NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Alliance (UK). Cystic Fibrosis: Diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2017 Oct 25. (NICE Guideline, No. 78.)
K.1. Literature review
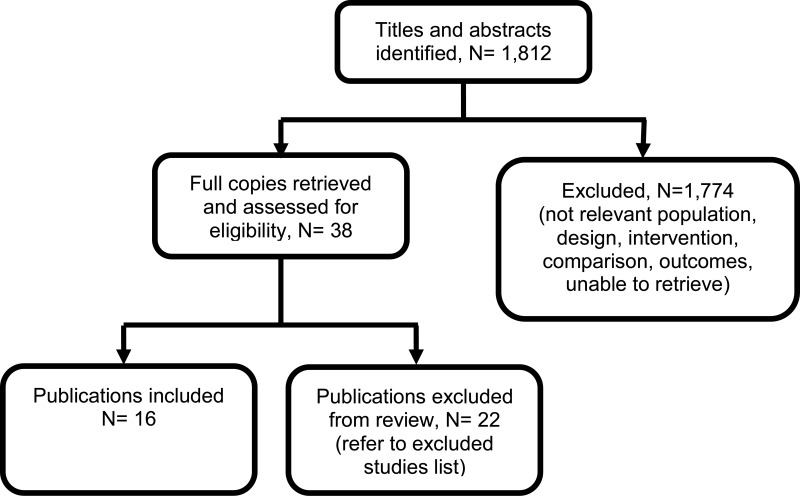
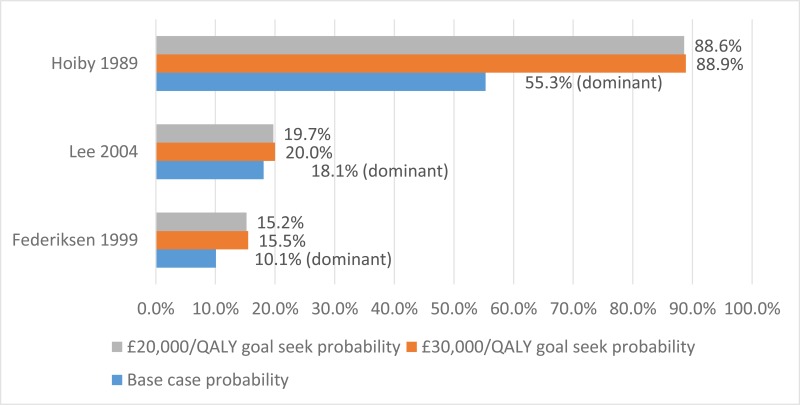
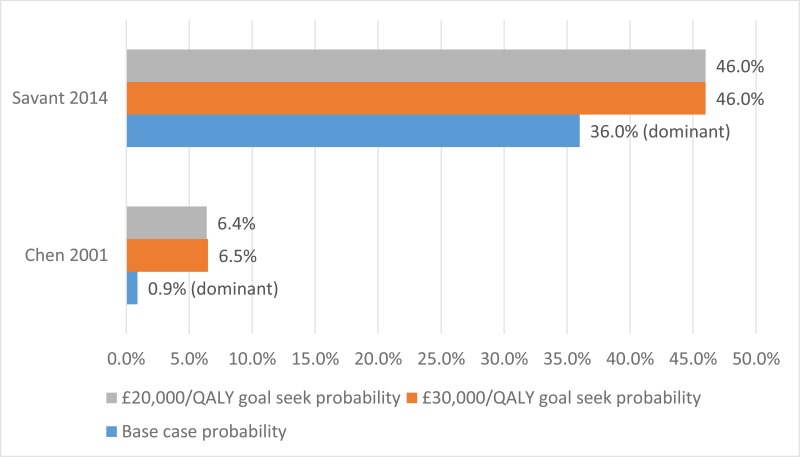
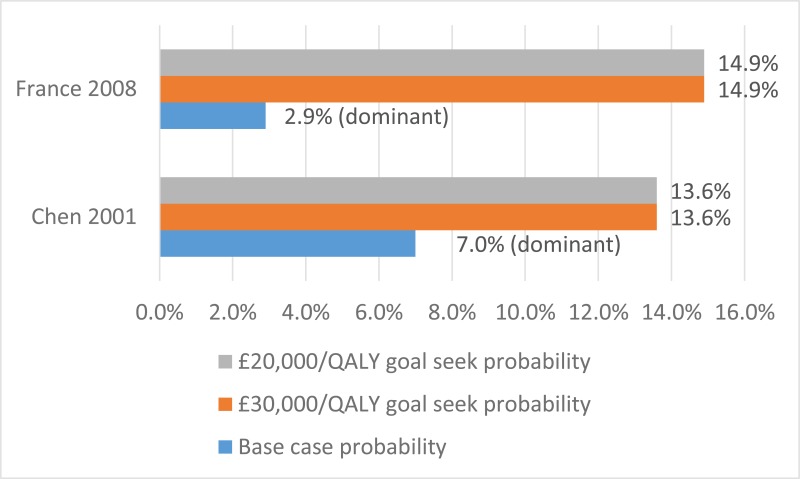
The final search of economic evidence relating to all treatments for cystic fibrosis identified 1,812 papers. Of those, 38 were ordered for full-text review. An additional 9 papers that were ordered for full-text review were unavailable. Of those 38 papers retrieved, 22 were excluded following a full-text review, the reasons for which are provided in Appendix H. The remaining 16 papers were considered to be relevant to one of the review questions in this guideline.
Figure 1 below provides an illustration of the process used to select those papers and Table 1 presents the number of papers identified according to the area in the guideline. Full details of the search strategies are presented in Appendix E.
Table 1Number of included studies by area
| Area | Include |
|---|---|
| Airway clearance | 1 |
| Monitoring pulmonary disease | 2 |
| Monitoring liver disease | 0 |
| DIOS | 0 |
| PERT | 0 |
| Nutrition | 0 |
| Mucoactive or mucolytic agents | 6 |
| Antimicrobials | 4 |
| Service configuration | 3 |
| Cross-infection | 0 |
| Immunomodulatory agents | 0 |
| UDCA | 0 |
| Psychological and behavioural assessment | 0 |
| Exercise | 0 |
| BMD | 0 |
| CFRD | 0 |
| Clinical manifestations | 0 |
| Information and support | 0 |
| Complications of CF | 0 |
| Transition | 0 |
| Total | 16 |
BMD, bone mineral density; CF, cystic fibrosis; DIOS, distal ileal obstruction syndrome; PERT, Pancreatic enzymes for exocrine pancreatic insufficiency; UDCA, ursodeoxycholic acid
The methods and results for each of those 16 economic evaluations will be presented in the appropriate sections below, whilst data extraction tables and quality assessments can be found in Appendix L and M, respectively.
K.2. Airway clearance
K.2.1. Literature review
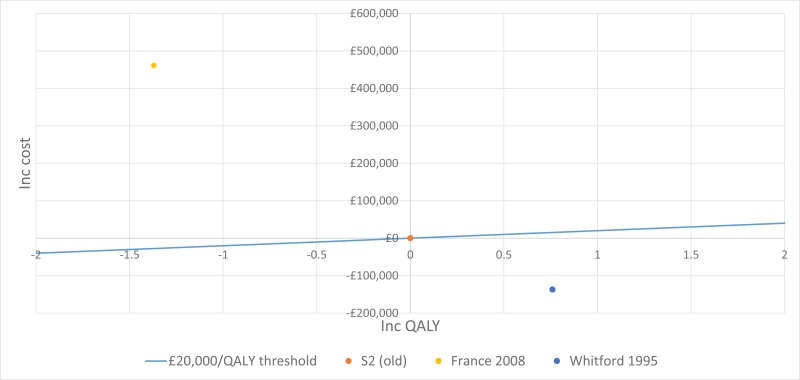
No published health economic evaluations were identified in the literature search that were relevant to this review question. However, one conference abstract compared positive expiratory pressure (PEP) to high frequency chest wall oscillation (HFCWO) vests in 107 people with cystic fibrosis in Canada (McIlwaine 2104).
Conference abstracts rarely contain enough information to allow confident judgements about the quality and results of a study. However, they can be important in interpreting evidence in the absence of full published studies. Prior to title and abstract screening, it was decided that conference abstracts would be considered for inclusion from 1st January 2014 as high-quality studies reported in abstract form before 2014 were expected to have been published in a peer-reviewed journal. For these reasons McIllwaine 2014 was included.
In their analysis, the medical costs of PEP are compared with those for HFCWO by comparing the cost of equipment and costs associated with managing exacerbations (number of hospital days, antibiotic treatment either IV, inhaled, or oral, and number of days on home IV). They concluded that PEP was less expensive and more effective (dominant) at reducing the number of exacerbations than HFCWO. The methods and results from this analysis are summarised in Table 2. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1. Data extraction tables and quality assessments of included studies can be found in Appendix L and M, respectively.
Table 2Summary of McIlwaine 2014
| Study | Limitations | Applicability | Other comments | Inc. costs | Inc. effects | Inc. cost-effectiveness | Uncertainty |
|---|---|---|---|---|---|---|---|
| McIlwaine 2014 | Seriousa,b,c | Partiallyd,e |
| Total medical cost/participant (including equipment cost) over 1 year:
|
| NR | Not assessed |
HFCWO, high frequency chest wall oscillation; NR, not reported; PEP, positive expiratory pressure
- (a)
Absence of detail regarding: cost build up for HFCWO equipment, specific sources of cost data, definition of an exacerbation, perspective and study dates
- (b)
Data in the paper is based on single values, there is no measure of data dispersion
- (c)
The cost of HFCWO equipment has not been annuitised over the equipment lifespan which over estimates the cost of the vest over one year
- (d)
Conference paper with limited details to assess with certainty
- (e)
QALY not used as an outcome measure
K.2.2. Background and methods
According to the committee, the techniques used in clinical practice vary. There are relatively new techniques, such as vests, that are available in some hospitals in the UK and available for people with cystic fibrosis to purchase themselves. It is particularly important to compare the vest to the other techniques given the relatively high initial capital outlay. Whilst the vest is not widely used within the NHS, it is widely used in other developed countries and, as such, is frequently asked for by people with cystic fibrosis, or their parents and carers.
It is also important to consider if there are potential cost saving to the NHS if techniques performed at home demonstrate equivalent, or greater, efficacy over techniques, such as manual chest physiotherapy, that utilise NHS resources each time they are performed.
Based on the clinical evidence it is unlikely recommendations would represent a significant change from current practice. Moreover, the effectiveness and side effect profiles do not vary between techniques, hence recommendations are unlikely to have large health benefits.
For these reasons, this review question was not ranked as a high priority by the committee for de novo modelling. Instead a cost description of the techniques was undertaken for the committee to aid considerations of cost-effectiveness.
K.2.3. Resource and cost use
The vest, oscillating devices, PEP devices and non-invasive ventilation (NIV) equipment incur a capital cost, requiring an up-front payment. There are 2 aspects to capital costs:
- Opportunity cost – this is the money spent on equipment that could have been invested in another venture. This cost is calculated by applying an interest rate on the sum invested in the capital.
- Depreciation cost – the equipment has a certain lifespan and depreciates over time, and will eventually need to be replaced.
The usual practice for economic evaluation is to calculate an ‘annual equivalent cost’. This is calculated by annuitising the initial capital outlay (including training costs) over the expected life of the equipment. Calculating the equivalent annual cost means making allowance for the differential timing of costs by discounting.
The formula for calculating the equivalent annual cost is:
- E = equivalent annual cost
- K = purchase price of the device
- T = training
- A(n,r) = annuity factor (n years at interest rate r)
- r = discount (interest) rate
- n = equipment lifespan (years)
Using this formula a cost/ person/ annum for use of a vest, oscillating device, NIV or PEP mask was calculated to allow for comparison. It is assumed the monitoring schedules do not differ hugely across the techniques as they would be reviewed at routine attendances to the clinic.
K.2.3.1. High frequency chest wall oscillation (HFCWO) vests
According to the committee, HillRom is the most widely used vest in the UK. In light of this, HillRom was approached to provide accurate costing information on the vest. According to HillRom, the vest has an upfront capital cost of £6,995 (excluding VAT), this cost also includes a garment and an additional larger garment for a child as they grow. HillRom advised that the vest should last for at least 10 years before it needs to be replaced. Table 3 below presents the parameters used to calculate the equivalent annual cost.
Table 3Equivalent annual cost of vests
| Parameter | Value | Source |
|---|---|---|
| K = purchase price of a vest | £6,995 | HillRom |
| T = training | £0 | committee assumption that training to use a vest would be minimal |
| r = discount (interest) rate | 3.5% | NICE reference case |
| n = equipment lifespan | 10 years | Assumption informed by committee |
| A (n,r) = annuity factor (n years at interest rate r) | 8.61 | Calculated |
| E = equivalent annual cost | £813 | Calculated |
NA, not applicable; NICE, National Institute for Health and Care Excellence
In addition to the initial capital outlay, the committee advised that the vest would be serviced annually. Conversely, HillRom stated that the vest does not need annual servicing, but out of warranty repairs would incur a cost of £395 (excluding VAT). HillRom added that the number of repairs a device will require in a lifetime is impossible to say as they vary from never to once a year. However, 1 repair every 3 years would be reasonable assumption. Furthermore, additional garments would cost £295 (excluding VAT), but HillRom noted that most people would have enough garment provision in their purchase package to last 10 years.
Assuming a vest is purchased by a hospital for use across patients at the cystic fibrosis centre, a unit cost can be calculated based on the typical use of the vest over a period of time. As a result, the unit cost would depend on the usage of the vest, for example the more the vest is used the lower the cost per use.
However, current practice in the UK would be for the person with cystic fibrosis to purchase the vest themselves for home use. The reasons for this are to minimise the risk of cross-infection, reduce staff time and reduce the burden of clinic visits to use the vest by enabling the vest to be used as-and-when required.
K.2.3.2. Manual chest physiotherapy
Manual chest physiotherapy can include a variety of techniques such as chest shaking / vibrations or chest percussion. However, these techniques would not differ hugely in the time required to perform. According to NHS Reference Costs 2015/16, 1 manual chest physiotherapy session would cost £45 (WF01A, Non-Consultant-Led, Non-Admitted, Follow-up, 650). However, the number of sessions over a time frame would be individualised to the person with cystic fibrosis according to their severity and other treatment schedules.
K.2.3.3. Active cycle of airway breathing techniques (ACBT)
ACBT would require 1 initial appointment with a physiotherapist to show the person with cystic fibrosis how to perform the technique at a cost of £57 (NHS Reference Costs 2015/16: WF01B, Non-Consultant-Led, Non-Admitted, First, 650). Following this, ACBT could be replicated at home. Assuming the monitoring schedules across the techniques are similar, ACBT would cost less than the alternative techniques that require ongoing resources from staff time and equipment.
K.2.3.4. Oscillating and positive expiratory pressure (PEP) devices
The committee advised that oscillating devices and PEP devices are normally included in hospital equipment budgets and provided by cystic fibrosis centre. However, some people with cystic fibrosis may choose to replace their device themselves which, with regular use, would be every 2 to 5 years.
Table 4 below presents the cost of the most widely used oscillating and PEP devices available to the NHS, but other manufacturers, such as Astra, are available. In addition to the device, people may require accessories and replacement parts, for example the PARI O-PEP may require a nose clip and pressure hose to optimise the technique. The cost of PARI O-PEP accessories, are reproduced in Table 5 to provide an estimate of the total cost.
Table 4Cost of oscillating and PEP devices
| Device | Cost | Source |
|---|---|---|
| Acapella | £40.50 | NHS Electronic drug tariff (part IXA, oscillating positive expiratory pressure devices) November 2016 |
| Flutter | £40.50 | NHS Electronic drug tariff (part IXA, oscillating positive expiratory pressure devices) November 2016 |
| Lungflute | £37.50 | NHS Electronic drug tariff (part IXA, oscillating positive expiratory pressure devices) November 2016 |
| PARI O-PEP | £27.28 | NHS Electronic drug tariff (part IXA, oscillating positive expiratory pressure devices) November 2016 |
| RC Cornet | £62.21 | NHS Supply Chain 2015 |
Table 5Total cost of PEP equipment
| Device | Cost | Source |
|---|---|---|
| Nose clip for PARI PEP system | £1.37 | NHS Supply Chain 2015 |
| Manometer 0–100Mbar with pressure hose for use with PARI PEP system | £42.02 | NHS Supply Chain 2015 |
| PARI O-PEP device | £27.28 | Table 4 |
| Total cost of PARI PEP (including device and accessories) | £70.67 | Calculated |
Assuming each new device requires a visit to a physiotherapist, to issue the device and teach them how to replicate the technique at home, the equivalent annual cost across a 5 year lifespan, ranges from £20.86 to £27.39, for oscillating devices and PARI O-PEP, respectively (Table 6). Further follow-up visits will be needed during the lifespan of the devices. However, it is assumed this is equivalent across the airway clearance techniques as there is no opportunity cost created by switching from one technique to another.
Table 6Equivalent annual cost of oscillating and PEP devices
| Parameter | Value | Source |
|---|---|---|
| Ko = purchase price of oscillating device | £40.50 | Table 4 |
| Kp = purchase price of PEP device | £70.67 | Table 5 |
| T = training | £57 | NHS Reference Costs 2015/16, WF01B, non-consultant-led, non-admitted, first physiotherapy attendance |
| r = discount (interest) rate | 3.5% | NICE reference case |
| n = equipment lifespan | 5 years | Best case scenario informed by committee |
| A (n,r) = annuity factor (n years at interest rate r) | 4.67 | Calculated |
| Eo = equivalent annual cost of oscillating devices | £20.86 | Calculated |
| Ep = equivalent annual cost of PEP | £27.39 | Calculated |
| n = equipment lifespan | 2 years | Worst case scenario informed by committee |
| A (n,r) = annuity factor (n years at interest rate r) | 1.97 | Calculated |
| Eo = equivalent annual cost of oscillating devices | £49.59 | Calculated |
| Ep = equivalent annual cost of PEP | £65.10 | Calculated |
NA, not applicable; NICE, National Institute for Health and Care Excellence; PEP, positive expiratory pressure
K.2.3.5. Non-invasive ventilation (NIV)
The cost of NIV depends upon the specification of the device and the consumables used. However, the committee noted that the most common device provided to people with cystic fibrosis in the UK is the NIPPY ventilator. The typical cost of a Nippy ventilator is £4,000 according to the NHS Supply Chain (Table 7). However, as previously stated, other manufacturers are available.
Table 7Cost of NIV equipment
| NIV description | Costa |
|---|---|
| NIPPY junior+ ventilator with internal battery & carry bag | £5,878 |
| NIPPY 3+ ventilator with carry bag (no internal battery) - supplied with each unit | £4,776 |
| NIPPY 3+ ventilator with internal battery and carry bag | £5,020 |
| NIPPY st+ ventilator with carry bag (no internal battery) | £3,184 |
| NIPPY st+ ventilator with internal battery and carry bag | £3,429 |
| NIPPY s+ ventilator with carry bag (no internal battery) | £2,571 |
| NIPPY s+ ventilator with internal battery and carry bag - no 0792 | £2,816 |
- (a)
Basic price, excluding VAT
For illustrative purposes a cost £4,000 has been used to calculate the equivalent annual cost (£465). In addition to that initial capital outlay, the equipment also requires consumables such as a mask, or mouthpiece, and filters. According to members of the committee these would be replaced annually at a cost of approximately £100, leading to a total annual cost of £565 (Table 8).
Table 8Equivalent annual cost of NIV
| Parameter | Value | Source |
|---|---|---|
| K = purchase price of NIV | £4,000 | Assumption based on Table 7 |
| T = training | £0 | NA - captured on a per patient basis |
| r = discount (interest) rate | 3.5% | NICE reference case |
| n = equipment lifespan | 10 years | Assumption informed by committee |
| A (n,r) = annuity factor (n years at interest rate r) | 8.61 | Calculated |
| E = equivalent annual cost | £465 | Calculated |
NA, not applicable; NICE, National Institute for Health and Care Excellence; NIV, non-invasive ventilation
The committee advised, in most cases, the cystic fibrosis centre would purchase NIV equipment and lend it to the person with cystic fibrosis for however long it was needed. They do not provide each person with their own personal device to keep over the equipment’s lifespan. Ideally, when NIV is required by someone with cystic fibrosis, it is initiated over a few days of coaching with a physiotherapist in an inpatient setting at a cost of £300/day (NHS Reference Costs 2015/16, DZ30Z, elective inpatient, chest physiotherapy attendance). The NIV equipment could then be used at home without assistance from a healthcare professional, but would be reviewed regularly at future attendances to the cystic fibrosis centre.
K.2.4. Conclusions
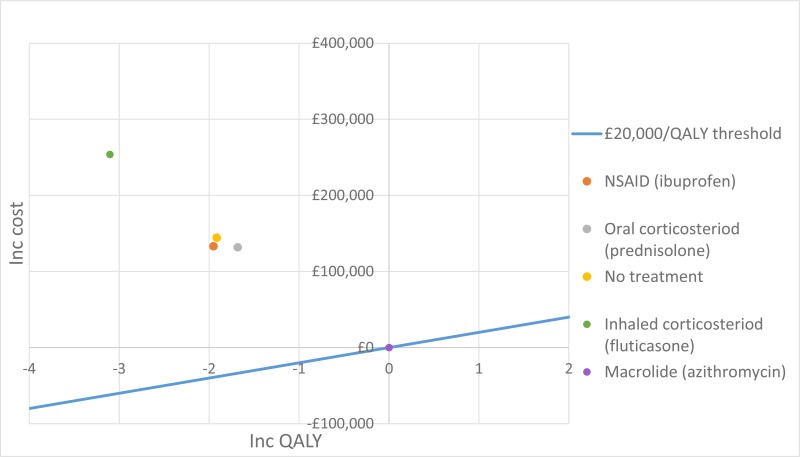
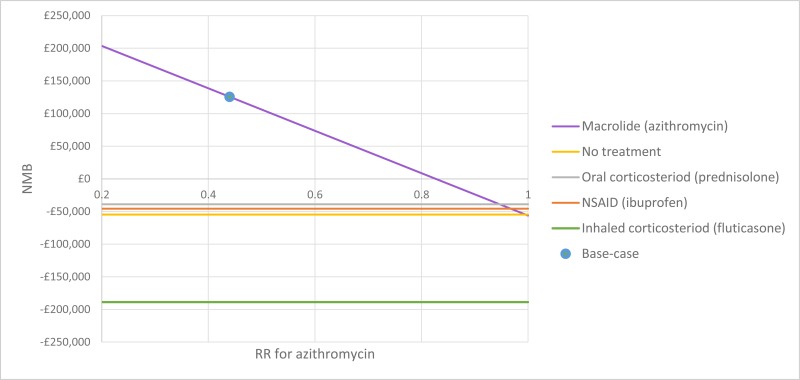
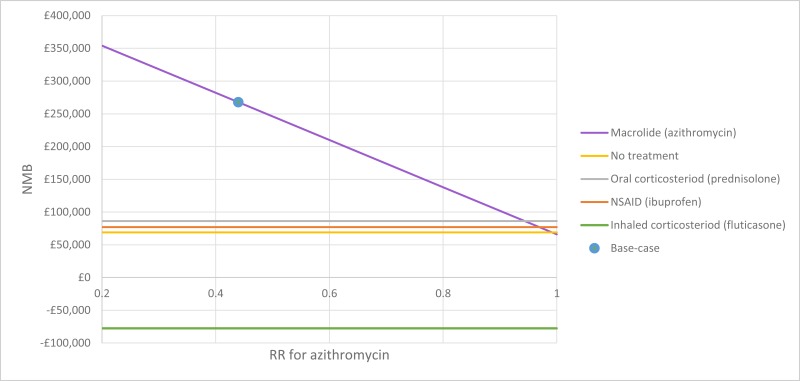
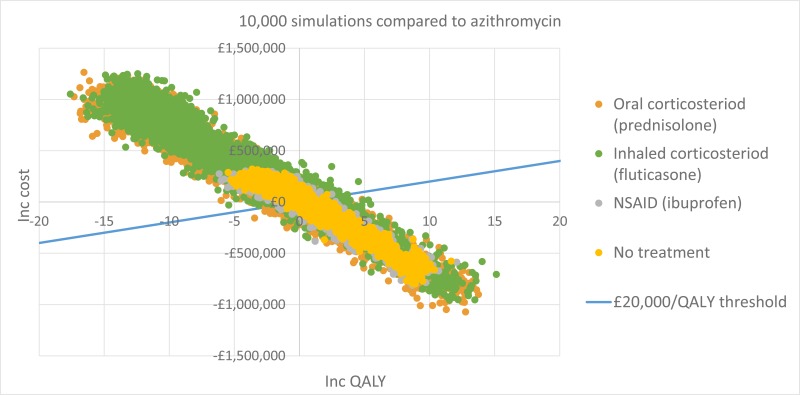
The only clinically significant finding demonstrated in the clinical evidence review was between PEP and HFCWO vests, where PEP reduced exacerbations by a greater amount. Given that exacerbations incur a treatment cost and negatively impact quality of life, vests should not be recommended as a cost-effective technique to improve airway clearance. There is clinical and cost-effectiveness evidence to suggest the vest is dominated (more expensive and less effective) by PEP. However, it is important to note that the clinical and cost effectiveness of the vest has not been reviewed in people with cystic fibrosis and neurodisabilties where other airway clearance techniques cannot be performed.
Techniques including ACBT, oscillating devices and PEP can be performed at home after an initial visit with a physiotherapist. As a result, the cost of recommending these techniques would be negligible compared to manual chest physiotherapy or HFWCO vests over the longer term. In addition, those techniques have no associated increase in cost if they are performed more frequently. However, it is important to consider the opportunity cost of the person’s time. Techniques may be free to deliver at home, but this does not necessarily mean they should be performed if they are not improving their health-related quality of life.
Overall, the recommendations are not likely to represent a change in current practice. In addition, the clinical evidence review did not produce any significant evidence in favour of one technique. Therefore, recommendations are likely to be for a stepwise escalation of techniques using the least resource intensive, and cheaper, options first (ACBT) and manual chest physiotherapy or NIV as a last resort.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 9.2.7.3 ‘Evidence to recommendations’.
K.3. Monitoring pulmonary disease
K.3.1. Literature review
Two studies that assessed pulmonary disease monitoring in people with cystic fibrosis were identified and included in the literature search conducted for this guideline (Table 9). One of those studies, summarised in section K.3.1.1, was directly relevant to the review question as it compared monitoring strategies in the protocol (bronchoalveolar lavage [BAL]-therapy versus standard therapy). The second, summarised in section K.3.1.2 however, was not considered to be applicable as it assessed the frequency of monitoring, rather than the type of testing. However, this paper was included given that the population included people with cystic fibrosis and the committee may consider recommendations on the type of monitoring and the frequency of monitoring.
Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1. Data extraction tables and quality assessments of included studies can be found in Appendix L and M, respectively.
Table 9Summary of included economic evaluations, monitoring pulmonary disease
| Study | Limitations | Applicability | Other comments | Inc. costs | Inc. effects | Inc. cost-effectiveness | Uncertainty |
|---|---|---|---|---|---|---|---|
| Etherington 2008 | Very seriousa | Not applicableb,c | New protocol to reduce the number of routine susceptibility tests | The projected savings of this intervention (cost year 2008) were €3,500 in consumables and 170 hours (costed at €6,500) of laboratory staff time per annum, a total annual saving of €10,000 (£6,500) | No significant differences in median change of FEV1, FVC, CRP, white cell count, weight or duration of IV antibiotics were observed. | NR | Not assessed |
| Moodie 2014 | Minord | Directlye | Data collected from a RCT | Mean total costs per child during the 5-year study period:
| NR | NR | 95% CIs reported |
A$, Australian dollars; BAL; bronchoalveolar lavage; CI, confidence interval; CF, cystic fibrosis; CRP, C-reactive protein; FEV, forced expiratory volume; FVC, forced vital capacity; IV, intravenous; MD, mean difference; NA, not applicable; NR, not reported; RCT, randomised controlled trial
- (a)
no detail regarding resource use and unit costs, only point estimates reported
- (b)
frequency of tests not a comparison of interest in the protocol, but considered useful for decision making in this area
- (c)
QALY not used as an outcome measure
- (d)
not all important and relevant outcomes included (health-related quality of life and adverse effects)
- (e)
This study does not include the preferred measure of effects (QALYs), but is still thought to be useful for decision making, given that all other criteria are relevant and the alternative outcome measure reported is unlikely to change the conclusions about cost-effectiveness.
K.3.1.1. Moodie 2014
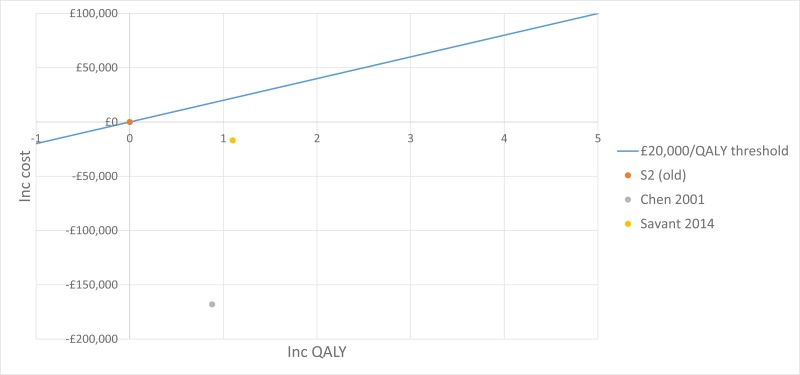
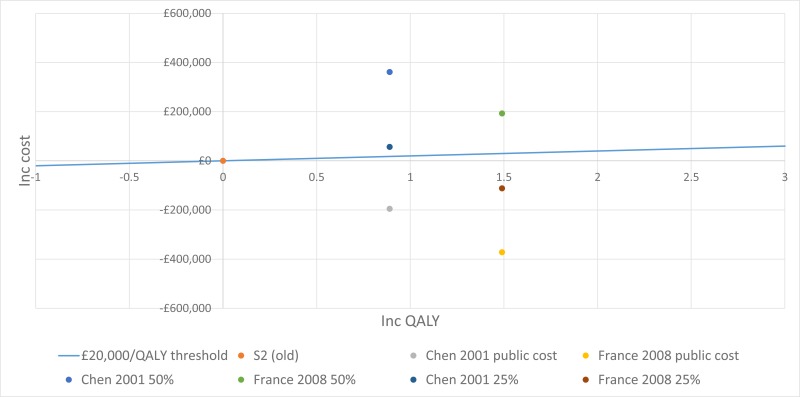
The trial by Wainwright 2011, included in the clinical evidence review, found no between-group difference for the 2 primary outcomes. Consequently, the original proposal for cost-effectiveness analysis (which addressed whether the incremental benefit of BAL-directed therapy was worth its incremental cost, measured against standard therapy) was no longer warranted.
Instead Moodie 2014 assessed the difference in costs between the 2 groups by using patient level data to ascertain whether BAL-directed therapy, rather than standard therapy, was still justified on the grounds of costs and whether BAL-directed therapy reduced treatment costs, by decreasing hospital days. Costs were valued in Australian dollars using a 2010 cost year. The costs included in the analysis were hospital admissions, BAL procedures, pharmaceutical costs, professional attendance, pathology tests and other procedures.
They found that the additional cost of BAL (A$11,880) was not offset by reductions in other health care expenditure. The mean total costs/child during the 5-year study period were A$92,860 in BAL-directed group and A$90,958 in standard group (mean difference [MD] A$1,902, 95% confidence interval [CI] −27,782 to 31,586, P = 0.90).
Moreover, there was no significant difference in mean hospital admission costs between the 2 groups. The mean hospital costs/child during the 5-year study period were A$ 57,302 in the BAL-directed group and A$66,590 in the standard group (MD A$-9,288; 95% CI −35,252 to 16,676, P =0.48).
It is important to note that both of these estimates are subject to wide Cis, reducing their credibility. However, considering that BAL-directed treatment offered no clinical advantage over standard therapy, BAL-directed therapy should not be recommended as a cost-effective monitoring strategy.
K.3.1.2. Etherington 2008
Etherington 2008 examined the cost and clinical impact of reducing the number of routine susceptibility tests conducted on isolates of P aeruginosa obtained from chronic infections in adults with cystic fibrosis.
This study was undertaken at a hospital in Leeds in 119 participants chronically infected with P aeruginosa. Their initial policy was to collect sputum samples at each clinic visit, every 8 weeks, and at the beginning and end of every course of intravenous (IV) antibiotics (routine therapy every 3 to 4 months). This is in accordance with the UK’s Cystic Fibrosis Trust recommendations that respiratory samples should be obtained every 4 to 8 weeks.
The application of a new protocol whereby isolates were only taken at the commencement of antibiotic therapy, there was evidence of clinical failure of therapy or routinely if not tested in the previous 3 months reduced the number of susceptibility tests by 56%.
No significant differences in median change of FEV1%, forced vital capacity (FVC), C-reactive protein, white cell count, weight or duration of IV antibiotics were observed following the new protocol. However, the projected savings (cost year 2008) were €3,500 in consumables and 170 hours (costed at €6,500) of laboratory staff time per annum, a total annual saving of €10,000 (£6,500). However, the study does not report the number of times samples were taken when there was evidence of clinical failure of therapy. For this reason, the savings reported may be overestimated because it is unclear which participants failed treatment and followed an alternative treatment strategy that could incur additional assessments. Moreover, details regarding resource and cost use were not provided beyond the results stated above.
Overall, this study showed that the number of routine susceptibility tests conducted on P aeruginosa isolates can be reduced without adversely affecting clinical outcomes of IV antibiotic therapy. However, the relevance of this analysis is questionable given that the study compared the number of susceptibility tests of P aeruginosa, rather than monitoring techniques for pulmonary disease.
K.3.2. Background and methods
Monitoring for pulmonary disease was not prioritised by the committee for de novo economic modelling as relatively cheap and non-invasive microbiological techniques are preferred. However, the frequency of monitoring will have resource implications and current practice may be cost-ineffective. Moreover, more costly and invasive techniques such as imaging and BAL can be used as alternative strategies.
Three clinical reviews were undertaken to compare monitoring strategies or combinations of monitoring strategies to identify pulmonary disease and to compare their effects on clinical outcomes, with a view to improving subsequent management. For each of those reviews there are the following economic considerations:
- 1)
Monitoring for pulmonary disease onset in people with cystic fibrosis without clinical signs or symptoms of lung disease
Young children with cystic fibrosis without clinical signs or symptoms of lung disease are often prescribed antibiotics as prophylaxis against Staphylococcus aureus colonisation or as acute treatment. Identifying the onset of pulmonary disease could initiate additional treatments, such as mucolytics and immunomodulatory agents, to prevent deterioration of health, lung function or tissue architecture. If the onset of pulmonary disease is identified promptly, the downstream costs to manage pulmonary disease, for example, from a reduction in exacerbations, could be reduced.
- 2)
Monitoring for evolving pulmonary disease in people with cystic fibrosis with established lung disease
People with established lung disease are likely to be receiving a mucoactive or mucolytic agent, immunomodulatory agent or prophylactic antibiotics, or a combination. If the person with cystic fibrosis becomes unresponsive to treatment, or demonstrates issues with adherence, monitoring informs the changes to existing treatment. Evolving pulmonary disease may require changes to the management strategy as the current strategy may no longer be cost-effective as the benefits and aims of treatment may change. For these reasons, monitoring would lead to more timely management and has, therefore, indirectly, potentially important resource implications.
- 3)
Monitoring the response to treatment following an acute exacerbation
Once the acute exacerbation has resolved, treatment should be discontinued. However, if the person with cystic fibrosis demonstrates an inadequate response to treatment, a different treatment strategy should be considered. Similarly to protocol 2, monitoring can stop ineffective treatments earlier, reducing the cost of acquisition and expected cost to manage treatment related adverse effects.
Overall, investigation techniques will not be considered cost-effective if there is not an effective treatment for the condition being monitored, or if management is not changed by the results of the investigation. In other words, if monitoring techniques do not add any additional information, and do not change the management strategy, they should not be recommended as a cost-effective use of resources.
There are relatively large differences in the costs of monitoring techniques under consideration, hence a cost description of the techniques was undertaken to aid recommendations.
The monitoring techniques under consideration are aggregated in section K.3.3 as they do not vary substantially between the 3 clinical reviews.
K.3.3. Resource and cost use
K.3.3.1. Non-invasive microbiological investigation
Non-invasive microbiological investigation techniques include an induced sputum sample, cough swab, throat swab and nasopharyngeal aspiration. Each of these investigations would be performed by a specialist nurse at the cystic fibrosis centre, with results available 2 to 3 days later.
According to NHS Reference Costs 2015/16 the average cost of directly accessed pathology services relating to microbiology is £8 (currency code DAPS07), whilst the cost of a nurses time is £33 for a 15 minute consultation (PSSRU 2016, Band 7, £130 per hour of patient contact) leading to a cost of £41 for a non-invasive microbiological investigation, excluding any subsequent visits for treatment.
K.3.3.2. Invasive microbiological investigation
BAL is an invasive microbiological investigation performed as an inpatient procedure. The results are not instantaneous and could take up to 5 days to process and report. The committee advised that BAL is performed in current UK clinical practice if the person with cystic fibrosis could not produce adequate quantities of mucus through coughing, as the procedure is invasive and costly. The cost of BAL is presented in Table 10.
Table 10Cost of BAL
| Service | National average unit cost | Source |
|---|---|---|
| Diagnostic Bronchoscopy, 19 years and over | £1,187 | NHS Reference Costs 2015/16, inpatient procedure, DZ69A |
| Diagnostic Bronchoscopy, 18 years and under | £2,605 | NHS Reference Costs 2015/16, inpatient procedure, DZ69B |
K.3.3.3. Lung physiological function tests
Lung physiological function tests include spirometry, lung clearance index (LCI) and cardio-pulmonary exercise testing (CPEX); all of which, provide instantaneous results.
The committee advised that spirometry is regularly performed at the annual review, whereas LCI and CPEX are not. The committee added that CPEX is only performed in current UK clinical practice in people with established lung disease.
The cost of a spirometry is relatively inexpensive and could be performed by a specialist nurse at the cystic fibrosis centre. A procedure using spirometry is not provided in NHS Reference Costs 2015/16, but the chronic obstructive pulmonary disease (COPD) costing model for spirometry and pulmonary rehabilitation estimated a cost of £5.53 (inflated to 2015/16 prices) based on the work-up reproduced in Table 11.
Table 11Cost of spirometry reproduced from DoH 2012a
| Assessment of airflow obstruction using spirometry | Time to complete (minutes) | HCPs time | Cost (cost year 2012) |
|---|---|---|---|
| Explain and demonstrate tests to patient | 2.5 | Respiratory technician Band 4, cost/minute £0.35 | £0.88 |
| Baseline RVC | 5 | £1.75 | |
| Baseline FVC | 5 | £1.75 | |
| Record baseline spirometry | 2.5 | £0.88 | |
| Total | 15 | - | £5.25b |
DoH, Department of Health; HCHS, Hospital and Community Health Services; HCP, healthcare professional; FVC, forced vital capacity; RVC, relaxed vital capacity
- (a)
Taken from: spirometry and pulmonary rehabilitation. Published by: Department of Health 2012. Available from: http://www
.respiratoryfutures .org.uk/knowledge-portal /department-of-healthdocuments /costing-model-spirometry-and-pulmonary-rehabilitation/ [last accessed 11/09–2015] - (b)
HCHS inflation factor 1.0513 (2011/12 PPI 282.5/2015/16 PPI 297.0) provides a 2015/16 cost of £5.53
However, committee communications with their cystic fibrosis centres found a much higher cost than that estimated by the Department of Health for COPD. The committee advised that the tariff for spirometry in people with cystic fibrosis (using a semi-portable machine as opposed to a hand-held device) is £42, but local Clinical Commissioning Groups (CCGs) could negotiate this price with their providers.
LCI and CPEX on the other hand, are more costly and would be performed by a pulmonary function technician who has expertise in performing and interpreting the tests (Table 12).
Table 12Cost of CPEX and LCI
| Service | National average unit cost | Source |
|---|---|---|
| CPEX | ||
| Paediatric visit | £412 | NHS Reference Costs 2015/16, 258 Paediatric Respiratory Medicine, DZ31Z, Outpatient procedure, Cardio Pulmonary Exercise Testing |
| Adult visit | £195 | NHS Reference Costs 2015/16, 340 Respiratory Medicine, DZ31Z, Outpatient procedure, Cardio Pulmonary Exercise Testing |
| LCI | ||
| Paediatric visit | £179 | NHS Reference Costs 2015/16, WF01C, Non-Admitted Face to Face Attendance, Follow-up, Non-Consultant led, 258 Paediatric Respiratory Medicine |
| Adult visit | £117 | NHS Reference Costs 2015/16, WF01A, Non-Admitted Face to Face Attendance, Follow-up, Non-Consultant led, 340 Respiratory Medicine |
CPEX, cardiopulmonary exercise testing, LCI, lung clearance index
K.3.3.4. Imaging techniques
According to the committee, imaging techniques, including chest x-rays and CT scans, are used to monitor people with cystic fibrosis without clinical signs or symptoms, or those experiencing an acute exacerbation, but not in people with established lung disease, where management is unlikely to be influenced from the scans. The unit costs of those imaging techniques are presented in Table 13.
Table 13Cost of chest x-rays and CT scans
| Service | National average unit cost | Source |
|---|---|---|
| CT scan | ||
| 19 years and over | £99 | NHS Reference Costs 2015/16, diagnostic imaging, RD20A, 1 area, without contrast |
| 6 to 18 years | £108 | NHS Reference Costs 2015/16, diagnostic imaging, RA20B, 1 area, without contrast |
| 5 years and under | £96 | NHS Reference Costs 2015/16, diagnostic imaging, RA20C, 1 area, without contrast |
| Chest x-ray | ||
| All ages | £30 | NHS Reference Costs 2015/16, Direct Access Plain Film, Directly Accessed - Diagnostic Services |
CT, computerised tomography
It is also important to note that generally, all children less than 4 years of age would require a general anaesthetic such as propofol (BNF November 2016: 0.5% emulsion for injection 20ml ampoules; 5 ampoules/£14.71) from an anaesthetist (NHS Reference Costs 2015/16: WF01B, 190, Non-Admitted Face to Face Attendance, First, Non-consultant led, £90) to produce good images of their lungs. The committee also noted that administering an anaesthetic can lead to longer than ideal waiting times which reduces the number of CT scans performed for a timely assessment.
K.3.4. Conclusions
BAL is the most expensive and invasive investigation under consideration. There is no clinical or cost-effectiveness evidence to suggest that the benefits of BAL can outweigh the costs. Therefore, the committee will need to provide exceptional justifications to recommend BAL over any of the other investigations included in this review.
One study included in the clinical evidence review found spirometry and CT scans accurately predicted future FEV1% and exacerbations. For this reason, spirometry and CT scans should continue to be used at the annual review to monitor for pulmonary disease onset, if those tests are subsequently used to inform the patient’s management strategy. However, if spirometry and CT scans are equally effective at informing management strategies, spirometry should be recommended ahead of a CT scan as it is cheaper and not subject to the negative effects from radiation.
As can be seen from Table 12, CPEX and LCI are relatively expensive compared to spirometry. However, cost data for these investigations have little use without associated benefits. Therefore, while the costs of these investigation are relatively expensive, without knowing the benefits, we cannot know if they will be cost-effective compared to current clinical practice.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 9.1.7.3 ‘Evidence to recommendations’.
K.4. Monitoring for the onset of CFRD
K.4.1. Literature review
No economic evaluations of strategies to monitor for the onset of cystic fibrosis-related diabetes (CFRD) were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.4.2. Background and methods
It is important to screen for diabetes as early treatment can protect against weight loss, deterioration in lung function and long-term complications. For these reasons, the costs incurred by some monitoring strategies may be offset if those downstream costs can be prevented. However, to fully address the cost-effectiveness of strategies to monitor for the onset of CFRD would require a model that also included the specialist management of CFRD, which is beyond the scope of this guideline.
The oral glucose tolerance test (OGTT) is currently the most common way to screen for CFRD. After an overnight fast, 2 or 3 blood samples are taken to measure blood glucose levels for up to 2 hours after drinking a prescribed amount of glucose solution. However, a more complete picture of blood glucose levels over a period of days can be obtained by using a continuous glucose monitor (CGM). This involves placing a small sensor under the skin and attaching a small recording device. The sensor measures glucose between the cells and gives a complete trace of what is happening 24 hours a day over a number of days.
The OGTT is a relatively simple and cheap test to undertake, whereas CGM requires an upfront capital cost and ongoing maintenance. As result, a recommendation in favour of CGM would lead to a change in clinical practice and additional resources to implement. To enable a cost comparison between CGM and OGTT, the NICE website was searched for any recently published guidance on diabetes that provided relevant cost data.
K.4.3. Resource and cost use
K.4.3.1. Oral glucose tolerance test (OGTT)
The costs of an OGTT test comprises of the laboratory test costs for each blood sample, the costs of the glucose solution and the costs of staff time in administering the OGTT. Although practice will not be the same everywhere, it was assumed that as part of the test it would be necessary to provide some explanation of the test, obtain patient consent, prepare the glucose solution, take blood samples and inform the patient of the result. The blood tests are often taken by a healthcare assistant but a diabetic specialist nurse will often be responsible for explaining the test and providing them with the results.
After consultation with the committee, it was assumed that a 2-sample OGTT will take 30 minutes of a healthcare assistant’s time and 5 minutes of a nurse’s time.
The committee advised that someone with cystic fibrosis would be seen in a dedicated clinic room for the whole of their attendance at the clinic for an OGTT (2 hours). Moreover, when OGTTs are taken in children a band 6 nurse would fit them with a cannula, which could take up to 30 minutes. Following this, committee members communicated a cost of approximately £50 per OGTT from their hospital for adults with cystic fibrosis and a cost of approximately £70 for children, if a cannula is fitted. The breakdown of those costs are provided in Table 14.
Table 14Cost of OGTT
| Item | Cost | Source |
|---|---|---|
| Health care assistant Band 3 | ||
| Cost/hour | £27.00 | PSSRU 2016 |
| 30 minute 2-sample OGTT | £13.50 | Calculated |
| Nurse Band 6a | ||
| Cost/hour | £44.00 | PSSRU 2016 |
| 5 minute 2-sample OGTT | £3.67 | Calculated |
| 30 minutes cannula fitting (paediatrics) | £22.00 | Calculated |
| Non-staff costs | ||
| Laboratory costs 2-sample OGTT | £8.18 | NG3 2014 NHS hospital trust personal communication (inflated to 2015/16 costs)b |
| Glucose solutionc | £3.48 | BNF November 2016 |
| Cannula (paediatrics) | £0.57 | NHS Supply Chain 2015: cannula intravenous infusion set £28.58/50 |
| Clinic room cost | £20.00 | committee estimate |
| Total costs | ||
| Adults | £48.83 | Calculated |
| Paediatrics | £71.40 | Calculated |
BNF, British National Formulary; OGTT, oral glucose tolerance test; PSSRU, Personal Social Services Research Unit
- (a)
The hourly cost of a Band 6 nurse is based on a cost/hour as opposed to a cost/patient hour, which assumes that only 41% of a nurse’s time is spent in direct contact with patients. It is assumed that the nurse’s time input reflects all OGTT related activity and not just patient contact time.
- (b)
Inflation factor 1.022 calculated from HCSC (2015/16 PPI 297.0/2013/14 PPI 290.5)
- (c)
BNF dose for OGTT: 75g oral solution; £3.48/300ml, glucose 250 mg per 1 ml
The committee noted that a single-point OGTT would not be sufficient to monitor for the onset of CFRD as diet and lifestyle choice are variable within individuals. To obtain a more complete picture of blood glucose levels, the committee agreed that the OGTT should be repeated after a few days leading to a total cost of £98 for adults and £143 for children who require a cannula.
K.4.3.2. Continuous glucose monitoring (CGM)
The cost of CGM used to inform NICE NG17 (August 2015, Type 1 diabetes in adults: diagnosis and management) was based on the average of 3 of the main technologies available in the UK: Dexcom G4, Abbott Freestyle, and Medtronic RT Guardian. The items included in the estimation of the annual cost were the receiver, sensors, transmitters, and calibration (self-blood tests). NICE DG21 (February 2016, Integrated sensor-augmented pump therapy systems for managing blood glucose levels in type 1 diabetes) also provided a cost analysis of those 3 CGM technologies.
One noteworthy discrepancy between those analyses was the cost of a Dexcom G4 receiver (NICE NG17, £1,750; DG21, £750) and the exclusion of calibration from DG21. Details of the analyses by NICE NG17 and NICE DG21 are reproduced in Table 15 and Table 16, respectively. Despite this, the annual cost to provide CGM is approximately £3,500.
Table 15Cost of CGM reproduced from NICE NG17
| Service | Unit cost | Units/year | Cost/year |
|---|---|---|---|
| Dexcom G4 | |||
| Receiver | £1,750 | 1/5 | £374a |
| Sensors | £63 (£250/4) | 52 | £3,250 |
| Transmitters | £275 | 2 | £550 |
| Calibration | £0.29 | 2*365b | £212 |
| Total | £4,386 | ||
| Abbott Freestyle | |||
| Receiver | £950 | 1/5 | £203a |
| Sensors | £48 (£288/6) | 60 | £2,880 |
| Transmitters | NAc | NA | £0 |
| Calibration | £0.29 | 1*365d | £106 |
| Total | £3,189 | ||
| Medtronic RT Guardian | |||
| Receiver | £1,059e | 1/5 | £227a |
| Sensors | £42 (£420/10) | 60 | £2,520 |
| Transmitters | £490f | 1f | £490 |
| Calibration | £0.29 | 2*365b | £212 |
| Total | £3,449 | ||
NA, not applicable
- (a)
Annual cost estimated assuming a five year life span and a discount (dis) of 3.5% using the formula: purchase cost/(1−1/(1+dis)^(life span −1))/dis)
- (b)
Assuming SMBG for calibration is performed twice a day
- (c)
Rechargeable
- (d)
On average calibration is performed once per day
- (e)
Total initial cost of £1,599 included also the cost of sensors, which has been subtracted by the initial cost.
- (f)
Except for the first year.
Table 16Cost of CGM reproduced from NICE DG21
| Service | Equipment cost | Units | Cost/year |
|---|---|---|---|
| Dexcom G4 | |||
| Receiver | £745.00 | 5 years of use | £149.00 |
| Transmitter | £335.00 | 0.5 years of use | £670.00 |
| Sensor | £46.50 | 52.14 units per year (7 days of use) | £2,424.64 |
| Total | £3,243.64 | ||
| Abbott Freestyle | |||
| Receiver | £950.00 | 5 years of use | £190.00 |
| Transmitter | £0.00 | 0 years of use | £0.00 |
| Sensor | £48.00 | 60.83 units per year (6 days of use) | £2,920.00 |
| Total | £3,110.00 | ||
| Medtronic RT Guardian | |||
| Receiver | £1,059.00 | 5 years of use | £211.80 |
| Transmitter | £228.70 | 1 years of use | £228.70 |
| Sensor | £42.05 | 60.83 units per year (6 days of use) | £2,558.04 |
| Total | £2,998.54 | ||
It is important to note that those costs in Table 15 and Table 16 are based on continuous use once diabetes has been diagnosed. When screening for CFRD, the committee advised that a person with cystic fibrosis would use a monitoring system for up to 1 week to clarify a diagnosis of CFRD. For this reason, the monitoring system would be shared across people at the clinic screened for CFRD; subsequently lowering the cost/person.
If the annual cost to provide a CGM system is approximately £3,500, the cost/person could be as low as £67 if one person with cystic fibrosis utilised one system a week. However, the committee added that administration to track the equipment (to facilitate returns and monitor lending history) would be required and this, combined with cleaning, could lead to a delay between uses of up to a week. As a result, the committee expected the equipment to be shared by up to 25 people with cystic fibrosis each year leading to a cost from £135/year to provide the CGM system.
Additional consultations to provide the CGM system and discuss the results would also be incurred (PSSRU 2016, Nurse advanced band 7, per hour of patient contact, £130) leading to a total cost of up to £200 if up to an additional 30 minutes of staff time is required.
It is important to note that for CGM to be viable, a centre would need access to several systems as to allow more than one person with cystic fibrosis to be monitored at any period. As a result, the implementation cost could be substantial if centres do not currently have access to CGM systems to monitor for the onset of CFRD.
K.4.4. Conclusions
It is clear that CGM is more expensive than a single OGTT. However, the cost of OGTT could overtake the cost of CGM if several visits are required to obtain a dynamic result. Without knowing the prognostic accuracy of CGM or OGTT to detect CFRD, we cannot know if the benefits of CGM outweigh its additional cost compared to OGTT, or vice versa. Overall, the committee will have to provide additional justifications if their recommendations increase current resource use and should consider a research recommendation if current practice could change upon such evidence.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 10.6.7.3 ‘Evidence to recommendations’.
K.5. Monitoring liver disease
K.5.1. Literature review
No economic evaluations of test to detect related liver disease in people with cystic fibrosis were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.5.2. Background and methods
Current practice is to offer all people with cystic fibrosis a clinical (annual) review to either test for liver disease or monitor the progression of liver disease using an ultrasound scan, clinical examination (hepatomegaly and splenomegaly) and liver function blood tests. However, the first thing that should reflect developing liver problems are liver function blood tests that can measure the amount of enzymes spilling into the blood. Furthermore, not all people with cystic fibrosis will develop liver disease or progressively worsening liver disease. For these reasons, there are potential cost savings to the NHS if liver function blood tests can replace ultrasound scans.
