NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Risk factors for predicting survival after abdominal aortic aneurysm rupture
Review question
Which signs, symptoms, risk factors (or combinations of these) and assessment tools predict survival in people with ruptured abdominal aortic aneurysms?
Introduction
This review question aims determine which risk factors or assessment tools are accurate in predicting survival and might therefore inform the decision to undertake surgery for a ruptured abdominal aortic aneurysm (AAA).
PICO table
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in Appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A single broad search was used to identify all studies that examine the diagnosis, surveillance or monitoring of AAAs. This was a ‘bulk’ search that covered multiple review questions. The database was sifted to identify all studies that met the criteria detailed in Table 1. The relevant review protocol can be found in Appendix A.
Table 1
Inclusion criteria.
Initially the review protocol outlined that prospective or retrospective observational studies that use multivariate logistic regression or Cox regression to explore the association between risk factors and mortality in people with ruptured AAA would be sought. However the protocol was subsequently amended to incorporate a sample size restriction to the inclusion criteria: only studies with more than 200 participants were included.
Studies were excluded if they:
- were not in English
- were not full reports of the study (for example, published only as an abstract)
Clinical evidence
Included studies
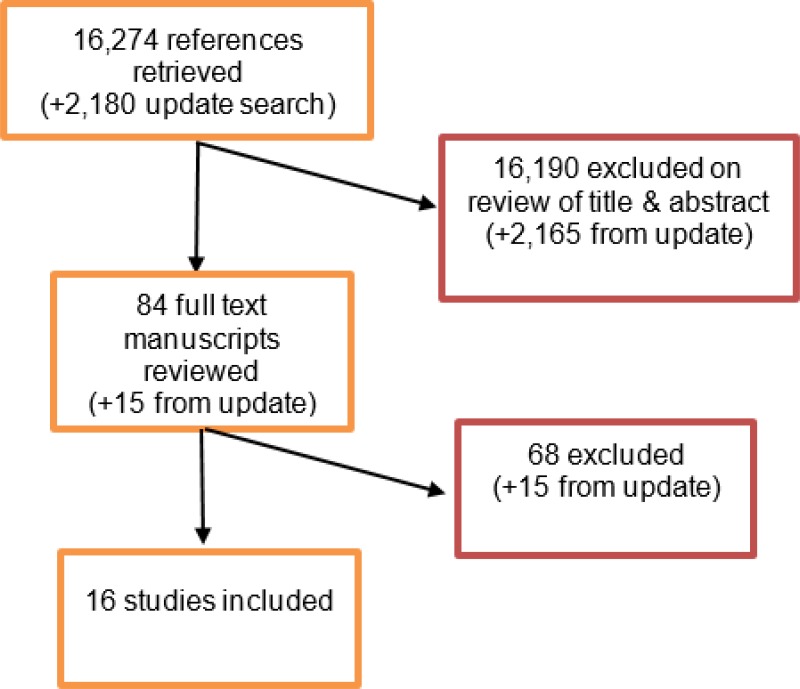
From an initial database of 16,274 abstracts, 84 were identified as being potentially relevant. Following full-text review of these articles, 16 studies were included. These included 3 prospective cohort studies and 13 retrospective cohort studies.
An update literature search was performed and provided by Cochrane, in December 2017. The search found a total of 2,180 abstracts; of which, 15 full manuscripts were ordered. Upon review of the full manuscripts, none of the studies met the inclusion criteria for this review question.
Excluded studies
The list of papers excluded at full-text review, with reasons, is given in Appendix G.
Summary of clinical studies included in the evidence review
A summary of the included studies is provided in the below table.
Table 2
Included studies.
See Appendix D for full evidence tables.
Quality assessment of clinical studies included in the evidence review
See Appendix E for full GRADE tables, highlighting the quality of evidence from the included studies.
Economic evidence
Included studies
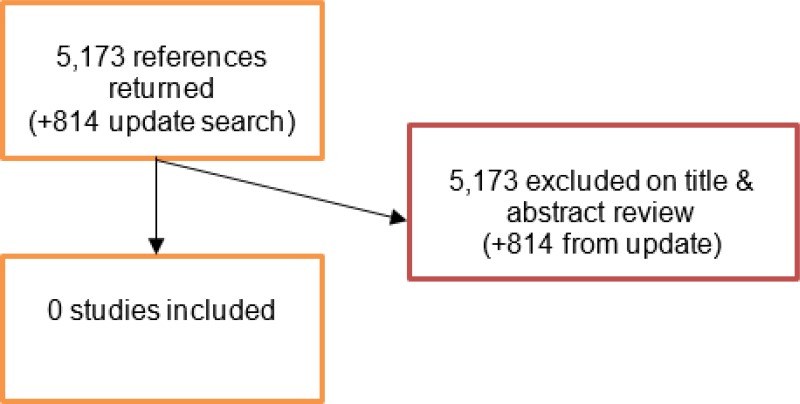
A literature search was conducted jointly for all review questions by applying standard health economic filters to a clinical search for AAA. This search returned a total of 5,173 citations. Following review of all titles and abstracts, no studies were identified as being potentially relevant to risk factors associated with aneurysm expansion or rupture. No full texts were retrieved, and so no studies were included as economic evidence.
An update search was conducted in December 2017, to identify any relevant health economic analyses published during guideline development. The search found 814 abstracts; all of which were not considered relevant to this review question. As a result no additional studies were included.
Excluded studies
No studies were retrieved for full-text review.
Evidence statements
Mortality within 48 hours
Moderate-quality evidence from 1 retrospective cohort study, including 309 people with ruptured AAA, indicated that increasing age increased the odds of death within 48 hours of open surgical repair.
30-day and in-hospital mortality
Low- to moderate-quality evidence from 1 prospective and 10 retrospective cohort studies, including 178,994 people with ruptured AAA, indicated that the following risk factors increased the odds of death within 30 days (or within hospital) after EVAR or open surgical repair:
- Increasing age
- Female gender
- Increasing aneurysm diameter
- Myocardial disease
- Liver disease
- Renal failure
- Cerebrovascular disease
- Increasing creatinine levels
- Increasing Glasgow Aneurysm Scale scores
- Increasing APACHE II scores
- Increasing American society of anaesthesiology (ASA) score
- Shock
- Cardiac arrest
- Haemoglobin <11mg/dL
Moderate- to high-quality evidence from 1 prospective and 1 retrospective cohort study, including 551 people with ruptured AAA, indicated that loss of consciousness and hypotension longer than 60 minutes increased the odds of death within 30 days (or in-hospital) of open surgical repair.
Low-quality evidence from 1 retrospective cohort study, including 27,750 people with ruptured AAA, indicated that hypertension and increasing haematocrit levels decreased the odds of death within 30 days (or in-hospital mortality) of EVAR or open surgery.
Very low-quality evidence from 3 retrospective cohort studies, including up to 30,551 people with ruptured AAA, reported inconsistent associations between COPD or congestive heart failure and the odds of death within 30 days (or in-hospital mortality) of EVAR or open surgery.
Very low- to low-quality evidence from 4 retrospective cohort studies, including up to 29,213 people with ruptured AAA, could not detect an association between coronary heart disease or diabetes and the odds of death within 30 days (or in-hospital mortality) of EVAR or open surgery.
Mortality within 5 years
Very low- to moderate-quality evidence from 2 retrospective cohort studies, including up to 2,567 people with ruptured AAA, indicated that increasing age, congestive heart disease, a history of cerebrovascular disease, cardiac arrest, loss of consciousness and a systolic blood pressure below 90 mmHg increased the odds of death within 5 years of EVAR or open surgical repair.
