NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Alliance (UK). Pancreatic cancer in adults: diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2018 Feb. (NICE Guideline, No. 85.)
13.1. Methods
13.1.1. Clinical data considered in the network meta-analyses
The Network Meta-Analysis (NMA) considered the effectiveness of treatments for unresectable locally advanced non-metastatic pancreatic cancer (LAPC). The NMA includes all studies, identified by the accompanying clinical evidence review, which are phase II or phase III randomised comparative trials that compared treatments which fit into the broad groups of:
- chemotherapy,
- chemoradiotherapy,
- combination of chemotherapy and chemoradiotherapy,
- radiotherapy
- biological therapies
with another treatment or to placebo, best supportive care or no treatment. Other local therapies (such as microwaves, radiofrequency ablation) were not considered in the NMA although it was unlikely that randomised evidence would be identified to allow inclusion. Treatments not in these broad groups (as well as the excluded interventions) were only considered if they provided indirect evidence to the network via a closed loop of treatment effects for included interventions. Studies in which all investigated treatments were not considered in any other study, and therefore could not be usefully statistically synthesised into either the main NMAs or a smaller alternative one were not considered in this analysis.
Only studies published in the year 2000 or later were included in the NMA as it was considered evidence published prior to this date would not adequately represent current practice. Studies were excluded from the NMA if they included cancers other than pancreatic cancer or included populations that had both locally advanced and metastatic disease and the locally advanced group were not analysed and reported separately. Studies which considered a previously treated patient group with responding or stable disease were also excluded from the NMA, unless they were randomised before receiving treatment, as it was considered that this patient group would have better outcomes than for studies which included treatment naïve patients.
All data were derived from trials identified in the accompanying systematic reviews.
13.1.2. Review Strategy and Evidence Synthesis
Inspection of the data in the accompanying clinical evidence review identified 9 trials involving 1294 patients considering 12 different treatments. The only outcome reported in all these trials was overall survival (OS). It was therefore decided that the primary NMA would consider OS. OS was inputted into the model in the form of a hazard ratio comparing the intervention to the control. Where hazard ratios had not been reported in the original paper these were calculated using methods outlined in Parmar et al. (2008). Consequently outcomes were also reported in terms of hazard ratio using gemcitabine as the control. This was because gemcitabine was the most widely used control treatment in the studies identified. It is also widely used within England for the treatment of LAPC and is covered by TA25 for use in the treatment of both locally advanced unresectable and metastatic pancreatic cancer.
Inspection of the other outcome measures reported, identified both progression-free survival (PFS) and objective response (complete response or partial response) as outcomes that would form usefully sized networks although these would be smaller (less participants and interventions) and would be considered as secondary NMAs. The NMA for PFS considered 7 studies looking at 10 treatments involving 1125 patients. The NMA for objective response looked at 6 studies involving 706 patients. As with OS, PFS was included in the NMA in the form of hazard ratios. Again where hazard ratios had not been reported these were calculated using the same methods as for OS. Outcomes were again reported in terms of hazard ratio with gemcitabine as control. All studies included in the objective response NMA reported this information or it was able to be easily calculated from the partial response and complete response data. However, there were differences in studies between what criteria was used to assess resectability or this was not reported. It was therefore difficult to say how strictly comparable this outcome was between studies. This data was included in the NMA as count data. Outcomes from this secondary analysis were reported in terms of odds ratios, again with gemcitabine as the control.
Treatment related adverse events were also reported widely in the literature. However, due to the definitions used for recording these and uncertainty about whether an unreported event had not occurred or had not been included in the data, it was decided that an NMA looking at adverse events would not be useful. Therefore, this analysis was not performed. Other outcomes identified by the committee in the clinical evidence review protocol were either too sparsely or inconsistently reported to make any sort of evidence synthesis worthwhile. Minimally important differences were not considered in any of the NMAs as the results of both the primary and secondary analyses fed directly into a cost effectiveness model.
The following studies were included in the accompanying clinical evidence review but were excluded in both the primary or secondary NMAs (Table 220):
- Chung et al. (2014) and Rich et al. (2012): these studies only included interventions which were not considered by other studies. It was therefore not possible to include them in a useful way in any of the NMA analyses.
- Mukherjee et al (2013), Khan et al. (2016) and the 2nd randomisation in Hammel et al. (2016): these randomisations only considered previously treated patients with responding or stable disease.
Table 220
List of studies included in the Clinical Evidence review but excluded from the primary and secondary NMA analyses.
Of the studies included in the primary analysis only Shinchi et al. (2001) was not included in any of the secondary analyses as both PFS and objective response were not reported. The list of studies included in the primary and secondary analyses are reported in Table 221. Where hazard ratios or counts have been inputted as not reported (NR) these studies have not been included in the corresponding secondary analysis. The sole reason for studies not being included in the secondary analysis was that the outcome of interest was not reported in the study.
Table 221
List of studies included in the primary NMA and where sufficient data has been reported the relevant secondary NMAs.
13.1.3. Network meta-analysis Model structure
The network for the primary and two secondary NMAs including studies which did not connect to the main network are shown in Figure 8 to Figure 10 The area of the nodes are in proportion with the number of patients, in the NMAs, receiving that treatment.
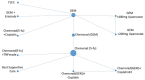
Figure 8
Network for overall survival.
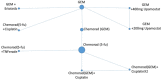
Figure 9
Network for Progression Free Survival.
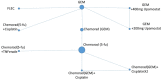
Figure 10
Network for Objective response.
Fixed effects models were run for all 3 NMAs considered. It was not possible to run an alternative random effects model, to compare goodness of fit, as no two trials in the NMA compared the same interventions and both random and fixed effect models would give identical results. The fixed effects model was created to estimate the hazard ratio for OS and PFS and the odds ratio for overall response compared to the reference treatment gemcitabine for use in the economic model.
