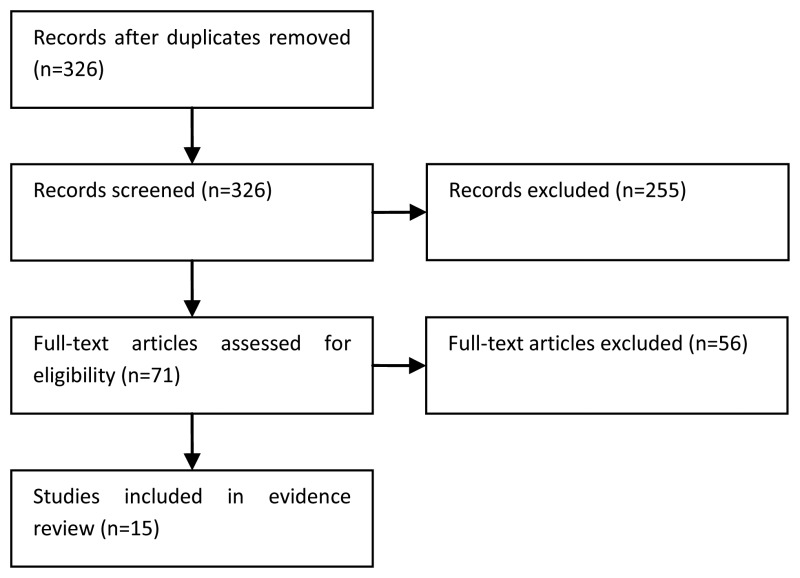
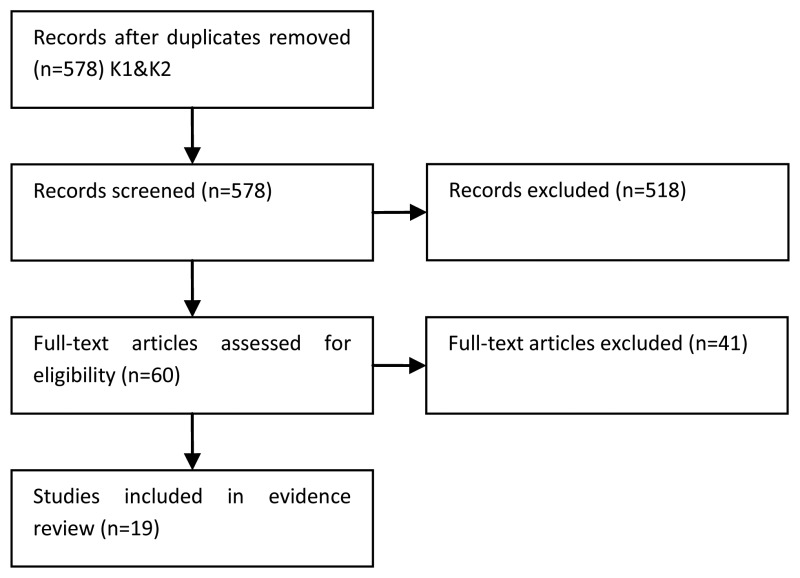
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Bladder Cancer: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2015 Feb. (NICE Guideline, No. 2.)
3.1. Risk Stratification
3.1.1. Prognostic markers in NMIBC
Review question: In addition to the factors specified in the EORTC risk tables, do TCC variants, differentiation of TCC and lymphovascular invasion predict recurrence and progression after treatment?
Rationale
In general, recurrence is a problem for patients (because any tumour recurrence raises the concern that the cancer will progress and/or spread) and for the NHS (because of the need to provide capacity for treatment of recurrence), but it does not threaten patients' lives. In contrast, progression does threaten patients' lives, because if the muscle coat of the bladder becomes involved with cancer, between 20 and 25 out of 100 such patients will have spread into their lymph glands, and their chance of cure falls sharply.
We have some pathological markers of the risks of recurrence and progression, such as stage, grade, and the presence of carcinoma in situ, and other clinical markers, such as tumour size, number and the presence of recurrence at three months from the initial resection. On the basis of EORTC chemotherapy study data, it was suggested many years ago that the management of LRNMIBC could be streamlined significantly by the use of two easily established clinical variables alone, namely whether the initial tumour is solitary or multifocal, and whether there was recurrence or not at three months. Despite the evidence base for this, and its ease of assessment, it has not become widely used in the NHS.
So the use of these factors remains unsatisfactory for an individual patient, and does not predict the individual risks of recurrence and progression. Molecular markers (such as EGFR) have been studied for over 20 years, to see if some laboratory studies are able to be useful in clinical practice, but none has emerged as useful to the NHS.
If we knew better for individual patients about their risk of recurrence and particularly progression, it would be possible to inform the discussion of the cancer risk, which is one of the pillars of the discussion about which treatment option is best for a given patient. Many patients would consider better forecasting of their own personal cancer risk to be a very useful step forward.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with newly diagnosed NMIBC | Prognostic factors: EORTC risk factors TCC variants (micropapillary and nested patterns) TCC differentiation (squamous, glandular and sarcomatoid) Lymphovascular invasion | N/A |
|
METHODS
Information sources
A literature search was conducted by the information specialist (EH).
Selection of studies
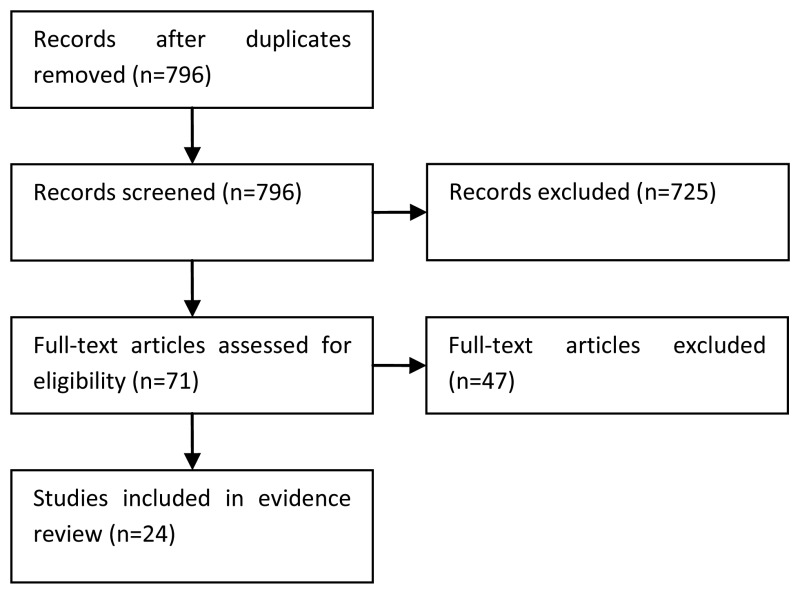
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. Validation studies of the EORTC risk calculator were selected if there were sufficient numbers of patients in each risk category to allow a meaningful validation. Prognostic studies of the other factors in the PICO (TCC variants, TCC differentiation, lymphovascular invasion) were also appraised.
Data synthesis
The results are presented by outcome and by prognostic factor. Hazard ratios and p values are provided as reported in the studies. The validation studies of the EORTC risk factors are also presented with c-indices and estimated and observed numbers of recurrences and progressions.
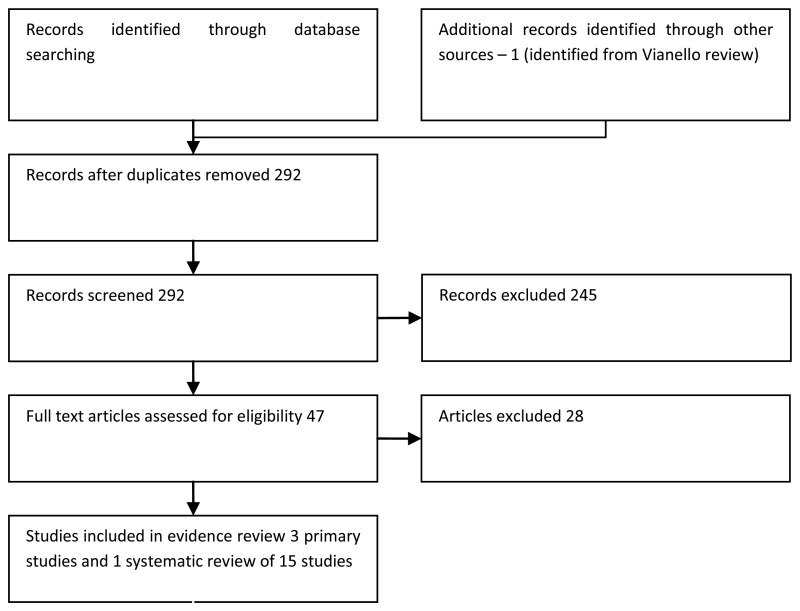
RESULTS
Study quality and results
The NICE prognostic studies methodological checklist was used to assess the quality of the prognostic studies. All studies were assessed as being of high quality as they included the population of interest, measured the outcome adequately, and used appropriate statistical analysis. However, validation studies of the EORTC risk tables were limited by heterogeneous patient populations and treatments received and by low numbers of progression events. Studies exploring the prognostic factors of lymphovascular invasion, TCC variants and TCC differentiation were limited by small sample sizes and few patients with the factor under investigation. The results of the study quality assessment is provided in Table 30. The results of the included studies are summarised in Tables 26-33 and Figures 31-35.
Table 30
Study quality assessment.
Table 31
Univariate and multivariate analyses of recurrence.
Table 32
Univariate and multivariate analyses of progression.
Table 33
Univariate and multivariate analyses of overall survival.
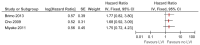
Figure 31
Univariate analyses of lymphovascular invasion on recurrence.
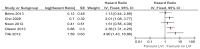
Figure 32
Multivariate analyses of lymphovascular invasion on recurrence.
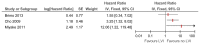
Figure 33
Univariate analyses of lymphovascular invasion on progression.
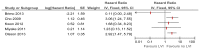
Figure 34
Multivariate analyses of lymphovascular invasion on progression.
Narrative summary of evidence
EORTC risk factors: Recurrence & Progression
The European Organization for Research and Treatment of Cancer (EORTC) Genito-Urinary Group published risk tables that provide the probabilities that a patient with superficial bladder cancer (Ta,T1) will recur or progress to muscle-invasive disease after transurethral resection of the bladder tumour (TURBT) (Sylvester, 2006). Seven randomised trials including 2596 patients and with a maximum follow-up of 15 years were included in the analysis by Sylvester (2006). 6% of patients were randomised to intravesical bacillus Calmette-Guérin (BCG) and none of the patients received maintenance therapy. The EORTC scoring system was derived based on six clinical and pathological factors: number of tumours, tumour size, prior recurrence rate, T category, carcinoma in situ (CIS), and grade. Fernandez-Gomez (2011) reported a validation of the EORTC risk tables in a cohort of 1062 patients treated with BCG from 4 randomised trials (CUETO studies). 73% of this cohort received 10-12 BCG instillations. The EORTC risk tables successfully divided CUETO patients into four risk groups for recurrence and progression.
The c-indices for recurrence were similar in the EORTC and CUETO series. For recurrence the PSEP in the CUETO series was lower than the EORTC at 1-year (0.3/0.26 vs. 0.46), similar results were found at 5-years (0.49/0.51 vs. 0.47). For progression, the c-index in the CUETO cohort was 0.69 at 1-year and 0.68 at 5-year, which are lower than the EORTC c-indices for progression at 1-year (0.74) and 5-years (0.75). The PSEP in the CUETO series was lower than the EORTC for progression at 5-years (0.25 vs. 0.442). Xylinas (2013) presented a validation study of 4689 patients, and reported c-indices which demonstrated poor discrimination of the EORTC risk models for recurrence and progression.
In 7 validation studies (Fernandez-Gomez 2011; Seo 2010; Altieri 2012; Hernandez 2011; van Rhijn 2010; Xu 2013; Lammers 2014), the EORTC risk tables generally overestimated the risk of recurrence in all risk groups and the risk of progression at 5-years especially in high risk groups. However, many of these studies were limited by a low number of progression events. In an earlier report from the CUETO cohort, Fernandez-Gomez (2008) reported that, in multivariate analysis, female gender (HR=1.71) compared to male gender, recurrent tumours (HR=1.9) compared to primary tumours, multiplicity, and presence of associated tumour in situ (TIS) (HR=1.55), were significant independent factors for recurrence. For progression, recurrent tumours (HR=1.62) compared to primary tumours, high-grade tumours (HR=5.64) compared to G1 tumours, T1 tumours (HR=2.15) compared to Ta tumours, and recurrence at 3-month cystoscopy (HR=4.6) were independent predictive factors.
One study of 592 Japanese patients, half of whom received no intravesical therapy after TURBT, attempted to validate the EORTC risk scores for recurrence (Sakano, 2010). There was only a significant difference in recurrence-free survival when the low-risk and intermediate-low risk groups were combined into one group, and the intermediate-high risk and high risk groups were considered as another group. Multivariate analysis showed that prior recurrence rate, number of tumours, and T category were independent predictors for time to first recurrence.
In another validation study including 230 patients with primary non-muscle invasive bladder cancer (van Rhijn, 2010), EORTC risk scores for progression and recurrence were significant factors in multivariate analysis. However, none of the patients in this cohort were at high risk of recurrence and all patients had primary NMIBC, which limits the usefulness of this study. One study of patients with T1 bladder cancer, all of whom were treated with BCG, reported that EORTC risk scores were not significant predictors of progression or recurrence (van Rhijn, 2012). Multiplicity was the most important variable for predicting recurrence, whilst sub-stage (T1m/T1e), female gender and CIS were the most important variables for progression in multivariate analysis.
One prognostic study of 146 patients with T1G3 NMIBC treated with an induction course of BCG reported that female gender and presence of CIS in the prostatic urethra were associated with an increased risk of recurrence, progression and disease-specific mortality (Palou, 2012).
Lymphovascular invasion: Recurrence and progression
Seven studies included lymphovascular invasion as a prognostic factor for recurrence or progression (Brimo 2013; Miyake 2011; Kwon 2012; Cho 2009; Tilki 2012; Park 2009; Olsson 2013). Some studies reported that the presence of lymphovascular invasion was a prognostic factor for recurrence or progression and some studies reported no significant effect in univariate and multivariate analyses (see Figures 31-35 below for forest plots of reported hazard ratios from univariate and multivariate analyses). Analysis of this factor was limited by the low number of patients with invasion. Park (2009) reported that lymphovascular invasion was not a predictor of recurrence or progression in patients with T1G3 bladder cancer who received BCG therapy (HRs were not reported so could not be included in the forest plots).
Lymphovascular invasion: Disease-specific survival
Two studies (Lopez, 1995; Tilki, 2012) reported that lymphovascular invasion was an independent prognostic factor for disease-specific survival and one study reported no significant effect (Olsson, 2013) (see Figure 35).
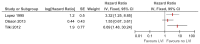
Figure 35
Multivariate analyses of lymphovascular invasion on disease-specific survival.
Lymphovascular invasion: Overall survival
One study of 108 patients (Branchereau 2013) reported that lymphovascular invasion was an independent prognostic factor for overall survival for patients with high grade T1 tumours (p=0.003, HR not reported).
Histological subtype (‘usual TCC vs. micropapillary/sarcomatoid TCC): Recurrence and progression
One study (Brimo, 2013) reported that adverse histological variants were significantly associated with progression and recurrence on univariate analysis but were insignificant on multivariate analysis. Only 4 tumours were not ‘usual TCC. 3 had features of micropapillary TCC and 1 had features of sarcomatoid TCC.
Histological subtype (TCC vs. squamous cell carcinoma): Overall survival and disease specific survival
Scosyrev (2009) reported that squamous cell histologic features were associated with overall mortality and disease-specific mortality compared to TCC in patients who did not undergo cystectomy, but was not associated with increased mortality in those who were treated with cystectomy.
Micropapillary pattern (MPP): Progression
One study (Alkibay, 2009), reported that progression rates increased in patients with NMIBC and MPP compared with MPP-negative patients but this difference was not statistically significant (p=0.064). This analysis was based on only 6 patients with T1 bladder cancer and MPP, and 125 TaT1 MPP-negative patients.
Evidence statements
The EORTC risk tables (Sylvester et al., 2006) have been validated in several studies, which report that the tables successfully stratify patients into risk groups for recurrence and progression, but generally overestimate the risk of recurrence in all risk groups and the risk of progression in high risk groups (Fernandez-Gomez et al., 2011; Seo et al., 2010; Altieri et al., 2012; Hernandez et al., 2011; van Rhijn et al., 2010; Xu et al., 2013; Lammers et al., 2014).
There is some low quality evidence to suggest that the presence of lymphovascular invasion increases the risk of recurrence, progression and disease-specific survival. However, this is based on low numbers of patients with evidence of lymphovascular invasion.
One study (Brimo et al., 2013) reported that adverse histological variants were significantly associated with progression and recurrence on univariate analysis but were insignificant on multivariate analysis. Only four tumours were not ‘usual TCC. Three had features of micropapillary TCC and one had features of sarcomatoid TCC.
One study (Scosyrev et al., 2009) reported that squamous cell histologic features were associated with overall mortality and disease-specific mortality compared to TCC in patients who did not undergo cystectomy, but was not associated with increased mortality in those who were treated with cystectomy.
One study (Alkibay et al., 2009), reported that progression rates increased in patients with NMIBC and micropapillary pattern (MPP) compared with MPP-negative patients but this difference was not statistically significant (p=0.064). This analysis was based on only six patients with T1 bladder cancer and MPP, and 125 TaT1 MPP-negative patients.
Table 34
Univariate and multivariate analyses of disease-specific survival.
Table 37
Probabilities of recurrence according to EORTC risk tables and validation studies at 1-year and 5-year.
Table 38
Probabilities of progression according to EORTC risk tables and validation studies at 1-year and 5-year.
References to included studies
- Alkibay T, et al. Micropapillary pattern in urothelial carcinoma: a clinicopathological analysis. Urologia Internationalis. 2009;83(3):300–305. [PubMed: 19829030]
- Altieri VM, et al. Recurrence and progression in non-muscle-invasive bladder cancer using EORTC risk tables. Urologia Internationalis. 2012;89(1):61–66. [PubMed: 22722366]
- Branchereau J, et al. Prognostic value of the lymphovascular invasion in high-grade stage pT1 bladder cancer. Clinical Genitourinary Cancer. 2013;11(2):182–188. [PubMed: 23276589]
- Brimo F, et al. Prognostic factors in T1 bladder urothelial carcinoma: the value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion. Human Pathology. 2013;44(1):95–102. [PubMed: 22939956]
- Cho KS, et al. Lymphovascular invasion in transurethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. Journal of Urology. 2009;182(6):2625–2630. [PubMed: 19836779]
- Fernandez-Gomez J, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: Multivariate analysis of data from four randomized CUETO trials. European Urology. 2008;53(5):992–1002. [PubMed: 17950987]
- Fernandez-Gomez J, et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: external validation of the EORTC risk tables. European Urology. 2011;60(3):423–430. [PubMed: 21621906]
- Hernandez V, et al. External validation and applicability of the EORTC risk tables for non-muscle-invasive bladder cancer. World Journal of Urology. 2011;29(4):409–414. [PubMed: 21190023]
- Kwon DH, Song PH, Kim HT. Multivariate analysis of the prognostic significance of resection weight after transurethral resection of bladder tumor for non-muscle-invasive bladder cancer. Korean Journal of Urology. 2012;53(7):457–462. [PMC free article: PMC3406190] [PubMed: 22866215]
- Lammers RJ, et al. Comparison of expected treatment outcome provided by risk models and international guidelines with observed treatment outcome in a cohort of Dutch non-muscle-invasive bladder cancer patients treated with intravesical chemotherapy. BJU Int. 2014 [PubMed: 24304638]
- Lopez JI, Angulo JC. The prognostic significance of vascular invasion in stage T1 bladder cancer. Histopathology. 1995;27(1):27–33. [PubMed: 7557903]
- Miyake M, et al. Clinical significance of subepithelial growth patterns in non-muscle invasive bladder cancer. BMC Urology. 2011;11(1):17. [PMC free article: PMC3167754] [PubMed: 21816111]
- Olsson H, et al. Population-based study on prognostic factors for recurrence and progression in primary stage T1 bladder tumours. Scandinavian Journal of Urology. 2013;47(3):188–195. [PubMed: 22954205]
- Palou J, et al. Female Gender and Carcinoma In Situ in the Prostatic Urethra Are Prognostic Factors for Recurrence, Progression, and Disease-Specific Mortality in T1G3 Bladder Cancer Patients Treated With Bacillus Calmette-Guerin. European Urology. 2012;62(1):118–125. [PubMed: 22101115]
- Park J, et al. Prognostic significance of non-papillary tumor morphology as a predictor of cancer progression and survival in patients with primary T1G3 bladder cancer. World Journal of Urology. 2009;27(2):277–283. [PubMed: 19020879]
- Sakano S, et al. Risk group stratification to predict recurrence after transurethral resection in Japanese patients with stage Ta and T1 bladder tumours: validation study on the European Association of Urology guidelines. BJU International. 2011;107(10):1598–1604. [PubMed: 21087393]
- Scosyrev E, Yao J, Messing E. Urothelial carcinoma versus squamous cell carcinoma of bladder: is survival different with stage adjustment? Urology. 2009;73(4):822–827. [PubMed: 19193403]
- Seo KW, et al. The efficacy of the EORTC scoring system and risk tables for the prediction of recurrence and progression of non-muscle-invasive bladder cancer after intravesical bacillus calmette-guerin instillation. Korean Journal of Urology. 2010;51(3):165–170. [PMC free article: PMC2855454] [PubMed: 20414391]
- Sylvester RJ, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology. 2006;49(3):466–475. [PubMed: 16442208]
- Tilki D, et al. Lymphovascular invasion is independently associated with bladder cancer recurrence and survival in patients with final stage T1 disease and negative lymph nodes after radical cystectomy. BJU International. 2013;111(8):1215–1221. [PubMed: 23181623]
- van Rhijn BW, et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. European Urology. 2010;58(3):433–441. [PubMed: 20646825]
- van Rhijn BW, et al. Prognostic value of molecular markers, sub-stage and European Organisation for the Research and Treatment of Cancer risk scores in primary T1 bladder cancer. BJU International. 2012;110(8):1169–1176. [PubMed: 22448597]
- Xu T, et al. Predicting recurrence and progression in Chinese patients with nonmuscle-invasive bladder cancer using EORTC and CUETO scoring models. Urology. 2013;82(2):387–393. [PubMed: 23759377]
- Xylinas E, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. British Journal of Cancer. 2013;109(6):1460–1466. [PMC free article: PMC3776972] [PubMed: 23982601]
References to excluded studies (with reasons for exclusion)
- Roychowdhury DF, Hayden A, Liepa AM. Health-related quality-of-life parameters as independent prognostic factors in advanced or metastatic bladder cancer. Journal of Clinical Oncology. 2003;21(4):673–678. Reason: not relevant to PICO (MIBC) [PubMed: 12586805]
- Jeon SH, Jeon SH, Chang SG. Clinical prognostic factors for radical cystectomy in bladder cancer. Cancer Research & Treatment. 2005;37(1):48–53. Reason: population not relevant to PICO (RC cohort) [PMC free article: PMC2785423] [PubMed: 19956510]
- Samaratunga H, Khoo K. Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathology. 2004;45(1):55–64. Reason: not study of prognosis . [PubMed: 15228444]
- Pich A, et al. Proliferative activity is the most significant predictor of recurrence in noninvasive papillary urothelial neoplasms of low malignant potential and grade 1 papillary carcinomas of the bladder. Cancer. 2002;95(4):784–790. Reason: prognostic factors not in PICO . [PubMed: 12209722]
- Oosterlinck W. Diagnostic and Prognostic Factors in Non-Muscle-Invasive Bladder Cancer and Their Influence on Treatment and Outcomes. European Urology, Supplements. 2008;7(7):516–523. Reason: expert review .
- van Rhijn BW. Combining molecular and pathologic data to prognosticate non-muscle-invasive bladder cancer. Urologic Oncology. 2012;30(4):518–523. [Review] Reason: expert review . [PubMed: 22742564]
- van den Bosch S, Alfred WJ. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. European Urology. 2011;60(3):493–500. [Review] Reason: not a prognostic study . [PubMed: 21664041]
- Herrmann E, et al. The prognostic impact of pelvic lymph node metastasis and lymphovascular invasion on bladder cancer. International Journal of Urology. 2008;15(7):607–611. Reason: population not relevant to PICO (MIBC) [PubMed: 18462352]
- Kim SP, et al. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. Journal of Urology. 2012;188(2):405–409. Reason: population not relevant to PICO (RC cohort) [PubMed: 22704101]
- Ehdaie B, et al. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. Journal of Urology. 2012;187(1):74–79. Reason: population not relevant to PICO (RC cohort) [PMC free article: PMC3692007] [PubMed: 22088332]
- Abdollah F, et al. Survival after radical cystectomy of non-bilharzial squamous cell carcinoma vs. urothelial carcinoma: a competing-risks analysis. BJU International. 2012;109(4):564–569. Reason: population not relevant to PICO (RC cohort) [PubMed: 21810161]
- Rodriguez FO, et al. Clinical predictive factors of poor outcome in patients with stage pT0 disease at radical cystectomy. Journal of Urology. 2011;186(2):442–447. Reason: population not relevant to PICO (RC cohort) [PubMed: 21679981]
- Resnick MJ, et al. Longitudinal evaluation of the concordance and prognostic value of lymphovascular invasion in transurethral resection and radical cystectomy specimens. BJU International. 2011;107(1):46–52. Reason: population not relevant to PICO (MIBC and NMIBC not reported separately) [PubMed: 20880163]
- Wasco MJ, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology. 2007;70(1):69–74. Reason: population not relevant to PICO (MIBC) [PubMed: 17656211]
- Wang J, et al. Clinical features of sarcomatoid carcinoma (carcinosarcoma) of the urinary bladder: analysis of 221 cases. Sarcoma. 2010;2010:2010. Reason: not relevant to PICO (not prognostic study) [PMC free article: PMC2913791] [PubMed: 20706685]
- Shariat SF, et al. International validation of the prognostic value of lymphovascular invasion in patients treated with radical cystectomy. BJU International. 2010;105(10):1402–1412. Reason: population not relevant to PICO (MIBC, RC cohort) [PubMed: 20132195]
- Ploeg M, et al. Clinical epidemiology of nonurothelial bladder cancer: analysis of the Netherlands Cancer Registry. Journal of Urology. 2010;183(3):915–920. Reason: population not relevant to PICO (MIBC) [PubMed: 20083267]
- Bolenz C, et al. Lymphovascular invasion is an independent predictor of oncological outcomes in patients with lymph node-negative urothelial bladder cancer treated by radical cystectomy: a multicentre validation trial. BJU International. 2010;106(4):493–499. Reason: population not relevant to PICO (MIBC) [PubMed: 20067452]
- Chang WC, Chang YH, Pan CC. Prognostic significance in substaging ofT1 urinary bladder urothelial carcinoma on transurethral resection. American Journal of Surgical Pathology. 2012;36(3):454–461. Reason: prognostic factors not relevant to PICO . [PubMed: 22261706]
- Van Der Meijden A, et al. The role and impact of pathology review on stage and grade assessment of stages Ta and T1 bladder tumors: a combined analysis of 5 European Organization for Research and Treatment of Cancer Trials. Journal of Urology. 2000;164(5):1533–1537. Reason: not prognostic study . [PubMed: 11025698]
- Rogers CG, et al. Clinical outcomes following radical cystectomy for primary nontransitional cell carcinoma of the bladder compared to transitional cell carcinoma of the bladder. Journal of Urology. 2006;175(6):2048–2053. Reason: RC cohort, NMIBC and MIBC not reported separately . [PubMed: 16697800]
- Wasco MJ, et al. Nested variant of urothelial carcinoma: a clinicopathologic and immunohistochemical study of 30 pure and mixed cases. Human Pathology. 2010;41(2):163–171. Reason: population not relevant to PICO (MIBC) [PubMed: 19800100]
- Wright JL, et al. Differences in survival among patients with sarcomatoid carcinoma, carcinosarcoma and urothelial carcinoma of the bladder. Journal of Urology. 2007;178(6):2302–2306. Reason: population not relevant to PICO (MIBC and NMIBC not reported separately) [PubMed: 17936803]
- Pillai R, Wang D, Abel P. Is the proposed EORTC prognostic algorithm for pTa/pT1 bladder transitional cell cancer (TCC) valid in a routine clinical setting? European Urology Supplements. 2007;6(2):172–172. Reason: duplicate, abstract only .
- Streeper NM, et al. The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. BJU International. 2009;103(4):475–479. Reason: population not relevant to PICO (stage 1+2 reported together) [PubMed: 18990174]
- May M, et al. Pathological upstaging detected in radical cystectomy procedures is associated with a significantly worse tumour-specific survival rate for patients with clinical T1 urothelial carcinoma of the urinary bladder. Scandinavian Journal of Urology & Nephrology. 2011;45(4):251–257. Reason: population not relevant (T2 and RC cohort) [PubMed: 21388337]
- Tilki D, et al. Characteristics and outcomes of patients with clinical carcinoma in situ only treated with radical cystectomy: an international study of 243 patients. Journal of Urology. 2010;183(5):1757–1763. Reason: population not relevant (CIS refractory to BCG only, RC cohort) [PubMed: 20299059]
- Cho KS. Differences in Tumor Characteristics and Prognosis in Newly Diagnosed Ta, T1 Urothelial Carcinoma of Bladder According to Patient Age. Urology. 2009;73(4):828–832. Reason: outcomes not relevant to PICO . [PubMed: 19195693]
- Rosevear HM. Usefulness of the Spanish Urological Club for Oncological Treatment scoring model to predict nonmuscle invasive bladder cancer recurrence in patients treated with intravesical bacillus Calmette-Guerin plus interferon-alpha. Journal of Urology. 2011;185(1):67–71. Reason: not relevant to PICO (CUETO prognostic factors) [PubMed: 21074202]
- Manoharan M, et al. Lymphovascular invasion in radical cystectomy specimen: is it an independent prognostic factor in patients without lymph node metastases? World Journal of Urology. 2010;28(2):233–237. Reason: not relevant to PICO (RC cohort, includes MIBC) [PubMed: 19597735]
- Gaya JM, et al. The case for conservative management in the treatment of patients with non-muscle-invasive micropapillary bladder carcinoma without carcinoma in situ. Canadian Journal of Urology. 2010;17(5):5370–5376. Reason: not prognostic study / mostly MIBC . [PubMed: 20974029]
- Comperat E, et al. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology. 2010;42(7):650–654. Reason: not relevant to PICO (mostly MIBC) [PubMed: 21080874]
- Mulders PF, et al. Prognostic factors in pTa-pT1 superficial bladder tumours treated with intravesical instillations. The Dutch South-Eastern Urological Collaborative Group. British Journal of Urology. 1994;73(4):403–408. Reason: prognostic factor not relevant to PICO . [PubMed: 8199828]
- Pillai R, et al. Do standardised prognostic algorithms reflect local practice? Application of EORTC risk tables for non-muscle invasive (pTa/pT1) bladder cancer recurrence and progression in a local cohort. Thescientificworldjournal. 2011;11:751–759. Reason: insufficient validation cohort, no patients in some groups . [PMC free article: PMC5720000] [PubMed: 21479347]
- Ather MH, Zaidi M. Predicting recurrence and progression in non-muscle-invasive bladder cancer using European organization of research and treatment of cancer risk tables. Urology Journal. 2009;6(3):189–193. Reason: insufficient validation cohort, no patients in some risk groups . [PubMed: 19711273]
- Alkhateeb SS, et al. Long-term prognostic value of the combination of EORTC risk group calculator and molecular markers in non-muscle-invasive bladder cancer patients treated with intravesical Bacille Calmette-Guerin. Urology annals. 2011;3(3):119–126. Reason: insufficient validation cohort, no patients in some risk groups . [PMC free article: PMC3183702] [PubMed: 21976923]
- Sylvester R, et al. Prognostic factors in patients with intermediate and high risk stage Ta T1 papillary carcinoma of the bladder treated with maintenance epirubicin or maintenance bacillus Calmette-Guerin. Results of EORTC GU group study 30911. Journal of Urology. 2008;179(4):586–586. Reason: abstract only, insufficient information for inclusion .
