All rights reserved. NICE copyright material can be downloaded for private research and study, and may be reproduced for educational and not-for-profit purposes. No reproduction by or for commercial organisations, or for commercial purposes, is allowed without the written permission of NICE.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Alliance (UK). Non-Hodgkin's Lymphoma: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2016 Jul. (NICE Guideline, No. 52.)
A.1. Background
To date, there is no consensus on the optimal treatment strategies for people with relapsed follicular lymphoma. While there is some prospectively collected (pre-rituximab) evidence to suggest that autologous stem cell transplantation (ASCT) might be superior compared to conventional chemotherapy (Schouten et al.2003), the only prospective trial comparing allogeneic transplantation (allo-HSCT) to ASCT had to close prematurely due to insufficient patient recruitment (Tomblyn et al. 2011). Furthermore, no full economic evaluations have been published that would address the question of the optimal treatment strategy for people with relapsed follicular lymphoma. While ASCT is associated with acceptable toxicity and relatively low treatment-related mortality (TRM), concerns about late relapses and secondary malignancies remain. Allo-HSCT, on the other hand, offers the possibility of lasting remissions and curative potential with low late relapse rates but its high toxicity and TRM rates are limiting its application to a selected patient population in which the disease risk has to be outweighed against the procedure-related morbidity and mortality. Similarly, in the post-rituximab era, R-chemotherapy may also provide an appropriate treatment option for patients, particularly when not suitable for transplantation options e.g. based on a patient's co-morbidities. Considering the long natural history of the disease and the generally slow progression, it is important to evaluate the effectiveness of the treatment options against both risk and costs. As summarised in the clinical evidence review, the evidence base is of generally low quality consisting of mostly observational studies which report contradictory results on the clinical effectiveness of the different strategies at different time points. As well as the uncertainty around clinical effectiveness, the cost-effectiveness of these strategies in the UK context is as yet unknown.
A.1.1. Health Economics Priority
As decisions about the use of different transplantation strategies will significantly impact on NHS resources and patient benefits, this topic was identified as a high economic priority by the guideline committee (GC).
A.1.2. Existing Economic Evidence
No existing economic evidence as defined under the PICO for this guideline topic was identified after a systematic search of the literature.
A.2. De novo economic model (overview)
A.2.1. Aim
The aim of the economic evaluation was to estimate the cost-effectiveness of autologous transplantation and allogeneic transplantation compared to no transplantation (R-chemotherapy) for people with relapsed follicular lymphoma.
A.2.2. Population
The population for the economic analysis comprised adults and young people aged 16 years or over with a confirmed diagnosis of follicular Non- Hodgkin Lymphoma after first relapse. People with Grade IIIB, transformed or composite/discordant FL were excluded from the analysis. The economic analysis was concerned with treatment strategies at first and second relapse after initial first-line chemotherapy treatment for FL. No sub-groups were considered in the economic analysis.
A.2.3. Interventions and comparator
Table 1 summarises the interventions and comparator at first and second relapse.
Table 1
Summary of comparators included in the economic analysis.
In the base case, R-chemotherapy in second and third line was assumed to be R-CHOP due to limited data availability for other regimens.
A.2.4. Model structure
Since no current economic literature could be found to address the decision problem, a de novo economic evaluation was undertaken to assess cost-effectiveness. An individual patient simulation model was developed using Microsoft Excel with coding in Visual Basic for Applications (VBA). VBA was chosen due to the complexity of the disease progression that had to be modelled. The fact that transition probabilities change with increasing length of remission and that there are certain constraints on the treatment options pushes the boundaries of the capabilities of a conventional Markov model and VBA patient level simulation deals well with any level of complexity.
Figure 1 illustrates the modelled treatment pathway, while Figure 2 shows the possible health states and transitions (Markov states).
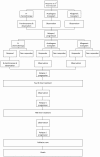
Figure 1
Modelled treatment pathway.
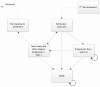
Figure 2
Possible health states and transitions (Markov states).
A decision tree model followed a cohort of 100,000 people with a starting age of 50 years suffering from follicular lymphoma who required second-line treatment after first relapse. They were assumed to have received R-chemotherapy in first line and responded to second-line induction chemotherapy.
The first decision in the model was the allocation of each person to their second-line treatment. They either received more R-chemotherapy, had an autologous transplantation or an allogeneic transplantation. Once allocated to their second-line treatment, they were assigned an initial health state for the next year (remission, death from treatment, death from cancer, all-cause death or relapse). This initial health state determined the clinical pathway that each patient would take. Transitions between health states were evaluated annually for a total modelling horizon of 35 years. Before moving onto the next time period, it was ascertained whether a patient experienced any adverse events linked with their treatment. These adverse events were counted and noted at each stage of treatment.
The model assumes that, if the patient was in remission after second-line treatment, they would remain on that same treatment pathway and receive either rituximab maintenance for two years after R-chemotherapy or if they were allocated to a transplantation arm they would be monitored over a period of two years. At any time during the two year maintenance/observation period, the patient could relapse or die of natural causes. If, after the two years, they still remained in remission they would receive no further treatment irrespective of treatment arm and would be monitored until they relapsed again or died.
