NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Bladder Cancer: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2015 Feb. (NICE Guideline, No. 2.)
A.1. Background
Non-muscle invasive bladder cancer (NMIBC) tumours can be surgically removed using transurethral resection of bladder tumour (TURBT). However, these tumours are likely to return on the urothelium. This high risk of recurrence is a problem for patients because it raises the concern that the cancer will progress and so the patient will need to undergo further treatment (either another TURBT or diathermy).
The risk of recurrence can be reduced by the administration of chemotherapy medication into the bladder (intravesical chemotherapy), which can be done immediately, or shortly after TURBT. However, there are disadvantages to using intravesical chemotherapy as it is associated with some side effects and comes at an additional cost.
There is currently debate about which NMIBC patients should be treated with intravesical chemotherapy, including whether patients with small or very small tumours should be treated.
A.2. Aim of analysis
To estimate the cost-effectiveness of a single instillation of intravesical chemotherapy in addition to TURBT in comparison to TURBT alone in patients with NMIBC.
A.3. Existing Economic Evidence
A systematic literature review was performed to assess the current economic literature in this area. The review identified 515 possibly relevant economic papers relating to bladder cancer. Of these, 50 full papers were obtained for appraisal. One paper was identified that related to the topic at hand; Green et al. 2013.
In the study, the authors utilised a decision analytic model to estimate the cost-effectiveness of a single instillation of chemotherapy given after a TURBT, with effectiveness estimated in terms of quality adjusted life years (QALYs). Thus, the study met the inclusion criteria as it was a relevant cost-utility analysis.
Green et al. 2013 sought to examine the cost-effectiveness of fulguration compared to TURBTs with and without perioperative intravesical chemotherapy in patients with low risk NMIBC. The authors concluded that fulguration without perioperative intravesical chemotherapy was the most cost-effective strategy for treating low-risk NMIBC. However, unusually, the authors based this conclusion upon individual cost-effectiveness calculations rather than the standard incremental calculations. When following the more standard cost-effectiveness methodology using incremental cost-effectiveness ratios (ICERs), it appears that perioperative intravesical chemotherapy plus fulguration would be the most cost-effective strategy. This strategy has an ICER of $4,169 per QALY, which is likely to fall below the cost-effectiveness thresholdk. The authors also conducted sensitivity analysis, which showed that the effectiveness of perioperative intravesical chemotherapy and the cost of TURBT were likely to be key drivers of the cost-effectiveness result.
However, Green et al. 2013 can only be deemed partially applicable to the decision problem this guideline seeks to address. The analysis considered the US healthcare system, which differs substantially from the UK system. In addition, the study only partially addressed our decision problem as it only evaluated cost-effectiveness in low risk NMIBC patients, whereas we are interested in all NMIBC risk groups. Furthermore, some potential limitations were identified in the analyses with uncertainty over some of the input values that were utilised and some concerns over the interpretation of the results.
Overall, it was considered that the current economic literature was partially useful but further analysis would be required to robustly estimate the cost-effectiveness. It should also be noted that the existing economic literature was useful for informing the development of our own economic model.
A.4. De Novo Economic Model
Since the current economic literature didn't adequately address the decision probleml, a de novo economic evaluation was undertaken to assess cost-effectiveness. A Markov decision model was developed using Microsoft Excel. The basic model structure is shown in Figure 39.
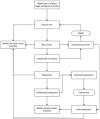
Figure 39
Basic model structure.
The patient enters the model in a ‘disease free’ state following an initial transurethral resection of the bladder tumour (TURBT) with or without a single instillation of chemotherapy (depending upon modelled treatment arm). At each 3-monthly model cycle the patient may experience a bladder cancer recurrence. If the recurrence is detected, the patient will undergo a further TURBT (or fulguration of the tumour) and return to a disease free state. However, if the recurrence is not detected, then the patient will be at risk of progression and will have to undergo further treatment once this progression is eventually detected (cystectomy and possibly neo-adjuvant chemotherapy). The patient may also die from bladder cancer related mortality after experiencing progression and may die from other cause mortality from any health state.
Estimated total costs and quality adjusted life years (QALYs) are collected over the modelled 10 year time horizon for each follow-up strategy. The total costs will include all costs associated with initial treatment, surveillance, further treatment and management and are described in more detail in the cost section of this report. QALYs are calculated by multiplying the life years that patients spend in each health state by the associated quality of life (QoL) weighting, which represent the patient's valuation of their health state. QALYs and QoL values are discussed in more detail in later sections of the report.
Future costs and benefits were discounted at a rate of 3.5% per year as recommended by NICE.