- Wang JK, et al. Outcomes following radical cystectomy for micropapillary bladder cancer versus pure urothelial carcinoma: a matched cohort analysis. World Journal of Urology. 2012;30(6):801–806. Reason: population not relevant to PICO (MIBC) [PubMed: 23132611]
- Rodriguez FO, Palou J. Predictive factors for recurrence progression and cancer specific survival in high-risk bladder cancer. Current Opinion in Urology. 2012;22(5):415–420. [Review] Reason: expert review . [PubMed: 22825460]
- Yamazaki K, Kumamoto Y, Tsukamoto T. Expression of squamous cell carcinoma-associated antigen in grade 3 pT1 transitional cell carcinoma of the bladder and prediction of its progression and intravesical recurrence. Cancer. 1993;72(12):3676–3684. Reason: prognostic factor not relevant to PICO, no SCC component in carcinoma . [PubMed: 7902777]
- Van Der Aa MNM. Clinical and pathological prognostic factors for recurrence, progression and mortality in non-muscle invasive bladder cancer: A meta-analysis. Current Urology. 2009;3(3):113–123. Reason: prognostic factors not relevant to PICO .
- Pan CC, et al. Constructing prognostic model incorporating the 2004 WHO/ISUP classification for patients with non-muscle-invasive urothelial tumours of the urinary bladder. Journal of Clinical Pathology. 2010;63(10):910–915. Reason: prognostic factors not relevant to PICO . [PubMed: 20876324]
- Lee CT, et al. Lymphovascular invasion is an independent predictor of survival in cT1 bladder cancer. Journal of Urology. 2005;173(4):246–246. Reason: abstract only .
- Kohjimoto Y. External validation of eortc and cueto scoring models to predict recurrence and progression in patients with nonmuscle invasive bladder cancer treated with bacillus calmette-guerin. Journal of Urology. 2012;187(4):E716–E717. Reason: abstract only .
- Ajili F, et al. The efficiency of the EORTC scoring system for the prediction of recurrence and progression of non-muscle-invasive bladder cancer treated by bacillus Calmette-Guerin immunotherapy. Ultrastructural Pathology. 2013;37(4):249–253. Reason: insufficient validation study . [PubMed: 23899093]
- Borkowska EM, et al. EORTC risk tables - their usefulness in the assessment of recurrence and progression risk in non-muscle-invasive bladder cancer in Polish patients. Central European Journal of Urology. 2013;66(1):14–20. Reason: insufficient validation study . [PMC free article: PMC3921849] [PubMed: 24578979]
- Walczak R, Bar K, Walczak J. The value of EORTC risk tables in evaluating recurrent non-muscle-invasive bladder cancer in everyday practice. Central European Journal of Urology. 2014;66(4):418–422. Reason: insufficient data for inclusion – outcomes reported not relevant to PICO . [PMC free article: PMC3992449] [PubMed: 24757531]
Evidence tables
Download PDF (369K)
3.2. Managing non-muscle-invasive bladder cancer
3.2.1. Intravesical therapy
Review question: What are the most effective adjuvant intravesical therapy (chemotherapy or immunotherapy) regimens for low-risk, intermediate and high-risk non-muscle invasive bladder cancer?
Rationale
The risk of recurrence can be reduced by the administration of chemotherapy medication, in liquid form, into the bladder (intravesical chemotherapy). This can be done immediately, or shortly after telescopic removal of the tumour (transurethral resection), and subsequently, as a planned outpatient procedure. Several different chemotherapy drugs have been used, and studied.
There is debate (and variation) about which patients with which sort of LRNMIBC should be treated with intravesical chemotherapy, including whether patients with small or very small tumours should be treated, and what sort of recurrent tumours should be treated.
The advantage of not being treated is that no side effects of treatment are suffered, whereas the benefit of being treated may be that recurrence becomes less likely. The disadvantage of not being treated is that there is no reduction in the risk of recurrence, and the disadvantage of being treated is that side effects (such as urine infection, bladder pain, and genital rashes) are suffered.
Instillation of BCG vaccine is also offered to some patients who have recurrence of LRNMIBC following previous intravesical chemotherapy. The side effects of BCG include irritation of the bladder, urine infection, occasional rare consequences probably related to the effects of BCG on the body's immune system, and very rare infections with the BCG bacteria. These side effects need to be considered in a consideration of the advantages and disadvantages of BCG equivalent to the consideration of the advantages and disadvantages of intravesical chemotherapy.
The topic is being considered because LRNMIBC is common, recurrence is common, and because intravesical chemotherapy has significant efficacy, but the pattern of disease is not homogeneous, meaning the grade, size, number and recurrence history of tumours can combine to present a significantly mixed group of patients and tumours, so that determining which patients with which tumours should be treated is an important area for guidance.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with newly diagnosed NMIBC following first TUR Subgroups: Male/female Low/intermediate-risk NMIBC High-risk NMIBC | Intravesical chemotherapy/BCG Single installation/ Induction course/ Maintenance BCG Mitomycin C Epirubicin Doxorubicin (adriamycin) Gemcitabine Eoquin | Each other None |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH) using a systematic review and randomised trials filter, with no date limit.
Selection of studies
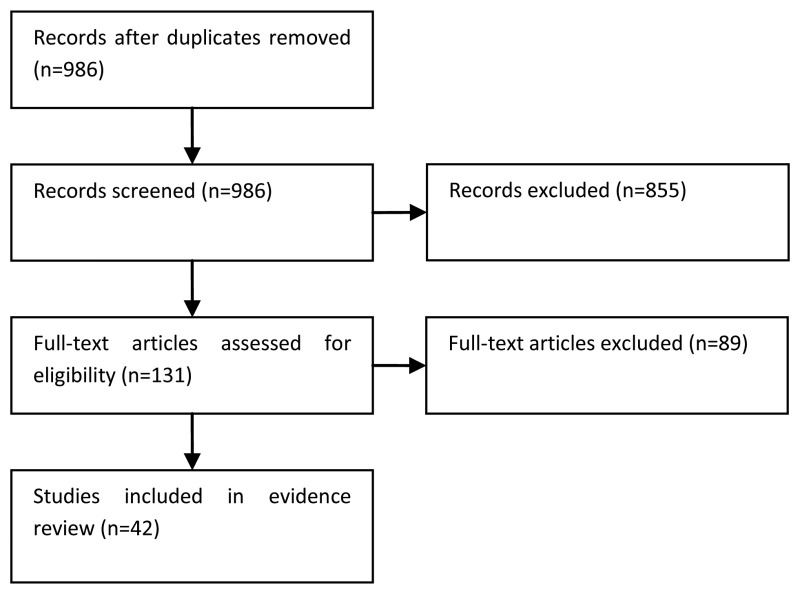
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. Systematic reviews and randomised trials were selected for this review.
Data synthesis
Dichotomous data (e.g. number of events and number of participants) from systematic reviews and randomised trials were presented in RevMan when possible. Overall risk ratios are presented in GRADE and forest plots are also provided. The evidence was analysed by gender and risk subgroups where appropriate. Consideration was given to immediate single installation therapy, induction therapy and maintenance therapy. Intravesical chemotherapy agents were analysed together with specific agents included as subgroups.
RESULTS
Study quality and results
The quality and results of the included studies are summarised in GRADE evidence profiles (Tables 39-65).
Table 39
GRADE evidence profile: TUR + BCG versus TUR alone.
Table 40
GRADE evidence profile: TUR + BCG versus TUR + other treatment (chemotherapy or other immunotherapy) or TUR alone.
Table 41
GRADE evidence profile: TUR + BCG versus TUR + other treatment (chemotherapy or other immunotherapy) of TUR alone for T1G3 bladder cancer.
Table 42
GRADE evidence profile: TUR + chemotherapy versus TUR alone.
Table 43
GRADE evidence profile: TUR+ one single post-operative chemotherapy instillation versus TUR alone.
Table 44
GRADE evidence profile: TUR + single dose epirubicin (100mg) versus TUR + double dose epirubicin (2×100mg).
Table 45
GRADE evidence profile: TUR + 2×20mg/40ml epirubicin versus TUR + 2×50mg/100ml epirubicin versus TUR only.
Table 46
GRADE evidence profile: Adriamycin versus Epirubicin.
Table 47
GRADE evidence profile: TUR + chemotherapy versus TUR + BCG.
Table 48
GRADE evidence profile: TUR + chemotherapy versus TUR + BCG for CIS only.
Table 49
GRADE evidence profile: BCG versus MMC.
Table 50
GRADE evidence profile: BCG versus Epirubicin.
Table 51
GRADE evidence profile: BCG versus Gemcitabine.
Table 52
GRADE evidence profile: Maintenance BCG versus induction BCG.
Table 53
GRADE evidence profile: Standard dose BCG (81mg) versus reduced dose BCG (27mg).
Table 54
GRADE evidence profile: Low dose BCG (27mg) versus very low dose BCG (13.5mg).
Table 55
GRADE evidence profile: Standard dose BCG (81mg) versus reduced dose BCG (27mg).
Table 56
GRADE evidence profile: Standard dose BCG (81mg) versus reduced dose BCG (54mg).
Table 57
GRADE evidence profile: 120mg BCG versus 80mg BCG versus 40mg BCG.
Table 58
GRADE evidence profile: One immediate instillation chemotherapy versus one instillation plus maintenance.
Table 59
GRADE evidence profile: One immediate instillation followed by short-term versus long-term instillations during 12 months.
Table 60
GRADE evidence profile: One immediate instillation chemotherapy versus delayed instillations to month 12.
Table 61
GRADE evidence profile: One immediate instillation chemotherapy + additional instillations during 6 mo versus delayed instillations during 6 mo.
Table 62
GRADE evidence profile: One immediate instillation chemotherapy + additional instillations during 12 mo versus delayed instillations during 12 mo.
Table 63
GRADE evidence profile: Short-term delayed instillations versus long-term delayed instillations.
Table 64
GRADE evidence profile: Less intense or frequent schedule of chemotherapy versus more intense or frequent schedule of chemotherapy.
Table 65
GRADE evidence profile: Intravesical chemotherapy + BCG versus maintenance BCG alone.
Narrative summary of evidence
TUR + BCG versus TUR alone
Moderate quality evidence from a meta-analysis (Shelley et al., 2000) of 585 medium-high risk patients from six randomised trials (all published prior to 1999) produced an overall HR of 0.44 (95% CI 0.34 to 0.56), indicating a 56% reduction in the possibility of tumour recurrence for TUR+BCG compared to TUR alone.

Figure 37
TUR+BCG versus TUR alone. Outcome: recurrence-free survival (Shelley 2000)
29% (79/275) of the BCG group presented with a recurrence at 12 months, compared to 56% (144/257) in the TUR only group with a risk ratio (RR) of 0.54 (95% CI 0.44 to 0.66), indicating a 46% reduced risk of recurrence at 12 months with BCG compared to TUR alone.

Figure 38
TUR+BCG versus TUR alone. Outcome: Recurrence at 12 months (Shelley 2000)
Another meta-analysis (Han, 2006) provided high quality evidence from 9 RCTs and controlled observational cohort studies (1100 patients) published between 1997 and 2005. BCG+TUR was associated with a lower risk of recurrence compared to TUR alone (RR 0.59, 95% CI 0.45 to 0.78).
The systematic review by Shelley (2000) reported that the main toxicities associated with BCG were urinary frequency (71%), cystitis (67%), haematuria (23%), and fever (25%). No BCG sepsis or deaths were reported.
TUR + BCG versus TUR alone or TUR + another treatment
Moderate quality evidence from a meta-analysis (Pan, 2014) of 48 RCTs and observational cohort studies (9482 patients) reported a pooled random effects OR for recurrence of 0.59 (95% CI 0.49 to 0.71) for TUR + BCG compared to those treated with resection alone or TUR plus another treatment other than BCG, with significant heterogeneity across studies (p<0.01). Evidence from an earlier meta-analysis (Han, 2006) suggested that the effect of BCG is less conclusive when induction BCG only is given compared to control groups (RR 0.99, 95% CI 0.77 to 1.28). In the maintenance BCG subgroup the combined random effect RR is 0.65 (95% CI 0.48 to 0.88), suggesting that maintenance BCG reduces the risk of recurrence by 35%. There were no differences when studies were stratified by BCG strain. Another meta-analyses (Pan, 2008) of 13 trials or controlled studies, which compared maintenance BCG versus no maintenance BCG for T1G3 bladder cancer, reported that overall 41% of the maintenance BCG group recurred compared to 45% in the control group (RR 0.73, 95% CI 0.61, 0.88).
High quality evidence from one meta-analysis (Sylvester 2002) of 24 randomised trials with 4863 patients reported that the risk of progression was 27% lower for patients treated with BCG compared to those treated with either resection alone or TUR plus another treatment other than BCG (HR 0.73, 95% CI 0.60 to 0.88). There was no difference in the size of treatment effects across the different control groups (see figure 39 below) or according to the strain of BCG used.

Figure 39
TUR+BCG versus TUR+other treatments. Outcome: Progression (Sylvester, 2002)
No reduction in the risk of progression was seen in the four trials where maintenance BCG was not used (HR 1.28, 95% CI 0.82 to 1.98). In trials where maintenance BCG was used, the risk of progression was lower for those treated with BCG compared to the control groups (HR 0.57, 95% CI 0.44 to 0.75).
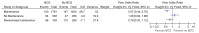
Figure 40
TUR+BCG versus TUR+another treatment. Outcome: Progression (Sylvester, 2002)
There were no significant differences in overall survival (HR 0.89, 95% CI 0.75 to 1.06) or disease-specific survival (HR 0.81, 95% CI 0.57 to 1.13) between those treated with BCG and those in the control groups. The two meta-analyses by Han (2006) and Pan (2008) both reported that drug-related and systemic toxicities were significantly more frequent in the BCG groups than chemotherapy or immunotherapy groups.
TUR + chemotherapy versus TUR alone
One systematic review and meta-analysis of 11 studies and 3703 patients with primary bladder cancer reported a Peto Odds Ratio (pOR) of 0.56 (95% CI 0.48 to 0.65) for 1-year recurrence in favour of adjuvant intravesical chemotherapy compared to TUR alone (Huncharek, 2000). However, significant statistical heterogeneity was reported and sensitivity analyses were conducted. The data were stratified by duration of treatment, which indicated that short-term therapy (≤2 months duration) reduced recurrence at 1-year (pOR 0.70, 95% CI 0.55 to 0.90) and 2-years (pOR 0.68, 95% CI 0.54 to 0.85) by approximately 30%, as compared to TUR alone (moderate quality evidence). The pooled pOR for 5 trials where patients received 2 years of chemotherapy was 0.27 (95% CI 0.19 to 0.39), indicating a 73% reduction in the risk of recurrence at 2 years for those treated with chemotherapy.
One systematic review and meta-analysis of 8 studies and 1609 patients with recurrent bladder cancer reported a pooled pOR for 1-year recurrence of 0.62 (95% CI 0.51 to 0.76), in favour of chemotherapy over TUR alone, with no evidence of statistical heterogeneity (moderate quality evidence). For the 2- and 3-year recurrence rates, significant statistical heterogeneity was reported, which was not accounted for by treatment duration. Therefore, moderate quality evidence was reported from the data when stratified by drug type (adriamycin versus other drugs). The pOR for 2-year recurrence of studies using adriamycin was 0.57 (95% CI 0.43 to 0.75), with no significant heterogeneity, indicating that drug type was a major contributor to outcome heterogeneity. Drugs other than adriamycin showed a reduction in 2-year recurrence of 73% (versus 43% for adriamycin) with an pOR of 0.27 (95% CI 0.19 to 0.37). The non-overlapping CIs indicate a significant difference in tumour reduction effect, with adriamycin appearing less effective than other drugs (e.g. thiotepa, MMC).
A systematic review and meta-analysis (Pawinski, 1996) provided moderate quality evidence from 6 randomised trials, which suggests there is uncertainty about the effect of intravesical chemotherapy on progression (HR 1.19, 95% CI 0.97 to 1.47), overall survival (HR 1.1, 95% CI 0.95 to 1.27), and disease-specific survival (HR 1.1, 95% CI not reported but effect size was non-significant), compared to TUR alone.
TUR + one post-operative instillation of chemotherapy versus TUR alone
Low to moderate quality evidence was provided from a systematic review and meta-analysis of 18 trials comparing one post-operative dose of chemotherapy with TUR alone (Abern, 2013). 36.6% (577/1576) of those in the TUR + chemotherapy group experienced a recurrence compared with 50.4% (769/1527) of those treated with TUR alone (RR 0.67, 95% CI 0.56 to 0.79), with significant statistical heterogeneity. This corresponds to a number needed to treat of 7.2 patients to avoid one recurrence. Gemcitabine and interferon α-2b did not show a benefit on recurrence, whereas the other chemotherapy agents did. The pooled RR for mitomycin C and epirubicin was 0.71 (95% CI 0.64 to 0.78), in favour of chemotherapy, with no clear dose-response relationship. Individual tumour risk factors such as recurrence, multiplicity, stage, and grade, did not appear to alter the efficacy of a single dose of chemotherapy. Funnel plots suggested the existence of publication bias with small trials contributing disproportionately to the protective effect of chemotherapy. A meta-analysis (Sylvester, 2004) of 7 trials (1476 patients) reported mild, transient, irritative bladder symptoms including dysuria, frequency and macroscopic haematuria, in approximately 10% of patients treated with intravesical chemotherapy.
TUR + chemotherapy versus TUR + BCG
One systematic review of 9 trials and 2261 patients (Huncharek, 2003) reported low quality evidence of an overall OR for 1-year recurrence of 0.89 (95% CI 0.74 to 1.07), with significant heterogeneity. Heterogeneity persisted despite stratification by chemotherapy drug type. A sensitivity analysis was therefore performed stratifying by previous intravesical chemotherapy. Pooling all studies that enrolled patients with prior chemotherapy (1480 patients) provided moderate quality evidence, with an OR of 0.54 (95% CI 0.43 to 0.69), in favour of BCG. This reflects a 46% reduction in tumour recurrence at 1-year among patients treated with BCG versus chemotherapy, and a lack of statistical heterogeneity. Pooling data from 2 studies which excluded patients previously treated with chemotherapy gave an OR of 1.82 (95% CI 1.37 to 2.41), in favour of chemotherapy. This suggests that amongst patients not previously treated, intravesical chemotherapy (MMC) reduces tumour recurrence by 82% versus BCG. Similar results were found for 2-year and 3-year recurrence when stratified by previous therapy.
One systematic review of 8 randomised trial and 2427 patients (Huncharek, 2004) randomised to either adjuvant intravesical BCG or chemotherapy provided moderate quality evidence of an OR for progression of 1.24 (95% CI 0.95 to 1.61), in favour of BCG. The confidence intervals include the value of no effect which suggests uncertainty of a difference between the two treatments in terms of progression. The total number of events in each arm was not reported. Subgroup analyses of MMC vs. BCG (4 trials, 1478 patients) provided an OR of 1.04 (0.76 to 1.42) suggesting no difference in risk of progression. The pooled OR of the two trials (781 patients) which excluded patients who had previously been treated with intravesical chemotherapy was 0.75 (0.45 to 1.25) in favour of MMC. In trials which included patients previously treated with chemotherapy the OR was 1.49 (1.09 to 2.03) in favour of BCG.
One meta-analysis (Sylvester 2005) of 9 randomised trials and 700 patients with CIS provided moderate quality evidence that 34% of complete responders treated with BCG and 50% of complete responders treated with chemotherapy recurred during follow-up (HR 0.47, 95% CI 0.31 to 0.73) in favour of BCG. 47% of patients treated with BCG and 26% treated with chemotherapy had no evidence of disease during follow-up, an absolute difference of 20% and a relative reduction of 59% in the odds of treatment failure on BCG (HR 0.41, 95% CI 0.30 to 0.56). BCG only appeared to be superior to MMC in the trials where maintenance BCG was given (see Figure 41). Data on progression was less conclusive with an HR of 0.74 (95% CI 0.45 to 1.22). Overall survival was reported in three studies (407 patients). 35.9% of patients treated with chemotherapy and 34.2% treated with BCG therapy died from any cause. Two trials reported disease-specific survival. 13.3% of patients treated with chemotherapy and 10.5% of patients treated with BCG died due to bladder cancer.

Figure 41
BCG versus MMC according to BCG maintenance. Outcome: no evidence of disease (Sylvester, 2005)
BCG vs. MMC
Moderate quality evidence was reported from one systematic review and meta-analysis (Bohle 2003) of 2749 patients from 9 prospective trials and 2 observational studies. A further trial of 92 patients was indentified and added to the pooled analysis for overall recurrence and recurrence by maintenance therapy (Mangiarotti 2008). The overall RR for recurrence was 0.77 (95% CI 0.63 to 0.95) in favour of BCG over MMC. BCG maintenance showed superiority over MMC with an RR or 0.68 (95% CI 0.55 to 0.83) (Figure 42). A dose response relationship was observed, where at least 12 instillations of BCG are required for its relevant superiority over MMC. The studies using BCG strain RIVM or RIVM plus TICE reported much weaker efficacy results for BCG than any other study in the meta-analysis. Cystitis was more frequent in the BCG group compared to the MMC group (53.8% vs. 39.2%, p<0.001). Local and systemic toxicities were more frequent in the BCG group, except for allergy and skin reactions which were more common in MMC group. The risk of cystitis was no different between maintenance BCG and no maintenance BCG. No deaths from sepsis were reported in either arm.

Figure 42
BCG versus MMC by maintenance. Outcome: Recurrence (Bohle, 2003)
Moderate quality evidence from one meta-analysis including 1277 patients (Bohle, 2004) reported no difference between BCG and MMC in terms of disease progression. Overall, 7.7% (98/1127) of the BCG group progressed versus 9.4% (107/1133) of the MMC group (RR 0.79, 95% CI 0.61 to 1.03). However, BCG did show superiority over MMC in the subgroup of BCG maintenance trials (RR 0.70, 95% CI 0.52 to 0.94). There were no significant confounding effects when stratified by BCG strain, BCG dose, risk group, MMC dose, number of MMC instillations, follow-up duration, or year of publication.

Figure 43
BCG versus MMC by BCG maintenance. Outcome: Progression (Bohle, 2004)
High quality evidence from a meta-analysis of individual patient data (Malmstrom, 2009) including 9 trials (2820 patients) reported that in trials with BCG maintenance, there was a 32% reduction in the risk of recurrence with BCG compared to MMC (HR 0.68, 95% CI 0.58 to 0.8), whilst there was a 28% risk increase for BCG trials without maintenance (HR 1.28, 95% CI 1.07 to 1.52) (see Figure 44). Maintenance BCG was more effective than MMC in both patients previously treated and those not previously treated with intravesical chemotherapy.

Figure 44
BCG versus MMC, by BCG maintenance. Outcome: Time to first recurrence (Malmstrom, 2009)
Moderate quality evidence from 7 trials (1880 patients) in the IPD meta-analyses reported that after a median follow-up of 4.8 years, 12% of patients progressed to MIBC and 24% died (of those 30% died from bladder cancer). There were no significant differences between MMC and BCG for these end-points, even when stratified by BCG maintenance and patient risk groups.
BCG versus Epirubicin (EPI)
One systematic review of 5 randomised trials (Shang, 2011), reported that the risk of recurrence was reduced in patients treated with BCG (35.5%) compared to EPI (51.4%) with a RR of 0.69 (95% CI 0.60 to 0.79), in favour of BCG. Subgroup analyses demonstrated that two trials which treated patients with Pasteur strain BCG found no significant difference in recurrence between BCG and EPI (RR 0.78, 95% CI 0.56 to 1.10).

Figure 45
BCG versus EPI. Outcome: Recurrence (Shang, 2011)
There was no significant difference between BCG and EPI for disease progression (RR 0.78, 95% CI 0.54 to 1.13). No differences were found for overall mortality (2 studies) or disease-specific mortality (2 studies). However, overall mortality was less frequent in the TICE BCG group compared to the EPI group in the study by Sylvester (2010) (RR 0.79, 95% CI 0.62 to 0.99) (see Figure 46). Drug-induced cystitis (54% versus 32%), haematuria (31% versus 16%), and systemic side-effects (35% versus 1%) were significantly more frequent with BCG than EPI. However, there was significant heterogeneity between trials for systemic side-effects due to the frequency of BCG administration. There were no significant differences for delayed or terminated treatment due to adverse events between BCG and EPI (9% versus 7%) (RR 0.91, 95% CI 0.41 to 2.04).

Figure 46
BCG versus EPI. Outcome: Overall survival (Shang, 2011)
BCG versus Gemcitabine
One systematic review by Jones (2012) reported 3 studies comparing Gemcitabine with BCG (one of these trials and the trial comparing BCG with MMC included patients who had failed BCG therapy which is covered in another topic). Heterogeneity between trials prevented pooling of data. One trial of 80 patients at intermediate risk of recurrence (primary Ta-T1, no CIS) provided low quality evidence that BCG (no maintenance) and Gemcitabine showed similar rates of recurrence (25% vs. 30%) and progression, with significantly more adverse effects with BCG (Bendary 2011). Moderate quality evidence was provided by one trial of 64 high risk patients, which reported that recurrence rate was higher for Gemcitabine than BCG (53% vs. 28%) and time to recurrence was shorter with Gemcitabine (25.6 months vs. 39.4 months). No patients in either group had disease progression at a mean follow-up of 44 months. Local and systemic toxicity were similar between groups. In this trial maintenance therapy for non-recurring patients in each group was up to 36 months duration (Porena, 2010).
Duration of BCG
In 6 meta-analyses (Sylvester, 2002; Han, 2006; Sylvester, 2005; Malmstrom, 2009; Bohle, 2003; Bohle 2004) BCG was superior to chemotherapy only if a maintenance schedule was used.
Six trials of maintenance versus induction BCG were indentified which varied in the population included and the schedule and duration of maintenance therapy. High quality evidence from five of these trials demonstrated that 53.9% of patients in the BCG induction arm had a recurrence, compared to 37.6% in the maintenance BCG arm (RR 0.70, 95% CI 0.60 to 0.81). Moderate quality evidence from 5 trials showed that there were no overall differences in progression (27.6% versus 31.8%). However, this data should be interpreted with caution due to the variation in BCG maintenance schedules and the duration of follow-up across studies.

Figure 47
Maintenance versus induction BCG. Outcome: Recurrence
Two controlled trials published in 1987 (Hudson 1987; Badalament 1987) showed no significant benefit of maintenance BCG therapy on recurrence. A study of 384 patients with recurrent bladder cancer or CIS were randomised to BCG induction alone or BCG induction plus 3-week maintenance schedule for up to 3-years (Lamm, 2000). With a median follow-up of 7 years, maintenance BCG significantly improved median recurrence-free survival (from 36 months to 77 months, p<0.0001). 5-year survival also increased from 78% to 83% with BCG maintenance, but this difference was non-significant (p=0.08). A Japanese Cooperative study (Hinotsu, 2010) of 115 patients with multiple or recurrent NMIBC without CIS, reported that 2-year recurrence-free survival was significantly longer in the combined BCG groups compared with 9 weeks Epirubicin therapy, and for BCG maintenance versus induction BCG only (Recurrence rate: 12% versus 33%). No difference in progression was reported between BCG maintenance and non-maintenance, although there were no cases of progression in the maintenance arm. A further study randomised 53 patients (88% with CIS) who had achieved a complete response after induction BCG therapy into maintenance (4 instillations) therapy or observation (Koga, 2010). The 2-year recurrence free survival was higher in the maintenance group (95.8%) than the observation group (74.1%), although this was not significant. Two patients in each group died during follow-up. There were no significant changes in quality of life scores (EORTC-QLQ) in either group from induction treatment to 14 months after randomisation. Very low quality evidence from one observational study reported that overall quality of life was moderate, and more patients rated it as good during maintenance than during induction therapy (Mack 1996). Drug-related toxicities, such as dysuria, haematuria and fever, were generally more prevalent with maintenance BCG than with induction BCG.
Dose of BCG
Two trials provided moderate quality evidence of no difference in recurrence, progression, overall survival and disease-specific survival between one-third (27mg) dose and full dose (81mg) BCG. One trial (Martinez-Pineiro, 2002) included 500 patients (Ta/T1/CIS, G1-G3) and the other trial (Martinez-Pineiro, 2005) included 155 patients with T1G3 disease or CIS. Martinez-Pineiro (2002) reported that in patients with multifocal disease the standard dose was more effective against recurrences and progression than the reduced dose. Local toxicity was significantly reduced in the low dose BCG arm (53% versus 67%), and fewer patients had delayed instillations or withdrew from treatment. There were no differences between groups for severe systemic toxicities (3.8% versus 2.7%).
Moderate quality evidence from another CUETO group trial (Ojea, 2007) reported that there were no differences in recurrence-free survival between low dose BCG (27mg) and very-low dose BCG (13.5mg) in intermediate risk patients. There were no differences in time to progression and cancer-specific survival between the two BCG treatment groups. Rates of local (65.5% vs. 64.1%) and systemic (11.3% vs. 10.8%) adverse events were also similar between the two groups.
Moderate quality evidence was reported in one trial of 1355 patients randomised into 4 trial arms (Oddens, 2012). With a median follow-up of 7.1 years, there were no differences in recurrence, progression, overall survival and toxicity between one-third (27mg) dose and full dose (81mg) BCG. When results were stratified by maintenance and dose, one-third dose BCG with 1-year maintenance was suboptimal compared to full-dose BCG with 3-year maintenance (HR for disease-free interval 0.75, 95% CI 0.59 to 0.94). In intermediate-risk patients, 3 years of maintenance was more effective than 1 year in patients receiving one-third dose (HR 1.35, 95% CI 1.03 to 1.79) but not in patients receiving full-dose (HR 0.88, 95% CI 0.64 to 1.21). In high-risk patients, 3 years of maintenance was more effective than 1 year in patients receiving full dose (HR 1.61, 95% CI 1.13 to 2.30) but not in patients receiving one-third dose BCG (HR 1.01, 95% CI 0.69 to 1.47). There were no significant differences between treatment groups for the time to progression or overall survival.

Figure 48
1-year of maintenance versus 3-year of maintenance BCG according to dose and risk group. Outcome: Disease-free interval (Oddens, 2012)
Evidence statements
TUR + BCG versus TUR alone
Moderate quality evidence from a meta-analysis (Shelley et al., 2000) of 585 medium to high risk patients from six randomised trials produced an overall hazard ratio (HR) for recurrence-free survival of 0.44 (95% CI 0.34 to 0.56), indicating a 56% reduction in the risk of tumour recurrence for TUR+BCG compared to TUR alone. The main toxicities associated with BCG are urinary frequency (71%), cystitis (67%), haematuria (23%), and fever (25%). No BCG sepsis or deaths are reported.
TUR + BCG versus TUR + other treatment (chemotherapy or immunotherapy) or TUR alone
Moderate quality evidence from a meta-analysis (Pan et al., 2014) of 48 RCTs and observational cohort studies (9,482 patients) reported a pooled random effects OR for recurrence of 0.59 (95% CI 0.49 to 0.71) for TUR + BCG compared to those treated with resection alone or TUR plus another treatment other than BCG, with significant heterogeneity across studies (p<0.01). Evidence from an earlier meta-analysis (Han & Pan, 2006) suggested that the effect of BCG is less conclusive when induction BCG only is given compared to control groups (RR 0.99, 95% CI 0.77 to 1.28). In the maintenance BCG subgroup the combined random effect RR is 0.65 (95% CI 0.48 to 0.88), suggesting that maintenance BCG reduces the risk of recurrence by 35%. Moderate quality evidence from a meta-analysis of 13 trials or controlled studies comparing maintenance BCG versus no maintenance BCG for T1G3 bladder cancer, reports that overall 41% of the maintenance BCG group recurred compared to 45% in the control group (RR 0.73, 95% CI 0.61, 0.88) (Pan et al., 2008).
High quality evidence from one meta-analysis of 24 randomised trials with 4863 patients, suggests that the risk of progression was 27% lower for patients treated with BCG compared to those treated with either resection alone or TUR plus another treatment other than BCG (HR 0.73, 95% CI 0.60 to 0.88) (Sylvester et al., 2002). No reduction in the risk of progression was seen in the four trials where maintenance BCG was not used (HR 1.28, 95% CI 0.82 to 1.98). There is uncertainty of any difference for overall survival (HR 0.89, 95% CI 0.75 to 1.06) and disease-specific survival (HR 0.81, 95% CI 0.57 to 1.13) between those treated with BCG and those in the control groups. Moderate quality evidence from the two meta-analyses by Han & Pan (2006) and Pan et al. (2008) both report that drug-related and systemic toxicities are significantly more frequent in the BCG groups than chemotherapy or immunotherapy groups.