For the OS and PFS models the log hazard ratio for each trial j comprised a normal likelihood:
Where γik represents the log hazard ratio of treatment k relative to the control arm of trial i, seik represents the standard error of the log hazard ratios and θik represents the trial-specific log hazard ratio. As the data used in the NMA is relative to other treatments, no baseline values can be predicted and the linear predictor is reduced to:
θik = δi,bk
Where δi,bk is the trial specific log hazard ratio for treatment k compared to a control of treatment b in trial i. As fixed effects are assumed then:
δi,bk = d12
Where d12 is the log hazard ratio of treatment 2 compared to a baseline of treatment 1.
For the objective response model, the data for each trial j comprised a binomial likelihood:
rjk ~ Bin (pjk, njk)
where pjk is the probability of an objective response in trial j under treatment k, rjk is the number of people experiencing the event in trial j under treatment k, and njk is the total number of people at risk of the event in trial j under treatment k.
Since the parameters of interest, pjk, are probabilities and therefore can only take values between 0 and 1, a transformation (link function) was used that mapped these probabilities into a continuous measure between plus infinity and minus infinity. Also, since this was a binomial likelihood the logit link function was used. The probabilities of success pjk were modelled on the logit scale as:
logit(pik) = μi + d12 × I{k ≠ 1}
where
In the fixed effects model the between-trial heterogeneity σ2 was set to 0 which was equivalent to assuming homogeneity of the underlying true treatment effects.
The analysis was undertaken following Bayesian statistical principles. The goodness of fit of the models was tested using the total residual deviance in the model. All models were created in WinBUGS 14 and the code for the OS and PFS models is provided in Table 222 and the objective response model in Table 223. All code was based on that reported by Dias et al. (2016).
Table 222
WinBUGS code used to estimate the hazard ratio for overall survival and progression free survival for all treatment options compared to gemcitabine for people with LAPC - fixed effects model.
Table 223
WinBUGS code used to estimate the odds ratio for objective response for all treatment options for people with LAPC - fixed effects model.
13.2. Network Meta-analysis Results
13.2.1. Estimated Hazard Ratios and Odds Ratios
Table 224 to Table 226 show the results of the three NMAs compared to gemcitabine as the reference case. In all three analyses only 1 treatment, chemoradiotherapy with gemcitabine, reported a hazard ratio or odds ratio, which had a 95% credible interval that did not pass the line of no effect. This effect would have been completely driven by 1 trial, Loehrer et al. (2012). Table 227 shows the direct trial results and the NMA indirect results in the form of a matrix. Given that there were no independent closed loops in the NMA and only 1 trial identified for each comparison, where both indirect and direct evidence is available the HR is identical although inverted.
The results presented for progression free survival in Table 225 may seem counterintuitive with PFS being most favourable for the gemcitabine and gemcitabine and upamostat therapies. This is despite them performing relatively more poorly in the OS NMA. It may be expected that interventions which delay progression in cancer also lead to an increase in overall survival and there is strong evidence in advanced pancreatic cancer of a strong correlation between OS and PFS (Hamada 2016). The great uncertainty with the PFS NMA should be noted in that all of the 95% credible intervals for all interventions in this NMA passed the line of no effect and could all plausibly have higher or lower PFS than the reference treatment gemcitabine.
Table 224
Estimated Hazard Ratios and Credible Intervals for overall survival compared to gemcitabine.
Table 225
Estimated Hazard Ratios and Credible Intervals for progression free survival compared to gemcitabine.
Table 226
Estimated Odds ratio and Credible Intervals for objective response.
Table 227
Indirect and direct comparisons for overall survival.
13.2.2. Model Fit
The goodness of model fit, evaluated using total residual deviance, for the OS NMA was 12.01 almost identical to the number of data points. The same is seen with the PFS NMA (9.003 for 9 data points) this suggested a good model fit. For the objective response NMA the residual deviance (16.08) is much larger than the number of data points suggesting a poor model fit. Given this and the wide credible intervals (given the large number of 0 or small number of events in the data) around the estimates it would be difficult make any strong conclusions around this NMA.
13.3. Economic Model
13.3.1. Interventions Considered
An economic model was created to consider the interventions identified by and connected in the primary network meta-analysis for overall survival described above. Given its wide use across England in NHS settings for the treatment of LAPC, FOLFIRINOX was also included in a secondary economic analysis despite no evidence being identified which matched the inclusion criteria for it to be included in any of the NMAs or the clinical evidence review. Gemcitabine was chosen as the comparator for the included interventions in the economic model for identical reasons for using it as the comparator in the NMAs.
Best supportive care was not considered by the economic model. Where there are already established treatments for a disease it is not deemed appropriate to recommend a no treatment strategy based on cost effectiveness alone. If best supportive care is deemed to be the optimal treatment strategy, on clinical effectiveness grounds, it is likely to be both cost saving as well as health improving making the need for economic modelling redundant. Interventions which had components of TNFerade and Upamostat were also not considered in the analysis. This is because they were seldom or never used in the NHS for any condition and did not appear in either the BNF or EMIT database of drug prices. The review of the costing literature failed to identify any costs for these two interventions for any condition in any country. It was therefore agreed that any meaningful estimate of cost effectiveness would be near impossible and of little use in making recommendations. Given both these drugs are ‘on patent’ they are likely to be associated with drug costs much higher than other drug interventions considered in this analysis. These interventions are therefore unlikely to have strong evidence of cost effectiveness without strong evidence of clinical effectiveness. This was not provided by the accompanying NMA.
