NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Goal-directed therapy (GDT) during repair of unruptured abdominal aortic aneurysms
Review questions
Is goal-directed therapy effective during the surgical repair of an unruptured abdominal aortic aneurysm?
Is goal-directed therapy effective during the surgical repair of a ruptured abdominal aortic aneurysm?
Introduction
Goal directed therapy (GDT) refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation. The intention of the technique is to reduce the risk of perioperative (30-day) morbidity and mortality after major surgery. Review questions 14 and 26 aim to assess the use of goal-directed therapy in people undergoing surgery for unruptured and ruptured abdominal aortic aneurysms (AAAs), respectively.
PICO table
Table 1
PICO table for GDT for unruptured or ruptured AAA.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in Appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A ‘bulk’ search strategy was used to cover 2 review questions and identify studies that assessed the efficacy of goal-directed therapy for improving outcomes of surgical repair of unruptured and ruptured AAAs. The first literature search used a randomised controlled trial (RCT) and systematic review (SR) filter while the second search used an observational study filter to identify potentially relevant studies.
The reviewer sifted the RCT database first to identify systematic reviews, RCTs or quasi-randomised controlled trials that assessed whether goal-directed therapy improved outcomes of people undergoing surgical repair of unruptured or ruptured AAAs. Since limited evidence was identified from the RCT and systematic review literature search, the observational study database was sifted to identify non-randomised controlled trials which were potentially relevant to the review questions.
Studies were excluded if they:
- were not in English
- were not full reports of the study (for example, published only as an abstract)
- were not peer-reviewed
- were cohort studies, case series or case-control studies
Clinical evidence
Included studies
From an initial RCT database of 1,518 abstracts, 11 studies were identified as being potentially relevant to review question 14 and no questions were identified for review question 26. Following full-text review of the 14 ordered articles, 3 studies were included. No studies were identified as being potentially relevant to review questions 14 or 26 in an initial observational study database of 831 abstracts.
Update searches were conducted in December 2017, to identify any relevant studies published during guideline development. The update RCT and the observational study databases contained 42 and 45 abstracts, respectively. None of these were considered relevant. As a result no additional studies were identified.
Excluded studies
The list of papers excluded at full-text review, with reasons, is given in Appendix H.
Summary of clinical studies included in the evidence review
A summary of the included studies is provided in the tables below.
Table 2
Summary of included studies on GDT during repair of unruptured AAA.
See Appendix D for full evidence tables.
GDT during repair of ruptured AAA
No studies were identified as being potentially relevant.
Quality assessment of clinical studies included in the evidence review
See Appendix F for full GRADE tables, highlighting the quality of evidence from the included studies related to GDT during repair of unruptured AAA.
Economic evidence
Included studies
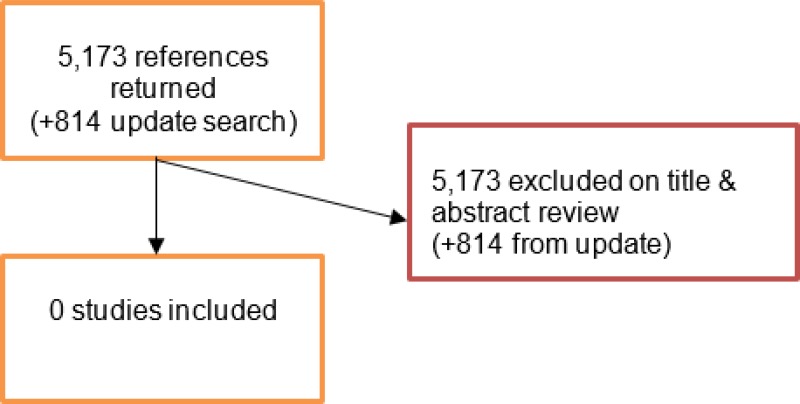
A literature search was conducted jointly for all review questions by applying standard health economic filters to a clinical search for AAA. This search returned a total of 5,173 citations. Following review of all titles and abstracts, no studies were identified as being potentially relevant to this review.
An update search was conducted in December 2017, to identify any relevant health economic analyses published during guideline development. The search found 814 abstracts; all of which were not considered relevant to this review. As a result no additional studies were included.