TUR + chemotherapy versus TUR alone
One systematic review and meta-analysis of 11 studies and 3,703 patients with primary bladder cancer provides a Peto Odds Ratio (pOR) of 0.56 (95% CI 0.48 to 0.65) for one-year recurrence in favour of adjuvant intravesical chemotherapy compared to TUR alone (Huncharek et al., 2000). However, significant statistical heterogeneity is reported and sensitivity analyses were conducted. The data were stratified by duration of treatment, which indicates that short-term therapy (≤2 months duration) reduces recurrence at one-year (pOR 0.70, 95% CI 0.55 to 0.90) and two-years (pOR 0.68, 95% CI 0.54 to 0.85) by approximately 30%, as compared to TUR alone (moderate quality evidence). The pooled pOR for five trials where patients received two years of chemotherapy is 0.27 (95% CI 0.19 to 0.39), indicating a 73% reduction in the risk of recurrence at two-years for those treated with chemotherapy.
Moderate quality evidence from one meta-analysis of eight studies and 1,609 patients with recurrent bladder cancer provides a pooled OR for one-year recurrence of 0.62 (95% CI 0.51 to 0.76), in favour of chemotherapy over TUR alone, with no evidence of statistical heterogeneity (Huncharek et al., 2001). For the two- and three-year recurrence rates, significant statistical heterogeneity was reported, which was not accounted for by treatment duration. Therefore, moderate quality evidence is provided from the data when stratified into drug type (adriamycin versus other drugs). The OR for two-year recurrence of studies using adriamycin is 0.57 (95% CI 0.43 to 0.75), with no significant heterogeneity, indicating that drug type was a major contributor to outcome heterogeneity. Drugs other than adriamycin showed a reduction in two-year recurrence of 73% (versus 43% for adriamycin) with an OR of 0.27 (95% CI 0.19 to 0.37).
Another systematic review and meta-analysis provides moderate quality evidence from six randomised trials, which suggests there is uncertainty about the effect of intravesical chemotherapy on progression (HR 1.19, 95% CI 0.97 to 1.47), overall survival (HR 1.1, 95% CI 0.95 to 1.27), and disease-specific survival (HR 1.1, 95% CI not reported but effect size was non-significant), compared to TUR alone (Pawinski et al., 1996).
TUR + one post-operative instillation of chemotherapy versus TUR alone
Low to moderate quality evidence is reported from a systematic review and meta-analysis of 18 trials comparing one post-operative dose of chemotherapy with TUR alone (Abern et al., 2013). 36.6% (577/1576) of those in the TUR + chemotherapy group experienced a recurrence compared with 50.4% (769/1527) of those treated with TUR alone (RR 0.67, 95% CI 0.56 to 0.79), with significant statistical heterogeneity. This corresponds to a number needed to treat of 7.2 patients to avoid one recurrence. Gemcitabine and interferon α-2b does not show a benefit on recurrence, whereas the other chemotherapy agents do. The pooled RR for mitomycin C and epirubicin is 0.71 (95% CI 0.64 to 0.78), in favour of chemotherapy, with no clear dose-response relationship. Funnel plots suggest publication bias with small trials contributing disproportionately to the protective effect of chemotherapy. Progression and survival are not reported. A meta-analysis (Sylvester et al., 2004) of seven trials (1476 patients) reports mild, transient, irritative bladder symptoms including dysuria, frequency and macroscopic haematuria, in approximately 10% of patients treated with one single post-operative dose of intravesical chemotherapy.
TUR+ single dose epirubicin versus TUR + double dose Epirubicin
Low quality evidence from one randomised trial of 143 patients without CIS suggests no difference in recurrence or progression between patients treated with a single dose of 100mg epirubicin within six hours of TUR and those given a second dose of 100mg epirubicin 12-18 hours after TUR (Turkeri et al., 2010).
Moderate quality evidence from one trial of 270 patients without CIS reports that two instillations of 50mg epirubicin within 24 hours of TUR is associated with longer recurrence-free survival than TUR alone (38 months versus 13 months, p=0.004). Recurrence-free survival with two instillations of lower dose epirubicin (20mg/40ml) is not significantly longer than TUR alone (24 months versus 13 months, p=0.163). There are no significant differences between 2×50mg and 2×20mg epirubicin (p=0.146). Local grade one toxicity was reported in 22.9% of the low dose epirubicin group and 35.6% of high dose epirubicin group (RR 0.63, 95% CI 0.39 to 1.02).
Intravesical Adriamycin versus Epirubicin
Moderate quality evidence is provided by two randomised trials comparing one year treatment with adriamycin with the same schedule of epirubicin (Eto et al., 1994; Shuin et al., 1994). There were no differences in recurrence rate (RR 1.31, 95% CI 0.72 to 2.4) or local toxicities (RR 0.73, 95% CI 0.46 to 1.15) between the two treatment arms.
Adjuvant intravesical BCG versus adjuvant intravesical chemotherapy
One systematic review of nine trials and 2,261 patients (Huncharek et al., 2003) reports low quality evidence of an overall OR for one-year recurrence of 0.89 (95% CI 0.74 to 1.07), with significant heterogeneity. Heterogeneity persisted despite stratification by chemotherapy drug type. A sensitivity analysis was therefore performed stratifying by previous intravesical chemotherapy. Pooling all studies that enrolled patients with prior chemotherapy (1480 patients) provides moderate quality evidence, with an OR of 0.54 (95% CI 0.43 to 0.69) in favour of BCG. This reflects a 46% reduction in tumour recurrence at one-year among patients treated with BCG versus chemotherapy, and a lack of statistical heterogeneity. Pooling data from two studies which excluded patients previously treated with chemotherapy gives an OR of 1.82 (95% CI 1.37 to 2.41), in favour of chemotherapy. This suggests that amongst patients not previously treated, intravesical chemotherapy (MMC) reduces tumour recurrence by 82% versus BCG. Similar results were found for two-year and three-year recurrence when stratified by previous therapy.
One systematic review of eight randomised trials and 2,427 patients (Huncharek et al., 2004) randomised to either adjuvant intravesical BCG or chemotherapy provides moderate quality evidence of an OR for progression of 1.24 (95% CI 0.95 to 1.61), in favour of BCG. The confidence intervals include the value of no effect which reflects uncertainty about a difference in progression between the two treatments. The total number of events in each arm is not reported. The pooled OR of the two trials (781 patients) which excluded patients who had previously been treated with intravesical chemotherapy is 0.75 (0.45 to 1.25) in favour of MMC. In trials which included patients previously treated with chemotherapy the OR is 1.49 (1.09 to 2.03) in favour of BCG.
One meta-analysis (Sylvester et al., 2005) of nine randomised trials and 700 patients with CIS provides moderate quality evidence that 34% of complete responders treated with BCG and 50% of complete responders treated with chemotherapy recurred during follow-up (HR 0.47, 95% CI 0.31 to 0.73, in favour of BCG). 47% of patients treated with BCG and 26% treated with chemotherapy had no evidence of disease during follow-up, relating to an absolute difference of 20% and a relative reduction of 59% in the odds of treatment failure on BCG (HR 0.41, 95% CI 0.30 to 0.56). BCG is only superior to MMC in the trials where maintenance BCG was given. Data on progression was less conclusive with a HR of 0.74 (95% CI 0.45 to 1.22). Overall survival is reported in three studies (407 patients). 35.9% of patients treated with chemotherapy and 34.2% treated with BCG therapy died from any cause. Two trials reported disease-specific survival. 13.3% of patients treated with chemotherapy and 10.5% of patients treated with BCG died due to bladder cancer.
BCG versus Mitomycin C (MMC)
Moderate quality evidence is reported from one meta-analysis (Bohle et al., 2003) of 2,749 patients from nine prospective trials and two observational studies. A further trial of 92 patients was indentified and added to the pooled analysis for recurrence (Mangiarotti et al., 2008). The overall RR for recurrence is 0.77 (95% CI 0.63 to 0.95) in favour of BCG over MMC. High quality evidence from a meta-analysis of individual patient data (Malmstrom et al., 2009) including nine trials (2,820 patients) reported that in trials with BCG maintenance, there is a 32% reduction in the risk of recurrence with BCG compared to MMC (HR 0.68, 95% CI 0.58 to 8), whilst there is a 28% risk increase for BCG trials without maintenance (HR 1.28, 95% CI 1.07 to 1.52). Maintenance BCG is more effective than MMC in both patients previously treated and those not previously treated with intravesical chemotherapy.
Moderate quality evidence from one meta-analysis including 1,277 patients (Bohle et al., 2004) reports no difference between BCG and MMC in terms of disease progression (RR 0.79, 95% CI 0.61 to 1.03). However, BCG does show superiority over MMC in the subgroup of BCG maintenance trials (RR 0.70, 95% CI 0.52 to 0.94). Moderate quality evidence from seven trials (1,880 patients) in the IPD meta-analyses reports that after a median follow-up of 4.8 years, 12% of patients progressed and 24% died (of those 30% died from bladder cancer). There are no significant differences between MMC and BCG for these end-points, even when stratified by BCG maintenance and patient risk groups.
Cystitis was more frequent in the BCG group compared to the MMC group (53.8% vs. 39.2%, p<0.001). Local and systemic toxicities were more frequent in the BCG group, except for allergy and skin reactions which were more common in MMC group. The risk of cystitis was no different between maintenance BCG and no maintenance BCG. No deaths from sepsis were reported in either arm (Bohle et al., 2003).
BCG versus Epirubicin (EPI)
Moderate quality evidence from one meta-analysis of five randomised trials (Shang et al., 2011), reports that the risk of recurrence was reduced in patients treated with BCG (35.9%) compared to EPI (51.4%) with a RR of 0.69 (95% CI 0.60 to 0.79), in favour of BCG. Low quality evidence from a subgroup analysis demonstrates no significant difference in recurrence between BCG and EPI in two trials using Pasteur strain BCG (RR 0.78, 95% CI 0.56 to 1.10). Low quality evidence for disease progression demonstrated that there are no significant differences between BCG and EPI (RR 0.78, 95% CI 0.54 to 1.13). No differences are reported for overall mortality (two studies) or disease-specific mortality (two studies). However, overall mortality is less frequent in the TICE BCG group compared to the EPI group in the study by Sylvester et al. (2010) (RR 0.79, 95% CI 0.62 to 0.99). Drug-induced cystitis (54% versus 32%), haematuria (31% versus 16%), and systemic side-effects (35% versus 1%) are significantly more frequent with BCG than EPI. However, there is significant heterogeneity between trials for systemic side-effects due to the frequency of BCG administration across studies. Moderate quality evidence from four randomised trials suggests there are no significant differences for delayed or terminated treatment due to adverse events between BCG and EPI (9% versus 7%) (RR 0.91, 95% CI 0.41 to 2.04).
BCG versus Gemcitabine
One systematic review by Jones et al. (2012) includes three studies comparing Gemcitabine with BCG. Heterogeneity between trials prevented pooling of data. One trial of 80 patients at intermediate risk of recurrence (primary Ta-T1, no CIS) provides low quality evidence that BCG (no maintenance) and Gemcitabine showed similar rates of recurrence (25% vs. 30%) and progression, with significantly more adverse effects with BCG. Moderate quality evidence is provided by one trial of 64 high risk patients, which reports that recurrence rate is higher for Gemcitabine than BCG (53% vs. 28%) and time to recurrence is shorter with Gemcitabine (25.6 months vs. 39.4 months). No patients in either group had disease progression at a mean follow-up of 44 months. Local and systemic toxicity are similar between groups. In this trial, maintenance therapy for non-recurring patients in each group was up to 36 months duration. No evidence about survival is reported.
Maintenance BCG versus induction BCG
Six trials of maintenance versus induction BCG were indentified which vary in the population included and the schedule and duration of maintenance therapy. High quality evidence from five of these trials reports that 53.9% of patients in the BCG induction arm had a recurrence, compared to 37.6% in the maintenance BCG arm (RR 0.70, 95% CI 0.60 to 0.81). Moderate quality evidence from five trials suggests that there are no overall differences in progression (27.6% versus 31.8%). However, this data should be interpreted with caution due to the variation in BCG maintenance schedules and the duration of follow-up across studies. There are no differences between groups in terms of overall survival and disease-specific survival. Moderate quality evidence from two trials suggests that dysuria is more frequent in the maintenance arm (88.9% versus 68.3%). Rates of fever/chills are not different between groups (RR 1.47, 95% CI 0.88 to 2.44).
One trial reported moderate quality evidence that there are no significant changes in quality of life scores (EORTC-QLQ) in either group from induction treatment to 14 months after randomisation (Koga et al., 2010). Very low quality evidence from one observational study reports that overall quality of life was moderate, and more patients rated it as good during maintenance than during induction therapy (Mack et al., 1996).
Dose of BCG
Low dose versus standard dose BCG
Two trials provide moderate quality evidence of no difference in recurrence, progression, overall survival and disease-specific survival between one-third (27mg) dose and full dose (81mg) BCG. One trial (Martinez-Pineiro et al., 2002) included 500 patients (Ta/T1/CIS, G1-G3) and the other trial (Martinez-Pineiro et al., 2005) included 155 patients with T1G3 disease or CIS. Martinez-Pineiro et al. (2002) reports that, in patients with multifocal disease, standard dose BCG is more effective against recurrences and progression than reduced dose BCG. Local toxicity is significantly reduced in the low dose BCG arm (53% versus 67%), and fewer patients have delayed instillations or withdraw from treatment. There are no differences between groups for severe systemic toxicities (3.8% versus 2.7%).
One trial of 80 patients provides low quality evidence of no difference in recurrence, progression or cystitis between patients receiving 81mg BCG versus those receiving 54mg BCG (Yalcinkaya et al., 1998). One trial of 128 patients randomised into three arms, provides low quality evidence of no difference in recurrence rates between 120mg BCG, 80mg BCG and 40mg BCG. No patients had disease progression. Both local toxicity and systemic toxicity were reduced with lower dose of BCG (Agrawal et al., 2007).
Low dose versus very low dose BCG
Moderate quality evidence from one trial (Ojea et al., 2007) suggests that there are no differences in recurrence-free survival between low dose BCG (27mg) and very-low dose BCG (13.5mg) in intermediate risk patients. There are no differences in time to progression and cancer-specific survival between the two BCG treatment groups. Rates of local (65.5% vs. 64.1%) and systemic (11.3% vs. 10.8%) adverse events are also similar between the two groups.
Low dose and standard dose with 1 year or 3 year maintenance
Moderate quality evidence is provided by one trial of 1,355 patients randomised into four trial arms (Oddens et al., 2012). With a median follow-up of 7.1 years, no differences are reported for recurrence, progression, overall survival and toxicity between one-third (27mg) dose and full dose (81mg) BCG. When results are stratified by maintenance and dose, one-third dose BCG with one-year maintenance is suboptimal compared to full-dose BCG with three-year maintenance (HR for disease-free interval 0.75, 95% CI 0.59 to 0.94). In intermediate-risk patients, three years of maintenance is more effective than one year in patients receiving one-third dose (HR 1.35, 95% CI 1.03 to 1.79) but not in patients receiving full-dose (HR 0.88, 95% CI 0.64 to 1.21). In high-risk patients, three years of maintenance is more effective than one year in patients receiving full dose (HR 1.61, 95% CI 1.13 to 2.30) but not in patients receiving one-third dose BCG (HR 1.01, 95% CI 0.69 to 1.47). No significant differences are reported between treatment groups for the time to progression or overall survival.
The schedule and duration of intravesical chemotherapy
One systematic review of randomised trials (Sylvester et al., 2008) which compared intravesical instillations with respect to their number, frequency, timing, duration, dose, or dose intensity concludes that the optimal schedule and duration of intravesical chemotherapy after an immediate instillation remains unknown. In low-risk patients, one immediate instillation of epirubicin may not be less effective than a delayed course of multiple instillations. In patients with multiple tumours, one immediate instillation is insufficient treatment. Additional instillations may further reduce the recurrence rate; however, there is no conclusive evidence regarding their optimal duration. A short intensive schedule of instillations within the first 3-4 months after an immediate instillation may be as effective as longer-term treatment schedules. Instillations during ≥1 year in intermediate-risk patients seem effective only when an immediate instillation has not been given. Higher drug concentrations and optimization of the drug's concentration in the bladder may provide better results.
Chemotherapy + maintenance BCG versus maintenance BCG alone
Low quality evidence is provided by a systematic review of four randomised trials (801 patients) comparing sequential chemotherapy added to maintenance BCG with maintenance BCG alone (Houghton et al., 2012). A further study of 96 patients with CIS which compared MMC and BCG with BCG alone was also identified and added to the meta-analysis (Oosterlinck et al., 2011). The dose and duration of intravesical therapies used and the average length of follow-up varies across trials. Meta-analysis of five trials provides low quality evidence of uncertainty of a difference in recurrence between the combination arms (42.6%) and the BCG-alone arms (46.7%) (RR 0.92, 95% CI 0.79 to 1.08), but significant heterogeneity (p=0.03). Sub-group analyses provides moderate quality evidence that adding chemotherapy to maintenance BCG was associated with lower recurrence than BCG alone for Ta or T1 disease (RR 0.75, 95% CI 0.61 to 0.92), but not for CIS (RR 1.13, 95% CI 0.93 to 1.37).
Meta-analysis of five trials provides low quality evidence of no significant difference in progression between the combination arms (11.1%) and the BCG-alone arms (13%) (RR 0.84, 95% CI 0.59 to 1.20), but significant heterogeneity (p=0.03). Sub-group analyses provide moderate quality evidence that adding chemotherapy to maintenance BCG is associated with lower progression than BCG alone for Ta or T1 disease (RR 0.45, 95% CI 0.25 to 0.81), but not for CIS (RR 1.33, 95% CI 0.83 to 2.13). Three studies report drug-related toxicity, with no differences in cystitis, haematuria or fever between groups. The numbers of adverse events in each arm is not reported.
References to included studies
- Abern MR, et al. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. Journal of the National Comprehensive Cancer Network. 11(4):477–84. [PubMed: 23584348]
- *. Barghi MR, et al. Immediate intravesical instillation of mitomycin C after transurethral resection of bladder tumor in patients with low-risk superficial transitional cell carcinoma of bladder. Urology Journal. 2006;3(4):220–224. [PubMed: 17559045]
- *. De NC, et al. Long-term experience with early single mitomycin C instillations in patients with low-risk non-muscle-invasive bladder cancer: prospective, single-centre randomised trial. World Journal of Urology. 2011;29(4):517–521. [PubMed: 21594708]
- *. El-Ghobashy S, et al. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term follow-up. Journal of Egyptian National Cancer Institute. 2007;19(2):121–126. [PubMed: 19034342]
- *. Gudjónsson S, et al. Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. European urology. 2009;55:773–780. [PubMed: 19153001]
- *. Berrum-Svennung I, et al. A single instillation of epirubicin after transurethral resection of bladder tumors prevents only small recurrences. Journal of Urology. 2008;179(1):101–105. [PubMed: 17997459]
- *. Bohle A, et al. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. European Urology. 2009;56(3):495–503. [PubMed: 19560257]
- Agrawal MS, et al. The safety and efficacy of different doses of bacillus Calmette Guérin in superficial bladder transitional cell carcinoma. Urology. 2007;70:1075–1078. [PubMed: 18158020]
- Badalament RA, et al. A prospective randomized trial of maintenance versus nonmaintenance intravesical bacillus Calmette-Guerin therapy of superficial bladder cancer. Journal of Clinical Oncology. 1987;5(3):441–449. [PubMed: 3546618]
- Bohle A, Bock PR. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63:682–686. (Provisional abstract) [PubMed: 15072879]
- Bohle A, et al. The quality of life during intravesical bacillus Calmette-Guerin therapy. Journal of Urology. 1996;155(4):1221–1226. [PubMed: 8632536]
- Bohle A, Jocham D, Bock PR. Intravesical Bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. Journal of Urology. 2003;169:90–95. (Provisional abstract) [PubMed: 12478111]
- Eto H, et al. Comparison of the prophylactic usefulness of epirubicin and doxorubicin in the treatment of superficial bladder cancer by intravesical instillation: a multicenter randomized trial. Kobe University Urological Oncology Group. Cancer Chemotherapy & Pharmacology. 1994;35 Suppl:S46–S51. [PubMed: 7994786]
- Han RF, Pan JG. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer: a meta-analysis of randomized trials. Urology. 2006;67:1216–1223. (Structured abstract) [PubMed: 16765182]
- Hinotsu S, et al. Maintenance therapy with bacillus Calmette-Guerin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJU International. 2011;108(2):187–195. [PubMed: 21176079]
- Houghton B. Intravesical chemotherapy plus BCG in non-muscle invasive bladder cancer. A systematic review with meta-analysis. BJU International. 2012;111:977–983. [PubMed: 23253618]
- *. Ali-El-Dein B, et al. Sequential bacillus Calmette-Guerin and epirubicin versus bacillus Calmette-Guerin alone for superficial bladder tumors: a randomized prospective study. Journal of Urology. 1999;162(2):339–342. [PubMed: 10411034]
- *. Cai T, et al. Can early single dose instillation of epirubicin improve bacillus Calmette-Guerin efficacy in patients with nonmuscle invasive high risk bladder cancer? Results from a prospective, randomized, double-blind controlled study. The Journal of urology. 2008;180:110–115. [PubMed: 18485394]
- Hudson MA, et al. Single course versus maintenance bacillus Calmette-Guerin therapy for superficial bladder tumors: a prospective, randomized trial. Journal of Urology. 1987;138(2):295–298. [PubMed: 3298694]
- Huncharek M, Kupelnick B. Impact of intravesical chemotherapy versus BCG immunotherapy on recurrence of superficial transitional cell carcinoma of the bladder: metaanalytic reevaluation. American Journal of Clinical Oncology. 2003;26(4):402–407. [PubMed: 12902895]
- Huncharek M, Kupelnick B. The influence of intravesical therapy on progression of superficial transitional cell carcinoma of the bladder: a metaanalytic comparison of chemotherapy versus bacilli Calmette-Guerin immunotherapy. American journal of clinical oncology. 2004;27:522–528. (Provisional abstract) [PubMed: 15596924]
- Huncharek M, et al. Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: a meta-analysis of 3703 patients from 11 randomized trials. Journal of Clinical Epidemiology. 2000;53:676–680. (Structured abstract) [PubMed: 10941943]
- Huncharek M, McGarry R, Kupelnick B. Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer research. 2001;21:765–769. (Structured abstract) [PubMed: 11299841]
- Jones G, et al. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews. 2012 [PubMed: 22259002]
- Koga H, et al. Maintenance intravesical bacillus Calmette-Guérin instillation for Ta, T1 cancer and carcinoma in situ of the bladder: randomized controlled trial by the BCG Tokyo Strain Study Group. International journal of urology : official journal of the Japanese Urological Association. 2010;17:759–766. [PubMed: 20604814]
- Lamm DL, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. Journal of Urology. 2000;163(4):1124–1129. [PubMed: 10737480]
- Mack D. Quality of life in patients undergoing bacille Calmette-Guerin therapy for superficial bladder cancer. British Journal of Urology. 1996;78(3):369–371. [PubMed: 8881944]
- Malmstrom PU, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus Bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. European urology. 2009;56:247–256. (Structured abstract) [PubMed: 19409692]
- *. Friedrich MG, et al. Long-term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short-term intravesical chemotherapy and short-term therapy with Bacillus Calmette-Guerin (BCG) in patients with non-muscle-invasive bladder carcinoma. European urology. 2007;52:1123–1130. [PubMed: 17383080]
- *. Gardmark T, et al. Analysis of progression and survival after 10 years of a randomized prospective study comparing mitomycin-C and bacillus Calmette-Guerin in patients with high-risk bladder cancer. BJU International. 2007;99(4):817–820. [PubMed: 17244282]
- *. Jarvinen R, et al. Long-term efficacy of maintenance Bacillus Calmette-Guerin versus maintenance mitomycin C instillation therapy in frequently recurrent TAT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised Finnbladder I study with a 20-year follow-up. European urology. 2009;56:260–265. [PubMed: 19395154]
- Mangiarotti B, et al. A randomized prospective study of intravesical prophylaxis in non-musle invasive bladder cancer at intermediate risk of recurrence: mitomycin chemotherapy vs. BCG immunotherapy. Archivio italiano di urologia, andrologia : organo ufficiale [di] Società italiana di ecografia urologica e nefrologica / Associazione ricerche in urologia. 2008;80:167–171. [PubMed: 19235434]
- Martínez-Piñeiro JA, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. The Journal of urology. 2005;174:1242–1247. [PubMed: 16145378]
- Martínez-Piñeiro JA, et al. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guérin with a reduced dose of 27 mg in superficial bladder cancer. BJU international. 2002;89:671–680. [PubMed: 11966623]
- Oddens J, et al. Final Results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guerin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. European Urology. 2013;63(3):462–472. [PubMed: 23141049]
- Ojea A, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. European urology. 2007;52:1398–1406. [PubMed: 17485161]
- Oosterlinck W, et al. Sequential intravesical chemoimmunotherapy with mitomycin C and bacillus Calmette-Guérin and with bacillus Calmette-Guérin alone in patients with carcinoma in situ of the urinary bladder: results of an EORTC genito-urinary group randomized phase 2 trial (30993). European urology. 2011;59:438–446. [PubMed: 21156335]
- *. Kirkali Z. Sequential chemo-immunotherapy with mitomycin C (MMC) and bacillus calmette-guerin (BCG) versus BCG alone in patients with carcinoma in situ (CIS) of the urinary bladder. RESULTS of eortc GU group randomized phase II study 30993. Journal of Urology. 2010.:4. Conference(var.pagings)
- Palou J, et al. Control group and maintenance treatment with bacillus Calmette-Guerin for carcinoma in situ and/or high grade bladder tumors. Journal of Urology. 2001;165(5):1488–1491. [PubMed: 11342902]
- Pan J, Zhou X. A meta-analysis of randomized trials of maintenance bacillus Calmette-Guerin instillation efficacy against recurrence of T1G3 bladder tumour. Front Med China. 2008;2(3):259–263.
- Pawinski A, Sylvester R, Kurth KH, et al. A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. J Urol. 1996;156:1934. [PubMed: 8911360]
- Saika T, et al. Two instillations of epirubicin as prophylaxis for recurrence after transurethral resection of Ta and T1 transitional cell bladder cancer: a prospective, randomized controlled study. World Journal of Urology. 2010;28:413–418. [PubMed: 20054553]
- Serretta V, et al. A 1-year maintenance after early adjuvant intravesical chemotherapy has a limited efficacy in preventing recurrence of intermediate risk non-muscle-invasive bladder cancer. BJU International. 2010;106(2):212–217. [PubMed: 20070299]
- Shang PF, et al. Intravesical Bacillus Calmette-Guérin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews. 2011 [PubMed: 21563157]
- *. Sylvester RJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. European Urology. 2010;57(5):766–773. [PMC free article: PMC2889174] [PubMed: 20034729]
- *. Meijden AP, et al. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. European urology. 2003;44:429–434. [PubMed: 14499676]
- *. Meijden AP, et al. Intravesical instillation of epirubicin, bacillus Calmette-Guerin and bacillus Calmette-Guerin plus isoniazid for intermediate and high risk Ta, T1 papillary carcinoma of the bladder: a European Organization for Research and Treatment of Cancer genito-urinary group randomized phase III trial. The Journal of urology. 2001;166:476–481. [PubMed: 11458050]
- Shelley M, et al. Intravesical Bacillus Calmette-Guérin in Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews. 2000 [PMC free article: PMC7017976] [PubMed: 11034738]
- Shelley MD, et al. A systematic review of intravesical Bacillus Calmette-Guerin plus transurethral resection vs. transurethral resection alone in Ta and T1 bladder cancer (Brief record). BJU international. 2001;88:209–216. [PubMed: 11488731]
- Shuin T, et al. A phase II study of prophylactic intravesical chemotherapy with 4'-epirubicin in recurrent superficial bladder cancer: comparison of 4'-epirubicin and adriamycin. Cancer Chemotherapy & Pharmacology. 1994;35 Suppl:S52–S56. [PubMed: 7994787]
- Sylvester RJ, et al. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. Journal of Urology. 2005;174(1):86–91. [PubMed: 15947584]
- Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. Journal of Urology. 171(6 Pt 1):2186–2190. [PubMed: 15126782]
- Sylvester RJ, Oosterlinck W, Witjes JA. The schedule and duration of intravesical chemotherapy in patients with non-muscle-invasive bladder cancer: a systematic review of the published results of randomized clinical trials. European Urology. 2008;53(4):709–719. [Review] [41 refs] [PMC free article: PMC2587437] [PubMed: 18207317]
- *. Hendricksen K, et al. Comparison of three schedules of intravesical epirubicin in patients with non-muscle-invasive bladder cancer. European urology. 2008;53:984–991. [PubMed: 18248876]
- Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. Journal of Urology. 2002;168(5):1964–1970. [PubMed: 12394686]
- Türkeri L, et al. Comparison of the efficacy of single or double intravesical epirubicin instillation in the early postoperative period to prevent recurrences in non-muscle-invasive urothelial carcinoma of the bladder: prospective, randomized multicenter study. Urologia internationalis. 2010;85:261–265. [PubMed: 20332605]
- Yalcinkaya F, et al. Prospective randomized comparison of intravesical BCG therapy with standard dose versus low doses in superficial bladder cancer. International Urology & Nephrology. 1998;30(1):41–44. [Erratum appears in Int Urol Nephrol 1998;30(6):following 821] [PubMed: 9569110]
- Pan J, Liu M, Zhou X. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with non-muscle invasive bladder cancer? An update and cumulative meta-analysis. Front Med. 2014;8(2):241–249. [PubMed: 24810644]
References to excluded studies (with reasons for exclusion)
- Addeo R, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. Journal of Clinical Oncology. 2010;28(4):543–548. Reason: Relevant to another topic . [PubMed: 19841330]
- Zlotta AR, et al. What is the optimal regimen for BCG intravesical therapy? Are six weekly instillations necessary? European Urology. 2000;37(4):470–477. Reason: Not randomised trial/outcomes not relevant to PICO . [PubMed: 10765079]
- Yari H. Comparison of full-dose intravesical BCG versus half dose BCG and Mitomycin-C in treatment of patients with superficial bladder cancer. European Urology, Supplements. 2010.:6–596. Conference(var.pagings) Reason: Abstract only, insufficient data to be included .
- Witjes JA, et al. Use of maintenance intravesical bacillus Calmette-Guerin (BCG), with or without intradermal BCG, in patients with recurrent superficial bladder cancer. Long-term follow-up of a randomized phase 2 study. Urologia Internationalis. 1993;51(2):67–72. Reason: Comparison not relevant to PICO . [PubMed: 8351757]
- Wang Y. Sequential Bacillus Calmette-guerin plus Chemotherapy for Prevention of Post-operative Recurrence of Superficial Bladder Cancer: A Systematic Review. Chinese Journal of Evidence-Based Medicine. 2007;7(9):650–657. Reason: Foreign language .
- Shelley MD, et al. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials (Brief record). BJU international. 2004;93:485–490. Reason: Superseded by IPD meta-analysis by Malmstrom 2009. [PubMed: 15008714]
- Shelley M, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database of Systematic Reviews. 2003. Reason: Superseded by IPD meta-analysis by Malmstrom 2009. [PubMed: 12917955]
- Serretta V, et al. A randomised study evaluating maintenance schedule in early adjuvant chemotherapy for intermediate risk non-muscle-invasive bladder cancer. European Urology Supplements. 2008;7(3):298–298. Reason: Duplicate of Serretta 2010 .