Interventions in patients with stable and responding disease having been previously treated were explicitly excluded from the NMA. However, subsequent different (or further) treatment of patients with stable and responding disease form a vital part of treatment and widely happens in practice for treatment of LAPC across the NHS. Therefore, a secondary analysis was included in the economic model to compare treatments for stable disease. Three interventions (chemoradiotherapy (gemcitabine), chemoradiotherapy (capecitabine) and continued gemcitabine) were considered for this economic model. This covered all interventions that were investigated in studies which were solely excluded from the NMA on account of being in people with responding or stable disease. The model was configured so that change in treatment happened 12 weeks into the model. This analysis was performed using the same methodology as for all other interventions but treatment was only altered in patients with disease that had not progressed during initial treatment. Given a paucity of evidence around the topic the outcomes of this secondary analysis were independent of the initial treatment received. For the purposes of modelling this secondary analysis was performed in people with stable disease from the gemcitabine alone group although given the assumptions made above the results would be identical for any initial treatment. Continued gemcitabine was used as the basecase comparative treatment in this analysis
13.3.2. Model Structure
A partitioned survival analysis was developed to estimate the expected life time quality adjusted life years (QALYs) and costs associated with the competing interventions in the patient population. A partitioned survival analysis divides the model cohort between different health states based on the parametric survival functions derived in the NMAs for OS and PFS. The expected OS and PFS are then calculated from the area under the respective curves. For our model three mutually exclusive health states were derived for the cohort to be partitioned into:
- Alive without progressed disease (equal to the difference between area under the PFS curve)
- Alive with progressed disease (equal to the area between the PFS curve and OS curve)
- Death (area above the OS curve)
An illustrative example of the structure of the partitioned survival analysis is shown in Figure 11.
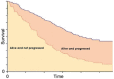
Figure 11
Illustrative example of partitioned survival analysis.
A partitioned survival analysis approach was chosen over other modelling approaches, for example a state transition model. This approach is not widely used in models of the cost-effectiveness of oncology interventions. A review of recent oncology NICE Technology Appraisals found that this approach was used in 73% of submissions (Woods 2017). This approach was chosen given the properties of the accompanying NMAs. As both hazard ratios for OS and PFS were estimated in separate mutually exclusive NMAs these values were independent of each other. Consequently, as the survival functions of the included interventions in the model are informed by these hazard ratios the survival curves were also independent of each other. In the absence of evidence of the relationship between OS and PFS a partitioned survival analysis approach allowed for these estimates to feed directly into the model. Given the modelling assumptions made about other events in the model, such as adverse events and receiving resection, do no impact upon OS and PFS, the curves do not need to account for these factors. Such events are a potential source of bias in partitioned survival analysis.
Whilst not a consideration in choosing the most appropriate modelling approach, a partitioned survival analysis is a more intuitive modelling approach for LAPC. Evidence from trials and observational studies where survival is a key outcome are almost exclusively reported as median overall and progression free survival with accompanying hazard ratio and Kaplan Meier survival curves. As these are the primary inputs for partitioned survival analysis the inputs can be easily compared with those observed in the included trials and other external sources.
A partitioned survival analysis was performed for each intervention considered in the economic evaluation and total time spent in each health state for the model cohort was recorded. Each health state was assigned a quality of life weighting so that QALYs could be calculated.
A proportion of the cohort (informed by the secondary NMA) will have an objective response to treatment and will have a probability of becoming eligible for and receive resection of the pancreas with curative intent. This will incur costs associated with the surgical procedure. Surgery will have no impact upon health outcomes in the model as any benefit of surgery would have been picked up in the OS and PFS of the studies included in the NMA and thus any inclusion in the economic model will lead to double counting and overestimation of the costs and effectiveness of treatments.
Independently of the partitioned survival analysis the model cohort also has a probability of having treatment-related adverse events. The model considered four adverse events which were the most widely reported in the clinical evidence used to inform the NMA and economic model. These were neutropenia, thrombocytopenia, diarrhoea and fatigue. Adverse events were only considered by the model if they were either rated grade III or grade IV as these were considered the severity in which significant costs and quality of life (QoL) detriments were likely to occur. People in the cohort with treatment-related adverse events were given both quality of life detriment and cost at the start of the model. It was acknowledge by the committee that other adverse events were likely to be associated with both QoL detriments and costs, however as these were not consistently reported across the literature it was difficult to include in the model. However, sensitivity analysis was performed to test the robustness of this structural assumption.
The economic component of the model was built and run in Microsoft Excel 2013.
13.3.3. Model Parameters
13.3.3.1. Overall and Progression Free Survival
OS and PFS hazard ratios used in the economic model were estimated in the NMA. As the outcomes of the NMA were reported as relative and not absolute values, an assumption had to be made around absolute overall survival and progression free survival for 1 of the interventions. As gemcitabine is the reference treatment in both the NMA and economic evaluation it was deemed most appropriate to assign an absolute value of OS and PFS for this treatment. OS and PFS hazard ratios used in the economic model were estimated in the NMAs. As the outcomes of the NMA were reported as relative and not absolute values, an assumption had to be made around absolute overall survival and progression free survival for 1 of the interventions. As gemcitabine is the reference treatment in both the NMA and economic evaluation it was deemed most appropriate to assign an absolute value of OS and PFS for this treatment. For the base case analysis a survival curve was fitted based on the summary Kaplan Meier curves reported in Hammel 2016. This trial was chosen for modelling the baseline OS and PFS as it was both the most recent and largest trial reporting OS and PFS for gemcitabine treatment in patients with LAPC. The curve was fitted using methods detailed in Hoyle 2011. The curves were fitted in R Statistical package using code made publicly available by the authors. The shape and scale parameters were taken directly from the R package results and added to the excel model. The covariance for these parameters were also calculated in the form of a Cholesky Decomposition Matrix and used to inform the probabilistic sensitivity analysis (PSA). These parameters are summarised in Table 228. Weibull and exponential models were considered using Akaike Information Criteria with weibull distribution estimated to be the best fit for both the OS and PFS data.