A.4.1. Natural history of disease - risk of recurrence and progression
The risk of recurrence and progression in patients with NMIBC was estimated using risk equations based on an analysis of 2,596 patients from seven EORTCm trials (Sylvester et al. 2006). Patients are ‘scored’ based on a number of risk factors, such as number of tumours, tumour size, prior recurrence rate, T category, presence of CIS and grade. The scores associated with the risk factors are shown in table 158.
Table 158
EORTC scores associated with risk factors.
The overall recurrence and progression risk scores computed from the above table have an associated one year and five year risk of recurrence and progression. The one year and five year risks of recurrence and progression are shown in tables 159 and 160.
Table 159
EORTC recurrence probabilities for recurrence score groups.
Table 160
EORTC recurrence probabilities for recurrence score groups.
For the purposes of the economic model, it was necessary to convert these five year and one year risks into 3-monthly risks to match the model cycle length used. In order to capture the higher risk of recurrence and progression in the first year, separate 3 monthly risks were used in the first year and in subsequent years (based on the one year risk and five year risk, respectively).
The EORTC risk equations consider recurrence and progression independently but, for the purposes of this analysis, a relationship between recurrence and progression was assumed. This relationship was estimated from the EORTC data by calculating the probability of progression given recurrence in each of the risk groups.
Note that the risk group classifications used in clinical practice do not translate neatly to any one set of recurrence and progression risk. There are multiple permutations of recurrence and progression risk that are possible in each of the clinical risk groups as shown in table 161
Table 161
Recurrence and progression risk scores for each risk group variant.
In the base case analysis, the recurrence and progression risk combinations that are likely to best reflect the majority of patients within each clinical risk group were selected. Variations in the recurrence and progression score are assessed in sensitivity analysis. Table 162 shows the three monthly risks of recurrence, progression and progression given recurrence applied for each of the risk groups in the base case analysis.
Table 162
Three monthly recurrence and progression risk applied in the model.
Note that since the modelled time horizon of 10 years exceeds the predicted risk estimates from the EORTC trials (5 years), it was also necessary to make some assumptions about the risk profile of patients in years 5-10. In the base case, it was assumed that the estimated subsequent year rate (i.e. years 2-5) would be maintained in years 6-10 except in the case of low-risk patients in whom it was assumed that risk would be zero after 5 years (reflecting the clinical practice of discharging low-risk patients from follow-up protocols after 5 years).
It should also be noted that, in accordance with the EORTC risk scores, modelled low risk and intermediate risk patients that experience a recurrence will thereafter be subject to the higher risk of recurrence and progression associated with the risk level above. For example, low risk patients that have a recurrence are thereafter subject to the recurrence and progression risk scores associated with intermediate risk patients. However, there are nuances to this increased risk which cannot be accurately captured in the model as it does not model changes in tumour characteristics directly. For example, it is not always the case that a recurrence would place an intermediate risk patient into a higher risk group as it would depend on the patient's initial score.
A.4.2. Key clinical effectiveness data
A.4.2.1. Effectiveness of single instillation of chemotherapy
The key effectiveness data utilised in the model is the reduction in recurrence risk associated with a single instillation of intravesical chemotherapy following a TURBT. According to the systematic review of the clinical evidence, the use of a single instillation of intravesical chemotherapy in addition to TURBT has a relative risk of 0.67 in comparison to TURBT alone. This treatment effect was assumed to last for two years reflecting the general consensus around its possible duration. Thereafter, the risk of recurrence was assumed to be equal to that with TURBT only. In addition, the treatment effect is not assumed to affect future recurrences if the patient has a recurrence during the two years after the single chemotherapy instillation.
Note that the single instillation of chemotherapy does not directly reduce the rates of progression. This is in line with the evidence base, which suggests that there is no treatment effect on the rates of progression. However, it should be noted that because of the model structure, a lower rate of recurrences would lead to a lower rate of progression because progression is dependent upon recurrence. Therefore, an indirect treatment effect on progression is essentially included in the model. This assumption is relaxed in a sensitivity analysis where the rates of recurrence and progression are assumed to be independent.
A.4.2.2. Treatment related morbidity
No comparative data on morbidity were identified in the systematic review of the clinical evidence. However a meta-analysis (Sylvester 2004) of seven trials suggested that mild irritative bladder symptoms (including dysuria, frequency and macroscopic haematuria) would occur in approximately 10% of patients treated with a single post-operative dose of intravesical chemotherapy. In addition, allergic skin reactions were reported in 1-3% of patients in two studies.
Since no data were available on morbidity in patients treated with TURBT, it was conservatively assumed that 5% would have irritative bladder symptoms and there would be no skin reactions. The treatment related morbidity rates applied in the model are shown in table 163.
