NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Risk factors associated with abdominal aortic aneurysm growth or rupture
Review question
What risk factors are associated with abdominal aortic aneurysm a) expansion and b) rupture?
Introduction
The management of small abdominal aortic aneurysms (AAA) vary considerably. An important aspect of management of AAAs is understanding how often people should be monitored for aneurysm growth. Furthermore, it is important to identify which patients are more likely to experience aneurysm rupture. As a result, this review question aims to determine which risk factors (or combinations of these) may suggest the need for more frequent monitoring of patients with AAA and inform the decision about when to offer intervention.
PICO table
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in Appendix A.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy.
A single broad search was used to identify all studies that examine the diagnosis, surveillance or monitoring of AAAs. This was a ‘bulk’ search that covered multiple review questions. The database was sifted to identify all studies that met the criteria detailed in Table 1. The relevant review protocol can be found in Appendix A.
Table 1
Inclusion criteria.
Prospective observational studies that explored the association between potential risk factors and the occurrence of aneurysm growth or rupture, using multivariate logistic regression or Cox regression were considered for inclusion. Ideally, prospective cohort studies with sample sizes of more than 500 participants were included. In the absence of prospective cohort studies, retrospective cohort studies in which all individuals in a cohort were followed up to examine whether they developed aneurysm growth or rupture, were included. For example, all patients included in a disease register or screening programme, established in the past, who were followed up prospectively.
Studies were excluded if they:
- were case-controls or cross-sectional studies
- were not in English
- were not full reports of the study (for example, published only as an abstract)
- were not peer-reviewed.
Clinical evidence
Included studies
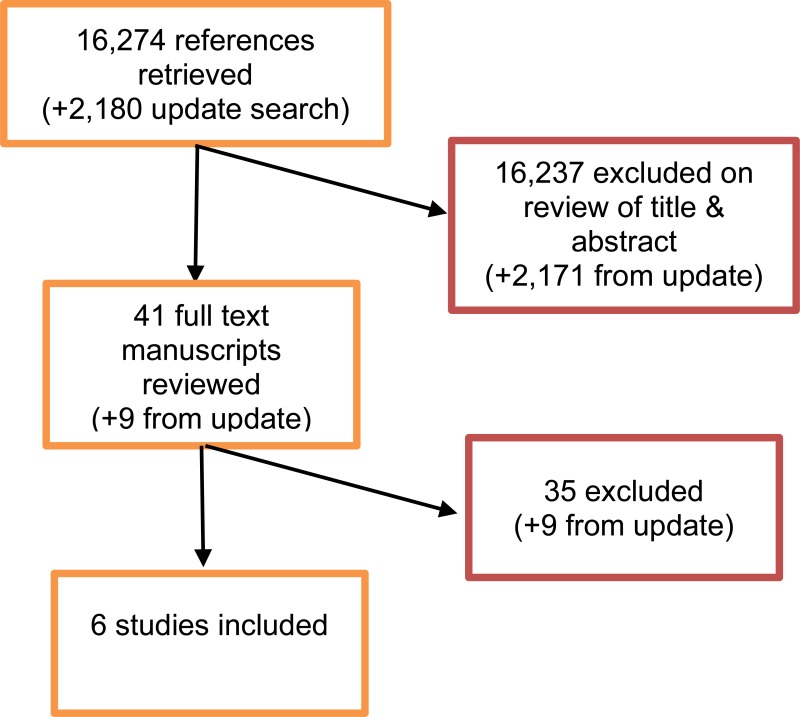
From a database of 16,274 abstracts, 41 were identified as being potentially relevant. Following full-text review of these articles, 6 studies were included. These included 2 prospective cohort studies, 3 retrospective cohort studies and 1 individual patient data (IPD) meta-analysis which did not include data from any of the other studies which have been included individually. The IPD meta-analysis was considered as 1 large cohort study on the basis that analysis was performed pooling data from individual patients, as opposed to pooling study level data.
An update literature search was performed and provided by Cochrane, in December 2017. The search found a total of 2,180 abstracts; of which, 9 full manuscripts were ordered. Upon review of the full manuscripts, none of the studies met the inclusion criteria for this review question.
Excluded studies
The list of papers excluded at full-text review, with reasons, is given in Appendix G.
Summary of clinical studies included in the evidence review
A summary of the included studies is included in the table below.
Table 2
Summary of included studies.
See Appendix D for full evidence tables.
Quality assessment of clinical studies included in the evidence review
See Appendix E for full GRADE tables, highlighting the quality of evidence from the included studies
Economic evidence
Included studies
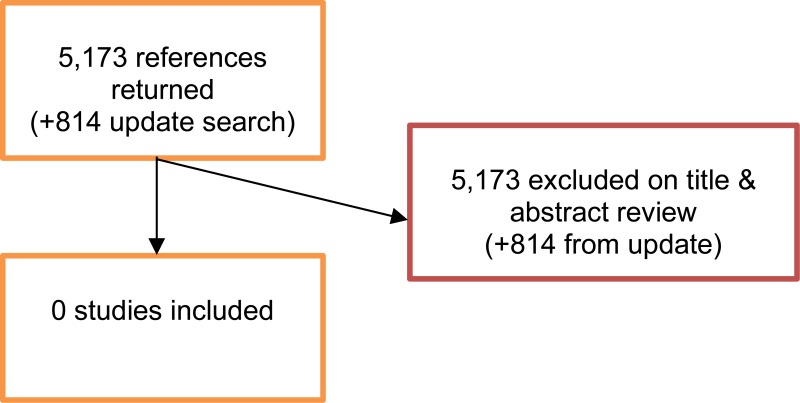
A literature search was conducted jointly for all review questions by applying standard health economic filters to a clinical search for AAA. This search returned a total of 5,173 citations. Following review of all titles and abstracts, no studies were identified as being potentially relevant to risk factors associated with AAA expansion or rupture. No full texts were retrieved, and so no studies were included as economic evidence.
An update search was conducted in December 2017, to identify any relevant health economic analyses published during guideline development. The search found 814 abstracts; all of which were not considered relevant to this review question. As a result no additional studies were included.
Excluded studies
No studies were retrieved for full-text review.
Evidence statements for aneurysm growth
History of cardiovascular disease
Very low-quality evidence from a retrospective cohort study, including 665 people with AAA, could not differentiate aneurysm growth between people with and without a family history of cardiovascular disease. Conversely, low- to high-quality evidence from 1 retrospective cohort study and 1 prospective cohort study, including up to 665 people with AAA, indicated that people with coronary artery disease were less likely to experience aneurysm growth than those without coronary artery disease.
Hypertension
Very low- to moderate-quality evidence from 1 retrospective cohort study and 1 prospective cohort study, including up to 665 people with AAA, could not differentiate aneurysm growth between people with and without hypertension. Conversely, very low-quality evidence from 1 retrospective cohort study, including 790 people with AAA, indicated that people with hypertension were more likely to experience aneurysm growth than those without hypertension.
Diabetes
Very low-quality evidence from 1 retrospective cohort study, including 665 people with AAA, could not differentiate aneurysm growth between people with and without diabetes. Conversely, very low- to high-quality evidence from 1 retrospective cohort study and 1 prospective cohort study, including up to 790 people with AAA, indicated that people with diabetes were less likely to experience aneurysm growth than those without diabetes.
Claudication
Very low-quality evidence from 1 retrospective cohort study, including 790 people with AAA, indicated that people with claudication were less likely to experience aneurysm growth than those without claudication.
Initial aneurysm diameter
Moderate- to high-quality evidence from 1 retrospective cohort study and 1 prospective cohort study, including up to 652 people with AAA, indicated that increasing aneurysm diameters, at the time of diagnosis, increased the odds of aneurysm growth.
Medication use
Very low- to moderate-quality evidence from 1 retrospective cohort study and 1 prospective cohort study, including up to 665 people with AAA, aspirin, beta-blocker, ace inhibitor, angiotensin receptor blocker, calcium-channel blocker or statin use had no impact on aneurysm growth. Moderate-quality evidence from 1 retrospective cohort study, including 665 people with AAA, indicated that people taking statins had lower odds of aneurysm growth than those who were not taking statins.