- Perdona S, et al. Is gemcitabine an option in BCG-refractory nonmuscle-invasive bladder cancer? A single-arm prospective trial. Anti-Cancer Drugs. 2010;21(1):101–106. Reason: Relevant to another topic . [PubMed: 19858710]
- Pansadoro V, et al. Long-term follow-up of G3T1 transitional cell carcinoma of the bladder treated with intravesical bacille Calmette-Guerin: 18-year experience. Urology. 2002;59(2):227–231. Reason: Not randomised trial . [PubMed: 11834391]
- Palou J, et al. Intravesical bacille Calmette-Guerin in the treatment of carcinoma in situ or high-grade superficial bladder carcinoma after radiotherapy for bladder carcinoma. BJU International. 1999;83(4):429–431. Reason: Not randomised trial . [PubMed: 10210566]
- Muto S, et al. Maintenance Therapy with Intravesical Bacillus CalmetteGurin in Patients with Intermediate- or High-risk Non-muscle-invasive Bladder Cancer. Japanese Journal of Clinical Oncology. 2013;43(3):305–313. Reason: Not randomised trial . [PubMed: 23303841]
- Nilsson SR. A systematic overview of chemotherapy effects in urothelial bladder cancer. Acta Oncologica. 2001;40(2-3):371–390. Reason: Not systematic review of randomised trials . [PubMed: 11441942]
- Mohanty NK, et al. Intravesicle gemcitabine in management of BCG refractory superficial TCC of urinary bladder-our experience. Urologic Oncology-Seminars and Original Investigations. 2008;26(6):616–619. Reason: Relevant to another topic . [PubMed: 18367121]
- Mohanty NK, et al. Management of BCG non-responders with fixed dose intravesical gemcitabine in superficial transitional cell carcinoma of urinary bladder. Indian Journal of Urology. 2008;24(1):44–47. Reason: Relevant to another topic . [PMC free article: PMC2684230] [PubMed: 19468358]
- Montie JE. Intravesical bacille calmette-guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Journal of Urology. 2005;173(3):730–731. Reason: Comment on Bohle 2003 . [PubMed: 15711255]
- Li J, Chen GJ. Intravesical therapy with Bacillus Calmette-Guerin following transurethral resection in patients with superficial bladder cancer: a meta-analysis of randomized controlled trials. Chinese Journal of Cancer Biotherapy. 2011;18:70–74. (Provisional abstract) Reason: foreign language .
- Lorenzo G, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116:1893–1900. Reason: Relevant to another topic . [PubMed: 20162706]
- Barlow L, et al. A single-institution experience with induction and maintenance intravesical docetaxel in the management of non-muscle-invasive bladder cancer refractory to bacille Calmette-Guerin therapy. BJU International. 2009;104(8):1098–1102. Reason: Relevant to another topic . [PubMed: 19389012]
- Barlow LJ, et al. Novel intravesical therapies for non-muscle-invasive bladder cancer refractory to BCG. Urologic Oncology. 2010;28(1):108–111. Reason: Relevant to another topic . [PubMed: 20123359]
- Barlow LJ, McKiernan JM, Benson MC. Long-Term Survival Outcomes with Intravesical Docetaxel for Recurrent Nonmuscle Invasive Bladder Cancer After Previous Bacillus Calmette-Guerin Therapy. Journal of Urology. 2013;189(3):834–839. Reason: Relevant to another topic . [PubMed: 23123371]
- Bassi BPF. Paclitaxel plus hyaluronic acid bioconiugated for intravesical therapy of bcg refractory carcinoma in situ of the bladder: Results of a phase study. European Urology, Supplements. 2010.:2. Conference(var.pagings) Reason: Relevant to another topic .
- Cho IC, et al. Adjuvant Intravesical Instillation for Primary T1G3 Bladder Cancer: BCG versus MMC in Korea. Anticancer Research. 2012;32(4):1493–1498. Reason: Not randomised trial . [PubMed: 22493392]
- Duchek M, et al. Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferon-alpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. European Urology. 2010;57(1):25–31. Reason: Excluded from systematic review Shang 2011 (interferon alpha2b not in PICO) [PubMed: 19819617]
- Khanna OP, et al. Multicenter study of superficial bladder cancer treated with intravesical bacillus Calmette-Guerin or adriamycin. Results of long-term follow-up. Urology. 1991;38(3):271–279. Reason: Not randomised trial . [PubMed: 1887543]
- De JR, et al. Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. Urology. 1991;38(6):507–513. Reason: Not systematic review of randomised trials . [PubMed: 1836081]
- Erol A, et al. Trial with Bacillus-Calmette-Guerin and Epirubicin Combination in the Prophylaxis of Superficial Bladder-Cancer. Urologia Internationalis. 1994;52(2):69–72. Reason: Not randomised trial . [PubMed: 8178379]
- Herr HW, Dalbagni G, Donat SM. Bacillus Calmette-Guerin Without Maintenance Therapy for High-Risk Non-Muscle-Invasive Bladder Cancer. European Urology. 2011;60(1):32–36. Reason: Not a randomised trial . [PubMed: 21497431]
- Jakse G, et al. Intravesical BCG in patients with carcinoma in situ of the urinary bladder: long-term results of EORTC GU Group phase II protocol 30861. European Urology. 2001;40(2):144–150. Reason: Not randomised trial . [PubMed: 11528191]
- Dalbagni G, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guerin-refractory transitional cell carcinoma of the bladder. Journal of Clinical Oncology. 2006;24(18):2729–2734. Reason: Relevant to another topic . [PubMed: 16782913]
- Yates DR. Treatment options available for bacillus Calmette-Guerin failure in non-muscle-invasive bladder cancer. European Urology. 2012;62(6):1088–1096. Reason: Relevant to another topic . [PubMed: 22959049]
- Green DA, et al. Cost-effective treatment of low-risk carcinoma not invading bladder muscle. BJU International. 2013;111(3B):E78–E83. Reason: Health economics . [PubMed: 22958598]
- Lee CT, et al. Economic and Humanistic Consequences of Preventable Bladder Tumor Recurrences in Nonmuscle Invasive Bladder Cancer Cases. Journal of Urology. 2012;188(6):2114–2119. Reason: Health economics . [PubMed: 23083857]
- Sylvester RJ. Bacillus Calmette-Guerin treatment of non-muscle invasive bladder cancer. International Journal of Urology. 2011;18(2):113–120. [Review] Reason: Expert review . [PubMed: 21091799]
- Gontero P, et al. The role of bacillus Calmette-Guerin in the treatment of non-muscle-invasive bladder cancer. European Urology. 2010;57(3):410–429. [Review] [92 refs] Reason: Expert review . [PubMed: 19969411]
- van den Bosch S, Witjes JA. Long-term Cancer-specific Survival in Patients with High-risk, Non-muscle-invasive Bladder Cancer and Tumour Progression: A Systematic Review. European Urology. 2011;60(3):493–500. Reason: Not relevant to PICO . [PubMed: 21664041]
- Bohle A. Bladder cancer: meta-analysis of BCG versus mitomycin C--a deeper insight? Nature Reviews Urology. 2010;7(1):8–10. Reason: Comment on Malmstrom 2009 . [PubMed: 20062070]
- Okamura T, et al. Single monthly bacillus Calmette-Gu,rin intravesical instillation is effective maintenance therapy to prevent recurrence in Japanese patients with non-muscle-invasive bladder cancer. International Journal of Clinical Oncology. 2012;17(5):477–481. Reason: Retrospective study . [PubMed: 21947596]
- Brausi MA, et al. Can Gemcitabine Instillation Ablate Solitary Low-Risk Non-Muscle-Invasive Bladder Cancer? Results of a Phase II Marker Lesion Study. Urologia Internationalis. 2011;87(4):470–474. Reason: Non comparative study . [PubMed: 22086229]
- Volpe A, et al. Thermochemotherapy for Non-Muscle-Invasive Bladder Cancer: Is There a Chance to Avoid Early Cystectomy? Urologia Internationalis. 2012;89(3):311–318. Reason: Relevant to another topic . [PubMed: 22965159]
- Badalato GM, et al. Maximizing intravesical therapy options: is there an advantage to the administration of perioperative mitomycin C prior to an induction course of BCG? Canadian Journal of Urology. 2011;18(5):5890–5895. Reason: Retrospective study . [PubMed: 22018151]
- Hilton WM, et al. Efficacy of combined intravesical immunotherapy and chemotherapy for non-muscle invasive bladder cancer. Expert Review of Anticancer Therapy. 2011;11(6):949–957. Reason: Expert review . [PubMed: 21707292]
- Racioppi M, et al. Intensive Intravesical Mitomycin C Therapy in Non-Muscle-Invasive Bladder Cancer: A Dose Intensity Approach. Urologia Internationalis. 2010;85(3):266–269. Reason: Non-randomised trial . [PubMed: 20516670]
- Zarogianni C, Tsiamis C. Immunological treatment of superficial bladder carcinoma. Journal of BUOn. 2004;9(1):41–46. Reason: Includes non intravesical therapy . [PubMed: 17385826]
- Moutzouris G, et al. Prospective, randomized, comparative study of high dose intravesical epirubicin versus BCG for prophylaxis in intermediate risk superficial bladder tumors. European Urology Supplements. 2007;6(2):171–171. Reason: Abstract only, Not included in systematic review by Shang 2011 .
- Sylvester RJ, et al. The side effects of Bacillus Calmette-Guerin in the treatment of Ta T1 bladder cancer do not predict its efficacy: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. European Urology. 2003;44(4):423–428. Reason: Same study as Sylvester 2010 . [PubMed: 14499675]
- Rocchini L. Neoadjuvant short-term intensive intravesical mitomycin C regimen compared to standard schedule for low-risk non-muscle invasive bladder cancer. A randomized phase ii study. Journal of Urology. 2012.:4–e715. Conference(var.pagings) Reason: Same study as Colombo (2012)
- Colombo R, et al. Neoadjuvant short-term intensive intravesical mitomycin C regimen compared with weekly schedule for low-grade recurrent non-muscle-invasive bladder cancer: preliminary results of a randomised phase 2 study. European Urology. 2012;62(5):797–802. Reason: Not relevant to PICO (neoadjuvant therapy) [PubMed: 22633362]
- Witjes JA, et al. Clinical Practice Recommendations for the Prevention and Management of Intravesical Therapy-Associated Adverse Events. European Urology Supplements. 2008;7(10):667–674. Reason: Expert review .
- Boorjian SA, Zhu F, Herr HW. The effect of gender on response to bacillus Calmette-Guerin therapy for patients with non-muscle-invasive urothelial carcinoma of the bladder. BJU International. 2010;106(3):357–361. Reason: Non randomised trial (case series) [PubMed: 20002665]
- Witjes JA. Management of the first recurrence of T1G3 bladder cancer: Does intravesical chemotherapy deserve a chance? Urologic Oncology-Seminars and Original Investigations. 2009;27(3):322–324. Reason: Expert review . [PubMed: 19414122]
- Lerner SP, et al. Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urologic Oncology. 2009;27(2):155–159. Reason: Comparison not relevant to PICO . [PMC free article: PMC2695968] [PubMed: 18367117]
- Lerner SP, et al. Patterns of recurrence and outcomes following induction bacillus Calmette-Guerin for high risk Ta, T1 bladder cancer. Journal of Urology. 2007;177(5):1727–1731. Reason: Comparison not relevant to PICO . [PubMed: 17437798]
- Djulbegovic M. Advancing comparative effectiveness research through network meta-analysis: Intravesical therapy in bladder cancer. Journal of Urology. 2012.:4. Conference(var.pagings) Reason: Abstract only: Cochrane review in progress .
- Cheng CW, et al. 17-year follow-up of a randomized prospective controlled trial of adjuvant intravesical doxorubicin in the treatment of superficial bladder cancer. International Braz J Urol. 2005;31(3):204–211. Reason: Not included in Abern 2013 . [PubMed: 15992422]
- Jeong CW, et al. Comparison of 30 mg and 40 mg of mitomycin C intravesical instillation in Korean superficial bladder cancer patients: prospective, randomized study. Cancer Research & Treatment. 2005;37(1):44–47. Reason: Not included in Sylvester 2008 . [PMC free article: PMC2785421] [PubMed: 19956509]
- Ferakis N, et al. A Randomized Study of Single Dose Intravesical Mitomycin-C in the Treatment of Superficial Bladder Cancer. European Urology Supplements. 2010;9(6):594–594. Reason: Abstract only, not included in Abern 2013 .
- Ayres BE, Crew JP., Action for Bladder Cancer. Is immediate postoperative intravesical chemotherapy beneficial in non-muscle- invasive bladder cancer? BJU International. 2010;105 Suppl 2:14–17. [Review] [33 refs] Reason: Expert review . [PubMed: 20089093]
- Holmang S. Early Single-Instillation Chemotherapy Has No Real Benefit and Should Be Abandoned in Non-Muscle-Invasive Bladder Cancer. European Urology Supplements. 2009;8(5):458–463. Reason: Expert review .
- Mostafid AH, et al. Immediate administration of intravesical mitomycin C after tumour resection for superficial bladder cancer. BJU International. 2006;97(3):509–512. Reason: Not randomised trial . [PubMed: 16469017]
- Bartoletti R, et al. Is early single-dose instillation of epirubicin able to improve BCG efficacy in non-muscle invasive high-risk bladder cancer patients? Results from a prospective, randomised, double-blind and controlled study. European Urology Supplements. 2008;7(3):177–177. Reason: Same study as Cai 2008 .
- Gulpinar O, et al. The value of perioperative mitomycin C instillation in improving subsequent bacillus calmette-guerin instillation efficacy in intermediate and high-risk patients with non-muscle invasive bladder cancer: a prospective randomized study. International Braz J Urol. 2012;38(4):474–479. Reason: Not included in Houghton 2012 (no maintenance BCG) [PubMed: 22951160]
- Lammers RJ, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. European Urology. 2011;60(1):81–93. [Review] Reason: Not relevant to PICO (hyperthermia) [PubMed: 21531502]
- Damiano R, et al. Short-term administration of prulifloxacin in patients with nonmuscle-invasive bladder cancer: an effective option for the prevention of bacillus Calmette-Guerin-induced toxicity? BJU International. 2009;104(5):633–639. Reason: Comparison not relevant to PICO (toxicity covered in another topic) [PubMed: 19298412]
- Hinotsu S, et al. Sustained prophylactic effect of intravesical bacille Calmette-Guerin for superficial bladder cancer: a smoothed hazard analysis in a randomized prospective study. Urology. 2006;67(3):545–549. Reason: Number of events not reported, insufficient data to add to existing meta-analyses . [PubMed: 16527576]
- O'Brien TS, et al. A Prospective Randomised Trial of Hexylaminolevulinate (Hexvix) Assisted Transurethral Resection (Turbt) Plus Single Shot Intravesical Mitomycin (Mmc) Versus Conventional White Light Turbt Plus Single Shot Mmc in Newly Presenting Bladder Cancer. European Urology Supplements. 2011;10(2):150–150. Reason: Not relevant to PICO .
- Weiss C, et al. Treatment options for high-risk T1 bladder cancer: status quo and future perspectives of radiochemotherapy. Strahlentherapie und Onkologie. 2008;184(9):443–449. [Review] [67 refs] Reason: Expert review . [PubMed: 19016022]
- Gontero P, et al. The impact of intravesical gemcitabine and 1/3 dose Bacillus Calmette-Guerin instillation therapy on the quality of life in patients with nonmuscle invasive bladder cancer: results of a prospective, randomized, phase II trial. Journal of Urology. 2013;190(3):857–862. Reason: Possibly relevant to Cochrane review update (Jones et al) [PubMed: 23545101]
- Zhu S, et al. Optimal schedule of bacillus calmette-guerin for non-muscle-invasive bladder cancer: a meta-analysis of comparative studies. BMC Cancer. 2013;13:332. Reason: No further studies presented/includes non-RCTs) [PMC free article: PMC3722001] [PubMed: 23829273]
- Sengiku A, et al. A prospective comparative study of intravesical bacillus Calmette-Guerin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. Journal of Urology. 2013;190(1):50–54. Reason: Comparison not relevant to PICO . [PubMed: 23376145]
- Perlis N, et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. European Urology. 2013;64(3):421–430. [Review] Reason: Same studies as meta-analysis by Abern (2013) [PubMed: 23830475]
- Inamoto T. Comparable effect with minimal morbidity of low-dose Tokyo 172 strain compared with regular dose Connaught strain as an intravesical bacillus Calmette-Guerin prophylaxis in nonmuscle invasive bladder cancer: Results of a randomized prospective comparison. Urology Annals. 2013;5(1):7–12. Reason: Comparison not relevant to PICO . [PMC free article: PMC3643329] [PubMed: 23662001]
- Ehdaie B, Sylvester R, Herr HW. Maintenance bacillus Calmette-Guerin treatment of non-muscle-invasive bladder cancer: a critical evaluation of the evidence. European Urology. 2013;64(4):579–585. [Review] Reason: Expert review . [PubMed: 23711538]
Evidence tables
Download PDF (530K)
Health Economic Evidence: What are the comparative patient outcomes for treating low-risk non-muscle invasive bladder cancer with: Intravesical chemotherapy
Background
Non-muscle invasive bladder cancer (NMIBC) tumours can be surgically removed using transurethral resection of bladder tumour (TURBT). However, these tumours are likely to return on the urothelium. This high risk of recurrence is a problem for patients because it raises the concern that the cancer will progress and so the patient will need to undergo further treatment (either another TURBT or diathermy).
The risk of recurrence can be reduced by the administration of chemotherapy medication into the bladder (intravesical chemotherapy), which can be done immediately, or shortly after TURBT. However, there are disadvantages to using intravesical chemotherapy as it is associated with some side effects and comes at an additional cost.
Aim of analysis
To estimate the cost-effectiveness of a single instillation of intravesical chemotherapy in addition to TURBT in comparison to TURBT alone in patients with NMIBC.
Existing Economic Evidence
A systematic literature review identified one paper related to the decision problem, a cost-utility analysis by Green et al. 2013. In the study, a decision analytic model was utilised to estimate the cost-effectiveness of fulguration compared to TURBTs with and without perioperative intravesical chemotherapy in patients with low risk NMIBC.
The authors concluded that fulguration without perioperative intravesical chemotherapy was the most cost-effective strategy for treating low-risk NMIBC. However, unusually, the authors based this conclusion upon individual cost-effectiveness calculations rather than the standard incremental calculations. When following the more standard cost-effectiveness methodology using incremental cost-effectiveness ratios (ICERs), it appears that perioperative intravesical chemotherapy plus fulguration would be the most cost-effective strategy. This strategy has an ICER of $4,169 per QALY, which is likely to fall below the cost-effectiveness threshold3. The authors also conducted sensitivity analysis, which showed that the effectiveness of perioperative intravesical chemotherapy and the cost of TURBT were likely to be key drivers of the cost-effectiveness result.
However, Green et al. 2013 can only be deemed partially applicable to the decision problem this guideline seeks to address. The analysis considered the US healthcare system, which differs substantially from the UK system. In addition, the study only partially addressed our decision problem as it only evaluated cost-effectiveness in low risk NMIBC patients, whereas we are interested in all NMIBC risk groups.
Overall, it was considered that the current economic literature was partially useful but further analysis would be required to robustly estimate the cost-effectiveness. It should also be noted that the existing economic literature was useful for informing the development of our own economic model.
De Novo Economic Model
Since the current economic literature did not adequately address the decision problem, a de novo economic evaluation was undertaken to assess cost-effectiveness. A Markov decision model was developed using Microsoft Excel.
The patient enters the model in a ‘disease free state following an initial transurethral resection of the bladder tumour (TURBT) with or without a single instillation of chemotherapy (depending upon modelled treatment arm). At each 3-monthly model cycle the patient may experience a bladder cancer recurrence. If the recurrence is detected, the patient will undergo a further TURBT (or fulguration of the tumour) and return to a disease free state. However, if the recurrence is not detected, then the patient will be at risk of progression and will have to undergo further treatment once this progression is eventually detected (cystectomy and possibly neo-adjuvant chemotherapy). The patient may also die from bladder cancer related mortality after experiencing progression and may die from other cause mortality from any health state.
Estimated total costs and quality adjusted life yefars (QALYs) are collected over the modelled 10 year time horizon for each follow-up strategy. Future costs and benefits were discounted at a rate of 3.5% per year as recommended by NICE.
The risk of recurrence and progression in patients with NMIBC was estimated using risk equations based on an analysis of 2,596 patients from seven EORTC4 trials (Sylvester et al. 2006). Patients are ‘scored based on a number of risk factors, such as number of tumours, tumour size, prior recurrence rate, T category, presence of CIS and grade. An individual's one year and five year risks of recurrence and progression can then be estimated based upon these scores.
For the purposes of the economic model, it was necessary to convert these five year and one year risks into 3-monthly risks. The higher risk of recurrence and progression in the first year was captured by calculating separate 3 monthly risks for the first year and subsequent years (based on the one year risk and five year EORTC risks). Furthermore, since the EORTC risk equations consider recurrence and progression independently, it was necessary to link the progression rates to the recurrence rate i.e. estimate the probability of progression given recurrence in each of the risk groups.
Table 66
Three Monthly Recurrence And Progression Risk Applied In The Model.
As the modelled time horizon of 10 years exceeds the predicted risk estimates from the EORTC trials (5 years), it was also necessary to make some assumptions about the risk profile of patients in years 5-10. In the base case, it was assumed that the subsequent year rate (i.e. years 2-5) would be maintained in years 6-10 except in the case of low-risk patients in whom it was assumed that risk would be zero after 5 years (reflecting clinical practice of discharging low-risk patients from follow-up after 5 years).
The key effectiveness data utilised in the model is the reduction in recurrence risk associated with a single instillation of intravesical chemotherapy following a TURBT. According to the systematic review of the clinical evidence, the use of a single instillation of intravesical chemotherapy in addition to TURBT has a relative risk of 0.67 in comparison to TURBT alone. This treatment effect was assumed to last for two years reflecting the general consensus around its possible duration. Thereafter, the risk of recurrence was assumed to be equal to that with TURBT only. In addition, the treatment effect is not assumed to affect future recurrences if the patient has a recurrence during the two years after the single chemotherapy instillation.
Note that the single instillation of chemotherapy does not directly reduce the rates of progression. This is in line with the evidence base, which suggests that there is no treatment effect on the rates of progression. However, it should be noted that because of the model structure, a lower rate of recurrences would lead to a lower rate of progression because progression is dependent upon recurrence. Therefore, an indirect treatment effect on progression is essentially included in the model. This assumption is relaxed in a sensitivity analysis where the rates of recurrence and progression are assumed to be independent.
No comparative data on morbidity were identified in the systematic review of the clinical evidence. However a meta-analysis (Sylvester 2004) of seven trials suggested that mild irritative bladder symptoms (including dysuria, frequency and macroscopic haematuria) would occur in approximately 10% of patients treated with a single post-operative dose of intravesical chemotherapy. In addition, allergic skin reactions were reported in 1-3% of patients in two studies.
Since no data were available on morbidity in patients treated with TURBT, it was conservatively assumed that 5% would have irritative bladder symptoms and there would be no skin reactions. The treatment related morbidity rates applied in the model are shown in the table below.
The diagnostic accuracy data for flexible cystoscopy were sourced from the systematic review of the clinical evidence conducted for this guideline, with most data being sourced from a systematic review by Mowatt et al. 2010.
Bladder cancer related mortality rates were estimated using data from a systematic review by Van den Bosch et al. 2011. Using the data in the study, separate three mortality rates were estimated for patients that progressed to muscle invasive disease and those that remained non-muscle invasive following a cystectomy (3.6% and 0.5%, respectively). The lower rate in NMIBC patients reflects an assumption that patients would have to first progress to MIBC before dying of bladder cancer.
Death from other causes was captured using 2009-2011 life tables for England and Wales from the office of national statistics (ONS). These life tables give an estimate of the annual probability of death given a person's age and gender with the model assuming that 50% of patients were female and that the average age was 60 years old. These annual probabilities were converted to three-monthly probabilities for use in the model.
Costs and utilities
Modelled patients accrue costs associated with any treatment, monitoring or management strategy that they are undergoing. The costs considered in the model reflect the perspective of the analysis, thus only costs that are relevant to the UK NHS & PSS were included. These costs include drug costs, treatment costs and any other resource use that may be required (e.g. GP visit). Where possible, all costs were estimated in 2012-13 prices.
The majority of costs were sourced from NHS reference costs 2012/13 by applying tariffs associated with the appropriate HRG code. Drug costs were calculated using dosages from the British National Formulary (BNF) and unit cost information from the electronic market information tool (eMit). Where unit costs for drugs were not available from eMit, prices from the BNF were used. Resource use and cost information were obtained from the Personal Social Services Research Unit (PSSRU) and the advice of the GDG.
The model estimates effectiveness in terms of quality adjusted life years (QALYs). QALYs were estimated by combining the life year estimates with utility values (or QOL weights) associated with being in a particular health state. These utility values were identified through a search of the available literature.
Base case results
The base case results of the analysis are presented in the table below for patients in each risk category. It can be seen that, in every risk category, a strategy of TURBT plus a single instillation of chemotherapy is more effective than a strategy of TURBT alone.
In the case of low and intermediate risk patients, it can also be seen that the addition of a single instillation of chemotherapy is cost saving over the modelled time horizon. This shows that the initial additional costs associated with the single chemotherapy instillation are outweighed by the cost savings associated with a reduction in recurrences (recurrence reductions of 17% and 10% were estimated over the modelled time horizon in the low and intermediate risk groups, respectively). Therefore in low and intermediate risk patients, a single instillation of chemotherapy can be considered dominant i.e. more effective and cost saving.
However, in the case of high risk patients, it can be seen that this is not the case. In high risk patients, the single instillation of chemotherapy is more costly than TURBT alone, suggesting that the potential cost savings are not as large in this group. However, it can also be seen that the addition of a single chemotherapy instillation provides an additional QALY at a cost of £6,432 and thus would be considered cost-effective using the NICE threshold (i.e. <£20,000 per QALY).
Table 67
Base Case Results Of The Model.
Sensitivity analysis
A series of one-way sensitivity analyses were conducted, whereby the value of an input parameter is changed and its effect on the overall outcome is recorded and assessed.
The analyses showed that the conclusion of the model is insensitive to changes in the input parameters over plausible ranges i.e. TURBT plus a single instillation of chemotherapy remains cost-effective in the all the analyses across all the risk groups.
The variations in the treatment effect duration are perhaps particularly notable as this is one of the uncertainties around the effectiveness of the single instillation of chemotherapy. The analysis shows, unsurprisingly, that the intervention is less cost-effective when the treatment effect duration is decreased. However, crucially, the single instillation of chemotherapy remains cost-effective in all analyses, even when making very pessimistic assumptions about the likely treatment effect duration (i.e. even when assuming that the chemotherapy instillation only reduces recurrences in the first 3 months after administration).
In addition to the core cost-utility analysis, the GDG were also interested in a cost analysis comparing the cost of delivering the single instillation of chemotherapy on the ward against the cost of delivering it in theatre. It was found that delivering the single instillation of chemotherapy in theatre was the cheaper of the two approaches (delivery by nurse estimated to cost an additional £23.83). This was primarily a result of the longer amount of time taken to deliver the instillation in the ward setting compared to in theatre.
A probabilistic sensitivity analysis was also conducted to assess the combined parameter uncertainty in the model. In this analysis, the mean values that were utilised in the base case were replaced with values drawn from distributions around the mean values. It was found that, at a threshold of £20,000 per QALY, TURBT plus a single instillation of chemotherapy has a very high probability of being cost-effective in the low and intermediate risk groups (100%). However, the probability is substantially lower in high risk patients at 66%, although still very much in favour of TURBT plus a single instillation of chemotherapy.
Conclusion
The results of the analysis suggest that the use of a single instillation of chemotherapy after a TURBT, in comparison to a TURBT alone, was found to be strongly cost-effective in all risk groups. It was found to be particularly cost-effective in low and intermediate risk groups, in which the strategy was cost saving as well as more effective (dominant). Furthermore, this result was found to be robust in alternative scenario analyses, one-way and probabilistic sensitivity analysis.
References
- Green DA. Cost-effective treatment of low-risk carcinoma not invading bladder muscle. BJU International. 2013;111(3B):E78–E83. [PubMed: 22958598]
- Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DWW, Kurth Karlheinz. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. European Urology. 2006;49:466–477. [PubMed: 16442208]
- Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. Journal of Urology. 2004;171(6 Pt 1):2186–2190. [PubMed: 15126782]
- Mowatt G, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technology Assessment. 2010:1. (Structured abstract) [PubMed: 20082749]
- Van den Bosch S, Alfred WJ. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. European Urology. 2011;60(3):493–500. [Review] [PubMed: 21664041]
- NHS reference costs 2012-13. London: UK Department of Health; [database on the Internet]
- Joint Formulary Committee. British National Formulary (online). London: BMJ Group and Pharmaceutical Press;
- Curtis L. Unit Costs of Health and Social Care. Personal Social Services Research Unit (PSSRU), University of Kent; Canterbury: 2013.
- Hall RR, Parmar MKB, Richards AB, Smith PH. Proposal for changes in cystoscopic follow–up of patients with bladder cancer and adjuvant intravesical chemotherapy. BMJ. 1994;308:257–260. [PMC free article: PMC2539314] [PubMed: 8179678]
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The Average Body Surface Area of Adult Cancer Patients in the UK: A Multicentre Retrospective Study. PLoS ONE. 2010;5(1):e8933. [PMC free article: PMC2812484] [PubMed: 20126669] [CrossRef]
- Kulkarni GS, Finelli A, Fleshner NE, Jewett MAS, Lopushinsky SR, Alibhai SMH. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4:1538–49. [PMC free article: PMC1989749] [PubMed: 17896857]
- Yoshimura K, et al. Impact of superficial bladder cancer and transurethral resection on general health-related quality of life: an SF-36 survey. Urology. 2005;65(2):290–94. [PubMed: 15708040]
- Ara R, Brazier J. Deriving an Algorithm to Convert the Eight Mean SF-36 Dimension Scores into a Mean EQ-5D Preference-Based Score from Published Studies (Where Patient Level Data Are Not Available). Value in Health. 2008;11(7):1131–1143. [PubMed: 18489495]
3.2.2. The role of biopsy in people with recurrent non-muscle invasive bladder cancer
Review question: In patients with recurrent bladder cancer and previous low risk bladder cancer does treatment without histological sampling affect outcome?
Rationale
Treatment of low risk bladder cancer recurrences may be with endoscopic resection to remove the cancer, fulguration by electrocautery or laser energy to destroy the cancer in situ (with or without biopsy), intravescial chemotherapy (also known as chemoresection) or merely observation (so called active surveillance). The former allows pathological evaluation of the cancer and may be necessary to remove tissue from large tumors, but requires regional or general anaesthesia and a rigid cystoscopy and bladder resection. Consequently, the risks of intervention are higher than for fulguration (which may performed under local anaesthesia), chemotherapy or active surveillance. However, these other approaches do not sample the tissue of the cancer recurrence and could miss the minority of cases in which the cancer is becoming more aggressive. Also these approaches are less effective at removing the cancer and so could lead to higher recurrence (or residual cancers) rates and more post-treatment symptoms.
In this review we will evaluate each approach to treating recurrence within the bladder following a previous low risk bladder cancer. We will attempt to determine in which patients the benefits of transurethral resection outweigh the risks from the treatment and from the cancer. We will attempt to identify low risk cancers in which the rate of disease progression is higher and so the evaluation of tissue is necessary for patient safety. We will look to identify tumors in which less intensive intervention is sufficient and to compare the outcomes of the different approaches.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with recurrent bladder cancer and previous low risk NMIBC | Treatment with histological sampling e,g, cystocopy & biopsy or TUR | Treatment without histological sampling e.g cystodiathermy |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. Comparative evidence was looked for, but only one study was identified. Therefore, evidence from non-comparative observational studies was included.
Data synthesis
Data was presented using GRADE. Meta-analysis was not possible for this review question.
RESULTS
Study quality and results
Very low quality evidence was obtained from seven observational studies. Evidence is presented in Table 68.
Table 68
GRADE evidence profile: Treatment with histological sampling versus treatment without histological sampling (e.g. cystodiathermy).
Evidence statements
Very low quality evidence from one retrospective observational study reported on 42 patients who underwent fulguration for recurrent Ta bladder cancer and 42 matched patients who underwent TURBT. 12 patients in the fulguration group and 11 patients in the TURBT group had a recurrence during follow-up (RR 0.92, 95% CI 0.46 to 1.84) (Park et al., 2013).
Very low quality evidence from one prospective cohort study of outpatient laser ablation (OLA) in an elderly population (n=54) reported that the procedure was well tolerated with pain scores of 0-2 out of 10. The 3-month recurrence rate was 10.6% with white light OLA and 4.3% with PDD OLA (Wong et al., 2013).
One study of electromotive drug administration (EMDA) of local anaesthetic (LA) for outpatient flexible cystoscopy biopsy and cystodiathermy of recurrent low grade pTaG1-2 (Biers et al., 2009) reported that there were no recurrences at the site of cystodiathermy and there were no progression events. 19% (3/16) of those with benign pathology at biopsy had a recurrence after a mean follow-up of 16.4 months. 9% (1/11) of those with TCC pathology at biopsy had a recurrence, with a time to recurrence of 15 months. Mean pain score was one, on a scale of one (no pain) to 10 (worst pain). There were no intraoperative complications (Very low quality evidence).