Table 163
Treatment related morbidity rates applied in the model.
A.4.2.3. Follow-up test diagnostic accuracy data
The diagnostic accuracy data for flexible cystoscopy (sensitivity and specificity) that was applied in the model are shown in table 164. The data were sourced from the systematic review of the clinical evidence conducted for this guideline, with most data being sourced from a systematic review by Mowatt et al. 2010.
Table 164
Diagnostic accuracy of flexible cystoscopy.
A.4.2.4. Bladder cancer related mortality
Bladder cancer related mortality rates were estimated using data identified in the systematic review of the clinical evidence. A systematic review by Van den Bosch et al. 2011 was utilised, which estimated survival rates in high risk NMIBC patients that have progressed to MIBC. In the report, the assumption was made that patient that die from bladder cancer must first progress to muscle invasive disease and then to metastatic cancer. The same assumption was made in the economic model.
Van den Bosch et al. 2011 reported a disease specific survival rate of 35% in NMIBC patients that have undergone a cystectomy and experienced progression over a median follow-up time of 48-123 months. This was converted to an estimated 3 monthly disease specific mortality rate of 3.6% in patients that have progressed to MIBC in the model. In NMIBC patients, the estimated disease specific mortality rate applied in the model was 0.5%. This lower rate reflects that patients would have to first progress to MIBC before dying of bladder cancer (based on the 21.3% progression rate reported in Van den Bosch et al. 2011).
It should also be noted that patients with undetected progression are assumed to be subject to the mortality rate associated with MIBC.
A.4.2.5. Other cause mortality
Death from other causes was captured using 2009-2011 life tables for England and Wales from the office of national statistics (ONS). These life tables give an estimate of the annual probability of death given a person's age and gender. In the base case, the model was run with an average age of 60 and was assumed to be 50% female (note that these parameters only influence other cause mortality in the model). The annual probabilities of other mortality were converted to three-monthly probabilities for use in the model.
A.4.3. Cost data
Modelled patients accrue costs associated with any treatment, monitoring or management strategy that they are undergoing. The costs considered in the model reflect the perspective of the analysis, thus only costs that are relevant to the UK NHS & PSS were included. These costs include drug costs, treatment costs and any other resource use that may be required (e.g. GP visit). Where possible, all costs were estimated in 2012-13 prices.
The majority of costs were sourced from NHS reference costs 2012/13 by applying tariffs associated with the appropriate HRG code. Drug costs were calculated using dosages from the British National Formulary (BNF) and unit cost information from the electronic market information tool (eMit). Where unit costs for drugs were not available from eMit, prices from the BNF were used. Resource use and cost information were obtained from the Personal Social Services Research Unit (PSSRU) and the advice of the GDG.
Costs for each aspect of the treatment pathway are detailed in the relevant sections below.
A.4.3.1. Cost of initial TURBT and single instillation of chemotherapy
The cost of a TURBT was estimated to be £1,267.59, which was based on the cost of an ‘Intermediate Endoscopic Bladder Procedure’ from NHS reference costs. The cost of delivering the single instillation of chemotherapy is dependent upon the setting in which it is given; in theatre or ward. If it is given in the theatre then the delivery cost will be the cost of using the Mito-In system (estimated to be £4.00) and the surgical consultant time (£4.67). Whereas, if it is delivered by a nurse then the costs incurred will be the cost of an advanced nurse consultation (includes clinical nurse specialist), the cost of the Mito-in system and the additional costs of gloves, syringes and other sundries (estimated to be around £6.50) (Table 165).
Table 165
Initial TURBT and single instillation costs.
In the base case it was assumed that intravesical chemotherapy was delivered immediately after surgery in theatre in 25% of cases with the remaining 75% delivered later by a nurse.
A.4.3.2. Adverse event costs
The GDG felt that, in most instances, there would not be any additional costs associated with the treatment related morbidity that could be experienced as no treatment would be administered. However, it was thought that antihistamines and antibiotics were sometimes used to treat a skin rash and irritative bladder symptoms, respectively. Thus, we conservatively assumed (i.e. biasing against the intervention being tested) that all irritative bladder symptoms and skin reactions would be treated, with the drugs being prescribed after a consultation with the urologist (cost of ‘Non-admitted face to face attendence, follow-up in Urology’ from NHS reference costs). The treatment related morbidity costs applied in the model are detailed in table 166.
Table 166
Adverse event costs.
A.4.3.3. Follow-up costs
Post resection follow-up
Following the initial resection, patients were assumed to be followed up in the manner that best reflects current practice. However, there is variation in current practice and the strategy most commonly used is not definitively known. The GDG adjudged that the strategies described by Hall et al. 1994 best reflect current practice and so these were used in the analysis. The strategies are summarised in table 167 for each risk group:
Table 167
Current practice follow-up strategies.
