Health, 12(2): 245–52. [PubMed: 18783394] | Non-UK (Canada) |
| Bosch et al. (2002). Abdominal aortic aneurysms: cost-effectiveness of elective endovascular and open surgical repair. Radiology, 225(2): 337–44. [PubMed: 12409564] | Non-UK (US) |
| Bowen et al. (2005). Systematic review and cost-effectiveness analysis of elective endovascular repair compared to open surgical repair of abdominal aortic aneurysms. Interim report. Ontario Ministry of Health & Long-term Care. | Interim results of Tarride et al. (2008) |
| Burgers et al. (2016). Cost-effectiveness of Elective Endovascular Aneurysm Repair Versus Open Surgical Repair of Abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg, 52: 29–40. [PubMed: 27118618] | Non-UK (Netherlands) |
| Hynes et al. (2007). A prospective clinical, economic, and quality-of-life analysis comparing endovascular aneurysm repair (EVAR), open repair, and best medical treatment in high-risk patients with abdominal aortic aneurysms suitable for EVAR: The Irish patient trial. J Endocasc Ther, 14: 763–76. [PubMed: 18052596] | Non-UK (Republic of Ireland) |
| Lederle et al. (2016). Long-term cost-effectiveness in the vetereans Affairs Open vs Endovascular Repair Study of aortic abdominal aneurysm: a randomised clinical trial. JAMA Surg, 151(12): 1139–1144. [PubMed: 27627802] | Non-UK (US) |
| McCarron et al. (2013). The impact of using informative priors in a Bayesian cost-effectiveness analysis: an application of endovascular versus open surgical repair for abdominal aortic aneurysms in high-risk patients. Med Decis Mak, 33(3): 437–50. [PubMed: 23054366] | Non-UK (Canada) |
| Patel et al. (1999). The cost-effectiveness of endovascular repair versus open surgical repair of abdominal aortic aneurysms: a decision analysis model. J Vasc Surg, 29(6): 958–72. [PubMed: 10359930] | Non-UK (US) |
| Prinssen et al. (2007). Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: Results of a randomized trial. J Vasc Surg, 46: 883–90. [PubMed: 17980274] | Non-UK (Netherlands) |
| Sousa et al. (2014). Cost-effectiveness of the endovascular repair of abdominal aortic aneurysm in Portugal. Angiol Cir Vasc, 10(2): 41–8. | Non-UK (Portugal) |
| Sultan & Hynes (2011a). Clinical efficacy and cost per quality-adjusted life years of pararenal endovascular aortic aneurysm repair compared with open surgical repair. J Endovasc Ther, 18: 181–96. [PubMed: 21521058] | Non-UK (Republic of Ireland) |
| Takayama (2017). A Cost-Utility Analysis of Endovascular Aneurysm Repair for Abdominal Aortic Aneurysm. Ann Vasc Dis, 10(3): 185–91. [PMC free article: PMC5684165] [PubMed: 29147166] | Non-UK (Japan) |
| Tarride et al. (2008). Cost-effectiveness analysis of elective endovascular repair compared with open surgical repair of abdominal aortic aneurysms for patients at a high surgical risk: A 1-year patient-level analysis conducted in Ontario, Canada. J Vasc Surg, 48: 779–87. [PubMed: 18639421] | Non-UK (Canada) |
| Excluded based on study selection criteria | |
| Armstrong et al. (2014). The use of fenestrated and branched endovascular aneurysm repair for juxtarenal and thoracoabdominal aneurysms: a systematic review and cost-effectiveness analysis. HTA, 18(70). [PMC free article: PMC4780914] [PubMed: 25522080] | Not a CUA |
| Badger et al. (2014). Endovascular treatment for ruptured abdominal aortic aneurysm (review). Cochrane Database of Systematic Reviews, 7. [PubMed: 25042123] | Review article, no additional CUAs |
