NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Accuracy of imaging techniques in identifying complications after surgery
Review question
When monitoring people after they have had EVAR or open repair of an abdominal aortic aneurysm, which imaging techniques are most useful for detecting postoperative complications, further aneurysm expansion and aneurysm rupture?
Introduction
Endovascular aneurysm repair (EVAR) and open repair of abdominal aortic aneurysms (AAAs) are associated with a number of postoperative complications such as endoleak and graft occlusion, as well as further aneurysm expansion and aneurysm rupture. Due to these complications, surveillance is required. This review question aims to determine which imaging technique is most accurate in identifying postoperative complications. This review also aims to determine which imaging techniques are most acceptable to people with AAA and clinicians, taking into account the safety profiles of the approaches.
PICO table
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in Table 1.
Table 1
Inclusion criteria.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A broad search strategy was used to pull in all studies that examine the diagnosis, surveillance or monitoring of AAAs. This was a ‘bulk’ search that covered multiple review questions. The reviewer sifted the database to identify all studies that assessed the accuracy, safety and acceptability of imaging techniques in the diagnosis of AAAs, including asymptomatic aneurysms, symptomatic unruptured aneurysms, and ruptured aneurysms. An available Cochrane review (Abraha 2017) was used as an additional source of studies which examined the diagnostic accuracy of CDUS in the identification of endoleaks.
Cross sectional studies and systematic reviews of this study design, examining diagnostic accuracy using sensitivity, specificity and likelihood ratios were considered for inclusion. Studies examining adverse events after surgery and acceptability of approach to people with AAA and clinicians were also considered.
Ideally, studies using imaging techniques such as colour duplex ultrasound (CDUS) and computed tomography angiography (CTA) as the reference standard were included. If studies did not report diagnostic test accuracy measures, 2×2 tables of true positives, false positives, true negatives and false negatives were derived from raw data or calculated from the set of test accuracy statistics. These measures were presented as calculated test accuracy measures within the evidence review. Studies from which 2×2 tables could not be calculated were excluded.
Studies were also excluded if they were:
- Not in English
- Abstracts or non-published data
- Published before 2000.
Clinical evidence
Included studies
From a database of 12,786 studies, 188 studies were identified as being potentially relevant. An update search was conducted in December 2017, during which 8 further studies were included for consideration. Following full text review of the 196 studies, 37 studies of cross-sectional study design were included.
Overall, included studies explored the diagnostic accuracy of colour duplex ultrasound (CDUS), contrast enhanced duplex ultrasound (CEUS), 3D contrast enhanced duplex ultrasound (3D CEUS), 4D contrast enhanced duplex ultrasound (4D CEUS) and magnetic resonance imaging (MRI). Data was identified for complications such as endoleaks, graft occlusion and change in aneurysm size. All included studies compared the index tests to computed tomography angiography (CTA). Where possible, the diagnostic accuracy of the tools in the identification of Type I and III endoleaks as well as Type II endoleak was explored.
No studies were included which examined which imaging techniques were most acceptable to people with AAA and clinicians, taking into account the safety profiles of the approaches.
Excluded studies
The list of papers excluded at full text review, with reasons, is given in Appendix H.
Summary of clinical studies included in the evidence review
A summary of the included studies is provided in the table below. See Appendix D for full evidence tables.
Table 2
Summary of included studies.
Quality assessment of clinical studies included in the evidence review
See Appendix F for full GRADE tables, highlighting the quality of evidence from the included studies
Economic evidence
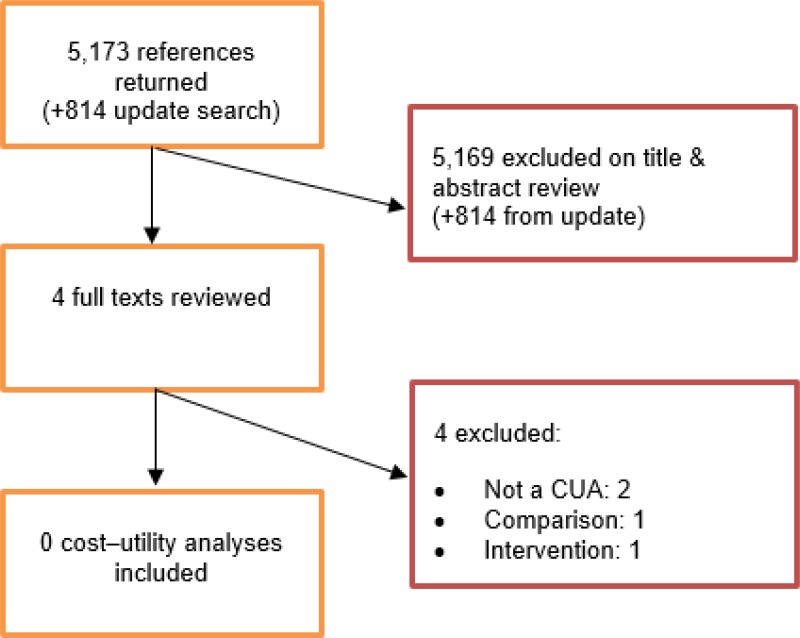
A systematic review of economic literature was conducted jointly for all review questions in this guideline by applying standard health economic filters to a clinical search for AAA (see Appendix B). A total of 5,173 studies was identified. The studies were reviewed to identify economic evaluations in the form of cost–utility analyses evaluating imaging techniques for detecting postoperative complications, further aneurysm expansion and aneurysm rupture. Studies that met the eligibility criteria were assessed using the quality appraisal criteria as outlined in the Guidelines Manual (2014).
Included studies
Following an initial review of titles and abstracts, the full texts of 4 studies were retrieved for detailed consideration. Following full-text review, none of the 4 studies were judged to be potentially applicable cost–utility analyses.
An update search was conducted in December 2017, to identify any relevant cost–utility analyses that had been published during guideline development. This search returned 814 studies. Following review of titles and abstracts, no studies were ordered for detailed consideration.
No studies were therefore included as economic evidence for this review question.
Excluded studies
Studies that were excluded after full-text review, and reasons for exclusion, are provided in Appendix H – Excluded studies.
Evidence statements
Diagnostic test accuracy
Thirty-seven studies were identified which examined the diagnostic accuracy of different diagnostic tools (CDUS, CEUS and MRI) after EVAR. Two of these studies examined 3D and 1 study examined 4D CEUS. Diagnostic accuracy of the tools was evaluated using positive and negative likelihood ratios. The following schema, adapted from the suggestions of Jaeschke et al. (1994), was used to interpret the likelihood ratio findings from diagnostic test accuracy reviews.
| Likelihood ratio | Value of likelihood ratio | Interpretation |
|---|---|---|
| Negative likelihood ratio | LR ≤ 0.1 | Very large decrease in probability of disease |
| 0.1 < LR ≤ 0.2 | Large decrease in probability of disease | |
| 0.2 < LR ≤ 0.5 | Moderate decrease in probability of disease | |
| 0.5 < LR ≤ 1.0 | Slight decrease in probability of disease | |
| Positive likelihood ratio | 1.0 < LR < 2.0 | Slight increase in probability of disease |
| 2.0 ≤ LR < 5.0 | Moderate increase in probability of disease | |
| 5.0 ≤ LR < 10.0 | Large increase in probability of disease | |
| LR ≥ 10.0 | Very large increase in probability of disease |
The schema above has the effect of setting a minimal important difference for positive likelihoods ratio at 2, and a corresponding minimal important difference for negative likelihood ratios at 0.5. Likelihood ratios (whether positive or negative) falling between these thresholds were judged to indicate no meaningful change in the probability of disease.
Evidence statements were formed to reflect the different complications associated with AAA repair as well as people undergoing infrarenal and complex EVAR.
No studies were identified which assessed the diagnostic accuracy of different diagnostic tools after open surgical repair of AAA.
Identification of an any type of endoleak
Interpretation of positive test results
A positive finding on the following tools increases the probability that an endoleak is present to a degree that is likely to be very large:
- CEUS after complex EVAR (high-quality evidence from 1 study with 62 participants; 95% CI ranged from large to very large increase)
- 4D CEUS after complex EVAR (low-quality evidence from 1 study with 22 participants; 95% CI ranged from slight to very large increase)
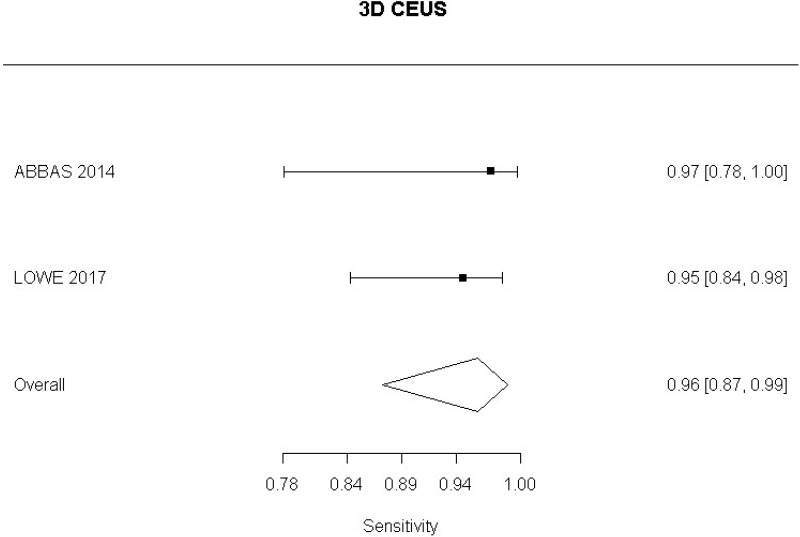
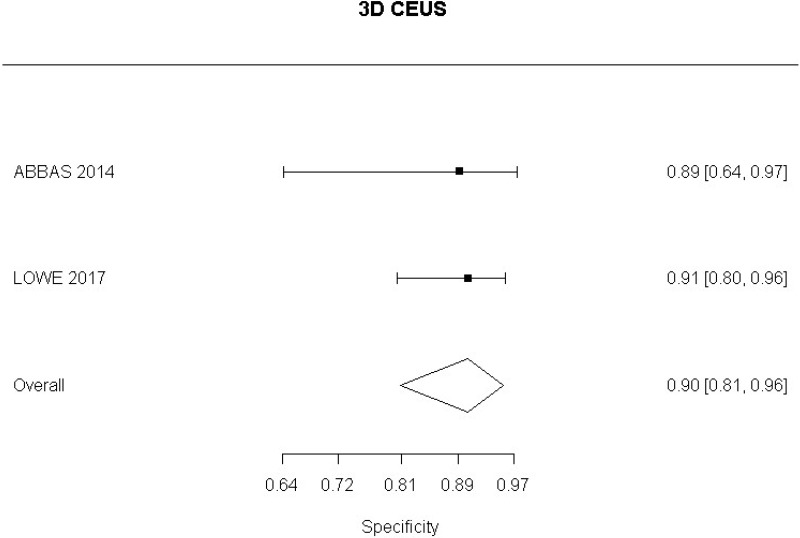
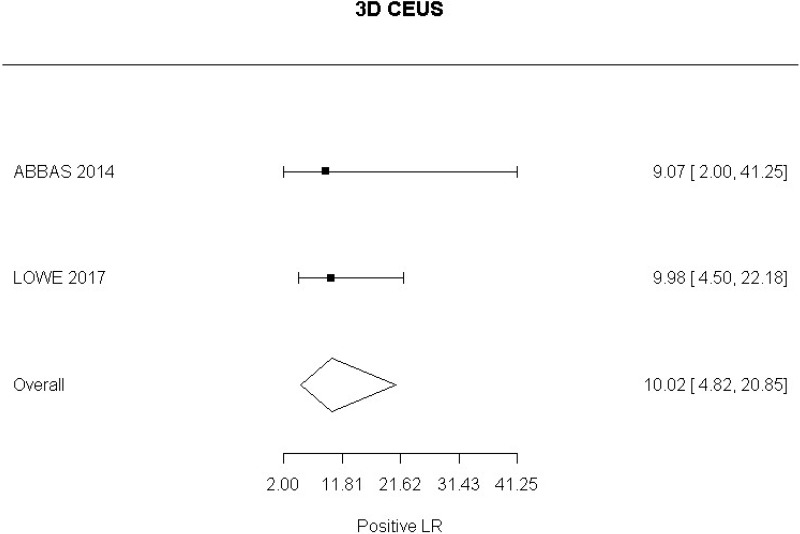
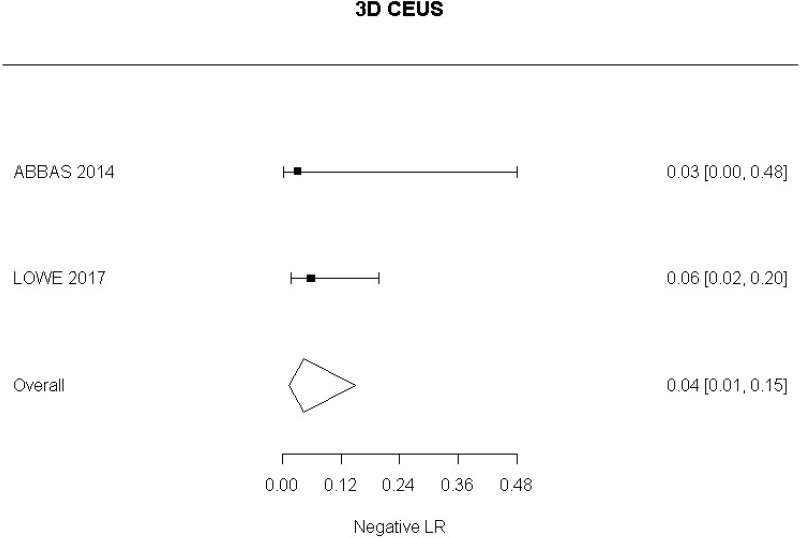
- 3D CEUS after EVAR (Moderate-quality evidence from 2 studies with 130 measurements; 95% CI ranged from moderate to very large increase)
- MRI after EVAR (very low-quality evidence from 1 study with 46 participants; 95% CI ranged from moderate to very large increase).