FibroScan® is a relatively new non-invasive imaging system that if implemented, requires an upfront capital cost, staff training and annual maintenance. Current practice in the UK would be to offer people with cystic fibrosis an ultrasound scan for imaging purposes. Therefore, a recommendation in favour of FibroScan® would lead to a change in clinical practice. To enable a cost comparison between ultrasound and FibroScan® the equivalent annual cost will need to be estimated.
The most recognised gold standard test is a liver biopsy, but the procedure is costly, painful and invasive and has the potential for life-threatening complications and sampling errors. For these reasons, a new definition of liver disease has come into practise which is based on the monitoring tests performed at the clinical (annual) review.
Overall, diagnostic procedures will not be considered cost-effective if there is not an effective treatment for the condition being diagnosed, or if the person’s management is not changed by the results of the procedure. In other words, if the tests do not add any additional information to the clinical assessment, and do not change the person’s management strategy, they should not be recommended. However, to fully address the cost-effectiveness of tests to detect related liver disease would require a model that also included treatment that lies outside the scope of this guideline. To aid considerations of cost-effectiveness a cost description of the tests included in the review has been undertaken.
K.5.3. Resource and cost use
K.5.3.1. Tests currently performed at annual review
As described in the clinical evidence review, a new definition of cystic fibrosis-related liver disease has come into practice using recommendations based on the tests performed at the annual review. These tests include an ultrasound scan, clinical examination (hepatomegaly, splenomegaly) and liver function blood tests (Table 17).
Table 17Cost of tests performed at the annual review to monitor for liver disease
| Service | National average unit cost | Source |
|---|---|---|
| Clinical assessment | £33 | PSSRU 2016, Nurse advanced (Band 7) per 15 minute consultation (cost/hour of patient contact £130) |
| Liver function blood tests | £3 | NHS Reference Costs 2015/16, DAPS05, direct access, haematology |
| Ultrasound scan | £60 | NHS Reference Costs 2014/15, diagnostic imaging, RA42Z, outpatient, 20 minutes and over |
PSSRU, Personal Social Services Research Unit
As can be seen from Table 17, ultrasound scans are more costly than liver function blood tests. Moreover, if ultrasound scans produce unclear images, the additional information they provide, may be reduced. This was highlighted by Mueller-Abt 2008, who reported that the highest kappa values (a statistical measure of agreement between observers) were obtained for nodularity, attenuation and spleen size (0.76–0.94). However, kappa values for hepatic homogeneity/coarseness were relatively low, indicating high variance in interpretation.
K.5.3.2. Transient elastography (FibroScan®)
Purchasing the FibroScan® is a capital cost, requiring an up-front payment. The National Horizon Screening Centre estimated the cost of FibroScan® to be £49,950 in 2008 prices (excluding VAT).
The unit cost of FibroScan® according to the Resource Impact Report for NICE NG50 (Cirrhosis in over 16s: assessment and management) is £164; comprising an ultrasound scan more than 20 minutes (£56; Health Resource Group, HRG code RA24Z) and a follow-up appointment as a hepatology outpatient (£108, HRG code WF01B).
For completeness, the equivalent annual cost (Table 18) has also been calculated, given that many centres may need an injection of resources to implement FibroScan® in their centre.
Table 18Equivalent annual cost of FibroScan®
| Parameter | Value | Source |
|---|---|---|
| K = purchase price of FibroScan® | £55,562 | Taken from the NHCS paper, inflated from 2008 prices (£49,950)a |
| T= training | £0 | committee advised training is relatively quick and easy and take place within the hospital. Witters 2009 also stated FibroScan® is easy to learn, independent of professional training (i.e. a nurse could do it) |
| r = discount (interest) rate | 3.5% | NICE reference case |
| n = equipment lifespan | 10 years | Assumption informed by committee |
| A (n,r) = annuity factor (n years at interest rate r) | 8.61 | Calculated |
| E = equivalent annual cost | £6,455 | Calculated |
HCSC, Hospital and Community Health Services; NA, not applicable; NHCS, National Horizon Scanning Centre; NICE, National Institute of Health and Care Excellence.
- (a)
HCHS pay & price index (2008/09 PPI [267.0]/2015/16 PPI [297.0]) inflation factor 1.112
In addition to the initial capital outlay there are several other parameters to consider when estimating the unit cost (i.e. total cost/scan), these are presented in Table 19.
Table 19Total cost per scan (FibroScan®)
| Parameter | Value | Source |
|---|---|---|
| E = equivalent annual cost | £6,455 | Table 18 |
| M = annual maintenance cost | £3,337 | Inflated from the NHCS paper, inflated from 2008 prices (£3,000)a |
| Nf = number of FibroScan® per centre | 1 | Assumption |
| Np = number of CF patients per centre | 150 | Assumption based on CF Trust Standard of Care 2011 for a medium sized centre |
| D = disposables | £0 | committee assumption that lubricating jelly is readily available in hospitals at a negligible cost |
| C = consultation cost | £33 | PSSRU 2016, Nurse advanced (Band 7) per 15 minute surgery consultation. Band and time based on information provided in the NHCS paper, in consultation with the committee |
| Total cost/scan in a mediumsized centre including staff costs | £98 | Estimatedb |
CF, cystic fibrosis; HCHS, Hospital and Community Health Services; NA, not applicable; NHSC, National Horizon Scanning Centre; NHSRC, National Health Service Reference Costs; PSSRU, Personal Social Services Research Unit.
- (a)
HCHS pay & prices index (2008/09 PPI [267.0]/2015/16 PPI [297.0]) inflation factor 1.112
- (b)
Total cost/scan: ((E+M)*Nf / Np) +D +C
The cost/scan will vary depending on the usage of the machine, for example, the more the machine is used the lower the cost/person. The CF Trust Standard of Care 2011 advise a minimum of 75 adults or children to be managed by a specialist CF centre. However, a small number of ‘tertiary’ cystic fibrosis centres may manage 200 to 250 people with cystic fibrosis.
According to the NHCS paper, and members of the committee, FibroScan® can be performed by trained medical or paramedical staff within 15 minutes and would not require any additional disposables. Based on this, one scan could be performed by a specialist nurse within one surgery consultation at a cost of £33. Therefore, the total cost/scan in centre managing 150 people with cystic fibrosis would be £98. On the other hand, if a hepatologist (£108 HRG code WF01B) is believed to be more appropriate, a cost of £173 would closely reflect the cost reported by NG50.
It is important to note that FibroScan® could be used to diagnose liver fibrosis outside of people with cystic fibrosis, subsequently lowering the average cost/scan. However, more FibroScan® machines may be required, for supply to equal demand.
K.5.3.3. CT and MRI
Although CT and MRI scans are not invasive and are significantly cheaper than a liver biopsy, the committee noted that they are not routinely performed to detect liver disease. This was reflected in the clinical evidence review that found no evidence for MRI or CT scanning used as the reference standard.
In addition to the costs reported in Table 20, the committee advised that young children would require a general anaesthetic, such as propofol (BNF November 2016: 0.5% emulsion for injection 20ml ampoules; 5 ampoules/£14.71), from an anaesthetist (NHS Reference Costs 2015/16, WF01B, 190, Non-Admitted Face to Face Attendance, First, Non-consultant led, £90) to produce good images and this could lead to longer than ideal waiting times.
The committee stated that the images obtained from CT and MRI are not always clear-cut and may be subject to variance in interpretation, which questions their cost-effectiveness relative to liver function blood tests that are cheaper and objective.
Table 20Cost of CT and MRI scans
| Service | National average unit cost | Source |
|---|---|---|
| MRI scan | ||
| 19 years and over | £146 | NHS Reference Costs 2015/16, diagnostic imaging, RD01A, 1 area, without contrast |
| 6 to 18 years | £143 | NHS Reference Costs 2015/16, diagnostic imaging, RD01B, 1 area, without contrast |
| 5 years and under | £115 | NHS Reference Costs 2015/16, diagnostic imaging, RD01C, 1 area, without contrast |
| CT scan | ||
| 19 years and over | £99 | NHS Reference Costs 2015/16, diagnostic imaging, RA20A, 1 area, without contrast |
| 6 to 18 years | £108 | NHS Reference Costs 2015/16, diagnostic imaging, RA20B, 1 area, without contrast |
| 5 years and under | £96 | NHS Reference Costs 2015/16, diagnostic imaging, RA20C, 1 area, without contrast |
CT, computerised tomography; MRI, magnetic resource imaging
K.5.3.4. Liver biopsy
Liver biopsies are generally performed under local anaesthesia and require a short hospital stay. The committee also advised that this invasive technique is associated with serious adverse events due to bleeding and other complications. For those reasons, liver biopsies should only be performed when the benefits outweigh the risks in terms of changing the disease outcome.
Unlike clinical examinations and imaging, the results from a biopsy are not instantaneous and could take up to 2 weeks. Moreover, the results could be inconclusive resulting in the need for a repeat biopsy. Some clinicians would take a “dual pass” to reduce this uncertainty, this was demonstrated by Lewindon 2011 who reported that a dual pass biopsy improved the detection of fibrosis in their trial. Their first pass detected liver fibrosis in 26 people with cystic fibrosis and the second detected liver fibrosis in another 5.
For these reasons, liver biopsies can have a negative impact on quality of life due to their invasiveness, potential scarring and delayed results which can cause anxiety and distress. Moreover, if complications or subsequent procedures are incurred, those costs would be greater. The cost of a single biopsy is reported in Table 21.
Table 21Cost of liver biopsy
| Service | National average unit cost | Source |
|---|---|---|
| Percutaneous biopsy of lesion of liver | £1,592 | NHS Reference Costs 2015/16, elective inpatient, YG10Z |
If biopsies do not provide additional diagnostic information to inform the patient’s management strategy, because the aetiology (secondary biliary cirrhosis) is generally, already known, it is clear that biopsies would be dominated (more expensive and less effective) by the current annual review as they would not produce any additional benefit to outweigh the expected cost and expected QALY losses associated with the procedure itself and potential adverse effects of the procedure.
However, the cost-effectiveness of a biopsy is less certain if there was reason to suggest a non-cystic fibrosis cause of liver disease as their management strategy may differ to that for secondary biliary cirrhosis.
K.5.4. Conclusions
The main concern for people incorrectly diagnosed with liver disease (false positives) is the psychological effect, rather than the cost of treatment, as ursodeoxycholic acid is relatively inexpensive (NHS Electronic Drug Tariff November 2016 price, £0.79 per 300mg tablet; BNF dose, 12–16mg/kg once daily) and often prescribed as a prophylactic with minimal side effects. However, most of the studies included in the clinical evidence review found specificity to be greater than sensitivity to detect early stage liver disease which implies that tests are more accurate at ruling out liver disease than ruling in liver disease.
Sensitivity was only found to be consistently greater than specificity when detecting oesophageal vices and, in some studies, when detecting cirrhosis. However, these estimates were subject to very serious imprecision. This implied that in later stages of liver disease, diagnostic accuracy was greater at identifying true cases of liver disease, but this is to be expected because later stages would be diagnosed at higher thresholds.
The economic harms associated with an incorrect diagnosis are much smaller for early stage liver disease than late stage liver disease. If the majority of false negatives are likely to be picked up at their next annual review as true positives, this questions if additional monitoring to those standard reviews is cost-effective. On the other hand, the cost of monitoring for late stage liver disease would be relatively insignificant when compared with the losses in quality of life and “downstream” costs associated with people who have developed liver cirrhosis, and subsequent portal hypertension, and require a liver transplant.
With regards to the monitoring strategies, it is evident that liver biopsies are costly, invasive and, occasionally, inconclusive which can cause substantial anxiety and distress. As a result, cost savings could be made if their use is restricted to when the aetiology of liver disease is unknown as this is when they can provide additional information to the other strategies to justify their additional cost and risk.
If abnormal liver function blood tests are the first indication of liver disease, there are potential cost savings to the NHS if liver function blood tests replace ultrasound scans at the annual review, especially in adults who are unlikely to develop liver disease without prior suspicion. Furthermore, if ultrasound or FibroScan® do not add any additional information to those liver function blood test and do not change the patient’s management strategy, ultrasound or FibroScan® should not be recommended. However, if ultrasound and FibroScan® can detect cirrhosis and portal hypertension that cannot always be identified by a clinical assessment and liver function blood tests, the cost of those procedure may be outweighed.
Overall, the clinical evidence review demonstrated difficulty in assessing the best reference standard based on the available evidence. Given that there is no reason currently to prefer one test over the other in terms of their accuracy, then the cheapest and least invasive option should be considered first.
The committee’s discussion regarding the associated economic benefits and harms are reported in Section 10.4.7.3 ‘Evidence to recommendations’.
K.6. Distal intestinal obstruction syndrome (DIOS)
K.6.1. Literature review
No economic evaluations of strategies for the treatment or secondary prevention of DIOS were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.6.2. Background and methods
Treatment of DIOS is still largely empirical according to the committee, as there are few randomised controlled trials (RCTs) to guide therapy. As a previous episode of DIOS is a risk factor for recurrence, maintenance laxative and reassessment of adequate pancreatic enzyme dosage i.e. pancreatic enzyme replacement therapy (PERT) are often considered for secondary prevention.
The clinical evidence review did not identify any relevant evidence for this review question. Despite this, the committee agreed that DIOS treatments are frequently prescribed to people with cystic fibrosis. To aid consideration of the costs, a cost description of DIOS treatments was undertaken.
K.6.3. Resource and cost use
Drug acquisition costs are taken from the NHS Electronic Drug Tariff November 2016, unless unreported and otherwise stated. Dosages reflect those reported in the BNF according to age. When dose ranges were reported the mid-point was taken for costing purposes. For a cost description of PERT, please refer to Section K.7.
K.6.3.1. Acetylcysteine
The recommended dosages reported in the BNF for acetylcysteine according to indication and age are presented below:
- Treatment of DIOS:
- Child 1 month–2 years 0.4–3 g as a single dose;
- Child 2–7 years 2–3 g as a single dose;
- Child 7–18 years 4–6 g as a single dose.
- Prevention of DIOS:
- Child 1 month–2 years 100–200 mg tds (3 times daily);
- Child 2–12 years 200 mg tds;
- Child 12–18 years 200–400 mg tds.
Table 22 presents the cost of acetylcysteine for the treatment of DIOS based the cheapest available manufacturer for tablet and capsule preparations. However, other forms are available from special-order manufacturers such as granules and oral solution.
Table 22Acquisition cost of acetylcysteinea
| Population | Cost/day | Cost/week | Cost/month |
|---|---|---|---|
| Treatment of DIOS | |||
| Child 2–7 yearsb | £2.00 | £14.00 | £60.80 |
| Child 7–18 yearsc | £4.50 | £31.50 | £136.80 |
| Prevention of DIOS | |||
| Child 2–12 yearsd | £0.50 | £3.50 | £106.40 |
| Child 12–18 yearse | £1.00 | £7.00 | £212.80 |
- (a)
600mg tablet £0.50 (quantity, 30; basic price, £15.00)
- (b)
assume 4 tablets/day (2.4g/day)
- (c)
assume 9 tablets/day (5.4g/day)
- (d)
assume 1 tablet/day (600mg/day)
- (e)
assume 2 tablets/day (1.2g/day)
K.6.3.2. Osmotic laxative containing polyethylene glycol
The recommended dosages reported in the BNF for lactulose to treat chronic constipation are presented below, according to age:
- Movicol-Paediatric®:
- Child 2–5 years: 1 sachet daily, adjust dose to produce regular soft stools; maximum 4 sachets per day
- Child 6–11 years: 2 sachets daily, adjust dose to produce regular soft stools; maximum 4 sachets per day.
- Macrogol:
- Child 12–17 years: 1–3 sachets daily in divided doses usually for up to 2 weeks; maintenance 1–2 sachets daily
- Adult: 1–3 sachets daily in divided doses usually for up to 2 weeks; maintenance 1–2 sachets daily.
Based on those dosages, Table 23 presents the cost of Movicol-Paediatric® oral powder and Macrogol oral powder compound for children and adult, respectively.
Table 23Acquisition cost of osmotic laxative containing polyethylene glycol (Macrogol)
| Population | Unit cost (quantity, basic price) | Cost/day | Cost/week | Cost/month |
|---|---|---|---|---|
| Movicol-Paediatric®, oral powdera | ||||
| Child 2 to 11 yearsb | £0.15 (30, £4.38) | £0.29 | £2.04 | £8.88 |
| Macrogol oral powder compound sachets, sugar free | ||||
| Over 12 yearsb | £0.14 (30, £4.27) | £0.28 | £1.99 | £8.65 |
- (a)
cost taken from the BNF
- (b)
assume 2 sachets daily
K.6.3.3. Sodium meglumine diatrizoate (Gastrogafin®)
The cost of sodium meglumine diatrizoate (Gastrografin®) is reported in Table 24 for an indication of DIOS in children with cystic fibrosis.
Table 24Acquisition cost of sodium meglumine diatrizoate (Gastrografin®)
| Population | Unit cost (quantity, basic price) | Cost/day | Cost/week | Cost/month |
|---|---|---|---|---|
| Gastrografin® | ||||
| Body weight 15–25kga | £0.18/ml (1,000ml, £175.00) | £8.75 | £61.25 | £266.00 |
| Body weight >25kgb | £17.50 | £122.50 | £532.00 | |
- (a)
BNF: 50ml as a single dose
- (b)
BNF: 100ml as a single dose
K.6.3.4. Lactulose
The recommended dosages reported in the BNF for lactulose to treat constipation according to age are presented below:
- Child 1–11 months: 2.5 mL bd, adjusted according to response;
- Child 1–4 years: 2.5–10 mL bd, adjusted according to response;
- Child 5–17 years: 5–20 mL bd, adjusted according to response;
- Adult: Initially 15 mL bd, adjusted according to response.
Table 25 summaries those dosages in to 2 categories, to illustrate the plausible costs.
Table 25Acquisition cost of lactulose
| Population | Unit cost (quantity, basic price) | Cost/day | Cost/week | Cost/month |
|---|---|---|---|---|
| Lactulose 10g/15ml oral solution 15ml sachets sugar free | ||||
| Child 1 to 4 yearsa | £0.25 (10, £2.50) | £0.25 | £1.75 | £7.60 |
| Over 5 yearsb | £0.50 | £3.50 | £15.20 | |
| Lactulose 3.1–3.7g/5ml oral solution | ||||
| Child 1 to 4 years | £0.02/5ml (500ml, £2.47) | £0.06c | £0.43 | £1.88 |
| Over 5 years | £0.15d | £1.04 | £4.51 | |
- (a)
assume one 15ml sachet/day
- (b)
assume two 15ml sachets/day
- (c)
assume 12.5ml/day
- (d)
assume 30ml/day
K.6.3.5. Phosphates enema
Enemas are relatively more invasive than oral preparations requiring application to the rectum. The committee noted that enemas are often administered under radiological supervision to ensure the required site is reached, at a cost of £89 (NHS Reference Costs 2015/16, WF01A, Non-Admitted Non-Face to Face Attendance, Non-consultant led, Follow-up, Colorectal Surgery, 104).
Table 26 presents the acquisition cost of phosphates enema according to the dosages reported in the BNF:
- Child 3–6 years: 45–65 ml once daily;
- Child 7–11 years: 65–100 ml once daily;
- Child 12–17 years: 100–128 ml once daily;
- Adult: 128ml daily.
Table 26Acquisition cost of phosphates enema
| Population | Unit cost (quantity, basic price) | Cost/day | Cost/week | Cost/month |
|---|---|---|---|---|
| Phosphates enema (Formula B) 128ml standard tube | ||||
| Child 3 to 7 years | £0.03/ml (128ml, £3.98) | £1.71a | £11.97 | £51.99 |
| Child 7 to 12 years | £2.57b | £17.96 | £77.98 | |
| Over 12 years | £3.98c | £27.86 | £120.99 | |
- (a)
assume 55ml/day
- (b)
assume 82.5ml/day
- (c)
assume 128ml/day
K.6.3.6. Stimulant laxatives
Stimulant laxatives include bisacodyl, sodium picosulfate, and members of the anthraquinone group, senna and dantron, but the committee regarded sodium picosulfate and senna to be the most common laxatives prescribed to people with cystic fibrosis to treat DIOS. For this reason only senna and sodium picosulfate are presented in Table 27 based on the following dosages reported in the BNF for constipation:
- Senna:
- Child 2–3 years: 3.75–15 mg once daily, adjusted according to response;
- Child 4–5 years: 3.75–30 mg once daily, adjusted according to response;
- Child 6–17 years: 7.5–30 mg once daily, adjusted according to response;
- Adult: 7.5–15 mg daily (max. per dose 30 mg daily), dose usually taken at bedtime; initial dose should be low then gradually increased, higher doses may be prescribed under medical supervision.
- Sodium picosulfate:
- Child 1 month–3 years: 2.5–10 mg once daily, adjusted according to response;
- Child 4–17 years: 2.5–20 mg once daily, adjusted according to response;
- Adult: 5–10 mg once daily, dose to be taken at bedtime.
Table 27Stimulant laxatives
| Population | Unit cost (quantity, basic price) | Cost/day | Cost/week | Cost/month |
|---|---|---|---|---|
| Senna 7.5mg tablets | ||||
| Child 2 to 3 yearsa | £0.05 (60, £2.98) | £0.05 | £0.35 | £1.51 |
| Child 4 to 5 yearsb | £0.10 | £0.70 | £3.02 | |
| Over 6 yearsc | £0.15 | £1.04 | £4.53 | |
| Senna 7.5mg/5ml oral solution sugar free | ||||
| Child 2 to 3 yearsa | £0.01/ml (500ml, £3.99) | £0.04 | £0.28 | £1.21 |
| Child 4 to 5 yearsb | £0.08 | £0.56 | £2.43 | |
| Over 6 yearsc | £0.12 | £0.84 | £3.64 | |
| Sodium picosulfate 5mg/5ml oral solution sugar free | ||||
| Child 1 month to 4 yearsd | £0.02/ml (300ml, £7.10) | £0.14 | £0.99 | £4.32 |
| Over 4 yearse | £0.24 | £1.66 | £7.19 | |
- (a)
assume 7.5mg/day
- (b)
assume 15mg/day
- (c)
assume 22.5mg/day
- (d)
assume 6ml/day
- (e)
assume 10ml/day
K.6.3.7. Surgery (distal ileal resection)
The committee advised that surgery is currently limited to very specific cases and only when first and second line treatments have failed. The cost of an elective inpatient procedure according to NHS Reference Costs 2015/16 is presented in Table 28 for the possible complexities.
Table 28Cost of surgery to manage DIOS
| Currency description | National average unit cost |
|---|---|
| Major Small Intestine Procedures, 19 years and over, with CC Score 0–1, FZ67Fa | £4,171 |
| Very Major Small Intestine Procedures, 19 years and over, with CC Score 0–1, FZ66F | £5,488 |
| Very Major or Major, Small Intestine Procedures, between 2 and 18 years, with CC Score 0–1, FZ68H | £7,359 |
| Very Major or Major, Small Intestine Procedures, 1 year and under, with CC Score 0, FZ68L | £5,038 |
- (a)
Cost not reported for <19 years
K.6.4. Conclusions
Osmotic laxatives are relatively inexpensive and the clinical evidence review did not produce any evidence to justify additional resource use in this area. According to guidance produced by Colombo 2011, a stepwise approach to DIOS treatment is readily adopted in clinical practice. As a result, recommendations are likely to be for a stepwise escalation of treatment using the least invasive, and cheaper, options first and surgery only as a last resort.
As an aside, in addition to using PERT for the secondary prevention of DIOS, PERT may also be used to manage exocrine pancreatic insufficiency, potentially providing a cost-effective treatment when both of those indications require treatment. However, the costs of lifetime maintenance treatment could be significant. Without knowing the benefits of those treatments we cannot know for certain if they will be cost-effective. For this reason, the committee may consider a research recommendation to mitigate current uncertainty in this area.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 10.3.7.3 ‘Evidence to recommendations’.
K.7. Pancreatic enzymes for exocrine pancreatic insufficiency (PERT)
K.7.1. Literature review
No economic evaluations of PERT were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.7.2. Background and methods
This review question was not prioritised for de novo economic modelling. However, it is important to consider the additional cost of adding ant-acid drugs to PERT, and the cost difference between low-dose and high-dose PERT, if the recommended regimens are likely to increase the cost of PERT to the NHS.
There are several preparations of pancreatin available; these can be low dose (Creon® 10,000, Creon® Micro, Pancrex® and Pancrex V®), or high dose (Creon® 25,000, Creon® 40,000, Nutrizym 22® and Pancrease HL®). Moreover, the proportions of pancreatic enzymes (protease, lipase and amylase) that make up these therapies differs and this could impact the response to PERT. As a result, titrating should be done as part of regular review at the cystic fibrosis centre.
K.7.3. Resource and cost use
A cost description of 3 anti-acid drugs (cimetidine, omeprazole and ranitidine) and 8 PERTs routinely prescribed to people with cystic fibrosis was undertaken to aid consideration of the costs. Cost data were taken from the NHS Electronic Drug Tariff November 2016 unless unreported and otherwise stated.
K.7.3.1. Anti-acid drugs
The committee considered the addition of acid suppression such as H2 receptor antagonists (ranitidine or cimetidine) or proton pump inhibitors (omeprazole) in those with persistent symptoms of malabsorption. Table 29 presents the cost of anti-acid drugs over the course of 1 week and a typical monthly cost of continued use based on the following dosages reported by the BNF:
- Omeprazole: 20 mg once daily increased to 40 mg once daily if necessary, child over 1 year can receive up to 40 mg once daily;
- Rantidine: 150 mg bd (morning and night) or 300 mg at night, child 3–12 years can receive up to 150mg bd;
- Cimetidine: 400 mg bd (morning and night) or 800 mg at night; when necessary the dose may be increased to a maximum of 400 mg qds, child 1–12 years can receive up to 400 mg qds.
Table 29Acquisition cost of anti-acid drugs
| Drug (quantity, basic price) | Unit cost | Cost/week | Cost/month |
|---|---|---|---|
| Omeprazole | |||
| 20mg dispersible gastro-resistant tablets (28, £11.60) | £0.41 | £2.90a | £12.59 |
| 20mg gastro-resistant capsules (28, £0.91) | £0.03 | £0.23a | £0.99 |
| 20mg gastro-resistant tablets (28, £5.96) | £0.21 | £1.49a | £6.47 |
| 40mg dispersible gastro-resistant tablets (7, £5.80) | £0.83 | £5.80b | £25.19 |
| 40mg gastro-resistant capsules (7, £0.75) | £0.11 | £0.75b | £3.26 |
| 40mg gastro-resistant tablets (7, £5.98) | £0.85 | £5.98b | £25.97 |
| Ranitidine | |||
| 150mg effervescent tablets (60, £34.88) | £0.58 | £4.07c | £17.67 |
| 150mg tablets (60, £1.31) | £0.02 | £0.15c | £0.66 |
| 300mg effervescent tablets (30, £34.88) | £1.16 | £8.14d | £35.35 |
| 300mg tablets (30, £1.29) | £0.04 | £0.30d | £1.31 |
| 75mg/5ml oral solution sugar free (300ml, £6.45) | £0.11/5ml | £0.75e | £3.27 |
| Cimetidine | |||
| 200mg/5ml oral solution (600ml, £28.49) | £0.24/5ml | £1.66f | £7.22 |
| 200mg/5ml oral solution sugar free (300ml, £14.25) | £0.24/5ml | £1.66f | £7.22 |
| 400mg tablets (60, £15.18) | £0.25 | £1.77g | £7.69 |
| 800mg tablets (30, £9.09) | £0.30 | £2.12g | £9.21 |
- (a)
assume 20mg daily
- (b)
assume 40mg daily
- (c)
assume 150mg daily
- (d)
assume 300mg daily
- (e)
assume 300mg/20ml daily
- (f)
assume 400mg daily
- (g)
assume 800mg daily.
The preparation of anti-acid drugs received can impact the cost of the therapy, for example, oral solutions and effervescent (rapid dissolving) tablets, are more costly than standard tablets and capsules. As can be seen from Table 29, anti-acids provided over a short time-period can be relatively inexpensive if the lowest cost preparation is chosen.
However, the clinical review does not compare the efficacy according to the preparation; hence, the preparation received must be informed by clinical judgment. Moreover, the dose will depend on response, age and weight.
K.7.3.2. High-dose versus low dose PERT
Table 30 presents the cost of PERT over the course of 1 week and a typical monthly cost of continued use. Cost data were taken from the BNF November 2016, when dose ranges were reported the mid-point was taken for costing purposes. The clinical evidence suggested that there was a dose response, but in clinical practice the optimal dose may depend on the size of the person and how well the drug mixes with food. As a result, it is important to consider the concentration of enzymes, also reported in Table 30.
Table 30Cost of PERT
| Drug | Concentration of enzymes | BNF recommended dose | Unit cost | Cost/weeka | Cost/montha |
|---|---|---|---|---|---|
| Low-dose | |||||
| Creon® 10,000 (capsules) | Protease 600 units, lipase 10,000 units, amylase 8,000 units | Adult and child 1–2 capsules with each meal | £0.13 | £4.07b | £17.69 |
| Creon® Micro (granules) | Protease 200 units, lipase 5,000 units, amylase 3,600 units per 100mg | Adult and child 100 mg with each meal | £0.16c | £3.31 | £14.36 |
| Pancrex® (granules) | Protease 300 units, lipase 5,000 units, amylase 4,000 units/g | Adult and child 5–10 g just before meals | £0.19d | £29.93e | £129.96 |
| Pancrex V® (capsules) | Protease 430 units, lipase 8000 units, amylase 9000 units | Adult and child over 1 year 2–6 capsules with each meal | £0.18 | £14.90g | £64.69 |
| Pancrex V® (capsules 125) | Protease 160 units, lipase 2950 units, amylase 3300 units | Neonate contents of 1–2 capsules mixed with feeds | £0.14 | £4.42b | £19.18 |
| Pancrex V® (tablets) | Protease 110 units, lipase 1900 units, amylase 1700 units | Adult and child 5–15 tablets before each meal | £0.13 | £27.15h | £117.92 |
| Pancrex V® (tablets forte) | Protease 330 units, lipase 5600 units, amylase 5000 units | Adult and child 6–10 tablets before each meal | £0.16 | £26.94i | £117.00 |
| Pancrex V® (powder) | Protease 1400 units, lipase 25 000 units, amylase 30 000 units/g | Adult and child over 1 month, 0.5–2 g before each meal | £0.20j | £4.12k | £17.90 |
| High-dose | |||||
| Creon® 25,000 (capsules) | Protease (total) 1,000 units, lipase 25,000 units, amylase 18,000 units | Adult and child 1–2 capsules with meals | £0.28 | £8.90f | £38.65 |
| Creon® 40,000 (capsules) | Protease (total) 1,600 units, lipase 40,000 units, amylase 25,000 units | Adult and child 1–2 capsules with meals | £0.42 | £13.17f | £57.18 |
| Nutrizym 22® (capsules) | Protease 1,100 units, lipase 22,000 units, amylase 19,800 units | Adult and child over 15 years, 1–2 capsules with meals and 1 capsule with snacks | £0.33 | £10.83f | £47.04 |
| Pancrease HL® (capsules) | Protease 1,250 units, lipase 25,000 units, amylase 22,500 units | Adult and child over 15 years, 1–2 capsules during each meal and 1 capsule with snacks | £0.40 | £13.12f | £56.99 |
- (a)
assume 3 meals per day
- (b)
assume 1.5 capsules with each meal
- (c)
20g= £31.50, 100mg=£0.16
- (d)
300g=£57.00, 1g=£0.18
- (e)
assume 7.5g with each meal
- (f)
based on 1.5 capsules with meals and 1 capsules with 3 meals and 1 snack per day
- (g)
assume 4 capsules with each meal
- (h)
assume 10 tablets with each meal
- (i)
assume 8 tablets with each meal
- (j)
300g=£58.88, 1g= £0.20
- (k)
assume 1g with each meal for an adult and child over 1 month
Taking PERT can be burdensome if it is taken before every meal or snack. As a result, choosing a PERT regimen that will have less of an impact lifestyle may increase adherence and effectiveness. However, this is not necessarily the cheapest option or the most effective.
As can be seen in Table 30 Creon® Micro granules and Creon® 10,000 capsules are the cheapest preparation of low-dose PERT at a cost of £14.36 per month and £17.69 per month, respectively. The cost of high-dose PERT is greater than Creon® Mirco granules and Creon® 10,000 capsules, ranging from £38.65 to £56.99 per month, but without knowing the benefits of high-dose PERT for certain we cannot know if high-dose is cost-effective compared to low-dose.
K.7.4. Conclusions
Tablet preparations (non-dispersible and non-effervescent) of anti-acid drugs and capsule preparations of PERT can be relatively inexpensive. As a result, when tablets or capsules can be tolerated, they should be offered over oral solutions if there is no evidence to suggest they are any less effective. A dose-response may justify the additional cost of high-dose PERT over low-dose PERT, but this may be overridden if the concentration of enzymes in PERT is paramount. For these reasons, clinical judgement is necessary to provide the optimal dose and concentration of enzymes based on weight and drug adherence.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 10.2.7.3 ‘Evidence to recommendations’.
K.8. Nutrition
K.8.1. Literature review
No economic evaluations of nutritional interventions were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.8.2. Background and methods
This review question was not prioritised for de novo economic modelling. However, the interventions under consideration vary in the resources and costs required. For example, lifestyle changes could be implemented at home, whereas tube feeding may require an invasive procedure and greater monitoring. To aid considerations of cost-effectiveness, relevant resource and cost use data are presented.
K.8.3. Resource and cost use
K.8.3.1. Tube feeding
Tube feeding can be used as an adjunct to oral feeding, or if there is clinical concern about the safety of swallowing they can replace oral feeding. Long term interventions to optimise nutritional status include gastrostomy or jejunostomy tube feeding, whereas nasogastric tube feeding would be used on a shorter term basis. The former are surgical procedures associated with a high cost, whereas the latter can be performed by a nurse as an outpatient procedure. However, there are specific clinical implications for long term nasogastric tube placement that mean they are not the preferred route of enteral feeding beyond short term use.
The costs associated with long-term nutritional supplementation via gastrostomy or nasogastric tube feeding, are outside the scope of NHS Reference Costs and should remain within primary medical services (Department of Health, Reference Costs Guidance 2015–16). For this reason, currency codes related to endoscopic insertions from NHS Reference Costs 2015/16 are presented in Table 31 as a proxy. With regards to nasogastric tube feeding, costs were reported solely for babies under special care (XA03Z); these were considered irrelevant to this review and are not reported.
Table 31Cost of tube feeding procedures
| Procedure | Cost |
|---|---|
| Endoscopic Insertion of, Gastrojejunostomy or Jejunostomy Tube, elective inpatient, FZ94Z | £1,137 |
| Endoscopic Insertion of, Gastrojejunostomy or Jejunostomy Tube, day case, FZ94Z | £665 |
| Endoscopic Insertion of Gastrostomy Tube, 19 years and over, elective inpatient, FZ93A | £1,014 |
| Endoscopic Insertion of Gastrostomy Tube, 18 years and under, elective inpatient, FZ93B | £2,313 |
| Endoscopic Insertion of Gastrostomy Tube, 19 years and over, day case, FZ93A | £572 |
| Endoscopic Insertion of Gastrostomy Tube, 18 years and under, day case, FZ93B | £1,074 |
The randomised study by Corry 2008 was identified as a relevant source of costing data on tube feeding through ad-hoc searches. This study was included in the Cochrane review on tube feeding for adults with swallowing disturbances. However, it is important to note that Corry 2008 was based on patients with head and neck cancer who required enteral feeding. These costs may not be generalisable to people with cystic fibrosis. They stated that the insertion costs are significantly different as nasogastric tubes are inserted by nursing staff as an outpatient attendance, including the cost of chest X-ray. Whereas, percutaneous endoscopic gastrostomy tubes are inserted by surgeons in theatre. Table 32 below reports the costs by Corry 2008 alongside inflated sterling prices.
Table 32Tube feeding resource and cost use reported by Corry 2008
| Resource | NGT | PEG |
|---|---|---|
| Feeding tube cost, 2008 prices | $26 | $110 |
| Insertion costs, 2008 prices | $50 | $626 |
| Total cost of procedure, 2008 prices | $76 | $736 |
| Total cost of procedure, sterlinga | £57 | £555 |
| Total cost of procedure, 2015/16 pricesb | £66 | £641 |
NGT, nasogastric tube; PEG, percutaneous endoscopic gastrostomy
- (a)
- (b)
1.325 USD = 1 GBP
1 GBP = 0.755
Inflator to 2015/16 prices 1.1556, based on the hospital & community health services (HCHS) index (297.0 [2015/16 PPI] / 257 [2007/8 PPI])
In addition to the procedure, the committee advised that some people with cystic fibrosis would undergo an intense monitoring schedule during the first few days or weeks with a specialist. Thereafter, they would be monitored on a similar frequency to those receiving oral supplementation or appetite stimulants, with gastrostomy or jejunostomy incurring 1 additional visit with their surgeon each year at a cost of £132 (NHS Reference Costs 2015/16, 301, Consultant-Led, Non-Admitted Face to Face attendance, Follow-Up, Gastroenterology, WF01A).
In addition to the monetary cost of tube feeding, the committee advised that some qualitative reviews show tube feeding can negatively impact a person’s quality of life by affecting social interactions at meal times. Moreover, if the procedure and use of tube feeding is associated with adverse effects, they can incur further treatment costs and decrements in quality of life.
Nasogastric tubes frequently fall out and require the cost of a healthcare professional to reapply the tube if the family or carer were unable to do so. The committee added that there are a number of clinical concerns to their long term usage. Equally, gastrostomy and jejunostomy tubes need routine and, on occasion, emergency replacement which may need professional, rather than family, intervention. It was noted that tube feeding, when used appropriately, positively impacts on clinical wellbeing and health, improving quality of life and so justifying the high costs tube feeding can entail in those cases.
Compared to usual care, 1 cohort study (Bradley 2012) included in the clinical evidence review found a clinically significant difference in the indices of nutrition and growth for weight and BMI. However, there was no clinically significant difference in height, or FEV1%, between the group receiving gastrostomy and those who received usual care at 6 month and 1 year follow-up. Based on this, gastrostomy could be considered cost-effective, when the aim is to improve weight and BMI, as improvements may reduce later downstream costs from hospital attendances related to malnutrition. However, gastrostomy is unlikely to be considered cost-effective when the aim is to improve lung function as the same benefits can be obtained from usual care which is a lot less costly and not subject to the decrements in quality of life previously outlined.
K.8.3.2. Oral supplementation
Table 33 reports the cost of high-energy feed supplements for fat, carbohydrate and protein and other nutritional supplements that may be considered as first line supplements for people with cystic fibrosis.
Table 33Acquisition cost of oral supplements
| Oral supplement | Quantity | Price | Notes |
|---|---|---|---|
| Feed supplements: high-energy supplements; fat, protein & carbohydrate | |||
| Calogen® Extra emulsion | 200ml | £4.98 | Formulation Liquid/100 mL Energy (kJ) 1650 kJ / 400 kcal Protein 5 g cows’ milk Carbohydrate 4.5 g (sugars 3.5 g) Fat 40.3 g Fibre Nil Special characteristics Gluten-free, Residual lactose, Contains vitamins and minerals |
| Calogen® Extra Shots emulsion | 240ml | £5.75 | Formulation Liquid/100 mL Energy (kJ) 1650 kJ / 400 kcal Protein 5 g cows’ milk Carbohydrate 4.5 g (sugars 3.5 g) Fat 40.3 g Fibre Nil Special characteristics Gluten-free, Residual lactose, With vitamins and minerals |
| Calshake® powder | 609g | £17.01 | Formulation Powder/87 g Energy (kJ) 1841 kJ / 439 kcal Protein 4.1 g cows’ milk Carbohydrate 56.4 g (sugars 20 g) Fat 22 g Fibre Nil Special characteristics Contains lactose, Gluten-free |
| Enshake® oral powder 96.5g sachets | 6 sachets | £12.93 | Formulation Powder/100 g Energy (kJ) 1893 kJ / 450 kcal Protein 8.4 g cows’ milk, soy protein isolate Carbohydrate 69 g (sugars14.5 g) Fat 15.6 g Fibre Nil Special characteristics Residual lactose, Contains vitamins and minerals |
| MCT Procal® oral powder 16g sachets | 30 sachets | £24.21 | Formulation Powder/100 g Energy (kJ) 2742 kJ / 657 kcal Protein 12.5 g cows’ milk Carbohydrate 20.6 g (sugars 3.1 g) Fat 63.1 g (MCT 99%) Fibre Nil Special characteristics Contains lactose |
| Pro-Cal® powder | 375g | £16.13 | Formulation Powder/100 g Energy (kJ) 2787 kJ / 667 kcal Protein 13.6 g cows’ milk Carbohydrate 28.2 g (sugars 16 g) Fat 55.5 g Fibre Nil Special characteristics Contains lactose, Gluten-free |
| 510g | £14.95 | ||
| 1500g | £30.45 | ||
| 3000g | £71.88 | ||
| 12500g | £216.41 | ||
| Pro-Cal® Shot | 120ml | NR | Formulation Liquid/100 mL Energy (kJ) 1385 kJ / 334 kcal Protein 6.7 g cows’ milk Carbohydrate 13.4 g (sugars 13.3 g) Fat 28.2 g Fibre Nil Special characteristics Contains lactose, Gluten-free, Contains soya |
| 720ml | £14.71 | ||
| Pro-Cal® Singles | NR | NR | Formulation Liquid/100 mL Energy (kJ) 1385 kJ / 334 kcal Protein 6.7 g cows’ milk, soya Carbohydrate 13.4 g (sugars 13.3 g) Fat 28.2 g Fibre Nil Special characteristics Contains lactose, Gluten-free |
| Scandishake® Mix oral powder 85g sachets | 6 sachets | £15.00 | Formulation Powder/100 g Energy (kJ) 2099 kJ / 500 kcal Protein 4.7 g cows’ milk Carbohydrate 65 g (sugars 14.3 g) Fat 24.7 g Fibre Nil Special characteristics Gluten-free, Contains lactose |
| Vitasavoury® 200 powder | NR | NR | Formulation Powder/100 g Energy (kJ) 2562 kJ / 619 kcal Protein 12 g cows’ milk Carbohydrate 22.5 g (sugars 1.4 g) Fat 52 g Fibre 6.4 g Special characteristics Contains lactose, Contains soya (chicken flavour) |
| Nutritional supplements: 5 g (or more) protein/100 mL | |||
| Altraplen® Protein | 800ml | £5.96 | Formulation Liquid (sip feed) per 100 mL Energy (kJ) 632 kJ / 150 kcal Protein 10 g cows’ milk, soya protein Carbohydrate 15 g (sugars 4.6 g) Fat 5.6 g Fibre Nil Special characteristics Gluten-free, Residual lactose |
| Ensure® Plus Fibre | 200ml | £2.02 | Formulation Liquid (sip or tube feed) per 100 mL Energy (kJ) 652 kJ / 155 kcal Protein 6.25 g cows’ milk, soya protein isolate Carbohydrate 20.2 g (sugars 5.5 g) Fat 4.92 g Fibre 2.5 g Special characteristics Gluten-free, Residual lactose |
| Ensure® Plus Milkshake style Ensure® Plus Savoury Ensure® Plus Yoghurt style | 220ml | £1.40 | Formulation Liquid (sip or tube feed) per 100 mL Energy (kJ) 632 kJ / 150 kcal Protein 6.25 g cows’ milk, soya protein isolate Carbohydrate 20.2 g (Milkshake style and Savoury sugars 6.89 g, Yoghurt style sugars 11.7 g) Fat 4.92 g Fibre Nil Special characteristics Gluten-free, Residual lactose |
| Fortisip® Bottle | 200ml | £1.40 | Formulation Liquid (sip feed) per 100 mL Energy (kJ) 630 kJ / 150 kcal Protein 6 g cows’ milk Carbohydrate 18.4 g Fat 5.8 g Fibre Nil Special characteristics Gluten-free, Residual lactose |
| Fortisip® Yoghurt Style | 200ml | £2.06 | Formulation Liquid (sip feed) per 100 mL Energy (kJ) 630 kJ / 150 kcal Protein 6 g cows’ milk Carbohydrate 18.7 g (sugars 10.8 g) Fat 5.8 g Fibre 200 mg Special characteristics Gluten-free, Contains lactose |
| Fortisip® Savoury Multi Fibre | NR | NR | Formulation Liquid (sip feed), per 100 mL Energy (kJ) 625 kJ / 150 kcal Protein 7.5 g cows’ milk Carbohydrate 12.8 g (sugars 900 mg) Fat 7 g Fibre 2.3 g Special characteristics Gluten-free, Residual lactose |
| Fresubin® Protein Energy Drink | 200ml | £2.08 | Formulation Liquid (sip feed) per 100 mL Energy (kJ) 630 kJ / 150 kcal Protein 10 g cows’ milk Carbohydrate 12.4 g (sugars 6.4 g) Fat 6.7 g Fibre Nil Special characteristics Gluten-free, Residual lactose, Contains fish gelatin |
| Fresubin® Thickened | 800ml | £9.40 | Formulation Liquid (sip feed) per 100 mL Energy (kJ) 630 kJ / 150 kcal Protein 10 g cows’ milk Carbohydrate 12.2 g (sugars 7.1 g) Fat 6.7 g Fibre 480 mg Special characteristics Gluten-free, Residual lactose |
| Fresubin® YOcrème | 500 gram | £8.16 | Formulation Semi-solid per 100 g Energy (kJ) 630 kJ / 150 kcal Protein 7.5 g whey protein Carbohydrate 19.5 g (sugars 16.8 g) Fat 4.7 g Fibre Nil Special characteristics Gluten-free, Contains lactose |
Costs are taken from the BNF (July 2017). NR, not reported
The cost of supplements undoubtedly varies by brand, but each brand can provide a different make up of nutrients. Consequently, the cheapest brand may not be suitable for all deficiencies; hence, supplements should be individualised to the person with cystic fibrosis.
The cost of supplements will also depend on the frequency those supplements are administered. If they were used to substitute rather than complement diet at home, the cost could be substantial. However, the person’s diet should be reviewed and modified prior to consideration of supplements and the healthcare professional should determine the appropriate quantity and frequency.
Compared to usual care, the clinical evidence review found no clinically significant difference for any indices of nutrition and growth. Moreover, 1 of 2 RCTs (Hanning 1993, Poustie 2006) showed a clinically significant decrease in FEV1% in the group of participants receiving oral calorie supplementation compared to the participants in the control group receiving usual care at 3 month follow-up, but no significant difference was found by that study at 1-year follow-up. Based on this evidence the benefits of oral supplementation are unlikely to justify the costs in most cases.
Compared to nutritional advice, the clinical evidence review found 1 study (Kalnins 2005) that showed no clinically significant difference for any indices of nutrition and growth. However, the same study showed a clinically significant decrease in FEV1% in the group of participants receiving oral calorie supplementation for 3 months compared to the participants receiving nutritional advice at 3 month follow-up and at 6 month follow-up.
K.8.3.3. Appetite stimulants
The acquisition cost of appetite stimulants are reported in Table 34 over a course of 1 day and 1 month of continued use. It is evident from Table 34 that cyproheptadine hydrochloride is half the cost of megace; therefore, if there is no evidence to suggest cyproheptadine hydrochloride is any less effective, cyproheptadine hydrochloride should be offered instead of megace if stimulants can improve quality of life and reduce the downstream costs associated with a reduced appetite.
Table 34Acquisition cost of appetite stimulants
| Stimulant | Dose | Unit cost | Cost/day | Cost/month |
|---|---|---|---|---|
| Cyproheptadine hydrochloride, periactin 4mg tablets (30, £5.99)a | 4mg qds (Homnick 2004) | £0.20 | £0.80 | £24.28 |
| Megace 160mg tablets (30, £19.52)b | 10 mg/kg/day (Eubanks 2002 & Marchand 2000) | £0.65 | £1.95c | £59.34c |
| Megestrol 200mg/5ml oral suspension (NR, NR) | NR | NC | NC | NC |
NC, not calculable; NR, not reported
- (a)
Taken from the BNF November 2016
- (b)
Taken from the NHS Electronic Drug Tariff November 2016
- (c)
Approximate for a weight of 50kg requiring 3 tablets to provide 480mg
Compared to placebo, 3 RCTs (Homnick 2004, Eubanks 2002, Marchand 2000) showed a clinically significant benefit in the indices of nutrition and growth. However, none of the RCTs found a clinically significant difference in FEV1%, the number of exacerbations or adverse events (constipation). Therefore, if improvements in the indices of nutrition and growth are considered to be of greater importance than improvements in lung function or the number of exacerbations, appetite stimulants could be considered cost-effective.
K.8.3.4. Psychological and behavioural interventions
According to NHS Reference Costs 2015/16 the cost of a psychotherapy attendance is £158 (WF01A, 713, Consultant-Led, Non-Admitted Face to Face Attendance, Follow-Up). The cost of psychotherapy will ultimately depend on the number of sessions the person requires. The committee advised that sessions would be performed intensively for the first few months on a weekly basis, with further follow up sessions as required. Due to the difficulties of accessing these service promptly on the NHS, teaching psychological strategies to families to support the person with nutritional difficulties after the course of intensive healthcare professional input would be advantageous. However, the committee noted that not all families could engage with this.
Compared to usual care, the clinical evidence review found 1 RCT (Stark 1996) that showed no clinically significant difference for any indices of nutrition and growth or lung function. Based on these findings, the cost of psychological or behavioural interventions cannot be justified if usual care can provide the same benefits at a much cheaper cost. However, this is 1 small RCT with 9 participants that may not be representative of the population with cystic fibrosis in the UK today.
K.8.4. Conclusions
The clinical evidence showed that enteral tube feeding and appetite stimulants are effective in improving nutritional status and growth in people with cystic fibrosis. However, because of their additional high cost and invasive nature, dietary modifications through nutritional advice should be considered as the first line treatment for undernutrition or faltering growth.
No evidence was found showing that the benefits of oral calorie supplements would outweigh their cost. However, if the committee believe people with faltering growth have larger scope to benefit from oral calorie supplements, a research recommendation should be considered to identify for which people with cystic fibrosis, oral calorie supplements would be cost-effective.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 10.1.7.3 ‘Evidence to recommendations’.
K.9. Mucoactive or mucolytic agents
K.9.1. Literature review
Six economic evaluations of mucoactive or mucolytic agents to facilitate expectoration in people with cystic fibrosis were identified in the literature search conducted for this guideline.
All 6 of those studies took a UK, NHS, non-societal perspective. However, 3 of those (Christopher 1999; Menzin 1996 and McIntyre 1999) used clinical effectiveness data from US clinical trials (Oster 1995 and Fuchs 1994) that may reflect outdated practices and practices that may not be generalisable to the UK.
Oster 1995 undertook a RCT in the US to inform their cost-benefit analysis that compared the costs of respiratory tract infection-related inpatient and outpatient care for daily dornase alfa, alternate day dornase alfa and placebo. However, the cost of dornase alfa was not included in their analysis, therefore, we cannot know if the cost of dornase alfa treatment is offset by the cost savings from improved clinical outcomes.
The NICE 2014 Guidelines manual states “weaker studies are more likely to be excluded when cost-effectiveness (or lack of it) can be readily established without them”. Following this, Oster 1995 was excluded as higher quality UK evidence on the cost-effectiveness of dornase alfa has been included. As an aside, Fuchs 1994 was not included as this was a clinical trial, rather than an economic evaluation.
Five of the 6 economic evaluations included dornase alfa as an intervention, compared with either no dornase alfa, or hypertonic saline (Section K.9.1.1). The remaining economic evaluation assessed mannitol (with and without dornase alfa) against the control - best supportive care (BSC) (Section K.9.1.2). The results from these studies are summarised in Table 35. No economic evaluations were identified that included N-acetylcysteine.
Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1. Data extraction tables and quality assessments of included studies can be found in Appendix L and M, respectively.
K.9.1.1. Dornase alfa
Menzin 1996 presented a cost-benefit analysis for daily dornase alfa versus placebo, based on clinical evidence in the US from Fuchs 1994. They suggested that dornase alfa therapy may reduce the cost of respiratory tract infection-related care. However, the cost of dornase alfa was not included in their analysis so we cannot know if the cost of dornase alfa treatment is completely offset from those savings.
Christopher 1999, also informed by Fuchs 1994, undertook a cost-effectiveness analysis for daily dornase alfa versus placebo. They found that the cost/life year gained in the subgroup of participants with initial FEV1 <70% was a lot less (£16,000) than the whole population (£52,500). However, they noted that the short term evidence used to inform their analysis questioned the credibility of their findings.
McIntyre 1999 presented a cost-benefit analysis for daily dornase alfa versus placebo based on assumptions for disease progression, survival and cost savings from reduced respiratory tract infection-related care inferred from several clinical trials. In the best (worst) case scenario dornase alfa might maintain a person with cystic fibrosis in a mild state of disease for an additional 4 years and extend their life by up to 7 (2) years at a cost of £6,084 (£45,234) per life year gained. Suri 2002 undertook a clinical crossover trial to compare the total health service costs of daily dornase alfa with alternate day dornase alfa and hypertonic saline. They found that daily dornase alfa was more effective than hypertonic saline, but significantly increased health care costs. Moreover, administering dornase alfa on alternate days, rather than daily, was as effective, with a potential for cost savings.