Other potential risk factors
Very low- to moderate-quality evidence from 1 retrospective cohort study and 1 prospective cohort study, including up to 665 people with AAA, could not identify any associations between the following factors and aneurysm growth:
- Age
- Sex
- Smoking status
- BMI
- A family history of AAA
- Presence of COPD
- Presence of peripheral artery disease
- Presence of cerebral artery disease
- Presence of dyslipidaemia
- Ischaemic changes on ECG
- Haemodialysis
- Creatinine levels
Evidence statements for aneurysm rupture
Age
Moderate-quality evidence from 1 prospective cohort study, including 2,256 people with AAA, could not find any association between increasing age and aneurysm rupture. Conversely, low-quality evidence from 1 individual patient data meta-analysis, including 15,745 people with AAA, indicated that increasing age increased the odds of aneurysm rupture.
Sex
High-quality evidence from 1 prospective cohort study, including 2,256 people with AAA, indicated that women were more likely than men to experience aneurysm rupture. Additional low-quality evidence from 1 individual patient data meta-analysis, including 15,745 people with AAA, highlighted that women were more likely to experience aneurysm rupture than men.
Smoking status
Moderate- to high-quality evidence from 1 prospective cohort study, including 2,242 people with AAA, indicated that ex-smokers were less likely to experience aneurysm rupture than current smokers. The same study reported that people who never smoked were less likely to experience rupture than current smokers; however, the differences between groups were not significant. Low-quality evidence from 1 individual patient data meta-analysis, including 15,745 people with AAA, highlighted that current smokers were more likely experience aneurysm rupture than ex-smokers or those who never smoked.
BMI
Moderate-quality evidence from 1 prospective cohort study, including 2,242 people with AAA, could not differentiate aneurysm rupture rates of people with different BMI measurements. Conversely, low-quality evidence from 1 individual patient data meta-analysis, including 15,745 people with AAA, indicated that increasing BMI decreased the odds of aneurysm rupture.
Diabetes
Very low-quality evidence from 1 individual patient data meta-analysis, including 15,475 people with AAA, could not differentiate aneurysm rupture rates of people with and without diabetes.
Blood pressure
Low-quality evidence from 1 individual patient data meta-analysis, including 15,475 people with AAA, highlighted that both increasing arterial blood pressure and increasing pulse pressure increased the odds of aneurysm rupture. High-quality evidence from 1 prospective cohort study, including 2,146 people with AAA, could not differentiate aneurysm rupture rates of people with different ankle–brachial pressure index measurements.
Cholesterol levels
Moderate-quality evidence from 1 prospective cohort study, including 2,107 people with AAA, could not differentiate aneurysm rupture rates in people with different cholesterol level measurements.
History of cardiovascular disease
Very low-quality evidence from 1 individual patient data meta-analysis, including 15,475 people with AAA, could not differentiate aneurysm rupture rates between people with and without a history of cardiovascular disease.
Initial aneurysm diameter
High-quality evidence from 1 prospective cohort study, including 2,257 people with AAA, indicated that increasing aneurysm diameters, at the time of diagnosis, increased the odds of aneurysm rupture.
The committee’s discussion of the evidence.
Interpreting the evidence
The outcomes that matter most
The committee considered various types of risk factors, including modifiable and non-modifiable risk factors. It was agreed that modifiable risk factors mattered most as they would support people with AAA to decrease their chances of experiencing aneurysm growth or rupture.
The quality of the evidence
The committee noted that the quality of evidence ranged from very low to high. Evidence from retrospective cohort studies was considered lower in quality than that of prospective cohort studies because of the inability to accurately monitor confounders during follow-up. Nakayama et al. (2012) was considered to be at high risk of selection bias because the study population only comprised people who underwent surgery. This means that data from patients who had growing aneurysms which did not reach the threshold for surgical repair or patients who opted not to receive intervention would not have been considered in any analyses. The study by Santilli et al. (2002) was considered to be prone to responder bias because participants were asked to complete a brief questionnaire asking whether they had ever been told by a physician that they had any risk factors of interest.
The committee noted that statistical heterogeneity (I2) ranged from 0 to 98% in the IPD meta-analysis by Thompson et al. (2013). There was some variation in baseline AAA diameters across included studies, making comparisons between the studies difficult. Furthermore, there was some heterogeneity in the imaging techniques and paramaters used in included studies in the meta-analysis. Most studies from which data were obtained used ultrasound imaging to measure aneurysm diameters; however, a few of the studies used CT. Some studies measured external (outer-to-outer) wall diameters, whereas others measured internal diameters. Finally, study-specific thresholds for surgical intervention varied from 4.5 cm up to 6.0 cm.
The committee suspected that atheromatous coronary artery disease would be associated with aneurysm growth and was surprised that the identified evidence indicated that coronary artery disease may decrease the odds of growth. It was noted that the studies did not specify the nature of the coronary artery disease. Therefore, in the absence of this information, the committee refrained from making any recommendations.
Benefits and harms
The committee noted that the identified evidence highlighted no association between the following factors and the occurrence of aneurysm growth: increasing age, sex, BMI and a family history of AAA. The committee noted that the majority of these factors were non-modifiable and interpreted the evidence as an indication that little could be done in relation to these factors to alter the course of aneurysm growth.
The committee agreed to focus recommendations on modifiable risk factors associated with aneurysm growth or rupture because targeting these factors would help people with AAA to decrease the chances of aneurysm growth or rupture.
Evidence from the IPD meta-analysis identified being a current smoker as a clear predictor of risk of aneurysm rupture. This was supported by evidence from the prospective cohort study by Brown et al. (2013) which indicated that ex-smokers are less likely to experience aneurysm rupture than current smokers. The committee therefore agreed that smoking cessation was likely to reduce the odds of rupture.
The committee discussed the evidence suggesting that women are approximately 3 times more likely to experience AAA rupture than men; however, it was noted that there is currently no published evidence indicating that women with AAA should be treated differently to men with AAA. The committee were aware that there is ongoing observational research (in the form of cohort studies) on aneurysms in women which might inform sex-specific recommendations in the future.
Cost effectiveness and resource use
The committee considered that a cross-referral to NICE Public Health guidance relating to stop smoking services was unlikely to have a direct impact on costs. This is because current practice already outlines that all people who smoke should be offered access to a stop smoking service. The committee noted that not all clinicians are able to provide smoking cessation advice but there is usually an avenue to refer patients on to a stop smoking service.
Other factors the committee took into account
The committee agreed that referral pathways to hypertension management services between primary and secondary vary across the NHS. As a result, it was considered that the recommendation would help address the variability. The committee believed that specifying which clinicians should provide hypertension management services would be too prescriptive. As a result, it was decided that a cross-referral to existing NICE guidance was appropriate.
Upon consideration of the evidence highlighting that women had a higher risk of experiencing aneurysm rupture than men, the committee discussed whether it was possible to make recommendations specific to monitoring of women. They agreed that it was not possible to specify shorter follow-up intervals in women without evidence to support such a recommendation. The committee noted that they made a research recommendation, in a separate review assessing thresholds for surgery, which explicitly mentioned that subgroup analyses should be stratified by sex to determine whether sex-specific monitoring frequencies are possible. As a result, the committee decided not to make a recommendation until additional evidence is available.
