NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Benjamin DK Jr., Lewandowski A, Anand R. Ampicillin Pharmacokinetics and Safety in Infants [Internet]. Bethesda (MD): National Institute of Child Health and Human Development (US); 2014 Nov 24.
11.1. Data Sets Analyzed
A total of 75 participants were enrolled in this study under the drug of interest ampicillin. All enrolled participants had at least one available plasma PK sample. Of these 75 participants, 73 (97%) were included in the PK analysis. Reasons for removing these two participants from the analysis are provided in Section 11.4.1.
11.2. Summary of Demography, Dosing, and Data of Special Interest for Overall Study Population
11.2.1. Demographic and Other Baseline Characteristics
Summary of baseline demographics for the safety population are presented in Table 11-1 and Section 14.1.2. Out of 75 participants in the safety population, forty (53%) participants were male, 58 (77%) were of Caucasian descent and 14 (19%) were Hispanic or Latino. The median PNA was 6 days (range 0 to 30 days) and median weight was 2500 g (range 500 to 5400 g) for the total population.
Table 11-1
Summary of Demographic Characteristics.
The summary of baseline medical history of participants is presented in Section 14.1.2. One participant was diagnosed with cystic fibrosis, and 2 participants were diagnosed with congenital heart disease.
11.2.2. Drug Dose
All 75 participants in this study had ampicillin administered per standard of care. Table 14.2.1 summarizes doses by PNA and GA group. Overall, the average number of recorded doses was 7.7 per participant. Thirty (40%) participants had a single sampling dose in which an available plasma PK sample was drawn after the dose. Thirty-seven (49%) participants had 2 sampling doses, and the remaining 8 (11%) participants had 3 sampling doses.
The maximum sampling dose amount for the 75 participants were distributed with a median of 99 mg/kg (range, 8-129 mg/kg). This amount was consistent across PNA and GA groups. Only one sampling dose was not administered through IV infusion. This sampling dose was administered via an intramuscular injection.
11.2.3. Data Related to the PK Analysis
No administrations of probenecid, the only concomitant medication of interest, were reported.
The distribution of lab values is summarized in Table 14.2.5. Only the closest lab measurements within 24 hours of a plasma sample are included in this summary. Participants had lab draws for serum creatinine (57 participants), total bilirubin (54 participants), direct bilirubin (28 participants), albumin (15 participants), AST (11 participants), and ALT (11 participants).
11.2.4. Sample Collection and Drug Concentration
11.2.4.1. PK Sampling
Table 14.2.2 summarizes PK sampling by PNA and GA group. One hundred sixty available plasma PK samples were collected from the 75 participants, which is an average of 2.1 per participant. Seventeen (23%) participants had more than two samples collected. All plasma samples were available and had recorded drug concentrations.
Ten (6%) plasma samples were collected within an hour before a dose, 4 (3%) were collected before the end of flush, and an additional 47 (29%) samples were not collected within a sampling window. The remaining samples were collected within one of the post-dose sampling windows defined in Table 9-2.
11.2.4.2. PK Drug Concentration
Table 14.2.3 summarizes the recorded drug concentration by PNA and GA group for each sampling window. The window with the highest median concentration was the window that includes samples taken immediately after the end of IV dose and flush. The median level in this window was 233.4 ng/mL. The highest recorded concentration was 777.1 ng/mL, which was recorded in the 1-4 hour window after a 72 mg/kg QID dose.
11.3. Measurement of Treatment Compliance
All participants were under the care of a primary treating caregiver; therefore each dose of drug administration was given and monitored by clinical staff, then reviewed by study personnel. The prescribing of drugs to children was not part of this protocol.
11.4. Summary of PK Analysis
11.4.1. Excluded Data
PK results that were excluded from the final NONMEM dataset were categorized into 4 groups and filtered from the analysis using the IGNORE function. The categories included:
- Concentrations (DV) < 0.05 that were below BQL
- Missing times
- Samples drawn during infusion or flush
- Results poorly characterized by the model with high WRES and large differences between IPRED and measured concentrations, suggesting sample contamination or other collection or dosing error.