Excluded studies
No studies were retrieved for full-text review.
Evidence statements
GDT during repair of unruptured AAA
Low-quality evidence from 2 RCTs, including 104 people undergoing elective open repair of AAA, indicated that people who received GDT during surgery needed more postoperative colloids than those who received traditional fluid administration during surgery.
Very low- to moderate-quality evidence from 3 RCTs, including 204 people who received goal-directed therapy or traditional fluid administration during elective open repair of AAA, could not differentiate the following outcome measures between groups:
- Mortality
- Cardiac complications (acute coronary syndrome, congestive heart failure arrhythmia and dysrhythmia)
- Pulmonary complications (pulmonary oedema and respiratory failure)
- Renal complications (acute kidney injury and
- Gastrointestinal complications (ischaemic gut and gastrointestinal paralysis)
- Cerebral thrombosis
- Lower limb paresis
- Infection (sepsis, pneumonia, wound infections and urinary tract infection)
- Blood loss
- Resource use (postoperative use of fluids, length of ICU or hospital stay, reoperations, the need for acute dialysis and the need for mechanical intervention)
GDT during repair of ruptured AAA
No studies assessing the efficacy of GDT during repair of ruptured AAA were identified.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The committee considered that the outcomes which matter most are perioperative morbidity and mortality. The committee considered that perioperative outcomes were more important than long-term outcomes because their clinical experience highlighted that people undergoing AAA surgery are more likely to experience negative outcomes immediately after surgery.
The quality of the evidence
The committee noted that the term GDT encompasses a broad range of practices, and the studies included in this review adopted different complex strategies for optimising cardiac output in people undergoing AAA surgery.
The committee considered that the identified evidence was very-low-to-moderate in quality. The main reason for downgrading evidence was imprecision of effect estimates: 95% confidence intervals of pooled estimates crossed 1 or 2 lines of predefined minimal important differences. Another reason for downgrading evidence was the statistical heterogeneity observed in some of the meta-analyses performed in this review.
The committee noted that pooled results from 2 RCTs indicated that people who receive GDT need more colloids after surgery; however, the committee considered that this was not a clinically important outcome. No differences in the remaining outcome measures were observed between people who received GDT and those treated by traditional haemodynamic management strategies.
The committee discussed whether it was appropriate to make a “do not offer GDT” recommendation. As GDT encompasses a broad range of different practices, it would be problematic to disentangle these differing elements and they agreed that it was not possible to point to 1 or 2 specific practices that should not be performed. It was also noted that haemodynamic monitoring and management are essential during major surgery. The committee agreed that a “do not offer” recommendation was too restrictive and could potentially limit the use of some basic techniques for managing people during surgery. As a result, the committee decided not to make any recommendations.
Benefits and harms
The committee noted that, although the identified evidence reported no differences in clinically important outcomes between people who received GDT and those managed using traditional approaches, the evidence comprised 3 small RCTs. As a result, the committee concluded that the evidence failed to show any benefit in performing GDT on people undergoing surgical repair of unruptured AAA.
Cost effectiveness and resource use
The committee noted that GDT is a complex and highly technical procedure that needs more staff and additional training compared with standard practice. These factors result in considerably higher costs associated with GDT. With this in mind, the committee believed that GDT was unlikely to be cost effective in AAA surgery; especially since the specialty generally has low operative mortality rates.
Other factors the committee took into account
The committee noted that studies had previously been published highlighting that GDT reduces morbidity but not mortality in people undergoing vascular surgery. The committee considered that these studies were not suitable for consideration because they included heterogeneous groups of people with different vascular conditions, making it impossible to extrapolate whether there were any benefits to people with AAA.