People who experienced a relapse were once again assigned to one of the three treatment options(R-chemotherapy, autologous transplant or allogeneic transplant). The exact combinations which were allowed are outlined in Table 2.
Table 2
Possible treatment combinations.
Once the third line treatment was allocated, before determining the initial health state for the next time period, it had to be established whether the patients would respond to the third-line treatment or not (this was not required in second line as people only entered the model after initial response to induction). If they did not respond, they would drop out of the usual treatment pathway and be counted and noted. If they did respond they would then be allocated the initial health state for their treatment which was assumed to be the same as in second-line.
Upon relapse after third-line treatment at any stage (either during the treatment, maintenance or monitoring periods), they proceeded directly to fourth-line treatments. Data availability for fourth line treatments was limited and therefore the treatments were combined into one option (with one cost and utility attached to them). From here they could go into remission, relapse or die from any cause. No further rituximab maintenance was assumed at this stage and patients were monitored until relapse or death. Once they relapsed they would proceed directly to fifth-line treatments. Again, the simplified treatment option was utilised with the same health states as for fourth-line treatment.
Once a patient relapsed after fifth-line treatment, they progressed directly to palliative care, where they were assumed to remain for one year before death.
A UK NHS &PSS perspective has been adopted in the analysis, in line with NICE methodological recommendations. Health outcomes have been expressed in terms of quality-adjusted life years (QALYs) and costs and benefits were discounted at a rate of 3.5%. The analysis undertaken was a cost-utility analysis producing incremental cost-effectiveness ratios (ICERs) expressed as cost/QALY gained.
A.2.5. Key Model Assumptions
- The analysis follows current UK standard practice for treatment and surveillance post-treatment as advised by the GC.
- At model entry, all patients have a confirmed FL relapse, i.e. are in an existing cancer state based on their diagnosis after relapse from first-line treatment.
- All patients are assumed to have received and responded to induction therapy prior to the initial treatment strategy at model entry (ASCT, allo-HSCT or 3 cycles of R-chemotherapy).
- Total Body Irradiation (TBI) is not considered as a conditioning treatment pretransplantation.
- All Allo-HSCT patients are assumed to be given a reduced-intensity conditioning regimen prior to transplant.
- Patients who complete R-chemotherapy are assumed to receive 8 cycles of rituximab maintenance therapy over a period of 2 years.
- After relapse from allo-HSCT, patients do not receive further transplantation and receive R-chemotherapy as the only treatment option (due to lack of outcome data).
- After first relapse from ASCT, patients can receive either allo-HSCT or R-chemotherapy but no second autologous transplantation (due to lack of outcome data).
- ASCT and allo-transplantations are only considered in second and third treatment line.
- After relapse following third-line treatment, all people are assumed to receive R-chemotherapy in fourth and fifth line.
- Secondary malignancies were not included in the model as an adverse event following GC advice.
- Serious adverse events included in the model are febrile neutropenia for all treatment strategies and graft versus host disease for patients who received allo-HSCT.
- After relapse following fifth-line treatment, all patients are assumed to be receiving palliative care until death.
- All patients remain in the model until death from any cause (disease-related, treatment-related or natural causes)
A.3. Cost-effectiveness analysis: inputs (base case)
The cost-effectiveness analysis required relevant clinical evidence, health utilities, health care resources associated with the treatment pathways and associated costs for the two transplant options and R-chemotherapy. Where possible, the clinical evidence review was used to provide data inputs with structured searches undertaken to identify other required parameters (e.g. utilities, risk increase and certain costs).
A.3.1. Clinical data
A considerable challenge during economic modelling was the paucity of high quality clinical evidence, as summarised in the evidence review. Of particular note is thereby the lack of randomised controlled trials providing direct comparisons of ASCT vs. allo-HSCT or allo-HSCT vs. R-chemotherapy. The strongest clinical evidence to inform the economic analysis was provided by Schouten et al. (2003), who compared ASCT to chemotherapy after first relapse in a randomised controlled trial. However, the study was conducted in the pre-rituximab era and therefore would not be fully reflective of current clinical practice and sample size was small (n=86). We used observational data reported by Robinson et al. (2013) for the direct comparative data of ASCT vs. allo-HSCT. This study was chosen due to its high quality methodology, large sample size (n=875) and completeness of reported outcome data. We utilised the ‘best available’ evidence from the clinical review and additional literature searches to populate parameters not covered by these studies to compare the three treatment options. All data inputs underwent full validation by the GC and uncertainty was considered within the sensitivity analysis.
A.3.1.1. Relapse rates
Robinson et al. (2013) report 1-year, 3-year and 5-year cumulative relapse incidence for people who underwent autologous and allogeneic transplantations. The number of prior treatment lines in this retrospective review was >3 for 45% of ASCT patients and 63% of allo-HSCT patients, respectively. Since transplantation was modelled only in second and third line, it was considered that the reported data was more representative of third-line treatment than second-line treatment. Data was adapted to second-line by applying a 20% risk increase per additional treatment line reported by Kothari et al. (2014) and also used in a recent comparable model by Prica et al. (2015). Relapse rates were converted into annual probability of relapse and, following GC advice, were staggered (see Table 3) to reflect the curative potential of ASCT and allo-HSCT apparent in the cumulative relapse incidence curves which show a decrease in relapse rate after year one and then again after year 3 for ASCT and a marked decrease of relapse rate after year 1 for allo-HSCT3. Annual probability of relapse for allo-HSCT as a second transplant option could not be staggered as only 3-year CRI was reported. Annual probability of relapse for R-chemotherapy was calculated by applying the hazard ratio of 0.3 reported by Schouten et al. (2003) to the values for ASCT used in the model (see Table 3). While this RCT was conducted before the introduction of rituximab and the relapse rate for chemotherapy (CHOP) could be considered too high when applied for R-CHOP, the GC was of the opinion that it was appropriate for the higher risk population that would be considered for transplantation.
