NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Bladder Cancer: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2015 Feb. (NICE Guideline, No. 2.)
Bladder cancer is the seventh most common cancer in the UK, with just over 10,000 cases diagnosed each year (CRUK, 2013a). These are unevenly split between men (fourth most common cancer) and women (11th most common cancer).
Around 5,000 people each year die from bladder cancer, making it the seventh most common cause of cancer death (CRUK, 2013b). As with new diagnoses these are unevenly split between men (sixth most common cancer death) and women (12th most common cancer death).
There are a number of well-known risk factors for bladder cancer, with the main risk being increasing age. Smoking is also a key risk and the chance of developing bladder cancer is about three times higher in smokers (Parkin, 2011a). There are also certain industrial chemicals linked to bladder cancer: these chemicals are now controlled but it is estimated they account for about 7% of males and 2% of female bladder cancers (Parkin, 2011b).
1.1. Methods
Incident cases were extracted from the National Cancer Registration Service (NCRS) in England, and the Welsh Cancer Intelligence and Surveillance Unit (WCISU) in Wales. The following codes were used to identify cases:
| ‘Malignant neoplasm of bladder’ |
| ‘Carcinoma in situ of bladder’ |
| ‘Neoplasm uncertain/unknown behaviour of bladder’ |
All deaths in England and Wales are certified by a medical professional and then processed by the Office for National Statistics (ONS). The ONS derive a single underlying cause of death which is used to identify bladder cancer deaths.
Deprivation in England has been measured using the income deprivation component of the English Indices of Deprivation (DCLG, 2012). In Wales the Welsh Index of Multiple Deprivation (WIMD) is used (Welsh Government, 2014).
1.2. Incidence
It is only valid to analyse data from the year 2000 onwards for England, and 2007 onwards for Wales. This is due to a change of coding.
Since 2000 the age-standardised rate (ASR) in men in England has decreased by 1.7% each year on average, and the ASR in women has decreased by 1.3% each year. The ASR in men is over three times that in women: 17.8 per 100,000 in men and 5.3 per 100,000 in women. In 2012 6,457 men in England were diagnosed with bladder cancer, compared to 2,453 women (Figure 1).

Figure 1
Incidence of bladder cancer (ICD-10 code C67), age-standardised rate per 100,000 by sex, England 1995-2012. Source: NCRS; ONS
In Wales, since 2007, the ASR in men has decreased by an average of 4.1% each year. The ASR in 2012 was 16.8 per 100,000 in men and 5.6 per 100,000 in women. In 2012 393 men in Wales were diagnosed with bladder cancer, compared to 160 women (Figure 2).
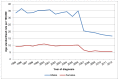
Figure 2
Incidence of bladder cancer (ICD-10 code C67), age-standardised rate per 100,000 by sex, Wales 1995-2012. Source: WCISU; ONS
The majority of bladder cancers are urothelial carcinoma but there are differences by sex. In both England and Wales urothelial carcinomas are more common in men (p<0.001 for both) and squamous cell cancers more common in women (p<0.001 for both). In England sarcomas are more common in women (p=0.003), however there are very few cases so the magnitude of the difference is small.
The rate of bladder cancer incidence increases with age in both males and females, with the highest rates occurring in those aged 80 and over (Figures 3 and 4). In England in 2012 34% of cases in men were diagnosed in those aged 80+ (2,200 cases) and 43% of cases in women were diagnosed in those aged 80+ (1,048 cases). This proportion has increased steadily since the year 2000, when 25% of cases in men and 38% of cases in women were in those aged 80 and over. This is likely to be a result of an aging population, but also a cohort effect of those who may have been exposed via industry in the 1950s/60s or had higher smoking prevalence (Figures 3 and 4).