OS and PFS for the interventions were calculated from the hazard ratios reported in the NMA relative to the survival for gemcitabine. The usual proportional hazard assumptions were made about the hazard ratios for both OS and PFS. During PSA these hazard ratios were drawn at random from the iterations of the NMA to reflect uncertainty. PFS was constrained in the model so that it could not be greater than OS and cause a logistical inconsistency. Whilst this might constrict the range of PFS, potentially underestimating the true endpoint for PFS, this logical inconsistency happens in only a tiny number of cases and is unlikely to impact upon the conclusions of the model.
Where PFS was not reported for an intervention and therefore could not be calculated in the NMA it was assumed to be identical to PFS for gemcitabine in the absence of the alternative. As no values for OS and PFS for FOLFIRINOX had been calculated by the NMAs, excluded papers from the clinical evidence were searched for the best available evidence to inform this parameter. In the absence of randomised comparative evidence in a pure LAPC population, observational data was considered. From this, 1 systematic review and patient level meta-analysis of the use of FOLFIRINOX in people with LAPC was identified (Suker et al. 2016). The study identified 13 studies of 653 patients, 355 of which had LAPC. No studies were identified which were both randomised and comparative. The meta-analysis reported a median OS of 24.2 months (95% CI 21.7-26.8) and a median PFS of 15.0 months (95% CI 13.8-16.2). As FOLFIRINOX was the only intervention considered in this meta-analysis no comparative analysis was performed with any other intervention and therefore a hazard ratio was not and could not be calculated. FOLFIRINOX was therefore incorporated into the secondary analysis using the summary Kaplan Meier curves reported in Suker 2016. Identical methods were used for estimating the survival curves for FOLFIRINOX as used for gemcitabine and again a Weibull distribution was estimated to be the most appropriate fit for both OS and PFS. Shape, scale and Cholesky Decomposition Matrix parameters are reported in Table 231.
The shape and scale of both the OS and PFS curves for gemcitabine and FOLFIRINOX were varied during PSA using the estimated Cholesky Decomposition Matrices calculated above. This uncertainty is again estimated using methods discussed in Hoyle 2011.
The model used a time horizon of 5 years at which point over 99% of the cohort had died. This meant the survival curves were extrapolated out past three years reported by both Hammel 2016 and Suker 2016 using the shape and scale parameters estimated. It is difficult to say how accurate this extrapolation is in the absence of longer term follow-up data although any uncertainty should be picked up in the PSA. The extrapolation is only relevant to a small proportion of the trial cohort so the impact of any inaccuracy should be limited.
13.3.3.2. Proportion Adverse Events
The proportion of treatment related adverse events were taken from the accompanying clinical evidence review using the combined estimate for adverse events from the summary forest plots. Where the adverse events considered by the model were not reported in the clinical evidence they were assumed to be equal to that of gemcitabine. The proportion of adverse events for FOLFIRINOX were taken from Suker et al. (2016). During probabilistic sensitivity analysis, adverse events were varied using a binomial distribution when reported by the evidence. Where adverse events where not reported they were given a wide uniform distribution between 0% and 100% to reflect the large uncertainty.
13.3.3.3. Proportion receiving resection
The model assumed that a patient would go on to receive resection if their cancer had had an objective response to treatment. Given the difficulties discussed above with different criteria being used to estimate objective response it was difficult to give any weight to the absolute estimates of objective response estimated by the model and these were disregarded by the committee as they had little face validity. Therefore, the proportion of patients receiving gemcitabine becoming eligible for resection was assumed to be 3% based upon the committee’s clinical opinion. The resection rate for other treatments were then estimated using the Odds Ratios estimated in the objective response NMA. During PSA these hazard ratios were drawn at random from the iterations of the NMA to reflect uncertainty. Where an intervention was not included in the objective response NMA it was assumed to have an objective response rate equal to that of gemcitabine but was varied over a uniform distribution between 0% and 6% during PSA.
The proportion receiving resection following FOLFIRINOX was again taken from Suker et al (2016). During probabilistic sensitivity analysis the proportion receiving resection was randomly drawn from the iterations of the NMA. Where this had not reported a wide uniform distribution was assigned around this variable ranging from 0% to 25%. The estimates for FOLFIRINOX were varied along a beta distribution.
Whilst it was acknowledge that the results of initial treatment may influence further treatment; not only with resection but also by chemotherapy and radiotherapy these were not considered in the base case analysis. The economic model considers chemoradiotherapy (gemcitabine), chemoradiotherapy (capecitabine) and continued treatment with gemcitabine in patients with stable and responding disease although the model will assume the effectiveness of this is independent of the previous treatment received. It will be the case that those patients receiving interventions with greater effectiveness will be more likely to receive further treatment downstream whether considered by the model or not. The model will underestimate both effectiveness and costs for the interventions. There is a paucity of evidence around 2nd and 3rd line treatments and the relationship with first line treatment, therefore any relationship between the two could not be accurately modelled and was therefore not considered in the analysis. As the bias will be in both costs and health outcomes it is not possible to say in which direction the bias will be on the overall cost effectiveness. Given the relatively short life expectancy of the cohort and the small number of patients able to receive 2nd and 3rd line treatments, in practice the more effective treatments will likely be given without consideration of future treatment.
13.3.4. Costs
13.3.4.1. Treatment costs
All chemotherapy and radiotherapy were costed in line with the trial protocols identified in the accompanying clinical evidence review. These are presented in the clinical evidence review. Patients were assumed to have a body surface area of 1.79m^2 based on a retrospective study of 3,613 adult cancer patients in the UK (Sacco et al., 2010). All patients in the cohort were assumed to complete the regimens as per the trial protocols. Given the relatively low life expectancy of the model cohort, the high probability of progression and the potential for serious adverse events this assumption was likely to be an unrealistic assumption. However it was likely to bias against interventions with the lower adverse events and higher OS and PFS for example, the more clinically effective interventions.