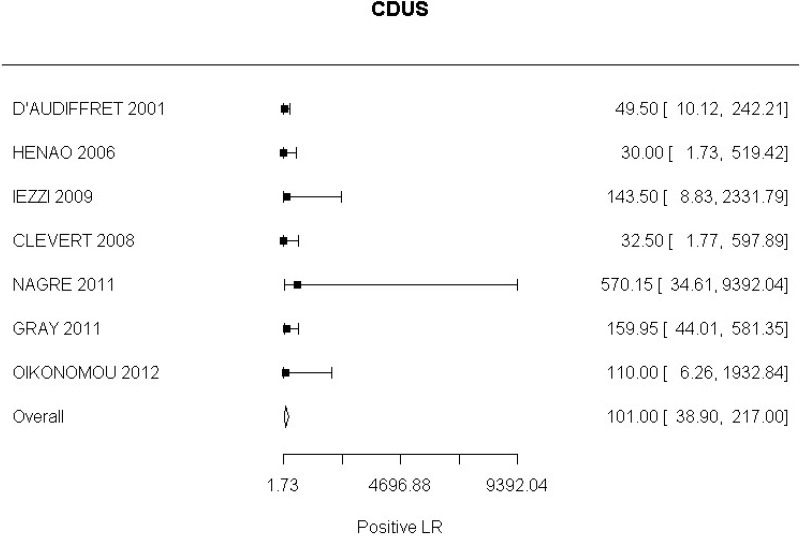
A positive finding on the following tools increases the probability that an endoleak is present (based on positive likelihood ratio) to a degree that is likely to be large:
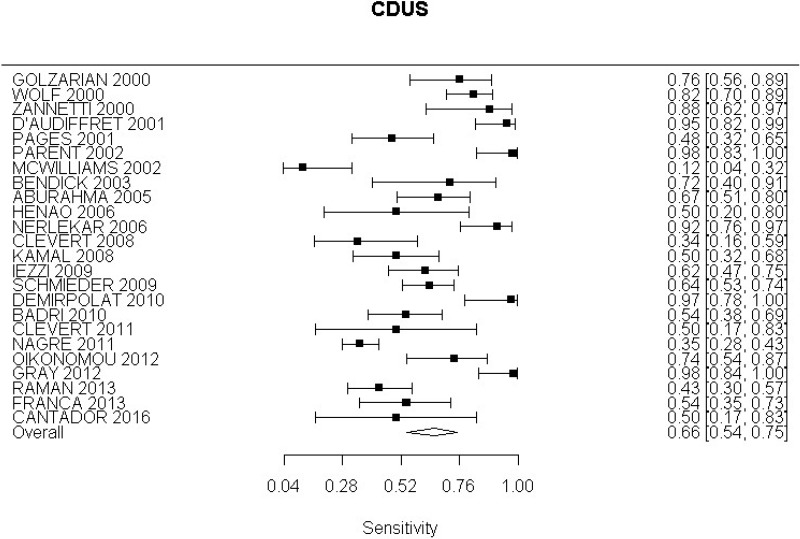
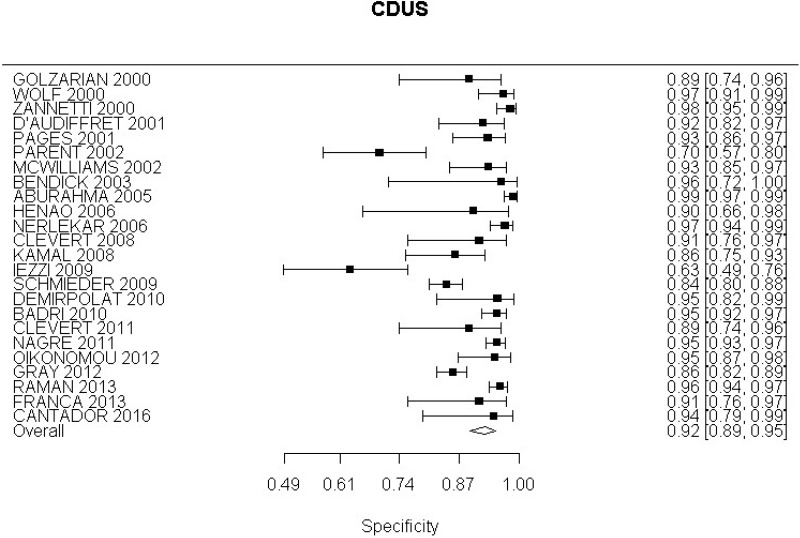
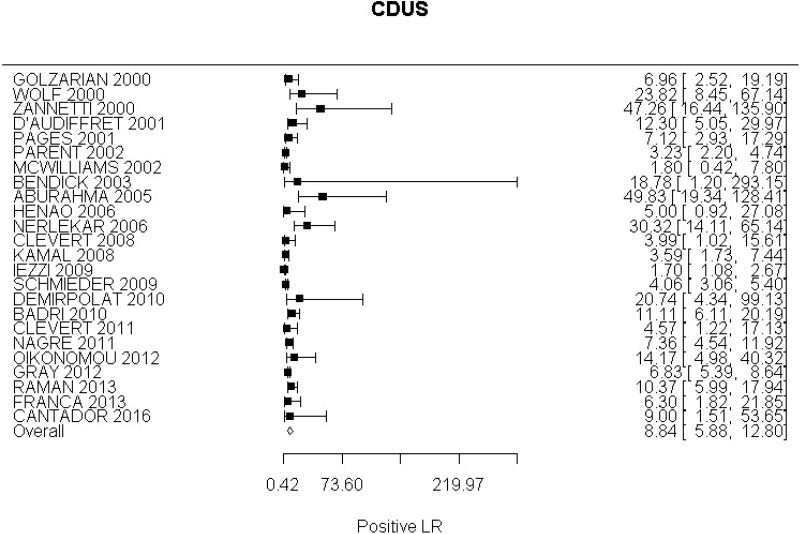
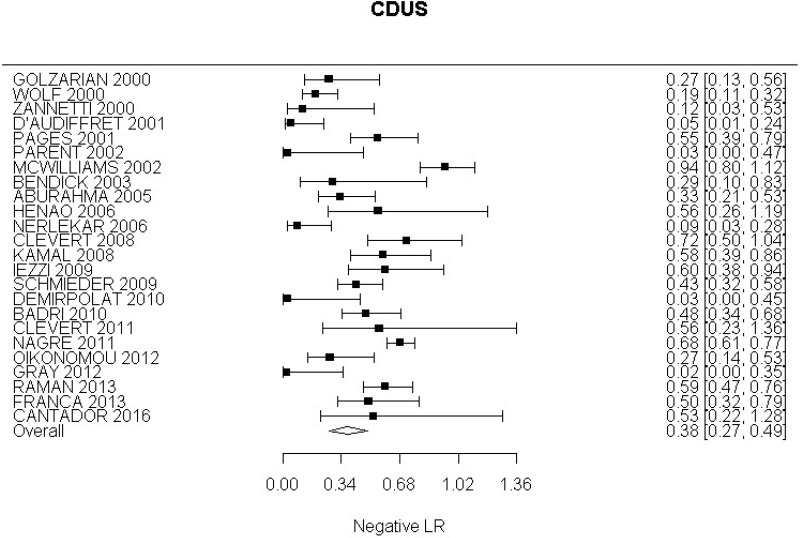
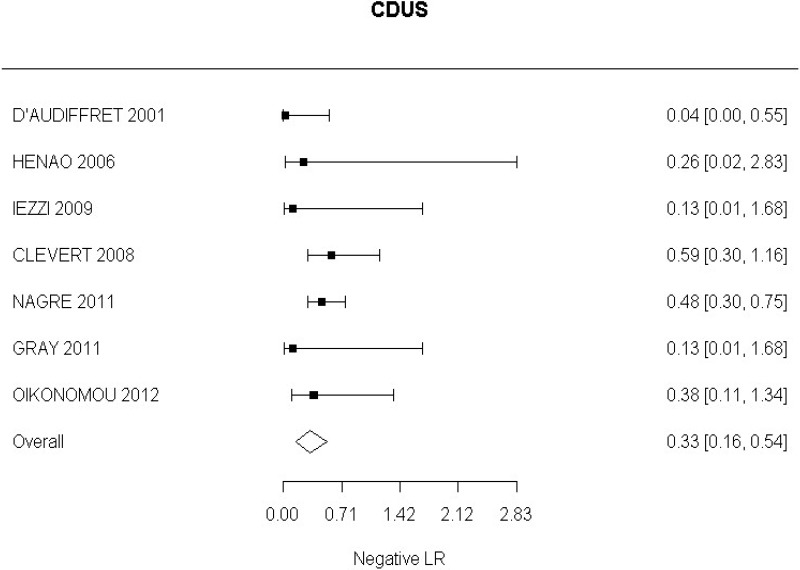
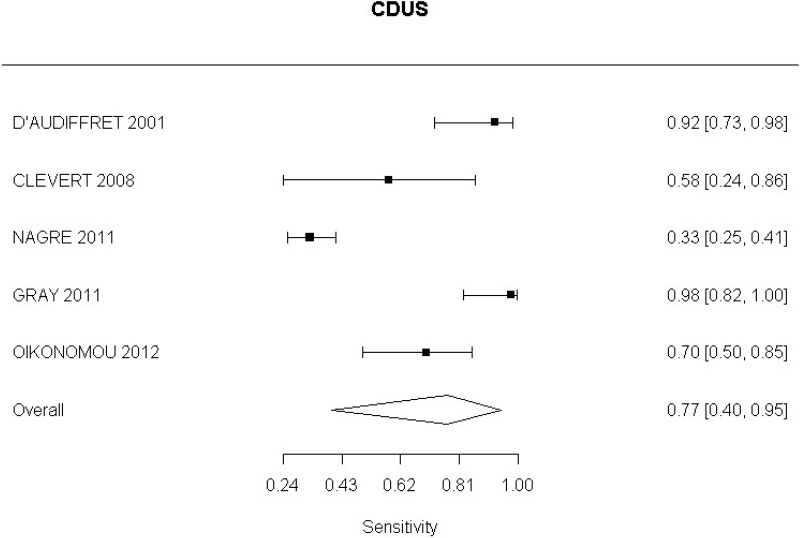
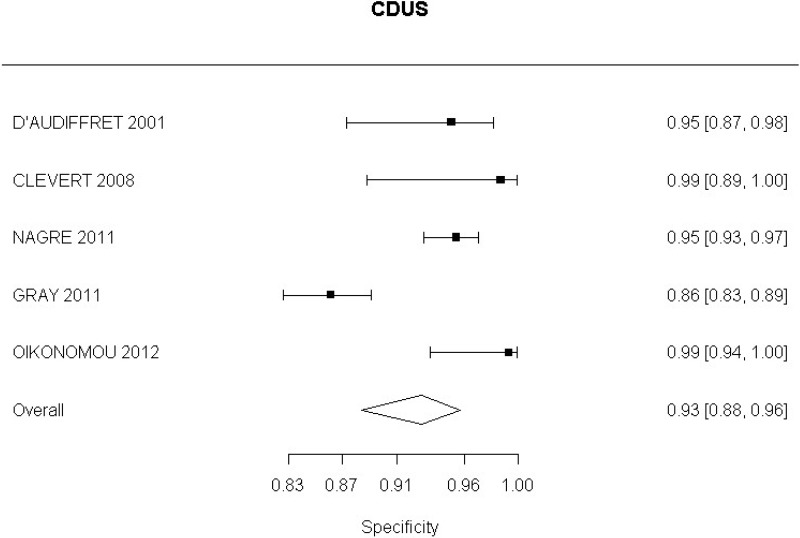
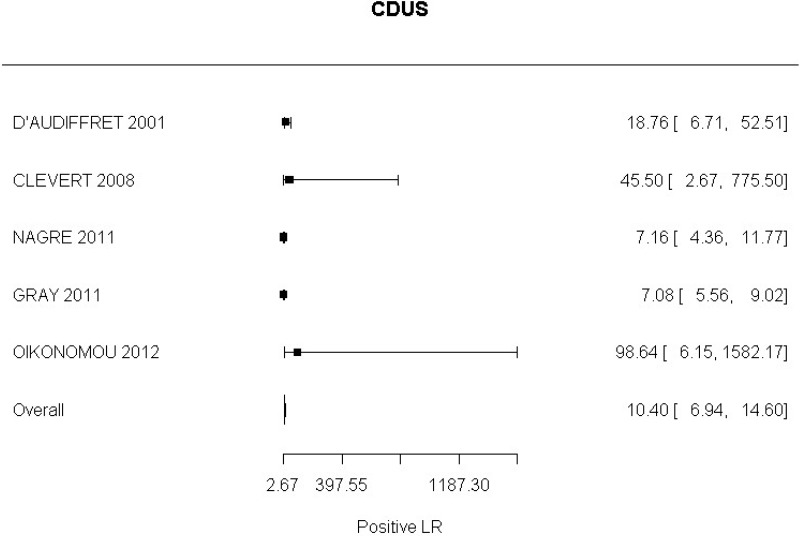
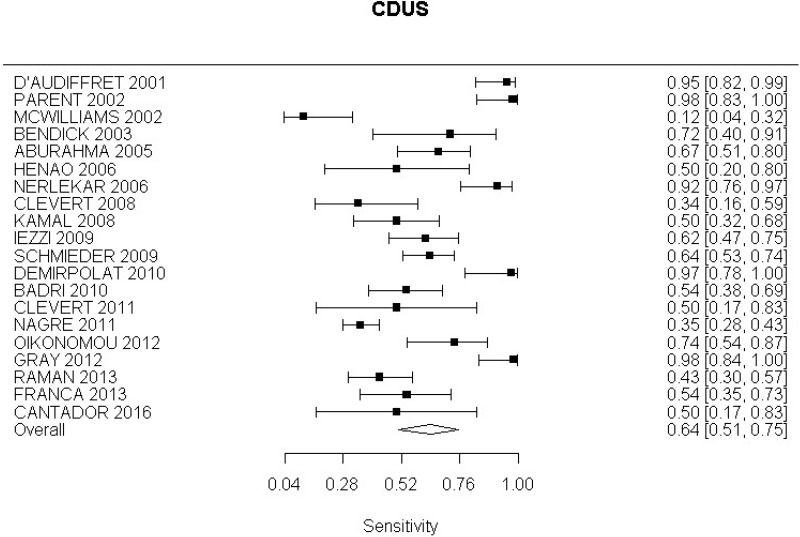
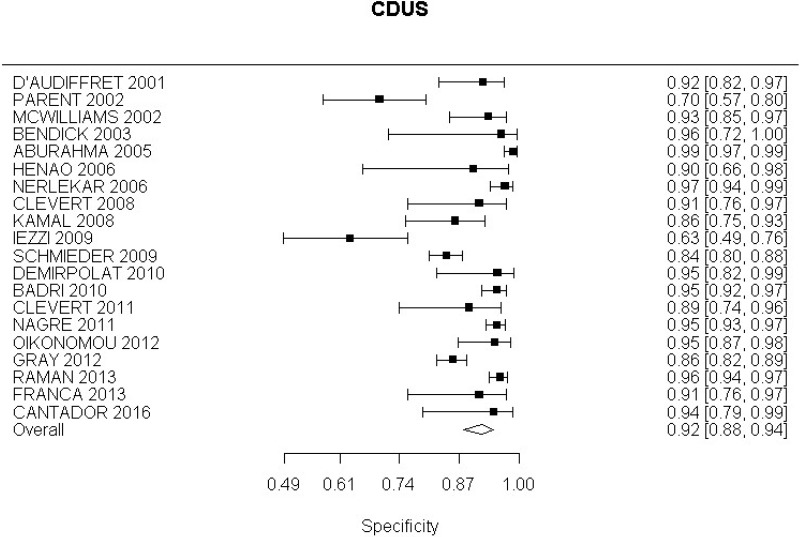
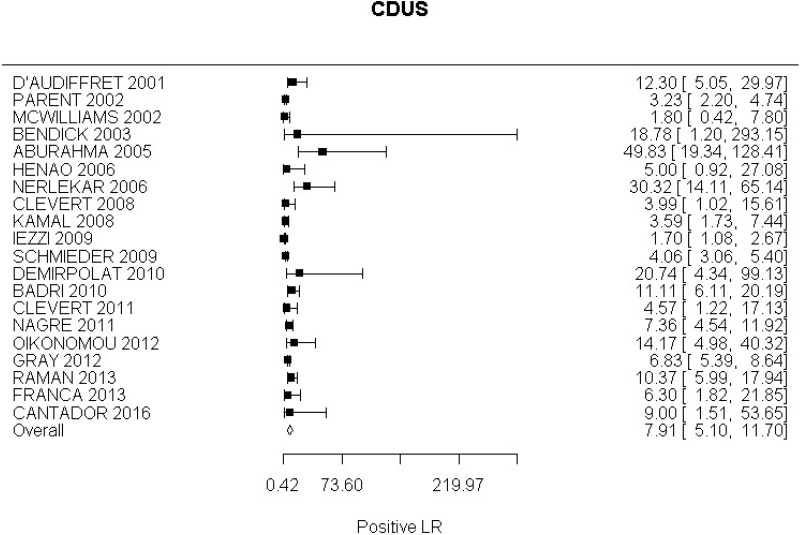
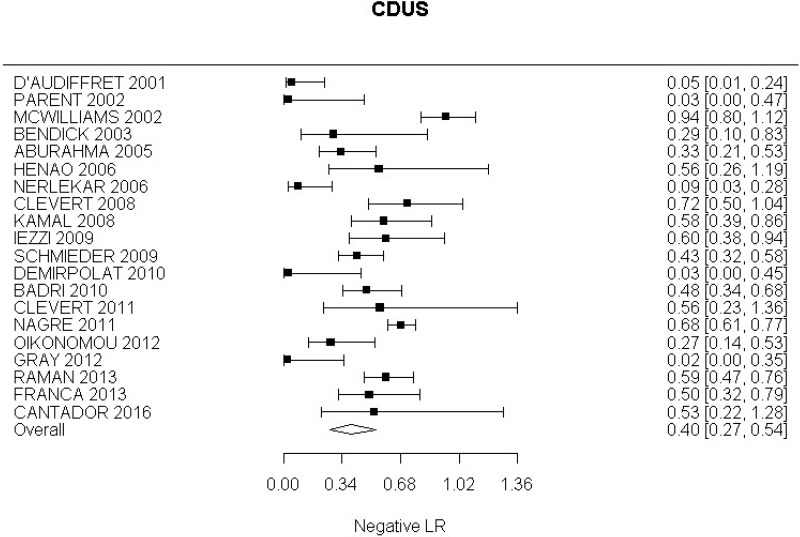
- CDUS after EVAR (very low-quality evidence from 24 studies including 4,198 measurements; 95% CI ranged from large to very large increase)
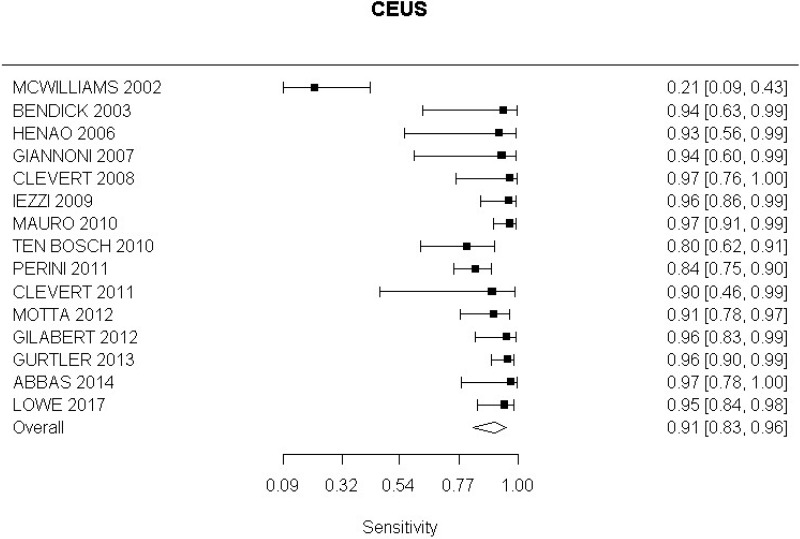
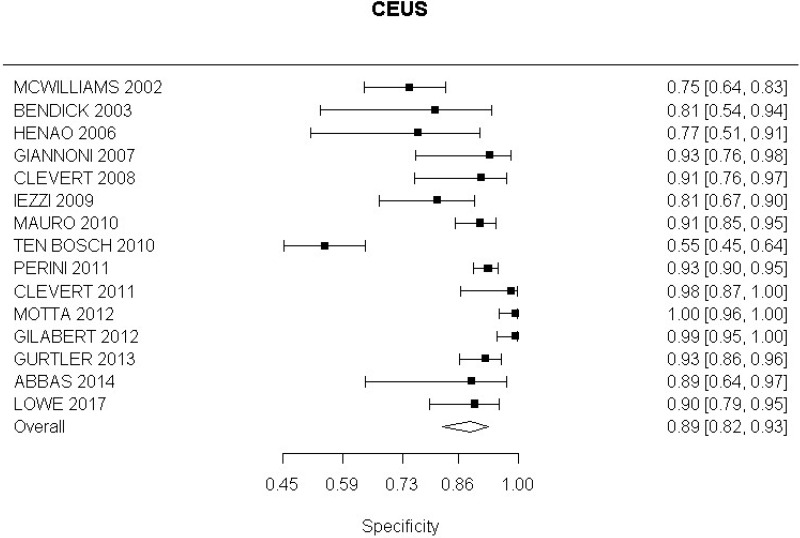
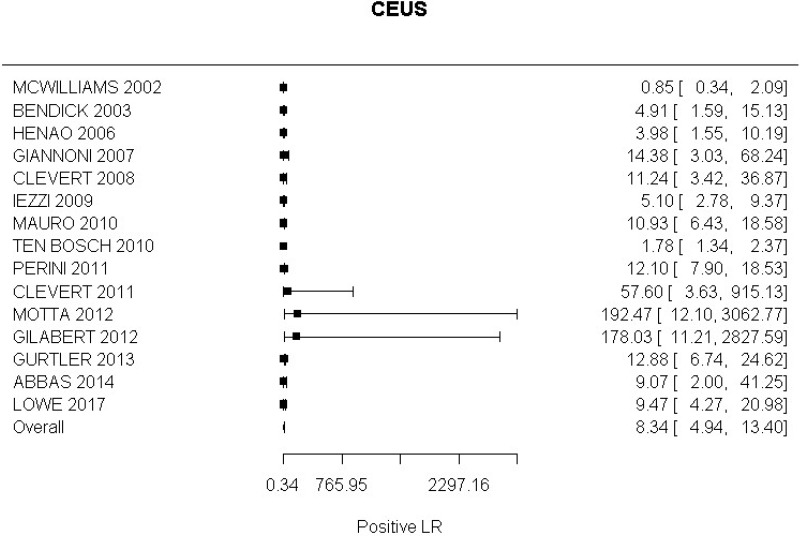
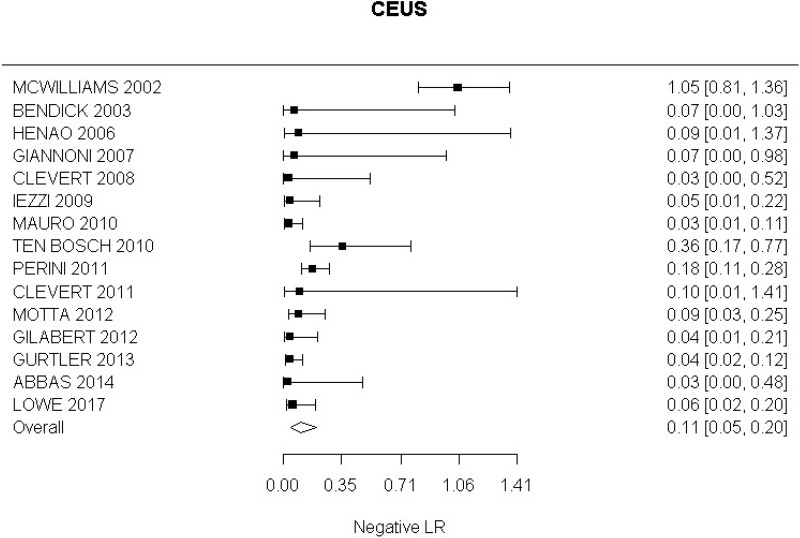
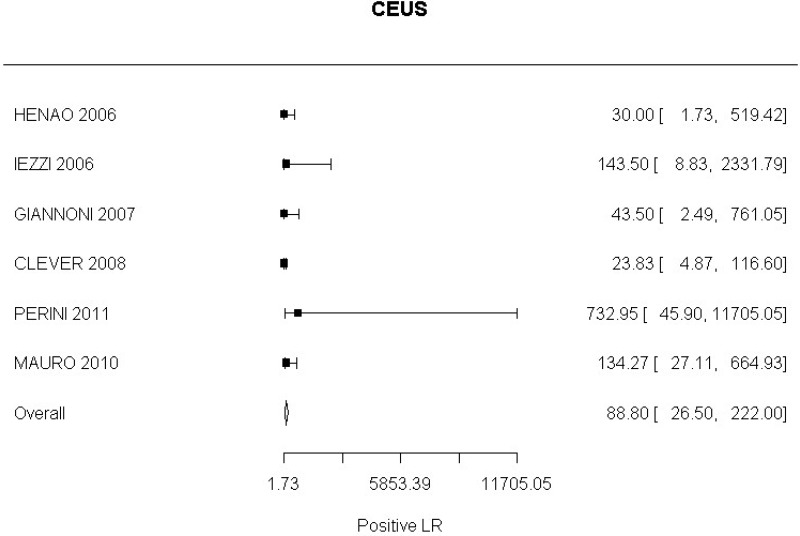
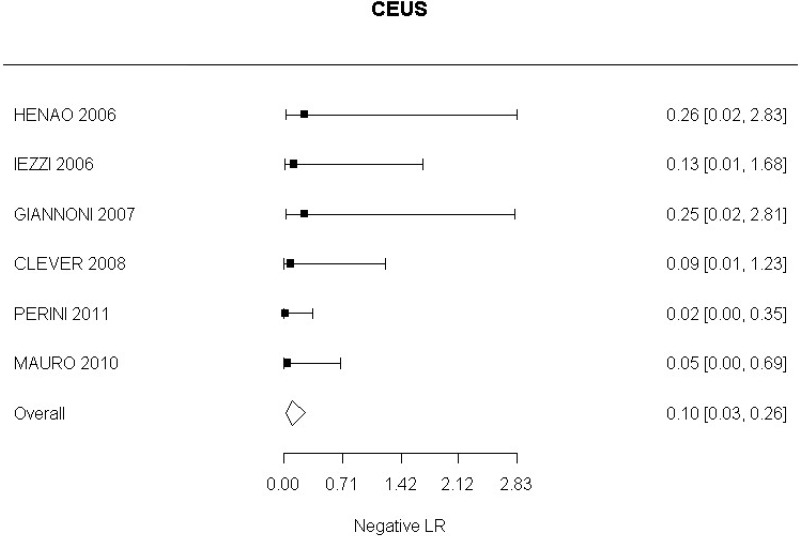
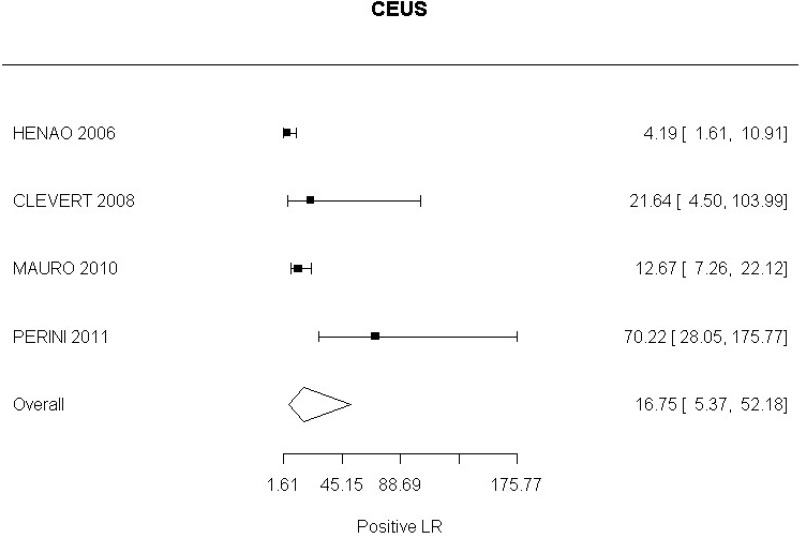
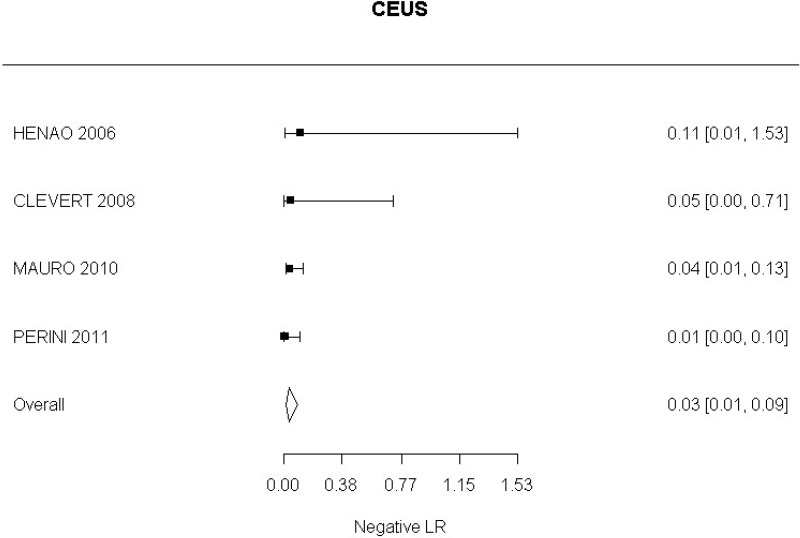
- CEUS after EVAR (very low-quality evidence from 15 studies including 1,667 measurements; 95% CI ranged from moderate to very large increase).