Appendices
Appendix A. Review protocols
Review protocol for review question 14: GDT during repair of unruptured abdominal aortic aneurysms
| Review question 14 | Is goal-directed therapy effective during the surgical repair of an unruptured abdominal aortic aneurysm? |
|---|---|
| Objectives | To assess the use of goal-directed therapy in people undergoing surgery for an unruptured abdominal aortic aneurysm |
| Type of review | Intervention |
| Language | English only |
| Study design | Systematic reviews of study designs listed below Randomised controlled trials Quasi-randomised controlled trials If insufficient evidence identified, non-randomised controlled trials |
| Status | Published papers only (full text) No date restrictions |
| Population | People undergoing surgery for a confirmed unruptured abdominal aortic aneurysm |
| Intervention | Goal-directed fluid and/or ionotrope administration ‘Goal-directed’ refers to the use of cardiac output monitoring to tailor fluid administration to a maximal cardiac output |
| Comparator | Standard practice Fluid and/or ionotrope administration based on static preload parameters and traditional hemodynamics |
| Outcomes |
|
| Other criteria for inclusion / exclusion of studies | Exclusion: Non-English language Abstract/non-published Pharmacological interventions not available in the UK |
| Baseline characteristics to be extracted in evidence tables | Age Sex Size of aneurysm Comorbidities |
| Search strategies | See Appendix B |
| Review strategies | Appropriate NICE Methodology Checklists, depending on study designs, will be used as a guide to appraise the quality of individual studies. Data on all included studies will be extracted into evidence tables. Where statistically possible, a meta-analytic approach will be used to give an overall summary effect. All key findings from evidence will be presented in GRADE profiles and further summarised in evidence statements. |
| Key papers | Funk DJ, HayGlass KT, Koulack J, Harding G, Boyd A, Brinkman R. A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Crit Care. 2015 Jun 10;19:247 [PMC free article: PMC4479246] [PubMed: 26062689] |
Review protocol for review question 26: GDT during repair of ruptured abdominal aortic aneurysms
| Review question 26 | Is goal-directed therapy effective during the surgical repair of a ruptured abdominal aortic aneurysm? |
|---|---|
| Objectives | To assess the use of goal-directed therapy in people undergoing surgery for a ruptured abdominal aortic aneurysm |
| Type of review | Intervention |
| Language | English only |
| Study design | Systematic reviews of study designs listed below Randomised controlled trials Quasi-randomised controlled trials If insufficient evidence identified, non-randomised controlled trials |
| Status | Published papers only (full text) No date restrictions |
| Population | People undergoing surgery for a ruptured abdominal aortic aneurysm |
| Intervention | Goal-directed fluid and/or ionotrope administration ‘Goal-directed’ refers to the use of cardiac output monitoring to tailor fluid administration to a maximal cardiac output |
| Comparator | Standard practice Fluid and/or ionotrope administration based on static preload parameters and traditional hemodynamics |
| Outcomes |
|
| Other criteria for inclusion / exclusion of studies | Exclusion: Non-English language Abstract/non-published Pharmacological interventions not available in the UK |
| Baseline characteristics to be extracted in evidence tables | Age Sex Size of aneurysm Comorbidities |
| Search strategies | See Appendix B |
| Review strategies | Appropriate NICE Methodology Checklists, depending on study designs, will be used as a guide to appraise the quality of individual studies. Data on all included studies will be extracted into evidence tables. Where statistically possible, a meta-analytic approach will be used to give an overall summary effect. All key findings from evidence will be presented in GRADE profiles and further summarised in evidence statements. |
| Key papers | Funk DJ, HayGlass KT, Koulack J, Harding G, Boyd A, Brinkman R. A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Crit Care. 2015 Jun 10;19:247 [PMC free article: PMC4479246] [PubMed: 26062689] |
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review questions 14 and 26
Medline Strategy, searched 27th June 2017 Database: Ovid MEDLINE(R) 1946 to June Week 3 2017 Search Strategy: |
|---|
| 1 Aortic Aneurysm, Abdominal/ |
