All rights reserved. NICE copyright material can be downloaded for private research and study, and may be reproduced for educational and not-for-profit purposes. No reproduction by or for commercial organisations, or for commercial purposes, is allowed without the written permission of NICE.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Alliance (UK). Non-Hodgkin's Lymphoma: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2016 Jul. (NICE Guideline, No. 52.)
4.1. Follicular lymphoma
Follicular lymphoma is a relatively common lymphoma subtype that typically has a chronic, relapsing and remitting disease course with a long overall survival. Significant heterogeneity in disease behaviour exists, however, and there is risk of transformation to more aggressive disease subtypes. Molecular testing has already added to the understanding of disease behaviour and is highly likely to complement or supplant existing clinical prognostic scoring systems.
4.1.1. First line treatment for early stage
In stage IA follicular lymphoma a large proportion of patients can be cured by local radiotherapy and in some cases after complete excision observation only may be considered.
In stage IIA disease there is considerable controversy as to most appropriate therapy varying from watchful waiting to radiotherapy or immunochemotherapy.
This topic focuses on the most effective first line treatment for stage IIA disease in the PET era. Relatively low dose radiotherapy delivering 24Gy is effective in follicular lymphoma and if the disease is truly localised and encompassed in the radiation field then cure is possible. Acute toxicity is low. There are limited data on long term effects. Most cases will involve irradiation to the neck, axilla or supraclavicular fossa. Localised mediastinal or abdominopelvic presentations of follicular lymphoma are rare and so the more serious long term effects of radiotherapy such as cardiac deaths and second malignancies of the breast and lung are not major concerns.
Clinical question: What is the most effective first-line treatment for people with stage IIA follicular lymphoma?
4.1.1.1. Clinical evidence (see section 4.1.1 in Appendix G)
The evidence included ten studies: two randomised controlled trials, four retrospective cohort studies and four case series.
4.1.1.1.1. Radiotherapy alone
Very low quality evidence of overall survival rates came from three studies (two retrospective case series and one prospective cohort study: MacManus et al. 1996; Sack et al. 1998; Wilder et al. 2001) including 189 patients. These studies reported overall survival rates of 86% (5-8 years), 65% (10 years) and 43% (15 years) in patients with stage I (<50% of total sample size) and II follicular lymphoma. MacManus et al. (1996) and Sack et al. (1998) also reported relapse free survival rates between 69% and 88.8%. Recurrence rate at 5 years was 31% and at 7 years was 44% (Sack et al. 1998) with a 45% freedom from relapse rate at 10 years (MacManus et al. 1996). Wilder et al. (2001) reported a 15 year progression free survival rate of 26% and a cancer specific survival rate of 54%.
4.1.1.1.2. Radiotherapy versus no radiotherapy
One observational study (Pugh et al. 2010) including 2140 patients provided very low quality evidence of an overall survival benefit (higher disease specific survival: Hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.65-0.94 p=0.01; higher overall survival rates: HR 0.78, 95% CI 0.65-0.94 p=0.01) in 505 patients with stage II follicular lymphoma treated with radiotherapy (external beam radiation therapy) compared to 1,635 patients treated with no radiotherapy (no information provided on type of treatments used in the comparison group).
4.1.1.1.3. Radiotherapy versus radiotherapy and chemotherapy
One observational study (Besa et al. 1995) provided very low quality evidence of a survival benefit (higher rate of freedom from relapse, p=0.008 [15 year rate]) in 80 patients with stage I (∼30%) and II follicular lymphoma treated with radiotherapy and chemotherapy compared to 45 patients treated with radiotherapy alone. Overall survival (15 years) did not significantly differ according to treatment group (63% in the chemotherapy and radiotherapy group compared to 53% in the radiotherapy group) but no statistical analyses were presented to assess significant differences in relapse rates (0 at 7.5 years in the chemotherapy and radiotherapy group compared to 1 at beyond 15 years in the radiotherapy group) or the incidence of acute leukaemia (5 cases in the chemotherapy and radiotherapy group compared to 6 in the radiotherapy group).
4.1.1.1.4. Radiotherapy and chemotherapy alone
Very low quality evidence came from one non-comparative study (Seymour et al. 2003) which reported 10 year overall survival and freedom from treatment failure rates of 87% and 70% in 47 stage II follicular lymphoma patients treated with chemotherapy and involved field radiotherapy.
4.1.1.1.5. Radiotherapy versus rituximab versus radiotherapy and rituximab
One observational study (Mondello et al. 2014) provided very low quality evidence of lower relapse rates, higher progression free survival rates and longer time to next treatment in patients with stage I (47%) and II treated with either rituximab (n=38) or rituximab and radiotherapy (n=34) compared to patients treated with radiotherapy alone (n=36). Complete response rates were not significantly different according to the three treatment groups.
4.1.1.1.6. Chemotherapy versus chemotherapy and rituximab
Very low quality evidence came from one randomised controlled trial (RCT: Bachy et al. 2013) which compared rituximab plus CHVP to CHVP alone in 39 patients with stage II follicular lymphoma. This trial reported uncertainty about the relative effectiveness of the treatments in terms of event free survival (HR: 0.855 95% CI: 0.330-2.217; where HR < 1 favours chemo+rituximab).
4.1.1.1.7. Chemotherapy versus watch and wait
One randomised controlled trial (RCT: Ardeshna et al. 2014) provided low quality evidence of uncertainty about the relative median time to start of new treatment in 19 patients with stage IIA follicular lymphoma treated with rituximab compared to 17 patients with stage IIA follicular lymphoma who were randomised to a watch and wait programme (HR: 0.55 95%CI: 0.18-1.63).
4.1.1.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Offer local radiotherapy as first-line treatment to people with localised stage IIA follicular lymphoma. Consider ‘watch and wait’ (observation without therapy) as first-line treatment for people with stage IIA follicular lymphoma who are asymptomatic and for whom treatment with a single radiotherapy volume is not suitable. Offer the same treatments that might be offered to people with advanced-stage (stages III and IV) symptomatic follicular lymphoma to people with stage IIA follicular lymphoma who are symptomatic and for whom radiotherapy is not suitable. Rituximab maintenance therapy is recommended as an option for the treatment of people with follicular non-Hodgkin's lymphoma that has responded to first-line induction therapy with rituximab in combination with chemotherapy. [This recommendation is from Rituximab for the first-line maintenance treatment of follicular non-Hodgkin's lymphoma (NICE technology appraisal guidance 226).] |
|---|---|
| Relative value placed on the outcomes considered | The critical outcomes for this topic were disease specific survival and overall survival. Other important outcomes of interest included progression free survival, treatment related mortality and morbidity, health related quality of life and patient preference, although no evidence was found for treatment related mortality, treatment related morbidity, health related quality of life or patient preference. |
| Quality of the evidence | The evidence for this topic was assessed using GRADE and ranged from very low to low quality overall. The evidence was downgraded due to imprecision (small sample sizes and low event rates), limited descriptions of methods and indirectness of populations (limited data on stage IIA). The lack of comparative studies for some of the interventions meant that non-comparative studies were also included as evidence. It was not possible to compare outcomes across studies as each study compared different interventions, thus making it difficult to summarise across the evidence base. Although there was an absence of high quality, randomised trial evidence, the GC thought that radiotherapy should be recommended strongly because it has low toxicity, potential curative benefit (indicated by a large SEER dataset showing a 9% improvement in overall survival at ten years with radiotherapy for stage II follicular lymphoma) and further trials are unlikely in this area. Evidence about ‘watch and wait’ from the randomised trial was of low quality due to imprecision and the GC could not make a strong recommendation about this intervention. |
| Trade off between clinical benefits and harms | The GC considered that there was a potential for cure with radiotherapy in a minority of patients. Although not reported in the evidence the GC acknowledge the potential harms of radiotherapy including low risk of second malignancies, and location specific toxicity, but they thought that current radiotherapy techniques were likely to help minimise this. The GC made a separate recommendation for patients whose asymptomatic disease could not be treated by a single radiotherapy volume. The GC thought that for asymptomatic disease deferring chemotherapy toxcity by using a ‘watch and wait’ approach was a reasonable option. For those with symptomatic stage IIA disease not suitable for treatment with radiotherapy the GC consensus supported a strong recommendation to offer the same treatment as for advanced stage follicular lymphoma, given that active treatment is required for symptomatic disease. The GC acknowledged that patients treated with watch and wait may experience anxiety; although the evidence about the effectiveness of watch and wait was inconclusive the GC thought that such patients would benefit from delaying or avoiding treatment toxicity. |
| Trade off between net health benefits and resource use | No health economic evidence was identified and no health economic model was built for this topic. The recommendations are likely to result in increased resources spent on radiotherapy as it will be used more while watch and wait or immediate chemotherapy will be used less. In terms of costs, radiotherapy is likely to be similar to chemotherapy (possibly slightly cheaper) but more expensive than watch and wait. However, in terms of effectiveness, radiotherapy is appears to be superior with a possibility for cure in a minority of patients. Therefore, even if the use of radiotherapy is more costly, it was thought likely to be cost-effective in cost per QALY terms. |
| Other considerations | The GC consider recommendations will lead to a change in practice in some centres, who use either watch and wait or standard chemotherapy for people with localised stage IIA follicular lymphoma. The recommendation for rituximab maintenance therapy as an option after first line induction therapy with rituximab in combination with chemotherapy came from NICE technology appraisal guidance 226. |
4.1.2. Consolidation therapy in follicular lymphoma
Follicular lymphoma is a comparatively indolent disorder in most patients, and the majority will respond to salvage therapies. Nevertheless, conventional immuno-chemotherapy is not curative and many patients will be considered candidates for some form of transplant procedure at some point in the treatment pathway. Escalation to high dose therapy with autologous stem cell transplantation (ASCT) offers improved progression free survival in selected patients, with a significant fraction achieving longer term disease stability, which has been equated to ‘functional’ cure. Given the older age of patients with follicular lymphoma (median age of onset 60 years), it has also been argued that cure should not be the therapeutic goal for most patients with the disorder, as control of the disease and maintenance of quality of life may allow patients to live with their disease until other medical issues intervene.
There are, however, groups of patients that can be identified with worse overall prognoses. Such patients are often best identified according to the level and duration of response to prior therapies, and by prognostic indices at relapse or progression. When high dose consolidation and ASCT is contemplated, the question also arises as to whether allogeneic transplantation (alloHSCT) – which is generally held to offer the best chance of overall cure but at the expense of an increased risk of morbidity and mortality – should be considered, or whether this should be reserved for those relapsing after ASCT.
In most patients ASCT or alloHSCT are reserved for second or subsequent response. The published data supporting such strategies come largely from single arm studies and registry data. Comparison between the two modalities is technically difficult as patient groups being offered either modality are generally not well matched for disease characteristics, age or co-morbidities. Current practice therefore varies widely across the UK.
This is one area in which pharmaco-economic analyses may help to define future practice given the often closely balanced clinical issues. Current improvements in pharmacological therapies also complicate the picture. Whilst on the one hand they may offer improved rates of progression free survival, making transplantation strategies less appealing, this will undoubtedly come at considerable financial cost and may just delay transplantation.
Clinical question: Is autologous transplantation, allogeneic transplantation or no transplantation the most effective treatment for people with follicular lymphoma at various time points?
4.1.2.1. Clinical evidence (see section 4.1.2 in Appendix G)
4.1.2.1.1. Transplantation in previously untreated people with follicular lymphoma
Using autologous transplantation with high-dose chemotherapy may significantly improve PFS when compared to allogeneic transplantation but not when overall survival is considered in patients at first line who have responded to chemotherapy. Similarly, auto-transplantation + high dose chemotherapy showed significantly better PFS compared to rituximab + chemotherapy but this did not remain significant when overall survival is compared, in examination of a meta-analysis reported by Schaaf et al. (2012).
This meta-analysis of 4 randomised control trials (RCTs) evaluated high dose chemotherapy + autologous transplantation (HDCT +ASCT) compared to chemotherapy or chemo-immunotherapy. This review provided low quality evidence, from 1093/1105 evaluable people, that significantly increased progression-free survival (PFS) was seen after HDCT+ASCT compared to chemotherapy (HR=0.42, 95% CI 0.33-0.54; p=<0.00001) but no significant difference seen in overall survival (OS) (HR=0.97, 95%C) 0.76-1.24, p=0.81). No significant differences were seen in treatment related mortality (TRM), onset of secondary myeloid leukemia/myelodysplasia syndromes or solid cancers. Adverse events were seldom reported and reporting differed between trials which did not allow for meta-analysis. However, they were generally higher in people in the HDCT +ASCT arm. When HSCT +ASCT was compared to rituximab +chemotherapy; PFS remained advantageous in the HDCT +ASCT group (HR= 0.36, p=0.001); with no significant difference in OS (RR=0.88, p=0.75).
4.1.2.1.2. First transplantation after relapse
Autologous transplantation versus chemotherapy
In their review, Schaaf et al (2012) reported on one trial in which 70 relapsed people were treated with HDCT + ASCT versus chemotherapy with no prior rituximab (Schouten et al. 2003). Schouten et al. (2012) provided low quality evidence of a survival advantage of HDCT +ASCT compared to chemotherapy in terms of progression-free survival (HR=0.3); 95%CI 0.15-0.61, and overall survival HR=0.4 95%CI 0.18-0.89) but no other outcomes were reported.
Autologous transplantation versus Immuno-chemotherapy
There is limited evidence on long-term QOL outcome with one study providing evidence. That people with FL reported have lower QOL when compared to the general population.
The impact of treatment on QOL outcomes when measured by different instruments (cancer-specific versus general QOL measures) is inconsistent.
Very low quality evidence came from a cross-sectional study (Andresen et al. 2012) from Germany which compared the quality of life (QOL) of 124 long-term survivors after HDCT+ASCT compared to R-CHOP using the EORTC QLQ-C30 and EQ-5D. The study reported QOL differences between the two groups (HDCT+ ASCT versus R-CHOP) with significant differences seen in the social functioning scale and pain (p=0.04 and 0.01) and index score of the EQ-5D (p=0.049) in favour of HDCT +ASCT. However, for both groups, QOL scores were lower than the general population with a significant decrease in QoL for the HDCT group in four of five subcategories of the EORTC QLQ-C30 functional state (physical, role, cognitive and social functioning and six of the nine subcategories of the symptomatic state (fatigue, dyspnea, insomnia, constipation, diarrhea and financial difficulties)(p<0.05).
Autologous transplantation following rituximab treatment
One observational study compared rituximab status prior to autologous transplantion in 194 relapsed FL patients (Phipps et al 2015). Rituximab status was categorised as rituximab-sensitive (RS) (n= 35), rituximab- refractory (RR) (n=65) and no rituximab (noR) (n=94). This study provided very low quality evidence that 3 year PFS was better for RS patients compared to RR and no R patients (85% vs 35% vs 49%, p=0.004) and OS (97% vs. 63% 73.4%, p=0.03). On multivariate analysis, only RS was associated with improved OS and PS (HR 0.24, p=0.01 and HR 0.35, p=0.006) respectively.
Autologous transplantation versus allogeneic transplantation (mixed conditioning regimens)
The evidence comparing autologous and allogeneic transplantation, from studies where patients received a variety of different conditioning regimens is inconsistent.
Three studies provided very low quality evidence about the use of ASCT versus alloHSCT with mixed conditioning regimens. Evens et al. (2013) reported on a review of the National Comprehensive Cancer Network NHL Outcomes database in the USA. No significant difference in 3 year EFS reported in the ASCT group (n=135) vs. alloHSCT (n=49) of 57% vs 52%, p=0.14. Eighty-nine percent of people received prior rituximab-based therapy. However, statistical significant differences were reported in 3 year OS (87%vs 61%, p= <0.0001) and 100 day and 3 year non-relapse mortality (1% vs 6% and 3% vs 24%, p=<0.001) in favour of auto-transplantation In the ASCT group, 69% of deaths were due to progressive disease compared to 38% in the alloHSCT; with deaths due to second malignancy 15% vs 10% respectively.
Grauer et al (2009) reviewed 117 people from a single cancer centre in the USA receiving ASCT (n=81) vs alloHSCT (n=36) with rituximab therapy not reported. 5 year OS was reported as 53% vs 49% for those with relapsed or refractory disease; with higher non-relapsed mortality (NRM) in alloHSCT (25% vs 11%) with OS for all people favouring alloHSCT (67% vs 57%). 5 year PFS was higher in alloHSCT (46% vs 38%).
A retrospective cohort study of 35 people at a single USA centre transplant programme assessed outcomes following ASCT or alloHSCT of which 7% and 33% respectively received prior rituximab)(Reddy et al 2012). No significant difference as reported in 5 year PFS (73.3% vs 43%) or rate of relapse (26.6% vs 22.5%), but significant differences in 5 year OS (91.7 vs 53.9%, P=0.01) in favour of auto-transplantation. Non-relapse mortality was 42% in the alloHSCT group and 0% in the ASCT group. No adverse events were reported.
BEAM-Conditioning Transplantation
There is limited evidence on the use of the BEAM- conditioning (BiCNU, etoposide, ara-C and melphalan) regimen in auto and allogeneic transplantation.
One study (Noriega et al 2014) was graded as very low quality in which a retrospective analysis of outcomes for 171 people (of which 65% received prior rituximab) receiving BEAM-auto hematopoietic stem cell transplantation or BEAM-alemtuzumab allogeneic hematopoietic stem cell transplantation was undertaken in 2 UK centres. The median follow up was 6.5 (0.4 -18.2 years). A separate analysis of 59 and 38 people with non-transformed FL was reported. A 10 year cumulative relapse rate was reported at 61.6% vs. 30.5% in ASCT vs. alloHSCT, p=0.018, with all other reported outcomes including 71 people with transformed FL.
Myeloablative allogeneic transplantation vs. autologous transplantation
There was inconsistent evidence when myeloablative allogeneic transplantation is compared to autologous transplantation.
Two studies provided very low quality evidence about ASCT versus alloHSCT where myeloablative conditioning regimens were used. Deshpande (2004) reported a US-based retrospective analysis of people receiving ASCT (n=186) or alloHSCT (n=18) with a conditioning regimen of cyclosphamide and TBI in 54% and 72% of people respectively with no reporting of rituximab therapy. In a median follow up of 7.8 years (range 1.7-1.92 years); the 5 year EFS was reported as 41% vs. 71%, p=0.034 in favour of myeloablative allo-transplantation; and 5 year OS as 61% vs. 76% p=0.18, again in favour of allo-transplantation
van Besien et al. (2003) reported on a retrospective analysis of 904 people registered with the International Bone Marrow Transplant Registry and Autologous Blood and Marrow Transplant Registry, followed up for a median of 36 months for allogeneic transplantation (n=176), 49 months for purged autologous transplantation (n=131) and 41 months for unpurged allogeneic transplantation (n= 597) with no prior rituximab therapy reported. Five year overall survival was 51%, 62% and 55% for purged auto-transplantation, unpurged auto transplantation and allogeneic transplantation respectively. With regard to causes of non-relapse mortality, death was recorded in 50 (28%) , 18 (13.7%) and 45 (7.5%) of people for purged auto-transplantation, unpurged auto transplantation and allogeneic transplantation respectively; with new malignancies reported as cause of death in 5 and 9 people receiving purged and unpurged autologous transplantation and 10 cases attributed to GVHD.
Allogeneic transplantation vs. autologous transplantation (unknown conditioning regimen)
De Fontbrune (2009) reported a retrospective cohort study of 143 people which provided very low quality evidence on outcomes comparing ASCT or alloHSCT. Median follow up was 4.4 years and 4 years respectively in each group. Five year EFS and OS were reported as 46% vs. 58% and 73% vs 58% (ASCT versus alloHSCT); and after propensity score matching; 52.4% vs. 66% and 77% vs. 67% which were not statistically significant.
Klyuchinikov et al 2015 reported 5 year outcomes following reduced intensity conditioning allogeneic transplantationor. ASCT, in 518 patients. This study provided very low quality evidence on the probability of NRM, relapse/progression, PFS and OS was 5% vs. 26% (p<0.001); 54% vs.20% (p<0.001), 41% vs.58% (p<0.001) and 74% vs. 66% (P=0.05) in favour of alloHSCT. On multivariate analysis, ASCT was associated with reduced NRM (RR 0.21, p<0.0001) and time varying effects seen in other outcomes.
Non-myeloablative allogeneic transplantation vs. autologous transplantation
There is inconsistent and very low quality evidence about the use of non-myeloablative allo-transplantation on outcomes when compared to auto-transplantation. The role of adding rituximab to conditioning regimens prior to transplantation was assessed in one retrospective observational study from the USA (Khouri et al 2005) which compared autologous versus non-myeloablative allogeneic transplantation after high-dose rituximab containing conditioning regimens for chemo sensitive FL. This study included 68 people who were followed up for a median of 34 months. Three year DFS and OS were reported as 84% vs. 85% (auto versus allo) and 84% and 88% (p=0.8); with risk of progression reported as 5% and 3% respectively. In those that had previously failed auto-SCT (n=8), a 4 year DFS of 87% was reported.
A retrospective cohort study of 40 people at a single cancer centre in the USA who underwent BEAM conditioning ASCT (n=20) and alloHSCT with conditioning regimen of cyclophosphamide, fludarabine and TBI provided very low quality evidence on outcomes reported at median time of 34 months follow up (Lunning 2012). No report of prior rituximab use was given. Three year EFS and OS were reported as 60%vs 79% and 62% and 85% (not statistically significant) respectively, In people whose previous remission duration was <12 months (11/20 and 20/20); 3 year EFS was reported as 36% vs. 79%, p= <0.03).
Reduced-intensity Conditioning Allogeneic Transplantation vs. autologous transplantation
A retrospective review of 875 people in the European Bone Marrow Transplant Registry (Robinson et al 2013) provided low quality evidence on outcomes of people who underwent ASCT (n=726) versus alloHSCT in order to compare outcomes of reduced intensity alloHSCT with median follow up of 59 months (range 3-108 months). 53% and 61% received prior rituximab in each respective group. The NRM was significantly worse for people undergoing reduced-intensity alloHSCT, with 100 days, 1 year and 5 year NRM reported as 2%vs6%, 3%vs 17% and 5%vs 22%, p<0.001). For PFS, there was a survival benefit at 1 year PFS favouring ASCT (77%v 68%) but in 3 and 5 years this PFS benefit favoured alloHSCT 57% vs. 62% and 48% vs. 57%, all these differences statistically significant. p=<0.001,. Non-significant differences were reported for OS with 1, 3 and 5 year rates reported as 90% vs. 80%, 78% vs68% and 72% vs. 67%, (p=0.84), respectively in favour of ASCT . The number of non-relapse deaths were 37 (5%) in the ASCT group and 32 (21%) in the alloHSCT group.
Further very low quality evidence was provided by an observational study of long-term outcomes of RIC alloHSCT compared to ASCT in Grade I/II patients FL patients (Klyuchnikov et al, 2015). The 5 year adjusted probabilities of NRM, relapse/progression, PFS and OS of ASCT vs. alloHSCT groups were 5% vs. 26% (p<0.001); 54% vs. 20% (p<0.0001); 41% vs. 58% (p <0.001) and 74% vs. 66% (p=0.05) respectively. On multivariate analysis, ASCT was associated with reduced NRM (RR=0.21, p<0.0001) and time varying effects were seen on other outcomes.
Autologous transplantation (no comparator)
Very low quality evidence froma single centre, non–comparative study of, Jagadesh et al, (2014) reported that in 127 patients in whom 93% had prior exposure to rituximab, 10 year PFS and OS were 33.2% and 52.4% respectively, with age at transplant and number of prior therapies (>3 vs. 1-3) significant prognostic factors in both univariate and multivariate analysis (Higher age HR1.76, 95% CI 1.23-2.52, p=0.002) and >3 prior therapies (HR 2.58,95% CI 1.21-5.12,p=0.006). Oh et al 2014 reported outcomes of 180 patients following relapse of chemotherapy. Very low quality evidence from this study indicated that, in univariate analysis, 5 year OS was significantly higher in patients receiving ASCT at 1st/2nd Line compared to no ASCT and ASCT beyond second relapse (92.4% v 66.5% v62.5%, p=<0.001). Allogeneic transplantation did not affect OS (p=0.62). In a multivariate analysis, ASCT at 1st/2nd relapse was associated with improved OS (HR=4.55, p=0.002) independent of Follicular Lymphoma International Prognostic Index (FLIPI) score 0-2 at diagnosis, no transformation and ever use or rituximab with chemotherapy or as maintenance.
An observational study of 640 patients undergoing HDT/ASCT between 1989-2007from the Grupo Español de Linfomas/Trasplante Autólogo de Médula Ósea (GELTAMO) registry provided very low quality evidence on outcomes with a median follow up of 12.2 years from transplantation (Ubito et al, 2014). The median PFS and OS were 9.4and 21.3 years with patients transplanted at first complete response achieving a significantly better PFS (68%) and OS (74%) then those transplanted at 2nd complete response, p=0.005
In another longer term follow up of outcomes with HDCT+ ASCT, Arcani et al (2015) report on 117 patients with relapsed/refractory follicular lymphoma. This study provided low quality evidence on the 5 year PFS and OS of patients after a median follow up of 6.7 years, with median time to relapse of 17months in 46 patients who relapsed after treatment. For the 117 patients, 5 year PFS was 54% (95% CI: 45-63%) and 5 years OS was 83% (95% CI: 74-89%). For patients who were in first relapse, the 5 year OS was 85.3% (95%CI: 74.4-91.9%) and 74% (95%CI:54.5-86./1%) for patients who underwent ASCT after 3 or more lines (p=0.05).
Allogeneic transplantation (no comparator)
A US based observational study (Khouri et al 2008) of 47 patients who receivedalloHSCT with non-myeloablative conditioning with fludarabine, cyclophosphamide and rituximab provided very low quality evidence on outcomes after a median follow up of 60 months after transplantation. Five year PFS and OS was 85% and 83%. The incidence of grade 2 acute GVHD was 11% and chronic and chronic extensive GVHD was 60% and 36% respectively. Seven patients died (6 due to infection), with no cases due to recurrent lymphoma. Transplantation at second relapse (including relapse following prior autologous transplantation)
Robinson et al (2013) provided very low quality evidence of subsequent outcomes for people who relapsed after their ASCT (n=292); with 17 (6%) receiving a second autologous transplantation and 56 (19%) proceeding to an alloHSCT. Only 1 of the 29 patients relapsing in the ASCT received a second transplant (myeloablative alloHSCT). In 56 patients receiving an alloHSCT, the 3 year NRM, disease progression, PFS and OS rates were 30%, 30%, 39% and 50% respectively.
Very low quality evidence came from a retrospective observational study (Okoroji et al, 2010) in a single cancer centre in the USA which reported outcomes for 50 people after receiving non-myeloablative allogeneic stem cell transplantation or conventional treatment (single agent rituximab, combination chemo-antibodies or unknown treatment), with reporting that this followed the introduction of rituximab). The median follow up was 49 (range 23-113) months for people receiving alloHSCT and 37 months (range 17-130) months for those not allo-transplanted. Four year actuarial survival was reported as 73% vs. 71%, p=0.9.
Very low quality evidence came from a retrospective analysis of 146 patients in the Germany Registry for Stem Cell Transplantation, Heinzelman et al (2015) which reported survival outcomes. This included 90/146 patients who received a prior ASCT (data not reported separately), with a median follow-up of 9.1 years (range 3.6-15.7 years). The estimated 1, 2, 5 and 10 year OS was 67%, 60%, 53% and 48% respectively. The EFS was estimated at 63%, 53%, 47% and 40%. Multivariate analysis suggested treatment-sensitive disease, limited chronic GvHD and TBI-based conditioning in treatment refractory patients as independent prognostic factors for OS (data not reported).
4.1.2.2. Cost-effectiveness evidence (see also Appendix A)
4.1.2.2.1. Background
To date, there is no consensus on the optimal treatment strategies for people with relapsed follicular lymphoma. As summarised in the clinical evidence review, the evidence base is of generally low quality, consisting of mostly observational studies which report contradictory results on the clinical effectiveness of the different strategies at different time points. While there is some prospectively collected (pre-rituximab) evidence to suggest that autologous stem cell transplantation (ASCT) might be superior compared to conventional chemotherapy (Schouten at al. 2003), the only prospective trial comparing allogeneic transplantation (allo-HSCT) to ASCT had to close prematurely due to insufficient patient recruitment.
Furthermore, no full economic evaluations have been published that address the question of the optimal treatment strategy for people with relapsed follicular lymphoma. Thus, as well as the uncertainty around clinical effectiveness the cost-effectiveness of these strategies in the UK context is as yet unknown.
4.1.2.2.2. Aim
The aim of the economic evaluation was to estimate the cost-effectiveness of autologous transplantation and allogeneic transplantation compared to no transplantation (R-chemotherapy) for people with relapsed follicular lymphoma.
4.1.2.2.3. Existing Economic Evidence
No existing economic evidence as defined under the PICO for this guideline topic was identified after a systematic search of the literature.
4.1.2.2.4. De novo economic model
Since no current economic literature could be found to address the decision problem, a de novo economic evaluation was undertaken to assess cost-effectiveness. An individual patient simulation model was developed using Microsoft Excel with coding in Visual Basic for Applications (VBA).
Clinical data used in model
The strongest clinical evidence to inform the economic analysis was provided by Schouten et al. (2003), who compared ASCT to chemotherapy after first relapse in a randomised controlled trial. We used observational data reported by Robinson et al. (2013) for the direct comparative data of ASCT vs. allo-HSCT. We utilised the best available evidence from the clinical review and additional literature searches to populate parameters not covered by these studies to compare the three treatment options. All data inputs underwent full validation by the GC and uncertainty was considered within the sensitivity analysis. A 20% risk increase per additional treatment line was applied to all clinical inputs where no data specific to treatment line was available.
Relapse rates
Relapse rates were converted into annual probability of relapse and, following GC advice, were staggered to reflect the curative potential of ASCT and allo-HSCT apparent in the cumulative relapse incidence curves which show a decrease in relapse rate after year one and then again after year 3 for ASCT and a marked decrease of relapse rate after year 1 for allo-HSCT (Table 8). Annual probability of relapse for allo-HSCT as a second transplant option could not be staggered as only 3-year CRI was reported. Annual probability of relapse for R-chemotherapy was calculated by applying the hazard ratio of 0.3 reported by Schouten et al. (2003) to the values for ASCT used in the model. While this RCT was conducted before the introduction of rituximab and the relapse rate for chemotherapy (CHOP) could be considered too high when applied for R-CHOP, the GC was of the opinion that it was appropriate for the higher risk population that would be considered for transplantation.
Table 8
Annual probability of relapse after third-line treatment.
The model was initially designed to calculate the cost-effectiveness of the treatment options in second and third line separately but due to lack of available data this could not be done. However, it was still considered more intuitive to use different relapse rate after different treatment strategies in subsequent treatment lines. This means that people who received an initial second-line R-chemotherapy course, relapsed and then underwent third-line transplantation were re-assigned a new relapse probability after their transplantation which reflected the efficacy of the last undergone treatment. This approach was chosen to reflect the very different effect on relapse rates observed for R-chemotherapy and transplantation options. However, since the relapse data available was based on cumulative relapse incidence, this approach might introduce bias as second and third relapses might be double-counted and relapse rates overestimated. The effect of this potential bias on the results has therefore been assessed in sensitivity analysis by applying the same relapse rate based on the first treatment throughout the model horizon.
Disease-related mortality
Disease-related mortality was estimated using combined data from both treatment arms of Robinson et al. (2013). This equated to an annual estimate of disease-related mortality of 42.36%. The model links disease-related mortality to rate of relapse/progression and the annual probability of disease-related death applies only to people who have previously relapsed or progressed rather than the general cohort. Linking disease-related mortality to relapse rate resulted in staggered values for disease-related death which followed the relapse probabilities for each treatment arm.
Non-cancer mortality
Death from other causes was captured using 2012-2014 life tables for England and Wales from the Office of National Statistics (ONS). These life tables give an estimate of the annual probability of death given a person's age and gender. A starting age of 50 years and a male proportion of 55% were applied in the model based on patient demographics from Robinson et al. (2013).
Treatment- related mortality
The high treatment-related mortality of allo-HSCT and to a lesser extent ASCT was considered a crucial parameter that could influence the potential cost-effectiveness of transplantation strategies compared to R-chemotherapy to a significant degree. Treatment-related mortality for ASCT and allo-HSCT was extrapolated from 1-year and 3-year non-relapse mortality (NRM) rates reported by Robinson et al. (2013), adjusted for the appropriate non-cancer mortality for the cohort (50 years, 55% male) and converted into annual probabilities. Following the NRM curves, probability of treatment-related death was staggered with a higher rate in year 1 and lower rates in years 2 and 3 (Table 9). No treatment-related mortality was assumed beyond year 3 following transplantation.
Table 9
Annual probability of treatment-related death after third-line treatment.
Adverse events
Febrile neutropenia was identified by the GC as the adverse event that was most likely to result in significant costs of treatment. Probability of febrile neutropenia after transplantation was based on Leger et al. 2006 who reported that 98.3% of patients (n=60) undergoing ASCT were treated for febrile neutropenia post-transplant. This was assumed to be transferable to allo-HSCT. Reporting of febrile neutropenia rates for R-chemotherapy was found to be rare and thus was assumed to be 20% based on chemotherapy values reported in literature and GC advice. Febrile neutropenia rate for rituximab maintenance was assumed to be 5%. Sensitivity analysis was performed to assess the effect of the uncertainty surrounding these values on the results. Febrile neutropenia rates were only applied in the year of treatment.
In the allo-HSCT arm, we applied a probability of grade 3/4 acute graft versus host disease (GVHD) of 12.08% based on 18 out of 149 people reported by Robinson et al. (2013) to have developed acute GVHD in the year of transplantation only. Additionally, an annual probability of chronic extensive GVHD of 13.69% was applied in years 2 and 3 only based on 38 of 149 affected people over 2 years reported by Robinson et al. (2013) and converted to annual probability.
Costs
Modelled patients accrue costs associated with any treatment, monitoring or management strategy that they are undergoing. The costs considered in the model reflect the perspective of the analysis, thus only costs that are relevant to the UK NHS & PSS were included. These costs include drug costs, treatment costs and any other resource use that may be required (e.g. adverse events or death). Where possible, all costs were estimated in 2013-14 prices.
The majority of costs were sourced from NHS reference costs 2013/14 by applying tariffs associated with the appropriate HRG code. Drug costs were calculated using dose information from the British National Formulary (BNF) and unit costs from the Electronic Market Information Tool (eMit). Other costs were estimated using the advice of the guideline committee.
Costs of R-chemotherapy and rituximab maintenance
Cost of second and third-line R-chemotherapy was assumed to be the cost of R-CHOP based on the outcome data being mainly reported for this regimen. The drug costs of R-CHOP and rituximab maintenance were estimated using dosages and unit costs from the British National Formulary (BNF) and the Electronic Market Information Tool (eMit). The cost associated with delivering rituximab and chemotherapy was estimated using cost codes for the delivery of chemotherapy (weighted for outpatient and daycase) from NHS reference costs 2013/14. It was assumed that granulocyte-colony stimulating factor (GCSF) would be used in 50% of patients receiving chemotherapy. The unit costs associated with GCSF agents (lenograstim or filgrastim, including biosimilars) were sourced from the BNF as unit costs were not available from eMIT. It was assumed that GCSFs would be administered for seven days based on guidelines for the use of GCSF from St Luke's Cancer Alliance at a cost of £414.10 per patient.
In second line, all patients entered the model after response to induction chemotherapy, so it was assumed that R-chemotherapy patients would receive a further 3 cycles of R-CHOP at a total cost of £6,758.29 (including GCSF). In third-line, people received 6 cycles of R-CHOP costing £13,516.58 (including GCSF) per patient.
The annual cost of rituximab maintenance was based on 6 cycles per year amounting to £9,583.28 and was applied for 2 years. No GCSF was assumed to be given to patients during rituximab maintenance treatments and delivery cost was applied for first attendance only.
Costs of transplantation
The cost of the autologous and allogeneic transplantation procedure was estimated to be £34,000 and £82,000, respectively based upon the tariff utilised by the transplanting haematologist on the guideline committee. It should be noted that alternative values of £16,359 and £36,288 were available from NHS Reference costs but they were thought to be considerable underestimates of the true cost and so were not used in the base case analysis. However, the impact of utilising the lower costs was explored in sensitivity analysis.
It was assumed that patients undergoing a transplant would first receive three cycles of salvage chemotherapy. Numerous chemotherapy regimens are used for this purpose in clinical practice but the guideline committee thought that the most commonly used regimens were R-ESHAP, R-DHAP, R-GDP or R-ICE. Therefore, the average cost of these chemotherapy regimens was applied in the economic analysis (assuming an equivalent weighting for each option i.e. a crude average).
The costs associated with delivering chemotherapy were sourced from NHS Reference costs. Based on the advice of the guideline committee, it was further assumed that R-ESHAP or R-DHAP would be delivered in an inpatient setting whereas R-GDP or R-ICE would be delivered in an outpatient setting. The costs associated with delivering outpatient chemotherapy were sourced from NHS Reference costs (using the same proportions as those used in the sections above). Following NHS Reference costs methodology the cost of inpatient chemotherapy was estimated using bed day costs (as there is no specific code for inpatient chemotherapy delivery). Therefore, inpatient chemotherapy costs were estimated using the average cost of an excess bed day in patients with malignant Lymphoma, including Hodgkin's and non-Hodgkin's (£348.88) multiplied by the number of days where chemotherapy is delivered. The unit costs of drugs were sourced from Emit. Where eMIT costs were not available, BNF costs were used.