Grieve 2003 performed a cost-effectiveness analysis based on the 12 week trial by Suri 2002. Benefits were based on the change in effectiveness measured by FEV1% which was similar between daily dornase alfa (14% change) and alternate day dornase alfa (12% change) and absent from hypertonic saline (0% change). Using this data they estimated the cost/ 1% gain in FEV1% and presented cost-effectiveness acceptability curves (CEACs) and net benefit statistics for each comparison. They showed that unless decision makers are willing to pay over £200/ 1% gain in FEV1%, the probability of daily dornase alfa proving more cost-effective than alternate day dornase alfa was less than 50%. Moreover, the mean net benefit for the daily regimen compared to alternate days at that threshold was negative. As a result, they concluded that it may be more cost-effective if dornase alfa was prescribed on an alternate day basis rather than a daily basis.
The findings from those 5 studies suggest that daily dornase alfa is more costly and more effective than no dornase alfa and hypertonic saline. Moreover, if the effectiveness of daily and alternate day dornase alfa is similar, and alternate day dornase alfa is less expensive than daily dornase alfa, alternate day dornase alfa would be considered cost-effective compared to daily dornase alfa. However, those 5 studies do not report cost per QALY ICERs which makes their interpretation relative to NICE’s threshold difficult.
It is important to note that all 5 economic evaluations were subject to short trial durations. Therefore, if the effectiveness of dornase alfa is expected to decrease over time, the cost savings relative to no dornase alfa or hypertonic saline reported by the studies may be overestimated when longer time horizons are considered.
K.9.1.2. Mannitol dry powder for inhalation (Bronchitol®)
The sixth and final study included in this review was the cost-effectiveness evidence for a NICE Health Technology Appraisal (HTA, NICE TA266) submitted by Pharmaxis, who intended to make the following 2 comparisons:
- mannitol versus BSC;
- mannitol + rhDNase (dornase alfa) versus BSC + rhDNase (dornase alfa).
The cost-utility analysis developed for the HTA was a patient-level simulation Markov model. Here, the progression of each individual patient is modelled, rather than the progression of a whole patient cohort at once. As a result, individual patient characteristics are taken into account when determining the transition probabilities, hence the path through the tree. A schematic presentation of the relationship between treatment (black), time (black), clinical endpoints (grey) and economic endpoints (blue) is show in Figure 2. A green arrow indicates a positive relationship and a red arrow indicates a negative relationship.
Figure 2Relationships in the model, reproduced from the submission
Source: Manufacturer’s submission for TA266, February 2011, Figure 15
The effect of mannitol was assumed to be the same in rhDNase users and non-users. The only difference in the model between the 2 populations relates to the cost of rhDNase. The manufacturer concluded that mannitol treatment for people with cystic fibrosis in addition to BSC and regardless of rhDNase use was effective. There was no clear statement regarding the cost-effectiveness, but the ICERs (cost per QALY gained) for each comparison were similar and above NICE’s threshold for cost-effectiveness:
- mannitol versus BSC: £41,074;
- mannitol + rhDNase versus BSC + rhDNase: £47,095.
The Technology Assessment Group (TAG, Riemsma 2011) questioned the assumption that mannitol use was completely independent of rhDNase use. This means that any benefit of mannitol did not depend on whether a patient used, or did not use, rhDNase.
As a result, the TAG suggested during clarification that the economic results should be reported separately for the 2 comparisons according to rhDNase suitability, where the rate ratios (RRs) for exacerbations are population specific (according to rhDNase suitability). The manufacturer subsequently provided the following results to the TAG:
- mannitol + rhDNase versus rhDNase + BSC for people with cystic fibrosis using rhDNase (i.e. mannitol as add-on therapy): £74,140;
- mannitol versus BSC for people with cystic fibrosis who are ineligible, intolerant or inadequately responsive to rhDNase (i.e. mannitol as second-line therapy): £19,828.
Based on the manufacturer’s initial submission, the findings of the TAG, and manufacturer’s response to clarification, the TAG presented an alternative base case with the following amendments:
- the cost-effectiveness of mannitol is analysed separately for the 2 populations/comparisons according to rhDNase suitability where RRs for exacerbations are population specific;
- treatment independent and improvement specific values for costs and utilities are used to value health states.
These amendments led to the following estimates of cost-effectiveness for the 2 populations:
- mannitol + rhDNase versus rhDNase + BSC for people with cystic fibrosis using rhDNase (i.e. mannitol as add-on therapy): £80,098;
- mannitol versus BSC for people with cystic fibrosis who are ineligible, intolerant or inadequately responsive to rhDNase (i.e. mannitol as second-line therapy): £29,883.
In the manufacturer’s base case analysis, mannitol is almost equally cost-effective for both comparisons. However, under the TAG’s scenario, mannitol would only be considered cost-effective in people with cystic fibrosis who are ineligible, intolerant or inadequately responsive to rhDNase (i.e. mannitol as second-line therapy).
Box 1 below presents the Appraisal Committee’s discussion of cost-effectiveness based on the manufacturer’s submission and TAG’s report.
Box 1Mannitol dry powder for inhalation (Bronchitol®), Final Appraisal Determination for NICE TA266
“Noting that the ICERs for the subgroup of people using rhDNase were between £50,000 and £80,000 per QALY gained, the committee concluded that mannitol was not cost effective for people using rhDNase, and could not be recommended for this subgroup.
The committee concluded that the ICERs for mannitol in people who cannot use rhDNase because of ineligibility, intolerance or inadequate response to rhDNase were underestimates because mortality in the model was underestimated, and also associated with several uncertainties because of the lack of validity in the model (for example, the duration of the effect long term).
Therefore, the committee concluded that the ICERs for mannitol were likely to be above £30,000 per QALY gained in people who cannot use rhDNase because of ineligibility, intolerance or inadequate response to rhDNase, and that mannitol could not be recommended for this subgroup.”
Note rhDNase is referred to as dornase alfa since this publication
Following the discussion of the evidence, the Appraisal committee explored if there was a group of adults with cystic fibrosis in which treatment with mannitol would provide a cost-effective use of NHS resources. They believed that if mannitol treatment was offered only to people with cystic fibrosis with a rapid decline in lung function, the ICER would most likely be lower than in the whole population because of this group’s lower quality of life and lung function, and a greater potential to improve. The committee concluded that the ICER for mannitol in people with cystic fibrosis for whom hypertonic saline is not considered appropriate, who cannot use rhDNase because of ineligibility, intolerance or inadequate response to rhDNase, and whose lung function is rapidly declining would be under £30,000 per QALY gained; hence, they concluded that mannitol should be recommended as an acceptable use of NHS resources for those people with cystic fibrosis.
Table 35Summary of included economic evaluations, mucoactive or mucolytic agents
| Study | Limitations | Applicability | Other comments | Inc. costs | Inc. effects | Inc. cost-effectiveness | Uncertainty |
|---|---|---|---|---|---|---|---|
| Christopher 1999 | Very seriousa,b | Partiallyc,d,e | Considered 2 populations:
| Daily dornase alfa may lead to average savings of £1,746 per person from reduced hospitalisations over a 6-month period compared to placebo | Continued use of dornase alfa over a lifetime may increase life expectancy by 2 years in all patients, or 7 in years in the subgroup, compared to placebo | All: £52,550 per LYG Subgroup: £16,110 per LYG | Explored changing the rate of decline in FEV1, initial FEV1, and the mean % improvement in FEV1 with dornase alfa treatment. Varied the length of treatment and discount rate for costs and benefits. CIs not reported. |
| McIntyre 1999 | Very seriousa,b,f | Partiallyc,d,e | None | Assumed cost savings from RTI-related care would offset between 18.3% and 37.5% of the acquisition cost of dornase alfa based on Oster 1995. | FEV1% improvement with daily dornase alfa assumed to be either 8%, 4.3%, or 20% based on different literature sources. Based on assumptions for disease progression and survival, 3 additional LYs would be gained by a patient on dornase alfa compared to no dornase alfa | Cost per LYG varying FEV1% improvement as a result of dornase alfa and the cost offset possible with dornase alfa use. Improvement with daily dornase alfa: 8%; 4.3%; 20%:
| Improvement with dornase alfa and cost offset varied to provide a range of results. An increase in the cost of annual care for CF severe patients (FEV1<40%) from £19,995 (Robson 1992) to £30,000 (Fogarty 1996) explored. CIs not reported. |
| Menzin 1996 | Very seriousa,g,h,i | Partiallyc,j | Measures of physical resource use were compared between participants who received daily dornase alfa versus placebo in the US trial (Oster 1995). Differences in RTI-related resource use were then evaluated using local (country specific) estimates of unit costs | Difference in the mean costs of RTI-related care over 24 weeks (placebo – daily dornase alfa)
| Difference in mean health care utilisation over 24 weeks (placebo – daily dornase alfa)
| NR | Not assessed. CIs not reported. |
| Suri 2002 | Minork | Partiallyc | Undertook a prospective, open, randomised, crossover trial completed by 43 children aged 5 to 18 years, this trial included a 2 week wash-out period. Clinical effectiveness data from this trial was also used to inform Grieve 2003 | Daily dornase alfa - HS, mean costs over 12 weeks Intervention: +£1,718 Total non-interventional drugs: −£90 Total hospital care: −£212 Total community care: −£3 Grand total: £1,409 Daily dornase alfa - alternate day dornase alfa over 12 weeks Intervention: +£892 Total non-intervention drugs: +£18 Total hospital care: −£397 Total community care: £0 Grand total: +£513 | Mean FEV1 increase at 12 weeks from baseline
| NR | Mean incremental costs and benefits reported with 95% CIs. Scenarios reducing the price of dornase alfa reported by the BNF by 10–30% and 20th and 80th percentiles of the costs per occupied bed day. |
| Grieve 2003 | Minorl | Directlyc,m | Clinical effectiveness data taken from Suri 2002 | Over 12 weeks
| FEV1% over 12 weeks
| £ per 1% gain in FEV
| Mean incremental costs and benefits reported with 95% CIs. Using 2,000 samples CE planes and CEACs are presented. Scenario reducing the price of dornase alfa reported by the BNF by 10–30%. Net benefits were calculated for a range of ceiling ratios per 1% increase in FEV1 |
| NICE TA266 | Seriousn,o,p | Directlyq | Results are based on 100,000 simulations over a lifetime horizon |
|
|
|
|
BNF, British National Formulary; CE plane, cost-effectiveness plane; CEAC, cost-effectiveness acceptability curve; CI, confidence interval; CF, cystic fibrosis; FEV, forced expiratory volume; HS, hypertonic saline; LYG, life years gained; NR, not reported; PSA, probabilistic sensitivity analysis; RTI, respiratory tract infection; control, best supportive care
Note: dornase alfa was referred to as rhDNase in the studies, updated here to reflect the term used in current UK practice
- (a)
Clinical effectiveness data taken from old US trial(s) that may reflect outdated practices and practices that may not be generalisable to the UK, the short trial duration(s) questions if those effects can be sustained, resource utilisation recorded in the trial(s) did not include the full scope of possible costs
- (b)
Assumptions on survival and disease progression may reflect outdated practices, lung transplants were also not considered as part of the lifetime analysis
- (c)
QALY not used as an outcome measure
- (d)
Discount rate 6% for costs and 0% for benefits, whereas the NICE reference case specifies 3.5% for both costs and benefits
- (e)
Cost-effectiveness analysis based on outdated US practice
- (f)
Cost year unclear and costs are not reported to be inflated to the same year, insufficient detail on how costs taken from Robson 1992 were estimated
- (g)
Little detail regarding sources used for cost build up
- (h)
Uncertainty not assessed
- (i)
The cost of dornase alfa therapy was not included, as it was not being marketed at the time the assessment was undertaken, therefore we cannot know of the cost of treatment is offset by cost savings from improved clinical outcomes
- (j)
Practice-adjustment analyses were only undertaken for Italy and France in the likelihood of hospitalisation for a RTI as these patients were believed to be treated as outpatients rather than inpatients - the authors do not justify if this difference applies to the UK, overall, adjustments to reflect UK clinical practice are not sufficiently described
- (k)
Effectiveness based on a crossover trial
- (l)
Population not described, but said to be found in Suri 2002 who undertook a crossover trial
- (m)
Cost-effectiveness analysis informed from a UK clinical trial and methods closely follow the NICE reference case. The preferred measure of effects (QALYs) is not used, but is still thought to be useful for decision making, given that all other criteria are relevant and the alternative outcome measure reported is unlikely to change the conclusions about cost-effectiveness.
- (n)
Both comparisons use clinical effectiveness data taken from the whole adult population, irrespective of dornase alfa use which underestimates the effectiveness of dornase alfa use
- (o)
Due to a short trial duration they assumed the benefits if mannitol would be maintained over the patient’s life if they remained on treatment, this would affect the ICER favourably, but there is uncertainty around this assumption
- (p)
Costs and utilities applied in the model are treatment specific rather than health state specific which is inaccurate as this removes the dependency on time in each health state
- (q)
UK cost-utility analysis that closely follows the NICE reference case
K.9.2. Background and methods
Following the literature searches for clinical and cost-effectiveness evidence, the committee agreed that de novo economic analysis in this area would be superfluous as the published economic evidence was sufficient to justify current clinical practice.
For completeness, a cost description of all interventions specified in the protocol has been undertaken. To enable a common comparison across the mucoactive and mucolytic agents, the cost/day is presented alongside the unit cost. Dosages are taken from the BNF and drug acquisition costs are taken from the NHS Electronic Drug Tariff November 2016, unless unreported and otherwise stated.
K.9.3. Resource and cost use
In addition to the acquisition cost of inhaled mucoactive and mucolytic agents, the use of inhalers or nebulisers for their delivery is associated with fixed costs related to equipment purchase and ongoing costs associated with maintenance. Inhalers are relatively inexpensive such as the Spacer anti-static with mouthpiece at a cost of £7.73 (NHS Supply Chain 2015). Nebulisers on the other hand, cost substantially more (Table 36).
Table 36Cost of nebuliser reproduced from the NHS Supply Chain 2015
| Product | Cost |
|---|---|
| PARI SINUS inhalation device with pulsating aerosol for the nasal sinuses | £108.27 |
| Paediatric nebuliser system JuniorBoy SX | £89.90 |
| BOY mobile S Portable multi-voltage nebuliser with LC SPRINT nebuliser adult mask 12v cable battery & carry bag | £284.91 |
| Adult nebuliser system TurboBoy SX | £84.64 |
| Eflow rapid with 2 handsets batteries international power adapter carry case | £718.95 |
All treatments would be administered at home without assistance of a healthcare professional; hence no administration costs are incurred. Prescription services are excluded because people with cystic fibrosis are assumed to receive prescriptions at their regular visits to the clinic at no additional cost. There is likely to be some ongoing monitoring, but it is reasonable to assume this is equivalent across all treatments, as there is no opportunity cost created by switching from one treatment to another.
K.9.3.1. Mannitol dry powder for inhalation (Bronchitol®)
Table 37 reproduces the manufacturer’s expected unit costs of Bronchitol®, whilst Table 38 provides the latest acquisition cost from the BNF November 2016.
Table 37Unit costs of Bronchitol®, reproduced from the manufacturer’s submission
| Variable | Description |
|---|---|
| Pharmaceutical formulation | Bronchitol® is encapsulated in a size 3 hard gelatine capsules as 40mg of spray-dried mannitol powder for inhalation with no excipients |
| Acquisition cost (excluding VAT) | The cost for 14 day carton of 160 capsules and 2 inhaler devices is expected to be around £236.25a The initiation dose carton which contains 10 capsules and 1 inhaler device will be free of charge |
| Method of administration | Inhalation |
| Doses | The recommended dose of Bronchitol® is 400mg |
| Dosing frequency | Twice a day |
| Average length of a course of treatment | Lifetime |
| Average cost of a course of treatment | Average daily cost (800mg) is expected to be around £16.88 |
| Anticipated average interval between courses of treatment | NA |
| Anticipated number of repeat courses of treatments | Treatment is for a chronic condition and is likely to be continuous |
| Dose adjustments | NA |
NA, not applicable
- (a)
“The NICE Single Technology Appraisal process triggered for Bronchitol precedes marketing authorisation approval expected early next year. Pharmaxis has provided tentative costs of Bronchitol however the final acquisition costs are contingent on the final label text approved by EMA”
Table 38Acquisition cost of Bronchitol®
| Mannitol | Unit cost | Cost/day | Cost/year |
|---|---|---|---|
| Bronchitol® 40mg inhalation powder capsules with 2 devices (280 capsules, £231.66) | £0.83 | £16.55a | £6,039.90 |
- (a)
BNF: By inhalation of powder: adult, maintenance 400 mg bd, an initiation dose assessment must be carried out under medical supervision, for details of the initiation dose regimen, consult product literature
K.9.3.2. Dornase alfa
Table 39 below presents the acquisition cost (NHS Electronic Drug Tariff November 2016) of dornase alfa, alongside the cost/day and cost/year. The dose to calculate those costs was taken from the BNF for an indication to manage people with cystic fibrosis with FVC >40% of predicted to improve pulmonary function. Dornase alfa would also incur the cost of a nebuliser (Table 36) if the person with cystic fibrosis did not already acquire one.
Table 39Acquisition cost of dornase alfa
| Dornase alfa (quantity, basic price) | Unit cost | Cost/day | Cost/year |
|---|---|---|---|
| Pulmozyme 2.5mg nebuliser liquid 2.5ml ampoules, Dornase alfa 1mg per 1ml (30 ampoules, £496.43) | £16.55 | £16.55a | £6,039.90 |
- (a)
BNF: by inhalation of nebulised solution, child 5–17 years: 2,500 units once daily, administered by jet nebuliser
K.9.3.3. Nebulised hypertonic sodium chloride
Table 40 below present the acquisition cost of nebulised sodium chloride for each available concentration reported in the NHS Electronic Drug Tariff November 2016. The cost/day and cost/year are also presented based on BNF recommendations for an indication to mobilise lower respiratory tract secretions in mucous consolidation (e.g. cystic fibrosis). Nebulised sodium chloride would also incur the cost of a nebuliser if the person with cystic fibrosis did not already acquire one (Table 36).
Table 40Acquisition cost of nebulised sodium chloride
| Nebulised sodium chloride (quantity, basic price) | Unit cost | Cost/day | Cost/year |
|---|---|---|---|
| MucoClear 3% inhalation solution 4ml ampoules (20 ampoule, £12.98) | £0.65 | £1.95a | £710.66 |
| MucoClear 3% inhalation solution 4ml ampoules (60 ampoule, £27.00) | £0.45 | £1.35a | £492.75 |
| MucoClear 6% inhalation solution 4ml ampoules (20 ampoule, £12.98) | £0.65 | £1.30b | £473.77 |
| MucoClear 6% inhalation solution 4ml ampoules (60 ampoule, £27.00) | £0.45 | £0.90b | £328.50 |
| Nebusal 7% inhalation solution 4ml ampoules (60 vials, £27.00) | £0.45 | £0.90c | £328.50 |
- (a)
child 4ml bd to qds, costing based on tds
- (b)
child 4ml bd
- (c)
child 4ml up to bd, costing based on bd
K.9.3.4. Acetylcysteine
The BNF does not report an indication or dose for acetylcysteine relevant to cystic fibrosis and the committee noted that acetylcysteine is generally no longer used as a mucolytic agent in cystic fibrosis. Where acetylcysteine has been used, the committee advised:
- Adults, 20% (200mg/mL) injection solution diluted (50–100mg/mL) and nebulised bd to qds;
- Children, 3 to 5mL of 20% (200mg/mL) injection solution diluted to 100mg/mL and nebulised bd to qds.
Table 41 below present the unit cost of acetylcysteine (NHS Electronic Drug Tariff November 2016) and the cost/day and cost/year based on 1 ampoule per dose, on the assumption that ampoules cannot be carried over. Acetylcysteine would also incur the cost of a nebuliser (Table 36) if the person with cystic fibrosis did not already acquire one.
Table 41Acquisition cost of acetylcysteine
| Acetylcysteine (quantity, basic price) | Unit cost | Cost/day | Cost/year |
|---|---|---|---|
| Acetylcysteine 2g/10ml solution for infusion ampoules (20 ampoule, £21.26) | £1.06 | £3.19a | £1,163.99 |
- (a)
Costing based on three doses, one ampoule/dose
K.9.4. Conclusions
Hypertonic saline solution is the cheapest treatment under consideration. However, the economic evaluations have provided evidence that dornase alfa is more effective. The clinical evidence review also showed significant differences in FEV1% in favour of dornase alfa compared to placebo at 1, 3, 6 and 24 month follow-up and compared to hypertonic saline at 3 months. On the other hand, a lack of significant differences in FEV1% was demonstrated by dornase alfa compared to hypertonic saline at 3 weeks and compared to placebo in people with severe lung disease at 1 month.
Furthermore, the analysis by Suri 2002 and Grieve 2003 demonstrated that it may be more cost-effective if dornase alfa is prescribed on an alternate day basis as opposed to routine UK practice where it is prescribed daily, given the potentially large cost savings from a small decrease in effectiveness.
However, those economic evaluations that included dornase alfa did not report cost per QALY ICERs, this makes cost-effectiveness subject to the committee’s interpretation. No economic evaluations were identified that included acetylcysteine - the cheapest treatment following hypertonic saline. However, the clinical evidence review found no clinically significant differences in FEV1% between acetylcysteine and placebo at 4, 12 and 24 week follow-up. Likewise, there were no differences in need for additional intravenous antibiotics for pulmonary exacerbation at 24 weeks follow-up. As a result, the committee will need to provide additional justifications to recommend acetylcysteine as a cost-effective treatment, as other more costly treatment have provided additional benefits compared to placebo, to justify their additional cost.
The Appraisal Committee for NICE TA266 identified a subgroup where the ICER for mannitol would be under NICE’s threshold of £30,000 per QALY. As a result, those recommendations will be adopted for this guideline to promote a cost-effective use of resources. It is also important to note that those recommendations state dornase alfa and osmotic agents should be an option prior to mannitol. The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 9.3.7.3 ‘Evidence to recommendations’.
K.10. Antimicrobial prophylaxis
K.10.1. Literature review
No economic evaluations of antibiotics to prevent pulmonary bacterial colonisation with S aureus in people with cystic fibrosis were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.10.2. Background and methods
This review question was not ranked as a high priority by the committee for de novo modelling. Instead a cost description of the antibiotics specified in the protocol has been undertaken. Administration costs were assumed to be equivalent across the interventions under consideration, for this reason they have not been included.
Drug acquisition costs are taken from the NHS Electronic Drug Tariff November 2016. Dosages reflect those reported in the BNF according to age, unless unreported and otherwise stated. For this cost description, BNF dosages were the preferred costing method because trial dosages may not reflect UK clinical practice. Moreover, not all interventions have been included in the clinical evidence review.
K.10.3. Resource and cost use
K.10.3.1. Combination antibiotics
The committee advised the following dosages of co-amoxiclav and co-trimoxazole to prevent pulmonary bacterial colonisation with S aureus in people with cystic fibrosis:
- As co-amoxiclav
- Child 1 month – 1 year: 0.5mL/kg tds of 125/31mg/5mL suspension;
- Child 1 – 6 years: 5mL tds of 250/62mg/5mL suspension;
- Child 6 – 12 years: 10mL tds of 250/62mg/5mL suspension or 625mg tablet 3 times a day;
- Child >12 years and adult: 625mg tds.
- As Augmentin Duo® preparation (400/57mg/5mL)
- Child 2 month – 2 years: 0.3mL/kg bd;
- Child 2–6 years (13 – 21kg): 5mL bd;
- Child 7 – 12 years (22 – 40kg): 10mL bd;
- Child >12 years (>40kg): 10mL tds.
- Co-trimoxazole
- Child 6 weeks – 5 months: 120mg bd;
- Child 6 months – 5 years: 240mg bd;
- Child 6 – 12 years: 480mg bd;
- Child > 12 years and adult: 960mg bd.
Table 42 below presents the cost of 2 combination antibiotics: co-amoxiclav and cotrimoxazole.
For adults, the cost of trimoxazole would be £0.46/day (960mg tablet ×2) for a tablet preparation, or £3.98 for a sugar free oral solution (40ml). Co-amoxiclav would be cheaper at a cost £0.28/day for tablets (625mg tablet ×3) and £2.48/day for oral solution (10ml tds).
Table 42Acquisition cost of combination antibiotics
| Antibiotic (basic price, quantity) | Unit cost |
|---|---|
| Co-amoxiclav | |
| 125mg/31mg/5ml oral suspension sugar free (£2.19, 100ml) | £0.11/5ml |
| 250mg/125mg tablets (£2.03, 21) | £0.10 |
| 250mg/62mg/5ml oral suspension sugar free (£2.25, 100ml) | £0.11/5ml |
| 400mg/57mg/5ml oral suspension sugar free (£4.13, 35ml) | £0.59/5ml |
| 400mg/57mg/5ml oral suspension sugar free (£5.79, 70ml) | £0.41/5ml |
| 500mg/125mg tablets (£1.98, 21) | £0.09 |
| 875mg/125mg tablets (£8.60, 14) | £0.61 |
| Co-trimoxazole | |
| 160mg/800mg tablets (£23.46, 100) | £0.23 |
| 80mg/400mg tablets (£2.29, 28) | £0.08 |
| Co-trimoxazole 80mg/400mg/5ml oral suspension (£10.55, 100ml) | £0.53/5ml |
| Co-trimoxazole 40mg/200mg/5ml oral suspension sugar free (£9.95, 100ml) | £0.50/5ml |
K.10.3.2. Beta-lactam antibiotics
The recommended dosages reported in the BNF for beta-lactam antibiotics according to indication and age are:
- Tetracyline for susceptible infections:
- Child 12–17 years: 250 mg qds, increased if necessary to 500 mg tds to qds, increased dose used in severe infections.
- Adult: 250 mg qds, increased if necessary to 500 mg tds to qds, increased dose used in severe infections.
- Cefradine for prevention of S aureus lung infection in cystic fibrosis
- Child 7–17 years: 2 g bd.
- Ceflexin for susceptible infections:
- Child 1–4 years: 12.5 mg/kg bd, alternatively 125 mg tds;
- Child 5–11 years: 12.5 mg/kg bd, alternatively 250 mg tds;
- Child 12–17 years: 500 mg bd to tds;
- Adult: 250 mg every 6 hours, alternatively 500 mg every 8–12 hours; increased to 1–1.5 g every 6–8 hours, increased dose to be used for severe infections.
Table 43 below presents the cost of 3 beta-lactam antibiotics specified in the protocol: tetracycline, cefradrine and cephalexin. Based on the dosages above, for adults, the cost of tetracycline would be £0.28/day (250mg tablet ×4), cefradine £1.08/day (500mg capsule ×8) and cefalexin £0.14/day if the cheapest preparation is chosen (500mg capsules ×2) or £0.46/day if the more expensive oral solution is chosen (20ml).
Table 43Acquisition cost of Beta-lactam antibiotics
| Antibiotic (basic price, quantity) | Unit cost |
|---|---|
| Tetracycline | |
| 250mg tablets (£2.05, 28) | £0.07 |
| Cefradrine | |
| 250mg capsules (£1.80, 20) | £0.09 |
| 500mg capsules (£2.71, 20) | £0.14 |
| Cefalexin | |
| 125mg/5ml oral suspension (£0.84, 100ml) | £0.04/5ml |
| 250mg capsules (£1.38, 28) | £0.05 |
| 250mg tablets (£1.80, 28) | £0.06 |
| 250mg/5ml oral suspension (£2.35, 100ml) | £0.12/5ml |
| 500mg capsules (£1.46, 21) | £0.07 |
| 500mg tablets (£1.92, 21) | £0.19 |
K.10.3.3. Flucloxacillin
The BNF advised the following indications and dosages of flucloxacillin for people with cystic fibrosis:
- Prevention of S aureus lung infection in cystic fibrosis—primary prevention
- Child 1 month–3 years: 125 mg bd.
- Prevention of S aureus lung infection in cystic fibrosis—secondary prevention
- Child and adult: 50 mg/kg bd (max. per dose 1 g bd).
From Table 44 it is evident that capsules are cheaper than oral solutions, and non-sugar free solutions are cheaper than sugar free. For adults receiving 1g bd, the cost would range from £10.59 (40ml, 250mg/5ml oral solution sugar free) to £0.31 (500mg capsule ×4).
Table 44Acquisition cost of flucloxacillin
| Flucloxacillin (basic price, quantity) | Unit cost |
|---|---|
| 125mg/5ml oral solution (£5.60, 100ml) | £0.28 |
| 125mg/5ml oral solution sugar free (£21.97, 100ml) | £1.10 |
| 250mg capsules (£1.35, 28) | £0.05 |
| 250mg/5ml oral solution (£26.04,100ml) | £1.30 |
| 250mg/5ml oral solution sugar free (£26.48, 100ml) | £1.32 |
| 500mg capsules (£2.14, 28) | £0.08 |
K.10.3.4. Azithromycin
Azithromycin can be used to prevent pulmonary bacterial colonisation with S aureus, and also, as an immunomodulator.
The committee advised the following for children over 6 years and adults for this indication:
- <40kg: 250mg 3 times a week;
- >40kg: 500mg 3 times a week (250mg daily may also be used if necessary).
It is evident that from Table 45 that tablets are cheaper than capsules. For adults the cost would be £1.32/week for a tablet preparation and £15.13 for a capsule preparation.
Table 45Acquisition cost of azithromycin
| Azithromycin (basic price, quantity) | Unit cost |
|---|---|
| 250mg capsules (£15.13, 6) | £2.52 |
| 250mg tablets (£1.40, 4) | £0.35 |
| 500mg tablets (£1.32, 3) | £0.44 |
K.10.4. Conclusions
Overall, the preparation of a drug varies its cost substantially. If the lowest cost preparation was chosen, cephalexin, flucloxacillin (capsules) and azithromycin (tablets) would be the cheapest drugs under consideration at a cost of less than £2/week.
The clinical evidence review found a significant difference in the number of children from whom S aureus was identified at least once, favouring cephalexin and flucloxacilin over “as required”. If cephalexin and flucloxacilin are believed to be equivalent in terms of efficacy, people with cystic fibrosis should be offered the cephalexin oral solution rather than the flucloxacilin oral solution as the former is significantly cheaper. In addition, when capsules can be tolerated they should be offered instead of oral solutions because they are evidently cheaper.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 9.4.1.7.3 ‘Evidence to recommendations’.
K.11. Service configuration
K.11.1. Literature review
Three economic evaluations were identified that compared home-care IV antibiotic therapy to hospital IV antibiotic therapy. Two of those studies utilised the same data to produce the same cost estimates; hence, only the cost-effectiveness analysis is discussed in Section K.11.1.2. The results from these 3 studies are summarised in Table 46, whilst data extraction tables and quality assessments of included studies can be found in Appendix L and M, respectively.
Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.11.1.1. Wolter 1998
Wolter 1998 conducted a cost–consequence analysis in Australia based on an RCT that included 17 adult participants. The perspective of the study was not clearly stated, but authors appear to include costs incurred by the hospital and costs incurred by people with cystic fibrosis and their families. Costs were valued in Australian dollars (A$) at 1992 prices. Hospital-based treatment costs were calculated using inpatient costs from the Prince Charles Hospital and from projected diagnostic-related group reimbursement figures. Home-care IV therapy costs were calculated based on hospital acquisition costs and consumption of resource. Staff costs spent on education and home visits were calculated from hourly wages. Travel costs were determined according to a standard allowance per km. Other patient and family costs were determined by interview. Mean total costs included the costs of home physiotherapy, home visits, training, equipment, drugs and bed occupancy.
The authors concluded that home-care IV therapy was considerably less expensive for families than hospitalisation per day of hospitalisation (A$15.08 versus A$23.77). Moreover, the estimated cost saving for managing exacerbations at home compared with hospital was estimated to be A$2,552. These estimates should be approached with some caution owing to the small sample size within the study. It is also unclear whether or not these findings would hold in a UK setting today.
K.11.1.2. Thornton 2005
Thornton 2005 performed a cost-effectiveness analysis based on data collected retrospectively from 116 adults with cystic fibrosis at the Manchester cystic fibrosis unit over 1 year. Participants were categorised as belonging to the ‘home’ or ‘hospital’ group if they received >60% of treatment at home or in hospital, respectively. The study was conducted from a NHS non-societal perspective and costs were valued in UK pounds sterling at 2002 prices.
Unit costs were calculated from the NHS Trust, the cystic fibrosis unit’s budget, the BNF and the hospital-supplied catalogue. Resource use and costs were estimated for IV antibiotics, disposable equipment, home kits, sputum microbiology and sensitivity and blood drug level assays. The time spent with each person with cystic fibrosis was estimated using a time sheet completed by each staff member attending to the patient. Staff costs were obtained from the CF Unit budget. Clinical records were used to determine the number of days people with cystic fibrosis spent in hospital relating to IV antibiotic treatment. Fixed costs for the ward and outpatient clinic were calculated from the cystic fibrosis unit’s budget; these were used to estimate a fixed cost / hour related to an inpatient stay or clinic visit. A standard time per home visit was determined by interviewing staff. Travel time from the clinic to each patient’s home was estimated using data from the Automobile Association. The cost of travel for each home visit was calculated using a standard mileage allowance obtained from the hospital payroll department.
Treatment was defined as effective if lung function was maintained at the baseline ‘best’ FEV1% level i.e. percentage decline in FEV1 was ≤0%. An additional analysis with a less stringent definition of effectiveness of percentage decline in FEV1 of ≤2% was also performed.
The authors reported that hospital-based care was more effective in terms of FEV1 but also more expensive compared with home-care IV therapy. Hospital-based care may be cost-effective with a 95% probability at a willingness to pay of £262,500 for 1 extra patient with a decline in FEV1 of ≤2%. However, using a stricter definition of lung function (decline in FEV1 of ≤0%) the probability that hospital-based care is cost-effective at a willingness to pay of £10 million per patient is <0.05.
Table 46Summary of included economic evaluations, models of care
| Study | Limitations | Applicability | Other comments | Inc. costs | Inc. effects | Inc. cost-effectiveness | Uncertainty |
|---|---|---|---|---|---|---|---|
| Wolter 1997 | Seriousa,b | Partiallyc,d,e | Data collected from a prospective RCT that included 17 participants with colonisation of P. aeruginosa |
| FEV1%, mean (SD): Home: Day 0, 39 (17) Day 10, 45 (22) Day 21 43 (19) Hospital: Day 0, 44 (20) Day 10, 50 (21) Day 21, 51 (21) | NR | SDs reported |
| Elliot 2005 | Minorb,f | Partiallyd,g | Data used to inform Thornton 2005 analysis | Total mean (95% CI) costs over the 1 year study period Home-care IV therapy: £13,528 (£9,989 to £17,068) Hospital: £22,609 (£17,648 to £27,569) | NR | NR | 95% CIs reported |
| Thornton 2005 | Minorh,f | Directlyg,i |
| Total mean (95% CI) costs over the 1 year study period Home-care IV therapy: £13,528 (£9,989 to £17,068) Hospital: £22,609 (£17,648 to £27,569) | Effectiveness at the end of the 1 year study period compared with baseline “average” FEV1, n (%):
| Mean ICER (95% CI) hospital IV therapy versus home-care IV therapy
| Two outcomes for effectiveness presented 95% CIs, bootstrapped ICERs with percentiles, CE planes and CEACs also presented |
Abbreviations used in the table: BNF, British National Formulary; CE plane, cost-effectiveness plane; CEAC, cost-effectiveness acceptability curve; CI, confidence interval; FEV, forced expiratory volume; ICER, incremental cost-effectiveness ratio; IV, intravenous; NR, not reported; RCT, randomised controlled trial
- (a)
Small sample size (17 out of 54 were considered eligible to include in the trial)
- (b)
Uncertainty not assessed beyond CIs or SDs
- (c)
Clinical effectiveness data taken from an old Australian trial that may reflect outdated practices and practices that may not be generalisable to the UK
- (d)
QALY not used as an outcome measure
- (e)
Perspective unclear
- (f)
Retrospective data used to inform the analysis
- (g)
The models of care are not exclusive as participants were categorised as belonging to the ‘home’ or ‘hospital’ group if they received >60% of treatment at home or in hospital, respectively
- (h)
Authors state that some participants used hospital transport but it is unclear if this has been costed
- (i)
This study does not include the preferred measure of effects (QALYs), but is still thought to be useful for decision making given that all other criteria are applicable and the alternative outcome measure reported is unlikely to change the conclusions about cost-effectiveness.
K.11.2. Background and methods
NICE recommend that each review question relating to service guidance should have a linked conceptual model. This is a simplified, diagrammatical representation of the care/service pathway that describes the resources, processes and interactions in the delivery of healthcare interventions.
The conceptual model should be able to contextualise and describe the various models of care in terms of the following areas:
- who is using the service;
- interventions being delivered;
- current service models being used;
- regional or national variations;
- key decision makers;
- key outcomes for the service;
- assumed strengths of the service;
- assumed weaknesses of the service;
- data identification;
- potential trade-offs between options such as effectiveness, volume and impact on travelling times;
- waiting list issues.
Subsequently, a conceptual map (Figure 3) was developed in consultation with the committee and used to inform the data requirements for a costing exercise.
In order to estimate the costs of providing each model of care it is important to know the numbers of people anticipated to use such models. It is then necessary to define the resources and MDT required. In addition to staffing and equipment, this may also include travel costs.
The conceptual map (Figure 3) clearly shows that the models of care are not exclusive. Only the Specialist Centre has access to the core and extended MDT to deliver all aspects of care related to cystic fibrosis. Moreover, all people with cystic fibrosis in UK clinical practice must be seen by the MDT from a Specialist Centre at least twice a year. Therefore, people with cystic fibrosis, or the MDT, are required to move between the models, if the person with cystic fibrosis does not receive all their care at the Specialist Centre.
The CF Trust Standards of Care 2011 advise a Specialist Centre to treat between 100 to 250 adults or children. However, staffing numbers should also take into account time spent by staff from the Specialist Centre seeing people with cystic fibrosis in a local hospital clinic for people with cystic fibrosis (Outreach Care) and their homes. Therefore, it is important to consider if the models of care lead to a shuffle in staff organisation, or a reduction or increase in staff required.
K.11.3. Resource and cost use
Two costing tools were developed that utilised a “what-if” approach. One tool, reported in Section K.11.3.1, enables the user to explore the cost of providing a MDT of various compositions. The second tool, reported in Section K.11.3.2 compares the cost of providing the different models of care, utilising those assumptions from the previous tool on the composition of the MDT for a given clinic size.
The PSSRU considers the following costs when calculating the unit cost for health and social care professionals:
- wages;
- salary on-costs (employer’s national insurance plus contribution to superannuation;
- staff overheads (administration and estates staff);
- non-staff overheads (costs to the provider for office, publishing, training courses and conferences, supplies and services for clinical and general use, and utilities such as water, gas and electricity);
- capital overheads (based on the new-build and land requirements of NHS hospital facilities);
- travel (no information is available on average mileage covered per visit, the NHS reimbursement rate should be used).
A description of how those costs may vary according to the model of care are provided below.
Wages, salary on-costs and staff overheads
Wages, salary on-costs and staff overheads will be incurred by each model of care under consideration. However, if supplementary models of care require a substantial increase in administration due to scheduling and communication issues across the models, staff overheads may increase. Providing an additional administrator would incur a mean annual basic pay of £27,134 (PSSRU 2016: non-medical occupational groupings, administration and estates, full-time equivalent).
Non-staff overheads and capital overheads
Home-care and telemedicine do not require an on-site attendance. For this reason, non-staff overheads and capital overheads may be reduced under these models of care.
Travel
Home-care (excluding self-administered treatment) and Outreach Care incur an opportunity cost in terms of travel, as it is necessary for the MDT from the Specialist Centre to travel off-site. Shared Care will also involve travel when visits include the MDT from the Specialist Centre. There is also the opportunity cost of staff time in making a visit, as the number of people they can see in a given time frame will be reduced.
The mean travel cost/visit can be estimated by multiplying the distance by a mileage allowance. From July 2014, NHS reimbursement has been based on a single rate for the first 3,500 miles travelled of 56p/mile, and a reduced rate thereafter of 20p/mile, irrespective of the type of car or fuel use. This approach can be used to explore how the geographical spread of a region would influence the costs of a model as rural areas would typically involve longer travel distances.
The committee advised that nurses and physiotherapists travel within a 40 mile radius on an almost daily basis to see individual people with cystic fibrosis. This was regarded as a stretch for those healthcare professionals as it would take up most of their day. In terms of time, the committee reported an average duration of 2 hours for a home-visit, with a range of 1 to 3 hours depending on geographics.
K.11.3.1. Multidisciplinary teams of various compositions
MDTs are of various compositions including a core team and, on occasion, an extended team. The cost of providing healthcare professionals within the core and extended MDT are presented in Table 47.
Table 47Cost of providing the MDT at the Specialist Centre
| HCP | Cost/annum | Cost/hour | Source (bands informed by the committee) |
|---|---|---|---|
| Core MDT | |||
| Specialist CF Clinician | £190,408a | £105b | PSSRU 2016: Hospital-based doctors, medical consultant |
| Specialist Nurse | £83,628c | £53d | PSSRU 2016: Band 7, hospital-based nurses |
| Specialist Dietitian | £85,739f | £54e | PSSRU 2016: Band 7, scientific and professional staff |
| Specialist Physiotherapist | £87,381g | £55e | PSSRU 2016: Band 7, scientific and professional staffh |
| Specialist Pharmacist | £101,367i | £64e | PSSRU 2016: Band 8a, scientific and professional staff |
| Specialist Psychologist | £101,367i | £64e | PSSRU 2016: Band 8a, scientific and professional staff |
| Specialist Social worker | £61,730j | £40k | PSSRU 2016: Social worker (adult services) |
| Specialist Social worker | £58,947l | £39k | PSSRU 2016: Social worker (children’s services) |
| Extended MDT | |||
| Paediatric Diabetic Medicine | £253 per attendance | NHS Reference Costs 2015/16: WF01A, Non-Admitted Face to Face Attendance Follow-up, Consultant-led, Paediatric Diabetic Medicine 263 | |
| Diabetic Medicine | £159 per attendance | NHS Reference Costs 2015/16: WF01A, Non-Admitted Face to Face Attendance Follow-up, Consultant-led, Diabetic Medicine 307 | |
| Paediatric ENT surgeon | £103 per attendance | NHS Reference Costs 2015/16: WF01A, Non-Admitted Face to Face Attendance, Follow-up, Consultant led, Paediatric Ear Nose And Throat 215 | |
| ENT surgeon | £89 per attendance | NHS Reference Costs 2015/16: WF01A, Non-Admitted Face to Face Attendance, Follow-up, Consultant led, Ear Nose And Throat 120 | |
| Obstetrician | £121 per attendance | NHS Reference Costs 2015/16: WF01A, Non-Admitted Face to Face Attendance, Follow-up, Consultant-led, Obstetrics 501 | |
| General surgeon | £123 per attendance | NHS Reference Costs 2015/16: WF01A, Non-Admitted Face to Face Attendance, Follow-up, Consultant led, General Surgery 100 | |
| Gastroenterologist/hepatologist | £253 per attendance | NHS Reference Costs 2015/16: WF01A, Non-Admitted Face to Face Attendance, Follow-up, Consultant led, Hepatology 306 | |
CF, cystic fibrosis; ENT, ear, nose and throat; HCP, heath care professional; MDT, multidisciplinary team; PSSRU, Personal Social Services Research Unit
- (a)
Including wages/salary £87,449; salary oncosts £23,198; management, admin and estates staff overheads £26,777; non-staff overheads £47,689 and capital overheads £5,295
- (b)
Working time 42.3 weeks (1,838 hours) per year 43.3 hours per week
- (c)
Including wages/salary £38,550; salary oncosts £9,605; management, admin and estates staff overheads £11,653; non-staff overheads £20,755 and capital overheads £3,065
- (d)
Working time 42 weeks (1,572 hours) per year, 37.5 hours per week
- (e)
Working time 42.7 weeks (1,603 hours) per year, 37.5 hours per week
- (f)
Band 7 Dietitian. Including wages/salary £38,786; salary oncosts £9,670; management, admin and estates staff overheads £11,726; non-staff overheads £20,885 and capital overheads £4,672
- (g)
Band 7 Physiotherapist. Including wages/salary £38,786; salary oncosts £9,670; management, admin and estates staff overheads £11,726; non-staff overheads £20,885 and capital overheads £6,314
- (h)
Specialist Centre must be led by a Principal CF Physiotherapy Practitioner (Band 8)
- (i)
Band 8a. Including wages/salary £46,095; salary oncosts £11,702; management, admin and estates staff overheads £13,987; non-staff overheads £24,911 and capital overheads £4,672
- (j)
Including wages/salary £31,288; salary oncosts £9,463; direct overheads £11,818; indirect overheads £6,520 and capital overheads £2,641
- (k)
Working time 41 weeks (1,517) 37 hours per week
- (l)
Including wages/salary £29,854; salary oncosts £8,978; direct overheads £11,261; indirect overheads £6,213 and capital overheads £2,641
The composition of the MDT will depend on the model of care and the number of people with cystic fibrosis managed by that model. For these reasons, the costing tool allows the user to define the composition of the MDT, number of whole time equivalents (WTEs) and number of people with cystic fibrosis they manage. Figure 4 below provides an example of the user form which allows the user to determine how the MDT composition is to be specified. The number of WTEs utilised in Figure 4 is based on the CF Trust Standards of 2011 for a clinic managing 250 people with cystic fibrosis.
Figure 4MDT configuration taken from the costing tool
Note: specialist pharmacist WTE increased from 1 to 2 to reflect committee consensus on current clinical practice in England, a specialist centre is led by a principal physiotherapist (band 8) and all remaining physiotherapists in the MDT are band 7
K.11.3.2. Models of care
Estimating the annual cost/person for each model requires the following inputs for each model of care:
- clinic size;
- number of clinic visits/ person/ year;
- core members of the MDT;
- WTEs for the size of the clinic.
In addition to their work at the Specialist Centre, core members of the MDT would be responsible for providing Outreach Care and telemedicine. Home-care (excluding self-administered treatment), on the other hand, would only be provided by dietitians, physiotherapists and nurses. Therefore, it is evident telemedicine and home-care alone cannot provide sufficient care for all people with cystic fibrosis (the models of care are not mutually exclusive). Therefore, supplementary models of care should not be compared to the recognised models of care as all people with cystic fibrosis will continue to need the support of the full MDT. Moreover, the frequency of healthcare professional contact via the supplementary models will vary considerably from person to person which is difficult to represent in the costing tool. For these reasons, the only valid comparison (annual cost) is between the Specialist Centre, Shared Care and Outreach Care.
Based on the committee’s feedback, it was agreed the composition of the MDT would be the same for the Specialist Centre and Shared Care, and reflect the core members in their recommendations for that review question.
In the base case, a clinic size of 150 was utilised for Shared Care and 250 for the Specialist Centre, according to committee opinion that Shared Care would usually manage a smaller number of people with cystic fibrosis than the Specialist Centre.
However, a scenario using a clinic size of 250 for both Shared Care and the Specialist Centre was also undertaken, as the committee stated that a smaller clinic size may be driven by restricted resources rather than ideal practice.
WTE recommendations reported in the CF Trust Standards of Care 2011 are presented in Table 48 alongside the estimated annual cost to provide full-time MDTs at Shared Care and the Specialist Centre. This does not include the cost of providing joint-clinics.
Table 48Core MDT compositions
| HCP | WTE | |||
|---|---|---|---|---|
| Paediatrics (Shared Care) n=75 | Paediatrics (Shared Care) n=150 | Paediatrics (Shared Care) n=250 | Adults (Specialist Centre) n=250 | |
| Specialist Clinician | 1a | 2c | 3e | 3e |
| Specialist Nurse | 2.5b | 3.5d | 5f | 6g |
| Specialist Dietitian | 0.5 | 1 | 1.5 | 2 |
| Specialist Physiotherapist* | 2 | 3 | 4 | 6 |
| Specialist Pharmacist | 0.5 | 2h | 2h | 2h |
| Specialist Psychologist | 0.5 | 1 | 1.5 | 2 |
| Specialist Social workeri | 0.5 | 1 | 1 | 2 |
| Total costj | ||||
| Total cost/year | £747,951 | £1,384,445 | £1,881,229 | £2,311,670 |
| Total cost/person/year | £9,973 | £9,230 | £7,525 | £9,247 |
HCP, health care professional; MDT, multidisciplinary team; SpR, Specialist Registrar; WTE, whole time equivalent
- *
a CF specialist centre is led by a band 8 physiotherapist and all remaining physiotherapists in that MDT are band 7, all physiotherapists in the Shared Care MDT are band 7
- (a)
increased from 0.8 to 1 to include 0.5 WTE Staff grade/fellow and 0.3 SpR
- (b)
increased from 2 to 2.5 to include 0.5 WTE Staff grade/fellow and 0.3 SpR
- (c)
increased from 1.5 to 2 to include 1 WTE Staff grade/fellow and 0.5 SpR
- (d)
increased from 3 to 3.5 to include 1 WTE Staff grade/fellow and 0.5 SpR
- (e)
increased from 2.5 to 3 to include 1 WTE Staff grade/fellow and 1 SpR
- (f)
increased from 4 to 5 to include 1 WTE Staff grade/fellow and 1 SpR
- (g)
increased from 5 to 6 to include 1 WTE Staff grade/fellow and 1 SpR
- (h)
increased from 1 to 2 to reflect committee consensus on current clinical practice in England
- (i)
adult social worker assumed for the specialist centre, children’s social worker for shared care
- (j)
estimated from Table 47
The committee noted that the increasing complexity and cost of treatments, coupled with increasing longevity, polypharmacy, chronic use of potentially toxic drugs and the increasing medicines optimisation agenda all supported the need for additional pharmacist resource. For these reasons, the committee regarded the ‘economy of scale’ for pharmacist time seen in the current CF Trust Standards of Care 2011 to no longer be appropriate. Instead, the committee suggested that pharmacist resource should increase in line with other healthcare professionals, such as physiotherapists and dietitians, who have a mandate to see all people with cystic fibrosis regularly. For this reason, the committee agreed it was necessary to increase the WTE of pharmacists from 1 to 2, for clinic sizes ≥150.
The committee advised that “sick” people with cystic fibrosis and babies in their first year of life would need to be seen more frequently than those who are “well”. However, for simplicity, the committee agreed 6 clinics/year would be reasonable to inform the model.
Under the Specialist Centre model, all 6 of those clinics would be conducted at the Specialist Centre. Based on the adult WTEs reported in Table 48, this would cost £2,311,670/year (£9,247/person) for a clinic size of 250.
To calculate the cost of Shared Care (n=150), it is assumed one half of people with cystic fibrosis (n=75) attend the Specialist Centre whilst the other half (n=75) are managed under Shared Care. The Specialist Centre would incur the cost for all 150 people with cystic fibrosis as they supervise Shared Care. Based on the paediatric WTEs reported in Table 48, the Specialist Centre MDT would cost £1,384,445 /year, whilst the Shared Care MDT managing 75 people with cystic fibrosis would cost an additional £747,951 /year.
As previously stated, every person with cystic fibrosis is seen approximately 6 times a year by their MDT. Under a Shared Care model, 4 of those reviews would be undertaken by the local clinic (the Shared Care MDT) and 2 would be undertaken by the Shared Care MDT and Specialist Centre MDT together as a joint clinic.
Each visit to Shared Caremade by the Specialist Centre MDT (assuming 1 of each MDT speciality takes part) costs approximately £470/day in travel costs (120 miles/healthcare professional/visit @ 56p/mile).
Assuming the Specialist Centre MDT can review 12 people/day at Shared Care, a centre managing 75 people with cystic fibrosis would require 13 days (150/12) from the Specialist Centre MDT, to conduct 2 reviews for each person, incurring travel costs of £6,115 (£470 ×13).
The committee also advised that Shared Care would incur additional administration due to the communication with the Specialist Centre. As a result, the committee agreed the cost of an administrator (0.5 WTE) (£13,567/year) should be added.
Including the cost of the Specialist Centre MDT (£1,398,431), Shared Care MDT (£747,951), additional administration (£13,567) and reviews undertaken by the Specialist Centre MDT at the Shared Care Centre (£6,115), the cost to provide Shared Care is £2,166,064/year, or £14,440/person based on a clinic size of 150.
For Outreach Care, the committee advised that the core MDT from the Specialist Centre (assuming 1 of each MDT speciality takes part) would travel 120–200 miles (including return travel) 6 times/year to perform a cystic fibrosis clinic in a local hospital. If the MDT can perform up to 12 reviews each day, it would take approximately 0.5 days to undertake 6 reviews for a person with cystic fibrosis each year. Therefore, the MDT would incur travel costs of £314/year (£627*0.5) if they travelled 160 miles/day to the local clinic for each person with cystic fibrosis.
It is important to note that Outreach Care requires economies of scale to make it viable as the resources and costs to provide Outreach Care for a single person with cystic fibrosis would be substantial. For this reason, the clinic should be organised to ensure several people with cystic fibrosis attend the clinic on the day the MDT are scheduled to visit. Ideally, the committee advised a minimum of 12 people/day.
Similarly to Shared Care, the committee agreed Outreach Care would increase administration costs. For each person with cystic fibrosis this would cost an additional £60/year (£121/day/administrator). Outreach care would also incur additional capital costs and non-staff overheads as additional clinic space is needed. Based on the non-staff overheads and capital overheads reported in the PSSRU for each healthcare professional attending the clinic, this would cost an additional £440 /person /year.