Figure 3
Incidence of bladder cancer (ICD-10 code C67) in men, age-specific rate per 100,000, England 1995-2012. Source: NCRS; ONS
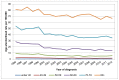
Figure 4
Incidence of bladder cancer (ICD-10 code C67) in women, age-specific rate per 100,000, England 1995-2012. Source: NCRS; ONS
In Wales the highest age-specific rates are also in those aged 80 and over. In 2012 31% of cases in men were diagnosed in those aged 80+ (123 cases) and 40% of cases in women were diagnosed in those aged 80+ (64 cases). This proportion is largely unchanged since 2007 (Figures 5 and 6).
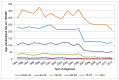
Figure 5
Incidence of bladder cancer (ICD-10 code C67) in men, age-specific rate per 100,000, Wales 1995-2012. Source: WCISU; ONS
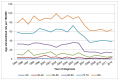
Figure 6
Incidence of bladder cancer (ICD-10 code C67) in women, age-specific rate per 100,000, Wales 1995-2012. Source: WCISU; ONS
The incidence of bladder cancer in England is higher in the most deprived population compared to the least deprived population (p<0.001).
Analysis of trends in data for England indicate that incidence of bladder cancer is decreasing more quickly in the least deprived populations. Therefore the inequality between least and most deprived is growing (Figures 7 and 8).

Figure 7
Incidence of bladder cancer (ICD-10 code C67) in men, age-standardised rate per 100,000 by deprivation quintile, England 1995-2010. Source: NCRS; ONS; DCLG

Figure 8
Incidence of bladder cancer (ICD-10 code C67) in women, age-standardised rate per 100,000 by deprivation quintile, England 1995-2010. Source: NCRS; ONS; DCLG
The numbers of cases in each deprivation quintile in Wales is small, and there are fewer years available for analysis. Therefore we cannot be sure of any trends by deprivation quintile, or if rates are truly higher in the most deprived areas (Figure 9 and 10).
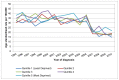
Figure 9
Incidence of bladder cancer (ICD-10 code C67) in men, age-standardised rate per 100,000 by deprivation quintile, Wales 1995-2010. Source: WCSIU; ONS
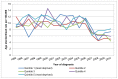
Figure 10
Incidence of bladder cancer (ICD-10 code C67) in women, age-standardised rate per 100,000 by deprivation quintile, Wales 1995-2010. Source: WCSIU; ONS
Stage at diagnosis is not recorded in all cases. In England in 2012, 35% of diagnoses had a valid TNM stage recorded. Of these 34% were stage I, 29% stage II, 6% stage III and 30% stage IV. Recording is better in Wales, with 78% of cases in 2012 having a valid TNM stage. Of these cases, 46% were stage I, 34% stage II, 12% stage III and 9% stage IV.
Stage at diagnosis is related to sex, age and deprivation. A logistic regression analysis on data in England and Wales indicates that the greatest difference in odds for being diagnosed with more advanced cancer is between men and women. Women have between 15% and 45% higher odds of advanced disease depending on the country and whether non-malignant bladder tumours (D41.4, D09.0) are included in analysis. Increasing age decreases the odds of being diagnosed with both MIBC and stage IV disease when considering bladder cancer (C67) diagnoses alone. Whilst there is some interaction with deprivation, the magnitude of the change in odds is generally small compared to the effect of sex or age. Increasing age decreases the odds of being diagnosed with both MIBC and stage IV disease when considering bladder cancer (C67) diagnoses alone. When all bladder tumours (C67, D41.4, D09.0) are included in the analysis the odds of being diagnosed with MIBC increases with age, however the odds of stage IV disease continue to be lower with increasing age.
Analysis of data at Clinical Commissioning Group (CCG) or Health Board level shows that CCGs with higher than average rates are located in all areas of the country but there is a distinct group around Liverpool, Manchester and Leeds. London has a number of CCGs with lower than average ASRs, plus there are several areas in the Midlands (Figures 11 and 12).