Table 3
Annual probability of relapse after third-line treatment.
The model was initially designed to calculate the cost-effectiveness of the treatment options in second and third line separately but due to lack of available data this could not be done. However, it was still considered more intuitive to use different relapse rate after different treatment strategies in subsequent treatment lines. This means that people who received an initial second-line R-chemotherapy course, relapsed and then underwent third-line transplantation were re-assigned a new relapse probability after their transplantation which reflected the efficacy of the last undergone treatment. This approach was chosen to reflect the very different effect on relapse rates observed for R-chemotherapy and transplantation options. However, since the relapse data available was based on cumulative relapse incidence, this approach might introduce bias as second and third relapses might be double-counted and relapse rates overestimated. The effect of this potential bias on the results has therefore been assessed in sensitivity analysis by applying the same relapse rate based on the first treatment throughout the model horizon.
A.3.1.2. Mortality
Disease-related mortality
Disease-related mortality was estimated using combined data from both treatment arms of Robinson et al. (2013). This equated to an annual estimate of disease-related mortality of 42.36%. The model links disease-related mortality to rate of relapse/progression and the annual probability of disease-related death applies only to people who have previously relapsed or progressed rather than the general cohort. Linking disease-related mortality to relapse rate resulted in staggered values for disease-related death which followed the relapse probabilities for each treatment arm and was again adapted to second-line treatment using a 20% risk increase per additional treatment line.
Non-cancer mortality
Death from other causes was captured using 2012-2014 life tables for England and Wales from the Office of National Statistics (ONS). These life tables give an estimate of the annual probability of death given a person's age and gender. A starting age of 50 years and a male proportion of 55% were applied in the model based on patient demographics from Robinson et al. (2013).
Treatment- related mortality
The high treatment-related mortality of allo-HSCT and to a lesser extent ASCT was considered a crucial parameter that could influence the potential cost-effectiveness of transplantation strategies compared to R-chemotherapy to a significant degree. Treatment-related mortality for ASCT and allo-HSCT was extrapolated from 1-year and 3-year non-relapse mortality (NRM) rates reported by Robinson et al. (2013), adjusted for the appropriate non-cancer mortality for the cohort (50 years, 55% male) and converted into annual probabilities. Following the NRM curves, probability of treatment-related death was staggered with a higher rate in year 1 and lower rates in years 2 and 3 (table 4). No treatment-related mortality was assumed beyond year 3 following transplantation.
Table 4
Annual probability of treatment-related death after third-line treatment.
Due to lack of comparative data, treatment-related mortality for R-chemotherapy was taken from vanOers et al. (2006) where 1 of 234 participants died from treatment-related toxicity in the R-CHOP arm. Based on no treatment-related deaths in the rituximab maintenance arm of the same trial (vanOers et al. 2010), probability of treatment-related death was assumed to be 0.
A.3.1.3. Adverse events
Febrile neutropenia
Febrile neutropenia was identified by the GC as the adverse event that was most likely to result in significant costs of treatment. Probability of febrile neutropenia after transplantation was based on Leger et al. (2006) who reported that 98.3% of patients (n=60) undergoing ASCT were treated for febrile neutropenia post-transplant. This was assumed to be transferable to allo-HSCT. Reporting of febrile neutropenia rates for R-chemotherapy was found to be rare and thus was assumed to be 20% based on chemotherapy values reported in literature (Zinzani et al. 2006) and GC advice. Febrile neutropenia rate for rituximab maintenance was assumed to be 5%. Sensitivity analysis was performed to assess the effect of the uncertainty surrounding these values on the results.
Febrile neutropenia rates were only applied in the year of treatment.
Graft versus host disease
Graft versus host disease (GVHD) is a severe complication that is possible after an allogeneic transplantarion whereby T-cells in the donated bone marrow (‘the graft’) attack the patient's body (‘the host’). In the allo-HSCT arm, we applied a probability of grade 3/4 acute GVHD of 12.08% based on 18 out of 149 people reported by Robinson et al. (2013) to have developed acute GVHD in the year of transplantation only. Additionally, an annual probability of chronic extensive GVHD of 13.69% was applied in years 2 and 3 only based on 38 of 149 affected people over 2 years reported by Robinson et al. (2013) and converted to annual probability.
A.3.1.4. Third-line treatment and probability of response
After having received R-chemotherapy, ASCT or allo-HSCT in second line, once people relapsed, they were eligible for third-line treatment according to predefined transition probabilities based on the previous treatment and values reported in literature (Table 5).