A negative finding on the following tools decreases the probability that an endoleak is present (based on negative likelihood ratio) to a degree that is likely to be very large:
- 3D CEUS after EVAR (Moderate-quality evidence from 2 studies including 130 people; 95% CI ranged from large to very large decrease)
- MRI after EVAR (very low-quality evidence from 1 study including 46 people; 95% CI ranged from moderate to very large decrease).
Interpretation of negative test results
Very low-quality evidence from 15 studies, including 1,667 measurements, indicated a negative finding on CEUS after EVAR decreases the probability that an endoleak is present to a degree that is likely to be large (95% CI ranged from large to very large decrease).
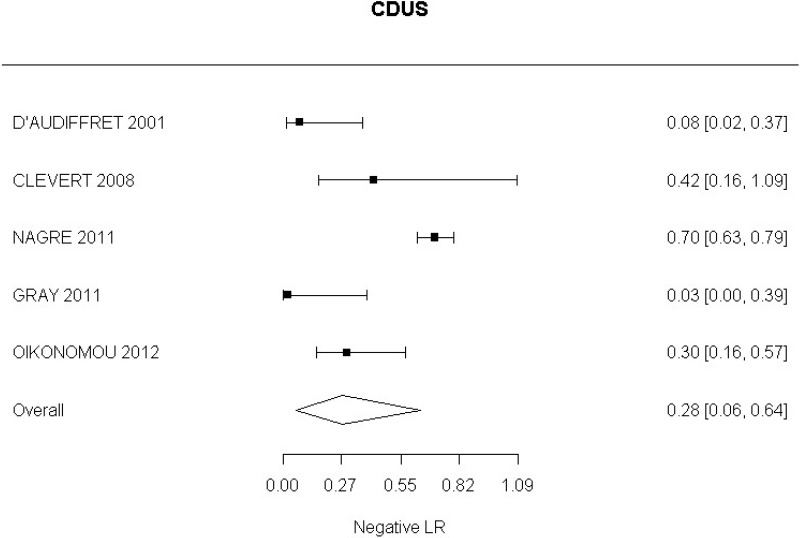
Very low-quality evidence from 24 studies, including 4,198 measurements, indicated a negative finding on CDUS after EVAR decreases the probability that an endoleak is present to a degree that is likely to be moderate.
The following tools could not demonstrate whether a negative finding altered the probability that an endoleak is present:
- CEUS after complex EVAR (moderate-quality evidence from 1 study including 62 people; 95% CI ranged from very large decrease to slight increase)
- 4D CEUS after complex EVAR (low-quality evidence from 1 study including 22 people; 95% CI ranged from large decrease to slight increase).