| 2 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco-abdom* or aort* or spontan* or juxtarenal* or juxta-renal* or juxta renal* or paraerenal* or para-renal* or para renal* or suprarenal* or supra renal* or supra-renal* or short neck* or short-neck* or shortneck* or visceral aortic segment*)).tw. |
| 3 Aortic Rupture/ |
| 4 (AAA or RAAA).tw. |
| 5 (endovascular* adj4 aneurysm* adj4 repair*).tw. |
| 6 (endovascular* adj4 aort* adj4 repair*).tw. |
| 7 (EVAR or EVRAR or FEVAR or F-EAVAR or BEVAR or B-EVAR).tw. |
| 8 (Anaconda or Zenith Dynalink or Hemobahn or Luminex* or Memoth-erm or Wallstent).tw. |
| 9 (Viabahn or Nitinol or Hemobahn or Intracoil or Tantalum).tw. |
| 10 or/1–9 |
| 11 X-Rays/ |
| 12 (x-ray* or x ray* or xray* or x-radiation* or x radiation* or roentgen ray* or grenz ray* or radiograph*).tw. |
| 13 Aortography/ |
| 14 aortograph*.tw. |
| 15 Tomography, X-Ray Computed/ |
| 16 (cat scan* or ct scan* or cine ct or cine-ct or tomodensitomet*).tw. |
| 17 ((computed or computer assisted or computeriz* or computeris* or electron beam* or axial*) adj4 tomograph*).tw. |
| 18 Four-Dimensional Computed Tomography/ |
| 19 (4d ct or 4dct or 4-dimensional CT or four dimensional CT).tw. |
| 20 exp Tomography, Spiral Computed/ |
| 21 ((helical or spiral) adj4 ct*).tw. |
| 22 exp Magnetic Resonance Imaging/ |
| 23 (nmr tomograph* or mr tomograph* or nmr imag* or mri scan* or functional mri* or fmri* or zeugmatograph* or cine-mri* or cinemri*).tw. |
| 24 (proton spin adj4 tomograph*).tw. |
| 25 ((chemical shift or magnetic resonance or magneti* transfer) adj4 imag*).tw. |
| 26 exp Angiography/ |
| 27 (angiograph* or arteriograph*).tw. |
| 28 exp Ultrasonography/ |
| 29 (ultrasound* or ultrason* or sonograph* or echograph* or echotomograph*).tw. |
| 30 exp Echocardiography/ |
| 31 echocardiograph*.tw. |
| 32 Finite element analysis/ |
| 33 (finite adj4 element* adj4 analys*).tw. |
| 34 (finite adj4 element* adj4 comput*).tw. |
| 35 FEA.tw. |
| 36 ((wall adj4 stress adj4 analys*) or (wall adj4 stress adj4 comput*)).tw. |
| 37 exp Computer simulation/ |
| 38 Software/ |
| 39 Image interpretation, computer-assisted/ or Radiographic image interpretation, computer-assisted/ |
| 40 Imaging Three-Dimensional/ |
| 41 exp Image enhancement/ |
| 42 Stress, mechanical/ |
| 43 (stress* adj4 mechanical*).tw. |
| 44 (scan* or imag*).tw. |
| 45 Watchful waiting/ |
| 46 (watchful adj4 waiting*).tw. |
| 47 Mass screening/ |
| 48 screen*.tw. |
| 49 Population surveillance/ |
| 50 surveillan*.tw. |
| 51 ((period* or test* or frequen* or regular* or routine* or rate or optimal* or optimis* or optimiz* or repeat* or interval*) adj4 (test* or monitor* or observ* or measur* or assess* or screen* or re-screen* or rescreen* or exam* or evaluat*)).tw. |
| 52 ((aneursym* or sign* or diameter or risk*) adj4 (grow* or siz* or measur* or expan* or ruptur* or tear* or progress* or enlarg* or dilat* or bulg* or evaluat*)).tw. |
| 53 Patient Selection/ |
| 54 ((patient or subject or criteria or treatment*) adj4 select*).tw. |
| 55 ((follow-up or follow up) adj4 (visit* or repeat* or monitor* or assess* or care*)).tw. |
| 56 Aftercare/ |
| 57 (aftercare or after-care).tw. |
| 58 Disease progression/ |
| 59 ((disease or illness or condition) adj4 (progress* or worsen* or exacerbat* or deterior* or course or duration or trajector* or improv* or recur* or relaps* or remission)).tw. |
| 60 endosure*.tw. |
| 61 ((endosensor* or intrasac*) adj4 (monitor* or transduc*)).tw. |
| 62 or/11–61 |
| 63 10 and 62 |
| 64 animals/ not humans/ |
| 65 63 not 64 |
| 66 limit 65 to english language |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom*.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov*.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree* or analys*)).tw. |
| 20 (cost or costs or costing* or costly or costed).tw. |
| 21 (price* or pricing*).tw. |
| 22 budget*.tw. |
| 23 expenditure*.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic* or (pharmaco adj economic*)).tw. |
| 26 or/1–25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly* or qald* or qale* or qtime*).tw. |
| 7 disability adjusted life.tw. |
| 8 daly*.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health* year* equivalent*.tw. |
| 19 utilit*.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili*.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble*.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1–30 |
Appendix D. Clinical evidence tables
GDT during repair of unruptured AAA
Download PDF (170K)
GDT during repair of ruptured AAA
No studies were identified as being relevant to review question 26.