Table 5
Probability of third-line treatment options.
As now outcome data could be identified in the literature for second ASCT after previous ASCT or allo-HSCT after allo-HSCT, the model assumes that each transplantation option would only be performed once.
Due to the lack of relevant outcome data, length of previous remission and other clinical factors could not be taken into account when determining third-line treatment. However, it is assumed that the published mean data used would incorporate these parameters to a certain extent.
After third-line R-chemotherapy, 85.1% of people were assumed to achieve a response based on the overall response rate in relapsed patients after R-CHOP treatment reported by vanOers et al. (2010). Similarly, 98.5% of ASCT patients were considered to proceed with transplant after successful stem cell mobilisation as reported by Derenzini et al. (2013). No data could be identified for allo-HSCT and a base case value of 99% of people achieving transplant was assumed. Patients who did not respond to chemotherapy or proceed to transplantation were removed from the model and counted.
A.3.2. Costs
Modelled patients accrue costs associated with any treatment, monitoring or management strategy that they are undergoing. The costs considered in the model reflect the perspective of the analysis, thus only costs that are relevant to the UK NHS & PSS were included. These costs include drug costs, treatment costs and any other resource use that may be required (e.g. adverse events or death). Where possible, all costs were estimated in 2013-14 prices.
The majority of costs were sourced from NHS reference costs 2013/14 by applying tariffs associated with the appropriate HRG code. Drug costs were calculated using dose information from the British National Formulary (BNF) and unit costs from the Electronic Market Information Tool (eMit). Other costs were estimated using the advice of the guideline committee.
A.3.2.1. Costs of treatment
R-chemotherapy and rituximab maintenance
Cost of second and third-line R-chemotherapy was assumed to be the cost of R-CHOP based on the outcome data being mainly reported for this regimen. The drug costs of R-CHOP and rituximab maintenance were estimated using dosages and unit costs from the British National Formulary (BNF) and the Electronic Market Information Tool (eMit). The cost associated with delivering rituximab and chemotherapy was estimated using cost codes for the delivery of chemotherapy (weighted for outpatient and daycase) from NHS reference costs 2013/14. The cost of R-CHOP and rituximab maintenance is shown in Table 6.
Table 6
Cost of second and third-line R-chemotherapy and rituximab maintenance.
Based on the advice of the guideline committee, it was assumed that granulocyte-colony stimulating factor (GCSF) would be used in 50% of patients receiving chemotherapy. The unit costs associated with GCSF agents (lenograstim or filgrastim, including biosimilars) were sourced from the BNF as unit costs were not available from eMIT. It was assumed that GCSFs would be administered for seven days based on guidelines for the use of GCSF from St Luke's Cancer Alliance (Table 7).
Table 7
Cost of GCSF.
In second line, all patients entered the model after response to induction chemotherapy, so it was assumed that R-chemotherapy patients would receive a further 3 cycles of R-CHOP at a total cost of £6,758.29 (including GCSF). In third-line, people received 6 cycles of R-CHOP costing £13,516.58 (including GCSF) per patient.
The annual cost of rituximab maintenance was based on 6 cycles per year amounting to £9,583.28 and was applied for 2 years. No GCSF was assumed to be given to patients during rituximab maintenance treatments and delivery cost was applied for first attendance only.
Costs of transplantation
The cost of the autologous and allogeneic transplantation procedure was estimated to be £34,000 and £82,000, respectively based upon the tariff utilised by the transplanting haematologist on the guideline committee. It should be noted that alternative values of £16,359 and £36,288 were available from NHS Reference costs but they were thought to be considerable underestimates of the true cost and so were not used in the base case analysis. However, the impact of utilising the lower costs was explored in sensitivity analysis.
It was assumed that patients undergoing a transplant would first receive three cycles of salvage chemotherapy. Numerous chemotherapy regimens are used for this purpose in clinical practice but the guideline committee thought that the most commonly used regimens were R-ESHAP, R-DHAP, R-GDP or R-ICE. Therefore, the average cost of these chemotherapy regimens was applied in the economic analysis (assuming an equivalent weighting for each option i.e. a crude average).
The costs associated with delivering chemotherapy were sourced from NHS Reference costs. Based on the advice of the guideline committee, it was further assumed that R-ESHAP or R-DHAP would be delivered in an inpatient setting whereas R-GDP or R-ICE would be delivered in an outpatient setting. The costs associated with delivering outpatient chemotherapy were sourced from NHS Reference costs (using the same proportions as those used in the sections above). Following NHS Reference costs methodology the cost of inpatient chemotherapy was estimated using bed day costs (as there is no specific code for inpatient chemotherapy delivery). Therefore, inpatient chemotherapy costs were estimated using the average cost of an excess bed day in patients with malignant Lymphoma, including Hodgkin's and non-Hodgkin's (£348.88) multiplied by the number of days where chemotherapy is delivered.
The unit costs of drugs were sourced from Emit. Where eMIT costs were not available, BNF costs were used (Table 8).
Table 8
Cost of the chemotherapy regimens before transplant.
As above, the cost of GCSF was added to the chemotherapy cost for 50% of the patients resulting in a cost per patient of £10,032.17 for chemotherapy prior to transplant.
