Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Pembrolizumab (Keytruda): CADTH Reimbursement Review: Therapeutic area: Classical Hodgkin lymphoma [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021 Dec.

Pembrolizumab (Keytruda): CADTH Reimbursement Review: Therapeutic area: Classical Hodgkin lymphoma [Internet].
Show detailsExecutive Summary
An overview of the submission details for the drug under review is provided in Table 1.
Table 1
Submitted for Review.
Introduction
The purpose of this report is to summarize the evidence regarding the use of pembrolizumab as monotherapy in adult and pediatric patients with refractory or relapsed classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) or who are not candidates for salvage chemotherapy and ASCT. Pembrolizumab is an immune checkpoint inhibitor dosed at 200 mg every 3 weeks in adults and 2 mg/kg every 3 weeks in pediatrics.
The term Hodgkin lymphoma (HL) refers to a group of lymphoid proliferations that share clinical and morphological features that distinguish them from other types of lymphoma. It is estimated that in 2020, 1,000 Canadians were diagnosed with cHL and 100 died from the disease.1 Clinically, HL presents most commonly with enlarged cervical lymph nodes, and spread is generally between contiguous nodal areas. Mediastinal masses and B symptoms (fever, weight loss, and night sweats) are common. A bimodal age distribution is appreciated for HL, with most patients diagnosed between the ages of 15 years and 39 years. A second peak is seen in individuals over the age of 70 years.2 HL is diagnosed by biopsy of an affected tissue or organ. On histopathology, large, atypical, and malignant cells, termed Reed-Sternberg or Hodgkin cells, are observed in a heterogeneous background consisting of non-neoplastic inflammatory cells.3 Subclassification of HL into cHL (nodular sclerosis, mixed cellularity, lymphocyte rich, and lymphocyte depleted) and nodular lymphocyte-predominant HL is based on the degree of atypia of the malignant cells, their immunophenotype, and the features of the inflammatory background.
Standards of Therapy
The treatment of cHL is guided by careful assessment of stage- and disease-specific risk factors. 18F-fluorodeoxyglucose PET is considered the gold standard for staging of HL and the Cotswold modification of the Ann Arbor staging system is applied to determine the numerical stage. In 1998, Hasenclever et al. published a prognostic score for adult patients with advanced HL consisting of 7 clinical (sex, age, and stage) and laboratory (anemia, leukocytosis, reduced serum albumin, and lymphopenia) factors. This score predicts freedom from progression (84% for patients with no risk factors to 42% for those with 5 or more factors) and overall survival (OS; 89% for patients with no risk factors to 56% for those with 5 or more factors) at 5 years.4 Treatment is risk-adapted, with low-risk patients (stage I to II, few risk factors) receiving a limited number of cycles of ABVD (doxorubicin-bleomycin-vinblastine-dacarbazine) chemotherapy, often with low-dose involved field radiotherapy5,6 while those with higher-risk disease receiving more extensive chemotherapy. Intensive chemotherapy, in the form of escalated BEACOPP (bleomycin-etoposide-doxorubicin-cyclophosphamide-vincristine-procarbazine-prednisone), is reserved for patients with the highest risk of adverse outcome.7 Response-adapted therapy with the use of interim PET restaging is used to de-escalate treatment for patients who are likely cured8,9 and to escalate treatment of patients who are not responding as expected.8,10 Risk- and response-adapted combined modality therapies are similarly used in pediatric protocols with some differences in chemotherapy backbones.11 Recognizing the shared clinical-pathological features of HL in the most affected age groups, North American adult and pediatric study groups have amalgamated efforts to study new agents using a common chemotherapy backbone.12 The outcome of HL has improved significantly over time and today more than 80% of patients with cHL are cured with initial therapy.2,13
Most patients who do not respond to or who relapse after first-line treatment for HL are treated with high-dose chemotherapy and autologous cell transplantation.14 This approach is supported by 2 randomized trials,15,16 several phase II17-20 and registry21 studies, and results in progression-free survival (PFS) in 50% to 60% of patients and OS in 60% to 80% of patients. Careful patient selection is required for successful ASCT as the presence of multiple or severe comorbidities may make the treatment-related mortality of high-dose therapy prohibitive. Patients may also not undergo ASCT if they fail to mobilize sufficient numbers of hematopoietic stem cells to support the use of high-dose chemotherapy, if they fail to respond to salvage chemotherapy, or for reasons of conscience as in the case of Jehovah’s Witnesses. Overall, approximately 85% of patients with relapsed or refractory cHL undergo ASCT.