The cost of chemotherapy drugs were taken from the Drugs and Pharmaceutical Electronic Market Information Tool (eMIT). All regimens were costed assuming no wastage. Where the cost of the chemotherapy regimens were not available on eMIT the drugs were costed using the BNF (BNF 72). It was noted that this was likely to overestimate the true cost paid by the NHS for these drugs. The costs of drug procurement and administration were based on NHS reference costs. Chemotherapy regimens which required a longer infusion were costed at the higher complex tariff.
Radiotherapy and surgery were also costed using NHS reference costs. For radiotherapy the model cohort were assumed to complete the regimen specified in the trial protocols. The cost for radiotherapy included an initial set-up cost followed by a cost per fraction administered. Two costs are presented in the NHS reference costs for resection surgery, for surgeries with and without complications. The cost of surgery was estimated assuming a probability of complications of 39.6% based on the value estimated, from the literature, of a previous costing for a UK economic evaluation of preoperative biliary drainage in pancreatic cancer (Morris et al. 2014).
Total resource use, in line with the trial protocols are reported in Table 231. These were not varied during the PSA. All treatment costs were varied using a gamma distribution and the reported standard deviations during the PSA.
Table 228
Total resource use assumed by the model for each intervention considered.
13.3.4.2. Cost of adverse events
No UK costs were identified for the specific adverse events considered by the economic model. In the absence of this evidence it was assumed that the adverse events could be treated during 1 face-to-face consultant follow-up meeting and was costed as such using NHS reference costs. Only 1 cost was assumed for any combination of the four considered adverse events included as part of the model structure. Again this assumption was likely to bias against treatments with a lower proportion of adverse events. The cost of adverse events was varied during PSA using a gamma distribution.
13.3.4.3. Cost of death
Studies of resource use in cancer show a peak in costs towards the final months of life. Given that over 99% of the model cohort died during the time horizon of the model no terminal cost was assigned to death in the model as this cost was likely to be borne by all patients regardless of intervention received. As costs after year 1 in the model are discounted this assumption again is likely to bias against the clinically effective interventions with the higher OS.
13.3.5. Quality of Life
As above three different, mutually exclusive health states were created in the partitioned survival analysis:
- Alive without progressed disease
- Alive with progressed disease
- Death
Each of these health states were given a quality of life (QoL) weighting based on those reported in a previous economic evaluation of LAPC (Murphy et al. 2012). This study used expert opinion to estimate a utility weight of 0.68 for patients without progressed disease. Based on a review of the literature a detriment of 0.12 was estimated for disease progression. This gave an estimate of 0.56 for patients with progressed disease. As these estimates were based on expert opinion and were considered very low quality evidence for informing this parameter, QoL weightings were given a large uniform distribution during sensitivity analysis, under the assumption that the QoL without progressed disease was higher or equal to that of progressed disease.
No evidence was identified around adverse events and they were therefore difficult to accurately build into the model. These adverse events were relatively easy to treat and only occurred for a short period of time and therefore the overall impact on QoL was likely to be small. Therefore, in the base case analysis no QoL detriment was assigned to adverse events. The committee acknowledged however that such adverse events are not negligible for patients receiving treatment for LAPC and some effort should be made to capture these in the QoL measures. Therefore, during probabilistic sensitivity analysis a 0.1 QoL weight detriment was assigned to all adverse events. During PSA this value was varied along a uniform distribution between this value and zero.
13.3.6. Discounting
All health outcomes were discounted at a rate of 3.5% per annum in line with the NICE guidelines manual. The way the model is structured no costs are consider after year 1. Therefore no discounting is necessary around costs.
13.3.7. Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was also conducted to assess the combined parameter uncertainty in the model. In this analysis, the mean values that are utilised in the base case are replaced with values drawn from distributions around the mean values. The distributions used are presented in Table 229
13.3.8. Net Monetary Benefit
All results are presented as incremental net monetary benefit (INMB). INMB is a representation of cost effectiveness where incremental QALY gains, compared to the comparator intervention, are converted into a monetary value by multiplying by a willingness to pay per QALY. For example if an intervention had a QALY gain of 0.5 compared to the comparator and the willingness to pay per QALY was £20,000, the monetary value of the QALY gain would equal £10,000. INMB is then calculated by subtracting total incremental cost from this incremental monetary value of the QALYs gained. For our analysis the ‘willingness to pay’ per QALY is set equal to £20,000 the cost per QALY below which NICE conventionally recommends interventions and £50,000, a higher willingness to pay which NICE consider for interventions which increase life expectancy by at least three months in people in their final 24 months of life relative to current treatment. Interventions which report a positive INMB are cost effective compared to the comparator (gemcitabine) with those reporting a negative value not being cost effective. The ‘preferred’ intervention would be the one which reports the highest INMB.
Table 229
List of parameters used in the economic model and PSA distribution.
13.4. Results Economic Model
13.4.1. Overall and Progression Free Survival
Figure 12 and Figure 13 show the estimated OS and PFS estimated by the model for the interventions considered. FOLFIRINOX has greater OS up to 27 months and greater PFS throughout. This result is expected given the greater median OS and PFS reported by Suker 2016. The committee did not expect OS to be higher at any time point for the non-FOLFIRINOX interventions. This may be because the proportional hazard assumptions made for survival may not hold for the tail end of the survival curves. Of the other interventions considered in the primary analysis of interventions included in the NMA, chemoradiotherapy with gemcitabine had the greatest OS and gemcitabine the greatest PFS. This is consistent with the magnitude of the hazard ratios estimated by the NMAs.