Identification of Type I and III endoleaks
Interpretation of positive test results
A positive finding on the following tools increases the probability that Type I and III endoleaks are present (based on positive likelihood ratio) to a degree that is likely to be very large:
- CDUS after EVAR (low-quality evidence from 7 studies including 1,346 measurements)
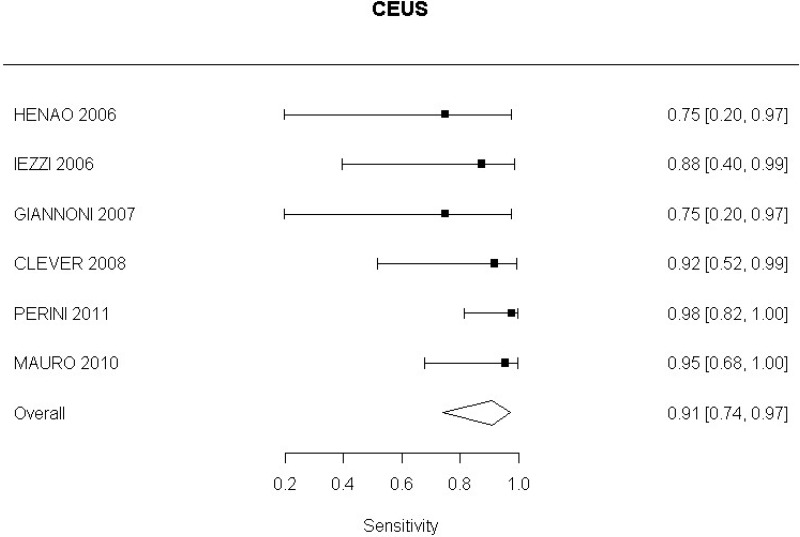
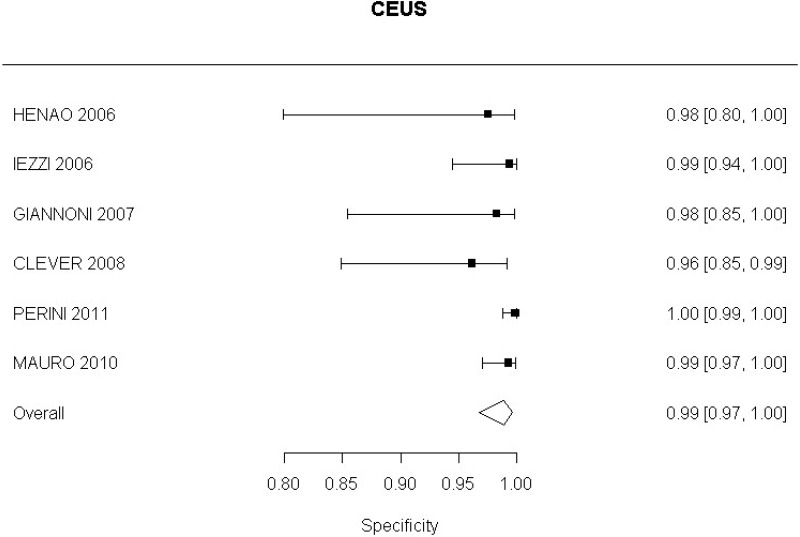
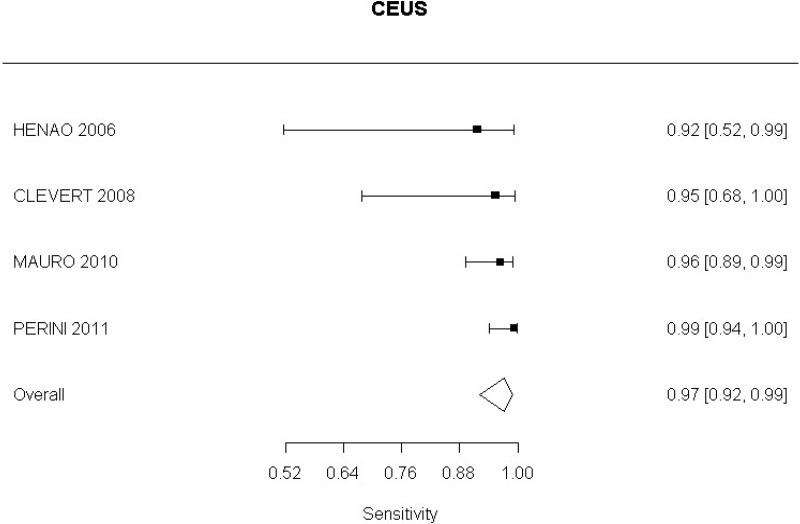
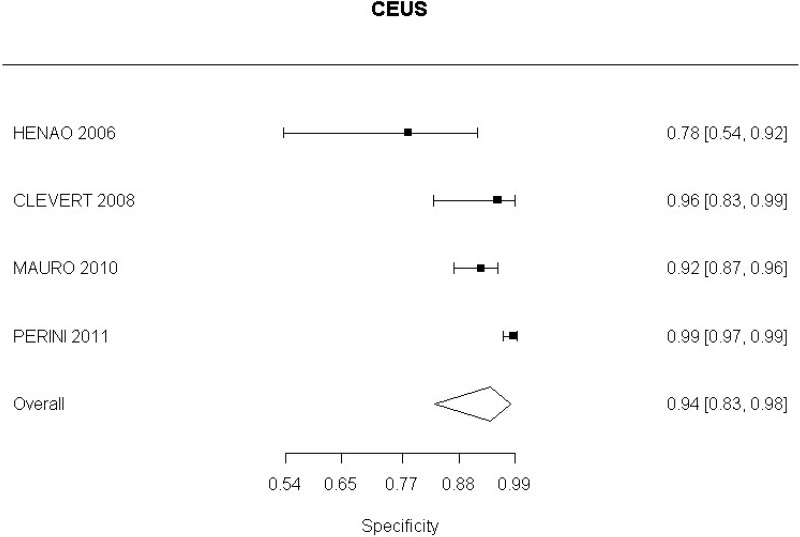
- CEUS after EVAR (high-quality evidence from 6 studies including 791 measurements)
- CEUS after complex EVAR (high-quality evidence from 1 study including 62 people; 95% CI ranged from large to very large increase).
Interpretation of negative test results
High-quality evidence from 6 studies, including 791 measurements, indicated a negative finding on CEUS after EVAR decreases the probability that a Type I or III endoleak is present to a degree that is likely to be large (95% CI ranged from moderate to very large decrease).
High-quality evidence from 7 studies, including 1,346 measurements, indicated a negative finding on CDUS after EVAR decreases the probability that a Type I or III endoleak is present to a degree that is likely to be moderate (95% CI ranged from slight to large decrease).
Low-quality evidence from 1 study, including 62 people, could not demonstrate whether a negative finding on CEUS after complex EVAR alters the probability that a Type I endoleak is present (95% CI ranged from very large decrease to moderate increase).
Identification of Type II endoleak
Interpretation of positive test results
A positive finding on the following tools increases the probability that a Type II endoleak is present (based on positive likelihood ratio) to a degree that is likely to be very large:
- CDUS after EVAR (very low-quality evidence from 5 studies including 1,242 measurements; 95% CI ranged from large to very large)
- CEUS after EVAR (low-quality evidence from 4 studies including 678 measurements)
- CEUS after complex EVAR (high-quality evidence from 1 study including 62 people; 95% CI ranged from moderate to very large increase).
Interpretation of negative test results
High-quality evidence from 4 studies, including 678 measurements, indicated a negative finding on CEUS after EVAR decreases the probability that a Type II endoleak is present to a degree that is likely to be very large (95% CI ranged from large to very large decrease).
High-quality evidence from 5 studies, including 1,242 measurements, indicated a negative finding on CDUS after EVAR decreases the probability that a Type II endoleak is present to a degree that is likely to be moderate (95% CI ranged from slight to very large decrease).
Moderate-quality evidence from 1 study, including 62 people, could not demonstrate whether a negative finding on CEUS after complex EVAR alters the probability that a Type II endoleak is present (95% CI ranged from very large decrease to slight increase).
Identification of overall change in aneurysm size
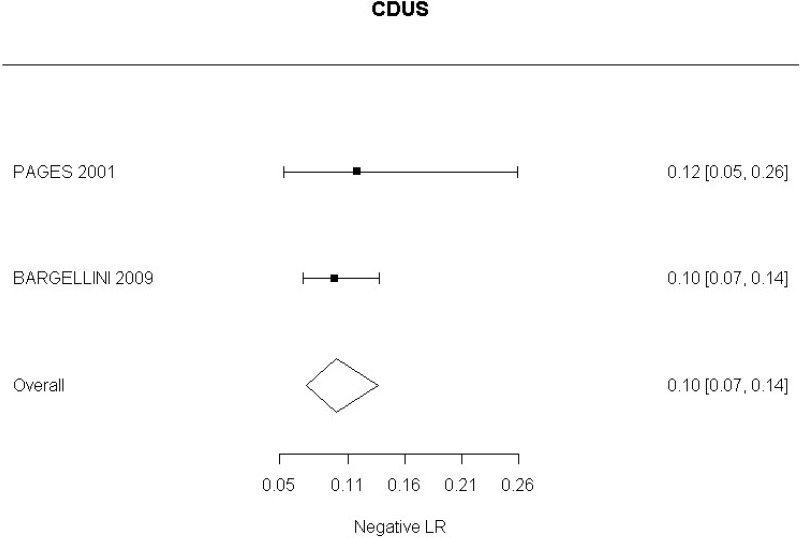
Low-quality evidence from 2 studies, including 773 measurements, could not demonstrate whether a positive finding on CDUS after alters the probability of a change in aneurysm size (95% CI ranged from slight decrease to very large increase).
Low-quality evidence from 2 studies, including 773 measurements, indicated a negative finding on CDUS after EVAR decreases the probability of a change in aneurysm size to a degree that is likely to be large (95% CI ranged from large to very large decrease).
Identification of aneurysm expansion
Moderate-quality evidence from 1 study, including 180 measurements, indicated a positive finding on CDUS after EVAR increases the probability that an aneurysm has grown to a degree that is likely to be very large (95% CI ranged from large to very large decrease).
Low-quality evidence from 1 study, including 180 measurements, indicated a negative finding on CDUS after EVAR decreases the probability that an aneurysm has grown to a degree that is likely to be slight (95% CI ranged from slight to moderate decrease).
Identification of aneurysm reduction
Moderate-quality evidence from 1 study, including 657 measurements, indicated a positive finding on CDUS after EVAR increases the probability that an aneurysm has become smaller to a degree that is likely to be moderate.
Moderate-quality evidence from 1 study, including 657 measurements, indicated a negative finding on CDUS after EVAR decreases the probability that an aneurysm has become smaller to a degree that is likely to be very large.
Identification of graft occlusion
Moderate-quality evidence from 1 study, including 134 measurements, indicated a positive finding on CEUS after EVAR increases the probability that graft occlusion is present to a degree that is likely to be very large (95% CI ranged from large to very large increase).
Low-quality evidence from 1 study, including 134 measurements, indicated a negative finding on CEUS after EVAR decreases the probability that that graft occlusion is present to a degree that is likely to be moderate (95% CI ranged from slight to moderate decrease).
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
In the evidence review, sensitivity, specificity and likelihood ratios were calculated for each index text. The committee took into consideration the likelihood ratios but also examined the sensitivity of index tests in the identification of different complications.
While no evidence on patient and clinician acceptability was identified, the committee took these outcomes into consideration when making recommendations.
The quality of the evidence
Overall, the evidence ranged from very low to moderate quality. The studies had varying follow-up periods and were conducted in a number of different settings. Only 4 studies were conducted in the UK.
In a number of studies, methodological limitations were identified. Firstly, a number of studies did not specify if the results from the reference standard were blinded from the results of the index test. Due to this uncertainty in blinding, these studies were downgraded for risk of bias. A number of studies were downgraded for risk of bias because the time interval between the reference standard and index test was not specified. Studies in which the time interval between the 2 tests spanned more than 1 month were also downgraded for serious risk of bias, as disease progression during this time interval could have had an impact on the results.
A number of studies did not adequately provide a definition for a positive identification of the complication of interest. Studies in which definition of a positive test was not provided or unclear were downgraded for risk of bias. The committee also further discussed the studies in which a definition was provided. In relation to the identification of endoleak with CDUS, a number of studies defined the complication as the persistent blood flow outside the lumen of the graft. The committee agreed that this imaging sign is insufficiently sensitive to detect all endoleaks.
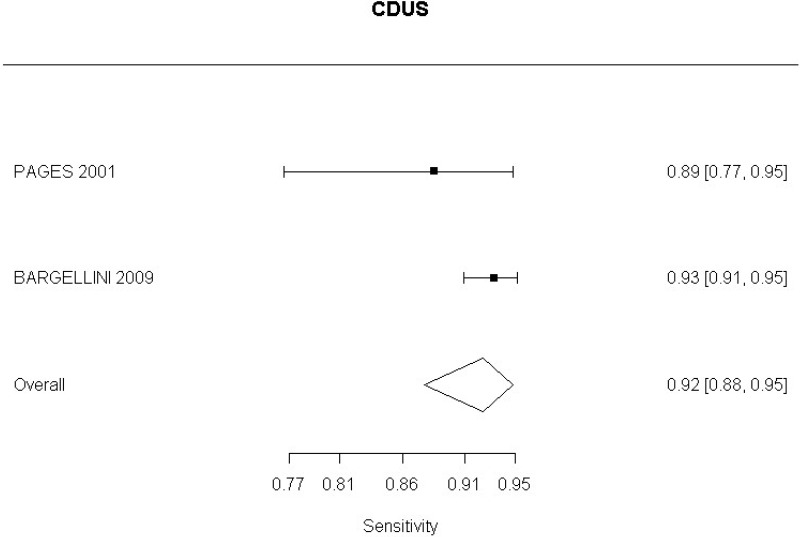
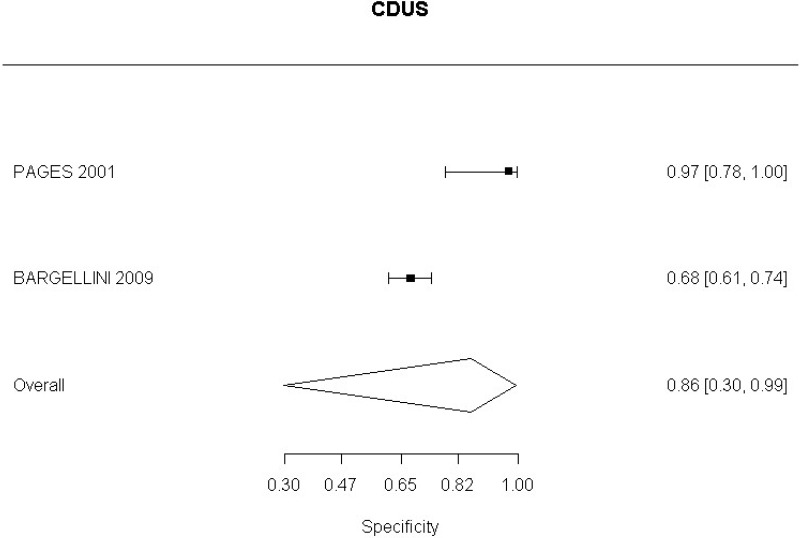
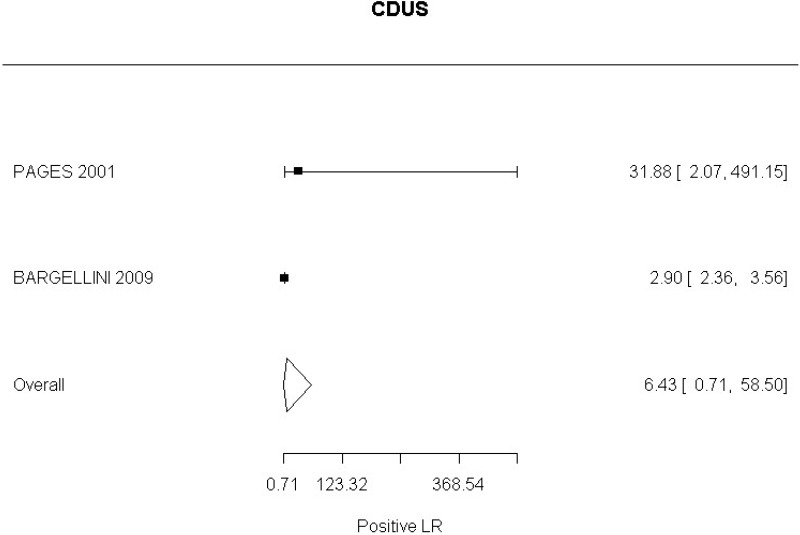
In relation to aneurysm expansion, 2 studies (Bargellini et al., 2009 and Pages et al., 2001) defined sac expansion on CDUS as an increase in the maximum anteroposterior or transverse sac diameter. The committee noted that measuring transverse diameter using CDUS was challenging. Similarly, 1 study (Motta et al., 2012) was identified which examined accuracy of CEUS in the identification of graft occlusion. The study defined graft occlusion as the presence of endograft partial thrombosis. The committee did not find this to be an adequate indicator of the risk of graft occlusion.
A number of studies were downgraded for indirectness. One study was identified which examined the diagnostic accuracy of unenhanced MRI. This study specifically examined unenhanced 2D motion sensitised-driven equilibrium (MSDE)-prepared balanced turbo filled echo (BTFE) sequences. The committee noted that this is not a sequence commonly used in practice. Due to this, the study was downgraded for indirectness, as it was not viewed as being representative of conventional MRI. Due to the quality of the study, the committee did not make recommendations for the use of MRI.
The review protocol specified studies published since 2000. All studies included in the review met this criterion, though 4 studies (Golzarian et al., 2002; Pages et al., 2001; Wolf et al., 2000 and Zannetti et al., 2000), whilst published after 2000, were undertaken before this date. A sensitivity analysis was conducted in which these studies were removed from the assessment of the diagnostic accuracy of CDUS. This resulted in a slight decrease in the overall estimate of CDUS diagnostic accuracy. These 4 studies were downgraded for partial indirectness. The committee were mindful that ultrasound technology has advanced considerably over the past decade. Furthermore, they noted that in some of the included studies the identification of endoleaks was based on ultrasound images acquired by technicians and retrospectively reviewed by radiologists. In light of this, a second sensitivity analysis was performed to consider only studies published from 2008 onwards in which the presence of endoleaks was determined in real-time by the person who was performing the scan. This sensitivity analysis indicated a slight increase in the diagnostic accuracy of CDUS, however the increase was not significant enough to change the committee’s conclusions.
Benefits and harms
The committee agreed on the importance of early identification of complications, particularly aneurysm expansion and endoleaks. Based on their experience, the committee agreed that there is considerable variation in monitoring strategies adopted across the NHS. Some centres use CTA as the primary imaging tool to detect sac expansion and endoleaks - CT is widely accepted as a reference standard due to its high diagnostic accuracy. In other centres, ultrasound is used as the main imaging modality to observe changes in sac size and detect large high flow endoleaks. Where changes in sac size are found on ultrasound further imaging with imaging tools that have higher discriminatory power is then undertaken. Since the evidence demonstrated that CDUS had a satisfactory sensitivity and specificity in identifying changes in sac size, the committee considered that this was a reasonable approach, so long as any abnormalities are subsequently explored with contrast-enhanced CTA or CEUS to exclude the presence of endoleaks.
