NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Effectiveness of endovascular aneurysm repair compared with open surgical repair of ruptured abdominal aortic aneurysms
Review question
What is the effectiveness of EVAR compared to open repair surgery in repairing ruptured abdominal aortic aneurysms?
Introduction
This review question aims to assess the advantages and disadvantages of emergency endovascular aneurysm repair in comparison with conventional open surgical repair for the treatment of ruptured abdominal aortic aneurysms (AAAs). Furthermore, this question aims to explore the subgroup effects of various patient characteristics, leading to more tailored recommendations.
PICO table
Table 1
Inclusion criteria.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A recent update of a Cochrane systematic review (Badger et al. 2017) comparing EVAR and open surgical repair of ruptured infrarenal AAAs was identified as a source of randomised controlled trials (RCTs) relevant to this review question. Data were extracted from the systematic review, and individual RCTs within it, to compare the efficacy of emergency EVAR with that of open surgical repair of ruptured infrarenal aneurysms. Since the Cochrane systematic review did not consider complex aneurysm anatomies (such as juxtarenal and suprarenal type IV aneurysms) a supplementary literature search was performed. Non-randomised comparative studies, and prospective cohort studies comparing EVAR and open surgical repair of ruptured complex AAAs were included.
Studies were excluded if they:
- were not in English
- were not full reports of the study (for example, published only as an abstract)
- were not peer-reviewed.
Clinical evidence
Included studies
Standard EVAR
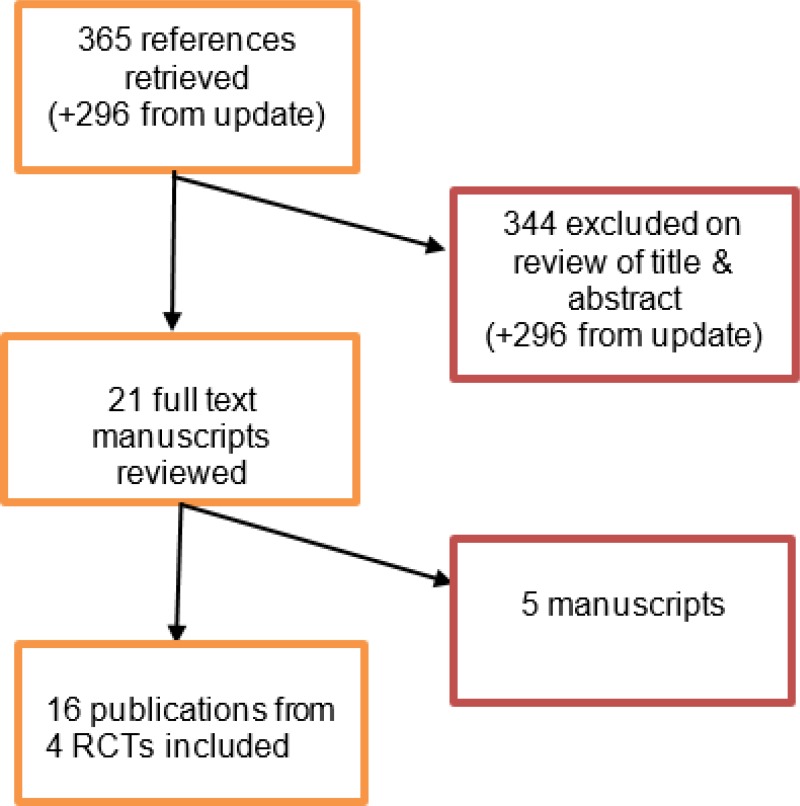
In relation to standard EVAR, searches for the initial 2014 Cochrane review and the 2017 update yielded a total of 365 abstracts. Of these, 21 were identified as being potentially relevant. Following full-text review 4 RCTs (published across 16 publications) were included. An update literature search was performed and provided by Cochrane, in December 2017. The search yielded a total of 296 abstracts. None of which were identified as potentially relevant.
Complex EVAR
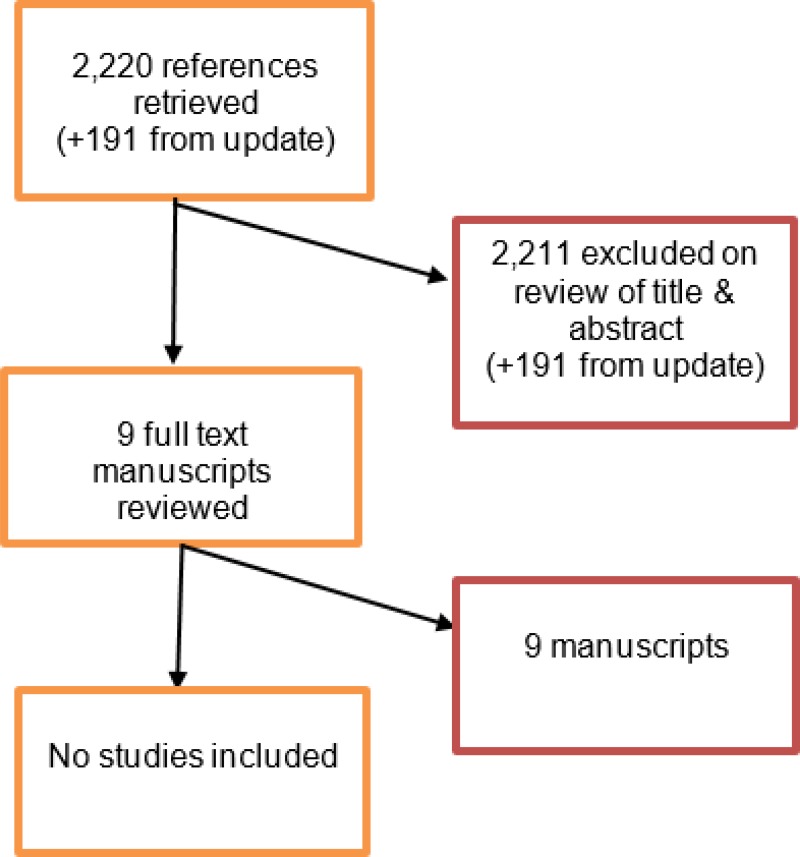
Since the Cochrane systematic review did not include complex aneurysms, a supplementary literature search was conducted by NICE in August 2017. The search yielded 2,220 abstracts. Of these, 9 studies were identified as being potentially relevant. Following full-text review none of these studies were included. An update search was conducted by NICE in December 2017. The search yielded 191 abstracts; of which, none of which were considered relevant.
Excluded studies
The list of papers excluded at full-text review, with reasons, is given in Appendix J – Excluded studies.
Summary of clinical studies included in the evidence review
A summary of the included studies is provided in the table below.
Table 2
Included studies.
See appendix D for full evidence tables.
Quality assessment of clinical studies included in the evidence review
See appendix F for full GRADE tables, highlighting the quality of evidence from the included studies.
Economic evidence
Included studies
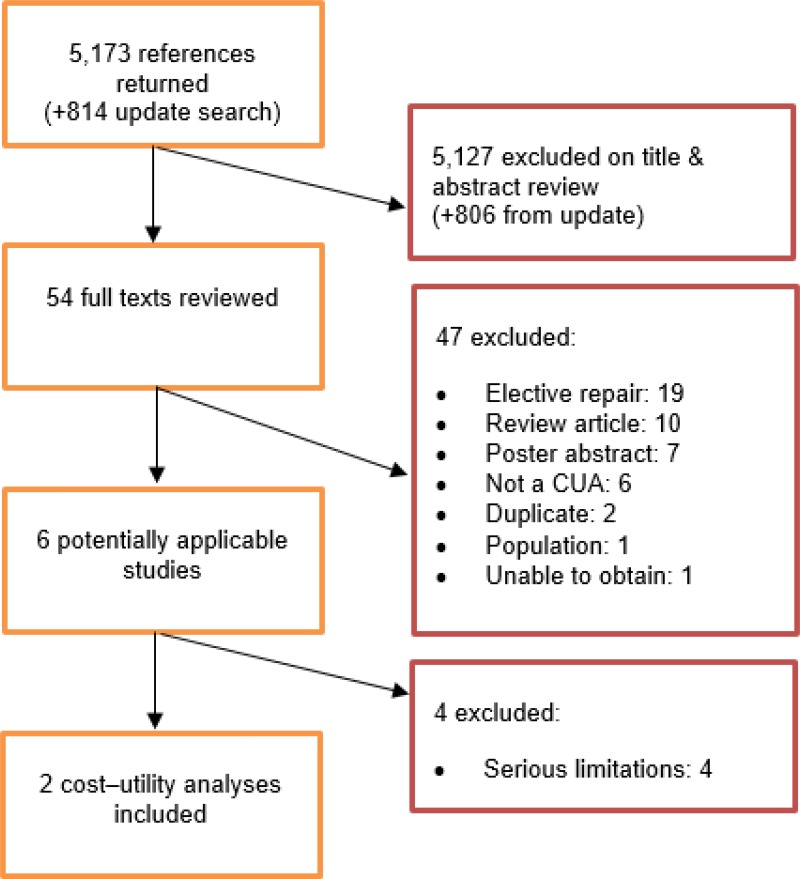
A systematic review of economic literature was conducted jointly for all review questions in this guideline by applying standard health economic filters to a clinical search for AAA (see Appendix B). A total of 5,173 studies was identified. The studies were reviewed to identify economic evaluations in the form of cost–utility analyses exploring the costs and effects of emergency procedures to repair ruptured AAA. Studies that met the eligibility criteria were assessed using the quality appraisal criteria as outlined in the Guidelines Manual (2014).