The total cost for three cycles of R-ESHAP, R-DHAP, R-GDP and R-ICE was estimated to be £11,380.19, £9,161.62, £7,763.82 and £9,338.43, respectively. As above, the cost of GCSF was added to the chemotherapy cost for 50% of the patients resulting in an average cost per patient of £10,032.17 for chemotherapy prior to transplant.
Cost of subsequent lines of chemotherapy
As described in a previous section above, patients that experience a relapse after third-line treatment or beyond were assumed to receive further treatment with another immunochemotherapy regimen. The guideline committee provided a list of eleven immunochemotherapy regimens that might be used in this setting including R-CHOP, R-CVP, R-Bendamustine, R-ESHAP, R-DHAP, R-GDP, R-ICE, R-GEMP, R-FC, R-GCVP or R-Mini-BEAM. The average cost associated with this basket of regimens was estimated (assuming an equivalent proportion of each regimen was used i.e. a crude average) and applied for each subsequent relapse.
As above, the costs associated with delivering chemotherapy were sourced from NHS Reference costs, with different costs used depending on whether the regimen is delivered on an outpatient, day case or inpatient basis (using the same methodology as above). The unit costs of drugs were sourced from Emit or the BNF (where eMIT costs were not available). However, in the case of carmustine, unit costs were not available from eMIT or the BNF. The guideline committee advised that this was due to a recent lack of availability of the drug, which is now only available through specialist importers. A pharmacy colleague of one of the guideline committee members provided the previous price paid for the drug (£358.80 for 100mg), which was utilised in the analysis. An alternative and much higher estimate was provided by the pharmacy colleague of another guideline committee member (£1,000 per 100mg), suggesting that there is considerable variability in the price of the drug. In order to address this uncertainty, a wide uniform distribution between the guideline committee's lower (£200) and upper estimates (£1,000) was utilised in the probabilistic sensitivity analysis.
The total costs for the regimens not already specified above were estimated to be £11,932.05 for six cycles of R-CVP, £14,212.38 for six cycles of R-bendamustine, £8,366.64 for four cycles of R-GEMP, £8,102.06 for four cycles of R-FC, £7,896.05 for three cycles of R-GCVP, £11,383.98 for two cycles of R-Mini-BEAM delivered on an inpatient basis and £8,138.32 for two cycles of R-Mini-BEAM delivered as an outpatient procedure. The overall average cost of the subsequent immunotherapy regimens was estimated to be £9,996. Cost of GCSF was added to the chemotherapy costs as described above resulting in a total average cost of chemotherapy in fourth and fifth line of £10,772.34.
Costs of surveillance/follow-up
It was assumed that, at each follow-up visit, the patient would undergo a physical examination and enquiry about symptoms as well as various tests (£156.41), full blood count (£6.92), full profile- U&E, LFT, Ca (£18.85), serum IgG, lgA, IgM and electropheresis (£27.67). It was also assumed that patients would receive a CT scan if relapse/progression was suspected or to evaluate the response to treatment (e.g. to evaluate the response to rituximab at 12 months).
While there is likely to be some variation in clinical practice, the follow-up frequency reported in the BJH Guidance by McNamara et al. 2011 was thought to provide a good estimate of current UK practice and was therefore used as a basis in the economic model. People were assumed to receive a follow-up examination 3-monthly in year 1, 4 to 6-monthly in year 2 and 3 (equating to an average 2.47 follow-up visits per year) and annually thereafter.
Costs of adverse events
The cost of febrile neutropenia with malignancy was taken from NHS reference costs 2012/13 and inflated to 2015 prices and amounted to £6,226.29 per episode.
No reference costs could be found for graft versus host disease. All costs associated with transplantation up to 100 days post-transplant are included in the tariff. The cost of acute GVHD was therefore assumed to be £0 to avoid double counting.
Khera et al. 2014 analysed the medical costs of 311 patients who underwent allo-HSCT in the USA and found that extensive chronic GVHD increased the overall cost of allogeneic transplantation by 45%. Based on a transplant cost of £82,000, cost of extensive chronic GVHD was assumed to be £36,900 per patient in the economic evaluation.
Cost of disease-related death
The cost of disease-related death was based on the cost of palliative care using estimates from a costing report by the Nuffield Trust (Georghiou et al. 2014, ‘Exploring the cost of care at the end of life’). A cost of £7,287 was applied based on the average resource use of patients with cancer in the last three months of life.
It should be noted that this cost is generic to all cancers and is not specifically related to follicular lymphoma. However, in the absence of more robust data, it has been assumed that the costs in follicular lymphoma would not differ substantially.
Cost of non-disease specific death
Cost of non-disease specific death was considered an unrelated cost and was omitted from the analysis.
Cost of treatment-related death
Cost of treatment-related death was assumed to be from septicaemia following infections due to treatment toxicity and costed using NHS reference costs at £4,211.
Cost of palliative care
After fifth-line treatment, the model assumes that people will receive palliative care or best supportive care for one year until death. The cost of £12,028.18 was taken from Prica et al. (2015) (converted to £ Sterling and inflated to 2015 prices).
Health-related quality of life
The model estimates effectiveness in terms of quality-adjusted life years (QALYs) so that both the quantity and quality of life are taken into account. QALYs were estimated by combining the life year estimates with utility values (or QoL weights) associated with being in a particular health state. For the purposes of this economic evaluation, the QoL data shown in Table 10 below were utilised.
Table 10
Quality of life values applied in the model.
The model assumes that quality of life is worst in the initial treatment stage and then increases the longer the patient remains progression free. This means that people who have been progression free for more than 3 years are assumed to have a higher QoL (0.88) compared to people whose remission length is still shorter than 3 years (0.8050). Furthermore, quality of life is assumed to be generally lower in fourth and fifth line compared to second and third line. Most QoL data were sourced from an unpublished Oxford Outcomes study (Wild et al. 2005) that was utilised in the NICE technology appraisal for rituximab in the first-line treatment of stage III-IV follicular lymphoma. Further details of the study were subsequently published in the accompanying technology assessment report by ScHARR. For QoL beyond fourth line, we followed the approach used by Prica et al. 2015 who assumed a deterioration of QoL in subsequent treatment lines and based utility values beyond second line on a cost-effectiveness analysis performed by Fagnoni et al. 200916 which was using data from the GOELAMS 072 study.
It should be noted that both, the Wild et al. 2005 and Fagnoni et al. 2009 studies have limitations. Wild et al. 2005 is unpublished and full details of the study are unavailable. Furthermore, the patient numbers are relatively small (particularly for the disease free health state) and in some cases it is not clear how values have been estimated. The GOELAMS 072 study was investigating ASCT as first-line treatment and did not produce QALYs as an outcome measure. For their economic evaluation, Fagnoni et al. 2009 weighted utility values from literature according to health state duration from the GOELAMS study which could introduce bias. However, as there is no better alternative data available, the use of this QoL data was thought to be appropriate. Both studies have also been used in previous economic evaluations making this analysis consistent with the existing economic literature. The effect of using alternative QoL values was explored in sensitivity analysis.
The model applies utility decrements of 0.075 for R-chemotherapy and 0.1 for transplants as well as 0.018 for adverse events, 0.05 for grade 3/4 acute GVHD and 0.1 for chronic extensive GVHD.
Base case results
The model was run over a 35-year time horizon with total costs and QALYs estimated for each treatment strategy with future costs and benefits discounted at a rate of 3.5% per year as recommended by NICE.
The base case results of the analysis are presented in Tables 11 and 12 below. It can be seen that, in comparison to R-chemotherapy, both autologous and allogeneic transplantation were found to be cost-effective with ICERs of £4,814 and £12,246 per QALY gained, respectively. Using dominance rank to ascertain the optimal strategy overall, it can be seen that autologous transplantation is the most cost-effective strategy. Allogeneic transplantation was found to be slightly less effective with a substantially increased cost which means it is dominated by autologous transplantation as a first transplant option in second and third line.
Table 11
Base case cost-effectiveness results against common baseline (R-chemotherapy).
Table 12
Base case cost-effectiveness results using dominance rank.
Deterministic sensitivity analysis
A series of deterministic sensitivity analyses were conducted, whereby an input parameter is changed, the model is re-run and the new cost-effectiveness result is recorded. This analysis is a useful way of estimating uncertainty and determining the key drivers of the model result. The results of the one-way sensitivity analysis are shown in the Table 13 below.
Table 13
One-way sensitivity analysis results.
It can be seen that the conclusion of the analysis is unchanged in most of the modelled scenarios i.e. autologous transplantation is the optimal strategy. In scenarios where relapse rates of ASCT are considerably higher compared to allo-HSCT the latter emerges as the optimal strategy being-cost-effective against both R-chemotherapy and ASCT.
Probabilistic sensitivity analysis (PSA)
Probabilistic sensitivity analysis was also conducted to assess the combined parameter uncertainty in the model. In this analysis, the mean values that are utilised in the base case are replaced with values drawn from distributions around the mean values.
The results of 10,000 runs of the probabilistic sensitivity analysis are shown using the cost-effectiveness acceptability curve (CEAC) below (Figure 4), which shows the probability of each diagnostic strategy being considered cost-effective at various thresholds on the x axis.
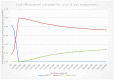
Figure 4
Cost-effectiveness acceptability curve (CEAC) of management strategies for relapsed follicular lymphoma.
In the CEAC presented in Figure 4 where all interventions are considered, it can be seen that, at a willingness to pay threshold of £20,000 per QALY, ASCT has a 94.8% probability of being cost-effective, while allo-HSCT has a 5.2% probability of being cost-effective and R-chemotherapy has 0% probability of being cost-effective.
Summary
The base case results suggest that both ASCT and allo-HSCT are cost-effective compared to R-chemotherapy with ICERs of £4,812 and £12,244, respectively. Allo-HSCT is more expensive and less effective compared to ASCT and is therefore dominated. Sensitivity analyses confirm these results. However, allo-HSCT does emerge as the optimal strategy in scenarios where ASCT relapse rates are increased compared to allo-HSCT. The base case result was also strengthened in the probabilistic sensitivity analysis where ASCT was found to be the optimal strategy in 94.8% of runs with allo-HSCT being the optimal strategy in the remaining 5.2% of runs. It can therefore be concluded that the economic evaluation provides robust evidence that ASCT is the most cost-effective treatment strategy for people with relapsed follicular lymphoma in second and third line. Furthermore, ASCT is the most cost-effective transplantation strategy at the point of first transplant. However, allo-HSCT can be cost-effective compared to ASCT in cases where ASCT is not expected to be successful.
| Recommendations | Offer consolidation with autologous stem cell transplantation for people with follicular lymphoma in second or subsequent remission (complete or partial) who have not already had a transplant and who are fit enough for transplantation. Consider consolidation with allogeneic stem cell transplantation for people with follicular lymphoma in second or subsequent remission (complete or partial):
|
|---|---|
| Relative value placed on the outcomes considered | The key outcomes for this recommendation were overall survival, progression free survival, treatment related morbidity or mortality and health-related quality of life, although health-related quality of life was not reported in the evidence. |
| Quality of the evidence | The quality of the evidence ranged from low to very low using GRADE. Quality was downgraded for the following main reasons: non-randomised study design, inclusion of some patients with stage IIIB disease and imprecision. There was a lack of randomised trial evidence comparing allogeneic transplantation with other treatments. |
| Trade-off between clinical benefits and harms | The evidence indicates that consolidation with allogeneic stem cell transplantation improves progression free and overall survival when compared to conventional chemotherapy, but with increased treatment toxicity. The GC judged that the recommendations could improve both quality of life and survival which would offset any acute and late transplantation related toxicity. The GC noted that autologous or allogeneic transplantation is never appropriate as a first line treatment (i.e. first remission) and this is why their recommendations specify second or subsequent remission. The GC noted that consolidation with autologous transplantation would not be appropriate for some patients – for example when stem cell harvesting was not possible, but these patients might still benefit from allogeneic transplantation. |
| Trade-off between net health benefits and resource use | No published health economic evidence was found but a de novohealth economic model was developed, which was used to inform the recommendations. The model was used to estimate the cost-effectiveness of autologous transplantation, allogeneic transplantation and R-Chemotherapy in patients with follicular lymphoma. The results of the analysis indicated that both autologous and allogeneic transplantation were cost-effective compared to R-chemotherapy. In the base case, autologous transplantation had an ICER of £4,814 per QALY compared to R-chemotherapy alone, whereas allogeneic transplantation was dominated. At a willingness to pay threshold of £20,000 per QALY, probabilistic sensitivity analysis indicated that autologous transplantation had a 95% probability of being cost effective compared with 5% for allogeneic transplantation and 0% for R-chemotherapy alone. The results from this model informed the recommendation to offer autologous transplantation. The recommendation to consider allogeneic transplantation where the use of autologous transplantation is not appropriate or where its use has not resulted in remission was also informed using the results of the economic model. When the use of autologous transplantation was removed from the analysis, allogeneic transplantation was found to be the most cost-effective option with an ICER of £12,246 per QALY compared to R-chemotherapy. It was anticipated that the recommendation may have a substantial resource impact through an increased use of autologous transplantation. However, as stated above, autologous transplantation is expected to be cost-effective and so this is an appropriate use of resources. |
| Other considerations | The GC considered that the recommendations would lead to increased autologous transplantation and may result in decreased use of allogeneic transplantation, but would eventually result in more uniform practice. The GC considered that patients with pre-existing co-morbidities are unlikely to be candidates for autologous transplantation and that this will disproportionately affect older patients. The GC therefore based their recommendations on patient fitness rather than age. |
4.1.3. Treating advanced-stage asymptomatic follicular lymphoma
Follicular lymphoma has a long natural history. The conventional view is that apart from very localised disease which may be ablated by local radiotherapy there is no advantage in terms of survival for immediate treatment compared to a watch and wait approach. This delays treatment until either the patient develops significant symptoms or there is risk of or actual dysfunction of a major organ system.
The evidence supporting this approach is based on data from the pre-rituximab era and there have been significant changes in the management of follicular lymphoma since then. In particular: immunochemotherapy achieves a higher number of responses and prolonged relapse free survival compared to chemotherapy alone; more intensive chemotherapy (CHOP) is more effective than previous approaches using oral chlorambucil or CVP; bendamustine has high activity in follicular lymphoma and may now rival CHOP as the chemotherapy agent of choice; maintenance treatment continuing for two years beyond completion of immunochemotherapy further prolongs relapse free survival; a recent large trial of watch and wait compared to immediate immunotherapy with rituximab has found that twice as many patients in the watch and wait group required treatment after three years compared to those who received a short course of rituximab. However it remains the case that 15-20% of patients may never need intervention over a period of 10-15 years for whom early therapy would be unnecessary
Diagnostic procedures have also improved. It is recognised that follicular lymphoma may transform to a more aggressive lymphoma, usually diffuse large B cell lymphoma (DLBCL), and also that some cases of follicular lymphoma will have coexisting DLBCL. In both of these settings watch and wait would not be considered.
This topic will address the most effective first line strategy in the management of asymptomatic follicular lymphoma.
Clinical question: Is immediate treatment or deferred chemotherapy (watch and wait) the more effective treatment for people with advanced asymptomatic follicular lymphoma?
4.1.3.1. Clinical evidence (see section 4.1.3 in Appendix G)
Three randomised controlled trials and two retrospective cohort studies were included as evidence.
4.1.3.1.1. Chlorambucil versus ‘watch and wait’
Very low quality evidence from one randomised trial in 309 patients (Ardeshna et al 2003) reported that time to second line chemotherapy (HR = 1.422, 95% CI 1.086-1.861), but not overall survival (HR = 1.026, 95% CI 0.798-1.319) was longer after treatment with chlormabucil compared to ‘watch and wait’.
4.1.3.1.2. Rituximab induction versus ‘watch and wait’
Very low quality evidence from one randomised trial in 167 patients (Ardeshna et al 2014) reported that the need for new treatment (HR = 0.35, 95% CI 0.22-0.56) and progression-free survival (HR = 0.55, 95% CI 0.37-0.83), but not overall survival (HR not reported), time to transformation (HR not reported) and quality of life (HR not reported) were superior after treatment with rituximab induction compared to ‘watch and wait’.
4.1.3.1.3. Rituximab imaintenance versus ‘watch and wait’
Low quality evidence from one randomised trial in 379 patients (Ardeshna et al 2014) reported that the need for new treatment (HR = 0.21, 95% CI 0.14-0.31) and progression-free survival (HR = 0.23, 95% CI 0.16-0.32), but not overall survival (HR = 0.73, 95% CI 0.34-1.54) or time to transformation (HR = 0.62, 95% CI 0.3-1.26) were superior after treatment with rituximab maintenance compared to ‘watch and wait’. Quality of life was either superior or similar after treatment with rituximab maintenance compared to ‘watch and wait’ (HRs not reported).
4.1.3.1.4. Prednimustine versus ‘watch and wait’
Very low quality evidence on ‘Freedom from treatment’/‘freedom from treatment failure’ (HR not reported) and overall survival (HR not reported) was reported in one randomised trial with 130 patients (Brice et al 1997) with no difference reported after treatment with prednimustine compared to ‘watch and wait’.
4.1.3.1.5. Interferon alfa versus ‘watch and wait’
Very low quality evidence from one randomised trial with 129 patients (Brice et al, 1997) reported that ‘freedom from treatment’/‘freedom from treatment failure’ (HR not reported) and overall survival (HR not reported) did not differ after treatment with interferon alfa compared to ‘watch and wait’.
4.1.3.1.6. Chemotherapy ± rituximab (NOS) versus ‘watch and wait’
Very low quality evidence from one retrospective cohort study with 79 patients (Pereira et al 2014) reported that time to next treatment (HR not reported) and progression-free survival (HR not reported), but not overall survival (HR not reported) were superior after treatment with chemotherapy ± rituximab (NOS) compared to ‘watch and wait’.
4.1.3.1.7. Immunochemotherapy (NOS) versus ‘watch and wait’
Very low quality evidence from one retrospective cohort study with 116 patients (Stemmelin et al, 2014) reported that overall survival (HR not reported) did not differ after treatment with immunochemotherapy (NOS) compared to ‘watch and wait’.
4.1.3.2. Cost-effectiveness evidence (see also Appendix B)
4.1.3.2.1. Background
Follicular lymphoma has a long natural history. The conventional view is that apart from localised stage I disease, which may be ablated by local radiotherapy there is no advantage in terms of survival for immediate treatment compared to a watch and wait approach. This delays treatment until either the patient develops significant symptoms or there is risk of, or actual dysfunction of, a major organ system.
The evidence supporting this approach is based on data from the pre-rituximab era and there have been significant changes in the management of follicular lymphoma since then. In particular: immunochemotherapy achieves a higher number of responses and prolonged relapse free survival compared to chemotherapy alone; more intensive chemotherapy (CHOP) is more effective than previous approaches using oral chlorambucil or CVP; bendamustine is a new drug to the UK with high activity in follicular lymphoma which may now rival CHOP as the chemotherapy agent of choice; maintenance treatment continuing for two years beyond completion of immunochemotherapy further prolongs relapse free survival; a recent large trial of watch and wait compared to immediate immunotherapy with rituximab has found that twice as many patients in the watch and wait group required treatment after three years compared to those who received a short course of rituximab.
The availability of more effective treatment and the ability to identify those cases harbouring more aggressive lymphoma have led to uncertainty with regard to the role of a watch and wait approach. However it remains the case that 15-20% of patients may never need intervention over a period of 10-15 years for whom early chemotherapy would be unnecessary.
4.1.3.2.2. Aims
To estimate the cost-effectiveness of the following management strategies for people with advanced asymptomatic follicular lymphoma:
- Watchful waiting
- Rituximab induction
- Rituximab induction and maintenance
4.1.3.2.3. Existing Economic Evidence
A systematic literature review identified one paper that was deemed to be partially applicable to the current decision problem. Prica et al. 2015 published a Canadian study assessing the cost-effectiveness of frontline rituximab monotherapy induction (with or without maintenance) versus a watch and wait approach for asymptomatic advanced stage follicular lymphoma.
The results of the analysis showed that rituximab induction without maintenance was the preferred strategy. It was found to be both cheaper and more effective than watchful waiting (which was therefore dominated). Rituximab induction with maintenance was found to be marginally more effective than rituximab induction alone but also more costly and not cost-effective with an ICER of $62,350 per QALY.
While the analysis was thought to be of generally high quality, it was not deemed sufficient to address the decision problem in the UK context.
4.1.3.2.4. De Novo Economic Model
Since the current economic literature didn't adequately address the decision problem, a de novo economic evaluation was undertaken to assess cost-effectiveness. A Markov decision model was developed using Microsoft Excel.
Clinical data
Need for new treatment
The key clinical data utilised in the economic model was the number of patients receiving new treatment from Ardeshna et al. 2014. This outcome captures the number of patients in the watchful waiting arm that eventually require treatment or the number of patients initially treated with rituximab that require further treatment. The most likely reason for requiring treatment was disease relapse/progression but other reasons would also be captured in this measure including patient preference.
Ardeshna et al. 2014 reported that 54% of patients in the watchful waiting arm required new treatment after 3 years. The use of rituximab induction was shown to reduce the number of patients requiring new treatment with a HR of 0.35 [0.22-0.56] in comparison to watchful waiting (equating to 11% needing new treatment after 3 years). The use of rituxmab induction with maintenance was shown to further reduced the numbers of patients requiring new treatment with a HR of 0.21 [0.14-0.31] in comparison to watchful waiting (equating to 19% needing new treatment after 3 years).
For the purposes of the model, these values were converted to annual recurrence rates of 22.8%, 6.7% and 3.9% for the watchful waiting, rituximab induction and rituximab maintenance arms (assuming a constant rate of recurrence over the study period). In the base case, these values were maintained over the time horizon of the model but variations in recurrences after 3 years were extensively explored in sensitivity analysis.
Subsequent relapse/progression rates
Patients may also experience a relapse/progression following subsequent lines of treatment. For simplicity, a constant rate of relapse after subsequent treatments has been assumed in the model. An annual progression rate of 12.8% has been applied based on Van Oers et al. 2010.
Disease related and other cause mortality
Ardeshna et al. 2014 reported no statistically significant difference in survival between the watchful waiting and rituximab arms. Therefore it has been assumed in the model that there is no difference in survival between the strategies.
Disease related mortality was captured in the model using combined data from the watchful waiting and rituximab arms from Ardeshna et al. (2014). The combined NHL–related mortality rate over three years was 3.7%, this was converted to an annual estimate of 1.2% in the model (assuming a constant rate of mortality over the study period).
Death from other causes was captured using 2011-2013 life tables for England and Wales from the office of national statistics (ONS). These life tables give an estimate of the annual probability of death given a person's age and gender. A starting age of 60 and a male proportion of 46% were applied in the model based on averages from Ardeshna et al. (2014).
Costs
Modelled patients accrue costs associated with any treatment, monitoring or management strategy that they are undergoing. The costs considered in the model reflect the perspective of the analysis, thus only costs that are relevant to the UK NHS & PSS were included. These costs include drug costs, treatment costs and any other resource use that may be required (e.g. GP visit). Where possible, all costs were estimated in 2013-14 prices.
The majority of costs were sourced from NHS reference costs 2013/14 by applying tariffs associated with the appropriate HRG code. Drug costs were calculated using dose information from the British National Formulary (BNF) and unit costs from the Electronic Market Information Tool (eMit). Other costs were estimated using resource use and cost information from the Personal Social Services Research Unit (PSSRU) and the advice of the guideline committee.
Rituximab induction with and without maintenance
The drug costs of rituximab induction and maintenance were estimated using dosages and unit costs from the British National Formulary (BNF). The cost associated with delivering rituximab was estimated using cost codes associated with the delivery of chemotherapy at first attendance on an outpatient or day case basis (a weighted average of outpatient and day case costs was estimated using the number of procedures in NHS reference costs). The costs of rituximab induction and maintenance were estimated to be £6,388.85 and £9,583.28, respectively.
Watchful waiting and follow-up costs
The only costs associated with watchful waiting are the costs of monitoring patients. Such costs would also be incurred in the active treatment arms as patients require regular follow-up after treatment in order to detect recurrences. Based on the advice of the guideline committee, it was assumed that the frequency and duration of monitoring as well as the investigations used would be the same in the watchful waiting and rituximab arms.
While there is likely to be some variation in clinical practice, the follow-up frequency reported in the BJH Guidance by McNamara et al. 2011 was thought to provide a good estimate of current UK practice and was therefore used in the economic model.
It was assumed that, at each follow-up visit, the patient would undergo a physical examination and enquiry about symptoms as well as various tests (£156.41); full blood count (£6.92), full profile- U&E, LFT, Ca (£18.85), serum IgG, lgA, IgM and electropheresis (£27.67) and lactate dehydrogenate (£13.99). It was also assumed that patients would receive a CT scan if relapse/progression was suspected or to evaluate the response to treatment (e.g. to evaluate the response to rituximab at 12 months). The costs of follow-up investigations applied in the model are shown in the table below.
Second and third line treatment
As described in an earlier section above, patients will receive immunochemotherapy as second-line treatment and may receive autologous transplant (if they are less than 65 years old) or an alternative immunochemotherapy regimen as third line treatment.
Chemotherapy ± rituximab
Most patients experiencing a recurrence are likely to be treated with chemotherapy in combination with rituximab. Based on the advice of the guideline committee, it was assumed that patients would receive R-CHOP, R-Bendamustine or R-CVP. The costs associated with delivering chemotherapy were sourced from NHS Reference costs, with a weighted average of outpatient and daycase delivery costs estimated using the number of procedures in NHS reference costs. The unit costs of drugs were sourced from eMIT. Where eMIT costs were not available, BNF costs were used.
The total cost for six cycles of R-CHOP, R-CVP and R-Bendamustine was estimated to be £12,274.27, £11,932.05 and £14,212.38, respectively.
Autologous transplant
It was assumed that patients undergoing an autologous transplant would first receive three cycles of salvage chemotherapy. Numerous chemotherapy regimens are used for this purpose in clinical practice but the guideline committee thought that the most commonly used regimens were R-ESHAP, R-DHAP, R-GDP or R-ICE. Therefore, the average cost of these chemotherapy regimens was applied in the economic analysis (assuming an equivalent weighting for each option i.e. a crude average).
The costs associated with delivering chemotherapy were sourced from NHS Reference costs. Based on the advice of the guideline committee, it was assumed that R-ESHAP or R-DHAP would be delivered in an inpatient setting whereas R-GDP or R-ICE would be delivered in an outpatient or day case setting (using the same proportions as those used in the sections above). Following NHS Reference costs methodology the cost of inpatient chemotherapy was estimated using bed day costs (as there is no specific code for inpatient chemotherapy delivery). Therefore, inpatient chemotherapy costs were estimated using the average cost of an excess bed day in patients with malignant lymphoma, including Hodgkin's and non-Hodgkin's subtypes (£348.88) multiplied by the number of days where chemotherapy is delivered. The unit costs of drugs were sourced from Emit. Where eMIT costs were not available, BNF costs were used.
The total cost for three cycles of R-ESHAP, R-DHAP, R-GDP and R-ICE was estimated to be £11,380.19, £9,161.62, £7,763.82 and £9,338.43, respectively.
The cost of the autologous transplantation procedure was estimated to be £34,000 based upon the current tariff from NHS England Specialised Services Clinical Reference Group for Blood and Marrow Transplantation (tariff identified by transplanting haematologist on the guideline committee). It should be noted that an alternative value of £16,359 was available from NHS Reference costs but it was thought to be a considerable underestimate of the true cost and so was not used in the base case analysis. However, the impact of utilising the lower cost was explored in sensitivity analysis.
Subsequent immunochemotherapy treatment
As described in a previous section above, patients that experience a relapse after third-line treatment or beyond were assumed to receive further treatment with another immunochemotherapy regimen. The guideline committee provided a list of eleven immunochemotherapy regimens that might be used in this setting; R-CHOP, R-CVP, R-Bendamustine, R-ESHAP, R-DHAP, R-GDP, R-ICE, R-GEMP, R-FC, R-GCVP OR R-Mini-BEAM. The average cost associated with this basket of regimens was estimated (assuming an equivalent proportion of each regimen was used i.e. a crude average) and applied for each subsequent relapse.
As above, the costs associated with delivering chemotherapy were sourced from NHS Reference costs, with different costs used depending on whether the regimen is delivered on an outpatient, day case or inpatient basis (using the same methodology as above). The unit costs of drugs were sourced from eMIT or the BNF (where eMIT costs were not available). However, in the case of carmustine, unit costs were not available from eMIT or the BNF. The guideline committee advised that this was due to a recent lack of availability of the drug, which is now only available through specialist importers. A pharmacy colleague of one of the guideline committee members provided the previous price paid for the drug (£358.80 for 100mg), which was utilised in the analysis.
The total costs for the regimens not already specified above were estimated to be £8,366.64 for four cycles of R-GEMP, £8,102.06 for four cycles of R-FC, £7,896.05 for three three cycles of R-GCVP, £11,383.98 for two cycles of R-Mini-BEAM delivered on an inpatient basis and £8,138.32 for two cycles of R-Mini-BEAM delivered as an outpatient. The overall average cost of the subsequent immunotherapy regimens was estimated to be £9,996.
GCSF costs
Based on the advice of the guideline committee, it was assumed that granulocyte-colony stimulating factor (GCSF) would be used in 50% of patients receiving chemotherapy. The unit costs associated with GCSF agents (lenograstim or filgrastim, including biosimilars) were sourced from the BNF as unit costs were not available from eMIT. It was assumed that GCSFs would be administered for seven days based on guidelines for the use of GCSF from St Luke's Cancer Alliance. The average cost for seven days of GCSF was estimated to be £414.10.
Palliative care costs
The cost of palliative care was estimated using estimates from a costing report by the Nuffield Trust (Georghiou et al. 2014, ‘Exploring the cost of care at the end of life’). A cost of £7,287 was applied based on the average resource use of patients with cancer in the last three months of life.
It should be noted that this cost is generic to all cancers and is not specifically related to follicular lymphoma. However, in the absence of more robust data, it has been assumed that the costs in follicular lymphoma would not differ substantially. The influence of changing the cost of palliative care was explored in sensitivity analysis.
Health related quality of life (QoL) values
The model estimates effectiveness in terms of quality adjusted life years (QALYs) so that both the quantity and quality of life are taken into account. QALYs were estimated by combining the life year estimates with utility values (or QoL weights) associated with being in a particular health state. For the purposes of this economic evaluation, the QoL data shown in Table 14 were utilised.
Table 14
Quality of life values applied in the economic model.
The QoL data were sourced from an unpublished Oxford Outcomes study (Wild et al. 2005) that was utilised in the NICE technology appraisal for rituximab in the first-line treatment of stage III-IV follicular lymphoma. Further details of the study were subsequently published in the accompanying technology assessment report by ScHARR.
There was no suitable QoL data that was directly applicable to the asymptomatic follicular lymphoma health state. Therefore, it was assumed that the QoL value associated with this health state would be equivalent to ‘disease free’ patients from the Wild et al. 2005 study (utility value of 0.880 based on 27 patients).
The QoL values associated with symptomatic follicular lymphoma and progressive disease were estimated to be 0.8050 and 0.7363, respectively. This was based upon the Wild et al. 2005 QoL study, using the approach adopted in the ScHARR technology assessment report whereby aggregated utility values for a ‘progression free’ (n=84) and ‘disease progression’ (n=132) health state were used.
Base case results
The model was run over a 40 year time horizon with total costs and QALYs estimated for each treatment strategy with future costs and benefits discounted at a rate of 3.5% per year as recommended by NICE.
The base case results of the analysis for are presented in Tables 15 and 16. It can be seen that, in comparison to watchful waiting, both rituximab induction and rituximab maintenance were found to be cost-effective and indeed dominant (i.e. more effective and cost saving). Using dominance rank to ascertain the optimal strategy overall, it can be seen that rituximab induction is the most cost-effective strategy with rituximab maintenance found to be more effective but at a substantially increased cost that means it's not cost-effective with an ICER of £69,406 well above the NICE threshold.
Table 15
Base case cost-effectiveness results against common baseline (watchful waiting).
Table 16
Base case cost-effectiveness results using dominance rank.
Deterministic sensitivity analysis
A series of deterministic sensitivity analyses were conducted, whereby an input parameter is changed, the model is re-run and the new cost-effectiveness result is recorded. This analysis is a useful way of estimating uncertainty and determining the key drivers of the model result. The results of the one-way sensitivity analysis are shown in Table 17.
Table 17
One-way sensitivity analysis results.
It can be seen that the conclusion of the analysis is unchanged in most modelled scenarios i.e. rituximab induction was found to be the optimal strategy in most analyses. The notable exceptions were the upper hazard ratio for starting new treatment after rituximab induction (making it less effective) and the lower hazard ratio for starting new treatment after rituximab induction plus maintenance (making it more effective). In these scenarios, it was found that rituximab maintenance became the optimal strategy as its relative effectiveness in comparison to rituximab induction was improved.
Threshold analysis
One of the distinguishing features of this analysis in comparison to previous economic evaluations of watchful waiting and active treatment in other disease areas, was that there was assumed to be no QoL benefit for patients on watchful waiting (in comparison to active treatment). While there is fairly strong evidence for this assumption from Ardeshna et al. 2014, it was thought to be an area worthy of further exploration.
Therefore, a threshold analysis was conducted to ascertain the QoL improvement required in patients on watchful waiting, over and above active treatment with a rituximab strategy, for watchful waiting to become cost-effective at a threshold of £20,000 per QALY.
It was found that watchful waiting becomes cost-effective when it was assumed that QoL is 0.105 lower for patients on receiving rituxiumab in comparison to watchful waiting strategies.
Probabilistic sensitivity analysis (PSA)
Probabilistic sensitivity analysis was also conducted to assess the combined parameter uncertainty in the model. In this analysis, the mean values that are utilised in the base case are replaced with values drawn from distributions around the mean values.
The results of 10,000 runs of the probabilistic sensitivity analysis are shown using the cost-effectiveness acceptability curve (CEAC) below (Figure 5), which shows the probability of each diagnostic strategy being considered cost-effective at various thresholds on the x axis.
Figure 5
Cost-effectiveness acceptability curve (CEAC) for management strategies for asymptomatic follicular lymphoma.
It can be seen that, at a willingness to pay threshold of £20,000 per QALY, rituximab induction has a 68% probability of being cost-effective, while rituximab maintenance has a 21% probability of being cost-effective and watchful waiting has 11% probability of being cost-effective.
Conclusion
The results of the base case analysis suggest that rituximab induction alone is the optimal strategy to adopt in patients with asymptomatic follicular lymphoma. This result was shown to be robust in one-way sensitivity analysis, where rituximab induction remained cost-effective in the vast majority of scenarios. The result was further strengthened in probabilistic sensitivity analysis (PSA) where the strategy was found to have a 68% probability of being cost-effective at a threshold of £20,000 per QALY. Furthermore, rituximab maintenance was shown to have the next highest probability of being cost-effective with a 21% probability of being cost-effective at the £20,000 per QALY threshold, suggesting that there is a strong case for active treatment (i.e. 89% probability of active treatment being cost-effective) rather than a watchful waiting approach.
| Recommendation | Offer rituximab induction therapya to people with advanced-stage (stages III and IV) follicular lymphoma who are asymptomatic. |
|---|---|
| Relative value placed on the outcomes considered | Overall survival was considered the critical clinical outcome when drafting recommendations. |
| Quality of the evidence | The quality of the evidence for this topic was moderate to very low as assessed using GRADE. The main issues with the evidence were: imprecision and outcome assessment was not blinded. Although time to next treatment is an unusual primary endpoint due to its subjective component, the results for progression free survival were similar, giving the GC more confidence in the evidence. The rituximab induction treatment arm was stopped early in Ardesha (2014) due to the publication of other rituximab induction and maintenance studies affecting recruitment and resulting in a loss of equipoise. The GC, however, still considered this trial as useful evidence. |
| Trade off between clinical benefits and harms | The GC considered that the delayed time to next treatment following rituximab induction compared to watchful waiting would result in fewer patients needing further chemotherapy, because their disease would not progress within their lifetime. Rituximab induction treatment would probably result in a reduction in anxiety in those patients receiving active treatment instead of watchful waiting. Although rituximab induction plus maintenance was also effective – it involved significantly more rituximab with associated increased costs and possible increased toxicity compared with induction alone. There are limited, low risk side effects due to induction rituximab which would be an additional harm for some patients whose disease would not have progressed. It is theoretically possible that induction rituximab could reduce the effectiveness of subsequent rituximab. Ardeshna (2014) follow-up has been extended to capture this however these data are not yet available. PRIMA data suggests no impact of prior rituximab maintenance on effectiveness of subsequent rituximab containing therapy. The GC concluded that the risk of harm from rituximab induction was low (and in some cases theoretical) compared with the tangible benefit of reducing the need for further treatment. The evidence suggested that other reported therapies (chlorambucil, prednimustine and interferon alpha) were less effective than rituximab when compared to watch and wait and so the GC did not make recommendations about these treatments. |
| Trade off between net health benefits and resource use | A cost-utility analysis by Prica et al. (2015) was identified. However, the study was only partially applicable to our decision problem as it did not consider the UK health care setting. Therefore this evidence was not used by the GC when agreeing their recommendations. A health economic model was developed for this topic and the results of the analysis were used to inform the recommendations. The base case results showed that rituximab induction was the most cost-effective strategy. In comparison to a watchful waiting strategy, rituximab induction was found to be less expensive and more effective (i.e. dominant). Rituximab induction plus maintenance was found to be marginally more effective than rituximab induction alone but it was not found to be cost-effective (ICER of £52,047 per QALY, well above the NICE threshold of £20,000 per QALY). It should be noted that the superior effectiveness of the rituximab strategies observed in the model were not based on a survival benefit but rather QoL benefits associated with delaying the use of intensive treatments. Uncertainty in the clinical evidence as well as the evidence used to inform cost and QoL values was assessed in one-way and probabilistic sensitivity analyses. This result was shown to be robust in one-way sensitivity analysis, where rituximab induction remained cost-effective in the vast majority of modelled scenarios. The result was further strengthened in probabilistic sensitivity analysis (PSA) where the strategy was found to have a 63% probability of being cost-effective at a threshold of £20,000 per QALY. Furthermore, rituximab maintenance was shown to have the next highest probability of being cost-effective (28%), suggesting that there is a strong case for active treatment rather than a watchful waiting approach i.e. 91% probability of active treatment being cost-effective. Thus, despite the clinical data inputs being assessed as low to very low quality, the GC were able to make strong recommendations as the model predicted a high likelihood of rituximab being cost-effective. It should be noted that there may be short term cost increases associated with the increased use of rituximab. However, as shown in the economic model, the use of rituximab is thought to be cost saving in the long term. |
| Other considerations | This recommendation will result in a major change in practice because rituximab induction is not routinely given in this setting (or licensed for this use). There is likely to be a short term impact on the chemotherapy day care units delivering rituximab induction but in the long term this recommendation should reduce throughput. This is an off license recommendation. |
- a
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information. The evidence reviewed for the guideline supports the standard monotherapy dosage of 4 doses of 375 mg/m2 at weekly intervals.