The committee believed that CEUS was appropriate for exclusion of endoleaks because the addition of contrast agent improves the performance of ultrasound considerably when compared with CDUS alone.
While CDUS is used in the surveillance of post-operative complications following EVAR in some settings, the committee recommended against its use as a definitive imaging tool. The committee formed these conclusions based on the evidence which showed CDUS to have insufficient sensitivity for certain kinds of endoleak. This led the committee to agree that the use of CDUS as the sole imaging tool would not allow some complications, in particular type I and III endoleaks, to be adequately excluded.
Two studies of low quality were identified that demonstrated 3D CEUS to increase the probability of identifying endoleaks in people who have undergone fenestrated EVAR. One study was also identified which demonstrated 4D CEUS increases the probability of identifying endoleaks in people who have undergone EVAR. It was discussed that 3D imaging allows clinicians to form a complete picture of the complication and 4D imaging allows movement over time to be captured. However, the committee noted that, while these imaging techniques showed promise, additional software is required compared with standard 2D CEUS. Taking into consideration the quality of the evidence and cost and training implications, the committee did not make any recommendations on 3D and 4D CEUS.
Cost effectiveness and resource use
The committee advised that current practice in this area varies extensively, with some centres using only ultrasound, others using only CTA, and some using a mixture of techniques. It is the committee’s experience that CEUS has not been widely adopted in practice as a replacement for duplex ultrasound; it is mainly used only in large, specialist centres. The primary reason for this is that CEUS requires important new skills for sonographers – requiring cannulation of the patient and administration of contrast. These are skills that, in many cases, would require additional training and, potentially, medical staff to deal with any complications that may occur. In addition, the committee cited a perceived patient preference for not being cannulated, and a perception that CEUS does not materially influence subsequent decision-making compared with duplex ultrasound.
The committee discussed the resource implications of using CEUS over duplex ultrasound, and of using CTA over either ultrasound technique. CEUS requires administering contrast agent (approximately £40–60 per vial), a one-off software cost, as well as cannulation and contrast delivery, with the associated training and staff needs described above. CTA has a higher unit cost than both ultrasound techniques.
The committee discussed the cost implications of a hypothetical situation in which CTA was used as the main imaging modality for detecting postoperative complications. Using conservative assumptions, the resource impact of using CTA-alone as a first-line post-EVAR surveillance technique was estimated to have an upper limit of £860,000 per year, based on NHS reference costs data (3,875 EVARs in England, 2016–17). This assumes that current practice is 100% duplex ultrasound, to be replaced by CTA in 100% of cases, using NHS tariff costs of £85 for CTA and £48 for ultrasound. It assumes that newly-repaired aneurysms require 2 scans during their first year after AAA repair, and that in any given year there will be the same number of aneurysms receiving a single scan in years 2, 3, 4 and 5 after EVAR, giving a total of 23,250 scans per year. The resulting figure of £860,000 represents the maximum possible resource impact of using CTA in all cases.
In reality the resource impact of the recommendation is likely to be small, primarily because complimentary imaging regimens based on US assessment of sac size as an initial surveillance modality are widely adopted.
Furthermore, elsewhere in this guideline the committee have recommended against the use of EVAR for the repair of unruptured infrarenal AAA for patients who are suitable for OSR, and outside of a clinical trial for those who are not. EVAR for unruptured complex AAA should also only be undertaken in the context of an RCT. The alternative surgical procedure, open surgical repair, requires less follow-up surveillance, typically a single 1 consultation. Given that these elective procedures make up the large majority of AAA repair procedures performed in the UK, a shift in practice from EVAR towards open surgery will reduce the number of EVARs per year significantly from the 3,875 figure identified above, removing most elective EVARs and leaving mainly emergency cases. This would reduce the overall resource required for post EVAR surveillance.
Other factors the committee took into account
It was identified that studies included in this review predominantly enrolled men, which raised questions about the generalisability of the results to women. In the absence of evidence, it was agreed that the accuracy of the diagnostic tools would not be expected vary between these 2 groups.
Along with assessing diagnostic test accuracy, this question aimed to determine which imaging techniques are most acceptable to people with AAAs and clinicians, taking into account the safety profiles. While no studies which examined the acceptability of diagnostic tools were identified, the committee did take this into consideration when making recommendations.
The committee noted that CEUS involves intravenous administration of microbubble contrast material, but this is generally well tolerated by people. The need for cannulation and administration of contrast could have an impact on clinician acceptability. However, the committee noted that CEUS is a sensitive tool and would allow clearer visualisation of complications, particularly endoleaks.
For CTA, there is a small but non-zero risk of contrast-induced nephropathy associated with administering iodinated contrast agents, especially in people with established renovascular disease (which is common in the population undergoing AAA repair). Some people are also allergic to the contrast agent used. The committee also discussed that CTA is associated with exposure to ionising radiation. Since CTA was not recommended as the sole imaging tool for detecting complications, the committee agreed that it was unlikely to be used often enough in individual patients to result in a meaningful increase in the occurrence of malignancies. They were in agreement that the average life expectancy following EVAR is too short for radiation-induced cancer to develop in most people undergoing endoleak surveillance. Taking the safety profile of CTA into consideration, the committee recommended the use of CEUS in people with contraindications to contrast-enhanced CTA.
No studies were identified that examined the diagnostic accuracy of imaging tools in the follow-up of people undergoing open surgical repair. The committee noted that complications do occur following open surgical repair; however these tend to be clinically manifested earlier, which means that a comprehensive follow-up programme is not required. Therefore, recommendations were confined to imaging follow-up after EVAR.
Appendices
Appendix A. Review protocols
Review protocol for the effectiveness of tests in predicting poor and good surgical outcomes
| Review Question 28 | When monitoring people after they have had EVAR or open repair of an abdominal aortic aneurysm, which imaging techniques are most useful for detecting postoperative complications, further aneurysm expansion and aneurysm rupture? |
|---|---|
| Objectives |
To determine which imaging technique is most accurate in identifying complications (endoleak, graft migration, graft kinking, graft occlusion and aortic neck expansion), further aneurysm expansion and aneurysm rupture in people who have undergone surgical repair of an AAA To determine which imaging techniques are most acceptable to patients and clinicians, taking into account the safety profiles of the approaches |
| Type of review | Diagnostic |
| Language | English only |
| Study design |
Systematic reviews of study designs listed below Cross-sectional studies |
| Status |
Published papers only (full text) No date restrictions |
| Population |
People who have undergone surgical repair of an abdominal aortic aneurysm Subgroup: position of aneurysm |
| Index test |
Ultrasound, including colour duplex ultrasound (CDUS), contrast-enhanced CDUS Plain film radiography, aortography/angiography CT Helical CT technology MRI Intrasac pressure monitoring |
| Reference standard | Computed tomographic angiography, preferably with post-processing techniques/workstations – dual or triple or venous phase |
| Endpoints |
Diagnostic accuracy (sensitivity and specificity for endoleak, graft migration, graft kinking, graft occlusion, aortic neck expansion) Adverse events Acceptability of approach to patients and clinicians |
| Other criteria for inclusion / exclusion of studies |
Exclusion: Non-English language Abstract/non-published Diagnostic accuracy measures for which both sensitivity and specificity are not available/ cannot be calculated Publication before the year 2000 |
| Baseline characteristics to be extracted in evidence tables |
Age Sex Size of aneurysm Comorbidities Date of first investigation |
| Search strategies | See Appendix B |
| Review strategies |
Appropriate NICE Methodology Checklists, depending on study designs, will be used as a guide to appraise the quality of individual studies. Available Cochrane review (Abraha, 2013) will be used as a ‘seed review’ for the identification of endoleak. Abraha’s Cochrane review (ongoing at the time of protocol development) will be used as the evidence base for ultrasound for endoleak in people who have undergone EVAR for AAA Data on all included studies will be extracted into evidence tables. Where statistically possible, a meta-analytic approach will be used to give an overall summary effect. All key findings from evidence will be presented in GRADE profiles and further summarised in evidence statements. |
| Key papers | Endoleak: Armerding MD, Rubin GD, Beaulieu CF, Slonim SM, Olcott EW, Samuels SL, Jorgensen MJ, Semba CP, Jeffrey RB Jr, Dake MD. Aortic aneurysmal disease: assessment of stent-graft treatment-CT versus conventional angiography. Radiology. 2000 Apr;215(1):138–46 [PubMed: 10751479] Ayuso JR, de Caralt TM, Pages M, Riambau V, Ayuso C, Sanchez M, Real MI, Montaña X. MRA is useful as a follow-up technique after endovascular repair of aortic aneurysms with nitinol endoprostheses. J Magn Reson Imaging. 2004 Nov;20(5):803–10 [PubMed: 15503334] Bendick PJ, Bove PG, Long GW, Zelenock GB, Brown OW, Shanley CJ. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg. 2003 Feb;37(2):381–5 [PubMed: 12563210] Elkouri S, Panneton JM, Andrews JC, Lewis BD, McKusick MA, Noel AA, Rowland CM, Bower TC, Cherry KJ Jr, Gloviczki P. Computed tomography and ultrasound in follow-up of patients after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg. 2004 May;18(3):271–9 [PubMed: 15354627] Gargiulo,M., Gallitto,E., Serra,C., Freyrie,A., Mascoli,C., Bianchini Massoni,C., et al. Could four-dimensional contrast-enhanced ultrasound replace computed tomography angiography during follow up of fenestrated endografts? Results of a preliminary experience. Eur J Vasc Endovasc Surg 2014;48(5):536–42 [PubMed: 25023904] Giannoni MF, Palombo G, Sbarigia E, Speziale F, Zaccaria A, Fiorani P. Contrast-enhanced ultrasound imaging for aortic stent-graft surveillance. J Endovasc Ther. 2003 Apr;10(2):208–17. [PubMed: 12877601] Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, Lumsden AB, Bush RL. Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg. 2006 Feb;43(2):259–64 [PubMed: 16476596] Iezzi R, Cotroneo AR, Filippone A, Di Fabio F, Quinto F, Colosimo C, Bonomo L. Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: are unenhanced and delayed phase enhanced images effective for endoleak detection? Radiology. 2006 Dec;241(3):915–21 [PubMed: 17032909] Sandford RM, Bown MJ, Fishwick G, Murphy F, Naylor M, Sensier Y, Sharpe R, Walker J, Hartshorn T, London NJ, Sayers RD. Duplex ultrasound scanning is reliable in the detection of endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2006 Nov;32(5):537–41 [PubMed: 16875850] van der Laan MJ, Bartels LW, Viergever MA, Blankensteijn JD. Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. Eur J Vasc Endovasc Surg. 2006 Oct;32(4):361–5 [PubMed: 16630731] Wolf YG, Johnson BL, Hill BB, Rubin GD, Fogarty TJ, Zarins CK. Duplex ultrasound scanning versus computed tomographic angiography for postoperative evaluation of endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2000 Dec;32(6):1142–8 [PubMed: 11107086] |
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review question 28
Abraha Iosief, Luchetta Maria Laura, De Florio , Rita , Cozzolino Francesco, Casazza Giovanni, Duca Piergiorgio, Parente Basso, Orso Massimiliano, Germani Antonella, Eusebi Paolo, and Montedori Alessandro (2017) Ultrasonography for endoleak detection after endoluminal abdominal aortic aneurysm repair. The Cochrane database of systematic reviews 6, CD010296 [PMC free article: PMC6481872] [PubMed: 28598495]
|
Medline Strategy, searched 13th April 2016 Database: Ovid MEDLINE(R) 1946 to March Week 5 2016 Search Strategy: |
|---|
| 1 Aortic Aneurysm, Abdominal/ |
| 2 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco-abdom* or aort* or spontan* or juxtarenal* or juxta-renal* or juxta renal* or paraerenal* or para-renal* or para renal* or suprarenal* or supra renal* or supra-renal* or short neck* or short-neck* or shortneck* or visceral aortic segment*)).tw. |
| 3 Aortic Rupture/ |
| 4 (AAA or RAAA).tw. |
| 5 (endovascular* adj4 aneurysm* adj4 repair*).tw. |
| 6 (endovascular* adj4 aort* adj4 repair*).tw. |
| 7 (EVAR or EVRAR or FEVAR or F-EAVAR or BEVAR or B-EVAR).tw. |
| 8 (Anaconda or Zenith Dynalink or Hemobahn or Luminex* or Memoth-erm or Wallstent).tw. |
| 9 (Viabahn or Nitinol or Hemobahn or Intracoil or Tantalum).tw. |
| 10 or/1–9 |
| 11 X-Rays/ |
| 12 (x-ray* or x ray* or xray* or x-radiation* or x radiation* or roentgen ray* or grenz ray* or radiograph*).tw. |
| 13 Aortography/ |
| 14 aortograph*.tw. |
| 15 Tomography, X-Ray Computed/ ( |
| 16 (cat scan* or ct scan* or cine ct or cine-ct or tomodensitomet*).tw. |
| 17 ((computed or computer assisted or computeriz* or computeris* or electron beam* or axial*) adj4 tomograph*).tw. |
| 18 Four-Dimensional Computed Tomography/ |
| 19 (4d ct or 4dct or 4-dimensional CT or four dimensional CT).tw. |
| 20 exp Tomography, Spiral Computed/ |
| 21 ((helical or spiral) adj4 ct*).tw. |
| 22 exp Magnetic Resonance Imaging/ |
| 23 (nmr tomograph* or mr tomograph* or nmr imag* or mri scan* or functional mri* or fmri* or zeugmatograph* or cine-mri* or cinemri*).tw. |
| 24 (proton spin adj4 tomograph*).tw. |
| 25 ((chemical shift or magnetic resonance or magneti* transfer) adj4 imag*).tw. |
| 26 exp Angiography/ |
| 27 (angiograph* or arteriograph*).tw. |
| 28 exp Ultrasonography/ |
| 29 (ultrasound* or ultrason* or sonograph* or echograph* or echotomograph*).tw. |
| 30 exp Echocardiography/ |
| 31 echocardiograph*.tw. |
| 32 Finite element analysis/ |
| 33 (finite adj4 element* adj4 analys*).tw. |
| 34 (finite adj4 element* adj4 comput*).tw. |
| 35 FEA.tw. |
| 36 ((wall adj4 stress adj4 analys*) or (wall adj4 stress adj4 comput*)).tw. |
| 37 exp Computer simulation/ |
| 38 Software/ |
| 39 Image interpretation, computer-assisted/ or Radiographic image interpretation, computerassisted/ |
| 40 Imaging Three-Dimensional/ |
| 41 exp Image enhancement/ |
| 42 Stress, mechanical/ |
| 43 (stress* adj4 mechanical*).tw. |
| 44 (scan* or imag*).tw. |
| 45 Watchful waiting/ |
| 46 (watchful adj4 waiting*).tw. |
| 47 Mass screening/ |
| 48 screen*.tw. |
| 49 Population surveillance/ |
| 50 surveillan*.tw. |
| 51 ((period* or test* or frequen* or regular* or routine* or rate or optimal* or optimis* or optimiz* or repeat* or interval*) adj4 (test* or monitor* or observ* or measur* or assess* or screen* or rescreen* or rescreen* or exam* or evaluat*)).tw. |
| 52 ((aneursym* or sign* or diameter or risk*) adj4 (grow* or siz* or measur* or expan* or ruptur* or tear* or progress* or enlarg* or dilat* or bulg* or evaluat*)).tw. |
| 53 Patient Selection/ |
| 54 ((patient or subject or criteria or treatment*) adj4 select*).tw. |
| 55 ((follow-up or follow up) adj4 (visit* or repeat* or monitor* or assess* or care*)).tw. |
| 56 Aftercare/ |
| 57 (aftercare or after-care).tw. |
| 58 Disease progression/ |
| 59 ((disease or illness or condition) adj4 (progress* or worsen* or exacerbat* or deterior* or course or duration or trajector* or improv* or recur* or relaps* or remission)).tw. |
| 60 or/11–59 |
| 61 10 and 60 |
| 62 animals/ not humans/ |
| 63 61 not 62 |
| 64 limit 63 to english language |
Note: RCT, Systematic Review and Observational study filters appended to strategy.