Following an initial review of titles and abstracts, the full texts of 46 studies were retrieved for detailed consideration. Following full-text review, 5 of the 46 studies were judged to be potentially applicable cost–utility analyses for emergency AAA repair. Three of the 5 studies were excluded because they were judged to be subject to very serious limitations.
An update search was conducted in December 2017, to identify any relevant cost–utility analyses that had been published during guideline development. This search returned 814 studies. Following review of titles and abstracts, the full texts of 8 studies were retrieved for detailed consideration. Two were determined to be potentially applicable. One of these (Powell et al. 2017) was an analysis of the IMPROVE trial, using more recent data than another IMPROVE analysis that was identified by the original search (Powell et al. 2015). The earlier study was therefore excluded. The other potentially relevant study from the update search was excluded as it had very serious limitations. A total of 2 studies was therefore included as economic evidence for emergency repair of ruptured AAA.
Excluded studies
Studies that were excluded after full-text review, and reasons for exclusion, are provided in Appendix J – Excluded studies.
Summary of studies included in the economic evidence review
Kapma et al. (2014)
Kapma et al. performed a cost–utility analysis alongside the AJAX trial, an RCT comparing EVAR with open surgical repair for the repair of 116 ruptured AAAs conducted in 2 centres in the Netherlands. No extrapolation beyond the 6-month data was conducted. Hospital resource use data collected in AJAX included primary procedure, reintervention and subsequent care resources, costed at 2010 prices. The EQ-5D questionnaire was used to elicit health-related quality of life data, with general population quality of life assumed to prevail prior to aneurysm rupture. Bootstrapping was performed to characterise uncertainty in the estimates of incremental costs and QALYs, generating 25,000 samples.
Base-case results found that EVAR patients typically accrued 0.026 additional QALYs than open surgical repair patients, though, at a 95% confidence level, the data were consistent with no difference. EVAR was €10,189 more expensive than open surgical repair in terms of total costs, mainly due to the primary procedure cost and a higher incidence of subsequent hospital resource use. The ICER for EVAR was €391,885 per QALY gained, with a probability of less than 25% that the true ICER is under €80,000. Results were not sensitive to scenario analyses. The primary limitation of this analysis is its short time horizon. Additionally, the AJAX study is a relatively small trial, with its results based on 57 EVAR patients and 59 open surgical repair patients.
Table 3
Kapma et al. (2014) cost–utility model results.
Powell et al. (2017)
A within-trial cost–utility analysis was also undertaken for the IMPROVE study (Powell et al., 2017), a pragmatic trial randomising people with suspected ruptured AAAs to either open surgical repair or a strategy in which EVAR was used for anatomically suitable AAAs (and OSR used if EVAR was not possible). This was the only UK economic evaluation identified that was informed by trial-based effectiveness evidence for ruptured AAA repair. Resource-use data included the primary procedure and subsequent use of critical, specialist or routine care, including staff time, costed using standard UK sources (2011–12 prices). The EQ-5D-3L questionnaire was used to elicit quality of life data, with elective repair baseline quality of life assumed to prevail prior to AAA rupture. Bootstrapping was performed to characterise uncertainty in the estimates of incremental costs and QALYs.
Base-case results suggest that participants randomised to the ‘EVAR if possible’ strategy typically accrued 0.166 additional QALYs than open surgical repair at 3 years. The mean total cost of EVAR study subjects was lower than open surgical repair, due to fewer days spent in critical care and a lower incidence of transfer to a different hospital. EVAR was therefore found to dominate open surgical repair, with more than a 90% likelihood of being cost effective if QALYs are valued at £20,000 each. This result was found to be robust to a number of sensitivity analyses around costs and how the trial data were analysed (e.g. unadjusted vs. adjusted for baseline variables, and adjusting for compliance to the randomised intervention). Like the Kapma et al. (2014) analysis, the study is limited by its relatively short time horizon. It is based on 3-year data from the IMPROVE study with no extrapolation, though 6-year Kaplan-Meier plots are presented, depicting a higher mortality rate for trial participants who were randomised to EVAR than those randomised to open surgical repair beyond 3 years.
Table 4
Powell et al. (2017) cost–utility analysis results.
Economic model
The effectiveness of EVAR compared with open surgical repair for the repair of ruptured AAAs was identified as an area of priority for new economic analysis. New clinical evidence has become available since the existing technology appraisal (TA 167) was published, particularly the IMPROVE trial, in the UK setting, and the European AJAX and ECAR trials. A new economic model was therefore developed to support decision-making in this area.
Methods
The model took a state-transition structure, from the point at which an individual arrives at hospital with a ruptured AAA. The analysis perspective on costs was those incurred by the NHS and Personal Social Services (PSS), and the perspective on outcomes was the direct health effects for people using AAA services. Two distinct populations were modelled: (1) those for whom open surgical repair is a suitable intervention, comparing EVAR with open surgical repair; and (2) those for whom open surgical repair is not a suitable intervention, because their operative risk is considered to be too high, comparing EVAR with no intervention. The main outcomes were incremental costs and QALYs, and the resulting ICER. The model time horizon was the lifetime of the patient (to a maximum age of 100), from a baseline cohort age of 76 years, composed of 1-month cycles. All outcomes were discounted by 3.5% per year (Developing NICE guidelines 2014).
First, modelled patients were at risk of perioperative (30-day) death, for 1 cycle. In the base-case model, this was informed by the National Vascular Registry (2017) data on open surgical repair for ruptured AAA (41.2%), representing a current snapshot of UK practice outcomes. The relative perioperative mortality rate with EVAR was informed by a Cochrane systematic review of emergency AAA repair trials (odds ratio: 0.88; Sweeting et al. 2017), leading to an estimated perioperative mortality of 37.0%. In the population for whom open surgical repair is an unsuitable intervention, there is no directly relevant randomised comparative data to inform EVAR perioperative mortality. To do so, the 30-day EVAR mortality rates in the EVAR-2 trial (open repair not suitable) and the EVAR-1 trial (open repair suitable) were compared, and the difference between them was used to estimate an ‘unfit for OSR’ mortality effect. The model applies this effect to the IMPROVE EVAR 30-day mortality rate, thereby estimating a mortality rate associated with emergency EVAR in people for whom OSR is not suitable. For this population, a strategy of ‘no intervention’ is associated with a 100% mortality rate.
Surviving patients move into the post-perioperative survival phase of the model, capturing their long-term mortality hazard after surviving the AAA repair procedure and full 30-day perioperative period. Long-term survival outcomes were informed by the IMPROVE trial, for which anonymised patient-level survival data were obtained. General population mortality rates were calibrated to post-perioperative mortality, on the OSR arm of IMPROVE, using a piecewise hazard ratio to produce a curve that fits the data accurately. Beyond the 7-year IMPROVE data, projecting the relative effect produces a notable long-term survival benefit from OSR; however, the true difference in long-term survival is not known. In the base case, therefore, at the point at which the IMPROVE follow-up data is exhausted, the hazard ratio between EVAR and OSR is informed by the more-mature post-perioperative data in the elective setting (see Evidence review K). Throughout the model, patients are at risk of complications leading to reintervention, informed by the IMPROVE trial.
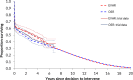
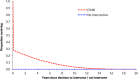
In order to explore subgroup effects, the model was configured so that both perioperative and long-term survival estimates could be influenced by effect modifiers; in particular, age, AAA diameter and sex were tested by logistic regression analysis using the IMPROVE data. For perioperative mortality, age was a significant predictor of death. Being female was a significant predictor of death with open surgical repair, while females were less likely than males to experience perioperative death with EVAR. For post-perioperative mortality, multivariable Cox regressions using the IMPROVE dataset found EVAR to be associated with improved survival for up to 3 years, while being female was associated with worse survival beyond 3 years. The effect of age was implicitly captured in this by our use calibrated of general population survival data. Base case overall survival curves are presented in Figure 1 and Figure 2.
Complex aneurysms were not simulated for the emergency repair of ruptured AAA (unlike elective, unruptured cases; see Evidence review K). This is because complex grafts, which usually need to be custom-made for the individual, are less likely to be option for emergency AAA repair. Additionally, there is an absence of clinical evidence for emergency repair outcomes in the complex population.
Resource use was obtained from the published IMPROVE data (Powell et al. 2015; Powell et al. 2017), to which up-to-date national unit costs were applied. The cost of an EVAR graft was obtained from NHS Trusts by members of the guideline development committee. Following advice from the committee, a strategy of ‘no intervention’ is assumed to incur non-zero costs, associated with a further outpatient attendance and CT scan. Quality of life was primarily informed by the published IMPROVE 3-year EQ-5D data, supplemented by decrements for complications identified by informal searches. In the IMPROVE study, the EVAR arm can be interpreted as an ‘EVAR if possible’ arm; EVAR was used where it was determined to be anatomically suitable (infrarenal) by CT scan, and 40% of participants randomised to it went on to receive open surgical repair instead. Its resource use and quality-of-life data reflect this, as does its survival data, and therefore much of our model. Our analysis should therefore be interpreted as comparing a world that permits emergency EVAR, where anatomically appropriate, with a world in which EVAR is not permitted at all (i.e. ‘open surgical repair only’ or ‘no intervention’ only).