Cost of subsequent lines of chemotherapy
As described in a previous section above, patients that experience a relapse after third-line treatment or beyond were assumed to receive further treatment with another immunochemotherapy regimen. The guideline committee provided a list of eleven immunochemotherapy regimens that might be used in this setting including R-CHOP, R-CVP, R-Bendamustine, R-ESHAP, R-DHAP, R-GDP, R-ICE, R-GEMP, R-FC, R-GCVP OR R-Mini-BEAM. The average cost associated with this basket of regimens was estimated (assuming an equivalent proportion of each regimen was used i.e. a crude average) and applied for each subsequent relapse.
As above, the costs associated with delivering chemotherapy were sourced from NHS Reference costs, with different costs used depending on whether the regimen is delivered on an outpatient, day case or inpatient basis (using the same methodology as above). The unit costs of drugs were sourced from Emit or the BNF (where eMIT costs were not available). However, in the case of carmustine, unit costs were not available from eMIT or the BNF. The guideline committee advised that this was due to a recent lack of availability of the drug, which is now only available through specialist importers. A pharmacy colleague of one of the guideline committee members provided the previous price paid for the drug (£358.80 for 100mg), which was utilised in the analysis. An alternative and much higher estimate was provided by the pharmacy colleague of another guideline committee member (£1,000 per 100mg), suggesting that there is considerable variability in the price of the drug. In order to address this uncertainty, a wide uniform distribution between the guideline committee's lower (£200) and upper estimates (£1,000) was utilised in the probabilistic sensitivity analysis.
The costs associated with each of the regimens as well as the overall average (£8,669) are shown in table 9 below. Note that full cost details are not shown for R-CHOP as it has already been presented in previous sections.
Table 9
Cost of subsequent lines of chemotherapy used in the model.
Cost of GCSF was added to the chemotherapy costs as described above resulting in a total average cost of chemotherapy in fourth and fifth line of £10,772.34.
A.3.2.2. Costs of surveillance/follow-up
It was assumed that, at each follow-up visit, the patient would undergo a physical examination and enquiry about symptoms as well as various tests including full blood count, full profile (U&E, LFT, Ca), serum IgG, lgA, IgM and electropheresis. It was also assumed that patients would receive a CT scan if relapse/progression was suspected or to evaluate the response to treatment (e.g. to evaluate the response to rituximab at 12 months). The cost of follow-up investigations applied in the model are shown in Table 10.
Table 10
Cost of follow up.
While there is likely to be some variation in clinical practice, the follow-up frequency reported in the BJH Guidance by McNamara et al. 201114 was thought to provide a good estimate of current UK practice and was therefore used as a basis in the economic model. People were assumed to receive a follow-up examination 3-monthly in year 1, 4 to 6-monthly in year 2 and 3 (equating to an average 2.47 follow-up visits per year) and annually thereafter.
A.3.2.3. Costs of adverse events
The cost of febrile neutropenia with malignancy was taken from NHS reference costs 2012/13 and inflated to 2015 prices and amounted to £6,226.29 per episode.
No reference costs could be found for graft versus host disease. All costs associated with transplantation up to 100 days post-transplant are included in the tariff. The cost of acute GVHD was therefore assumed to be £0 to avoid double counting.
Khera et al. (2014) analysed the medical costs of 311 patients who underwent allo-HSCT in the USA and found that extensive chronic GVHD increased the overall cost of allogeneic transplantation by 45%. Based on a transplant cost of £82,000, cost of extensive chronic GVHD was assumed to be £36,900 per patient in the economic evaluation.
A.3.2.4. Cost of death
Cost of disease-related death
The cost of disease-related death was based on the cost of palliative care using estimates from a costing report by the Nuffield Trust (Georghiou et al. (2014), ‘Exploring the cost of care at the end of life’). A cost of £7,287 was applied based on the average resource use of patients with cancer in the last three months of life (Table 11).
Table 11
Palliative care costs.
It should be noted that this cost is generic to all cancers and is not specifically related to follicular lymphoma. However, in the absence of more robust data, it has been assumed that the costs in follicular lymphoma would not differ substantially.
It should also be noted that the costs of local authority-funded care may be an overestimate of the true cost because the data may include some patients that have made private contributions to partly cover the cost of care. However, since this aspect only makes up a small proportion of the overall average cost, the effect of this overestimate was thought to be negligible.
Cost of non-disease specific death
Cost of non-disease specific death was considered an unrelated cost and was omitted from the analysis.
Cost of treatment-related death
Cost of treatment-related death was assumed to be from septicaemia following infections due to treatment toxicity and costed using NHS reference costs at £4,211 (WA03A).
Cost of palliative care
After fifth-line treatment, the model assumes that people will receive palliative care or best supportive care for one year until death. The cost of £12,028.18 was taken from Prica et al. (2015) (converted to £ Sterling and inflated to 2015 prices).
A.3.3. Health-related quality of life
The model estimates effectiveness in terms of quality-adjusted life years (QALYs) so that both the quantity and quality of life are taken into account. QALYs were estimated by combining the life year estimates with utility values (or QoL weights) associated with being in a particular health state. For the purposes of this economic evaluation, the QoL data shown in Table 12 below were utilised.