Very low-quality evidence from 2 retrospective cohort studies, including 2,567 people with ruptured AAA, could not detect an association between being female and the odds of death within 5 years of EVAR or open surgical repair.
Very low-quality evidence from 1 retrospective cohort study, including up to 1,463 people with ruptured AAA, could not detect an association between ischaemic heart disease and death within 5 years of EVAR or open surgical repair.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The committee considered that the most important outcome is equitable access of a patient to appropriate assessment and a balanced decision of care.
The quality of the evidence
The committee noted that some of the evidence came from retrospective cohort studies, and these studies may have been prone to selection bias because study samples (people with ruptured AAA) were determined by retrospective review of disease classification codes from data disease registers, hospital records, and health insurance provider databases. It was agreed that bias may have been introduced in some studies as investigators ascertained the presence or absence of risk factors (covariates) by retrospectively reviewing data from these data sources. Furthermore, the committee noted that studies which included mixed populations of people treated by EVAR or open surgical repair did not use type of treatment as a confounding factor in their analyses. The committee agreed there was reasonable evidence that some symptoms, signs, risk factors and assessment tools were associated with postoperative outcomes but they could not identify specific factors that were strong enough to inform the decision on whether or how to operate on people with ruptured AAA. Additionally, the committee considered that there was no evidence to demonstrate that use of these factors as decision-making tools affects care and subsequent outcomes of people with ruptured AAA. In light of this, the committee agreed that recommendations were needed to highlight that individual factors should not be used to inform treatment decisions.
The committee were mindful that AAA is a clinical area in which there is disproportionately more evidence on men than women. The committee discussed the studies highlighting that women were more likely to die from aneurysm rupture than men, and agreed that female sex could not be used as a sole factor to inform the treatment decisions. Moreover, the committee could not identify additional factors that, when combined with female sex, could be used to inform the decision to operate. As a result, the committee decided that it was not possible to make a recommendation specific to women.
Benefits and harms
The committee agreed that the evidence identified on risk assessment tools failed to show that individual tools could be used determine how a person with a ruptured AAA should be treated. The committee emphasised that there would be some potential for harm if clinicians made their decision to operate solely on the basis of risk assessment tool scores because some people would be inappropriately denied treatment. Thus, they decided to make a separate recommendation specific to risk assessment tools. The committee were aware that a few clinicians have been using the Hardman index in practice; however, it was noted that none of the identified studies assessing the Hardman index met inclusion criteria for this review (mainly due to small sample sizes). The committee were aware that the excluded evidence on the Hardman index generally highlighted that the risk assessment tool had insufficient prognostic power to inform decisions to operate.
Cost effectiveness and resource use
The committee considered that the recommendations were unlikely to have an impact on cost effectiveness and resource use.
Other factors the committee took into account
The committee discussed whether the recommendations needed to list all the risk factors that were not useful on their own for deciding whether or how to intervene in a person with a ruptured AAA. It was agreed that such a list would be cumbersome. Moreover, the committee did not want to give the impression of having identified signs, symptoms and risk factors that should not be taken into account at all; rather, they wanted to emphasise that any single factor, on its own, should not be used to define decision-making..
Appendices
Appendix A. Review protocols
Review protocol for risk factors predicting survival after abdominal aortic aneurysm rupture
| Review question 21 | Which signs, symptoms, risk factors (or combinations of these) and assessment tools predict survival in people with ruptured abdominal aortic aneurysms? |
|---|---|
| Objectives | To determine which risk factors or assessment tools are accurate in predicting survival and might therefore inform the decision to undertake surgery for a ruptured abdominal aortic aneurysm |
| Type of review | Prognostic |
| Language | English |
| Study design | Initially, the following study designs were included in the review protocol:
|
| Status | Published papers only (full text) No date restrictions |
| Population | People with a ruptured abdominal aortic aneurysm |
| Index test / factors of interest | Respiratory failure Intubation Cardiac arrest Myocardial ischaemia on ECG Hypoxia Hypotension Altered consciousness Glasgow aneurysm score Hardman index Position and anatomy of aneurysm, including morphology scores Cardiovascular disease Renal disease COPD Obesity Ethnicity Blood pressure Presence of shock |
| Endpoint | Mortality Quality of life Resource use, including length of hospital or intensive care stay, and costs |
| Other criteria for inclusion / exclusion of studies | Exclusion: Non-English language Abstract/non-published |
| Baseline characteristics to be extracted in evidence tables | Age Sex Size of aneurysm Position of aneurysm Comorbidities |
| Search strategies | To be developed |
| Review strategies | Double-sifting of randomly selected 20%. Appropriate NICE Methodology Checklists, depending on study designs, will be used as a guide to appraise the quality of individual studies. 20% will be appraised by a second reviewer. Data on all included studies will be extracted into evidence tables. Where statistically possible, a meta-analytic approach will be used to give an overall summary effect. All key findings from evidence will be presented in GRADE profiles and further summarised in evidence statements. |
| Key papers | Cadili A, Turnbull R, Hervas-Malo M, Ghosh S, Chyczij H. Identifying patients with AAA with the highest risk following endovascular repair. Vasc Endovascular Surg. 2012 Aug;46(6):455–9 [PubMed: 22717782] von Meijenfeldt,G.C.I., Ultee,K.H.J., Eefting,D., Hoeks,S.E., ten Raa,S., Rouwet,E.V., et al. Differences in mortality, risk factors, and complications after open and endovascular repair of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2014;47(5):479–86 [PubMed: 24560648] |
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review question 21
Medline Strategy, searched 29th September 2016 Database: 1946 to September Week 3 2016 Search Strategy: |
|---|
| 1 Aortic Aneurysm, Abdominal/ |
| 2 Aortic Rupture/ |
| 3 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco-abdom* or aort* or spontan* or juxtarenal* or juxta-renal* or juxta renal* or paraerenal* or para-renal* or para renal* or suprarenal* or supra renal* or supra-renal* or short neck* or short-neck* or shortneck* or visceral aortic segment*)).tw. |
| 4 or/1–3 |
| 5 prognosis.sh. |
| 6 diagnosed.tw. |
| 7 cohort.mp. |
| 8 predictor:.tw. |
| 9 death.tw. |
| 10 exp models, statistical/ |
| 11 or/5–10 |
| 12 (sensitiv: or predictive value:).mp. or accurac:.tw. |
| 13 11 or 12 |
| 14 “signs and symptoms”/ |
| 15 ((sign or signs) adj5 symptom*).tw. |
| 16 Risk Factors/ |
| 17 factor*.tw. |