The cost of a flexible cystoscopy applied in the model was £401.88, which was based upon the cost of a “Diagnostic Flexible Cystoscopy, 19 years and over” as a day case procedure from NHS reference costs. However, there is variation in current practice as to whether cystoscopies are coded as an outpatient or day case procedure. Day case procedures were thought to be more common and thus were selected for the base case analysis but the cost associated with flexible cystoscopies given as outpatient procedures (£164.00) was applied in a sensitivity analysis.
The consequences of cystoscopic inaccuracy should also be noted. True negative and false negative results would only incur the cost of the initial investigation itself whereas true positive and false positive results would incur the cost of the initial investigation and the cost of performing a biopsy (‘unnecessarily’ in the case of false positive patients, at which point the error would be realised).
A.4.3.4. Recurrence costs
The costs associated with treating recurrences are shown in table 168.
Table 168
TURBT and diathermy costs used to treat recurrences.
Patients that have a recurrence would need further treatment; either another TURBT or diathermy in assumed proportions of 33% and 67%, respectively. The cost of a TURBT was estimated to be £1,267.59, which was based on the cost of an ‘Intermediate Endoscopic Bladder Procedure’ from NHS reference costs. The cost of diathermy was estimated to be equivalent to the cost of a flexible cystoscopy (£401.88 from NHS reference costs).
A.4.3.5. Further treatment costs
Mitomycin C course
Patients with intermediate risk bladder cancer are assumed to receive a course of Mitomycin C (once weekly for 6 weeks) at a cost of £479.28 (sourced from the BNF). The cost of administering Mitomycin C was obtained from NHS reference costs 2012/13 (‘Introduction of Therapeutic Substance into Bladder’ – LB17Z). In clinical practice, the therapy is either delivered as an outpatient or day case procedure. Thus, a weighted average cost was calculated based on the number of outpatient and day case admissions listed in NHS reference costs (57% were day case and 43% were outpatient). The average weighted cost of delivering Mitomycin C was estimated to be £220.74 per instillation.
In current clinical practice, some low risk patients may receive a course of Mitomycin c following a recurrence. To capture this in the model it was assumed that 50% of low risk patients would receive a course of Mitomycin C after a recurrence. This assumption was informed by the clinical opinion of the GDG.
Bacillus Calmette-Guérin (BCG) therapy
Patients with high risk bladder cancer and initially low and intermediate risk patients that have had multiple recurrences are assumed to receive Bacillus Calmette-Guérin (BCG) therapy. These patients will first receive induction BCG therapy, which consists of six doses of BCG given once a week over a six week period. After a six week off-period, patients that have not had a recurrence or progression will then go onto receive maintenance BCG therapy. This consists of a further three doses given once a week over a three week period at six monthly intervals for a maximum of three years.
Patients that progress to muscle invasive disease while receiving BCG therapy are classed as ‘BCG failures’ and are assumed to undergo a cystectomy. In addition, in an attempt to reflect the clinical practice of classifying high risk recurrences as BCG failures, it has been assumed that a proportion of recurrences in patients receiving BCG therapy would be BCG failures. In high risk patients it is assumed that 50% of patients with a first recurrence and all patients with two recurrences on BCG therapy would be classed as BCG failures. In low and intermediate risk patients it is assumed that 50% of patients with a first or second recurrence and all patients with three recurrences on BCG therapy would be classed as BCG failures.
The cost of the BCG therapy is based on the average cost of ImmuCyst and OncoTICE with costs sourced from the BNF. The cost of delivering BCG was estimated to be £220.74 and was based on the same NHS reference cost codes used for the MMC course (see above).
The costs associated with bladder instillations (Mitomycin c and BCG) are shown in table 169.
Table 169
Intravesical instillation costs – Mitomycin C and BCG courses.
Cystectomy and neo-adjuvant chemotherapy
Patients that progress to muscle invasive disease or experience BCG failure are assumed to undergo a cystectomy. The cost associated with a cystectomy was estimated to be £9,538.29 based on the cost of a ‘Cystectomy with Urinary Diversion and Reconstruction, with CC Score 0-2’ from NHS reference costs.
It was further assumed that 80% of patients undergoing a cystectomy would receive neo-adjuvant chemotherapy. In current clinical practice the majority of patients receiving neoadjuvant chemotherapy receive a regimen of gemcitabine and cisplatin (GemCis) but a minority also receive accelerated MVAC (methotrexate, vinblastine, adriamycin and cisplatin). The proportion of patients receiving each regimen in the model was based on the clinical opinion of the GDG, with 90% receiving GemCis and 10% receiving accelerated MVAC.