Appendix E. Forest plots
GDT during repair of unruptured AAA
GDT during repair of ruptured AAA
No studies were identified as being relevant to review question 26.
Appendix F. GRADE tables
GDT during repair of unruptured AAA
Mortality
| Quality assessment | No of patients | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Need for reoperation (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
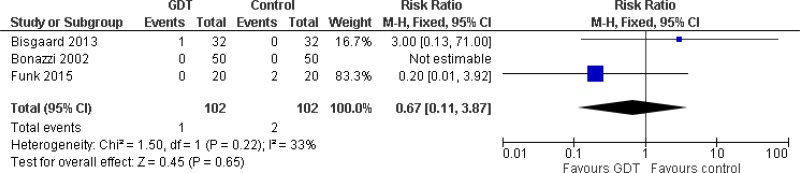
| 3, (Bisgaard 2013, Bonazzi 2002, Funk 2015) | RCT | Not serious | Not serious | Not serious | Very serious1 | 102 | 102 | RR 0.67 (0.11, 3.87) | Low |
- 1
Confidence interval crosses 2 lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Intraoperative blood loss
| Quality assessment | No of patients | Difference in medians (intervention minus control group) | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Estimated blood loss (ml; lower numbers favour intervention) | |||||||||
| 3 (Bisgaard 2013, Bonazzi 2002, Funk 2015) | RCT | Not serious | Not serious | Not serious | Very serious1 | 102 | 102 | Bisgaard diff in medians: −10 Bonazzi diff in medians: −100 Funk diff in medians: 200 (All non-significant according to the Mann-Whitney or Wilcoxon rank test) | Low |
- 1
Only median values were reported, downgrade 2 levels.
Postoperative complications: any complications
| Quality assessment | No of patients | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Any complications (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard 2013 | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 1.13 (0.69, 1.85) | Low |
- 1
Confidence interval crosses 2 lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Postoperative complications: cardiovascular complications
| Quality assessment | No of patients | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Acute coronary syndrome (including myocardial infarction; lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| 3 (Bisgaard 2013, Bonazzi 2002, Funk 2015) | RCT | Not serious | Not serious | Not serious | Very serious1 | 102 | 102 | RR 0.27 (0.05, 1.58) | Low |
| Arrhythmia (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
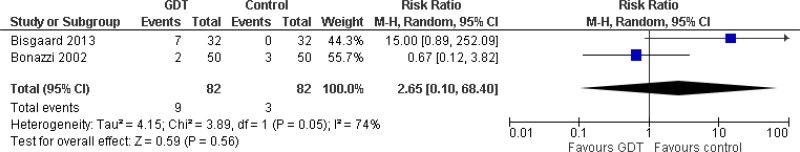
| 2 (Bisgaard 2013, Bonazzi 2002) | RCT | Not serious | Not serious | Very serious2 | Very serious1 | 82 | 82 | RR 2.65 (0.10, 68.40) | Very low |
| Dysrhythmia (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Funk (2015) | RCT | Serious3 | Not serious | N/A | Very serious1 | 20 | 20 | RR 0.67 (0.12, 3.57) | Very low |
| Congestive heart failure (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bonazzi (2002) | RCT | Not serious | Not serious | N/A | Very serious1 | 50 | 50 | RR 0.33 (0.01, 7.99) | Low |
- 1
Confidence interval crosses 2 lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
- 2
I2 value >66.7%, downgrade 2 levels.
- 3
Unclear whether an appropriate approach was used to perform randomisation, downgrade 1 level.