From July 2014, NHS reimbursement has been based on a single rate for the first 3,500 miles travelled of 56p/mile, and a reduced rate thereafter of 20p/mile. Given that the MDT from the Specialist Centre MDT will not travel a distance greater than 3,500 miles under a Shared Care or Outreach Care model, the reimbursement rate is set at 56p/mile. Conversely, healthcare professionals performing home-care will travel more frequently. Assuming a healthcare professional providing home-care travels 40 miles each working day (225 days), the first 3,500 miles would be reimbursed at 56p a day whilst the remaining 5,500 miles would be reimbursed at 20p a day leading to a total annual travel cost of £3,060.
It is assumed each healthcare professional incurs a travel cost, but in reality they may car share or use alternative transport, this will, for example, reduce the cost from £470 for a MDT travelling 120 miles with seven cars to £67 with 1 car, or even less under a car sharing scheme. For a clinic managing 250 people with cystic fibrosis, this is a small cost/person (£1.90 versus £0.30) so is unlikely to change our decision.
Based on the assumptions outlined above, Table 49 shows that the cheapest model of care is the Specialist Centre (£9,247) followed by Outreach Care (£10,126) and Shared Care (n=150, £14,440; n=250, £13,220). However, it is important to note that if demand increases for Shared Care or Outreach Care, the WTEs utilised in the model may underestimate their numbers, as the current WTEs may not consider the time staff spend outside of the Specialist Centre.
Table 49Annual cost/person with cystic fibrosis across the recognised models of carea
| Model of care | Clinic size | Annual travel costs incurred by the Specialist Centre MDT | Annual MDT staff costs | Total annual cost/clinic | Total annual cost/person |
|---|---|---|---|---|---|
| Specialist Centre | 250 | None | £2,311,670 (Specialist Centre MDT) | £2,311,670 | £9,247 |
| Shared Care | 250 | £11,760 (25 days of travel totalling 3,000 miles @ 56p/mile for 7 HCPs) | £1,384,445 (paediatric Shared Care MDT for 150 people) + £1,895,215 (Specialist Centre for 250 peopleb) + £13,567 (0.5 administrator) | £3,304,987 | £13,220 |
| Shared Care | 150 | £6,115 (13 days of travel totalling 1,560 miles @ 56p/mile for 7 HCPs) | £747,951 (paediatric Shared Care MDT for 75 people) + £1,398,431 (Specialist Centre for 150 peopleb) + £13,567 (0.5 administrator) | £2,166,064 | £14,440 |
| Outreach Care | 1 | £627 (1 day of travel totalling 160 miles @ 56p/miles for 7 HCPs) shared by 2 people | £9,247 (Specialist Centre MDT divided by a clinic size of 250) + £60 (administrator, 0.5 days) + £440 (capital costs, 0.5 days) | NC | £10,126 |
CP, health care professional; NC, not calculable; MDT, multidisciplinary team
- (a)
Excluding diagnosis and treatment costs
- (b)
A Specialist Centre led by a band 8 physiotherapist, made up of the WTE figures reported in Table 48 for a Shared Care model
Recommending the Specialist Centre model alone would require the person with cystic fibrosis, and their families or carers, to travel greater distances than they would under a Shared Care or Outreach Care model. However, the NICE reference case states that economic analyses should only include costs borne by the NHS. Therefore, costs incurred by people with cystic fibrosis, and their families or carers, using services that are not reimbursed by the NHS should not be included. This position is based on the argument that including the costs of lost productivity discriminates between people based on their capacity for work and income.
However, if the committee felt that the journeys were sufficiently burdensome to negatively impact quality of life, or reduce hospital attendance resulting in later downstream costs, this should be acknowledged. To quantify this reduction in a person’s quality of life we can estimate the QALY gain necessary to determine the additional (incremental) benefit that would be needed for each of the models to be considered as the most cost-effective option.
The 2014 NICE Guidelines Manual advises that an intervention will generally be considered cost-effective if the ICER is £20,000 per QALY or less. The cost per QALY (incremental cost-effectives ratio, ICER) is given by:
Incremental cost ÷ incremental QALY gain = incremental cost per QALY
Or, rearranging:
Incremental cost ÷ £20,000 = incremental QALY gain
To estimate the QALY gain necessary for an intervention to be considered the first step is to calculate the incremental cost of the models being compared. If we compare Shared Care to the Specialist Centre, the incremental cost is £3,973 (£13,220 - £9,247) and if we compare Outreach Care to the Specialist Centre, the incremental cost is £879 (£10,126 - £9,247).
Table 50 suggests what additional benefit each model is required to provide in order to be considered cost-effective relative to the comparator. Hence, despite higher costs, a model could be considered cost-effective if those QALY gains can be achieved. This is not to say that a model is cost-effective, but rather it gives the level of clinical effectiveness relative to the comparator that would be necessary given the current differential in cost and NICE’s threshold of £20,000 per QALY.
Table 50QALY gain necessary, models of care
| Comparison | Additional cost/patient/year | Additional QALY gain | Interpretation |
|---|---|---|---|
| Outreach Care versus Specialist Centre | £879 | 0.04 | As long as person gains at least 0.04 additional QALYs as a result of having the more expensive Outreach Care, or losses at least 0.04 QALYs as a result of having the cheaper Specialist Centre, Outreach Care would still be considered cost-effective relative to the Specialist Centre |
| Shared Care (n=250) versus Specialist Centre | £3,973 | 0.20 | As long as a person gains at least 0.20 additional QALYs as a result of having the more expensive Shared Care, or losses at least 0.20 QALYs as a result of having the cheaper Specialist Centre, Shared Care would still be considered cost-effective relative to the Specialist Centre. |
QALY, quality adjusted life year
To interpret those QALY gains in Table 50 with regards to patient travel and potential time off work, we can take the usual activities domain of the EQ-5D as a proxy. The EQ-5D is NICE’s preferred measure of health-related quality of life in adults comprising the following 5 dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has 3 levels: no problems, some problems, extreme problems. The resulting value sets (disutilities) for usual activities estimated from a representative sample of the UK population are summarised in Table 51.
Table 51Disutility for each level of usual activities
| Usual activities | Disutility |
|---|---|
| Level 1: I have no problems with performing my usual activities | 0 |
| Level 2: I have some problems with performing my usual activities | −0.036 |
| Level 3: I am unable to perform my usual activities | 0.094 |
If the Specialist Centre negatively impacts usual activities at either level 2 or level 3, Outreach Care could be considered cost-effective compared to the Specialist Centre, as the disutilities in Table 51 could offset the QALY gains in Table 50. However, neither level 2 or level 3 would be sufficient to justify Shared Care on the basis of usual activities alone, as the disutilities in Table 51 do not offset a QALY gain of 0.20 (Table 50).
K.11.4. Conclusion
A core MDT with a cost-effective composition should include healthcare professionals with cystic fibrosis expertise in those specialities regularly sought by all people with cystic fibrosis. The extended MDT can sometimes play an important role in the management of people with cystic fibrosis with complications or comorbidities not typically seen in all people with cystic fibrosis. Therefore, when we consider the opportunity cost of their services outside of cystic fibrosis, the MDT should have access to the extended MDT on a case-by-case basis as opposed to full-time. However, it is important to reiterate that the cost-effectiveness of MDT compositions cannot be ascertained in the absence of clinical effectiveness data.
According to the economic literature, if we can accept a small decrease in effectiveness to provide cost savings, home-IV antibiotic therapy could be considered cost-effective, relative to hospital IV therapy. Moreover, the clinical evidence review found no significant difference in lung function between people with cystic fibrosis receiving therapy at home or at hospital. However, more people with cystic fibrosis treated in hospital did not require a further course of antibiotics at 12 weeks compared to those receiving antibiotics at home. Therefore, to ensure effectiveness is not compromised, suitable home-IV patients, in terms of, for example their severity and ability to self-administer, must be identified and IV training should be provided.
Given that the opportunity cost of travel time is more attendances, it is reasonable to assume home-care and Outreach Care will perform less attendances/day and as a result, cost more to provide than telemedicine, or the Specialist Centre. Furthermore, if non face-to-face attendances (telemedicine) take less time to perform than face-to-face attendances (traditional clinics) then it would be reasonable to assume telemedicine can see the greatest number of people with cystic fibrosis per day and potentially incur fewer costs and resources, relative to the other models of care. However, if telemedicine takes relatively more organisation than traditional clinics, and because it will often be carried out at a time convenient for patients, it may not be possible to group them all into 1 virtual clinic.
Wilkinson 2008 identified from the clinical evidence review, compared telemedicine to usual care. They reported no significant differences in the number of visits, general practitioner attendances, courses of IV antibiotics, length of hospital inpatient stay, or visits to hospital between telemedicine and usual care, demonstrating that the costs savings from the initial appointment may not be outweighed by additional face-to-face appointments. Wilkinson 2008 also showed no significant difference in lung function between the participants using telemedicine and participants receiving usual care. Therefore, if telemedicine does not lead to additional face-to-face appointments, telemedicine may be considered as a cost-effective supplementary model for routine monitoring.
The estimated annual cost for each person with cystic fibrosis inferred that the Specialist Centre is the cheapest recognised model of care. Consequently, if the committee want to recommend Shared Care and Outreach Care above the Specialist Centre, they will need to show that they can provide additional benefits to outweigh their additional costs.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Sections 7.2.6.3 and 7.3.6.3 ‘Evidence to recommendations’.
K.12. Strategies to prevent cross-infection
K.12.1. Literature review
No economic evaluations of strategies to prevent cross-infection were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.12.2. Background
All people with cystic fibrosis require routine reviews carried out in a cystic fibrosis clinic, which puts them at risk of cross-infection from other users. Infection with pathogens such as B cepacia complex and P aeruginosa are associated with important cost and quality of life implications, but the risk of their transmission at cystic fibrosis clinics can be reduced when effective strategies are put in place. The committee agreed further economic analysis would help to reduce their uncertainty with regards to cost-effectiveness as the strategies can entail high costs.
K.12.3. Model structure
A decision tree model was developed in Microsoft Excel® (2013) (Error! Reference source not found.) from the perspective of the UK NHS and using 2015/16 costs. The time horizon for the model was 1 year as this reflected the incidence rates reported in the clinical evidence review. Moreover, strategies may change over time as the prevalence of a pathogen changes. The committee also stated that despite segregation policies, an inevitable baseline incidence of new acquisition from the environment will exist, which explains the upward (unavoidable) trend in prevalence over time that was demonstrated in the clinical evidence review. For these reasons, extrapolation to a lifetime horizon was considered to be inappropriate. Following this decision, no discount rate was applied.
It is assumed that people with cystic fibrosis who die from their infection will die half way through the model incurring 50% of the utility they would if they were alive With regards to costs, those with an intermittent infection incur the cost of their full treatment course before they die, as the treatment course is relatively short, whereas those with a chronic infection incur 50% of the cost.
K.12.4. Clinical effectiveness
No RCTs (including cluster RCTs) identified from the clinical search matched the protocol. Consequently, only before and after type studies were included. Those studies reported incidence data, or prevalence data, or both. However, incidence data is preferred for modelling to ensure that the effectiveness of the new strategy is not influenced by the number of cases that were transmitted during the old strategy. A control in the model for prevalence was considered to include those studies that reported prevalence, but given the variation in study demographics and volume of prevalence data required, this was not considered further.
The prevalence of long term infections usually increases year after year because prevalence includes existing cases and new cases. Incidence data on the other hand, only represent the number of new cases. Therefore, new strategies to prevent cross-infections would never be considered cost-effective relative to old strategies based on prevalence data as prevalence would always be greater following the new strategy that is implemented at a later point in time. For this reason, studies that report prevalence data were only included in the model if there were no studies that reported on the same strategy, or if the studies that considered the same strategy were too heterogeneous for one to displace the results of another. Overall, studies that report prevalence data alone should be interpreted with caution.
To estimate the cost of a strategy it is important the strategy is accurately defined. The type of segregations defined in the clinical evidence review provided little detail on how those strategies were achieved. To inform the economic model those types of segregation/strategies need to be translated into costs. However, the studies included in the clinical evidence review did not provide comprehensive descriptions of their strategies. Consequently, assumptions were made to fit those studies into a pragmatic number of strategies.
Following this, the committee agreed that the following 4 strategies were sufficiently representative of those studies included in the clinical evidence review and the model:
- Cohorting outpatient clinics by pathogen (effectively reducing the number of people attending the clinic)
- Protective equipment
- Individual inpatient segregation (single inpatient rooms versus beds on shared wards)
- Incomplete cohort segregation including en suite bathroom facilities versus no cohort segregation including shared bathroom facilities (to reflect Jones 2005)
The studies also assessed different pathogens, and given that those pathogens incur different quality of life impacts and treatment costs, it was necessary to categorise the studies and strategies by the type of pathogen they aimed to prevent. For simplicity, intermittent infections were assumed if a chronic infection was not stated in the study. The following 4 pathogens were included in the model:
- Intermittent B cepacia complex
- Intermittent P aeruginosa
- Chronic P aeruginosa
- Superinfection with chronic P aeruginosa
Table 52 below summarises how the studies included in the clinical evidence review fitted into the model according to the strategy and pathogen. It is important to note that not all studies included in the clinical evidence review were included in the model, either because they did not report incidence data, or assessed pathogens or strategies that were not considered applicable to UK practice today.
Table 52Studies included in the model according to strategy and pathogen
| Study | Pathogen | Clinical evidence review comparison |
|---|---|---|
| Cohorts by pathogen | ||
| Federiksen 1999 | Chronic PA & Intermittent PA | Comparison 6. Cohort segregation versus no cohort segregation (inpatient and outpatient) |
| France 2008a | Intermittent BCC | Comparison 6. Cohort segregation versus no cohort segregation (inpatient and outpatient) |
| Hoiby 1989b | Chronic PA | Comparison 6. Cohort segregation versus no cohort segregation (inpatient and outpatient) |
| Lee 2004 | Chronic PA & Intermittent PA | Comparison 2. Cohort segregation by location versus no cohort segregation (outpatient) |
| Whitford 1995 | Intermittent BCC | Comparison 6. Cohort segregation versus no cohort segregation (inpatient and outpatient) |
| Protective equipment | ||
| Chen 2001a | Intermittent BCC | Comparison 10. Cohort segregation + individual segregation + protective equipment versus usual care (inpatient and outpatient) |
| Savant 2014 | Intermittent PA | Comparison 3. Combination of protective equipment + individual segregation versus incomplete protective equipment + incomplete individual segregatione (outpatient) |
| Single inpatient room versus ward bed | ||
| Chen 2001a | Intermittent BCC | Comparison 9. Cohort segregation + individual segregation versus cohort segregation (inpatient and outpatient) |
| France 2008a | Intermittent BCC | Comparison 7. Complete cohort segregation versus incomplete cohort segregation (inpatient and outpatient) |
| Incomplete cohort segregation including en suite bathroom facilities versus no cohort segregation | ||
| Jones 2005 | Superinfection with chronic PAc & Intermittent PAd | Comparison 6. Cohort segregation versus no cohort segregation (inpatient and outpatient) |
BCC, B cepacia complex; PA, P aeruginosa
- (a)
Intermittent assumed as chronic not reported
- (b)
Multiply resistant pseudomonas assumed to have a similar treatment cost and effect on quality of life as chronic pseudomonas
- (c)
Incidence of superinfection by transmissible strains among people with cystic fibrosis already infected with chronic P aeruginosa
- (d)
New cases of P aeruginosa infection with transmissible strain among people with cystic fibrosis without chronic P aeruginosa infection assumed to have a similar treatment cost and effect on quality of life as intermittent P aeruginosa
- (e)
Individual segregation achieved through a “no-waiting” room policy incurring no additional cost to incomplete segregation
Annual probabilities used to inform the model are presented in Table 53 for intermittent infections and Table 54 for chronic infections.
Table 53Annual probability of infection with intermittent pathogen
| Study | Intermittent pathogen | Strategy 1 (new) probability | Strategy 2 (old) probability |
|---|---|---|---|
| Cohorts by pathogen | |||
| Federiksen 1999 | PA | 22.5% | 33.3% |
| France 2008 | BCC | 16.3% | 4.0% |
| Lee 2004 | PA | 34.5% (30%)a | 26.2% (28%)a |
| Whitford 1995b | BCC | 2.1% | 9.0% |
| Protective equipment | |||
| Chen 2001 | BCC | 0.9% | 8.8% |
| Savant 2014c | PA | 36% | 46% |
| Single inpatient room versus ward bed | |||
| Chen 2001 | BCC | 7.0% | 15.0% |
| France 2008 | BCC | 2.9% | 16.3% |
| Incomplete cohort segregation including en suite bathroom facilities versus no cohort segregation | |||
| Jones 2005d | PA | 0.0% | 9.7% |
BCC, B cepacia complex; PA, P aeruginosa
- (a)
Prevalence data (incidence data) 1990 versus 2000
- (b)
6 month probability translated into a 12 month probability using rates
- (c)
Prevalence data as incidence data not calculable
- (d)
2000 data compared to 2001 data as these are the dates when incidence is reported, the clinical evidence review compared 1999 to 2001 based on prevalence
Table 54Annual probability of infection with chronic P aeruginosa
| Study | Strategy 1 (new) probability | Strategy 2 (old) probability |
|---|---|---|
| Cohorts by pathogen | ||
| Federiksen 1999 | 10.1% | 20.0% |
| Lee 2004a | 18.1% | 24.5% |
| Hoiby 1989b | 55.3% | 93.7% |
| Incomplete cohort segregation including en suite bathroom facilities versus no cohort segregation | ||
| Jones 2005c | 3.3% | 4.4% |
- (a)
Prevalence data used as incidence not reported or calculable
- (b)
1 month probability translated into a 12 month probability
- (c)
Superinfection with chronic P aeruginosa, 2000 data compared to 2001 data as these are the dates when incidence is reported, the clinical evidence compared 1999 to 2001 based on prevalence
The incidence of infection reported in Jones 2005 following the new strategy, for the years 2000 to 2003, were somewhat random, illustrating no downward (or upward) trend. For this reason, an analysis was conducted that uses an average of 2001 to 2003, whilst another compares 2000 to 2001 to reflect the post-strategy year used in the clinical evidence review.
Table 55Incidence data reproduced from Jones 2005
| Year | Incidence of superinfection by transmissible strains among people with CF already infected with chronic PA | New cases of PA infection with transmissible strain among people with CF without chronic PA |
|---|---|---|
| 1999 | NR | NR |
| 2000 | 4.4% | (3/31) 9.7% |
| 2001 | 3.3% | (0/30) 0% |
| 2002 | 4.6% | (0/37) 0% |
| 2003 | 5.9% | (0/45) 0% |
| Average of 2001–2003 | 4.6% | 0% |
CF, cystic fibrosis; NR, not reported; PA, P aeruginosa
For Lee 2004, the fall in chronic P aeruginosa infection was associated with a rise in those classified as intermittent. The annual incidence of new growths of P aeruginosa while fluctuating, showed no downward trend, despite segregation of people with cystic fibrosis with chronic P aeruginosa infection, potentially as they are acquired from the community.
Lee 2004 reported incidence data narratively and graphically, whereas they provided quantitative prevalence data. For completeness, both data are utilised in the model, where incidence is taken arbitrarily from the graph illustrated in the paper (reproduced in Table 56 and Figure 6). Similarly to Jones 2005, one analysis was conducted that compares the incidence in 1990 to the average incidence across 1991 to 2000, and another analysis compares the incidence in years 1990 to 2000, to reflect the post-strategy year (2000) used in the clinical evidence review. However, regardless of whether incidence or prevalence data is used to inform the model for intermittent P aeruginosa, it is evident that the number of intermittent cases rises following the new strategy to segregate people with cystic fibrosis.
Table 56Incidence of P aeruginosa estimated from Lee 2004
| Year | Incidence |
|---|---|
| 1990 | 28% |
| 1991 | 40% |
| 1992 | 30% |
| 1993 | 37% |
| 1994 | 34% |
| 1995 | 48% |
| 1996 | 55% |
| 1997 | 34% |
| 1998 | 29% |
| 1999 | 49% |
| 2000 | 30% |
| Average of 1991–2000 | 39% |
Figure 6Figure reproduced from Lee 2004
Source: Lee 2004, figure 4: Incidence of new growth of P aeruginosa in patients described as “never” or “free” in period 1990–2000. Incidence is expressed as a percentage of those “at risk” i.e. those classed as “never” or “free” in each successive year
K.12.5. Resource and cost use
K.12.5.1. Strategy costs
Cohorts by pathogen
The committee agreed that once a segregation plan was set-up by their administrator (according to pathogen status) it would take no additional time to follow and a negligible amount of time to update thereafter. Therefore, rather than additional administration to segregate a cohort, the committee stated that the number of people with cystic fibrosis seen by the clinic would be reduced, potentially halving the number that could be reviewed. The committee considered if additional cleaning would be required following a segregation plan, but concluded that cleaning between people with cystic fibrosis should be common practice, regardless of whether cohort segregation was in place, therefore no additional cleaning costs would be incurred.
Based on the estimates derived for the review on service configuration (Section K.11.3.2), it costs £2,311,670 to employ the MDT at the clinic for 250 people with cystic fibrosis. Therefore, it would cost £10,274 to employ the MDT each day, based on 225 working days.
As described in the review on service configuration (Section K.11.3.2) each person with cystic fibrosis undergoes approximately 6 reviews each year by the MDT. If the MDT can perform routine reviews for 20 people with cystic fibrosis each day, it would cost £514 for each review and approximately £3,082 patient/year ([£10,274/20] *6) based on a Specialist Centre managing 250 people with cystic fibrosis. If the number of reviews was reduced to 10/day the cost would increase, but the cost would not necessarily double, as the proportion or clinics that are cohorted depends on the prevalence of the pathogen the clinic wants to segregate. In the worst case scenario, cohorting would take place on each clinic day (5 days/week) leading to a cost £1,027 for each review, or £6,164 patient/year ([£10,274/10] *6). Table 57 below presents the prevalence of intermittent P aeruginosa, intermittent P aeruginosa and B cepacia complex reported by the CF Registry 2014.
Table 57Lung infections in 2014 taken from the CF Registry 2014
| Infectiona | Children, % | Adults, % |
|---|---|---|
| BCC | 1.3% | 5.0% |
| Chronic PA | 8.0% | 48.3% |
| Intermittent PA | 21.3% | 15.4% |
BCC, B cepacia complex; PA, P aeruginosa
- (a)
The definition for chronic on the Registry is three or more growths in a year, and is only reported for Pseudomonas aeruginosa and Staphylococcus aureus. Other bacteria are reported if they grow at all in the year.
Based on the data in Table 57 it is evident that cohort segregation by pathogen would be greater for adults than children and also greater for an infection with P aeruginosa than B cepacia complex.
In the base case, the model will illustrate a Specialist Centre model managing 250 people with cystic fibrosis to reflect the committee’s recommendations on service configuration. This recommendation stated how care should be delivered by a Specialist Centre unless the MDT think outreach or Shared Care is a justifiable model due to, for example, geographics. Moreover, only 2 of the 5 studies included in this comparison explicitly included children alone. For completeness a sensitivity analysis has been conducted on those 2 studies (Whiteford 1995 and Lee 2004) based on a Shared Care centre model managing 250 people with cystic fibrosis. For the study that segregated by P aeruginosa the number of clinic days is reduced to 1 a week to reflect the lower prevalence in children (Table 57).
Combined with the committee’s clinical experience, it was agreed separate clinics for people with cystic fibrosis infected with P aeruginosa would take place twice a week (£4,315 /patient/year), whereas separate clinics for people with cystic fibrosis infected with B cepacia complex would take place once every 2 weeks (£3,390 /patient /year). With regards to the clinical evidence review, the former assumption for P aeruginosa applies to Federiksen 1999, Hoiby 1989 and Lee 2004, whereas the latter assumption for B cepacia complex applies to Whitford 1995 and France 2008.
It is important to note that the model does not make further assumptions on the resource use required to cohort a pathogen within an inpatient setting as the committee believed this was often achieved via individual inpatient segregation. Moreover the committee were unable to quantify the opportunity cost of inpatient segregation as this would depend on the size of the wards within the hospital and the number of patients with cystic fibrosis they managed. However, if the strategy was cost-ineffective when resources to segregate inpatients are excluded, the strategy would undoubtedly cost-ineffective if additional resources were needed.
Protective equipment
The use of masks or gloves were considered by Chen 2001 and Savant 2014 as part of their new strategies. Chen 2001 advised inpatients colonised with B cepacia complex to wear masks and gloves when out of their rooms, whilst Savant 2014 requested all people with cystic fibrosis in their outpatient clinic to wear masks.
The cost of masks and gloves taken from the NHS Supply Chain 2015 are presented in Table 58.
Table 58Cost of protective equipment
| Protective equipment (quantity, basic price) | Description | Unit cost |
|---|---|---|
| Mask (50, £1.76) | Ear loop latex free facemask | £0.04 |
| Gloves (100, £7.11) | NitraFine, Gloves protective non-latex unisex nitrile powder free disposable | £0.07 |
Given that people with cystic fibrosis visit their clinic approximately 6 times/year, the cost to provide facemasks on an outpatient basis (1 mask/visit) would be relatively cheap at a cost of £0.24 /patient/year.
The cost to provide protective equipment during an inpatient stay depends largely on the duration of those stays. According to data obtained from the CF Registry, the average length of an inpatient stay for people with cystic fibrosis without a chronic infection is 17.8 days.
If inpatients were required to wear a mask and gloves when out of their rooms they would usually use new equipment each time. It would be reasonable to assume patients make 2 or 3 trips/day when they are first admitted and 6 or 7 towards the end of their stay. Assuming they made on average 5 trips/day the cost to provide masks and gloves would be £9.79 (17.8 × 5 × £0.11). Given that outpatients were also required to wear masks (£0.24/ patient/ year) the total cost would be £10.03.
Inpatient beds and bathroom facilities
To cost inpatient strategies in the model, the duration of a hospital stay/ patient/ year is required. According to data obtained from the CF Registry, the average length of an inpatient stay for people with cystic fibrosis without a chronic infection is 17.8 days and with a chronic infection 20.5 days.
The following findings were extracted from the NIHR 2015 report that evaluated the workforce implications and impact on patient and staff experiences of all single room hospital accommodation:
- Cleaning costs were 69% higher for single rooms than shared rooms (£7.88/ room/ day versus £5.44/ bed/ day) according to Maidstone and Tunbridge Wells NHS Trust 2012–13 and up to 75% higher according to Whitehead 2010
- Moving from multiple occupancy to single rooms was reported to increase ward staffing by only 2 to 4% (Whitehead 2010)
- Construction costs/bed were 14% higher for an all single room hospital than for one with 50% single rooms (£66,333 versus £58,324) (NHS Estates 2005)
Those papers referenced by NIHR 2015 were subsequently acquired for further review. In addition to those points above, NHS Estates 2005 reported that the additional space cost/bed/day and additional cleaning cost/bed/day relative to shared rooms was £3.07 and £5.14, respectively. This led to a total additional cost of £8.30/bed/day in 2005 costs and £10.61/bed/day when inflated to 2015/16 costs (inflator to 2015/16 prices 1.279, based on the hospital & community health services (HCHS) index (297.0 [2015/16 PPI] / 232.3 [2004/5 PPI).
NHS Estates 2005 do not report the baseline cost for a bed in a shared room. However, as stated above, the additional (incremental) cost of single rooms can be calculated. Moreover, it is the incremental cost that is needed to calculate cost-effectiveness using the incremental cost-effectiveness ratio (ICER). If a person with cystic fibrosis was admitted for 17.8 days they would incur an additional cost of £189/year (2015/16 costs).
South Devon Healthcare NHS Foundation Trust report an average tariff price of £412/night for inpatient single overnight accommodation and £275/night for ward inpatient accommodation to UK private patients, oversea visitors and insurance companies (effective from 1st April 2015 to 31st March 2016). If a person with cystic fibrosis was admitted for 17.8 days they would incur a cost of £7,334 for a single room and £4,895 for a ward bed. Even though the absolute charges would be inappropriate (and potentially overestimated) for UK NHS patients, there is no evidence to suggest that the relative numbers would differ greatly. Based on this, single rooms cost approximately 50% higher than a bed in a shared room. However, under a private market, there might be reason to suspect that third degree price discrimination is in place, if the single room market is relatively inelastic. To account for this uncertainty, a 50% uplift (£412 versus £275) and 25% uplift (£344 versus £275) using those figures reported by South Devon Healthcare NHS Foundation Trust has been explored in sensitivity analysis (Section K.12.7).
The Healthcare Premises Cost Guides 2010 help to estimate the cost of healthcare buildings at the strategic outline case stage and outline business case stage. Part of this guide costs rooms (public, clinic or staff spaces) within a department and provides a worked example to illustrate their applications. The facilities relevant to this review are presented in Table 59 for a bed and en suite room. These costs include construction, space and capital, but do not include staff or cleaning costs that are incurred once they are constructed. However, a single inpatient room is almost 20% higher than a ward bed which reflects the relative difference in construction costs reported by NHS Estates 2005 (14%).
Table 59Inpatient bed and bathroom costs
| Strategy | Cost (2010) | Cost (2015/16)a | Source |
|---|---|---|---|
| Inpatient en suite shower room | £16,488 | £18,231 | Healthcare Premises Cost Guides: In-patient shower room (en suite), 40 rooms @£659,520 |
| Shared inpatient shower room | £5,954 | £6,584 | Healthcare Premises Cost Guides: In-patient shower room, 2 rooms @£47,632, shared by 8 beds |
| Single inpatient room with en suite | £86,104 | £95,208 | Single inpatient room without en suite (£69,616) + inpatient en suite facilities on ward (£16,488) |
| Single inpatient room | £69,616 | £76,977 | Healthcare Premises Cost Guides: In-patient single-bed room, 40 beds @£2,784,640 |
| Standard shared ward bed | £58,624 | £64,823 | Healthcare Premises Cost Guides: In-patient multi bed room, 8 beds @£468,992 |
| Standard shared ward bed plus shared shower room | £64,578 | £71,406 | Standard shared ward bed (£58,624) + shared inpatient shower room (£5,954) |
- (a)
Inflator to 2015/16 prices 1.11, based on the hospital & community health services (HCHS) index (297.0 [2015/16 PPI] / 268.6 [2009/10 PPI])
The costs in Table 59 relate specifically to new builds and should not be used to estimate the cost of maintaining and providing the facilities once they are constructed. For this reason, the additional (incremental) cost (£189/year) of single rooms compared to beds in shared rooms estimated from NHS Estates 2005 has been used to inform the model in the base case. However, this input is tested in sensitivity analysis (Section K.12.7).
With regards to bathroom facilitates, no alternative sources to the Healthcare Premises Cost Guides 2010 were identified, except for those related to maternity. For example, East and North Hertfordshire NHS trust advertise a cost of £90/night for a side room without an en suite and £175/night for amenity rooms with an en suite. Similarly, Poole Hospital NHS Foundation Trust advertise a cost of £75/night for an amenity room without en suite facilities and £175/night for an amenity room with en suite facilities.
Due to the difficulty in estimating the cost of inpatient bathroom facilities, an analysis that identifies the additional cost that would be accepted given the incremental benefit and NICE’s cost-effective threshold has been undertaken.
K.12.5.2. Cost to treat infection
When the committee advised on the appropriate management strategies for each of the infections they considered the recommendations they made on antibiotics for the treatment of acute pulmonary infection or exacerbation for consistency. Drug costs are taken from the November 2016 BNF Drug Tariff, unless unreported and otherwise stated. People with cystic fibrosis who become infected with a pathogen are assumed to visit their specialist nurse (band 7) for a 20 minute consultation at the clinic at a cost of £43 (£130 per hour of patient contact PSSRU 2016) and receive microbiology tests at a cost of £17 (£8, NHS Reference Costs 2015/16, DAPS07, microbiology; £9 /10 minutes, Band 7 microbiologist, £54 /hour, PSSRU 2016).
In addition to the acquisition cost of drugs, the use of nebulisers for their delivery is associated with fixed costs related to equipment purchase and ongoing costs associated with maintenance. For example a Pari BOY mobile S nebuliser has an upfront cost of £285 (Table 36), but many people with cystic fibrosis would already possess a nebuliser for other treatments. Clinical experts advised Tappenden 2013 and Tappenden 2014 (see Section K.14.1) that the cost to cover replacement aerosol heads and filters would be approximately £200 /year. In the base case, it is assumed people with cystic fibrosis already possess a nebuliser, but a sensitivity analysis has been explored that includes this cost (Section K.12.7).
Intermittent B cepacia complex
The committee advised that the average person infected with intermittent B cepacia complex would receive 3 weeks of treatment with IV co-trimoxazole and ceftazadime, incurring a cost of £398 (Table 60).
Table 60Drug acquisition cost for intermittent B cepacia complex
| Drug (quantity, basic price) | Unit cost | Cost/day | Cost/week |
|---|---|---|---|
| Septrin for Infusion 80mg/400mg/5ml solution for infusion ampoules (10, £17.76)a | £1.78 | £10.66 | £74.59 |
| Ceftazidime 2g powder for solution for injection vials (10, £27.70)b | £2.77 | £8.31 | £58.17 |
| Total cost | NA | £18.97 | £132.76 |
- (a)
Cost taken from the November 2016 BNF, dose (bd) informed by the committee
- (b)
Cost taken from the November 2016 BNF, note the lowest cost brand has been used to inform the model, dose (2g tds) informed by the committee
The committee also noted that the course of IV treatment is usually administered in hospital, although it is possible for this treatment to be administered at home without healthcare professional supervision in a small number of cases. Following this, the committee agreed it was reasonable to assume 100% of people with intermittent B cepacia complex receive IV treatment as an inpatient (NHS Reference Costs 2015/16, WH50B, elective inpatient, £574 /day) leading to an average cost administration cost of £12,054 over 3 weeks.
Including the drug acquisition cost (£398), initial consultation cost (£60) and IV administration cost (£12,054), the cost applied in the model to treat intermittent B cepacia complex is £12,512.
Intermittent P aeruginosa
The committee advised that the average person infected with intermittent P aeruginosa would receive 12 weeks of treatment with oral ciprofloxacin and nebulised colistimethate sodium, incurring a cost of £579 (Table 61). The committee also noted that it would be reasonable to assume that the course of treatment is administered at home without health care supervision, following their initial consultation to confirm their pathogen (£60) and their subsequent visit for their first administration of treatment (£43).
Table 61Drug acquisition cost for intermittent P aeruginosa
| Drug (quantity, basic price) | Unit cost | Cost/day | Cost/week |
|---|---|---|---|
| Ciprofloxacin 250mg tablets (10, £0.74)a | £0.07 | £0.37 | £2.59 |
| Colomycin 2million unit powder for solution for injection vials (10, £32.40)b | £3.24 | £6.48 | £45.36 |
| Total cost | NA | £6.85 | £47.95 |
- (a)
NHS Electronic Drug Tariff November 2016, dose (400mg three times daily) informed by the committee
- (b)
Cost and dose (2MU twice daily) taken from the November 2016 BNF
Including the drug acquisition cost (£575) and initial consultation and administration (£103), the cost applied in the model to treat intermittent P aeruginosa is £678.
Chronic P aeruginosa
The committee advised that the average person infected with chronic P aeruginosa would receive monthly alternate treatment at home with colistimethate sodium and tobramycin indefinitely (Table 62). However, a sensitivity analysis has been conducted based on colistimethate sodium alone as many people with cystic fibrosis start chronic treatment on a single antibiotic.
Table 62Drug acquisition cost for chronic P aeruginosa
| Drug (quantity, basic price) | Unit cost | Cost/day | Cost/month |
|---|---|---|---|
| Bramitob® 300mg/4ml nebuliser solution 4ml ampoules (56, £1,187.00)a | £21.20 | £42.39 | £1,187.00 |
| Colomycin® 2million unit powder for solution for injection vials (10, £32.40)b | £3.24 | £6.48 | £181.44 |
- (a)
Cost and dose (300mg bd) taken from the November 2016 BNF
- (b)
Cost and dose (2MU bd) taken from the November 2016 BNF
People chronically infected with P aeruginosa would also experience exacerbations each year, requiring 2 weeks of IV treatment with 2 antibiotics to manage each exacerbation. The committee agreed that 2 exacerbations/year would be reasonable to inform the model, adding that around half of exacerbations seen in clinical practice are severe and managed on an inpatient basis, whilst the remaining would be less severe and managed on an outpatient basis. These assumptions reflect the participants included in the study by Thornton 2005 who estimated the cost of home-based and hospital-based treatment with IV antibiotics for respiratory exacerbations in adults with cystic fibrosis.
Over the 1 year study period, 116 participants received 454 courses of IV antibiotics. In 213 (46.9%) of these courses, the intention had been to treat the person with cystic fibrosis in hospital and in the other 214 (47.1%) courses, the intention had been to treat the person with cystic fibrosis at home. However, 71 (15.6%) of the courses included a mixture of home and hospital treatment.
The mean total length of courses classified as hospital treatment was 15 days, with a mean of 12 days in hospital and 3 days at home. On the other hand, the mean total length of courses classified as home treatment was 16 days, with a mean of 14 days at home and 2 days in hospital. In both cases, the mean length of treatment was approximately 2 weeks, reflecting the views of the committee.
Thornton 2005 calculated unit costs from the NHS Trust, their cystic fibrosis unit’s budget, the BNF and the hospital-supplied catalogue, for IV antibiotics, disposable equipment, home kits, laboratory testing, clinic appointments and hospital stays. Table 63 below shows the mean cost of treatment per participant with IV antibiotics over 1 year, based on all study participants, where 47 were treated at home, 51 were treated in hospital and 18 were treated in both settings.
Assuming each person with cystic fibrosis receives 3.9 courses (454 exacerbations / 116 people with cystic fibrosis) each year, the cost/course is £6,827. This cost was subsequently accepted by the committee as a representative approximation of the costs to manage exacerbations in people with cystic fibrosis. Combined with their assumption that people with chronic P aeruginosa experience 2 exacerbations each year in UK clinical practice today, the cost applied in the model is £13,654.
It is important to note that the models developed by Tappenden 2013 and Tappenden 2014 to inform NICE TA276 used asthma complications reported in NHS Reference Costs as a proxy for the cost of a cystic fibrosis related exacerbation (major exacerbation, £1,500; minor exacerbation, £403) which is substantially cheaper than the costs estimated by Thornton 2005. As a result, the committee agreed the cost based on Thornton 2005 may overestimate the cost for some people, whilst the cost used by Tappenden 210 and Tappenden 2014 may underestimate the cost for others, concluding that the cost to manage an exacerbation should be explored in sensitivity analysis.
Table 63Mean cost of resources/participant over 1 year (all participants, n=116) for respiratory exacerbations
| Resource | Cost |
|---|---|
| IV antibiotics and disposables | £8,974 |
| Home kits | £25 |
| Laboratory testing | £101 |
| Clinic appointments | £546 |
| Hospital stay | £8,856 |
| Total mean cost/patient, 2002 prices | £18,513 |
| Total mean cost/patient, 2015 pricesa | £26,626 |
| Total mean cost per exacerbationb | £6,827 |
- (a)
Inflator to 2015/16 prices 1.438, based on the hospital & community health services (HCHS) index (297.0 [2015/16 PPI] / 206.5 [2001/2 PPI])
- (b)
3.9 courses per patient
Based on the assumptions outlined above, the cost to treat a chronic infection with P aeruginosa is £22,106 /year (Table 64).
Table 64Cost to manage P aeruginosa
| Resource | Cost |
|---|---|
| Initial consultation | £60 |
| Drug cost to manage P aeruginosa | £8,392 |
| Cost to manage 2 exacerbations | £13,654 |
| Total cost | £22,106 |
Superinfection with chronic P aeruginosa
The committee advised that the average person infected with a superinfection with chronic P aeruginosa would receive monthly alternate treatment at home with colistimethate sodium and tobramycin indefinitely, but treatment could be escalated to nebulised aztreonam lysine. As with chronic infection, people with a superinfection would also experience exacerbations each year that would each require 2 weeks of IV treatment to manage each exacerbation. The committee agreed that superinfections would cause an additional 2 exacerbations/year, compared to chronic P aeruginosa alone, leading to a total of 4/year.
Based on the assumptions outlined above, the cost to treat a chronic infection with P aeruginosa is £35,760/year (Table 65).
Table 65Cost to manage superinfection with chronic P aeruginosa
| Resource | Cost |
|---|---|
| Initial consultation | £60 |
| Drug cost to manage P aeruginosa | £8,392 |
| Cost to manage 4 exacerbations | £27,308 |
| Total cost | £35,760 |
K.12.6. Health-related quality of life
The QALY is NICE’s preferred measure of benefit for economic evaluation. This is because it can be seen as a generic measure of health which allows a comparison across treatments which affect different dimensions of health.
The QALY reflects the 2 principle objectives of health care:
- increase longevity;
- increase quality of life.
Estimating a QALY involves placing a quality of life weight on a particular event. This quality weight lies between 0 and 1, where 1 denotes full or ‘perfect health’ and 0 denotes death.
In the model there are the following scenarios to consider when estimating the quality of life in people with cystic fibrosis:
- utility of people with cystic fibrosis in the absence of infection
- utility of people with cystic fibrosis who experience an intermittent B cepacia complex infection
- utility of people with cystic fibrosis who experience an intermittent P aeruginosa infection
- utility of people with cystic fibrosis who experience a chronic P aeruginosa infection
- utility of people with cystic fibrosis who experience a superinfection with chronic P aeruginosa
A separate systematic search to identify utility values for people with cystic fibrosis was not undertaken. Instead, a search was conducted on the CEA Registry (https://research.tufts-nemc.org/cear4/) using the terms “cepacia”, “pseudomonas”, “B.cepacia” and “P. aeruginosa” in July 2016. This search identified no studies with health states relevant to the pathogens of interest that could be used to inform the model with regards to a point estimate.
Quality of life in people with cystic fibrosis is linked loosely to their lung function, which declines over time, and the number and severity of exacerbations they experience (Solem 2014, Whitting 2014, Bradley 2010, Yi 2003). For these reasons, it was difficult to find a point estimate to inform the model.
For chronic P aeruginosa infection the committee stated that the impact on a person’s quality of life is often driven by their exacerbations, as people with cystic fibrosis adapt well to their condition. This was also demonstrated by the small differences in utility across the lung function strata (strata >40% FEV1%) in the literature.
The committee agreed that for chronic P aeruginosa infection and superinfection with P aeruginosa it would be reasonable to apply the decrements for exacerbations alone.
Tappenden 2013 and Tappenden 2014 applied disutilities associated with exacerbations from Bradley 2010. This observational study conducted at 5 UK hospitals, recruited 94 participants with cystic fibrosis aged ≥ 16 years, infected with P aeruginosa.
Participants included in the study, completed the EQ-5D. Those without an exacerbation had a baseline utility of 0.85 (95% 0.80 to 0.89).The disutilities incurred by an exacerbation, adjusted for the duration of exacerbations are presented in Table 66. Based on committee consensus that approximately half of exacerbations are minor and half are severe, the average disutility from an exacerbation is 0.095.
Table 66Exacerbation disutility, taken from Bradley 2010
| Health state | Disutility | SD |
|---|---|---|
| Severe exacerbation requiring hospitalisation | 0.174 | 0.341 |
| Mild exacerbation (no hospitalisation) | 0.015 | 0.048 |
| Disutility used in the model/exacerbation | 0.095 | NA |
If people with chronic P aeruginosa experience 2 exacerbations/ year this would incur a disutility of 0.19. Also, if superinfections incurred an additional 2 exacerbations/ year, 4 exacerbations would incur a disutility of 0.38.
For intermittent infections, the committee stated that colonisation (infection) with a pathogen has little impact on a person’s quality of life as they are often asymptomatic. The committee considered the burden of treatment and potential increase in exacerbations to estimate percentage decrements in quality of life. The resulting utility values applied in the model according to the pathogen are summarised in Table 67.
Table 67Utility values applied in the model
| Infection | Description of disutility | Disutility | Resulting utility |
|---|---|---|---|
| No infection | None | 0.00 | 0.85 |
| Intermittent BCC | 5% of utility without infection | 0.04 | 0.81 |
| Intermittent PA | 5% of utility without infection | 0.04 | 0.81 |
| Chronic PA | 2 exacerbations (50% severe, 50% minor) | 0.19 | 0.66 |
| Superinfection with chronic PA | 4 exacerbations (50% severe, 50% minor) | 0.38 | 0.47 |
BCC, B cepacia complex; PA, P aeruginosa
According to the committee, the pathogens under consideration can also affect the longevity of life as they increase the risk of death. Similarly to quality of life, the risk of morality depends on lung function, exacerbations, comorbidities and complications. The literature reflects those dependent factors which goes beyond the structure of this model. Based on their own clinical expertise, the committee provided the estimates in Table 68 to inform the model.
Table 68Probability of death from infection applied in the model (1 year)
| Infection | Probability of death |
|---|---|
| Intermittent BCC | 0.5% |
| Intermittent PA | 0.5% |
| Chronic PA | 1.0% |
| Superinfection with chronic PA | 2.0% |
BCC, B cepacia complex; PA, P aeruginosa
K.12.7. Sensitivity analysis
A series of sensitivity analyses were undertaken in order to test how sensitive the results were to uncertainty in individual parameters. Parameters varied in the sensitivity analysis were chosen on the basis of uncertainty in their estimation or the potential impact that they had on the results. The values varied, along with their rationale are shown in Table 69.
Table 69Description of sensitivity analysis, cross-infection
| Analysis, parameter(s) to be changed | Default parameter value | Value tested | Rationale |
|---|---|---|---|
| 1. Drug to treat chronic infection | Alternate treatment with tobramycin & colistimethate sodium | Colistimethate sodium | Treatment is usually initiated with a single antibiotic unless it is not suitable or has not worked well enough. |
| 2. Nebuliser cost | Not included | £200/year | Not all people with cystic fibrosis will possess a nebuliser to administer their treatment. |
| 3. Utility of intermittent infection | 5% | 10% | The committee agreed that a 5% decrement may underestimate the decrement for some people with cystic fibrosis who feel a greater burden from treatment or who are symptomatic. |
| 4. Cost to treat an exacerbation | £6,827 | 50% | Tappenden 2013 and Tappenden 2014 used asthma complications reported in NHS Reference Costs as a proxy which was substantially less, the committee agreed their cost underestimated the cost for the average person with cystic fibrosis but considered it was a useful scenario to explore. |
| 5. Service delivery model | Specialist Centre clinic | Shared Care clinic | 2 studies included children which may be managed under a Shared Care model which is more costly, both cost estimates reflect those estimated in the review on service configuration. |
| 6. Inpatient bed cost | Single rooms cost an additional £10.61/night compared to beds in shared rooms (NHS Estates 2005) | 50% and 25% uplift inferred from South Devon NHS charges | There is a large difference in the absolute cost difference between the sources identified. However, there is no reason to suggest that the relative difference charged for private patients is unreasonable (50% uplift) unless third degree price discrimination is applied to the private market; for this reason another scenario using a lower 25% uplift was explored. |
| 7. Drug to treat superinfection | Alternate treatment with tobramycin & colistimethate sodium | Nebulised aztreonam lysine | In some cases, treatment will be escalated to aztreonam on the assumption that the former became ineffective. |
| 8. Probability of infection | Table 53 and Table 54 | Value required to achieve an ICER of £30,000 | Model results are sensitive to the difference in infection incidence between 2 strategies and the ICER will change substantially when varied. Threshold analysis would be useful to quantify this uncertainty. |
In addition to the sensitivity analysis described, a number of ICERs will be produced for each strategy, as each strategy includes more than 1 study. As stated previously, studies within a strategy could not be meta-analysed due to heterogeneity. However, if those studies lead to similar estimates of cost-effectiveness, confidence in the generalisability of that strategy will increase.
Given that the values of model inputs were generally well known and one-way sensitivity analysis was undertaken to assess extreme scenarios, probabilistic analysis was not considered useful, particularly when the results were robust to those changes (Section K.12.9.2).
Moreover, the clinical evidence review did not always produce evidence which allowed a probability distribution of effect size (incidence) to be estimated. For this reason, threshold analysis on infection probabilities was conducted to address that uncertainty (Section K.12.9.2).
K.12.8. Model validation
Provided in K.15.
K.12.9. Results
If there is strong evidence that an intervention dominates the alternatives (that is, it is both more effective and less costly), it should normally be recommended. However, if 1 intervention is more effective but also more costly than another, then the ICER should be considered. Here the ICER is the difference in the mean costs divided by the differences in QALYs gained.
The cost-effectiveness of a healthcare intervention is determined by the opportunity cost of the health foregone on the basis that, with a fixed health care budget, any newly funded intervention would displace the least cost-effective treatment currently provided. In the UK, NICE typically uses a threshold of £20,000 to £30,000 per QALY as a benchmark for the opportunity cost of health foregone from the least cost-effective treatment currently provided on the NHS. It is important to note that NICE’s threshold represents the opportunity cost rather than a willingness to pay (WTP), although WTP is how it is often represented elsewhere.
An ICER below £20,000 would generally be considered cost-effective, whereas an ICER above £30,000 would generally not be considered cost-effective without additional justifications. The committee may want to consider:
- the degree of certainty around the ICER;
- limitations to the generalisability of the evidence for effectiveness;
- the assessment of the change in quality of life has been inadequately captured, and may therefore misrepresent, the health gain;
- if the intervention is an innovation that adds demonstrable and distinct substantial benefits that may not have been adequately captured in the measurement of health gain.
Section K.12.9.1 below presents the base case results whilst Section K.12.9.2 presents the results based on the sensitivity analysis outlined in Section K.12.7. The total costs and total QALYs represent those for the clinic when 250 people with cystic fibrosis enter the model, as the strategy would be implemented across the clinic as opposed to individual patients.
K.12.9.1. Base case
It is important to note that the total costs incorporate the cost of the strategy to prevent cross-infections with transmissible pathogens (Section K.12.5.1) plus the expected cost to treat the infection (Section K.12.5.2).
As the strategies are applied to a clinic that manages many people with cystic fibrosis the results are reported based on a clinic size of 250. Regardless of the number of people with cystic fibrosis, the incremental costs and QALYs will produce the same ICER, as the relative difference is the same.
Cohort segregation
Segregating a cohort would be considered cost-effective to prevent the transmission of chronic P aeruginosa, as the new strategy is less expensive and more effective than the old strategy, subsequently dominating the old strategy. The cost-effectiveness plane illustrated in Figure 7 shows that the ICERs for each study lie in the south-east quadrant of the cost-effectiveness plane.
However, cohort segregation to prevent intermittent P aeruginosa would not be considered cost-effective as the ICER is substantially greater than NICE’s threshold or dominated (less effective and more effective than no cohort segregation). This is illustrated in Figure 8 with points in the north quadrants above NICE’s advisory threshold of £20,000 per additional QALY.
The cost-effectiveness with regards to intermittent BCC is uncertain with ICERs above and below NICE’s threshold in Figure 9. Based on the probability of infection reported by Whitford 1995, cohort segregation is more effective and less expensive than no cohort segregation; however, according to France 2008, cohort segregation is less effective and more expensive which is expected given that the probability of transmission in France 2008 is greater following the new strategy (16.3% versus 3 to 5%).
Table 70Cohort segregation results (new strategy versus old strategy)
| Study | Pathogen | Total £ S1 | Total £ S2 | Total QALYs S1 | Total QALYs S2 | Inc. costs | Inc. QALYs | ICER |
|---|---|---|---|---|---|---|---|---|
| France 2008 | Intermittent BCC | £1,357,488 | £895,680 | 210.69 | 212.05 | £461,808 | −1.37 | Dominated |
| Whitford 1995 | Intermittent BCC | £914,521 | £1,050,954 | 212.26 | 211.50 | −£136,433 | 0.76 | Dominant |
| Federiksen 1999 | Intermittent PA | £1,116,939 | £827,033 | 210.00 | 208.79 | £289,906 | 1.20 | £241,185 |
| Lee 2004 (prevalence) | Intermittent PA | £1,137,291 | £814,992 | 208.66 | 209.58 | £322,299 | −0.92 | Dominated |
| Lee 2004 (incidence 1990 versus 2000) | Intermittent PA | £1,19,659 | £818,045 | 209.16 | 209.38 | £311,615 | −0.22 | Dominated |
| Lee 2004 (incidence 1990 versus average 1991–2000) | Intermittent PA | £1,144,923 | £818,045 | 208.16 | 209.38 | £326,879 | −1.22 | Dominated |
| Federiksen 1999 | Chronic PA | £1,639,441 | £1,870,334 | 207.60 | 202.84 | −£230,893 | 4.76 | Dominant |
| Hoiby 1989 | Chronic PA | £4,136,118 | £5,922,151 | 185.77 | 167.23 | −£1,786,033 | 18.54 | Dominant |
| Lee 2004 (prevalence) | Chronic PA | £2,079,079 | £2,117,784 | 203.75 | 200.66 | −£38,705 | 3.09 | Dominant |
BCC, B cepacia complex; ICER, idomncremental cost-effectiveness ratio; PA, P aeruginosa; QALYs, quality-adjusted life years; S1, new strategy; S2, old strategy
Protective equipment
Equipment such as masks, gloves and aprons are relatively inexpensive. According to Chen 2001 and Savant 2014 the probability of infection decreased following their new strategies that included protective equipment. It is important to note that Chen 2001 also cohorted their patients according to B cepacia infection; hence the cost of this strategy has also been included. Savant 2014 on the other hand, did not cohort their patients according to pathogen status, but explicitly stated that a “no-waiting” room policy was applied. Given that Chen’s strategy was a lot less costly than Savant’s with little differences in their effectiveness, Chen’s strategy would be preferable.