Table 12
Quality of life values applied in the model.
The model assumes that quality of life is worst in the initial treatment stage and then increases the longer the patient remains progression free. This means that people who have been progression free for more than 3 years are assumed to have a higher QoL (0.88) compared to people whose remission length is still shorter than 3 years (0.8050). Furthermore, quality of life is assumed to be generally lower in fourth and fifth line compared to second and third line. Most QoL data were sourced from an unpublished Oxford Outcomes study (Wild et al. 2005) that was utilised in the NICE technology appraisal for Rituximab in the first-line treatment of stage III-IV follicular lymphoma. Further details of the study were subsequently published in the accompanying technology assessment report by ScHARR. For QoL beyond fourth line, we followed the approach used by Prica et al. (2015) who assumed a deterioration of QoL in subsequent treatment lines and based utility values beyond second line on a cost-effectiveness analysis performed by Fagnoni et al. 200916 which was using data from the GOELAMS 072 study.
It should be noted that both, the Wild et al. (2005) and Fagnoni et al. (2009) studies have limitations. Wild et al. 2005 is unpublished and full details of the study are unavailable. Furthermore, the patient numbers are relatively small (particularly for the disease free health state) and in some cases it is not clear how values have been estimated. The GOELAMS 072 study was investigating ASCT as first-line treatment and did not produce QALYs as an outcome measure. For their economic evaluation, Fagnoni et al. 2009 weighted utility values from literature according to health state duration from the GOELAMS study which could introduce bias. However, as there is no better alternative data available, the use of this QoL data was thought to be appropriate. Both studies have also been used in previous economic evaluations making this analysis consistent with the existing economic literature. The effect of using alternative QoL values was explored in sensitivity analysis.
The model applies utility decrements for all three treatment options as well as for adverse events which were taken from literature (Table 13).
Table 13
Quality of life decrements.
Data availability for utility decrements was limited which led to some assumptions having to be made. Furthermore, the utility decrement values reported by Hornberger et al. (2008) were derived from utility registries and little is known about the methodology. While these limitations may introduce bias, it was considered important to account for the toxic effects of treatments and potentially severe adverse events in the model and uncertainty around the data was explored in sensitivity analysis.
A.4. Sensitivity analysis
Deterministic (one-way) and probabilistic sensitivity analyses were conducted to test the robustness of the results of the economic model.
A.4.1. One-way sensitivity analysis
Table 14 presents the range of parameter estimates applied to the comparison of autologous transplantation, allogeneic transplantation and R-chemotherapy during one-way sensitivity analysis.
Table 14
Parameter variation during one-way sensitivity analysis.
A.4.2. Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was performed to test the robustness of the modelling conclusions in the face of uncertainty surrounding the choice of modelling inputs. Parameter values were varied within a reasonable range in each of 10,000 runs and the results averaged across runs. Costs were sampled from gamma distributions, utilities from beta distributions and rates and probabilities from log normal or beta distributions. Due to the limitations of available data and the large number of parameters, the standard error of the mean was assumed to be 50% of the mean for all parameters where no uncertainty data (standard error, standard deviation, sample size, 95% confidence intervals) could be obtained.
A.5. Base case results
The model was run over a 35-year time horizon with total costs and QALYs estimated for each treatment strategy with future costs and benefits discounted at a rate of 3.5% per year as recommended by NICE.
The base case results of the analysis are presented in tables 15 and 16 below. It can be seen that, in comparison to R-chemotherapy, both autologous and allogeneic transplantation were found to be cost-effective with ICERs of £4,812 and £12,244 per QALY gained, respectively. Using dominance rank to ascertain the optimal strategy overall, it can be seen that autologous transplantation is the most cost-effective strategy. Allogeneic transplantation was found to be slightly less effective with a substantially increased cost which means it is dominated by autologous transplantation as a first transplant option in second and third line.
Table 15
Base case cost-effectiveness results against common baseline (R-chemotherapy).
Table 16
Base case cost-effectiveness results using dominance rank.
A.6. Sensitivity analysis
A.6.1. Deterministic sensitivity analysis
A series of deterministic sensitivity analyses were conducted, whereby an input parameter is changed, the model is re-run and the new cost-effectiveness result is recorded. This analysis is a useful way of estimating uncertainty and determining the key drivers of the model result. The results of the one-way sensitivity analysis are shown in the Table 17 below.
Table 17
One-way sensitivity analysis results.
It can be seen that the conclusion of the analysis is unchanged in most of the modelled scenarios i.e. autologous transplantation is the optimal strategy. In scenarios where relapse rates of ASCT are considerably higher compared to allo-HSCT the latter emerges as the optimal strategy being-cost-effective against both R-chemotherapy and ASCT.
Probabilistic sensitivity analysis (PSA)
Probabilistic sensitivity analysis was conducted to assess the combined parameter uncertainty in the model. In this analysis, the mean values that are utilised in the base case are replaced with values drawn from distributions around the mean values.
Tables 18 and 19 summarise the point estimate results of the probabilistic sensitivity analysis. The results of 10,000 runs of the probabilistic sensitivity analysis are shown using ICER scatterplot and a cost-effectiveness acceptability curve (CEAC) in Figures 3 and 4. The ICER scatter plot shows the incremental costs and QALYs associated with each of the 10,000 runs of the PSA along with the mean result. The CEAC graph shows the probability of each diagnostic strategy being considered cost-effective at various cost-effectiveness thresholds.