Results
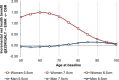
In the base-case model, in a cohort for whom open surgical repair is a suitable option, a strategy that uses EVAR where possible was found to have an ICER of £7,228per QALY gained (Table 5) compared with open surgical repair. Probabilistic sensitivity analysis showed that its ICER was £20,000 per QALY gained or better in 76% of 1,000 model iterations (Figure 3). The only individual parameters that reversed this result were the perioperative mortality odds ratio, post-perioperative mortality hazard ratios and the total critical care costs for emergency EVAR; if they were at the 95% confidence limits that most favoured open surgical repair, unlikely based on the available evidence, then the EVAR strategy would not be cost effective. The ICER in women was £3,725, and in men was £12,799 this difference reflects the significantly higher perioperative mortality rate among women with open surgical repair. In men, the strategy that permits EVAR had an ICER below £20,000 at ages 71 and above (Figure 4). It was cost-effective at all ages (50 to 100) in women, to such an extent that it is cost-effective, on average, in a population that matches the IMPROVE cohort.
Table 5
NICE cost–utility model results, population for whom open surgical repair is an option.
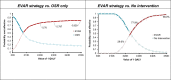
Figure 3
Cost-effectiveness acceptability results from 5,000 probabilistic sensitivity analysis runs
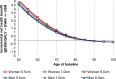
In the population for whom open surgical repair is not a suitable option, an EVAR strategy was compared with offering no AAA repair (with a 100% mortality rate). The ICER was found to be £22,945 per QALY gained (Table 6). For this population, the NICE ‘end of life criteria’ are applicable: life expectancy without intervention is less than 24 months; intervention is expected to produce at least 3 additional months of life; and the expected patient population is small. In probabilistic sensitivity analysis, 29.6% of iterations had an EVAR ICER below £20,000, while 98% were below £50,000 (Figure 3). The only parameter that caused the ICER to exceed £50,000 per QALY gained was age; namely, in men aged 85 or over, and in women aged 86 or over, due to the high risk of perioperative death and limited long-term survival thereafter (Figure 5). Note that this analysis shows a small influence of aneurysm diameter because post-perioperative survival is simulated using EVAR-2 data, in which diameter was a covariate of outcome. This is different to the ‘fit for OSR population’, in which we use specific post-rupture long-term survival data from IMPROVE; here, we do not find that diameter affects survival prospects in those people who survive surgery.
Table 6
NICE cost–utility model results, population for whom open surgical repair is not an option.
For detailed results, sensitivity analyses and discussion, including limitations and comparison with published analyses, please see the separate health economics appendix.
Evidence statements
Clinical evidence
Ruptured infrarenal AAA
- All-cause mortality:
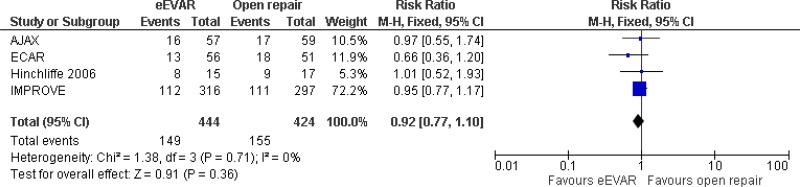
- Perioperative mortality (30-day or in-hospital mortality) cannot be differentiated between emergency EVAR and open repair (moderate-quality evidence from 4 RCTs, including 868 people).
- All-cause mortality is lower with emergency EVAR than open repair at 3–36 months (moderate-quality evidence from 1 RCT including 613 people).
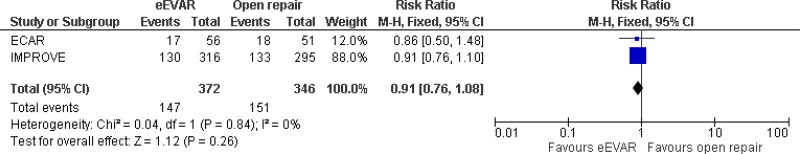
- All-cause mortality cannot be differentiated between emergency EVAR and open repair at 0–6 months (low-quality evidence from 1 RCT including 116 people), 0–1 year (moderate-quality evidence from 2 RCTs including 718 people) and a mean follow-up of 0–4.9 years (moderate-quality evidence from 1 RCT including 613 people).
- Moderate-quality evidence from 1 RCT, including 613 people with ruptured AAAs, could not differentiate AAA-related mortality rates between patients treated by emergency EVAR and those treated by open repair at a mean follow-up of 4.9 years.
- Low-quality evidence from 2 RCTs, including up to 223 people with ruptured AAAs, could not differentiate major complication rates between patients treated by emergency EVAR and those treated by open repair at 30-day and 1-year follow-up.
- Low-quality evidence from 2 RCTs, including up to 223 people with ruptured AAAs, reported lower rates of bowel ischaemia in patients treated by emergency EVAR compared to those treated by open repair at 30-day follow-up. Very low- to low-quality evidence from 4 RCTs, including up to 255 people with ruptured AAAs, could not differentiate rates of myocardial infarction, stroke, renal complications, cardiac complications, respiratory failure, spinal cord ischaemia, and amputation between patients treated by emergency EVAR and those treated by open repair at 30-day follow-up.
- Moderate-quality evidence from 1 RCT, including up to 223 people with ruptured AAAs, reported lower renal complication rates in patients treated by emergency EVAR compared to those treated by open repair at 6-month follow-up. Low-quality evidence from 1 RCT, including to 106 people with ruptured AAAs, could not differentiate rates of stroke, cardiac complications, bowel ischaemia, spinal cord ischaemia, and amputation between patients treated by emergency EVAR and those treated by open repair at 6-month follow-up.
- Low-quality evidence from 3 RCTs, including up to 613 people with ruptured AAAs, could not differentiate reintervention rates between patients treated by emergency EVAR compared with those treated by open repair at 30-day, 6-month and 3-year follow-up.
- High-quality evidence from 1 RCT, including 317 people with ruptured AAAs, reported better quality of life outcomes (measured by EQ-5D scores) in patients treated by emergency EVAR compared with those treated by open repair at 3-month follow-up. Moderate-quality evidence from the same trial could not differentiate EQ-5D scores between groups at 1-year and 3-year follow-up.
- Moderate-quality evidence from 3 RCTs, including up to 255 people with ruptured AAAs, could not differentiate length of stay in intensive care and length of hospital stay between patients treated by emergency EVAR and those treated by open repair.
Ruptured complex AAA
No evidence was identified comparing the efficacy of EVAR with open surgical repair of ruptured complex AAA.
Economic evidence
Published evidence
Ruptured infrarenal AAA
- One directly applicable cost–utility analysis with potentially serious limitations, based on data from the IMPROVE trial, found that a strategy of using EVAR where anatomically appropriate, otherwise open repair, was associated with QALY gains and lower costs compared with using open repair only, over 3 years, with at least a 90% probability of having an ICER of £20,000 per QALY gained or better.
- One partially applicable cost–utility analysis with potentially serious limitations, based on data from the AJAX trial, found that EVAR was associated with an ICER of €391,885 per QALY gained compared with open repair over 6 months.
Ruptured complex AAA
No evidence was identified comparing the efficacy of EVAR with open surgical repair of ruptured complex AAA.
NICE model
- One directly applicable cost–utility analysis with minor limitations found that allowing EVAR where anatomically suitable, otherwise using open repair, was associated with an ICER of £7,228 per QALY gained, compared with using open repair in all cases, in people for whom open repair is a suitable intervention, based on a cohort composed of 78% men with a mean age of 76. The ICER had a 76% probability of being lower than £20,000. This result was sensitive to sex: in men, EVAR had a net health benefit only at ages 714 and over; in women, EVAR had a net health benefit at all ages from 50 to 100.
- One directly applicable cost–utility analysis with minor limitations found that EVAR was associated with an ICER of £22,945 per QALY gained, compared with no surgical intervention, in people for whom open repair is not a suitable intervention, based on a cohort composed of 78% men with a mean age of 76. The ICER had a 30% probability of being £20,000 or lower, and a 98% probability of being £50,000 or lower. This result was sensitive to age: at ages above 85 in men, and 86 in women, the ICER for EVAR was higher than £50,000 per QALY gained.
Research recommendation
RR10. What is the effectiveness and cost-effectiveness of complex EVAR versus open surgical repair in people with a ruptured AAA for whom open surgery is a suitable option?
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The committee agreed that the outcomes that matter most are long-term survival, as well as a reduction in the need for reintervention. This is because committee members believed that, apart from the fundamental need for any intervention to increase the immediate chances of a person surviving a ruptured AAA, the intervention should also ensure that they live as long as possible and have the best quality of life possible following rupture.
The quality of the evidence
The committee had no serious concerns about the overall quality of the evidence retrieved from literature searches but noted that no long-term data were available. All but 1 trial (ECAR trial) were considered to have a low risk of bias. The committee noted that the ECAR trial may have been prone to selection bias as patients were allocated to groups by week; patients were treated by open repair during the first week and subsequent odd numbered weeks. The committee considered that this study did not sway the results of most meta-analyses because it was allocated a small weighting.
The committee noted that, from a clinician’s point of view, the design of the IMPROVE RCT could be considered confusing, as a large proportion of people with suspected ruptured AAA who were randomised to the ‘EVAR’ arm actually underwent open repair (because their AAA was anatomically unsuitable for standard EVAR). However, it agreed that this design reflected the decision problem at a commissioning level – that is, whether a service should offer emergency EVAR where possible – and, therefore, it would not be appropriate to downgrade the evidence for providing a biased estimate of effect.
Although the review protocol outlined that data from the National Vascular Registry, and testimony from expert witnesses would be considered in this review question, no such evidence was available to inform committee discussions.