Appendices
Appendix A. Review protocols
Review protocol for risk factors associated with aneurysm growth or rupture
| Review question 3 | What risk factors are associated with abdominal aortic aneurysm a) expansion and b) rupture? |
|---|---|
| Objectives | To determine which risk factors (or combinations of these) may suggest the need for more frequent monitoring of patients with AAA, and to inform management decisions |
| Type of review | Prognostic |
| Language | English |
| Study design |
|
| Status |
|
| Population |
People with a confirmed abdominal aortic aneurysm >3cm in diameter Subgroups: by aneurysm diameter, age, sex, comorbidities |
| Index test / factors of interest |
Aneurysm size (different approaches to measurement) Abdominal pain Back pain Abdominal palpation Pulsatile abdominal mass/pulsation Age Sex Other cardiovascular disease (existing or previous) – other aneurysms, atherosclerotic disease, vascular claudication Inflammatory disease Smoking Blood pressure/hypertension Dislipidaemia Hypercholesterolaemia Family history of abdominal aortic aneurysms, other aneurysms, collagen disorders Ethnicity Diabetes COPD BMI/weight/obesity Chemotherapy Other surgery, particularly abdominal or urological Finite element method rupture index (FEARI) (risk of rupture based on geometry, blood pressure, gender-specific strength of wall) Stiffness of the aorta (pulse wave velocity = surrogate marker) AAA wall stress Vessel asymmetry Rupture potential index (RPI) Severity parameter (SP) Growth of intraluminal thrombus Rate of expansion |
| Endpoint |
Radiological diagnosis of abdominal aortic aneurysm expansion; single test within a study Surgically- or radiologically-confirmed rupture of an abdominal aortic aneurysm |
| Other criteria for inclusion / exclusion of studies |
Exclusion: Non-English language Abstract/non-published (i only) |
| Baseline characteristics to be extracted in evidence tables |
Age Sex Size of aneurysm Comorbidities |
| Search strategies | See Appendix B |
| Review strategies |
Available Cochrane review (Filardo, 2015) will be used as a ‘seed review’; studies published since 2014 and studies with outcomes of interest not reported in the Cochrane review will be added Data on all included studies will be extracted into evidence tables. Where statistically possible, a meta-analytic approach will be used to give an overall summary effect. All key findings from evidence will be presented in GRADE profiles.
i and ii) All key findings will be summarised in evidence statements. |
| Key papers |
Bhak, Rachel H., Wininger, Michael, Johnson, Gary R., Lederle, Frank A., Messina, Louis M., Ballard, David J., Wilson, Samuel E.. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg 2015;150(1):44–50 [PubMed: 25389641] Thompson SG, Brown LC, Sweeting MJ, Bown MJ, Kim LG, Glover MJ, Buxton MJ, Powell JT. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess. 2013 Sep;17(41):1–118 [PMC free article: PMC4781118] [PubMed: 24067626] |
Appendix B. Literature search strategies
Clinical search literature search strategy
Main searches
Bibliographic databases searched for the guideline
- Cumulative Index to Nursing and Allied Health Literature - CINAHL (EBSCO)
- Cochrane Database of Systematic Reviews – CDSR (Wiley)
- Cochrane Central Register of Controlled Trials – CENTRAL (Wiley)
- Database of Abstracts of Reviews of Effects – DARE (Wiley)
- Health Technology Assessment Database – HTA (Wiley)
- EMBASE (Ovid)
- MEDLINE (Ovid)
- MEDLINE Epub Ahead of Print (Ovid)
- MEDLINE In-Process (Ovid)
Identification of evidence for review questions
The searches were conducted between November 2015 and October 2017 for 31 review questions (RQ). In collaboration with Cochrane, the evidence for several review questions was identified by an update of an existing Cochrane review. Review questions in this category are indicated below. Where review questions had a broader scope, supplement searches were undertaken by NICE.
Searches were re-run in December 2017.
Where appropriate, study design filters (either designed in-house or by McMaster) were used to limit the retrieval to, for example, randomised controlled trials. Details of the study design filters used can be found in section 4.
Search strategy review question 3
|
Medline Strategy, searched 29th September 2016 Database: 1946 to September Week 3 2016 Search Strategy: |
|---|
| 1 Aortic Aneurysm, Abdominal/ |
| 2 Aortic Rupture/ |
| 3 (aneurysm* adj4 (abdom* or thoracoabdom* or thoraco-abdom* or aort* or spontan* or juxtarenal* or juxta-renal* or juxta renal* or paraerenal* or para-renal* or para renal* or suprarenal* or supra renal* or supra-renal* or short neck* or short-neck* or shortneck* or visceral aortic segment*)).tw. |
| 4 or/1-3 |
| 5 prognosis.sh. |
| 6 diagnosed.tw. |
| 7 cohort.mp. |
| 8 predictor:.tw. |
| 9 death.tw. |
| 10 exp models, statistical/ |
| 11 or/5-10 |
| 12 (sensitiv: or predictive value:).mp. or accurac:.tw. |
| 13 11 or 12 |
| 14 “signs and symptoms”/ |
| 15 ((sign or signs) adj5 symptom*).tw. |
| 16 Risk Factors/ |
| 17 factor*.tw. |
| 18 predict*.tw. |
| 19 or/14-18 |
| 20 13 or 19 |
| 21 4 and 20 |
| 22 animals/ not humans/ |
| 23 21 not 22 (12444) |
| 24 limit 23 to english language |
Health Economics literature search strategy
Sources searched to identify economic evaluations
- NHS Economic Evaluation Database – NHS EED (Wiley) last updated Dec 2014
- Health Technology Assessment Database – HTA (Wiley) last updated Oct 2016
- Embase (Ovid)
- MEDLINE (Ovid)
- MEDLINE In-Process (Ovid)
Search filters to retrieve economic evaluations and quality of life papers were appended to the population and intervention terms to identify relevant evidence. Searches were not undertaken for qualitative RQs. For social care topic questions additional terms were added. Searches were re-run in September 2017 where the filters were added to the population terms.
Health economics search strategy
| Medline Strategy |
|---|
| Economic evaluations |
| 1 Economics/ |
| 2 exp “Costs and Cost Analysis”/ |
| 3 Economics, Dental/ |
| 4 exp Economics, Hospital/ |
| 5 exp Economics, Medical/ |
| 6 Economics, Nursing/ |
| 7 Economics, Pharmaceutical/ |
| 8 Budgets/ |
| 9 exp Models, Economic/ |
| 10 Markov Chains/ |
| 11 Monte Carlo Method/ |
| 12 Decision Trees/ |
| 13 econom*.tw. |
| 14 cba.tw. |
| 15 cea.tw. |
| 16 cua.tw. |
| 17 markov*.tw. |
| 18 (monte adj carlo).tw. |
| 19 (decision adj3 (tree* or analys*)).tw. |
| 20 (cost or costs or costing* or costly or costed).tw. |
| 21 (price* or pricing*).tw. |
| 22 budget*.tw. |
| 23 expenditure*.tw. |
| 24 (value adj3 (money or monetary)).tw. |
| 25 (pharmacoeconomic* or (pharmaco adj economic*)).tw. |
| 26 or/1-25 |
| Quality of life |
| 1 “Quality of Life”/ |
| 2 quality of life.tw. |
| 3 “Value of Life”/ |
| 4 Quality-Adjusted Life Years/ |
| 5 quality adjusted life.tw. |
| 6 (qaly* or qald* or qale* or qtime*).tw. |
| 7 disability adjusted life.tw. |
| 8 daly*.tw. |
| 9 Health Status Indicators/ |
| 10 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 11 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 12 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 13 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 14 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 15 (euroqol or euro qol or eq5d or eq 5d).tw. |
| 16 (qol or hql or hqol or hrqol).tw. |
| 17 (hye or hyes).tw. |
| 18 health* year* equivalent*.tw. |
| 19 utilit*.tw. |
| 20 (hui or hui1 or hui2 or hui3).tw. |
| 21 disutili*.tw. |
| 22 rosser.tw. |
| 23 quality of wellbeing.tw. |
| 24 quality of well-being.tw. |
| 25 qwb.tw. |
| 26 willingness to pay.tw. |
| 27 standard gamble*.tw. |
| 28 time trade off.tw. |
| 29 time tradeoff.tw. |
| 30 tto.tw. |
| 31 or/1-30 |
Appendix D. Clinical evidence tables
Download PDF (227K)
Appendix E. GRADE tables
Risk factors associated with aneurysm growth
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| Over 65 vs. under 65 | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Serious4 | 665 | HRa 0.84 (0.38, 1.85) | Very low |
| Age (continuous) | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 1.10 (0.93, 1.30) | Moderate |
| Sex | |||||||||
| Males vs. females | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Serious4 | 665 | HRa 1.88 (0.89, 3.96) | Very low |
| Males vs. females | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 0.77 (0.376, 1.56) | Moderate |
| Smoking status | |||||||||
| Ex-smoker vs. lifelong smoker | 1 Norman (2004) | Retrospective cohort | Serious1 | N/A | Not serious | Serious4 | 545 | ORa 0.9 (0.4, 1.8) | Low |
| Current smoker vs. lifelong smoker | 1 Norman (2004) | Retrospective cohort | Serious1 | N/A | Not serious | Serious4 | 545 | ORa 1.8 (0.8, 4.1) | Low |
| Ex-smoker vs. non-smoker | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 1.1 (0.7, 1.7) *estimated from a graph | Very low |
| Current smoker vs. non-smoker | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 | HRa 1.77 (0.97, 3.22) | Very low |
| Ex-smoker vs. non smoker | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 0.75 (0.47, 1.20) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Retrospective cohort in which confounding was not adequately assessed, downgrade 1 level.