4.1.4. Treating advanced-stage symptomatic follicular lymphoma
NICE has developed a suite of technology appraisal guidance on non-Hodgkin's lymphoma. It has not been possible to develop recommendations on treating advanced stage symptomatic follicular lymphoma in this guideline due to published technology appraisals or those in development.
Recommendations in this guideline will complement the existing technology appraisals.
For more information on the relationship between the technology appraisal and clinical guidelines programmes please see Updating technology appraisals in the context of clinical guidelines.
| Recommendations | Rituximab, in combination with:
The guideline committee did not assess evidence or develop recommendations on bendamustine for treating people with follicular lymphoma, because a NICE technology appraisal on ‘the clinical and cost effectiveness of bendamustine in combination with rituximab within its licensed indication for the first-line treatment of advanced indolent non-Hodgkin's lymphoma’ was in development. This technology appraisal is currently suspended. |
|---|---|
| These recommendations are from Rituximab for the first-line treatment of stage III-IV follicular lymphoma (NICE technology appraisal guidance 243). They were formulated by the technology appraisal and not by the guideline developers. They have been incorporated into this guideline in line with NICE procedures for developing clinical guidelines, and the evidence to support these recommendations can be found at www |
4.1.5. Treating advanced-stage relapsed or refractory follicular lymphoma
NICE has developed a suite of technology appraisal guidance on non-Hodgkin's lymphoma. It has not been possible to develop recommendations on treating advanced stage relapsed or refractory follicular lymphoma in this guideline due to published technology appraisals or those in development.
For more information on the relationship between the technology appraisal and clinical guidelines programmes please see Updating technology appraisals in the context of clinical guidelines.
| Recommendations | The recommendations in this section are from Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin's lymphoma (NICE technology appraisal guidance 137). Rituximab, within its marketing authorisation, in combination with chemotherapy, is recommended as an option for the induction of remission in people with relapsed stage III or IV follicular non-Hodgkin's lymphoma. Rituximab monotherapy as maintenance therapy, within its marketing authorisation, is recommended as an option for the treatment of people with relapsed stage III or IV follicular non-Hodgkin's lymphoma in remission induced with chemotherapy with or without rituximab. Rituximab monotherapy, within its marketing authorisation, is recommended as an option for the treatment of people with relapsed or refractory stage III or IV follicular non-Hodgkin's lymphoma, when all alternative treatment options have been exhausted (that is, if there is resistance to or intolerance of chemotherapy). |
|---|---|
| These recommendations are from Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin's lymphoma (NICE technology appraisal guidance 137). They were formulated by the technology appraisal and not by the guideline developers. They have been incorporated into this guideline in line with NICE procedures for developing clinical guidelines, and the evidence to support these recommendations can be found at www |
4.1.6. Treating transformed follicular lymphoma
There is an approximately 2% per year risk of a patient with follicular lymphoma transforming to high grade lymphoma. In the pre-rituximab era this event was associated with a poor prognosis, with median survival rates of 7 to 20 months. Many centres therefore adopted high dose therapy with autologous stem cell rescue (ASCT) as standard treatment for transformed lymphoma after response to first-line chemotherapy. Results from observational studies suggest that in the rituximab era, the outcome for transformed follicular lymphoma is more favourable. Other registry studies suggest that ASCT can prolong survival in these patients. Subsequently, practise across the UK is highly variable with some units uniformly consolidating transformation with ASCT, whereas others restrict this to patients who had a high international prognostic index (IPI) score at transformation, or indeed not at all.
The role of allogeneic stem cell transplantation is even less clear. Research suggests that high grade lymphoma arise, not as a sequential step from the low grade lymphoma but rather as a separate lymphoma derived from a common lymphoma progenitor cell. Theoretically, by targeting this cell the graft-versus-lymphoma effect may therefore cure both the high grade and the low grade components, unlike ASCT which is generally held to offer more potential to cure only the high grade component. Some small series report successful allogeneic stem cell transplantation of multiply relapsed high grade lymphoma, and subgroup analyses of those with transformed disease have suggested somewhat superior outcomes compared to those with de novo disease, although experience remains limited.
Sometimes patients present with both high and low grade disease at the same time. This can be:
- With both histologies present within the same biopsy (composite lymphoma)
- With high grade disease in the lymph node and low grade lymphoma in the bone marrow (discordant bone marrow involvement)
Traditionally patients with composite lymphoma are usually treated in the same way as other high grade transformation events. However, when the low grade component is in the bone marrow the outcome with immunochemotherapy alone is very encouraging.
Clinical question: What is the effectiveness of first-line consolidation with high-dose therapy with autologous or allogeneic transplantation in people with histological transformation of follicular lymphoma to diffuse large B-cell lymphoma or concurrent presentation with follicular lymphoma & diffuse large B-cell lymphomas, compared with other strategies?
4.1.6.1. Clinical evidence (see section 4.1.4 in Appendix G)
Six retrospective observational studies provided evidence comparing the effectiveness of the two types of transplantation (allogeneic versus autologous), five retrospective observational studies provided evidence comparing the effectiveness of transplantation to other strategies and four single arm retrospective observational studies provided additional evidence of the use of autologous transplantation in patients with transformed lymphoma.
4.1.6.1.1. Autologous versus allogeneic
Overall survival
Five retrospective observational studies (Ban Hoefen et al. 2013; Micallef et al. 2006; Reddy et al. 2012; Villa et al. 2013a; Wirk et al. 2014) provided very low quality evidence about overall survival rates after autologous versus allogeneic transplantation in 393 patients with histological transformation of indolent lymphoma (76-100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL) . Reporting overall survival rates (range 2-5 years; follow-up range 0.25 – 7.5 years) of 50-83% in the autologous group compared to 22-68.5% in the allogeneic group. Micallef et al. (2006) reported median overall survival time of 3 years in the autologous group compared to 9 months in the allogeneic group. Villa et al. (2013a) and Reddy et al. (2012) reported no significant difference in overall survival rates in the two groups (Ban Hoefen et al. 2013, Micallef et al. 2006 and Wirk et al. 2014 provided no statistical analysis comparing the two groups).
Progression free survival
Three retrospective observational studies (Reddy et al. 2012; Villa et al. 2013a; Wirk et al. 2014) provided very low quality evidence about 5-year progression free survival rates after autologous versus allogeneic transplantation in 297 patients with histological transformation of indolent lymphoma (100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL) . Reporting 5-year progression-free survival rates (follow-up range 0.25 – 7.5 years) of 35-55% in the autologous group compared to 18-46% in the allogeneic group. Villa et al. (2013) and Reddy et al. (2012) reported no significant difference in 5-year progression free survival rates in the two groups (Wirk et al. 2014 provided no statistical analysis comparing the two groups).
Response rates
Two retrospective observational studies (Reddy et al. 2012; Villa et al. 2013a) provided very low quality evidence about response rates after autologous versus allogeneic transplantation in 156 patients with histological transformation of indolent lymphoma (100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL) . The autologous group had complete (56.7%) and partial response rates (23.4%) comparable to those in the allogeneic group (complete: 55.2%, partial: 17.2%).
Adverse events
Five retrospective observational studies (Ban Hoefen et al. 2013; Micallef et al. 2006; Reddy et al. 2012; Villa et al. 2013; Wirk et al. 2014) provided very low quality evidence about adverse events after the treatment of autologous or allogeneic transplantation in 393 patients with histological transformation of indolent lymphoma (76-100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL) .
Two studies (Ban Hoefen et al. 2013; Villa et al. 2013a) reported higher rates of death due to treatment related toxicity (follow-up median: 3.4-7.5 years) in the allogeneic group (27.5%) compared to the autologous group (4.1%). Villa et al. (2013a) reported that this difference was significant at 1 year post transplantation (p=0.01) and at 4-years post transplantation (p=0.001). Death due to disease progression was comparable between the autologous group (18%) and the allogeneic group (22%) (Ban Hoefen et al. 2013). However, non-relapse mortality rates were higher in the allogeneic group (31.4-41%) compared to the autologous group (4.6-8%) (Reddy et al. 2012: 0.06; no statistical analysis reported by Wirk et al. 2014).
4.1.6.1.2. Autologous versus no transplantation
Overall survival
Two retrospective observational studies (Ban Hoefen et al. 2013; Villa et al. 2013b) provided very low quality evidence about overall survival rates after autologous versus no transplantation in 250 patients with histological transformation of indolent lymphoma (86-94% follicular lymphoma) to aggressive B-cell lymphoma (94-100% DLBCL) . Reporting overall survival rates (range 2-3 years; follow-up range 3.3-3.4 years) of 54-83% in the autologous group compared to 7-65% in the no treatment group (Villa et al. 2013b reported that patients not treated with autologous transplantation due to progressive disease had an overall survival rate of 7% whilst patients with other reasons [e.g. age ≥65 years; declined the transplant] for not receiving a transplantation had an overall survival rate of 65%). Neither study reported on whether the overall survival rates were significantly different.
Response rates
One retrospective observational study (Villa et al. 2013b) provided very low quality evidence about response rates following autologous versus no transplantation in 150 patients with histological transformation of indolent lymphoma (94% follicular lymphoma) to aggressive B-cell lymphoma (94-100% DLBCL). The autologous group had a better complete (14%) and partial response rate (82%) compared to those in the no treatment group (complete: 6%, partial: 15%, p<0.001).
Adverse events
Two retrospective observational studies (Ban Hoefen et al. 2013; Villa et al. 2013b) provided very low quality evidence about adverse events after the treatment of either autologous or other treatment in 250 patients with histological transformation of indolent lymphoma (76-94% follicular lymphoma) to aggressive B-cell lymphoma (94-100% DLBCL) . The rate of death due to treatment related toxicity (follow-up median: 3.3-3.4 years) was comparable in the two groups (autologous: 3.8% versus no transplantation: 5%). However, Villa et al. 2013b reported that there were significantly more late deaths [>100 days] in the autologous group [6%] compared to the no treatment group [0%; p<0.01]). Death due to disease progression in the autologous group (8%) was not reported to be significantly different to the rate reported in the no transplantation group (10%, Ban Hoefen et al. 2013).
4.1.6.1.3. Allogeneic versus no transplantation
Overall survival
One retrospective observational study (Ban Hoefen et al. 2013) provided very low quality evidence about 2-year overall survival ratse after allogeneic versus no transplantation in 68 patients with histological transformation of indolent lymphoma (86% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL). Reporting a 2-year overall survival rate (follow-up 3.4 years) of 65% (95% confidence interval: 39-83%) in the allogeneic group compared to 53% (95% confidence interval: 39-68%) in the no treatment group. It was not reported if these survival rates were significantly different in the two groups.
Adverse events
One retrospective observational study (Ban Hoefen et al. 2013) provided very low quality evidence about adverse events after allogeneic transplantation or other treatment in 68 patients with histological transformation of indolent lymphoma (86% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL). The rate of death due to treatment related toxicity (follow-up 3.4 years) was 22% in the allogeneic group compared to 10% in the no transplantation group. Death due to disease progression (follow-up 3.4 years) was 22% in the allogeneic group compared to 34% in the no transplantation group. It was not reported if these adverse events were significantly different in the two groups.
4.1.6.1.4. Autologous versus chemotherapy plus rituximab
Overall survival
Two retrospective observational studies (Madsen et al. 2013; Villa et al. 2013a) provided very low quality evidence about5-year overall survival rates after autologous transplantation versus chemotherapy plus rituximab in 245 patients with histological transformation of indolent lymphoma (100% follicular lymphoma in Villa et al. 2013a; Madsen et al. 2013 did not provide breakdown of indolent lymphomas) to diffuse large B-cell lymphoma (DLBCL; Madsen et al. 2013 did not provide detail on transformation diagnosis). Reporting 5-year overall survival rates (follow-up 7.5 years reported by Villa et al. 2013a only) of 57-65% in the autologous group compared to 36-61% in the chemotherapy plus rituximab group. Both reported that patients receiving autologous transplantation had a significantly improved overall survival compared with those who received chemotherapy plus rituximab (p=0.09; p<0.001).
One retrospective observational study (Madsen et al. 2013) provided very low quality evidence about 5-year overall survival rates after autologous transplantation versus chemotherapy plus rituximab in 95 patients with a primary diagnosis of transformed indolent lymphoma (composite lymphoma). Reporting that the 5-year overall survival rates in the autologous group (80%) did not significantly differ compared to the chemotherapy plus rituximab group (67%).
Progression free survival
Two retrospective observational studies (Madsen et al. 2013; Villa et al. 2013a) provided very low quality evidence about 5-year progression free survival rates after autologous transplantation versus chemotherapy plus rituximab in 245 patients with histological transformation of indolent lymphoma (100% follicular lymphoma in Villa et al. 2013a; Madsen et al. 2013 did not provide breakdown of indolent lymphomas) to diffuse large B-cell lymphoma (DLBCL; Madsen et al. 2013 did not provide detail on transformation diagnosis). Reporting 5-year progression free survival rates (follow-up 7.5 years reported by Villa et al. 2013a only) of 47-55% in the autologous group compared to 6-40% in the chemotherapy plus rituximab group. Madsen et al. (2013) reported that patients receiving autologous transplantation had a significantly improved progression free survival compared with those who received chemotherapy plus rituximab (p=0.003).
One retrospective observational study (Madsen et al. 2013) provided very low quality evidence about 5-year progression free survival rates after autologous versus allogeneic transplantation in patients with a primary diagnosis of transformed indolent lymphoma (composite lymphoma). Reporting that the 5-year progression free survival rates in the autologous group (75%) did not significantly differ compared to the chemotherapy plus rituximab group (61%).
4.1.6.1.5. Allogeneic versus chemotherapy plus rituximab
Overall survival
One retrospective observational study (Villa et al. 2013a) provided very low quality evidence about 5-year overall survival rates after allogeneic transplantation versus chemotherapy plus rituximab in 119 patients with histological transformation of indolent lymphoma (100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL). Villa et al. (2013a) reported no significantly difference 5-year overall survival rates (follow-up 7.5 years) in the allogeneic group (46%: standard error: 11) compared to the chemotherapy plus rituximab group (61%: standard error: 7).
Progression free survival
One retrospective observational study (Villa et al. 2013a) provided very low quality evidence about 5-year progression free survival rates after allogeneic transplantation versus chemotherapy plus rituximab in 119 patients with histological transformation of indolent lymphoma (100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL). Villa et al. (2013a) reported no significantly different 5-year progression free survival rates (follow-up 7.5 years) in the allogeneic group (46%: standard error: 11) compared to the chemotherapy plus rituximab group (40%: standard error: 7).
4.1.6.1.6. Autologous transplantation
Overall survival
Two non comparative retrospective observational studies (Eide et al. 2011; Williams et al. 2001) provided very low quality evidence about 5-year overall survival rates after autologous transplantation in 80 patients with histological transformation of indolent lymphoma (91-100% follicular lymphoma) to any high grade lymphoma (76-100% DLBCL). Reporting 5-year overall survival rates (follow-up 4.92 years, reported in Williams et al. 2001 only) of 47-51%.
Progression free survival
Two non comparative retrospective observational studies (Eide et al. 2011; Williams et al. 2001) provided very low quality evidence about 5-year progression free survival rates after autologous transplantation in 80 patients with histological transformation of indolent lymphoma (91-100% follicular lymphoma) to any high grade lymphoma (76-100% DLBCL). Reporting 5-year progression free survival rates (follow-up 4.92 years, reported in Williams et al. 2001 only) of 30-32%.
Response rates
Two non comparative retrospective observational studies (Eide et al. 2011; Williams et al. 2001) provided very low quality evidence about response rates after autologous transplantation in 80 patients with histological transformation of indolent lymphoma (91-100% follicular lymphoma) to any high grade lymphoma (76-100% DLBCL). Complete response rates were 76.3% and partial response rates were 31.3%, with Eide et al. (2011) reporting a relapse rate of 43.3%.
Adverse events
Two non comparative retrospective observational studies (Eide et al. 2011; Williams et al. 2001) provided very low quality evidence about adverse events after autologous transplantation in 80 patients with histological transformation of indolent lymphoma (91-100% follicular lymphoma) to any high grade lymphoma (76-100% DLBCL). Death due to disease progression (follow-up 4.92 years, reported in Williams et al. 2001 only) was reported in both observational studies at a rate of 21.3% with procedure related death reported in one study (Williams et al. 2001) at 18%.
4.1.6.1.7. Exposure to rituximab prior to transplantation
Five retrospective observational studies assessed prior exposure to rituximab and use of transplantation (Ban Hoefen et al. 2013; Calvo-Villas et al. 2011; Muccilli et al. 2009; Villa et al. 2013b; Wirk et al. 2014).
Overall survival
Two non comparative retrospective observational studies (Calvo-Villas et al. 2011; Muccilli et al. 2009) provided very low quality evidence about 5-year overall survival rates after autologous transplantation in 125 patients with histological transformation of indolent lymphoma (100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL, transformation diagnosis not reported in Muccilli et al. 2009). Reporting 5-year overall survival rates (follow-up 61 months, reported in Calvo-Villas et al. 2011) of 36-66.4%. These two studies also reported the overall survival rates according to prior exposure to rituximab, finding that overall surival rates in the autologous with no prior exposure group were between 36-48.2% compared to 51-66.4% in the autologous patients who had prior exposure to rituximab. Calvo-Villas et al. (2011) reported that there was no significant difference between the two groups, however, Muccilli et al (2009) reported a trend for prior exposure to improved overall survival compared to no prior exposure(p=0.11). Wirk et al. (2014) reported that 37% of their sample (n=141) had prior exposure to rituximab finding that exposure prior to transplantation had no impact on overall survival rates in the patients receiving autologous or allogeneic transplantations. Ban Hoefen et al. (2013) reported that 70% of their sample (n=118) had prior exposure to rituximab finding that there was no survival difference based on rituximab exposure prior to transplantation. Villa et al. (2013b) reported that 77% of their sample (n=105) had prior exposure to rituximab finding that there was no survival difference based on rituximab exposure prior to transplantation.
Progression free survival
Two non comparative retrospective observational studies (Calvo-Villas et al. 2011; Muccilli et al. 2009) provided very low quality evidence about 5-year progression free survival rates after autologous transplantation in 125 patients with histological transformation of indolent lymphoma (100% follicular lymphoma) to diffuse large B-cell lymphoma (DLBCL, transformation diagnosis not reported in Muccilli et al. 2009). Reporting 5-year progression free survival rates (follow-up 61 months, reported in Calvo-Villas et al. 2011) of 22-67.2%. These two studies also reported the progression free survival rates according to prior exposure to rituximab, finding that the rates in the autologous with no prior exposure group were between 22-48.4% compared to 55-67.2% in the autologous patients who had prior exposure to rituximab. Calvo-Villas et al. (2011) reported that there was no significant difference between the two groups, however, Muccilli et al (2009) reported a significant difference for prior exposure to improved progression free survival compared to no prior exposure (p=0.04). Wirk et al. (2014) reported that 37% of their sample (n=141) had prior exposure to rituximab finding that exposure prior to transplantation had no impact on overall progression free survival rates in the patients receiving autologous or allogeneic transplantations.
4.1.6.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Consider consolidation with autologous stem cell transplantation for people with transformation of previously diagnosed follicular lymphoma that has responded to treatment and who are fit enough for transplantation. Consider consolidation with autologous or allogeneic stem cell transplantation for people with transformation of follicular lymphoma who need more than 1 line of treatment for a response and who are fit enough for transplantation. Do not offer consolidation with high-dose therapy and autologous or allogeneic stem cell transplantation to people presenting with concurrent diagnoses of follicular lymphoma and diffuse large B-cell lymphoma that have responded to first-line treatment. |
|---|---|
| Relative value placed on the outcomes considered | The critical outcomes when drafting the recommendations included overall survival, progression free survival and toxicity (treatment related morbidity). There was no evidence relating to health related quality of life (HRQoL), patient satisfaction or patient preference or diagnosis at relapse. |
| Quality of the evidence | All the evidence for each outcome was rated very low quality as assessed using GRADE and NICE quantitative checklists. The primary reason for downgrading studies was imprecision due to small sample sizes and low frequency of events. Additionally evidence was downgraded for indirectness due to a mix of populations (five studies used populations of patients with an original diagnosis of any indolent lymphomas not specifically follicular lymphoma; four studies used populations of patients with transformation to any aggressive lymphoma not specifically diffuse large B-cell lymphoma) and there was a lack of clarity regarding whether patients were receiving consolidation therapy receiving first-line therapy or at first response (which might be achieved after multiple lines of therapy). Only one study included patients with a concurrent diagnosis of follicular lymphoma and diffuse large B-cell lymphoma. The definition of transformed lymphoma varied across studies with some studies only including patients for which the transformed diagnosis occurred six months after the initial diagnosis of follicular lymphoma. Because the evidence base concerned different populations of patients who were receiving consolidation therapy after first-line therapy or at first response (which might be achieved after multiple lines of therapy) the GC made separate recommendations according to the following groups:
|
| Trade-off between clinical benefits and harms | The GC thought that the recommendation to use consolidation therapy would optimise survival rates in patients with transformed follicular lymphoma to diffuse large B-cell lymphoma. The evidence base suggested that such patients with either response after first-line immunochemotherapy or first response after multiple-lines of immunochemotherapy stood to benefit from consolidation therapy. The evidence indicated autologous stem cell transplantation was associated with a treatment related mortality of about 3%, due primarily to neutropenic sepsis. Allogeneic stem cell transplantation was associated with considerably higher treatment related mortality. The GC considered that the recommendation to not use consolidation therapy in patients with a diagnosis of concurrent follicular lymphoma and diffuse large B-cell lymphoma would reduce unnecessary treatment related toxicity. For patients with a concurrent diagnosis of follicular lymphoma and diffuse large B-cell lymphoma there was one study, reporting very low quality evidence. The GC expressed their concerns regarding the use of highly toxic consolidation therapies in these patients with no evidence of survival benefit compared to no consolidation therapy and therefore the GC made a ‘do not offer’ recommendation. |
| Trade-off between net health benefits and resource use | No relevant health economic evidence was identified and no health economic model was built for this topic The resource impact associated with the majority of these recommendations was thought to be minimal as they are a consolidation of what is widely regarded to be best practice. However, there could be resource implications where best practice is not currently implemented. In particular, it was thought that there may be reduction in the use of autologous stem-cell transplants (and associated costs) for patients with a diagnosis of concurrent follicular lymphoma and diffuse large B-cell lymphoma. The recommendations were also thought likely to be cost-effective as specified below. In comparison to the alternatives, the recommendation to consider consolidation therapy would increase costs but it would also improve survival rates and it was thought that it would be cost-effective in cost per QALY terms. The recommendation to not offer consolidation therapy in patients with a diagnosis of concurrent follicular lymphoma and diffuse large B-cell lymphoma will reduce costs and will also reduce treatment related toxicity (through a reduction in unnecessary treatment). Thus this recommendation would also be highly likely to be cost-effective and in this case even dominant (cost saving and more effective). |
| Other considerations | The GC noted that the recommendations would lead to a minor change in practice through the reinforcement of current best practice. The GC noted that the recommendations will provide uniformity in practice by reducing uncertainty in which treatment regimen to use in patients with transformed follicular lymphoma. The GC noted that the drafted recommendations are in-line with the EBMT and BSBMT transplantation indication tables. The GC noted there was insufficient evidence about whether biological and clinical factors can be used to identify which patients with high-grade transformation of follicular lymphoma can be treated with immunochemotherapy alone. For this reason they made a research recommendation. |
| Research recommendation | In people with high-grade transformation of follicular lymphoma, which biological and clinical factors predict good outcomes with immunochemotherapy alone? |
|---|---|
| Why this is important | Before rituximab, it was accepted that high-grade transformation of follicular lymphoma to diffuse large B-cell lymphoma portended a poor prognosis. Recent data suggests that although transformation remains an important clinical event, outcomes have improved. It is unclear which people are likely to do well with conventional treatment (such as R-CHOP) and which people may benefit from intensive treatment with, for example, high-dose therapy and autologous stem cell transplantation. Many factors are likely to influence outcome, including clinical factors (such as age, stage at transformation and extranodal involvement at transformation), radiological findings (such as early improvement of disease identified using an interim FDG-PET CT scan) and molecular factors (such as certain driver mutations present at transformation, the presence of MYC translocation and response of circulating tumour DNA to treatment). A better understanding of which factors are associated with high-risk or low-risk disease would enable therapy to be tailored to the person's needs, reducing unnecessary toxicity for people at low risk and reserving intensive therapy for people at high risk. Outcomes of interest include progression-free survival and overall survival in subgroups defined by clinical factors, radiological findings and molecular analyses. |
4.2. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma
4.2.1. First line treatment
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (or MALT lymphoma) is the third most common type of non-Hodgkin's lymphoma in the UK, by annual incidence figures. The stomach is the most commonly involved extra-nodal organ; half of all gastric lymphomas are MALT lymphomas and there is an important association with chronic Helicobacter pylori infection in the majority of gastric MALT cases.
Other sites that may be involved by MALT lymphoma include the salivary glands, orbit, lung, intestinal tract, and thyroid gland, breast tissue, the dura, and genitourinary tract. Autoimmune disease has been linked to the development of non-gastric MALT lymphoma.
MALT lymphomas usually demonstrate an indolent clinical behaviour. Very rarely they may demonstrate features of high-grade histology at the time of initial presentation; transformation may occur throughout the disease course.
Diagnosis is based on history, physical examination, radiology, histopathological and immunohistochemical evaluation of the biopsy specimen, and special molecular laboratory techniques.
Treatment is based on the site of disease and severity of symptoms at presentation. Surgery, radiation therapy, immunotherapy and chemotherapy have all been studied. Unlike many other lymphomas, anti-microbial therapy is an important consideration in H pylori associated gastric lymphomas- eradication therapy is the mainstay of treatment for localised H pylori-positive gastric MALT lymphoma. It remains controversial as to whether other infectious agents may have a pathogenic role in the development of MALT lymphomas at other disease sites.
It may be possible to define a group of patients with disease that is less likely to respond to antibiotic therapy and more likely to require chemo-immunotherapy e.g. Helicobacter pylori-negative patients, tumours with a t(11;18)(q21;q21) translocation and those with disease extending through the sub-mucosa.
The effectiveness of endoscopic follow-up of response to treatment has been reported in many clinical trials. Endoscopy also allows for multiple biopsies to be taken and is generally performed every 3-6 months following the end of treatment for up to two years to assess the response to treatment. For patients with disease localised to the stomach, concomitant follow-up with imaging (e.g. with computerised tomography) offers no additional benefit in the majority of cases.
Response rates to antibiotic therapy can be slow. Therefore, escalation to chemotherapy or radiotherapy may not be necessary unless there are specific risks (extensive disease, significant ulceration).
Clinical question: What is the most effective first-line treatment for people with MALT lymphoma?
4.2.1.1. Clinical evidence (see section 4.2.1 in Appendix G)
Three randomised trials and six retrospective cohort studies provided evidence about chemotherapy, surgery or radiotherapy as first line treatment for MALT lymphoma. Evidence about antibiotic therapy in positive gastric MALT lymphoma came from 36 case series (26 prospective) in patients with helicobacter positive disease and 11 case series (4 prospective) in those with helicobacter negative disease. One randomised trial and 29 observational studies (21 prospective) provided evidence about treatment after antibiotic therapy for gastric MALT lymphoma.
4.2.1.1.1. What is the most effective first-line treatment in patients with MALT lymphoma?
Four observational studies (Kalpadakis et al., 2009; Oh et al., 2010; Papaxoinis et al., 2006; Olszewski et al., 2014) and one randomised control trial (Zucca et al., 2013) assessed the use of chemotherapy, rituximab and radiotherapy as first-line treatment in patients with MALT lymphoma. Overall survival rates ranged from 65-100%.
One observational study (Papaxoinis et al., 2006) compared the use of anthracycline chemotherapy (AC, e.g. CHOP, CEOP, CNOP) to non-anthracycline chemotherapy (C) in 97 patients with MALT lymphoma (in more than 12 body sites) provided very low quality evidence. The study reported complete response rate in the AC group of 73% compared to 68% in the C group. The 5-year progression free survival (AC: 37% versus C: 51%) and overall survival rates (AC: 80% versus C: 65%) were not significantly different between the two groups.
The role of adding rituximab to treatment regimens (chlorambucil, CVP, CHOP, other) was assessed in two retrospective observational studies (Kalpadakis et al., 2009; Oh et al., 2010 [stage IV MALT]) and one randomised control trial (RCT) (Zucca et al., 2013). Zucca et al. (2013) reported a randomised control trial in which 227 patients with MALT lymphoma previously untreated (apart from prior local therapy) were randomly assigned to either receive chlorambucil plus rituximab (n=116) or chlorambucil alone (n=115). With a median follow-up of 62 months the RCT provided low quality evidence for a higher overall response rate (94% versus 87%), complete response rate (78% versus 65%), 5-year event free survival rate (68% versus 50%), 5-year progression free survival rate (71% versus 62%) and a lower partial response rate (16% versus 22%) in the chlorambucil plus rituximab group compared to the chlorambucil only group. However, only the 5-year event free survival rate was significantly different in the two groups (p<0.01).
Kalpadakis et al. (2009) compared the use of chlorambucil plus rituximab compared to chlorambucil alone in 44 patients with MALT lymphoma (7 body sites, no gastric MALT). The study reported very low quality evidence of an overall response rate of 95% in the chlorambucil plus rituximab group compared to 79% in the chlorambucil only group. The other observational study (Oh et al., 2010) compared the use of chemotherapy plus rituximab to chemotherapy alone in 62 patients with MALT lymphoma. Both observational studies reported very low quality evidence of a higher complete response rates and partial response rates in the chlorambucil plus rituximab group (complete response: 61.3-90%; partial response: 22.6%) versus the chlorambucil only group (complete response: 35.5-75%; partial response: 19.4%) with Oh et al. (2010) reporting that the complete response rates were significantly different (p<0.05). The 5-year event-free survival rates were higher in the chlorambucil only group (68%) compared to the chlorambucil plus rituximab group (52%) but this group had lower 10-year progression free survival rates (74% versus 94%) and 5-year overall survival rates (90% versus 100%).
The use of radiotherapy compared to other treatments (predominately surgery) was reported in two observational studies (Olszewski et al., 2014 and Wohrer et al, 2014). Olszewski et al. (2014) reported on over 7000 patients with MALT lymphoma (>10 body sites) using the SEER database. The study reported very low quality evidence of an overall lymphoma related death rate ranging from 0-9.3% in the radiotherapy group compared to 4-12.8% in the other treatments group. Olszewski et al. (2014) reported no significant differences in the treatment groups and an overall relative survival rate at 10 years of 85.7%. Wohrer et al, (2014) reported a retrospective comparison of outcomes according to treatment in a series of 185 patients with extra-gastric MALT. Treatment response ranged from 100% with surgery to 33% with antibiotics, this was very low quality evidence because treatment choice was related to disease stage and site leading to baseline differences in patient characteristics. Five year progression free survival ranged from 68% with surgery to less than 40% with antibiotics.
4.2.1.1.2. Regardless of helicobacter infection, what is the most effective first-line treatment in patients with Gastric MALT lymphoma?
Four studies reported data on the use systemic treatment as first-line treatment in patients with gastric MALT lymphoma. Avilés et al. (2005) reported a randomised control trial in which 241 patients with stage I or IIE (according to the Lugano Conference criteria, 1994) low grade Gastric MALT previously untreated were randomly assigned to either receive radiotherapy (n=78), chemotherapy (n=83) or surgery (n=80). With a median follow-up of 7.5 years (range 4.8-11.6 years) the RCT provided high quality evidence for complete response rates of 100% in each treatment arm. The 10-year event-free survival rate was significantly higher in the chemotherapy arm (87%) compared to the radiotherapy (n=52%) and the surgery (n=52%) arms (p=0.01). In addition, 10-year overall survival was highest in the chemotherapy arm (87%) compared to the radiotherapy (75%) and the surgery (80%) arms (p=0.04). There were no treatment related deaths and late toxicity was reported to be mild in all arms.
One observational study (Amiot et al. 2014) compared the use of alkylating agents to rituximab and chemotherapy plus rituximab in 107 patients with gastric MALT lymphoma. The study reported very low quality evidence of significantly higher overall response rates in the chemotherapy plus rituximab group (100%) compared to the rituximab alone group (73%, p<0.01) ) and the alkylating agents group (68%, p<0.05). The chemotherapy plus rituximab group had higher complete response (92%) compared to the rituximab only group (64%, p<0.05) and the alkylating agents group (66%, p<0.05). In multivariate analysis the 5-year progression free survival rates were higher in the chemotherapy plus rituximab group (89%) compared to the rituximab group (70%) and the alkylating agents group (68%, p<0.04). However, overall survival rates did not differ between the three groups (chemotherapy plus rituximab: 96% versus rituximab: 95% versus alkylating agents: 91%). Toxic events were significantly more frequent in the two groups treated with alkylating agents (p=0.04 for the comparison of alkylating agents versus rituximab and p<0.001 for the comparison of combination therapy versus rituximab).
One observational study (Olszewski et al., 2013) compared the use of radiotherapy and chemotherapy in 347 patients with MALT lymphoma and the use of chemotherapy plus rituximab versus chemotherapy in 102 patients with MALT lymphoma. The study reported very low quality evidence of no difference in lymphoma related death rates between the rituximab (17.7%) and the chemotherapy plus rituximab groups (22.4%) but a significantly lower rate of lymphoma related deaths when comparing radiotherapy (5.3%) to chemotherapy (19.1%, P<0.001). Increased rate of neutropenic infection was reported in the chemotherapy plus rituximab group compared to the rituximab alone group (p<0.01)
One randomised control trial reported the use of systemic treatment as first-line treatment in patients with high grade MALT lymphoma. Aviles et al. (2006) reported a randomised control trial in which 108 patients with stage I or IIE (according to the Lugano Conference criteria, 1994) B-cell CD10+ high grade primary gastric lymphoma (diagnosis according to the criteria of Isaacson, 1994) previously untreated were randomly assigned to either receive combined therapy of surgery and chemotherapy (n=52) or chemotherapy alone (n=50). With a mean follow-up of 88.6 months (range: 61-132 months) the RCT provided high quality evidence for complete response rates in the combined therapy group (94%, 95% CI: 88-99%) that were no different to those in the chemotherapy alone group (96%, 95% CI: 89-100%) (p=0.5). In addition, there were no differences in the 5-year event free survival (combined therapy: 70% versus chemotherapy alone: 67%, p=0.5) and 5-year overall survival rates (combined therapy: 78% versus chemotherapy alone: 76%, p=0.8). There were no treatment related deaths and late toxicity related to surgery was reported to be mild.
4.2.1.1.3. What is the effectiveness of antibiotic therapy in patients with Gastric MALT lymphoma, positive for helicobacter infection?
One systematic review (Zullo et al., 2009) and two observational studies provided evidence from 36 observational studies reporting very low quality evidence for the use of eradication therapy for helicobacter pylori in patients with low graded gastric MALT (and DLBCL-MALT [n=56; 4.7%]) lymphoma positive for the helicobacter pylori infection. The 36 studies (26 prospective and 10 retrospective) provided data from 1495 participants (median sample size = 30, range: 4-189) treated most frequently with a standard triple therapy with a proton pump inhibitor plus two antibiotics twice daily (a combination of two of the following: amoxicillin, clarithromycin, metronidazole/tinidazole), administered for 7-28 days. The pooled overall lymphoma regression rate for the 34 observational studies included in the Zullo et al. (2009) systematic review was 77.8% and in the Zucca et al. (2000) observational study it was 70%. Zucca et al. (2000) and Vrieling et al. (2008) reported complete remission rates of 66.1% and partial remission rates of 13.4%, with Zucca et al. (2000) reporting lymphoma relapse in 7% of their sample (follow-up median: 26 months). Finally, Vrieling et al. (2008) reported a 5-year overall survival rate of 89% in their sample.