Table 18
PSA cost-effectiveness results against common baseline (R-chemotherapy).
Table 19
PSA cost-effectiveness results using dominance rank.
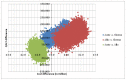
Figure 3
ICER scatterplot of pairwise comparisons.
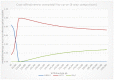
Figure 4
Cost-effectiveness acceptability curve (CEAC) of management strategies for relapsed follicular lymphoma.
The ICER scatterplot depicted in Figure 3 shows the incremental cost-effectiveness of pairwise comparisons between the different treatment strategies. It can be seen that the majority of the results for R-chemotherapy vs. allo-HSCT reside in the South West quadrant showing that R-chemotherapy was found to be less expensive but also less effective than allo-HSCT with most ICERs around £12,000 per QALY. For the comparison of allo-HSCT and ASCT, most results are located in the South East quadrant with allo-HSCT more expensive but less effective compared to ASCT (i.e. allo-HSCT is dominated). When comparing R-chemotherapy to ASCT, it can be seen that R-chemotherapy was found to be less expensive but less effective in some cases and more costly and less effective in other cases (i.e. R-chemotherapy is dominated).
In the CEAC presented in Figure 4 where all interventions are considered, it can be seen that, at a willingness to pay threshold of £20,000 per QALY, ASCT has a 94.8% probability of being cost-effective, while allo-HSCT has a 5.2% probability of being cost-effective and R-chemotherapy has 0% probability of being cost-effective.
A.7. Summary
The base case results suggest that both ASCT and allo-HSCT are cost-effective compared to R-chemotherapy with ICERs of £4,812 and £12,244, respectively. Allo-HSCT is more expensive and less effective compared to ASCT and is therefore dominated. Sensitivity analyses confirm these results. However, allo-HSCT does emerge as the optimal strategy in scenarios where ASCT relapse rates are increased compared to allo-HSCT. This result was also strengthened in the probabilistic sensitivity analysis where ASCT was found to be the optimal strategy in 94.8% of runs with allo-HSCT being the optimal strategy in the remaining 5.2% of runs. It can therefore be concluded that the economic evaluation provides robust evidence that ASCT is the most cost-effective treatment strategy for people with relapsed follicular lymphoma in second and third line. Furthermore, ASCT is the most cost-effective transplantation strategy at the point of first transplant. However, allo-HSCT can be cost-effective compared to ASCT in cases where ASCT is not expected to be successful.
A.8. Limitations of the analysis
While the model provides robust evidence for the cost-effectiveness of transplantation strategies for people with relapsed follicular lymphoma, the analysis is limited by the scarcity and quality of the available data used to populate the economic model.
Ideally, an indirect comparison would have been the method of choice to enable a comparison of allo-HSCT, ASCT and R-chemotherapy; however, due to the significant heterogeneity in the evidence, this was deemed unfeasible. Thus, the analysis focused on undertaking a pair-wise comparison (ASCT vs. R-Chemotherapy; ASCT vs. allo-HSCT; allo-HSCT vs. R-chemotherapy); with an additional analysis based on 3-way comparisons (ASCT vs. allo-HSCT vs. R Chemotherapy) based on the best available published pairwise comparisons and hazard and risk ratios.
Another challenge was the paucity of evidence regarding the length of remission time with no estimates available to provide robust and reliable estimates of the impact of length of remission on subsequent relapse and mortality rates. Thus, the analysis did not formally take into account the impact of previous length of remission on subsequent cancer outcomes. However, the impact of length of remission was indirectly taken into account by the staggering of relapse rates for ASCT and allo-HSCT (see above).
Due to the lack of available data, it was impossible to provide distinction between patients who achieved a CR or PR; thus, the model only distinguishes responders and non-responders in third line (all people are considered responders in second line at model entry) and relapse and mortality rates represent an average comprising both people achieving CR and PR.
Furthermore, lack of available data made it impossible to estimate the cost-effectiveness of ASCT and allo-HSCT in second and third line separately. The results therefore need to be interpreted with this in mind.
The model does not account for treatment discontinuation due to treatment toxicity. It is assumed that treatment discontinuation is incorporated in the non-responder rate which could underestimate this value.
The main data sources, Robinson et al. (2013) and Schouten et al. (2003), have limitations themselves. Especially, neither study reports UK specific data but is based on data from European centres. Robinson et al. (2013) is an observational study and gives little information about previous treatments and Schouten et al. (2003) reports data from the pre-rituximab era. Therefore, the data reported may not be entirely reflective of UK figures based on potential differences current clinical practice which could introduce bias. However, the GC was of the opinion that the data used in the model was reflecting UK practice to a satisfactory degree.