Chemotherapy drug costs were estimated using unit costs from the BNF with doses and schedules as recommended by the GDG. Drug doses were estimated using an average body surface area of 1.91m2 for men and 1.71m2 for women as reported in a study by Sacco et al. 2010. In addition to the drug costs, the costs associated with delivering chemotherapy were also captured using tariffs from NHS reference costs, which vary depending upon the complexity of delivering the chemotherapy (principally the time required to deliver the chemotherapy). In the case of accelerated MVAC, patients also receive the G-CSF, Pegylated filgrastim at a cost of £686.38 for a 6mg prefilled syringe.
The costs per cycle of chemotherapy are shown in table 170 for a schedule of GemCis and accelerated MVAC. Patients receiving neoadjuvant chemotherapy are assumed to receive three cycles of chemotherapy as recommended by the GDG.
Table 170
Chemotherapy cost per cycle of GemCis and accelerated MVAC.
Post cystectomy follow-up
Patients that have undergone a cystectomy are assumed to be followed up in the manner reflecting current practice with a combination of urological consultations, urethroscopies, CT scans and blood tests (kidney function and PSA). The patient is assumed to be followed up by the urological consultant at three, six and twelve months and annually thereafter at a cost of £94.11 per consultation based on the cost of a ‘Non-admitted face to face attendence, follow-up in Urology’ from NHS Reference Costs. Urethroscopies are assumed to be used annually at an estimated cost of £672.53, based on the cost associated with a ‘Minor or Intermediate Urethra Procedure, 19 years and over’ as a day case procedure from NHS Reference Costs. CT scans are assumed to be used on a six monthly basis for the first year and annually thereafter at a cost of £83.85 (NHS Reference Costs). Blood tests are assumed to be done on a six monthly basis at an assumed cost of £20.00. The follow-up costs applied in the model are shown in table 171.
Table 171
Post-cystectomy follow-up costs.
Systemic chemotherapy and palliative care
A metastatic bladder cancer state was not explicitly modelled as such. However, it was assumed that patients that die from bladder cancer related mortality after progressing to muscle invasive disease were likely to have developed metastatic disease. Thus, the costs associated with treating metastatic disease as well as the cost of palliative care were applied to these patients.
It was assumed that the patient would have received systemic chemotherapy, which, as was the case in neoadjuvant chemotherapy, was assumed to be either GemCis or accelerated MVAC in assumed proportions of 90% and 10%, respectively. The chemotherapy doses were the same as in the neoadjuvant setting and so the cost per cycle is the same as in the table above for neoadjuvant chemotherapy. However, more cycles of chemotherapy are administered in systemic chemotherapy with patients assumed to receive six cycles of chemotherapy (based on the advice of the GDG).
The cost of palliative care in bladder cancer patients was sourced from a report on deaths from urological cancers in England, 2001-10 by the National End of Life Care Intelligence Network. The palliative care cost was estimated to be £8,502, based on an average length of stay of 11.4 days and an average of 3.1 admissions.
A.4.4. Health-related quality of life data
The model estimates effectiveness in terms of quality adjusted life years (QALYs). QALYs are estimated by combining the life year estimates with utility values (or QOL weights) associated with being in a particular health state. These utility values were identified through a search of the available literature.
There is a paucity of high quality of life (QoL) data available in bladder cancer. In particular, there is a shortage of data on patients with NMIBC with most of the available QoL data focusing on post-cystectomy patients. However, it is recognised that QALYs need to be estimated in order to assess cost-effectiveness using the thresholds employed by NICE (£20,000 - £30,000 per QALY) and thus it is useful to utilise QoL data, even if they are of relatively poor quality. It is however recognised as a limitation of the analysis and the QoL values were subjected to sensitivity analysis to assess how influential they are on the final decision.
For the purposes of this economic evaluation, the following QoL data were utilised (Table 172).
Table 172
Health related quality of life weights.
The baseline QoL for patients undergoing monitoring for bladder cancer recurrence (after an initial TURBT) was estimated to be 0.78. This value was sourced from a HTA by Mowatt et al. 2010.
A decrement was utilised for patients that underwent treatment for a bladder cancer recurrence. This was estimated using a study by Yoshimura et al. 2005 that measured QoL in patients with superficial bladder cancer that underwent TURBT. This study measured quality of life using the Short-Form 36-item survey (SF-36), which is not the measure preferred by NICE. Therefore, a mapping algorithm by Ara et al. 2008 was utilised to convert the SF-36 data into EuroQol 5-dimension (EQ-5D) data (the measure preferred by NICE). Using this methodology, the QoL decrement for a bladder cancer recurrence was estimated to be 0.033 for a primary recurrence and 0.057 for a subsequent recurrence.