- 2
Only patients who underwent elective surgical repair were included. Data from patients who had growing aneurysms that did not reach the threshold for surgical repair or patients who opted not to receive surgery were not included, downgrade 1 level
- 3
Results were reported graphically, downgrade 1 level.
- 4
95% CI crosses the line of no effect, downgrade 1 level.
- 5
95% CI not reported, downgrade 2 levels.
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| BMI | |||||||||
| BMI >25 vs. BMI <25 | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Serious4 | 665 | HRa 0.82 (0.45, 1.50) | Very low |
| Family history of AAA | |||||||||
| History vs. no history | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 1.2 (0.5, 2.9) *estimated from a graph | Very low |
| Coronary artery disease | |||||||||
| Presence vs. absence | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Not serious | 652 | ORa 0.67 (0.46, 0.97) | High |
| Presence vs. absence | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Not serious | 665 | HRa 0.55 (0.32, 0.94) | Low |
| Family history vs. no history | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 0.8 (0.3, 1.75) *estimated from a graph | Very low |
| Peripheral artery disease | |||||||||
| Presence vs. absence | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 0.96 (0.62, 1.48) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Retrospective cohort in which confounding was not adequately assessed, downgrade 1 level.
- 2
Only patients who underwent elective surgical repair were included. Data from patients who had growing aneurysms that did not reach the threshold for surgical repair or patients who opted not to receive surgery were not included, downgrade 1 level
- 3
Results were reported graphically, downgrade 1 level.
- 4
95% CI crosses the line of no effect, downgrade 1 level.
- 5
95% CI not reported, downgrade 2 levels.
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| COPD | |||||||||
| Presence vs. absence | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 1.4 (0.75, 2.3) *estimated from a graph | Very low |
| Hypertension | |||||||||
| Presence vs. absence | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Serious4 | 665 | HRa 0.97 (0.52, 1.81) | Very low |
| Presence vs. absence | 1 Santilli (2002) | Retrospective cohort | Serious1 | N/A | Not serious | Very serious5 | 790 |
ORa 2.5 *Significant: 95% CI not reported | Very low |
| Presence vs. absence | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 0.92 (0.64, 1.31) | Moderate |
| Dyslipidaemia | |||||||||
| Presence vs. absence | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Serious4 | 665 | HRa 1.02 (0.58, 1.80) | Very low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Retrospective cohort in which confounding was not adequately assessed, downgrade 1 level.
- 2
Only patients who underwent elective surgical repair were included. Data from patients who had growing aneurysms that did not reach the threshold for surgical repair or patients who opted not to receive surgery were not included, downgrade 1 level
- 3
Results were reported graphically, downgrade 1 level.
- 4
95% CI crosses the line of no effect, downgrade 1 level.
- 5
95% CI not reported, downgrade 2 levels.
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Diabetes | |||||||||
| Presence vs. absence | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Serious4 | 665 | HRa 0.88 (0.49, 1.58) | Very low |
| Presence vs. absence | 1 Santilli (2002) | Retrospective cohort | Serious1 | N/A | Not serious | Very serious5 | 790 |
ORa 0.60 *Significant: 95% CI not reported | Very low |
| Presence vs. absence | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Not serious | 652 | ORa 0.37 (0.22, 0.62) | High |
| Claudication | |||||||||
| Presence vs. absence | 1 Santilli (2002) | Retrospective cohort | Serious1 | N/A | Not serious | Very serious5 | 790 |
ORa 0.35 *Significant: 95% CI not reported | Very low |
| Haemodialysis | |||||||||
| Presence vs. absence | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2 | N/A | Not serious | Serious4 | 665 | HRa 1.85 (0.48, 7.2) | Very low |
| Cerebral artery disease | |||||||||
| Presence vs. absence | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 1.7 (0.85, 3.2) *estimated from a graph | Very low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Retrospective cohort in which confounding was not adequately assessed, downgrade 1 level.
- 2
Only patients who underwent elective surgical repair were included. Data from patients who had growing aneurysms that did not reach the threshold for surgical repair or patients who opted not to receive surgery were not included, downgrade 1 level
- 3
Results were reported graphically, downgrade 1 level.
- 4
95% CI crosses the line of no effect, downgrade 1 level.
- 5
95% CI not reported, downgrade 2 levels.
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Ischaemic changes on ECG | |||||||||
| Changes vs. no changes | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 0.45 (0.1, 1.5) *estimated from a graph | Very low |
| Initial AAA diameter | |||||||||
| 4.0-5.4 cm vs. 3.0-3.9 cm | 1 Norman (2004) | Retrospective cohort | Serious1 | N/A | Not serious | Not serious | 545 | ORa 7.2 (4.3, 12.2) | Moderate |
| Per 4.3 mm (continuous) | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Not serious | 652 | ORa 1.78 (1.49, 2.14) | High |
| C-reactive protein levels (mg/L) | |||||||||
| 1.2-2.1 vs. <1.2 | 1 Norman (2004) | Retrospective cohort | Serious1 | N/A | Not serious | Serious4 | 545 | ORa 1.3 (0.6, 2.9) | Low |
| 2.2-3.5 vs. <1.2 | 1 Norman (2004) | Retrospective cohort | Serious1 | N/A | Not serious | Serious4 | 545 | ORa 0.9 (0.4,2.2) | Low |
| 3.6-6.2 vs. <1.2 | 1 Norman (2004) | Retrospective cohort | Serious1 | N/A | Not serious | Serious4 | 545 | ORa 1.0 (0.4, 2.4) | Low |
| ≥ 6.3 vs. <1.2 | 1 Norman (2004) | Retrospective cohort | Serious1 | N/A | Not serious | Serious4 | 545 | ORa 1.9 (0.9, 4.1) | Low |
| Creatinine levels (mg/L) | |||||||||
| >1.5 vs <1.5 | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 1.65 (0.7, 3.7) *estimated from a graph | Very low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Retrospective cohort in which confounding was not adequately assessed, downgrade 1 level.
- 2
Only patients who underwent elective surgical repair were included. Data from patients who had growing aneurysms that did not reach the threshold for surgical repair or patients who opted not to receive surgery were not included, downgrade 1 level
- 3
Results were reported graphically, downgrade 1 level.
- 4
95% CI crosses the line of no effect, downgrade 1 level.
- 5
95% CI not reported, downgrade 2 levels.
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Aspirin | |||||||||
| Taking vs. not taking | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 1.10 (0.78, 1.56) | Moderate |
| Beta-blockers | |||||||||
| Taking vs. not taking | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 1.9 (0.5, 1.4) *estimated from a graph | Very low |
| Taking vs. not taking | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 1.13 (0.76, 1.67) | Moderate |
| ACE inhibitors | |||||||||
| Taking vs. not taking | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 0.8 (0.4, 1.7) *estimated from a graph | Very low |
| Taking vs. not taking | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 0.91 (0.64, 1.31) | Moderate |
| Angiotensin receptor blockers | |||||||||
| Taking vs. not taking | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 0.75 (0.45, 1.15) *estimated from a graph | Very low |
| Calcium-channel blockers | |||||||||
| Taking vs. not taking | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Serious4 | 665 |
HRa 1.0 (0.6, 1.4) *estimated from a graph | Very low |
| Statins | |||||||||
| Taking vs. not taking | 1 Nakayama (2012) | Retrospective cohort | Very serious1,2,3 | N/A | Not serious | Not serious | 665 |
HRa 0.65 (0.3, 0.9) *estimated from a graph | Very low |
| Taking vs. not taking | 1 Ferguson (2010) | Prospective cohort | Not serious | N/A | Not serious | Serious4 | 652 | ORa 1.23 (0.86, 1.76) | Moderate |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
Retrospective cohort in which confounding was not adequately assessed, downgrade 1 level.