| 18 predict*.tw. |
| 19 or/14–18 |
| 20 13 or 19 |
| 21 4 and 20 |
| 22 animals/ not humans/ |
| 23 21 not 22 (12444) |
| 24 limit 23 to english language |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom*.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov*.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree* or analys*)).tw. |
| 20 (cost or costs or costing* or costly or costed).tw. |
| 21 (price* or pricing*).tw. |
| 22 budget*.tw. |
| 23 expenditure*.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic* or (pharmaco adj economic*)).tw. |
| 26 or/1–25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly* or qald* or qale* or qtime*).tw. |
| 7 disability adjusted life.tw. |
| 8 daly*.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health* year* equivalent*.tw. |
| 19 utilit*.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili*.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble*.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1–30 |
Appendix D. Clinical evidence tables
Download PDF (357K)
Appendix E. GRADE tables
Mortality within 48 hours
Age
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Open surgery only | |||||||||
| Age: ≥70 vs. <70 years | Van Dongen (1998) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 309 | ORa 7.2 (2.1, 25.1) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level
30-day or in-hospital mortality
Age
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR only | |||||||||
| Age: ≥75 vs. <75 | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Very serious3,4 | 221 | ORa 1.5 (Not significant; CI not reported) | Very low |
| Open surgery only | |||||||||
Age: 69–75 >75 years, All vs. <69 years | Van Beek (2014) | Prosp. cohort | Not serious | N/A | Not serious | Serious3 | 208 | ORa 1.52 (0.63, 3.66) ORa 2.05 (0.95, 4.47) | Moderate |
| Age: >76 vs. ≤76 | Robinson (2013) | Prosp. cohort | Not serious | N/A | Not serious | Not serious | 242 | ORa 5.3 (2.8, 10.1) | High |
| Age: ≥75 vs. <75 | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Serious4 | 221 | ORa 2.8 (Significant; CI not reported) | Very low |
| Age: >80 years vs. <70 years | Van Dongen (1998) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 309 | HRa 5.1 (2.1, 11.6) | Moderate |
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Age: per year increase | 3 (Mureebe 2010, Noel 2001, Schlosser 2010) | Retro. cohort | Very serious1,5 | Not serious | Not serious | Not serious | 52,876 | ORa 1.08 (1.07, 1.08) ORa 1.05 (1.02, 1.09) HRa 1.04 (1.03, 1.05) | Moderate |
| Age: per 5 year increase | Dueck (2004) | Retro. cohort | Very serious1,5 | N/A | Not serious | Not serious | 2,601 | HRa 1.2 (1.16, 1.25) | Low |
| Age: per 10 year increase | Visser (2009) | Prosp. cohort | Not serious | N/A | Not serious | Not serious | 201 | ORa 2.21 (1.18, 4.13) | Low |
Age: 65–79, ≥80 years All vs. <65 years | Trenner 2015 | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 4,859 | ORa 1.82 (1.46, 2.28) ORa 3.75 (2.94, 4.78) | Moderate |
Age: 60–70, 70–80, ≥80 years All vs. <60 | Giles (2009) | Retro. cohort | Serious5 | N/A | Not serious | Not serious | 28,429 | ORa 1.6 (1.3, 2.1) ORa 2.4 (1.9, 3.1) ORa 4.2 (3.2, 5.4) | Moderate |
| Age > 70 Vs. <70 | Heller (2000) | Retro. cohort | Very serious1,2,5 | N/A | Not serious | Not serious | 67,751 | ORa 4.8 (3.0, 78.0) | Low |
| Age: >80 vs. <80 | McPhee (2009) | Retro. cohort | Very serious1,5 | N/A | Not serious | Not serious | 27,750 | ORa 1.95 (1.72, 2.22) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models; downgrade 1 level.
- 3
95% CI crosses the line of no effect (1); downgrade 1 level.
- 4
95% CI not reported; downgrade 1 level
- 5
Patients with ruptured AAA (study sample) was determined by retrospectively reviewing classification codes (such as ICD 9 codes) in disease registers and health insurance provider databases; downgrade 1 level.
Sex
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Open surgery only | |||||||||
| Female | Van Beek (2014) | Prosp. cohort | Not serious | N/A | Not serious | Serious1 | 208 | ORa 1.53 (0.79, 2.99) | Moderate |
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Female | 6 (Dueck 2004, Giles 2009, McPhee 2009, Mureebe 2010, Schlosser 2010, Heller 2000) | Retro. cohort | Very serious2,3 | Not serious | Not serious | Not serious | 178,994 | HRa 1.2 (1.05, 1.38) ORa 1.3 (1.1, 1.4) ORa 1.41 (1.23, 1.61) ORa 1.53 (1.47, 1.58) HRa 1.11 (0.92, 1.34) ORa 3.0 (1.7, 5.2) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
95% CI crosses the line of no effect (1); downgrade 1 level.
- 2
Patients with ruptured AAA (study sample) was determined by retrospectively reviewing classification codes (such as ICD 9 codes) in disease registers and health insurance provider databases; downgrade 1 level.
- 3
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
Aneurysm diameter & anatomy
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR or open surgery (or surgical approach not specified) | |||||||||
Diameter: 50–59 60–69 ≥70 mm All vs. <50mm | Trenner 2015 | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 4,859 | ORa 1.33 (0.94, 1.87) ORa 1.56 (1.16, 2.09) ORa 2.31 (1.37, 2.36) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
Comorbid conditions
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Open surgery only | |||||||||
| COPD | Van Beek (2014) | Prosp. cohort | Not serious | N/A | Not serious | Not serious | 208 | ORa 2.33 (1.17, 4.64) | High |
| Cardiac comorbidity (arrhythmia, cardiac surgery or myocardial infarction) | Van Beek (2014) | Prosp. cohort | Not serious | N/A | Not serious | Serious1 | 208 | ORa 1.23 (0.70, 2.24) | Moderate |
| Renal comorbidity (history of chronic kidney failure or dialysis) | Van Beek (2014) | Prosp. cohort | Not serious | N/A | Not serious | Serious1 | 208 | ORa 1.39 (0.60, 3.25) | Moderate |
| Cerebrovascular comorbidity previous history of transient ischemic attack or stroke) | Van Beek (2014) | Prosp. cohort | Not serious | N/A | Not serious | Serious1 | 208 | ORa 1.29 (0.60, 2.77) | Moderate |
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| COPD | 2 (Brahmbhatt 2016, McPhee 2009) | Retro. cohort | Very serious2,3 | Not Serious4 | Not serious | Not serious | 30,551 | ORa 2.4 (1.7, 3.4) ORa 0.74 (0.62, 0.89) | Very low |
| Congestive heart failure | 2 (McPhee 2009, Schlosser 2010) | Retro. cohort | Very serious2,3 | Not Serious4 | Not serious | Not serious | 29,213 | ORa 0.83 (0.57, 1.23) HRa 1.52 (1.03, 2.25) | Very low |
| Ischaemic/coronary heart disease | Schlosser (2010) | Retro. cohort | Very serious2,3 | N/A | Not serious | Serious1 | 1,463 | HRa 1.01 (0.78, 1.30) | Very low |
| Myocardial disease (myocardial infarction and/or angina pectoris) | Visser (2009) | Prosp. cohort | Not serious | N/A | Not serious | Not serious | 201 | ORa 1.18 (1.13, 2.89) | High |
| Hypertension | McPhee (2009) | Retro. cohort | Very serious2,3 | N/A | Not serious | Not serious | 27,750 | ORa 0.72 (0.60, 0.87) | Low |
| Liver disease | McPhee (2009) | Retro. cohort | Very serious2,3 | N/A | Not serious | Not serious | 27,750 | ORa 3.35 (1.94, 5.78) | Low |
| Renal failure | 3 (McPhee 2009, Heller 2000, Visser 2009) | 2 Retro. and 1 prosp. cohort | Very serious2,3 | N/A | Not serious | Not serious | 95,702 | ORa 1.07 (0.84, 1.37) ORa 2.5 (1.3, 4.9) ORa 3.03 (1.55, 5.89) | Low |
| Diabetes | 2 (McPhee 2009, Schloesser 2010) | Retro. cohort | Very serious2,3 | N/A | Not serious | Serious1 | 29,213 | ORa 1.17 (0.92, 1.48) HRa 1.09 (0.73, 1.62) | Very low |
| Cerebrovascular disease | 2 (Schlosser 2010, Visser 2009) | 1 Retro. and 1 prosp. cohort | Very serious2,3 | Not serious | Not serious | Not serious | 1,664 | HRa 1.44 (1.01, 2.06) HRa 2.20 (1.19, 4.07) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
95% CI crosses the line of no effect (1); downgrade 1 level.