QoL values for patients in a post-cystectomy state and a metastatic state with palliative care (0.743 and 0.600, respectively) were sourced from a health economic study by Kulkarni et al. 2007
Note that, in the base case, it was assumed that there would be no further QoL decrements associated with irritative bladder symptoms or skin reactions. This assumption was made after discussion with the GDG and, in particular, the patient representatives, who felt that the QoL impact of these side effects would be negligible when considering the QoL decrement associated with TURBTs themselves. However, this assumption was relaxed in sensitivity analysis where QoL decrements were applied for treatment-related adverse events.
A.4.5. Sensitivity analysis
To estimate uncertainty and determine the key drivers of the model, a series of one-way sensitivity analysis were conducted. One-way sensitivity analysis involves changing one input parameter, re-running the model and recording and interpreting the new cost-effectiveness result.
To further estimate uncertainty in the model, probabilistic sensitivity analysis was performed. Probabilistic sensitivity analysis involves running a series of simulations where the values of the model's input parameters are randomly sampled from a distribution around their mean value. This analysis is useful for assessing the uncertainty around all parameter values simultaneously.
The standard errors, distribution type and distribution parameters (alpha and beta values) used to inform the distributions used in the probabilistic sensitivity analysis are shown in each of the input tables in this report. Where possible, the PSA distributions were informed by the standard deviations or standard errors reported in the study or data source. Where data on uncertainty were not available, the distribution parameters were estimated by assuming that the upper and lower quartiles were equal to ±50% of the mean value.
Note that, in general, gamma distributions were used for cost inputs, beta distributions were used for utility values and probabilities, dirichlect distributions were used for conditional variables and normal distributions were used for all other variables.
A.4.6. Results
The results of the economic model are presented as expected costs and QALYs for intervention along with an incremental cost-effectiveness ratio (ICER) for each comparison. The ICER is used to measure the cost-effectiveness of one intervention over another; it is calculated as shown in figure 40.
Figure 40
Calculation of the incremental cost-effectiveness ratio (ICER).
It can be seen that by dividing the difference in costs of each intervention by the difference in benefits (in QALY terms), a cost per QALY can be calculated for each comparison. NICE typically has a threshold of £20,000 for one additional QALY gained. Thus, an intervention with ICER < £20,000 can usually be considered cost-effective. Interventions with ICER values above £30,000 are not typically considered cost-effective. For ICER values between £20,000 and £30,000, an intervention may be considered cost-effective if it is associated with significant benefits.
The model was run over a time horizon of ten years as this was expected to be the time period over which the outcomes were most likely to differ for patients undergoing each of the follow-up strategies.
A.4.6.1. Base case results
The base case results of the analysis are presented in table 173 for patients in each risk category. It can be seen that, in every risk category, a strategy of TURBT plus a single instillation of chemotherapy is more effective than a strategy of TURBT alone.
Table 173
Base case results of the model.
In the case of low and intermediate risk patients, it can also be seen that the addition of a single instillation of chemotherapy is cost saving over the modelled time horizon. This shows that the initial additional costs associated with the single chemotherapy instillation are outweighed by the cost savings associated with a reduction in recurrences (recurrence reductions of 17% and 10% were estimated over the modelled time horizon in the low and intermediate risk groups, respectively). Therefore in low and intermediate risk patients, a single instillation of chemotherapy can be considered dominant i.e. more effective and cost saving.
However, in the case of high risk patients, it can be seen that this is not the case. In high risk patients, the single instillation of chemotherapy is more costly than TURBT alone, suggesting that the potential cost savings are not as large in this group. However, it can also be seen that the addition of a single chemotherapy instillation provides an additional QALY at a cost of £6,432 and thus would be considered cost-effective using the NICE threshold (i.e. <£20,000 per QALY).
A.4.6.2. Risk score variants
As mentioned in an earlier section of the report, the EORTC risk equations suggest that multiple permutations of recurrence and progression risk are possible within each clinical risk group. For the base case analysis (above) the recurrence and progression risk combinations that were thought to best reflect the majority of patients were used. Table 174 shows the cost-effectiveness results using alternative combinations of recurrence and progression risk for low, intermediate and high risk patients.
Table 174
Cost-effectiveness results using variants on the clinical risk groups.
It can be seen that, despite changes in the cost, QALY and ICER values, the conclusions regarding cost-effectiveness are unchanged from the base case analysis. In low and intermediate risk patients, TURBT plus a single instillation of chemotherapy is still dominant i.e. more effective and cost saving. In high risk patients, TURBT plus a single instillation of chemotherapy is still more effective and expensive than TURBT alone and it remains cost-effective in all risk variants.