- 2
Only patients who underwent elective surgical repair were included. Data from patients who had growing aneurysms that did not reach the threshold for surgical repair or patients who opted not to receive surgery were not included, downgrade 1 level
- 3
Results were reported graphically, downgrade 1 level.
- 4
95% CI crosses the line of no effect, downgrade 1 level.
- 5
95% CI not reported, downgrade 2 levels.
Risk factors associated with aneurysm rupture
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| Years per tertile group (59-66 vs. 67-71 vs. 72-77) | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Serious1 | 2,256 | HRa 1.03 (0.98, 1.08) | Moderate |
| Per year (continuous) | 1 Thompson (2013) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Not serious | 15,475 | HRa 1.04 (1.01, 1.07) | Low |
| Sex | |||||||||
| Females vs males | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Not serious | 2,256 | HRa 3.0 (1.99, 4.53) | High |
| Females vs. males | 1 Thompson (2013) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Not serious | 15,475 | HRa 3.76 (2.58, 5.47) | Low |
| Smoking status | |||||||||
| Ex-smokers vs. current smoker | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Not serious | 2,242 | HRa 0.59 (0.39, 0.89) | High |
| Never-smokers vs. current smoker | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Serious1 | 2,242 | HRa 0.65 (0.27, 1.53) | Moderate |
| Current smokers vs. ex/never smokers | 1 Thompson (2013) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Not serious | 15,475 | HRa 2.02 (1.33, 1.53) | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
95% CI crosses the line of no effect, downgrade 1 level.
- 2
Authors did not use a risk of bias assessment tool to assess the quality of included studies, downgrade 1 level.
- 3
Inconsistency between included studies: Most studies used ultrasound imaging to measure the diameters of aneurysms; however, a few of the studies used computed-tomography. Some studies measured external (outer-to-outer) wall diameters, whereas others measured internal diameters. Study-specific thresholds for surgical intervention varied from 4.5 cm to 6.0 cm.
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| BMI | |||||||||
| BMI by tertile group (15-23.3 vs. 23.4-26.3 vs. 26.4-42.1) | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Serious1 | 2,242 | HRa 0.99 (0.94,1.04) per kg/m2 | Moderate |
| BMI (continuous) | 1 Thompson (2013) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Not serious | 15,475 | HRa 0.93 (0.88, 0.99) per kg/m2 | Low |
| Diabetes | |||||||||
| Presence vs. absence | 1 Thompson (2013) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Serious1 | 15,475 | HRa 1.27 (0.45, 3.54) | Very low |
| Arterial blood pressure | |||||||||
| Mean blood pressure by tertile group (57-102 vs. 103-116 vs. 117-193) | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Not serious | 2,222 | HRa 1.02 (1.00, 1.03) per mmHg | High |
| Mean blood pressure (continuous) | 1 Thompson (1999) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Not serious | 15,475 | HRa 1.32 (1.11, 1.56) per 10 mmHg | Low |
| Pulse pressure | |||||||||
| Pulse pressure (continuous) | 1 Thompson (2013) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Not serious | 15,475 | HRa 1.11 (1.02, 1.22) per 10 mmHg | Low |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
95% CI crosses the line of no effect, downgrade 1 level.
- 2
No risk of bias tool was used to assess the quality of included studies, downgrade 1 level
- 3
Inconsistency between included studies: Most studies used ultrasound imaging to measure the diameters of aneurysms; however, a few of the studies used computed-tomography. Some studies measured external (outer-to-outer) wall diameters, whereas others measured internal diameters. Study-specific thresholds for surgical intervention varied from 4.5 cm to 6.0 cm.
| Predictor | No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | No. of participants | Effect size (95% CI) | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Ankle-brachial pressure index measurement (ABPI) | |||||||||
| Mean ABPI by tertile group (0.02-0.86 vs. 0.87-1.03 vs. 1.04-1.90) | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Serious1 | 2,146 | HRa 0.93 (0.34, 2.58) per unit | Moderate |
| Cholesterol levels | |||||||||
| mmoL by tertile group (1.6-5.6 vs. 5.7-6.6 vs. 6.7-16.9) | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Serious1 | 2,107 | HRa 0.92 (0.78, 1.08) per mmol/L | Moderate |
| History of cardiovascular disease | |||||||||
| History vs. no history | 1 Thompson (2013) | IPD meta-analysis | Serious2 | Serious3 | Not serious | Serious1 | 15,475 | HRa 1.32 (0.77, 2.27) | Very low |
| Initial AAA diameter | |||||||||
| Diameter ranges (3.0-3.9 vs. 4.0-5.5 vs. 5.6-9.7) | 1 Brown (1999) | Prospective cohort | Not serious | N/A | Not serious | Not serious | 2,257 | HRa 2.97 (2.49, 3.48) | High |
- a
As multivariate analyses were performed, hazard and odds ratios were reported adjusting for confounders or other factors.
- 1
95% CI crosses the line of no effect, downgrade 1 level.
- 2
No risk of bias tool was used to assess the quality of included studies, downgrade 1 level.
- 3
Inconsistency between included studies: Most studies used ultrasound imaging to measure the diameters of aneurysms; however, a few of the studies used computed-tomography. Some studies measured external (outer-to-outer) wall diameters, whereas others measured internal diameters. Study-specific thresholds for surgical intervention varied from 4.5 cm to 6.0 cm.
Appendix G. Excluded studies
Clinical studies
| No. | Study | Reason for exclusion |
|---|---|---|
| 1 | Behr-Rasmussen C, Grondal N, Bramsen M B, Thomsen M D, and Lindholt J S (2014) Mural thrombus and the progression of abdominal aortic aneurysms: A large population-based prospective cohort study. European Journal of Vascular and Endovascular Surgery 48(3), 301–307 [PubMed: 24969094] | Although study abstract indicates that 615 patients had AAA, only 416 were included in the analysis. |
| 2 | Bhak Rachel H, Wininger Michael, Johnson Gary R, Lederle Frank A, Messina Louis M, Ballard David J, Wilson Samuel E, Aneurysm Detection, Management Study, and Group (2015) Factors associated with small abdominal aortic aneurysm expansion rate. JAMA surgery 150(1), 44–50 [PubMed: 25389641] | No data of interest: aneurysm growth rates were calculated by linear regression analysis. This is a different outcome to that specified in the review protocol: “radiological diagnosis of abdominal aortic aneurysm expansion; single test within a study” |
| 3 | Brady Anthony R, Thompson Simon G, Fowkes F Gerald R, Greenhalgh Roger M, Powell Janet T, and Participants U K. Small Aneurysm Trial (2004) Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 110(1), 16–21 [PubMed: 15210603] | No data of interest: aneurysm growth rates were calculated by linear regression analysis. This is a different outcome to that specified in the review protocol: “radiological diagnosis of abdominal aortic aneurysm expansion; single test within a study” |
| 4 | Brown M J, Sweeting M J, Brown L C, Powell J T, and Thompson S G (2013) Surveillance intervals for small abdominal aortic aneurysms: A meta-analysis. JAMA - Journal of the American Medical Association 309(8), 806–813 [PubMed: 23443444] | This meta-analysis of individual patient data, estimates aneurysm growth rates (mm/year) and rupture rates (per 1000 patient years) according to aneurysm diameter at diagnosis. Although partially applicable, multivariate analysis was not performed to assess risk factors for aneurysm growth or rupture. |
| 5 | Brown Peter M, Sobolev Boris, and Zelt David T (2003) Selective management of abdominal aortic aneurysms smaller than 5.0 cm in a prospective sizing program with gender-specific analysis. Journal of vascular surgery 38(4), 762–5 [PubMed: 14560227] | Multivariate analysis was not performed to assess risk factors associated with aneurysm expansion or rupture. |
| 6 | Brunner-Ziegler Sophie, Hammer Alexandra, Seidinger Daniela, Willfort-Ehringer Andrea, Koppensteiner Renate, and Steiner Sabine (2015) The role of intraluminal thrombus formation for expansion of abdominal aortic aneurysms. Wiener klinische Wochenschrift 127(13-14), 549–54 [PubMed: 25994875] | The study had a sample size of less than 500 participants (n=116). |