One study of 48 patients who were suitable for cystodiathermy under LA reported a local recurrence rate of 6% (n=3) and 15 recurrences (31%) at a different site after a median of 15 weeks follow-up (80% subsequently treated with LA cystodiathermy and 20% referred for GA cystodiathermy). No progressions were reported (Davenport et al., 2004) (Very low quality evidence).
Two studies of 192 patients (515 tumours) undergoing treatment for NMIBC recurrences with Ho:YAG laser ablation under LA with a flexible cystoscope reported a local recurrence rate of 12% (37/304) and an off-site recurrence rate of 50% (Syed et al. 2001; 2013). One study (Syed et al., 2013) reported complication rates of dysuria (4.2%), frequency (1.5%), haematuria (1.9%) and no UTIs. Mean visual pain score was one, on a scale of 0 (no pain) to 10 (worst pain) (Very low quality evidence).
In one study of 267 patients, 103 had small, low grade papillary recurrence and negative cytology and underwent office cystodiathermy at least once during the study period (Donat et al., 2004). No significant differences were seen in progression of disease for patients undergoing cystodiathermy (n=103) compared to those never fulgurated in the office (n=164) (p=0.86) (Very low quality evidence).
References to included studies
- Biers SM, Mostafid AH. Electromotive drug administration of local anesthesia for biopsy and cystodiathermy of recurrent low grade bladder tumors. Current Urology. 2009;3(1):15–18.
- Davenport K, Keeley FX Jr., Timoney AG. Audit of safety, efficacy, and cost-effectiveness of local anaesthetic cystodiathermy. Annals of the Royal College of Surgeons of England. 2010;92(8):706–709. [PMC free article: PMC3229385] [PubMed: 20615299]
- Donat SM, et al. Efficacy of office fulguration for recurrent low grade papillary bladder tumors less than 0.5 cm. Journal of Urology. 2004;17(2:Pt 1) t-9. [PubMed: 14713776]
- Park DS, et al. An Analysis of the Efficacy, Safety, and Cost-Effectiveness of Fulguration Under Local Anesthesia for Small-Sized Recurrent Masses: A Comparative Analysis to Transurethral Resection of Bladder Tumors in a Matched Cohort. Journal of Endourology. 2013;27(10):1240–1244. [PubMed: 23964922]
- Syed HA, et al. Flexible cystoscopy and Holmium:Yttrium aluminum garnet laser ablation for recurrent nonmuscle invasive bladder carcinoma under local anesthesia. Journal of Endourology. 2013;27(7):886–891. [PubMed: 23537221]
- Syed HA, et al. Holmium:YAG laser treatment of recurrent superficial bladder carcinoma: initial clinical experience. Journal of Endourology. 2001;15(6):625–627. [PubMed: 11552789]
- Wong KA, et al. Outpatient laser ablation of non-muscle-invasive bladder cancer: is it safe, tolerable and cost-effective? BJU International. 2013;112(5):561–567. [PubMed: 23819486]
References to excluded studies (with reasons for exclusion)
- de la Pena E, et al. Safety and efficacy of an active surveillance program in recurrent non-muscle invasive bladder tumors. Results of an extended series. European Urology, Supplements. 2012;11(1):e1041–e1041a. Reason: not relevant to PICO .
- de la Pena E, et al. Oncological safety of an active surveillance program in recurrent non muscle-invasive bladder tumor. Journal of Urology. 2013;18(4 SUPPL. 1):e731. Reason: not relevant to PICO .
- Soloway MS, Bruck DS, Kim SS. Expectant management of small, recurrent, noninvasive papillary bladder tumors. Journal of Urology. 2003;17(2:Pt 1) t-41. Reason: not relevant to PICO . [PubMed: 12853794]
- Herr HW, Donat SM, Reuter VE. Management of Low Grade Papillary Bladder Tumors. Journal of Urology. 2007;178(4):1201–1205. Reason: not relevant to PICO . [PubMed: 17698090]
- Gofrit ON, Shapiro A. Active surveillance of low grade bladder tumors. Archivio Italiano di Urologia, Andrologia. 2008;80(4):132–135. Reason: not relevant to PICO . [PubMed: 19235428]
- Linton KD, et al. Disease specific mortality in patients with low risk bladder cancer and the impact of cystoscopic surveillance. Journal of Urology. 2013;189(3):828–833. Reason: not relevant to PICO . [PubMed: 23017513]
- Finch WJG, Habib MR, Banerjee GK. Holmium laser ablation of recurrent superficial bladder tumours under topical anaesthesia using a flexible cystoscope in anticoagulated patients. European Urology, Supplements. 2009;8(4):374. Reason: abstract only, unclear if population relevant to PICO .
- Hernandez V, et al. Safety of active surveillance program for recurrent nonmuscle-invasive bladder carcinoma. Urology. 2009;73(6):1306–1310. Reason: not relevant to PICO . [PubMed: 19375783]
- Pruthi RS, et al. Conservative management of low risk superficial bladder tumors. Journal of Urology. 1990;179(1):87–90. Reason: not relevant to PICO . [PubMed: 17997444]
- Gofrit ON, et al. Watchful waiting policy in recurrent Ta G1 bladder tumors. European Urology. 49(2):303–306. 306. Reason: not relevant to PICO . [PubMed: 16413659]
- Zhang Y, Denton BT, Nielsen ME. Comparison of surveillance strategies for low-risk bladder cancer patients. Medical Decision Making. 2013;33(2):198–214. Reason: not relevant to PICO (health economics) [PubMed: 23178638]
- Gao X, et al. Thulium laser resection via a flexible cystoscope for recurrent non-muscle-invasive bladder cancer: initial clinical experience. BJU International. 2008;102(9):1115–1118. Reason: not relevant to PICO (all patients sampled) [PubMed: 18565172]
- Jonler M, Lund L, Bisballe S. Holmium : YAG laser vaporization of recurrent papillary tumours of the bladder under local anaesthesia. BJU International. 2004;94(3):322–325. Reason: outcomes not relevant to PICO . [PubMed: 15291860]
- MacDonald D, Jones R, Syed H. A prospective study of the efficacy and tolerability of ambulatory holmium laser ablation of recurrent non-muscle-invasive transitional cell carcinoma with flexible cystoscope under local anaesthesia. Journal of Endourology. 2009;23:A128. Reason: abstract only, maybe same study as Syed (2013)
- Chandrasekar P, et al. Efficacy of managing small recurrent bladder tumours by diathermy using the CYF-4 olympus flexible cystoscope under local anaesthesia as an office setup. Journal of Urology. 2009;18(4 SUPPL. 1):639. Reason: abstract only .
- Siddiqui M, et al. The use of KTP laser for ablation of small, superficial transitional cell carcinoma of bladder in outpatient, office setting. Journal of Endourology. 2009;23:A67. Reason: abstract only .
- Sabir EF. TaG1 bladder tumours: One third of all primary tumours and 80 % of all recurrent tumours can be treated in the office under local anaesthesia. European Urology, Supplements. 2013.:1. Conference(var.pagings): Reason: abstract only .
- Liu H, et al. Comparison of the safety and efficacy of conventional monopolar and 2-micron laser transurethral resection in the management of multiple nonmuscle-invasive bladder cancer. Journal of International Medical Research. 2013;41(4):984–992. Reason: comparison not relevant to PICO . [PubMed: 23760914]
Evidence tables
Download PDF (259K)
Health Economic Evidence: What are the comparative patient outcomes for treating low-risk non-muscle invasive bladder cancer with transurethral resection
Review questions
In patients with recurrent bladder cancer and previous low risk bladder cancer does treatment without histological sampling affect outcome?
Table 69Pico Table For Treatment With And Without Histological Sampling In Patients With Recurrent Bladder Cancer And Previous Low Risk Bladder Cancer
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with recurrent bladder cancer and previous low risk NMIBC | Treatment with histological sampling e,g, cystocopy & biopsy or TUR | Treatment without histological sampling e.g cystodiathermy |
|
Information sources and eligibility criteria
The following databases were searched for economic evidence relevant to the PICO: MEDLINE, EMBASE, COCHRANE, NHS EED and HEED. Studies conducted in OECD countries other than the UK were considered.
Studies were selected for inclusion in the evidence review if the following criteria were met:
- Both cost and health consequences of interventions reported (i.e. true cost-effectiveness analyses)
- Conducted in an OECD country
- Incremental results are reported or enough information is presented to allow incremental results to be derived
- Studies that matched the population, interventions, comparators and outcomes specified in PICO
- Studies that meet the applicability and quality criteria set out by NICE, including relevance to the NICE reference case and UK NHS
Note that studies that measured effectiveness using quality of life based outcomes (e.g. QALYs) were desirable but, where this evidence was unavailable, studies using alternative effectiveness measures (e.g. life years) were considered.
Selection of studies
The literature search results were screened by checking the article's title and abstract for relevance to the review question. The full articles of non-excluded studies were then attained for appraisal and compared against the inclusion criteria specified above.
Results
Three searches for economic evidence were run over the development of the guideline; one at the start of the process, an update midway through and a further update at the end of the process. The diagram below shows the combined results of the three searches and illustrates the sifting process.
It can be seen that, in total, 1,189 possibly relevant papers were identified. Of these, 1,124 papers were excluded at the initial sifting stage based on the title and abstract while 65 full papers were obtained for appraisal. A further 56 papers were excluded based on the full text as they were not applicable to the PICO or did not include an incremental analysis of both costs and health effects. Therefore, nine papers were included in the systematic review of the economic evidence for this guideline.
Two of these nine papers related to the topic at hand and were thus included in the review of published economic evidence for this topic; Green et al. 2013 and Wong et al. 2013. The studies included a cost-effectiveness analysis where effectiveness was measured using quality adjusted life years (QALYs) i.e. a cost-utility analysis.
Quality and applicability of the included study
Green et al. 2013 was deemed only partially applicable to the guideline. This was primarily because it considered the US health care system, which differs substantially from the UK system. Wong et al. 2013 was also deemed to be only partially applicable despite being based in the UK. This was because of uncertainty over the applicability of some model inputs (QoL values and discount rates), details of which were omitted in the report.
Potentially serious limitations were identified in the study by Green et al. 2013. There was uncertainty over the treatment effect that had been applied in the model and there were concerns about the conclusions that had been drawn by the authors when interpreting the cost-effectiveness results. Very serious limitations were identified in the study by Wong et al. 2013. Omissions in the study report make it difficult to assess the quality of many of the input parameters used in the model e.g. the value and source of unit costs and resource use in the model are not fully reported.
Table 70table showing methodological quality and applicability of the included studies
| Methodological quality | Applicability | |
|---|---|---|
| Directly applicable | Partially applicable | |
| Minor limitations | ||
| Potentially serious limitations | Green et al. 2013 | |
| Very serious limitations | Wong et al. 2013 | |
Modified GRADE table
The primary results of the analyses by Green et al. 2013 and Wong et al. 2013 are summarised in the modified GRADE table below.
Table 71
modified grade table showing the included evidence (Green et al. 2013 and Wong et al. 2013) for the treatment of recurrent bladder cancer and previous low risk bladder cancer with and without histological sampling.
Evidence statements
Green et al. 2013 concluded that fulguration without perioperative intravesical chemotherapy was the most cost-effective strategy for treating low-risk NMIBC. However, unusually, the authors based this conclusion upon individual cost-effectiveness calculations rather than the standard incremental calculations. When following the more standard cost-effectiveness methodology using incremental cost-effectiveness ratios (ICERs), the strategy of perioperative intravesical chemotherapy (PIC) plus fulguration would most likely be considered the most cost-effective strategy with an ICER of $4,169 per QALY.
Of particular relevance to the topic at hand, was the finding that fulguration was more cost-effective than TURBT when both were used alone or when both were used in combination with intravesical chemotherapy. In both instances fulguration was found to be more effective and cheaper than TURBT alone i.e. dominant. However, as the study is US based, these results may lack applicability to the UK healthcare system.
Wong et al. 2013 found that outpatient laser ablation was cost-effective in comparison to inpatient cystodiathermy for the treatment of NMIBC, especially in elderly patients. In the base case, outpatient laser ablation was found to be cheaper (cost reduction of $2,526) and more effective (0.12 QALYs) than inpatient cystodiathermy and is thus dominant. A further analysis showed that using PDD in addition to outpatient laser ablation was also cost-effective and indeed dominant in comparison to inpatient cystodiathermy.
Probabilistic sensitivity analysis showed that, at a threshold of £30,000 per QALY, outpatient laser ablation had approximately an 80%5 probability of being cost-effective in comparison to intravesical chemotherapy. With the addition of PDD to OLA, the strategy was more cost-effective than IC in 79.2% of simulations.
However, while the study is of some interest, it does not directly address the decision problem at hand because TURBT is not used as a comparator. The study instead compares two alternatives to TURBT and thus the key aspect of our decision problem remains unanswered by this study.
While both of these studies are somewhat useful, their lack of direct applicability to the decision problem under consideration makes it difficult to draw firm conclusions. As such, the cost-effectiveness of perioperative intravesical chemotherapy remains, to a large extent, uncertain.
References
- 1.
- Green DA, Rink M, Cha EK, Xylinas E, Chughtai B, Scherr DS, Shariat SF, Lee RK. Cost-effective treatment of low-risk carcinoma not invading bladder muscle. BJU Int. 2013;111(3B):E78–E83. [PubMed: 22958598]
- 2.
- Wong KA, Zisengwe G, Athanasiou T, O'Brien T, Thomas K. Outpatient laser ablation of non-muscle invasive bladder cancer: is it safe, tolerable and cost-effective? BJU Int. 2013;112(5):561–7. Epub. [PubMed: 23819486]
Full evidence table
The full details of the studies included in the evidence review are presented in the evidence table below.
Table 72
full evidence table showing the included evidence (Green et al. 2013 and Wong et al. 2013) for the treatment of recurrent bladder cancer and previous low risk bladder cancer with and without histological sampling.
3.3. Re-resection in high risk non-muscle invasive bladder cancer
Review question: Does re-resection in high risk NMIBC influence outcomes?
Rationale
High-grade non-muscle invasive (HGNMI) bladder cancer is an aggressive disease. The natural history of these cancers can be difficult to predict. Around 1 in 4 will progress to invade the bladder wall and may eventually spread beyond the bladder. Radical treatment, by either bladder removal (cystectomy) or radiotherapy, is necessary for tumours invading the bladder wall if cure is to be obtained. Whilst all patients with HGNMI bladder cancer are followed closely after initial treatment, a proportion of tumours progress to invasion and spread without detection. The risk of progression to invasion, or recurrence of another HGNMI cancer within the bladder, is related to several factors. These include pathological features of the tumour, patient factors and the practice of endoscopic transurethral resection. Whilst most surgeons agree on the need for an initial tumour resection, there is controversy regarding the role of an early, planned re-resection. This normally occurs within 6 weeks of the initial transurethral resection. It should reassess the site of the initial cancer and sample the urothelium within the bladder/prostatic fossa.
Advocates of re-resection report that a proportion of HGNMI tumours are found to actually be invasive upon re-assessment, and that pathological features missed in the initial resection may be detected. Furthermore, residual disease at re-resection is known to be a poor prognostic feature for the patient and may alter treatment plans. However, in many patients re-resection does not influence their treatment and adds cost to the healthcare provider and the risks of further surgery to the patient. Furthermore, some surgeons feel that the emphasis should be on an initial high-quality resection, so that all pathological factors and all invasive tumours are identified at this time. They argue that the re-resection delays the time to reaching a final pathological diagnosis.
This review will assess the evidence for re-resection in HGNMI bladder cancer and identify in which patients and tumours it is beneficial. It will identify measures of high quality re-resection that should be achieved by this procedure.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with newly confirmed high risk NMIBC following first TUR | Re -resection | No –re-resection |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (NB) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. The full articles were then obtained for potentially relevant studies and checked against the inclusion criteria. A second sift of the literature was conducted by another reviewer (JH) and any disagreement between reviewers was discussed. Data from randomised trials and one systematic review of observational studies was identified.
Data synthesis
Evidence was synthesised using RevMan and pooled effect sizes are reported in a GRADE evidence profile (Table 73) and forest plots (see Figures 52-53).
Table 73
GRADE Profile for re-resection versus no re-resection in people with high risk NMIBC.

Figure 52
Recurrence free survival forest plot.

Figure 53
Cystectomy rate forest plot.
RESULTS
Study quality and results
Low quality evidence was identified. The quality and results are summarised with GRADE in Table 73.
Evidence statements
Low quality evidence (Divrik et al., 2010; Kim et al., 2012) suggests a benefit for repeat transurethral resection in patients with high risk non muscle invasive bladder cancer in terms of bladder cancer recurrence, disease progression and bladder cancer specific mortality.
Using event free survival rates from the no re-resection group in Divrik et al. (2010) trial combined with the hazard ratios reported in Table 73 we could expect five year recurrence free survival rates of 63% following re-resection versus 33% without no re-resection. Estimated five-year progression-free survival would be 92% following re-resection group versus 76% without re-resection.
Low quality evidence (Divrik et al., 2010) suggests re-resection is associated with minor complications in approximately 9% of cases, including prolonged bleeding, epididymitis and transient urinary retention. Such complications could be avoided in patients who do not undergo re-resection
A systematic review of observational studies (Vianello et al., 2011) provided evidence of upstaging and tumour persistence rates at re-resection. For patients with stage T1 tumours at initial TURB, approximately 32% were found to have persistent tumour of the same or lower stage at repeated TURB. Around 9% of patients with T1 tumours at initial TURB were upstaged at repeat TURB.
No evidence was found about the impact of re-resection on health related quality of life in this population.
References to included studies
- Divrik RT, et al. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. European Urology. 2010;58(2):185–190. [PubMed: 20303646]
- Kim W, et al. Value of immediate second resection of the tumor bed to improve the effectiveness of transurethral resection of bladder tumor. Journal of Endourology. 2012;26:1059–1064. [PubMed: 22390720]
- Skolarus TA, et al. Use of restaging bladder tumor resection for bladder cancer among Medicare beneficiaries. Urology. 2011;78:1345–1349. [PubMed: 21996111]
- Vianello A, et al. Repeated white light transurethral resection of the bladder in nonmuscle-invasive urothelial bladder cancers: systematic review and meta-analysis. Journal of Endourology. 2011;25:1703–1712. [PubMed: 21936670]
References to excluded studies (with reasons for exclusion)
- Divrik RT, Sahin AF, Ergor G. Reply from authors re: Marko Babjuk. Second resection for non-muscle-invasive bladder carcinoma: current role and future perspectives. Eur Urol 2010;58:191-2 and Giacomo Novara, Vincenzo Ficarra. Does routine second transurethral resection affect the long-term outcome of patients with T1 bladder cancer? Why a flawed randomized controlled trial cannot address the issue. Eur Urol 2010;58:193-4. European Urology. 2010;58:195–196. Reason: Authors reply to comment . [PubMed: 20471156]
- Divrik RT, Yildirim U, Zorlu F, Ozen H. Re: The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: A prospective, randomized clinical trial. European Urology. 2007;51:1753. Reason: Comment on the Divrik et al trial . [PubMed: 16600720]
- Angbein S, Guzman S, Haecker A, Weib C, Michel MS, Alken P. [The influence of “differentiated trandurethral resection” in the recurrence and progression of superficial bladder cancer]. Archivos Espanoles de Urologia. 2006;59:25–30. [Spanish] Reason: Spanish language – is differentiated resection the same as repeated resection? [PubMed: 16568690]
- Aning JJ. Early re-resection for T1 transitional cell carcinoma of the bladder-A study of current practice in the South West of England. British Journal of Medical and Surgical Urology. 2011;4:18–23. Reason: Non comparative, possibly relevant to update Vianello review .
- Jahnson S, Wiklund F, Duchek M, Mestad O, Rintala E, Hellsten S, et al. Results of second-look resection after primary resection of T1 tumour of the urinary bladder. Scandinavian Journal of Urology & Nephrology. 2005;39:206–210. Reason: All patients had early second-look resection – non comparative study . [PubMed: 16127800]
- Katumalla FS, Devasia A, Kumar R, Kumar S, Chacko N, Kekre N. Second transurethral resection in T1G3 bladder tumors - Selectively avoidable? Indian Journal of Urology. 2011;27:176–179. Reason: Only patients with second TUR are reported – non comparative study . [PMC free article: PMC3142825] [PubMed: 21814305]
- Klan R, Loy V, Huland H. Residual tumor discovered in routine second transurethral resection in patients with stage T1 transitional cell carcinoma of the bladder. Journal of Urology. 1991;146:316–318. Reason: Non – comparative, results are not reported for the 23 patients who did not have repeat TUR . [PubMed: 1856924]
- Kohrmann KU, Woeste M, Kappes J, Rassweiler J, Alken P. The Value of Secondary Transurethral Resection for Superficial Bladder-Tumors. Aktuelle Urologie. 1994;25:208–213. Reason: German language non comparative .
- Langbein S, Badawi K, Haecker A, Weiss C, Hatzinger M, Alken P, et al. Persistence, recurrence, and progression rates of superficial bladder tumours after resection using the differentiated technique. Medical Principles & Practice. 2006;15:215–218. Reason: All patients had second resection – the comparison was between differentiated and non-differentiated technique . [PubMed: 16651838]
- Lopatkin NA, Martov AG, Gushchin BL, Gnatiuk AP, Ergakov DV, Serebrianyi SA. [Diagnosis and treatment of recurrent surface cancer of the urinary bladder (early repeated cystoscopy and biopsy)]. Urologiia (Moscow, Russia). 2003:45–49. [Russian] Reason: Russian language . [PubMed: 14658273]
- Ojea CA, Nunez LA, Alonso RA, Rodriguez IB, Benavente DJ, Barros Rodriguez JM, et al. [Value of a second transurethral resection in the assessment and treatment of patients with bladder tumor]. Actas Urologicas Espanolas. 2001;25:182–186. [Spanish] Reason: Spanish language non comparative . [PubMed: 11402530]
- Orsola A, Cecchini L, Raventos CX, Trilla E, Planas J, Landolfi S, et al. Risk factors for positive findings in patients with high-grade T1 bladder cancer treated with transurethral resection of bladder tumour (TUR) and bacille Calmette-Guerin therapy and the decision for a repeat TUR. BJU International. 2010;105:202–207. Reason: Repeat TUR done in all T1b or greater cases – no matched comparison group, possibly relevant to update Vianello review . [PubMed: 19558557]
- Parkin J. G3T1 bladder cancer: Is early re-resection necessary? British Journal of Medical and Surgical Urology. 2011;4:13–17. Reason: Non-comparative study, possibly relevant to update Vianello review .
- Richterstetter M, Wullich B, Amann K, Haeberle L, Engehausen DG, Goebell PJ, et al. The value of extended transurethral resection of bladder tumour (TURBT) in the treatment of bladder cancer. BJU International. 2012;110:E76–E79. Reason: Non-comparative study, possibly relevant to update Vianello review . [PubMed: 22313727]
- Rigaud J, Karam G, Braud G, Glemain P, Buzelin JM, Bouchot O. [T1 bladder tumors: value of a second endoscopic resection]. Progres En Urologie. 2002;12:27–30. [French] Reason: All patients had early second TUR – non comparative study . [PubMed: 11980011]
- Rodriguez-Rubio Cortadellas FI, Garrido IS, Rivas AD, Hens PA, Bachiller BJ, Beltran AV, et al. [Second resection in patients with Ta-T1 bladder tumors]. Actas Urologicas Espanolas. 2001;25:553–558. [Spanish] Reason: Spanish language non comparative . [PubMed: 11692797]
- Schulze M, Stotz N, Rassweiler J. Retrospective analysis of transurethral resection, second-look resection, and long-term chemo-metaphylaxis for superficial bladder cancer: indications and efficacy of a differentiated approach. Journal of Endourology. 2007;21:1533–1541. Reason: Repeat TUR done in all high risk cases – no matched comparison group . [PubMed: 18186695]
- Shen H-B. Clinical analysis of re-transurethral resection in management of non-muscle invasive bladder urothelial cancer. Journal of Shanghai Jiaotong University (Medical Science). 2012;32:491–494. Reason: Chinese language – no mention of randomization .
- Shen YJ, Ye DW, Yao XD, Zhang SL, Dai B, Zhu YP, et al. [Repeat transurethral resection for non-muscle invasive bladder cancer]. Chung-Hua Wai Ko Tsa Chih. [Chinese Journal of Surgery]. 2009;47:725–727. [Chinese] Reason: Exclude – Chinese language, non comparative . [PubMed: 19615201]
- Vasdev N. The role of early re-resection in pTaG3 transitional cell carcinoma of the urinary bladder. British Journal of Medical and Surgical Urology. 2011;4:158–165. Reason: Non randomized study – historical comparison group .
- Vogeli TA, Grimm MO, Simon X, Ackermann R. [Prospective study of effectiveness. Reoperation (re-TUR) in superficial bladder carcinoma]. Urologe (Ausg, A). 2002;41:470–474. [German] Reason: German language non comparative . [PubMed: 12426865]
- Wilby D. Comparison of re-resection rates for new G3pT1 bladder cancer, in patients randomised to initial blue light or white light resection: 1 year follow up data. Journal of Endourology, Conference. 2009:A67. Reason: Abstract only – compares blue with white light resection .
- Wong SSW. Pathological staging of superficial high-grade bladder transitional cell carcinoma at re-resection. Journal of Urology, Conference. 2009 var- Reason: Abstract only – non comparative all had re-resection .
- Yucel M, Hatipoglu NK, Atakanli C, Yalcinkaya S, Dedekarginoglu G, Saracoglu U, et al. Is repeat transurethral resection effective and necessary in patients with T1 bladder carcinoma? Urologia Internationalis. 2010;85:276–280. Reason: All patients had early second TUR – non comparative study, possibly relevant to update Vianello review . [PubMed: 20733272]
- Holmang S. High-grade non-muscle-invasive bladder cancer: is re-resection necessary in all patients before intravesical bacillus Calmette-Guerin treatment? Scandinavian Journal of Urology. 2013;47(5):363–369. Reason: Non randomized study . [PubMed: 23394122]
- Sfakianos JP, et al. The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette-Guerin. Journal of Urology. 2014;191(2):341–345. Reason: Non randomized study . [PMC free article: PMC4157345] [PubMed: 23973518]
- Suer E, et al. Time between first and second transurethral resection of bladder tumors in patients with high-grade T1 tumors: is it a risk factor for residual tumor detection? Urologia Internationalis. 2013;91(2):182–186. Reason: Non-comparative study, possibly relevant to update Vianello review . [PubMed: 23751593]
- Lazica DA, et al. Second transurethral resection after ta high-grade bladder tumor: a 4.5-year period at a single university center. Urologia Internationalis. 2014;92(2):131–135. Reason: Non-comparative study, possibly relevant to update Vianello review . [PubMed: 23988813]
Evidence tables
Download PDF (243K)
3.3.1. BCG or primary cystectomy in high risk non-muscle invasive bladder cancer
Review question: For which patients with non-muscle invasive bladder cancer would primary cystectomy produce better outcomes than BCG?
Rationale
High-grade non-muscle invasive (HGNMI) bladder cancer is an aggressive disease. The natural history of these cancers is difficult to predict. Around 1 in 4 will eventually progress to invade the bladder wall and may spread beyond the bladder to cause death. Invasion marks a dramatic worsening in prognosis for the patient and needs aggressive treatment if cure is to be obtained. Whilst various pathological factors can be used to guide the risk of developing invasion from a HGNMI tumour, none offer absolute certainty to the patient.
Currently, many urologists offer an initial treatment of BCG immunotherapy for HGNMI bladder cancer. BCG may reduce the chance of a tumour progressing to invasion but has side effects and can delay the identification of worsening cancers. This delay may affect the cure rate for aggressive cancers. Advocates of BCG suggest this treatment may reduce progression rates for individual tumours, allows the identification of patients with non-progressing cancers (and so these patients do not receive radical treatment) and is safe if the bladder is monitored closely. In contrast, other physicians claim that BCG is not effective at reducing progressing and delays the identification of worsening disease such that it reduces the chances of cure in the patients. An alternate approach to BCG is primary radical treatment (usually cystectomy) for HGNMI cancers. This may be the safest option for patients, but will lead to over treatment for those whose cancers would not progress to invasion and carries the risks of major surgery or radiotherapy. Although radical radiotherapy / chemo-radiotherapy is used to treat muscle invasive bladder cancer, evidence to support its use in the HGNMI disease is less compelling. Various pathological and clinical factors may be used to guide the risk of progression and the treatment options.
This review will look at the evidence of BCG and primary radical treatment (cystectomy) for HGNMI bladder cancer. It will estimate the risks and benefits of each approach and try to identify factors that would be useful in aiding patient choice.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients diagnosed with high risk NMIBC with no prior BCG therapy Subgroups:
| Primary Cystectomy Primary Radiotherapy/ chemoradiotherapy | BCG therapy |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
Randomised trials and comparative studies were included in the evidence review. After discussion with the GDG it was decided to also include the two largest series of patients (one cohort of patients treated with BCG and one treated with cystectomy) in order to benchmark the survival data from comparative studies. The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. Studies comparing primary treatments were included, as were studies comparing primary versus deferred cystectomy, in order to assess the clinical outcomes of undergoing initial bladder-sparing treatment.
Data synthesis
Data from comparative studies were pooled using RevMan to provide overall effect estimates. The case series studies are summarised in Table 74-78.
Table 75
GRADE evidence profile: Primary cystectomy versus conservative treatment (surveillance or intravesical therapy) for high-risk non muscle invasive bladder cancer.
Table 76
GRADE evidence profile: Early cystectomy versus deferred cystectomy for high-risk non-muscle invasive bladder cancer.
Table 77
Disease-specific survival (DSS) in patients with high-risk NMIBC and progression after initial conservative treatment (reported in systematic review by van den Bosch, 2011).
RESULTS
Study quality and results
The quality of the evidence was assessed using GRADE. The evidence is summarised in Tables 74-78 and Figures 52-53.
Table 74
GRADE evidence profile: Radiotherapy versus control (observation or intravesical therapy) for T1G3 bladder cancer.
Table 78
Recurrence, disease-specific mortality and overall mortality of 1136 T1G3 NMIBC patients treated with radical cystectomy and bilateral lymphadenectomy (Fritsche, 2011) (51% of patients were upstaged to pT2 or higher).
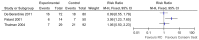
Figure 52
Primary cystectomy versus primary conservative therapy; Outcome, Disease-specific mortality rate.

Figure 53
Early cystectomy versus deferred cystectomy; Outcome, 5-yr disease-specific mortality rate.
Evidence statements
Radiotherapy versus observation or BCG therapy
Moderate quality evidence from one randomised trial of 204 patients (Harland et al., 2007) suggests uncertainty over whether radiotherapy is more or less effective than observation or BCG therapy in terms of recurrence-free survival, progression-free survival and overall survival. 5/102 (5%) of patients in the radiotherapy arm experienced long-term toxicity. 18% of the radiotherapy arm and 13% of the control arm underwent cystectomy due to recurrence or progression.
Primary cystectomy versus primary conservative treatment
Very low quality evidence from two retrospective studies suggests uncertainty over whether primary cystectomy is more or less effective than primary conservative treatment (observation or intravesical therapy) in terms of progression or overall survival. Conservative treatment was associated with better five-year disease-specific survival than primary cystectomy in three studies (Badalato et al., 2012; Park et al., 2009; Patard et al., 2001). However, in one study (Park et al., 2009) patients undergoing cystectomy were older, more likely to have proper muscle absent in the TUR specimen and included a higher proportion of gross non-papillary tumours, all of which were associated with reduced disease-specific survival. Three studies reported disease-specific mortality rates in 337 patients. There were no differences in disease-specific mortality in two studies. Low quality evidence from six studies reported a subsequent cystectomy rate of 26% in patients initially treated by conservative therapy.
Early cystectomy versus deferred cystectomy
Very low quality evidence from one study suggests uncertainty of a difference in five-year overall survival between patients treated with early cystectomy compared with patients undergoing deferred cystectomy after BCG failure (72.2% versus 73.2% five-year survival, p=0.75). Three studies suggest reduced disease-specific survival in patients undergoing deferred cystectomy, with five-year disease-specific survival ranging from 78% to 84% across studies for early cystectomy and from 67% to 75% across studies for deferred cystectomy. Ten-year disease-specific survival ranged from 69% to 79% across studies for early cystectomy and from 51% to 64% for deferred cystectomy. Denzinger et al. (2009) reported that concomitant CIS was related to a decrease in disease-specific survival in the deferred cystectomy group only. One systematic review reported that disease-specific survival after progression from high-risk NMIBC in initially conservatively treated patients was 35% after a median follow-up of 48-123 months (van den Bosch et al., 2011). The disease-specific mortality rate in 1136 clinical T1G3 patients who underwent radical cystectomy was 29.8% at five years (Fritsche et al., 2010). 50% of this cohort were upstaged to pT2 or higher at cystectomy.