| Forbes et al. (2002). A cost-effectiveness analysis of standard versus endovascular abdominal aortic aneurysm repair. J Can Chir, 45(6): 420–4. [PMC free article: PMC3684656] [PubMed: 12500916] | Not a CUA |
| Greenhalgh et al. (2005). Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. The Lancet, 365(9458): 2179–86. [PubMed: 15978925] | Not a CUA |
| Hayes et al. (2010). Cost-effectiveness analysis of endovascular versus open surgical repair of acute abdominal aortic aneurysms based on worldwide experience. J Endovasc Ther, 17: 174–82. [PubMed: 20426633] | Population (emergency repair) |
| Jonk et al. (2007). Cost-effectiveness of abdominal aortic aneurysm repair: a systematic review. Int J Tech Assess Health Care, 23(2): 205–15. [PubMed: 17493306] | Review article, no additional CUAs |
| Kapma et al. (2007). Emergency abdominal aortic aneurysm repair with a preferential endovascular strategy: mortality and cost-effectiveness analysis. J Endovasc Ther, 14: 777–84. [PubMed: 18052593] | Not a CUA |
| Kapma et al. (2014). Cost-effectiveness and cost–utility of endovascular versus open repair of ruptured abdominal aortic aneurysm in the Amsterdam Acute Aneurysm Trial. Br J Surg, 101(3): 208–15. [PubMed: 24469619] | Population (emergency repair) |
| Lederle. (2009). Repair of nonruptured abdominal aortic aneurysm: a systematic review of randomized trials. Vascular, 17: S71. | Poster abstract |
| Lederle et al. (2012). Cost-effectiveness at two years in the VA open versus endovascular repair trial. Eur J Vasc Endovasc Surg, 44: 543–8. [PubMed: 23116986] | Non-UK (US) |
| Luebke et al. (2014). Cost-effectiveness of endovascular versus open repair of acute complicated type B aortic dissections. J Vasc Surg, 59: 1247–55. [PubMed: 24418638] | Population (thoracic aortic dissection) |
| Mandavia et al. (2015). The role of cost-effectiveness for vascular surgery service provision in the United Kingdom. J Vasc Surg, 61: 1331–9. [PubMed: 25925543] | Review article, no additional CUAs |
| Medical Advisory Secretariat Ontario (2002). Endovascular repair of abdominal aortic aneurysm: an evidence-based analysis. Ontario HTA Series, 2(1). [PMC free article: PMC3387737] [PubMed: 23074438] | Review article, no additional CUAs |
| Michaels et al. (2014). Long-term cost-effectiveness analysis of endovascular versus open repair for abdominal aortic aneurysms based on four randomized clinical trials. Br J Surg, 101(6): 632. [PubMed: 24723016] | Commentary, no additional CUAs |
| Patel et al. (2000). The cost-effectiveness of repairing ruptured abdominal aortic aneurysms. J Vasc Surg, 32: 247–57. [PubMed: 10917983] | Population (emergency repair) |
| Perras et al. (2009). Elective endovascular abdominal aortic aneurism repair versus open surgery: a review of the clinical and cost-effectiveness. | Review article, no additional CUAs |
| Powell et al. (2015). Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J, 35: 2061–9. [PMC free article: PMC4553715] [PubMed: 25855369] | Population (emergency repair) |
| Powell et al. (2017). Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ, 359. [PMC free article: PMC5682594] [PubMed: 29138135] | Population (emergency repair) |
| Rollins et al. (2014). Mid-term cost-effectiveness analysis of open and endovascular repair for ruptured abdominal aortic aneurysm. Br J Surg, 101: 225–31. [PubMed: 24469621] | Population (emergency repair) |