- 2
Patients with ruptured AAA (study sample) was determined by retrospectively reviewing classification codes (such as ICD 9 codes) in disease registers and health insurance provider databases; downgrade 1 level.
- 3
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
- 4
95% CIs of one of two similar sized studies crosses line of no effect (non-significant result), making it difficult to ascertain the overall significance of the evidence as a whole, downgrade 1 level.
Preoperative clinical tests
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR only | |||||||||
| Haemoglobin <11mg/dL | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Serious3 | 221 | ORa 3.24 (significant; CI not reported) | Very low |
| eGFR <60 | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Very serious3,4 | 221 | ORa 1.5 (Not significant; CI not reported) | Very low |
| Open surgery only | |||||||||
| Haemoglobin <11mg/dL | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Serious3 | 221 | ORa 3.4 (significant; CI not reported) | Very low |
| eGFR <60 | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Very serious3,4 | 221 | ORa 1.5 (Not significant; CI not reported) | Very low |
| Duration of hypotension: >60 mins vs. <30 mins | Van Dongen (1998) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 309 | HRa 2.2 (1.0, 5.0) *significant | Moderate |
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Creatinine: per g/dL increase | Brahmbhatt (2016) | Retro. cohort | Very serious1,2,5 | N/A | Not serious | Not serious | 2,761 | ORa 1.2 (1.0, 1.2) *significant | Low |
| Haematocrit: per percentage increase | 2 (Brahmbhatt2016, Noel 2001) | Retro. cohort | Very serious1,2,5 | Not serious | Not serious | Not serious | 3,192 | ORa 0.94 (0.91, 0.97) ORa 0.98 (0.95, 1.0) *significant | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models; downgrade 1 level.
- 3
95% CI not reported; downgrade 1 level
- 4
95% CI crosses the line of no effect (1); downgrade 1 level.
- 5
Patients with ruptured AAA (study sample) was determined by retrospectively reviewing classification codes (such as ICD 9 codes) in disease registers and health insurance provider databases; downgrade 1 level.
Shock, cardiac arrest, and loss of consciousness
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR only | |||||||||
| Shock | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Serious3,4 | 221 | ORa 0.73 (Not significant; CI not reported) | Very low |
| Open surgery only | |||||||||
| Shock | Von Meijenfeldt (2014) | Retro. cohort | Very serious1,2 | N/A | Not serious | Serious3 | 221 | ORa 2.4 (significant; CI not reported) | Very low |
| Cardiac arrest | Robinson (2013) | Prosp. cohort | Not serious | N/A | Not serious | Not serious | 242 | ORa 4.3 (1.6, 12.0) | High |
| Loss of consciousness | Robinson (2013) | Prosp. cohort | Not serious | N/A | Not serious | Not serious | 242 | ORa 2.7 (1.2, 6.0) | High |
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Shock | 2 (Korhonen 2004, Visser 2009) | 1 retro. and 1 prop. cohort | Serious1 | Not serious | Not serious | Not serious | 1,037 | ORa 2.13 (1.45, 3.11) ORa 3.82 (2.29, 6.38) | Moderate |
| Cardiac arrest | Noel (2001) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 413 | ORa 3.14 (1.39, 7.11) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
- 2
Stepwise regression was not performed. Instead, variables found to be significant in univariate analyses were input into logistic regression models; downgrade 1 level.
- 3
95% CI not reported; downgrade 1 level
- 4
95% CI crosses the line of no effect (1); downgrade 1 level.
Risk assessment tool score
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Per 10 unit increase in GAS score | Korhonen (2004) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 836 | ORa 1.81 (1.54, 2.12) | Low |
| Per unit increase in APACHE II score | Noel (2001) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 413 | ORa 1.05 (1.01, 1.09) | Moderate |
ASA score: 3 4–5 All vs 1–2 ASA: American Society of Anaesthesiologists | Trenner (2015) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 4,859 | ORa 1.86 (1.41, 2.45) ORa 4.95 (3.73, 6.56) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
Long term (5-year) mortality
Age
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Age: per year increase | Schlosser 2010 | Retro. cohort | Very serious1,2 | N/A | Not serious | Not serious | 1,463 | HRa 1.05 (1.04, 1.06) | Low |
| Age: per 5 year increase | Robinson (2016) | Retro. cohort | Serious2 | N/A | Not serious | Not serious | 1,104 | ORa 1.4 (1.3, 1.4) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Patients with ruptured AAA (study sample) was determined by retrospectively reviewing classification codes (such as ICD 9 codes) in disease registers and health insurance provider databases; downgrade 1 level.
- 2
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
Sex
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Female | 2 (Robinson 2016, Schlosser 2010) | Retro. cohort | Very serious1,2 | N/A | Not serious | Serious3 | 2,567 | ORa 1.3 (1.03, 1.6) HRa 1.14 (0.96, 1.35) | Very low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Patients with ruptured AAA (study sample) was determined by retrospectively reviewing classification codes (such as ICD 9 codes) in disease registers and health insurance provider databases; downgrade 1 level.
- 2
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
- 3
Visual inspection of point estimates and 95% CIs across studies indicates inconsistent findings, downgrade 1 level.
Comorbid conditions
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Congestive heart disease | Schlosser (2010) | Retro. cohort | Very serious1,2 | N/A | Not serious | Not serious | 1,463 | HRa 1.55 (1.06, 2.26) | Low |
| Ischaemic heart disease | Schlosser (2010) | Retro. cohort | Very serious1,2 | N/A | Not serious | Serious3 | 1,463 | HRa 1.05 (0.84, 1.32) | Very low |
| History of cerebrovascular disease | 2 (Robinson 2016, Schlosser 2010) | Retro. cohort | Very serious1,2 | Not serious | Not serious | Not serious | 2,567 | ORa 1.7 (1.1, 2.7) HRa 1.60 (1.16, 2.21) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Patients with ruptured AAA (study sample) was determined by retrospectively reviewing classification codes (such as ICD 9 codes) in disease registers and health insurance provider databases; downgrade 1 level.
- 2
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
- 3
95% CI crosses the line of no effect (1); downgrade 1 level.
Preoperative clinical tests
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Systolic blood pressure: <90mmHG vs. >90mmHG | Robinson (2016) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 1,104 | ORa 1.4 (1.1, 1.7) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level.
Cardiac arrest, loss of consciousness, shock
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| EVAR or open surgery (or surgical approach not specified) | |||||||||
| Cardiac arrest | Robinson (2016) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 1,104 | ORa 2.9 (2.2, 3.9) | Moderate |
| Loss of consciousness | Robinson (2016) | Retro. cohort | Serious1 | N/A | Not serious | Not serious | 1,104 | ORa 1.7 (1.3, 2.2) | Moderate |
- a
As multivariate analyses were performed hazard, and odds ratios were reported adjusting for confounders or other factors.