One study reported that 7% of patients had major surgical complications which were distributed equally between early and deferred cystectomy groups, including two fatal pulmonary embolias and one fatal cardiac ishaemia.
One study (Kamat et al., 2006) provides very low quality evidence from 30 patients with micropapillary bladder cancer. 12 patients undergoing cystectomy as initial therapy had ten-year disease-specific survival of 72%, whilst in 18 patients who underwent cystectomy after progression the median disease-specific survival was 61.7 months with no patient surviving ten years. Very low quality evidence from one study (Cheng et al., 1999) of patients with primary CIS suggests uncertainty about a difference in 15-year progression-free survival and disease-specific survival between those treated with immediate cystectomy and those that were not (some deferred cystectomy, some intravesical therapy). Radical cystectomy performed within three months after the initial diagnosis was associated with improved overall survival, but this was not significant after controlling for age.
References to included studies
- Ali-El-Dein B, et al. Survival after primary and deferred cystectomy for stage T1 transitional cell carcinoma of the bladder. Urology annals. 2011;3(3):127–132. [PMC free article: PMC3183703] [PubMed: 21976924]
- Badalato GM, et al. Immediate radical cystectomy vs conservative management for high grade cT1 bladder cancer: is there a survival difference? BJU International. 2012;110(10):1471–1477. [PubMed: 22487512]
- Canter D, et al. Use of radical cystectomy as initial therapy for the treatment of high-grade T1 urothelial carcinoma of the bladder: A SEER database analysis. Urologic Oncology-Seminars and Original Investigations. 2013;31(6):866–870. [PubMed: 21906968]
- Cheng L, et al. Survival of patients with carcinoma in situ of the urinary bladder. Cancer. 1999;85:2469–2474. [PubMed: 10357420]
- Dalbagni G, et al. Clinical outcome in a contemporary series of restaged patients with clinical T1 bladder cancer. European Urology. 2009;56(6):903–910. [PMC free article: PMC8177014] [PubMed: 19632765]
- De BE, et al. T1G3 high-risk NMIBC (non-muscle invasive bladder cancer): conservative treatment versus immediate cystectomy. International Urology & Nephrology. 2011;43(4):1047–1057. [PubMed: 21442469]
- Denzinger S, et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? European Urology. 2008;53(1):146–152. [PubMed: 17624657]
- Fritsche HM, et al. Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. European Urology. 2010;57(2):300–309. [PubMed: 19766384]
- Harland SJ, et al. A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. The Journal of urology. 2007;178:807–813. [PubMed: 17631326]
- Hautmann RE, Volkmer BG, Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World Journal of Urology. 2009;27(3):347–351. [PubMed: 19319539]
- Iida S, et al. Clinical outcome of high-grade non-muscle-invasive bladder cancer: a long-term single center experience. International Journal of Urology. 2009;16(3):287–292. [PubMed: 19207115]
- Kamat AM, et al. The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. Journal of Urology. 2006;175(3 Pt 1):881–885. [Erratum appears in J Urol. 2006 May;175(5):1967] [PubMed: 16469571]
- Park J, et al. Prognostic significance of non-papillary tumor morphology as a predictor of cancer progression and survival in patients with primary T1G3 bladder cancer. World Journal of Urology. 2009;27(2):277–283. [PubMed: 19020879]
- Patard J, et al. Tumor progression and survival in patients with T1G3 bladder tumors: multicentric retrospective study comparing 94 patients treated during 17 years. Urology. 2001;58(4):551–556. [PubMed: 11597537]
- Thalmann GN, et al. Primary T1G3 bladder cancer: organ preserving approach or immediate cystectomy? Journal of Urology. 2004;172(1):70–75. [PubMed: 15201740]
- van den Bosch S, Alfred WJ. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. European Urology. 2011;60(3):493–500. [Review] [PubMed: 21664041]
- Wong SW. Immediate versus delayed cystectomy for high-grade PT1 Transitional Cell Carcinoma of the bladder. BJU International. :4. Conference(var.pagings)
References to excluded studies (with reasons for exclusion)
- Droller MJ. Tumor progression and survival in patients with T1G3 bladder tumors: multicentric retrospective study comparing 94 patients treated during 17 years. Journal of Urology. 2002;168(2):855–856. Reason: Comment on Patard 2002 . [PubMed: 12134840]
- Sternberg IA. The role of immediate radical cystectomy in the treatment of patients with residual T1 on restaging transurethral resection. Journal of Urology. 2012.:4. Conference(var.pagings) Reason: Abstract only, unclear if relevant to PICO . [PubMed: 23146082]
- Kulkarni GS, et al. Cost-effectiveness analysis of immediate radical cystectomy versus intravesical Bacillus Calmette-Guerin therapy for high-risk, high-grade (T1G3) bladder cancer. Cancer. 2009;115:5450–5459. (Structured abstract) Reason: Health Economics . [PubMed: 19685529]
- Kulkarni GS, et al. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Medicine / Public Library of Science. 2007;4(9):e284. Reason: Health Economics . [PMC free article: PMC1989749] [PubMed: 17896857]
- Jager W, et al. Early vs delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: delay of cystectomy reduces cancer-specific survival. BJU International. 2011;108(8 Pt 2):E284–E288. Reason: Comparison not relevant to PICO Not reported if other primary treatment received before delayed cystectomy Time to cystectomy as continuous variable . [PubMed: 21244611]
- May M. Survival Rates after Radical Cystectomy according to Tumor Stage of Bladder Carcinoma at First Presentation. Urologia Internationalis. 2004;72(2):103–111. Reason: Comparison not relevant to PICO . [PubMed: 14963349]
- Norming U. Prognostic significance of mucosal aneuploidy in stage Ta/T1 grade 3 carcinoma of the bladder. Journal of Urology. 1992;148(5 I):1420–1427. Reason: Not relevant to PICO RC preceded by RT . [PubMed: 1433541]
- Trinchieri A, et al. Conservative treatment of high grade superficial bladder tumours. Archivio Italiano di Urologia, Andrologia. 2005;77(4):215–218. Reason: Not relevant to PICO No primary RC . [PubMed: 16444936]
- Takaoka E, et al. Risk factors for intravesical recurrence in patients with high-grade T1 bladder cancer in the second TUR era. Japanese Journal of Clinical Oncology. 2013;43(4):404–409. Reason: Non-comparative . [PubMed: 23444116]
- Dalbagni G, et al. Variability of treatment selection among surgeons for patients with cT1 urothelial carcinoma. BJU International. 2010;106(10):1502–1507. Reason: Not relevant to PICO Doesn't compare BCG and RC treated patients . [PubMed: 20367633]
- Bolenz C, et al. Management of elderly patients with urothelial carcinoma of the bladder: guideline concordance and predictors of overall survival. BJU International. 2010;106(9):1324–1329. Reason: Population not relevant to PICO, includes MIBC . [PubMed: 20500510]
- Nielsen ME, et al. A delay in radical cystectomy of >3 months is not associated with a worse clinical outcome. BJU International. 2007;100(5):1015–1020. Reason: Comparison not relevant to PICO Includes MIBC . [PubMed: 17784888]
- Lambert EH, et al. The increasing use of intravesical therapies for stage T1 bladder cancer coincides with decreasing survival after cystectomy. BJU International. 2007;100(1):33–36. Reason: Non-comparative All patients had RC, no primary IVT . [PubMed: 17552951]
- Kulkarni JN, Gupta R. Recurrence and progression in stage T1G3 bladder tumour with intravesical bacille Calmette-Guerin (Danish 1331 strain). BJU International. 2002;90(6):554–557. Reason: Non-comparative . [PubMed: 12230616]
- Rodel C, et al. Radiotherapy is an effective treatment for high-risk T1-bladder cancer. Strahlentherapie und Onkologie. 2001;177(2):82–88. Reason: Non-comparative . [PubMed: 11233839]
- Villar A, et al. External beam irradiation for T1, T2-3 and T4 transitional cell carcinoma of the urinary bladder. Radiotherapy & Oncology. 1987;9(3):209–215. Reason: Non-comparative, unclear if high-risk NMIBC . [PubMed: 3114833]
- Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? Journal of Urology. 2001;166(4):1296–1299. Reason: Not relevant to PICO All previous BCG before RC . [PubMed: 11547061]
- Zietman AL. Selective bladder conservation using transurethral resection, chemotherapy, and radiation: Management and consequences of TA, T1, and TIS recurrence within the retained bladder. Urology. 2001;58(3):380–385. Reason: Population not relevant (MIBC) [PubMed: 11549485]
- Shahin O, et al. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade 3 bladder cancer: recurrence, progression and survival. Journal of Urology. 2003;169(1):96–100. Reason: Comparison not relevant to PICO (BCG vs no BCG) [PubMed: 12478112]
- Masood S, et al. T1G3 bladder cancer--indications for early cystectomy. International Urology & Nephrology. 2004;36(1):41–44. Reason: Non-comparative . [PubMed: 15338671]
- Solsona E, et al. The optimum timing of radical cystectomy for patients with recurrent high-risk superficial bladder tumour. BJU International. 2004;94(9):1258–1262. Reason: Non-comparative . [PubMed: 15610101]
- Chang SS. Non-muscle-invasive bladder cancer: The role of radical cystectomy. Urology. 2005;66(5):917–922. Reason: Expert review . [PubMed: 16286095]
- Nieder AM, et al. Radical cystectomy after bacillus Calmette-Guerin for high-risk Ta, T1, and carcinoma in situ: defining the risk of initial bladder preservation. Urology. 2006;67(4):737–741. Reason: Not relevant to PICO – all previous BCG . [PubMed: 16618564]
- Stockle M, et al. Radical cystectomy--often too late? 1987. European Urology. 2006;50(6):1132–1138. Reason: Population not relevant –MIBC . [PubMed: 17183625]
- Weiss C, et al. Radiochemotherapy after transurethral resection for high-risk T1 bladder cancer: an alternative to intravesical therapy or early cystectomy? Journal of Clinical Oncology. 2006;24(15):2318–2324. Reason: Non-comparative . [PubMed: 16710030]
- Gautam G, Kumar R. T1 bladder cancer on restaging transurethral resection should be treated with immediate cystectomy. Indian Journal of Urology. 2007;23(2):218. Reason: Not relevant to PICO . [PMC free article: PMC2721545] [PubMed: 19675813]
- Raj GV, et al. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. Journal of Urology. 2007;177(4):1283–1286. Reason: Not relevant to PICO . [PubMed: 17382713]
- Inoue M, et al. Clinical outcome of chemoradiotherapy for T1G3 bladder cancer. International Journal of Urology. 2008;15(8):747–750. Reason: Non-comparative . [PubMed: 18786198]
- Steen-Banasik E, et al. Brachytherapy versus cystectomy in solitary bladder cancer: a case control, multicentre, East-Netherlands study. Radiotherapy & Oncology. 2009;93(2):352–357. Reason: Not relevant to PICO – all T2 in RC group . [PubMed: 19457565]
- Montgomery JS, Weizer AZ, Montie JE. T1 bladder cancer: advocating early cystectomy to improve oncologic control. Urologic Oncology. 2010;28(5):466–468. Reason: Expert review . [PubMed: 20451423]
- Denzinger S, et al. Prognostic value of histopathological tumour growth patterns at the invasion front of T1G3 urothelial carcinoma of the bladder. Scandinavian Journal of Urology & Nephrology. 2009;43(4):282–287. Reason: Comparison not relevant to PICO . [PubMed: 19382004]
- Park J. Prognostic significance of the presence of proper muscle in the resected specimens of primary T1G3 bladder cancer. Korean Journal of Urology. 2006;47(2):137–142. Reason: Foreign language .
- Morelli B. Which is the most suitable treatment of transitional bladder epithelium carcinoma G3 T1? Acta Urologica Italica. 1992;6(SUPPL. 4):155–156. Reason: Foreign language .
- Dunst J, et al. Radiochemotherapy for T1G3 bladder cancer. Frontiers of Radiation Therapy & Oncology. 2002;36:151–158. [Review] [16 refs] Reason: Expert review . [PubMed: 11842745]
- Hollenbeck BK, Montie JE. Early cystectomy for clinical stage T1 bladder cancer. Nature Clinical Practice Urology. 2004;1(1):4–5. Reason: Expert review . [PubMed: 16474441]
- Kanayama H-O. Bladder preservation therapy and total cystectomy for primary carcinoma in situ of the urinary bladder. Nishinihon Journal of Urology. 1998;60(5):407–412. Reason: Foreign language .
- Yates DR, Catto JW. Time to change our approach to high-risk nonmuscle-invasive bladder cancer management in the United Kingdom? Observations from the British Association of Urological Surgeons Cancer Registry. BJU International. 2010;106(5):593–594. Reason: Outcomes not relevant to PICO . [PubMed: 21105261]
- Chang SS. The adverse consequences of delaying radical cystectomy. Nature Clinical Practice Urology. 2006;3(6):300–301. Reason: Comment on study not relevant to PICO . [PubMed: 16763637]
- Dinh T. Compa rative effectiveness of conservative therapy versus cystectomy for non-muscle invasive bladder cancer patients. Value in Health. 2013.:7. Conference(var.pagings) Reason: Abstract only .
- Huang Y. Conservative treatment versus early radical cystectomy for T1G3 bladder cancer: A Meta-analysis. Tumor. 2013;33(10):903–908. Reason: Foreign language .
- Yu L. Immediate cystectomy or conservative management for T1G 3 bladder cancer: A meta-analysis of general survival rate. Chinese-German Journal of Clinical Oncology. 2013;12(5):243–245. Reason: Meta-analysis not appropriate .
Evidence tables
Download PDF (400K)
Health Economic Evidence: What are the comparative patient outcomes for treating high-risk non-muscle invasive bladder cancer with radiotherapy, intravesical BCG or radical cystectomy with urinary stoma or bladder reconstruction?
Review question
For which patients with non-muscle invasive bladder cancer would primary cystectomy produce better outcomes than BCG?
Table 79Pico Table For Treating High Risk Non-Muscle Invasive Bladder Cancer
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients diagnosed with high risk NMIBC with no prior BCG therapy Subgroups:
| Primary Cystectomy Primary Radiotherapy/chemoradiotherapy | BCG therapy |
|
Information sources and eligibility criteria
The following databases were searched for economic evidence relevant to the PICO: MEDLINE, EMBASE, COCHRANE, NHS EED and HEED. Studies conducted in OECD countries other than the UK were considered.
Studies were selected for inclusion in the evidence review if the following criteria were met:
- Both cost and health consequences of interventions reported (i.e. true cost-effectiveness analyses)
- Conducted in an OECD country
- Incremental results are reported or enough information is presented to allow incremental results to be derived
- Studies that matched the population, interventions, comparators and outcomes specified in PICO
- Studies that meet the applicability and quality criteria set out by NICE, including relevance to the NICE reference case and UK NHS
Note that studies that measured effectiveness using quality of life based outcomes (e.g. QALYs) were desirable but, where this evidence was unavailable, studies using alternative effectiveness measures (e.g. life years) were considered.
Selection of studies
The literature search results were screened by checking the article's title and abstract for relevance to the review question. The full articles of non-excluded studies were then attained for appraisal and compared against the inclusion criteria specified above.
Results
Three searches for economic evidence were run over the development of the guideline; one at the start of the process, an update midway through and a further update at the end of the process. The diagram below shows the combined results of the three searches and illustrates the sifting process.
It can be seen that, in total, 1,189 possibly relevant papers were identified. Of these, 1,124 papers were excluded at the initial sifting stage based on the title and abstract while 65 full papers were obtained for appraisal. A further 56 papers were excluded based on the full text as they were not applicable to the PICO or did not include an incremental analysis of both costs and health effects. Therefore, nine papers were included in the systematic review of the economic evidence for this guideline.
One of these nine papers related to the topic at hand and was thus included in the review of published economic evidence for this topic; Kulkarni et al. 2009. The study included a cost-effectiveness analysis where effectiveness was measured using quality adjusted life years (QALYs) i.e. a cost-utility analysis.
Quality and applicability of the included study
The study was only partially applicable to the decision problem that we are evaluating, primarily because it was a Canadian study and as such the estimated costs and benefits might not apply to the UK health care setting. In addition, quality of life values were not all reported directly from patients and were often not drawn from bladder cancer patients (data from prostate, lung and breast cancer patients)
Potentially serious limitations were also identified with the analysis. Although a systematic literature review was conducted, the evidence identified and utilised was not always of high quality. The costs applied in the model were not always sourced from patients with bladder cancer e.g. chemotherapy costs were based on patients with non-small cell lung cancer. In addition, while PSA and scenario analyses were performed, further sensitivity analyses could have been conducted to better explore uncertainty.
Table 80Table Showing Methodological Quality And Applicability Of The Included Study
| Methodological quality | Applicability | |
|---|---|---|
| Directly applicable | Partially applicable | |
| Minor limitations | ||
| Potentially serious limitations | Kulkarni et al. 2009 | |
| Very serious limitations | ||
Modified GRADE table
The primary results of the analysis by Kulkarni et al. 2009 are summarised in the modified GRADE table below.
Table 81
Modified Grade Table Showing The Included Evidence For Treatments For High Risk Non-Muscle Invasive Bladder Cancer.
Evidence statements
The base case results of the cost-effectiveness analysis showed that immediate cystectomy was cheaper and more effective than conservative therapy (BCG with possible delayed cystectomy) i.e. immediate cystectomy was found to be the dominant strategy.
Scenario analyses, in which age and co-morbid status were varied, showed that the optimal strategy is likely to be different for different patient subgroups. The analysis showed that immediate cystectomy was dominant in patients aged ≤55 years old regardless of co-morbid status. For patients ≥70 years old, conservative therapy was either dominant or had an ICER that was likely to be considered cost-effective (≤$32,700 per QALY). For patients between ages 60 and 70 years old, the optimal choice was dependent upon co-morbidities, with increased co-morbid burden making conservative therapy more cost-effective.
The probabilistic sensitivity analyses (PSA) showed that immediate cystectomy was found to be cost-effective in 70% and 67% of simulations at thresholds of $20,000 and $50,000 per QALY, respectively.
The results suggest that, compared with a conservative strategy using BCG, immediate radical cystectomy yielded better outcomes and lower costs for the average patient. Furthermore, the results suggest that tailoring therapy based on patient age and co-morbidity may increase survival and yield significant costs savings for the health care system.
However, there are reservations about the applicability of the analysis because it considered the Canadian health care system which may not reflect the UK setting. There were also concerns about the quality of life data that were used as they were not all patient reported and were often not drawn from patients with bladder cancer (data from prostate, lung and breast cancer were used). Potentially serious limitations were also identified as, although a systematic literature review was conducted, some of the evidence informing the model was not considered to be of high quality. Furthermore, costs were not always sourced from patients with bladder cancer, such as chemotherapy costs, which were based on patients with non-small cell lung cancer.
References
- 1.
- Kulkarni GS, et al. Cost-effectiveness analysis of immediate radical cystectomy versus intravesical Bacillus Calmette-Guerin therapy for high-risk, high-grade (T1G3) bladder cancer. Cancer. 2009;115(23):5450–59. (Structured abstract) [PubMed: 19685529]
Full evidence table
The full details of the study included in the evidence review are presented in the evidence table below.
Table 82
full evidence table showing the included evidence (Kulkarni et al. 2009) for treatments for high risk non-muscle invasive bladder cancer.
4.3.2. Treatment following failure of BCG
Review question: What is the optimum treatment for patients with non-muscle invasive bladder cancer who have failed BCG?
Rationale
Intravesical BCG is an immunotherapy used to treat intermediate and high-risk non-muscle invasive bladder cancer. This therapy may be administered as either a single 6 week course (known as “induction BCG”) or as repeated instillations episodically for several years (known as “maintenance BCG”). Each treatment includes the instillation of live BCG bacteria, of which various strains are known to exist, into the bladder. Failure to respond to BCG occurs when a further bladder cancer arises following or during BCG treatment. These cancers may be better, similar or worse to the original tumour, and may be detected during, shortly after, or many years following BCG treatment. Therefore the term BCG failure includes a wide spectrum of events. It can also include patients who did not complete their treatment due to BCG related side effects (called BCG intolerant). In general most physicians agree that the development of tumour with muscle invasion following or during BCG treatment requires radical treatment - either bladder removal (cystectomy) or radiotherapy, if cure is to be obtained. In contrast, there is less consensus regarding the treatment of BCG failure when the disease is not muscle invasive. Some physicians feel that the timing of failure (early versus late) is important, whilst other feel that failure at any time requires more aggressive treatment.
Whilst radical cystectomy is perceived as the gold standard treatment for BCG failure, it may be over treatment in some patients and other patients are keen to avoid bladder removal regardless of risks. Therefore “bladder-sparing” treatments are reported for use in this context. These include immunotherapies (e.g. repeated BCG instillations with or without additional immune modulator), intravesical chemotherapy (such as gemcitabine), device assisted intravesical chemotherapy (e.g. mitomycin-c administration using EDMA or hyperthermia) and radiotherapy. These approaches avoid removal of the bladder, but carry the risk that the tumour may not respond and will progress to invasion or spread beyond the bladder. They also have side effects and toxicity. Given the spectrum of events encompassed by the term BCG-failure, it is possible that different treatments will be better for different types of failure.
This review will compare different treatments for patients who fail BCG. It will identity the risks and benefits of each treatment and try to identify if some are more suited to certain types of BCG failure.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients diagnosed with NMIBC who have failed BCG Subgroups:
| Intravesical chemotherapy Radiotherapy/chemoradiotherapy Cystectomy BCG therapy Interferon Cystoscopy | Each other |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (DJ) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. A second sift of the literature was conducted by another reviewer (JH) and any disagreement between reviewers was discussed. Randomised trials and comparative studies were selected.
Data synthesis
Data was synthesised using GRADE. Meta-analysis was not possible for this review.
RESULTS
Study quality and results
The evidence is summarised in GRADE evidence profiles (Tables 83-86)
Table 83
GRADE evidence profile: mitomycin C compared to gemcitabine for patients diagnosed with NMIBC who have failed BCG.
Table 84
GRADE evidence profile: gemcitabine compared to BCG for patients diagnosed with NMIBC who have failed BCG.
Table 85
GRADE evidence profile: BCG compared to chemotherapy for patients diagnosed with NMIBC who have failed BCG.
Table 86
GRADE evidence profile: BCG alone compared to BCG plus interferon α2B for patients diagnosed with NMIBC who have failed BCG.
Evidence statements
Gemcitabine versus Mitomycin C
Moderate quality evidence from one randomised trial (Addeo et al., 2009) of 109 patients suggests uncertainty over the incidence of tumour recurrence in gemcitabine- versus mitomycin C-treated patients. Although incidence of tumour recurrence was lower in gemcitabine treated patients after 36 months of follow up, the 95% confidence interval around the estimated effect included both no effect and considerable benefit for gemcitabine.
Moderate quality evidence from one randomised trial of 109 patients (Addeo et al., 2009) suggests uncertainty over the incidence of tumour progression in gemcitabine- versus mitomycin C-treated patients. Incidence of tumour progression was lower in gemcitabine treated patients after 36 months of follow up, but the 95% confidence interval around the estimated effect was wide and included considerable harm, no effect and considerable benefit for gemcitabine.
Moderate quality evidence from one randomised trial of 109 patients (Addeo et al., 2009) suggested that gemcitabine treatment was associated with fewer adverse events than mitomycin C.
Gemcitabine versus BCG
Two studies (Di Lorenzo et al., 2009; Gacci et al., 2006) compared the effectiveness of gemcitabine to BCG. Meta-analysis of the results was not possible due to differences in study design and outcome definitions.
Moderate quality evidence from one randomised trial of 80 patients (Di Lorenzo et al., 2009) suggests that the incidence of tumour recurrence after 12 months is lower in patients treated with gemcitabine compared to treatment with BCG. In patients experiencing recurrence (n=56), there was no significant difference between treatment groups in the incidence of cystectomy due to disease progression. The incidence of grade two and grade three adverse events was similar for both treatments.
Very low quality evidence from one observational trial of 19 patients (Gacci et al., 2006) found no significant difference in tumour recurrence, overall survival, bladder preservation rates or adverse events between gemcitabine and BCG treatment.
BCG versus chemotherapy (MMC or epirubicin)
Very low quality evidence from one observational trial of 183 patients (Matsumoto et al., 2012) suggests that rates of recurrence-free survival (after five years of follow up) are greater in patients treated with BCG than in patients treated with chemotherapy (MMC or epirubicin).
BCG versus BCG plus interferon α2B
Very low quality evidence from one observational trial of 139 patients (Prasad et al., 2009) suggests that the incidence of disease recurrence is lower in patients treated with BCG alone compared with BCG in combination with interferon α2B.
References to included studies
- Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, Miragliuolo A, Guarrasi R, Lanna M, Cennamo G, Faiola V, Prete S. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(4):543–548. [PubMed: 19841330]
- Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, Ascierto P, Simeone E, De Sio M, Autorino R. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116(8):1893–1900. [PubMed: 20162706]
- Gacci M, Bartoletti R, Cai T, Nerozzi S, Pinzi N, Repetti F, Viggiani F, Ghezzi P, Nesi G, Carini M. TUR (Toscana Urologia) Group. Intravesical gemcitabine in BCG-refractory T1G3 transitional cell carcinoma of the bladder: a pilot study. Urologia Internationalis. 2006;76(2):106–111. [PubMed: 16493208]
- Matsumoto K, Kikuchi E, Shirakawa H, Hayakawa N, Tanaka N, Ninomiya A, Miyajima A, Nakamura S, Oya M. Risk of subsequent tumour recurrence and stage progression in bacille Calmette-Guerin relapsing non-muscle-invasive bladder cancer. BJU International. 2012;110(11 Pt B):E508–E513. [PubMed: 22574662]
- Prasad SM. Durability of response: The achilles heel of salvage combination immunotherapy with intravesical bacillus calmette-guerin and interferon-alpha 2B in bladder cancer. Journal of Urology. 2009. Conference abstract.
References to excluded studies (with reasons for exclusion)
- Autorino R. Gemcitabine versus BCG after initial BCG failure in non muscle-invasive bladder cancer: A prospective randomized trial. European Urology, Supplements. 2009;8(4):283–283. Reason for exclusion: Conference abstract only Same study as Di Lorenzo 2009; no extra results reported by this article .
- Bohle A, Bock PR. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63(4):682–686. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 15072879]
- Böhle A, Leyh H, Frei C, Kühn M, Tschada R, Pottek T, Wagner W, Knispel HH, Pokrzywnitzki W, Zorlu F, Helsberg K, Lübben B, Soldatenkova V, Stoffregen C, Büttner H. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. European Urology. 2009;56(3):495–503. Reason for exclusion: Patients not relevant to PICO . [PubMed: 19560257]
- Cervenakov I, Szoldova K, Mardiak J, Chovan D, Mala M, Slavov D. Alpha 2-b interferon and farmarubicin in the prophylaxis of recurrence of superficial transitional cell carcinoma of the urinary bladder. Bratislavské lekárske listy. 2000;101(6):317–320. Reason for exclusion: Patients not relevant to PICO . [PubMed: 11039202]
- Colombo R, Brausi M, Da Pozzo L, Salonia A, Montorsi F, Scattoni V, Roscigno M, Rigatti P. Thermo-chemotherapy and electromotive drug administration of mitomycin C in superficial bladder cancer eradication. a pilot study on marker lesion. European Urology. 2001;39(1):95–100. Reason for exclusion: Patients not relevant to PICO . [PubMed: 11173946]
- Colombo R, Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, Caldarera E, Pavone Macaluso M. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(23):4270–4276. Reason for exclusion: Patients not relevant to PICO . [PubMed: 14581436]
- Debruyne FM, Meijden AP, Geboers AD, Franssen MP, Leeuwen MJ, Steerenberg PA, Jong WH, Ruitenberg JJ. BCG (RIVM) versus mitomycin intravesical therapy in superficial bladder cancer. First results of randomized prospective trial. Urology. 1988;31(3 Suppl):20–25. Reason for exclusion: Patients not relevant to PICO . [PubMed: 3279698]
- Esuvaranathan K. BCG & interferon alpha: Defining its benefits & limitations in high risk non-muscle invasive urothelial carcinoma after long follow-up. International Journal of Urology. 2010. Conference(Republic of China) var- Reason for exclusion: Conference abstract only Results not adequately reported .
- Gardmark T, Jahnson S, Wahlquist R, Wijkstrom H, Malmstrom PU. Analysis of progression and survival after 10 years of a randomized prospective study comparing mitomycin-C and bacillus Calmette-Guerin in patients with high-risk bladder cancer. BJU International. 2007;99(4):817–820. Reason for exclusion: Patients not relevant to PICO . [PubMed: 17244282]
- Hasner F. Combined thermochemotherapy (Synergo) in Non Muscle Invasive Bladder Cancer (NMIBC): 8 year follow up of a prospective monocentric cohort study. Urology. 2009.:4. Conference(var.pagings) Reason for exclusion: Conference abstract only Results not adequately reported .