Benefits and harms
The committee noted that medium-term follow up data from the IMPROVE trial indicated that EVAR offered a significant survival benefit (lower mortality rates) between 3 months and 3 years after surgery. However, no benefit was observed between EVAR and open surgery at the mean follow-up of 4.9 years. It was also noted that no differences in 3-year reintervention rates or quality of life (measured by EQ-5D scores) were observed between the groups. Upon consideration of these data, the committee concluded that there was substantial uncertainty about the relative long-term benefits and harms of EVAR and open surgery. When considering short-term outcomes, the committee noted that patients treated by EVAR and open surgery were not significantly different in terms of 30-day mortality, reintervention rates, and all complications apart from renal complications. The committee were surprised by the results of the AJAX trial, which reported that fewer renal complications occurred in patients treated by EVAR than those treated by open repair and agreed that this observation was inconsistent with their own clinical experience. Upon consideration of the clinical evidence, as a whole, the committee agreed that there was insufficient evidence demonstrating that EVAR was superior to open surgery. As a result, the committee drafted a recommendation highlighting that either approach could be considered for people with ruptured infrarenal AAA whose anatomy made EVAR a suitable option for them.
In the absence of evidence relating to complex EVAR for ruptured AAA, the committee discussed the potential for harm if patients who were suitable for open surgical repair were offered complex EVAR instead. Committee members agreed that, compared with infrarenal EVAR, complex EVAR is more technically demanding and less frequently available. However, they were mindful that the potential benefit of EVAR had been shown when limited to infrarenal cases, so it is plausible that an endovascular approach would prove to be reasonable in some complex emergency cases. Therefore, while the committee were clear that it would be inappropriate to recommend the use of complex EVAR as standard practice, it agreed that it would be valuable to explore the benefits, harms and costs of the approach in an RCT. This will ensure that data would be collected to inform future updates of the guideline.
Cost effectiveness and resource use
The committee discussed the published cost-effectiveness evidence for the repair of ruptured infrarenal AAA. It noted that a within-trial UK cost–utility analysis alongside the IMPROVE trial found the pragmatic EVAR strategy to dominate an open surgical repair strategy over a 3-year period, whereas a partially applicable study in the Dutch setting determined that EVAR was not cost-effective over a 6-month period. The committee agreed that the time horizons of both analyses were too short to accurately reflect the cost-effectiveness of EVAR, particularly because 6-year IMPROVE follow-up data have been published showing that the EVAR survival benefit over the first 3 years is eroded thereafter. This trend suggests that the long-term outcomes of EVAR relative to open surgical repair for ruptured AAA may be similar to those observed in elective cases for unruptured AAA. The committee agreed that the published evidence should be supplemented by new modelling, in particular to capture the population for whom open surgical repair is not a suitable intervention, and complex AAA repair, and the longer-term data from the IMPROVE trial. The committee therefore considered evidence from the new economic model developed for this guideline.
The committee were satisfied with the modelling approach of: (1) using the UK National Vascular Registry data to inform baseline perioperative mortality; (2) using a Cochrane meta-analysis of RCTs to inform relative perioperative mortality rates; (3) projecting long-term survival by calibrating general population mortality to IMPROVE survival data, conditional on surviving the intervention, and; (4) applying long-term relative survival estimates based on mature elective repair data, from the point at which the IMPROVE follow-up expires. The committee understood that the economic model evaluating the population for whom open surgical repair is not a suitable option provides weaker evidence, as there is no RCT evidence for emergency repair in this population, and it was therefore supplemented with evidence from the EVAR-2 trial.
The committee discussed the appropriateness of using the IMPROVE trial to inform much of the model; in particular, the fact that it is a pragmatic RCT, in which 40% of participants randomised to the EVAR arm actually received open surgical repair. It agreed that this approach does not provide a direct comparison of EVAR with open surgical repair in the emergency repair population, but that it does provide a comparison of a strategy that permits EVAR if the AAA is anatomically suitable, and open repair if it is not, with one that uses open surgical repair for all cases.
The committee agreed that the new economic model provides evidence that, on average across a population of people for whom open surgical repair is a suitable option, a strategy that permits EVAR where anatomically suitable – otherwise open surgical repair – is likely to be cost effective compared with using open repair in all cases. The base-case model results suggest that, for the average person, the EVAR strategy produces more QALYs than open surgery, at an additional cost to the NHS and PSS that represents good value for money. The base-case ICER is £7,228 per QALY gained, with a 76% probability of this being less than £20,000. This positive ICER reflects that the EVAR strategy was more costly, per person, than the open surgical repair strategy, whereas the published IMPROVE cost–utility analysis estimated that EVAR was less costly than open repair. The committee understood that this was because the NICE model used publicly available UK cost sources (alongside the published IMPROVE resource data), rather than the unit costs from the IMPROVE trial centres, and captured reintervention costs over a longer period. Results of the NICE model were sensitive to age and sex: the ICER is above £20,000 per QALY gained in men below 714 years old, but in women it remains £20,000 or better at all ages. This is primarily because of worse perioperative survival from open surgical repair in women. The committee agreed to reflect that EVAR may confer greater benefits in women in their recommendations.
The ICER for EVAR compared with ‘no intervention’, in the population for whom open surgical repair is not a suitable option, was £22,945 per QALY gained. The committee were aware that the ‘end of life criteria’ may be applicable for this population: life expectancy without intervention for a ruptured AAA is 0 years; EVAR is expected to gain more than 3 months of additional life (0.768 QALYs); and the population affected is likely to be small. The committee therefore considered that the base-case ICER provided acceptable value for money to the NHS and PSS, noting that the ICER had a 98% probability of being less than £50,000 per QALY gained.
These results were sensitive to age, with the EVAR ICER exceeding £50,000 in men aged 85 or over, and women aged 86 or over; however, the committee advised that this represents only a small subgroup of the relevant population. The committee also agreed that if an older person has a ruptured AAA and open surgical repair is not a suitable option, but the person is still deemed to be a candidate for emergency EVAR, then they are likely to be systematically different to the ‘average’ individual captured by the model. In practice, if emergency AAA repair is being considered then the treating clinician must believe that the person has a reasonable probability of surviving the procedure and life expectancy thereafter. In this way, the committee advised that the model results in this population at older ages are less likely to reflect clinical reality, and that EVAR is more likely to be cost effective at older ages than the model results suggest. The committee advised that there may be some costs associated with choosing to provide ‘no intervention’ for people presenting with ruptured aneurysms – an outpatient attendance for everyone and a proportion of people who will require a CT scan – which reduces the ICER for EVAR at all ages.
Other factors the committee took into account
While the committee agreed that permitting EVAR for the emergency repair of ruptured infrarenal AAAs is likely to be cost effective, based on the available evidence, it recognised that there may be practical difficulties in implementing such a recommendation alongside the more compelling evidence that EVAR is not cost effective for the elective repair of unruptured AAAs. In particular, the committee recognised that it may be difficult to retain EVAR capacity and expertise for use in the relatively small number of infrarenal AAA ruptures seen in practice, without being able to conduct EVAR relatively frequently in the elective setting. The committee agreed that there is no simple solution to this implementation difficulty and that the current evidence is clear that EVAR should be retained as a cost-effective option for emergency infrarenal AAA repair. However, the committee were clear that maintaining capacity to provide emergency EVAR is, on its own, an insufficient reason to offer elective EVAR, as the QALYs forgone by retaining elective EVAR would outweigh the QALYs saved by having it available in the emergency setting.
The committee discussed the use of complex EVAR in the context of emergency AAA repair, noting that the new economic model had not captured this population, owing to the lack of clinical evidence. Complex EVAR is not typically used in the emergency setting, as shown by the IMPROVE study protocol. To repair a complex AAA, EVAR devices must be custom-designed for the individual and ordered in advance, and this is not possible in the emergency setting. The committee advised that complex emergency EVAR occasionally does happen in practice using physician-modified grafts or advanced adjuncts to standard, infrarenal EVAR devices. The committee agreed that such practice is speculative, and saw no evidence to advise on the effectiveness or cost effectiveness of this approach, compared with open surgical repair. The committee therefore decided to recommend complex emergency EVAR only within the context of an RCT.