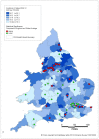
Figure 11
Incidence of bladder cancer (ICD-10 code C67) in men, age-standardised rate per 100,000, Clinical Commissioning Groups (England) and Health Boards (Wales) 2008-2012. Source: NCRS; WCSIU; ONS
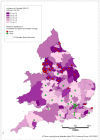
Figure 12
Incidence of bladder cancer (ICD-10 code C67) in women, age-standardised rate per 100,000, Clinical Commissioning Groups (England) and Health Boards (Wales) 2008-2012. Source: NCRS; WCSIU; ONS
The National Cancer Intelligence Network (NCIN) ran a project to analyse how cancer patients came to be diagnosed with cancer. This project was called ‘Routes to Diagnosis’ (NCIN, 2013). 16% of men and 24% of women diagnosed with bladder cancer (C67) in 2006-10 were diagnosed via an emergency route. The proportion of cases diagnosed as an emergency increased with age and deprivation, while the proportion diagnosed via a Two Week Wait referral decreased accordingly.
The same study showed that the one-year relative survival was worst in those diagnosed via an emergency route at 34%. In contrast those diagnosed via a Two Week Wait had a one-year survival of 84%.
1.3. Non-malignant bladder tumours
As with bladder cancer, uncertain behaviour tumours and carcinoma in situ are more common in men. In England in 2012 the age-standardised rate of carcinoma in situ was 4.8 times higher in men than women (p<0.001). The age-standardised rate of uncertain behaviour tumours (papilliary tumours) was 3.3 times higher in men than women (p<0.001). In Wales in 2012 the age-standardised rate of carcinoma in situ was 7.2 times higher in men than women (p<0.001) and the age-standardised rate of uncertain behaviour tumours was 2.9 times higher in men than women (p<0.001).
In England there were 1,701 diagnoses of carcinoma in situ in men in 2012, and 420 in women. The corresponding number of uncertain behaviour tumours was 4,601 and 1,611. In Wales in 2012 there were 74 diagnoses of carcinoma in situ in men and 10 in women, and 319 diagnoses of uncertain behaviour tumours in men and 123 in women.
Between 2000 and 2012 there was no increase or decrease in the ASR of carcinoma in situ in either men or women in England. The ASR of uncertain behaviour tumours increased by 3.7% each year in men and by 4.5% each year in women over the same time period. There was no evidence of an increase or decrease in the ASR of carcinoma in situ or uncertain behaviour tumour in Wales post 2007.
1.4. Mortality
The code change which affects bladder cancer incidence data in 2000/2007 does not have an effect on deaths data because virtually no people were registered as dying from non-malignant bladder tumours. Therefore it is possible to compare mortality rates over a longer time period.
Deaths from bladder cancer are more common in men – reflective of the higher incidence rates. In 2012, age-standardised mortality rates (ASMRs) were nearly three times higher in men then in women (p<0.001). In English men the ASMR was 7.6 per 100,000 and in English women it was 2.8 per 100,000 (Figure 13). In Welsh men the ASMR was 6.8 per 100,000 and in Welsh women it was 2.5 per 100,000 (Figure 14).
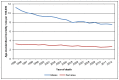
Figure 13
Mortality from bladder cancer (ICD-10 code C67), age-standardised rate per 100,000 by sex, England 1995-2012. Source: ONS
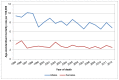
Figure 14
Mortality from bladder cancer (ICD-10 code C67), age-standardised rate per 100,000 by sex, Wales 1995-2012. Source: ONS
In 2012 2,918 men in England died from bladder cancer, compared to 1,399 women. The equivalent figures in 1995 were 3,075 and 1,488. In Wales in 2012 172 men died from bladder cancer compared to 88 women. The equivalent figures in 1995 were 166 and 93.
Although the number of deaths has only varied slightly, the ASMRs have fallen consistently over the time studied. In English men, rates decreased more quickly from 1997 to 2005 (2.5% each year) than from 2005 to 2012 (1.3% per year) (Figure 13). In English women the rate has fallen steadily from 1995 to 2012 at 1.3% each year (Figure 13). In men in Wales the ASMR has decreased steadily at 1.8% from 1995 to 2012, but in women there was not enough evidence to say that the rate has fallen (Figure 14). This will be affected by the smaller number of deaths.