Both of these studies show that protective equipment dominates no protective equipment (more effective and less expensive). This is illustrated by the points in Figure 10 that lie in the south-east quadrant of the cost-effectiveness plane. However, it is important to note that it is unclear as to whether the addition of protective equipment is driving the reduction in transmissible pathogens.
Table 71Protective equipment results (new strategy versus old strategy)
| Study | Pathogen | Total £ S1 | Total £ S2 | Total QALYs S1 | Total QALYs S2 | Inc costs | Inc QALYs | ICER |
|---|---|---|---|---|---|---|---|---|
| Chen 2001a | Intermittent BCC | £878,272 | £1,045,827 | 212.40 | 211.52 | −£167,554 | 0.88 | Dominant |
| Savant 2014b | Intermittent PA | £61,116 | £78,016 | 208.49 | 207.38 | −£16,900 | 1.11 | Dominant |
BCC, B cepacia complex; ICER, incremental cost-effectiveness ratio; PA, P aeruginosa; QALYs, quality-adjusted life years; S1, new strategy; S2, old strategy
- (a)
Includes cohort segregation
- (b)
Does not include cohort segregation
Inpatient beds
It is clear that single rooms are more effective than beds in shared rooms as the ICERs lie in the east quadrants of the cost-effectiveness plane in Figure 11. However, cost-effectiveness depends largely on the cost used to inform the additional cost of single beds, relative to beds in shared rooms.
As stated in Section K.12.5.1 the additional cost of a single inpatient room compared to a bed in a shared room is uncertain. When the model is informed by the additional cost reported by NHS Estates 2005, single rooms dominate beds in shared rooms as they are more effective and less expensive with points in the south-east quadrant of the cost-effectiveness plane in Figure 11.
However, when the costs are based on the charges by South Devon NHS (50% greater) the ICERs are substantially above NICE’s advisory cost-effectiveness threshold of £20,000 per additional QALY.
When a 25% uplift is considered, the ICER based on France 2008 is dominant (single beds are less expensive and more effective) whereas the ICER based on Chen 2001 is above NICE’s upper threshold for cost-effectiveness. This is unsurprising given the greater reductions in transmission reported by France 2008 than Chen 2001 following the introduction of single rooms (16.3% to 2.9% versus 15% to 7%).
The total costs and QALYs for these comparisons are provided in Table 72. Estimating the cross-over point for a threshold of £20,000 per QALY, the additional cost of single rooms to be considered cost-effective would need to be less than £100/day for France 2008 and less than an additional £60/day for Chen 2001.
Table 72Single inpatient room versus ward bed results
| Study | Pathogen | Total £ S1 | Total £ S2 | Total QALYs S1 | Total QALYs S2 | Inc costs | Inc QALYs | ICER |
|---|---|---|---|---|---|---|---|---|
| NHS Estates 2005 costs (single rooms cost an additional £10/night relative to beds in shared rooms) | ||||||||
| Chen 2001 | Intermittent BCC | £1,113,792 | £1,316,823 | 211.72 | 210.83 | −£203,031 | 0.89 | Dominant |
| France 2008 | Intermittent BCC | £985,541 | £1,357,488 | 212.18 | 210.69 | −£371,947 | 1.49 | Dominant |
| 50% uplift (beds in shared rooms £275/night versus single rooms £413/night) | ||||||||
| Chen 2001 | Intermittent BCC | £1,678,452 | £1,316,823 | 211.72 | 210.83 | £361,629 | 0.89 | £406,154 |
| France 2008 | Intermittent BCC | £1,550,201 | £1,357,488 | 212.18 | 210.69 | £192,713 | 1.49 | £129,218 |
| 25% uplift (beds in shared rooms £275/night versus single rooms £344/night) | ||||||||
| Chen 2001 | Intermittent BCC | £1,373,627 | £1,316,823 | 211.72 | 210.83 | £56,804 | 0.89 | £63,798 |
| France 2008 | Intermittent BCC | £1,245,376 | £1,357,488 | 212.18 | 210.69 | −£112,112 | 1.49 | Dominant |
BCC, B cepacia complex; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years; S1, new strategy; S2, old strategy
Incomplete cohort segregation including en suite bathroom facilities versus no cohort segregation including shared bathroom facilities
The 2014 NICE Guidelines Manual advises that an intervention will generally be considered cost-effective if the ICER is £20,000 per QALY or less. In other words, the NHS is willing to pay up to at least £20,000 per QALY gained. The cost per QALY (ICER) is given by:
Incremental cost ÷ incremental QALY gain = incremental cost per QALY
Or, rearranging:
Incremental cost = incremental QALY gain × £20,000
To estimate the accepted additional cost for a strategy to be considered cost-effective, the first step is to calculate the incremental QALY gain of the strategies. If we look at intermittent P aeruginosa, the incremental QALY gain is 1.08 (212.50 – 211.42) for a clinic managing 250 people with cystic fibrosis.
Table 73 suggests what the new strategy can cost above the old strategy in order for the new strategy to be considered cost-effective. Hence, despite higher costs, a strategy could be considered cost-effective if those QALY gains can be achieved. This is not to say that a strategy is cost-effective, but rather it gives the relative cost that would be accepted given the current differential in QALYs and NICE’s willingness to pay threshold of £20,000.
Table 73Accepted additional cost of “bathroom facilities”/year based on the clinical effectiveness reported by Jones 2005
| Comparison | Pathogen | QALY gain | Accepted additional cost | Interpretation |
|---|---|---|---|---|
| Jones 2005 | Intermittent PA | 1.08/clinic (0.004/person) | £21,600/clinic | As long as en suite facilities cost the clinic less than an additional £21,600/year, en suite facilities would still be considered cost-effective relative to shared facilities |
| Jones 2005 (2000, 4.4% versus 2001, 3.3%) | Super infection with chronic PA | 1.05/clinic (0.004/person) | £21,000/clinic | As long as en suite facilities cost the clinic less than an additional £21,000/year, en suite facilities would still be considered costeffective relative to shared facilities |
| Jones 2005 (2000, 4.4% versus average 2001–03, 4.6%) | Super infection with chronic PA | −0.19/clinic (−0.001/person) | −£3,800/clinic | En suite facilities are less effective than shared facilities, as long as en suite cost the clinic less than £3,800/year than shared facilities, the cost saving could outweigh the QALY loss. The ICER would lie in the SW quadrant of the costeffectiveness plane |
ICER, incremental cost-effectiveness ratio; SW, south-west quadrant of the cost-effectiveness plane (less effective and less expensive than the comparator); PA, P aeruginosa; QALY, quality-adjusted life year
K.12.9.2. Sensitivity analysis
Sensitivity analysis regarding the cost of inpatient beds has already been reported in the previous sub-section for ease of reference with the base case. For Lee 2004, sensitivity analysis was only conducted on prevalence data since prevalence data was utilised in the clinical evidence review and the same ICERs were found for incidence data (dominated). Due to the large number of comparisons only ICERs are reported in Table 74.
Overall, the results were robust to the analyses undertaken.
Using a cheaper treatment to treat the infection (scenario 1) or reducing the cost to treat an exacerbation (scenario 4) favours the less effective strategy and increases the incremental cost between the old and new strategy. For Lee 2004 this increases the ICER from dominant to positive (£19,021 and £21,525, respectively). However, the ICERs are still around NICE’s advisory lower threshold for cost-effectiveness; hence, cohort segregation could still be recommended as a cost-effective strategy to prevent cross-infections.
Conversely, including the cost of a nebuliser (scenario 2) increases the cost of the less effective strategy, favouring the more effective strategy by decreasing the incremental costs. Given that the new strategy is more effective, the incremental cost decreases, producing a lower ICER, strengthening our decision that the new strategy is cost-effective. Overall, the results are not sensitive to the additional cost of a nebuliser.
Increasing the disutility from an intermittent infection (scenario 3) favours the more effective strategy by increasing the incremental QALYs. However, the ICER produced for Federiksen 1999 remains substantially above NICE’s upper threshold. All remaining comparisons were robust to a change in the disutility.
Shared Care is more expensive to provide than a Specialist Centre, consequently the cost of the new strategy increases when the former is assumed (if the same number of days are segregated in both models). However, given that the prevalence of P aeruginosa is lower in children than adults, less days at the clinic will be segregated under a Shared Care model. Overall, the service delivery model does not change the ICER from the base case.
Scenario 6 has not been undertaken in the model because the QALY gain from Jones 2005 have been used to estimate the acceptable additional cost given NICE’s threshold of £20,000 per QALY. However, it is clear that increasing the cost to treat the infection will favour the more effective strategy. This may reduce the incremental cost between en suite bathroom facilitates and shared bathroom facilitates increasing the likelihood that en suite bathroom facilities are cost-effective (given than en suite bathroom facilities are more effective).
Scenario 8 has identified the scope in the probability of infection (associated with the new strategy), required for an ICER to be on the boarder of NICE’s threshold for cost-effectiveness (£20,000 to 30,000 per QALY). The results of this analysis are illustrated in Figure 12, Figure 13, Figure 14 and Figure 15. Large changes in the probability (associated with the new strategy) from the base case, provide greater confidence in the base case ICER, as the ICER is unlikely to change when alternative, plausible probabilities are applied. Smaller changes on the other hand, show that the probability of infection is a key driver of cost-effectiveness.
From Figure 12 it is clear that substantial changes from the study are needed for intermittent P aeruginosa (Federiksen 1999, Lee 2004) to be considered cost-effective. It is important to note that absolute changes can appear small when considered on a 0 to 100% scale, but the relative changes from the base case probability are somewhat larger; for example, for Whitford 1995 a change in probability from 2.1% to 6.7% is over 3 times more likely, but only 4.5% points greater.
Those studies that require less than a 50% relative change from the base case which could potentially question the generalisability of the cost-effectiveness estimates in clinical practice include Lee 2004 (cohorts by pathogen, chronic infection) and Savant 2014 (protective equipment, intermittent B cepacia complex). However, the relative change required to be considered certain or uncertain, is subjective.
Table 74Description of sensitivity analysis (new strategy versus old strategy)
| Study/strategy | Pathogen | ICER | |||||
|---|---|---|---|---|---|---|---|
| Base case | 1. Drug to treat chronic infection | 2. Nebuliser cost | 3. Utility of intermittent infection | 4. Cost to treat an exacerbation | 5. Shared Care model | ||
| Cohort segregation | |||||||
| France 2008 | iBCC | Dominated | NA | NA | Dominated | NA | NA |
| Whitford 1995 | iBCC | Dominant | NA | NA | Dominant | NA | Dominant |
| Federiksen 1999 | iPA | £241,185 | NA | £236,693 | £123,541 | NA | NA |
| Lee 2004 (prevalence) | iPA | Dominated | NA | Dominated | Dominated | NA | Dominated |
| Federiksen 1999 | cPA | Dominant | Dominant | Dominant | NA | Dominant | NA |
| Hoiby 1989 | cPA | Dominant | Dominant | Dominant | NA | Dominant | NA |
| Lee 2004 (prevalence) | cPA | Dominant | £19,021 | Dominant | NA | £22,128 | Dominant |
| Protective equipment | |||||||
| Savant 2014 | iPA | Dominant | NA | Dominant | Dominant | NA | NA |
| Chen 2001 | iBCC | Dominant | NA | NA | Dominant | NA | NA |
| Inpatient beds | |||||||
| Chen 2001 | iBCC | Dominant | NA | NA | Dominant | NA | NA |
| France 2008 | iBCC | Dominant | NA | NA | Dominant | NA | NA |
iBCC, intermittent B cepacia complex; iPA, intermittent P aeruginosa; ICER, incremental cost-effectiveness ratio; NA, not applicable
Figure 12Threshold analysis, cohorts by pathogen, intermittent infection
Note: Excel produces negative percentages to obtain the requested ICERs, given that negative percentages are not plausible with regards to the incidence of infection, 0% is produced as the lower limit
K.12.10. Discussion
This is the first cost-effectiveness analysis of strategies to prevent the transmission of pathogens in cystic fibrosis. Using utilities and subsequently QALYs, as the measure of effectiveness incorporates changes in morbidity and mortality and enables comparisons across the studies that analyse different strategies and infections. This in turn, allows broad comparisons across all health care interventions provided by the NHS.
Despite clinics undertaking strategies to reduce the risk of transmissible pathogens, pathogens can be acquired outside the clinic, from the community. Only Chen 2001, Jones 2005 and France 2008 genotyped the participants in their studies. The findings from the remaining studies are less reliable as the pathogens may not be transmissible strains which may overestimate the risk of cross-infection. Furthermore, the pathogens assessed by the studies varied widely and a representative and pragmatic number of pathogens were included in the model that admittedly, may not follow their ideal treatment pathway. Despite this, the deviations were not considered large enough to warrant additional pathogens in the model.
In addition to genotyping participants, future research in this area that compares strategies targeted at populations, should consider a cluster RCT design to control for the prevalence of a pathogen and “contamination” across individuals. This would address those uncertainties inherent in individually RCTs and before and after type studies this review was exposed to.
The studies included in the clinical evidence review did not explicitly define how their strategies were undertaken which has to be acknowledged as a limitation that stems from insufficient reporting in the studies. Consequently, the groupings used in the model may be unrepresentative of the studies.. However, this enabled the committee to consider how those strategies would be implemented in UK centres today, to inform the cost of those strategies in the model. Given that the effects of those strategies were assumed to hold when those inputs were specified, the applicability of the analysis to inform changes to current UK practice was considered to increase.
For reasons previously outlined, the clinical evidence was not meta-analysed as the studies were too heterogeneous. Instead, each of the studies was used to provide a separate measure for cost-effectiveness (ICER) to provide a range of plausible ICERs, but those studies most applicable to UK clinical practice today, such as France 2008 and Jones 2005, should be given greater weight in decision making.
Individual facilities (rooms or bathrooms) can have a lower opportunity cost than shared facilities because they can be filled by anybody, regardless of their infection status. Conversely, shared facilities can have a higher opportunity cost if their supply exceeds their demand. As a result, clinics must take into account the expected prevalence of pathogens in the area they service, to reduce the opportunity cost of their layout; when building plans, or changes to their construction are proposed.
Assuming demand for inpatient care equals supply, the additional cost of single facilities compared to shared facilities, following their construction, is driven by additional cleaning costs and capital such as rent space and utility bills. This additional cost was sourced from NHS Estates 2005 (additional £10.61/day), but a 25% uplift and 50% uplift was explored due to the much higher costs charged by hospitals to the private market for single rooms.
The cost and hence cost-effectiveness of en suite bathroom facilities is uncertain. If en suite bathroom facilities are part of a single inpatient room, it is logical they should be used. However, according to the study by Jones 2005 undertaken in Manchester, not all rooms are designed with en suite facilities.
K.12.11. Conclusion
The economic model showed that cohort segregation was cost-effective compared to no cohort segregation, to reduce the transmission of chronic P aeruginosa according to 3 studies, but not intermittent P aeruginosa according to 2 studies. Lee 2004 assessed both intermittent and chronic P aeruginosa and found that a fall in the number of cases with chronic infection was associated with a rise in those classified as intermittent. As a result, the applicability of this finding to clinical practice, or the classification of “intermittent” used by the study, must be questioned.
The new strategies implemented by Savant 2014 and Chen 2001 that included protective equipment to prevent cross-infections were cost-effective (dominant), but this may not be driven by the addition of protective equipment as Chen 2001 also cohorted their participants according to B cepacia complex infection and Savant 2014 applied a “no-waiting” room policy. If the benefits from protective equipment are out-ruled, this increases the confidence in cohort segregation as a cost-effective strategy to prevent cross-infection.
Single rooms would be considered cost-effective compared to beds in shared rooms based on the additional cost reported by NHS Estates (£10.61/night) as they are the dominant strategy. Based on a threshold of £20,000 per QALY the accepted additional costs would be less than £100/night based on the reductions in transmission reported by France 2008 and less than £60/night for those reported by Chen 2001.
The cost-effectiveness of cohorting people with cystic fibrosis according to B cepacia status is uncertain as the studies lie in different quadrants on the cost-effectiveness plane. However, Chen 2001 compared a strategy that looked at single inpatient rooms to beds in shared rooms and cohorted people with cystic fibrosis according to B cepacia status. This strategy dominated the shared room strategy providing evidence that the combination of cohort segregation and individual segregation is cost-effective. France 2008 also looked at the same comparisons as Chen 2001 (according to B cepacia) reiterating that cost-effectiveness was increased when both strategies were undertaken.
Table 75 below presents the results from the model for each of the strategies according to each pathogen.
Table 75Summary of cost-effectiveness estimates, cross-infection
| Strategy | Infection with transmissible pathogen | |||
|---|---|---|---|---|
| Intermittent BCC | Intermittent PA | Chronic PA | Super infection with chronic PA | |
| Cohort segregation | Cost-effectiveness uncertain (dominant to dominated) | Not Cost-Effective (ICER £241,185 to dominated) | Cost-effective (dominant) | NC |
| Protective equipment | Cost-effective (dominant) | Cost-effective (dominant) | NC | NC |
| Single inpatient rooms versus beds in shared rooms | Cost-effective (dominant) | NC | NC | NC |
| Incomplete cohort segregation including en suite bathroom facilities versus no cohort segregation including shared bathroom facilities | NC | Cost-effective if en suite facilities cost less than an additional £21,600/clinic/year | NC | Cost-effectiveness uncertain |
BCC, B cepacia complex; ICER, incremental cost-effectiveness ratio; NC, not calculable; PA, P aeruginosa
Finally, the committee should consider strategies outside of this model that could be considered cost-effective. For example, strategies that incur negligible costs and time to follow, such as closing clinic room doors, should be recommended if they have a perceived benefit, as it is evident they are cost-effective.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 11.7.3 ‘Evidence to recommendations’.
K.13. Immunomodulatory agents
K.13.1. Literature review
No economic evaluations of immunomodulatory agents in the management of lung disease for people with cystic fibrosis were identified in the literature search conducted for this guideline. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
K.13.2. Background
The committee stated that it was crucial the adverse effects of immunomodulatory agents were taken into consideration when making their recommendations, as they may outweigh the benefits related to lung function or exacerbations they can provide. As a result, this question was prioritised for de novo economic modelling, to assess those trade-offs.
Interventions with insufficient clinical effectiveness data (budesonide, beclomethasone, omalizumab and IV methylprednisolone) have not been included in the model. For completeness, a cost description of all interventions specified in the protocol has been undertaken to aid consideration of the costs.
K.13.3. Resource and cost use
Drug acquisition costs are presented over a typical month of continued use. Basic prices are taken from the NHS Electronic Drug Tariff September 2016, unless unreported and otherwise stated.
Dosages reported by the BNF or the committee were the preferred costing method, because trial dosages my not reflect UK clinical practice. Moreover, not all interventions (budesonide, beclomethasone, omalizumab and IV methylprednisolone) have been included in the clinical evidence review. When ranges of dosages are reported according to either age or weight, a mid-point has been taken to represent the cost of a typical person within that population.
There is likely to be some on-going monitoring for all immunomodulatory agents. According to the committee this would involve a full blood count, renal function tests and liver function tests, but it would be reasonable to assume this is equivalent across all treatments, as there is no opportunity cost created by switching from one treatment to another.
K.13.3.1. Omalizumab
The dose of omalizumab received is based on the level of immunoglobulin E antibodies (IgE level) and bodyweight and is given as subcutaneous injections every 2 to 4 weeks, but the maximum recommended dose is 600 mg every 2 weeks (8× 150 mg prefilled syringe, £2,049 per month). Table 76 presents the recommended dosages taken from the Summary of Product Characteristics (SPC) for a 4 week administration schedule, and Table 77 presents the cost of syringes available. In addition to the acquisition cost, a day case visit would be required to administer the injection at a cost of £570 (NHS Reference Costs 2015/16, DZ19K, Other Respiratory Disorders with Single Intervention, with CC Score 0–4). The committee stated that a day case tariff would be appropriate as the patient may require access to a bed for the duration of the administration and would require observations before and after administration; hence, an outpatient clinic attendance would not be appropriate.
Table 76Omalizumab doses (milligrams per dose) administered by subcutaneous injection every 4 weeks
| IgE (IU/ml) | Bodyweight (kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| ≥20–25 | >25–30 | >30–40 | >40–50 | >50–60 | >60–70 | >70–80 | >80–90 | |
| ≥30–100 | 75 | 75 | 75 | 150 | 150 | 150 | 150 | 150 |
| >100–200 | 150 | 150 | 150 | 300 | 300 | 300 | 300 | 300 |
| >200–300 | 150 | 150 | 225 | 300 | 300 | 450 | 450 | 450 |
| >300–400 | 225 | 225 | 300 | 450 | 450 | 450 | 600 | 600 |
| >400–500 | 225 | 300 | 450 | 450 | 600 | 600 | NA | NA |
| >500–600 | 300 | 300 | 450 | 600 | 600 | NA | NA | NA |
| >600–700 | 300 | NA | 450 | 600 | NA | NA | NA | NA |
NA, not applicable administration every 2 weeks required
Table 77Drug acquisition cost of omalizumab
| Omalizumaba | Unit cost |
|---|---|
| Injection, 150 mg/mL, 0.5-mL (75-mg) prefilled syringe | £128.07 |
| Injection, 150 mg/mL, 1-mL (150-mg) prefilled syringe | £256.15 |
- (a)
Cost taken from BNF (not reported in the Electronic Drug Tariff)
K.13.3.2. Intravenous corticosteroids
The dose of IV methylprednisolone as an immunomodulatory agent for people with cystic fibrosis has been informed by the committee. Unlike oral corticosteroids and inhaled corticosteroids, IV methylprednisolone is administered monthly over 3 days.
For illustrative purposes, the cost of IV methylprednisolone calculated for 20kg and 60kg bodyweights are presented in Table 78. In addition to the acquisition cost, a day case visit would be required, each day to administer the injection, for the same reasons previously outlined for omalizumab.
Table 78Drug acquisition costs for IV corticosteroids
| IV Methylprednisolone (basic price, quantity) | Unit cost | Cost/montha | |
|---|---|---|---|
| 240mgb (approx. 20kg) | 720mgc (approx. 60kg) | ||
| 120mg/3ml suspension (£8.96, 1) | £8.96 | £53.76 | £161.28 |
| 40mg/1ml suspension (£3.44, 1) | £3.44 | £61.92 | £185.76 |
| 80mg/2ml suspension (£6.18, 1) | £6.18 | £55.62 | £166.86 |
- (a)
10–15mg/kg for 3 days (max. 1000 mg) repeated monthly
- (b)
240mg/administration, 720mg/month
- (c)
720mg/administration, 2,160g/month
K.13.3.3. Inhaled corticosteroids
In addition to the acquisition cost of inhaled corticosteroids, the use of inhalers or nebulisers for the delivery of immunomodulatory agents is associated with fixed costs related to equipment purchase and ongoing costs associated with maintenance and replacement parts. Tappenden 2013 and Tappenden 2014 (see Section K.14.1) included costs associated with nebuliser use when they constructed their economic model, based on expert opinion that it would cost approximately £100 per year to cover replacement heads and filters. The committee also advised that inhalers would be included in the majority of prescriptions, but if inhalers were not included, the cost would be negligible. For example, inhalers such as the Spacer cost of £7.73 (NHS Supply Chain 2015).
The committee advised that the dosages reported in the BNF for an indication of asthma would also apply when they are prescribed as immunomodulatory agents. The acquisition cost of budesonide, beclomethasone and fluticasone are presented in Table 79 according to age and method of inhalation.
Table 79Drug acquisition costs for inhaled corticosteroids
| Inhaled corticosteroid (basic price, dose) | Unit cost |
|---|---|
| Budesonide, by inhalation of dry powdera | |
| 100ug (£8.86, 200) | £0.04 |
| 200ug (£17.71, 200) | £0.09 |
| 400ug (£17.71, 100) | £0.18 |
| Population | Cost/month |
| Child 6 to 12 years, assume 400ug/day | £5.39 |
| Adult and child over 12 years, assume 800ug/day | £10.77 |
| Budesonide, by inhalation of nebuliser suspensionb | |
| 500ug/2ml nebuliser liquid unit dose vials (4×5) (£26.42, 20) | £1.32 |
| 1mg/2ml nebuliser liquid unit dose vials (£40.00, 20) | £2.00 |
| Population | Cost/month |
| Child 6 months to 12 years, assume 500ug/day | £40.16 |
| Adult and child over 12 years, assume 1mg/day | £60.80 |
| Beclomethasone dipropionate, by inhalation of dry powderc | |
| 100ug (£5.36, 100) | £0.05 |
| 200ug (£9.89, 100) | £0.10 |
| 200ug (£14.93, 200) | £0.07 |
| 400ug (£19.61, 100) | £0.20 |
| Population | Cost/month |
| Child under 12 years, assume 100ug tds | £4.89 |
| Adult and child over 12 years, assume 500ug/day (2× 200ug [quantity, 200] + 1× 100ug) | £6.17 |
| Fluticasone, Accuhaler®, by inhalation of dry powderd | |
| 50ug/blister with Accuhaler® device (£6.38, 60) | £0.11 |
| 100ug/blister with Accuhaler® device (£8.93, 60) | £0.15 |
| 250ug/blister with Accuhaler® device (£21.26, 60) | £0.35 |
| Population | Cost/month |
| Child 5 to 16 years, assume 100ug bd (2× 100ug) | £9.05 |
| Adult and child over 16 years, assume 500ug bd (4× 250ug) | £43.09 |
| Fluticasone, Evohaler®, by aerosol inhalatione | |
| 50ug/metered inhalation (£5.44, 120) | £0.05 |
| 125ug/metered inhalation (£21.26, 120) | £0.18 |
| 250ug/metered inhalation (£36.14, 120) | £0.30 |
| Population | Cost/month |
| Child 4 to 16 years, assume 100ug bd (4× 50ug) | £5.51 |
| Adult and child over 16 years, assume 250ug bd (2× 250ug) | £18.31 |
| Fluticasone, Nebules® (= single-dose units for nebulisation), by inhalation of nebuliser suspensionf | |
| 250ug/ml (£9.34, 10) | £0.93 |
| 1mg/ml (£37.35, 10) | £3.71 |
| Population | Cost/month |
| Child 4 to 16 years, assume 1mg bd (2× 1mg) | £227.09 |
| Adult and child over 16 years, assume 2mg bd (4× 1mg) | £454.18 |
Costs taken from the BNF (not reported in the Electronic Drug Tariff) ug, micrograms
- (a)
Child 6–12 years 100–400 micrograms bd, adjusted as necessary; alternatively, in mild to moderate asthma, 200–400 micrograms as a single dose each evening if stabilised on daily dose given in 2 divided doses. Adult and child over 12 years 100–800 micrograms bd, adjusted as necessary; alternatively, in mild to moderate asthma, 200–400 micrograms (max. 800 micrograms) as a single dose each evening if stabilised on daily dose given in 2 divided doses.
- (b)
Child 6 months–12 years 125–500 micrograms bd, adjusted according to response (max. 2 mg daily). Adult and child over 12 years 0.25–1 mg bd adjusted according to response (usual max. 2 mg daily, but higher doses may be used in severe disease).
- (c)
Standard dose for inhaled beclomethasone: Child under 12 years 100–200 micrograms bd. Adult and child over 12 years 100–400 micrograms bd.
- (d)
Child 5–16 years 50–100 micrograms bd adjusted as necessary; max. 200 micrograms bd. Adult and child over 16 years 100–500 micrograms bd, adjusted as necessary; max. 1 mg bd (doses above 500 micrograms bd initiated by a specialist).
- (e)
Child 4–16 years 50–100 micrograms bd adjusted as necessary; max. 200 micrograms bd. Adult and child over 16 years 100–500 micrograms bd adjusted as necessary; max. 1 mg bd (doses above 500 micrograms bd initiated by a specialist).
- (f)
Child 4–16 years 1 mg bd. Adult and child over 16 years 0.5–2 mg bd
Fluticasone, specifically by inhalation of dry powder, was the only inhaled corticosteroid included in the model as no clinical effectiveness data on the remaining inhaled corticosteroids in the protocol was identified.
Fluticasone by inhalation of dry powder was included in the NMA, based on the effectiveness reported by Boeck 2007. They administered 500ug bd which mirrors the dosages in the BNF (Table 79d).
K.13.3.4. Macrolides
According to the committee, azithromycin can be used as an immunomodulatory agent and as an antibiotic to prevent pulmonary bacterial colonisation with S aureus, using similar dosages:
- Bodyweight 25–40kg, 250mg 3 times/week;
- Bodyweight >40kg, 500mg 3 times/week.
Those dosages were also administered by the trials included in the NMA (Clement 2006 and Saiman 2010) and subsequently, the dosages in the model. Based on those dosages the cost/month is presented in Table 80.
Table 80Drug acquisition cost of azithromycin
| Azithromycin (basic price, quantity) | Unit cost | Cost/month ≤40kg | Cost/month >40kg |
|---|---|---|---|
| 250mg capsules (£15.13, 6) | £2.52 | £30.26 | NA |
| 250mg tablets (£1.38, 4) | £0.35 | £4.14 | NA |
| 500mg tablets (£1.34, 3) | £0.45 | NA | £5.36 |
K.13.3.5. Nonsteroidal anti-inflammatory drugs (NSAIDs)
The following dosages were provided by the committee to represent ibuprofen prescribed as an immunomodulatory agent for people with cystic fibrosis:
- Child 1 to 2 years, 50mg bd to qds (2 to 4 times daily);
- Child 3 to 7 years, 100mg bd to qds;
- Child 8 to 12 years, 200mg bd to qds;
- Adults, 400mg tds.
Similarly, the trials included in the NMA (Konstan 1995 and Lands 2007) administered 20 to 30 mg/ kg/ day to a maximum of 1600mg /day. All available preparations of oral ibuprofen are presented in Table 81 for dosages of 800mg/day and 1,200mg/day.
Table 81Drug acquisition cost of ibuprofen
| Ibuprofen (basic price, quantity) | Unit cost | Cost/month 800mg/day | Cost/month 1,200mg/day |
|---|---|---|---|
| 100mg/5ml oral suspension (£8.88, 500ml) | £0.09/5ml | £21.60 | £32.39 |
| 100mg/5ml oral suspension sugar free (£1.33, 100ml) | £0.07/5ml | £16.17 | £24.26 |
| 200mg tablets (£1.00, 24) | £0.04 | £5.07 | £7.60 |
| 200mg tablets (£3.50, 84) | £0.04 | £5.07 | £7.60 |
| 400mg tablets (£1.07, 24) | £0.04 | £2.71 | £4.07 |
| 400mg tablets (£3.75, 84) | £0.04 | £2.71 | £4.07 |
| 600mg effervescent granules sachets (£6.80, 20) | £0.34 | £20.67a | £20.67 |
| 600mg tablets (£5.66, 84) | £0.07 | £3.27b | £4.10 |
- (a)
costed using two sachets on the assumption that the remaining contents is discarded
- (b)
600mg plus 200mg
K.13.3.6. Oral corticosteroids
Two trials included in the clinical evidence review (prednisone, Auberch 1985; prednisolone, Greally 1984) administered up to 60mg of oral corticosteroids daily. However, the committee stated that in clinical practice today, the maximum dose is 40mg, due to concerns on drug toxicity.
The committee agreed that there was no evidence to suggest prednisolone and prednisone differ in their effects and given that prednisolone in its non-proprietary form, is a lot cheaper than prednisone, prednisolone is used to inform the model, to reflect the best price available to the NHS (NICE 2013 Guides to the methods of technology appraisal). Available preparations of prednisolone are presented in Table 82 for dosages of 30mg/day and 40mg/day.
Table 82Drug acquisition costs for oral corticosteroids
| Prednisolone (basic price, quantity) | Unit cost | Cost/month 30mg/day | Cost/month 40mg/day |
|---|---|---|---|
| 10mg tablets (£1.90, 30) | £0.06 | £5.78 | £7.70 |
| 20mg tablets (£3.80, 30) | £0.13 | £5.78 | £7.70 |
| 5mg gastro-resistant tablets (£1.21, 28) | £0.04 | £7.88 | £10.51 |
| 5mg soluble tablets (£53.48, 30) | £1.78 | £325.16 | £432.90 |
| 5mg tablets (£0.88, 28) | £0.03 | £5.67 | £7.56 |
| 5mg/5ml oral solution unit dose (10ml, £11.41) | £5.71/5ml | £1,041.50 | £1,388.67 |
| 10mg/ml oral solution sugar free (30ml, £55.00) | £1.83/ml | £166.90 | £222.93 |
K.13.4. Model structure
A decision analytic model was developed in Microsoft Excel® (2013) from the perspective of the UK NHS and using 2015/16 costs. The model takes the form of a state transition model to estimate transitions between 3 lung function (FEV1%) strata. Transition probabilities between these 3 strata are taken from the NMA using the trials identified in the clinical evidence review. The first cycle is 9 months long and subsequent cycles are 12 months, to reflect the short- and long-term NMA. The first cycle is 9, as opposed to 10 in the NMA protocol, given that the longest trial duration included in the short-term network was 6 months. It was considered plausible that those short term effects could last beyond the 6-month trial duration; hence, an assumption was made that they would last between 6 and 12 months, before the long-term effect commenced.
The model takes a lifetime horizon based on the assumption that immunomodulatory agents are given on this basis. People with cystic fibrosis enter the model aged 5 as the committee considered this to be the age when treatment is started in clinical practice.
Cost-effectiveness results should reflect the present value of the stream of costs and benefits accruing over the time horizon of the analysis. NICE considers that it is usually appropriate to discount costs and health effects at the same annual rate of 3.5%, based on the recommendations of the UK Treasury for the discounting of costs. Consequently the model has adopted a discount rate of 3.5% for both costs and benefits (QALYs), but this input can be varied by the user in the model.
During each cycle people with cystic fibrosis may remain in their current FEV1% state, or transition to a worsened FEV1% state, experience a treatment related adverse effect, or die. Additional costs and quality of life decrements are applied to people with cystic fibrosis who experience an exacerbation in the FEV1% health states. People with cystic fibrosis in the worst FEV1% health state (<40%) may undergo a lung transplant, subsequently they will go off-treatment.
A conceptual form of the model is presented in Figure 16 and a further description of the model health states is provided in Table 83.
Table 83Description of the health states included in the model
| Health state | Description |
|---|---|
| FEV1% >70 |
|
| FEV1% 40–70 |
|
| FEV1% <40% |
|
| Post lung transplant |
|
| Treatment related adverse event (TRAE) |
|
| Death |
|
BMD, bone mineral density; CF, cystic fibrosis; FEV, forced expiratory volume; NMA, network meta-analysis; NSAID, nonsteroidal anti-inflammatory drugs TRAE, treatment related adverse event
The treatment related adverse events included in the model were considered to be the most important adverse events that impact costs and quality of life to demonstrate the differences between treatments (Table 84). It assumed no adverse events are caused by inhaled corticosteroids (fluticasone).
Table 84Description of TRAEs included in the model
| Adverse event | Chronic | Mortality | Cost | Disutility | Treatment following TRAE |
|---|---|---|---|---|---|
| Oral corticosteroids (prednisolone) | |||||
| Reduced BMD | Yes | No | Yes (lifetime) | No | Oral corticosteroid |
| Cataracts | No | No | Yes (one-off) | Yes (one-off) | Oral corticosteroid |
| Diabetes | Yes | Yes | Yes (lifetime) | Yes (lifetime) | Oral corticosteroid |
| NSAIDs (ibuprofen) | |||||
| Abdominal pain | No | No | No | Yes (1 week) | Macrolide |
| Abdominal bleed | No | No | Yes (one-off) | Yes (4 weeks) | Macrolide |
| Renal impairment | Yes | Yes | Yes (lifetime) | Yes (lifetime) | Macrolide |
| Macrolides (azithromycin) | |||||
| Hearing impairment | No | No | Yes (one-off) | Yes (one-off) | None |
BMD, bone mineral density; NSAID, nonsteroidal anti-inflammatory drugs TRAE, treatment related adverse event
K.13.5. Resource and cost use included in the model
K.13.5.1. Drug acquisition costs
People with cystic fibrosis enter the model aged 5; hence it is important to consider their weight as they get older because this will influence the dose and hence cost of treatment they receive. Three of the 4 immunomodulatory agents included in the model rely primarily on body weight to determine the appropriate dose:
- azithromycin ≤40kg, 250mg; >40kg, 500mg;
- prednisolone 1–2mg/kg;
- ibuprofen 20–30mg/kg.
The CF Registry 2014 found in relation to UK growth data, that the median weight percentiles among children and adults (reported up to 19 years of age) with cystic fibrosis reduces, as age increases. Therefore general population estimates may overestimate the weight of people with cystic fibrosis, and more so as they age. In the absence of data, it is assumed the data at 19 years can be used to approximate adults over the age of 19. Even though their BMI may not differ, their weight (and height) would still be lower than average.
The median percentiles over the age of 15 years in the CF Registry 2014 were around the 30th percentile. The 30th percentile implies 30% of the general population at the same age are the same weight or lighter than someone with cystic fibrosis, 70% of the general population weigh more than someone with cystic fibrosis. In the model people with cystic fibrosis over the age of 15 are assumed to weigh 60kg based on the average 30th percentile reported in the GIRLSUK Growth chart 2–20 years and BOYSUK Growth Chart 2–20 years at 20 years of age. The weight associated with children aged 14 to 15 was also adjusted using equivalent methods.
For ages 5 to 13, the percentiles reported in the CF Registry 2014 were not considered large enough to warrant adjustments from the general population as the median percentile did not drop below 40. Table 85 below summarises the weight of children in the general population based on World Health Organisation (WHO) Child Growth Standards (50th percentile) and the median percentiles among children and adults (reported up to 19 years of age) with cystic fibrosis used to inform the body weights in the model.
Table 85Weight for age assumed in the model
| Age (years) | General population weight (kg, 50th percentile) | CF Registry 2014 median percentile | Weight (kg) used in the model |
|---|---|---|---|
| 5 to 6 | 18 | 41 to 46.9 | 18 |
| 7 to 9 | 23 | 50.3 to 48.8 | 23 |
| 10 to 11 | 34 | 41 to 44.8 | 34 |
| 12 to 13 | 43 | 45.6 to 47.8 | 43 |
| 14 to 15 | 53 | 40.2 to 35.6 | 50 |
| Adult male | 70a | 31.3b | 60c |
| Adult female | 58a | 28.1b | 60c |
- (a)
20 years used as a proxy for adults, maximum age reported
- (b)
19 years used as a proxy for adults, maximum age reported
- (c)
30th percentiles of 65kg and 54kg for males and females, respectively, providing an average of 60kg
The model also uses a lower dose of fluticasone in children under 16 years of age (200ug bd) to reflect prescribing practices. Table 86 below presents the acquisition cost of immunomodulatory agents for adults in the model, assuming 100% adherence to treatment.
More than 1 preparation of ibuprofen and prednisolone is available, and those preparations can vary in their cost. As outlined in the NICE 2013 Guide to the methods of technology appraisal, the reduced price should be used in the base-case analysis to best reflect the price available to the NHS. Therefore, the lowest cost preparation (tablets) has been used to inform the model for prednisolone and ibuprofen. For completeness, oral solution preparations of ibuprofen and prednisolone are explored in sensitivity analysis.
Table 86Drug acquisition costs included in the model, adults
| Antibiotic | Dose | Cost/day | Cost/month | Cost/year |
|---|---|---|---|---|
| Fluticasone by inhalation of dry powder | ||||
| 250 ug/blister with Accuhaler® device (£21.26/60, blister/£0.35) | 500ug bd | £1.42 | £43.09 | £517.04 |
| Prednisolone | ||||
| 5mg tablets (£0.88/28, £0.03) | 40mg /day | £0.25 | £7.56 | £90.68 |
| 10mg/ml oral solution sugar free (30ml/£55.00, 1ml/£1.83) | 40mg/day | £7.33 | £222.93 | £2,675.20 |
| Ibuprofen | ||||
| 400mg tablets (£1.07/24, £0.04) | 1,500mg/daya | £0.18 | £5.42 | £65.06 |
| 100mg/5ml oral suspension sugar free (100ml/£1.33, 5ml/£0.07) | 1,500mg/day | £1.00 | £30.32 | £363.89 |
| Azithromycin | ||||
| 500mg tablets (£1.34/3, £0.45) | 500mg 3/week | NA | £5.36 | £64.32 |
- (a)
25mg/kg = 25mg × 60kg. Costing based on 1,600mg/day on the assumption that tablets cannot be carried over
K.13.5.2. Treatment related adverse events
Oral corticosteroids (prednisolone)
Reduced bone mineral density (BMD)
The committee advised that people with reduced BMD would usually receive vitamin D supplementation and bisphosphonates. It is assumed people with cystic fibrosis receive 500 nanograms alfacalcidol daily and 35mg risedronate weekly to reflect the dosages reported in the BNF. People with reduced BMD are also assumed to visit their endocrinologist every 6 months and undergo a dual energy X-ray absorptiometry (DXA) scan once a year, leading to a total cost of £440.19/year (Table 87).
Table 87Cost of treating reduced BMD
| Service | Unit cost | Cost/year |
|---|---|---|
| Drug acquisition cost (quantity, basic price) | ||
| Risedronate 35mg (4, £1,13) | £0.28 | £14.69 |
| Vitamin D, alfacalcidol, 500 nanogram capsule (30, £4.64) | £0.15 | £56.45 |
| Monitoring cost | ||
| NHS Reference Costs 2015/16, DIAGIMDA, RD50Z, dexa scan | £68.29 | £68.29 |
| NHS Reference Costs 2015/16, WF01A Non-Admitted Face to Face Attendance, Follow-up, Endocrinology 302 | £150.38 | £300.76 |
| Total ongoing annual cost | NA | £440.19 |
People with reduced BMD are at higher risk of a bone fracture; however, the committee agreed that the incidence of fractures would be negligible if they are treated according to the suggested treatment schedule. Therefore it was agreed that fractures should not be added to the model.
Cataracts
A cataracts procedure, performed as a day case procedure costs £961 according to NHS Reference Costs 2015/16 (BZ31B). The committee advised that both eyes would usually be treated when the cause of cataracts is oral corticosteroid use and 2 separate operations (1 for each eye) would be carried out a few weeks apart to give the first eye time to heal and time for vision to return. As a result, the cost to perform the operation in both eyes is £1,922.
Diabetes
The Global Diabetes Community reported that annual inpatient care, to treat short and long term complications of diabetes, is estimated at between £1,800 and £2,500 per person. Whilst annual outpatient costs, which includes the cost of medications and monitoring supplies, is estimated at between £300 and £370 per person. However, the committee advised that people with cystic fibrosis related diabetes would be seen quarterly by a diabetes specialist with a knowledge of cystic fibrosis rather than their community GP. According to NHS Reference Costs 2015/16 the cost of a follow-up appointment with a multi-professional in diabetes medicine is £190 (WF02A, 307).
Based on the upper cost of annual inpatient care estimates from the global diabetes community (£2,500), assumed to represent the increased complexity of diabetes in cystic fibrosis and outpatient costs inferred by the committee (£570), the ongoing annual cost of diabetes included in the model is £3,070.
Macrolides (azithromycin)
Hearing impairment
It is assumed a hearing impairment is diagnosed following a consultation at a cost of £112 (NHS Reference Costs 2015/16, Non-Admitted Face to Face Attendance, First, WF01B, 120). It is assumed that the hearing impairment will be resolved once the drug is discontinued, hence no further costs are incurred.
NSAIDs (ibuprofen)
Renal impairment
Most often, the diagnosis of a renal impairment is made via a routine blood or urine test followed by a visit to a kidney specialist. The economic model for NICE CG169 on acute kidney injury assumed that people with moderate to severe disease incur the cost of 3-monthly consultations with a nephrologist and this would include an eGFR measurement. The cost of an eGFR measurement was considered to be the cost of lab resources combined with the cost of 5 minutes of phlebotomist time. In order to consider the proportion of people requiring diuretics the NICE CG169 committee assumed that 60% would be on 40mg of furosemide daily. Based on those assumptions the cost to manage renal impairments in the model is £638.08/ person/ year (Table 88).
Table 88Ongoing cost to manage renal impairment
| Resource | Source | Unit cost | Cost/year |
|---|---|---|---|
| Clinical Biochemistry | NHS Reference Costs 2015/16, DAPS04 | £1.18 | £4.72 |
| Phlebotomist | PSSRU 2016, Band 6, 5 minutes of fixed salary cost, £44/hour | £3.67 | £14.67 |
| Nephrology attendance | NHS Reference Costs 2015/16, Non-Admitted Face to Face Attendance, Nephrology 361, WF01A | £153.01 | £612.04 |
| Diuretics | 40mg furosemide daily | £0.03 | £6.65 |
| Total ongoing annual cost | £638.08 | ||
Abdominal bleed
Abdominal bleeds incur a one-off treatment cost of £1,406 based on the cost reported in NHS Reference Costs 2015/16 for a gastrointestinal bleed without Interventions (FZ38P, non-elective inpatient).
K.13.5.3. Exacerbations
NHS Reference Costs do not report costs specific to cystic fibrosis related exacerbations. As a result, Tappenden 2013 and Tappenden 2014 (see Section K.14.1) took the cost of asthma complications as a proxy to inform their economic model. They assumed that the Reference Costs (2010/11) for asthma with major complications without intubation (DZ15D, long stay, £1,500) reflected the cost of major exacerbations due to cystic fibrosis, whilst the cost of asthma complications without intubation (DZ15E, short stay, £403) reflected the cost of minor exacerbations. Table 89 below presents the latest currency codes related to asthma complications from NHS Reference Costs 2015/16.
Table 89Cost of treating exacerbations using asthma as a proxy
| Currency code description | National average cost |
|---|---|
| Non-elective, short stay, minor | |
| Asthma with Interventions, DZ15M | £765 |
| Asthma without Interventions, with CC Score 9+, DZ15N | £490 |
| Asthma without Interventions, with CC Score 6–8, DZ15P | £459 |
| Asthma without Interventions, with CC Score 3–5, DZ15Q | £436 |
| Asthma without Interventions, with CC Score 0–2, DZ15R | £413 |
| Non-elective, long stay, major | |
| Asthma with Interventions, DZ15M | £2,886 |
| Asthma without Interventions, with CC Score 9+, DZ15N | £2,310 |
| Asthma without Interventions, with CC Score 6–8, DZ15P | £1,750 |
| Asthma without Interventions, with CC Score 3–5, DZ15Q | £1,461 |
| Asthma without Interventions, with CC Score 0–2, DZ15R | £1,229 |
In light of those costs, the committee agreed that they would underestimated the cost of cystic fibrosis related exacerbations, as a cystic fibrosis related inpatient stay would often be longer and require more costly treatment. As a result, an alternative source for the cost to manage an exacerbation was sought.
As described in Section K.12.5.2 the committee agreed a cost of £6,827 estimated by Thornton 2005, was more appropriate, but lower costs to reflect the TAG’s analysis for NICE TA276 are explored in sensitivity analysis.
K.13.5.4. Lung transplant
Lung transplants were included in the model, even though these were not performed during the studies identified in the clinical evidence review. This was due to the short duration of the studies, but in real life it is likely that several people with cystic fibrosis will receive a lung transplant.
According to NHS Reference Costs 2015/16, the national average cost of a lung transplant is £39,689 (elective inpatient, DZ01Z).
The follow-up cost after a lung transplant were taken from a UK study (Anyanwu 2002) which reported the mean cost up to 15 years after lung transplant in 1999 UK pounds sterling at an annual discount rate of 6%.
Several sources, published and unpublished, were used to obtain their cost data. These included peer-reviewed literature (Sharples 2001), accounts departments in transplantation units, the Department of Health, the regional transplant coordinator’s office, and cost data from various health authorities.
Cost were adjusted to 2015/16 prices using the HCHS pay and price index reported by the PSSRU 2016, and corrected to the 3.5% inflation rate.
In the model it is assumed each person with cystic fibrosis undergoing a lung transplant survives for 5 years incurring a follow-up cost of £62,944, estimated from the sum of years 1 to 5, where years 4 and 5 are equal to year 3 (Table 90).
Table 90Lung transplant monitoring costs taken from Anyanwu 2002
| Post-transplant follow-up | Cost year 1999, discount rate 6% | Cost year 2015/16, discount rate 3.5%a |
|---|---|---|
| Year 1 | £14,818 | £24,395 |
| Year 2 | £5,824 | £9,820 |
| Year 3 | £5,358 | £9,576b |
| Year 4–10 | £26,263 | £48,073 |
| Year 11–15 | £10,818 | £20,280 |
| Total | £63,081 | £112,145 |
- (a)
Inflator to 2015/16 prices 1.646, based on the hospital & community health services (HCHS) index (297.0 [2015 PPI] / 180.4 [1999 PPI])
- (b)
Assumed to equal year 4 and year 5
K.13.6. Clinical effectiveness
K.13.6.1. Lung function
Natural history
It is well-documented that lung function declines over time in people with cystic fibrosis. The assumed natural rate of decline in lung function is age dependent and was taken from a large, prospective, multicentre, encounter-based, observational study of US and Canadian people with cystic fibrosis (N=4,161 adults, 1994–2005; N=1,359 children, 1997).
This study used repeated-measures, mixed-model linear regression analysis to assess risk factors for decline in FEV1% and estimate the mean rate of change in FEV1% across 2 age groups.
The mean change in FEV1% for the 18–24 year and ≥25 year groups over the observation period were −1.92% (95%CI −2.04 to −1.81) and −1.45% (95% CI −1.62 to −1.27) predicted per year, respectively.
The committee agreed that the decline in FEV1% is generally faster at higher levels (younger people). However, the committee noted that a decline of −1.45% per year may overestimate the decline in later years as this estimate includes young adults; despite this, the committee felt unable to provide a third category for older people with cystic fibrosis, subsequently agreeing that the results reported by Konstan 2012 would be reasonable to inform the model for all ages. For completeness, the committee tested alternate values in the model, but the difference in the results was found to be negligible.
Method
To adequately implement decline of FEV1% in the model the between-individual variability is required in addition to the uncertainty around the mean of the regression coefficient, to ensure that the model accounts for how much each individual varied in their decline over time. However, Konstan 2012 do not report this data; hence, it is assumed the 95% CI around the regression coefficient sufficiently accounts for both sources of variability through the use of a mixed effects model. In probabilistic analysis the starting FEV1% is varied to reflect between-individual variability, as it unlikely people with cystic fibrosis will have the same FEV1% value, and also to reflect the possibility that they could start with an FEV1% less than 70%.
The mean differences (MDs) from the NMA can be added to the mean baseline FEV1% to obtain the added benefit of each treatment. FEV1% predicted is assumed to follow a normal distribution with a standard deviation dependent on age, informed by the 95% CI around the regression coefficients reported by Konstan 2012.
The proportion of the population in each FEV1% strata for each treatment can then be estimated by calculating the proportion of the normal distribution that is above and below the cut-offs for the FEV1% strata.
Firstly, using the equation below, we can calculate the FEV1% CI for any age at each cycle:
- y= FEV1% for each cycle
- α = FEV1% upon model entry (assumed to be 94.4% at age 5 based on the UK CF Registry 2014)
- β = upper or lower CI
- x = cycle
For example, at 12 years of age the mean FEV1% for “no treatment” is 80.96, based on an annual mean decline of −1.92. Similarly, the 95% CI at 12 years is 80.12 to 81.73 using the upper (−2.04) and lower (−1.81) 95% CI annual declines in FEV1%, respectively.
Given that 95% of the area of a normal distribution is within 1.96 standard deviations of the mean, the difference between the mean and lower or upper CI can be divided by 1.96, to calculate the SD, which at 12 years of age is 0.43 ((80.96 – 80.12)/1.96)).
The number of SDs away from the 70% and 40% cut-offs for each FEV1% strata could then be estimated using:
(mean-SD)/cut-off
Using this formula, the number of SDs away from 70% and 40%, at 12 years of age are 25.6 and 95.6, respectively.
The normal cumulative distribution function at those values provides the probability of remaining above 70% and below (1-above) 40%. As the time horizon increases, the probability of remaining above 70% decreases, whilst the probability of remaining below 40% increases. During those cycles where both cut-offs have a 0% probability, patients remain in the 40 to 70% strata. This is illustrated in the (deterministic) trace for “no treatment” below.
Treatment effect
The MDs produced from the NMA are reported in Table 91 and illustrated in Figure 18 when they are applied to the natural history of FEV1%. Five studies of 511 participants were included in long-term the network, 3 of those reported data at 12 months (clement 206, De Boeck 2006 and Lands 2007), 1 at 48 months (Eigan 1995) and 1 at 4 years (Konstan 1995). Evidently, the extrapolation of this data to a lifetime horizon is considerably longer than the duration of the trials.
The MDs in FEV1% are assumed to continue indefinitely due to insufficient evidence to suggest otherwise. Ideally, future research in this area should consider longer follow-up times, where outcomes are measured at several intervals, to analyse if the treatment effect is independent of time. To explore the uncertainty surrounding the extrapolation of the 24-week efficacy data to a lifetime horizon, a “within-trial” analysis has been explored in sensitivity analysis.