Appendices
Appendix A. Review protocol
Review protocol for assessing the effectiveness of endovascular aneurysm repair compared with open surgical repair of ruptured abdominal aortic aneurysms
| Review question 23 | What is the effectiveness of EVAR compared to open repair surgery in repairing ruptured abdominal aortic aneurysms? | |||||
|---|---|---|---|---|---|---|
| Objectives | To assess the advantages and disadvantages of emergency endovascular aneurysm repair in comparison with conventional open surgical repair for the treatment of ruptured abdominal aortic aneurysms To explore the subgroup effects of various patient characteristics, leading to more tailored recommendations | |||||
| Type of review | Intervention | |||||
| Language | English | |||||
| Study design | i) Systematic reviews of study designs listed below Randomised controlled trials Quasi-randomised controlled trials Non-randomised controlled trials for comparisons in people eligible for complex EVAR only Prospective cohort studies for comparisons in people eligible for complex EVAR only ii) Analysis of UK registry data (National Vascular Registry) | |||||
| Interventions | ||||||
| Standard (on-IFU) EVAR | Complex EVAR | |||||
| Off-IFU use of standard EVAR | Other complex EVAR | |||||
| Infrarenal |
Systematic reviews RCTs Quasi-RCTs |
Systematic reviews RCTs Quasi-RCTs Non-randomised controlled trials Prospective cohort studies UK registry data (National Vascular Registry) |
Systematic reviews RCTs Quasi-RCTs Non-randomised controlled trials Prospective cohort studies UK registry data (National Vascular Registry) | |||
| Juxtarenal |
Systematic reviews RCTs Quasi-RCTs |
Systematic reviews RCTs Quasi-RCTs Non-randomised controlled trials Prospective cohort studies UK registry data (National Vascular Registry) |
Systematic reviews RCTs Quasi-RCTs Non-randomised controlled trials Prospective cohort studies UK registry data (National Vascular Registry) | |||
| Suprarenal / ‘type IV’ | - | - |
Systematic reviews RCTs Quasi-RCTs Non-randomised controlled trials Prospective cohort studies UK registry data (National Vascular Registry) | |||
| Status | Published papers only (full text) No date restrictions | |||||
| Population | People undergoing surgery for a ruptured abdominal aortic aneurysm Subgroups: fitness for surgery, age, sex, comorbidities (including cardiovascular disease, renal disease, COPD, obesity), ethnicity | |||||
| Intervention | Emergency standard (on-IFU) EVAR for infrarenal and juxtarenal abdominal aortic aneurysms Emergency complex EVAR for infrarenal, juxtarenal and suprarenal abdominal aortic aneurysms, including: fenestrated EVAR EVAR with chimneys EVAR with snorkels branched grafts ‘CHIMPS’ (CHIMneys, Periscopes, Snorkels) infrarenal devices used for juxtarenal AAA – that is, off-IFU use of standard devices Open repair Summary: | |||||
| No surgery | Open repair | Standard (on-IFU) EVAR | Off-IFU use of standard EVAR | Other complex EVAR | ||
| Infrarenal | ✓ | ✓ | ✓ | ✓ | Iliac-branched only | |
| Juxtarenal | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Suprarenal / ‘type IV’ | ✓ | ✓ | - | - | ✓ | |
| Comparator | Each other | |||||
| Outcomes | Mortality/survival Peri- and post-operative complications Successful exclusion of the aneurysm, aneurysm rupture, or further aneurysm growth Need for reintervention Quality of life Resource use, including length of hospital or intensive care stay, and costs | |||||
| Other criteria for inclusion / exclusion of studies |
Exclusion: Non-English language Abstract/non-published | |||||
| Baseline characteristics to be extracted in evidence tables |
Age Sex Size of aneurysm Comorbidities | |||||
| Search strategies | See Appendix B | |||||
| Review strategies | i) Appropriate NICE Methodology Checklists, depending on study designs, will be used as a guide to appraise the quality of individual studies. The update of Badger et al’s 2014 Cochrane review (ongoing at the time of protocol development) comparing endovascular and open surgical repair of ruptured AAAs will be used as the RCT evidence base for this review question Data on all included studies will be extracted into evidence tables. Where statistically possible, a meta-analytic approach will be used to give an overall summary effect. All key findings from evidence will be presented in GRADE profiles. ii) Expert witnesses will attend a Committee meeting to answer questions from members of the Committee. They will be invited to present their evidence at a Committee meeting in the form of expert testimony based on a written paper. The Developer will write up the expert testimony and agree this with the witness after the meeting. i and ii) All key findings will be summarised in evidence statements. | |||||
| Key papers | None identified. | |||||
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review question 23
Badger S, Bedenis R, Blair PH et al. (2017) Endovascular treatment for ruptured abdominal aortic aneurysm. Cochrane Database Syst Rev;(5):CD005261. doi: 10.1002/14651858.CD005261.pub4 [PMC free article: PMC6481849] [PubMed: 28548204] [CrossRef]
|
Medline Strategy, searched 22nd June 2016 Search Strategy: |
|---|
| #1 MESH DESCRIPTOR Aneurysm, Ruptured EXPLODE ALL TREES |
| #2 MESH DESCRIPTOR Aneurysm, Dissecting |
| #3 MESH DESCRIPTOR Aorta EXPLODE ALL TREES WITH QUALIFIERS SU |
| #4 ((aneurysm* or abdom* or thoracoabdom* or thoraco-abdom* or aort*) near (ruptur* or tear or bleed* or trauma) ):TI,AB,KY |
| #5 RAAA:TI,AB,KY |
| #6 #1 OR #2 OR #3 OR #4 OR #5 |
| #7 MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES |
| #8 MESH DESCRIPTOR Stents EXPLODE ALL TREES |
| #9 MESH DESCRIPTOR Vascular Surgical Procedures |
| #10 MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES |
| #11 MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES |
| #12 endovasc*:TI,AB,KY |
| #13 endostent*:TI,AB,KY |
| #14 endoluminal:TI,AB,KY |
| #15 endoprosthe*:TI,AB,KY |
| #16 (graft or endograft*):TI,AB,KY |
| #17 percutaneous*:TI,AB,KY |
| #18 stent*:TI,AB,KY |
| #19 (Palmaz or Zenith or Dynalink or Hemobahn or Luminex* or Memotherm or Wallstent):TI,AB,KY |
| #20 (Viabahn or Nitinol or Intracoil or Tantalum):TI,AB,KY |
| #21 EVAR:TI,AB,KY |
| #22 EVRAR:TI,AB,KY |
| #23 TEVAR:TI,AB,KY |
| #24 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 |
| #25 #6 AND #24 |
| #26 * NOT SR-PVD:CC AND 31/03/2014 TO 31/07/2016:DL |
| #27 #25 AND #26 |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom*.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov*.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree* or analys*)).tw. |
| 20 (cost or costs or costing* or costly or costed).tw. |
| 21 (price* or pricing*).tw. |
| 22 budget*.tw. |
| 23 expenditure*.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic* or (pharmaco adj economic*)).tw. |
| 26 or/1–25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly* or qald* or qale* or qtime*).tw. |
| 7 disability adjusted life.tw. |
| 8 daly*.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health* year* equivalent*.tw. |
| 19 utilit*.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili*.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble*.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1–30 |
Appendix C. Clinical evidence study selection
Appendix D. Clinical evidence tables
Download PDF (501K)
Studies included in the systematic review by Badger et al
Download PDF (487K)
Appendix E. Forest plots
Note: all data reported in GRADE tables relate to ruptured infrarenal AAA. No evidence comparing EVAR with open surgical repair of ruptured complex AAA were identified.
Appendix F. GRADE tables
Note: all data reported in GRADE tables relate to ruptured infrarenal AAA. No evidence comparing EVAR with open surgical repair of ruptured complex AAA were identified.
Mortality
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| All-cause Perioperative mortality (30-day or in-hospital mortality); effect sizes below 1 favour EVAR | |||||||||
| 4 (AJAX, ECAR, IMPROVE & Hinchcliffe trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 444 | 424 | RR 0.92 (0.77, 1.10) | Moderate |
| All-cause mortality at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Very serious2 | 57 | 59 | RR 0.92 (0.52, 1.62) | Low |
| All-cause mortality at 1 year; effect sizes below 1 favour EVAR | |||||||||
| 2 (IMPROVE & ECAR trials) | RCTs | Not serious | Not serious | Not serious | Serious1 | 372 | 346 | RR 0.91 (0.76, 1.08) | Moderate |
| All-cause mortality between 3 months and 3 years; effect sizes below 1 favour EVAR | |||||||||
| 1 IMPROVE trial | RCT | Not serious | Not serious | N/A | Serious1 | 316 | 297 | HR 0.57 (0.36, 0.90) | Moderate |
| All-cause mortality at mean follow-up of 4.9 years; effect sizes below 1 favour EVAR | |||||||||
| 1 IMPROVE trial | RCT | Not serious | Not serious | N/A | Serious3 | 316 | 297 | HRa 0.90 (0.73, 1.10) | Moderate |
| AAA-related mortality at mean follow-up of 4.9 years; effect sizes below 1 favour EVAR | |||||||||
| 1 IMPROVE trial | RCT | Not serious | Not serious | N/A | Serious3 | 316 | 297 | HRa 0.88 (0.68, 1.15) | Moderate |
- a
Hazard ratios were reported adjusting for age, sex, Hardman index, and lowest systolic blood pressure.
- 1
Confidence interval crosses one line of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 1 level.
- 2
Confidence interval crosses two lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
- 3
Non-significant result (95% confidence interval crosses the line of no effect), downgrade 1 level.
Major complications
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Major complications at 30 days; effect sizes below 1 favour EVAR | |||||||||
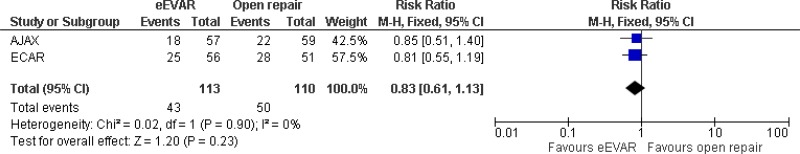
| 2 (AJAX & ECAR trials) | RCT | Serious1 | Not serious | Not serious | Serious2 | 113 | 110 | RR 0.83 (0.61, 1.13) | Low |
| Major complications at 1 year; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Very serious3 | 57 | 59 | RR 0.89 (0.55, 1.47) | Low |
- 1
Method of randomisation was not reported a study (ECAR trial) which had a high weighting (over 33%) in the meta-analysis, downgrade 1 level
- 2
Confidence interval crosses one line of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 1 level.