There is currently no standard of care for patients with cHL who relapse after ASCT or who are ineligible for ASCT for 1 of the reasons noted above. Options to treat these patients include chemotherapy, radiotherapy (for those with localized recurrences), targeted therapy with brentuximab vedotin (BV), and immune checkpoint inhibitors. Although responses to standard-dose chemotherapy occur frequently in later lines of treatment, the use of conventional-dose salvage chemotherapy is unlikely to lead to a cure in these patients.22 Good palliation can be achieved with oral single-agent or combination chemotherapy regimens.23,24 BV is an antibody-drug conjugate that targets CD30-positive cells, delivering the antimitotic agent MMAE into the cytoplasm of these cells by endocytosis.25 The utility of BV in relapsed or refractory cHL was demonstrated in a pivotal phase II study in 102 patients who failed ASCT. The overall response rate was 75% with complete responses seen in 34% of patients and toxicity was manageable, although peripheral neuropathy was frequently dose limiting.26 After 5 years of follow-up, OS in this cohort of 102 patients was 41% and PFS was 22%; median OS and PFS were higher among those patients who achieved complete remission to BV.27 Real-world experience with BV in transplant-ineligible relapsed or refractory cHL was provided in a phase II study of 136 patients with a median age of 70 years at diagnosis. The most common reasons for transplant ineligibility in this cohort were comorbidities and age. A median of 8 cycles was given and overall and complete responses were observed in 74.3% and 34.6% of patients, respectively, similar to the results seen in patients who had previously undergone ASCT. Median PFS and OS were 15.1 and 17.8 months, respectively.28
Immune checkpoint inhibitors affect the PD1/PD-L1 axis and lead to increased immune reactivity against cancers that have exploited this mechanism to escape immune control. Nivolumab is a human IgG monoclonal antibody that targets PD1. Nivolumab is licensed for treatment of patients with advanced hepatocellular carcinoma, non–small cell lung cancer, advanced renal cell carcinoma, and certain cases of colorectal carcinoma or malignant melanoma. It is also approved for treatment of patients with cHL who have progressed after ASCT and BV or 3 or more lines of systemic therapy including hematopoietic cell transplantation.29 The indication in cHL is based on the results of the CHECKMATE-205 and CHECKMATE-039 studies, which enrolled a total of 95 patients, demonstrating an overall response rate of 66% and complete and partial remission of 6% and 60%, respectively. Median duration of response (DOR) was 13.1 months. Toxicity was manageable, although immune-mediated toxicity was observed.30,31 A second immune checkpoint inhibitor, pembrolizumab, is the subject of this CADTH review.
Stakeholder Perspectives
The information in this section is a summary of input provided by the patient groups who responded to CADTH’s call for patient input and from clinical experts consulted by CADTH for the purpose of this review.
Patient Input
Lymphoma Canada was the only patient group to provide input and did so after conducting 2 online surveys which yielded 128 responses. Patients often experienced fatigue, trouble breathing, fever/chills, loss of appetite, itching, anxiety, problems concentrating, loss of sexual desire, and memory loss. Many patients had quit working or school due to their diagnosis. Patients sought treatments that would provide disease control or remission with fewer side effects than current treatment options and valued longer survival and remission.
Clinician Input
Input From Clinical Experts Consulted by CADTH
The clinical experts highlighted that patients with cHL who relapse after ASCT or who are not fit for multi-agent chemotherapy and ASCT have limited treatment options. The available treatments can be associated with significant side effects and are seldom curative. Both pediatric and adult experts expect pembrolizumab to be effective earlier in the treatment paradigm but that it is also appropriate for use in patients who have failed or are ineligible for ASCT. However, patients recently on therapy for autoimmune disease, patients with poor performance status, patients with organ failure, or at high risk of autoimmune side effects may not be suited for pembrolizumab. A response to therapy would be marked by resolution of disease symptoms, radiologic evidence of disease improvement, improved ability to perform activities of daily living, reduction in size of lymph nodes and other disease sites, and in some patients, becoming eligible for an allogeneic or ASCT. Patients receiving pembrolizumab are assessed clinically every 3 weeks and radiologically every 3 to 4 cycles. Treatment should be discontinued if there is disease progression, severe immune-related adverse event (AE), or severe infusion or hypersensitivity reactions.
Clinician Group Input
Twelve clinicians from the Ontario Health Hematology Disease Site Drug Advisory Committee, Lymphoma Canada Scientific Advisory Board, and the Pediatric Oncology Group of Ontario (POGO) all provided feedback for this review. The input provided aligned with the advice provided from the CADTH clinical experts.
Drug Program Input
Some drug plan questions were regarding retreating patients with pembrolizumab who had already received it. The clinical experts identified limited evidence to provide guidance, but there is some evidence of retreating patients with pembrolizumab who have already received 35 cycles if disease progression is observed. The clinical experts were hesitant to treat patients with pembrolizumab if they had already been treated with a PD-1 or PD-L1 inhibitor unsuccessfully, believing pembrolizumab should be stopped if there is evidence of disease progression or intolerable side effects. The clinical experts were also hesitant to switch a patient from BV to pembrolizumab if the patient is responding to BV.