There was insufficient data to estimate the short-term MD in FEV1% for prednisolone and ibuprofen. As a result it is assumed the long-term difference is equal to the short-term difference. Over a lifetime horizon, the short-term benefit is negligible in the model as only the long-term benefit is extrapolated. However, as previously stated, there is an option in the model to vary the time horizon which will put a higher weight on the short-term benefit when the duration is shortened.
Table 91MD in FEV1%, immunomodulatory agents
| Intervention | MD |
|---|---|
| First cycle, 9 months | |
| Oral corticosteroid (prednisolone) | 4.07a |
| Inhaled corticosteroid (fluticasone) | −2.00 |
| Macrolides (azithromycin) | 2.16 |
| NSAID (ibuprofen) | 2.26a |
| Subsequent annual cycles | |
| Oral corticosteroid (prednisolone) | 4.07 |
| Inhaled corticosteroid (fluticasone) | 7.17 |
| Macrolides (azithromycin) | −2.81 |
| NSAIDs (ibuprofen) | 2.26 |
MD, mean difference
- (a)
Assumed to equal long term in the absence of evidence
K.13.6.2. Lung transplant
Lung transplants were included in the model, even though these were not performed during the studies identified in the clinical evidence review. This was due to the short duration of the studies, but in clinical practice some people with cystic fibrosis will receive a lung transplant.
A person with cystic fibrosis is usually eligible for a lung transplant when their FEV1% falls below 30%. Based on this criteria, the UK CF Registry 2014 stated that 247 people with cystic fibrosis had been evaluated for bilateral lung transplants and 146 (59%) of those were accepted onto the transplant waiting list.
However, given that the worst FEV1% health state includes FEV1% up to 40%, the probability reported above would overestimate the number of lung transplants in the model. Following this, a probability of 0.92% (per 24 weeks) estimated on data from the CF Registry 2014 and data from the US Cystic Fibrosis Foundation by Tappenden 2013 and Tappenden 2014 associated with an FEV1<40%, has been applied in the model and adjusted to reflect a 1-year cycle (1.66%).
K.13.6.3. Exacerbations
In the model exacerbations do not form a separate health state, instead people with cystic fibrosis in each lung function health state will experience a number of exacerbations each cycle.
The number of exacerbations experienced by people receiving immunomodulatory agents was calculated by applying the appropriate rate-ratios (RRs) to the baseline number of exacerbations in the placebo arm. These RRs were estimated from the NMA.
The NMA generates a measure of treatment effect for each drug class relative to placebo. It was assumed that placebo could be used to represent a “no treatment” option. However, the event rate in the placebo arm varies considerably from trial to trial and may not necessarily reflect the current baseline risk in England and Wales.
Therefore, alternative sources of baseline data were sought. The baseline number of exacerbations for associated with placebo was taken from the CF Registry following a data request. The CF Registry receives the number of days in hospital and number of days on home IV antibiotics during 1 year. As a result, they advised that it would be reasonable to assume that each course of exacerbation treatment would last 14 days, either at home, or in hospital. The number of total hospital days averaged 17.78 days whilst the number of home days on treatment averaged 13.06 days, leading to 30.84 days of treatment a year, translating into approximately 2.20 exacerbation a year.
No data on the rate of exacerbations was identified from the clinical evidence review for ibuprofen. The committee agreed that ibuprofen is unlikely to reduce the rate of exacerbations compared to “no treatment”. Following this, the committee concluded that assuming equal effectiveness to placebo would be reasonable in the base case given that a threshold analysis to assess the number of exacerbations required to change their decision, or to reach a cost-effective decision in favour of ibuprofen is undertaken.
There was insufficient data to estimate the short-term rate of exacerbations for corticosteroids and ibuprofen. As a result, long-term data was used to inform the first cycle and subsequent cycles for all treatments in the model, including azithromycin. Using short-term data for azithromycin alone may introduce bias, as the committee believed treatments work better initially and the absence of evidence cannot infer lack of effect. Even though azithromycin was better than placebo in the short-term (RR 0.75, see Section 9.5.4.1.3 in the full guideline) it was less effective than the longer term data which the committee questioned. For these reasons, the short-term data on azithromycin was not used to inform the model.
Table 92Probability of exacerbations, immunomodulatory agents
| Comparison | Baseline number of exacerbations/cycle | RR | Number of exacerbations for the comparator treatment/cycle |
|---|---|---|---|
| First cycle, 9 months | |||
| Oral corticosteroids (prednisolone) versus placebo | 1.65 | 0.91a | 1.65 |
| Inhaled corticosteroids (fluticasone) versus placebo | 1.34a | 2.21 | |
| Macrolides (azithromycin) versus placebo | 0.44b | 0.73 | |
| NSAIDs (ibuprofen) versus placebo | 1.00c | 1.65 | |
| Subsequent annual cycles | |||
| Oral corticosteroids (prednisolone) versus placebo | 2.20 | 0.91 | 2.00 |
| Inhaled corticosteroids (fluticasone) versus placebo | 1.34 | 2.95 | |
| Macrolides (azithromycin) versus placebo | 0.44 (0.37d) | 0.97 (0.73) | |
| NSAIDs (ibuprofen) versus placebo | 1.00b | 2.20 | |
- (a)
Assumed to equal long term in the absence of evidence
- (b)
RR 0.79 estimated from the NMA rejected by the committee and assumed to equal the longer-term estimate of 0.44
- (c)
Exacerbation data not available, assumed to equal placebo in the base case
- (d)
Hazard ratio
Upon reflection of the NMA results, the committee regarded the time to next exacerbation for azithromycin (expressed as a hazard ratio at 6- and 12-month follow-up) as more reliable than the rate of exacerbations, particularly as the study reporting this outcome was of a higher quality than those used to estimate the rate of exacerbations. Given that a HR is linked to time it would be inaccurate to use the 6-month HR to inform the cycles in the model, but assumptions can be made to include the HR linked to a 12-month time frame.
At month 12, the time to remain free of exacerbations during the study was significantly longer in those receiving azithromycin (median time 8.7 months) than in those given placebo (median time 2.9 months); providing a HR of 0.37 (95% CI 0.22 to 0.63). This implies 37% as many people receiving azithromycin will have an exacerbation compared to those receiving placebo in the next unit of time. In other words, a HR of 0.37 is equal to a 63% reduction in the risk compared with someone in the placebo group.
Based on a 12-month time frame, those receiving azithromycin would experience 1.38 exacerbations whilst those receiving placebo would experience 4.14. Adjusting this result to the baseline rate (2.20 for placebo) azithromycin is associated with 0.73 exacerbations a year.
Hazards may vary with time, but the proportional hazard assumption means the proportion is constant. Given that we are extrapolating the long-term data to a lifetime horizon, assuming the treatment effect is maintained we can also assume proportional hazards. As a result, a scenario using the HR was explored for azithromycin, but the RR from the NMA was used to inform the base case.
Not all studies included in the clinical evidence review distinguished between minor and major exacerbations even though the severity of an exacerbation impacts the cost of treating the exacerbation and the person’s quality of life. Hence, for completeness the model allows a proportion of major and minor exacerbations to influence costs and utilities. Based on committee opinion, one half of exacerbations are major and require hospitalisation, whilst the remaining half are minor and treated on an outpatient basis.
K.13.6.4. Treatment related adverse events
The probability of treatment related adverse events included in the model are summarised in Table 93.
Table 93Probability of TRAEs included in the model
| TRAE | 1-year probability | Source |
|---|---|---|
| Oral corticosteroids (prednisolone) | ||
| Cataracts | 2.42% | Eigan 1995 (calculated from 1mg 4-year follow-up and 2mg 3-year follow-up) |
| Reduced BMD | 2.42% | Assumed to equal cataracts based on similar risks (very rare) reported in the eMC |
| Diabetes | 1.48% | Eigan 1995 (calculated from 1mg 4-year follow-up and 2mg 3-year follow-up) |
| Macrolides (azithromycin) | ||
| Hearing impairment | 1.10% | Saimen 2003 & Saimen 2010 |
| NSAIDs (ibuprofen) | ||
| Abdominal pain | 4.33% | Gabrial 1991 |
| Abdominal bleed | 0.70% | Lands 2007 |
| Renal impairment | 0.70% | Assumed to equal abdominal bleed based on similar risks (very rare) reported in the eMC |
BMD, bone mineral density; eMC, electronic Medicines Compendium; NSAID, nonsteroidal antiinflammatory drugs; TRAE, treatment related adverse event
Risks reported in the eMC were considered to underestimate the risk of the events when the drugs are given on a long-term basis and at a higher dose compared to the general population. On the other hand, the committee agreed that the relative difference between the events could be used to assume equivalent risks in the absence of data.
Oral corticosteroids (prednisolone)
Cataracts
In clinical practice people with cataracts may have to wait until both eyes are affected which can take several years. However, for simplicity, it is assumed cataracts is treated immediately and cannot occur more than once. This was considered as a reasonable assumption given the aims of the model and the importance of this adverse effect.
Eigan 1995 identified from the clinical evidence review conducted a RCT on 285 people with cystic fibrosis. At 4 years follow-up they found 3.2% probability of cataracts in participants who received 1mg prednisone and 11.6% probability at 3 years follow-up in participants who received 2mg prednisone. Translating these results into a 1-year probability results in values of 0.81% and 4.03%, respectively. Taking the average of these 2 results leads to an annual probability of 2.42%. Given the low probability of the event, relative to the number of cycles, this extrapolation was considered to be justifiable.
It is assumed that people who experience cataracts will remain on oral corticosteroid treatment.
Reduced BMD
According to the eMC, cataracts and reduced BMD are both very rare adverse events of prednisolone treatment. The literature was searched to inform the risk of BMD; however no relevant studies could be identified. Consequently the probability of reduced BMD was assumed to equal the probability of cataracts (2.42% per year).
It is assumed that people who experience reduced BMD will remain on oral corticosteroid treatment.
Diabetes
Eigan 1995 identified from the clinical evidence review conducted an RCT on 285 people with cystic fibrosis. At 4 years follow-up they found a 3.2% probability of diabetes in participants who received 1mg prednisone and a 6.3% probability at 3 years follow-up in participants who received 2mg prednisone. Translating these results into a 1 year probability results in values of 0.81% and 2.15%, respectively. Taking the average of these 2 results leads to an annual probability of 1.48%.
It is assumed that people who experience diabetes will remain on oral corticosteroid treatment.
Macrolides (azithromycin)
Hearing impairment
Seimen 2003 identified from the clinical evidence review conducted an RCT on 185 people with cystic fibrosis. At 6 month follow-up they found that 1.1% (1/87) of people treated with azithromycin experienced a hearing impairment and 1.1% (1/87) experienced tinnitus.
The committee stated that some people may remain on azithromycin treatment, or receive a lower dose, if their hearing impairment is tolerable. However, the committee agreed that this practice should not be followed without specialist advice, concluding that it would be reasonable to make the simplifying assumption that all people who experience hearing impairments go off-treatment in the model.
NSAIDs (ibuprofen)
Abdominal pain
As highlighted in the clinical evidence review on comorbidities, abdominal pain is more prevalent in people with cystic fibrosis than the general population, and the risk is increased with NSAID use. For economic modelling we are interested in the difference between treatments, i.e. the additional risk of abdominal pain from NSAID use compared to no NSAID use.
Konstan 1995 identified from the clinical evidence review conducted a RCT on 85 people with cystic fibrosis. At 3 years follow-up they found a 12.2% probability of abdominal pain in participants who received 20–30mg/kg ibuprofen and a 16.3% probability in those receiving placebo. Lands 2007 also identified from the clinical evidence review, conducted a RCT on 142 people with cystic fibrosis. At 2 years follow-up they found a 1.4% probability of abdominal pain in participants who received 20–30mg/kg ibuprofen and a 5.6% probability in those receiving placebo.
Those trials included in the clinical evidence review found no significant difference between participants receiving NSAID or placebo. This finding was not supported by the committee’s experience, or the increased risk of pain reported in the eMC for NSAIDs (uncommon: ≥1/1000 and <1/100).
Following this, the literature was searched to identify a study that aimed to assess the gastrointestinal effects of NSAID use. The RCT by Silverstein 2000 was subsequently identified where 8,059 participants were randomised to receive celeoxib 400mg bd or ibuprofen 800mg tds. During the 6-month treatment period 10.1% (321/3,169) participants receiving ibuprofen withdrew from treatment due to gastrointestinal adverse effects including abdominal pain, dyspepsia and constipation. Their analyses over 12-months using the cox proportional hazards models suggested a rate of 13.98 discontinuations due to gastrointestinal abdominal pain per 100 person-years. However, this study may overestimate the number of events when a lower dose is used for an immunomodulatory indication in people with cystic fibrosis (max. 1600mg/day versus 800mg bds).
A meta-analysis on adverse gastrointestinal events related to NSAID use by Gabriel 1991 was also identified. The overall odds ratio (OR) of the risk for adverse gastrointestinal events, summarised from 16 studies (9 case-control and 7 cohort) was 1.92 (95% Cl, 1.19 to 3.13) for more than 3 months of exposure. Applying this to the 1-year probability of abdominal pain found by Lands 2007 (2.84%) and Konstan 1995 (4.35%) led to 1-year probabilities of 5.95% and 9.57% from NSAID use.
In the model a health state for abdominal pain is only included for NSAID treatment; hence rates were calculated to estimate the probability of abdominal pain from NSAID use in the absence of cystic fibrosis. Following this, a 1-year probability of 4.33% was used to inform the model (Lands 2007: NSAID rate 0.061 – placebo rate 0.029).
It is assumed that people who experience abdominal pain will switch to macrolide (azithromycin) treatment.
Abdominal bleed
Lands 2007 identified from the clinical evidence review conducted a RCT on 142 people with cystic fibrosis. At 2 years follow-up they found a 1.4% probability of an abdominal bleed in participants who received 20–30mg/kg ibuprofen and no cases in those receiving placebo. Translating this result into a 1 year probability leads to a value of 0.7%.
Lesko and Mitchel 1995 was later identified through ad hoc searches in this area. They undertook a RCT to assess if ibuprofen increased the risk of hospitalisation among children. They found that 4 out of 55,785 participants receiving ibuprofen experienced an abdominal bleed (0.01%). However, the dose of ibuprofen was much lower (5–10mg/kg) than the dose used for an immunomodulatory indication in people with cystic fibrosis.
A third study by Silverstein 2000 was identified who found that 0.5% (20/3,981) of participants receiving NSAIDs experienced a gastrointestinal bleed over the 6-month treatment period leading to an annual probability of 1.00%. Other studies identified from ad-hoc searches and reference list searches that report the increased risk of bleeding compared from NSAID use include Garcia 1994, Perez 1997 and Henry 1996 who showed NSAIDs increased the risk of bleeds, but ibuprofen was one of the lowest NSAID risks.
Overall, given that Lands 2007 has a population relevant to the modelled population, the committee agreed a value of 0.7% would be reasonable to inform the model.
It is assumed that people who experience an abdominal bleed will switch to macrolide (azithromycin) treatment.
Renal impairment
As highlighted in the clinical evidence review on comorbidities, renal problems are more prevalent in people with cystic fibrosis than the general population, and the risk is increased with oral corticosteroid use. However, none of the studies included in the clinical evidence review on immunomodulatory agents reported the incidence of renal function; hence, the literature was searched.
Silverstein 2000 found that 1% (32/3,169) of participants receiving NSAIDs withdrew due to renal impairments during the 6-month treatment period translating into an annual probability of 1.99%. However, as stated previously, this study may overestimate the number of events as participants received a higher dose than that recommended for an immunomodulatory indication in people with cystic fibrosis.
Schneider 2006 undertook a matched case-control study in Canada on 121,772 new NSAID users >65 years from 1999 to 2002 to assess the association of NSAIDs with acute renal failure. They also found that NSAIDs increased the rate of acute renal failure with an adjusted rate of 2.30 for users compared to non-users.
Conversely, Lesko and Mitchel 1995 found that no participants receiving ibuprofen experienced acute renal failure (injury), but their dose of ibuprofen was much lower (5–10mg/kg).
Overall, given that the eMC reported both renal impairments and abdominal bleeds as very rare (<1/10,000) a probability of 0.7% was used to inform the probability in the model for both of those events.
It is assumed that people who experience a renal impairment will switch to macrolide (azithromycin) treatment.
K.13.7. Mortality
K.13.7.1. Cystic fibrosis related
The committee considered how mortality related to cystic fibrosis, could be influenced by exacerbations and lung function. However, the systematic review undertaken by Tappenden 2013 suggested that the evidence did not show that such a relationship would hold (Box 2). Given that there is no up-to-date evidence on such a relationship, alternative methods were sought.
Box 2Relationship between FEV1% and survival reported by Tappenden 2013
“On the basis of this review, it is reasonable to suggest that there exists Level 1/2 evidence to support the hypothesis that a change in FEV1% directly leads to a change in mortality, and therefore FEV1% alone is unlikely to represent a valid independent surrogate for patient survival. As such, the assumption of a direct linear relationship between FEV1% alone and mortality risk, without adjustment for other confounding factors, as assumed within the Forest Laboratories analysis, should be approached with considerable caution…
On the basis of the weaknesses in the evidence associated with the potential relationship between FEV1% and mortality (see Methodological issues surrounding the economic evaluation of cystic fibrosis treatments), this relationship was not considered within the Assessment Group model.”
No publications of complete survival curves for people with cystic fibrosis in England were identified. However, a manufacturer (Vertex) developed partial curves using data from the UK CF Registry, to derive background mortality hazard for people with cystic fibrosis in the UK, to inform survival in their submission for NICE TA398.
Due to complete survival data not being available in the UK CF Registry annual report (as data collection is ongoing) Vertex used parametric survival analysis to fit a parametric function to the observed curves from the registry, in order to extrapolate the survival over the entire lifespan of all members of the population. Within their submission, it is stated parametric survival analysis was conducted in accordance with NICE guidance on survival analysis in economic evaluations.
The analyses were based on the most recent published Kaplan-Meier curves of cystic fibrosis survival in the UK, which reported survival for 6,082 people grouped into birth cohorts ranging from 1980 to 2008. Various parametric functions were tested to arrive at the best parametric fit that is visually and statistically credible, as well as clinically plausible and the face validity of long-term projections was also considered.
The Weibull fit was considered to produce more plausible projections with the curve reaching 0% alive near 60 years of age, and a predicted median of 41 years and was subsequently chosen by Vertex, to inform survival in a UK population with cystic fibrosis. The coefficients of the Weibull function selected to conduct the analyses, reproduced from their submission and applied in the model developed for this review are summarised in Table 94 and illustrated in Figure 19.
Table 94Parameters for Weibull distribution used to derive CF survival projections, reproduced from Vertex’s submission for NICE TA398
| Parameter | Value/formula |
|---|---|
| λ | 3.938E-07a |
| γ | 3.2577 |
| S(t) | exp (-λt^γ) |
| h(t) | λγ^t-1 |
| H(t) | λt^γ |
- a)
corrected from 3.938E-06
Figure 19Survival projections using a Weibull distribution reproduced from Vertex’s submission for NICE TA398
K.13.7.2. Renal impairment
A cost-utility analysis, over a lifetime horizon, was developed by NICE CG169 on the prevention, detection and management of acute kidney injury. They took mortality associated with moderately reduced kidney function from the study by Eriksen 2006. This study provided age and sex-dependent standardised mortality ratios for people under 69 years of age. Because our model does not distinguish between genders the total ratio of 3.1 reported by the study will be multiplied by the age dependent mortality for cystic fibrosis.
K.13.7.3. Diabetes
No data specific to people with cystic fibrosis with diabetes was identified. As a result, The National Diabetes Audit Mortality Analysis 2012–2013 was retrieved who report age-specific mortality RRs for diabetes. The committee advised that diabetes in cystic fibrosis is neither type 1 nor type 2; hence the average RRs presented in Table 95 for type 1 and type 2 diabetes, combined, was used to inform the model.
Table 95Diabetes mortality rate
| Age | RR |
|---|---|
| 15–34 | 4.18 |
| 35–64 | 2.67 |
| 65–74 | 2.00 |
| 75–84 | 1.74 |
| 85 and over | 1.25 |
RR (rate ratio) for the general population equals 1
K.13.7.4. Lung transplant
Anyanwu 2002 undertook a cost-utility analysis of lung transplantation in the UK. They estimated survival data from the UK Cardiothoracic Transplant Audit 2000 and found lung transplants to provide an additional 2.5 (3.3) discounted (undiscounted) life years.
The latest audit on lung transplant survival was conducted on people who received a transplant between 1st July 1995 and 31st March 2012. The audit reported that the majority of children who received a lung transplant had cystic fibrosis. For this reason, the paediatric lung transplant mortality rates are reproduced in Table 96 from this audit, as opposed to the adult population.
Table 96Lung transplant mortality
| Years after LT | Survival | 95% CI |
|---|---|---|
| 0 | 100.0% | NA |
| 1 | 83.4% | 74.7% to 89.4% |
| 3 | 74.7% | 64.8% to 82.2% |
| 5 | 62.7% | 51.6% to 72.0% |
| 10 | 40.0% | 26.4% to 53.8% |
CI, confidence interval; LT, lung transplant; NA, not applicable
Due to the memoryless feature of the Markov model we cannot know when each person in the post-lung transplant health state received their transplant to implement the survival reported in Table 96. Consequently, a simplifying assumption was made with the committee to assume people with cystic fibrosis who receive a lung transplant survive for 5 years following their transplant.
K.13.8. Health-related quality of life
As stated in Section K.12.6, the QALY is NICE’s preferred measure of benefit for economic evaluation.
K.13.8.1. Lung function
Bradley 2010 was a health utility study in people with cystic fibrosis aged ≥ 16 years, infected with P. aeruginosa. This observational study conducted at 5 UK hospitals recruited 94 participants and classified them whether they had an exacerbation at the day of study entry. Participants included in the study performed spirometry tests for FEV1% and completed the CFQ-R and the EQ-5D questionnaire. These participants had a mean age of 28.5 years, and mean FEV1% of 58.7%; 50% were male and 91% were Caucasian.
Additional analyses to estimate the mean EQ-5D utility across 3 FEV1% strata (Table 97) was presented within the Novartis Pharmaceuticals submission for NICE TA276, as the utility values reported by Bradley 2010 related specifically to exacerbations (severe, mild or none) rather than FEV1% levels per se. These additional analyses were subsequently used to inform the economic model developed by the TAG (Tappenden 2013 and Tappenden 2014). However, it is important to note that these estimates were regarded as potentially unreliable by the TAG.
Table 97Utility values according to FEV1% used to inform Tappenden 2013 and Tappenden 2014
| FEV1% health state | Utility | SD |
|---|---|---|
| >70% | 0.864 | 0.165 |
| 40 to 70% | 0.810 | 0.216 |
| <40% | 0.641 | 0.319 |
To account for this uncertainty, alternative sources of utility values used by recent NICE TAs in people with cystic fibrosis are explored in sensitivity analysis.
K.13.8.2. Exacerbations
As described in K.12.6 the disutility incurred by the typical 2-week exacerbation is 0.095.
K.13.8.3. Lung transplant
Anyanwu 2001 used the EQ-5D to assess quality of life in UK participants before and after lung transplantation. They found that prior to transplant, utility on the waiting list was 0.31, increasing to 0.75 (0 to 6 months), 0.83 (7 to 18 months), 0.81 (19 to 36 months) and 0.82 (>36 months) over time. People with cystic fibrosis in the model are assumed to achieve the average utility reported by this study (0.83) until they die following the methods used by the models developed for NICE TA266 and NICE TA276.
K.13.8.4. Treatment related adverse events
The utility decrement is calculated by subtracting the utility value with the condition from the general population utility, where the general population utility reflects the age of study participants. Those population norms for the EQ-5D using the UK weighted index score were taken from Kind 1999 (Table 98).
Table 98Kind 1999 population norm EQ-5D values
| Age | Utility |
|---|---|
| 25–34 | 0.8684 |
| 35–44 | 0.8656 |
| 45–54 | 0.8203 |
| 55–64 | 0.7974 |
| 65–74 | 0.7732 |
| 75+ | 0.7366 |
Oral corticosteroids (prednisolone)
The disutility, source, and duration of disutility applied in the model for cataracts and diabetes are presented in Table 99.
Table 99Utility values applied to corticosteroid related adverse events
| Adverse event | Utility reported | Source | Participant age | Decrement applied in model | Duration of disutility |
|---|---|---|---|---|---|
| Cataracts | 0.821 | Van Gestel 2010 | 55 | −0.059 | 1 cycle |
| Diabetes | 0.81 | Lee 2011 | 38 | −0.056 | Lifetime |
Reduced BMD
The committee believed the majority of people with reduced BMD would not experience problems that affected their mobility or usual activities, and very few would be in pain or discomfort. Instead the disutility would be driven from the treatment they received, but this was also considered to be negligible. For this reason, a disutility has not been included in the model for this event.
Cataracts
The disutility applied for cataracts (−0.059) was obtained from Van Gestel 2010. This study used a multivariable linear regression model to estimate utility values using the National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) and Health Utilities Index Mark 3 (HUI3). These estimates were derived from observational research among 531 ocular hypertension and glaucoma patients in the University Eye Clinic Maastricht and 5 other Dutch ophthalmology centres.
This led to the following formula in their model:
- MD = mean deviation.
- SE = presence of side-effects, 0 = no, 1 = yes.
- Cataract = presence of cataract, 0 = no, 1 = yes.
Diabetes
The U.K. Prospective Diabetes Study (UKPDS) Group directly assessed utility scores in type 2 diabetes by applying the EQ-5D questionnaire. This source was used to inform the economic analysis in NICE CG87 and several other cost-utility analysis including Tunis and Minishall 2010, Tunis 2010, Pollock 2010, Farmer 2009. However, a utility value of 0.73 may be confounded in an older population (60 years), especially as the committee advised that diabetes in cystic fibrosis should not be classified into type 1 or type 2.
Lee 2011 was subsequently identified who asked 213 adults in the US with type 1 diabetes to self-report their health status using the time trade-off (TTO) and HUI. The disutility associated with diabetes in the model was estimated by subtracting the utility with diabetes estimated from the TTO (0.81) from the population norm according to the age of study participants (mean 38 years, 0.8656).
Ericsson 2013 was also identified who reported a baseline utility of 0.83 and 0.87 for type 1 and type 2 diabetes, respectively. This study was not used to inform the model as the characteristics of participants used to derive those estimate was not reported and could not be validated with the participants in the original study. However, this study provides evidence that the utility values are not substantially different between type 1 and type 2.
Overall, the disutility associated with diabetes in cystic fibrosis is unknown, and the committee should consider a research recommendation in this area to aid future cost-utility analysis in this population. Until such a study is published, the committee agreed a decrement of −0.056 would be reasonable to inform the model.
NSAIDs (ibuprofen)
Disutilities were applied to abdominal pain, abdominal bleed and renal impairments. The disutility, source, and duration of disutility applied within the model for each complication are presented in Table 100.
Table 100Utility values applied to NSAID related adverse events
| Adverse event | Utility reported | Source | Participant age | Decrement applied in model | Duration of disutility |
|---|---|---|---|---|---|
| Abdominal pain | NA | Dolan 1997 | NA | −0.002 | 1 week |
| Abdominal bleed | 0.54 | Lee 2013 | 52 years | −0.019 | 4 weeks |
| Renal impairment | 0.672a | Tajima 2010 | 61 years | −0.125 | Lifetime |
NA, not applicable; NSAID, nonsteroidal anti-inflammatory drugs
- (a)
adjusted value obtained from the analysis in CG192
Abdominal pain
The methodology used to estimate the disutility associated with abdominal pain was adopted from a model-based economic evaluations that were undertaken to inform relevant NICE diagnostics guidance. In those economic models, people who underwent a diagnostic test experienced some level of disutility due to the associated anxiety of waiting for test results; this disutility was imputed by using the EQ-5D health state valuation equation for the UK reported by Dolan (1997) which allows estimation of a person’s utility based on their responses to EQ-5D classification system. The system has 5 dimensions (mobility, self-care, ability to perform usual activities, pain/discomfort, and anxiety/depression) and in the version used by Dolan each dimension had 3 levels of response (no problems, moderate problems, and severe problems).
Those diagnostic evaluations used only the utility decrement due to anxiety/depression, but the same methodology can be used to estimate utility decrements due to abdominal pain. This can be is expressed by the following equation:
- α = 0.081 is the constant applied to any level of disutility in any of the 5 EQ-5D dimensions
- PD = 0.123 [for each level of disutility associated with pain/discomfort]
- P2 = 0.140 [for severe pain/discomfort]
- N3 = 0.269 [when any of the 5 dimensions of EQ-5D is severe]
It is assumed that people (with cystic fibrosis) already have a utility less than 1 (so the α value was not applied at the estimation of the utility decrement due to PD) and that they moved from a state of no pain/discomfort to moderate pain/discomfort resulting in a disutility of −0.123.
This disutility of was applied for only 1 week in the model, as clinical experts advised that abdominal pain is unlikely to last longer. This gave a 1-week disutility of −0.002 attributed to abdominal pain from NSAIDs.
Abdominal bleed
Given that no NICE guidance was identified to provide a disutility associated with an abdominal bleed, a search was undertaken in the CEA Tufts registry to identify utility values. A value of 0.54 assumed from McNamara 1997 was used by several studies including Pignone 2006, Pignone 2007 and Lee 2013. In the absence of bleeding Lee 2013 used a baseline utility of 0.79 derived from the EQ-5D taken from participants with a median age of 52 years. This value was applied to a 4-week cycle in their model.
The disutility for an abdominal bleed in the model was estimated by subtracting the utility associated with an abdominal bleed (utility 0.54) from the baseline utility in the absence of bleeding (0.79). To reflect a 4-week duration the 1-year disutility (0.25) is divided by 13 to produce a 4-week disutility of 0.019.
Renal impairment
A cost-utility analysis was developed for NICE CG169 on the prevention, detection and management of acute kidney injury. They identified a utility of 0.883 for CKD stages 3–4 in a Japanese study by Tajima 2010 and chose that study as it was the largest (n=569) EQ-5D based study for this indication. In order to make the utilities more relevant to a UK population, the utilities reported by Tajima 2010 were multiplied by the UK population utility averages from Kind 1999. To calculate the decrement the adjusted utility value (0.672) is subtracted from the utility norm of study participants (0.7974) to produce a decrement of −0.125.
Macrolides (azithromycin)
Hearing impairment
The disutility, source, and duration of disutility applied to a hearing impairment is presented in Table 101.
Table 101Utility values applied to macrolide related adverse events
| Adverse event | Utility reported | Source | Participant age | Decrement applied in model | Duration of disutility |
|---|---|---|---|---|---|
| Hearing impairment | 0.64 | Iris 2011 | 45–54 years | −0.18 | 1 cycle |
The TAG (PenTAG 2007) for NICE TA166 undertook a systematic search of the literature to identify studies that reported utility values with deafness, or living with cochlear implants. They concluded that the best study that estimates the utility associated with being a severely or profoundly deaf adult or with unilateral cochlear implantation in deaf adults is that by the UK Cochlear Implant Study Group. Table 102 below presents the utility values elicited values in that study from 311 deafened adults (mean age 50.8 years) who completed the HUI-3 instrument after cochlear implantation.
Table 102Mean utilities (measured using HUI-3) reported by the UK Cochlear Implant Study Group
| Type of candidate | Utility |
|---|---|
| All | 0.630 |
| All traditional candidates | 0.624 |
| Non-benefiting traditional candidates | 0.597 |
| Benefiting traditional candidates | 0.666 |
| All marginal hearing aid users | 0.645 |
| Non-scoring marginal hearing aid users | 0.627 |
| Scoring marginal hearing aid users | 0.676 |
Definitions: Traditional candidates – scored zero on BKB Sentence Test with each ear aided acoustically; Non benefiting traditional candidates – also no significant improvement on CUNY Sentence Test when lip reading was supplemented by acoustical aiding; Benefiting traditional candidates – also significant improvement on CUNY Sentence Test when lip reading was supplemented by acoustical aiding; Nonscoring marginal hearing aid users – were implanted in an ear which scored zero when aided; Scoring marginal hearing aid users – were implanted in an ear which scored above zero when aided, often their better ear.
Iris 2011 was also identified who administered the EQ-5D and HUI-3 to 429 people with tinnitus. Mean utility scores for EQ-5D (0.77; SD 0.22) and HUI-3 (0.64; SD 0.28) were significantly different. However, the HUI-3 is more responsive than the EQ-5D to hearing impairments, and therefore preferred in this population. Moreover a utility of 0.64 reflects the utility values found by the Cochlear Implant Study Group.
The disutility for a hearing impairment was estimated by subtracting the utility with a hearing impairment (0.64) from the population norm of study participants in Iris 2011 aged 45–54 years without a hearing impairment (0.8203).
K.13.9. Sensitivity analysis
K.13.9.1. Deterministic
A series of scenario analyses were undertaken in order to test how sensitive the results were to uncertainty in individual parameters. Parameters varied in the scenario analysis were chosen on the basis of uncertainty in their estimation or the potential impact that they had on the results. The values varied, along with their rationale are shown in Table 103.
Table 103Description of sensitivity analysis, immunomodulatory agents
| Analysis, parameter(s) to be changed | Default parameter value | Value tested | Rationale |
|---|---|---|---|
| 1 FEV1% utility values | Novartis analyses of Bradley 2010 | Solem 2014: FEV1%>70, 0.949 FEV1% 40–70, 0.918 FEV1% < 40%, 0.881 | Solem 2014 is a larger and more recent RCT that used data from a 48-week, Phase 3, multicentre study (STRIVE) to evaluate the relationship between EQ5D measures and FEV1% in 161 participants with CF. Solem 2014 was also used to inform a recent NICE TA398. |
| 2 Azithromycin exacerbation data | RR | HR | The committee regarded the time to next exacerbation for azithromycin (expressed as a HR) as more reliable than the rate of exacerbations, particularly as the study reporting this outcome was of a higher quality than those used to estimate the rate of exacerbations. |
| 3 Exacerbation cost | £6,827 | £1,220 | The cost used to inform the models developed for NICE TA276 based on asthma complications was a lot cheaper than the cost estimated from Thornton 2005. |
| 4 Number of baseline exacerbations reduced | 2.20/year inferred from the number of days in hospital and number of days on home IV antibiotics | 1.27/year inferred from the number of days in hospital | Not all home IV antibiotic treatment may relate to exacerbation treatment, so hospital treatment alone should be explored to assess the impact any potential inaccuracy. |
| 5 Ibuprofen preparation | Tablets | Oral solution | The best price available to the NHS is used to inform the base case, but more expensive preparations are available (NICE 2013 Guides to the methods of technology appraisal). |
| 6 Prednisolone preparation | Tablets | Oral solution | The best price available to the NHS is used to inform the base case, but more expensive preparations are available (NICE 2013 Guides to the methods of technology appraisal). |
| 7 Probability of ibuprofen TRAEs | Abdominal bleed 0.7% Renal impairment 0.7% | Probabilities doubled | The committee stated the risk of abdominal bleeds and renal impairments related to long-term ibuprofen use could be higher. |
| 8 No TRAE | Table 93 | 0% | To assess the impact of TRAEs |
| 9 Within-trial (time horizon reduced to minimise extrapolation) | Lifetime (60 years) | 2 cycles from age 12 | There is considerable uncertainty surrounding the extrapolation of trial data to a lifetime horizon. |
| 10 Exacerbation cost and disutility (minor) | £6,827 and −0.095 | £1,220 and −0.015 | This scenario would be less favourable to the more effective treatments. If those treatments are still cost-effective, we can have greater confidence in the decision. |
| 11 RR for azithromycin | 0.44 | 0.2, 0.4, 0.6, 0.8, 1 over a lifetime horizon | Potentially unreliable studies were included in the NMA, particularly for azithromycin. This analysis will determine the rate of exacerbations required to change our decision regarding the cost-effectiveness of azithromycin. |
CF, cystic fibrosis; FEV, forced expiratory volume; HR, hazard ratio, IV, intravenous; RCT, randomised controlled trial; RR, rate ratio; TA, technology appraisal; TRAE, treatment related adverse effect
K.13.9.2. Probabilistic
Probabilistic sensitivity analysis (PSA) was conducted in the model to take account of the simultaneous effect of uncertainty relating to model parameter values. Key parameters in the model relating to costs, utility values and clinic effectiveness, were varied by sampling from probability distributions. The model was run for 10,000 simulations to generate estimates of total costs and total QALYs for azithromycin, ibuprofen, prednisolone, fluticasone and “no treatment” by varying those parameters simultaneously. The model structure and model settings were kept constant.
A beta probability distributions was employed for probabilities and utilities, whilst a gamma or normal probability distribution was employed for costs. A recommended arbitrary starting point for unknown data was to assume the value of the SD will be 20% of the expected input parameter mean.
RRs and MDs, used to inform the model for exacerbations and FEV1%, respectively, were estimated from NMA performed in WinBUGS. Coda output from WinBUGs lists the values generated from the full posterior distribution which can be used to inform each PSA simulation. When coda output is used, it is important that the correlations in the parameter estimates are preserved. This was done by ensuring that all parameter values are sampled from the same Bayesian Markov chain Monte Carlo iteration. When the coda output is stored as separate columns for each parameter with iteration values along the rows, this corresponds to sampling all the parameter values in 1 row, each time.
NHS Reference Costs give a mean cost and an upper and lower quartile range (UQR and LQR). They also provide data on the number of data submissions on which these summary statistics are based. The NCC-WCH (now NGA) developed a spreadsheet tool which estimates parameters for a probabilistic sensitivity analysis. The ‘front end’ of this is shown below (Figure 20).
The user is asked to input the mean, UQR, LQR and number of data submissions for the cost to be sampled as part of a PSA. The user then hits the ‘run’ button and is asked for a low and high value for SD (e.g. £50 and £450). The user is then asked how many different SDs they wish to fit. The default assumes that the user will wish to try SDs at £1 intervals, and therefore in the example with a low SD of £50 and a high SD of £450, a total of 401 different SDs will be ‘fitted’.
The spreadsheet tool estimates which distribution out of log-normal, gamma or normal best fits the population distribution. For each distribution a ‘goodness of fit’ statistic is calculated for each fitted SD. For each distribution and SD the model calculates the inverse of the cumulative probability density function at a probability of 0.25 and 0.75, in order to indicate the actual UQR and LQR range associated with the fitted distribution.
The goodness of fit statistic is then estimated by summing the square of the difference between the actual UQR and the UQR of the fitted distribution and the actual LQR and the LQR of the fitted distribution.
The fitted distribution which has the lowest goodness of fit statistic is that which has the closest fit to the NHS Reference Cost data. This is done for the 3 types of distribution and the distribution which has the lowest goodness of fit is deemed to be the one that best matches the NHS Reference cost. The best fit distribution came therefore has a best fit SD.
Most NHS Reference Costs have a number of data submission points and therefore it is reasonable to assume according to central limit theorem that the sampling distribution is approximately normally distributed. Therefore, the PSA parameters estimated from the spreadsheet tool are normal distribution with a mean equal to the NHS Reference Cost mean and a standard error given by the best fit SD divided by the square root of the number of data submissions.
This tool was used to estimate PSA parameters for the cost of a lung transplant, visit for hearing impairments, cataracts procedure and abdominal bleed procedure based on the costs in NHS Reference Costs 2015/16 and the starting FEV1% value obtained from the CF Registry 2014. PSA parameters for the cost of an exacerbation based on the mean and range reported by Thornton 2005 were also estimated. To use this tool for a reported range, rather than IQR, the LQR (0.25) was replaced with 1/number of submissions and the UQR (0.75) with (number of submissions – 1)/number of submissions.
Parameters varied in PSA are provided in Table 104. Survival was not varied in PSA as no evidence was provided to allow a probability distribution of effect size to be estimated. The committee also agreed that the survival analysis used in the model was reflective of mortality to date.
An additional PSA was performed using asthma complications as a proxy for the cost to treat an exacerbation, to reflect the possibility of a cheaper input used by Tappenden 2013.
Table 104PSA parameters, immunomodulatory agents
| Parameter | Distribution | Mean | SD | Source |
|---|---|---|---|---|
| Costs | ||||
| Lung transplant procedure | Normal | £39,689 | £4,330a | NHSRC 2015/16 DZ01Z |
| Lung transplant monitoring | Gamma | £62,944 | £6,423b | Anyanwu 2002 |
| Exacerbation cost | Normal | £6,827 | £715a | Thornton 2005 |
| Exacerbation cost (minor for additional analysis) | Normal | £459 | £11 | Tappenden 2013 inflated to 2015/16 prices |
| Exacerbation cost (major for additional analysis) | Normal | £1,610 | £35 | Tappenden 2013 inflated to 2015/16 prices |
| Hearing impairment | Normal | £112 | £1a | NHSRC 2015/16 WF01B |
| Cataracts (per eye) | Normal | £961 | £2a | NHSRC 2015/16 BZ31B |
| Abdominal bleed | Normal | £1,406 | £3a | NHSRC 2015/16 FZ38P |
| Reduced BMD | Gamma | £440 | £45b | committee opinion |
| Renal impairment | Gamma | £638 | £65b | NICE CG169 |
| Diabetes | Gamma | £3,070 | £313b | Global Diabetes Community, committee opinion |
| Utility | ||||
| Disutility minor | Beta | 0.015 | 0.048 | Bradley 2010/Tappenden 2013 |
| Disutility major | Beta | 0.174 | 0.341 | Bradley 2010/Tappenden 2013 |
| FEV1% >70 | Beta | 0.864 | 0.165 | Bradley 2010/Tappenden 2013 |
| FEV1% 40–70 | Beta | 0.810 | 0.216 | Bradley 2010/Tappenden 2013 |
| FEV1% <40 | Beta | 0.641 | 0.319 | Bradley 2010/Tappenden 2013 |
| Lung transplant | Beta | 0.830 | 0.180 | Anyanwu 2001 |
| Hearing impairment | Beta | 0.640 | 0.280 | Iris 2011 |
| Cataracts | Beta | 0.821 | 0.083b | Van Gestel 2010 |
| Diabetes | Beta | 0.810 | 0.250 | Lee 2011 |
| Abdominal pain | Beta | 0.002 | 0.001b | committee opinion |
| Abdominal bleed | Beta | 0.540 | 0.055b | Lee 2004 |
| Renal impairment | Beta | 0.672 | 0.027 | NICE CG169/Tajima 2010 |
| Lung function (FEV1%) | ||||
| Starting FEV1% for natural history | Normal | 94.4 | 15.11a | CF Registry 2014 |
| Probabilities | ||||
| Exacerbation data | NA | NA | NA | NMA coda output |
| FEV1% data | NA | NA | NA | NMA coda output |
| Cataracts | Beta | 2.42% | 0.25%b | Eigan 1995 |
| BMD | Beta | 2.42% | 0.25%b | Assumption |
| Diabetes | Beta | 1.48% | 0.15%b | Eigan 1995 |
| Hearing impairment | Beta | 1.10% | 0.11%b | Saimen 2003 & Saimen 2010 |
| Abdominal pain | Beta | 4.33% | 0.44%b | Gabrial 1991 |
| Abdominal bleed | Beta | 0.70% | 0.07%b | Lands 2007 |
| Renal impairment | Beta | 0.70% | 0.07%b | Assumption |
| Lung transplant | Beta | 1.66% | 0.17%b | Tappenden 2013 |
BMD, bone mineral density; CG, clinical guideline; CF, cystic fibrosis; FEV, forced expiratory volume; NHSRC, NHS Reference Costs; SD, standard deviation; TA, Technology Appraisal; PSA, probabilistic sensitivity analysis
- (a)
Estimated using the spreadsheet tool that identifies the best fitting SD according to the range and number of data submissions
- (b)
When the evidence did not allow a probability distribution of effect size to be estimated an arbitrary starting point was to assume the value of the SD will be 20% of the expected input parameter mean.
K.13.10. Model validation
Provided in K.15.
K.13.11. Results
A discussion of NICE’s threshold for cost-effectiveness and uncertainty regarding the estimate is provided in K.12.9.
K.13.11.1. Base case
When comparing multiple mutually exclusive options, a fully incremental approach should be adopted that compares the interventions sequentially in rank order of cost.
When a fully incremental analysis is performed the interventions are sequentially ranked in order of cost from the least expensive (azithromycin) to the most expensive (fluticasone). Interventions that are followed by more expensive and less effective alternatives are excluded as they are dominated. ICERs are then re-calculated for the remaining interventions. However, azithromycin dominates all of the alternative treatments in the base case, so no ICERs are calculated (Table 105).
Table 105Base case results, immunomodulatory agents
| Treatment | Total costs | Total QALYs | ICER |
|---|---|---|---|
| Macrolide (azithromycin) | £158,404 | 14.20 | - |
| Oral corticosteroid (prednisolone) | £289,619 | 12.53 | Dominated |
| NSAID (ibuprofen) | £291,035 | 12.26 | Dominated |
| “No treatment” | £302,045 | 12.37 | Dominated |
| Inhaled corticosteroid (fluticasone) | £411,046 | 11.11 | Dominated |
ICER, incremental cost-effectiveness ratio; NSAID, nonsteroidal anti-inflammatory drugs; QALYs, qualityadjusted life years
Azithromycin is the cheapest and most effective treatment in the base case, consequently dominating the alternatives. Despite decreasing lung function, azithromycin incurs a relatively cheap acquisition cost and relatively low rate of exacerbations compared to the other treatments in the model.
Fluticasone has the most expensive acquisition cost out of the treatments included in the model. Although it is not associated with any adverse events and improves FEV1% by a greater amount than the other treatments, the RR of exacerbations compared to “no treatment” is high. Given that exacerbations that have a cost and QALY impact it is unsurprising fluticasone is the most expensive and least effective treatment in the model.
The results for ibuprofen are based on an exacerbation rate equal to “no treatment” (RR 1) due to insufficient data to suggest otherwise. For this reason, it is unsurprising “no treatment” produces more QALYs than ibuprofen, given that ibuprofen is associated with adverse events that negatively impact morbidity and mortality. For ibuprofen to be considered cost-effective under a threshold of £30,000 per additional QALY relative to azithromycin, the long-term exacerbation RR would need to equal azithromycin (0.44). In other words, ibuprofen would need to reduce exacerbations, at the same rate as azithromycin.
In Figure 21, the point estimates for all remaining agents lie in the north-west quadrant as they are less effective and more expensive than azithromycin (dominated).
K.13.11.2. Deterministic sensitivity analysis
Table 106Results of SA, immunomodulatory agents
| Treatment | Total costs | Total QALYs | ICER |
|---|---|---|---|
| 1: FEV1% utility | |||
| Macrolide (azithromycin) | £158,404 | 16.42 | - |
| Oral corticosteroid (prednisolone) | £289,619 | 14.57 | Dominated |
| NSAID (ibuprofen) | £291,035 | 14.32 | Dominated |
| “No treatment” | £302,045 | 14.52 | Dominated |
| Inhaled corticosteroid (fluticasone) | £411,046 | 13.11 | Dominated |
| 2: Azithromycin exacerbation data | |||
| Macrolide (azithromycin) | £132,480 | 14.56 | - |
| Oral corticosteroid (prednisolone) | £289,619 | 12.53 | Dominated |
| NSAID (ibuprofen) | £291,035 | 12.26 | Dominated |
| “No treatment” | £302,045 | 12.37 | Dominated |
| Inhaled corticosteroid (fluticasone) | £411,046 | 11.11 | Dominated |
| 3: Exacerbation cost | |||
| Macrolide (azithromycin) | £33,076 | 14.20 | - |
| NSAID (ibuprofen) | £55,785 | 12.26 | Dominated |
| “No treatment” | £57,220 | 12.37 | Dominated |
| Oral corticosteroid (prednisolone) | £67,160 | 12.53 | Dominated |
| Inhaled corticosteroid (fluticasone) | £81,318 | 11.11 | Dominated |
| 4: Number of baseline exacerbations reduced | |||
| Macrolide (azithromycin) | £93,787 | 15.10 | - |
| NSAID (ibuprofen) | £169,745 | 13.95 | Dominated |
| Oral corticosteroid (prednisolone) | £174,924 | 14.13 | Dominated |
| “No treatment” | £175,818 | 14.12 | Dominated |
| Inhaled corticosteroid (fluticasone) | £241,045 | 13.48 | Dominated |
| 5: Ibuprofen preparation | |||
| Macrolide (azithromycin) | £158,404 | 14.20 | - |
| Oral corticosteroid (prednisolone) | £289,619 | 12.53 | Dominated |
| NSAID (ibuprofen) | £293,107 | 12.26 | Dominated |
| “No treatment” | £302,045 | 12.37 | Dominated |
| Inhaled corticosteroid (fluticasone) | £411,046 | 11.11 | Dominated |
| 6: Prednisolone preparation | |||
| Macrolide (azithromycin) | £158,954 | 14.20 | - |
| NSAID (ibuprofen) | £291,035 | 12.26 | Dominated |
| “No treatment” | £302,045 | 12.37 | Dominated |
| Oral corticosteroid (prednisolone) | £340,317 | 12.53 | Dominated |
| Inhaled corticosteroid (fluticasone) | £411,046 | 11.11 | Dominated |
| 7: Probability of ibuprofen TRAEs increased | |||
| Macrolide (azithromycin) | £158,404 | 14.20 | - |
| NSAID (ibuprofen) | £287,655 | 12.08 | Dominated |
| Oral corticosteroid (prednisolone) | £289,619 | 12.53 | Dominated |
| “No treatment” | £302,045 | 12.37 | Dominated |
| Inhaled corticosteroid (fluticasone) | £411,046 | 11.11 | Dominated |
| 8: No TRAEs | |||
| Macrolide (azithromycin) | £138,541 | 14.48 | - |
| Oral corticosteriod (prednisolone) | £277,353 | 12.80 | Dominated |
| No treatment | £302,045 | 12.37 | Dominated |
| NSAID (ibuprofen) | £302,255 | 12.37 | Dominated |
| Inhaled corticosteriod (fluticasone) | £411,046 | 11.11 | Dominated |
| 9: Within-trial | |||
| Macrolide (azithromycin) | £9,583 | 1.31 | - |
| Oral corticosteroid (prednisolone) | £24,914 | 1.13 | Dominated |
| NSAID (ibuprofen) | £25,514 | 1.13 | Dominated |
| “No treatment” | £27,005 | 1.10 | Dominated |
| Inhaled corticosteroid (fluticasone) | £37,072 | 0.98 | Dominated |
| 10: Exacerbation (minor) | |||
| Macrolide (azithromycin) | £33,076 | 15.99 | - |
| NSAID (ibuprofen) | £55,785 | 15.62 | Dominated |
| “No treatment” | £57,220 | 15.86 | Dominated |
| Oral corticosteroid (prednisolone) | £67,160 | 15.70 | Dominated |
| Inhaled corticosteroid (fluticasone) | £81,318 | 15.82 | Dominated |
FEV, forced expiratory volume; ICER, incremental cost-effectiveness ratio; NSAID, nonsteroidal antiinflammatory drugs; QALYs; quality-adjusted life years; SA, sensitivity analysis; TRAE, treatment related adverse effect
Using the utility values reported by Solem 2014 reduces the range in quality of life between the FEV1% strata from 0.169 (0.949 – 0.881) to 0.068 (0.81 – 0.641). The total QALYs for each treatment increase as more QALYs are gained from those higher utility values, but this scenario favours the less effective treatments as the incremental QALY gains will be reduced. Reducing the cost of an exacerbation or reducing the number of baseline exacerbations also favours the less effective treatments as the incremental costs and QALYs gains will be reduced. In each of these scenarios that do not favour azithromycin, azithromycin still dominates its comparators which increases the confidence in our decision that azithromycin is the most cost-effective immunomodulatory agent. Similarly, the 2-way analysis using a lower exacerbation cost and lower disutility does not change our decision, as azithromycin remains to dominate the alternatives.
Given that the cheaper preparations of ibuprofen and prednisolone resulted in a dominated decision, oral solution preparations that are more costly, would only decrease their cost-effectiveness further.
Increasing the probability of ibuprofen related adverse events reduces the total QALYs for ibuprofen due to the decrements in quality of life associated with those events and the increase in mortality. The total costs reduce despite the cost of those adverse events due to the increase in mortality.
On the other hand, removing treatment related adverse events from the model increases the total QALYs for azithromycin, prednisolone and ibuprofen as the disutilties associated with treatment related adverse events are removed. The total costs also reduce for azithromycin and prednisolone as there is no cost to manage treatment related adverse events. For ibuprofen, the total costs increase, potentially as the rate of mortalities is reduced, increasing the number of people incurring the cost of ibuprofen treatment and the number of people eligible for a lung transplant. Overall, the conclusions do not change as azithromycin remains the dominant option.
Using the time to next exacerbation rather than the rate of exacerbations for azithromycin reduces the number of exacerbations associated with azithromycin treatment, subsequently reducing the total cost and increasing the total QALYs. Therefore, this scenario provides further evidence that azithromycin dominates the alternatives. Reducing the time horizon to a “within-trial” analysis with a starting age of 12 years means the effects on lung function will not be realised as it takes many years for a person to transition between the FEV1% strata (>70, 40–70, <40). As a result, there will be little or no difference in FEV1% in terms of quality of life, so the difference will be driven by exacerbations, drug acquisition costs, and treatment related adverse events.
The effect of varying the RR for azithromycin (ceteris paribus) is illustrated in Figure 22 and Figure 23 using the net monetary benefit (total QALYs × WTP− total costs). This approach requires QALYs to be rescaled using NICE’s threshold of £20,000 to £30,000 per QALY, where the treatment with the highest NMB is inferred as the most cost-effective option.