- Houghton BB. Intravesical chemotherapy plus bacille Calmette-Guerin in non-muscle invasive bladder cancer: A systematic review with meta-analysis. BJU International. 2013;111(6):977–983. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 23253618]
- Huncharek M. Impact of intravesical chemotherapy versus BCG immunotherapy on recurrence of superficial transitional cell carcinoma of the bladder: Metaanalytic reevaluation. American Journal of Clinical Oncology: Cancer Clinical Trials. 2003;26(4):402–407. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 12902895]
- Huncharek M, Kupelnick B. The influence of intravesical therapy on progression of superficial transitional cell carcinoma of the bladder: a metaanalytic comparison of chemotherapy versus bacilli Calmette-Guerin immunotherapy. American Journal of Clinical Oncology. 2004;27(5):522–528. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 15596924]
- Huncharek M, McGarry R, Kupelnick B. Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer Research. 2001;21(1B):765–769. (Structured abstract) Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 11299841]
- Ibrahiem EH, Ghoneim MA, Nigam V, Brailovsky C, Elhilali MM. Prophylactic maltose tetrapalmitate and bacillus Calmette-Guerin immunotherapy of recurrent superficial bladder tumors: preliminary report. The Journal of urology. 1988;140(3):498–500. Reason for exclusion: Patients not relevant to PICO . [PubMed: 3411660]
- Isbarn H, Budäus L, Pichlmeier U, Conrad S, Huland H, Friedrich MG. [Comparison of the effectiveness between long-term instillation of mitomycin C and short-term prophylaxis with MMC or bacille Calmette-Guérin. Study of patients with non-muscle-invasive urothelial cancer of the urinary bladder]. Der Urologe Ausg A. 2008;47(5):608–615. Reason for exclusion: Non English publication . [PubMed: 18317718]
- Jarvinen R, Kaasinen E, Sankila A, Rintala E. Long-term efficacy of maintenance Bacillus Calmette-Guerin versus maintenance mitomycin C instillation therapy in frequently recurrent TAT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised Finnbladder I study with a 20-year follow-up. European Urology. 2009;56(2):260–265. Reason for exclusion: Patients not relevant to PICO . [PubMed: 19395154]
- Jimenez-Cruz JF, Vera-Donoso CD, Leiva O, Pamplona M, Rioja-Sanz LA, Martinez Lasierra M, Flores N, Unda M. Intravesical immunoprophylaxis in recurrent superficial bladder cancer (Stage T1): multicenter trial comparing bacille Calmette-Guérin and interferon-alpha. Urology. 1997;50(4):529–535. Reason for exclusion: Patients not relevant to PICO . [PubMed: 9338727]
- Jones G, Cleves A, Wilt TJ, Mason M, Kynaston HG, Shelley M. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database of Systematic Reviews. 2012;1 [Review] CD009294. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 22259002]
- Kaasinen E, Rintala E, Hellström P, Viitanen J, Juusela H, Rajala P, Korhonen H, Liukkonen T. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. European Urology. 2002;42(2):167–174. Reason for exclusion: Patients not relevant to PICO . [PubMed: 12160589]
- Kaasinen E, Rintala E, Pere AK, Kallio J, Puolakka VM, Liukkonen T, Tuhkanen K. Weekly mitomycin C followed by monthly bacillus Calmette-Guerin or alternating monthly interferon-alpha2B and bacillus Calmette-Guerin for prophylaxis of recurrent papillary superficial bladder carcinoma. The Journal of urology. 2000;164(1):47–52. Reason for exclusion: Patients not relevant to PICO . [PubMed: 10840422]
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, Colombo R. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. European Urology. 2011;60(1):81–93. [Review] Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 21531502]
- Luciani LG, Neulander E, Murphy WM, Wajsman Z. Risk of continued intravesical therapy and delayed cystectomy in BCG-refractory superficial bladder cancer: an investigational approach. Urology. 2001;58(3):376–379. Reason for exclusion: Non comparative study . [PubMed: 11549484]
- Lundholm C, Norlén BJ, Ekman P, Jahnson S, Lagerkvist M, Lindeborg T, Olsson JL, Tveter K, Wijkstrom H, Westberg R, Malmström PU. A randomized prospective study comparing long-term intravesical instillations of mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. The Journal of urology. 1996;156(2 Pt 1):372–376. Reason for exclusion: Patients not relevant to PICO . [PubMed: 8683682]
- Mallstrom PU. A randomized comparative dose-ranging study of interferon-alpha and mitomycin-C as an internal control in primary or recurrent superficial transitional cell carcinoma of the bladder. BJU International. 2002;89(7):681–686. Reason for exclusion: Patients not relevant to PICO . [PubMed: 11966624]
- Malmström PU, Wijkström H, Lundholm C, Wester K, Busch C, Norlén BJ. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. The Journal of urology. 1999;161(4):1124–1127. Reason for exclusion: Patients not relevant to PICO . [PubMed: 10081852]
- Mangiarotti B, Trinchieri A, Nero A, Montanari E. A randomized prospective study of intravesical prophylaxis in non-musle invasive bladder cancer at intermediate risk of recurrence: mitomycin chemotherapy vs BCG immunotherapy. Archivio italiano di urologia, andrologia : organo ufficiale [di] Società italiana di ecografia urologica e nefrologica / Associazione ricerche in urologia. 2008;80(4):167–171. Reason for exclusion: Patients not relevant to PICO . [PubMed: 19235434]
- Melekos MD. Intravesical bacillus Calmette-Guerin versus epirubicin in the prophylaxis of recurrent and/or multiple superficial bladder tumours. Oncology. 1996;53(4):281–288. Reason for exclusion: Patients not relevant to PICO . [PubMed: 8692531]
- Merz VW, Marth D, Kraft R, Ackermann DK, Zingg EJ, Studer UE. Analysis of early failures after intravesical instillation therapy with bacille Calmette-Guerin for carcinoma in situ of the bladder. British Journal of Urology. 1995;75(2):180–184. Reason for exclusion: Patients not relevant to PICO . [PubMed: 7850322]
- Mukamel E, deKernion JB. Conservative treatment of diffuse carcinoma in situ of the bladder with repeated courses of intravesical therapy. British Journal of Urology. 1989;64(2):143–146. Reason for exclusion: Non comparative study . [PubMed: 2504435]
- Okamura T. Non muscle invasive bladder cancer cases initially failing to respond to bacillus Calmette-Guerin intravesical instillation therapy. Current Urology. 2010;4(1):18–24. Reason for exclusion: Non comparative study .
- Oosterlinck W. Sequential chemo-immunotherapy with mitomycin C (MMC) and bacillus calmette-guerin (BCG) versus bcg alone in patients with carcinoma in situ (CIS) of the urinary bladder results of eortc GU group randomized phase II study 30993. European Urology, Supplements. 2010.:2. Conference(var.pagings) Reason for exclusion: Patients not relevant to PICO .
- Pinsky CM, Camacho FJ, Kerr D, Geller NL, Klein FA, Herr HA, Whitmore WF, Oettgen HF. Intravesical administration of bacillus Calmette-Guérin in patients with recurrent superficial carcinoma of the urinary bladder: report of a prospective, randomized trial. 1985;69(1):47–53. Cancer treatment reports. Reason for exclusion: Patients not relevant to PICO . [PubMed: 3881177]
- Racioppi M. Thermo-chemotherapy for intermediate or high-risk recurrent non muscle-invasive bladder cancer patients after first-line therapy failure. Anticancer Research. 2010.:4–1444. Conference(var.pagings) Reason for exclusion: Patients not relevant to PICO .
- Rintala E, Jauhiainen K, Alfthan O, Hansson E, Juusela H, Kanerva K, Korhonen H, Permi J, Sotarauta M, Vaalasti T. Intravesical chemotherapy (mitomycin C) versus immunotherapy (bacillus Calmette-Guérin) in superficial bladder cancer. European Urology. 1991;20(1):19–25. Reason for exclusion: Patients not relevant to PICO . [PubMed: 1743226]
- Rintala E, Jauhiainen K, Kaasinen E, Nurmi M, Alfthan O. Alternating mitomycin C and bacillus Calmette-Guerin instillation prophylaxis for recurrent papillary (stages Ta to T1) superficial bladder cancer. Finnbladder Group. The Journal of urology. 1996;156(1):56–59. Reason for exclusion: Patients not relevant to PICO . [PubMed: 8648837]
- Said MT, Abomelha MS, Orkubi SA. Intravesical immunotherapy for superficial bladder cancer. Saudi Medical Journal. 2002;23(12):1458–1461. Reason for exclusion: Patients not relevant to PICO . [PubMed: 12518191]
- Sawczuk IS, Munver R, Fromer DL, Volfson IA, Sawczuk A, Galli B, Hackensack NJ, McKiernan JM, Goluboff ET, Hoke G. Intravesical BCG and interferon-alpha 2b therapy prior to cystectomy does not impact negatively on survival in patients with recurrent superficial bladder cancer. Journal of Urology. 2006;175(4):404–404. Reason for exclusion: Non comparative study .
- Serretta V. Choice of adjuvant intravesical therapy in recurring intermediate-risk NMI-BC. Anticancer Research. 2011.:5–1819. Conference(var.pagings) Reason for exclusion: Patients not relevant to PICO .
- Serretta V. Re-treatment by intravesical therapy in recurring patients affected by intermediate risk non-muscle invasive bladder cancer (NMI-BC). European Urology, Supplements. 2011.:2–149. Conference(var.pagings) Reason for exclusion: Patients not relevant to PICO .
- Shelley MD, Jones G, Cleves A, Wilt TJ, Mason MD, Kynaston HG. Intravesical gemcitabine therapy for non-muscle invasive bladder cancer (NMIBC): a systematic review. BJU International. 2012;109(4):496–505. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 22313502]
- Shelley MD, Mason MD, Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treatment Reviews. 2010;36(3):195–205. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 20079574]
- Sylvester RJ, Oosterlinck W, Witjes JA. The schedule and duration of intravesical chemotherapy in patients with non-muscle-invasive bladder cancer: A systematic review of the published results of randomized clinical trials. European Urology. 2008;53(4):709–719. Reason for exclusion: Review No included studies relevant to PICO . [PMC free article: PMC2587437] [PubMed: 18207317]
- Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. Journal of Urology. 2005;174(1):86–91. Reason for exclusion: Review No included studies relevant to PICO . [PubMed: 15947584]
- Vegt PD, Witjes JA, Witjes WP, Doesburg WH, Debruyne FM, Meijden AP. A randomized study of intravesical mitomycin C, bacillus Calmette-Guerin Tice and bacillus Calmette-Guerin RIVM treatment in pTa-pT1 papillary carcinoma and carcinoma in situ of the bladder. The Journal of urology. 1995;153(3 Pt 2):929–933. Reason for exclusion: Patients not relevant to PICO . [PubMed: 7853577]
- Witjes JA, Fransen MP, Meijden AP, Doesburg WH, Debruyne FM. Use of maintenance intravesical bacillus Calmette-Guérin (BCG), with or without intradermal BCG, in patients with recurrent superficial bladder cancer. Long-term follow-up of a randomized phase 2 study. Urologia Internationalis. 1993;51(2):67–72. Reason for exclusion: Patients not relevant to PICO . [PubMed: 8351757]
- Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urologic Oncology. 2013;31(8):1635–1642. Reason for exclusion: Non-comparative study . [PubMed: 22575238]
Evidence tables
Download PDF (314K)
Download PDF (226K)
Download PDF (223K)
Download PDF (224K)
Download PDF (216K)
3.4. Managing side effects of treatment of non-muscle-invasive bladder cancer
Review question: What is the most effective intervention for bladder toxicity following radiotherapy or BCG therapy for bladder cancer?
Rationale
Radiotherapy and intravesical BCG (BCG vaccine inserted into the bladder), treatments used for high risk bladder cancer that is confined to the bladder can result in patients being cured of their cancer and with their bladder preserved but with significant side effects which can result in patients having a poor quality of life.
Irritative urinary symptoms (urinary frequency, urgency, and pain when passing urine) are usually experienced by most patients for approximately 48 hours following intravesical BCG and for some weeks after radiotherapy. However for some patients these side effects continue long-term.
The cause of long term side effects of radiotherapy to the bladder or intravesical BCG may include bladder inflammation, abnormal blood vessel development within the bladder or scarring in the bladder. Consequently the bladder may be unable to store significant quantities of urine resulting in patients passing small volumes of urine frequently and urgently during the day and night, pain passing urine and blood in urine. These symptoms can develop up to 20 years after completion of radiotherapy to the bladder.
It is expected that this review will identify effective methods to reduce the risk of long term side effects of radiotherapy to the bladder and make recommendations for the standardisation of treatment for significant long term side effects which occur as a result of radiotherapy to the bladder or intravesical BCG.
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients who develop bladder toxicity following radiotherapy or BCG therapy for bladder cancer | Interventions for bladder toxicity: Cystectomy Isoniazid Ofloxacin Cystistat Elmiron Anticholinergics Botox Alum Formalin Embolisation Catherisation Hyperbaric oxygen Reduced dose of intravesical BCG Increased time between treatments of intravesical BCG | Each other No intervention |
|
METHODS
Information sources
A literature search was performed by the information specialists (EH and SA).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO. Randomised trial and comparative studies were included when available. Non-comparative data was considered for interventions where there were no comparative studies.
Data synthesis
Evidence was presented using GRADE. Meta-analysis was not possible for this review.
RESULTS
Study quality and results
No evidence was identified for health-related quality of life across any of the interventions. No evidence was identified for the following interventions specified in the PICO: cystectomy, botox, alum, embolisation, catheterisation, increased time between treatments of BCG, elmiron. Evidence is summarised in Tables 87-93.
Table 87
GRADE evidence profile: Ofloxacin for the prevention of BCG-induced toxicity in superficial bladder cancer.
Table 88
GRADE evidence profile: Isoniazid for the prevention of BCG-induced bladder toxicity in superficial bladder cancer.
Table 89
GRADE evidence profile: Oxybutynin for the prevention of BCG-induced toxicity in superficial bladder cancer.
Table 90
GRADE evidence profile: Reduced BCG dose for BCG-induced toxicity: 1/3 dose versus standard dose.
Table 91
GRADE evidence profile: Formalin for the treatment of bladder haemorrhage secondary to radiation-induced cystitis.
Table 92
GRADE evidence profile: Hyperbaric oxygen therapy (HBOT) for the treatment of radiation-induced hemorrhagic cystitis.
Table 93
GRADE evidence profile: Sodium hyaluronate for the treatment of chemical-induced cystitis.
Evidence statements
Ofloxacin
One randomised trial (115 participants) of moderate quality was identified comparing BCG therapy plus ofloxacin with BCG therapy plus placebo in patients with superficial bladder cancer. Treatment with 2 × 200mg ofloxacin with each BCG instillation resulted in a lower rate of mild to moderate adverse events compared to placebo between instillations four and six, and a lower rate of severe adverse events between instillations one and nine. However, the proportion of participants specifically with bladder toxicity was not reported, as the outcome of adverse events included both local and systemic symptoms.
Isoniazid
Two randomised trials (997 participants) provided moderate quality evidence on the efficacy of isoniazid for the prevention of BCG-induced bladder toxicity. In both studies the 95% confidence intervals of the effect sizes (risk ratios) included the null value, so there is no strong evidence that isoniazid has an effect on the rate of chemical cystitis, frequency or haematuria (van der Meijden et al., 2001) or bladder toxicity (including haematuria, dysuria, and frequency) (Al Khalifa et al., 2000). When toxicity was sub-grouped by severity, participants receiving isoniazid were more likely to experience mild toxicity and less likely to experience severe toxicity than the placebo group. However, it should be noted that this data was based on a low number of participants.
Oxybutynin
One randomised trial (Johnson et al., 2013) of 50 participants provided low quality evidence of an increase in urinary symptoms (frequency and burning) and systemic symptoms (fever, dry mouth) in those treated with oxybutynin alongside BCG treatment compared to those in the placebo group.
Reduced BCG dose
High quality evidence from one trial of reduced dose BCG reported by Brausi et al. (2014) stated that there were no differences between rates of local and systemic BCG side effects between the 1/3 dose BCG group and the full-dose BCG group (RR 0.95, 95% CI 0.86 to 1.06). Reducing the dose of BCG did not decrease the percentage of patients who discontinued treatment due to side effects.
Formalin
Two case series studies (12 participants) reported the effects of intravesical formalin for treating bladder haemorrhage secondary to radiation-induced cystitis. Both studies reported that all patients had a good response to treatment with cessation of bleeding observed for three to five months (very low quality evidence).
Hyperbaric oxygen therapy (HBOT)
Seven case series studies (153 participants) provided very low quality evidence on the efficacy of HBOT for treating radiation-induced cystitis. Overall 94/153 (61%) participants showed a complete resolution of haematuria, with effectiveness ranging from 27% to 100% across studies. In most studies patients had received previous treatment for cystitis, such as alum or formalin, without success.
Sodium hyaluronate
One case series (54 patients) provided very low quality evidence on the efficacy of intravesical sodium hyaluronate for the treatment of chemical-induced cystitis in bladder cancer patients treated with Mitomycin C or BCG therapy. It is not stated whether Cystistat was the treatment used. Bladder capacity increased in all patients after treatment (mean difference 226.1 ml, 95% CI 207.1 to 245 ml). Patient-reported pain as measured by the Visual Analogue Scale (VAS) decreased in all patients (mean difference -7.7, 95% CI -8.12 to -7.31). VAS scores range from 1 to 10, with 10 indicating maximum pain tolerated.
References to included studies
- Al Khalifa M, et al. The effect of isoniazid on BCG-induced toxicity in patients with superficial bladder cancer. European Urology. 2000;37(Suppl 1):26–30. [PubMed: 10575269]
- Brausi M, et al. Side Effects of Bacillus Calmette-Guerin (BCG) in the Treatment of Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Bladder: Results of the EORTC Genito-Urinary Cancers Group Randomised Phase 3 Study Comparing One-third Dose with Full Dose and 1 Year with 3 Years of Maintenance BCG. European Urology. 2014;65(1):69–76. [PubMed: 23910233]
- Colombel M, et al. The effect of ofloxacin on bacillus calmette-guerin induced toxicity in patients with superficial bladder cancer: results of a randomized, prospective, double-blind, placebo controlled, multicenter study. Journal of Urology. 2006;176(3):935–939. [PubMed: 16890660]
- Corman JM, et al. Treatment of radiation induced hemorrhagic cystitis with hyperbaric oxygen. Journal of Urology. 2003;169(6):2200–2202. [PubMed: 12771749]
- Del Pizzo JJ, et al. Treatment of radiation induced hemorrhagic cystitis with hyperbaric oxygen: Long-term followup. Journal of Urology. 1998;160(3):731–733. [PubMed: 9720533]
- Johnson MH, et al. Randomized controlled trial of oxybutynin extended release versus placebo for urinary symptoms during intravesical Bacillus Calmette-Guerin treatment. Journal of Urology. 2013;189(4):1268–1274. [PMC free article: PMC4150693] [PubMed: 23123375]
- Kumar S, Rosen P, Grabstald H. Intravesical Formalin for Control of Intractable Bladder Hemorrhage Secondary to Cystitis Or Cancer. Journal of Urology. 1975;114(4):540–543. [PubMed: 1235376]
- Lee HC, et al. Hyperbaric-Oxygen Therapy in Hemorrhagic Radiation Cystitis - A Report of 20 Cases. Undersea & Hyperbaric Medicine. 1994;21(3):321–327. [PubMed: 7950806]
- Likourinas M, et al. Intravesical Formalin for the Control of Intractable Bladder Hemorrhage Secondary to Radiation Cystitis Or Bladder-Cancer. Urological Research. 1979;7(2):125–126. [PubMed: 573008]
- Mathews R, et al. Hyperbaric oxygen therapy for radiation induced hemorrhagic cystitis. Journal of Urology. 1999;161(2):435–437. [PubMed: 9915420]
- Parra C, et al. Management of Hemorrhagic Radiation Cystitis with Hyperbaric Oxygen Therapy. Actas Urologicas Espanolas. 2011;35(3):175–179. [PubMed: 21334100]
- Rijkmans BG, et al. Successful treatment of radiation cystitis with hyperbaric oxygen. European Urology. 1989;16(5):354–356. [PubMed: 2776806]
- Sommariva ML, Sandri SD, Ceriani V. Efficacy of sodium hyaluronate in the management of chemical and radiation cystitis. Minerva Urologica e Nefrologica. 2010;62(2):145–150. [PubMed: 20562794]
- van der Meijden AP, et al. Intravesical instillation of epirubicin, bacillus Calmette-Guerin and bacillus Calmette-Guerin plus isoniazid for intermediate and high risk Ta, T1 papillary carcinoma of the bladder: a European Organization for Research and Treatment of Cancer genito-urinary group randomized phase III trial. Journal of Urology. 2001;166(2):476–481. [PubMed: 11458050]
- Weiss JP, et al. Primary-Treatment of Radiation-Induced Hemorrhagic Cystitis with Hyperbaric-Oxygen - 10-Year Experience. Journal of Urology. 1994;151(6):1514–1517. [PubMed: 8189559]
References to excluded studies (with reasons for exclusion)
- Witjes JAP. Clinical Practice Recommendations for the Prevention and Management of Intravesical Therapy-Associated Adverse Events. European Urology, Supplements. 2008;7(10):667–674.
- O'Donnell M, O'Donnell M. Does ofloxacin protect against BCG-related toxic effects in patients with bladder cancer? Nature Clinical Practice Urology. 2007;4(6):304–305. [PubMed: 17426721]
- Wammack R. Long-term results of ileocecal continent urinary diversion in patients treated with and without previous pelvic irradiation. Journal of Urology. 2002;167(5):2058–2062. [PubMed: 11956438]
- Kowalski M, et al. A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCG-refractory and BCG-intolerant patients. Drug Design Development and Therapy. 2010;4:313–320. [PMC free article: PMC2998804] [PubMed: 21151619]
- Harris V. Planning outcomes and acute toxicity of intensity modulated radiotherapy (IMRT) used for simultaneous treatment of bladder and pelvic nodes. Radiotherapy and Oncology. 2010 September; Conference(var.pagings)
- Grant T, Donaldson I. Natural history and outcome of patients with high risk non-muscle invasive bladder cancer treated with Bacille Calmette Guerin: A contemporary UK series. Journal of Urology. 2012.:e676. Conference(var.pagings)
- Ferrandez MLJ. Effectiveness of hyaluronic acid in prevention of acute urinary toxicity in HDR brachytherapy. Radiotherapy and Oncology. 2010 September; Conference(var.pagings)
- Damiano R. Short-term administration of prulifloxacin in patients with nonmuscle-invasive bladder cancer: An effective option for the prevention of bacillus Calmette-Guerin-induced toxicity? BJU International. 2009;104(5):633–639. [PubMed: 19298412]
- Harris V. Planning outcomes and acute toxicity of intensity-modulated radiotherapy (IMRT) for treatment of bladder and pelvic lymph nodes. Journal of Clinical Oncology. 2011. Conference(var.pagings)
- Paterson DL, Patel A. Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: review of complications and their treatment. Australian and New Zealand Journal of Surgery. 1998;68(5):340–344. [PubMed: 9631906]
- Denton AS, Clarke N, Maher J. Non-surgical interventions for late radiation cystitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database of Systematic Reviews. 2002;(3) [PMC free article: PMC7025765] [PubMed: 12137633]
- Nabi G, et al. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews. 2006;(4) [PMC free article: PMC8729219] [PubMed: 17054185]
- Madhuvrata P, et al. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database of Systematic Reviews. 2012;(1) [PubMed: 22258963]
- Huddart R. Updated results of the BC2001 phase III randomized trial of standard vs reduced high dose volume radiotherapy for muscle invasive bladder cancer (ISCRTN:68324339): Tumour control, toxicity and quality of life. European Journal of Cancer, Supplement. 2009.:423. Conference(var.pagings)
- Huddart RAJ. BC2001: A multicenter phase III randomized trial of standard versus reduced volume radiotherapy for muscle invasive bladder cancer (ISCRTN:68324339). Journal of Clinical Oncology. 2009.:5022. Conference(var.pagings)
- Mangar SA, et al. Evaluating the effect of reducing the high-dose volume on the toxicity of radiotherapy in the treatment of bladder cancer. Clinical Oncology. 2006;18(6):466–473. [PubMed: 16909970]
- Plenk HP. Hyperbaric Radiation-Therapy - Preliminary Results of A Randomized Study of Cancer of Urinary-Bladder and Review of Oxygen Experience. American Journal of Roentgenology. 1972;114(1):152. &. [PubMed: 4333187]
- Dische S. Hyperbaric-Oxygen Chamber in Radiotherapy of Carcinoma of Bladder. British Journal of Radiology. 1973;46(541):13–17. [PubMed: 4566856]
- Cade IS, et al. Hyperbaric-Oxygen and Radiotherapy - Medical-Research-Council Trial in Carcinoma of Bladder. British Journal of Radiology. 1978;51(611):876–878. [PubMed: 361143]
- Suvorova YV, Tarazov PG. Transcatheter embolization vs surgical ligation in the treatment of bleeding bladder neoplasms. European Journal of Cancer. 1997;33:149–149.
- Losa A, Hurle R, Lembo A. Low dose bacillus Calmette-Guerin for carcinoma in situ of the bladder: long-term results. Journal of Urology. 2000;163(1):68–71. [PubMed: 10604316]
- Mack D, et al. The ablative effect of quarter dose bacillus Calmette-Guerin on a papillary marker lesion of the bladder. Journal of Urology. 2001;165(2):401–403. [PubMed: 11176382]
- Mugiya S, et al. Long-term outcome of a low-dose intravesical bacillus calmette-guerin therapy for carcinoma in situ of the bladder: Results after six successive instillations of 40 mg BCG. Japanese Journal of Clinical Oncology. 2005;35(7):395–399. [PubMed: 15976065]
- Shindel AW, et al. Ureteric embolization with stainless-steel coils for managing refractory lower urinary tract fistula: a 12-year experience. BJU International. 2007;99(2):364–368. [PubMed: 17026590]
- Bui Q-C. The efficacy of hyperbaric oxygen therapy in the treatment of radiation-induced late side effects. International Journal of Radiation Oncology Biology Physics. 2004;60(3):871–878. [PubMed: 15465205]
- Safra T. Improved Quality of Life with Hyperbaric Oxygen Therapy in Patients with Persistent Pelvic Radiation-induced Toxicity. Clinical Oncology. 2008;20(4):284–287. [PubMed: 18222656]
- Shao Y, Lu GL, Shen ZJ. Comparison of intravesical hyaluronic acid instillation and hyperbaric oxygen in the treatment of radiation-induced hemorrhagic cystitis. BJU International. 2012;109(5):691–694. [PubMed: 21895939]
- Lamm DL, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. Journal of Urology. 1992;147(3):596–600. [PubMed: 1538436]
- Rawls WH, et al. Fatal sepsis following intravesical bacillus Calmette-Guerin administration for bladder cancer. Journal of Urology. 1990;144(6):1328–1330. [PubMed: 2231917]
- Mack D. Low-dose bacille Calmette-Guerin (BCG) therapy in superficial high-risk bladder cancer: A phase II study with the BCG strain connaught Canada. British Journal of Urology. 1995;75(2):185–187. [PubMed: 7850323]
- Whillis D, et al. Radical radiotherapy with salvage surgery for invasive bladder cancer: results following a reduction in radiation dose. Journal of the Royal College of Surgeons of Edinburgh. 1992;37(1):42–45. [PubMed: 1573607]
- Takashi M, et al. Evaluation of a low-dose intravesical bacillus Calmette-Guérin (Tokyo strain) therapy for superficial bladder cancer. International Urology and Nephrology. 1995;27(6):723–733. [PubMed: 8725038]
- Oddens J, et al. Final Results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guerin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. European Urology. 2012 Nov 2; epub ahead of print. [PubMed: 23141049]
- Pagano F, et al. A low dose bacillus Calmette-Guerin regimen in superficial bladder cancer therapy: is it effective? The Journal of urology. 1991;146(1):32–35. [PubMed: 2056600]
- Goswami AK, et al. How Safe Is 1-Percent Alum Irrigation in Controlling Intractable Vesical Hemorrhage. Journal of Urology. 1993;149(2):264–267. [PubMed: 8426397]
- Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. Journal of Urology. 2006;175(6):2004–2010. [PubMed: 16697786]
- Agrawal MS, et al. The safety and efficacy of different doses of bacillus Calmette Guérin in superficial bladder transitional cell carcinoma. Urology. 2007;70(6):1075–1078. [PubMed: 18158020]
- Ojea A, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guerin (27 mg) versus very low-dose bacillus Calmette-Guerin (13.5 mg) versus mitomycin C. European Urology. 2007;52(5):1398–1406. [PubMed: 17485161]
- Martinez-Pineiro JAF. Long-term follow-up of a randomized prospective trial comparing a standard 81 mg dose of intravesical bacille Calmette-Guerin with a reduced dose of 27 mg in superficial bladder cancer. BJU International. 2002;89(7):671–680. [PubMed: 11966623]
- Irie A, et al. Sufficient prophylactic efficacy with minor adverse effects by intravesical instillation of low-dose bacillus Calmette-Guerin for superficial bladder cancer recurrence. International Journal of Urology. 2003;10(4):183–189. [PubMed: 12657096]
- Martinez-Pineiro JA, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. Journal of Urology. 2005;174(4 Pt 1):1242–1247. [PubMed: 16145378]
- Yoneyama T, et al. Low-dose instillation therapy with bacille Calmette-Guerin Tokyo 172 strain after transurethral resection: historical cohort study. Urology. 2008;71(6):1161–1165. [PubMed: 18279920]
- Yalçinkaya F, et al. Prospective randomized comparison of intravesical BCG therapy with standard dose versus low doses in superficial bladder cancer. International Urology and Nephrology. 1998;30(1):41–44. [PubMed: 9569110]
- Fergany AF, Moussa AS, Gill IS. Laparoscopic Cystoprostatectomy for Radiation-Induced Hemorrhagic Cystitis. Journal of Endourology. 2009;23(2):275–278. [PubMed: 19187011]
- Pagano F, et al. Improving the efficacy of BCG immunotherapy by dose reduction. European Urology. 1995;27 Suppl 1:19–22. [PubMed: 7750527]
- Malhotra P, et al. Isoniazid resistance among Bacillus Calmette Guerin strains: implications on bladder cancer immunotherapy related infections. Canadian Journal of Urology. 2011;18(3):5671–5675. [Review] [PubMed: 21703038]
- Brausi MAO. Bacillus Calmette-Guerin: One third dose versus full dose and one year versus three years of maintenance. Final results of an EORTC GU Cancers Group randomized trial in non muscle invasive bladder cancer. European Urology, Supplements. 2012.:e1050–e1050a. Conference(var.pagings)
- Vegt PD, et al. Does isoniazid reduce side effects of intravesical bacillus Calmette-Guerin therapy in superficial bladder cancer? Interim results of European Organization for Research and Treatment of Cancer Protocol 30911. Journal of Urology. 1997;157(4):1246–1249. [PubMed: 9120912]
- Van der Meijden APM. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: Results from a European organisation for research and treatment of cancer genito-urinary group phase III trial. European Urology. 2003;44(4):429–434. [PubMed: 14499676]
- Norkool DM, et al. Hyperbaric-Oxygen Therapy for Radiation-Induced Hemorrhagic Cystitis. Journal of Urology. 1993;150(2):332–334. [PubMed: 8326555]
- Mayr M, Sommerhuber A, Loidl W. Dose Reduction of Bacillus Calmette-Guerin (Bcg) in the Treatment of Carcinoma in Situ (Cis) of the Bladder: Does A Less Intense and Shorter Maintenance Regime Have An Impact on Efficacy and Patient Compliance? A 14 Year Experience. European Urology Supplements. 2009;8(4):284–284.
- Yari H, et al. Comparison of Full-Dose Intravesical Bcg Versus Half Dose Bcg and Mitomycin-C in Treatment of Patients with Superficial Bladder Cancer. European Urology Supplements. 2010;9(6):595–596.
- Fotouhi GA. Hyperbaric oxygen treatment, alpha-tocopherol, and ascorbic acid are effective for reducing late radiation-induced side effects of pelvic radiation therapy. International Journal of Radiation Oncology Biology Physics. 2012.:3–S653. Conference(var.pagings)
- A randomized controlled trial of standard dose BCG versus low dose BCG and interferon alpha in patients with superficial bladder cancer - A five-year series. European Urology Supplements. 2005;4(3):221–221.
- Esuvaranathan K. Five-year data of a randomized controlled trial of standard dose BCG versus low dose BCG and interferon alpha in patients with superficial bladder cancer. Journal of Urology. 2004;171(4):73–73.
- Serretta V, et al. [Prevention of topic toxicity of BCG with single-dose prulifloxacin. Preliminary results of a randomized pilot study]. Urologia. 2010;77(4):240–247. [PubMed: 21234866]
Reason: expert review/commentary
Reason: not relevant to PICO
Reason: duplicate of included study
Reason: meeting abstract only – insufficient information for inclusion
Reason: foreign language
Evidence tables
Prevention of BCG toxicity (PDF, 313K)
Reduced dose BCG (PDF, 309K)
Hyperbaric oxygen therapy (HBOT) for radiation-induced hemorrhagic cystitis (PDF, 428K)
Intravesical formalin for radiation-induced hemorrhagic cystitis (PDF, 312K)
Sodium hyaluronate for chemical-induced cystitis (PDF, 314K)
3.5. Follow-up after treatment for non-muscle-invasive bladder cancer
Review question: What are the optimal follow-up protocols for low/intermediate risk and high-risk non-muscle invasive bladder cancer?
Rationale
Currently all patients with NMIBC require regular cystoscopic surveillance of their bladder and high risk patients may require additional imaging to look for progression. Long term cystoscopic surveillance is expensive and may not be necessary in low risk cases.
Although there is general agreement that NMIBC patients require cystoscopic surveillance to detect recurrence, there are variations in frequency and length of follow-up. The optimal tests for detecting progression are unknown. It is also difficult to co-ordinate current surveillance protocols with concurrent treatment e.g. with intravesical therapy.
Cystoscopic surveillance could be rationalised into low, intermediate and high risk group. Defining the optimal length of follow-up in low risk patients would allow many to be safely discharged whilst high risk patients would benefit from an integrated follow-up that is synchronised with treatment and includes imaging for progression.
Alternative approaches could include non invasive follow up using ultrasound for some risk groups and/or defining a group of patients in whom invasive surveillance may not be appropriate.
Patients with NMIBC are at increased risk of developing upper tract TCC. Tests to detect upper tracts tumour in these patients are variably performed at present but should be considered within follow up protocols
Question in PICO format
| Population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients who have undergone curative treatment for NMIBC Subgroups:
| Follow up: Cystoscopy intervals (rigid/flexi) Intravenous urography (IVU) CT Ultrasound Urine tests (Cytology, NMP22, UroVysion, ImmunoCyt) | No follow-up Each other (including frequency and duration of follow-up) |
|
METHODS
Information sources
A literature search was performed by the information specialist (EH).
Selection of studies
The information specialist (EH) did the first screen of the literature search results. One reviewer (JH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO.
Data synthesis
One randomised trial was identified. No other comparative data was found. Therefore, data is presented from observational studies about recurrence rates during follow-up for non-muscle invasive bladder cancer. No meta-analysis was possible for this review.
RESULTS
Study quality and results
Evidence is summarised in Tables 94-96.
Table 94
GRADE evidence profile: Frequent versus less frequent follow-up for TaG1-2 bladder cancer.