Both the number of deaths and the ASMR is highest in those aged 80 and over. In men the rate in those aged 80 and over is 3.5 times (England) or 4.3 times (Wales) the rate in those aged 70-79. In women it is 2.8 times (England) or 3.3 times (Wales) higher (p<0.001 for all) (Figures 15-18).

Figure 15
Mortality from bladder cancer (ICD-10 code C67) in men, age-specific rate per 100,000, England 1995-2012. Source: ONS
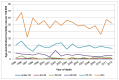
Figure 18
Mortality from bladder cancer (ICD-10 code C67) in women, age-specific rate per 100,000, Wales 1995-2012. Source: ONS
In men in England, there has been a decreasing trend in age-specific mortality at all ages 40 and over. The largest proportional decrease has been in those men aged 60-69, where the age-specific rate has decreased by 3.3% yearly from 1995 to 2012 (Figure 15). In Welsh men, there is no evidence of a decrease outside ages 60-79. In both men aged 60-69 and men aged 70-79 the rate has steadily decreased by 2.5% each year from 1995 to 2012 (Figure 16).
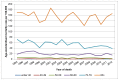
Figure 16
Mortality from bladder cancer (ICD-10 code C67) in men, age-specific rate per 100,000, Wales 1995-2012. Source: ONS
The number of deaths in women is smaller so there is less power to detect trends in age-specific rates. In England only those women aged 60-69 and 70-79 show statistically significant decreases. In those aged 60-69 the rate has decreased by 2.6% each year from 1995 to 2012, and in those aged 70-79 the rate has decreased by 2.5% each year from 1998 to 2012 (Figure 17). In women in Wales there was a statistically significant decrease only in those aged 60-69, with an annual average decrease of 3.4% from 1995 to 2012 (Figure 18).
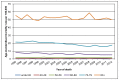
Figure 17
Mortality from bladder cancer (ICD-10 code C67) in women, age-specific rate per 100,000, England 1995-2012. Source: ONS
There is a consistent pattern across England of higher mortality rates in people living in more deprived areas. In 2012 the ASMR in men in the most deprived quintile was 40% higher than in the least deprived; the ASMR in quintile 5 was 9.0 per 100,000 and in quintile 1 was 6.4 per 100,000 (p<0.001). In women the ASMR in the most deprived quintile was 65% higher than in the least deprived; the ASMR in quintile 5 was 3.4 per 100,000 and in quintile 1 was 2.0 per 100,000 (p<0.001).
In Wales this pattern is not apparent and there is no statistically significant difference between the most and least deprived groups.
ASMRs have fallen in all deprivation groups in England, but there is evidence that the decrease has been larger in the least deprived populations. In men the ASMR in the least deprived quintile decreased by 2.2% each year between 1995 and 2010, compared to 1.1% each year in the most deprived quintile between 1998 and 2010. In women the ASMR in the least deprived quintile decreased by 1.6% each year between 1997 and 2010, compared to 1.1% each year in the most deprived quintile between 1995 and 2010
Those Clinical Commissioning Groups (CCGs) which have a bladder cancer ASMR higher than the England and Wales average tend to be in the north and north-west of England. In contrast the CCGs with lower ASMRs tend to be in the south and south-east of England (Figures 19 and 20).
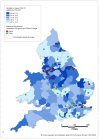
Figure 19
Mortality from bladder cancer (ICD-10 code C67) in men, age-standardised rate per 100,000, Clinical Commissioning Groups (England) and Health Boards (Wales) 2008-2012. Source: ONS
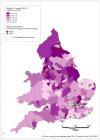
Figure 20
Mortality from bladder cancer (ICD-10 code C67) in women, age-standardised rate per 100,000, Clinical Commissioning Groups (England) and Health Boards (Wales) 2008-2012. Source: ONS
1.5. Survival
Data presented here are for five-year rolling averages as this is necessary for the period survival calculations. Survival data are also affected by the recoding of tumours in the year 2000/2007. As this recoding reduced incidence but had little effect on mortality there was a corresponding reduction in survival. Therefore in England only survival data post-2000 should be assessed. In Wales only one time-period (2007-2011) is after the coding change so no trends can be analysed.