It is important to note that NICE’s threshold for cost-effectiveness represents the opportunity cost, rather than a willingness to pay (WTP). However, to coincide with the literature on net monetary benefits, WTP terminology is also used here as it is assumed the NHS’s WTP is equal to the opportunity cost at the margin.
It is clear from Figure 22 that azithromycin has the highest NMB up to a RR of 0.95. In this case, prednisolone is more effective than azithromycin and would become the most-cost-effective option with an ICER of £12,007 in the north-east quadrant of the cost-effectiveness plane. However, this ICER is only plausible if there is reason to reject the evidence that azithromycin has a lower rate of exacerbations compared to “no treatment”.
Figure 22NMB (£20,000 per QALY threshold) varying the RR for azithromycin
NMB, net monetary benefit at a £20,000 threshold: no treatment, −£54,700; prednisolone, −£39,018; ibuprofen, −£45,767; fluticasone, −£188,751; azithromycin, dependent on the RR
Figure 23NMB (£30,000 per QALY threshold) varying the RR for azithromycin
NMB, net monetary benefit at a £30,000 threshold: no treatment, £68,973; prednisolone, £86,283; ibuprofen, £76,867; fluticasone, −£77,603; azithromycin, dependent on the RR
Overall, the results were robust to the scenarios undertaken with azithromycin dominating the alternative in each scenario.
K.13.11.3. Probabilistic analysis
Table 107 below presents the average probabilistic results obtained from 10,000 simulations.
Table 107Probabilistic results, immunomodulatory agents
| Treatment | Total costs | Total QALYs | ICER |
|---|---|---|---|
| Macrolide (azithromycin) | £225,381 | 13.08 | - |
| NSAID (ibuprofen) | £292,035 | 12.05 | Dominated |
| “No treatment” | £299,917 | 12.18 | Dominated |
| Oral corticosteroid (prednisolone) | £416,561 | 10.61 | Dominated |
| Inhaled corticosteroid (fluticasone) | £503,392 | 9.59 | Dominated |
Compared to the results in the deterministic base case, the total costs are higher for azithromycin, ibuprofen, prednisolone and fluticasone and the total QALYs are lower for all treatments. Given that the coda values, particularly for exacerbations, varied so widely, it was necessary to restrict the number of exacerbations a person with cystic fibrosis could experience each cycle, as the coda inferred numbers that would not be plausible. The restriction to 8 exacerbations/year was considered to be conservative and intended to remove extreme “outliers”.
The simulations for ibuprofen do not vary widely, relative to the other treatments, as the exacerbation data was assumed equal to “no treatment” (i.e. deterministic).
Prednisolone is more expensive and less effective than “no treatment” in this analysis. Given that prednisolone had one of the widest 95% credible interval this is not unexpected. The ordering for all remaining treatments is unchanged and azithromycin is still the most cost-effective treatment as it dominates the alternatives.
In Figure 24 the simulations are distributed predominantly across the north-west and south-east quadrant of the cost-effectiveness plane. To aid interpretation of those simulations the proportion of simulations in each quadrant are provided in Table 108. From Table 108 it is evident that the majority of simulations (60–77%) compared to azithromycin were more expensive and less effective; however a notable proportion were less expensive and more effective (20–29%) than azithromycin that suggests azithromycin is not the most cost-effective option in all cases.
Table 108Proportion of simulations in each quadrant
| Treatment | NW | NE | SE | SW |
|---|---|---|---|---|
| Oral corticosteroid (prednisolone) | 6,169 (60%) | 690 (7%) | 2,782 (29%) | 359 (4%) |
| Inhaled corticosteroid (fluticasone) | 7,360 (73%) | 618 (6%) | 1,920 (20%) | 102 (1%) |
| NSAID (ibuprofen) | 7,533 (76%) | 221 (2%) | 2,049 (20%) | 196 (2%) |
| “No treatment” | 7,611 (77%) | 235 (2%) | 2,093 (20%) | 61 (1%) |
NW, north-west, more expensive and less effective than azithromycin; NE, north-east, more expensive and more effective than azithromycin; SW, south-west, less expensive and less effective than azithromycin; SE, south-east, less expensive and more effective than azithromycin
The net monetary benefit (total QALYs × WTP− total costs) has also been calculated to find the probability of being the most cost-effective treatment (Table 109). From Table 109 it is clear that azithromycin has the highest probability of being the most cost-effective option, with a probability of 52.3%, followed by prednisolone with a probability of 25.8%. The CEAC illustrated Figure 25 also shows that azithromycin is the most optimal treatment for all WTP thresholds estimated up to £100,000/QALY.
Table 109Probability of being the most cost-effective treatment
| Treatment | Probability |
|---|---|
| Macrolide (azithromycin) | 52.3% |
| Oral corticosteroid (prednisolone) | 25.8% |
| Inhaled corticosteroid (fluticasone) | 14.7% |
| “No treatment” | 4.3% |
| NSAID (ibuprofen) | 2.9% |
Figure 25CEAC, immunomodulatory agents
The principle of the willingness-to-pay (WTP) threshold is that it represents at the margin the cost per QALY of the last NHS pound allocated.
The second PSA using the cost to treat asthma complications as a proxy to treat an exacerbation led to lower total costs, but similar conclusions. The results from 10,000 simulations are presented in Table 110, Table 111, Figure 26 and Figure 27.
Table 110Additional probabilistic analysis using an alternative, cheaper exacerbation cost input, immunomodulatory agents
| Treatment | Total cost | Total QALYs | ICER |
|---|---|---|---|
| Macrolide (azithromycin) | £65,346 | 13.05 | - |
| NSAID (ibuprofen) | £81,814 | 12.04 | Dominated |
| “No treatment” | £83,861 | 12.12 | Dominated |
| Oral corticosteroid (prednisolone) | £122,981 | 10.65 | Dominated |
| Inhaled corticosteroid (fluticasone) | £140,961 | 9.61 | Dominated |
Table 111Probability of being the most cost-effective treatment (alternative, cheaper exacerbation cost)
| Treatment | Probability |
|---|---|
| Macrolide (azithromycin) | 49.4% |
| Oral corticosteroid (prednisolone) | 27.5% |
| Inhaled corticosteroid (fluticasone) | 16.0% |
| “No treatment” | 4.8% |
| NSAID (ibuprofen) | 2.3% |
K.13.12. Discussion
This is the first cost-effectiveness analysis of immunomodulatory agents in people with cystic fibrosis. Using QALYs, as the measure of effectiveness, incorporates changes in morbidity and mortality and allows broad comparisons across all health care interventions provided by the NHS. In addition, undertaking cost-utility analysis was of upmost importance, given the need to assess the trade-offs from various treatment related adverse events.
A key strength of this analysis was that clinical and economic systematic reviews were conducted to a high standard, including comprehensive search strategies, and study selection, data extraction and quality assessment according to pre-defined protocols.
The economic model developed for this review was based on committee opinion regarding current treatment pathways and systematic reviews of evidence relating to the plausibility of relationships between treatment outcomes such as lung function and exacerbations. The model was populated using the best available evidence and was peer reviewed by several individuals with clinical and methodological expertise.
NMAs were undertaken for this review question, to allow the treatments identified in the review to be compared to a single comparator and enable the economic model to perform a fully incremental analysis that compares all treatments simultaneously, to identify the most cost-effective treatment. However, in the network, there were a lot of indirect comparisons coming from a small number of head-to-head trials, and for most comparisons where direct evidence was available, it came from a single trial. Consequently, there are some concerns regarding the robustness of the NMAs and care should be taken not to over-interpret them.. To account for this uncertainty, an analysis varying the rate of exacerbations associated with of azithromycin was undertaken. The results of this analysis inferred that if the RR for azithromycin is less than 0.95 (relative to “no treatment”) we can be confident that azithromycin provides the greatest (positive) NMB. Furthermore, calculating the NMB for each treatment showed that at a threshold of £20,000 per QALY, all treatments except for azithromycin had a negative NMB. This suggests that those treatments should not be recommended as their costs are higher than the value of benefit achieved.
An important assumption in the model included extrapolation of the trial data to a lifetime horizon. On the one hand, this was useful to assess all important differences in costs and outcomes that would be possible from lifetime immunomodulatory treatment and any potential chronic conditions, but on the other, potentially misleading if the treatment effect is time dependent. To account for this uncertainty, the time horizon in the model can be varied. Furthermore, the ‘within-trial’ analysis and lifetime analysis led to the same conclusions which increases the confidence in the extrapolation. As with most analyses that take a lifetime horizon, the committee stated that it was important to note that future mortality used to inform the model is overestimated because survival will increase in the future due to advances in technology and research. It is also important to note that the additional risk of mortality associated with diabetes or a renal impairment, may be double counted in the model, if those mortalities are already captured within the general cystic fibrosis survival estimates. However, the impact of this limitation is considered to be negligible, given the small proportion of people who are potentially affected.
The model is insensitive to FEV1% as it takes many years for a person to transition between the FEV1% strata (>70, 40–70, <40). The starting FEV1% value in the model was varied in PSA which meant people in the model could transition sooner if they started with a lower value; however, the conclusions from PSA were similar to the deterministic analysis. For this reason, the results are driven by exacerbations, drug costs and treatment related adverse events. In the model, fluticasone was found to improve FEV1% by a greater amount than the other treatments, whilst incurring the highest risk of exacerbations. Given that exacerbations have a high cost and negative QALY impact, it is unsurprising fluticasone was the most expensive and least effective treatment in the model. Conversely, azithromycin was found to weaken FEV1%, but reduce exacerbations, providing a cost-effective treatment overall.
The model assumes that mortality is independent of exacerbations and lung function due to insufficient, reliable data to suggest otherwise. If a decline in lung function increased the rate of mortality, this would favour fluticasone. To incorporate such a relationship in the model old require patient level data, or a published analysis of that data, which unfortunately, was not available.
This area was prioritised for economic modelling as ibuprofen and prednisolone are subject to treatment related adverse events which increases their uncertainty regarding cost-effectiveness. Despite the inclusion of those important adverse events in the model, prednisolone dominates “no treatment”, NSAIDs and fluticasone in the base case, providing a rationale to accept such a trade-off when azithromycin is contraindicated or no longer effective. However, mucoactive agents, mucolytics agents, antimicrobials and immunomodulatory agents aim to stabilise (or improve) lung function or reduce the number of exacerbations. For this reason, the ordering and preference of treatments is not clear cut. Current clinical practice would offer azithromycin as the first line immunomodulatory agent, but this may be used alongside other drugs such as mucoactive or mucolytic agents, so it is difficult to observe their effects exclusively.
Unfortunately there was insufficient data on how ibuprofen affected the rate of exacerbations. For this reason, the RR required for ibuprofen to be considered cost-effective was estimated from the model. However, given that ibuprofen was not expected to reduce the rate of exacerbations compared to “no treatment”, it was reasonable to make the rate equal to “no treatment” in the base case. When PSA was undertaken this issue was more pronounced as the simulations compared to the remaining immunomodulatory agents was more concentrated.
Finally, this model did not examine the effects of adherence to treatment outcomes which could potentially vary across the treatments under consideration. Adherence with treatment in general is recognised as poor in people with cystic fibrosis and would be important to include when data are available.
K.13.13. Conclusion
The economic model has demonstrated that azithromycin is the cheapest and most effective agent as is dominates the alternatives. In addition, if inhaled corticosteroids such as fluticasone are no longer used as immunomodulatory agents in clinical practice, the model can be used to justify the current position and provide further evidence for a “do not” recommendation, given that they were the most expensive and least effective treatment in the model. This result was also reiterated by the extensive sensitivity analysis and probabilistic analysis. Furthermore, this is the first cost-effectiveness analysis on immunomodulatory agents in people with cystic fibrosis to date, providing evidence that current clinical practice should continue to be recommended as a cost-effective use of NHS resources.
However, future research should consider how immunomodulatory agents, mucoactive and mucolytic agents and antibiotics can complement each other to reduce treatment burden and reduce unnecessary treatment costs, given that there is uncertainty regarding their combined clinical and cost-effectiveness.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 9.5.7.3 ‘Evidence to recommendations’.
K.14. Chronic antimicrobials
K.14.1. Literature review
Four economic evaluations of antibiotic agents to suppress chronic infection were identified in the literature search conducted for this guideline. The methods and results from those analyses are summarised in Table 112 and described in more detail in Appendix L and M. Full details of the search can be found in Appendix E and the economic article selection flow chart is illustrated in Figure 1.
In the cost–benefit analysis by Iles 2003, the impact of nebulised tobramycin on the usage of healthcare resource in the UK are compared with those prior to nebulised tobramycin treatment in 41 participants with chronic P. aeruginosa infection. They concluded that the cost of nebulised tobramycin is reduced by fewer hospital attendances and parenteral antibiotics, but not completely offset by improved clinical outcomes.
In response to the Health Technology Appraisal (HTA) submission by Forest Laboratories (NICE TA276), the TAG (Tappenden 2013) developed a de novo probabilistic state transition model to compare the cost-effectiveness of colistimethate sodium dry powder inhalation with nebulised tobramycin in people with cystic fibrosis who had chronic P. aeruginosa infection. The model estimates treatment specific transitions between 3 FEV1% strata. Each strata is associated with different utility values and additional utility decrements are applied to the minor or major exacerbations experienced within each of those 3 health states. People with cystic fibrosis who enter the most severe FEV1% health state may undergo a lung transplant and will not receive any further antibiotic treatment. The structure of the model is described in more detail in the Appendix L.
Given the uncertainty surrounding the extrapolation of the 24-week efficacy data to a lifetime horizon, both a lifetime horizon and a “within-trial” analysis was presented. The TAG also presented results across 6 colistimethate sodium dry powder pricing scenarios to reflect the range of conceivable prices charged by the manufacturer.
The “within-trial” analysis resulted in smaller incremental differences in both costs and QALYs, but both time horizons examined by the TAG led to the same conclusions. If colistimethate sodium dry powder is priced lower than that of nebulised tobramycin the ICER lies in the south-west quadrant of the cost-effectiveness plane reflecting a QALY loss and cost savings for colistimethate sodium dry powder compared with nebulised tobramycin. However, if colistimethate sodium dry powder is priced higher than that of nebulised tobramycin the incremental cost is positive, and colistimethate sodium dry powder is dominated by nebulised tobramycin.
The TAG did not initially include tobramycin dry powder in the model because patient-level FEV1% data were not available from Forest Laboratories, at the time the report was produced. This was subsequently published by Tappenden 2014 who also incorporated the revised Patient Access Scheme (PAS) for colistimethate sodium dry powder agreed with the Department of Health (details of which cannot be reproduced here due to confidentiality reasons).
Only the price of colistimethate sodium dry powder was amended in the model by Tappenden 2014; all other assumptions and parameters in the model remained unchanged. The methods and results from both analysis are summarised in Table 112 and described in more detail in Appendix L.
When the revised PAS discount for colistimethate sodium dry powder was incorporated over a lifetime horizon, the incremental QALY was −0.13, and the incremental cost was – £37,946 for colistimethate sodium dry powder compared to nebulised tobramycin (list price). These results demonstrated that colistimethate sodium dry powder was less effective and less expensive than nebulised tobramycin, with an ICER of £288,563.
Considering the second comparison, tobramycin dry powder consistently dominated nebulised tobramycin with inclusion of the PAS for tobramycin dry powder, that is, there was a cost saving and QALY gain for tobramycin dry powder compared to nebulised tobramycin.
The probabilistic sensitivity analysis associated with these analyses estimated that with the revised PAS, colistimethate sodium dry powder and tobramycin dry powder had a probability of 1.0 of being cost-effective at the £20,000 per QALY gained threshold compared with nebulised tobramycin.
The PAS price discount had a significant impact upon the cost-effectiveness, changing from a clear reason to not recommend colistimethate sodium dry powder (dominated by tobramycin dry powder) to a decision on disinvestment (cost saving and QALY loss). Sensitivity analysis demonstrated that the source of utility values was a key driver of cost-effectiveness due to the small QALY gains for tobramycin dry powder compared with nebulised tobramycin and for colistimethate sodium dry powder compared with nebulised tobramycin.
The fourth and most recent study by Schechter 2015 was identified during re-run searches, post development of the economic model described in Section K.14.4. Similarly to Tappenden 2013 and Tappenden 2014 they developed a Markov state transition model, defined principally by FEV1% health states. Their cost-utility analysis with a 3-year time horizon was performed to compare nebulised aztreonam lysine to nebulised tobramycin from the perspective of a third party payer in the US. Clinical data from the trial by Asseal 2013 was used to estimate transition probabilities between FEV1% health states and the probability of hospitalisations. They concluded that aztreonam was associated with a cost saving as well as greater QALYs and total life years than nebulised tobramycin. However, it is important to note that the cost inputs used in this study differ noticeably from this model in favour of aztreonam.
Table 112Summary of included economic evaluations, chronic antimicrobials
| Study | Limitations | Applicability | Other comments | Incremental | Uncertainty | ||
|---|---|---|---|---|---|---|---|
| Costs | Effects | Cost-effectiveness | |||||
| Iles 2003 | Seriousa,b | Directlyc | Cost-benefit analysis estimated from 41 participants who received NT, comparing 12 months before and 12 months during the use of NT. | Post NT versus pre NT
| Post NT versus pre NT
| NA | 95% CIs reported |
| Tappenden 2013 | Minord | Directly | Study employed a Markov DAM with a lifetime horizon. A within-trial analysis was also examined. Six pricing scenarios for Coli DPI were presented. | List price: Inc. cost Coli DPI versus NT
| QALYs gained Coli DPI versus NT −0.13 |
| OWSA and PSA undertaken. Results sensitive to alternative utility values, this leads to positive ICERs that were previously dominated. |
| Tappen den 2014 | Minord | Directly | Study employed a Markov DAM with a lifetime horizon, analyses including DPI PAS discount also examined in response to Tappenden 2013 | List price: Inc cost
Coli DPI versus NT −£37,946
| QALYs gained Coli DPI versus NT −0.13 Tobi DPI versus NT +0.34 | List price: ICER
| OWSA (list price) and PSA undertaken. Results sensitive to alternative utility values and equal FEV1% trajectories for Coli DPI versus NT, this leads to positive ICERs that were previously dominated. Results sensitive to equal FEV1% trajectories for Tobi DPI versus NT, this led to NT dominating Tobi DPI |
| Schechter 2015 | Seriouse,f | Partiallyg,h | Cost-utility analysis estimated using a Markov DAM where clinical effectiveness was informed by Assael 2013 | Aztreonam versus NT - $41,947 | Aztreonam versus NT 0.0286 QALYs | Aztreonam dominates NT | Assessed using scenario analysis, univariate sensitivity analysis and PSA. Results robust to changes in key assumptions. |
OWSA, One way sensitivity analysis; PSA, Probabilistic sensitivity analysis; Markov DAM, Markov Decision Analytic Model; ICER, Incremental Cost-effectiveness ratio; NT, nebulised tobramycin; aztreonam = nebulised aztreonam lysine
- (a)
Sources for cost components and drug costs not reported and absence of detail regarding cost build up for: NT costs, drug costs, ward costs and ICU costs
- (b)
Observational before and after study design
- (c)
This study does not include the preferred measure of effects (QALYs), but is still thought to be useful for decision making given that all other criteria are applicable and the alternative outcome measure reported is unlikely to change the conclusions about cost-effectiveness
- (d)
Limitations of the model noted, but stem from insufficient data rather than the approach of the authors, these include: exclusion of treatment related AEs, resistance to tobramycin and treatment sequencing
- (e)
Insufficient detail on cost sources and the methods used to estimate the number of hospitalisations (exacerbations) by FEV1 status
- (f)
Supported by Gilead Sciences (the manufacturer of aztreonam)
- (g)
US third party payer perspective (includes payments from insurance) which is not representative of the UK public health system
- (h)
Cost inputs differ from those in a UK setting with an exacerbation here costing $29,205 and aztreonam inhalation solution costing less than tobramycin solution ($3,035/28 days versus $3,338/28 days).
K.14.2. Drug acquisition cost of antibiotics for pathogens other than P aeruginosa
A cost description of all interventions specified in the protocol has been undertaken. Dosages were informed by the committee, and drug acquisition costs are taken from the NHS Electronic Drug Tariff November 2016, unless unreported and otherwise stated. As outlined in the NICE 2013 Guide to the methods of technology appraisal, the reduced price should be used in the reference-case analysis to best reflect the price to the NHS. For this reason, the lowest cost brand is presented.
In addition to the acquisition cost of inhaled antibiotics, the use of inhalers or nebulisers for their delivery is associated with fixed costs, related to equipment purchase and ongoing costs, associated with maintenance. Inhalers are relatively inexpensive such as the Spacer anti-static with mouthpiece at a cost of £7.73 (NHS Supply Chain 2015), nebulisers on the other hand cost substantially more (Table 113).
Table 113Cost of nebuliser reproduced from the NHS Supply Chain 2015
| Product | Cost |
|---|---|
| PARI SINUS inhalation device with pulsating aerosol for the nasal sinuses | £108.27 |
| Paediatric nebuliser system JuniorBoy SX | £89.90 |
| BOY mobile S Portable multi-voltage nebuliser with LC SPRINT nebuliser adult mask 12v cable battery & carry bag | £284.91 |
| Adult nebuliser system TurboBoy SX | £84.64 |
| Eflow rapid with 2 handsets batteries international power adapter carry case | £718.95 |
All treatments would be administered at home without the assistance of a healthcare professional, therefore, no administration costs are incurred. Prescription services are also excluded because people with cystic fibrosis are assumed to receive prescriptions at their regular visits to the clinic at no additional cost.
There is likely to be some on-going monitoring for long-term use, this would also involve a full blood count and liver function tests, but it reasonable to assume this is equivalent across all treatments, as there is no opportunity cost created by switching from one treatment to another.
K.14.2.1. Burkholderia cepacia
Based on the dosages provided by the committee below, the most expensive antibiotic for adults are imipenem and temocillin at a cost of £60.40 /day and £50.90/day whilst co-trimoxazole and trimethoprim cost <£1/day if the cheapest preparation is chosen (tablets). The unit cost of all antibiotics to manage B cepacia are provided in Table 114.
- Ceftazdime (nebulised):
- Child >1 month and adult: 1g bd.
- Co-trimoxazole (by mouth):
- Child 6 weeks – 5 months: 120mg bd;
- Child 6 months – 5 years: 240mg bd;
- Child 6 – 12 years: 480mg bd;
- Child > 12 years and adult: 960mg bd.
- Meropenem (nebulised)
- Child 6 – 12 years: 125mg bd;
- Child >12 years and adult: 250mg bd.
- Imipenem (IV)
- Child 1 month – 18 years: 25mg/kg (max. 1g) qds;
- Adult: 1g qds.
- Trimethoprim (by mouth)*
- Child 6 weeks–5 months: 4 mg/kg bd (max. per dose 200 mg), alternatively 25 mg bd;
- Child 6 months–5 years: 4 mg/kg bd (max. per dose 200 mg), alternatively 50 mg bd;
- Child 6–11 years: 4 mg/kg bd (max. per dose 200 mg), alternatively 100 mg bd;
- Child 12–17 years: 200 mg bd;
- Adult: 200 mg bd.
- Temocillin (nebulised)
- Adult: 1g bd.
Table 114Acquisition cost of antibiotics to suppress B cepacia
| Antibiotic (quantity, basic price) | Unit cost |
|---|---|
| Ceftazidimea | |
| 500mg powder for solution for injection vials (1, £4.25) | £4.25 |
| 1g powder for solution for injection vials (10, £13.90) | £1.39 |
| 2g powder for solution for injection vials (10, £27.70) | £2.77 |
| Co-trimoxazole | |
| 160mg/800mg tablets (100, £23.46) | £0.23 |
| 80mg/400mg tablets (28, £2.29) | £0.08 |
| 40mg/200mg/5ml oral suspension sugar free (100ml, £9.95) | £0.50/5ml |
| 80mg/400mg/5ml oral suspension (100ml, £10.95) | £0.55/5ml |
| Meropenema | |
| 500mg powder solution for injection vials (10, £76.90) | £7.69 |
| 1g powder solution for injection vials (10, £153.50) | £15.35 |
| Imipenema | |
| 500mg powder for solution for infusion vials (10, £75.45) | £7.55 |
| Trimethoprim | |
| 100mg tablets (28, £1.27) | £0.05 |
| 200mg tablets (14, £1.71) | £0.12 |
| 50mg/5ml oral suspension (100ml, £2.00) | £0.10/5ml |
| Temocillina | |
| Negaban 1g powder for solution for injection vials (1, £25.45) | £25.45 |
* Dose taken from the BNF for respiratory tract infections
- (a)
BNF NHS indicative price
K.14.2.2. Staphylococcus aureus
Table 115 below presents the cost of oral antibiotics to suppress S aureus for each preparation. It is evident that flucloxacillin oral solution is the most expensive antibiotic for this indication at a cost of over £20/day for adults. However, capsule preparations of flucloaxacillin are relatively inexpensive at a cost of approximately £1/day. The cost of all remaining antibiotics are <£3/day when the dosages advised by the committee below are utilised.
- Flucloxacillin (by mouth):
- Child >1 month: 25mg/kg (max. 1g) four times daily (total daily dose, 100mg/kg, may be given in 3 divided doses (max. 4g/day));
- Adult: 1 – 2g qds.
- Co-trimoxazole (by mouth):
- Child 6 weeks – 5 months: 120mg bd;
- Child 6 months – 5 years: 240mg bd;
- Child 6 – 12 years: 480mg bd;
- Child > 12 years and adult: 960mg bd.
- Cefradine (by mouth)*:
- Child 7–11 years: 25–50 mg/kg daily in 2–4 divided doses.
- Child 12–17 years: 250–500 mg qds, alternatively 0.5–1 g bd; increased if necessary up to 1 g qds, increased dose may be used in severe infections.
- Adult: 250–500 mg qds, alternatively 0.5–1 g bd; increased if necessary up to 1 g qds, increased may be used in severe infections.
- Doxycycline (by mouth):
- Child > 12 years and adult: 200mg on day 1 then 100 – 200mg daily thereafter (100mg bd or 200mg once daily)
Table 115Acquisition cost of antibiotics to suppress S aureus
| Antibiotic (quantity, basic price) | Unit cost |
|---|---|
| Flucloxacillin | |
| 250mg/5ml oral solution (100ml, £26.04) | £1.30/5ml |
| 250mg/5ml oral solution sugar free (100ml, £26.48) | £1.32/5ml |
| 250mg capsules (28, £1.35) | £0.05 |
| 500mg capsules (28, £2.14) | £0.08 |
| Co-trimaxazole | |
| 160mg/800mg tablets (100, £23.46) | £0.23 |
| 80mg/400mg tablets (28, £2.29) | £0.08 |
| 40mg/200mg/5ml oral suspension sugar free (100ml, £9.95) | £0.50/5ml |
| 80mg/400mg/5ml oral suspension (100ml, £10.95) | £0.55/5ml |
| Cefradine | |
| 250mg capsules (20, £1.80) | £0.09 |
| 500mg capsules (20, £2.71) | £0.14 |
| Doxycycline | |
| 100mg capsules (8, £0.87) | £0.11 |
| 100mg dispersible tablets sugar free (8, £4.91) | £0.61 |
| 20mg tablets (56, £17.30) | £0.31 |
| 40mg modified-release capsules (14, £7.99) | £0.57 |
| 50mg capsules (28, £1.39) | £0.05 |
* Taken from the BNF for susceptible infections due to sensitive Gram-positive and Gram-negative bacteria
K.14.2.3. Aspergillus fumigatus
Table 116 below presents the cost of oral antibiotics (itraconazole, voriconazole and posaconizole) and inhaled antibiotics (amphotericin) to suppress A. fumigatus. This pathogen includes the most expensive antibiotics, with voriconazole tablets costing £78.76/day (adults, 200mg bd) and posaconazole tablets costing £74.61/day (adults, 300mg/day). However, itraconazole and amphotericin (Fungizone®) are substantially cheaper at costs of £0.92 (itraconazole tablets, 200mg bd), £15.52 (itrazconazole oral solution, 200mg tice daily) and £3.88 (Fungizone®, 25mg bd). The dosages advised by the committee for this indication are reported below:
- Itraconazole (by mouth):
- Child >1 month: 5mg/kg (max. 200mg) bd;
- Adult: 200mg bd, increased if necessary according to trough plasma concentration.
- Voriconazole (by mouth):
- Child 2 – 12 years; and 12 – 14 (body weight <50kg):
- –
Loading dose: Not recommended;
- –
Maintenance dose: 9mg/kg (max. 350mg) bd;
- –
If response inadequate, dose may be increased in 1mg/kg steps (or 50mg if starting dose 350mg) as tolerated;
- –
Reduce in steps of 1mg/kg (or 50mg if starting dose 350mg) if not tolerated.
- Child 12 – 14 (body weight >50kg), >15 years and adult:
- –
<40kg: 200mg bd first 24h then 100mg bd;
- –
>40kg: 400mg bd first 24h then 200mg bd;
- –
If response inadequate, increase to 150mg (<40kg) or 300mg (>40kg);
- –
Higher doses may be required to achieve therapeutic levels.
- Posaconazole:
- Oral tablets:
- –
Adult: 300mg bd for 24h then 300mg OD thereafter.
- Oral suspension
- –
Child: Safety and efficacy not established <18 years; limited manufacturers data suggest 400mg bd safe in 8 – 17 year olds, with similar pharmacokinetics to those observed in 18 – 64 year olds;
- –
Adult: 400mg bd.
- Amphotericin (Fungizone®, nebulised):
- Child <10 years: 5mg bd;
- Child >10 years: 10mg bd;
- Adult: 5 – 10mg bd, increased to max. 25mg bd depending on response and tolerability.
Table 116Acquisition cost of antibiotics to suppress A. fumigatus
| Antibiotic (quantity, basic price) | Unit cost |
|---|---|
| Itraconazole | |
| 100mg capsules (15, £3.44) | £0.23 |
| 50mg/5ml oral solution sugar free (150, £58.34) | £1.94/5ml |
| Voriconazolea | |
| 50mg tablets (28, £275.68) | £9.85 |
| 200mg tablets (28, £1,102.74) | £39.38 |
| 40mg/5ml oral suspension (75ml, £551.37) | £7.35/5ml |
| Posaconizolea | |
| 40mg/ml oral suspension (105ml, £491.20) | £4.68/ml |
| 100mg gastro-resistant tablets (24, £596.96) | £24.87 |
| 100mg gastro-resistant tablets (96, £2,387.85) | £24.87 |
| Amphotericina | |
| 50mg powder for solution for infusion vials (10, £821.87) | £82.19 |
| Intravenous 50mg powder for solution for infusion vials (Fungizone®) (1, £3.88) | £3.88 |
| 100mg/20ml concentrate for suspension for infusion vials (10, £775.04) | £77.50 |
- (a)
BNF NHS indicative price
K.14.3. Model structure
Similarly to the model developed for immunomodulatory agents (Section K.13, Figure 16: Markov state transition model), the model for a chronic indication takes the form of a Markov state transition model to estimate transitions between 3 lung function (FEV1%) strata, from the perspective of the UK NHS and using 2015/16 costs. Transition probabilities between these 3 strata are taken from the clinical evidence review. The first cycle is 28 days long to correspond to the cyclical “on-off” regimen used in the prescription for aztreonam and tobramycin, whilst subsequent cycles last 24 weeks (6 × 28 days) to reflect the short and long term benefits of antibiotic treatment estimated by the NMA. These cycle lengths were also used by Schechter 2015, Tappenden 2013 and Tappenden 2014 for comparable reasons.
The model takes a lifetime horizon based on the assumption that chronic antibiotic treatment is given on a long-term basis, but this can be varied by the user in the model. Cost-effectiveness results should reflect the present value of the stream of costs and benefits accruing over the time horizon of the analysis. NICE considers that it is usually appropriate to discount costs and health effects at the same annual rate of 3.5%, based on the recommendations of the UK Treasury for the discounting of costs. For this reason, the model has adopted a discount rate of 3.5% for both costs and benefits (QALYs), but this input can be varied by the user in the model.
During each cycle people with cystic fibrosis may remain in their current FEV1% state, or transition to a worsened FEV1% state, experience a treatment related adverse effect (related to tobramycin), or die. Additional costs and quality of life decrements are applied to people who experience an exacerbation in the FEV1% strata. People with cystic fibrosis in the worst FEV1% strata (<40%) may undergo a lung transplant, subsequently they will go off-treatment. This pathway reflects that used previously for immunomodulatory agents described and illustrated in K.13.4.
K.14.4. Comparisons
Multiple studies reported FEV1%, but the studies were too heterogeneous to allow for the ‘gold standard’ synthesis of a NMA, despite extensive investigations to try to explain the heterogeneity. On the advice of the TSU, these results were not meta-analysed and have been presented separately in the clinical evidence report (see Full Guideline Section 9.4.3).
One approach to assess cost-effectiveness is a ‘fully incremental’ approach that compares all treatment to each other. This approach is more in line with the NICE Reference Case, but involves the assumption that the studies are homogenous, which as stated above, cannot be accepted.
The second approach involves ‘multiple pairwise’ comparisons, where each comparison is informed by a different study:
- colistimethate sodium dry powder versus nebulised tobramycin (Schuster 2013);
- nebulised colistimethate sodium versus nebulised tobramycin (Hodson 2002).
Using a ‘multiple pairwise’ approach allows much more confidence in the coherence of the results, but the output will not be ‘incremental’ meaning it will be almost impossible to tell if colistimethate sodium dry powder or nebulised colistimethate sodium are cost-effective relative to any other treatment – only if they are cost-effective relative to the common baseline (nebulised tobramycin).
In the initial analysis, a third pairwise comparison was developed for the study by Asseal 2013, who compared aztreonam to nebulised tobramycin. Following re-run searches, the study by Flume 2016 was added to this analysis to provide a comparison between aztreonam, nebulised tobramycin and a combination treatment (28 days aztreonam lysine (nebulised) alternating with 28 days tobramycin (nebulised)).
A ‘fully-incremental’ comparison can also be presented that compares several chronic antibiotics (nebulised tobramycin, tobramycin dry powder, nebulised colistimethate sodium) to placebo that utilises the NMA data on exacerbations. Unlike the previous ‘multiple pairwise’ comparisons, where each comparison is only assessed by 1 study, there are multiple studies. As a result, the most representative and well-conducted studies would inform the base case for each treatment compared to placebo:
- nebulised tobramycin versus placebo (Chuchalin 2007);
- tobramycin dry powder versus placebo (Galeva 2013);
- nebulised colistimethate sodium versus placebo (Jensen 1988).
However, other plausible studies (Konstan 2011, Lenoir 2007) would be considered in sensitivity analysis.
Overall it was agreed the ‘multiple pairwise’ approach including those 4 comparisons outlined above would be most appropriate, as the uncertainty in clinical effectiveness from an indirect comparison outweighs the ability to tell if a particular treatment is cost-effective relative to any other treatment than the common baseline.
K.14.5. Clinical effectiveness
K.14.5.1. Lung function
Natural history
It is well-documented that lung function declines over time in people with cystic fibrosis. The assumed natural rate of decline in lung function is age dependent and was taken from a large, prospective, multicentre, encounter-based, observational study of US and Canadian people with cystic fibrosis (N=4,161 adults, 1994–2005; N=1,359 children, 1997).
This study used repeated-measures, mixed-model linear regression analysis to assess risk factors for decline in FEV1% and estimate the mean rate of change in FEV1% across 2 age groups.
The mean rates of FEV1% decline for the 18–24 year and ≥25 year groups over the observation period were −1.92% (95%CI −2.04 to −1.81) and −1.45% (95% CI −1.62 to −1.27) predicted per year, respectively. One risk factor included in their model was “positive for mucoid P. aeruginosa” associated with a decline of −0.15 per year. In the model this risk factor is included to represent the decline in chronic P aeruginosa infection.
The committee agreed that the decline in FEV1% is generally faster at higher levels (younger people). However, the committee noted that a decline of −1.45% per year may overestimate the decline in later years as this estimate includes young adults; despite this, the committee felt unable to provide a third category for older people with cystic fibrosis, subsequently agreeing that the results reported by Konstan 2012 would be reasonable to inform the model for all ages.
The committee also agreed chronic infection with P aeruginosa may increase the decline in FEV1% referring to a recent paper by Qvist 2015 on 141 Danish people with cystic fibrosis chronically infected with P aeruginosa. Qvist 2015 found chronic infection with P aeruginosa in cystic fibrosis impacted the rate of decline in FEV1% by −0.95 per year. However, it is unclear if this analysis considered the natural decline in FEV1%, or age, limiting the applicability of the results to the model.
As a result, Konstan 2012 was chosen to inform the natural history of FEV1% in the model. These results are illustrated in Figure 28 based on a starting FEV1% of 83.1 for a child aged 12 (UK CF Registry 2014).
Method
People with cystic fibrosis enter the model at 12 years with a starting FEV1% 83.1% based on the UK CF 2014 Annual Data Report. The methods used to estimate lung function in the model are described in K.13.6.1.
Treatment effect
Colistimethate sodium is given every day, whereas each 28-day treatment cycle of tobramycin or aztreonam is followed by a 28-day period that does not include the use of these drugs. However, the committee has noted that in clinical practice, people with cystic fibrosis may receive 28 days of treatment using another antibiotic, followed by 28 days of treatment using tobramycin or aztreonam as an ongoing repeated sequence.
Trials included in the clinical evidence review followed a ‘month-on, month-off’ regimen when they included tobramycin or aztreonam. One exception was Hodson 2002, who compared nebulised tobramycin to nebulised colistimethate sodium over 4 weeks (28 days). Clinical effectiveness in the former trials will not be confounded by sequencing if we can assume the treatment effect during the month-off tobramycin or aztreonam treatment is equivalent, in other words, the month-off will have no effect on the incremental change in costs or QALYs between treatments because the costs or QALY gain will change proportionally for each treatment. However, this is acknowledged as a limitation of the model, particularly in relation to face validity as people may switch to another antibiotic treatment during the “month-off” in clinical practice.
Tappenden 2013 also stated that treatment switching can occur in clinical practice due to apparent treatment failure, or as part of a planned regimen. However, they did not consider combination strategies or treatment switching in their assessment of cost-effectiveness for tobramycin or colistimethate sodium due to a lack of clinical efficacy and safety. The Appraisal committee’s discussion surrounding this issue reproduced from the Final Appraisal Determination in Box 3.
Box 3Appraisal committee’s discussion on antibiotic sequencing regimens reproduced from the Final Appraisal Determination for NICE TA276
“The committee heard from the Assessment Group that information from their clinical experts suggested that less than 25% of people with cystic fibrosis and chronic P. aeruginosa lung infection would receive an alternating therapy regimen. The committee therefore concluded that some people with cystic fibrosis and chronic pseudomonas lung infection may receive alternating tobramycin and colistimethate sodium treatment in clinical practice. The committee noted the increased cost of such alternating antibiotic regimens and that it had not been presented with any evidence as to the clinical effectiveness of this approach by the Assessment Group or the manufacturers or during consultation. It concluded that because there was no evidence of the clinical effectiveness of using these antibiotics in an alternating regimen, it could not consider this issue further.”
Given the uncertainty surrounding the extrapolation of trial data to a lifetime horizon they presented a lifetime analysis and a “within-trial” analysis that does not include extrapolation. For completeness, the model for this review enables the user to adjust the time horizon to explore the impact on the results.
With regards to Hodson 2002, the consequence of assuming a ‘month-on, month-off’ regimen is that the modelled treatment benefits reflect those associated with the continued use of nebulised tobramycin at only half of the cost of generating those benefits. Unless nebulised tobramycin is priced at parity with the cost of nebulised colistimethate sodium, or a month-off cycle is applied where the benefits from nebulised tobramycin reflect placebo, a substantial bias in favour of tobramycin may exist. For these reasons, 3 scenarios are explored in the model:
- the benefits reported for nebulised tobramycin and nebulised colistimethate sodium over 28 days are maintained over the time horizon applied in the model;
- the month-off nebulised tobramycin use follows the treatment effect for placebo, whilst the benefit from nebulised colistimethate sodium is maintained;
- the cost of nebulised tobramycin is priced continuously.
As noted above, more than 1 trial included in the clinical evidence review reported FEV1% for the treatments included in the ‘fully incremental’ comparison (comparison 1). As a result, the most representative and well-conducted trials were chosen to inform the base case in the model (Table 117), whilst other plausible trials are used in sensitivity analysis.
Table 117MD in FEV1% used to inform the base case, chronic antibiotic treatment
| Treatment | MD | Source |
|---|---|---|
| Comparison 1 | ||
| Tobramycin dry powder | 4.40a | Galeva 2013 |
| Nebulised tobramycin | 6.38a | Chuchalin 2007 |
| Nebulised colistimethate sodium | 6.00a | Jensen 1988 |
| Placebo | 0.00 | NH taken from Konstan 2012 |
| Comparison 2 | ||
| Nebulised colistimethate sodium | 0.37b | Hodson 2002 |
| Nebulised tobramycin | 6.70b | Hodson 2002 |
| Comparison 3 | ||
| Colistimethate sodium dry powder | 0.96b | Schuster 2013 |
| Nebulised tobramycin | 0.99b | Schuster 2013 |
| Comparison 4 | ||
| Nebulised aztreonam lysine | 2.05b | Assael 2013 |
| Nebulised tobramycin | −0.66b | Assael 2013 |
| Nebulised aztreonam lysine & nebulised tobramycin | 1.39c | Assael 2013 |
MD, mean difference, NH, natural history
- (a)
versus placebo
- (b)
pre-treatment versus post-treatment
- (c)
assumed to equal the effect of aztreonam plus nebulised tobramycin.
Similarly to the immunomodulatory model, the MDs in FEV1% are assumed to continue indefinitely due to insufficient evidence to suggest otherwise. Ideally, future research in this area should consider longer follow-up times, where outcomes are measured at several intervals, to analyse if the treatment effect is independent of time. To explore the uncertainty surrounding the extrapolation of the 24-week efficacy data to a lifetime horizon, a “within-trial” analysis has been explored in sensitivity analysis.
For reasons previously outlined, FEV1% outcome data could not be meta-analysed. Flume 2106 reported a mean change in FEV1% from baseline of 1.37 for the combination treatment and 0.04 for nebulised tobramycin. However, these results were calculated as the average of weeks 4, 12 and 20 which may skew the results if, for example, the treatment does very well at 4 weeks. For this reason, the MD for the combination treatment was calculated from the values reported in Assael 2013. Using this method the mean change is similar to that reported by Flume 2016 (1.37 versus 1.39), increasing the confidence and application of this method.
K.14.5.2. Exacerbations
In the model exacerbations do not form a separate health state, instead people in each lung function health state have a probability to experience an exacerbation each cycle, associated with a disutility and treatment cost, lasting 2 weeks.
The probability of experiencing at least 1 exacerbation for each treatment was calculated by applying the appropriate odds-ratios (ORs) to the baseline probability of experiencing at least 1 exacerbation in the placebo arm. These ORs were estimated from the NMA for short-term (4–10 weeks) treatment and long-term (>10 weeks) treatment, informing the first (28 days month) and subsequent cycles (24 weeks) in the model, respectively.
There was insufficient data to estimate the short-term rate of exacerbations for tobramycin dry powder. Therefore 2 solutions were presented to the committee:
- assume treatment specific long-term rates equal short-term rates; or,
- assume the rate of tobramycin dry powder versus placebo is equivalent to nebulised tobramycin versus placebo.
The committee advised that assuming long-term rates equal short-term rates may underestimate the treatment effects compared to placebo, as the greatest benefit is usually seen during the first few months of treatment. Despite this, the committee agreed this assumption was more reasonable than the latter that assumed a negligible difference in effectiveness between preparations.
Unfortunately no exacerbation data was available for nebulised colistimethate sodium (Hodson 2002) or colistimethate sodium dry powder (Schuster 2013). Consequently, exacerbations ratios were set equal to the comparator (nebulised tobramycin) in the base case. However, a threshold analysis has been conducted to estimate the probability required for colistimethate sodium to alter our decision from a decision of cost-effective to cost-ineffective, or vice versa.
In the short- and long-term NMA, aztreonam was found to have the highest probability of being the best treatment to reduce the odds of experiencing at least 1 exacerbation. This is also shown in Table 118 where aztreonam is associated with the lowest exacerbation probability compared to the treatments included in the model.
Table 118Probability of exacerbations in the model, chronic antibiotic treatment
| Comparison | Baseline probability | OR | Treatment related probabilitya |
|---|---|---|---|
| First cycle (28 days) | |||
| Nebulised tobramycin versus placebo | 6%b | 3.00 | 16% |
| Tobramycin dry powder versus placebo | 1.10b | 6%c | |
| Nebulised aztreonam lysine versus placebo | 0.30 | 2% | |
| Subsequent cycles (24 weeks) | |||
| Nebulised tobramycin versus placebo | 40%d | 0.88 | 37% |
| Tobramycin dry powder versus placebo | 1.01 | 40% | |
| Nebulised aztreonam lysine versus placebo | 0.40 | 21% | |
| Combinatione versus placebo | 0.67 | 31% | |
- (a)
Calculated by transforming the baseline probability into an odds and transforming back into a probability
- (b)
6% reported for placebo by Ramsey 1993 over 28 days and 14% by Retsch-Bogart 2009 over 42 days
- (c)
Long-term assumed to equal short-term in the absence of data, also adjusted from a 6-month probability to a 1-month probability
- (d)
40% reported for placebo by Chuchalin 2007 over 24 weeks
- (e)
Nebulised aztreonam plus nebulised tobramycin
In the base case it assumed that the probability relates to 1 exacerbation, but a sensitivity analysis has been explored that considers 2 exacerbations as the former may underestimate the true number. A discussion regarding this outcome measure is provided in Section K.14.12.
The NMA did not distinguish between minor and major exacerbations due to insufficient reporting in the included studies. However, the severity of an exacerbation impacts a person’s quality of life and the cost of treating the exacerbation. As stated previously (Section K.12.5.2), the committee advised that one half exacerbations are major and require hospitalisation, whilst the other half are minor and treated on an outpatient basis.
As described in Section K.13.6.3, the event rate in placebo arms may not necessarily reflect the current baseline risk in England and Wales. For this reason, alternative sources of baseline data were sought. However, none were considered to be relevant, hence the baseline probability from the trials was used.
K.14.5.3. Lung transplant
As described Section K.13.6.2, a probability of 0.92% (per 24 weeks) is applied.
K.14.5.4. Treatment related adverse effects
Ramsey 1999 included in the clinical evidence review was a RCT that included 520 people with cystic fibrosis. They found a clinically significant higher occurrence of tinnitus in the group of participants who received nebulised tobramycin (300 mg daily) compared to those who received placebo (8/258, 3.1% versus 0/262, 0%) at 24 weeks follow-up. In the base case, a probability of 3.1% is applied to each 24-week cycle, whilst a probability of 0.5% is applied to the first cycle. Alternative sources such as the eMC were explored, but the difference was found to be negligible to warrant further sensitivity analysis.
Following a hearing impairment, treatment is switched from tobramycin to aztreonam, based on committee opinion that an alternative antibiotic would be offered. The clinical effectiveness following this switch remains the effectiveness for tobramycin on the assumption that they would have been initiated on that treatment if it was more effective. A placebo effect for aztreonam treatment was rejected by the committee as they would not offer an alternative antibiotic that is considered to be ineffective.
All remaining treatment related adverse effects identified in the clinical evidence review were considered to have a negligible treatment cost or impact on health-related quality of life, or were considered as equally likely across the treatments included in the model; for these reasons, no further adverse effects were included.
As an aside Schechter 2015, Tappenden 2013 and Tappenden 2014 did not include any treatment related adverse effects in their models. The rationale provided by Tappenden 2013 is reproduced in Box 4. For completeness a scenario excluding treatment related adverse events has been explored.
Box 4Simplifications and exclusions from the economic analysis reported by Tappenden 2013
“The model does not include utility adjustments to account for the incidence of AEs. Although the incidence of cough, productive cough and dysgeusia were markedly higher for colistimethate sodium DPI than nebulised tobramycin, some AEs were less common for colistimethate sodium DPI. As a consequence, it is unclear whether the inclusion of health utility decrements associated with the incidence of AEs would improve or worsen the economic case for colistimethate sodium DPI.
Although Forest Laboratories kindly provided detailed AE data for each treatment group at each visit, the considerable gaps in the available EQ-5D evidence (…) relating to the disutility of these events precluded the inclusion of these effects within the model.
It should also be noted that the model does not include the potential impact of resistance to tobramycin. This exclusion is reasonable, as it is unclear how this phenomenon would manifest in terms of reduced treatment effect.”
AE, adverse event; DPI, dry powder inhalation
K.14.6. Mortality
As described in Section K.13.7.
K.14.7. Resource and cost use included in the model
K.14.7.1. Drug costs
Tappenden 2013 and Tappenden 2014 included costs associated with nebuliser use when they constructed their economic model. Clinical experts advised the TAG that it would cost approximately £200/year to cover replacement aerosol heads and filters. Similarly, the model for this review includes this assumption for nebulised tobramycin and Colomycin® (nebulised colistimethate sodium), but not for Cayston® (nebulised aztreonam lysine) or Promixin® (nebulised colistimethate sodium) as a nebuliser is provided when those drugs are purchased.
There is likely to be some on-going monitoring for all antibiotics used to suppress P aeruginosa. According to the committee, this would involve a full blood count, renal function tests and liver function tests, but it would be reasonable to assume this is equivalent across all treatments. For this reason, monitoring costs have not been included in the model as there is no opportunity cost created by switching from one treatment to another.
Table 119 below presents the drug doses and acquisition costs used to inform the model. Doses administered in the trials generally followed those advised by the BNF and the committee for this indication. The only exception to this was Hodson 2002 and Jensen 1987 who administered nebulised colistimethate sodium 1MU (80mg) bd, which is up to half of the dose received by adults in clinical practice today (2MU/ 160mg/ bd). In the base case, the dose administered in the trial is used to inform the model on the assumption that the dose can influence the treatment effect. However, for completeness, a sensitivity analysis using the upper dose has been explored.
Table 119Drug acquisition costs included model, chronic antibiotic treatment
| Antibiotic (quantity, basic price, unit cost) | Dose/day | Cost/day | Cost/28 days |
|---|---|---|---|
| Nebulised aztreonam lysine | |||
| Cayston® 75mg powder and solvent for nebuliser solution vials with Altera Nebuliser Handset (84, £2,181.53, £25.97) | 225mga | £77.91 | £2,181.53 |
| Colistimethate sodium | |||
| Dry powder inhalation | |||
| Colobreathe® 1,662,500 unit (125mg) inhalation powder capsules (56, £968.80, £17.30) | 250mg (3.325MU) | £34.60 | £968.80 |
| Nebulised | |||
| Promixin® 1million unit powder for nebuliser solution unit dose vials (30, £168.00, £5.60) | 2MU | £11.20 | £313.60 |
| Colomycin® 2million unit powder for solution for injection vials (10, £32.40, £3.24) | 2MU | £3.24 | £90.72 |
| Tobramycin | |||
| Dry powder inhalation | |||
| Tobi Podhaler® 28mg inhalation powder capsules with device (244, £1,790.00, £7.34) | 224mga | £58.69 | £1,643.28 |
| Nebulised | |||
| Tobi® / Tymbrineb® 300mg/5ml nebuliser solution 5ml ampoules (56, £1,305.92, £23.32) | 600mga | £46.64 | £1,305.92 |
| Bramitob® 300mg/4ml nebuliser solution 4ml ampoules (56, £1,187.00, £21.20) | 600mga | £42.39 | £1,187.00 |
- (a)
Alternate months
As shown in Table 119, more than 1 form of tobramycin and colistimethate sodium is available, and those forms can vary in their cost. As outlined in the NICE 2013 Guide to the methods of technology appraisal, the reduced price should be used in the base-case analysis to best reflect the price available to the NHS. For this reason, the lowest cost drugs (tobramycin, Bramitob®; colistimethate sodium, Colomycin®) are presented in the base case, but all drugs can be explored in the model using the user defined options.
Furthermore, there is an option in the model to apply discounts to the acquisition costs as the Department of Health has agreed Patient Access Schemes (PAS) with some manufacturers. Details of those discounts will not appear in any public facing documents to ensure confidentiality is not breached. Instead, the direction of the effect is presented in sensitivity analysis.
K.14.7.2. Exacerbations
The cost of hospitalisation used by Schechter 2015 ($29,205), inflated from the US paper by Briesacher 2011, was considerably greater than the costs estimated by Thornton 2005 and Tappenden 2013 for an exacerbation managed as an inpatient. For these reasons, the Schechter 2015 was considered to overestimate the cost of hospitalisations in the UK and was consequently not used to inform the model.
As described in Sections K.12.5.2 and K.13.5.3 the committee agreed a cost of £6,827 estimated by Thornton 2005, was reasonable to inform the model. However, to account for the lower cost used to inform the model by Tappenden 2013 and Tappenden 2014 for NICE TA276, a sensitivity analysis was conducted.
K.14.7.3. Lung transplant
As described in Section K.13.5.4.
K.14.7.4. Tinnitus
It is assumed a tinnitus is diagnosed following a consultation at a cost of £112 (NHS Reference Costs 2015/16, Non-Admitted Face to Face Attendance, First, WF01B, 120). It is assumed that tinnitus will be resolved once the drug is discontinued, hence no further costs are incurred.
K.14.8. Health-related quality of life
As stated in Section K.12.6, the QALY is NICE’s preferred measure of benefit for economic evaluation.
K.14.8.1. Lung function
As described in Section K.13.8.1 utility decreases with decreasing FEV1%.