- 1
Investigators ascertained the presence/absence of risk factors (covariates) by retrospectively reviewing data from disease registers, health insurance provider databases, or hospital records; downgrade 1 level
Appendix G. Excluded studies
Clinical studies
| No. | Study | Reason for exclusion |
|---|---|---|
| 1 | Aburahma A F, Woodruff B A, Stuart S P et al. (1991) Early diagnosis and survival of ruptured abdominal aortic aneurysms. The American journal of emergency medicine 9(2), 118–21 [PubMed: 1994936] | Sample size less than 200 participants. |
| 2 | AbuRahma A F, Woodruff B A, Lucente F et al. (1991) Factors affecting survival of patients with ruptured abdominal aortic aneurysm in a West Virginia community. Surgery Gynecology and Obstetrics 172(5), 377–382 [PubMed: 2028372] | Sample size less than 200 participants. |
| 3 | Acosta S, Lindblad B, and Zdanowski Z (2007) Predictors for Outcome after Open and Endovascular Repair of Ruptured Abdominal Aortic Aneurysms. European Journal of Vascular and Endovascular Surgery 33(3), 277–284 [PubMed: 17097899] | Sample size less than 200 participants. |
| 4 | Ahn Hyo Yeong, Chung Sung Woon, Lee Chung Won et al. (2012) Factors affecting the postoperative mortality in the ruptured abdominal aortic aneurysm. The Korean journal of thoracic and cardiovascular surgery 45(4), 230–5 [PMC free article: PMC3413827] [PubMed: 22880167] | Abstract states multivariate was performed but there is no mention of such analysis in the full manuscript: only univariate analysis is mentioned. As a result, it was not possible to ascertain which results were obtained from multivariate analysis. |
| 5 | Alexander S, Bosch J L, Hendriks J M et al. (2008) The 30-day mortality of ruptured abdominal aortic aneurysms: influence of gender, age, diameter and comorbidities. The Journal of cardiovascular surgery 49(5), 633–7 [PubMed: 18670381] | Sample size less than 200 participants. |
| 6 | Alonso-Perez M, Segura R J, Sanchez J et al. (2001) Factors increasing the mortality rate for patients with ruptured abdominal aortic aneurysms. Annals of vascular surgery 15(6), 601–7 [PubMed: 11769139] | Sample size less than 200 participants. |
| 7 | Anain Paul M, Anain Joseph M, Sr ,Tiso Michael et al. (2007) Early and mid-term results of ruptured abdominal aortic aneurysms in the endovascular era in a community hospital. Journal of vascular surgery 46(5), 898–905 [PubMed: 17980277] | Sample size less than 200 participants. |
| 8 | Antonello M, Frigatti P, Maturi C et al. (2009) Open repair for ruptured abdominal aortic aneurysm: is it possible to predict survival?. Annals of vascular surgery 23(2), 159–66 [PubMed: 18834704] | Sample size less than 200 participants. |
| 9 | Antonopoulos Constantine N, Kakisis John D, Andrikopoulos Vasilios, et al. (2014) Predictors affecting in-hospital mortality of ruptured abdominal aortic aneurysms: a Greek multicenter study. Annals of vascular surgery 28(6), 1384–90 [PubMed: 24517989] | Sample size less than 200 participants. |
| 10 | Aranson Nathan J, Lancaster Robert T, Ergul Emel, et al. (2016) Chronic Kidney Disease Class Predicts Mortality After Abdominal Aortic Aneurysm Repair in Propensity-matched Cohorts From the Medicare Population. Annals of surgery 264(2), 386–91 [PubMed: 27414155] | Not specific to ruptured AAA: all patients underwent elective AAA repair. |
| 11 | Baderkhan H, Goncalves F M. B, Oliveira N G, et al. (2016) Challenging Anatomy Predicts Mortality and Complications after Endovascular Treatment of Ruptured Abdominal Aortic Aneurysm. Journal of Endovascular Therapy 23(6), 1–9 [PubMed: 27385153] | Sample size less than 200 participants |
| 12 | Biancari F, Venermo M, Finnish Arterial Disease, and Investigators (2011) Open repair of ruptured abdominal aortic aneurysm in patients aged 80 years and older. The British journal of surgery 98(12), 1713–8 [PubMed: 22034180] | Sample size less than 200 participants. |
| 13 | Bonardelli Stefano, Cervi Edoardo, Maffeis Roberto et al. (2011) Open surgery in endovascular aneurysm repair era: simplified classification in two risk groups owing to factors affecting mortality in 137 ruptured abdominal aortic aneurysms (RAAAs). Updates in surgery 63(1), 39–44 [PMC free article: PMC3047051] [PubMed: 21336876] | Sample size less than 200 participants. |
| 14 | Botha J A, Tiruvoipati R, Last G C et al. (2008) Predictors of outcome of ruptured aortic aneurysms in a metropolitan hospital. Anaesthesia and intensive care 36(4), 560–4 [PubMed: 18714626] | Sample size less than 200 participants. |
| 15 | Bown M J, Cooper N J, Sutton A J, et al. (2004) The post-operative mortality of ruptured abdominal aortic aneurysm repair. European Journal of Vascular and Endovascular Surgery 27(1), 65–74 [PubMed: 14652840] | Sample size less than 200 participants. |
| 16 | Cadili Ali, Turnbull Robert, Hervas-Malo Marilou et al. (2012) Identifying patients with AAA with the highest risk following endovascular repair. Vascular and endovascular surgery 46(6), 455–9 [PubMed: 22717782] | Not specific to ruptured AAA: investigators assessed all patients undergoing EVAR at a single centre, regardless of whether their aneurysms had ruptured or not. Additionally, the study included less than 200 participants. |
| 17 | Chagpar Ryaz B, Harris Jeremy R, Lawlor D Kirk et al. (2010) Early mortality following endovascular versus open repair of ruptured abdominal aortic aneurysms. Vascular and endovascular surgery 44(8), 645–9 [PubMed: 20675315] | Sample size less than 200 participants. |
| 18 | Chen J C, Hildebrand H D, Salvian A J et al. (1996) Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. Journal of Vascular Surgery 24(4), 614–623 [PubMed: 8911410] | Sample size less than 200 participants. |
| 19 | Cho Jae-Sung, Kim Jang Yong, Rhee Robert Y et al. (2008) Contemporary results of open repair of ruptured abdominal aortoiliac aneurysms: effect of surgeon volume on mortality. Journal of vascular surgery 48(1), 10–8 [PubMed: 18515039] | Sample size less than 200 participants. |
| 20 | De Rango , P , Simonte G, Manzone A, et al. (2016) Arbitrary Palliation of Ruptured Abdominal Aortic Aneurysms in the Elderly is no Longer Warranted. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 51(6), 802–9 [PubMed: 27055926] | Sample size less than 200 participants. |
| 21 | De Rango , P , Simonte G, Manzone A, et al. (2017) Mortality Risk for Ruptured Abdominal Aortic Aneurysm in Women. Annals of vascular surgery 39, 143–151 [PubMed: 27789318] | Sample size less than 200 participants |
| 22 | Dingemans Siem A, Jonker Frederik H. W, Moll Frans L, et al. (2016) Aneurysm Sac Enlargement after Endovascular Abdominal Aortic Aneurysm Repair. Annals of vascular surgery 31, 229–38 [PubMed: 26627324] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 23 | Goncalves F B, Ultee K H. J, Hoeks S E, et al. (2016) Life expectancy and causes of death after repair of intact and ruptured abdominal aortic aneurysms Presented in the Plenary Rapid Pace Session at the 2015 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, Ill, June 17–20, 2015. Journal of vascular surgery 63(3), 610–6 | Not specific to ruptured AAA: authors pooled data from patients with unruptured and ruptured aneurysms. |