| 7 | Chang J B, Stein T A, Liu J P, and Dunn M E (1997) Risk factors associated with rapid growth of small abdominal aortic aneurysms. Surgery 121(2), 117–122 [PubMed: 9037221] | The population of interest for this review question is “people with a confirmed AAA greater than 3.0 cm in diameter. In this study 50.5% (260/514) of participants had AAAs less than 3.0 cm in diameter. |
| 8 | Cronin Oliver, Walker Philip J, and Golledge Jonathan (2013) The association of obesity with abdominal aortic aneurysm presence and growth. Atherosclerosis 226(2), 321–7 [PubMed: 23137825] | Systematic review including studies which employed various study designs (including case-controls, screening programs and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 9 | De Rango, P, Farchioni L, Fiorucci B, and Lenti M (2014) Diabetes and abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery 47(3), 243–261 [PubMed: 24447529] | Systematic review assessing the association between diabetes and AAAs. Population-based screening programmes, case-controls and prospective observational studies were included. Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 10 | Deeg Mark A, Meijer C Arnoud, Chan Lai Shan, Shen Lei, and Lindeman Jan H. N (2016) Prognostic and predictive biomarkers of abdominal aortic aneurysm growth rate. Current medical research and opinion 32(3), 509–17 [PubMed: 26636178] | Sample size less than 500 participants. |
| 11 | Harris P L, Vallabhaneni S R, Desgranges P, Becquemin J P, Van Marrewijk, C, and Laheij R J. F (2000) Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: The EUROSTAR experience. Journal of Vascular Surgery 32(4), 739–749 [PubMed: 11013038] | Authors reported that multivariate analysis was not possible because the number of observed aneurysm ruptures was too small. |
| 12 | Hatakeyama T, Shigematsu H, and Muto T (2001) Risk factors for rupture of abdominal aortic aneurysm based on three-dimensional study. Journal of vascular surgery 33(3), 453–61 [PubMed: 11241112] | No sample size data were available in the study abstract. Assessment of the full manuscript reveals that 39 patients with an atherosclerotic AAA met the inclusion criteria for this study. |
| 13 | Hendy K, Gunnarson R, and Golledge J (2014) Growth rates of small abdominal aortic aneurysms assessed by computerised tomography - A systematic literature review. Atherosclerosis 235(1), 182–188 [PubMed: 24848928] | Systematic review including prospective and retrospective observational studies. All included studies had sample sizes of less than 200 participants |
| 14 | Jalalzadeh H, Indrakusuma R, Planken R N, Legemate D A, Koelemay M J. W, and Balm R (2016) Inflammation as a Predictor of Abdominal Aortic Aneurysm Growth and Rupture: A Systematic Review of Imaging Biomarkers. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 52(3), 333–42 [PubMed: 27283346] | Systematic review of cohort studies which were out of scope of this review question. Studies assessed the diagnostic utility of inflammatory imaging biomarkers using advanced imaging techniques. Furthermore, none of the studies had sample sizes more than 500 participants. |
| 15 | Johnsen S H, Forsdahl S H, Solberg S, Singh K, and Jacobsen B K (2013) Carotid atherosclerosis and relation to growth of infrarenal aortic diameter and follow-up diameter: The tromso study. European Journal of Vascular and Endovascular Surgery 45(2), 135–140 [PubMed: 23267698] | Only 132 people with AAAs were included in the multivariate logistic regression model |
| 16 | Kleinstreuer Clement, and Li Zhonghua (2006) Analysis and computer program for rupture-risk prediction of abdominal aortic aneurysms. Biomedical engineering online 5, 19 [PMC free article: PMC1421417] [PubMed: 16529648] | Not primary research. This study outlines how a computer program can be used to develop an AAA risk assessment tool using data from previously published studies (effectively secondary data analysis). |
| 17 | Lederle Frank A, Wilson Samuel E, Johnson Gary R, Reinke Donovan B, Littooy Fred N, Acher Charles W, Ballard David J, Messina Louis M, Gordon Ian L, Chute Edmund P, Krupski William C, Busuttil Steven J, Barone Gary W, Sparks Steven, Graham Linda M, Rapp Joseph H, Makaroun Michel S, Moneta Gregory L, Cambria Robert A, Makhoul Raymond G, Eton Darwin, Ansel Howard J, Freischlag Julie A, Bandyk Dennis, Aneurysm Detection, Management Veterans Affairs Cooperative Study, and Group (2002) Immediate repair compared with surveillance of small abdominal aortic aneurysms. The New England journal of medicine 346(19), 1437–44 [PubMed: 12000813] | Multivariate analysis was not performed to assess risk factors associated with aneurysm expansion or rupture. |
| 18 | Lederle F A, Noorbaloochi S, Nugent S, Taylor B C, Grill J P, Kohler T R, and Cole L (2015) Multicentre study of abdominal aortic aneurysm measurement and enlargement. The British journal of surgery 102(12), 1480–7 [PubMed: 26331269] | Case-control: patients with AAA growth were identified via medical records and imaging reports, and were subsequently assessed for risk factors. |
| 19 | Louridas G, Reilly K, and Perry M O (1990) The role of the aortic aneurysm diameter aortic diameter ratio in predicting the risk of rupture. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde 78(11), 642–3 [PubMed: 2251606] | The study had a sample size of less than 500 participants (n=130). |
| 20 | Matthews E O, Rowbotham S E, Moxon J V, Jones R E, Vega de Ceniga, M, and Golledge J (2017) Meta-analysis of the association between peripheral artery disease and growth of abdominal aortic aneurysms. The British journal of surgery 104(13), 1765–1774 [PubMed: 29044481] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 21 | McCarthy R J, Shaw E, Whyman M R, Earnshaw J J, Poskitt K R, and Heather B P (2003) Recommendations for screening intervals for small aortic aneurysms. The British journal of surgery 90(7), 821–6 [PubMed: 12854107] | Multivariate analysis was not performed to assess risk factors associated with aneurysm expansion or rupture. |
| 22 | Mofidi R, Goldie V J, Kelman J, Dawson A R. W, Murie J A, and Chalmers R T. A (2007) Influence of sex on expansion rate of abdominal aortic aneurysms. The British journal of surgery 94(3), 310–4 [PubMed: 17262754] | Multivariate analysis was not performed to assess risk factors associated with aneurysm expansion or rupture. |
| 23 | Newby D (2017) Aortic Wall Inflammation Predicts Abdominal Aortic Aneurysm Expansion, Rupture and Need for Surgical Repair. Circulation (no pagination), [PMC free article: PMC5571881] [PubMed: 28720724] | The study had a sample size of less than 500 participants (n=342). |
| 24 | Parkinson Fran, Ferguson Stuart, Lewis Peter, Williams Ian M, Twine Christopher P, South East Wales Vascular, and Network (2015) Rupture rates of untreated large abdominal aortic aneurysms in patients unfit for elective repair. Journal of vascular surgery 61(6), 1606–12 [PubMed: 25661721] | Systematic review including cohort studies and RCTs; none of which had sample sizes of 500 participants, or larger. |
| 25 | Powell Janet T, Brown Louise C, Greenhalgh Roger M, and Thompson Simon G (2008) The rupture rate of large abdominal aortic aneurysms: is this modified by anatomical suitability for endovascular repair?. Annals of surgery 247(1), 173–9 [PubMed: 18156938] | Systematic review including studies which employed prospective and retrospective study designs; none of which had sample sizes of 500 participants, or larger. |
| 26 | Powell J T, Gotensparre S M, Sweeting M J, Brown L C, Fowkes F G. R, and Thompson S G (2011) Rupture rates of small abdominal aortic aneurysms: A systematic review of the literature. European Journal of Vascular and Endovascular Surgery 41(1), 2–10 [PubMed: 20952216] | Systematic review including studies which employed prospective and retrospective study designs. Individual studies were assessed to determine whether they met inclusion criteria for this review question. |