Table 95
GRADE evidence profile: Follow-up for non-muscle invasive bladder cancer.
Table 96
Patient experience and preference for follow-up of NMIBC.
Evidence statements
Moderate quality evidence from one randomised trial of 97 patients (Olsen et al., 1995) suggests uncertainty over whether follow up frequency of three months is more or less effective than follow up with a frequency of six months in terms of recurrence, progression or overall survival.
Low quality evidence from five observational studies of patients with low-grade superficial bladder cancer report recurrence rates over long-term follow-up. Two studies including 470 patients suggest that tumour detection at the first follow-up cystoscopy is associated with a greater risk of recurrence during subsequent follow-up compared to those who are tumour-free at the first cystoscopy (Holmang et al., 2002; Mariappan & Smith, 2005). All studies report a reduction in the risk of recurrence over time. Some studies suggest the risk of recurrences is greatly reduced after a tumour-free period of five years or more (Mariappan & Smith, 2005; Zieger et al., 2000). In Mariappan & Smith (2005) only one (0.9%) patient had a first recurrence after being tumour-free for five years, whereas LeBlanc et al. (1999) reports recurrence rates of approximately 30% in patients after remaining tumour-free for two to ten years. Another study reports that of 20 primary Ta-T1 patients who were tumour-free for five years, seven (35%) had muscle-invasive disease (Thompson et al., 1993).
One retrospective observational study of 542 intermediate-high risk patients who had received BCG treatment reports that 338/542 (62%) patients were not tumour-free for five years or more. 22/204 (10.8%) patients had a recurrence after being tumour-free for five years or more (Holmang et al., 2012). During the first five-years after BCG, 57 patients (10.5%) died from bladder cancer and between years six and 25, 32 patients (5.9%) died from bladder cancer.
Five observational studies report rates of upper urinary tract (UUT) recurrence ranging between 2.6% and 5.5%. Median times to UUT recurrence vary from 22 to 33 months in three studies (Miyake et al., 2005; Canales et al., 2006; Holmang et al., 1998) and one study (Hession et al., 1999) reports a mean time to recurrence of 78 months. In one study, two out of 18 UUT cancers were diagnosed by routine intravenous urography, and the other 18 presented with symptoms suggesting UUT recurrence before IVU (Miyake et al., 2006). Holmang et al. (1998) reported that IVU performed 0 to ten months before the UUT cancer was diagnosed failed to raise suspicion of a tumour in eight out of 16 patients (including three patients with initial muscle-invasive bladder cancer).
Two studies provide low quality evidence of the accuracy of ultrasound compared with cystoscopy for the detection of recurrent tumours in patients with superficial bladder cancer. In one study, three tumours detected by cystoscopy were missed by ultrasound (Stamatiou et al., 2011, and in the second study 15 patients with recurrence were not detected by ultrasound (Vallencien et al., 1986).
Low quality evidence for health-related quality of life is provided by three studies (503 patients) which report that most patients experience minimal pain (Yossepowitch et al., 2007) from undergoing cystoscopic follow-up, although the introduction of the cystoscope is rated as the most painful part of the procedure (van der Aa et al., 2008). Waiting for test results is rated as the most distressing part of follow-up by urine testing (van der Aa et al., 2008).
References to included studies
- Aa MN, et al. Patients' perceived burden of cystoscopic and urinary surveillance of bladder cancer: a randomized comparison. BJU International. 2008;101:1106–1110. [PubMed: 17888042]
- Canales BK, et al. Risk factors for upper tract recurrence in patients undergoing long-term surveillance for stage ta bladder cancer. Journal of Urology. 2006;175(1):74–77. [PubMed: 16406874]
- Hession P, et al. Intravenous urography in urinary tract surveillance in carcinoma of the bladder. Clinical Radiology. 1999;54(7):465–467. [PubMed: 10437700]
- Holmang S, Johansson SL. Stage Ta-T1 bladder cancer: the relationship between findings at first followup cystoscopy and subsequent recurrence and progression. Journal of Urology. 2002;167(4):1634–1637. [PubMed: 11912378]
- Holmang S, Strock V. Should follow-up cystoscopy in bacillus Calmette-Guerin-treated patients continue after five tumour-free years? European Urology. 2012;61(3):503–507. [PubMed: 22119022]
- Holmang S, et al. Long-term followup of a bladder carcinoma cohort: routine followup urography is not necessary. Journal of Urology. 1998;160(1):45–48. [PubMed: 9628602]
- Leblanc B, et al. Long-term followup of initial Ta grade 1 transitional cell carcinoma of the bladder. Journal of Urology. 1999;162(6):1946–1950. [PubMed: 10569544]
- Mariappan P, Smith G. A similar surveillance schedule for G2Ta and G1Ta bladder tumours permits safe discharge at 5 years: results of a 25-year prospective database. BJU International. 2005;95:53–53. [PubMed: 15758711]
- Mariappan P, Smith G. A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. Journal of Urology. 2005;173(4):1108–1111. [PubMed: 15758711]
- Miyake H, et al. Limited significance of routine excretory urography in the follow-up of patients with superficial bladder cancer after transurethral resection. BJU International. 2006;97(4):720–723. [PubMed: 16536761]
- Oge O, et al. Proposal for changes in cystoscopic follow-up of patients with low-grade pTa bladder tumor. European Urology. 2000;37(3):271–274. [PubMed: 10720851]
- Olsen LH, Genster HG. Prolonging follow-up intervals for non-invasive bladder tumors: a randomized controlled trial. Scandinavian Journal of Urology and Nephrology.Supplementum. 1995;172:33–36. [PubMed: 8578253]
- Stamatiou K, et al. Accuracy of modern ultrasonographic techniques in the follow up of patients with superficial bladder carcinoma. Medical Ultrasonography. 2011;13(2):114–119. [PubMed: 21655537]
- Sternberg IA, et al. Upper tract imaging surveillance is not effective in diagnosing upper tract recurrence in patients followed for nonmuscle invasive bladder cancer. Journal of Urology. 2013;190(4):1187–1191. [PubMed: 23680310]
- Thompson RA Jr., et al. Late invasive recurrence despite long-term surveillance for superficial bladder cancer. Journal of Urology. 1993;149(5):1010–1011. [PubMed: 8483201]
- Vallancien G, et al. Can transabdominal ultrasonography of the bladder replace cystoscopy in the followup of superficial bladder tumors? Journal of Urology. 1986;136(1):32–34. [PubMed: 3520026]
- Vriesema JL, et al. Patient opinion of urinary tests versus flexible urethrocystoscopy in follow-up examination for superficial bladder cancer: a utility analysis. Urology. 2000;56(5):793–797. [PubMed: 11068304]
- Yossepowitch O, Herr HW, Donat SM. Use of urinary biomarkers for bladder cancer surveillance: patient perspectives. Journal of Urology. 2007;177(4):1277–1282. [PubMed: 17382711]
- Zieger K, et al. Long-term follow-up of noninvasive bladder tumours (stage Ta): recurrence and progression. BJU International. 2000;85(7):824–828. [PubMed: 10792160]
References to excluded studies (with reasons for exclusion)
- Hernandez V, et al. Safety of active surveillance program for recurrent nonmuscle-invasive bladder carcinoma. Urology. 2009;73(6):1306–1310. Reason: not relevant to PICO – active surveillance . [PubMed: 19375783]
- Anastasiadis A, et al. Follow-up procedures for non-muscle-invasive bladder cancer: an update. Expert Review of Anticancer Therapy. 2012;12(9):1229–1241. Reason: expert review . [PubMed: 23098122]
- Ceylan K, et al. Comparison of cystoscopy with diffusion-weighted magnetic resonance images used in the diagnosis and follow-up of patients with bladder tumors. Asian Pacific Journal of Cancer Prevention: Apjcp. 2010;11(4):1001–1004. Reason: population not relevant – non follow-up patients . [PubMed: 21133614]
- David KA, et al. Surveillance of urothelial carcinoma: stage and grade migration, 1993-2005 and survival trends, 1993-2000. Cancer. 2009;115(7):1435–1447. Reason: not relevant to PICO . [PubMed: 19215030]
- Forsyth I, Shaikh S, Gunn I. The nurse cystoscopist. Extending the role. British Journal of Perioperative Nursing. 2005;15(8):342–345. Reason: outcomes not relevant to PICO . [PubMed: 16128420]
- Kent DL, et al. Evaluation of nonlinear optimization for scheduling of follow-up cystoscopies to detect recurrent bladder cancer. The Bladder Cancer follow-up Group. Medical Decision Making. 1991;11(4):240–248. Reason: outcomes not relevant to PICO . [PubMed: 1662739]
- Nam RK, et al. Comparison of molecular and conventional strategies for followup of superficial bladder cancer using decision analysis. Journal of Urology. 2000;163(3):752–757. Reason: not relevant to PICO – health economics . [PubMed: 10687970]
- Schmidbauer J, Lindenau G. Follow-up of nonmuscle invasive transitional cell carcinoma of the bladder: how and how often? Current Opinion in Urology. 2008;18(5):504–507. [Review] [32 refs] Reason: expert review . [PubMed: 18670275]
- Schrag D, et al. Adherence to surveillance among patients with superficial bladder cancer. Journal of the National Cancer Institute. 2003;95(8):588–597. Reason: outcomes not relevant to PICO . [PubMed: 12697851]
- Mazzonetto M. Postcystectomy follow-up in bladder neoplasm and continent urinary diversion. The role of CT. Italian Current Radiology. 1991;10(3-4):129–133. Reason: foreign language .
- Abel PD. Follow-up of patients with “superficial” transitional cell carcinoma of the bladder: the case for a change in policy. British Journal of Urology. 1993;72(2):135–142. [Review] [41 refs] Reason: expert review . [PubMed: 8402013]
- Hall RR, et al. Proposal for changes in cystoscopic follow up of patients with bladder cancer and adjuvant intravesical chemotherapy. BMJ. 1994;308(6923):257–260. Reason: expert review . [PMC free article: PMC2539314] [PubMed: 8179678]
- Dubernard JM, et al. Correlation between cytology and cystoscopy in the follow-up of patients with bladder tumours. European Urology. 1982;8(1):5–8. Reason: outcomes not relevant to PICO . [PubMed: 7060611]
- Gakis G, Kruck S, Stenzl A. Can the burden of follow-up in low-grade noninvasive bladder cancer be reduced by photodynamic diagnosis, perioperative instillations, imaging, and urine markers? Current Opinion in Urology. 2010;20(5):388–392. Reason: expert review . [PubMed: 20657287]
- Mathers MJ, et al. Is there evidence for a multidisciplinary follow-up after urological cancer? An evaluation of subsequent cancers. World Journal of Urology. 2008;26(3):251–256. Reason: not relevant to PICO . [PubMed: 18421461]
- Parmar MK, et al. Prognostic factors for recurrence and followup policies in the treatment of superficial bladder cancer: report from the British Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party). The Journal of urology. 1989;142:284–288. Reason: not relevant to PICO – prognostic factors . [PubMed: 2501516]
- Aa MN, et al. Sexual function of patients under surveillance for bladder cancer. BJU International. 2009;104:35–40. Reason: not relevant to PICO – questionnaire completed before starting surveillance program . [PubMed: 19154473]
- Gulliford MC, Petruckevitch A, Burney PG. Can efficiency of follow-up for superficial bladder cancer be increased? Annals of the Royal College of Surgeons of England. 1993;75(1):57–61. Reason: outcomes not relevant to PICO . [PMC free article: PMC2497742] [PubMed: 8422147]
- Rabbani F, et al. Upper-tract tumors after an initial diagnosis of bladder cancer: argument for long-term surveillance. Journal of Clinical Oncology. 2001;19(1):94–100. Reason: recurrence not reported separately for NMIBC and MIBC/metastatic disease . [PubMed: 11134200]
- Taylor J. An evaluation of a nurse-led cystoscopy surveillance service. Professional Nurse. 2003;18(10):580–583. Reason: not relevant to PICO . [PubMed: 12808860]
- Lee CT, et al. Early-stage bladder cancer surveillance does not improve survival if high-risk patients are permitted to progress to muscle invasion. Urology. 2007;69(6):1068–1072. Reason: not relevant to PICO – follow-up schedule not reported . [PubMed: 17572188]
- Chang SS, et al. Routine postoperative intensive care monitoring is not necessary after radical cystectomy. Journal of Urology. 2002;167(3):1321–1324. Reason: not relevant to PICO . [PubMed: 11832723]
- Tran W, et al. Longitudinal risk of upper tract recurrence following radical cystectomy for urothelial cancer and the potential implications for long-term surveillance. Journal of Urology. 2008;179(1):96–100. Reason: method of UUT tumour detection not reported . [PubMed: 17997449]
- Drake R. Bladder cancer: Routine follow-up after radical cystectomy improves survival. Nature Reviews Urology. 2010;7(8):419. Reason: comment on Giannarini 2010 .
- Vrooman OP, Witjes JA. Follow-up of patients after curative bladder cancer treatment: guidelines vs. practice. Current Opinion in Urology. 2010;20(5):437–442. Reason: expert review . [PubMed: 20657286]
- Wagner KR, et al. Prospective intermediate follow-up of carcinoma in situ involving the distal ureter at cystectomy: is there a role for ureteroscopy? Journal of Endourology. 2008;22(6):1241–1246. Reason: intervention not relevant to PICO . [PubMed: 18578657]
- Hellenthal NJ, et al. The role of surveillance in the treatment of patients with muscle-invasive bladder cancer after chemotherapy. BJU International. 2010;105(4):485–488. Reason: not relevant to PICO . [PubMed: 19849694]
- Gogus C, et al. 3-Dimensional computerized tomography in follow-up of patients with urinary diversion. International Urology & Nephrology. 2005;37(4):739–742. Reason: intervention not relevant to PICO, comparing 3D-CT with CT (no pathological findings) [PubMed: 16362591]
- Faithfull S, et al. Evaluation of nurse-led follow up for patients undergoing pelvic radiotherapy. British Journal of Cancer. 2001;85:1853–1864. Reason: Not relevant to PICO (nurse-led care versus control) [PMC free article: PMC2364007] [PubMed: 11747326]
- Soukup V, et al. Follow-up after surgical treatment of bladder cancer: a critical analysis of the literature. European Urology. 2012;62(2):290–302. Reason: Expert review . [PubMed: 22609313]
- Boorjian SA, et al. Detection of asymptomatic recurrence during routine oncological followup after radical cystectomy is associated with improved patient survival. Journal of Urology. 2011;186(5):1796–1802. Reason: Relevant to another topic . [PubMed: 21944088]
- Kuroda M, et al. Stage specific follow-up strategy after cystectomy for carcinoma of the bladder. International Journal of Urology. 2002;9(3):129–133. Reason: Relevant to another topic . [PubMed: 12010321]
- Slaton JW, et al. A stage specific approach to tumor surveillance after radical cystectomy for transitional cell carcinoma of the bladder. Journal of Urology. 1999;162(3 Pt 1):710–714. Reason: Relevant to another topic . [PubMed: 10458349]
- Yafi FA, et al. Surveillance guidelines based on recurrence patterns after radical cystectomy for bladder cancer: the Canadian Bladder Cancer Network experience. BJU International. 2012;110(9):1317–1323. Reason: Relevant to another topic . [PubMed: 22500588]
- Segal AJ, et al. Follow-up imaging of bladder carcinoma. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215 Suppl:765–772. Reason: Expert review . [PubMed: 11037498]
- Volkmer BG, et al. Oncological followup after radical cystectomy for bladder cancer-is there any benefit? Journal of Urology. 2009;181(4):1587–1593. Reason: Relevant to another topic . [PubMed: 19233433]
- Giannarini G, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? European Urology. 2010;58(4):486–494. Reason: Relevant to another topic . [PubMed: 20541311]
- Kamat AM, et al. Prospective trial to identify optimal bladder cancer surveillance protocol: reducing costs while maximizing sensitivity. BJU International. 2011;108(7):1119–1123. Reason: Outcomes not relevant to PICO (sensitivity and Health economics) [PubMed: 21426474]
- Tahoun NS, Abdel Maksoud AM, Mohamed DB. Evaluation of the Value of Combined Urine Cytology and Cystoscopy for Follow-up of Superficial Transitional Cell Carcinoma of the Urinary Bladder. Journal of Egyptian National Cancer Institute. 2010;22(2):105–111. Reason: Outcomes not relevant to PICO (sensitivity) [PubMed: 21860467]
- Perlis N, et al. Upper urinary tract and urethral recurrences following radical cystectomy: review of risk factors and outcomes between centres with different follow-up protocols. World Journal of Urology. 2013;31(1):161–167. Reason: Relevant to another topic . [PubMed: 22810052]
- Shinagare AB, Sadow CA, Silverman SG. Surveillance of patients with bladder cancer following cystectomy: yield of CT urography. Abdominal Imaging. 2013;38(6):1415–1421. Reason: Relevant to another topic . [PubMed: 23881008]
Evidence tables
Download PDF (319K)
Health Economic Evidence: What are the optimal follow-up protocols for low/intermediate risk and high-risk non-muscle invasive bladder cancer?
Background
There is general agreement that patients with non-muscle invasive bladder cancer (NMIBC) require regular cystoscopic surveillance of their bladder to check for recurrence. However, there is no agreement upon the optimal frequency and length of cystoscopic follow-up and, as such, there is significant variation in clinical practice.
Tailoring follow-up strategies based on risk could allow for follow-up to be safely reduced in the lower risk groups whilst ensuring that the higher risk patients are still monitored closely. In addition, the use of alternative tests to cystoscopy, such as urinary biomarkers and cytology, could have a useful role in reducing the burden of cystocopies. However, the effectiveness and cost-effectiveness of such approaches has never been reliably demonstrated.
Aims
To estimate the cost-effectiveness of reduced follow-up and/or follow-up using newer tests and techniques in comparison to the test and protocols used in current practice in NMIBC patients.
Existing Economic Evidence
A systematic literature review did not identify any cost-utility analyses that sufficiently addressed the current decision problem. However, three papers were identified that utilised modelling techniques to compare follow-up strategies; De Bekker Grob et al. 2009, Van Kessel et al. 2013 and Zhang et al. 2013. microsatellite analysis
De Bekker Grob et al. 2009 constructed a semi-Markov model to investigate two strategies; a conventional strategy consisting of cystoscopy every 3 months and a test arm consisting of microsatellite analysis of voided urine samples every 3 months with a control cystoscopy at 3, 12 and 24 months. The authors found that the probability of being without recurrence after 2 years was similar in the two groups but the total costs were higher in the test arm. Further analysis suggested that the test arm would be as effective and cost the same as the conventional arm if the sensitivity increased to ≥61%, the specificity was set to 73% and the costs were decreased from €158 to <€70. The authors concluded that cystoscopy could be partly replaced if the microsatellite analysis urine test had a higher sensitivity and its costs were reduced.
A similar analysis was conducted by Van Kessel et al. 2013, in which three surveillance strategies were compared using a Markov model; standard surveillance defined as cystoscopy every three months, minimal surveillance defined as cystoscopy at 3, 12 and 24 months and modified surveillance consisting of FGFR3 mutation analysis of voided urine samples every 3 months and cystoscopy at 3, 12 and 24 months. The authors found that the probability of no recurrence after two years of surveillance was higher for the modified surveillance than the standard or minimal surveillance arms. The total cost of surveillance was found to be lower for minimal and modified surveillance than for standard surveillance. The authors concluded that surveillance in which cystoscopy is partly replaced by FGFR3 mutation analysis of urine seems a safe, effective and cost-effective surveillance strategy.
The analysis conducted by Zhang et al. 2013 compared surveillance strategies for low risk NMIBC patients. The study was not a cost-effectiveness analysis and indeed did not even consider costs but it did estimate QALYs for each strategy. The authors developed a Markov model to compare surveillance strategies recommended in international guidelines and additional proposed strategies. The authors found that age and co-morbidities significantly affect the optimal surveillance strategy. The results suggested that younger patients should be screened more intensively than older patients and patients with co-morbidities should be screened less intensively.
De Novo Economic Model
Since the current economic literature did not adequately address the decision problem6, a de novo economic evaluation was undertaken to assess cost-effectiveness. A Markov decision model was developed using Microsoft Excel.
Patients were assumed to enter the model in a ‘disease free’ state following an initial transurethral resection of the bladder tumour (TURBT). At each 3-monthly model cycle the patient may experience a bladder cancer recurrence. If the recurrence is detected, the patient will undergo a further TURBT (or fulguration of the tumour) and return to a disease free state. However, if the recurrence is not detected, then the patient will be at risk of progression and will have to undergo further treatment once this progression is eventually detected (cystectomy and possibly neo-adjuvant chemotherapy). The patient may also die from bladder cancer related mortality after experiencing progression and may die from other cause mortality from any health state.
Estimated total costs and quality adjusted life years (QALYs) were collected over the modelled 10 year time horizon for each follow-up strategy. Future costs and benefits were discounted at a rate of 3.5% per year as recommended by NICE.
The risk of recurrence and progression in patients with NMIBC was estimated using risk equations based on an analysis of 2,596 patients from seven EORTC7 trials (Sylvester et al. 2006). Patients are ‘scored’ based on a number of risk factors, such as number of tumours, tumour size, prior recurrence rate, T category, presence of CIS and grade. An individual's one year and five year risks of recurrence and progression can then be estimated based upon these scores.
For the purposes of the economic model, it was necessary to convert these five year and one year risks into 3-monthly risks. The higher risk of recurrence and progression in the first year was captured by calculating separate 3 monthly risks for the first year and subsequent years (based on the one year risk and five year EORTC risks). Furthermore, since the EORTC risk equations consider recurrence and progression independently, it was necessary to link the progression rates to the recurrence rate i.e. estimate the probability of progression given recurrence in each of the risk groups.
The table below shows the three monthly risks of recurrence, progression and progression given recurrence applied for each of the risk groups in the base case analysis.
Table 97
Three Monthly Recurrence And Progression Risk Applied In The Model.
As the modelled time horizon of 10 years exceeds the predicted risk estimates from the EORTC trials (5 years), it was also necessary to make some assumptions about the risk profile of patients in years 5-10. In the base case, it was assumed that the subsequent year rate (i.e. years 2-5) would be maintained in years 6-10 except in the case of low-risk patients in whom it was assumed that risk would be zero after 5 years (reflecting clinical practice of discharging low-risk patients from follow-up after 5 years).
Bladder cancer related mortality rates were estimated using data from a systematic review by Van den Bosch et al. 2011. Using the data in the study, separate three mortality rates were estimated for patients that progressed to muscle invasive disease and those that remained non-muscle invasive following a cystectomy (3.6% and 0.5%, respectively). The lower rate in NMIBC patients reflects an assumption that patients would have to first progress to MIBC before dying of bladder cancer.
Death from other causes was captured using 2009-2011 life tables for England and Wales from the office of national statistics (ONS). These life tables give an estimate of the annual probability of death given a person's age and gender with the model assuming that 50% of patients were female and that the average age was 60 years old. These annual probabilities were converted to three-monthly probabilities for use in the model.
Follow-up strategies
The variations in the frequency of follow-up that were considered in the model are summarised below.
Table 98Variations In The Frequency Of Follow-Up That Are Considered In The Model
| Risk group | Follow-up strategy | ||
|---|---|---|---|
| Current practice | Slightly reduced frequency | Reduced frequency | |
| Low risk | Cystoscopy at 3 months, 1 year and annually thereafter | Cystoscopy at 3 months and annually thereafter | Cystoscopy at 3 months, 1 year and then discharge |
| Intermediate risk | Cystoscopy every 3 months for 2 years, then every 6 months for 2 years and annually thereafter | Cystoscopy every 3 months for 1 year, then 6 monthly for 2 years and annually thereafter | Escalating intervals up to 1 year, with cystoscopy at 3 months, 9 months, 18 months, 30 months and annually thereafter. |
| High risk | Cystoscopy every 3 months for 2 years, then every 6 months for 2 years and annually thereafter | Cystoscopy every 3 months for 2 years and annually thereafter | Cystoscopy every 3 months for 1 year, then 6 monthly for 1 year and annually thereafter |
In addition to these variations, the use of a urinary biomarker (FISH) or cytology as a safety net to detect recurrences at the time points that would normally be checked under current practice was also considered. The diagnostic accuracy of these tests as well as cystoscopy were estimated using data from the systematic review of the clinical evidence conducted for this guideline, with most data being sourced from a systematic review by Mowatt et al. 2010.
Costs and utilities
Modelled patients accrue costs associated with any treatment, monitoring or management strategy that they are undergoing. The costs considered in the model reflect the perspective of the analysis, thus only costs that are relevant to the UK NHS & PSS were included. These costs include drug costs, treatment costs and any other resource use that may be required (e.g. GP visit). Where possible, all costs were estimated in 2012-13 prices.
The majority of costs were sourced from NHS reference costs 2012/13 by applying tariffs associated with the appropriate HRG code. Drug costs were calculated using dosages from the British National Formulary (BNF) and unit cost information from the electronic market information tool (eMit). Where unit costs for drugs were not available from eMit, prices from the BNF were used. Resource use and cost information were obtained from the Personal Social Services Research Unit (PSSRU) and the advice of the GDG.
The model estimates effectiveness in terms of quality adjusted life years (QALYs). QALYs were estimated by combining the life year estimates with utility values (or QOL weights) associated with being in a particular health state. These utility values were identified through a search of the available literature.
Base Case Results
The base case results of the analysis for are presented in the table below for patients in each risk category. The results are shown in the ‘dominance rank’ format as it allows for the best overall strategy to be evaluated.
Table 99
Base Case Cost-Effectiveness Result Using Dominance Rank.
It can be seen that the optimal strategy in low and intermediate risk patients is the reduced frequency strategy. This strategy is the least effective of all the strategies but the difference is marginal and because it is substantially cheaper than the other strategies it was found to be cost-effective overall.
In the case of high risk patients, it can be seen that the reduced frequency strategy is again the cheapest strategy but it is no longer the preferred strategy in cost-effectiveness terms. Strategies of reduced frequency with a safety net using FISH or cytology were found to be more cost-effective than this strategy with the reduced frequency follow-up strategy with FISH found to be the most cost-effective (more cost-effective than cytology because of the superior sensitivity of FISH in the base case).
Sensitivity analysis
A series of one-way sensitivity analyses were conducted, whereby an input parameter is changed, the model is re-run and the new cost-effectiveness result is recorded. This analysis is a useful way of estimating uncertainty and determining the key drivers of the model result.
The analyses showed that, in low and intermediate risk patients, reduced frequency follow-up was the most cost-effective strategy in all modelled scenarios. In the case of high risk patients, the optimal strategy remains the same as in the base case (i.e. reduced frequency with FISH) in the vast majority of the analyses. However, there are two exceptions where the reduced frequency follow-up becomes the most cost-effective strategy; one where the modelled time horizon is reduced to five years and another where the bladder cancer specific mortality rates are equivalent for NMIBC and MIBC patients.
The GDG were also interested in an analysis where only variations in follow-up frequency were considered (i.e. variations in diagnostic tests were excluded from the analysis). As in the full analysis, it was found that the optimal strategy in low and intermediate risk patients was the reduced frequency strategy. However, in the case of high risk patients, the cystoscopy frequency used in current practice was found to be the most cost-effective strategy with a cost per QALY of £9,487 in comparison to the next based strategy (Slightly reduced follow-up).
A probabilistic sensitivity analysis was also conducted to assess the combined parameter uncertainty in the model. In this analysis, the mean values that were utilised in the base case were replaced with values drawn from distributions around the mean values. It was found that, at a threshold of £20,000 per QALY, the reduced frequency follow-up strategy had a 98% and 91% probability of being cost-effective in the low and intermediate risk group, respectively. In high risk patients it was found that, at a threshold of £20,000 per QALY, the reduced follow-up strategy in combination with FISH had a 79% probability of being cost-effective.
Conclusion
The results of the analysis suggest that reducing the frequency of cystoscopic follow-up in low and intermediate risk patients is cost-effective. Furthermore, the results show that the addition of cytology or FISH as a safety net was not cost-effective in these risk groups. In high risk patients, the results of the analysis suggest that reducing cystoscopic follow-up alone is not cost-effective in comparison to current practice. However, the addition of cytology or FISH as a safety net was found to be cost-effective with a reduced frequency follow-up strategy with FISH found to be the most cost-effective strategy.
However, there are concerns about the lack of comparative data that investigates variations in follow-up and further research is required to fully assess the safety, effectiveness and cost-effectiveness of the proposed follow-up strategies.
References
- Bekker-Grob EW, et al. Non-muscle-invasive bladder cancer surveillance for which cystoscopy is partly replaced by microsatellite analysis of urine: a cost-effective alternative? BJU International. 2009;104(1):41–47. (Provisional abstract) [PubMed: 19500328]
- Van Kessel KEM. FGFR3 mutation analysis in voided urine samples to decrease cystoscopies and cost in nonmuscle invasive bladder cancer surveillance: A comparison of 3 strategies. Journal of Urology. 2013;189(5):1676–81. [PubMed: 23142690]
- Zhang Y, Denton BT, Nielsen ME. Comparison of surveillance strategies for low-risk bladder cancer patients. Medical Decision Making. 2013;33(2):198–214. [PubMed: 23178638]
- Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DWW, Kurth Karlheinz. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. European Urology. 2006;49:466–477. [PubMed: 16442208]
- Hall RR, Parmar MKB, Richards AB, Smith PH. Proposal for changes in cystoscopic follow–up of patients with bladder cancer and adjuvant intravesical chemotherapy. BMJ. 1994;308:257–260. [PMC free article: PMC2539314] [PubMed: 8179678]
- Mowatt G, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technology Assessment. 2010:1. (Structured abstract) [PubMed: 20082749]
- Van den Bosch S, Alfred WJ. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. European Urology. 2011;60(3):493–500. [Review] [PubMed: 21664041]
- NHS reference costs 2012-13. London: UK Department of Health; [database on the Internet]
- Joint Formulary Committee. British National Formulary (online). London: BMJ Group and Pharmaceutical Press;
- Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al. Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation. Health Technol Asses. 2006;10(18) [PubMed: 16729917]
- Curtis L. Unit Costs of Health and Social Care 2013. Personal Social Services Research Unit (PSSRU), University of Kent; Canterbury:
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The Average Body Surface Area of Adult Cancer Patients in the UK: A Multicentre Retrospective Study. PLoS ONE. 2010;5(1):e8933. [PMC free article: PMC2812484] [PubMed: 20126669] [CrossRef]
- Kulkarni GS, Finelli A, Fleshner NE, Jewett MAS, Lopushinsky SR, Alibhai SMH. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4:1538–49. [PMC free article: PMC1989749] [PubMed: 17896857]
- Yoshimura K, et al. Impact of superficial bladder cancer and transurethral resection on general health-related quality of life: an SF-36 survey. Urology. 2005;65(2):290–94. [PubMed: 15708040]
- Ara R, Brazier J. Deriving an Algorithm to Convert the Eight Mean SF-36 Dimension Scores into a Mean EQ-5D Preference-Based Score from Published Studies (Where Patient Level Data Are Not Available). Value in Health. 2008;11(7):1131–1143. [PubMed: 18489495]
Footnotes
- 3
However, it should be noted that there is no official cost-effectiveness threshold used in the evaluation of treatments in the US health care system.
- 4
European Organisation for Research and Treatment of Cancer
- 5
Note that an approximate figure is used as two figures are presented for cost-effectiveness probability in the study (81.49% and 84.1%).
- 6
It should be noted that, while none of the above studies met the requirements for inclusion in the systematic review, they were nonetheless informative in helping to develop our own de novo economic model.
- 7
European Organisation for Research and Treatment of Cancer
- Manageing non-muscle-invasive bladder cancer - Bladder CancerManageing non-muscle-invasive bladder cancer - Bladder Cancer
- Key research recommendations - Bladder CancerKey research recommendations - Bladder Cancer
- Human helix-loop-helix proteins Id-1 (ID-1) and Id-1' (ID-1) genes, complete cdsHuman helix-loop-helix proteins Id-1 (ID-1) and Id-1' (ID-1) genes, complete cdsgi|1816511|gb|U57645.1|HSU57645Nucleotide
- maturase K, partial (chloroplast) [Thryptomene calycina]maturase K, partial (chloroplast) [Thryptomene calycina]gi|675400529|gb|AIL89065.1|Protein
- UNVERIFIED: Drosophila unipunctata even-skipped-like gene, partial sequenceUNVERIFIED: Drosophila unipunctata even-skipped-like gene, partial sequencegi|2086095276|gb|MW425268.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...