The model assumes that after relapse/progression and hence treatment failure, the benefits of the prior treatment are lost and patients continue through the model based on the benefits of the current treatment. This means that, for example, people who received ASCT in second line will transition through the model according to ASCT relapse rates until relapse but will change to allo-HSCT or R-chemotherapy relapse rate in third line depending on their third line treatment. This approach might introduce bias as the cumulative relapse incidence used to derive annual relapse probabilities would incorporate the possibility of several relapses and thus the relapse probability of subsequent treatments. However, it was considered by the GC that, based on the limitations of the data reported by Robinson et al. (2013) with a short median follow up of 60 months and a low number of events especially in the allo-HSCT arm (only 29 patients relapsed), this was the more intuitive and realistic approach. Sensitivity analysis was undertaken to estimate the effect of a constant relapse rate throughout the model horizon based on the rate of the initial treatment option on the results.
Febrile neutropenia was the only adverse event considered in the model (apart from graft versus host disease for allo-HSCT only). This approach was taken based on the GC's opinion that no other adverse event would cause significant costs to the NHS. Considering that treatment of adverse events up to 100 days following transplantation would be included in the tariff used in the base case, this omission will not affect the cost of transplantation but might slightly underestimate the cost of R-chemotherapy and at the same marginally overestimate the QALYs accumulated by all three treatments.
Due to the lack of comparative data (Schouten et al. 2003 does not report treatment-related mortality), TRM values for R-chemotherapy were taken from vanOers et al. (2006). While this has the potential to introduce bias, the GC considered the value to be a reasonable estimation.
References
- Derenzini E, Stefoni V, Maglie R, et al. Collection of Hematopoietic Stem Cells after Previous Radioimmunotherapy is Feasible and Does Not Impair Engraftment after Autologous Stem Cell Transplantation in Follicular Lymphoma. Biology of Blood and Marrow Transplantation. 2013;19(12):1695–1701. [PubMed: 24055654]
- Evens AM, Vanderplas A, Lacasce AS, et al. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: A comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119(20):3662–3671. [PubMed: 23921646]
- Fagnoni P, Milpied N, Limat S, et al. Cost effectiveness of high-dose chemotherapy with autologous stem cell support as initial treatment of aggressive non-Hodgkin's Lymphoma. Pharmacoeconomics. 2009;27(1):55–68. 2009. [PubMed: 19178124]
- Le Gouill S, de, Guibert S, Planche L, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica. 2011;96(8):1128–1135. [PMC free article: PMC3148906] [PubMed: 21486862]
- Hornberger J, Reyes C, Lubeck D, et al. Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma. Leukemia and Lymphoma. 2008;49(2):227–236. [PMC free article: PMC2430747] [PubMed: 18231908]
- Khera N, Emmert A, Storer BE, et al. Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. The Oncologist. 2014;19:1–6. [PMC free article: PMC4041670] [PubMed: 24797822]
- Kothari J, Peggs KS, Bird A, et al. Autologous stem cell transplantation for follicular lymphoma is of most benefit early in the disease course and can result in durable remissions, irrespective of prior rituximab exposure. British Journal of Haematology. 2014;165(3):334–340. [PubMed: 24438080]
- Leger C, Sabloff M, McDiarmid S, et al. Outpatient autologous hematopoietic stem cell transplantation for patients with relapsed follicular lymphoma. Annals of Hematology. 2006;85:723–729. [PubMed: 16832675]
- McNamara C, Davies J, Dyer M, et al. Guidelines on the investigation and management of follicular lymphoma. British Journal of Haematology. 2011;156:446–467. [PubMed: 22211428]
- Prica A, Chan K, Cheung M. Frontline rituximab monotherapy induction versus a watch and wait approach for asymptomatic advanced-stage follicular lymphoma: a cost-effectiveness analysis. Cancer. 2015;121(15):2637–45. [PubMed: 25877511]
- Robinson SP, Canals C, Luang JJ, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: An analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplantation. 2013;48(11):1409–1414. [PubMed: 23771004]
- Sacco JJ, Botten J, Macbeth F, et al. The Average Body Surface Area of Adult Cancer Patients in the UK: A Multicentre Retrospective Study. PLoS ONE. 2010;5(1):e8933. [PMC free article: PMC2812484] [PubMed: 20126669] [CrossRef]
- Schouten HC, Qian W, Kvaloy S, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: Results from the randomized European CUP trial. Journal of Clinical Oncology. 2003;21(21):3918–3927. [PubMed: 14517188]
- Tomblyn MR, Ewell M, Bredeson C, et al. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular Non-Hodgkin lymphoma beyond first complete response or first partial response. Biology of Blood and Marrow Transplantation. 2011;17(7):1051–1057. [PMC free article: PMC3114272] [PubMed: 21073974]
- vanOers MHJ, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108(10):3295–3301. [PubMed: 16873669]
- vanOers MHJ, van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. Journal of Clinical Oncology. 2010;28(17):2853–2858. [PMC free article: PMC2903319] [PubMed: 20439641]
- Zinzani PL. Salvage Chemotherapy in Follicular Non-Hodgkin's Lymphoma: Focus on Tolerability. Clinical Lymphoma & Myeloma. 2006;7(2):115–124. [PubMed: 17026822]
- A cost-utility analysis of autologous and allogeneic transplantation for people ...A cost-utility analysis of autologous and allogeneic transplantation for people with follicular lymphoma - Non-Hodgkin's Lymphoma: Diagnosis and Management
Your browsing activity is empty.
Activity recording is turned off.
See more...