| 24 | Guo Q, Du X, Zhao J, et al. (2017) Prevalence and risk factors of type II endoleaks after endovascular aneurysm repair: A meta-analysis. PLoS ONE 12(2), 0170600 [PMC free article: PMC5300210] [PubMed: 28182753] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 25 | Gutierrez-Morlote J, Llorca J, Ibanez de Elejalde, et al. (2002) Predictors of mortality in patients undergoing surgery for ruptured aortic aneurysm. Vasa - Journal of Vascular Diseases 31(4), 265–268 [PubMed: 12510552] | Sample size less than 200 participants. |
| 26 | Gwon JG, Kwon TW, Cho YP, et al. (2016) Analysis of in hospital mortality and long-term survival excluding in hospital mortality after open surgical repair of ruptured abdominal aortic aneurysm. Annals of surgical treatment and research 91(6), 303–308 [PMC free article: PMC5128376] [PubMed: 27904852] | Not specific to ruptured AAA: the study sample included patients with intact AAA (81.6%) and ruptured AAA (18.4%). |
| 27 | Halpern V J, Kline R G, D’Angelo A J, et al. (1997) Factors that affect the survival rate of patients with ruptured abdominal aortic aneurysms. Journal of Vascular Surgery 26(6), 939–948 [PubMed: 9423708] | Sample size less than 200 participants. |
| 28 | Hardman D T, Fisher C M, Patel M I et al. (1996) Ruptured abdominal aortic aneurysms: who should be offered surgery?. Journal of vascular surgery 23(1), 123–9 [PubMed: 8558727] | Sample size less than 200 participants. |
| 29 | Hashimoto Makoto, Ito Toshiro, Kurimoto Yoshihiko et al. (2013) Preoperative arterial blood lactate levels as a predictor of hospital mortality in patients with a ruptured abdominal aortic aneurysm. Surgery today 43(2), 136–40 [PubMed: 23212703] | Sample size less than 200 participants. |
| 30 | Healey CT, Neilson M, Clark D, et al. (2017) Predicting Mortality of Ruptured Abdominal Aortic Aneurysms in the Era of Endovascular Repair. Annals of vascular surgery 38, 59–63 [PubMed: 27794443] | Conference abstract |
| 31 | Ho Man-Fung, Chan Yiu-Che, Cheung Grace C et al. (2014) Multicenter audit of emergency endovascular repair of infrarenal aortic aneurysms. Annals of vascular surgery 28(3), 560–7 [PubMed: 24090827] | Sample size less than 200 participants. |
| 32 | Hultgren R, Granath F, and Swedenborg J et al. (2007) Different Disease Profiles for Women and Men with Abdominal Aortic Aneurysms. European Journal of Vascular and Endovascular Surgery 33(5), 556–560 [PubMed: 17239633] | Not specific to ruptured AAA: authors pooled data from patients with unruptured and ruptured aneurysms. |
| 33 | Janczyk Randy J, Howells Greg A, Bair Holly A et al. (2004) Hypothermia is an independent predictor of mortality in ruptured abdominal aortic aneurysms. Vascular and endovascular surgery 38(1), 37–42 [PubMed: 14760475] | Sample size less than 200 participants. |
| 34 | Jang HN, Park HO, Yang JH, Yang TW, et al. (2017) Evaluation of Preoperative Predictors of 30-Day Mortality in Patients with Ruptured Abdominal Aortic Aneurysm. Vascular specialist international 33(3), 93–98 [PMC free article: PMC5614377] [PubMed: 28955698] | Sample size less than 200 participants. |
| 35 | Johnston K W, Ameli F M, Au H H, Baird R J, Balachandra V K et al. (1994) Ruptured abdominal aortic aneurysm: Six-year follow-up results of a multicenter prospective study. Journal of Vascular Surgery 19(5), 888–900 [PubMed: 8170044] | Sample size less than 200 participants. |
| 36 | Karthikesalingam A, Holt P J, Vidal-Diez A, Ozdemir B A et al. (2014) Mortality from ruptured abdominal aortic aneurysms: Clinical lessons from a comparison of outcomes in England and the USA. The Lancet 383(9921), 963–969 [PubMed: 24629298] | Multivariate regression was not performed to assess risk factors associated with survival/mortality. |
| 37 | Kauvar David S, Sarfati Mark R, and Kraiss Larry W (2012) Intraoperative blood product resuscitation and mortality in ruptured abdominal aortic aneurysm. Journal of vascular surgery 55(3), 688–92 [PubMed: 22277689] | Sample size less than 200 participants. |
| 38 | Kim Sang Dong, Hwang Jeong Kye, Park Sun Cheol et al. (2012) Predictors of postoperative mortality of ruptured abdominal aortic aneurysm: a retrospective clinical study. Yonsei medical journal 53(4), 772–80 [PMC free article: PMC3381467] [PubMed: 22665345] | Sample size less than 200 participants. |
| 39 | Kordzadeh A, Malietzis G, Browne T et al. (2015) Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): A retrospective cohort study. International Journal of Surgery 15, 45–48 [PubMed: 25641718] | Sample size less than 200 participants. |
| 40 | Krenzien Felix, Matia Ivan, Wiltberger Georg et al. (2014) Early prediction of survival after open surgical repair of ruptured abdominal aortic aneurysms. BMC surgery 14, 92 [PMC free article: PMC4246487] [PubMed: 25403513] | Sample size less than 200 participants. |
| 41 | Kurc Erol, Sanioglu Soner, Ozgen Ayca et al. (2012) Preoperative risk factors for in-hospital mortality and validity of the Glasgow aneurysm score and Hardman index in patients with ruptured abdominal aortic aneurysm. Vascular 20(3), 150–5 [PubMed: 22393179] | Sample size less than 200 participants. |
| 42 | Lambert M E, Baguley P, and Charlesworth D (1986) Ruptured abdominal aortic aneurysms. The Journal of cardiovascular surgery 27(3), 256–61 [PubMed: 3958027] | Sample size less than 200 participants. |
| 43 | Li Hao-Jui, Kao Tsung-Chi, Liu Dah-Wel, et al. (2011) Predictors of outcome after open repair of ruptured abdominal aortic aneurysms. Chang Gung medical journal 34(5), 520–7 [PubMed: 22035897] | Sample size less than 200 participants. |
| 44 | Lo Albert, and Adams Dave (2004) Ruptured abdominal aortic aneurysms: risk factors for mortality after emergency repair. The New Zealand medical journal 117(1203), U1100 [PubMed: 15477924] | Sample size less than 200 participants. |
| 45 | Mathisen SR, and Abdelnoor M (2017) Beneficial effect of statins on total mortality in abdominal aortic aneurysm (AAA) repair. Vascular medicine (London, and England) 22(5), 406–410 [PubMed: 28835175] | Out of scope: this retrospective cohort study explores the efficacy of a postoperative pharmacological intervention on mortality rates after AAA repair. |
| 46 | Maynard N D, Taylor P R, Mason R C et al. (1996) Gastric intramucosal pH predicts outcome after surgery for ruptured abdominal aortic aneurysm. European Journal of Vascular and Endovascular Surgery 11(2), 201–206 [PubMed: 8616653] | Multivariate analysis was not performed to assess risk factors associated with survival/mortality. |
| 47 | McCready R A, Siderys H, Pittman J N et al. (1993) Ruptured abdominal aortic aneurysms in a private hospital: a decade’s experience (1980–1989). Annals of vascular surgery 7(3), 225–8 [PubMed: 8318385] | Sample size less than 200 participants. |