| 27 | Scott R Alan P, Kim Lois G, Ashton Hilary A, Multi-centre Aneurysm Screening Study, and Group (2005) Assessment of the criteria for elective surgery in screen-detected abdominal aortic aneurysms. Journal of medical screening 12(3), 150–4 [PubMed: 16156946] | Multivariate analysis was not performed to assess risk factors associated with aneurysm expansion or rupture. Instead, multivariate regression was performed to investigate the effect of aortic diameter and patient age on the decision to return a patient for surveillance (versus elective surgery). |
| 28 | Sweeting M J, Thompson S G, Brown L C, Powell J T, and collaborators Rescan (2012) Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. The British journal of surgery 99(5), 655–65 [PubMed: 22389113] | Duplication of data from the Health Technology Assessment by Thompson et al. (2013) which has been included in this review. |
| 29 | Takagi Hisato, Umemoto Takuya, and Group Alice (2016) Coronary artery disease and abdominal aortic aneurysm growth. Vascular medicine (London, and England) 21(3), 199–208 [PubMed: 26842623] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 30 | Takagi Hisato, Umemoto Takuya, and Group Alice (2016) Association of peripheral artery disease with abdominal aortic aneurysm growth. Journal of vascular surgery 64(2), 506–513 [PubMed: 27316409] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 31 | Takagi Hisato, Umemoto Takuya, and Group Alice (2016) Negative association of diabetes with rupture of abdominal aortic aneurysm. Diabetes & vascular disease research 13(5), 341–7 [PubMed: 27334484] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
| 32 | Takagi H, and Umemoto T (2017) Association of chronic obstructive pulmonary, coronary artery, or peripheral artery disease with abdominal aortic aneurysm rupture. International Angiology 36(4), 322–331 [PubMed: 27606806] | Systematic review of case-controls. |
| 33 | Takagi Hisato, and Umemoto Takuya (2016) The association between body mass index and abdominal aortic aneurysm growth: a systematic review. VASA. Zeitschrift fur Gefasskrankheiten 45(2), 119–24 [PubMed: 27058797] | Systematic review including studies which employed various study designs (including case-controls, screening programs and cohort studies). Individual studies were assessed to determine if they met inclusion criteria for this review question. |
| 34 | The Propranolol Aneurysm Trial Investigators (2002) Propranolol for small abdominal aortic aneurysms: results of a randomized trial. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, and North American Chapter 35(1), 72–79 | Study assessed whether propranolol reduced aneurysm growth rates. |
| 35 | Thompson S G, Ashton H A, Gao L, Buxton M J, Scott R A. P, Multicentre Aneurysm Screening Study, and Group (2012) Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. The British journal of surgery 99(12), 1649–56 [PMC free article: PMC3569614] [PubMed: 23034729] | Study did not assess risk factors associated with aneurysm rupture or growth. Instead, unadjusted Cox regression was used to compare deaths related to abdominal aortic aneurysm and all-cause mortality between individuals in two randomised groups. |
| 36 | Thompson S G, Ashton H A, Gao L, Scott R A. P, Multicentre Aneurysm Screening Study, and Group (2009) Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ (Clinical research ed.) 338, b2307 [PMC free article: PMC3272658] [PubMed: 19553269] | Study did not assess risk factors associated with aneurysm rupture or growth. Instead, unadjusted Cox regression was used to compare deaths related to abdominal aortic aneurysm and all-cause mortality between individuals in two randomised groups. |
| 37 | Thompson A R, Golledge J, Cooper J A, Hafez H, Norman P E, and Humphries S E (2009) Sequence variant on 9p21 is associated with the presence of abdominal aortic aneurysm disease but does not have an impact on aneurysmal expansion. European Journal of Human Genetics 17(3), 391–394 [PMC free article: PMC2986176] [PubMed: 18854858] | Case-control: patients with AAA growth were identified and were compared with controls to assess whether they had a variant of the 9p21 chromosome. |
| 38 | Thompson Andrew, Cooper Jackie A, Fabricius Michael, Humphries Steve E, Ashton Hilary A, and Hafez Hany (2010) An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. Journal of vascular surgery 52(1), 55–61.e2 [PubMed: 20620765] | No data of interest: aneurysm growth rates were calculated by linear regression analysis. This is a different outcome to that specified in the review protocol: “radiological diagnosis of abdominal aortic aneurysm expansion; single test within a study” |
| 39 | Urbonavicius S, Urbonaviciene G, Honore B, Henneberg E W, Vorum H, and Lindholt J S (2008) Potential circulating biomarkers for abdominal aortic aneurysm expansion and rupture--a systematic review. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 36(3), 273–2 [PubMed: 18639476] | Systematic review which aimed to summarise evidence on various systemic biomarkers for aneurysm rupture or expansion. Individual studies were assessed to determine whether they met inclusion criteria for this NICE review. |
| 40 | Vande Geest, Jonathan P, Wang David H. J, Wisniewski Stephen R, Makaroun Michel S, and Vorp David A (2006) Towards a noninvasive method for determination of patient-specific wall strength distribution in abdominal aortic aneurysms. Annals of biomedical engineering 34(7), 1098–106 [PubMed: 16786395] | Study did not assess risk factors associated with aneurysm growth or rupture. Instead investigators developed a statistical model for estimating AAA wall strength. |
| 41 | Vardulaki K A, Prevost T C, Walker N M, Day N E, Wilmink A B. M, Quick C R. G, Ashton H A, and Scott R A. P (1998) Growth rates and risk of rupture of abdominal aortic aneurysms. British Journal of Surgery 85(12), 1674–1680 [PubMed: 9876073] | Secondary data analysis of 2 population-based screening programmes. Multivariate analysis was not performed to assess risk factors associated with aneurysm growth or rupture. |
| 42 | Vardulaki K A, Walker N M, Day N E, Duffy S W, Ashton H A, and Scott R A. P (2000) Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. British Journal of Surgery 87(2), 195–200 [PubMed: 10671927] | Study employed a mixed methods design. Population-based screening (a cross-sectional approach) was used to assess the prevalence of AAAs. A prospective observational approach was used to assess aneurysm growth rates; however, multivariate analysis-regression was not performed. |
| 43 | Wanhainen Anders, Mani Kevin, Vorkapic Emina, De Basso, Rachel, Bjorck Martin, Lanne Toste, and Wagsater Dick (2017) Screening of circulating microRNA biomarkers for prevalence of abdominal aortic aneurysm and aneurysm growth. Atherosclerosis 256, 82–88 [PubMed: 27993388] | The study had a sample size of less than 500 participants (n=217). |
| 44 | Xiong Jiang, Wu Zhongyin, Chen Chen, Wei Yingqi, and Guo Wei (2016) Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: A meta-analysis. International journal of cardiology 221, 484–95 [PubMed: 27414727] | Systematic review which included studies that employed multiple study designs. Individual studies were assessed to establish if they met criteria for inclusion in this NICE review. |
Economic studies
No full text papers were retrieved. All studies were excluded at review of titles and abstracts.
Appendix H. Glossary
- Abdominal Aortic Aneurysm (AAA)
A localised bulge in the abdominal aorta (the major blood vessel that supplies blood to the lower half of the body including the abdomen, pelvis and lower limbs) caused by weakening of the aortic wall. It is defined as an aortic diameter greater than 3 cm or a diameter more than 50% larger than the normal width of a healthy aorta. The clinical relevance of AAA is that the condition may lead to a life threatening rupture of the affected artery. Abdominal aortic aneurysms are generally characterised by their shape, size and cause:
- Infrarenal AAA: an aneurysm located in the lower segment of the abdominal aorta below the kidneys.
- Juxtarenal AAA: a type of infrarenal aneurysm that extends to, and sometimes, includes the lower margin of renal artery origins.
- Suprarenal AAA: an aneurysm involving the aorta below the diaphragm and above the renal arteries involving some or all of the visceral aortic segment and hence the origins of the renal, superior mesenteric, and celiac arteries, it may extend down to the aortic bifurcation.