| Sala-Almonicil et al. (2017). Fenestrated and chimney endovascular aneurysm repair versus open surgery for complex abdominal aortic aneurysms. J Cardiovasc Surg, 58(6): 801–13. [PubMed: 28128541] | Not a CUA. |
| Stroupe et al. (2012). Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. J Vasc Surg, 56: 901–10. [PubMed: 22640466] | Duplicate of Lederle et al. (2012) |
| Silverstein et al. (2005). Abdominal aortic aneurysm (AAA): cost-effectiveness of screening, surveillance of intermediate-sized AAA, and management of symptomatic AAA. BUMC Proceedings, 18: 345–67. [PMC free article: PMC1255946] [PubMed: 16252027] | Review article, no additional CUAs |
| Sultan et al. (2009a). A prospective clinical and quality of life analysis of open repair (OR), endovascular repair (EVAR), and best medical treatment in high-risk patients: cost-effectiveness during global recession. Vascular, (17): S2. | Poster abstract |
| Sultan et al. (2009b). Five-year experience with EVAR without fenestration for juxtarenal AAA repair: clinical efficacy, reintervention rates, and cost-effectiveness. Vascular, 17: S74. | Not found |
| Sultan & Hynes (2010a). Five-year experience with pararenal endovascular aortic repair (PEVAR) without fenestration: clinical efficacy, reintervention rates & cost-effectiveness. J Vasc Surg, 51(6): S89. | Poster abstract |
| Sultan & Hynes (2010b). Five-year experience with pararenal endovascular aortic repair (PEVAR) without fenestration: clinical efficacy, reintervention rates & cost-effectiveness. J Vasc Surg, 51(4): 1068–9. | Poster abstract |
| Sultan & Hynes (2010c) | Poster abstract |
| Sultan & Hynes (2011b). A mid- to long-term experience of clinical efficacy and cost per quality-adjusted-life years with pararenal endovascular aortic repair (PEVAR) without fenestration for pararenal AAA compared with open surgical repair. Cardiovasc Interv Radiol, 3 (332/677). | Poster abstract |
| Sultan & Hynes (2012). Clinical efficacy and cost per quality-adjusted life years of para-renal endovascular aortic aneurysm repair compared with open surgical repair. JACC, 60(17): B38. | Poster abstract |
| Sweeting et al. (2015). Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg, 102: 1229–39. [PMC free article: PMC4744980] [PubMed: 26104471] | Review article, no additional CUAs |
| Tarride et al. (2011). Should endovascular repair be reimbursed for low risk abdominal aortic aneurysm patients? Evidence from Ontario, Canada. Int J Vasc Med, 2011. [PMC free article: PMC3124872] [PubMed: 21748018] | Not a CUA |
| Taylor et al. (2012). EVAR is now cost effective and should replace open surgery for all suitable patients: con. Cardiovasc Interv Radiol, 35: S48. | Review article, no additional CUAs |
| Tremont et al. (2016). Endovascular Repair for Ruptured Abdominal Aortic Aneurysms has Improved Outcomes Compared to Open Surgical Repair. Vasc Endovasc Surg, 50(3) 147–55. [PubMed: 26975604] | Population (emergency repair) |
| Van Bochove et al. (2016). Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm. J Vasc Surg, 63(3): 827–38. [PubMed: 26916588] | Review article, no additional CUAs |
| Weinkauf et al. (2017). Open versus endovascular aneurysm repair trial review. Surgery, 162(5): 974–78. [PubMed: 28602492] | Duplicate of Lederle et al. (2016) |
| Wilt et al. (2006). Comparison of endovascular and open surgical repairs for abdominal aortic aneurysm. Evid Rep Technol Assess, 144: 1–113. [PMC free article: PMC4780951] [PubMed: 17764213] | Review article, no additional CUAs |
Key: CUA, cost–utility analysis.