- 3
Confidence interval crosses two lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
Specific complications
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Endoleaks at final follow-up | |||||||||
| 3 (AJAX, ECAR & Hinchcliffe trials) | RCTs | Not serious | Not serious | Not serious | Very serious1 | 128 | N/A |
- 34.4% (44/128) Note: authors stated meta-analysis was not possible as endoleaks are only a result of EVAR. | Low |
| Myocardial infarction at 30 days; effect sizes below 1 favour EVAR | |||||||||
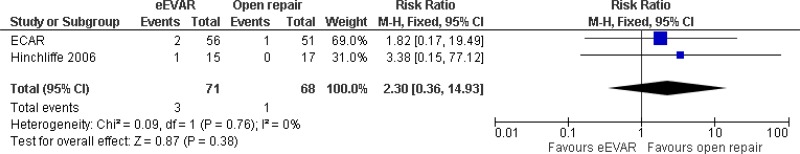
| 2 (ECAR & Hinchcliffe trials) | RCTs | Serious2 | Not serious | Not serious | Very serious3 | 71 | 68 | RR 2.30 (0.36, 14.93) | Very low |
| Stroke at 30 days; effect sizes below 1 favour EVAR | |||||||||
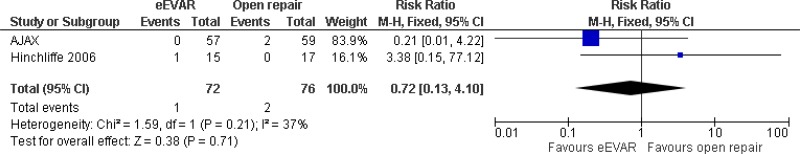
| 2 (AJAX, & Hinchcliffe trials) | RCTs | Not serious | Not serious | Not serious | Very serious3 | 72 | 76 | RR 0.72 (0.13, 4.10) | Low |
| Stroke at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCTs | Not serious | Not serious | N/A | Very serious3 | 57 | 59 | RR 0.21 (0.01, 4.22) | Low |
| Renal complications at 30 days; effect sizes below 1 favour EVAR | |||||||||
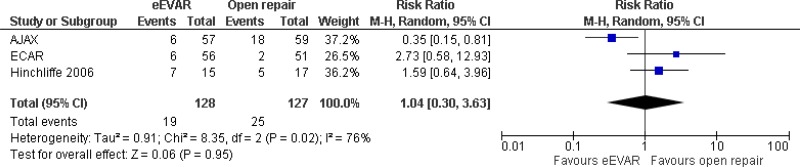
| 3 (AJAX, ECAR & Hinchcliffe trials) | RCTs | Not serious | Not serious | Very serious5 | Very serious3 | 128 | 127 | RR 1.04 (0.30, 3.63) | Very low |
| Renal complications at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Serious6 | 57 | 59 | RR 0.35 (0.15, 0.81) | Moderate |
| Cardiac complications at 30 days ; effect sizes below 1 favour EVAR | |||||||||
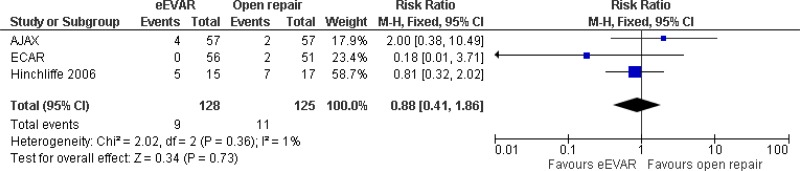
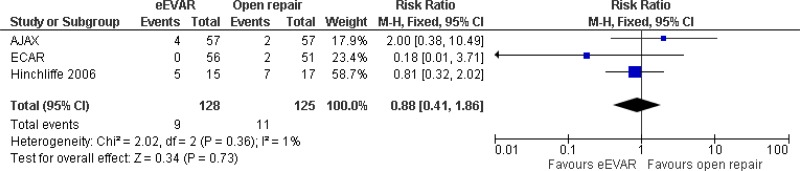
| 3 (AJAX, ECAR & Hinchcliffe trials) | RCTs | Not serious | Not serious | Not serious | Very serious3 | 128 | 125 | RR 0.88 (0.41, 1.86) | Low |
| Cardiac complications at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Very serious3 | 57 | 59 | RR 1.67 (0.42, 6.65) | Low |
| Respiratory failure at 30 days; effect sizes below 1 favour EVAR | |||||||||
| 1 Hinchliffe (2006) | RCT | Not serious | Not serious | N/A | Very serious3 | 15 | 17 | RR 3.38 (0.15, 77.12) | Low |
| Bowel ischaemia at 30 days; effect sizes below 1 favour EVAR | |||||||||
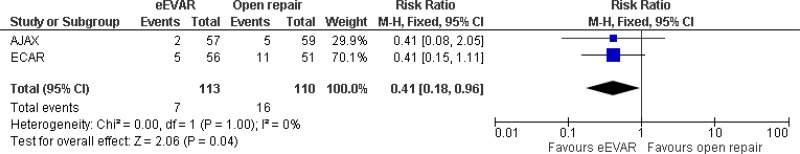
| 2 (AJAX & ECAR trials) | RCTs | Serious2 | Not serious | Not serious | Serious6 | 113 | 110 | RR 0.41 (0.18, 0.96) | Low |
| Bowel ischaemia at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Very serious3 | 57 | 59 | RR 0.41 (0.08, 2.05) | Low |
| Spinal cord ischaemia at 30 days; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Very serious3 | 57 | 59 | RR 3.10 (0.15, 74.64) | Low |
| Spinal cord ischaemia at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Very serious3 | 57 | 59 | RR 3.10 (0.15, 74.64) | Low |
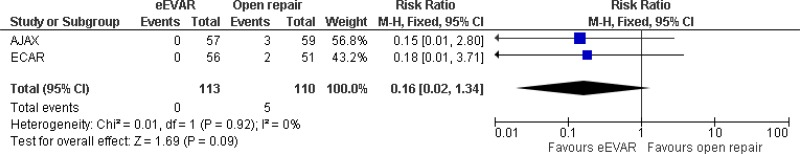
| Amputation at 30 days; effect sizes below 1 favour EVAR | |||||||||
| 2 (AJAX & ECAR trials) | RCTs | Serious2 | Not serious | Not serious | Very serious3 | 113 | 110 | RR 0.16 (0.02, 1.34) | Very low |
| Amputation at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT in Badger systematic review | Not serious | Not serious | N/A | Very serious3 | 57 | 59 | RR 0.15 (0.01, 2.80) | Low |
- 1
Effect sizes and measures of dispersion were not reported as meta-analysis was not possible, downgrade 2 levels.
- 2
Method of randomisation was not reported in a study (ECAR trial) which had a high weighting (over 33%) in the meta-analysis, downgrade 1 level
- 3
Confidence interval crosses two lines of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 2 levels.
- 4
I2value between 33.3% and 66.7%, downgrade 1 level.
- 5
I2value >66.7%, downgrade 2 levels.
- 6
Confidence interval crosses one line of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 1 level.
Need for reintervention
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Any reintervention at 30 days; effect sizes below 1 favour EVAR | |||||||||
| 3 (AJAX, ECAR & Hinchcliffe trials) | RCTs | Not serious | Not serious | Serious1 | Very serious2 | 128 | 125 | RR 0.88 (0.41, 1.86) | Very low |
| Any reintervention at 6 months; effect sizes below 1 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Very serious2 | 57 | 59 | RR 1.21 (0.61, 2.38) | Low |
| Any reintervention at 3 years; effect sizes below 1 favour EVAR | |||||||||
| 1 IMPROVE trial | RCT | Not serious | Not serious | N/A | Serious3 | 316 | 297 | HRa 1.04 (0.80, 1.35) | Moderate |
- a
Hazard ratios were reported adjusting for age, sex, Hardman index, and lowest systolic blood pressure.
- 1
I2value > 40%, downgrade 1 level.
- 2
Confidence interval crosses one line of a defined minimum clinically important difference (RR MIDs of 0.8 and 1.25), downgrade 1 level.
- 3
Non-significant result (95% confidence interval crosses the line of no effect), downgrade 1 level.
Quality of life
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| SF-36 Physical domain at 6 months; effect sizes below 0 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Serious1 | 29 | 27 | MD 3.56 (-2.0, 9.0) | Moderate |
| SF-36 mental domain at 6 months; effect sizes below 0 favour EVAR | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Serious1 | 29 | 27 | MD -5.25 (-11.0, 0) | Moderate |
| EQ-5D at 3 months; effect sizes below 0 favour EVAR | |||||||||
| 1 IMPROVE trial | RCT | Not serious | Not serious | N/A | Not serious | 167 | 150 | MD 0.087 (0.017, 0.158) | High |
| EQ-5D at 12 months; effect sizes below 0 favour EVAR | |||||||||
| 1 IMPROVE trial | RCT | Not serious | Not serious | N/A | Serious1 | 161 | 140 | MD 0.068 (-0.004, 0.140) | Moderate |
| EQ-5D at 3 years; effect sizes below 0 favour EVAR | |||||||||
| 1 IMPROVE trial | RCT | Not serious | Not serious | N/A | Serious3 | N=262 | MD 0.013 (-0.069, 0.096) | Moderate | |
- 1
Non-significant result (95% confidence interval crosses the line of no effect), downgrade 1 level.
Length of stay
| Quality assessment | No of patients | Effect estimate | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Indirectness | Inconsistency | Imprecision | EVAR | Open repair | Summary of results | |
| Length of stay in ICU (hours) | |||||||||
| 1 AJAX trial | RCT | Not serious | Not serious | N/A | Serious1 | 57 | 59 |
AJAX diff in medians: 18 (Non-significant according to the Mann-Whitney test) | Moderate |
| Length of Hospital stay (days) | |||||||||
| 3 (AJAX, ECAR & Hinchcliffe trials) | RCT | Not serious | Not serious | Not serious | Serious1 | 128 | 127 |
AJAX diff in medians: 4 ECAR diff in medians: 2.8 (Both non-significant according to the MannWhitney or Wilcoxon rank test) Hinchliffe diff in medians: 2 (statistical significance not reported) | Moderate |
- 1
Non-significant result, downgrade 1 level.