A.4.7. One-way sensitivity analysis
Table 175 shows the results of a range of one-way sensitivity analyses that were conducted.
Table 175
One-way sensitivity analysis results.
Table 175 shows that the conclusion of the model is insensitive to changes in the input parameters over plausible ranges i.e. TURBT plus a single instillation of chemotherapy remains cost-effective in the all the analyses across all the risk groups.
The variations in the treatment effect duration are perhaps particularly notable as this is one of the uncertainties around the effectiveness of the single instillation of chemotherapy. The analysis shows, unsurprisingly, that the intervention is less cost-effective when the treatment effect duration is decreased. However, crucially, the single instillation of chemotherapy remains cost-effective in all analyses, even when making very pessimistic assumptions about the likely treatment effect duration (i.e. even when assuming that the chemotherapy instillation only reduces recurrences in the first 3 months after administration).
A.4.8. Costing analysis
In addition to the core cost-utility analysis, the GDG were also interested in a cost analysis comparing the cost of delivering the single instillation of chemotherapy on the ward against the cost of delivering it in theatre. Table 176 shows the cost estimations for each approach.
Table 176
Cost comparison of methods for delivering an instillation of intravesical chemotherapy.
It can be seen that, according to the cost estimations, delivering the single instillation of chemotherapy in theatre was the cheaper of the two approaches (delivery by nurse estimated to cost an additional £23.83). This was primarily a result of the longer amount of time taken to deliver the instillation in the ward setting compared to in theatre.
A.4.9. Probabilistic sensitivity analysis
The results of 10,000 runs of the probabilistic sensitivity analysis are shown using a cost-effectiveness acceptability curve (CEAC) in figures 41, 42 and 43 for low, intermediate and high risk patients, respectively. The graph shows the probability of each diagnostic strategy being considered cost-effective at the various cost-effectiveness thresholds on the x axis.

Figure 41
Cost-effectiveness acceptability curves for low risk patients.
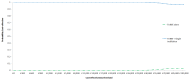
Figure 42
Cost-effectiveness acceptability curves for intermediate risk patients.
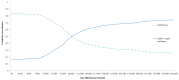
Figure 43
Cost-effectiveness acceptability curves for high risk patients.
It can be seen that at a threshold of £20,000 per QALY, TURBT plus a single instillation of chemotherapy has a very high probability of being cost-effective in the low and intermediate risk groups (100% in both risk groups). However, the probability is substantially lower in high risk patients at 66%, although still substantially in favour of TURBT plus a single instillation of chemotherapy.
A.4.10. Discussion
This analysis aimed to estimate the cost-effectiveness of administering a single instillation of intravesical chemotherapy immediately after a TURBT in comparison to a TURBT alone. The base case results of the model suggest that a single instillation immediately after a TURBT is a cost-effective strategy in low, intermediate and high risk patients with NMIBC.
The strategy was shown to be particularly cost-effective in low and intermediate risk patients where the cost savings driven by the reduction in recurrences were large enough to offset the initial higher costs associated with administering the chemotherapy. Thus, in low and intermediate risk groups, the administration of a single instillation of chemotherapy after a TURBT was shown to be cheaper and more effective and was thus considered dominant.
In high risk patients, cost savings from reduced recurrences are not large enough to completely offset the initial costs of administering the chemotherapy (i.e. not cost saving). However, while the strategy was more expensive, the QALY benefits obtained are substantial enough to make the single instillation of chemotherapy cost-effective. The base case estimate suggests that, in high risk patients, a single instillation of chemotherapy after TURBT provides one additional QALY at a cost of £6,432, which is well below the NICE threshold of £20,000 per QALY.
Furthermore, the results of the one-way sensitivity analysis suggested that the base case results were robust with the conclusion of the analysis remaining unchanged in all of the low, intermediate and high risk group analyses. Moreover, the probabilistic sensitivity analysis showed that, at a threshold of £20,000 per QALY, the probability of a TURBT plus a single instillation of chemotherapy being cost-effective in comparison to TURBT alone was high in all risk groups (100%, 100% and 66% in the low, intermediate and high risk groups, respectively).
However, it should be noted that there are numerous limitations to the analysis. As with most economic analyses, the analysis is highly dependent upon the clinical data upon which it is based. In this instance, the evidence for a reduction in the risk of recurrence is actually of high quality with numerous well conducted studies observing the effect. However, there are uncertainties elsewhere that have necessitated assumptions in the model.