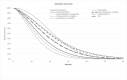
Figure 12
Estimated Overall Survival for all interventions in the model.
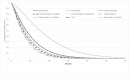
Figure 13
Estimated Progression Free Survival for all interventions in the model.
13.4.2. Deterministic Base Case Results
13.4.2.1. Clinical Outcomes
As expected given the magnitude of the hazard ratios estimated in the accompanying NMAs, chemoradiotherapy with gemcitabine had the largest mean OS and gemcitabine the largest mean PFS (Table 230). FLEC was estimated to have identical PFS to gemcitabine in the basecase analysis however given no evidence was identified to include FLEC in the PFS NMA this was directly as a result of the assumptions made in the model. The mean OS and PFS values are larger than the median values reported in the clinical evidence. Given the tails of the survival curves this is not unexpected.
FLEC resulted in the largest percentage of patients going on to receive resection, although these figures should be interpreted with caution given the large uncertainty and other weaknesses associated with the OR NMA highlighted above.
Table 230
Primary Base Case Analysis Results- Clinical Outcomes.
13.4.2.2. Economic Outcomes
Table 231 shows the base case results for the different interventions for LAPC considered by both the NMA and economic model. At the higher £50,000 per QALY threshold all interventions with a positive incremental QALY compared to gemcitabine returned a positive INMB and therefore could be considered cost effective compared to gemcitabine alone. Chemoradiotherapy with gemcitabine was the preferred option with an INMB of £7,299 per patient or a cost per QALY of £16,378 compared to gemcitabine alone. At a £20,000 per QALY threshold chemoradiotherapy with gemcitabine still remained the preferred option although of the interventions considered in the NMA. Using the means of the probabilistic results rather than deterministic results did not impact significantly upon the results and did not change the conclusions.
Table 231
Primary Base Case Analysis Results Economic Outcomes.
13.4.3. Deterministic one way sensitivity analysis
A number of one way sensitivity and scenario analyses were carried out to test the robustness of the model (Table 232). Broad scenarios were chosen for sensitivity analysis over individual sensitivity analyses as these are difficult to interpret for a large number of interventions and uncertainty is better displayed by the probabilistic results.
Chemoradiotherapy with gemcitabine remained the preferred option in the majority of scenarios. Importantly it was robust to assumptions around PFS and baseline OS.
Resection rates account for a large cost in the model with interventions with a large resection rate likely to have relatively larger costs. It was also acknowledged that estimates from the objective response NMA had great uncertainty and point estimates should be interpreted with caution. However, when resection rates and consequently costs are equal across all interventions the preferred intervention remained the same.
Only a handful of scenario analyses resulted in a different preferred therapy to the basecase. Halving the progressed disease state QALY resulted in gemcitabine becoming the preferred option. This is due largely to its point estimate performing well, comparative to other treatments, in the PFS NMA. Again these point estimates should be interpreted with caution given the large uncertainty and potentially counterintuitive results they produced.
When a one-off QALY detriment of 0.1 is added for adverse events, chemoradiotherapy with gemcitabine and cisplatin becomes the preferred option at a £20,000 willingness to pay threshold, reflecting its lower number of adverse events reported in the accompanying clinical evidence review. FLEC becomes the preferred option when treatment administration costs are not included although FLEC is a relatively complex chemotherapy to administer attracting higher tariffs, so it is not clear how realistic this scenario is.
Table 232
One Way Deterministic Sensitivity Analysis Results.
13.4.4. Secondary analysis of treatment for patients with stable or responding disease
In the secondary analysis, based on the results of the two trials identified during the clinical evidence review, continued gemcitabine alone dominated chemoradiotherapy, with gemcitabine being both health improving and less costly. Chemoradiotherapy with capecitabine was cost effective at a willingness to pay per QALY of both £20,000 and £50,000. Compared to continued treatment with gemcitabine it returned a cost per QALY of £13,052 again below both the £20,000 and £50,000 willingness to pay thresholds.
Table 233
Secondary analysis base case results.
13.4.5. Probabilistic Sensitivity Analysis
When only interventions included in the NMA are considered (Figure 14) chemoradiotherapy with gemcitabine and cisplatin becomes the preferred treatment option at the £20,000 per QALY threshold with a 24% chance of being the preferred option. Chemoradiotherapy with gemcitabine, the preferred choice in the deterministic analysis now has a 16% probability of being the most cost effective option. Gemcitabine alone had a 17% probability of being the preferred option in this scenario. As the only monotherapy in the analysis this corresponds to an 83% probability that some form of combination therapy is the most cost effective option.
At a £50,000 per QALY threshold chemoradiotherapy with gemcitabine becomes the preferred option with a 30% probability of being the most cost effective option. At this £50,000 per QALY threshold, gemcitabine has a 5% probability of being the preferred option corresponding to a probability of 95% that some form of combination therapy is the most cost effective option. The plateauing lines for all interventions suggests there is significant uncertainty around the clinical inputs for the model.
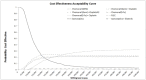
Figure 14
Cost effectiveness acceptability curve for all interventions included in the NMAs.
13.4.6. Secondary Analysis Including FOLFIRINOX
13.4.6.1. Clinical Outcomes
Values for FOLFIRINOX in the economic model were taken from Suker 2016 and no modelling was performed around these clinical outcomes (Table 234). When FOLFIRINOX was included as part of the secondary economic analysis the values for median OS and PFS were greater than for any intervention in any trial reported in the NMA. It was therefore expected that FOLFIRINOX would also report a greater mean OS and PFS. The reported 25.9% of patients receiving resection was much higher than anything predicted by the NMAs and economic model.
Table 234
Secondary Analysis Results- Clinical Outcomes.