Survival at both one and five years is higher in men than in women; which goes against the general trend for cancer. In England in 2006-10 one-year survival in men was 77% compared to 64% in women. In 2006-10 five-year survival in men was 58% compared to 47% (Figure 21). In Wales in 2007-11 one-year survival in men was 76% compared to 60% in women, and in 2007-11 five-year survival in men was 54% compared to 50% (Figure 22)
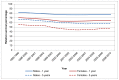
Figure 21
Relative survival from bladder cancer (ICD-10 code C67) by sex, England 1995-2010. Source: NCRS
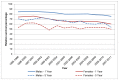
Figure 22
Relative survival from bladder cancer (ICD-10 code C67) by sex, Wales 1995-2011. Source: WCISU
In England - for both men and women - there was no difference in survival when comparing 2000-04 and 2006-10, and this is true for all subsequent analysis by separate groups.
Survival decreases with age for both men and women, even though relative survival takes into account increased overall mortality rates at older ages. This means that older people have proportionally worse survival as well as worse survival in absolute terms.
In the analysis by age it was not always possible to calculate survival for the youngest patients due to small numbers. This is indicated by gaps in the data.
In men in England in 2006-10 the highest one-year survival was in those aged under 40 at diagnosis at 90%, although the confidence intervals of the four youngest age groups (up to 69 years old) overlap indicating that observed variation is likely to be chance. Survival was worst in those aged 80 and over at diagnosis; where the relative survival was 65% (Figure 23). A similar pattern was seen in women where the one-year survival in under 40s was 77% but 51% in those aged 80 and over (Figure 24).
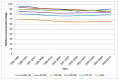
Figure 23
One-year relative survival from bladder cancer (ICD-10 code C67) by age, in men, England 1995-2010. Source: NCRS
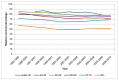
Figure 24
One-year relative survival from bladder cancer (ICD-10 code C67) by age, in women, England 1995-2010. Source: NCRS
Five-year survival for men was highest in the under 40s at 76%, compared to 42% in those aged 80 and over. As with one-year survival the rate in those aged under 70 was similar (Figure 25). In women a different pattern is seen, with the highest five-year survival in those aged 50-59 at diagnosis at 61%. The lowest survival was still in the 80+ age group at 32%. The confidence intervals on the youngest age groups overlap all others, likely due to small numbers of diagnoses (Figure 26).
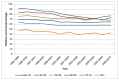
Figure 25
Five-year relative survival from bladder cancer (ICD-10 code C67) by age, in men, England 1995-2010. Source: NCRS
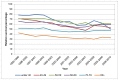
Figure 26
Five-year relative survival from bladder cancer (ICD-10 code C67) by age, in women, England 1995-2010. Source: NCRS
In Wales in 2007-11 one-year survival was highest in men aged under 40, at 87%, As with England data the confidence intervals on this rate overlap all others. Survival in those aged 80 and over is significantly lower than for those aged 50-79, at 60% (Figure 27). For women survival was also lowest in those aged 80 and over and was lower than men of the same age, at 45% (Figure 28).
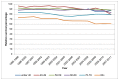
Figure 27
One-year relative survival from bladder cancer (ICD-10 code C67) by age, in men, Wales 1995-2011. Source: WCISU

Figure 28
One-year relative survival from bladder cancer (ICD-10 code C67) by age, in women, Wales 1995-2011. Source: WCISU
Five-year survival in Wales is lowest in those aged 80+. In men the rate was 40% and for women the rate was 35% (Figures 29 and 30). This is lower than the rate in 60-69 year olds, but confidence intervals in the oldest ages overlap.