The duration of the treatment effect is one such uncertainty. In the base case analysis it was assumed that the treatment effect (i.e. reduction in recurrence risk) would apply for two years after the administration of the chemotherapy (assuming that there are no recurrences during the 2 year period). This reflects the general consensus around the possible treatment effect duration but it's possible that it may be lower. However, the influence of the treatment effect duration was explored in sensitivity analysis and it was found that, while it is influential, the conclusions of the base case analysis were unchanged even in the most pessimistic scenario.
There was also found to be a paucity of quality of life data in this area. This is a common issue in cost-effectiveness evaluations but is nevertheless a significant one. The QoL values applied in the model are all of generally low quality and so the estimated QALYs may not be robustly estimated. However, the model is primarily driven by costs and the influence of this QoL values is likely to be limited.
A.4.11. Conclusion
The results of the analysis suggest that the use of a single instillation of chemotherapy after a TURBT, in comparison to a TURBT alone, was found to be strongly cost-effective in all risk groups. It was found to be particularly cost-effective in low and intermediate risk groups, in which the strategy was cost saving as well as more effective (dominant). Furthermore, this result was found to be robust in alternative scenario analyses, one-way and probabilistic sensitivity analysis.
A.5. References
- Ara R, Brazier J. Deriving an Algorithm to Convert the Eight Mean SF-36 Dimension Scores into a Mean EQ-5D Preference-Based Score from Published Studies (Where Patient Level Data Are Not Available). Value in Health. 2008;11(7):1131–1143. [PubMed: 18489495]
- Curtis L. Unit Costs of Health and Social Care 2013. Personal Social Services Research Unit (PSSRU), University of Kent; Canterbury: 2013.
- Green DA, et al. Cost-effective treatment of low-risk carcinoma not invading bladder muscle. BJU International. 2013;111(3B):E78–E83. 2013. [PubMed: 22958598]
- Hall RR, et al. Proposal for changes in cystoscopic follow–up of patients with bladder cancer and adjuvant intravesical chemotherapy. BMJ. 1994;308:257–260. [PMC free article: PMC2539314] [PubMed: 8179678]
- Joint Formulary Committee. British National Formulary (online). London: BMJ Group and Pharmaceutical Press;
- Kulkarni GS, et al. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4:1538–49. [PMC free article: PMC1989749] [PubMed: 17896857]
- Mowatt G, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technology Assessment. 2010;14(4):1–331. (Structured abstract) [PubMed: 20082749]
- NHS reference costs 2012-13. London: UK Department of Health; [database on the Internet]
- Sacco JJ, et al. The Average Body Surface Area of Adult Cancer Patients in the UK: A Multicentre Retrospective Study. PLoS ONE. 2010;5(1):e8933. [PMC free article: PMC2812484] [PubMed: 20126669] [CrossRef]
- Sylvester RJ, et al. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. Journal of Urology. 2004;171(6 Pt 1):2186–2190. [PubMed: 15126782]
- Sylvester RJ, et al. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. European Urology. 2006;49:466–477. [PubMed: 16442208]
- Van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. European Urology. 2011;60(3):493–500. [Review] [PubMed: 21664041]
- Yoshimura K, et al. Impact of superficial bladder cancer and transurethral resection on general health-related quality of life: an SF-36 survey. Urology. 2005;65(2):290–94. [PubMed: 15708040]
Footnotes
- k
However, it should be noted that there is no official cost-effectiveness threshold used in the evaluation of treatments in the US health care system.
- l
It should be noted that, while none of the above studies met the requirements for inclusion in the systematic review, they were nonetheless informative in helping to develop our own de novo economic model.
- m
European Organisation for Research and Treatment of Cancer
- The cost-effectiveness of a single instillation of chemotherapy immediately afte...The cost-effectiveness of a single instillation of chemotherapy immediately after transurethral resection of bladder tumour - Bladder Cancer
- UNVERIFIED: Drosophila cuaso even-skipped-like gene, partial sequenceUNVERIFIED: Drosophila cuaso even-skipped-like gene, partial sequencegi|2086095274|gb|MW425266.1|Nucleotide
- UNVERIFIED: Drosophila bandeirantorum even-skipped-like gene, partial sequenceUNVERIFIED: Drosophila bandeirantorum even-skipped-like gene, partial sequencegi|2086095279|gb|MW425271.1|Nucleotide
- AP-3 complex subunit sigma-1 isoform 9 [Homo sapiens]AP-3 complex subunit sigma-1 isoform 9 [Homo sapiens]gi|1396658779|ref|NP_001351051.1|Protein
- Arius arius voucher USNM:FISH:376608 ATPase subunit 8 and ATPase subunit 6 genes...Arius arius voucher USNM:FISH:376608 ATPase subunit 8 and ATPase subunit 6 genes, complete cds; mitochondrialgi|133843543|gb|DQ990681.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