Of 75 participants, two were excluded for the following reasons: (1) one subject had only one DV that was < 0.4 (BQL) and PNA at the time of the first PK sample was beyond the 28 day threshold, specifically 30 days; and (2) another subject received a recorded dose that was unreasonably low (i.e., 10 mg for 1290 gram participant) with a high concentration. Fourteen (9%) of 156 DV samples from the 73 participants were excluded: 6 were DV below BQL that were thought to be unreliable given the time after dose, 5 had levels drawn after 24 hrs which given the dosing interval were deemed to not be reliable, 1 had a sample drawn during infusion or flush, 1 had unusually high DV, and 1 had a sample drawn after intramuscular administration. A total of 73 participants with 142 observed drug concentrations were included in the PK analysis.
Of the 142 measured DV samples, 68 (48%) SCR values from 40 subjects were imputed using the closest SCR value available. A carry-forward or back-fill approach was used depending on which date was closest. If a subject did not have a SCR value for any time point, then the SCR was estimated using the population median value. Of the 142 measured DV samples, 62 (44%) WT values from 39 subjects were imputed using the closest value available. A carry-forward approach was primarily used to estimate WT from a preceding date.
11.4.2. Population PK Analysis
11.4.2.1. Demographics for PK Analysis Population
The median and range of demographic, baseline, and dosing variables at the time of first plasma pharmacokinetic sampling in participants with useable ampicillin concentration data are presented in Table 11-2.
Table 11-2
Demographic, baseline, and dosing summary for participants included in the population PK analysis.
The total daily dose was calculated from the recorded doses of ampicillin administered to each participant. This is the maximum recorded daily dose.
11.4.3. Population PK Covariate Selection and Model Summary
One-compartmental population PK models were fit as discussed in Section 9.8.1.4. As summarized in Table 11-3, the univariable screen identified SCR and PMA as potential covariates for CL and none for V.
Table 11-3
Summary of significant steps in the ampicillin model-building process.
The final model used the conditional estimation method with interaction (FOCE-I). The explicit formulas for the typical values of V and CL were:
CL (L/h) =θ(2) * WTKG * (0.6/SCR)θ (3) (PMA/37)θ (4)
where θ(1) =0. 399, θ(2)= 0.078, θ(3) =0.428, and θ(4) =1.34. WTKG is weight (kg), SCR is serum creatinine (mg/dL) and PMA is post-menstrual age (weeks). The between-subject variability for CL was 23%, and the residual variability was 34%. The estimated values for the population PK parameters, covariate and variances, along with the standard error of these estimates and bootstrap medians and the 95% confidence intervals for these values, are listed in Table 11-4. The ETA shrinkage value for CL was 21% while the EPS shrinkage value for CL was 13%.
Table 11-4
Final PK model parameters.
Individual subject post-hoc CL estimates appeared to increase with GA and PNA, as reflected by increasing CL with each group (i.e., Group 1 had the lowest CL and Group 4, the highest). Half-life decreased with increasing both components of PMA, GA and PNA, as would be expected with the increasing CL when V is constant (Figure 11-1).
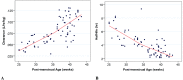
Figure 11-1
Clearance (A) and half-life (B) vs. PMA.
The goodness of fit plots demonstrated that the model generally fit the observed concentrations both for the population and the individual (Figure 11-2).
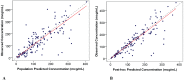
Figure 11-2
Observed versus Population (A) and Individual (B) Predictions, Final Model.