Figure 29
Five-year relative survival from bladder cancer (ICD-10 code C67) by age, in men, Wales 1995-2011. Source: WCISU
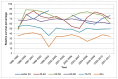
Figure 30
Five-year relative survival from bladder cancer (ICD-10 code C67) by age, in women, Wales 1995-2011. Source: WCISU
In England there is a consistent pattern of decreasing relative survival with increasing quintile of income deprivation. In men, one-year survival in 2006-10 was 78% in the least deprived quintile and 75% in the most deprived. In women, one-year survival in 2006-10 was 69% in the least deprived and 59% in the most deprived. Confidence intervals on these rates do not overlap, indicating that the differences are due to more than chance variation.
In men, five-year survival in 2006-10 was 60% in the least deprived quintile and 55% in the most deprived. However the confidence intervals overlap so we cannot be sure that this difference is not just chance variation. In women, five-year survival in 2006-10 was 51% in the least deprived and 42% in the most deprived. Here confidence intervals do not overlap, indicating that the differences are due to a true underlying difference.
In Wales in 2007-11 there is not a pattern of survival by deprivation; in contrast to England. The highest one-year survival for men was 79% in quintile 2, compared to 73% in the most deprived quintile. However, confidence intervals overlap on all quintiles. Survival for women was highest in quintile 2 at 72%, and lowest in quintile 4 at 52%. The confidence intervals do not overlap so this is likely to be a true difference.
Patterns are similar for five-year survival in Wales. Men in quintile 2 have the highest survival at 61% and men in quintile 4 the lowest at 42%, but confidence intervals overlap. Women in quintile 2 have the highest survival at 62% and women in quintile 4 the lowest at 36%. As with one-year survival the confidence intervals do not overlap so this is likely to be a true difference.
Survival decreases with increasing stage at diagnosis. This may help explain the poorer survival in women, as they are more likely to be diagnosed at an advanced stage. As described in the incidence section nearly 1 in 3 bladder cancer diagnoses in England and 1 in 10 in Wales are made at stage IV, which has poor outcomes.
In England in 2006-10 the relative survival at one year for stage IV disease was 42% in men and 34% in women, whilst five-year survival was 11% in men and 12% in women (Figures 31 and 32).
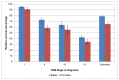
Figure 31
One-year relative survival from bladder cancer (ICD-10 code C67) by stage at diagnosis, England 2006-2010. Source: NCRS
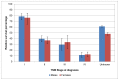
Figure 32
Five-year relative survival from bladder cancer (ICD-10 code C67) by stage at diagnosis, England 2006-2010. Source: NCRS
In Wales in 2007-11 one-year survival for stage IV disease was 56% for men and 54% for women. Five-year survival was 28% for men and 36% for women (Figures 33 and 34). The confidence intervals in these calculations are large, indicating a higher degree of uncertainty, and it is not possible to be sure that there is a survival difference between England and Wales.
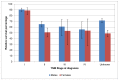
Figure 33
One-year relative survival from bladder cancer (ICD-10 code C67) by stage at diagnosis, Wales 2007-2011. Source: WCISU
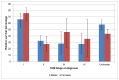
Figure 34
Five-year relative survival from bladder cancer (ICD-10 code C67) by stage at diagnosis, Wales 2007-2011. Source: WCISU
Non muscle-invasive disease (stage I) shows better outcomes then muscle-invasive disease (stage II-IV), with one-year survival of 95% in men and 91% in women in England (Figure 31). Five-year survival was 79% and 76% (Figure 32). The difference between NMIBC and MIBC is particularly apparent at five years of follow-up where the survival for stage II bladder cancer is nearly half that of stage I (Figure 32). In Wales one-year survival for stage I disease was 91% in men and 89% in women; five-year survival was 66% and 76% respectively (Figures 33 and 34).
One-year relative survival in men at CCG level varies from 60% to 96%, with the range for women 28% to 87%. There is greater uncertainty with survival calculations so fewer CCGs are statistically significantly different from the England average than with incidence or mortality data.