Postoperative complications: infection
| Quality assessment | No of patients | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Sepsis or septic shock (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
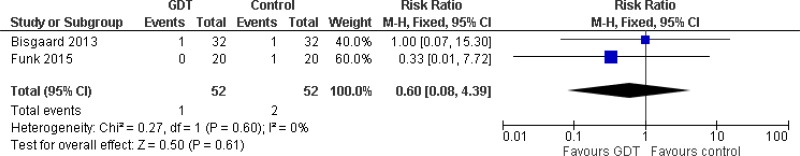
| 2 (Bisgaard 2013, Funk 2015) | RCT | Not serious | Not serious | Not serious | Very serious1 | 52 | 52 | RR 0.60 (0.08, 4.39) | Low |
| Pneumonia (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| 2 (Bonazzi 2002, Funk 2015) | RCT | Not serious | Not serious | Not serious | Very serious1 | 70 | 70 | RR 0.83 (0.27, 2.60) | Low |
| Abdominal infection (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 2.00 (0.08, 20.97) | Low |
| Deep wound infection (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 3.00 (0.13, 71.00) | Low |
| Superficial wound infection (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 0.50 (0.05, 5.24) | Low |
| Urinary tract infection (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 3.00 (0.13, 71.00) | Low |
- 1
Confidence interval crosses 2 lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Postoperative complications: other complications
| Quality assessment | No of patients | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Pulmonary oedema (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 0.20 (0.01, 4.01) | Low |
| Respiratory failure (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Funk (2015) | RCT | Serious2 | Not serious | N/A | Very serious1 | 20 | 20 | RR 0.33 (0.01, 7.72) | Very low |
| Acute kidney injury or renal failure (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
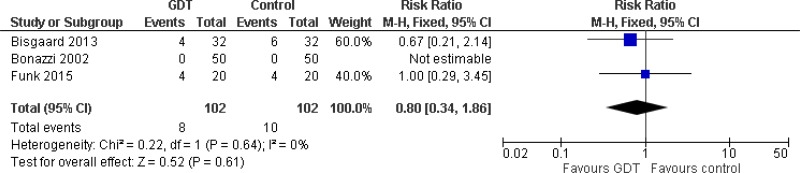
| 3 (Bisgaard 2013, Bonazzi 2002, Funk 2015) | RCT | Not serious | Not serious | Not serious | Very serious1 | 102 | 102 | RR 0.80 (0.34, 1.86) | Low |
| Creatinine kinase levels above 5000 U/I (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious2 | 32 | 32 | RR 1.00 (0.15, 6.67) | Low |
| Ischaemic gut | |||||||||
| Funk (2015) | RCT | Serious2 | Not serious | N/A | Very serious1 | 20 | 20 | RR 0.33 (0.01, 7.72) | Very low |
| Gastrointestinal paralysis (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 1.75 (0.57, 5.40) | Low |
| Severe upper gastrointestinal bleeding (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 0.33 (0.01, 7.89) | Low |
| Cerebral thrombosis (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 3.00 (0.13, 71.00) | Low |
| Lower limb paresis (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 0.33 (0.01, 7.89) | Low |
- 1
Confidence interval crosses 2 lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
- 2
Unclear whether an appropriate approach was used to perform randomisation, downgrade 1 level.
Need for additional intervention
| Quality assessment | No of patients | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Need for reoperation (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 0.82 (0.39, 1.70) | Low |
| Need for mechanical intervention (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 0.43 (0.12, 1.51) | Low |
| Need for acute dialysis (lower numbers favour intervention); effect sizes below 1 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious1 | 32 | 32 | RR 1.00 (0.15, 6.67) | Low |
| Postoperative blood transfusions (ml; lower numbers favour intervention); effect sizes below 0 favour GDT | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Serious2 | 32 | 32 | MD 77.00 (−38.76, 192.76) | Moderate |
| Postoperative use of crystalloids (Litres; lower numbers favour intervention); effect sizes below 0 favour GDT | |||||||||
| 2 (Bisgaard 2013, Funk 2015) | RCT | Not serious | Not serious | Not serious | Serious2 | 52 | 52 | MD 0.30 (0.00, 0.60 | Moderate |
| Postoperative use of colloids (Litres; lower numbers favour intervention); effect sizes below 0 favour GDT | |||||||||
| 2 (Bisgaard 2013, Funk 2015) | RCT | Not serious | Not serious | Very serious3 | Not serious | 52 | 52 | MD 0.39 (0.21, 0.58) | Low |
- 1
Confidence interval crosses 2 lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
- 2
Non-significant result, downgrade 1 level.