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom*.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov*.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree* or analys*)).tw. |
| 20 (cost or costs or costing* or costly or costed).tw. |
| 21 (price* or pricing*).tw. |
| 22 budget*.tw. |
| 23 expenditure*.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic* or (pharmaco adj economic*)).tw. |
| 26 or/1–25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly* or qald* or qale* or qtime*).tw. |
| 7 disability adjusted life.tw. |
| 8 daly*.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health* year* equivalent*.tw. |
| 19 utilit*.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili*.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble*.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1–30 |
Appendix D. Clinical evidence tables
Download PDF (732K)
Appendix E. Forest plots
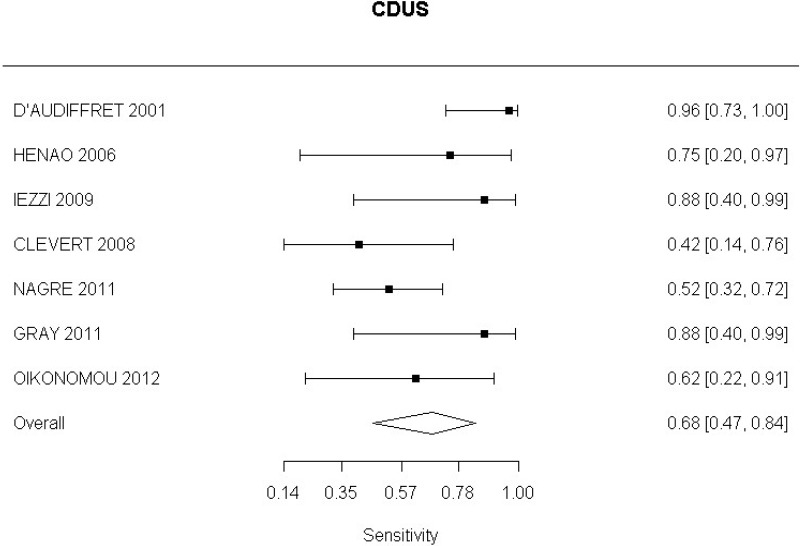
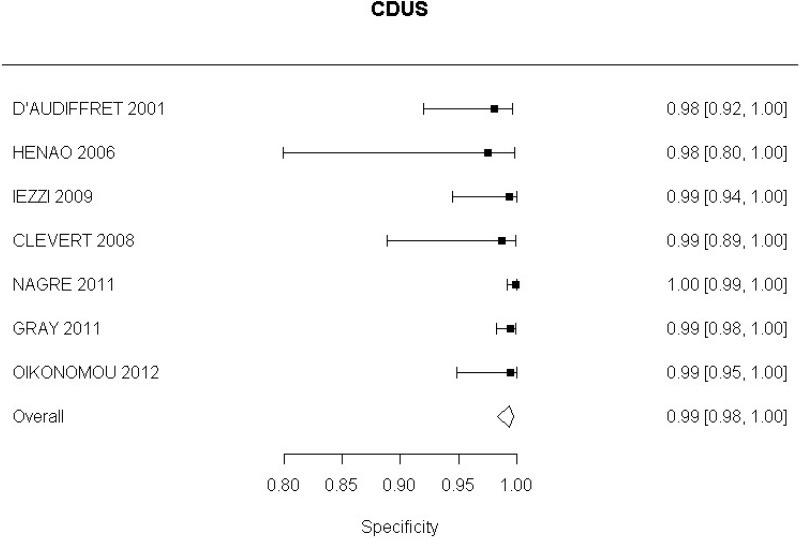
CDUS compared with angiography in the identification of endoleak
Sensitivity Analysis: CDUS compared to angiography in detection of change in aneurysm size
CDUS compared to angiography in detection of change in aneurysm size
CEUS compared with angiography in the identification of endoleak
Appendix F. GRADE tables
Complications
Identification of Endoleak
Colour Duplex ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; CDUS in the identification of all endoleaks in people undergoing EVAR | ||||||||||
| 241 | Cross Sectional |
4198 (combination of patients and scans) |
65.6% (54.5%, 75.2%) |
92.5% (89.3%, 94.8%) |
LR+ 8.84 (5.870, 12.80) | Serious4 | Very Serious5 | Not Serious | Not Serious | Very Low |
|
LR− 0.375 (0.268, 0.493) | Serious4 | Very Serious5 | Not Serious | Serious6 | Very Low | |||||
| Sensitivity analysis: Removal of studies conducted in 1990s. | ||||||||||
| 209 | Cross sectional |
3675 (combination of patients and scans) |
64.0% (50.8%, 75.4%) |
91.8 % (87.9%, 94.5%) |
LR+ 7.910 (5.10, 11.80) | Serious4 | Very Serious5 | Not Serious | Not Serious | Very Low |
|
LR− 0.395 (0.269, 0.539) | Serious4 | Very Serious5 | Not Serious | Serious6 | Very Low | |||||
| Sensitivity analysis: Removal of studies published before 2018, and those in which | ||||||||||
| 510 | Cross sectional |
914 (combination of patients and scans) |
77.2% (51.5%, 91.5%) |
93.6% (86.5%, 97.1%) |
LR+ 9.63 (6.26, 14.82) | Very Serious7 | Serious8 | Not Serious | Not Serious | Very Low |
|
LR− 0.29 (0.15, 0.60) | Very Serious7 | Serious8 | Not Serious | Serious6 | Very Low | |||||
| Reference Standard- CTA; CDUS in the identification of Type I and III endoleak in people undergoing EVAR | ||||||||||
| 72 | Cross Sectional |
1346 (combination of patients and scans) |
68.2% (46.6%, 84.15) |
99.3% (98.3%, 99.7%) |
LR+ 101.00 (38.80, 218.00) | Very Serious7 | Not Serious | Not Serious | Not Serious | Low |
|
LR− 0.328 (0.161, 0.538 | Very Serious7 | Not Serious | Not Serious | Serious6 | Very Low | |||||
| Reference Standard- CTA; CDUS in the identification Of Type II endoleak in people undergoing EVAR | ||||||||||
| 53 | Cross Sectional |
1242 combination of patients and scans) |
77.1% (39.6%, 94.5%) |
92.9% (88.4%, 95.7%) |
LR+ 10.40 (6.94, 14.60) | Very Serious7 | Serious8 | Not Serious | Not Serious | Very Low |
|
LR− 0.275 (0.0611,0.635) | Very Serious7 | Very Serious5 | Not Serious | Serious6 | Very Low | |||||
- 1
Oikonomou (2012), Golzarian (2000), D’Audiffret (2001), Bendick (2003), Clevert (2008), Clevert (2011), Iezzi (2009), Henao (2006), Parent (2002), Cantador (2016), Wolf (2000), Schmieder (2009), Gray (2012), Raman (2013), Aburahma (205), Franca (2013), Demirpolat (2010), Kamal (2008), Badri (2010), McWillams (2002), Pages (2001), Nagre (2011), Zannetti (2000), Nerlekar (2006)
- 2
Oikonomou (2012), D’Audiffret (2001), Henao (2006), Iezzi (2009), Clevert (2008), Nagre (2011), Gray (2012)
- 3
Clevert 2008, D’Audiffret 2001, Oikonomou 2012, Nagre 2011, Gray 2012
- 4
>33.3% of weighted data from studies at moderate or high risk of bias, downgrade 1 level. Studies rated moderate or high risk of bias due to unclear blinding between reference standard and index test and unclear time interval between two tests.
- 5
I2 greater than 66.7%, downgrade 2 levels
- 6
95% confidence interval for likelihood ratio crosses one end of a defined MID interval – (0.5, 2), downgrade 1 level
- 7
>33.3% of weighted data from studies at high risk of bias, downgrade 2 levels. Studies rated high risk of bias due to unclear blinding and interval between reference standard and index test.
- 8
I2 between 33.3% and 66.7%, downgrade 1 level
- 9
Oikonomou (2012), D’Audiffret (2001), Bendick (2003), Clevert (2008), Clevert (2011), Iezzi (2009), Henao (2006), Parent (2002), Cantador (2016), Schmieder (2009), Gray (2012), Raman (2013), Aburahma (205), Franca (2013), Demirpolat (2010), Kamal (2008), Badri (2010), McWillams (2002), Nagre (2011), Nerlekar (2006)
- 10
Badri (2010), Cantador (2016), Demirpolat (2010), Gray (2012), Oikonomou (2012)
Contrast Enhanced Colour Duplex ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; CEUS in identification of all endoleaks in people undergoing EVAR | ||||||||||
| 151 | Cross Sectional |
1667 (combination of patients and scans) |
91.0% (82.7%, 95.6%) |
88.0% (82.3%, 93.1%) |
LR+ 8.34 (4.94, 13.4) | Serious4 | Very Serious5 | Not Serious | Not Serious | Very Low |
| LR− 0.107(0.0491, 0.20) | Serious4 | Very Serious5 | Not Serious | Not Serious | Very Low | |||||
| Reference Standard – CTA, CEUS in identification of endoleaks in people undergoing complex EVAR | ||||||||||
| Perini 2012 | Cross sectional | 62 patients |
83.3% (36.9%, 97.7%) |
96.4% (86.8%, 99.1%) |
LR+ 23.33 (5.173, 95.304) | Not Serious | N/A6 | Not Serious | Not Serious | High |
|
LR− 0.173 (0.029, 1.035) | Not Serious | N/A6 | Not Serious | Serious7 | Moderate | |||||
| Reference Standard- CTA;CEUS, In identification of Type I and III endoleak in people undergoing EVAR | ||||||||||
| 62 | Cross Sectional |
791 (combination of patients and scans) |
91.0% (74.2%, 97.3%) |
98.8% (99.6%, 96.7%) |
LR+ 88.80 (26.40, 222.000) | Not Serious | Not Serious | Not Serious | Not Serious | High |
|
LR− 0.105 (0.027, 0.262) | Not Serious | Not Serious | Not Serious | Not Serious | High | |||||
| Reference Standard – CTA, CEUS in identification of type I endoleaks in people undergoing complex EVAR | ||||||||||
| Perini 2012 | Cross sectional | 62 patients |
75.0% (10.9%, 98.7%) |
99.2% (88.4%, 99.9%) |
LR+ 93.0 (5.251, 1647.197) | Not Serious | N/A6 | Not Serious | Not Serious | High |
|
LR− 0.252 (0.023, 2.780) | Not Serious | N/A6 | Not Serious | Very Serious8 | Low | |||||
| Reference Standard- CTA; CEUS, In identification of Type II endoleak in patients undergoing EVAR | ||||||||||
| 43 | Cross Sectional | 678 |
97.2% (92.2%, 99.0%) |
94.2% (83.35, 98.25) |
LR+ 16.746 (5.374, 52.180) | Not Serious | Very Serious5 | Not Serious | Not Serious | Low |
|
LR− 0.032 (0.011, 0.089) | Not Serious | Not Serious | Not Serious | Not Serious | High | |||||
| Reference Standard – CTA, CEUS in identification of type II endoleaks in people undergoing complex EVAR | ||||||||||
| Perini 2012 | Cross sectional | 62 patients |
66.7 % (26.8%, 91.65) |
98.2% (88.4%, 99.7%) |
LR+ 37.33 (4.937, 282.308) | Not Serious | N/A6 | Not Serious | Not Serious | High |
|
LR− 0.339 (0.109, 1.053) | Not Serious | N/A6 | Not Serious | Not Serious | Moderate | |||||
- 1
Perini (2011), Bendick (2003), Giannoni (2007), Clevert (2008), Henao (2006), Iezzi (2009), Clevert (2011), Ten Bosch (2010), Gurtler (2013), Abbas (2014), McWilliams (2002), Motta (2012), Gilbaert (2012), Mauro (2010), Lowe (2017).
- 2
Giannoni (2007), Clevert (2008), Henao (2006), Iezzi (2009), Perini (2011), Mauro (2010)
- 3
Clevert (2008), Henao (2006), Perini (2011), Mauro (2010)
- 4
>33.3% of weighted data from studies at moderate or high risk of bias, downgrade 1 level. Studies rated moderate or high risk of bias due to unclear blinding between reference standard and index test and unclear time interval between two tests.
- 5
I2 greater than 66.7%, downgrade 2 levels
- 6
Inconsistency not applicable as evidence from a single study
- 7
Downgrade 1 level as 95% confidence interval of likelihood ratio crosses one end of a defined MID interval (0.5, 2)
- 8
Downgrade 2 levels as 95% confidence interval of likelihood ratio crosses both ends of a defined MID interval (0.5, 2)
3D Contrast Enhanced Colour Duplex ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; 3D CEUS in identification of all endoleaks in people undergoing EVAR | ||||||||||
|
2 Abbas (2014) Lowe (2017) | Cross sectional | 130 scans |
96.0 % (87.2%, 98.8%) |
90.4% (80.8%, 95.5%) |
LR + 10.021 (4.817, 20.851) | Not serious | N/A1 | Not Serious | Not Serious | Moderate |
|
LR− 0.044 (0.013, 0.149) | Not serious | N/A1 | Not serious | Not serious | Moderate | |||||
- 1
Inconsistency not applicable as evidence from a single study.
4D Contrast Enhanced Colour Duplex ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; 3D CEUS in identification of all endoleaks in people undergoing complex EVAR | ||||||||||
| Gargiulo (2014) | Cross sectional | 22 patients | 62.5% (18%, 92.7%) |
97.5% (70.2%, 99.8%) |
LR+ 25 (1.460, 428.004) | Serious1 | N/A 2 | Not Serious | Serious3 | Low |
|
LR− 0.385 (0.108, 1.366) | Serious1 | N/A 2 | Not serious | Serious3 | Low | |||||
Downgrade 1 level for serious risk of bias due to unclear blinding between reference standard and index test
Inconsistency not applicable as evidence from a single study
Downgrade 1 level as 95% confidence interval of likelihood ratio crosses one end of a defined MID interval (0.5, 2)
Magnetic Resonance Imaging compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; MRI in identification of endoleaks in people undergoing EVAR | ||||||||||
|
1 Mori 2016 | Cross Sectional | 46 patients |
90.9% (56.1%, 98.7%) |
91.4% (76.6%, 97.2%) |
LR+ 10.606 (3.537, 31.799) | Serious1 | N/A2 | Very serious | Not serious | Very Low |
|
LR− 0.099 (0.015, 0.646) | Serious1 | N/A2 | Very serious | Serious3 | Very Low | |||||
Downgrade 1 level for serious risk of bias due to inadequate blinding between index test and reference standard.
Inconsistency not applicable as evidence from a single study
Downgrade 1 level as 95% confidence interval of likelihood ratio crosses one end of a defined MID interval (0.5, 2)
Downgrade 2 levels for serious indirectness. Unenhanced MRI sequence utilised in the study, is not routinely used in practice.
Identification of graft occlusion
Contrast Enhanced Colour Duplex ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; CEUS in the identification of graft occlusion in people undergoing EVAR | ||||||||||
|
1 Motta 2012 | Cross Sectional | 134 scans |
58.3% (35.4%, 78.1%) |
99.6% (93.6%, 100%) |
LR+ 137.667 (8.427, 2248.874) | Serious1 | N/A2 | Not serious | Not serious | Moderate |
|
LR− 0.418 (0.242, 0.723) | Serious1 | N/A2 | Not serious | Serious3 | Low | |||||
Downgrade 1 level for serious risk of bias. All paired scans not included in final analysis
Inconsistency not applicable as evidence from a single study
Downgrade 1 level as 95% confidence interval of likelihood ratio crosses one end of a defined MID interval (0.5, 2)
Change in aneurysm size
Overall change in aneurysm size
Colour Duplex Ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; CDUS In identification of aneurysm change in people undergoing EVAR | ||||||||||
|
2 Pages 2001 Bargellini 2009 | Cross Sectional | 773 scans |
92.4% (87.7%, 95.3%) |
85.7% (29.6%, 98.8%) |
LR+ 6.426 (0.706, 58.498) | Serious1 | Serious2 | Serious3 | Serious4 | Very low |
|
LR− 0.100 (0.073, 0.137) | Serious1 | Not serious | Serious3 | Not serious | Low | |||||
Downgrade 1 level for serious risk of bias. Studies rated moderate risk of bias due to unclear blinding and time interval between reference standard and index test.
I2 between 33.3% and 66.7%, downgrade 1 level
Downgrade 1 level for indirectness. Pages (2001) presented partially indirect data. While study was published prior to year 2000, study was conducted between 1996 and 1999, which does not match the review protocol.