Five-year relative survival in men at CCG level varies from 21% to 87%, with the range for women 0% to 76%.
There is no obvious geographical pattern in terms of CCGs which have higher or lower survival, although some CCGs with poorer one-year survival also have poorer five-year survival; as might be expected.
1.6. Treatment
Treatment data were only available for England.
Radical cystectomy is the complete removal of the bladder. It is one of the main treatments for muscle-invasive bladder cancer.
Numbers of radical cystectomies have risen in men from 935 in 1998 to 1,399 in 2012. In women the rise in number has been smaller; 300 operations were done in 1998 compared to 357 in 2012. As a proportion of cases diagnosed in that year the rate of radical cystectomy in men was 15% in 2000 compared to 22% in 2010 (p<0.001), with the proportion in women 11% and 15% respectively (p<0.001). Regression analysis indicates a linear increase in cystectomy rate of 4.2% each year for men and 3.5% for women (p<0.05 for both) (Figure 35).
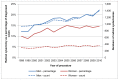
Figure 35
Radical cystectomy for bladder cancer (ICD-10 code C67), England 1998-2010. Source: HES; NCRS
The proportion of people aged under 70 who have cystectomy is similar; given the smaller numbers there is inherent instability in the rates for younger ages. The cystectomy rate is lowest in those aged 80 and over at diagnosis: 3% of men and 2% of women.
In men all age groups have shown a linear increase in cystectomy rate (p<0.05). The annual increase in rates ranged from 4.4%-8.7% but with fairly wide confidence intervals, so it is not possible to say that one age range increased more or less than another.
In women the cystectomy rate in those aged under 40 and 80+ did not change over the time period; although numbers in the youngest age group are very small. In women aged 60-69 analysis indicated that the data was best described by an increasing rate to 2002 followed by no change until 2010. The cystectomy rate in women aged 50-59 and 70-79 showed a linear increase of 3.2% and 5.6% respectively (p<0.05).
Cystectomy rates are higher in the least deprived men, compared to the most deprived (p<0.001). The proportion of men in the least deprived quintile who had cystectomy was 26% compared to 20%. However, women were equally likely to receive a cystectomy whichever deprivation group they were in.
The cystectomy rate increased linearly in each deprivation quintile for both men and women (p<0.05). This varied between 5.8%-7.3% in men and 4.4%-6.7% in women. There is no evidence that the rate increased more quickly or slowly with variation in deprivation.
Radiotherapy is also a frequently used treatment modality for muscle-invasive bladder cancer. Radiotherapy is also used for symptomatic relief of advanced bladder cancer, so it is important to differentiate between curative and palliative intent.
Data shown here is based on the number of radiotherapy treatment courses delivered in 2009 and 2010 as a proportion of diagnoses in those same years. This means that those diagnosed prior to 2009 are not represented, nor any treatment after 2010. This restriction is required as the radiotherapy data holds little demographic detail such as age and sex, so must be linked to diagnosis data.
The proportion of men having curative radiotherapy is higher than in women, but the difference is fairly small; 11.3% in men compared to 9.5% in women (p<0.001). The proportion having palliative radiotherapy is close to being statistically significant (p=0.06) but again the magnitude of any difference is small; 11.2% in men and 12.2% in women (Figure 36).

Figure 36
Radiotherapy for bladder cancer (ICD-10 code C67) by sex, England 2009-2010. Source: RTDS; NCDR
Data for radiotherapy by age are more difficult to interpret as numbers are smaller. In both sexes palliative radiotherapy is high in those aged 80+ with a corresponding dip in curative radiotherapy. In the three older age-bands, which include the majority of cases, the usage of palliative radiotherapy increases with age (Figure 37).
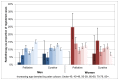
Figure 37
Radiotherapy for bladder cancer (ICD-10 code C67) by age and sex, England 2009-2010. Source: RTDS; NCDR
There is no strong evidence of any trend in radiotherapy use by quintile of deprivation.