- 3
I2 value >66.7%, downgrade 2 levels.
Resource use
| Quality assessment | No of patients | Difference in medians (intervention minus control group) | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | Intervention | Control | Summary of results | |
| Hospital length of stay (days; lower numbers favour intervention) | |||||||||
| 3 (Bisgaard 2013, Bonazzi 2002, Funk 2015) | RCT | Not serious | Not serious | Not serious | Very serious2 | 102 | 102 | Bisgaard diff in medians: 0 Bonazzi diff in medians: 1 Funk diff in medians: 0 (All non-significant according to the Mann-Whitney or Wilcoxon rank test) | Low |
| Intensive Care Unit length of stay (hours; lower numbers favour intervention) | |||||||||
| Bisgaard (2013) | RCT | Not serious | Not serious | N/A | Very serious2 | 32 | 32 | Difference in medians: −1 (non-significant) | Low |
- 1
Only median values were reported, downgrade 2 levels.
GDT during repair of ruptured AAA
No studies were identified as being relevant to GDT during repair of ruptured AAA.
Appendix H. Excluded studies
Clinical studies
GDT during repair of unruptured AAA
| No. | Study | Reason for exclusion |
|---|---|---|
| 1 | Dentz M E, Lubarsky D A, Smith L R, McCann R L, Moskop R J, Inge W, and Grichnik K P (1995) A comparison of amrinone with sodium nitroprusside for control of hemodynamics during infrarenal abdominal aortic surgery. Journal of cardiothoracic and vascular anesthesia 9, 486–90 [PubMed: 8547546] | It is unclear whether cardiac output monitoring was used to follow a goal-oriented protocol that tailored fluid administration in order to maintain maximal cardiac output. |
| 2 | Leijdekkers V J, Vahl A C, Mackaay A J. C, Huijgens P C, and Rauwerda J A (2006) Aprotinin does not diminish blood loss in elective operations for infrarenal abdominal aneurysms: A randomized double-blind controlled trial. Annals of Vascular Surgery 20, 322–329 [PubMed: 16779513] | This study assessed the use of a fibrinolysis inhibitor (aprotinin) during elective AAA repair. It is unclear whether cardiac output monitoring was used to follow a goal-oriented protocol that tailored fluid administration in order to maintain maximal cardiac output. |
| 3 | Kassim D Y, and Esmat I M (2016) Goal directed fluid therapy reduces major complications in elective surgery for abdominal aortic aneurysm: Liberal versus restrictive strategies. Egyptian Journal of Anaesthesia 32, 167–173 | This study does not compare the efficacy of GDT with that of standard practice. Instead the study compares 2 different GDT strategies in which patients were assigned to receive 6 ml/kg/h of crystalloid (restrictive strategy) or 12 ml/kg/h of crystalloid (liberal strategy). |
| 4 | McGinley J, Lynch L, Hubbard K, McCoy D, and Cunningham A J (2001) Dopexamine hydrochloride does not modify hemodynamic response or tissue oxygenation or gut permeability during abdominal aortic surgery. Canadian journal of anaesthesia = Journal canadien d’anesthesie 48, 238–44 [PubMed: 11305823] | Investigators assessed haemodynamic, biochemical and cardiovascular effects of using dopexamine during AAA repair. These types of outcome are not listed for inclusion in the review protocol. |
| 5 | Piper S N, Boldt J, Schmidt C C, Brosch C, Maleck W H, and Berchtold C (2000) Influence of dopexamine on hemodynamics, intramucosal pH, and regulators of the macrocirculation and microcirculation in patients undergoing abdominal aortic surgery. Journal of Cardiothoracic and Vascular Anesthesia 14, 281–287 [PubMed: 10890482] | Investigators assessed haemodynamic, biochemical and cardiovascular effects of using dopexamine during AAA repair. These types of outcome are not listed for inclusion in the review protocol. |
| 6 | Ragaller M, Muller M, Bleyl J U, Strecker A, Segiet T W, Ellinger K, and Albrecht D M (2000) Hemodynamic effects of hypertonic hydroxyethyl starch 6% solution and isotonic hydroxyethyl starch 6% solution after declamping during abdominal aortic aneurysm repair. Shock (Augusta, and Ga.) 13, 367–73 [PubMed: 10807011] | Study assessed the haemodynamic, and biochemical effects of using 2 different starch solutions during AAA surgery. These types of outcome are not listed for inclusion in the review protocol. |