- Abdominal compartment syndrome
Abdominal compartment syndrome occurs when the pressure within the abdominal cavity increases above 20 mm Hg (intra-abdominal hypertension). In the context of a ruptured AAA this is due to the mass effect of a volume of blood within or behind the abdominal cavity. The increased abdominal pressure reduces blood flow to abdominal organs and impairs pulmonary, cardiovascular, renal, and gastro-intestinal function. This can cause multiple organ dysfunction and eventually lead to death.
- Cardiopulmonary exercise testing
Cardiopulmonary Exercise Testing (CPET, sometimes also called CPX testing) is a non-invasive approach used to assess how the body performs before and during exercise. During CPET, the patient performs exercise on a stationary bicycle while breathing through a mouthpiece. Each breath is measured to assess the performance of the lungs and cardiovascular system. A heart tracing device (Electrocardiogram) will also record the hearts electrical activity before, during and after exercise.
- Device migration
Migration can occur after device implantation when there is any movement or displacement of a stent-graft from its original position relative to the aorta or renal arteries. The risk of migration increases with time and can result in the loss of device fixation. Device migration may not need further treatment but should be monitored as it can lead to complications such as aneurysm rupture or endoleak.
- Endoleak
An endoleak is the persistence of blood flow outside an endovascular stent - graft but within the aneurysm sac in which the graft is placed.
- Type I – Perigraft (at the proximal or distal seal zones): This form of endoleak is caused by blood flowing into the aneurysm because of an incomplete or ineffective seal at either end of an endograft. The blood flow creates pressure within the sac and significantly increases the risk of sac enlargement and rupture. As a result, Type I endoleaks typically require urgent attention.
- Type II – Retrograde or collateral (mesenteric, lumbar, renal accessory): These endoleaks are the most common type of endoleak. They occur when blood bleeds into the sac from small side branches of the aorta. They are generally considered benign because they are usually at low pressure and tend to resolve spontaneously over time without any need for intervention. Treatment of the endoleak is indicated if the aneurysm sac continues to expand.
- Type III – Midgraft (fabric tear, graft dislocation, graft disintegration): These endoleaks occur when blood flows into the aneurysm sac through defects in the endograft (such as graft fractures, misaligned graft joints and holes in the graft fabric). Similarly to Type I endoleak, a Type III endoleak results in systemic blood pressure within the aneurysm sac that increases the risk of rupture. Therefore, Type III endoleaks typically require urgent attention.
- Type IV– Graft porosity: These endoleaks often occur soon after AAA repair and are associated with the porosity of certain graft materials. They are caused by blood flowing through the graft fabric into the aneurysm sac. They do not usually require treatment and tend to resolve within a few days of graft placement.
- Type V – Endotension: A Type V endoleak is a phenomenon in which there is continued sac expansion without radiographic evidence of a leak site. It is a poorly understood abnormality. One theory that it is caused by pulsation of the graft wall, with transmission of the pulse wave through the aneurysm sac to the native aneurysm wall. Alternatively it may be due to intermittent leaks which are not apparent at imaging. It can be difficult to identify and treat any cause.
- Endovascular aneurysm repair
Endovascular aneurysm repair (EVAR) is a technique that involves placing a stent –graft prosthesis within an aneurysm. The stent-graft is inserted through a small incision in the femoral artery in the groin, then delivered to the site of the aneurysm using catheters and guidewires and placed in position under X-ray guidance.
- Conventional EVAR refers to placement of an endovascular stent graft in an AAA where the anatomy of the aneurysm is such that the ‘instructions for use’ of that particular device are adhered to. Instructions for use define tolerances for AAA anatomy that the device manufacturer considers appropriate for that device. Common limitations on AAA anatomy are infrarenal neck length (usually >10mm), diameter (usually ≤30mm) and neck angle relative to the main body of the AAA
- Complex EVAR refers to a number of endovascular strategies that have been developed to address the challenges of aortic proximal neck fixation associated with complicated aneurysm anatomies like those seen in juxtarenal and suprarenal AAAs. These strategies include using conventional infrarenal aortic stent grafts outside their ‘instructions for use’, using physician-modified endografts, utilisation of customised fenestrated endografts, and employing snorkel or chimney approaches with parallel covered stents.
- Goal directed therapy
Goal directed therapy refers to a method of fluid administration that relies on minimally invasive cardiac output monitoring to tailor fluid administration to a maximal cardiac output or other reliable markers of cardiac function such as stroke volume variation or pulse pressure variation.
- Post processing technique
For the purpose of this review, a post-processing technique refers to a software package that is used to augment imaging obtained from CT scans, (which are conventionally presented as axial images), to provide additional 2- or 3-dimensional imaging and data relating to an aneurysm’s, size, position and anatomy.
- Permissive hypotension
Permissive hypotension (also known as hypotensive resuscitation and restrictive volume resuscitation) is a method of fluid administration commonly used in people with haemorrhage after trauma. The basic principle of the technique is to maintain haemostasis (the stopping of blood flow) by keeping a person’s blood pressure within a lower than normal range. In theory, a lower blood pressure means that blood loss will be slower, and more easily controlled by the pressure of internal self-tamponade and clot formation.
- Remote ischemic preconditioning
Remote ischemic preconditioning is a procedure that aims to reduce damage (ischaemic injury) that may occur from a restriction in the blood supply to tissues during surgery. The technique aims to trigger the body’s natural protective functions. It is sometimes performed before surgery and involves repeated, temporary cessation of blood flow to a limb to create ischemia (lack of oxygen and glucose) in the tissue. In theory, this “conditioning” activates physiological pathways that render the heart muscle resistant to subsequent prolonged periods of ischaemia.
- Tranexamic acid
Tranexamic acid is an antifibrinolytic agent (medication that promotes blood clotting) that can be used to prevent, stop or reduce unwanted bleeding. It is often used to reduce the need for blood transfusion in adults having surgery, in trauma and in massive obstetric haemorrhage.
Final
Methods, evidence and recommendations
This evidence review was developed by the NICE Guideline Updates Team
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Endovascular aneurysm repair at 5 years: Does aneurysm diameter predict outcome?[J Vasc Surg. 2006]Endovascular aneurysm repair at 5 years: Does aneurysm diameter predict outcome?Zarins CK, Crabtree T, Bloch DA, Arko FR, Ouriel K, White RA. J Vasc Surg. 2006 Nov; 44(5):920-29; discussion 929-31.
- Incidence, follow-up, and outcomes of incidental abdominal aortic aneurysms.[J Vasc Surg. 2010]Incidence, follow-up, and outcomes of incidental abdominal aortic aneurysms.van Walraven C, Wong J, Morant K, Jennings A, Jetty P, Forster AJ. J Vasc Surg. 2010 Aug; 52(2):282-9.e1-2. Epub 2010 Jun 11.
- Variation in the elective management of small abdominal aortic aneurysms and physician practice patterns.[J Vasc Surg. 2019]Variation in the elective management of small abdominal aortic aneurysms and physician practice patterns.Davis FM, Jerzal E, Albright J, Kazmers A, Monsour A, Bove P, Henke PK. J Vasc Surg. 2019 Oct; 70(4):1089-1098. Epub 2019 Mar 2.
- Review Abdominal Aortic Aneurysms and Risk Factors for Adverse Events.[Cardiol Rev. 2016]Review Abdominal Aortic Aneurysms and Risk Factors for Adverse Events.Ahmed R, Ghoorah K, Kunadian V. Cardiol Rev. 2016 Mar-Apr; 24(2):88-93.
- Review The - Not So - Solid 5.5 cm Threshold for Abdominal Aortic Aneurysm Repair: Facts, Misinterpretations, and Future Directions.[Front Surg. 2016]Review The - Not So - Solid 5.5 cm Threshold for Abdominal Aortic Aneurysm Repair: Facts, Misinterpretations, and Future Directions.Kontopodis N, Pantidis D, Dedes A, Daskalakis N, Ioannou CV. Front Surg. 2016; 3:1. Epub 2016 Jan 25.
- Risk factors associated with abdominal aortic aneurysm growth or ruptureRisk factors associated with abdominal aortic aneurysm growth or rupture
Your browsing activity is empty.
Activity recording is turned off.
See more...