11.4.4. Pharmacodynamic (PD) Analysis
The most pathogenic infections treated with ampicillin in neonates, who are relatively immunodeficient, are Listeria monocytogenes with an MIC 90 of 2 mcg/mL and Escherichia coli with an MIC 90 of 8 mcg/mL. Streptococci are also common pathogens in neonates but sensitive to ampicillin with MIC <0.5 mcg/mL. Thus, this analysis was designed to determine the dose needed to provide exposure above a trough concentration for MIC of 2 mcg/mL and 8 mcg/mL in 90% of simulated participants.
Monte Carlo simulations (N=1920) were performed using the final population PK model to determine the distribution of steady-state ampicillin concentrations from the “typical” dose selected by clinicians for each age group in this study. The typical age group dose was determined to be the average total daily dose of the group divided by the median dose interval, rounded to the nearest 25mg/kg. For Groups 1 and 2 this was 100mg/kg every 12 hours, for Group 3 it was 75mg/kg every 8 hours and for Group 4 this was 100mg/kg every 8 hours.
In addition, simulations were performed using the dose recommendation from three references that are commonly used for neonatal doses: Neofax [11], Harriet Lane [23] and Pediatric Dosage Handbook [24]. Based on the relatively high concentrations seen with the typical current study dose used, a lower dosing strategy was also evaluated. This revised dosing was: Group 1 – 50 mg/kg every 12 hours, Group 2 – 75mg every 12 hours, Group 3 – 50 mg every 8 hours and Group 4 – 75mg every 8 hours. Simulations were performed to encompass the full range of gestational and postnatal ages across all four groups. 1920 virtual subjects, 480 in each age group, were included at the following gestational ages: 24, 26, 28, 30, 32, 34, 35, 36, 37, 38, 39 and 40 weeks and at the following postnatal ages: 1, 3, 7, 10 14, 21 and 28 days. Bodyweight and serum creatinine for each cohort were from a prior trial in premature participants [25]. An additional SCR variability of 30% (beyond fixed effects of GA and PNA) was included during the NONMEM simulation by including a random effect (ETA) on SCR with a variance (OMEGA) value of 0.09 (30% CV). Median, and 95% CI values were generated for the steady-state concentration time profiles of each age group using the various dosing strategies. In addition, the frequency of predicted concentrations greater than 2 and 8 mcg/mL were determined for 50% of the dose interval, 75% of the dose interval and 100% of the dose interval (trough concentrations). All participants had predicted concentrations >2 and 8 mcg/mL for 50% and 75% of the dose interval.
With standard of care ampicillin dosing, 100% of all participants had predicted trough concentrations at steady state > 2 mcg/mL; 100% of participants in Groups 1 and 2 and 89% in Group 3 and 4 had predicted trough concentrations ≥ 8 mcg/mL. All of the participants in Groups 3 and 4 who were below the 8 mcg/mL target were dosed every 12 hours as compared to every 8 hours. Because of variability in the primary caregiver’s dose selection we evaluated standardized dosing using Monte Carlo simulations. Based on the Monte Carlo simulations all 4 groups (with an average daily dose of 100 mg/kg every 12h in Groups 1 and 2; 75 mg/kg every 8h in Group 3; 100 mg/kg every 8h Group 4) had <3% of virtual participants with trough concentrations < 8 mcg/mL. In contrast, 10% of participants in at least 1 group failed to meet the surrogate PD target when dosing recommendations found in pediatric guidelines were used.
Additional Monte Carlo simulations were conducted, applying the final pharmacokinetic PMA-based model to the Pediatrix Database (N=132,966). The revised dosing regimens by GA/PNA and PMA (the latter using empirically-derived breakpoints) were simulated and compared to different pediatric dosing guidelines using the lowest recommended doses. The probabilities of target attainment for 50%, 75% and 100% of the dosing interval at MIC_90 of ≥2 and ≥8 mcg/mL were 95 to 100% based on dosing by GA/PNA or PMA. To achieve 75% of the dosing interval at the MIC_90 of ≥8 mcg/mL, ampicillin dosing recommendations from Neofax produced the lowest target attainment at 95%; however, >90% of patients achieved the surrogate efficacy target. In addition, while the target attainment was 100% based on current FDA recommendation of 25 mg/kg every 4 hours (i.e., 150-200 mg/kg/day divided q3-4 hours without delineation by GA, PNA, or PMA), the high frequency of dose administration may deter its clinical application. In all groups defined by GA and PNA, the proposed dosing regimen produced 100% target attainment for 75% of the dosing interval and >95% for 100% of the dosing interval at MIC ≥ 8 mcg/mL.