Chemotherapy may be used for bladder cancer before surgery or radiotherapy (neo-adjuvant) or afterwards (adjuvant). It may also be used for palliative care, but unlike the radiotherapy data this is not recorded in the dataset. Chemotherapy data here comes from outpatient HES data which is only available from 2003 onwards.
The proportion of patients who receive chemotherapy has risen since 2003. In 2003 2% of men and 1% of women had any chemotherapy recorded, but in 2010 this was 9% and 7% respectively. Figure 38 suggests that the increase has been faster since 2007, but small numbers and limited time period mean that there is no statistical evidence to confirm this. The Cochrane systematic review supporting the use of neo-adjuvant chemotherapy in bladder cancer was published in 2007. It is also important to bear in mind that recording of chemotherapy in HES may have variable completeness over time, and better evidence will be available with the upcoming Systemic Anti-Cancer Therapy (SACT) dataset.
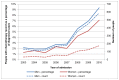
Figure 38
Chemotherapy for bladder cancer (ICD-10 code C67) by sex, England 2003-2010. Source: HES; NCDR
Analysis by age and deprivation group does not indicate a statistically significant difference in recorded chemotherapy use by these factors. Regression models indicate that all groups have shown an increase in recorded chemotherapy with time (p<0.05 for all). This increase has been between 24% and 45% in men by age group; 20% and 48% in women by age group; 30% and 34% in men by deprivation quintile; and, between 20% and 42% in women by deprivation quintile.
1.7. References
- CRUK. Bladder cancer incidence statistics. 2013. [17th March 2014]. (Online). Available from: http://www
.cancerresearchuk .org/cancer-info /cancerstats/types/bladder/incidence/ - CRUK. Bladder cancer mortality statistics. 2013. [17th March 2014]. (Online). Available from: http://www
.cancerresearchuk .org/cancer-info /cancerstats/types/bladder/mortality/ - DCLG. English indices of deprivation. 2012. [17th March 2014]. (Online). Available from: https://www
.gov.uk/government /collections /english-indices-of-deprivation. - NCIN. Routes to diagnosis. 2013. [19th May 2014]. Available from: http://www
.ncin.org.uk /publications/routes_to_diagnosis. - Parkin D. Tobacco-attributable cancer burden in the UK in 2010. British Journal of Cancer. 2011;105:S6–S13. [PMC free article: PMC3252064] [PubMed: 22158323]
- Parkin D. Cancers attributable to occupational exposures in the UK in 2010. British Journal of Cancer. 2011;105:S70–S72. [PMC free article: PMC3252066] [PubMed: 22158325]
- Welsh Government. Welsh Index of Multiple Deprivation (WIMD). 2014. [18th June 2014]. (Online). Available from: http://wales
.gov.uk/statistics-and-research /welsh-index-multiple-deprivation /?lang=en.
- Epidemiology - Bladder CancerEpidemiology - Bladder Cancer
- UNVERIFIED: Drosophila maculifrons even-skipped-like gene, partial sequenceUNVERIFIED: Drosophila maculifrons even-skipped-like gene, partial sequencegi|2086095277|gb|MW425269.1|Nucleotide
- Mus musculus Rous sarcoma oncogene (Src), transcript variant 4, mRNAMus musculus Rous sarcoma oncogene (Src), transcript variant 4, mRNAgi|2316837745|ref|NM_001412683.1|Nucleotide
- Mancoa foliosa tRNA-Leu (trnL) gene, partial sequence; trnL-trnF intergenic spac...Mancoa foliosa tRNA-Leu (trnL) gene, partial sequence; trnL-trnF intergenic spacer region, complete sequence; and tRNA-Phe (trnF) gene, partial sequence; chloroplast genes for chloroplast productsgi|18032647|gb|AF307552.1|Nucleotide
- Tribonema aequale voucher UTEX 50 photosystem II thylakoid membran protein (psbC...Tribonema aequale voucher UTEX 50 photosystem II thylakoid membran protein (psbC) gene, partial cds; plastidgi|354779965|gb|HQ710793.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