4.2.1.1.4. What is the effectiveness of antibiotic therapy in patients with Gastric MALT lymphoma, negative for helicobacter infection?
Eleven observational studies (data extracted from one systematic review: Zullo et al., 2013) reported very low quality evidence for the use of eradication therapy for helicobacter pylori in patients with early stage low grade (I, II) gastric MALT lymphoma negative for the helicobacter pylori infection. The 11 studies (4 prospective multicentre studies, 6 retrospective single-centre studies, 1 case report) provided data from 110 participants, treated with predominately standard triple therapy (10/11 studies), administered for 7-28 days. The majority of studies reported were from Asia (n=8; 72.7%), with the remaining from Europe (n=2; 18.2%) and the United States (n=1; 9.1%). Complete remission rate was 15.5% (17/110). Zullo et al. (2013) extracted data on lymphoma relapse at long-term follow-up in 3 studies (5.5%) with lymphoma relapse reported in 1 patient at 14 months, with the remaining 7 patients still in remission at 25-48 months follow-up.
4.2.1.1.5. What is the effectiveness of antibiotic therapy in patients with Gastric MALT lymphoma, regardless of helicobacter infection status?
Five observational studies reported very low quality evidence for the use of eradication therapy for helicobacter pylori in patients with early stage low grade gastric MALT lymphoma (staging systems reported: Blackledge modified Lugano; Ann Arbor). The 5 studies provided data from 455 participants, treated with predominately standard triple therapy (3/5 studies). The majority of patients were positive for the helicobacter pylori infection (n=279, 79%; H. pylori status was not reported in two studies). Complete remission rates ranging from 64-90% were reported in 4 observational studies (Choi et al., 2013; Park et al., 2010; Stathis et al., 2009; Ueda et al., 2013) and an overall lymphoma regression rate of 73% (Stathis et al., 2009; Yepes et al., 2012) with partial remission rates of 14.2% (Choi et al., 2013; Stathis et al. 2009). Lymphoma relapse was reported in 17% of two samples (Choi et al., 2013; Stathis et al., 2009) with a 10-year overall survival (follow-up median 6.3 years) of 83% (Stathis et al., 2009).
4.2.1.1.6. What is the most effective management strategy for patients with Gastric MALT lymphoma after treatment for helicobacter pylori infection eradication?
No response to antibiotic therapy
One systematic review (Zullo et al., 2010) provided evidence from 29 studies of low quality evidence assessing treatment of low-grade Gastric MALT lymphoma (stage IE1-IE2 or IIE1 according to Ann Arbor classification as modified by Musshof) unresponsive to helicobacter pylori eradication therapy. The 29 studies (21 prospective, 8 retrospective) provided evaluable data from 329 participants, of which 315 underwent oncologic therapy due to lymphoma persistence (successful eradicated patients n=233; infection persistence despite one or more antibiotic therapy n=45; lymphoma relapse at follow-up n=37). A total of 68 (21.6%) received chemotherapy, 112 (35.6%) received radiotherapy; 27 received rituximab (11.6%) and 80 underwent surgery (25.4%). Radiotherapy achieved a significantly higher remission rate (97.3%) compared to chemotherapy (85.3%, p=0.007). Remission rates for surgery (92.5%) were comparable to radiotherapy (p=0.2) and chemotherapy (p=0.2). Following monotherapy, lymphoma remission rate (59.3%) was significantly lower as compared with radiotherapy (p<0.001), surgery (p=0.004) and chemotherapy (p=0.006). When comparing the lymphoma remission rates achieved by a single therapy (overall considered: 287 patients) with that of combined treatments no statistically significant differences emerged (89.6% versus 96.4%, p=0.6). Zullo et al. (2010) report that radiotherapy alone was both the most frequently chosen therapy and the most effective in patients with low grade gastric MALT lymphoma unresponsive to anti-helicobacter therapy. However, Zullo et al. (2010) also reported that of the 329 evaluable patients 14 (4.2%) had a reported remission at follow-up without any further therapy following H. pylori eradiation.
Remission after antibiotic therapy
Hancock et al. (2008) reported a randomised control trial in which 110 stage I patients (Blackledge modified Lugano staging system) successfully treated for H. pylori infection were randomised to receive either chlorambucil (n=56, given for a median of 29 weeks [3-39 weeks]) or to be observed (n=54). The trial was stopped early due to slow recruitment (power calculations required a total of 173 patients). With a median follow-up of 58 months (4-115 months) the RCT reported moderate quality evidence for 5-year recurrence rates of 21% in the observation arm and 11% in the chlorambucil arm (95% CI: 9-29%; p=0.15). In total 22 patients (11 in each) had disease recurrence/progression or died with no difference between the two arms (Hazard Ratio [HR] =0.96, 95% CI: 0.41-2.2; p=0.91). The overall 5-year recurrence/progression free rate for all randomised patients was 79%. There was no overall survival difference between the two arms (HR=1.93, 95% CI: 0.39-9.58; p=0.42) with a 5-year overall survival rate for all randomised patients of 93%. As treatment was accepted as standard treatment in most European countries at the time of the study, toxicity data were not collected in detail without any cases of severe treatment-related toxicity were reported.
One observational study (Kondo et al., 2012) reported the follow-up of 61 patients who had responded to helicobacter pylori eradication therapy. All patients were underwent a watch and wait strategy involving upper gastrointestinal endoscopy, biopsy and abdominal CT every three months in the first year, every 4 months in the second year and at intervals of 6 months in the third year and beyond. With a median follow-up of 78.4 months the study reported very low quality evidence for 5-year overall survival rates of 100% and a lymphoma relapse rate of 14.8%.
4.2.1.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Gastric MALT lymphoma: localised disease Offer 1 or more lines of Helicobacter pylori eradication therapy, without any concurrent therapy, to people with H. pylori-positive gastric MALT lymphoma. (see the NICE guideline on gastro-oesophageal reflux disease and dyspepsia in adults). Consider H. pylori eradication therapy for people with H. pylori-negative gastric MALT lymphoma. (see the NICE guideline on gastro-oesophageal reflux disease and dyspepsia in adults). Consider ‘watch and wait’ (observation without therapy) for people with gastric MALT lymphoma that responds clinically and endoscopically to H. pylori eradication therapy but who have residual disease shown by surveillance biopsies of the stomach, unless high-risk features are present. For people with residual MALT lymphoma after H. pylori eradication therapy who are at high risk of progression [H. pylori-negative at initial presentation or t(11:18) translocation], consider a choice of the following, in discussion with the person:
Offer H. pylori eradication therapy to people with disseminated H. pylori-positive gastric MALT lymphoma (see the NICE guideline on gastro-oesophageal reflux disease and dyspepsia in adults). Offer chemotherapy (for example, chlorambucil or CVP) in combination with rituximabd to people with disseminated gastric MALT lymphoma who need treatment; for example, people who are symptomatic or with threatened vital organ function. Consider ‘watch and wait’ (observation without therapy) for people with disseminated gastric MALT lymphoma who are asymptomatic and do not have threatened vital organ function. Non-gastric MALT lymphoma For people with non-gastric MALT lymphoma, take into account the following before recommending any treatment: site of involvement and potential for organ dysfunction
Consider radiotherapy for people with localised disease sites of non-gastric MALT lymphoma, irrespective of stage. Consider ‘watch and wait’ (observation without therapy) for people with clinically non-progressive localised non-gastric MALT lymphoma that is unlikely to result in vital organ dysfunction, who are asymptomatic and for whom radiotherapy is not suitable. |
|---|---|
| Relative value placed on the outcomes considered | The GC considered progression free survival, toxicity (treatment related morbidity), response to first line helicobacter pylori eradication therapy, and overall survival to be the critical outcomes for this topic. Survival rates and level of toxicity associated with systemic therapies are of particular importance to people with MALT lymphoma. Response to first-line helicobacter pylori eradication therapy was used by the GC to assess the need for systemic therapies in patients with gastric MALT lymphoma. Health related quality of life (HRQoL) was considered an important outcome though no evidence was identified. |
| Quality of the evidence | The quality of the evidence ranged from very low to high quality for individual outcomes as assessed using GRADE. Evidence was downgraded for imprecision, indirectness and for study limitations. Specific issues with the evidence included:
|
| Trade off between clinical benefits and harms | Patients with non-gastric MALT lymphoma Due to lack of high quality evidence (small sample sizes) for antibiotic therapy for treatment of non-gastric MALT the GC were unable to make a recommendation. There was a lack of high quality evidence relating to patients with asymptomatic, non-progressive, localised disease that is unlikely to produce vital organ dysfunction and patients with localised symptomatic disease sites of non-gastric MALT irrespective of stage which meant the GC could not make strong recommendations. Despite this lack of evidence, the GC considered it important to make recommendations for this patient group around observation and treatment of this patient group. Despite a lack of available high quality evidence to recommend chemotherapy for patients for whom radiotherapy is unsuitable or patients with disseminated non-gastric MALT who require treatment, the GC made a strong recommendation for the use of chemotherapy. This was because there was no other treatment available so it was important that a recommendation on the use of chemotherapy was included for this patient group. Chlorambucil and CVP were given as examples of chemotherapy based on the evidence of their effectiveness. The evidence also indicated that the combination of chemotherapy with rituximab was more effective than chemotherapy alone. Patients with Gastric MALT lymphoma The GC made a strong recommendation for helicobacter pylori antibiotic eradication therapy in all patients with gastric MALT lymphoma because they thought it was important to reduce the use of toxic systemic therapies in some of these patients. There was evidence for the use helicobacter eradication therapy in patients with gastric MALT lymphoma positive for helicobacter pylori. However, the evidence base for the use of helicobacter eradication therapy in patients with gastric MALT lymphoma negative for helicobacter pylori was limited but suggested that in these patients around 15% will not require further treatment with systemic therapies. In addition, the GC considered that the detection of helicobacter pylori can vary depending on the diagnostic test used therefore, the GC used their clinical judgement to make a recommendation to use helicobacter eradication therapy in patients with gastric MALT lymphoma negative for helicobacter eradication therapy (in case this indicates a false negative). In patients with gastric MALT lymphoma who received antibiotic therapy, the GC considered that the recommendation for these patients needed to include assessment of response to antibiotic therapy in order to inform further treatment in these patients. However as the question had not investigated which assessment strategy (e.g., endoscopy, and imaging) was the most effective, the GC recommended endoscopy on the basis that the majority of the included evidence appraised used endoscopy to assess response to antibiotic therapy, and in their clinical opinion endoscopy is the gold standard for assessing response in these patients. The use of toxic systemic therapies is associated with treatment related morbidity and toxic side effects, and while the GC acknowledged that for some patients this is unavoidable due to the requirement for toxic systemic therapies, they considered that the recommendations for patients with gastric MALT lymphoma will reduce the number of patients needing to receive toxic systemic treatment overall. Specifically, the GC thought that the recommendation to use helicobacter antibiotic eradication therapy in all patients with gastric MALT would result in a reduction in the need for upfront toxic systemic therapies in some of these patients due to the high lymphoma regression rates after the eradication therapy. The GC acknowledged that in patients with gastric MALT lymphoma who have received helicobacter eradication therapy but no systemic therapy, there might be an increase in psychological distress associated with expectant management of the lymphoma. The GC suggested that a better defined treatment pathway for all patients with MALT lymphoma may help to negate any negative psychological impact of expectant management. The GC considered that in patients with gastric MALT lymphoma who receive helicobacter eradication therapy but do not respond or have progression in their lymphoma resulting in a need for systemic therapies, there was no evidence to suggest that the delay in starting intensive systemic therapies as a result of undergoing helicobacter eradication therapy first, is unlikely to impact on overall survival rates. |
| Trade off between net health benefits and resource use | No relevant health economic evidence was identified and no health economic model was built for this topic The resource impact associated with the majority of these recommendations was thought to be minimal as they are a consolidation of current practice. However, the recommendations to use antibiotic therapy differ from some current clinical recommendations and so there could be resource implications. However, it was thought that the use of antibiotic therapies would most likely be cost-effective because of its relatively low upfront cost as well as the resulting reduction in the the use of systemic therapies that would be expected. |
| Other considerations | The GC thought that the recommendations would eliminate variation in practice by providing a better defined treatment pathway for patients with all types of MALT lymphoma. The GC thought that the recommendations would consolidate current practice, providing clarity on the treatment pathway in the patient populations. The GC noted that there will need to be a re-organisation of practice for the treatment of patients with gastric MALT lymphoma and there will be a minor change in practice through the reinforcement of current best practice for the treatment of patients with non-gastric MALT lymphoma. The GC noted that the recommendations about the use of antibiotic therapy in patients with gastric MALT lymphoma negative for helicobacter pylori infection differ from current clinical recommendations (e.g. American Society for Haematology). However, the GC considered that the recommendations are justified considering the current evidence base, clinical opinion, and the low cost of antibiotic therapy. |
- b
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information.
- cc
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information.
- d
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information.
- e
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information.
4.3. Mantle cell lymphoma
Mantle cell lymphoma (MCL) accounts for 5-10% of NHL diagnoses, occurring predominantly in people over the age of 50 years. Historically MCL has been considered to combine adverse features of both low grade and high grade NHL in that cure is elusive despite attainment of apparent complete clinical responses following immunochemotherapy, but clinical progression is often relatively aggressive. Most patients present with advanced disease (stage IV), and bone marrow involvement is common. Median overall survival with immunochemotherapy is between 3 and 4 years. MCL is a distinct type of B-cell lymphoma genetically characterised by the t(11;14) translocation and cyclin D1 over-expression in the majority of cases. Although the median overall survival of patients has improved MCL is still has one of the poorest outcomes among the B-cell lymphomas
4.3.1. First line treatment
There is no accepted standard of care for patients with mantle cell lymphoma (MCL). The paucity of randomised control data, the relative infrequency of this lymphoma subtype, historical problems in identifying this entity correctly and finding trials with only MCL patients included have all contributed to this.
The majority of patients have advanced stage disease and require systemic treatment. The regimens that have been studied are mostly similar to those used in other B-cell lymphomas-chemotherapy with or without rituximab. In everyday practice the choice of therapy often depends on whether the patient is fit and considered for intensification with high-dose chemotherapy and autologous stem cell transplantation (ASCT). Several groups have demonstrated excellent activity of cytarabine (cytosine arabinoside)-based combinations, admittedly with greater toxicity than other chemotherapy options.
A small number of patients present with limited stage disease and are frequently considered for radiotherapy. There is also an ‘indolent’ form of MCL which may be indentified clinically.
It may be that newer agents will have a profound impact on the first-line treatment of MCL, on the basis of results of phase 1 studies reported in relapsed MCL patients. As mentioned, recommendations at this point in time are likely to be dependent on factors such as patient fitness, the MCL prognostic index and the intention of therapy.
Clinical question: What is the most effective first-line treatment for people with mantle-cell lymphoma?
4.3.1.1. Clinical evidence (see section 4.3.1 in Appendix G)
Evidence came from seven randomised controlled trials, 12 cohort studies (one prospective) and one retrospective case series.
4.3.1.1.1. Chemotherapy regimens
CHOP
One randomised control trial (RCT; evidence appraised at two time points: Lenz et al. 2005 and Hoster et al. 2008) comparing the use of CHOP+rituximab (RCHOP) to the use of CHOP alone in 123 patients with stage III/IV mantle cell lymphoma provided low quality evidence of higher response rates in the patients treated with RCHOP (complete: 33%, complete plus partial: 92%) compared to the patients treated with CHOP alone (complete: 8%, complete plus partial: 75%, p<0.05). The patients treated with RCHOP had a longer median time to treatment failure (28 months) and response duration (29 months) compared to the patients treated with CHOP alone (14 months, p<0.001; 29 months, p<0.01). However, there was no statistically significant difference in the 5 year overall survival rates (RCHOP: 59%, median not reached; CHOP: 46%, 59 months). Patients treated with RCHOP had higher rates of grade 3 and 4 granulocytopenia (63% versus 53%, p<0.01) and grade 1 and 2 allergic reactions (6% versus 0%, p<0.0001) compared to the patients treated with CHOP alone.
One observational comparative study (Bernard et al. 2001) compared the use of CHOP to C-VAD, CVP and Chlorambucil in 33 patients with blastic mantle cell lymphoma (85% stage IV, median age: 62, range: 29-80), reporting very low quality evidence of complete response rates of 57.9% in the CHOP group compared to 14.3% in the C-VAD group and 0% in the CVP and Chlorambucil groups. Treatment failure rates were 21.1% in the CHOP group, 71.4% in the C-VAD group, 75% in the CVP group and 100% in the Chlorambucil group. The patients in the CHOP group had a 90.9% rate of relapse after complete response. No statistical analyses were presented to compare the response rates in these patients.
One observational comparative study (Ying et al. 2012) compared the use of rituximab+CHOP (RCHOP) to conventional chemotherapy regimens in 30 patients with stage I-IV mantle cell lymphoma reporting very low quality evidence of uncertainty concerning any survival benefit of the addition of rituximab to CHOP (2 year progression free survival: 53%; 2 year overall survival: 59%) compared to those patients treated with conventional chemotherapy regimens not containing rituximab (PFS: 25%, p=0.083; OS: 72%, p=0.807). Response rates for the two groups did not differ significantly.
DHAP
Very low quality evidence from one phase II trial (Le Gouil et al. 2010) indicated an overall response rate of 92% and a complete response rate of 51% in 63 patients with mantle cell lymphoma (median age: 57 years, range: 30-65; 77% stage IV). One RCT (Hermine et al. 2012; 2013) comparing the use of CHOP+DHAP+rituximab+ARA-C versus the use of CHOP+rituximab in 455 patients with stage III-IV mantle cell lymphoma (median age 55 years, whole sample ≤65 years old) provided moderate quality evidence of significantly higher complete response rates of 36% in the CHOP+DHAP+rituximab+ARA-C compared to 25% in the CHOP+rituximab arm (p=0.012) but no difference in overall response rates (95% versus 90%), nor relapse rates after response (40% versus 81%). The patients treated with CHOP+DHAP+rituximab+ARA-C had significantly longer time to treatment failure rates (88 months versus 46 months: p=0.038) and better overall survival rates (median not reached versus 88 months; p=0.045) compared to patients treated with CHOP+rituximab (median follow-up of 51 months). Adverse events were comparable in the two groups with the exception of grade 3/4 haematological toxicity, which were higher in the CHOP+DHAP+rituximab+ARA-C compared to the CHOP+rituximab group (hemoglobin; white blood count; platelets: 30%; 75%; 74% versus 9%; 50%; 10%, no p values presented to assess significance).
FC
One RCT (Rule et al. 2011) comparing the use of FC+rituximab (FCR: Fludarabine, Cyclophosphamide) versus the use of FC alone in 370 patients with mantle cell lymphoma (median age: 66 years, range: 36-88) reported moderate quality evidence for better complete and overall response rates in patients treated with the addition of rituximab (complete: 64.7% versus 46.9%, p<0.01; overall: 90.6% versus 79.8%, p<0.01). There was no difference in progressive disease rates between the two groups (FCR: 5.8% versus FC: 11.9%). The patients treated with the addition of rituximab had significantly longer progression free (30.6 months versus 16.1 months: hazard ratio [HR]: 0.56, 95% confidence interval [CI] 0.43-0.73, p<0.001) and overall survival (45.7 months versus 37 months: HR: 0.72, CI: 0.54-0.97, p<0.05) rates (median follow-up 38.8 months) compared to those patients treated with FC alone.
One RCT (Kluin-Nelemans et al. 2012) comparing the use of FCR to CHOP+rituximab (RCHOP) in 485 patients with stage II-IV mantle cell lymphoma (median age: 66 years, range: 60-87) provided moderate quality evidence for higher overall response rates in the patients treated with RCHOP (86.2%) compared to the patients treated with FCR (78%) and lower rates of progressive disease (5% versus 14%) but higher complete response rates in the FCR group (39.8%) compared to the RCHOP group (33.9%). However, none of these comparisons were significantly different. Patients treated with RCHOP did have significantly higher overall survival rates (62%) compared to the patients treated with FCR (47%; HR: 1.50, CI: 1.13-1.99, p=0.005) (median follow-up 37 months). Rates of grade 1 and 2 anemia, leukocytopenia, constipation and neuropathy were higher in the RCHOP group (68%; 29%; 28%; 36%) compared to the FCR group (59%; 18%; 15%; 7%: p<0.05). Rates of grade 1 and 2 elevated bilirubin and nausea were higher in the FCR group (15%; 36%) compared to the RCHOP group (8%; 26%, p<0.05). Rates of grade 3 and 4 anaemia and leukocytopenia were higher in the FCR group (20%; 73%) compared to the RCHOP group (12%; 59%, p<0.05)
MCP
One RCT (Nickenig et al. 2006) comparing the use of MCP (Mitoxantrone, Chlorambucil and prednisolone) versus CHOP in 86 patients with stage III/IV mantle cell lymphoma (median age: 61, range: 35-79) provided low quality evidence of no difference between response rates (complete: 20% versus 15.2%; overall: 72.5% versus 87%) and treatment failures (90% versus 80.4%) in the patients treated with MCP versus those treated with CHOP. There was no significant difference in the 5-year time to treatment failure (MCP: 9% [CI: 0-19] versus CHOP: 20% [8-32], p=0.08) nor the overall survival rates (MCP: 48 months, 31% [CI: 15-47] versus CHOP: 61 months, 57% [43-72], p=0.058).
One RCT (Herold et al. 2007) comparing the use of MCP+rituximab (RMCP) versus MCP alone in 90 patients with mantle cell lymphoma (median age not reported) provided very low quality evidence of no difference between the two groups with regards to complete (RMCP: 31.8% versus MCP: 15.2%, p=0.082) and overall (RMCP: 70.5% versus MCP: 63%, p=0.51) response rates and progression free survival (RMCP: 20.5 months, 31% versus MCP: 19 months 14%, p=0.25), event free survival (RMCP: 19 months, 27% versus MCP: 14 months 11.5%, p=0.14) and overall survival rates at 42 months (RMCP: 56 months, 60% versus MCP: 50 months 61%, p=0.49) (median follow-up: 43 months).
FLU
One RCT (Zinzani et al. 2000) comparing the use of FLU-ID (Fludarabin and Idarubicin) to FLU alone in 29 patients with stage II-IV mantle cell lymphoma (median age not reported) provided low quality evidence of uncertainty in the value of adding Idarubicin to the regimen, with no difference in response rates (complete: FLU-ID: 33.3% versus FLU: 27.3%; FLU-ID: 27.8% versus FLU: 45.5%) or relapse rates after complete response (FLU-ID: 16.7% versus FLU: 33.3%) (median follow-up: 19 months). There were no fatalities resulting from drug-toxic effects.
R-HyperCVAD
Three observational comparative studies (LaCasce et al. 2012; Udvardy et al. 2012; Miura et al. 2011) compared the use of R-HyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, high dose methotrexate and cytarabine) to R-CHOP in 197 patients with stage I-IV mantle cell lymphoma (age range: 28 to >60). Two studies (Udvardy et al. 2012; Miura et al. 2011) provided very low quality evidence of higher complete response rates in the patients receiving R-HyperCVAD (80%) compared to the patients receiving RCHOP (42.3-49%, p<0.05 in the Miura et al. 2011 study). One study (LaCasce et al. 2012) provided very low quality evidence of lower progressive disease in the patients receiving R-HyperCVAD (37%) compared to the patients receiving RCHOP (72% relative risk: 0.52, CI: 0.36-0.74). Progression free survival was reported to be not significantly different in the Miura et al. (2011) study but significantly higher in the R-HyperCVAD group (58% [CI: 44-69]) compared to the RCHOP group (18% [CI: 6-36]) in the LaCasce et al. (2012) study (p<0.01). Overall survival rates between the two groups did not differ significantly in both the Miura et al. (2011, HR: 0.81, CI: 0.23-2.24) and the LaCasce et al. (2012, p=0.07) studies. Udvardy et al. (2012) reported that adverse events were significantly higher in R-HyperCVAD group (91.6%) compared to the RCHOP group (55.5%, p<0.05). However, LaCasce et al. (2012) reported no significant difference between the two groups concerning rates of febrile neutropenia or the rates of complications requiring hospital admission.
Nordic MCL2
One observational comparative study (Abrahamsson et al. 2014) compared seven chemotherapy regimens (CHOP, CHOP/cytarabine, FC, Chlorambucil, cytarabine, CVP, other) to the Nordic MCL2 regimen in 1015 patients with stage I-IV mantle cell lymphoma (median age: 70, range: 28-95) reporting low quality evidence of a poorer survival rate for the patients treated with CVP compared to patients treated with the Nordic MCL2 regimen (p<0.001).
Addition of rituximab to chemotherapy regimens
Three observational comparative studies (Leux et al. 2014, Kang et al. 2014, Griffiths et al. 2011) assessed the addition of rituximab to chemotherapy regimens in 897 patients with stage I-IV mantle cell lymphoma (age range: 26-78). Two studies reported an overall survival benefit from the addition of rituximab. Griffiths et al. (2011) provided low quality evidence that the addition of rituximab was associated with significantly lower cancer mortality rates at 2 years (HR for cancer mortality: 0.39, 95% CI: 0.23-0.67, p<0.001) but not non-cancer mortality rates (p=0.77). Patients treated with the addition of rituximab were more likely to be alive two years after beginning their first-line therapy (63%) compared to patients treated with chemotherapy alone (52%, p<0.001). Leux et al. (2014) provided very low quality evidence that patients treated with chemotherapy + rituximab had higher median overall survival rates (42 months) compared to those treated with chemotherapy alone (24 months, HR: 0.5, 95% CI: 0.1-0.7). However, Kang et al. (2014) provided very low quality evidence of uncertainty in the survival benefit for patients treated with rituximab regimens compared to those treated with non-rituximab containing regimens (Event free survival HR: 1.60, 95% CI: 0.93-2.75; Overall survival HR: 0.89, 95% CI: 0.51-1.54).
One observational comparative study (Abrahamsson et al. 2014) compared the addition of rituximab to eight chemotherapy regimens (Nordic MCL2, CHOP, CHOP/cytarabine, FC, Chlorambucil, cytarabine, CVP, other) to the Nordic MCL2 regimen in 1015 patients with stage I-IV mantle cell lymphoma (median age: 70, range: 28-95) reporting low quality evidence of a higher survival rate for the patients treated with regimens that included rituximab compared to patients treated with chemotherapy alone (p<0.001).
4.3.1.1.2. Radiotherapy
One observational comparative study (Leitch et al. 2003) compared the use of radiotherapy to no radiotherapy in 26 patients with stage I-II mantle cell lymphoma (median age not reported, <60:7; ≥60: 19) reporting very low quality evidence of a 5-year progression free survival benefit in patients receiving radiation therapy (73%) compared to those patients who received no radiation therapy (13%, p<0.05). Overall survival and response rates were not significantly different between the two groups (median follow-up time: 59 months, range: 5-85).
One observational comparative study (Dabaja et al. 2014) compared the use of radiotherapy and chemotherapy to either treatment alone in 160 patients with stage I-II mantle cell lymphoma (median age not reported ≤60: 70. >60: 90) reporting very low quality evidence of no survival benefit when combining the two treatments (10 year disease free survival rate: 44%; 10 year overall survival rate: 61%) compared to chemotherapy alone (DFS: 40%; OS: 70%) and radiotherapy alone (DFS: 54%, p=0.44; OS: 56%, p=0.68) (median follow-up time: 60 months, range: 4-245).
One observational study (Abrahamsson et al. 2014) provided low quality evidence of a 3 year overall survival rate of 93% in 43 patients with stage I-II mantle cell lymphoma receiving radiotherapy.
4.3.1.1.3. Watch and wait
One observational comparative study (Martin et al. 2009) compared 97 patients with stage I-IV mantle cell lymphoma receiving early treatment to those undergoing watch and wait (median age not reported, range: 40-89). With a median follow-up time of 42.5 months in the early treatment group and 55 months in the watch and wait group, the study provided very low quality evidence of a median overall survival rate of 64 months (CI: 45-85) in the early treatment group with the median overall survival rate not yet reached the watch and wait (p=0.004).
One observational study (Abrahamsson et al. 2014) provided low quality evidence of a 3 year overall survival rate of 79% in 29 patients with stage IV mantle cell lymphoma undergoing watch and wait.
4.3.1.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Offer chemotherapy in combination with rituximabf as first-line treatment for people with advanced-stage mantle cell lymphoma who are symptomatic. Take the person's fitness into account when deciding on the intensity of chemotherapy. Consider cytarabineg-containing immunochemotherapy for people with advanced-stage mantle cell lymphoma who are fit enough to tolerate an intensive approach. Consider radiotherapy for people with localised stage I or II mantle cell lymphoma. Consider ‘watch and wait’ (observation without therapy) until disease progression for people with clinically non-progressive mantle cell lymphoma who are asymptomatic and for whom radiotherapy is not suitable. The guideline committee did not assess evidence or develop recommendations on bendamustine for treating people with mantle cell lymphoma, because a NICE technology appraisal on ‘the clinical and cost effectiveness of bendamustine in combination with rituximab within its licensed indication for the first-line treatment of mantle cell lymphoma’ was is development. This technology appraisal is currently suspended. |
|---|---|
| Relative value placed on the outcomes considered | The critical outcomes for this topic included overall survival and treatment toxicity. Treatment toxicity was considered critical as it may impact on subsequent treatments. Although not listed in the review protocol, evidence was included for response rate. The GC considered this evidence useful as it is likely to be associated with better patient outcomes. |
| Quality of the evidence | The quality of the evidence for this topic ranged from very low to moderate for all outcomes as assessed using GRADE. Evidence was downgraded for indirectness, limitations in study design and imprecision. Specific issues with the evidence included:
The GC considered that although a recommendation on radiotherapy was necessary and supported by the evidence; the lack of high quality evidence from randomised trials precluded the inclusion of a strong recommendation. |
| Trade off between clinical benefits and harms | The GC considered the radiotherapy recommendation would spare patients toxicity of chemotherapy while offering a similar or, in some cases, better progression free survival. The GC considered this recommendation for watch and wait would delay the need for chemotherapy in this group of patients without compromising their outcomes. The GC acknowledged that some patients may experience more anxiety with watch and wait programs. When discussing the recommendation for rituximab in combination with chemotherapy the GC acknowledged that the evidence showed that treatment response, progression free survival and overall survival were improved by clinically significant amounts in patients treated with rituximab in combination with chemotherapy when compared to chemotherapy alone. The GC considered the potential harm of rituximab is that it is additionally immunosuppressive, although the published evidence suggested adding rituximab would increase the rate grade 3-4 infections or allergic reactions by around 1%. Based on the evidence the GC considered the primary benefit of cytarabine regimens is the better response rates (with approximately 10% more responders) although they did acknowledge that cytarabine has higher treatment related toxicity (particularly grade 3/4 haematological toxicity) than other appraised regimens. Overall the GC thought that the benefits associated with each of the individual treatment recommendations offset the potential harms, which the GC considered manageable. The guideline committee did not assess evidence or develop recommendations on bendamustine for treating people with mantle cell lymphoma, because a NICE technology appraisal on ‘the clinical and cost effectiveness of bendamustine in combination with rituximab within its licensed indication for the first-line treatment of mantle cell lymphoma’ was is development. This technology appraisal is currently suspended. |
| Trade off between net health benefits and resource use | No relevant health economic evidence was identified and no health economic model was built for this topic, The GC thought that the recommendations reflected current practice and so no resource impact was expected. In addition, the recommendations were all thought likely to be cost-effectiive for the reasons specified below: The recommendation to offer rituximab in combination with chemotherapy is thought to be cost-effective. In comparison to chemotherapy alone, it will be more costly because of the addition of the rituximab which is estimated to cost £1,222.41 per dose. However the evidence showed that the addition of rituximab improves treatment response, progression free survival and overall survival by clinically significant amounts. These improvements would be expected to translate into greater effectiveness in QALY terms. The GC thought that it was highly likely that the strategy would be cost-effective in cost per QALY terms. This view is supported by cost-effectiveness analyses undertaken as part of NICE technology appraisals on the addition of rituximab to chemotherapy regimens in other NHL subtypes (such as TA243 on the use of rituximab for the first-line treatment of stage III-IV follicular lymphoma). The recommendation to consider cytarabine regimens is thought likely to be cost-effective because of the better effectiveness associated with these regimens at a comparable cost to the alternative chemotherapy regimens. The recommendation to consider radiotherapy in localised stage I or II mantle cell lymphoma is expected to be cost-effective. This is because radiotherapy should be less costly than chemotherapy (the alternative) while being at least equivalent and possibly superior in effectiveness terms (as it has a similar or, in some cases, superior PFS without the toxicitiy of chemotherapy). The recommendation to consider watch and wait is expected to be cost-effective as the need for costly chemotherapy should be delayed without compromising effectiveness. |
| Other considerations | A number of ongoing NICE technology appraisals (Lymphoma (mantle cell, relapsed, refractory) - lenalidomide [ID739] and Lymphoma (mantle cell, relapsed, refractory) - ibrutinib [ID753]) meant that the GC did not make a strong recommendation for cytarabine and they did not recommend specific, named regimens. |
- f
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information.
- g
At the time of publication (July 2016) cytarabine did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information.
| Recommendation | Bortezomib is recommended, within its marketing authorisation, as an option for previously untreated mantle cell lymphoma in adults for whom haematopoietic stem cell transplantation is unsuitable. [This recommendation is from Bortezomib for previously untreated mantle cell lymphoma (NICE technology appraisal guidance 370).] |
|---|---|
| These recommendations are from Bortezomib for previously untreated mantle cell lymphoma (NICE technology appraisal guidance 370). They have been incorporated into this guideline in line with NICE procedures for developing clinical guidelines, and the evidence to support these recommendations can be found at www |
4.3.2. Consolidation therapy in mantle cell lymphoma
Since more intensive induction regimens are associated with higher overall response rates, strategies involving consolidation of first response with high-dose therapy followed by autologous transplantation (ASCT) have been investigated. This improves median overall survival >10 years. This approach has therefore become accepted standard of care for those deemed eligible for ASCT. Nevertheless, late relapses beyond 5 years do occur, with no clear plateau on survival curves suggestive of definitive cure. Furthermore, patient groups with worse prognoses can be identified, for example, those with high MIPI-B (mantle cell lymphoma international prognostic index-biological) scores have 10-year overall survival rates of <25%.
Treatment of mantle cell lymphoma with allogeneic stem cell transplantation (alloHCT) has been reported since the late 1990s, mostly in small series, in an attempt to define whether a graft-versus-lymphoma effect is present and can translate into the potential for cure. The introduction of reduced intensity conditioning strategies broadened availability to the generally older patient population with mantle cell lymphoma. More recent studies do suggest the possibility of cure in a portion of patients, but experience remains limited, and toxicities are not insignificant. AlloHCT have frequently been employed later in the disease process, for example following failure of ASCT, with more limited data in first-line usage. Given the higher procedural mortality associated with alloHCT, and the improved overall survival seen following the introduction of ASCT as a consolidation for first-line responses, significant controversy exists over any role in first line treatment strategies. Whilst an argument can be made for a role in patients with high MIPI/MIPI-B scores, or those with less than a complete response to induction, the ability of alloHCT to overcome these adverse prognostic features remains uncertain
Clinical question: What is the effectiveness of first-line consolidation of high-dose therapy with autologous or allogeneic transplantation in people with mantle-cell lymphoma?
4.3.2.1. Clinical evidence (see section 4.3.2 in Appendix G)
Evidence came from one randomised controlled trial and eleven retrospective cohort studies.
4.3.2.1.1. Progression free survival
Upfront consolidation with autologous stem-cell transplantation (ASCT) compared to no consolidation or maintenance therapy significantly improved progression free survival rates in patients with mantle cell lymphoma
One RCT (Dreyling et al. 2005) reported moderate quality evidence of a longer median progression free survival in 62 patients with mantle cell lymphoma receiving myeloablative radio-chemotherapy (12Gy) and ASCT (39 months, 54%) compared to 60 patients receiving interferon-α maintenance therapy (17 months, 25%) (p=0.01). Assessing sub-group analyses by induction therapies the author notes that the difference in progression free survival no longer remained significant when only assessing patients treated with R-CHOP as their induction therapy (p=0.73). Lenz et al. (2005) reported very low quality evidence of a significant progression free survival benefit of any consolidation therapy (ASCT or interferon-α) in 85 patients with mantle cell lymphoma compared to no post remission treatment in 8 patients with mantle cell lymphoma (p=0.0002).
Five retrospective comparative reviews provided very low quality evidence of a progression free survival benefit in 168 patients with mantle cell lymphoma receiving induction therapy and ASCT compared to 129 patients receiving induction therapy alone (Nastoupil et al. 2015; Frosch et al. 2015; Ahmadi et al. 2012; Schaffel et al. 2009; Hicks et al., 2006).
4.3.2.1.2. Overall survival
The value of consolidation with ASCT on overall survival rates in patients with mantle cell lymphoma varied between studies.