Appendix H. Economic evidence tables
Download PDF (510K)
Appendix J. Excluded studies
Clinical studies
| No. | Study | Reason for exclusion |
|---|---|---|
| 1 | Antoniou G A, Georgiadis G S, Antoniou S A et al. (2013) Endovascular repair for ruptured abdominal aortic aneurysm confers an early survival benefit over open repair. United States: Mosby Inc. (11830 Westline Industrial Drive, St. Louis MO 63146, United States) | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 2 | Braithwaite B, Greenhalgh R M, Grieve R, Hassan et al. (2015) Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: One-year outcomes from the IMPROVE randomized trial. European heart journal 36(31), 2061–2069-2069 [PMC free article: PMC4553715] [PubMed: 25855369] | Study is included in the Cochrane systematic review. |
| 3 | Desgranges P, Kobeiter H, Katsahian S, et al (2015) ECAR (Endovasculaire ou Chirurgie dans les Anevrysmes aorto-iliaques Rompus): A French Randomized Controlled Trial of Endovascular Versus Open Surgical Repair of Ruptured Aorto-iliac Aneurysms. : | Study is included in the Cochrane systematic review. |
| 4 | Improve trial, and investigators (2014) Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. British journal of surgery 101, 216–224 [PMC free article: PMC4164272] [PubMed: 24469620] | Study is included in the Cochrane systematic review. |
| 5 | Powell J T, Sweeting M J, Thompson M et al. (2014) Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. | A more recent publication of this study was available and is included in the Cochrane systematic review. |
| 6 | Qin C, Chen L, and Xiao Y B (2014) Emergent endovascular vs. open surgery repair for ruptured abdominal aortic aneurysms: a meta-analysis. | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 7 | Reimerink J J, Hoornweg L L, Vahl A C, et al. (2013) Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Annals of surgery 258(2), 248–256 [PubMed: 23549424] | Study is included in the Cochrane systematic review. |
| 8 | Sweeting M J, Balm R, Desgranges P, et al. (2015) Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. | Individual patient meta-analysis based on data from 3 RCTs. It is unclear whether a systematic approach was used to select and include the 3 studies. These studies have been included, in the Cochrane systematic review. |
| 9 | van Beek , S C, Conijn A P, Koelemay M J et al. (2014) Editor’s Choice - Endovascular Aneurysm Repair Versus Open Repair for Patients with a Ruptured Abdominal Aortic Aneurysm: a Systematic Review and Meta-analysis of Short-term Survival. | Systematic review including studies that employed various study designs. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
Economic studies
| Study | Primary reason for exclusion |
|---|---|
| Selectively excluded | |
| Hayes et al. (2010). Cost-effectiveness analysis of endovascular versus open surgical repair of acute abdominal aortic aneurysms based on worldwide experience. J Endovasc Ther, 17: 174–82. [PubMed: 20426633] | Very serious limitations |
| Patel et al. (2000). The cost-effectiveness of repairing ruptured abdominal aortic aneurysms. J Vasc Surg, 32: 247–57. [PubMed: 10917983] | Very serious limitations |
| Powell et al. (2015). Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J, 35: 2061–9. [PMC free article: PMC4553715] [PubMed: 25855369] | Population (emergency repair) |
| Rollins et al. (2014). Mid-term cost-effectiveness analysis of open and endovascular repair for ruptured abdominal aortic aneurysm. Br J Surg, 101: 225–31. [PubMed: 24469621] | Very serious limitations |
| Takayama (2017). A Cost-Utility Analysis of Endovascular Aneurysm Repair for Abdominal Aortic Aneurysm. Ann Vasc Dis, 10(3): 185–91. [PMC free article: PMC5684165] [PubMed: 29147166] | Very serious limitations |
| Excluded based on study selection criteria | |
| Armstrong et al. (2014). The use of fenestrated and branched endovascular aneurysm repair for juxtarenal and thoracoabdominal aneurysms: a systematic review and cost-effectiveness analysis. HTA, 18(70). [PMC free article: PMC4780914] [PubMed: 25522080] | Not a CUA |
| Badger et al. (2014). Endovascular treatment for ruptured abdominal aortic aneurysm (review). Cochrane Database of Systematic Reviews, 7. [PubMed: 25042123] | Review article, no additional CUAs |
| Blackhouse et al. (2009). A cost-effectiveness model comparing endovascular repair to open surgical repair of abdominal aortic aneurysm in Canada. Value in Health, 12(2): 245–52. [PubMed: 18783394] | Population (elective repair) |
| Bosch et al. (2002). Abdominal aortic aneurysms: costeffectiveness of elective endovascular and open surgical repair. Radiology, 225(2): 337–44. [PubMed: 12409564] | Population (elective repair) |
| Bowen et al. (2005). Systematic review and cost-effectiveness analysis of elective endovascular repair compared to open surgical repair of abdominal aortic aneurysms. Interim report. Ontario Ministry of Health & Long-term Care. | Population (elective repair) |
| Brown et al. (2012). The UK endovascular aneurysm repair (EVAR) trials: randomised trials of EVAR versus standard therapy. HTA, 16(9). [PubMed: 22381040] | Population (elective repair) |
| Burgers et al. (2016). Cost-effectiveness of Elective Endovascular Aneurysm Repair Versus Open Surgical Repair of Abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg, 52: 29–40. [PubMed: 27118618] | Population (elective repair) |
| Chambers et al. (2009). Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model. HTA, 13(48). [PubMed: 19849958] | Population (elective repair) |
| Epstein et al. (2008). Modelling the long-term costeffectiveness of endovascular or open repair for abdominal aortic aneurysm. Br J Surg, 95: 183–90. [PubMed: 17876749] | Population (elective repair) |
| Epstein et al. (2014). Long-term cost-effectiveness analysis of endovascular versus open repair for abdominal aortic aneurysm based on four randomized clinical trials. Br J Surg, 101(6): 623–31. [PubMed: 24664537] | Population (elective repair) |
| Forbes et al. (2002). A cost-effectiveness analysis of standard versus endovascular abdominal aortic aneurysm repair. J Can Chir, 45(6): 420–4. [PMC free article: PMC3684656] [PubMed: 12500916] | Not a CUA |
| Greenhalgh et al. (2005). Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. The Lancet, 365(9458): 2179–86. [PubMed: 15978925] | Not a CUA |
| Hynes et al. (2007). A prospective clinical, economic, and quality-of-life analysis comparing endovascular aneurysm repair (EVAR), open repair, and best medical treatment in high-risk patients with abdominal aortic aneurysms suitable for EVAR: The Irish patient trial. J Endocasc Ther, 14: 763–76. [PubMed: 18052596] | Population (elective repair) |
| Jonk et al. (2007). Cost-effectiveness of abdominal aortic aneurysm repair: a systematic review. Int J Tech Assess Health Care, 23(2): 205–15. [PubMed: 17493306] | Review article, no additional CUAs |
| Kapma et al. (2007). Emergency abdominal aortic aneurysm repair with a preferential endovascular strategy: mortality and cost-effectiveness analysis. J Endovasc Ther, 14: 777–84. [PubMed: 18052593] | Not a CUA |
| Lederle. (2009). Repair of nonruptured abdominal aortic aneurysm: a systematic review of randomized trials. Vascular, 17: S71. | Poster abstract |
| Lederle et al. (2012). Cost-effectiveness at two years in the VA open versus endovascular repair trial. Eur J Vasc Endovasc Surg, 44: 543–8. [PubMed: 23116986] | Population (elective repair) |
| Lederle et al. (2016). Long-term cost-effectiveness in the vetereans Affairs Open vs Endovascular Repair Study of aortic abdominal aneurysm: a randomised clinical trial. JAMA Surg, 151(12): 1139–1144. [PubMed: 27627802] | Population (elective repair) |
| Luebke et al. (2014). Cost-effectiveness of endovascular versus open repair of acute complicated type B aortic dissections. J Vasc Surg, 59: 1247–55. [PubMed: 24418638] | Population (thoracic aortic dissection) |
| Mandavia et al. (2015). The role of cost-effectiveness for vascular surgery service provision in the United Kingdom. J Vasc Surg, 61: 1331–9. [PubMed: 25925543] | Review article, no additional CUAs |
| McCarron et al. (2013). The impact of using informative priors in a Bayesian cost-effectiveness analysis: an application of endovascular versus open surgical repair for abdominal aortic aneurysms in high-risk patients. Med Decis Mak, 33(3): 437–50. [PubMed: 23054366] | Population (elective repair) |
| Medical Advisory Secretariat Ontario (2002). Endovascular repair of abdominal aortic aneurysm: an evidence-based analysis. Ontario HTA Series, 2(1). [PMC free article: PMC3387737] [PubMed: 23074438] | Review article, no additional CUAs |
| Michaels et al. (2005). Cost-effectiveness of endovascular abdominal aortic aneurysm repair. Br J Surg, 92(8): 960–7. [PubMed: 16034841] | Population (elective repair) |
| Michaels et al. (2014). Long-term cost-effectiveness analysis of endovascular versus open repair for abdominal aortic aneurysms based on four randomized clinical trials. Br J Surg, 101(6): 632. [PubMed: 24723016] | Commentary, no additional CUAs |
| Patel et al. (1999). The cost-effectiveness of endovascular repair versus open surgical repair of abdominal aortic aneurysms: a decision analysis model. J Vasc Surg, 29(6): 958–72. [PubMed: 10359930] | Population (elective repair) |