Appendix K. Research recommendation
| Research recommendation | What is the effectiveness and cost-effectiveness of complex EVAR versus open surgical repair in people with an unruptured AAA for whom open surgical repair is a suitable option? |
|---|---|
| Population |
People undergoing elective surgery for unruptured abdominal aortic aneurysm Sub-grouped by: age, sex, comorbidities (including cardiovascular disease, renal disease, COPD, obesity) and ethnicity |
| Intervention(s) |
|
| Comparator(s) | Open surgical repair |
| Outcomes |
|
| Study design | Randomised controlled trial (stratified by AAA anatomy |
| Potential criterion | Explanation |
|---|---|
| Importance to patients, service users or the population | EVAR is a widely performed non-invasive alternative to open surgical repair. However, it is more expensive. Although EVAR has been shown to produce no long-term benefit over open surgical repair in people with unruptured infrarenal aneurysms, it is less clear whether this is the same in people with unruptured or ruptured juxtarenal, pararenal, suprarenal, type IV thoracoabdominal aneurysms, and short-necked infrarenal aneurysms. As a result, research is needed to identify how effective complex EVAR is in these populations. |
| Relevance to NICE guidance | High priority: it is currently unclear whether EVAR can improve long-term outcomes of people with complex aneurysm anatomies. |
| Current evidence base | A single non-randomised controlled trial assessing the efficacy of chimney- and fenestrated-EVAR in 90 people was identified from literature searches. The study reported no significant differences in 30-day mortality, complication, and reintervention rates between patients treated by complex EVAR and those who received open surgery. The results of this study, coupled with data from a new health economic model produced by NICE led the committee to conclude that complex EVAR yielded no benefit over open surgery in the short-term. The committee considered that longer-term evidence from large RCTs was needed to clarify the clinical utility of complex EVAR, and inform health economic modelling. |
| Equality | No specific equality concerns are relevant to this research recommendation. |
| Feasibility | There is a sufficiently large and well defined population available that randomised controlled trials in this area should be feasible. |
Appendix L. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Please note that NICE amended recommendations 1.5.1 to 1.5.6 on repairing unruptured aneurysms, after the committee’s proposed recommendations were reviewed by NICE’s Board
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Comparison of the Short and Long-Term Outcomes of Endovascular Repair and Open Surgical Repair in the Treatment of Unruptured Abdominal Aortic Aneurysms: Meta-Analysis and Systematic Review.[Cureus. 2020]Comparison of the Short and Long-Term Outcomes of Endovascular Repair and Open Surgical Repair in the Treatment of Unruptured Abdominal Aortic Aneurysms: Meta-Analysis and Systematic Review.AlOthman O, Bobat S. Cureus. 2020 Aug 12; 12(8):e9683. Epub 2020 Aug 12.
- Outcomes for symptomatic abdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program.[J Vasc Surg. 2016]Outcomes for symptomatic abdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program.Soden PA, Zettervall SL, Ultee KH, Darling JD, Buck DB, Hile CN, Hamdan AD, Schermerhorn ML. J Vasc Surg. 2016 Aug; 64(2):297-305. Epub 2016 Apr 14.
- Regional variation exists in patient selection and treatment of abdominal aortic aneurysms.[J Vasc Surg. 2016]Regional variation exists in patient selection and treatment of abdominal aortic aneurysms.Zettervall SL, Buck DB, Soden PA, Cronenwett JL, Goodney PP, Eslami MH, Lee JT, Schermerhorn ML, Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2016 Oct; 64(4):921-927.e1. Epub 2016 Apr 8.
- Review Effectiveness of endovascular aneurysm repair compared with open surgical repair of ruptured abdominal aortic aneurysms: Abdominal aortic aneurysm: diagnosis and management: Evidence review T[ 2020]Review Effectiveness of endovascular aneurysm repair compared with open surgical repair of ruptured abdominal aortic aneurysms: Abdominal aortic aneurysm: diagnosis and management: Evidence review T. 2020 Mar
- Review Open Surgical Repair of Abdominal Aortic Aneurysms Maintains a Pivotal Role in the Endovascular Era.[Semin Intervent Radiol. 2020]Review Open Surgical Repair of Abdominal Aortic Aneurysms Maintains a Pivotal Role in the Endovascular Era.Blackstock CD, Jackson BM. Semin Intervent Radiol. 2020 Oct; 37(4):346-355. Epub 2020 Oct 1.
- Effectiveness of endovascular aneurysm repair, open surgical repair and non-surg...Effectiveness of endovascular aneurysm repair, open surgical repair and non-surgical management of unruptured abdominal aortic aneurysms
- H.sapiens mRNA for apoptosis specific proteinH.sapiens mRNA for apoptosis specific proteingi|2995197|emb|Y11588.1|Nucleotide
- Risk factors associated with abdominal aortic aneurysm growth or ruptureRisk factors associated with abdominal aortic aneurysm growth or rupture
Your browsing activity is empty.
Activity recording is turned off.
See more...