| 48 | Mell Matthew W, O’Neil Amy S, Callcut Rachael A et al. (2010) Effect of early plasma transfusion on mortality in patients with ruptured abdominal aortic aneurysm. Surgery 148(5), 955–62 [PubMed: 20378142] | Sample size less than 200 participants. |
| 49 | Mell Matthew W, Callcut Rachael A, Bech Fritz et al. (2012) Predictors of emergency department death for patients presenting with ruptured abdominal aortic aneurysms. Journal of vascular surgery 56(3), 651–5 [PubMed: 22560234] | Study assessed hospital-related risk factors such as, rural versus urban, teaching versus non-teaching, emergency department volume, and region. |
| 50 | Montan Carl, Johansson Fredrik, Hedin Ulf et al. (2015) Preoperative hypofibrinogenemia is associated with increased intraoperative bleeding in ruptured abdominal aortic aneurysms. Thrombosis research 135(3), 443–8 [PubMed: 25455998] | Sample size less than 200 participants. |
| 51 | Morisaki K, Yamaoka T, Iwasa K et al. (2017) Preoperative risk factors for aneurysm sac expansion caused by type 2 endoleak after endovascular aneurysm repair. Vascular 25(5), 533–541 [PubMed: 28395595] | Out of scope: study explores outcomes of patients with unruptured aneurysms who underwent AAA repair procedures |
| 52 | Nakayama Atsuko, Morita Hiroyuki, Miyata Tetsuro, Hoshina Katsuyuki, Nagayama Masatoshi, Takanashi Shuichiro, Sumiyoshi Tetsuya, Komuro Issei, and Nagai Ryozo (2014) Predictors of mortality after emergency or elective repair of abdominal aortic aneurysm in a Japanese population. Heart and vessels 29(1), 65–70 [PubMed: 23274579] | Sample size less than 200 participants. |
| 53 | Nie W, Wang Y, Yao K et al. (2016) Serum angiotensin-converting enzyme 2 is an independent risk factor for in-hospital mortality following open surgical repair of ruptured abdominal aortic aneurysm. Experimental and Therapeutic Medicine 12(3), 1412–1418 [PMC free article: PMC4998157] [PubMed: 27602068] | Sample size less than 200 participants. |
| 54 | Opfermann P, von Allmen , R , Diehm N et al. (2011) Repair of ruptured abdominal aortic aneurysm in octogenarians. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 42(4), 475–83 [PubMed: 21693385] | Sample size less than 200 participants. |
| 55 | Ouriel K, Geary K, Green R M, Fiore W et al. (1990) Factors determining survival after ruptured aortic aneurysm: the hospital, the surgeon, and the patient. Journal of vascular surgery 11(4), 493–6 [PubMed: 2325210] | Sample size less than 200 participants. |
| 56 | Overbey DM, Glebova NO, Chapman BC, et al. (2017) Morbidity of endovascular abdominal aortic aneurysm repair is directly related to diameter. Journal of vascular surgery 66(4), 1037–1047.e7 [PubMed: 28433338] | Not specific to ruptured AAA: all patients underwent elective AAA repair. |
| 57 | Ozen A, Hanedan M O, Songur C M et al. (2015) Risk factors for survival following open surgical repair of ruptured abdominal aortic aneurysms: A 13-year experience. Journal of Tehran University Heart Center 10(3), 117–121 [PMC free article: PMC4685366] [PubMed: 26697083] | Sample size less than 200 participants. |
| 58 | Piper Greta, Patel Nilesh A, Chandela Sweeta et al. (2003) Short-term predictors and long-term outcome after ruptured abdominal aortic aneurysm repair. The American surgeon 69(8), 703–10 [PubMed: 12953829] | Inadequate data presented: authors reported that multivariate analysis was performed; however, they did not report numerical outcomes of their analysis. They stated that core temperature was associated with mortality but did not report the direction of effects. Furthermore, the study included less than 200 participants. |
| 59 | Reimerink J J, van der Laan, M J, Koelemay M J et al. (2013) Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. The British journal of surgery 100(11), 1405–13 [PubMed: 24037558] | Systematic review of studies assessing mortality rates over different time periods. Risk factors associated with mortality were not assessed. |
| 60 | Ribeiro M, Oderich GS, Macedo T, et al. (2017) Assessment of aortic wall thrombus predicts outcomes of endovascular repair of complex aortic aneurysms using fenestrated and branched endografts. Journal of vascular surgery 66(5), 1321–1333 [PubMed: 28596039] | Study included people with different types of aneurysms. Less than 200 people in the sample had AAA. Results were not stratified according to type of aneurysm. |
| 61 | Richards T, Goode S D, Hinchliffe R et al. (2009) The importance of anatomical suitability and fitness for the outcome of endovascular repair of ruptured abdominal aortic aneurysm. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 38(3), 285–90 [PubMed: 19576803] | Sample size less than 200 participants. |
| 62 | San Norberto, Enrique M, Fuente Ruth, Garcia-Saiz Irene et al. (2016) New scale for predicting mortality in ruptured abdominal aortic aneurysms. Nueva escala de prediccion de mortalidad en los aneurismas de aorta abdominal rotos. 94(6), 339–45 [PubMed: 27060849] | Sample size less than 200 participants. |
| 63 | Sarac Timur P, Bannazadeh Mohsen, Rowan A F et al. (2011) Comparative predictors of mortality for endovascular and open repair of ruptured infrarenal abdominal aortic aneurysms. Annals of vascular surgery 25(4), 461–8 [PubMed: 21549913] | Sample size less than 200 participants. |
| 64 | Sasaki S, Yasuda K, Yamauchi H et al. (1998) Determinants of the postoperative and long-term survival of patients with ruptured abdominal aortic aneurysms. Surgery Today 28(1), 30–35 [PubMed: 9505314] | Sample size less than 200 participants. |
| 65 | Sasaki S, Sakuma M, Samejima M et al. (1999) Ruptured abdominal aortic aneurysms: Analysis of factors influencing surgical results in 184 patients. Journal of Cardiovascular Surgery 40(3), 401–405 [PubMed: 10412929] | Insufficient data reported in the study manuscript. Furthermore the study included less than 200 participants. |
| 66 | Scarcello Edoardo, Ferrari Mauro, Rossi Giuseppe et al. (2010) A new preoperative predictor of outcome in ruptured abdominal aortic aneurysms: the time before shock (TBS). Annals of vascular surgery 24(3), 315–20 [PubMed: 19900784] | Insufficient data reported in the study manuscript. Furthermore the study included less than 200 participants. |
| 67 | Shackleton C R, Schechter M T, Bianco R, and Hildebrand H D (1987) Preoperative predictors of mortality risk in ruptured abdominal aortic aneurysm. Journal of vascular surgery 6(6), 583–9 [PubMed: 3694756] | Sample size less than 200 participants. |
| 68 | Shahidi S, Schroeder T Veith, Carstensen M et al. (2009) Outcome and survival of patients aged 75 years and older compared to younger patients after ruptured abdominal aortic aneurysm repair: do the results justify the effort?. Annals of vascular surgery 23(4), 469–77 [PubMed: 19136232] | Sample size less than 200 participants. |
| 69 | Sharif M A, Lee B, Makar et al. (2007) Role of the Hardman index in predicting mortality for open and endovascular repair of ruptured abdominal aortic aneurysm. Journal of Endovascular Therapy 14(4), 528–535 [PubMed: 17696628] | Sample size less than 200 participants. |