13.4.6.2. Economic Outcomes
Table 235 shows the results of the secondary analysis which considers FOLFIRINOX as part of the secondary analysis. FOLFIRINOX has greater lifetime costs, other than gemcitabine with erlotinib, but also reports greater lifetime QALYs. FOLFIRINOX also becomes the preferred option for both a £20,000 and £50,000 per QALY willingness to pay thresholds.
Table 235
Secondary Analysis Results-Economic Outcomes.
13.4.7. Threshold Sensitivity Analysis around FOLFIRINOX
Given the potential biases discussed around the data used to populate FOLFIRINOX (Table 236) a range of threshold sensitivity analyses were performed to try to capture at which values for FOLFIRINOX the intervention is no longer the preferred option in the secondary analysis. FOLFIRINOX remains the preferred option for OS and PFS much below that reported in Suker 2016. Even if the identified biases do lead to a large overestimate of these important parameters FOLFIRINOX may still be a cost effective option.
FOLFIRINOX remains the preferred choice for all values of adverse events. FOLFIRNOX is a relatively toxic chemotherapy. Even if treatment does lead to a large number of patients experiencing adverse events it is still likely to remain the preferred option.
Table 236
Threshold sensitivity analyses for FOLFIRINOX.
13.4.8. Probabilistic Sensitivity Analysis
It can be seen from Figure 15 that the cost effective acceptability curve changes significantly when FOLFIRINOX is included as part of the analysis. FOLFIRINOX is now the most likely preferred option for all willingness to pay thresholds above £10,000 per QALY. The probability of FOLFIRINOX being the preferred option remains constant with a 51% and 56% chance of being cost effective at a willingness to pay per QALY of £20,000 and £50,000 respectively. At the same willingness to pay values there is only a few percentage points separating the other interventions (considered in the NMA) at both £20,000 and £50,000 with a less than 14% probability of any single intervention being the preferred option at both thresholds. Gemcitabine alone has a 3% and zero probability of being cost effective for a willingness to pay per QALY of £20,000 and £50,000 respectively. Again, this strongly suggests that a multimodal therapy approach is almost certainly the most cost effective treatment option.
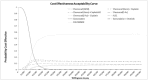
Figure 15
Cost effectiveness acceptability curve for all interventions including FOLFIRINOX.
13.4.9. Discussion
Of the interventions considered in the NMA chemoradiotherapy with gemcitabine was the preferred option during the deterministic base case results and, chemoradiotherapy with gemcitabine and cisplatin was the preferred option in the largest number of iterations in the PSA in line with the results of the NMA. However, it never had a greater than 25% probability compared to all other interventions at a willingness to pay per QALY values of £20,000 and £50,000 respectively. It was therefore difficult to strongly conclude for any intervention to be the preferred option from this group. The economic model suggested that gemcitabine alone only had a 17% probability of being the preferred option for any of the conventionally used willingness to pay thresholds suggesting strongly that multimodal therapy was likely to be cost effective.
FOLFIRINOX was the preferred option when added in the secondary analysis, being the preferred treatment in both the deterministic results and in over 50% of the iterations of the probabilistic sensitivity analysis. However, despite its prevalent usage for treatment of LAPC across England no direct, randomised comparative evidence was identified for this intervention solely in this patient group. The comparability of FOLFIRINOX to other interventions considered in the NMA and economic model is not strong. Whilst FOLFIRINOX was robust to the PSA, as the OS and PFS for FOLFIRINOX was reduced closer to those of other interventions in the NMA the strength of this conclusion was largely reduced. Comparative randomised evidence comparing FOLFIRINOX with other interventions in the NMA, would increase the comparability of this intervention and the strength of any conclusions drawn.
The plateauing of the lines in the CEACs suggest that most of the uncertainty around the model revolves around the clinical inputs. Additional randomised clinical trials which would strengthen and increase the power of the NMA would likely reduce this uncertainty and increase the strength of any recommendations made from the model.
The cost effectiveness evidence in TA25 compared 5-FU chemotherapy with gemcitabine chemotherapy. The two economic evaluations for this topic were largely based around 1 RCT (Burris et al. 1997) comparing gemcitabine monotherapy to 5-FU monotherapy in patients with either locally advanced or metastatic pancreatic cancer. The models submitted estimated a cost per QALY for gemcitabine compared to 5-FU of between £7,200 and £18,700.
It is difficult to draw comparisons with the NMA and economic model above given that 5-FU monotherapy was not used as a comparison in any of the identified evidence. Burris et al (1997) on which TA25 was based was not included as it was conducted before 2000 and had a mixed population of LAPC and metastatic cancer. Where evidence of 5-FU has been included in the NMA it is alongside radiotherapy, an intervention markedly different to 5-FU monotherapy. All regimens including 5-FU in the base case analysis are cost increasing and health decreasing compared to gemcitabine. This is mirrored by the PSA where again the 5-FU based regimens are rarely cost effective.
The costs of gemcitabine are also now likely to be much reduced compared to those considered in TA25 given that the treatment is now ‘off patent’ for this condition. The costs of gemcitabine and 5-FU are now likely to be very similar and that the total costs and costs per QALYs for gemcitabine are likely to be much lower than those reported in TA25 in 2001 even without taking account of inflation.
Despite the TA25 models not being strictly comparable to the bespoke economic model above the most pertinent difference is that gemcitabine monotherapy is now very unlikely to be the preferred option with the PSA estimating an almost 0% probability. This however is compared to regimens that were not considered by TA25. However, interventions that have a component of gemcitabine, in particular chemoradiotherapy with gemcitabine perform favourably in the bespoke economic model.