| Perras et al. (2009). Elective endovascular abdominal aortic aneurism repair versus open surgery: a review of the clinical and cost-effectiveness. | Review article, no additional CUAs |
| Prinssen et al. (2007). Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: Results of a randomized trial. J Vasc Surg, 46: 883–90. [PubMed: 17980274] | Population (elective repair) |
| Sala-Almonicil et al. (2017). Fenestrated and chimney endovascular aneurysm repair versus open surgery for complex abdominal aortic aneurysms. J Cardiovasc Surg, 58(6): 801–13. [PubMed: 28128541] | Not a CUA. |
| Sousa et al. (2014). Cost-effectiveness of the endovascular repair of abdominal aortic aneurysm in Portugal. Angiol Cir Vasc, 10(2): 41–8. | Population (elective repair) |
| Stroupe et al. (2012). Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. J Vasc Surg, 56: 901–10. [PubMed: 22640466] | Duplicate of Lederle et al. (2012) |
| Silverstein et al. (2005). Abdominal aortic aneurysm (AAA): cost-effectiveness of screening, surveillance of intermediate-sized AAA, and management of symptomatic AAA. BUMC Proceedings, 18: 345–67. [PMC free article: PMC1255946] [PubMed: 16252027] | Review article, no additional CUAs |
| Sultan et al. (2009a). A prospective clinical and quality of life analysis of open repair (OR), endovascular repair (EVAR), and best medical treatment in high-risk patients: cost-effectiveness during global recession. Vascular, (17): S2. | Poster abstract |
| Sultan et al. (2009b). Five-year experience with EVAR without fenestration for juxtarenal AAA repair: clinical efficacy, reintervention rates, and cost-effectiveness. Vascular, 17: S74. | Not found |
| Sultan & Hynes (2010a). Five-year experience with pararenal endovascular aortic repair (PEVAR) without fenestration: clinical efficacy, reintervention rates & cost-effectiveness. J Vasc Surg, 51(6): S89. | Poster abstract |
| Sultan & Hynes (2010b). Five-year experience with pararenal endovascular aortic repair (PEVAR) without fenestration: clinical efficacy, reintervention rates & cost-effectiveness. J Vasc Surg, 51(4): 1068–9. | Poster abstract |
| Sultan & Hynes (2010c) | Poster abstract |
| Sultan & Hynes (2011a). Clinical efficacy and cost per quality-adjusted life years of pararenal endovascular aortic aneurysm repair compared with open surgical repair. J Endovasc Ther, 18: 181–96. [PubMed: 21521058] | Population (elective repair) |
| Sultan & Hynes (2011b). A mid- to long-term experience of clinical efficacy and cost per quality-adjusted-life years with pararenal endovascular aortic repair (PEVAR) without fenestration for pararenal AAA compared with open surgical repair. Cardiovasc Interv Radiol, 3 (332/677). | Poster abstract |
| Sultan & Hynes (2012). Clinical efficacy and cost per qualityadjusted life years of para-renal endovascular aortic aneurysm repair compared with open surgical repair. JACC, 60(17): B38. | Poster abstract |
| Sweeting et al. (2015). Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg, 102: 1229–39. [PMC free article: PMC4744980] [PubMed: 26104471] | Review article, no additional CUAs |
| Tarride et al. (2008). Cost-effectiveness analysis of elective endovascular repair compared with open surgical repair of abdominal aortic aneurysms for patients at a high surgical risk: A 1-year patient-level analysis conducted in Ontario, Canada. J Vasc Surg, 48: 779–87. [PubMed: 18639421] | Population (elective repair) |
| Tarride et al. (2011). Should endovascular repair be reimbursed for low risk abdominal aortic aneurysm patients? Evidence from Ontario, Canada. Int J Vasc Med, 2011. [PMC free article: PMC3124872] [PubMed: 21748018] | Not a CUA |
| Taylor et al. (2012). EVAR is now cost effective and should replace open surgery for all suitable patients: con. Cardiovasc Interv Radiol, 35: S48. | Review article, no additional CUAs |
| Tremont et al. (2016). Endovascular Repair for Ruptured Abdominal Aortic Aneurysms has Improved Outcomes Compared to Open Surgical Repair. Vasc Endovasc Surg, 50(3) 147–55. [PubMed: 26975604] | Not a CUA |
| Van Bochove et al. (2016). Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm. J Vasc Surg, 3: 827–38. [PubMed: 26916588] | Population (elective repair) |
| Weinkauf et al. (2017). Open versus endovascular aneurysm repair trial review. Surgery, 162(5): 974–78. [PubMed: 28602492] | Population (elective repair) |
| Wilt et al. (2006). Comparison of endovascular and open surgical repairs for abdominal aortic aneurysm. Evid Rep Technol Assess, 144: 1–113. [PMC free article: PMC4780951] [PubMed: 17764213] | Review article, no additional CUAs |
Key: CUA, cost–utility analysis.
Appendix K. Research recommendation
| Research recommendation | What is the effectiveness and cost-effectiveness of complex EVAR versus open surgical repair in people with a ruptured AAA for whom open surgery is a suitable option? |
|---|---|
| Population |
People undergoing surgery for a ruptured abdominal aortic aneurysm Sub-grouped by: age, sex, comorbidities (including cardiovascular disease, renal disease, COPD, obesity) and ethnicity |
| Intervention(s) |
|
| Comparator(s) | Open surgical repair |
| Outcomes |
|
| Study design | Randomised controlled trial |
| Potential criterion | Explanation |
|---|---|
| Importance to patients, service users or the population |
EVAR is a widely performed non-invasive alternative to open surgical repair. However, it is more expensive and more difficult to perform. Although EVAR has been shown to produce comparable long-term outcomes to open surgical in people with ruptured infrarenal aneurysms, it is less clear whether these benefits are maintained in people with ruptured juxtarenal, suprarenal type IV, and branched infrarenal aneurysms. As a result, research is needed to identify how effective complex EVAR is in these populations. |
| Relevance to NICE guidance | High priority: it is currently not possible to make specific recommendations related to complex EVAR, other than to state that the procedure should not be performed on aneurysms that could be treated by open surgical repair, unless it is performed within the context of a randomised controlled trial. |
| Current evidence base | Randomised controlled trials have been performed to assess the efficacy of standard EVAR for unruptured or ruptured AAA, and complex EVAR of unruptured AAA. However, no RCTs have been performed to determine the efficacy of complex EVAR in people with ruptured juxtarenal, suprarenal type IV, and branched infrarenal aneurysms. In the absence of this type of evidence the committee recognised the potential for harm if patients who could receive open surgery were offered complex speculative EVAR for the wrong reasons. As a result, they agreed that complex EVAR should be performed in well-controlled environments, like that of an RCT to ensure that data will be collected to inform future updates of the guideline. |
| Equality | No specific equality concerns are relevant to this research recommendation. |
| Feasibility | There is a sufficiently large and well defined population available that randomised controlled trials in this area should be feasible. |
Appendix L. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Methods, evidence and recommendations
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Review Effectiveness of endovascular aneurysm repair, open surgical repair and non-surgical management of unruptured abdominal aortic aneurysms: Abdominal aortic aneurysm: diagnosis and management: Evidence review K[ 2020]Review Effectiveness of endovascular aneurysm repair, open surgical repair and non-surgical management of unruptured abdominal aortic aneurysms: Abdominal aortic aneurysm: diagnosis and management: Evidence review K. 2020 Mar
- Outcomes for symptomatic abdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program.[J Vasc Surg. 2016]Outcomes for symptomatic abdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program.Soden PA, Zettervall SL, Ultee KH, Darling JD, Buck DB, Hile CN, Hamdan AD, Schermerhorn ML. J Vasc Surg. 2016 Aug; 64(2):297-305. Epub 2016 Apr 14.
- Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis.[J Vasc Surg. 2006]Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis.Mehta M, Taggert J, Darling RC 3rd, Chang BB, Kreienberg PB, Paty PS, Roddy SP, Sternbach Y, Ozsvath KJ, Shah DM. J Vasc Surg. 2006 Jul; 44(1):1-8; discussion 8.
- Characteristics and outcomes of small abdominal aortic aneurysm rupture in the American College of Surgeons National Surgical Quality Improvement Program database.[J Vasc Surg. 2021]Characteristics and outcomes of small abdominal aortic aneurysm rupture in the American College of Surgeons National Surgical Quality Improvement Program database.Bellamkonda KS, Nassiri N, Sadeghi MM, Zhang Y, Guzman RJ, Ochoa Chaar CI. J Vasc Surg. 2021 Sep; 74(3):729-737. Epub 2021 Feb 19.
- Review Endovascular treatment for ruptured abdominal aortic aneurysm.[Cochrane Database Syst Rev. 2007]Review Endovascular treatment for ruptured abdominal aortic aneurysm.Dillon M, Cardwell C, Blair PH, Ellis P, Kee F, Harkin DW. Cochrane Database Syst Rev. 2007 Jan 24; (1):CD005261. Epub 2007 Jan 24.
- Effectiveness of endovascular aneurysm repair compared with open surgical repair...Effectiveness of endovascular aneurysm repair compared with open surgical repair of ruptured abdominal aortic aneurysms
Your browsing activity is empty.
Activity recording is turned off.
See more...