| 70 | Sharif M A, Arya N, Soong C V et al. (2007) Validity of the Hardman index to predict outcome in ruptured abdominal aortic aneurysm. Annals of vascular surgery 21(1), 34–8 [PubMed: 17349333] | Sample size less than 200 participants. |
| 71 | Stenbaek J, Granath F, and Swedenborg J (2004) Outcome after abdominal aortic aneurysm repair. Difference between men and women. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 28(1), 47–51 [PubMed: 15177231] | Authors stated that multivariate regression was performed; however, they did not provide the results of the multivariate analysis. |
| 72 | Stone Patrick A, Hayes J David, AbuRahma Ali F et al. (2005) Ruptured abdominal aortic aneurysms: 15 years of continued experience in a southern West Virginia community. Annals of vascular surgery 19(6), 851–7 [PubMed: 16200473] | Sample size less than 200 participants. |
| 73 | Tambyraja Andrew L, Murie John A, and Chalmers Roderick T. A (2008) Prediction of outcome after abdominal aortic aneurysm rupture. Journal of vascular surgery 47(1), 222–30 [PubMed: 17928187] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 74 | Treska V, and Novak M (2006) Rupture of abdominal aortic aneurysm--factors of mortality. Bratislavske lekarske listy 107(1–2), 22–5 [PubMed: 16771133] | Authors state that multivariate analysis was performed; however, the results (ORs) of the analysis were not reported. Furthermore, the study’s sample size was less than 200 participants. |
| 75 | Turton E P. L, Scott D J. A, Delbridge M et al. (2000) Ruptured abdominal aortic aneurysm: A novel method of outcome prediction using neural network technology. European Journal of Vascular and Endovascular Surgery 19(2), 184–189 [PubMed: 10727369] | Sample size less than 200 participants. |
| 76 | Ultee KH. J, Zettervall SL, Soden PA, et al. (2016) Incidence of and risk factors for bowel ischemia after abdominal aortic aneurysm repair. Journal of vascular surgery 64(5), 1384–1391 [PMC free article: PMC5079815] [PubMed: 27475466] | Not specific to ruptured AAA: the study sample included patients with intact AAA (91.2%) and ruptured AAA (8.8%). |
| 77 | Urwin S C, and Ridley S A (1999) Prognostic indicators following emergency aortic aneurysm repair. Anaesthesia 54(8), 739–744 [PubMed: 10460525] | Sample size less than 200 participants. |
| 78 | Van Beek , Sytse C, Legemate Dink A et al. (2014) Acute kidney injury defined according to the ‘Risk,’ ‘Injury,’ ‘Failure,’ ‘Loss,’ and ‘End-stage’ (RIFLE) criteria after repair for a ruptured abdominal aortic aneurysm. Journal of vascular surgery 60(5), 1159–1167.e1 [PubMed: 24998838] | Sample size less than 200 participants. |
| 79 | Visser Jacob J, Williams Martine, Kievit Jur et al. (2009) Prediction of 30-day mortality after endovascular repair or open surgery in patients with ruptured abdominal aortic aneurysms. Journal of vascular surgery 49(5), 1093–9 [PubMed: 19394540] | Sample size less than 200 participants. |
| 80 | von Meijenfeldt , G C I, van Beek , S C, Bastos Goncalves, F, et al. (2017) Development and External Validation of a Model Predicting Death After Surgery in Patients With a Ruptured Abdominal Aortic Aneurysm: The Dutch Aneurysm Score. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 53(2), 168–174 [PubMed: 27916478] | Study assesses a bespoke risk assessment tool that is not outlined in the review protocol |
| 81 | Vos CG, de Vries JP, Werson DA) Evaluation of five different aneurysm scoring systems to predict mortality in ruptured abdominal aortic aneurysm patients. (2016) Evaluation of five different aneurysm scoring systems to predict mortality in ruptured abdominal aortic aneurysm patients. Journal of vascular surgery 64(6), 1609–1616 [PubMed: 27575812] | Unclear whether multivariate analysis was performed. |
| 82 | Wallace Gabriel A, Starnes Benjamin W, Hatsukami Thomas S et al. (2013) Favorable discharge disposition and survival after successful endovascular repair of ruptured abdominal aortic aneurysm. Journal of vascular surgery 57(6), 1495–502 [PubMed: 23719035] | Sample size less than 200 participants. |
| 83 | Wise Eric S, Hocking Kyle M, and Brophy Colleen M (2015) Prediction of in-hospital mortality after ruptured abdominal aortic aneurysm repair using an artificial neural network. Journal of vascular surgery 62(1), 8–15 [PMC free article: PMC4484301] [PubMed: 25953014] | Sample size less than 200 participants. |
Economic studies
No full text papers were retrieved. All studies were excluded at review of titles and abstracts.
Appendix H. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Methods, evidence and recommendations
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Review Risk assessment tools for predicting surgical outcomes of patients who undergo elective abdominal aortic aneurysm repair: Abdominal aortic aneurysm: diagnosis and management: Evidence review H[ 2020]Review Risk assessment tools for predicting surgical outcomes of patients who undergo elective abdominal aortic aneurysm repair: Abdominal aortic aneurysm: diagnosis and management: Evidence review H. 2020 Mar
- The incidence, risk factors and in-hospital mortality of acute kidney injury in patients after abdominal aortic aneurysm repair surgery.[BMC Nephrol. 2017]The incidence, risk factors and in-hospital mortality of acute kidney injury in patients after abdominal aortic aneurysm repair surgery.Tang Y, Chen J, Huang K, Luo D, Liang P, Feng M, Chai W, Fung E, Lan HY, Xu A. BMC Nephrol. 2017 May 31; 18(1):184. Epub 2017 May 31.
- Comparison of long-term survival after repair of ruptured and non-ruptured abdominal aortic aneurysm.[Vasa. 1995]Comparison of long-term survival after repair of ruptured and non-ruptured abdominal aortic aneurysm.Soisalon-Soininen S, Salo JA, Takkunen O, Mattila S. Vasa. 1995; 24(1):42-8.
- Towards a Core Outcome Set for Abdominal Aortic Aneurysm: Systematic Review of Outcomes Reported Following Intact and Ruptured Abdominal Aortic Aneurysm Repair.[Eur J Vasc Endovasc Surg. 2021]Towards a Core Outcome Set for Abdominal Aortic Aneurysm: Systematic Review of Outcomes Reported Following Intact and Ruptured Abdominal Aortic Aneurysm Repair.Machin M, Ulug P, Pandirajan K, Bown MJ, Powell JT. Eur J Vasc Endovasc Surg. 2021 Jun; 61(6):909-918. Epub 2021 Mar 23.
- Review Quality of life after repair of ruptured abdominal aortic aneurysm.[Eur J Vasc Endovasc Surg. 2004]Review Quality of life after repair of ruptured abdominal aortic aneurysm.Tambyraja AL, Fraser SC, Murie JA, Chalmers RT. Eur J Vasc Endovasc Surg. 2004 Sep; 28(3):229-33.
- Risk factors for predicting survival after abdominal aortic aneurysm ruptureRisk factors for predicting survival after abdominal aortic aneurysm rupture
- AdherenceAdherence
- Eif3el1 eukaryotic translation initiation factor 3, subunit E like 1 [Rattus nor...Eif3el1 eukaryotic translation initiation factor 3, subunit E like 1 [Rattus norvegicus]Gene ID:100365062Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...