Dreyling et al. 2005 reported moderate quality evidence of no difference in the 3-year estimated overall survival rates in 122 patients with stage II-IV mantle cell lymphoma randomised to receive ASCT or interferon-α (p=0.18). When comparing consolidation to no further therapy two retrospective comparative studies provided very low quality evidence of no overall survival benefit of ASCT (Nastoupil et al., 2015; Schaffel et al., 2009) whereas four retrospective comparative studies provided very low quality evidence of an overall survival benefit of ASCT (Abrahamsson et al., 2014; Vose et al., 2012; Fieldman et al., 2010; Hicks et al., 2006). However, Fieldman et al. (2010) reported that ASCT provided an overall survival benefit only when comparing to patients treated with chemotherapy and rituximab and not when compared to patients treated with R-HyperCVAD. Finally, Cortelazzo et al. (2007) reported an increased overall survival rate in patients treated with doxorubicin or cisplatin, rituximab and ASCT compared to patients treated with Anthracycline or fludarabine alone but they did not report significance levels for these comparisons (conference abstract).
4.3.2.1.3. Adverse events
The majority of studies did not report any information concerning adverse events following ASCT. Dreyling et al. (2005) reported moderate quality evidence of a higher incidence of grade III and IV adverse events (e.g. Mucositis, anaemia, leukocytopenia, granulocytopenia, thrombocytopenia) in 60 patients treated with interferon-α compared to 62 patients treated with ASCT. However, patients treated with ASCT had a higher rate of infection related mortality (5%) compared to the patients treated with interferon-α (0%) (P value not reported). Nastoupil et al. (2015) and Mangel et al. (2004) provided very low quality evidence of no treatment related deaths in patients in their studies and Cortelazzo et al. (2007) reported 1.3% in their patients treated with ASCT compared to 0.8% in the patients receiving anthracycline or cyclophosphamide-fludarabine alone. Mangel et al. (2004) provided very low quality evidence of high rates of neutropenia (90%) and mucositis (60%) and moderate rates of pneumonititis (30% after ASCT) in patients treated with ASCT and rituximab maintenance but provided no comparison to the case controls who received induction therapy only. Finally, Frosch et al. (2015) provided very low quality evidence of significantly higher adverse events in patients treated with both R-HyperCVAD induction and ASCT (median 4) compared to R-CHOP and ASCT (median: 2, p=0.007), R-HyperCVAD alone (median: 1, p=0.008) and R-CHOP alone (median: 1.5, p=0.016)
There was no evidence to assess the effectiveness of upfront consolidation with allogeneic transplantation in patients with mantle cell lymphoma.
4.3.2.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendation | Consider consolidation with autologous stem cell transplantation for people with chemosensitive mantle cell lymphoma (that is, there has been at least a partial response to induction chemotherapy) who are fit enough for transplantation. |
|---|---|
| Relative value placed on the outcomes considered | Progression free survival was the critical outcome when drafting the recommendation. Other important outcomes for this topic included overall survival, disease free survival, progression free survival, treatment related mortality, treatment related morbidity and health related quality of life. No evidence for health related quality of life was identified. |
| Quality of the evidence | The quality of the evidence was very low to moderate as assessed using GRADE methodology. Evidence was downgraded for imprecision, study design limitations and indirectness. Apart from one randomised trial comparing autologous transplantation with interferon- α, the evidence came from non-randomised, comparative studies. For this reason the guideline committee were not able to make a strong recommendation. |
| Trade off between clinical benefits and harms | The GC thought the recommendation to consider autologous transplantation would prolong progression free survival; the evidence suggested a median progression free survival improvement of almost 2 years with autologous transplantation. The use of high dose therapy with autologous transplantation however was associated with toxicity including late effects and in some cases treatment related mortality. The GC considered that the increased progression free survival outweighed the harms due to late effects which can be managed and to some extent mitigated by surveillance. |
| Trade off between net health benefits and resource use | No economic evidence was identified and no economic model was built. The resource implications associated with the recommendation were thought to be negligible because the use of autologous transplantation as consolidation of induction chemotherapy is the current standard of care for people with chemosensitive mantle cell lymphoma. In comparison to the alternative courses of action, autologous transplantation was thought likely to be cost-effective. There would be increased costs associated with transplantation (in comparison to chemotherapy alone) with an autologous transplantation estimated to cost £34,000. However, the evidence suggested that progression free survival should be greatly improved with an autologous transplantation. These improvements would be expected to translate into superior effectiveness in QALY terms. The GC thought that it was likely that the strategy would be cost-effective in cost per QALY terms. This view is partially supported by extrapolating from the economic analysis conducted for this guideline in another NHL subtype. In patients with follicular lymphoma, it was found that autologous transplantation was cost-effective in comparison to chemotherapy with an ICER of £4,812 per QALY. Thus, it was demonstrated that autologous transplantation was cost-effective despite the high costs of initial therapy because of the substantial gains in effectiveness. |
| Other considerations | The GC considered that patients with pre-existing co-morbidities are unlikely to be candidates for autologous transplantation and that this will disproportionately affect older patients. The GC therefore based their recommendations on patient fitness rather than age. The GC chose not to make a research recommendation about upfront consolidation with allogeneic transplantation because a comparative study would not be feasibile. |
4.3.3. Maintenance strategies in mantle cell lymphoma
Choice of initial therapy for MCL is complex due to the lack of available randomised trials. The role of maintenance therapy remains unclear. Interferon alpha has been studied by various groups but the overall effect on MCL outcomes coupled with the side effect profile has meant that this treatment has not been widely adopted.
Maintenance therapy is topical in MCL for several reasons. Progression free survival is significantly prolonged by the use of maintenance with rituximab, with acceptable toxicity, in other lymphoma subtypes. A recent study in MCL demonstrated that maintenance rituximab almost doubled the duration of remission in older patients responding to RCHOP, compared with maintenance interferon-α, although this study administered rituximab maintenance until patients progressed (or withdrew due to toxicity or patient preference). In addition, overall survival was also significantly improved among patients who responded to R-CHOP chemotherapy, though this benefit could not be demonstrated in patients receiving nucleoside analogue therapy. A positive effect has also been demonstrated in younger patients following stem cell transplant.
Clinical question: What is the effectiveness of first-line maintenance strategies compared with observation for people with mantle-cell lymphoma?
4.3.3.1. Clinical evidence (see section 4.3.3 in Appendix G)
Evidence came from two randomised controlled trials and four retrospective cohort studies.
4.3.3.1.1. Efficacy of maintenance therapy post-induction
Three studies reported the effectiveness of rituximab maintenance after first-line induction therapies in 349 patients with mantle-cell lymphoma, suggesting that the use of rituximab maintenance significantly increases duration of remission (Kluin-Nelemans et al., 2012) and progression free survival (Ahmadi et al., 2012; Vokurka et al., 2014) compared to other types of maintenance therapy (interferon-α) or no maintenance therapy at all (p<0.05).
One randomised control trial (RCT; Kluin-Nelemans et al., 2012) comparing the use of rituximab maintenance therapy to the use of Interferon-α maintenance therapy in 316 patients with stage II-IV mantle cell lymphoma provided low quality evidence of longer durations of remission in the patients receiving rituximab compared to those receiving interferon-α (P<0.01). Overall survival rates did not differ significantly between the rituximab and interferon-α groups (79% versus 67%, respectively), however, the author reports that type of induction therapy influenced the survival benefit of rituximab maintenance. In the 111 patients treated with the induction regimen R-FC there was no difference in the rates of remission nor the overall 4-year survival rate in patients treated with rituximab maintenance compared to those treated with interferon-α. Patients (N=163) treated with the induction regimen R-CHOP did show survival benefits from the use of rituximab maintenance, with such patients having longer duration of remission (not yet reached versus 36 months, p<0.01) and better 4-year overall survival rates (not yet reached versus 64 months, p<0.01) compared to patients treated with interferon-α . Patients tolerated rituximab maintenance better than interferon-α, with a third of patients in the rituximab maintenance group stopping therapy at 4 years (28%) compared to nearly 50% of patients in the interferon-α group at 1 year. In addition, patients receiving interferon-α experienced significantly higher rates of grade 3 and 4 leukocytopenia (33% versus 19%), thrombocytopenia (15% versus 6%), fatigue (5% versus 1%) and Infection (11% versus 9%) (p<0.05) compared to patients receiving rituximab maintenance.
Two retrospective comparative studies compared rituximab maintenance therapy to no additional therapy (Ahmadi et al., 2012; Vokurka et al., 2014) and to autologous stem cell transplantation (ASCT) (Ahmadi et al., 2012) in 101 patients with mantle cell lymphoma reporting very low quality evidence of longer progression free survival in those receiving maintenance compared to no further therapy (P<0.05). Overall survival was not reported by either study with Vokurka et al. (2014) noting that the follow up was not yet long enough to assess overall survival. Ahmadi et al. (2012) reported no statistically significant difference between maintenance and consolidation therapy (3.9 years versus 4.5 years).
4.3.3.1.2. Efficacy of maintenance therapy post-consolidation
Three studies compared the effectiveness of rituximab maintenance after first-line consolidation therapy with autologous stem-cell transplantation, reporting a significant benefit, with patients receiving the additional maintenance therapy having an increased event free (Le Gouill et al., 2014) and progression free survival rate (Vokurka et al., 2014; Mangel et al., 2004 [update data from: Hicks et al, 2006]) compared to those who did not. There was variation in the overall survival benefit of rituximab maintenance in these studies.
One randomised control trial (RCT; Le Gouill et al., 2014; conference abstract) comparing the use of rituximab maintenance therapy to watch and wait in 238 patients with mantle cell lymphoma all treated with ASCT provided low quality evidence of significantly longer event free and progression free survival (p<0.05) in the patients receiving the maintenance therapy. There was however, no significant difference in the 2-year overall survival rates between the patients receiving maintenance (93.4%) compared to those patients undergoing watch and wait (93.9%).
One retrospective cohort study (Vokurka et al., 2014) provided very low quality evidence of a significant progression free survival benefit in 14 patients receiving rituximab maintenance (median not yet reached) compared to 12 patients receiving no maintenance therapy (46 months, p<0.05).
One retrospective cohort study (Mangel et al., 2004, updated data in conference abstract by Hicks et al., 2006) provided very low quality evidence of a significant progression free and overall survival benefit in 20 patients with mantle cell lymphoma receiving consolidation (ASCT) plus rituximab maintenance (5 year: 72%, 80%) compared to 40 patients with mantle cell lymphoma receiving conventional induction chemotherapy alone (19% P<0.001; 38%, P<0.01).
4.3.3.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Consider maintenance rituximabh, every 2 months until disease progression, for people with newly diagnosed mantle cell lymphoma who are not fit enough for high-dose chemotherapy and where there has been a response to R-CHOP-based immunochemotherapy. Consider maintenance rituximabi, every 2 months for 3 years, for people with newly diagnosed mantle cell lymphoma who are in remission after cytarabine-based induction and high-dose chemotherapy. |
|---|---|
| Relative value placed on the outcomes considered | Progression free survival and overall survival were considered the critical outcomes when drafting recommendations. Other important outcomes included disease free survival, treatment related mortality, treatment related morbidity and health related quality of life. Health related quality of life and treatment related mortality were not reported in the evidence. |
| Quality of the evidence | The quality of the evidence ranged from very low to low as assessed using GRADE. Evidence quality was downgraded for study design limitations, indirectness and imprecision. For some of the comparisons there were no randomised trials available so the evidence was drawn from non-randomised comparative studies. The GC recommended that rituximab maintenance be considered rather than offered, reflecting the low quality of the evidence. |
| Trade off between clinical benefits and harms | The GC considered the recommendations could result in improved progression free survival and overall survival for patients with mantle cell lymphoma The evidence indicated clinically significant improvements in overall survival and progression free survival with rituximab maintenance after first-line induction therapies, when compared to other maintenance therapy or no maintenance. Clinically signficiant improvements in progression free survival were also seen with rituximab maintenance after consoldation with autologous stem cell transplantation. The GC acknowledged that the potential harms of the recommendations were an increased number of hospital visits and a small increased risk of infection due to the immunosuppressive nature of rituximab. Evidence from one randomised trial indicated a grade 3 to 4 infection rate of around 9% with rituximab maintenance versus 11% with interferon-α maintenance, following first line induction. The GC noted that this patient group were already frequently visiting hospital at 3 to 6 monthly intervals and any extra visits would be outweighed by improved progression free survival. The recommended durations of rituximab maintenance therapy were drawn from the randomised trials. |
| Trade off between net health benefits and resource use | No relevant health economic evidence was identified and no economic model was built for this topic, It was thought that the recommendations would increase the number of patients receiving rituximab maintenance (and therefore increase the associated costs). Continuing rituximab maintenance until disease progression would mean around 2 years of treatment in the average patient. However, the overall resource impact was thought to be minimal because the absolute number of patients is small due to the rarity of the condition. Due to better progression free survival, cost savings could be made from the increased time to next treatment and avoiding the costs of second and third line therapies. These cost savings coupled with the QALY benefits that would be expected from the superior progression free survival mean that rituximab maintenance is likely to be cost-effective at at threshold of £20,000 per QALY. |
| Other considerations | The GC acknowledged that use of rituximab maintenance following autologous stem cell transplantation would lead to a significant change in practice. Stopping rituximab maintenance at disease progression (or at 3 years post-ASCT) would also be a change in practice for many centres. The GC acknowledged that while rituximab is not currently licensed for this indication, the evidence supports its use in this context. The GC considered that the recommendations should reduce variation in practice and promote consistency of care for patients with this rare condition. |
- h
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information. The evidence reviewed for the guideline supports a dosage of 375mg/m2 every 2 months until disease progression.
- i
At the time of publication (July 2016) rituximab did not have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council's Prescribing guidance: prescribing unlicensed medicines for further information. The evidence reviewed for the guideline supports a dosage of 375mg/m2 every 2 months for 3 years.
4.4. Diffuse large B-cell lymphoma (DLBCL)
4.4.1. Radiotherapy in first line treatment
In early stage diffuse large B-cell lymphoma (DLBCL) short course immunochemotherapy followed by radiotherapy is a standard treatment. In advanced stage DLBCL the role of radiotherapy after full course immunochemotherapy remains uncertain. The initial treatment of advanced stage DLBCL is immunochemotherapy and response rates to this are high. Radiotherapy is an effective treatment against DLBCL but limited by the distribution of disease which it can effectively cover. Advanced stage disease will by definition be multifocal and often bulky so that it could not feasibly be covered with conventional radiotherapy fields at presentation. Bulk is variably defined and is usually >7.5cm or 10cm. Furthermore there are concerns derived from data emerging from the treatment of Hodgkin's lymphoma related to the late effects of radiotherapy. In particular there is a risk of second cancers and after mediastinal irradiation cardiac deaths. This may be ameliorated by new techniques which use smaller volumes and lower doses.
Radiotherapy has been used in the past after primary chemotherapy for advanced DLBCL in cases where there is limited residual disease and to sites of bulk at presentation. These are most likely to be the focus for relapse in the future. In general a reduction in local relapse has been shown from this approach but no consistent effect upon survival is seen. The majority of published studies in this setting will reflect both the pre-rituximab era and the pre-positron emission tomography (PET) era. Computed tomography (CT) has conventionally been used for response assessment at treatment completion, however this anatomical technique cannot accurately discriminate remaining active lymphoma (residual disease) from post treatment necrosis or fibrosis. In contrast post-therapy metabolic imaging, e.g. PET-CT, has a high negative predictive value (the ability of a negative PET scan to exclude persistent disease or future relapse). The small false negative rate with PET is mostly related to its inability to detect microscopic disease which results in future relapse. Current practice following immunochemotherapy is for patients with residual disease to be considered for salvage intensive chemotherapy using an autograft or allograft. However there remains a subgroup of older patients or those with significant co-morbidity who will not be able to proceed with salvage chemotherapy to whom radiotherapy will be offered.
There are therefore two potential scenarios where radiotherapy may have a role after full course immunochemotherapy for advanced DLBCL. The first is when given as planned combined modality treatment to sites of original bulky disease for patients in complete remission and the second when given to patients with residual disease which can be encompassed within a radiation field. A recent prospective study has demonstrated a substantial benefit in elderly patients receiving radiotherapy to sites of original bulky disease with a hazard ratio of 4.3 for overall survival, although an important limitation of this study is that PET was not used for post immunochemotherapy response evaluation.
Clinical question: The role of consolidation radiotherapy in first-line treatment of diffuse large B-cell lymphoma.
4.4.1.1. Clinical evidence (see section 4.4.1 in Appendix G)
Evidence came from four cohort studies (one prospective).
Compared to immunochemotherapy alone, immunochemotherapy + consolidation radiotherapy is associated with similar or longer overall survival (4 observational studies [Dorth et al., 2012; Held et al., 2014; Marcheselli et al., 2011; Phan et al., 2010]; total N = 1200; very low quality evidence), longer event-free survival (3 observational studies [Dorth et al., 2012; Held et al., 2014; Marcheselli et al., 2011]; total N = 731; very low quality evidence), similar or longer progression-free survival (2 observational studies [Held et al., 2014; Phan et al., 2010]; total N = 939; very low quality evidence), similar or higher rates of complete response (1 observational study [Held et al., 2014]; total N = 470; very low quality evidence), similar or higher rates of treatment-related mortality (1 observational study [Held et al., 2014]; total N = 470; very low quality evidence), and similar or higher rates of treatment-related morbidity (1 observational study [Held et al., 2014]; total N = 470; very low quality evidence).
4.4.1.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendation | Consider consolidation radiotherapy delivering 30 Gy to sites involved with bulk disease at diagnosis for people with advanced-stage diffuse large B-cell lymphoma that has responded to first-line immunochemotherapy. For each person, balance the possible late effects of radiotherapy with the possible increased need for salvage therapy if it is omitted, and discuss the options with them. |
|---|---|
| Relative value placed on the outcomes considered | Progression free survival and treatment related morbidity and mortality were considered to be critical outcomes when agreeing the recommendations. These outcomes are crucial when considering the balance for each individual of the possible late effects of radiotherapy with the possible increased need for salvage therapy if radiotherapy is omitted. Overall survival was also considered an important outcome. No evidence was identified relating to health related quality of life, patient satisfaction or patient preference |
| Quality of the evidence | The evidence for each outcome was rated very low quality as assessed using GRADE and NICE checklists for quantitative studies. Evidence was downgraded for baseline differences between the comparison groups, indirect patient populations, and imprecision. For this reason, the GC did not make strong recommendations for the use of radiotherapy in patients who had responded to first-line immuno-chemotherapy. The GC noted that there was a lack of evidence as to whether any groups of patients with extranodal disease may benefit from radiotherapy after chemotherapy. The GC expressed concern that the current evidence base did not reflect contemporary practice due to the length of time for recruitment (>20 years) and the lack of consistency in the use of PET-CT to assess response to first-line therapy. The GC considered that the inconsistency in the low-quality evidence base and in the use of PET-CT to assess response to first-line therapy impacted on their ability to make a strong recommendation. |
| Trade-off between clinical benefits and harms | The GC thought that the recommendation may reduce the chance of relapse (and thus the need for intensive chemotherapy salvage therapy) and improve overall survival. The evidence indicated a clinically important improvement in overall survival and progression free survival with radiotherapy. The GC acknowledged the possible treatment related adverse events at the treatment site. There is a risk of both short term effects (e.g. transient skin, mucosal and gastrointestinal reactions) and potential late effects of radiotherapy (e.g. skin pigmentation, dry mouth, functional gastrointestinal disturbance). Evidence about treatment related toxcity was limited to a single study which suggested little effect on treatment toxicity when radiotherapy is added to treatment. The GC considered that the benefits of increased overall survival and potential reduction in the need for intensive chemotherapy salvage therapy for patients, outweighed the risks associated with radiotherapy, particularly as the short term morbidity would only occur during active treatment. The focus of the evidence review was not the optimal dose of radiotherapy, however the GC thought that it was important to state dose level in the recommendation in order to confirm best practice. Therefore, they used clinical consensus, experience and evidence from a trial comparing radiotherapy doses to recommend a radiotherapy dose of 30Gy (best practice based on a randomised controlled trial [Aviles et al, 2005] comparing varying dose levels of radiotherapy in the target population included in the PICO reporting no additional local control or survival benefit of doses above 30Gy). The GC considered that the recommendations would consolidate current practice, providing clarity on the treatment pathway in the patient populations and lead to reduced variation in practice. |
| Trade-off between net health benefits and resource use | No economic evidence was identified and no economic model was built for this topic. The recommendation was largely thought to be a consolidation of current practice although there may be increased costs associated with radiotherapy in places not currently providing this level of care. The use of radiotherapy is likely to be cost-effective as the upfront cost (which itself is relatively low in comparison to other treatment areas) would be expected to be justified by the improvements in longer progression free survival. The improved progression free survival would lead to QALY gains and would also offset the upfront cost (at least partially). Thus, the use of radiotherapy is likely to be cost-effective in cost per QALY terms. |
| Other considerations | The GC made a research recommendation for a trial in patients who had responded (PET-CT negative) to first-line immuno-chemotherapy. This was because there was an inconsistent and low-quality evidence base for this question and the GC noted that an RCT could be achieved in this area and warranted further investigation. In addition, the GC suggested that the outcome of the research recommendation may produce the required evidence to standardise clinical practice in this area. In relation to patients with extranodal disease the GC considered that the lack of patient numbers presenting would prevent success with a randomised trial and suggested that population based data may be the only way to assess use of radiotherapy in this patient population. |
| Research recommendation | In people presenting with diffuse large B-cell lymphoma and sites of bulky disease, are outcomes improved by radiotherapy to those sites following a full course of chemotherapy? |
|---|---|
| Why this is important | The role of radiotherapy to sites of original bulky disease in treating diffuse large B-cell lymphoma is uncertain. Some clinical teams will consider radiotherapy in this setting while others will not because of concerns about morbidity and late effects of treatment. In a recent randomised trial of chemotherapy in people over 60 years old with diffuse large B-cell lymphoma, people having radiotherapy were identified and compared with a cohort having no radiotherapy. Significant improvements in event-free, progression-free and overall survival were seen in the group having radiotherapy. These results have encouraged some teams to reconsider radiotherapy for bulky diffuse large B-cell lymphoma. A definitive randomised trial is needed to address this question. Outcomes of interest include overall survival, disease-free survival, progression-free survival, treatment-related mortality, treatment-related morbidity, health-related quality of life, patient satisfaction, patient preference and overall response rate (complete or partial remission). |
4.4.2. Central nervous system prophylaxis
Central Nervous System (CNS) relapse in patients with diffuse large B-cell lymphoma (DLBCL) occurs infrequently (approximately 5%), but is almost always fatal.
There is significant controversy regarding which factors most reliably identify patients at high risk of this complication. Clarification is also needed regarding the value of the various prophylaxis strategies when contemporary rituximab-containing chemotherapy regimens are used. Traditionally, involvement of > 1 extranodal site and an elevated lactate dehydrogenase level identifies individuals at highest risk (i.e. > 20% risk of the event). In addition, certain solitary extra-nodal sites (e.g. testis, kidney and breast) have been regarded as being higher risk. Due to the current lack of consensus, a wide variation of practise occurs across the UK with some centres only giving CNS directed prophylaxis to those with the highest risk (such as testicular involvement). Other centres would include patients with epidural disease, paranasal sinus involvement, bone marrow involvement and involvement of kidney or breast.
A high proportion of patients considered to be at high risk of CNS disease may already have occult or sub-clinical disease at the time of primary diagnosis. If these patients could be reliably identified one could separate patients into two risk groups- those with subclinical disease who require a CNS eradication strategy and those high risk patients without disease who may benefit from a prophylactic strategy.
Immunophenotyping by flow cytometry is a promising approach. Widespread use of this technique may redefine what risk and prophylaxis really mean. Intra-thecal and parenterally administered prophylaxis imparts small but definite risks to the patient. In addition, the administration of such prophylaxis is resource intensive. Intrathecal drug delivery requires an elaborate governance structure to avoid the wrong drug being administered, and intravenous administration requires an in-patient stay.
Although subgroups of patients with diffuse large B-cell lymphoma (DLBCL) with a relatively high risk of Central Nervous System (CNS) recurrence (i.e. ≥ 20%) can be identified, the current evidence base supporting the use of prophylactic strategies in patients receiving modern chemo-immunotherapy is limited.
There are also concerns over the efficacy of intra-thecal drugs in that they penetrate the brain substance very poorly and yet up to 40% of CNS lymphoma relapses occur in this way. The use of systemic (intravenous) prophylaxis in various forms is also limited and often confused by heterogeneity of entry criteria and the method of prophylaxis. Theoretically, intravenous prophylaxis would penetrate the brain substance more effectively as implied by results from patients with primary central nervous system lymphoma. Data of superiority in the prophylaxis setting however, are lacking.
The controversy surrounding CNS prophylaxis is unlikely to be answered in the form of a randomised clinical trial due to the rarity of CNS events in the DLBCL population. There are, however, a number of observational studies that may assist in the selection of both patients and strategies to be used to abrogate the risk of CNS disease in this patient group in the modern era.
Clinical question: What are the risk factors associated with central nervous system (CNS) relapse in patients with diffuse large B-cell lymphoma?
4.4.2.1. Clinical evidence (see section 4.4.2 in Appendix G)
Evidence came from one randomised controlled trial and seven retrospective case series.
The main challenges to the validity of the evidence as a whole concerned (1) variation in how the outcome (CNS relapse) was measured with two studies using clinical and neurological symptoms alone compared to radiographic and cerebrospinal fluid assessment as standard in the remaining studies; (2) a lack of information from conference abstracts about the prognostic factors included and statistical analyses, and (3) the included samples of participants representing a ‘reduced risk’ population, with those at highest risk of CNS relapse being treated up-front with prophylaxis under individual hospital protocols. Whilst, only a hypothesis, this could explain the lack of consistency in the results of relevant prognostic factors (because allocation to prophylaxis varied across hospital institutions) and the lack of evidence supporting known CNS relapse risk factors (e.g. involvement of the testis).
Six studies reported the prognostic value of clinical characteristics (age; performance status; lactate dehydrogenase; international prognostic index; involvement of extranodal sites/specific organ sties, MYC+BCL2+ and white blood cell count) on the development of secondary central nervous system relapse, in patients with diffuse large B-cell lymphoma. However, only two factors (involvement of the breasts, elevated LDH) were shown to be significantly independent in four of the studies (involvement of the breasts: Tomita et al. 2012, Deng et al. 2013; elevated LDH: Feugier et al. 2004, Morabito et al. 2005) with Yamamoto et al (2010) reporting no independent prognostic indicator in 375 patients with DLBCL and Tomita et al (2012) reporting that four out of seven factors assessed were independently associated with CNS relapse (age, involvement of the breasts, bone or adrenal glands) in 1221 patients with DLBCL.
Two studies (Schmitz et al. 2013; Savage et al. 2014a) reported the prognostic value of models (containing 5 or 6 factors), which group patients by the number of risk factors (low: 0-1 factors, moderate: 2-3 factors, higher: 4-5(6) factors) with a corresponding percent risk for developing a secondary CNS relapse within two years of diagnosis. Schmitz et al. (2013) reported a five factor model including age (>60 years), lactate dehydrogenase (>normal), stage (III or IV), Eastern Cooperative Oncology Group score (>1) and involvement of the kidneys. Savage et al. (2014a) reported the same five factor model included in the Schmitz et al. (2013) article (with the same cut-off points) but also included the factor extranodal sites (>1) and the involvement of kidneys or the adrenal glands. Both studies reported that an increase in the number of risk factors was associated with an increase risk of CNS relapse within two years of diagnosis with those reporting 0-1 factors having between a 0.6% (95% CI: 0.2-1.0%) and 0.8% (95% CI: 0.0-1.6%) risk for developing a CNS relapse within two-years, those with 2-3 risk factors having between a 3.9% (95% CI: 2.3-5.5%) and 4.1% (95% CI: 2.7-5.5%) risk for developing a CNS relapse within two-years and those with ≥4 factors having between 12% (4-6 risk factors; 95% confidence interval: 7.9-16.1%) and 17% (4-5 risk factors; 95% CI: 9.4-24.6%) risk for developing a CNS relapse within two-years. It is worthy to note that Savage et al. (2014a) reported that kidney/adrenal involvement was highly associated with CNS relapse (2 year CNS risk 33%), but no information of individual risk for CNS relapse for the other risk factors included in their factor model was provided because the article was a conference abstract. This could suggest that the risk factors included in the model do not carry equal weighting for CNS relapse risk and this may be problematic when considering a risk factor model that sums the risk factors because a patient with only kidney/adrenal gland involvement may have a higher risk for CNS relapse compared to a patient with 4 or more of the other risk factors.
4.4.2.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Explain to people with diffuse large B-cell lymphoma that they have an increased risk of central nervous system lymphoma if the testis, breast, adrenal gland or kidney is affected. Explain to people with diffuse large B-cell lymphoma that they may have an increased risk of central nervous system lymphoma if they have 2 or more of the following factors:
|
|---|---|
| Relative value placed on the outcomes considered | CNS disease rate was the critical outcome when agreeing the recommendation because CNS disease is associated with poor survival. CNS disease rate enabled the GC to assess which factors were associated with increased rates of CNS disease |
| Quality of the evidence | The evidence for this topic ranged from very low to low quality as assessed by the NICE checklist for prognostic studies. The issues with the evidence were: variation in how the outcome (CNS disease) was measured with two studies using clinical and neurological symptoms alone compared to radiographic and cerebrospinal fluid assessment as standard in the remaining studies; and a lack of information from conference abstracts about the prognostic factors included and statistically analysed. The GC considered that the included samples of participants may represent a ‘reduced risk’ population, with those at highest risk of CNS relapse being treated up-front with prophylaxis under individual hospital protocols. Whilst, only a hypothesis, this may explain the lack of consistency in the results of relevant prognostic factors (because allocation to prophylaxis varied across hospital institutions), and the lack of evidence supporting known CNS relapse risk factors (e.g., involvement of the testis). The GC accepted the possibility that these studies underestimated the baseline risk of CNS relapse, and the prognostic value of risk factors. The GC noted that the evidence base was potentially confounded by exclusion of high risk patients when assessing prognostic factors associated with CNS relapse (due to need to treat high risk patients), and the inclusion of low risk patients (unlikely to ever receive CNS prophylaxis) as the comparator in studies assessing the value of CNS prophylaxis. |
| Trade-off between clinical benefits and harms | The recommendations would help inform decisions about the need for CNS prophylaxis. The GC did not consider that there would be any harms from the recommendations made. A specific recommendation was made for patients with disease involvement of testis, breast, adrenal gland or kidney because the evidence suggested the risk of CNS replase was higher in such patients. The list of other risk factors is drawn from the evidenc,e which indicatesdpatients with 2 or more of these factors had around 4% risk of CNS relapse within 2 years. |
| Trade-off between net health benefits and resource use | No economic evidence was identified and no economic model was builtt for this topic. The GC considered that improved risk prediction for a CNS relapse may result in an increase in the number of patients receiving CNS prophylaxis. The GC noted that there are resource implications for the use of intrathecal CNS prophylaxis (costly drugs, special expertise and possible transfer to another hospital). However, the targeted use of CNS prophylaxis using the risk prediction criteria set out in the recommendations was thought likely to cost-effective. The increasd cost of CNS prophylaxis would be balanced agains a reduction in CNS relapse. CNS relapse is most often fatal and the costs of the intensive therapy (in a minority of CNS relapse patients who can tolerate the therapy) and/or palliative care costs (for the majority of CNS relapse patients) would be reduced if CNS relapse rates are lower after CNS prophylaxis. Therefore, despite a potential net increase in costs, it was thought that the strategy would be cost-effective in cost per QALY terms because of improvements in effectiveness (through a reduction in CNS relapse rates). |
Clinical question: What is the efficacy of central nervous system prophylaxis for people with diffuse large B-cell lymphoma?
4.4.2.3. Clinical evidence (see section 4.4.3 in Appendix G)
Evidence came from two randomised trials and 18 retrospective cohort studies.
4.4.2.3.1. Methotrexate
Intrathecal methotrexate versus no CNS prophylaxis
Eleven studies provided evidence concerning the use of intrathecal methotrexate (ITMTX) for central nervous system (CNS) prophylaxis (n=1084) compared to no CNS prophylaxis (n=4851) in patients with diffuse large B-cell lymphoma (6/11 studies samples were 100% DLBCL). The evidence base was inconsistent with six comparative observational studies reporting very low quality evidence of higher CNS relapse rates and relapse free survival rates in patients receiving ITMTX and four comparative observational studies reporting very low quality evidence of lower CNS relapse rates in these patients compared to patients receiving no CNS prophylactic therapy, but, none of these comparisons were significantly different (4/10 studies did not report significance values for CNS relapse rates). Only one randomized control trial reported the difference between the two groups to be statistically significant, with Tilly et al. (2003, 78.9% DLBCL) reporting very low quality evidence of a higher CNS relapse rate in 312 patients receiving no prophylaxis compared to 323 patients receiving ITMTX prophylaxis (8.3% versus 2.8%, p=0.002). However, patients receiving ITMTX had higher rates of treatment related adverse events (leucopenia, thrombocytopenia, infection and Mucositis, p<0.01) and a higher number of treatment related deaths (43%) compared to the patients receiving CHOP alone (23%, p=0.014).
Intravenous methotrexate (+/- intrathecal cytarabine) versus inadequate prophylaxis (IT chemotherapy only) or no CNS prophylaxis
One retrospective cohort study (Ferreri et al. 2015) provided very low quality evidence of significantly lower CNS relapse rates in 33 patients with high-risk DLBCL receiving IV MTX (0%) compared to 74 patients with high-risk DLBCL receiving either inadequate prophylaxis (IT chemotherapy only, n=7) or no prophylaxis at all (n=67) (12%; p=0.03). In addition, patients receiving IV MTX had significantly higher 5-year overall survival rates (87±6%) compared to the patients receiving inadequate or no prophylaxis (54±6%, p=0.001).
Intrathecal or intravenous methotrexate versus no CNS prophylaxis
Two cohort studies (Guirguis et al. 2012; Kumar et al., 2012) provided very low quality evidence of no significant reduction in CNS relapse rates or increased overall survival in 144 patients with diffuse large B-cell lymphoma receiving methotrexate either via intravenous or intrathecal prophylaxis compared to 1059 patients with DLBCL treated with no CNS prophylactic therapy.
Intrathecal methotrexate versus intravenous methotrexate versus HyperCVAD/CODOXM-IVAC
One comparative observational study (Cheah et al. 2014) provided very low quality evidence of lower CNS relapse rates in 43 patients receiving HyperCVAD or CODOXM-IVAC therapies (2.3%, 0.3-15.4%) compared to 125 patients receiving IV methotrexate (6.9%, 3.5-13.4%) and 49 patients receiving IT methotrexate (18.4%, 9.5-33.1%) (p=0.009). There was no reported significant difference in the 3-year relapse free survival rates between the three groups (p=0.051: IT: 65.5% [49.8-77.3%]; IV: 82.9% [74.7-88.6%]; HyperCVAD/CODOXM-IVAC: 70.6% [53.9-82.2%]), but the patients receiving HyperCVAD or CODOXM-IVAC had the highest rates of 3-year overall survival (89.2%) compared to the IT (68%) and IV (85.9%) methotrexate groups (p=0.029). The authors noted that in the patients receiving IV methotrexate there were high rates of renal impairment, occurring in 70% of cycles overall, although all patients recovered without the need for haemodialysis. No information regarding adverse events were reported for the other two treatment groups.
Consolidation with intrathecal methotrexate versus no CNS prophylactic consolidation
One randomised controlled trial (Récher et al. 2011) comparing the value of consolidative ITMTX in patients with aggressive B-cell lymphoma (97.5% DLBCL) who had been treated with ITMTX during their induction therapy (R-CHOP) provided very low quality evidence of no statistically significant difference in CNS relapse rates in the 196 patients treated with consolidative ITMTX (0%) compared to the 183 patients who received no ITMTX consolidation (1.09%). However, patients who received consolidation ITMTX had a significantly higher 3-year overall survival rate (92%) compared to those who received no consolidation therapy (84%, p=0.0071). Higher rates of adverse events were reported in the ITMTX consolidation group compared to the group not receiving ITMTX consolidation therapy, but significance values were not provided for these comparisons.
4.4.2.3.2. Any CNS prophylaxis
Five comparative observational studies (Aviles et al. 2013; Bernstein et al. 2009; Wilson et al. 2014; Ventre et al. 2013) compared the use of any CNS prophylaxis therapy in 1249 patients with DLBCL compared to 2552 patients with DLBCL receiving no CNS prophylaxis therapy. Aviles et al. (2013) and Bernstein et al. (2009) provided very low quality evidence of no significant benefit of CNS prophylaxis therapy on the CNS relapse rates in their patients. Aviles et al. (2013) further reported no relapse free or overall survival benefit from CNS prophylaxis. However, both Ventre et al. (2013) and Wilson et al. (2014) reported survival benefits in patients receiving CNS prophylaxis with Ventre et al. (2013) reporting very low quality evidence of an increased overall survival rate in 40 patients with DLBCL treated with CNS prophylaxis (94±7%) compared to 64 patients with DLBCL who received no CNS prophylaxis (46±6%, p=0.001) and Wilson et al. (2014) reporting very low quality evidence of a relapse free survival benefit in 132 patients with DLBCL who received more than 2 doses of intrathecal methotrexate, cytarabine or triple prophylactic therapy compared to 69 patients who received none, or less than 2 doses, of prophylactic therapy (p=0.025).