13.5. References
- Burris HA, Moore MJ, Andersen J et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology 15(6): 2403–13 [PubMed: 9196156]
- Cantore M, Fiorentini G, Luppi G et al. (2004) Gemcitabine versus FLEC regimen given intra-arterially to patients with unresectable pancreatic cancer: a prospective, randomized phase III trial of the Italian Society for Integrated Locoregional Therapy in Oncology. Journal of Chemotherapy 16(6): 589–94 [PubMed: 15700852]
- Chung HW, Bang SM, Park SW et al. (2004) A prospective randomized study of gemcitabine with doxifluridine versus paclitaxel with doxifluridine in concurrent chemoradiotherapy for locally advanced pancreatic cancer. International Journal of Radiation*Oncology*Biology* Physics 60(5): 1494–501 [PubMed: 15590180]
- Department of Health (2016) NHS reference costs 2015 to 2016. Reference costs 2015-2016. UK Government
- Department of Health (2016) eMit national database. UK Government
- Dias S, Welton NJ, Sutton AJ et al. (2016) NICE DSU technical support document 2:Gnereal Meta-analysis [Available at: http://www.bristol.ac.uk/media-library/sites/social-community-medicine/documents/mpes/TSD2%20General%20meta%20analysis%20Sep2016.pdf (accessed 27 April 2017)]
- Hamada T, Nakai Y, Isayama H et al. (2016) Progression-free survival as a surrogate for overall survival in first-line chemotherapy for advanced pancreatic cancer. European Journal of Cancer 65: 11–20 [PubMed: 27451020]
- Hammel P, Huguet F, van Laethem JL et al. (2016) Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 315(17): 1844–53 [PubMed: 27139057]
- Heinemann V, Ebert MP, Laubender RP et al. (2013) Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. British Journal of Cancer 108(4): 766–70 [PMC free article: PMC3590684] [PubMed: 23412098]
- Herman JM, Wild AT, Wang H et al. (2013) Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. Journal of Clinical Oncology 31(7): 886–94 [PMC free article: PMC4820756] [PubMed: 23341531]
- Hoyle MW and Henley W (2011) Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Medical Research Methodology 11(1): 139 [PMC free article: PMC3198983] [PubMed: 21985358]
- Joint Formulary Committee (2017) British National Formulary. 73rd ed. London, UK: BMJ Group and Pharmaceutical Press
- Khan K, Cunningham D, Peckitt C et al. (2016) miR-21 expression and clinical outcome in locally advanced pancreatic cancer: exploratory analysis of the pancreatic cancer Erbitux, radiotherapy and UFT (PERU) trial. Oncotarget 7(11): 12672–81 [PMC free article: PMC4914313] [PubMed: 26862857]
- Li CP, Chao Y, Chi KH et al. (2003) Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. International Journal of Radiation*Oncology*Biology* Physics 57(1): 98–104 [PubMed: 12909221]
- Loehrer PJ Sr, Feng Y, Cardenes H et al. (2011) Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. Journal of Clinical Oncology 29(31): 4105–12 [PMC free article: PMC3525836] [PubMed: 21969502]
- Morris S, Gurusamy KS, Sheringham J et al. (2015) Cost-effectiveness of preoperative biliary drainage for obstructive jaundice in pancreatic and periampullary cancer. Journal of Surgical Research 193(1): 202–9 [PMC free article: PMC4274324] [PubMed: 25172090]
- Mukherjee S, Hurt CN, Bridgewater J et al. (2013) Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncology 14(4): 317–26 [PMC free article: PMC3620899] [PubMed: 23474363]
- Murphy JD, Chang DT, Abelson J et al. (2012) Cost – effectiveness of modern radiotherapy techniques in locally advanced pancreatic cancer. Cancer 118(4): 1119–29 [PubMed: 21773972]
- NICE (2014) Developing NICE guidelines: the manual. London, UK: National Institute of Health and Care Excellence [PubMed: 26677490]
- NICE (2001) Pancreatic cancer - gemcitabine. TA25. London, UK: National Institute of Health and Care Excellence [Available at http://guidance.nice.org.uk/TA25 (accessed 27 April 2017)]
- Parmar MK, Torri V, Stewart L. (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics In Medicine 17(24): 2815–34 [PubMed: 9921604]
- Rich TA, Winter K, Safran H et al. (2012) Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer. Onco Targets and Therapy 5: 161–70 [PMC free article: PMC3430391] [PubMed: 22977306]
- Sacco JJ, Botten J, Macbeth F et al. (2010) The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PloS One 5(1): e8933 [PMC free article: PMC2812484] [PubMed: 20126669]
- Shinchi H, Takao S, Noma H et al. (2002) Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. International Journal of Radiation Oncology* Biology* Physics 53(1): 146–50 [PubMed: 12007953]
- Suker M, Beumer BR, Sadot E et al. (2016) FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. The Lancet Oncology 17(6): 801–10 [PMC free article: PMC5527756] [PubMed: 27160474]
- Wilkowski R, Boeck S, Ostermaier S et al. (2009) Chemoradiotherapy with concurrent gemcitabine and cisplatin with or without sequential chemotherapy with gemcitabine/cisplatin vs chemoradiotherapy with concurrent 5-fluorouracil in patients with locally advanced pancreatic cancer - a multi-centre randomised phase II study. British Journal of Cancer 101(11): 1853–9 [PMC free article: PMC2788265] [PubMed: 19904268]
- Woods B, Sideris E, Palmer S et al. (2017) NICE DSU Technical Support Document 19: Partitioned Survival Analysis for Decision Modelling in Health Care: A Critical Review. Report by the Decision Support Unit [Available at: http://www.nicedsu.org.uk (accessed 27 April 2017)]
- Network Meta-Analysis (Mixed Treatment Comparison) and Economic Model on treatme...Network Meta-Analysis (Mixed Treatment Comparison) and Economic Model on treatment of unresectable locally advanced non-metastatic pancreatic cancer - Pancreatic cancer in adults: diagnosis and management
- eggcsite.comBBW (0)BioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...