K.14.8.2. Exacerbations
As described in Section K.13.8.2 the disutility incurred by the typical 2-week exacerbation is 0.095.
K.14.8.3. Lung transplant
As described in Section K.13.8.3 people have a utility of 0.83 following a lung transplant.
K.14.8.4. Tinnitus
The relative decrement for tinnitus was estimated from Iris 2011 (described in Section K.13.8.4) by calculating the percentage change from a person with a hearing impairment (utility 0.64) to a person aged 45–54 years without a hearing impairment (0.8203): 0.64/0.8203 = 78.02%. To calculate the relative decrement this is subtracted from 100% to produce a relative utility decrement of −21.98%.
K.14.9. Sensitivity analysis
K.14.9.1. Deterministic
A series of sensitivity analyses were undertaken in order to test how sensitive the results were to uncertainty in individual parameters. Parameters varied in the sensitivity analysis were chosen on the basis of uncertainty in their estimation or the potential impact that they had on the results. The values varied, along with their rationale are shown in Table 120.
Table 120Description of sensitivity analysis, chronic antibiotic treatment
| Analysis, parameter(s) to be changed | Default parameter value | Value tested | Rationale |
|---|---|---|---|
| 1. FEV1% strata utility values | Novartis analyses of Bradley 2010 | Solem 2014: FEV1%>70, 0.949 FEV1% 40–70, 0.918 FEV1% < 40%, 0.881 | Solem 2014 is a larger and more recent RCT that used data from a 48-week, Phase 3, multicentre study (STRIVE) to evaluate the relationship between EQ5D measures and FEV1% in 161 participants with CF. Solem 2014 was also used to inform a recent NICE TA398 |
| 2. Tinnitus excluded | 3.1% | 0% | TRAEs have not been included in previous economic evaluations in this area |
| 3. Exacerbation cost | £6,827 | £1,220 | The cost used to inform the models for NICE TA276 based on asthma complications was a lot cheaper than the cost reported by Thornton 2005 |
| 4. Number of exacerbations | 1 | 2 | The NMA outcome (at least 1 exacerbation) does not specify the number of exacerbations experienced |
| 5. FEV1% MD, tobramycin dry powder versus placebo | 4.4 (Galeva 2013) | 13.3 (Konstan 2011) | The studies were too heterogeneous to perform NMA, but both populations could be applicable to a UK population today |
| 6. FEV1% MD, nebulised tobramycin versus placebo | 6.7 (Chucalin 2007) | 13.58 (Lenoir 2007) | The studies were too heterogeneous to perform NMA, but both populations could be applicable to a UK population today |
| 7. Within-trial (time horizon reduced) | Lifetime (60 years) | 2 cycles | There is uncertainty surrounding the extrapolation of the 24-week efficacy data to a lifetime horizon |
| 8. Hodson 2002 clinical effectiveness | Benefits for nebulised tobramycin and nebulised colistimethate sodium over 4-weeks are maintained over the time horizon applied in the model | The month-off nebulised tobramycin follows the treatment effect for placebo, whilst the benefit from nebulised colistimethate sodium is maintained | The consequence of assuming a ‘month-on, month-off’ regimen is that the modelled treatment benefits reflect those associated with the continued use of nebulised tobramycin at only half of the cost of generating those benefits. Unless nebulised tobramycin is priced at parity with the cost of nebulised colistimethate sodium, or a month-off cycle is applied where the benefits from nebulised tobramycin reflect placebo, a substantial bias in favour of tobramycin may exist. |
| 9. Hodson 2002 clinical effectiveness | The cost of nebulised tobramycin is priced continuously | ||
| 10. Nebulised colistimethate sodium drug cost | Colomycin® | Promixin® | The best price available to the NHS is used to inform the base case (NICE 2013 Guides to the methods of technology appraisal), but other more expensive brands and preparations are available |
| 11. Nebulised tobramycin drug cost | Tobi®/Tymbrineb® | Bramitob® | |
| 12. Nebulised colistimethate sodium dose | 2MU once daily | 2MU bd | The dose received by adults in clinical practice today is up to double that used in the studies, if dose is not linked to effectiveness, the base case will underestimate the cost of treatment |
| 13. Probabilities obtained from OR in WinBugs | Calculated externally | Calculated internally | Probabilities can be calculated from ORs directly from WinBUGS, or outside of WinBUGS. This scenario is to test the consistency of the modelling software rather than any specific assumption |
| 14. PAS prices | List price | Discounts | The DoH agrees discounts with the manufacturer to increase accessibility, these discounts are confidential but can be used to reassess cost-effectiveness for the NHS |
bd, twice daily, CF, cystic fibrosis; DoH, Department of Health; FEV, forced expiratory volume; OR, odds ratio; MD, mean difference; MU, million units; NMA, network meta-analysis; PAS, patient access scheme; TA, Technology Appraisal
K.14.9.2. Probabilistic
PSA was conducted in the model to take account of the simultaneous effect of uncertainty relating to model parameter values for the fourth comparison (aztreonam versus nebulised tobramycin). PSA was not undertaken for the other comparisons as current NICE HTA recommendations cannot be challenged. This is not to say that the deterministic analysis was superfluous, as additional recommendations to the NICE HTA can be made and the model can reduce the uncertainty inherent in the models undertaken by the manufacturer or the TAG.
Key parameters in the model relating to costs, utility values and clinic effectiveness, were varied by sampling from probability distributions. The model was run for 10,000 simulations to generate estimates of total costs and total QALYs for aztreonam, nebulised tobramycin and the combination treatment by varying those parameters simultaneously. The model structure and model settings were kept constant.
A beta probability distributions was employed for probabilities and utilities, whilst a gamma or normal probability distribution was employed for costs. A recommended arbitrary starting point for unknown data was to assume the value of the standard deviation will be 20% of the expected input parameter mean.
For exacerbations, ORs used to inform the model were estimated from a NMA performed in WinBUGS. Coda output from WinBUGs lists the values generated from the full posterior distribution which can be used to inform each PSA simulation. Coda output were on the log-odds scale compared with placebo, these were subsequently exponentiated to obtain ORs. For completeness, probabilities obtained directly from the NMA are also included in the model and can be used to inform PSA if specified by the user.
When coda output is used, it is important that the correlations in the parameter estimates are preserved. This was done by ensuring that all parameter values are sampled from the same Bayesian Markov chain Monte Carlo iteration. When the coda output is stored as separate columns for each parameter with iteration values along the rows, this corresponds to sampling all the parameter values in 1 row, each time.
NHS Reference Costs give a mean cost and an UQR and LQR. They also provide data on the number of data submissions on which these summary statistics are based. The spreadsheet tool developed by the NCC-WCH (now NGA) which estimates parameters for a probabilistic sensitivity analysis has been described previously in Section K.13.9.2.
Parameters varied in PSA are provided in Table 121. Survival was not incorporated in probabilistic analysis as the source did not provide evidence which allows a probability distribution of effect size to be estimated. The committee also agreed that the survival estimate used in the model was reflective of mortality to date.
Table 121PSA parameters, chronic antibiotic treatment
| Parameter | Distribution | Mean | SD | Source |
|---|---|---|---|---|
| Costs | ||||
| Lung transplant procedure | Normal | £39,689 | £4,330a | NHSRC 2015/16 DZ01Z |
| Lung transplant monitoring | Gamma | £62,944 | £6,423b | Anyanwu 2002 |
| Exacerbation cost | Normal | £6,827 | £715a | Thornton 2005 |
| Tinnitus | Normal | £112 | £1a | NHSRC 2015/16 WF01B |
| Utility | ||||
| Disutility minor | Beta | 0.015 | 0.048 | Bradley 2010/Tappenden 2013 |
| Disutility major | Beta | 0.174 | 0.341 | Bradley 2010/Tappenden 2013 |
| >70 | Beta | 0.864 | 0.165 | Bradley 2010/Tappenden 2013 |
| 40–70 | Beta | 0.810 | 0.216 | Bradley 2010/Tappenden 2013 |
| <40 | Beta | 0.641 | 0.319 | Bradley 2010/Tappenden 2013 |
| Lung transplant | Beta | 0.830 | 0.180 | Anyanwu 2001 |
| Tinnitus | Beta | 0.640 | 0.280 | Iris 2011 |
| Lung function (FEV1%) | ||||
| Starting FEV1% for natural history | Normal | 83.1 | 19.3a | CF Registry 2014 |
| Improvement from aztreonam | Normal | 2.05 | 0.69 | Assael 2013 |
| Improvement from nebulised tobramycin | Normal | −0.66 | 0.72 | Assael 2013 |
| Probabilities | ||||
| Tinnitus | Beta | 3.1% | 0.32%b | Ramsey 1999 |
| Lung transplant | Beta | 0.9% | 0.09%b | Tappenden 2013 |
CF, cystic fibrosis; FEV, forced expiratory volume; NHSRC, NHS Reference Costs; SD, standard deviation; TA, Technology Appraisal; PSA, probabilistic sensitivity analysis
- (a)
Estimated using the spreadsheet tool that identifies the best fitting SD according to the range and number of data submissions
- (b)
When the evidence did not allow a probability distribution of effect size to be estimated an arbitrary starting point was to assume the value of the SD will be 20% of the expected input parameter mean.
K.14.10. Model validation
Provided in K.15.
K.14.11. Results
K.14.11.1. Comparison 1
When a fully incremental analysis is performed the interventions are sequentially ranked in order of cost from the least expensive (placebo) to the most expensive (tobramycin dry powder). Interventions that are followed by more expensive and less effective alternatives are excluded as they are dominated.
In Table 122, tobramycin dry powder is dominated by nebulised tobramycin (and nebulised colistimethate sodium) and subsequently excluded. ICERs are then recalculated for the remaining interventions (nebulised colistimethate sodium versus placebo and nebulised tobramycin versus nebulised colistimethate sodium).
Table 122Comparison 1 results
| Treatment | Total costs | Total QALYs | Inc. costs | Inc. QALYs | ICER |
|---|---|---|---|---|---|
| Placebo | £92,040 | 11.25 | - | - | - |
| Nebulised colistimethate sodium | £105,872 | 11.52 | £13,833 | 0.33 | £52,168 |
| Nebulised tobramycin | £244,919 | 11.57 | £139,047 | 0.05 | £2,824,240 |
| Tobramycin dry powder | £274,658 | 11.43 | £29,739 | −0.14 | Dominated |
Given that tobramycin dry powder has a lower MD in FEV1% (Section K.14.5.1), higher long-term exacerbation OR (Section K.14.5.2) and higher drug acquisition cost (Section K.14.7.1) compared to nebulised colistimethate sodium and nebulised tobramycin, it is unsurprising that tobramycin dry powder is dominated.
In Figure 29 all comparators have ICERs above NICE’s advisory threshold of £20,000 to £30,000 per QALY which is illustrated with point estimates in the north-east quadrant above the threshold. Moreover, tobramycin dry powder lies to the north-west of nebulised tobramycin and nebulised colistimethate sodium as it is dominated (less effective and more expensive).
With regards to current NICE HTA recommendations, nebulised colistimethate sodium is recommended as the first line treatment for this indication, ahead of colistimethate sodium dry powder, nebulised tobramycin and tobramycin dry powder, which reflects the ordering of treatments in Table 122. However, the ICER for nebulised colistimethate sodium is above NICE’s upper advisory cost-effective threshold of £30,000.
It is important to note that the results presented here are not informed by PAS, which could provide a cost-effective decision in favour of antibiotic treatment.
In addition, the results for nebulised colistimethate sodium are based on an exacerbation ratio equal to nebulised tobramycin (short-term OR, 3.00; long-term OR, 0.88) due to insufficient data. For nebulised colistimethate sodium to be considered cost-effective under threshold of £30,000 per additional QALY relative to placebo, the long-term OR (to 2 decimal places) would need to be less than 0.82 (35% probability of exacerbations) and under a threshold of £20,000 less than 0.78 (34% probability of exacerbations).
Sensitivity analysis
Table 123 below presents the results of sensitivity analysis described in Table 120.
Table 123Comparison 1 SA results
| Treatment | Total costs | Total QALYs | ICER |
|---|---|---|---|
| FEV1% utility | |||
| Placebo | £92,040 | 12.91 | - |
| Nebulised colistimethate sodium | £105,872 | 13.06 | £93,317 |
| Nebulised tobramycin | £244,919 | 13.10 | £2,987,483 |
| Tobramycin dry powder | £274,658 | 12.99 | Dominated |
| Tinnitus excluded | |||
| Placebo | £92,040 | 11.25 | - |
| Nebulised colistimethate sodium | £105,872 | 11.52 | £52,168 |
| Nebulised tobramycin | £205,702 | 11.53 | £6,981,278 |
| Tobramycin dry powder | £254,328 | 11.39 | Dominated |
| Exacerbation cost | |||
| Placebo | £19,008 | 11.25 | - |
| Nebulised colistimethate sodium | £37,435 | 11.52 | £69,499 |
| Nebulised tobramycin | £177,274 | 11.57 | £2,840,324 |
| Tobramycin dry powder | £201,894 | 11.43 | Dominated |
| Number of exacerbations | |||
| Placebo | £180,963 | 10.02 | - |
| Nebulised colistimethate sodium | £189,200 | 10.37 | £24,043 |
| Nebulised tobramycin | £327,283 | 10.43 | £2,206,522 |
| Tobramycin dry powder | £363,255 | 10.20 | Dominated |
| Konstan 2011 FEV1% effect for tobramycin dry powder | |||
| Placebo | £92,040 | 11.25 | - |
| Nebulised colistimethate sodium | £105,872 | 11.52 | £52,168 |
| Nebulised tobramycin | £244,919 | 11.57 | £2,824,240a |
| Tobramycin dry powder | £274,077 | 11.65 | £355,982b |
| Lenoir 2007 FEV1% effect for nebulised tobramycin | |||
| Placebo | £92,040 | 11.25 | - |
| Nebulised colistimethate sodium | £105,872 | 11.52 | £52,168 |
| Nebulised tobramycin | £244,494 | 11.74 | £622,576 |
| Tobramycin dry powder | £274,658 | 11.43 | Dominated |
| Within-trial analysis | |||
| Placebo | £3,171 | 0.42 | - |
| Nebulised colistimethate sodium | £4,421 | 0.42 | Dominated |
| Nebulised tobramycin | £8,417 | 0.42 | £1,524,290 |
| Tobramycin dry powder | £9,641 | 0.43 | £179,805 |
| Nebulised colistimethate sodium cost | |||
| Placebo | £92,040 | 11.25 | - |
| Nebulised colistimethate sodium | £146,602 | 11.52 | £205,775 |
| Nebulised tobramycin | £244,919 | 11.57 | £1,996,961 |
| Tobramycin dry powder | £274,658 | 11.43 | Dominated |
| Nebulised tobramycin drug cost | |||
| Placebo | £92,040 | 11.25 | - |
| Nebulised colistimethate sodium | £105,872 | 11.52 | £52,168 |
| Nebulised tobramycin | £251,445 | 11.57 | £2,956,786 |
| Tobramycin dry powder | £274,658 | 11.43 | Dominated |
| Nebulised colistimethate sodium dose | |||
| Placebo | £92,040 | 11.25 | - |
| Nebulised colistimethate sodium | £123,783 | 11.52 | £119,716 |
| Nebulised tobramycin | £244,919 | 11.57 | £2,460,447 |
| Tobramycin dry powder | £274,658 | 11.43 | Dominated |
| WinBUGS | |||
| Placebo | £92,171 | 11.25 | - |
| Nebulised colistimethate sodium | £106,293 | 11.51 | £54,079 |
| Nebulised tobramycin | £245,335 | 11.56 | £2,820,226 |
| Tobramycin dry powder | £275,109 | 11.42 | Dominated |
| PAS | |||
| Placebo | No change | 11.25 | - |
| Nebulised colistimethate sodium | No change | 11.52 | No change |
| Nebulised tobramycin | Reduced | 11.57 | Reduced (>£30,000) |
| Tobramycin dry powder | Reduced | 11.43 | Dominated |
FEV, forced expiratory volume; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; PAS, patient access scheme; SA, sensitivity analysis
- (a)
Extended dominance
- (b)
ICER £1,293,885 when nebulised tobramycin excluded
Tobramycin dry powder is dominated in all scenarios except for when more favourable FEV1% data from Konstan 2011 is explored (13.3 versus 4.4) and when the model does not extrapolate data to a lifetime horizon. A higher MD compared to placebo means that people with cystic fibrosis will remain in a higher FEV1% strata for longer, increasing their QALY gains as their transition to lower strata associated with a lower quality of life is delayed. The favourable result for tobramycin dry powder is expected for a “within-trial” analysis, given that nebulised tobramycin and nebulised colistimethate sodium were associated with much higher short-term ORs than tobramycin dry powder (3.0 versus 1.1). However, in both scenarios the ICER for tobramycin dry powder remains above NICE’s advisory threshold.
Reducing the time horizon means the effects on lung function will not be realised as it takes many years for a person to transition between the FEV1% strata (>70, 40–70, <40). As a result, there will be little or no difference in FEV1% in terms of quality of life, so the difference will be driven by exacerbations and tobramycin related tinnitus.
Using the utility values reported by Solem 2014 reduces the range in quality of life between the FEV1% strata from 0.169 (0.949 – 0.881) to 0.068 (0.81 – 0.641). Consequently, this favours the less effective treatment (placebo) as the incremental QALY gains will be reduced.
It is evident that excluding tobramycin related tinnitus reduces the cost and increases the benefits obtained from tobramycin treatment, subsequently favouring tobramycin.
Reducing the cost of an exacerbation favours the least effective treatment, whereas, increasing the number of exacerbations favours the more effective treatment. The latter is the only scenario that reduces the ICER for nebulised colistimethate sodium, but the ICER remains above NICE’s £20,000 threshold for cost-effectiveness.
Overall, the results are not sensitive to the scenarios explored and none of the treatments would be considered cost-effective compared to placebo, unless PAS are in place, or the number of exacerbations in in the base case is underestimated.
K.14.11.2. Comparison 2
Nebulised tobramycin would not be considered cost-effective relative to nebulised colistimethate sodium as the ICER is substantially above NICE’s advisory threshold of £20,000 to £30,000 per additional QALY. This is illustrated in Figure 30 with a point estimate in the north-east quadrant above NICE’s threshold of £20,000 per QALY.
Table 124Comparison 2 results
| Treatment | Total costs | Total QALYs | Inc. costs | Inc. QALYs | ICER |
|---|---|---|---|---|---|
| Nebulised colistimethate sodium | £107,149 | 11.35 | - | - | - |
| Nebulised tobramycin | £244,890 | 11.58 | £137,741 | 0.23 | £602,472 |
With regards to current NICE HTA recommendations (NICE TA276), nebulised tobramycin would only be recommended when colistimethate sodium is contraindicated, is not tolerated or has not produced an adequate clinical response and the manufacturer provides tobramycin with the discount agreed as part of the PAS to primary, secondary and tertiary care in the NHS. Based on those criteria, the ICER may be cost-effective.
It is important to note that results for nebulised colistimethate sodium are based on an exacerbation ratio equal to nebulised tobramycin (short-term OR, 3.00; long-term OR, 0.88) due to insufficient data. For nebulised tobramycin to be considered cost-effective under a threshold of £30,000 per additional QALY, the long-term OR for exacerbations for nebulised colistimethate sodium (to 2 decimal places) would need to be at least 3.50.
Even though there is assumed to be no difference between the drugs with regards to exacerbations, nebulised tobramycin has a much higher MD in FEV1% (Section K.14.5.2) than nebulised colistimethate sodium, subsequently driving the incremental QALY gain for nebulised tobramycin.
Sensitivity analysis
Table 125 below presents the results of the sensitivity analysis described in Table 120. In all scenarios, the ICER for nebulised tobramycin remains substantially above NICE’s cost-effectiveness threshold.
Table 125Comparison 2 SA results
| Treatment | Total costs | Total QALYs | ICER |
|---|---|---|---|
| FEV1% utility | |||
| Nebulised colistimethate sodium | £107,149 | 12.99 | - |
| Nebulised tobramycin | £244,890 | 13.10 | £1,226,546 |
| Tinnitus excluded | |||
| Nebulised colistimethate sodium | £125,002 | 11.35 | - |
| Nebulised tobramycin | £205,664 | 11.54 | £416,476 |
| Within-trial analysis | |||
| Nebulised colistimethate sodium | £4,421 | 0.42 | - |
| Nebulised tobramycin | £8,417 | 0.42 | £1,524,290 |
| Hodson 2002 (FEV1%) | |||
| Nebulised colistimethate sodium | £107,149 | 11.35 | - |
| Nebulised tobramycin | £245,239 | 11.48 | £1,023,402 |
| Hodson 2002 (cost) | |||
| Nebulised colistimethate sodium | £107,149 | 11.35 | - |
| Nebulised tobramycin | £308,804 | 11.58 | £882,028 |
| Nebulised colistimethate sodium cost | |||
| Nebulised colistimethate sodium | £147,748 | 11.35 | - |
| Nebulised tobramycin | £244,890 | 11.58 | £424,896 |
| Nebulised tobramycin drug cost | |||
| Nebulised colistimethate sodium | £107,149 | 11.35 | - |
| Nebulised tobramycin | £251,416 | 11.58 | £631,016 |
| Nebulised colistimethate sodium dose | |||
| Nebulised colistimethate sodium | £125,002 | 11.35 | - |
| Nebulised tobramycin | £244,890 | 11.58 | £524,384 |
| WinBUGS | |||
| Nebulised colistimethate sodium | £107,568 | 11.34 | |
| Nebulised tobramycin | £245,306 | 11.57 | £602,328 |
| PAS | |||
| Nebulised colistimethate sodium | No change | 11.35 | - |
| Nebulised tobramycin | Reduced | 11.58 | Reduced (>£30,000) |
FEV, forced expiratory volume; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; PAS, patient access scheme; SA, sensitivity analysis
K.14.11.3. Comparison 3
Base case
Table 126 and Figure 31 show that colistimethate sodium dry powder is dominated by nebulised tobramycin as it is more expensive and less effective. The incremental QALYs are negligible, but colistimethate sodium dry powder would need to provide an additional 1.55 QALYs to be considered cost-effective under a £20,000 threshold (incremental cost ÷ £20,000 = incremental QALY gain). This result is unsurprising given the drugs similar effects on FEV1% (Section K.14.5.1) and greater drug acquisition cost of colistimethate sodium dry powder (Section K.14.7.1).
These results reflect those found by Tappenden 2014, summarised previously in Section K.14.1. To reiterate, when their model was informed using the list price, nebulised tobramycin dominated colistimethate sodium dry powder as it was cheaper and more effective. However, under the PAS, colistimethate sodium dry powder became less expensive than nebulised tobramycin with an ICER of £288,563 in the south-west quadrant of the cost-effectiveness plane.
Table 126Comparison 3 results
| Treatment | Total costs | Total QALYs | Inc. costs | Inc. QALYs | ICER |
|---|---|---|---|---|---|
| Colistimethate sodium dry powder | £276,593 | 11.37 | - | - | - |
| Nebulised tobramycin | £245,561 | 11.41 | -£31,032 | 0.04 | Dominant |
Sensitivity analysis
Table 127 below presents the results of applicable sensitivity analysis described in Table 120.
Table 127Comparison 3 SA results
| Treatment | Total costs | Total QALYs | ICER |
|---|---|---|---|
| FEV1% utility | |||
| Colistimethate sodium dry powder | £276,593 | 13.00 | - |
| Nebulised tobramycin | £245,561 | 13.04 | Dominant |
| Tinnitus excluded | |||
| Colistimethate sodium dry powder | £276,593 | 11.37 | - |
| Nebulised tobramycin | £206,574 | 11.37 | Dominant |
| Within-trial analysis | |||
| Colistimethate sodium dry powder | £10,563 | 0.42 | - |
| Nebulised tobramycin | £8,417 | 0.42 | Dominant |
| Nebulised tobramycin drug cost | |||
| Colistimethate sodium dry powder | £276,593 | 11.37 | - |
| Nebulised tobramycin | £252,082 | 11.41 | Dominant |
| WinBUGS | |||
| Colistimethate sodium dry powder | £277,013 | 11.36 | |
| Nebulised tobramycin | £245,976 | 11.40 | Dominant |
| PAS | |||
| Colistimethate sodium dry powder | Reduced | 11.37 | - |
| Nebulised tobramycin | Reduced | 11.41 | Increased (>£20,000) |
FEV, forced expiratory volume; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; PAS, patient access scheme; SA, sensitivity analysis
Overall, the results infer that nebulised tobramycin should be recommended above colistimethate sodium dry powder over the longer term if the population assessed by Schuster 2013 is representative of the UK population with cystic fibrosis. However, PAS could change this decision.
K.14.11.4. Comparison 4
Base case
Aztreonam is more effective and more costly than nebulised tobramycin, but the ICER is just above NICE’s upper advisory threshold of £30,000 per additional QALY (Table 128).
The combination treatment is dominated by aztreonam as it is more expensive and less effective. This is unsurprising given the higher acquisition cost to receive continuous antibiotic treatment as opposed to alternate months. Also, the probability of exacerbations obtained from the OR for the combination treatment lied in between nebulised tobramycin and aztreonam.
A result in favour of aztreonam is also to be expected as aztreonam was found to reduce exacerbations more than any of the treatments included in the NMA (Table 118). The study by Assael 2013 (Table 117) also showed that aztreonam increased FEV1% (2.05) whereas nebulised tobramycin reduced it (−0.66). Moreover, the trial by Flume 2016 found nebulised tobramycin provided little improvement in FEV1% (+0.04).
Figure 32 illustrates these estimates on a cost-effectiveness plane, where both point estimates compared to nebulised tobramycin lie in the north-east quadrant above NICE’s threshold of £20,000 per QALY.
Table 128Comparison 4 results
| Treatment | Total costs | Total QALYs | Inc. costs | Inc. QALYs | ICER |
|---|---|---|---|---|---|
| Nebulised tobramycin | £245,830 | 11.35 | - | - | - |
| Nebulised aztreonam lysine | £265,151 | 11.91 | £19,321 | 0.56 | £34,348 |
| Combinationa | £340,265 | 11.53 | £75,114 | −0.38 | Dominated |
ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years
- (a)
28 days aztreonam lysine (nebulised) alternating with 28 days tobramycin (nebulised))
Sensitivity analysis
In Table 129 all analyses except increasing the number of exacerbations and increasing the acquisition cost of nebulised tobramycin increase the ICER for aztreonam above the base case. Increasing the number of exacerbations favours the more effective treatment (aztreonam), whereas reducing the cost of exacerbations favours the less effective treatment (tobramycin).
Reducing the cost of exacerbations, excluding tobramycin related tinnitus and reducing the time horizon increases the ICER for aztreonam above NICE’s upper advisory cost-effectiveness threshold for reasons previously described. In all analyses, the combination treatment is dominated.
Table 129Comparison 4 SA results
| Treatment | Total costs | Total QALYs | ICER |
|---|---|---|---|
| FEV1% utility | |||
| Nebulised tobramycin | £245,830 | 13.02 | - |
| Nebulised aztreonam lysine | £265,151 | 13.52 | £38,946 |
| Combinationa | £340,265 | 13.16 | Dominated |
| Tinnitus excluded | |||
| Nebulised tobramycin | £206,944 | 11.32 | - |
| Nebulised aztreonam lysine | £265,151 | 11.91 | £97,589 |
| Combinationa | £407,141 | 11.58 | Dominated |
| Exacerbation cost | |||
| Nebulised tobramycin | £178,473 | 11.35 | - |
| Nebulised aztreonam lysine | £226,773 | 11.91 | £85,867 |
| Combinationa | £292,220 | 11.53 | Dominated |
| Number of exacerbations | |||
| Nebulised tobramycin | £327,843 | 10.21 | - |
| Nebulised aztreonam lysine | £311,879 | 11.27 | Dominant |
| Combinationa | £398,765 | 10.51 | Dominated |
| Within-trial analysis | |||
| Nebulised tobramycin | £8,417 | 0.42 | - |
| Nebulised aztreonam lysine | £10,456 | 0.45 | £66,459 |
| Combinationa | £14,631 | 0.44 | Dominated |
| Nebulised tobramycin drug cost | |||
| Nebulised tobramycin | £252,348 | 11.35 | - |
| Nebulised aztreonam lysine | £265,151 | 11.91 | £22,760 |
| Combinationa | £346,664 | 11.53 | Dominated |
| WinBUGs | |||
| Nebulised tobramycin | £246,244 | 11.34 | - |
| Nebulised aztreonam lysine | £266,532 | 11.89 | £36,947 |
| Combinationa | £341,972 | 11.51 | Dominated |
| PAS | |||
| Nebulised tobramycin | Reduced | 11.35 | - |
| Nebulised aztreonam lysine | Reduced | 11.91 | Reduced (<£30,000) |
| Combinationa | Reduced | 11.53 | Dominated |
FEV, forced expiratory volume; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; PAS, patient access scheme; SA, sensitivity analysis
- (a)
28 days aztreonam lysine (nebulised) alternating with 28 days tobramycin (nebulised)
Probabilistic analysis
Unlike the previous 3 comparisons, there are currently no NICE HTA recommendations with regards to aztreonam treatment to supress chronic P aeruginosa. For this reason, PSA was performed to represent the uncertainty in the parameter estimates.
The cost-effectiveness plane in Figure 33 illustrates 10,000 simulations. Those simulations for aztreonam and combination (both compared to nebulised tobramycin) are distributed predominantly across the north-east quadrant of the cost-effectiveness plane, where they are more expensive and more effective than nebulised tobramycin. To aid interpretation of those simulations in Figure 33, Table 130 presents the percentage of simulations in each quadrant when the model is run using list prices and PAS prices.
Table 130Proportion of simulations in each quadrant
| Treatment | NW | NE | SW | SE |
|---|---|---|---|---|
| List price | ||||
| Nebulised aztreonam lysine | 392 (3.9%) | 9,330 (93.3%) | 4 (1.1%) | 274 (2.7%) |
| Combinationa | 2,331 (23.3%) | 7,669 (76.7%) | 0 | 0 |
| PAS prices | ||||
| Nebulised aztreonam lysine | 261 (2.6%) | 9,398 (94.0%) | 4 (0.0%) | 337 (3.4%) |
| Combinationa | 1,699 (17.0%) | 8,301 (83.0% | 0 | 0 |
NW, north-west, more expensive and less effective than azithromycin; NE, north-east, more expensive and more effective than azithromycin; SW, south-west, less expensive and less effective than azithromycin; SE, south-east, less expensive and more effective than azithromycin
- (a)
28 days aztreonam lysine (nebulised) alternating with 28 days tobramycin (nebulised)
Using the list price, the average probabilistic ICER for aztreonam is £38,154 and the combination treatment ICER is dominated, as it more expensive and less effective than aztreonam. Conversely, the ICER for aztreonam falls below £30,000 when the model is run using PAS prices.
The CEAC illustrated in Figure 34 shows that nebulised tobramycin is the most optimal treatment up to a threshold of £30,000 to £40,000 per QALY, whilst the combination treatment has a 0% probability of being the most cost-effective option under the thresholds tested.
K.14.12. Discussion
The economic model developed for this review was based on committee opinion regarding current treatment pathways and systematic reviews of the evidence. The structure of the model closely followed existing models for this indication by using a Markov state transition model to estimate transitions between FEV1% strata.
A key strength of this analysis was that clinical and economic systematic reviews were conducted to a high standard, including comprehensive search strategies, and study selection, data extraction and quality assessment according to pre-defined protocols.
Similarly to the analysis undertaken by Tappenden 2013 and Tappenden 2014, the economic model provides results for a lifetime horizon and a reduced “within trial” time horizon to remove the uncertainty associated with the extrapolation of trial data. Reducing the time horizon of the model means the effects on lung function will not be realised as it takes many years for a person to transition between the FEV1% strata (>70, 40–70, <40). As a result, there will be little or no difference in FEV1% in terms of quality of life, so the difference will be driven by exacerbations. However, if there is no difference between the drugs in terms of exacerbations, the results will be driven by drug costs and treatment related adverse events.
As with most analyses that take a lifetime horizon, the committee stated that it was important to note that future mortality used to inform the model is overestimated because survival will increase in the future due to advances in technology and research.
The committee also raised that inhaled antibiotics can get less effective over time, which may overstate the effectiveness of drugs such as tobramycin in the longer term. This was also stated by Tappenden 2013, who added that their model did not include the potential impact of resistance to tobramycin as it is unclear how this phenomenon would manifest in terms of reduced treatment effect.
This model is not the first to assess the cost-effectiveness of antimicrobial agents. However, the model (comparison 3) can be used to strength the findings from Tappenden 2013 who found that colistimethate sodium dry powder is dominated by nebulised tobramycin according to list prices, and less expensive and less effective when the PAS discount is applied to colistimethate sodium dry powder. The model (comparison 1) also supports the findings from Tappenden 2014 that nebulised tobramycin is cost-effective compared to tobramycin dry powder using list prices, or PAS prices. Neither Tappenden 2013 nor Tappenden 2014 included nebulised colistimethate sodium or “no treatment” in their assessments. Despite this, the Appraisal committee for NICE TA276 concluded that nebulised colistimethate sodium should be offered first line. This result is reflected in the model developed for this review, as all remaining treatments included in that comparison (comparison 1) had ICERs above NICE’s advisory threshold for cost-effectiveness.
The cost-utility analysis by Schecter 2015 found aztreonam to dominate nebulised tobramycin, whereas aztreonam was more expensive than nebulised tobramycin in the model developed for this review. Schecter 2015 took a third party US perspective and applied a much greater cost to treat an exacerbation ($29,205 versus £6,738). Given that aztreonam is more effective at reducing exacerbations, a higher cost favours aztreonam by increasing the incremental cost of tobramycin. When a cost of £23,000 to treat an exacerbation is applied to the model, aztreonam dominates nebulised tobramycin, which demonstrates external consistency. It is also important to note that aztreonam was associated with a lower acquisition cost than nebulised tobramycin in the model by Schecter 2015 which is not reflective of current UK pricing.
In clinical practice, people with cystic fibrosis can switch antimicrobial treatments or receive a combination of antimicrobial treatments. As a result, this reduces the relevance of the comparisons identified from the clinical evidence review to inform the committee’s recommendations. Moreover, newer trials include participants that are not treatment naïve to tobramycin which reduces the reliability of their results compared to newer treatments that may work better initially. The trial by Flume 2015 who included a combination treatment was also subject to this limitation, reducing the reliability of their findings to make a strong recommendation regarding combination treatments. For these reasons, the committee may want to consider a research recommendation to assess the cost-effectiveness of different combinations in participants who are naive to treatment, if such a trial is plausible.
Unfortunately the studies that reported changes in FEV1% were too heterogeneous to perform a reliable NMA and subsequently, a fully incremental analysis. The NMA planned for exacerbations was also somewhat problematic as the studies reported different outcome measures related to an exacerbation, reducing the number of studies that could be included in the network, from the pool that met other eligibility criteria. As a result, one of the most commonly reported outcome was used, but exacerbation data remained unavailable for nebulised colistimethate sodium and inhaled colistimethate sodium, which meant cost-effectiveness was driven by changes in lung function. For completeness, threshold analyses was undertaken to identify the probability of experiencing exacerbations needed to change the decision from cost-ineffective to cost-effective.
The committee agreed that the outcome used for the NMA on exacerbations (number of people experiencing at least 1 exacerbation) was a useful clinical outcome measure, as any number of exacerbations would be a bad outcome for the person with cystic fibrosis and treatment should aim to remove the chance of experiencing all exacerbations. Given that the outcome was dichotomous, the exact number of exacerbations >1 was unknown. For this reason, 1 exacerbation was used to inform the base case, but 2 was explored in sensitivity analysis. As shown in the results of those analyses, increasing the number of exacerbations favours the more effective treatment.
Finally, this model did not examine the effects of adherence to treatment outcomes which could potentially vary across the treatments under consideration. Adherence with treatment in general is recognised as poor in people with cystic fibrosis and would be important to include when data are available.
K.14.13. Conclusion
Given that NICE HTA recommendations are in place for tobramycin and colistimethate sodium (NICE TA276), the ability of the first 3 comparisons in the model to inform the committee’s recommendations is limited. However, if “no treatment” is not an option, the results (comparison 1) reflect NICE TA276 to offer colistimethate sodium as first line.
The model has provided evidence that aztreonam could displace nebulised tobramycin based on PAS prices, as the deterministic and probabilistic ICERs are within NICE’s upper threshold. Within the same comparison, aztreonam was shown to dominate the combination treatment, using list price or PAS prices, inferring that the additional benefits provided by tobramycin do not outweigh its additional cost. However, the limitations of the trials used to inform this comparison mean the results should be interpreted with caution.
Overall, the model is limited by the small number of trials available, and limited comparability of evidence across the trials and comparability to UK clinical practice. To reduce this uncertainty, research recommendations should be considered, to assess the cost-effectiveness of combination strategies currently in place.
The committee’s discussion regarding the associated economic benefits and harms are reported in the Full Guideline Section 9.4.3.9.3 ‘Evidence to recommendations’.
K.15. Model validation
Validation was assessed using 2 primary criteria, internal (verification) and external consistency (validation). Internal validity addresses whether the model has been implemented correctly, and examines the extent to which the mathematical calculations are performed correctly and are consistent with the model’s specifications. Face validation helps to ensure a model is constructed and used in accord with the best available evidence. This process enhances credibility with experts and increases acceptance of results.
Internal validity was assessed by the primary modeller for each model, and a second health economist who also completed the Philips 2004 checklist for each model (Table 131, Table 132 and Table 133). The following areas of the models were checked:
- plausibility and accuracy of inputs and assumptions;
- programming of formulae and macros;
- efficacy and cost parameters were altered to check whether results changed in the expected direction;
- sensitivity analyses using zero and extreme values were undertaken to check whether results changed as expected;
- input parameters in all arms of the model were set at the same value to check whether outputs (costs and QALYs) in all arms became equal.
External consistency was assessed by assessing the face validity of the model, and comparing the results of the analysis against the clinical evidence review and other published data (cross validation). It was also assessed with members of the committee whether the setting, population, interventions, outcomes, assumptions, and time horizons correspond to those of decision problem.
Table 131Philips checklist for cross-infection
| Section | Pass/fail | Comments |
|---|---|---|
| Structure | ||
| Statement of decision problem / objective | P | There is a clear statement of the decision problem under consideration in the introduction section; specifically that the model will consider the economic impact vs no strategy of various strategies for the control of cross-infection in cystic fibrosis patients. Further detail on the population, interventions and pathogens is also included in the methods section. The objective of the analysis is consistent with this statement of the decision problem |
| Justification of modelling approach | P | There is a clear justification of the modelling strategy in the ‘methods: model structure’ section. No justification was given for the modelling framework selected, but this is consistent with Philips (2004) since “a model is simply and analytical framework with the purpose of synthesising the relevant evidence” |
| Statement of scope / perspective | P | The scope of the model is strictly defined by the NICE methods manual, although the author does highlight some key areas of uncertainty in the ‘methods: clinical effectiveness’ section. The model scope was heavily restricted by data availability, and this is reflected in the write-up |
| Structural assumptions | P | The structure of the model is consistent with a coherent theory of the natural history of the disease. The model structure does not describe a series of causal relationships between interventions and outcomes because of the confounding factor of non-clinic related infections, which is addressed in the model and therefore not relevant to the structural assumption check. The sources of data used to develop the model are clearly described and referenced and the model is independent of any particular model of service provision (although generic features of service provision are present in the model). |
| Strategies / Comparators | P | There is a clear definition of the data sources underpinning assumptions about the effectiveness of various strategies; the writeup notes that the data sources themselves are unclear on the exact procedure for enacting each different strategy. The strategies are not included in a statement of the decision problem, as there are multiple comparators. No detailed discussion of exclusions is recorded, but it is clearly implied that the evidence search was exhaustive and therefore feasible options which were not included in the model were not included for data availability reasons. |
| Model type | P | Deterministic decision tree is an entirely appropriate model for this decision question |
| Time horizon | P | The use of a non-standard one-year time horizon is clearly outlined in the text and justified strongly |
| Health states/disease pathways | P | The model uses paths in a decision tree model as the modelling substrate for disease states; the number and type of health states are clearly justified and recorded in the text |
| Cycle Length | N/A | Cycle length not relevant to a decision tree |
| Parsimony | P | In the view of the reviewer, the model is highly parsimonious, with the core decision tree being handled in a transparent way and the various costing ‘options boxes’ - although somewhat more complicated - clearly labelled and allowing important customisation options. |
| Data | ||
| Data Identification | P | Data identification is performed by a specialist information scientist |
| Data Synthesis | P | Data has been synthesised using standard methods for a deterministic decision tree. The only notable departure from standard methodology is the synthesis of incidence and prevalence results, which is justified in the write-up and made necessary by the data sources reporting different outcome measures. |
| Discounting | P | No discount rate was applied. This decision is justified in the write-up and consistent with the NICE Methods Manual |
| Analysis of trial data | P | It was not possible to analyse the trials included at the patient level. ITT-type considerations are not relevant to this model, although a discussion of similar issues occurs around the Thornton 2005 paper, where patients randomised to the ‘hospital’ group did not receive all of their care in hospital |
| Treatment effects | P | This is not strictly relevant as trials reported the absolute probability of infection (or absolute prevalence of infection, sometimes). Nevertheless the handling of these data are appropriate in the model. |
| Transition probabilities | P | Transition probabilities are simple to calculate in a decision tree, and are handled appropriately in this model. The probabilities are given in the write-up |
| Mortality | N/A | The time horizon of this model means that all-cause mortality is not relevant to the decision problem. This is not explained in the write-up. |
| Extrapolation | P | Several assumptions of this sort exist in the model (for example that patients with a terminal chronic infection will die at exactly halfway through their treatment costs). Most of these assumptions are justified with reference to the Guideline committee. This is appropriate, because the modelling supports their work and draws on their expert opinion. |
| Risk factors | N/A | No risk factors were included in the model as the data could not support such additions. The results indicate that such risk factors are probably not relevant to the decision problem. |
| Utilities | P | HRQoL, the sources of information on HRQoL and analysis of the different possible modelling choices are carefully described in the section ‘1.6 Methods: health-related quality of life’ |
| Charges and costs | P | Resource use is described in section ‘1.5 Methods: resource and cost use’. The model uses mostly entirely standard sources (PSSRU or NHS Reference Costs), with some nonstandard sources such as academic literature and NHS Estates data. The most uncertain resource tariff (single vs shared-occupancy rooms) was well grounded in the literature and the justification for using weak evidence was robust throughout. |
| Adjustment over time / between countries | P | No adjustment has been made between countries as this was not relevant. Adjustment between time periods has been made based on the hospital & community health services (HCHS) index, which is a standard method |
| Half-cycle correction | N/A | Half cycle correction would not be standard methodology for a decision tree |
| Data incorporation | P | The model appears to be internally consistent with respect to its choice of measurement units, time intervals and population characteristics. The sources of data are clearly described in the ‘methods’ sections (most explicitly in section ‘1.4 Methods: clinical effectiveness’), with sufficient discussion to allow for an intelligent assessment of the data quality. |
| Uncertainty | ||
| General statement regarding sensitivity analysis | P | The write-up includes a general statement outlining the strategy for sensitivity analysis |
| Structural | N/A | The general form of a deterministic decision tree is clearly the most appropriate for performing this kind of analysis, and so it would not improve the model to attempt re-analysis using a different structure |
| Methodological | N/A | Methodological uncertainty cannot systematically be explored in a NICE cost-effectiveness analysis; the analyst is constrained by the Reference Case |
| Parameter | P | The model is deterministic, and parameter uncertainty over estimates of effectiveness do not appear to have been translated into the model. Nevertheless, key values have been varied in sensitivity analysis, limiting the extent to which the model could be criticised for not incorporating parameter uncertainty. This sensitivity is largely univariate and / or scenario modelling, which explores a plausible range of parameters for particularly uncertain or important values. It is not clear from the write-up if the author has used results from the sensitivity analysis to identify areas where the value of future information is high, although this is not the principle point of a NICE guideline. |
| Consistency | ||
| Internal | P | The model behaves as theoretical predictions predict it should - values which should increase cost-effectiveness appear to do so and values which should do the opposite appear to do that. There do not appear to be any values with zero effect on the outcome, suggesting the model logic is piping through correctly. Extreme values - including zero values - do not produce contradictory or ridiculous results. |
| External | P | The model is extremely amenable to straightforward explanation and its structure is very clear. The output of the model appears to largely track committee opinion as to the relative costs of various interventions, although there is no health economic literature addressing the question this model answers so no independent way of corroborating this |
| Between-model | P | There is no health economics literature addressing this issue, so no independent between-model corroboration. A simple replication attempt produces consistent results with the final model, suggesting a high degree of between-model reliability |
| Predictive | N/A | There is no way to test whether the model has predictive validity before publication |
Table 132Philips checklist for immunomodulatory agents
| Section | Pass/fail | Comments |
|---|---|---|
| Structure | ||
| Statement of decision problem / objective | P | Decision problem stated clearly in title of review question, and clarified in the model structure section |
| Justification of modelling approach | P | Model structure justified with reference to clinical expert opinion |
| Statement of scope / perspective | P | No statement of scope, but table of contents makes scope explicitly clear so there is no risk of ambiguity |
| Structural assumptions | P | Assumptions justified in section ‘Model structure’, and clinical relevance confirmed with Guideline committee. Various structural assumptions relating to particular treatments or exacerbations explained in relevant sections. |
| Strategies / Comparators | P | Very nonstandard approach to transition probabilities (see below), but otherwise structure is highly consistent with other similar models in the area |
| Model type | P | Choice of Markov Model obvious. Limitations of this model type discussed and sensible attempts to address these limitations have been made |
| Time horizon | P | Lifetime time horizon, in keeping with Reference Case |
| Health states/disease pathways | P | Health states carefully considered - especially choice of FEV states |
| Cycle Length | P | Cycle length unusual (first cycle is 9 months, subsequent cycles annual) but justified with reference to literature on short-term effects of treatment. Subsequent annual cycle length standard. |
| Parsimony | P | Structural components of the model carefully chosen to aid understanding, especially the number and extent of health states. |
| Data | ||
| Data Identification | P | Systematic review of published literature |
| Data Synthesis | P | Synthesis strategy well justified and explained. Significant difficulty with integrating rates occurring across cycles that didn’t match the model cycle length, but approach to this explained and defensible. |
| Discounting | P | 3.5% as specified in Reference Case |
| Analysis of trial data | P | Data analysed at most appropriate level |
| Treatment effects | P | Odds ratios derived from trials and superimposed on population baseline risks generated through regression model |
| Transition probabilities | P | Derivation of transitions probabilities not standard as model attempts to map a continuous process onto a discrete-state model. Nevertheless the methodology employed here is well-described, and validated by NICE TSU |
| Mortality | P | Discussion of mortality in model write-up; CF has a very poor prognosis and so life tables not appropriate. Data from Vertex used to calculate ‘all cause CF’ mortality, and lung-transplant specific mortality appended to this. Model-specific mortality rates explained and justified. |
| Extrapolation | P | Significant extrapolation, but well justified in text with reference to committee expert opinion. Not possible to validate with reference to literature, as such literature does not exist |
| Risk factors | P | Evidence of nonlinear effect of risk factors on mortality sought and incorporated into model, for example by considering lung transplant as a separate state |
| Utilities | P | Utilities described in section on health-related quality of life, and justified with reference to literature. Model clear on baseline QoL and subsequent decrements |
| Charges and costs | P | Charges and costs described in section 1.3, and come from standard sources such as NHS Reference Costs and PSSRU |
| Adjustment over time / between countries | P | Costs inflated from historic values using standard sources |
| Half-cycle correction | F | No half-cycle correction undertaken. Defensible as cycle length much shorter than model time horizon, but half-cycle correction would be preferred |
| Data incorporation | P | Choice of data to incorporate and how the data are used is clear and well-justified |
| Uncertainty | ||
| General statement regarding sensitivity analysis | P | Described fully in sections on sensitivity analysis methods and results |
| Structural | N/A | Model structure not varied as committee opinion was that the structure was an effective one for investigating the review question |
| Methodological | P | Although discount rate not varied as per Philips (2004), substantial methodological variation examined and discussed |
| Parameter | P | Parameter uncertainty well investigated - Table 31 considers all relevant OWSAs that the committee suggested, and a PSA is further undertaken to reflect probabilistic uncertainty |
| Consistency | ||
| Internal | P | Model is highly robust to ‘stress testing’ such as putting extreme values into cells. Model behaves in an intuitive way, for example recommending treatments to which a substantial discount has been applied |
| External | P | Face validity confirmed with reference to Guideline committee. Additionally, values appear congruent with general clinical practice. |
| Between-model | P | Results consistent with literature on the topic, although literature is extremely sparse. |
| Predictive | N/A | Model not intended to be used predictively, and such predictive work would be well outside NICE methods manual |
Table 133Philips checklist for chronic antibiotic agents
| Section | Pass/fail | Comments |
|---|---|---|
| Structure | ||
| Statement of decision problem / objective | P | Decision problem stated clearly in title, and clarified in ‘comparisons’ section |
| Justification of modelling approach | P | Justified with reference to published literature on the same topic |
| Statement of scope / perspective | P | Comparisons’ section clearly delineates scope, and perspective explicitly described as being ‘Reference Case’ in section relating to model structure. |
| Structural assumptions | P | Assumptions justified in section ‘Model structure’, and clinical relevance confirmed with Guideline committee. Biggest assumption (of relationship between short and long-term treatment) described in relevant section |
| Strategies / Comparators | P | Very nonstandard approach to transition probabilities, but otherwise structure is highly consistent with other similar models in the area |
| Model type | P | Strategies selected with reference to available literature, especially pre-existing TAs. Choice of cut-offs for FEV states well justified as model is intended to match some of the work of prior economists in the area |
| Time horizon | P | Choice of Markov Model straightforward and well justified. Use of ‘fully incremental’ analysis slightly unusual, but well justified in the text with reference to heterogeneity of studies |
| Health states/disease pathways | P | Lifetime time horizon, in keeping with Reference Case |
| Cycle Length | P | Health states carefully considered and modelled to represent only critical transitions within disease pathway. |
| Parsimony | P | Cycle length unusual (first cycle is 28 days, subsequent cycles 24 weeks) but justified with reference to literature and committee consensus |
| Data | ||
| Data Identification | P | Systematic review of published literature |
| Data Synthesis | N/A | Unclear if any synthesis was appropriate |
| Discounting | P | 3.5% as specified in Reference Case |
| Analysis of trial data | P | Data analysed at most appropriate level |
| Treatment effects | P | Odds ratios derived from trials and superimposed on population baseline risks |
| Transition probabilities | P | Derivation of transitions probabilities not standard as model attempts to map a continuous process onto a discrete-state model. Nevertheless the methodology employed here is well-described, and validated by NICE TSU |
| Mortality | P | Discussion of mortality in model write-up; CF has a very poor prognosis and so life tables not appropriate. Data from Vertex used to calculate ‘all cause CF’ mortality, and lung-transplant specific mortality appended to this |
| Extrapolation | P | Significant extrapolation, but well justified in text with reference to committee expert opinion. Not possible to validate with reference to literature, as such literature does not exist |
| Risk factors | P | Evidence of nonlinear effect of risk factors on mortality sought and incorporated into model, for example by considering lung transplant as a separate state |
| Utilities | P | Utilities described in section on health related quality of life, and justified with reference to literature |
| Charges and costs | P | Charges and costs described in section on resource and cost use, and come from standard sources such as NHS Reference Costs and PSSRU |
| Adjustment over time / between countries | P | Costs inflated from historic values using HCHS index |
| Half-cycle correction | F | Half-cycle correction unlikely to be necessary as cycle length much shorter than time horizon of model |
| Data incorporation | P | Choice of data to incorporate and how the data are used is clear and well-justified |
| Uncertainty | ||
| General statement regarding sensitivity analysis | P | Described in sections on the method and results of sensitivity analysis |
| Structural | P | Model structure based on published and validated model, and deviating from this model would be methodologically unsound |
| Methodological | P | Although discount rate not varied as per Philips (2004), substantial methodological variation examined and discussed |
| Parameter | P | PSA undertaken for aztreonam comparison. Exclusion of other comparisons justified with reference to relative certainty of parametrisation for these comparisons (they have TAs from NICE which cannot be challenged) |
| Consistency | ||
| Internal | P | Model is highly robust to ‘stress testing’ such as putting extreme values into cells. Model behaves in an intuitive way, for example recommending treatments to which a substantial discount has been applied |
| External | P | Face validity confirmed with reference to Guideline committee. Additionally, values appear congruent with general clinical practice. |
| Between-model | P | Results consistent with literature on the topic |
| Predictive | N/A | Model not intended to be used predictively, and such predictive work would be well outside NICE methods manual |
- Literature review
- Airway clearance
- Monitoring pulmonary disease
- Monitoring for the onset of CFRD
- Monitoring liver disease
- Distal intestinal obstruction syndrome (DIOS)
- Pancreatic enzymes for exocrine pancreatic insufficiency (PERT)
- Nutrition
- Mucoactive or mucolytic agents
- Antimicrobial prophylaxis
- Service configuration
- Strategies to prevent cross-infection
- Immunomodulatory agents
- Chronic antimicrobials
- Model validation
- Health Economics - Cystic FibrosisHealth Economics - Cystic Fibrosis
- ATP2A2 ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 [Homo sapie...ATP2A2 ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 [Homo sapiens]Gene ID:488Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...