Since the final PMA-based model was mechanistically better than GA/PNA-based model with or without SCR and dosing by GA/PNA and PMA (the latter using empirically-derived thresholds for PMA and PNA) achieved similar PD target attainment, dosing regimen by GA/PNA was selected to offer the specificity needed to account for prematurity and developmental changes that occur with age, especially during the first 1 to 2 weeks of life. With the goal of achieving a trough concentration above the MIC of ≥8 mcg/mL in at least 90% of simulated infants, we were able to simplify the dosing regimens (Table 11-5) from several references and devise a simplified dosing regimen for ampicillin.
Table 11-5
Optimal dosing regimen based on PK analysis.
11.4.5. PK and Surrogate PD Discussion
Ampicillin is a commonly used drug in infants. However, the lack of pharmacokinetic studies in premature infants and lack of uniformity of dosing has led to a variety of doses being used based on factors including GA, PNA, weight and PMA. As this was an opportunistic study, the study did not control for dosing. Dosing ranged from 100-350 mg/kg/day and generally exceeded the recommended dosing in the most commonly pediatric dosing handbooks (Neofax, Harriet Lane and Pediatric Dosage Handbook). The high dose of prescribed ampicillin appears to stem from concerns for meningitis in the infants being treated.
The present study evaluated the population PK of ampicillin in 73 infants as young as 24 weeks gestation and up to 28 days postnatal age. This population PK model allowed us to characterize the CL and V of ampicillin in these infants but we were limited in looking at intra-variability because we had an average of only two samples per subject. A 1-compartment model appropriately described the data and was precise as evidenced by population CL and V point estimates nearly identical to the median bootstrap values and narrow 95% confidence intervals. A maturational change in ampicillin clearance was included in the final model through the PMA and SCR covariates. Given the low exponent value of 0.42 for SCR, it was not as important as the PMA (which is composed of PNA and GA) with an exponent value of 1.3.
The Monte Carlo simulation demonstrated that the higher dose of ampicillin currently being prescribed by most physicians, demonstrated by the average daily dose of ampicillin ordered by the primary caregiver for the infants in POPS, achieved the surrogate PD endpoint of trough concentrations at steady state ≥8 mcg/mL in >97% of virtual subjects as compared to 90% of virtual subjects with the current dosing references.
11.5. PK and Surrogate PD Conclusions
This population pharmacokinetics study of ampicillin in infants demonstrated the importance of PMA, composed of PNA and GA, in drug CL. The current dose used by most practitioners in infants appears to provide a higher dose than pharmacologically necessary. Given the goal of achieving a trough concentration above the MIC of ≥ in at least 90% of simulated infants, we were able to simplify the dosing regimens from several references and devise a simplified dosing regimen for ampicillin based on the 4 groups used in this study: 50 mg/kg every 12 hours for Group 1, 75 mg/kg every 12 hours for Group 2 and 50 mg/kg every 8 hours for Groups 3 and 4. Furthermore, although some references suggest every 6 hour dosing for some PNA and GA groups, adjusting the total dose would allow for every 8 hour dosing, simplifying the frequency of ampicillin administration.
- PHARMACOKINETIC EVALUATION - Ampicillin Pharmacokinetics and Safety in InfantsPHARMACOKINETIC EVALUATION - Ampicillin Pharmacokinetics and Safety in Infants
Your browsing activity is empty.
Activity recording is turned off.
See more...