| 7 | Waters J H, Gottlieb A, Schoenwald P, Popovich M J, Sprung J, and Nelson D R (2001) Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesthesia and analgesia 93, 817–822 [PubMed: 11574339] | This study does not explicitly compare the efficacy of GDT with that of standard practice. All patients undergoing were monitored via arterial or venous catheters but some were randomised to receive normal saline or lactated ringer’s solution for fluid management during surgery. |
| 8 | Valentine R J, Duke M L, Inman M H, Grayburn P A, Hagino R T, Kakish H B, and Clagett G P (1998) Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. Journal of vascular surgery 27, 203–11; discussion 211–2 [PubMed: 9510275] | Study is not specific to AAA: only 51% of the study sample were people with AAAs and results were not reported separately for this subgroup |
GDT during repair of ruptured AAA
No full text papers were retrieved. All studies were excluded at review of titles and abstracts.
Economic studies
No full text papers were retrieved for this review. All studies were excluded at review of titles and abstracts.
Appendix I. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life-threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Methods, evidence and recommendations
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Perioperative utility of goal-directed therapy in high-risk cardiac patients undergoing coronary artery bypass grafting: "A clinical outcome and biomarker-based study".[Ann Card Anaesth. 2016]Perioperative utility of goal-directed therapy in high-risk cardiac patients undergoing coronary artery bypass grafting: "A clinical outcome and biomarker-based study".Kapoor PM, Magoon R, Rawat R, Mehta Y. Ann Card Anaesth. 2016 Oct-Dec; 19(4):638-682.
- Goal-Directed Fluid Therapy Using Stroke Volume Variation for Resuscitation after Low Central Venous Pressure-Assisted Liver Resection: A Randomized Clinical Trial.[J Am Coll Surg. 2015]Goal-Directed Fluid Therapy Using Stroke Volume Variation for Resuscitation after Low Central Venous Pressure-Assisted Liver Resection: A Randomized Clinical Trial.Correa-Gallego C, Tan KS, Arslan-Carlon V, Gonen M, Denis SC, Langdon-Embry L, Grant F, Kingham TP, DeMatteo RP, Allen PJ, et al. J Am Coll Surg. 2015 Aug; 221(2):591-601. Epub 2015 Apr 7.
- Implementation and effects of pulse-contour- automated SVV/CI guided goal directed fluid therapy algorithm for the routine management of pancreatic surgery patients.[Technol Health Care. 2016]Implementation and effects of pulse-contour- automated SVV/CI guided goal directed fluid therapy algorithm for the routine management of pancreatic surgery patients.Kratz T, Simon C, Fendrich V, Schneider R, Wulf H, Kratz C, Efe T, Schüttler KF, Zoremba M. Technol Health Care. 2016 Nov 14; 24(6):899-907.
- Review Goal-directed fluid therapy in the perioperative setting.[J Anaesthesiol Clin Pharmacol....]Review Goal-directed fluid therapy in the perioperative setting.Kendrick JB, Kaye AD, Tong Y, Belani K, Urman RD, Hoffman C, Liu H. J Anaesthesiol Clin Pharmacol. 2019 Apr; 35(Suppl 1):S29-S34.
- Review Perioperative goal directed therapy using automated closed-loop fluid management: the future?[Anaesthesiol Intensive Ther. 2...]Review Perioperative goal directed therapy using automated closed-loop fluid management: the future?Joosten A, Alexander B, Delaporte A, Lilot M, Rinehart J, Cannesson M. Anaesthesiol Intensive Ther. 2015; 47(5):517-23. Epub 2015 Nov 18.
- Goal-directed therapy during repair of unruptured and ruptured abdominal aortic ...Goal-directed therapy during repair of unruptured and ruptured abdominal aortic aneurysms
Your browsing activity is empty.
Activity recording is turned off.
See more...