Downgrade 1 level as 95% confidence interval of likelihood ratio crosses one end of a defined MID interval (0.5, 2)
Reduction in aneurysm size
Colour Duplex Ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; CDUS in identification of reduction in aneurysm size in people undergoing EVAR | ||||||||||
|
1 Bargellini 2009 | Cross Sectional | 657 scans |
98.7 % (97.2%, 99.4%) |
68.2% (61.3%, 74.4%) |
LR+ 3.107 (2.525, 3.824) | Serious4 | N/A5 | Not serious | Not serious | Moderate |
|
LR− 0.019 (0.008, 0.042) | Serious4 | N/A5 | Not serious | Not serious | Moderate | |||||
Downgrade 1 level for serious risk of bias due to unclear blinding between reference standard and index test
Inconsistency not applicable as evidence from a single study
Increase in aneurysm size
Colour Duplex Ultrasound compared to angiography
| No. of studies | Study design | Sample size | Sensitivity (95%CI) | Specificity (95%CI) | Effect size (95%CI) | Risk of bias | Inconsistency | Indirectness | Imprecision | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard- CTA; CDUS In identification of increase in aneurysm size in people undergoing EVAR | ||||||||||
|
1 Bargellini 2009 | Cross Sectional | 180 scans |
41.7% (28.7%, 55.9%) |
99.2% (94.8%, 99.95) |
LR+ 55.00 (7.586, 398.753) | Serious1 | N/A2 | Not serious | Not serious | Moderate |
|
LR− 0.588 (0.463, 0.747) | Serious1 | N/A2 | Not serious | Serious3 | Low | |||||
Downgrade 1 level for serious risk of bias due to unclear blinding between reference standard and index test
Inconsistency not applicable as evidence from a single study
Downgrade 1 level as 95% confidence interval of likelihood ratio crosses one end of a defined MID interval (0.5, 2)
Appendix H. Excluded studies
Clinical studies
| Study ID | Title | Reason for Exclusion |
|---|---|---|
| AbuRahma (2006) | Fate of endoleaks detected by CT angiography and missed by color duplex ultrasound in endovascular grafts for abdominal aortic aneurysms. | Diagnostic accuracy measures were not available and could not be calculated. |
| Alerci (2009) | Prospective, intraindividual comparison of MRI versus MDCT for endoleak detection after endovascular repair of abdominal aortic aneurysms | Not a relevant intervention and/or comparator |
| Almaroof (2013) | Comparison of duplex ultrasound and computed tomography surveillance after endovascular aneurysm repair in determining proximal endograft location | No relevant outcomes reported |
| Antoniou (2013) | Plasma matrix metalloproteinase 9 levels may predict endoleaks after endovascular aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Arko (2004) | Duplex scanning after endovascular aneurysm repair: an alternative to computed tomography | Not a relevant study design |
| Armerding (2000) | Aortic aneurysmal disease: assessment of stent-graft treatment-CT versus conventional angiography. | Study did use adequate reference standard. In the study, CTA was compared to screen film or digital subtraction angiography. |
| Arsicot (2014) | Follow-up of aortic stent grafts: comparison of the volumetric analysis of the aneurysm sac by ultrasound and CT. | Case-control study design. |
| Ascenti (2011) | Dual-energy CT for detection of endoleaks after endovascular abdominal aneurysm repair: Usefulness of colored iodine overlay | Not a relevant intervention and/or comparator |
| Ashoke (2005) | Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review (Structured abstract). | Systematic review contained unpublished data and studies conducted before year 2000. |
| Ayuso (2004) | MRA is useful as a follow-up technique after endovascular repair of aortic aneurysms with nitinol endoprostheses. | Study population did not match review protocol. Study included patients after an endoleak was observed on a follow-up CTA. |
| Bakken (2010) | Long-term follow-up after endovascular aneurysm repair: is ultrasound alone enough? | Not a relevant study design |
| Balm (1996) | CT-angiography of abdominal aortic aneurysms after transfemoral endovascular aneurysm management | Published before 2000 or systematic review containing only papers published before 2000 |
| Balm (1997) | Use of spiral computed tomographic angiography: In monitoring abdominal aortic aneurysms after transfemoral endovascular repair | Published before 2000 or systematic review containing only papers published before 2000 |
| Balm (1997) | Computed tomographic angiographic imaging of abdominal aortic aneurysms: implications for transfemoral endovascular aneurysm management | Published before 2000 or systematic review containing only papers published before 2000 |
| Bastos (2011) | A multidetector tomography protocol for follow-up of endovascular aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Bastounis (1996) | The validity of current vascular imaging methods in the evaluation of aortic anastomotic aneurysms developing after abdominal aortic aneurysm repair | Published before 2000 or systematic review containing only papers published before 2000 |
| Baum (2000) | Diagnosis and treatment of inferior mesenteric arterial endoleaks after endovascular repair of abdominal aortic aneurysms | Not a relevant study design. No relevant outcomes reported |
| Baum (2001) | Diagnosis and management of type 2 endoleaks after endovascular aneurysm repair | Not a relevant study design |
| Baum (2003) | Endoleaks after endovascular repair of abdominal aortic aneurysms | Not a relevant study design |
| Baumueller (2011) | Maximum diameter measurements of aortic aneurysms on axial CT images after endovascular aneurysm repair: sufficient for follow-up? | Not a relevant intervention and/or comparator |
| Becker (2002) | Transluminal repair of abdominal aortic aneurysm: a call for selective use, careful surveillance, new device design, and systematic study of transrenal fixation | Not a peer-reviewed publication |
| Beeman (2009) | Duplex ultrasound imaging alone is sufficient for midterm endovascular aneurysm repair surveillance: a cost analysis study and prospective comparison with computed tomography scan | Not a relevant study design |
| Beeman (2010) | Duplex ultrasound factors predicting persistent type II endoleak and increasing AAA sac diameter after EVAR | Not a relevant intervention and/or comparator |
| Berman (1995) | Application of computed tomography for surveillance of aortic grafts | Published before 2000 or systematic review containing only papers published before 2000 |
| Bevis (2012) | Duplex ultrasound for surveillance after endovascular repair of abdominal aortic aneurysm. | No primary studies or no new primary studies extracted from this systematic review |
| Biasi (2009) | Intra-operative DynaCT improves technical success of endovascular repair of abdominal aortic aneurysms | Not a relevant intervention and/or comparator |
| Biasi (2009) | Intraoperative DynaCT detection and immediate correction of a type Ia endoleak following endovascular repair of abdominal aortic aneurysm | Not a relevant intervention and/or comparator |
| Binkert (2006) | Translumbar type II endoleak repair using angiographic CT | Not a relevant study design |
| Black (2009) | Long-term surveillance with computed tomography after endovascular aneurysm repair may not be justified | Not a relevant study design |
| Bley (2009) | Endovascular abdominal aortic aneurysm repair: nonenhanced volumetric CT for follow-up | Not a relevant study design |
| Blom (2012) | Duplex ultrasound imaging to detect limb stenosis or kinking of endovascular device | Not a relevant intervention and/or comparator. Not a relevant study design |
| Bobadilla (2013) | Clinical implications of non-contrast-enhanced computed tomography for follow-up after endovascular abdominal aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Bredahl (2013) | Volume estimation of the aortic sac after EVAR using 3-D ultrasound - a novel, accurate and promising technique | No relevant outcomes reported |
| Bredahl (2013) | Three-dimensional ultrasound improves the accuracy of diameter measurement of the residual sac in EVAR patients | No relevant outcomes reported |
| Cani (2012) | Volumetric analysis of the aneurysmal sac with computed tomography in the follow-up of abdominal aortic aneurysms after endovascular treatment | Not a relevant intervention and/or comparator. Not a relevant study design |
| Cantisani (2011) | Prospective comparative analysis of colour-doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. | Reference standard used in study did not match review protocol. CTA and MRA were used as the reference or angiography when available. |
| Cantisani (2015) | EVAR: Benefits of CEUS for monitoring stent-graft status | Not a relevant study design |
| Cantisani (2017) | Color Doppler Ultrasound with Superb Microvascular Imaging Compared to Contrast-enhanced Ultrasound and Computed Tomography Angiography to Identify and Classify Endoleaks in Patients Undergoing EVAR. | Reference standard used in study did not match review protocol. DSA used as reference standard. |
| Carnero (2006) | Aneurysm sac pressure measurement with a pressure sensor in endovascular aortic aneurysm repair | Not a relevant study design |
| Carrafiello (2006) | Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience | Not a relevant study design |
| Carrafiello (2008) | Endoleak detection and classification after endovascular treatment of abdominal aortic aneurysm: value of CEUS over CTA | Not a relevant study design |
| Carter (2005) | Color duplex ultrasound for the evaluation of endovascular stent grafts following endovascular repair of abdominal aortic aneurysm | Full text not obtained |
| Causey (2013) | Three-dimensional ultrasonography measurements after endovascular aneurysm repair | No relevant outcomes reported |
| Cejna (2002) | MR angiography vs CT angiography in the follow-up of nitinol stent grafts in endoluminally treated aortic aneurysms | Not a relevant intervention and/or comparator |
| Chandarana (2008) | Abdominal aorta: evaluation with dual-source dual-energy multidetector CT after endovascular repair of aneurysms--initial observations | Not a relevant intervention and/or comparator |
| Chernyak (2006) | Type II endoleak after endoaortic graft implantation: diagnosis with helical CT arteriography | Not a relevant intervention and/or comparator |
| Chisci (2012) | Surveillance imaging modality does not affect detection rate of asymptomatic secondary interventions following EVAR | Not a relevant intervention and/or comparator |
| Chung (2015) | Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. | No primary studies or no new primary studies extracted from this systematic review |
| Clevert (2009) | Imaging of endoleaks after endovascular aneurysm repair (EVAR) with contrast-enhanced ultrasound (CEUS). A pictorial comparison with CTA | Not a relevant study design |
| Clevert (2013) | Clevert D A, Gurtler V M, Meimarakis G, D’Anastasi M, Weidenhagen R, Reiser M F, and Becker C R (2013) Classification of endoleaks in the follow-up after EVAR using the time-to-peak of the contrast agent in CEUS examinations. Clinical hemorheology and microcirculation 55, 183–91 [PubMed: 23455839] | Study did not match the objectives of this review. Study evaluated if the time-to-peak is a helpful new feature in confirming the type of endoleak in uncertain cases. |
| Cohen (2008) | Time-resolved MR angiography for the classification of endoleaks after endovascular aneurysm repair | Not a relevant intervention and/or comparator |
| Collins (2007) | Ultrasound surveillance of endovascular aneurysm repair: a safe modality versus computed tomography. | Reference standard (CTA) was only offered to when ultrasound was positive for endoleaks. |
| Cornelissen (2010) | Detection of occult endoleaks after endovascular treatment of abdominal aortic aneurysm using magnetic resonance imaging with a blood pool contrast agent: preliminary observations | Not a relevant study design |
| Cornelissen (2011) | Use of multispectral MRI to monitor aneurysm sac contents after endovascular abdominal aortic aneurysm repair | Not a relevant intervention and/or comparator. Not a relevant study design |
| Czermak (2001) | Serial CT volume measurements after endovascular aortic aneurysm repair | Not a relevant intervention and/or comparator. Not a relevant study design |
| David (2016) | What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. | CTA not used as reference standard. |
| de Bucourt (2011) | Endoleak after endovascular aneurysm repair: evaluation of a single-acquisition CTA protocol using a prebolus | Not a relevant intervention and/or comparator |
| den (2009) | Comparison of ultrasonography with computed tomography in the diagnosis of incisional hernias | No relevant outcomes reported |
| Dias (2004) | Intra-aneurysm sac pressure measurements after endovascular aneurysm repair: differences between shrinking, unchanged, and expanding aneurysms with and without endoleaks | Not a relevant intervention and/or comparator |
| Diehm (2007) | Intraobserver and interobserver variability of 64-row computed tomography abdominal aortic aneurysm neck measurements | Not a relevant intervention and/or comparator. Not a relevant study design |
| Diehm (2008) | Commentary: Aneurysm Sac diameter measurement versus volume analysis in EVAR surveillance: out with the old and in with the new | Not a relevant study design |
| Dill-Macky (2006) | Aortic endografts: Detecting endoleaks using contrast-enhanced ultrasound | Not a relevant study design |
| Dindyal (2012) | Re: Single-centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR | Not a relevant study design |
| Dingemans (2016) | Aneurysm Sac Enlargement after Endovascular Abdominal Aortic Aneurysm Repair | Not a relevant study design |
| Donas (2013) | CT angiography at 24 months demonstrates durability of EVAR with the use of chimney grafts for pararenal aortic pathologies | Not a relevant intervention and/or comparator |
| Dudeck (2015) | Can early computed tomography angiography after endovascular aortic aneurysm repair predict the need for reintervention in patients with type II endoleak? | Not a relevant intervention and/or comparator |
| Elkouri (2004) | Computed tomography and ultrasound in follow-up of patients after endovascular repair of abdominal aortic aneurysm. | Diagnostic accuracy measures were not available and could not be calculated. |
| Engellau (1998) | Magnetic resonance imaging and MR angiography of endoluminally treated abdominal aortic aneurysms | Published before 2000 or systematic review containing only papers published before 2000 |
| Engellau (2003) | Patient preferences for follow-up methods after endovascular repair of abdominal aortic aneurysms. | Study examined patient experience of CT with MRI and DSA. These comparators were not listed in review protocol. |
| Engellau (2003) | Costs in follow-up of endovascularly repaired abdominal aortic aneurysms. Magnetic resonance imaging with MR angiography versus EUROSTAR protocols | Not a relevant study design |
| Ersoy (2004) | Blood pool MR angiography of aortic stent-graft endoleak | Not the correct population/condition of interest |
| Faries (2003) | Increased recognition of type II endoleaks using a modified intraoperative angiographic protocol: implications for intermittent endoleak and aneurysm expansion | Not a relevant intervention and/or comparator |
| Fearn (2003) | Follow-up after endovascular aortic aneurysm repair: the plain radiograph has an essential role in surveillance | Not a relevant study design |
| Figueroa (2010) | Preliminary 3D computational analysis of the relationship between aortic displacement force and direction of endograft movement | Not a relevant intervention and/or comparator. Not a relevant study design |
| Fletcher (2000) | Colour Doppler diagnosis of perigraft flow following endovascular repair of abdominal aortic aneurysm. | Diagnostic accuracy measures were not available and could not be calculated. |
| Franco (2000) | Endovascular repair of the abdominal aortic aneurysm with the ancure endograft: CT follow-up of perigraft flow and aneurysm size at 6 months. | Study did not compare index test (CTA) to a reference standard. |
| Gardet (2010) | Comparison of detection of F-18 fluorodeoxyglucose positron emission tomography and 99mTc-hexamethylpropylene amine oxime labelled leukocyte scintigraphy for an aortic graft infection | Not a relevant intervention and/or comparator |
| Georg (2005) | Aortic stentgraft movement detection using digital roentgen stereophotogrammetric analysis on plane film radiographs -- initial results of a phantom study | Not a relevant intervention and/or comparator |
| Giannoni (2003) | Contrast-enhanced ultrasound imaging for aortic stent-graft surveillance. | Reference standard used in study did not match review protocol. In the study, CTA and MRA were used as reference standards. |
| Giannoni (2012) | Role of contrast-enhanced ultrasound in the follow-up of endo-vascular aortic aneurysm repair: an effective and safe surveillance method | Not a relevant study design |
| Go (2008) | What is the clinical utility of a 6-month computed tomography in the follow-up of endovascular aneurysm repair patients? | Not a relevant intervention and/or comparator |
| Gorich (1999) | Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography, and radiography | Published before 2000 or systematic review containing only papers published before 2000 |
| Gurtler (2014) | Comparison of contrast-enhanced ultrasound and compression elastography in the follow-up after endovascular aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Habets (2013) | Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review (Provisional abstract) | Not a relevant intervention and/or comparator |
| Habets (2015) | Magnetic Resonance Imaging with a Weak Albumin Binding Contrast Agent can Reveal Additional Endoleaks in Patients with an Enlarging Aneurysm after EVAR | Not a relevant intervention and/or comparator |
| Han (2010) | Ultrasound-determined diameter measurements are more accurate than axial computed tomography after endovascular aortic aneurysm repair | No relevant outcomes reported |
| Hansen (2014) | Evaluation of low-dose CT angiography with model-based iterative reconstruction after endovascular aneurysm repair of a thoracic or abdominal aortic aneurysm | Not a relevant intervention and/or comparator |