4.4.2.3.3. Allocation of patients to prophylaxis
Unfortunately allocation to CNS prophylaxis in the majority of the studies was based on level of risk (which varied across studies) or physician discretion (which varied within studies), which may bring into question the value of the comparison of at risk (for CNS relapse) patients treated with prophylaxis to low risk patients not treated with prophylaxis. A non-significant difference when comparing high risk to low risk patients could lend support for the hypothesis that CNS prophylaxis is providing a benefit because the CNS relapse rates after prophylaxis become comparable to those CNS relapse rates in low risk patients where prophylaxis would rarely be considered. Only one study (Tilly et al. 2003) reported the value of prophylaxis in a randomised controlled trial, reporting a benefit of prophylaxis. However, these patients did not receive rituximab and whilst the aim of the present study was not to address the use of rituximab in relation to CNS relapse rates, there were no RCTs and only one of the observational studies post rituximab reported a benefit for the addition of prophylaxis when compared to no prophylaxis in patients who were matched on their risk for CNS relapse.
4.4.2.4. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendation | Offer central nervous system-directed prophylactic therapy to people with diffuse large B-cell lymphoma:
|
|---|---|
| Relative value placed on the outcomes considered | The critical outcome for this topic was CNS relapse, specifically: time to relapse, sites of relapse, isolated to CNS compared to systemic relapse, general relapse, parenchymal relapse and meningeal relapse. Additional important outcomes included, overall survival, treatment related mortality, treatment related morbidity and health related quality of life CNS relapse rate was considered critical when agreeing the recommendation because patients who have a CNS relapse have extremely poor survival rates, and therefore this outcome enabled the GC to assess the efficacy of CNS prophylaxis. There was no evidence for health related quality of life in patients undergoing CNS prophylaxis. The evidence relating to adverse events extracted from the evidence review was not considered useful in the assessment of the efficacy of CNS prophylaxis. This was because the GC noted that many of these events were a consequence of induction therapies, and were unlikely to be associated with the CNS prophylactic treatments. |
| Quality of the evidence | The quality of the evidence was very low quality for the outcome CNS relapse rate as assessed using GRADE. Two studies comparing induction regimens (not CNS prophylaxis) were downgraded due to indirectness (inclusion of patients with other types of NHL). Additionally it was unclear how CNS relapse was detected and no information was provided on allocation and detection biases. The remaining studies were downgraded for serious indirectness (sample included patients with other types of NHL; sample included patients with primary CNS DLBCL); serious limitations (unclear decision making for who received prophylactic treatment; allocation to prophylaxis based on risk level so comparison of groups at baseline differed; unclear rationale for detection of CNS relapse) and serious imprecision (due to low sample size and number of events). Allocation to CNS prophylaxis in the majority of the studies was based on level of risk (which varied across studies) or physician discretion (which also varied within studies). This may bring into question the value of the comparison of at risk (for CNS relapse) patients treated with prophylaxis to low risk patients not treated with prophylaxis. The GC acknowledged that such comparisons could underestimate the benefit of prophylaxis. In this situation, no apparent difference between high risk to low risk patients could lend support for the hypothesis that CNS prophylaxis is providing a benefit, because the CNS relapse rates after prophylaxis become comparable to those CNS relapse rates in low risk patients where prophylaxis would rarely be considered. There were no RCT's and only one observational study post rituximab that reported a benefit for the addition of prophylaxis when compared to no prophylaxis in patients who were matched on their risk for CNS relapse. The evidence base for the efficacy of CNS prophylaxis was consistent in terms of CNS relapse rates which the GC thought could reflect a value for the use of CNS prophylaxis (bringing the CNS relapse rate in high risk patients to similar to low risk patients). One study that did isolate comparisons to high risk versus high risk with prophylactic therapy, did report a clinically relevant benefit of prophyalxis. The GC noted that given the need to treat high risk patients with prophylaxis (to reduce the CNS relapse rate), it would be unlikely that evidence would ever be available to directly compare high risk to low risk.There is also little consensus across countries as to the best treatment pathway in these patients, so the evidence found in the review donefor this guideline, is the best available evidence. |
| Trade-off between clinical benefits and harms | The GC considered that the recommendation will result in a reduction in the CNS relapse rates (an often fatal manifestation of the lymphoma) in patients with DLBCL. The recommendation will provide uniformity of practice. The one randomised trial included reported a reduction of CNS relapse from 8.3% to 2.8% with CNS prophylaxis (although these patients did not receive rituximab). The GC acknowledged that patients will be exposed to an increase in toxicity, resulting in an increase rate of morbidity. The GC thought that the benefits of a reduction in a potential fatal relapse was offset by the manageable harms of increased toxicity from the recommended therapy. Separate recommendations were made for patients at high and moderate risk of CNS relapse. These risk groups were based on the evidence identified in the previous section (see section 4.4.3.2). The GC made an “offer” recommendation for the high risk group, despite the low evidence quality, because they judged there was a clear benefit with CNS prophylaxsis in the trade-off between benefits and harms for these patients. In addition, CNS relapse would almost always be fatal and this is the only treatment that can be given. For patients with a moderately raised risk of CNS relapse, the GC considered that on balance the benefits still outweighed the harms. However, given the lower baseline risk a greater number of patients would have to be treated to prevent each case of CNS relapse. Balancing the increased risk of CNS disease in older patients with the toxicity involved in repeat lumbar punctures meant that the GC thought that patients should be involved in these difficult decisions. |
| Trade-off between net health benefits and resource use | No health economic evidence was identified and no health economic model was developed for this topic. The GC considered that the recommendation may result in increased costs through an increase in the number of patients receiving CNS prophylaxis. The GC noted that there are resource implications for the use of intrathecal CNS prophylaxis (costly drugs, special expertise and possible transfer to another hospital). However, the use of CNS prophylaxis was thought likely to be cost-effective because the increased cost of CNS prophylaxis would be balanced against a reduction in CNS relapse. CNS relapse is most often fatal and the costs of the intensive therapy (in a minority of CNS relapse patients who can tolerate the therapy) and/or palliative care costs, (for the majority of CNS relapse patients), would be reduced if CNS relapse rates are lower after CNS prophylaxis. Therefore, despite a potential net increase in costs, it was thought that the strategy would be cost-effective in cost per QALY terms because of improvements in effectiveness (through a reduction in CNS relapse rates). |
| Other considerations | The GC considered that the recommendations may result in a minor change in practice and result in a small overall increase in the use of CNS prophylaxis by providing a more uniformed approach to treating patients presenting with DLBCL. The GC could not make a specific recommendation regarding the type of CNS prophylaxis to use (intrathecal versus intravenous methotrexate) due to a lack of evidence comparing the routes of administration. In addition, the GC noted that a research recommendation comparing the routes of administration of the therapy would be difficult to implement due to the predicted size of the sample required to power a study and the need for international collaboration, with little international consensus of treatment regimens and CNS prophylaxis eligibility criteria for categorising patients into high and low risk groups. |
4.4.3. Salvage therapy
Patients with diffuse large B cell lymphoma (DLBCL) who fail first-line therapy may be categorised into three distinct groups: (1) those relapsing after complete remission, (2) partial responders with persistent disease, and (3) refractory patients.
The survival outcomes are significantly different in each subgroup, becoming progressively worse from relapsed to refractory patients. For patients who are deemed candidates for high dose therapy, the standard strategy is salvage immunochemotherapy followed by autologous stem cell transplantation (ASCT). This approach is most effective in those with chemo-sensitive disease and is associated with prolonged survival in approximately 40% of relapsed patients who achieve at least a partial response to salvage as determined by conventional Computed Tomography (CT)-based criteria.
The main goal of salvage therapy is to minimise the disease burden and demonstrate continued chemo-sensitivity. Complete remission is not required, but demonstration of response is the most predictive factor of outcome after ASCT, and the best outcomes are reported in patients who achieve metabolic complete response before ASCT. The majority of favoured first-line salvage regimens include either one or both of a platinum compound or ifosfamide, and there is no clearly superior regimen. For patients who do not respond to first-line salvage, outcomes are extremely poor with 1-3 year survival rates of <10%. Although many clinicians attempt a second-line salvage regimen in this setting, the ultimate curability of these patients is quite limited.
Support for the role of ASCT in consolidation following salvage is based on one randomised study, and multiple single institution and registry studies confirming similar outcomes following ASCT. The landmark PARMA trial included only patients with relapsed DLBCL; all patients had attained a complete radiological (CT) response to initial induction therapy and were ≤ 60 years of age; patients with bone marrow or central nervous system involvement at relapse were excluded and patients had not received rituximab during induction or salvage. Both overall (OS) and event-free survival (EFS) were superior in the transplant group. Subsequent analyses have confirmed that IPI score at relapse and time to relapse are important prognostic variables. The approach to those excluded from this study (e.g. those with <complete response, those over 60 years, those with bone marrow or CNS involvement) remains more contentious.
Groups of patients with worse overall prognoses can be identified, for example ‘double hit’ lymphomas, those with primary resistant disease, or those failing to achieve a complete response to salvage. The role of allogeneic transplantation (alloHSCT) in these patients remains incompletely defined. The graft-versus-lymphoma effect is less well demonstrated in DLBCL than in other lymphomas. Furthermore, the non-relapse-related procedural mortality associated with such transplants is relatively high in patients with DLBCL (>20% in most series). Nevertheless, a number of published series indicate plateaus in the survival curves for patients undergoing alloHSCT, and it continues to be considered a clinical option in such cases. Some reserve alloHSCT for patients who have failed a prior ASCT or stem cell mobilisation enabling ASCT, recognising that only a minority will be salvaged to a position in which they can undergo such a procedure.
Clinical question: What is the most appropriate salvage strategy for people with relapsed/refractory diffuse large B-cell lymphoma?
4.4.3.1. Clinical evidence (see section 4.4.4 in Appendix G)
Evidence came from three randomised controlled trials, three retrospective cohort studies and four retrospective case series.
4.4.3.1.1. R-BEAM followed by ASCT versus B-BEAM followed by ASCT
Low quality evidence from one study of 224 patients reported that overall rate of grade 3-5 non-haematologic toxicities and grade 3-5 mucositis, but not other individual grade 3-5 non-haematologic toxicities, overall survival, progression-free survival, and treatment-related mortality were significantly lower in R-BEAM than B-BEAM (HRs not reported [BMT CTN 0401]).
4.4.3.1.2. R-ICE followed by ASCT versus R-DHAP followed by ASCT
One study (CORAL) with 477 patients provided moderate quality evidence that overall survival, progression-free survival, and event-free survival did not differ significantly between R-ICE and R-DHAP (HRs not reported).
4.4.3.1.3. (R-)GDP followed by ASCT versus (R-)DHAP followed by ASCT
One study with 619 patients (NCIC-CTG LY.12) provided low quality evidence that quality of life was significantly better or similar in (R-)GDP compared to (R-)DHAP and grade 3-4 nausea, febrile neutropenia and overall occurred significantly less in (R-)GDP than in (R-) DHAP, but the treatment groups did not differ in other individual grade 3-4 adverse events, overall survival, overall survival after transplantation, event-free survival, event-free survival after transplantation, overall response rate and rate of ASCT transplantation (HRs not reported).
4.4.3.1.4. R-ICE versus R-GDP as salvage chemotherapy
Low quality evidence from an indirect comparison of two randomised trials (CORAL and NCIC-CTG LY.12) suggested uncertainty about whether outcomes are better with R-GDP than with RICE.
4.4.3.1.5. R(if CD+)-ICE followed by ASCT (if < 66 years and response) versus R(if CD+)-DHAP followed by ASCT (if < 66 years and response) versus R(if CD+)-GDP followed by ASCT (if < 66 years and response)
Very low quality evidence from one study with 113 patients (Kusano et al, 2014) reported median second progression-free survival was longer in (R-)ICE than in two other two treatment groups combined and in (R-)ICE compared to (R-)DHAP alone, but not to (R-)GDP alone. There was significantly more grade 3-4 renal dysfunction with (R-)DHAP than in other two treatment groups, but the three treatment groups did not differ in overall or complete response, overall survival ((R-)ICE versus the other two treatment groups combined), median time from first progression to second progression or last follow up, and grade 3-4 haematological side effects (HRs not reported).
4.4.3.1.6. R-MICE versus R-DICEP
Oh et al (2015) provided very low quality evidence that median time to progression was significantly longer in R-MICE than R-DICEP (HR not reported; n=38).
4.4.3.1.7. R-GemOx versus RICE
Very low quality evidence from one study with 65 patients (Zhang et al, 2011) suggest that neutrocytopenia and gastrointestinal tract reactions occurred significantly more in RICE than R-GemOx (HR not reported).
4.4.3.1.8. Allogeneic transplantation
Very low quality evidence about outcomes following allogeneic transplantation came from 4 retrospective case series (Avivi et al, 2014; Rigacci et al, 2012; Sirvent et al, 2010 and van Kampen et al 2011) including 807 patients. Overall survival at five years after allogeneic stem cell transplant (allo-SCT) ranged from 34% to 43% and five year progression free survival ranged from 30% to 37%. The rates of non-relapse mortality ranged from 28% to 38%, rates of acute graft-versus-host disease ranged from 32% to 51% and rates of chronic graft-versus-host disease ranged from 35% to 42%.
4.4.3.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Offer salvage therapy with multi-agent immunochemotherapy to people with relapsed or refractory diffuse large B-cell lymphoma who are fit enough to tolerate intensive therapy:
Consider consolidation with allogeneic stem cell transplantation for people with chemosensitive diffuse large B-cell lymphoma (that is, there has been at least a partial response to chemotherapy):
|
|---|---|
| Relative value placed on the outcomes considered | The critical outcomes were treatment toxicity and overall survival. Important outcomes were health realted quality of life, progression free survival and response to chemotherapy Health related quality of life was not reported in the evidence. |
| Quality of the evidence | The quality of the evidence was moderate to very low using GRADE. Evidence comparing transplantation to non-transplantation strategies was lacking.The randomised trials involving autologous transplantation compared different salvage chemotherapy regimens Only non comparative studies were available for allogeneic transplantation. This limited the strength of the recommendation that the GC were able to make about allogeneic transplantation. |
| Trade-off between clinical benefits and harms | The GC considered that the recommendation to offer salvage therapy and consolidation with autologous transplantation would prolong overall survival. Evidence from trials comparing different salvage chemotherapies followed by autologous stem cell transplant indicated overall survival of around 40% and event free survival around 30%. The use of high dose therapy with autologous transplantation however is associated with toxicity including late effects and in some cases treatment related mortality. The GC considered that the increased overall survival outweighed the harms due to acute and late effects. The recommendation to consider salvage therapy R-GDP instead of R-DHAP, has the potential to reduce treatment related toxicity without adversely affecting overall survival. This recommendation was informed by a randomised trial which indicated R-GDP was as effective as R-DHAP with similar overall and event free survival, but with fewer serious adverse events (47% versus 60%). Evidence about allogeneic stem cell transplant indicated overall survival of around 40% at five years with similar rates of acute and chronic graft versus host disease. |
| Trade-off between net health benefits and resource use | No health economic evidence was identified for this topic and no health economic model was developed. The recommendation to offer high dose therapy with autologous transplantation is already the current standard of care for this patient group. Therefore there are unlikely to be significant changes in practice as a result of these recommendations and so the resource impact should be minimal. For places not currently providing this care, there could be resource implications. However, despite its high cost, the use of high dose therapy with autologous transplantation was thought likely to be cost-effective because it substantially prolongs overall survival. Thus, it is likely to be cost-effective in cost per QALY terms. The recommendation to use R-GDP instead of R-DHAP as salvage therapy may be a departure from current practice in some places. However, this recommendation was thought to be cost-effective and indeed cost saving. In QALY terms, R-GDP should be at least as effective as R-DHAP (and possibly more so given the potential to reduce treatment related toxicity). R-GDP is also less costly than R-DHAP with marginally cheaper drug costs and substantially cheaper delivery costs as R-GDP is delivered on a day case basis while R-DHAP is delivered on an inpatient basis. Costs for these regimens were estimated as part of the economic modelling execrcises conducted for the guideline. Three cycles of R-GDP were estimated to cost £8,437 while three cycles of R-DHAP were estimated to cost £9,783. The recommendation to consider allogeneic transplantation where autologous transplantation is not possible or where it has failed, is also likely to be cost-effective because it substantially improves survival in comparison to chemotherapy alone. Therefore, it is likely to be cost-effectiven in cost per QALY terms. |
| Other considerations | The GC noted that consolidation with autologous transplantation would not be appropriate for some patients – for example when stem cell harvesting was not possible, but these patients might still benefit from allogeneic transplantation. |
4.5. Burkitt lymphoma
4.5.1. First line treatment
Burkitt lymphoma (BL) is a rare and highly aggressive subtype of B-cell non-Hodgkin's lymphoma (NHL). Cure rates with intensive first line treatment are high in younger patients, and those with low risk disease (Castillo et al, Cancer 2013), although the outlook is generally very poor for patients who relapse as few patients respond to salvage therapy. Risk in Burkitt lymphoma is variably defined and in adults normal LDH, tumour size <10cm, limited stage, one or no extral nodal sites, no CNS or bone marrow disease, and good performance score are often considered features of low risk disease.
The Magrath regimen (Magrath et al, JCO, 1996; Mead et al, Ann Oncol, 2002; Wang et al, Cancer, 2003) - CODOX-M/IVAC - is widely used in the UK and like other intensive first-line approaches such as hyper-CVAD (Thomas et al, Cancer 2006; Cortes et al, Cancer 2002) and CALGB 9251 (Rizzieri et al, Cancer 2004), is highly effective but toxic, especially in older patients. The development of effective and less toxic therapy for BL is desirable. DA-EPOCH-R is emerging as a low intensity regimen which has demonstrated both efficacy and good tolerability in a non-randomised study including sporadic and HIV-associated subtypes of BL (Dunleavy et al, NEJM, 2013). Rituximab is frequently added to first-line regimens, such as CODOX-M/IVAC, but the survival benefit of doing so has not been evaluated in randomised trials (Barnes et al, Ann Oncol, 2011).
This topic will address the most effective initial therapy for BL.
Clinical question: What is the most effective first-line treatment for people with Burkitt lymphoma?
4.5.1.1. Clinical evidence (see section 4.5.1 in Appendix G)
Evidence came from one randomised controlled trial and seven cohort studies (one prospective).
4.5.1.1.1. Comparison of interventions
Five retrospective cohort observational studies including 650 patients reported comparisons of treatment regimens (HyperCVAD, CODOX-M/IVAC, CALGB9251, BFM and CHOP/CHOEP/MEVA/other). Overall survival rates were highest in the patient groups receiving HyperCVAD (82.8%), BFM (77.8-81.7%) and CODOX-M/IVAC (68.6-74.5%) and lowest in the patient groups receiving CHOP/CHOEP/mmCHOP/MEVA/Other regimens (35.5-38.8%). From the two observational studies reporting adverse events, the CHOP-like regimens reported lower rates of adverse events (treatment related mortality, neutropenia, nadir fever) but higher rates of CNS progression compared to the other treatment regimens (HyperCVAD, CODOX-M/IVAC, CALGB9251, BFM).
Overall survival
Three observational studies (Wästerlid et al., 2013; Walewski et al., 2001; Wang et al., 2000) including 376 patients provided very low quality evidence of overall survival rates on the effectiveness of CODOX-M/IVAC compared to CHOP/CHOEP/MEVA/other. Reporting overall survival (range 1-2 years; follow-up median 37.5 months) rates of 68.6-79% in the CODOX-M/IVAC group compared to 30-42% in the CHOP/CHOEP/MEVA/other group. Walewski et al. (2001) reported that difference in overall survival was significant in their population (p=0.0003).
Two observational studies (Wästerlid et al., 2013; Smeland et al. 2004) including 200 patients provided very low quality evidence of overall survival rates on the effectiveness of BFM compared to CHOP/CHOEP/mmCHOP. Overall survival (range 2-5 years, follow-up 13-247 months) rates ranged from 65-81.7% in the BFM group and from 23-38.8% in the CHOP/CHOEP/mmCHOP group. Wästerlid et al. (2013) reported that the difference between BFM and CHOP/CHEOP was significant at the univariate level (p<0.001) but did not remain significant at the multivariate analyses (p=0.1).
The Wästerlid et al. (2013) study also provided very low quality evidence of overall survival rates when comparing BFM to HyperCVAD and CODOX-M/IVAC, reporting that patients receiving BFM had a two year survival rate of 81.7% compared to 82.8% of patients receiving HyperCVAD and 68.6% of patients receiving CODOX-M/IVAC. The authors reported that these differences were not significantly different.
Complete remission and adverse events
One observational study (Smeland et al., 2004) including 49 patients comparing BFM to mmCHOP provided very low quality evidence of higher complete remission rates (73.7% versus 53.8%), higher rates of event free survival (73.7% versus 30.8%) and no events of central nervous system progression (0% versus 30.8%) in the BFM group. However, the BFM group reported more treatment related mortality (10.5% versus 0%) and higher rates of febrile neutropenia (52.6% versus 0%) compared to the mmCHOP group.
One observational study (Wang et al., 2000) including 38 patients comparing CODOX-M/IVAC to other treatment regimens (>60% CHOP) provided very low quality evidence of higher complete remission rates (8% versus 41.2%). The patients receiving CODOX-M/IVAC reported higher rates of neutropenia (95.2% versus 64.7%) and Nadir fever (90.5% versus 58.8%) compared to the patients receiving other treatment regimens (>60% CHOP). The author did not report significance level of these differences.
4.5.1.1.2. Role of rituximab
The role of adding rituximab to treatment regimens (HyperCVAD, CODOX-M/IVAC, CALGB 9251, BFM, CHOP/CHOEP, B-NHL86, LMBA) was assessed in four retrospective cohort observational studies (Wildes et al., 2014; Wästerlid et al., 2013; Dujmovic et al., 2012; Barnes et al., 2011) inclusing 393 patients and one randomised control trial (Ribrag et al. 2012) including 257 patients.
Overall survival
The four observational studies provided very low quality evidence of an overall survival (range 2-5 years; follow-up mean 29.4 months) range of 70.2-83% in the chemotherapy plus rituximab group versus 29.4-66% in the chemotherapy alone. The RCT assessed the addition of rituximab to LMBA reporting very low quality evidence of 3-year overall survival (follow-up median 38 months) of 82% compared to 71.33% in the group treated with LMBA only. Three of the four observational studies and the RCT reported a significant benefit of the addition of rituximab to chemotherapy in overall survival (in all studies p<0.05). The fourth observational study reported a trend in favour of the addition of rituximab. However, the addition of rituximab to chemotherapy failed to remain significant in three observational studies that reported multivariate analyses (Wildes et al., 2014; Wästerlid et al., 2013; Barnes et al., 2011). Age, performance ≥2 and central nervous system involvement were all factors that remained significant at the multivariate level.
Event free survival
Three of the four observational studies (Wildes et al., 2014; Dujmovic et al., 2012; Barnes et al., 2011) and the RCT provided very low quality evidence of higher event free survival (range 3-5 years) in the patients receiving chemotherapy plus rituximab (60.6-83% [observational studies]; 75.8% [RCT]) compared to the patients receiving chemotherapy alone (29.4-61% [observational], 64.3% [RCT]). One of the three observational studies and the RCT reported that the difference in event free survival was significant (p<0.05). However, neither of these papers reported multivariate statistical analyses.
Complete remission
Three of the four observational studies (Wildes et al., 2014; Dujmovic et al., 2012; Barnes et al., 2011) provided very low quality evidence of higher rates of complete remission (follow-up mean 29.7 months) in the chemotherapy plus rituximab group (83.3-94.4%) compared to the chemotherapy alone group (37.5-85%). Only one of these studies reported that this difference was significant (p=0.035: Dujmovic et al., 2012). This study did not report multivariate statistical analyses.
Adverse events
The addition of rituximab to the regimens was associated with very low quality evidence of lower incidence of tumour lysis syndrome reported in two of the observational studies (5.8% versus 14.6%: Dujmovic et al., 2012; Barnes et al., 2011) but a higher incidence of sepsis (12.5% versus 7.5%) reported in one observational study (Barnes et al., 2011). Very low quality evidence of higher rates of treatment related mortality in the chemotherapy plus rituximab group were reported in one observational study (10% versus 5%; Barnes et al., 2011) and the RCT (7% versus 5.4%). No statistical information was provided by the studies regarding these reported differences.
4.5.1.1.3. Da-Epoch-R
No comparative evidence was found for the use of Da-epoch-R. One prospective non-comparative study including 30 patients using the WHO 2008 modern diagnostic criteria (Dunleavy et al. 2013) provided very low quality evidence for the rate of freedom from progression of disease at medium follow up of 95% (confidence interval [CI]: 75-99%) in the Da-epoch-r group and 100% (CI: 72-100%) in the Sc-epoch-rr group and overall survival rates of 100% (CI: 82-100%) and 90% (CI: 60-98%), respectively. No treatment related deaths were reported but in 19% of the treatment cycles there was fever and neutropenia resulting in hospital admission. In addition, 17% of the patients experienced a neurological sensory impairment after treatment and 7% experienced a neurological motor impairment.
4.5.1.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendations | Offer intensive immunochemotherapy to people with Burkitt lymphoma who are fit enough to tolerate it. Consider using one of the following:
Offer less intensive immunochemotherapy to people with Burkitt lymphoma who are not fit enough to tolerate intensive chemotherapy. Consider using one of the following, alone or supplemented with intravenous and/or intrathecal methotrexate:
|
|---|---|
| Relative value placed on the outcomes considered | The GC considered overall survival to be the critical outcome when drafting recommendations. The GC also considered the balance between achieving a higher overall survival at an increased risk of treatment related morbidity. There was no evidence available for health related quality of life (HRQoL). |
| Quality of the evidence | The quality of the evidence was very low, as assessed GRADE. Specific issues with the evidence included:
These issues limited the recommendations that the GC was able to make and instead of recommending a specific treatment regimen, the GC made a strong recommendation to use one of a range of more intensive therapies. Although the GC considered there was insufficient evidence to select a single intensive regimen above the others they thought that a strong recommendation for intensive therapy in general was appropriate. This was because there was very low quality but consistent evidence of higher overall survival rates with intensive therapy when compared with less intensive therapies. The uncertainty in the evidence was largely due to small patient numbers (due to the rarity of the disease) and the inability to implement a comparative trial due to country variation in treatment regimens. Patients receiving treatments such as rituximab based chemotherapy have good overall survival rates so implementing comparison arms in trials by withholding such treatment would not be considered advisable. For these reasons the GC made no research recommendation for this topic. None of the recommendations were based solely upon clinical experience. No research recommendation was made for this topic. |
| Trade-off between clinical benefits and harms | The GC considered that the potential benefits of the recommendations would be an increased overall survival rate. For patients able to tolerate intensive therapy the GC recommended a list of immunochemotherapy regimens based on the evidence which indicated that the addition of rituximab improved overall survival by more than 10% at 3 years with less than 2% increase in treatment related mortality. For those unable to tolerate intensive therapy the GC considered that less intensive immunochemotherapy regimens were more appropriate given this group would be less able to tolerate treatment toxicty. The GC noted that while the evidence indicated that some patients' disease will respond to these less intensive regimens, they are less effective than intensive regimens. The GC made the recommendation to consider DA-EPOCH-R for those with low risk Burkitt lymphoma on the basis of low quality evidence suggesting it is highly effective. In this study selected patients also received intrathecal methotrexate. For this reason the recommendation includes possible supplementation with methotrexate. The recommendations made may potentially lead to an increase in treatment morbidity. However, the benefit of increased overall survival compared to lower treatment related morbidity as a result of using less intensive chemotherapy regimens, was considered the most important outcome to patients with Burkitt lymphoma. |
| Trade-off between net health benefits and resource use | No health economic evidence was identified and no economic model was developed for this topic. The GC considered that there may be potential costs from these recommendations in terms of increased hospital admissions due to the use of more intensive chemotherapy regimens (and an increased rate of treatment morbidity). However, the increased overall survival from the use of intensive chemotherapy regimens would make this strategy more effective than alternatives and it was thought likely to be cost-effective in cost per QALY terms. |
| Other considerations | The GC noted that the recommendations would provide reinforcement for current best practice and ensure consistency in care for patients with Burkitt lymphoma. |
4.6. Peripheral T-cell lymphoma
Peripheral T-cell lymphoma (PTCL) is a cancer of mature T cells and accounts for roughly 10% of all non-Hodgkin's Lymphomas (NHL). There are a number of subtypes although the most common are peripheral T-cell lymphoma Not Otherwise Specified (PTCL-NOS) and Angioimmunoblastic T-cell Lymphoma (AITL). The other subtypes are much less common and are therefore not included in this analysis.
The cure rate, and survival rates for PTCL are worse than for the more common high grade B-cell NHL with data from the International Peripheral T-cell Lymphoma project showing that at 5 years after diagnosis, only 30-40% of patients are still alive and only 20-30% of patients have not relapsed. First line treatment for these patients consists of combination chemotherapy. The most frequently used regimen is CHOP (cyclophosphamide, vincristine, doxorubicin and prednisolone) which although reasonably well tolerated is associated with infections, nerve damage and (more rarely) cardiac damage. The reason for this regimen being standard of care is historical. Before the routine use of immunohistochemistry in diagnostics, T-cell and B-cell high grade lymphomas were treated together. Randomised clinical trials confirmed that CHOP was superior to a number of other, more intensive, combination chemotherapy regimens. With improvement in diagnostics, T-cell lymphomas could be reliably identified as a subset. Until rituximab was available for routine use as part of therapy for B-cell lymphomas, some trials included high grade T-cell and B-cell lymphomas together although interpreting the results for T-cell lymphomas is difficult due to their relatively small number.
The German High Grade Study Group published an influential report which retrospectively looked at T-cell lymphoma patients entered into a number of different prospective randomised high grade lymphoma trials. They performed subgroup analysis which suggested that patients had improved survival rates if they received the drug etoposide as part of their front line treatment regimen. This has led some groups to use etoposide (usually in the form of CHOEP) for first line treatment although it is associated with additional toxicity. Retrospective data has suggested that the use of an anthracycline (e.g. doxorubicin) adds no survival benefit, so other groups have abandoned CHOP as first line treatment altogether. Gemcitabine is an attractive drug to use in combination for PTCL, because it is not affected by proteins which pump chemotherapy drugs out of cells (the so-called P-glycoprotein) which are present in a number of T-cell lymphoma subtypes. Single centre series suggest gemcitabine containing chemotherapy regimens are effective (such as GEM-P) but other results (for example using the PEGS regimen) are disappointing. In the UK, the use of CHOP, CHOEP and gemcitabine-containing regimens is highly variable.
The main question to ask, then, is should CHOP remain the standard of care, or is there sufficient evidence to support the addition of etoposide, or the use of a different chemotherapy backbone altogether?
4.6.1. First line treatment
The recommendation from this section should be read in conjunction with the recommendations in section 4.6.2.
Clinical question: What is the most effective first-line treatment for people with peripheral T-cell lymphoma?
4.6.1.1. Clinical evidence (see section 4.6.1 in Appendix G)
Twenty three studies (two randomised control trials; two retrospective cohort studies, two phase II trials and 17 non-comparative studies [1 systematic review of 16 non-comparative studies]) reported evidence of the effectiveness of six chemotherapy regimens in 2,080 patients with peripheral T-cell lymphoma (PTCL). Of the comparative studies the five chemotherapy regimens were all compared to CHOP/CHOP like regimens.
4.6.1.1.1. Intensive chemotherapy versus CHOP/CHOP like
One retrospective cohort study (Xie et al. 2013) provided very low quality evidence of overall survival rates in 276 patients with peripheral T-cell lymphoma (56% PTCL-Not Otherwise Specified [PTCL-NOS] or Angioimmunioblastic T-cell lymphoma [AITL]). Overall survival was 38.9% in patients receiving intensive chemotherapy (ECHOP, Ara-C, Mesna, MINE, ESHAP, GDP, DHAP or Hyper-CVAD) compared to 16.7% in patients receiving CHOP/CHOP like chemotherapy (p<0.001).
4.6.1.1.2. CHOEP versus CHOP
One retrospective cohort study of patients ≤60 years of age with either PTCL-U or AITL treated on protocols of the German High-Grade Non-Hodgkin Lymphoma Study Group between 1993 and 2007 provided low quality evidence of 3 year event free survival rates of 60.7% in patients receiving CHOEP compared to 48.3% in patients receiving CHOP (p=0.057) (Schmitz et al. 2010). The 3-year overall and event free survival rates for the PTCL-U patients (n=70) were 53.9% (95% confidence interval [CI]: 41.7-66.1) and 41.1% (CI: 29.5-52.7), respectively. The 3-year overall and event free survival rates for the AITL patients (n=28) were 67.5% (CI: 50.1-84.9) and 50.0% (CI: 31.6-68.4), respectively.
4.6.1.1.3. VIP-rABVD versus CHOP
One randomised control trial (Simon et al. 2010) compared the effectiveness of VIP-rABVD to CHOP in patients with peripheral T-cell lymphoma (PTCL-NOS n: 58; AITL n: 15) provided moderate quality evidence of no overall survival benefit in patients in the VIP-rABVD arm compared to the patients in the CHOP arm (both 43 months survival rate) nor in the 2-year event free survival rate (45 ±8 versus 41 ±7: p=0.70). Complete response rates in the VIP-rABVD and the CHOP arms (44% versus 33%) and number of deaths during follow-up (n=27 versus 25) did not significantly differ, however, haematological toxicities were significantly higher in the VIP-rABVD arm with 23% versus 8% suffering grade 3-4 neutropenia (p<0.001) and 20% versus 2% had grade 3-4 thrombocytopenia (p<0.001). In addition, red blood cell and platelet transfusions were more frequent in the VIP-rABVD arm (p<0.001). Finally, the overall proportion of cycles resulting in hospitalisation for toxicity were significantly higher in the VIP-rABVD arm compared to the CHOP arm (15% versus 8%, p=0.04).
4.6.1.1.4. CyclOBEAP versus CHOP
One retrospective cohort study (Niitsu et al. 2008) provided very low quality evidence of 5-year overall survival in 101 patients with peripheral T-cell lymphoma (PTCL-U n=59; AITL n=42) of 61.7% in patients receiving CyclOBEAP compared to 25.7% in patients receiving CHOP. The 5-year progression free survival rate for the patients receiving CyclOBEAP was 59% compared to 22% in the CHOP group. The authors did not report whether the reported survival rates were significantly different. Niitsu et al. (2011) conducted a prospective non-comparative study of the effectiveness of CyclOBEAP in 84 patients with peripheral T-cell lymphoma. In the whole sample the 5 year overall and event free survival rates were 72% (CI: 66-79) and 61% (CI: 56-68), respectively, with a complete response rate of 92%. The 5-year overall survival rate for the PTCL-NOS sample (n=43) was 63% and for the AITL sample (n=27) 74%. The rates of grade 3-4 neutropenia, anaemia, grade 3-4 thrombocytopenia and non-haematological adverse events in the whole sample (n=84) were 95%, 71%, 29% and 38%. There were no treatment related deaths (follow-up median: 82 months).
4.6.1.1.5. CMED versus CHOP
One randomised controlled trial (Avilés et al. 2008) compared the effectiveness of CMED to CHOP in 217 patients with peripheral T-cell lymphoma unspecified (PTCL-U) reporting moderate quality evidence of an increased overall survival benefit in patients in the CMED arm compared to the patients in the CHOP arm (64% [CI: 68-79] versus 34% [CI: 31-46]; p<0.01) and increased progression free survival (70% [CI: 58-70] versus 43% [CI: 21-32]; p<0.01). The CMED arm had higher complete response rates compared to the CHOP arm (76% [CI: 77-94] versus 57% [CI: 57-69]; p<0.05). There were no treatment related deaths. Grade 1 thrombocytopenia rates in the CMED arm were 16% compared to 12% in the CHOP arm. The rates of hospitalisation due to toxicity were similar in both arms (CMED: 9% versus CHOP: 10%). 4% of patients in the CMED group reported anaemia compared to none in the CHOP group. Finally, more patients in the CHOP group (23%) suffered from granulocytopenia compared to the CMED group (13%). The authors do not report if the numbers of adverse events differed significantly between the two arms.
4.6.1.1.6. Anthracycline-based chemotherapy
One systematic review (AbouYabis et al. 2011) reported 16 studies assessing the use of anthracycline-based chemotherapies in PTCL-NOS (n=432), AITL (n=169) and non-ALCL PTCL (n=417) patients. Pooled statistics for the AITL patients provided very low quality evidence of a 5-year overall survival rate of 32.1% (CI: 27.2-37.5%) and a complete response rate of 42.1% (CI: 33.9-50.9%). Due to heterogeneity the studies with PTCL-NOS or non-ALCL PTCL patients were not pooled. The range of 5 year overall survival rates in the PTCL-NOS sample were 32-45% for 3 retrospective non-comparative studies and for the non-ALCL PTCL sample were 26 (one retrospective study)-35% (one prospective study). Complete response rates in patients with PTCL-NOS ranged from 17.1-57.1% in three prospective studies and 47-69.6% in six retrospective studies. Complete response rates in patients with non-ALCL PTCL ranged from 41-49% in two prospective studies and 58-59% in two retrospective studies.