| Harrison (2011) | Surveillance after EVAR based on duplex ultrasound and abdominal radiography | Not a relevant study design |
| Haulon (2001) | Diagnosis and treatment of type II endoleak after stent placement for exclusion of an abdominal aortic aneurysm | Not a relevant intervention and/or comparator |
| Haulon (2001) | Prospective evaluation of magnetic resonance imaging after endovascular treatment of infrarenal aortic aneurysms | Not a relevant intervention and/or comparator |
| Haulon (2012) | Response to letter to the editor “Re: Single centre prospective comparison between contrast enhanced ultrasound and computed tomography angiography after EVAR” | Not a relevant study design |
| Heilberger (1997) | Postoperative color flow duplex scanning in aortic endografting | Published before 2000 or systematic review containing only papers published before 2000 |
| Hiramoto (2007) | The effect of magnetic resonance imaging on stainless-steel Z-stent-based abdominal aortic prosthesis | Not a relevant intervention and/or comparator |
| Hong (2008) | Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm | Not a relevant intervention and/or comparator |
| Hope (2009) | Initial experience characterizing a type I endoleak from velocity profiles using time-resolved three-dimensional phase-contrast MRI | Not a relevant study design |
| Houdek (2015) | Initial experience of follow up of patients after the endovascular treatment of abdominal aortic aneurysms using contrast-enhanced ultrasound. | Diagnostic accuracy measures were not available and could not be calculated. |
| Hovsepian (1999) | Tc-99m sulfur colloid scintigraphy for detecting perigraft flow following endovascular aortic aneurysm repair: A feasibility study | Published before 2000 or systematic review containing only papers published before 2000 |
| Huang (2013) | A prospective study of carbon dioxide digital subtraction versus standard contrast arteriography in the detection of endoleaks in endovascular abdominal aortic aneurysm repairs | Not a relevant intervention and/or comparator |
| Ichihashi (2013) | Preliminary experience with superparamagnetic iron oxide-enhanced dynamic magnetic resonance imaging and comparison with contrast-enhanced computed tomography in endoleak detection after endovascular aneurysm repair | Not a relevant intervention and/or comparator |
| Iezzi (2007) | MDCT angiography in abdominal aortic aneurysm treated with endovascular repair: diagnostic impact of slice thickness on detection of endoleaks | Not a relevant intervention and/or comparator |
| Iezzi (2008) | Multidetector-row computed tomography angiography in abdominal aortic aneurysm treated with endovascular repair: evaluation of optimal timing of delayed phase imaging for the detection of low-flow endoleaks | Not a relevant intervention and/or comparator. Not a relevant study design |
| Iezzi (2010) | Endoleaks after endovascular repair of abdominal aortic aneurysm: Value of CEUS | Not a relevant study design |
| Iezzi (2011) | Low-dose multidetector-row CT-angiography of abdominal aortic aneurysm after endovascular repair | Not a relevant intervention and/or comparator |
| Iino (2002) | Iino Misako, Kuribayashi Sachio, Imakita Satoshi, Takamiya Makoto, Matsuo Hiroshi, Ookita Yutaka, Ando Motomi, and Ueda Hatsue (2002) Sensitivity and specificity of CT in the diagnosis of inflammatory abdominal aortic aneurysms. Journal of computer assisted tomography 26, 1006–12 [PubMed: 12488751] | Study did not match objectives of review. Study assessed the diagnostic ability of CT in the diagnosis of inflammatory abdominal aortic aneurysm. |
| Inoue (2013) | Post-stress perfusion abnormalities detected on myocardial perfusion single-photon emission computed tomography predict long-term mortality after elective abdominal aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Insko (2003) | MR imaging for the detection of endoleaks in recipients of abdominal aortic stent-grafts with low magnetic susceptibility | Not a relevant intervention and/or comparator |
| Jawad (2016) | The role of contrast-enhanced ultrasound imaging in the follow-up of patients post-endovascular aneurysm repair | Not a relevant study design |
| Jung (2010) | Detection and characterization of endoleaks following endovascular treatment of abdominal aortic aneurysms using contrast harmonic imaging (CHI) with quantitative perfusion analysis (TIC) compared to CT angiography (CTA). | Study population did not match review protocol. Individuals with suspected endoleaks in routine follow-up were examined. |
| Kalman (1999) | The value of late computed tomographic scanning in identification of vascular abnormalities after abdominal aortic aneurysm repair | Published before 2000 or systematic review containing only papers published before 2000 |
| Karanikola (2014) | Duplex Ultrasound versus Computed Tomography for the Postoperative Follow-Up of Endovascular Abdominal Aortic Aneurysm Repair. Where Do We Stand Now? | Not a relevant study design |
| Karch (1999) | Algorithm for the diagnosis and treatment of endoleaks | Published before 2000 or systematic review containing only papers published before 2000 |
| Karthikesalingam (2012) | Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair (Structured abstract). | No primary studies or no new primary studies extracted from this systematic review |
| Kaspersen (2005) | Three-dimensional teleradiology for surveillance following endovascular aortic aneurysm repair: a feasibility study | Not a relevant intervention and/or comparator |
| Kirby (2007) | Computed tomography angiography in abdominal aortic endoleaks: what is the optimal protocol? | Not a relevant intervention and/or comparator |
| Kirkpatrick (2014) | Surveillance computed tomographic arteriogram does not change management before 3 years in patients who have a normal post-EVAR study | Not a relevant study design |
| Kranokpiraksa (2008) | Follow-up of Endovascular Aneurysm Repair: Plain Radiography, Ultrasound, CT/CT Angiography, MR Imaging/MR Angiography, or What? | Not a relevant study design |
| Lookstein (2004) | Time-resolved magnetic resonance angiography as a noninvasive method to characterize endoleaks: initial results compared with conventional angiography | Not a relevant intervention and/or comparator |
| Maggio (2001) | Colour duplex scanning using contrast medium in the follow-up of patients given endograft treatment for abdominal aorta aneurysms. | Diagnostic accuracy measures were not available and could not be calculated. |
| Manning (2009) | Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. | Study did not match objectives of this review. Index test and reference standard were not conducted concurrently. CDUS was conducted within 6 months of the CT scans. |
| Mattes (2012) | Evaluation of a new computerized analysis system developed for the processing of CT follow-up scans after EVR of infrarenal aneurysm | Not a relevant study design |
| McLafferty (2002) | The use of color-flow duplex scan for the detection of endoleaks. | Study did not match objectives of the review. Concurrent scans were not conducted. |
| McWilliams (1999) | Use of contrast-enhanced ultrasound in follow-up after endovascular aortic aneurysm repair | Published before 2000 or systematic review containing only papers published before 2000 |
| Megalopoulos (2008) | Reliability of selective surveillance colonoscopy in the early diagnosis of colonic ischemia after successful ruptured abdominal aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Mirza (2010) | Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis (Structured abstract). | No primary studies or no new primary studies extracted from this systematic review |
| Mita (2000) | Complications of endovascular repair for thoracic and abdominal aortic aneurysm: an imaging spectrum | Not a relevant intervention and/or comparator |
| Muller-Wille (2014) | Dual-energy computed tomography after endovascular aortic aneurysm repair: the role of hard plaque imaging for endoleak detection. | Reference standard used in study did not match review protocol. Triple phased CT and contrast enhanced ultrasound were used as reference standards. |
| Nakai (2015) | Utility of 99mTc-human serum albumin diethylenetriamine pentaacetic acid SPECT for evaluating endoleak after endovascular abdominal aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Nambi (2011) | Non-contrast computed tomography is comparable to contrast-enhanced computed tomography for aortic volume analysis after endovascular abdominal aortic aneurysm repair | Not a relevant intervention and/or comparator |
| Napoli (2004) | Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair | No relevant outcomes reported |
| Nordon (2010) | Secondary Interventions Following Endovascular Aneurysm Repair (EVAR) and the Enduring Value of Graft Surveillance | Not a relevant intervention and/or comparator. No relevant outcomes reported |
| Partovi (2015) | Contrast-enhanced ultrasound after endovascular aortic repair-current status and future perspectives | Not a relevant study design |
| Pfister (2009) | Contrast harmonic imaging ultrasound and perfusion imaging for surveillance after endovascular abdominal aneurysm repair regarding detection and characterization of suspected endoleaks. | Study population did not match review protocol. Study included individuals with clinically suspected endoleaks. |
| Pistolese (2002) | Postoperative regression of retroperitoneal fibrosis in patients with inflammatory abdominal aortic aneurysms: evaluation with spiral computed tomography | No relevant outcomes reported |
| Pitton (2005) | MRI versus helical CT for endoleak detection after endovascular aneurysm repair | Not a relevant intervention and/or comparator |
| Pitton (2006) | Diagnosis and management of endoleaks after endovascular aneurysm repair: role of MRI | Not a relevant intervention and/or comparator. Not a relevant study design |
| Raithel (1998) | Surveillance of patients after abdominal aortic aneurysm repair with endovascular grafting or conventional treatment | Not a relevant intervention and/or comparator. No relevant outcomes reported |
| Rand (2013) | Quality improvement guidelines for imaging detection and treatment of endoleaks following endovascular aneurysm repair (EVAR) | Not a relevant study design |
| Rozenblit (1995) | Endovascular repair of abdominal aortic aneurysm: value of postoperative follow-up with helical CT | Published before 2000 or systematic review containing only papers published before 2000 |
| Rydberg (2004) | Characterization of endoleaks by dynamic computed tomographic angiography | Not a relevant intervention and/or comparator |
| Saba (2008) | Diagnostic sensitivity of multidetector-row spiral computed tomography angiography in the evaluation of type-II endoleaks and their source: comparison between axial scans and reformatting techniques | Not a relevant intervention and/or comparator |
| Sandford (2006) | Duplex ultrasound scanning is reliable in the detection of endoleak following endovascular aneurysm repair. | Study did not match objective of this review. Index test and reference standard not conducted concurrently (conducted 6 months apart) |
| Sato (1998) | Endoleak after aortic stent graft repair: diagnosis by color duplex ultrasound scan versus computed tomography scan | Published before 2000 or systematic review containing only papers published before 2000 |
| Schnitzbauer (2017) | CT after Endovascular Repair of Abdominal Aortic Aneurysms: Diagnostic Accuracy of Diameter Measurements for the Detection of Aneurysm Sac Enlargement. | Voltmetery used as reference standard. |
| Sommer (2012) | Time-resolved CT angiography for the detection and classification of endoleaks. | Study included people who were suspected of having an endoleak at a previous imaging study or known to have postoperative endoleaks. |
| Stavropoulos (2005) | Use of CT angiography to classify endoleaks after endovascular repair of abdominal aortic aneurysms | Not a relevant intervention and/or comparator |
| Sueyoshi (2015) | Carbon dioxide digital subtraction angiography as an option for detection of endoleaks in endovascular abdominal aortic aneurysm repair procedure | Not a relevant intervention and/or comparator |
| Sun (2006) | Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm | Duplicate and/or already included within an included systematic review |
| Sun (2008) | CT virtual intravascular endoscopy in the visualization of fenestrated stent-grafts | Not a relevant intervention and/or comparator |
| Sun (2008) | Multislice CT angiography in the follow-up of fenestrated endovascular grafts: Effect of slice thickness on 2D and 3D visualization of the fenestration stents | Not a relevant intervention and/or comparator |
| Sun (2017) | A meta-analysis of ultrasound imaging in diagnosis of endoleak among patients after endovascular abdominal aortic aneurysm repair. | No primary studies or no new primary studies extracted from this systematic review |
| Thompson (1998) | Comparison of computed tomography and duplex imaging in assessing aortic morphology following endovascular aneurysm repair | Published before 2000 or systematic review containing only papers published before 2000 |
| Ustymowicz (2009) | Contrast-enhanced ultrasonography versus computed tomographic angiography in the monitoring of patients after endovascular repair of abdominal aortic aneurysm -- preliminary experience | No relevant outcomes reported |
| van der Laan (2006) | Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. | Study did not compare index tests (CTA and MRI) to a reference standard. |
| van Keulen (2009) | Potential value of aneurysm sac volume measurements in addition to diameter measurements after endovascular aneurysm repair | Not a relevant intervention and/or comparator |
| Wacker (2014) | C-Arm CT - An adjunct to DSA for endoleak classification in patients with endovascular repair of abdominal aortic aneurysms | Not a relevant intervention and/or comparator |
| Wicky (2003) | MR angiography of endoleak with inconclusive concomitant CT angiography | Not a relevant intervention and/or comparator |
| Wieners (2010) | Detection of type II endoleak after endovascular aortic repair: comparison between magnetic resonance angiography and blood-pool contrast agent and dual-phase computed tomography angiography | Not a relevant intervention and/or comparator |
| Wolstenhulme (2013) | Review of postoperative CT and ultrasound for endovascular aneurysm repair using Talent stent graft: can we simplify the surveillance protocol and reduce the number of CT scans? | Not a relevant study design |
| Zaiem (2017) | Surveillance after endovascular aortic repair. | Review did not contain new relevant papers. |
Economic studies
| Study ID | Title | Reason for Exclusion |
|---|---|---|
| Beeman (2009) | Duplex ultrasound imaging alone is sufficient for midterm endovascular aneurysm repair surveillance: a cost analysis study and prospective comparison with computed tomography. J Vasc Surg, 50: 1019–24. [PubMed: 19656651] | Not a CUA |
| Manta (2007) | Intense cardiac troponin surveillance for long-term benefits is cost-effective in patients undergoing open abdominal aortic surgery: a decision analysis model. Anesth Analg, 105: 134–56. [PubMed: 17959965] | Intervention (not imaging) |
| Post (2004) | Post PN, Kievit J, Van Bockel JH. Optimal follow-up strategies after aorto-iliac prosthetic reconstruction: a decision analysis and cost-effectiveness analysis. European journal of vascular and endovascular surgery. 2004 Sep 30;28(3):287–95. [PubMed: 15288633] | Comparison (not comparing alternative techniques) |
| Schuster (2009) | Schuster H, Dünser E, Osinger K, Bergmayr W, Fischer-Scholz U, Richter W, Mostbeck GH. Ultrasound imaging of abdominal aortic aneurysms: diagnosis of aneurysms and complications and follow-up after endovascular repair. Ultraschall in der Medizin (Stuttgart, Germany: 1980). 2009 Dec;30(6):528. [PubMed: 19998207] | Not a CUA |
Appendix I. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life-threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Methods, evidence and recommendations
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Comparison of midterm results of endovascular aneurysm repair for ruptured and elective abdominal aortic aneurysms.[J Vasc Surg. 2020]Comparison of midterm results of endovascular aneurysm repair for ruptured and elective abdominal aortic aneurysms.Oliveira-Pinto J, Soares-Ferreira R, Oliveira NFG, Bastos Gonçalves FM, Hoeks S, Van Rijn MJ, Raa ST, Mansilha A, Verhagen HJM. J Vasc Surg. 2020 May; 71(5):1554-1563.e1. Epub 2019 Oct 31.
- Endovascular aneurysm repair at 5 years: Does aneurysm diameter predict outcome?[J Vasc Surg. 2006]Endovascular aneurysm repair at 5 years: Does aneurysm diameter predict outcome?Zarins CK, Crabtree T, Bloch DA, Arko FR, Ouriel K, White RA. J Vasc Surg. 2006 Nov; 44(5):920-29; discussion 929-31.
- Endovascular aneurysm repair for symptomatic abdominal aortic aneurysms has comparable results to elective repair in the long term.[J Vasc Surg. 2020]Endovascular aneurysm repair for symptomatic abdominal aortic aneurysms has comparable results to elective repair in the long term.Abdulrasak M, Sonesson BJ, Vaccarino R, Singh BH, Resch TA, Dias NV. J Vasc Surg. 2020 Dec; 72(6):1927-1937.e1. Epub 2020 Apr 16.
- Review Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management.[Cardiovasc Diagn Ther. 2018]Review Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management.Daye D, Walker TG. Cardiovasc Diagn Ther. 2018 Apr; 8(Suppl 1):S138-S156.
- Review Postoperative surveillance after surgical repair of abdominal aortic aneurysms: Abdominal aortic aneurysm: diagnosis and management: Evidence review V[ 2020]Review Postoperative surveillance after surgical repair of abdominal aortic aneurysms: Abdominal aortic aneurysm: diagnosis and management: Evidence review V. 2020 Mar
- Accuracy of imaging techniques in identifying complications after surgeryAccuracy of imaging techniques in identifying complications after surgery
- proton-associated sugar transporter A isoform X1 [Homo sapiens]proton-associated sugar transporter A isoform X1 [Homo sapiens]gi|2462509673|ref|XP_054192808.1|Protein
- Profile neighbors for GEO Profiles (Select 89688682) (199)GEO Profiles
Your browsing activity is empty.
Activity recording is turned off.
See more...