4.6.1.1.7. CHOP + Avastin
One prospective non-comparative study (Advani et al. 2012) provided very low quality evidence for cardiac related adverse events in 44 patients treated with CHOP + Avastin. On average 20% of patients reported cardiac events (CI: 9.1-35.7) with 17% stopping the trial early due to congestive heart failure (CI: 5.6-34.7).
4.6.1.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendation | Consider CHOP chemotherapy as first-line treatment for people with peripheral T-cell lymphoma. |
|---|---|
| Relative value placed on the outcomes considered | Overall survival and treatment toxicity (treatment related mortality and morbidity) were considered the critical outcomes when drafting the recommendation. No evidence was identified for the outcome health related quality of life (HRQoL). |
| Quality of the evidence | The quality of evidence for the topic ranged from low to moderate as assessed using GRADE and NICE checklists for quantitative studies. Reasons for downgrading the quality included:
The GC noted that CMED is not currently used to treat patients with PTCL-NOS and AITL in the UK. In addition, the GC raised a number of issues concerning the RCT that compared CMED to CHOP:
|
| Trade off between clinical benefits and harms | The GC considered that the recommendation would discourage the use of more intensive/toxic induction therapies (e.g. CMED) when there is currently a lack of evidence to recommend they are better. Although evidence from one RCT showed a survival benefit in patients receiving CMED compared to patients receiving CHOP, with similar short term adverse event rates, the GC did not recommend CMED due to the issues with the trial listed above. The GC concluded the available evidence did not support a change in current practice and that the CHOP treatment regimen should continue to be used to treat patients with peripheral T-cell lymphoma, NOS, and angioimmunoblastic T-cell lymphoma. The GC noted that the recommendation to consider the use of CHOP is recognised within the clinical field as a treatment with limited success but that the current evidence base does not provide a suitable alternative. |
| Trade off between net health benefits and resource use | No health economic evidence was identified and no health economic model was built for this topic. The GC noted that the recommendation reflects current clinical practice and will not result in any additional costs, savings or change in practice. It was also thought that, in comparison to the alternatives, CHOP would be cost-effective. The cost of CHOP is similar to many of the alternative regimens and in terms of effectiveness, CHOP is thought to be the best option currently available (although, as mentioned above, it is recognised that CHOP is a treatment with limited success). |
| Other considerations | The GC noted that the recommendation is in line with the British Committee for Standards in Haematology. As the GC noted that there are current research activities in the target population (two ongoing trials, one currently recruiting within the UK) they agreed that a research recommendation for this topic was not necessary. |
4.6.2. Consolidation therapy in peripheral T-cell lymphoma
In an effort to improve the cure rate, high dose therapy with autologous stem cell transplantation (ASCT) in first remission has been employed for those who have responded to first-line chemotherapy. No randomised trials have been performed to investigate the role of either ASCT or allogeneic transplantation (alloHSCT) in PTCL. The best evidence comes from prospective, single arm studies, or from analyses of Registry data. Both have significant potential weaknesses, making definitive conclusions impossible and current practice contentious.
As with other lymphomas it is also possible to identify groups of patients with worse prognostic features. The possible role of alloHSCT has therefore been explored as consolidation either in those with higher risk features, or in younger patients in whom the toxicities and non-relapse-related procedural mortality are likely to be lower. The introduction of less toxic ‘reduced intensity’ alloHSCT regimens has more recently allowed evaluation of its role in older patients up to the age of 65 years.
The main alternative management strategy to transplantation is expectant observation following induction chemotherapy. Whilst this may appear economically favourable, it is important to acknowledge the subsequent costs of increasingly expensive salvage regimens in those destined to relapse, in many cases given with the intent to consolidate second remission by either ASCT or alloHSCT.
Clinical question: What is the effectiveness of high-dose consolidation of first-line therapy with autologous or allogeneic transplantation in people with peripheral T-cell lymphoma?
4.6.2.1. Clinical evidence (see section 4.6.2 in Appendix G)
Twenty studies (thirteen observational comparative studies [1 systematic review of 8 studies] and 7 non-comparative studies [1 systematic review of 5 non-comparative studies]) reported evidence of the effectiveness of consolidation therapy using stem-cell transplantation in 1,480 patients with peripheral T-cell lymphoma (PTCL).
4.6.2.1.1. Autologous transplantation versus chemotherapy alone
One systematic review (Yin et al. 2013) provided very low quality evidence of 3-year overall survival rates from two studies (PTCL-NOS and AITL, n=93) comparing patients who received either an autologous transplantation or chemotherapy alone after first line therapy finding no statistically significant difference between the two groups (Hazard ratio [HR]: 0.60; 95% confidence interval [CI]: 0.05-6.94). Six non-comparative studies of patients receiving consolidation therapy in first response (Mounier et al. 2004; 5 reported in Yin et al. 2013) provided very low quality evidence of 5-year overall survival rates between 52-62%. Finally Mounier et al. (2004) reported a 5 year disease free survival rate of 44% in patients receiving autologous transplantation.
One retrospective comparative observational study (Mehta et al. 2013) provided very low quality evidence of overall survival rates in 53 patients with peripheral T-cell lymphoma receiving consolidation therapy after first line therapy. In 32 patients with PTCL-Not Otherwise Specified (PTCL-NOS) the 4 year overall survival and progression free survival rates were 75% and 64.3% in the autologous group compared to 12.5% and 6.3% in the patients who received only chemotherapy. In 21 patients with angioimmunioblastic T-cell lymphoma [AITL] the 4 year overall survival and progression free survival rates were 62.8% and 48.2% in the autologous group compared to 66.7% and 33.3% in the patients who received only chemotherapy.
4.6.2.1.2. Complete response versus non-complete response
Very low quality evidence came from one systematic review (Yin et al. 2013) included three studies (n=149) comparing complete first response to non-complete first response prior to autologous transplantation. There was uncertainty about the difference in the overall survival of the two groups (HR: 3.17; 95% CI: 0.92-5.42). Three other studies (number of patients not provided by authors) compared complete response to partial response prior to autologous transplantation finding no statistically significant difference between the two groups (HR: 0.73; 95% CI: 0.36-1.48).
4.6.2.1.3. Allogeneic transplantation versus chemotherapy alone
Very low quality evidence about overall survival came from one retrospective cohort study (Mehta et al. 2013) in five patients with peripheral T-cell lymphoma receiving allogeneic consolidation therapy after first line therapy. In 4 patients with PTCL-NOS the 4 year overall survival and progression free survival rates were 100% and 50% in the allogeneic group compared to 12.5% and 6.3% in the patients who received only chemotherapy (n=26). One patient with AITL received an allogeneic transplantation but did not survive.
One non-comparative study (Le Gouill et al. 2008) provided very low quality evidence about complete response rates in PTCL-NOS (n=27) and AITL (n=11) patients receiving allogeneic transplantation of 22% and 9%, respectively and 5-year overall survival rates of 63% and 80%. The Le Gouill et al. (2008) study contained patients receiving consolidation therapy after more than one line of therapy although the exact numbers were not reported.
4.6.2.1.4. Allogeneic or autologous transplantation versus chemotherapy alone
One retrospective comparative study (Broussais-Guillaumot et al. 2013) compared peripheral T-cell lymphoma patients (PTCL-U n=81; AITL n=52; 19.7% complete first response) who had received either an autologous or allogeneic transplantation (n=75) to patients who received chemotherapy alone (n=133). rVery low quality evidence from this study indicated median overall survival of 51 months in the transplantation group compared to 15 months in the chemotherapy alone group.
4.6.2.1.5. Allogeneic versus autologous transplantation
One prospective comparative observational study (Corradini et al. 2014) of 61 patients with peripheral T-cell lymphoma (n=33 PTCL-NOS and n=14 AILT), of which 23 received an allogeneic stem cell transplant and 14 received an autologous stem cell transplant provided very low quality evidence about four-year overall and progression free survival rates of 92% and 70% in the autologous group versus 69% and 69% in the allogeneic group. The authors reported that there were no significant differences between transplant types.
One retrospective comparative observational study (Mehta et al. 2013) provided very low quality evidence about overall survival rates in five patients with peripheral T-cell lymphoma receiving allogeneic consolidation therapy after first line therapy compared to 34 patients receiving autologous consolidation therapy. In 32 patients with PTCL-Not Otherwise Specified (PTCL-NOS) the 4 year overall survival and progression free survival rates were 75% and 64.3% in the autologous group compared to 12.5% and 6.3% in the patients who received only chemotherapy. In 17 patients with AITL the 4 year overall survival and progression free survival rates were 62.8% and 48.2% in the autologous group (n=16) compared to 0% in the one patient who received allogeneic transplantation.
Very low quality evidence came from one retrospective comparative observational study (Smith et al. 2013) including 241 patients with peripheral T-cell lymphoma (PTCL-U n=102, AITL n=27), of which 24% were receiving transplantation in their first complete response. In 102 PTCL-U patients the one and three year progression free survival rates for the autologous transplantation group (n=39) were 60% (CI: 43-74%) and 29% (CI: 14-47) compared to the allogeneic group (n=63) 40% (CI: 28-52) and 33% (CI: 22-45). The one and three year overall survival rates for the autologous transplantation group (n=39) were 64% (CI: 46-77%) and 45% (CI: 27-62) compared to the allogeneic group (n=63) 52% (CI: 38-64) and 42% (CI: 30-55). The non-relapse mortality rates at one and three years in the autologous group were 3% (CI: 0-12) and 3% (CI: 0-12) compared to 16% (CI: 8-26) and 28% (CI: 17-39) in the allogeneic group. The three year chronic GVHD rate was 43% in the allogeneic group.
In 27 AITL patients the one and three year progression free survival rates for the autologous transplantation group (n=15) were 53% (CI: 26-74%) and 47% (CI: 21-69) compared to the allogeneic group (n=12) 67% (CI: 34-86) and 67% (CI: 34-86). The one and three year overall survival rates for the autologous transplantation group (n=15) were 60% (CI: 35-82%) and 51% (CI: 26-76) compared to the allogeneic group (n=12) 92% (CI: 70-100) and 83% (CI: 56-98).
The 3 year progression free survival rate for patients in their first complete response (n=40 was 58% with a one and three year overall survival rate of 80% and 70%, respectively.
4.6.2.1.6. Allogeneic transplantation versus high dose methotrexate
One retrospective comparative observational study (Iriyama et al. 2013) provided very low quality evidence about 3 and 5 year relapse rates in 28 patients with peripheral T-cell lymphoma (PTCL-NOS n=13, AITL n=11) receiving autologous transplantation (n=18) or high dose Methotrexate (n=10) consolidation therapy after first line therapy. The 3 and 5 year relapse rates were 68% and 53% versus 58% and 40%.
4.6.2.2. Cost-effectiveness evidence
A literature review of published cost-effectiveness analyses did not identify any relevant papers for this topic. Whilst there were potential cost implications of making recommendations in this area, other questions in the guideline were agreed as higher priorities for economic evaluation. Consequently no further economic modelling was undertaken for this question.
| Recommendation | Consider consolidation with autologous stem cell transplantation for people with chemosensitive peripheral T-cell lymphoma (that is, there has been at least a partial response to first-line chemotherapy) who are fit enough for transplantation. |
|---|---|
| Relative value placed on the outcomes considered | Overall survival and toxicity (treatment related mortality and morbidity) were considered to be the critical outcomes when wording the recommendation. No evidence was identified relating to health related quality of life (HRQoL) A number of the included articles presented outcomes (e.g. survival rates) by response rate to first line therapy and therefore the GC could establish the value of transplantation according to response to first line therapies. |
| Quality of the evidence | All the evidence for each outcome was rated as very low quality as assessed using GRADE and NICE checklists for quantitative studies. The primary reason for downgrading the quality of evidence was imprecision with wide confidence intervals as a resuit of small sample size or low event rate.. A number of studies were downgraded due to serious indirectness as a result of not providing a breakdown for each included subtype of peripheral T-cell lymphoma included in the PICO. Some studies also included a minority of patients with subtypes of peripheral T-cell lymphoma not included in the PICO. Other studies included populations of patients who had received more than one line of systemic therapy or included children (<16 years of age). These studies were included due to a lack of higher quality direct evidence, given the relative rarity of peripheral T-cell lymphoma. Due to the low quality of the evidence the GC could not make strong recommendations for the use of autologous transplantation. The GC did not make a recommendation concerning the use of allogeneic transplantation for patients with peripheral T-cell lymphoma as part of first-line therapy, since allogeneic transplantation has mainly been reserved for patients beyond first-line therapy. The only prospective study directly addressing this issue was relatively small and used a donor/no donor strategy to allocate transplant modality (Corradini et al, 2014). |
| Trade off between clinical benefits and harms | The evidence indicated better survival in patients receiving consolidation with autologous transplantation. As a result the main benefit associated with the recommendation is likely to be increased overall survival and progression free survival. The GC noted that they have recommended the use of a toxic treatment which may result in an increase in treatment related morbidity. The GC considered that the survival outcomes for patients with PTCL-NOS or AITL are poor with chemotherapy alone and therefore concluded that the potential for increased survival benefits in these patients using consolidation therapy is important and on balance outweighed the possible harms. |
| Trade off between net health benefits and resource use | No health economic evidence was identified and no health economic model was built. The recommendation reflects current practice, so the GC did not expect an increase in costs overall. Despite the higher upfront costs and potential adverse events associated with autologous transplantation, its use was considered likely to be cost-effective because of improvements in progression free survival and overall survival. These survival improvements should make the strategy more effective in QALY terms and should also produce downstream cost savings through a reduction in the need for further therapies (for example salvage therapy). Therefore, the GC thought the recommendation is likely to be cost-effective in cost per QALY terms. |
| Other considerations | The recommendation reflects current practice so the GC agreed there should be no change in practice. The GC noted that there is a need for research in the patients with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) and Angioimmunioblastic lymphoma (AITL) undergoing first line therapy, but considered that it would not be possible to address issues relating to transplant modality in a randomised fashion due to small patient populations. Although cohort studies are feasible, the GC noted that such studies have already been carried out and did not provide convincing evidence about transplant modality. The GC noted that the recommendation is in line with the British Committee for Standards in Haematology for the treatment of patients with peripheral T-cell lymphoma. |
References
- Abouyabis AN, Shenoy PJ, Sinha R, et al. systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral t-cell lymphoma. ISRN Hematology. 2011;2011 [PMC free article: PMC3197255] [PubMed: 22084700]
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood. 2014;124(8):1288–1295. [PubMed: 24859361]
- Abramson JS, Feldman T, Kroll-Desrosiers AR, et al. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Annals of Oncology. 2014;25(11):2211–2217. [PMC free article: PMC4481543] [PubMed: 25193992]
- Advani RH, Hong FX, Horning SJ, et al. Cardiac toxicity associated with bevacizumab (Avastin) in combination with CHOP chemotherapy for peripheral T cell lymphoma in ECOG 2404 trial. Leukemia & Lymphoma. 2012;53(4):718–720. [PMC free article: PMC3919492] [PubMed: 21916830]
- Ahmadi T, McQuade J, Porter D, et al. Potential prolongation of PFS in mantle cell lymphoma after R-HyperCVAD: auto-SCT consolidation or Rituximab maintenance. Bone Marrow Transplantation. 2012;47(8):1082–1086. [PubMed: 22080969]
- Arcarini L, Morello L, Tucci A, et al. Autologous stem cell transplantation with in vivo purged progenitor cell shows long-term efficacy in relapsed/refractory follicular lymphoma. Am,J Haematol. 2015;90(3):230–234. [PubMed: 25502635]
- Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362(9383):516–522. [PubMed: 12932382]
- Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. The Lancet Oncology. 2014;15(4):424–435. [PubMed: 24602760]
- Arkenau HT, Chong G, Cunningham D, et al. The role of intrathecal chemotherapy prophylaxis in patients with diffuse large B-cell lymphoma. Annals of Oncology. 2007;18(3):541–545. [PubMed: 17164228]
- Aviles A, Castaneda C, Neri N, et al. Results of a phase III clinical trial: CHOP versus CMED in peripheral T-cell lymphoma unspecified. Medical Oncology. 2008;25(3):360–364. [PubMed: 18247163]
- Aviles A, Jesus Nambo M, et al. Central nervous system prophylaxis in patients with aggressive diffuse large B cell lymphoma: an analysis of 3,258 patients in a single center. Medical Oncology. 2013;30(2):520. [PubMed: 23456620]
- Aviles A, Nambo MJ, Neri N, et al. Mucosa-associated lymphoid tissue (MALT) lymphoma of the stomach: results of a controlled clinical trial. Medical Oncology. 2005;22(1):57–62. [PubMed: 15750197]
- Aviles A, Neri N, Delgado S, et al. Residual disease after chemotherapy in aggressive malignant lymphoma: the role of radiotherapy. Medical Oncology. 2005;22(4):383–387. [PubMed: 16260856]
- Aviles A, Neri N, Nambo MJ, et al. Surgery and chemotherapy versus chemotherapy as treatment of high-grade MALT gastric lymphoma. Medical Oncology. 2006;23(2):295–300. [PubMed: 16720930]
- Avivi I, Canals C, Vernant JP, et al. Matched unrelated donor allogeneic transplantation provides comparable long-term outcome to HLA-identical sibling transplantation in relapsed diffuse large B-cell lymphoma. Bone Marrow Transplantation. 2014;49:671–678. [PubMed: 24510071]
- Bachy E, Houot R, Morschhauser F, et al. Long-term follow up of the FL2000 study comparing CHVP-interferon to CHVP-interferon plus rituximab in follicular lymphoma. Haematologica. 2013;98(7):1107–1114. [PMC free article: PMC3696615] [PubMed: 23645690]
- Ban-Hoefen M, Vanderplas A, Crosby-Thompson AL, et al. Transformed non-Hodgkin lymphoma in the rituximab era: analysis of the NCCN outcomes database. British Journal of Haematology. 2013;163(4):487–495. [PubMed: 24111533]
- Barnes JA, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt's lymphoma: a retrospective analysis. Annals of Oncology. 2011;22(8):1859–1864. [PubMed: 21339382]
- Barzenje DA, Cvancarova SM, Liestol K, et al. Radiotherapy Compared to Other Strategies in the Treatment of Stage I/II Follicular Lymphoma: A Study of 404 Patients with a Median Follow-Up of 15 Years. PLoS ONE. 2015;10:e0131158. [Electronic Resource] [PMC free article: PMC4492987] [PubMed: 26147646]
- Bernard M, Gressin R, Lefrere F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15(11):1785–1791. [PubMed: 11681422]
- Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin's lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. Journal of Clinical Oncology. 2009;27(1):114–119. [PMC free article: PMC4879698] [PubMed: 19047289]
- Besa PC, McLaughlin PW, Cox JD, et al. Long term assessment of patterns of treatment failure and survival in patients with stage I or II follicular lymphoma. Cancer. 1995;75(9):2361–2367. [PubMed: 7712449]
- Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113(17):3896–3902. [PubMed: 19144985]
- Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 1997;15(3):1110–1117. [PubMed: 9060552]
- Broussais-Guillaumot F, et al. Peripheral T-cell lymphomas: analysis of histology, staging and response to treatment of 208 cases at a single institution. Leukemia and Lymphoma. 2013;54(11):2392–2398. [PubMed: 23410100]
- Calvo-Villas JM, Martin A, Panizo C, et al. Impact of rituximab-based therapy after histological transformation on high-dose therapy and autologous stem cell transplantation in follicular transformed lymphomas. Blood. 2011;118(21)
- Cheah CY, Herbert KE, O'Rourke K, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. British Journal of Cancer. 2014;111(6):1072–1079. [PMC free article: PMC4453849] [PubMed: 25072255]
- Choi YJ, Kim N, Paik JH, et al. Characteristics of Helicobacter pylori-positive and Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma and their influence on clinical outcome. Helicobacter. 2013;18(3):197–205. [PubMed: 23305046]
- Corradini P, et al. Intensified chemo-immunotherapy with or without stem cell transplantation in newly diagnosed patients with peripheral T-cell lymphoma. Leukemia. 2014 published online: February 20th 2014. [PubMed: 24662801]
- Cortelazzo S, Magni M, Tarella C, et al. Update of a GITIL cohort study: Frontline high dose sequential chemotherapy with rituximab and autologous stem cell transplantation induces a high rate of long-term remissions in patients with mantle cell lymphoma. Blood. 2007;110(11):386A–386A.
- Crump M, Kuruvilla J, Couban S, et al. Gemcitabine, dexamethasone, cisplatin (GDP) comparedwith dexamethasone,cytarabine, cisplatin (DHAP) salvagechemotherapy prior to autologous stem cell transplantation for relapsed and refractory aggressive lymphomas: Final result of the phase III NCIC CTG study LY12; Hematological Oncology; Conference: 12th International Conference on Malignant Lymphoma; Lugano Switzerland. Conference Start: 20130619 Conference End: 20130622; Jun, 2013. Conference Publication: (var.pagings)
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. Journal of Clinical Oncology. 2014;32:01. [PubMed: 25267740]
- Cuccuini W, Briere J, Mounier N, et al. MYC#SUP#+#/SUP# Diffuse Large B Cell Lymphomas (DLBCL) treated in randomized prospective salvage therapy, RICE or RDHAP followed by beam plus Autologous Stem Cell Transplantation (ASCT). A BioCORAL report; Blood; Conference: 53rd Annual Meeting of the American Society of Hematology, ASH 2011; San Diego, CA United States. Conference Start: 20111210 Conference End: 20111213; 2011. p. 18. Conference Publication: (var.pagings)
- Cuccuini W, Briere J, Mounier N, et al. MYC+ diffuse large B-cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood. 2012;119:4619–4624. [PMC free article: PMC3815438] [PubMed: 22408263]
- Curtis L. Unit Costs of Health and Social Care 2014. Personal Social Services Research Unit (PSSRU), University of Kent; Canterbury: 2014.
- Dabaja B, Tsang RW, Qi S, et al. Favorable outcome in stage I-II mantle cell lymphoma: A report of 160 patients from the international lymphoma radiation oncology group (ILROG). International Journal of Radiation Oncology Biology Physics. 2014;90(1 SUPPL. 1):S151–S152.
- De Fontbrune F, Sibon D, Resche-Rigon M, et al. Allogeneic Versus Autologous Stem Cell Transplantation in Patients with Relapsing Follicular Lymphoma: Use of the Propensity Score to Reduce Recruitment Bias in A Retrospective Comparative Study. Haematologica-the Hematology Journal. 2009;94:290–290.
- Deng L, Song Y, Zhu J, et al. Secondary central nervous system involvement in 599 patients with diffuse large B-cell lymphoma: are there any changes in the rituximab era? International Journal of Hematology. 2013;98(6):664–671. [PubMed: 24234713]
- Deshpande AT, Bociek GR, Bierman PJ, et al. Long term outcome following autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Biology of Blood and Marrow Transplantation. 2004;10(2):28–28.
- Dorth JA, Prosnitz LR, Broadwater G, et al. Impact of consolidation radiation therapy in stage III-IV diffuse large B-cell lymphoma with negative post-chemotherapy radiologic imaging. International Journal of Radiation Oncology, Biology, Physics. 2012;84:762–767. [PubMed: 22420972]
- Dreyling M, Lenz G, Hoster E, V, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677–2684. [PubMed: 15591112]
- Drugs and pharmaceutical electronic market information (eMit). London: UK Department of Health; [database on the internet]
- Dujmovic D, et al. Addition of rituximab to high-dose methotrexate-based chemotherapy improves survival of adults with Burkitt lymphoma/leukemia. Acta Haematologica. 2012;127(2):115–117. [PubMed: 22212303]
- Eide MB, Lauritzsen GF, Kvalheim G, et al. High dose chemotherapy with autologous stem cell support for patients with histologically transformed B-cell non-Hodgkin lymphomas. A Norwegian multi centre phase II study. British Journal of Haematology. 2011;152(5):600–610. [Review] [PubMed: 21241276]
- Evens AM, Vanderplas A, Lacasce AS, et al. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: A comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119(20):3662–3671. [PubMed: 23921646]
- Fagnoni P, Milpied N, Limat S, et al. Cost effectiveness of high-dose chemotherapy with autologous stem cell support as initial treatment of aggressive non-Hodgkin's Lymphoma. Pharmacoeconomics. 2009;27(1):55–68. [PubMed: 19178124]
- Feldman T, Mato AR, Zielonka T, et al. Effect of front-line therapy with either high-dose therapy and autologous stem cell rescue (HDT/ASCR) or dose-intensive therapy (R-Hypercvad) on outcome in mantle cell lymphoma (MCL). Journal of Clinical Oncology. 2010;28(15 SUPPL. 1)
- Ferreri AJM, et al. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. British Journal of Haematology. 2015;168:654–662. [PubMed: 25312994]
- Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Annals of Oncology. 2004;15(1):129–133. [PubMed: 14679132]
- Frosch Z, Luskin MR, Landsburg DJ, et al. D. R-CHOP or R-HyperCVAD with or without autologous stem cell transplantation for older patients with mantle cell lymphoma. Clinical lymphoma, myeloma & leukemia. 2015;15(2):92–97. [PubMed: 25174772]
- Georghiou T, Bardsley M. Exploring the cost of care at the end of life. Nuffield Trust. 2014;2014
- Gisselbrecht C, Glass B, Mounier N, et al. R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by autologous stem cell transplantation: CORAL study; Journal of Clinical Oncology; Conference: 2009 Annual Meeting of the American Society of Clinical Oncology; ASCO Orlando, FL United States. Conference Start: 20090529 Conference End: 20090602; 2009. p. 20. Conference Publication: (var.pagings)
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of Clinical Oncology. 2010;28:4184–4190. [Erratum appears in J Clin Oncol. 2012 May 20;30(15):1896] [PMC free article: PMC3664033] [PubMed: 20660832]
- Gisselbrecht C, Glass B, Fournier M, et al. Salvage regimen with autologous stem cell transplantation with or without rituximab maintenance for relapsed diffuse large B-cell lymphoma (DLBCL): Coral final report; Annals of Oncology; Conference: 11th International Conference on Malignant Lymphoma; Lugano Switzerland. Conference Start: 20110615 Conference End: 20110618; Jun, 2011. Conference Publication: (var.pagings)
- Gisselbrecht C, Glass B, Laurent G, et al. Maintenance with rituximab after autologous stem cell transplantation in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL): CORAL final analysis; Journal of Clinical Oncology; Conference: ASCO Annual Meeting 2011; Chicago, IL United States. Conference Start: 20110603 Conference End: 20110607; 2011. p. 20. Conference Publication: (var.pagings)
- Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. Journal of Clinical Oncology. 2012;30:4462–4469. [PMC free article: PMC3646314] [PubMed: 23091101]
- Le Gouill S, de Guibert S, Planche L, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica. 2011;96(8):1128–1135. [PMC free article: PMC3148906] [PubMed: 21486862]
- Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab maintenance versus wait and watch after four courses of R-DHAP followed by autologous stem cell transplantation in previously untreated young patients with mantle cell lymphoma: First interim analysis of the phase iii prospective LyMa trial, a LYSA study; Blood; Conference: 56th Annual Meeting of the American Society of Hematology, ASH 2014; San Francisco, CA United States. Conference Start: 20141206 Conference End: 20141209; 2014. p. 06. Conference Publication: (var.pagings)
- Le Gouill, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Française de Greffe de Moëlle et de Thérapie Cellulaire. Journal of Clinical Oncology. 2008;26(14):2264–2271. [PubMed: 18390969]
- Gouill S, Thieblemont C, Gyan E, et al. High response rate after 4 courses of R-DHAP in untreated mantle cell lymphoma (MCL) patients in the ongoing phase III randomized GOELAMS and GELA LyMa trial. Blood. 2010;116(21) [Abstract No. 1758]
- Grauer A, Hamadani M, Blum KA, et al. Allogeneic Versus Autologous Stem Cell Transplantation (Sct) for Follicular Lymphoma (Fl). the James Comprehensive Cancer Center Experience. Biology of Blood and Marrow Transplantation. 2009;15(2):132–132.
- Griffiths R, Mikhael J, Gleeson M, et al. Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. 2011;118(18):4808–4816. [PMC free article: PMC3208292] [PubMed: 21873544]
- Guirguis HR, Cheung MC, Mahrous M, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. British Journal of Haematology. 2012;159(1):39–49. [PubMed: 22849793]
- Hagberg H, Gisselbrecht C., CORAL study group. Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: an update of the CORAL study. Annals of Oncology. 2006;17 Suppl-2 [PubMed: 16702182]
- Hancock BW, Qian W, Linch D, et al. Chlorambucil versus observation after anti-Helicobacter therapy in gastric MALT lymphomas: results of the international randomised LY03 trial. British Journal of Haematology. 2009;144(3):367–375. [PMC free article: PMC2659366] [PubMed: 19036078]
- Hasselblom S, Ridell B, Wedel H, et al. Testicular lymphoma--a retrospective, population-based, clinical and immunohistochemical study. Acta Oncologica. 2004;43(8):758–765. [PubMed: 15764222]
- Held G, Murawski N, Ziepert M, et al. Role of radiotherapy to bulky disease to elderly patients with aggressive B-cell lymphoma. Journal of Clinical Oncology. 2014 [PubMed: 24493716] [CrossRef]
- Henzelmann F, Bethgel W, Beelen DW, et al. Allogeneic haemopoetic cell transplantation as curative therapy for non-transformed follicular lymphomas: long term follow-up data of the German Registry for Stem Cell Transplantation (DRST); Bone Marrow Transplantation; Conference: 41st Annual Meeting of the European Society for Blood and Marrow Transplantation; Istanbul Turkey. 2015. pp. ppS66–67.
- Hermine OR, et al. Alternating courses of 3× chop and 3× dhap plus rituximab followed by a high dose ara-c containing myeloablative regimen and autologous stem cell transplantation (asct) increases overall survival when compared with six courses of chop plus rituximab followed by myeloablative radiochemotherapy and asct in mantle cell lymphoma: final analysis of the MCL younger trial of the European mantle cell lymphoma netword (MCL NET). Blood. 2012;120(21)
- Herold M, Haas A, Srock S, et al. Immunochemotherapy (R-MCP) is not superior to chemotherapy (MCP) alone in advanced mantle cell lymphoma - 42 months follow up results of the OSHO 39 study. Blood. 2007;110(11):189b. [Abstract No. 4474]
- Hicks L, Connors JM, Mangel J, et al. Autologous stem-cell transplant with a Rituximab purge and maintenance vs. standard chemotherapy for mantle cell lymphoma: Extended follow-up of a matched pair analysis. Blood. 2006;108(11):868A–869A.
- Hornberger J, Reyes C, Lubeck D, et al. Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma. Leukemia and Lymphoma. 2008;49(2):227–236. [PMC free article: PMC2430747] [PubMed: 18231908]
- Hoster E, Unterhalt M, Wormann B, et al. The Addition of Rituximab to First-Line Chemotherapy (R-CHOP) Results in Superior Response Rates, Time to Treatment Failure and Response Duration in Patients with Advanced Stage Mantle Cell Lymphoma: Long Term Results of a Randomized GLSG Trial. Blood. 2008;112(11):1048. [Abstract No. 3049]
- Iriyama N, Takahashi H, Hatta Y, et al. Efficacy of a dose-intensified CHOP (Double-CHOP) regimen for peripheral T-cell lymphomas. Oncology Reports. 2013;29(2):805–811. [PubMed: 23166041]
- Jagadeesh D, Rybicki L, Abounader DM, et al. Autologous Stem Cell Transplantation for Follicular Lymphoma in the Era of Rituximab: Cleveland Clinic Experience. Blood. 2014;124(21)
- Joint Formulary Committee. British National Formulary (online). London: BMJ Group and Pharmaceutical Press;
- Kalpadakis C, Pangalis GA, Vassilakopoulos TP, et al. Chlorambucil with or without rituximab is an effective therapy in non-gastric MALT lymphomas. Haematologica. 2009;94:398–399.
- Kang BW, Sohn SK, Moon JH, et al. Clinical features and treatment outcomes in patients with mantle cell lymphoma in Korea: Study by the Consortium for Improving Survival of Lymphoma. Blood Research. 2014;49(1):15–21. [PMC free article: PMC3974951] [PubMed: 24724062]
- Khera N, Emmert A, Storer BE, et al. Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. The Oncologist. 2014;19:1–6. [PMC free article: PMC4041670] [PubMed: 24797822]
- Khour iIF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–6. [PMC free article: PMC4624452] [PubMed: 18411419]
- Khouri IF, Saliba RM, Hosing CM, et al. Autologous stem cell (AUTO) vs. non-myeloablative allogeneic transplantation (NMT) after high-dose rituximab (HD-R) - Containing conditioning regimens for relapsed chemosensitive follicular lymphoma (FL). Blood. 2005;106(11):19A–19A.
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. New England Journal of Medicine. 2012;367(6):520–531. [PubMed: 22873532]
- Klyuchnikov E, Bacher U, Kroger N, et al. Reduced Intensity Conditionining (RIC) Allo Transplantation is Associated with Superior Long-term Disease Control in Relapsed/Refractory Grade I/II Folliculr Lymphoma; 20th Congress of the European Hematology Association; Vienna. 2014. p. pp13.
- Kondo S, Niwa Y, Tajika M, et al. Feasibility of watch-and-wait strategy for histological relapse of gastric MALT lymphoma after helicobacter pylori eradication therapy. Gastroenterology. 2012;142(5 SUPPL. 1):S761.
- Kumar A, Vanderplas A, LaCasce AS, et al. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer. 2012;118(11):2944–2951. [PubMed: 22006274]
- Kusano Y, Terui Y, Nishimura N, et al. ICE (ifosfamide, carboplatin, and etoposide) was the best salvage regimen in patients with relapsed or refractory malignant lymphoma; Blood; Conference: 56th Annual Meeting of the American Society of Hematology, ASH 2014; San Francisco, CA United States. Conference Start: 20141206 Conference End: 20141209; 2014. p. 06. Conference Publication: (var.pagings)
- LaCasce AS, Vandergrift JL, Rodriguez MA, et al. Comparative outcome of initial therapy for younger patients with mantle cell lymphoma: An analysis from the NCCN NHL Database. Blood. 2012;119(9):2093–2099. [PubMed: 22234679]
- Leger C, Sabloff M, McDiarmid S, et al. Outpatient autologous hematopoietic stem cell transplantation for patients with relapsed follicular lymphoma. Annals of Hematology. 2006;85:723–729. [PubMed: 16832675]
- Leitch HA, Gascoyne RD, Chhanabhai M, et al. Limited-stage mantle-cell lymphoma. Annals of Oncology. 2003;14(10):1555–1561. [PubMed: 14504058]
- Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Journal of Clinical Oncology. 2005;23(9):1984–1992. [PubMed: 15668467]
- Leux C, Maynadie M, Troussard X, et al. Mantle cell lymphoma epidemiology: A population-based study in France. Annals of Hematology. 2014;93(8):1327–1333. [PubMed: 24763513]
- Lunning MA, Maragulia JC, Moskowitz CH, et al. Remission duration < 12 months for early relapsed and refractory follicular lymphoma is predictive of early failures post-high dose therapy and autologous stem cell rescue. Blood. 2012;120(21)
- Mac Manus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. Journal of Clinical Oncology. 1996;14(4):1282–1290. [PubMed: 8648385]
- Marcheselli L, Marcheselli R, Bari A, et al. Radiation therapy improves treatment outcome in patients with diffuse large B-cell lymphoma. Leukemia & Lymphoma. 2011;52:1867–1872. [PubMed: 21663499]
- Madsen C, Pedersen MB, Segel EK, et al. Upfront autologous stem-cell transplantation in transformed indolent non-hodgkins lymphoma: An outcome analysis. Hematological Oncology. 2013;31:164.
- Madsen C, et al. Outcome determinants for transformed indolent lymphomas treated with or without autologous stem-cell transplantation. Annals of Oncology. 2015;26(2) [PubMed: 25411416]
- Mangel J, Leitch HA, Connors JM, et al. Intensive chemotherapy and autologous stem-cell transplantation plus Rituximab is superior to conventional chemotherapy for newly diagnosed advanced stage mantle-cell lymphoma: a matched pair analysis. Annals of Oncology. 2004;15(2):283–290. [PubMed: 14760123]
- Martin P, Chadburn A, Christos P, Weil K, Furman RR, Ruan J, Elstrom R, Niesvizky R, Ely S, Diliberto M, Melnick A, Knowles DM, Chen-Kiang S, Coleman M, Leonard JP. Outcome of deferred initial therapy in mantle-cell lymphoma. Journal of Clinical Oncology. 2009 Mar 10;27(8):1209–1213. [PubMed: 19188674]
- McNamara C, Davies J, Dyer M, Hoskin P, Illidge T, Lyttleton M, Marcus R, Montoto S, Ramsay A, Wong WL, Ardeshna K. Guidelines on the investigation and management of follicular lymphoma. British Journal of Haematology. 156:446–467. [PubMed: 22211428]
- Mehta N, Maragulia JC, Moskowitz A, Hamlin PA, Lunning MA, Moskowitz CH, Zelenetz A, Matasar MJ, Sauter C, Goldberg J, Horwitz SM. A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clinical lymphoma, myeloma & leukemia. 2013;13(6):664–670. [PubMed: 24035712]
- Mian M, Capello D, Ventre MB, Grazio D, Svaldi M, Rossi A, Tsang R, Gospodarowicz MK, Oldani E, Federico M, Luminari S, Marcheselli L, Pogliani EM, Rossini F, Cabrera ME, Martelli M, Gutierrez-Garcia G, Busetto M, Visco C, Fiegl M, Rossi D, Gaidano G, Cavalli F, Zucca E, Rambaldi A, Cortelazzo S. Early-stage diffuse large B cell lymphoma of the head and neck: Clinico-biological characterization and 18 year follow-up of 488 patients (IELSG 23 study). Annals of Hematology. 2014;93(2):221–231. [PubMed: 23959436]
- Micallef IN, Remstein ED, Ansell SM, Colgan JP, Inwards DJ, Johnston PB, Lewis JT, Markovic SN, Porrata LF, White WL, Witzig TE, Ristow K, Habermann TM. The International Prognostic Index predicts outcome after histological transformation of low-grade non-Hodgkin lymphoma. Leukemia & Lymphoma. 2006;47(9):1794–1799. [PubMed: 17064990]
- Miura K, Takasaki H, Tsujimura H, Kanno M, Maeda Y, Tomita N, Takai K, Masaki Y, Takizawa J, Mori H, Terasaki Y, Yoshida T, Takeuchi J, Motomura S. Does more intensive therapy have effects on mantle cell lymphoma? A clinical experience from the Lymphoma Treatment Study Group in Japan. International Journal of Hematology. 2011;93(5):684–686. [PubMed: 21479980]
- Mondello P, Steiner N, Wasle I, Pitini V, Mian M. Radiotherapy for stage I/II follicular lymphoma (FL): is it time for a re-appraisal? Anticancer Research. 2014;34(11):6701–6704. [PubMed: 25368277]
- Pereira D. Advanced stage follicular lymphoma-watch and wait or immunochemotherapy? Blood. 2014;(21) Conference(var.pagings)
- Morabito F, Stelitano C, Marcheselli L, Callea V, Di Renzo N, Gobbi P, Brugiatelli M, Federico M. Incidence and risk factors for central nervous system (CNS) occurrence in patients with diffuse large-B-cell lymphoma (DLBCL) homogenously treated with promacecytabom derived protocols: A GISL retrospective study. Annals of Oncology. 2005;16:172–172.
- Mounier N, Gisselbrecht C, Briere J, Haioun C, Feugier P, Offner F, Recher C, Stamatoullas A, Morschhauser F, Macro M, Thieblemont C, Sonet A, Fabiani B, Reyes F., Groupe d'Etude des Lymphomes de l'Adulte. Prognostic factors in patients with aggressive non-Hodgkin's lymphoma treated by front-line autotransplantation after complete remission: a cohort study by the Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology. 2004 Jul 15;22(14):2826–2834. [PubMed: 15254050]
- Muccilli AD, Doucette S, McDiarmid S, Huebsch LB, Sabloff M. The impact of prior exposure to rituximab on autologous stem cell transplantation in patients with follicular and transformed follicular lymphoma. Blood. 2009 Nov 20;114(22)
- Murawski N, Held G, Ziepert M, Kempf B, Viardot A, Hanel M, Witzens-Harig M, Mahlberg R, Rube C, Fleckenstein J, Zwick C, Glass B, Schmitz N, Zeynalova S, Pfreundschuh M. The role of radiotherapy and intrathecal CNS prophylaxis in extralymphatic craniofacial aggressive B-cell lymphomas. Blood. 2014 Jul 31;124(5):720–728. [PubMed: 24939657]
- Nastoupil LJ, Shenoy PJ, Ambinder A, Koff JL, Nooka AK, Waller EK, Langston A, Seward M, Kaufman JL, Bernal-Mizrachi L, King N, Lechowicz MJ, Lonial S, Sinha R, Flowers CR. Intensive chemotherapy and consolidation with high dose therapy and autologous stem cell transplant in patients with mantle cell lymphoma. Leukemia & Lymphoma. 2015;56(2):383–389. [PubMed: 24828864]
- National Life Tables. United Kingdom: Office for National Statistics; 1980-82 to 2011-13. (www
.ons.gov.uk) - NHS reference costs 2013-14. London: UK Department of Health; [database on the Internet]
- Nickenig C, Dreyling M, Hoster E, Pfreundschuh M, Trumper L, Reiser M, Wandt H, Lengfelder E, Unterhalt M, Hiddemann W., German Low-Grade Lymphoma Study Group. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study Group. Cancer. 2006 Sep 1;107(5):1014–1022. [PubMed: 16878325]
- Niitsu N, Hayama M, Yoshino T, Nakamura S, Tamaru J, Nakamine H, Okamoto M. Multicentre phase II study of the CyclOBEAP regimen for patients with peripheral T-cell lymphoma with analysis of biomarkers. British Journal of Haematology. 2011;153(5):582–588. [PubMed: 21492124]
- Niitsu N, Okamoto M, Nakamine H, Aoki S, Motomura S, Hirano M. Clinico-pathologic features and outcome of Japanese patients with peripheral T-cell lymphomas. Hematological Oncology. 2008;26(3):152–158. [PubMed: 18395866]
- Noriega V, Kaur H, Devereux S, Byrne J, Marcus R, Haynes A, Yallop D, McMillan A, Ingram W, Khan A, Kenyon M, Potter V, Russell N, Mufti GJ, Pagliuca A. Long term follow-up of BEAM-autologous and BEAM-alemtuzumab allogeneic stem cell transplantation in relapsed advanced stage follicular lymphoma. Leukemia Research. 2014;38(7):737–743. [PubMed: 24787231]
- Oh D, Duan Q, Peters A, Chua N, Stewart D. Autologous stem cell transplantation improves survival for patients with follicular lymphoma in first or second relapse: results of a comparative effectiveness instrumental analysis. Blood. 2014;124(21)
- Oh DH, Ghosh S, Chua N, Kostaras X, Tilley D, Chu M, Owen CJ, Stewart DA. Comparative effectiveness analysis of different salvage therapy intensities used for diffuse large B-cell lymphoma in Northern or Southern Alberta: an instrumental variable analysis. Leukemia & Lymphoma. 2015;56:1756–1762. [PubMed: 25284495]
- Oh SY, Kim WS, Kim JS, Kim SJ, Lee S, Lee DH, Won JH, Hwang IG, Kim MK, Lee SI, Chae YS, Yang DH, Kang HJ, Choi CW, Park J, Kim HJ, Kwon JH, Lee HS, Lee GW, Eom HS, Kwak JY, Suh C, Kim HJ. Stage IV marginal zone B-cell lymphoma - prognostic factors and the role of rituximab: Consortium for Improving Survival of Lymphoma (CISL) study. Cancer Science. 2010;101(11):2443–2447. [PubMed: 20831770]
- Okoroji G-J, De Padua Silva L, Saliba RM, Korbling M, Peter M, Hosing C, Anderlini P, Alousi A, De Lima M, Kebriaei P, Popat U, Qazilbash M, Fayad L, Samaniego F, Fowler N, Champlin R, Hagemeister F, Khouri IF. Outcome in follicular lymphoma (FL) patients (pts) relapsing after autologous stem cell transplantation (ASCT): Allografting Vs. Conventional therapy. Blood. 2010;116(21)
- Olszewski AJ, Castillo JJ. Comparative outcomes of oncologic therapy in gastric extranodal marginal zone (MALT) lymphoma: analysis of the SEER-Medicare database. Annals of Oncology. 2013;24(5):1352–1359. [PMC free article: PMC3629899] [PubMed: 23348804]
- Olszewski AJ, Desai A. Radiation Therapy Administration and Survival in Stage I/II Extranodal Marginal Zone B-Cell Lymphoma of Mucosa-Associated Lymphoid Tissue. International Journal of Radiation Oncology Biology Physics. 2014;88(3):642–649. [PubMed: 24411627]
- Papaioannou D, Rafia R, Rathbone J, Stevenson M, Buckley Woods H. Rituximab for the first-line treatment of stage III-IV follicular lymphoma (Review of TA 110). Health Technol Assess. [PubMed: 23021127]
- Papaxoinis G, Fountzilas G, Rontogianni D, Dimopoulos MA, Pavlidis N, Tsatalas C, Pectasides D, Xiros N, Economopoulos T. Low-grade mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of 97 patients by the Hellenic Cooperative Oncology Group (HeCOG). Annals of Oncology. 2008;19(4):780–786. [PubMed: 18156143]
- Park SH, Chi HS, Park SJ, Jang S, Park CJ, Huh JR. [Prognostic impact of Helicobacter pylori infection and eradication therapy in gastric mucosa-associated lymphoid tissue lymphoma]. Korean Journal Of Laboratory Medicine. 2010;30(6):547–553. [Korean] [PubMed: 21157137]
- Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, Rodriguez MA, Fayad L, Fowler N, Reed V, Horace P, Dabaja BS. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. Journal of Clinical Oncology. 2010;28:4170–4176. [PubMed: 20713859]
- Phipps C, Gopal AK, Storer BE, Cassaday RD, Press OW, Till BG, Pagel JM, Palanca-Wessels MC, Philip M, Bensinger WI, Holmberg LA, Shustove AR, Green DM, Chaucey T, Maloney DG, Libby EN. Autologous transplant for relapsed follicular lymphoma: impact of pre-transplant sensitivity. Leukemia and Lymphom. 2015;56(1):92–96. [PMC free article: PMC4269586] [PubMed: 24707941]
- Prica A, Chan K, Cheung M. Frontline rituximab monotherapy induction versus a watch and wait approach for asymptomatic advanced-stage follicular lymphoma: A cost-effectiveness analysis. Cancer. 2015;121:2637–2645. [PubMed: 25877511] [CrossRef]
- Pugh TJ, Ballonoff A, Newman F, Rabinovitch R. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2010 Aug 15;116(16):3843–3851. [PubMed: 20564102]
- Stemmelin GR. Therapeutic approach to advanced follicular lymphoma at diagnosis: An argentinian survey. Blood. 2014;(21) Conference(var.pagings)
- Récher C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNHO3-2B): an open-label randomized phase 3 trial. Lancet. 2011;378:1858–67. [PubMed: 22118442]
- Reddy N, Oluwole O, Greer JP, Goodman S, Engelhardt B, Jagasia MH, Savani BN. Superior long-term outcome of patients with early transformation of non-Hodgkin lymphoma undergoing stem cell transplantation. Clinical lymphoma, myeloma & leukemia. 2012;12(6):406–411. [PMC free article: PMC4845749] [PubMed: 22981964]
- Reddy N, Greer JP, Goodman S, Engelhardt B, Oluwole O, Jagasia MH, Savani BN. Long-term outcome after autologous or allogeneic stem cell transplantation in patients with recurrent follicular lymphoma. Bone Marrow Transplantation. 2012;47(10):1318–1320. [PMC free article: PMC4845748] [PubMed: 22327132]
- Ribrag V, et al. Addition of rituximab improves outcome of HIV negative patients with burkitt lymphoma treated with the lmba protocol: Results of the randomized intergroup (GRAALL-Lysa) LMBA02 protocol. (IGR sponsored LMBA02, NCT00180882). Blood. 2012;120(21)
- Rigacci L, Puccini B, Dodero A, et al. Allogeneic hematopoietic stem cell transplantation in patients with diffuse large B cell lymphoma relapsed after autologous stem cell transplantation: a GITMO study. Annals of Hematology. 2012;91:931–939. [PubMed: 22245922]
- Robinson SP, Canals C, Luang JJ, Tilly H, Crawley C, Cahn JY, Pohlreich D, le Gouill S, Gilleece M, Milpied N, Attal M, Biron P, Maury S, Rambaldi A, Maertens J, Capria S, Colombat P, Montoto S, Sureda A. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: An analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplantation. 2013;48(11):1409–1414. [PubMed: 23771004]
- Rule S, Smith P, Johnson PW, Bolam S, Follows GA, Gambell J, Hillmen P, Jack A, Johnson SAN, Kirkwood A, Kruger A, Seymour JF, Toncheva M, Walewski J, Linch DC. The addition of rituximab to Fludarabine and Cyclophosphamide (FC) improves overall survival in newly diagnosed Mantle Cell Lymphoma (MCL): Results of the randomised UK National Cancer Research Institute (NCRI) trial. Blood. 2011 Nov 18;118(21) [PMC free article: PMC4938327] [PubMed: 26611473]
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The Average Body Surface Area of Adult Cancer Patients in the UK: A Multicentre Retrospective Study. PLoS ONE. 2010;5(1):e8933. [PMC free article: PMC2812484] [PubMed: 20126669] [CrossRef]
- Sack H, Hoederath A, Stuschke M, Bohndorf W, Makoski HB, Muller RP, Potter R. Strahlentherapie und Onkologie. 1998;174(4):178–185. [PubMed: 9581177]
- Salles AG, Seymour JF, Feugier P, et al. Updated 6 Year Follow-Up Of The PRIMA Study Confirms The Benefit Of 2-Year Rituximab Maintenance In Follicular Lymphoma Patients Responding To Frontline Immunochemotherapy. Blood. 2013;122(21):509–509.
- Savage KJ, Sehn LH, Villa D, Kansara RR, Mottok A, Ennishi D, Ben-Neriah S, Kridel R, Steidl C, Tan KL, Johnson N, Slack GW, Connors JM, Farinha P, Scott DW, Gascoyne RD. The impact of concurrent MYC BCL2 protein expression on the risk of secondary central nervous system relapse in diffuse large B-Cell Lymphoma (DLBCL). Blood. 2014 Dec 6;124(21)
- Savage KJ, Zeynalova S, Kansara RR, Nickelsen M, Villa D, Sehn LH, Ziepert M, Scott DW, Pfreundschuh M, Gascoyne RD, Connors JM, Glass B, Loeffler M, Schmitz N. Validation of a prognostic model to assess the risk of CNS disease in patients with aggressive B-cell lymphoma. Blood. 2014 Dec 6;124(21)
- Schaaf M, Reiser M, Borchmann P, Engert A, Skoetz N. High-dose therapy with autologous stem cell transplantation versus chemotherapy or immuno-chemotherapy for follicular lymphoma in adults. Cochrane database of systematic reviews. 2012;1 (Online) CD007678. [PubMed: 22258971]
- Schaffel R, Hedvat CV, Teruya-Feldstein J, Persky D, Maragulia J, Lin D, Portlock CS, Moskowitz CH, Zelenetz AD. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Annals of Oncology. 2010;21(1):133–139. [PMC free article: PMC2795614] [PubMed: 20019090]
- Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010 Nov 4;116(18):3418–3425. [PubMed: 20660290]
- Schmitz N, Zeynalova S, Glass B, Kaiser U, Cavallin-Stahl E, Wolf M, Haenel M, Loeffler M, Shimazu Y, Notohara K, Ueda Y. Diffuse large B-cell lymphoma with central nervous system relapse: prognosis and risk factors according to retrospective analysis from a single-center experience. International Journal of Hematology. 2009;89(5):577–583. [PubMed: 19353238]
- Schmitz N, Zeynalova S, Nickelsen M, Ziepert M, Pfreundschuh M, Glass B, Loeffler M. A newprognosticmodel toassess the risk of CNS disease in patients with aggressive b-cell lymphoma. Hematological Oncology. 2013;31:111.
- Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnsen HE, Doorduijn JK, Sydes MR, Kvalheim G. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: Results from the randomized European CUP trial. Journal of Clinical Oncology. 2003;21(21):3918–3927. [PubMed: 14517188]
- Seymour JF, Pro B, Fuller LM, Manning JT, Hagemeister FB, Romaguera J, Rodriguez MA, Ha CS, Smith TL, Ayala A, Hess M, Cox JD, Cabanillas F, McLaughlin P. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2003 Jun 1;21(11):2115–2122. [PubMed: 12775737]
- Simon A, Peoch M, Casassus P, Deconinck E, Colombat P, Desablens B, Tournilhac O, Eghbali H, Foussard C, Jaubert J, Vilque JP, Rossi JF, Lucas V, Delwail V, Thyss A, Maloisel F, Milpied N, le Gouill S, Lamy T, Gressin R. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. British Journal of Haematology. 2010;151(2):159–166. [PubMed: 20738307]
- Sirvent A, Dhedin N, Michallet M, et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biology of Blood & Marrow Transplantation. 2010;16:78–85. [PubMed: 19744569]
- Smeland S, et al. Treatment of Burkitt's/Burkitt-like lymphoma in adolescents and adults: a 20-year experience from the Norwegian Radium Hospital with the use of three successive regimens. Annals of Oncology. 2004;15(7):1072–1078. [PubMed: 15205201]
- Smith SM, Burns LJ, van Besien K, Lerademacher J, He W, Fenske TS, Suzuki R, Hsu JW, Schouten HC, Hale GA, Holmberg LA, Sureda A, Freytes CO, Maziarz RT, Inwards DJ, Gale RP, Gross TG, Cairo MS, Costa LJ, Lazarus HM, Wiernik PH, Maharaj D, Laport GG, Montoto S, Hari PN. Hematopoietic cell transplantation for systemic mature T-cell non-hodgkin lymphoma. Journal of Clinical Oncology. 2013 Sep 1;31(25):3100–3109. [PMC free article: PMC3753702] [PubMed: 23897963]
- Stathis A, Chini C, Bertoni F, Proserpio I, Capella C, Mazzucchelli L, Pedrinis E, Cavalli F, Pinotti G, Zucca E. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Annals of Oncology. 2009;20(6):1086–1093. [PubMed: 19193705]
- Tai WM, Chung J, Tang PL, Koo YX, Hou X, Tay KW, Quek R, Tao M, Lim ST. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Annals of Hematology. 2011;90(7):809–818. [PubMed: 21229246]
- Thieblemont C, Briere J, Mounier N, Ullrich VH, Cuccini W, Hirchaud E, Rosenwald A, Jack A, Sundstrom C, Cogliatti S, Trougouboff P, Boudova L, Soulier J, Houlgatte R, Schmitz N, Gaulard P, Gisselbrecht C. Prognostic impact of Germinal Center (GC)/ Activated B-Cell (ABC) classification analysed by immunochemistry, FISH analysis and GEP, in relapsed/refractory diffuse large B-Cell lymphoma (DLBCL): The Bio-CORAL study; Blood; Conference: 52nd Annual Meeting of the American Society of Hematology, ASH 2010; Orlando, FL United States. Conference Start: 20101204 Conference End: 20101207; 2010. p. 19. Conference Publication: (var.pagings)
- Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, Rosenwald A, Jack A, Sundstrom C, Cogliatti S, Trougouboff P, Boudova L, Ysebaert L, Soulier J, Chevalier C, Bron D, Schmitz N, Gaulard P, Houlgatte R, Gisselbrecht C. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. Journal of Clinical Oncology. 2011;29:4079–4087. [PubMed: 21947824]
- Thieblemont C, Briere J, Mounier N, Voelker H, Cuccini W, Hirchaud E, Rosenwald A, Jack A, Sundstrom C, Cogliatti S, Trougouboff P, Boudova L, Ysebaert L, Gaulard P, Houlgatte R, Gisselbrecht C. The germinal center (GCB)/ activated B-cell (ABC) subclassifications have a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma (DLBCL). The bio-coral study; Annals of Oncology; Conference: 11th International Conference on Malignant Lymphoma; Lugano Switzerland. Conference Start: 20110615 Conference End: 20110618; Jun, 2011. Conference Publication: (var.pagings) [PubMed: 21947824]
- Tilly H, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102(13):4284–4289. [PubMed: 12920037]
- Tomblyn MR, Ewell M, Bredeson C, Kahl BS, Goodman SA, Horowitz MM, Vose JM, Negrin RS, Laport GG. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular Non-Hodgkin lymphoma beyond first complete response or first partial response. Biology of Blood and Marrow Transplantation. 2011;17(7):1051–1057. [PMC free article: PMC3114272] [PubMed: 21073974]
- Tomita N, Takasaki H, Ishiyama Y, Kishimoto K, Ishibashi D, Koyama S, Ishii Y, Takahashi H, Numata A, Watanabe R, Tachibana T, Ohshima R, Hagihara M, Hashimoto C, Takemura S, Taguchi J, Fujimaki K, Sakai R, Motomura S, Ishigatsubo Y. Intrathecal methotrexate is not effective in preventing central nervous system relapse in diffuse large B-cell lymphoma patients treated with R-CHOP. Haematologica. 2013 Jun 1;98:130.
- Tomita N, Yokoyama M, Yamamoto W, Watanabe R, Shimazu Y, Masaki Y, Tsunoda S, Hashimoto C, Murayama K, Yano T, Okamoto R, Kikuchi A, Tamura K, Sato K, Sunami K, Shibayama H, Takimoto R, Ohshima R, Hatta Y, Moriuchi Y, Kinoshita T, Yamamoto M, Numata A, Ishigatsubo Y, Takeuchi K. Central nervous system event in patients with diffuse large B-cell lymphoma in the rituximab era. Cancer Science. 2012;103(2):245–251. [PubMed: 22044593]
- Trneny M, Bosly A, Bouabdallah K, Ma D, Shpilberg O, Montoto S, Sebban C, Hagberg H, Moskowitz CH, Schmitz N, Gisselbrecht C. Independent predictive value of PET-CT pre transplant in relapsed and refractory patients with CD20 diffuse large b-cell lymphoma (DLBCL) included in the CORAL study; Blood; Conference: 51st Annual Meeting of the American Society of Hematology; ASH New Orleans, LA United States. Conference Start: 20091205 Conference End: 20091208; 2009. p. 20. Conference Publication: (var.pagings)
- Truemper L, Pfreundschuh M. CNS disease in younger patients with aggressive B-cell lymphoma: an analysis of patients treated on the Mabthera International Trial and trials of the German High-Grade Non-Hodgkin Lymphoma Study Group. Annals of Oncology. 2012;23(5):1267–1273. [PubMed: 21989328]
- Tsao C, Fisher K, Lee J-H, Chavez JC, Dalia S, Bello CM. Extranodal diffuse large B cell lymphoma in the rituximab era and the risk of central nervous system (CNS) relapse. A single center experience from 2008-2012. Blood. 2013 Oct 21;122(21)
- Udvardy M, Rosta A, Gasztonyi Z, Borbenyi Z, Radvanyi G, Egyed M, Gasztonyi B, Losonczy H, Matolcsy A, Andrasfalvy AH, Schneider TJ. Immunochemotherapy as induction (R-CHOP and R-HYPERC-VAD/R-MA) in mantle cell lymphoma, a hungarian multicenter open label phase II study (rituximab [MabThera] in mantle cell lymphoma, remeny). Blood. 2012 Nov 16;120(21)
- Ueda K, Terui Y, Yokoyama M, Sakajiri S, Nishimura N, Tsuyama N, Takeuchi K, Hatake K. Non-gastric advanced mucosa-associated lymphoid tissue (MALT) lymphoma has worse prognosis than gastric MALT lymphoma even when treated with rituximab-containing chemotherapy. Leukemia & Lymphoma. 2013;54(9):1928–1933. [PubMed: 23216271]
- van Besien K, Loberiza FR, Bajorunaite R, Armitage JO, Bashey A, Burns LJ, Freytes CO, Gibson J, Horowitz MM, Inwards DJ, Marks DI, Martino R, Maziarz RT, Molina A, Pavlovsky S, Pecora AL, Schouten HC, Shea TC, Lazarus HM, Rizzo JD, Vose JM. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102(10):3521–3529. [PubMed: 12893748]
- Van Den Neste E, Gisselbrecht C, Schmitz N, Mounier N, Gill S, Linch FD, Trneny M, Milpied N, Radford J, Ketterer N, Shpilberg O, Duhrsen U, Ma D, Briere J, Thieblemont C, Salles GA, Moskowitz CH, Glass B. Diffuse large B-cell lymphoma (DLBCL) patients failing second-line R-DHAP Or R-ICE chemotherapy included in the coral study; Blood; Conference: 55th Annual Meeting of the American Society of Hematology, ASH 2013; New Orleans, LA United States. Conference Start: 20131207 Conference End: 20131210; 2013. p. 21. Conference Publication: (var.pagings)
- Van Den Neste E, Gisselbrecht C, Schmitz N, Mounier N, Gill D, Lynch D, Trneny M, Milpied N, Radford J, Ketterer N, Shpilberg O, Duehrsen U, Ma D, Briere J, Thieblemont C, Salles G, Moskowitz C, Glass B. Outcomes in diffuse large B-cell lymphoma after failure to second-line chemotherapy: Analysis of patients included in the international coral study; Hematological Oncology; Conference: 12th International Conference on Malignant Lymphoma; Lugano Switzerland. Conference Start: 20130619 Conference End: 20130622; Jun, 2013. Conference Publication: (var.pagings)
- van Kampen RJ, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. Journal of Clinical Oncology. 2011;29:1342–1348. [PubMed: 21321299]
- vanOers MHJ, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, Jack A, van't Veer M, Vranovsky A, Holte H, van Glabbeke M, Teodorovic I, Rozewicz C, Hagenbeek A. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108(10):3295–3301. [PubMed: 16873669]
- Van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. Journal of Clinical Oncology. 2010;(28):2853–2858. [PMC free article: PMC2903319] [PubMed: 20439641]
- Ventre MB, Foppoli M, Citterio G, Donadoni G, Ponzoni M, Govi S, Scarfo L, Sassone M, Caligaris-Cappio F, Ferreri AJM. Risk-tailored CNS prophylaxis in 194 patients with diffuse large B-cell lymphoma (DLBCL) treated in the rituximab ERA: Risk definition by clinical variables and ontogenic stratification. Blood. 2013 Oct 21;122(21)
- Villa Da, Crump M, Panzarella T, Savage KJ, Toze CL, Stewart DA, MacDonald DA, Buckstein R, Lee C, Alzahrani M, Rubinger M, Foley R, Xenocostas A, Sabloff M, Muccilli A, Chua N, Couture F, Larouche JF, Cohen S, Connors JM, Ambler K, Al-Tourah A, Ramadan KM, Kuruvilla J. Autologous and allogeneic stem-cell transplantation for transformed follicular lymphoma: a report of the Canadian blood and marrow transplant group. Journal of Clinical Oncology. 2013 Mar 20;31(9):1164–1171. [PubMed: 23401459]
- Villa Db, Crump M, Keating A, Panzarella T, Feng B, Kuruvilla J. Outcome of patients with transformed indolent non-Hodgkin lymphoma referred for autologous stem-cell transplantation. Annals of Oncology. 2013;24(6):1603–1609. [PubMed: 23425946]
- Vockova P, Klener P, Pytlik R, Benesova K, Stritesky J, Velenska Z, Jaksa R, Campr V, Petrova M, Trneny M. Significant survival improvement of the elderly patients and women with low/intermediate risk MIPI mantle cell lymphoma over the period of 14 years. Haematologica. 2014 Jun 1;99:701.
- Vose JM, Carter SL, Burns LJ, Ayala E, Press OW, Moskowitz CH, Stadtmauer EA, Mineishi S, Ambinder RF, Fenske TS, Horowitz MM, Tomblyn M. Randomized phase III trial of #SUP#131#/SUP#iodine-tositumomab (Bexxar)/carmustine, etoposide, cytarabine, melphalan (BEAM) vs. rituximab/BEAM and autologous stem cell transplantation for relapsed Diffuse Large B-Cell Lymphoma (DLBCL): No difference in Progression-Free (PFS) or Overall Survival (OS); Blood; Conference: 53rd Annual Meeting of the American Society of Hematology, ASH 2011; San Diego, CA United States. Conference Start: 20111210 Conference End: 20111213; 2011. p. 18. Conference Publication: (var.pagings)
- Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, Stadtmauer EA, Mineshi S, Ambinder R, Fenske T, Horowitz M, Fisher R, Tomblyn M. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. Journal of Clinical Oncology. 2013;31:1662–1668. [PMC free article: PMC3635682] [PubMed: 23478060]
- Vose M, Loberiza R, Bierman J, Bociek G, Armitage O. The addition of stem cell transplantation following induction chemotherapy improves overall survival in mantle cell lymphoma patients who achieve a complete response. Haematologica. 2012 Jun 1;97:100–101.
- Vrieling C, de Jong D, Boot H, de Boer JP, Wegman F, Aleman BM. Long-term results of stomach-conserving therapy in gastric MALT lymphoma. Radiotherapy & Oncology. 2008;87(3):405–411. [PubMed: 18343513]
- Walewski J, et al. A major progress in outcome of Burkitt's and Burkitt-like lymphoma in a single institution; Evaluation of CHOP/MEVA and CODOX-M/IVAC chemotherapy programs in 80 consecutive patients over 20 years. Blood. 2001;98(11):252B–252B.
- Wang ES, et al. Intensive chemotherapy (CODOX-M/IVAC) compares favorably with other regimens for HIV positive and negative patients with Burkitt's lymphoma (BL). Blood. 2000;96(11):139A–139A.
- Wasterlid T, et al. Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic Lymphoma Group. Annals of Oncology. 2013;24(7):1879–1886. [PubMed: 23446093]
- Wild D, Pettengell R, Lewis G. Utility elicitation in patients with follicular lymphoma. 2005 Unpublished report by Oxford Outcomes prepared for Roche UK.
- Wilder RB, Jones D, Tucker SL, Fuller LM, Ha CS, McLaughlin P, Hess MA, Cabanillas F, Cox JD. Long-term results with radiotherapy for Stage I-II follicular lymphomas. International Journal of Radiation Oncology, Biology, Physics. 2001 Dec 1;51(5):1219–1227. [PubMed: 11728680]
- Wildes TM, et al. Rituximab is associated with improved survival in Burkitt lymphoma: A retrospective analysis from two US academic medical centers. Therapeutic Advances in Hematology. 2014;5(1):3–12. [PMC free article: PMC3891289] [PubMed: 24490019]
- Williams CD, Harrison CN, Lister TA, Norton AJ, Blystad AK, Coiffier B, Taghipour G, Schmitz N, Goldstone AH., European Bone Marrow Transplant Lymphoma Working Party. High-dose therapy and autologous stem-cell support for chemosensitive transformed low-grade follicular non-Hodgkin's lymphoma: a case-matched study from the European Bone Marrow Transplant Registry. Journal of Clinical Oncology. 2001 Feb 1;19(3):727–735. [PubMed: 11157024]
- Wilson WH, Bromberg JE, Stetler-Stevenson M, Steinberg SM, Martin-Martin L, Muniz C, Sancho JM, Caballero MD, Davidis MA, Brooimans RA, Sanchez-Gonzalez B, Salar A, Gonzalez-Barca E, Ribera JM, Shovlin M, Filie A, Dunleavy K, Mehrling T, Spina M, Orfao A. Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2014;99(7):1228–1235. [PMC free article: PMC4077085] [PubMed: 24727817]
- Wirk B, et al. Outcomes of hematopoietic cell transplantation for diffuse large B cell lymphoma transformed from follicular lymphoma. Biol Blood Marrow Transplant. 2014. Available online 15 March 2014. [PMC free article: PMC4060436] [PubMed: 24641828]
- Amiot A, Levy M, Copie-Bergman C, Dupuis J, Szablewski V, Le Baleur Y, Baia M, Belhadj K, Sobhani I, Leroy K, Haioun C, Delchier JC. Rituximab, alkylating agents or combination therapy for gastric mucosa-associated lymphoid tissue lymphoma: a monocentric non-randomised observational study. Alimentary Pharmacology & Therapeutics. 2014;39(6):619–628. [PubMed: 24467480]
- Wohrer S, Kiesewetter B, Fischbach J, Mullauer L, Troch M, Lukas J, Raderer M. Retrospective comparison of the effectiveness of various treatment modalities of extragastric MALT lymphoma: a single-center analysis. Annals of Hematology. 2014;93(8):1287–1295. [PubMed: 24633660]
- Xie W, Hu K, Xu F, Zhou D, Huang W, He J, Shi J, Luo Y, Zhang J, Lin M, Ye X, Cai Z, Huang H. Significance of clinical factors as prognostic indicators for patients with peripheral T-cell non-Hodgkin lymphoma: A retrospective analysis of 252 cases. 2013;1(5):911–917. Molecular and Clinical Oncology. [PMC free article: PMC3915658] [PubMed: 24649270]
- Andresen S, Brandt J, Dietrich S, Memmer M-L, Ho AD, Witzens-Harig M. The impact of high-dose chemotherapy, autologous stem cell transplant and conventional chemotherapy on quality of life of long-term survivors with follicular lymphoma. Leukemia and Lymphoma. 2012;53(3):386–393. [PubMed: 21864036]
- Yamamoto W, Tomita N, Watanabe R, Hattori Y, Nakajima Y, Hyo R, Hashimoto C, Motomura S, Ishigatsubo Y. Central nervous system involvement in diffuse large B-cell lymphoma. European Journal of Haematology. 2010;85(1):6–10. [PubMed: 20236301]
- Yepes S, Torres MM, Saavedra C, Andrade R. Gastric mucosa-associated lymphoid tissue lymphomas and Helicobacter pylori infection: A Colombian perspective. World Journal of Gastroenterology. 2012 Feb 21;18(7):685–691. [PMC free article: PMC3281227] [PubMed: 22363141]
- Yin J, Wei J, Xu JH, Xiao Y, Zhang YC. Autologous stem cell transplantation as the first-line treatment for peripheral T cell lymphoma: results of a comprehensive meta-analysis. Acta Haematologica. 2014;131(2):114–125. [Review] [PubMed: 24158006]
- Ying ZT, Zheng W, Wang XP, Xie Y, Tu MF, Lin NJ, Ping LY, Liu WP, Deng LJ, Zhang C, Zhu J, Song YQ. The clinical features, therapeutic responses, and prognosis of the patients with mantle cell lymphoma. Chinese Journal of Cancer. 2012;31(7):348–353. [PMC free article: PMC3777499] [PubMed: 22704490]
- Zhang H, Wang H, Fu K, Hou Y, Li W, Zhou S, Qiu L, Qian Z, Liu X. Comparative study of R-GemOx and RICE regimens as second-line treatments for refractory or relapsed DLBCL. Chinese Journal of Clinical Oncology. 2011;38:30.
- Zinzani PL, Magagnoli M, Moretti L, De Renzo A, Battista R, Zaccaria A, Guardigni L, Mazza P, Marra R, Ronconi F, Lauta VM, Bendandi M, Gherlinzoni F, Gentilini P, Ciccone F, Cellini C, Stefoni V, Ricciuti F, Gobbi M, Tura S. Randomized trial of fludarabine versus fludarabine and idarubicin as frontline treatment in patients with indolent or mantle-cell lymphoma. Journal of Clinical Oncology. 2000;18(4):773–779. [PubMed: 10673518]
- Zinzani PL. Salvage Chemotherapy in Follicular Non-Hodgkin's Lymphoma: Focus on Tolerability. Clinical Lymphoma & Myeloma. 2006;7(2):115–124. [PubMed: 17026822]
- Zucca E, Conconi A, Laszlo D, Lopez-Guillermo A, Bouabdallah R, Coiffier B, Sebban C, Jardin F, Vitolo U, Morschhauser F, Pileri SA, Copie-Bergman C, Campo E, Jack A, Floriani I, Johnson P, Martelli M, Cavalli F, Martinelli G, Thieblemont C. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. Journal of Clinical Oncology. 2013 Feb 10;31(5):565–572. [PubMed: 23295789]
- Zucca E, Roggero E, Smith P, Traulle C, Copie-Bergmann C, Delchier JC, Souhami R, Cavalli F, Hancock BW. Gastric MALT lymphoma: Response to anti-helicobacter therapy in the ongoing LY03 randomised co-operative trial of observation vs chlorambucil after anti-helicobacter therapy. British Journal of Cancer. 2000;83:15–15.
- Zullo A, Hassan C, Andriani A, Cristofari F, Bassanelli C, Spinelli GP, Tomao S, Morini S. Treatment of low-grade gastric MALT-lymphoma unresponsive to Helicobacter pylori therapy: a pooled-data analysis. Medical Oncology. 2010;27(2):291–295. [Review] [52 refs] [PubMed: 19308737]
- Zullo A, Hassan C, Andriani A, Cristofari F, De Francesco V, Ierardi E, Tomao S, Morini S, Vaira D. Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: a pooled data analysis. American Journal of Gastroenterology. 2009;104(8):1932–1937. [Review] [54 refs] [PubMed: 19532131]
- Zullo A, Hassan C, Ridola L, De Francesco V, Rossi L, Tomao S, Vaira D, Genta RM. Eradication therapy in helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: A systematic review. Journal of Clinical Gastroenterology. 2013;47(10):824–827. [PubMed: 23442842]
- Management - Non-Hodgkin's Lymphoma: Diagnosis and ManagementManagement - Non-Hodgkin's Lymphoma: Diagnosis and Management
- PsbK (chloroplast) [Cornus canadensis]PsbK (chloroplast) [Cornus canadensis]gi|1732475519|gb|QEJ84902.1|Protein
- Human gammaherpesvirus 8 isolate M23 K1 glycoprotein gene, partial cdsHuman gammaherpesvirus 8 isolate M23 K1 glycoprotein gene, partial cdsgi|1995959830|gb|MN782047.1|Nucleotide
- Homo sapiens X BAC RP13-314C10 (Roswell Park Cancer Institute Human BAC Library)...Homo sapiens X BAC RP13-314C10 (Roswell Park Cancer Institute Human BAC Library) complete sequencegi|25815264|gnl|bcmhgsc|project_hel lor|gb|AC131011.5|Nucleotide
- RecName: Full=Mitochondrial potassium channel; Short=MITOK; AltName: Full=Coiled...RecName: Full=Mitochondrial potassium channel; Short=MITOK; AltName: Full=Coiled-coil domain-containing protein 51; Flags: Precursorgi|148840310|sp|Q5PPN7.2|MITOK_RATProtein
Your browsing activity is empty.
Activity recording is turned off.
See more...