Clinical Evidence
Description of Studies
KEYNOTE-051
The KEYNOTE-05132 study was a nonrandomized, open-label, single-arm trial of pembrolizumab 2 mg/kg administered every 3 weeks in 7 pediatric patients aged 3 years to 18 years with relapsed or refractory cHL. A 28-day screening period was performed before patient enrolment to collect necessary laboratory, diagnostic, and demographic information and assess study eligibility. The KEYNOTE-051 study evaluated safety and efficacy including objective response rate (ORR), DOR, PFS, and OS for 35 cycles of treatment or until discontinuation due to disease progression or AEs. Post-treatment follow-up assessments occurred every 12 weeks. The study was funded by the sponsor and had a data cut-off date of January 2020.
KEYNOTE-087
The KEYNOTE-08733 study was a nonrandomized, single-arm study of pembrolizumab 200 mg administered every 3 weeks in adult patients with cHL. A 28-day screening period was performed before patient enrolment to collect necessary laboratory, diagnostic, and demographic information and assess study eligibility. The study evaluated ORR, PFS, DOR, health-related quality of life (HRQoL), and OS with a treatment duration up to 2 years, or until discontinuation of treatment due to disease progression, or occurrence of AEs. Post-treatment follow-up assessments occurred every 12 weeks. The study was funded by the sponsor with a data cut-off date of March 2019. The study consisted of 3 cohorts:
- Cohort 1: Patients who failed to respond to or progressed after ASCT and also relapsed after or failed to respond to treatment with BV after ASCT (N = 69)
- Cohort 2: Patients who were ineligible for ASCT and relapsed after or failed to respond to BV (N = 81)
- Cohort 3: Patients who failed to respond to or progressed after ASCT and had not yet received BV (N = 60)
KEYNOTE-204
The KEYNOTE-20434 study was a phase III, randomized (1:1 ratio), active controlled, open-label clinical trial comparing pembrolizumab 200 mg administered intravenously every 3 weeks (N = 151) with BV 1.8 mg/kg (maximum dose of 180 mg) administered intravenously every 3 weeks (N = 153) in adult patients with relapsed or refractory cHL. A 28-day screening period was performed before patient enrolment to collect necessary laboratory, diagnostic, and demographic information and assess study eligibility. The study evaluated PFS, OS, ORR, DOR, time to response, HRQoL, and safety for 35 cycles of treatment or until early discontinuation due to disease progression, unacceptable AEs, or other reasons to withdraw therapy. Post-treatment follow-up assessments occurred every 12 weeks. The study was funded by the sponsor with data cut-off date of February 2020. A diagram of the KEYNOTE-204 study design is provided in Figure 2.
Baseline Characteristics
Patients in the KEYNOTE-051 study had a median age of 15 years while the median age in the KEYNOTE-087 and KEYNOTE-204 studies ranged from 32.0 to 40.0. The proportion of female patients ranged from 41.2% among BV patients in the KEYNOTE-204 study to 47.8% among cohort 1 of the KEYNOTE-087 study. The proportion of patients with an Eastern Cooperative Oncology Group (ECOG) score of 0 ranged from 42.0% in cohort 1 of the KEYNOTE-087 study to 65.4% among BV patients from the KEYNOTE-204 study. The proportion of patients with an ECOG score of 0 was 54.3% and 48.3% in cohorts 2 and 3, respectively, of the KEYNOTE-087 study and 57.0% in the pembrolizumab arm of the KEYNOTE-204 study. Cohorts 1 and 3 of the KEYNOTE-087 study had higher rates of prior radiation use (46.4% and 40.0%, respectively) relative to either arm in the KEYNOTE-204 study (pembrolizumab: 38.4% and BV: 39.9%) while those in cohort 2 had lower rates (25.9%). Patients in either arm of the KEYNOTE-204 study had more bulky disease (pembrolizumab: 23.2% and BV: 16.3%) relative to any cohort in the KEYNOTE-087 study (cohort 1: 2.9%, cohort 2: 6.2%, and cohort 3: 1.7%). Baseline B symptoms were present in 30.4%, 33.3%, and 31.7% of patients in cohort 1, cohort 2, and cohort 3 of the KEYNOTE-087 study. Baseline B symptoms were also present in 28.5% and 23.5% of pembrolizumab and BV patients, respectively, in the KEYNOTE-204 study. The 2 arms within the KEYNOTE-204 study seem relatively balanced except that pembrolizumab patients had higher rates of bulky disease (23.2% versus 16.3%). Patients in the KEYNOTE-204 study were permitted to be treated with a subsequent anticancer medication after pembrolizumab or BV was discontinued.
Efficacy Results
Progression-Free Survival
In KEYNOTE-051, 3 patients (42.9%) experienced an event (disease progression or death). In the KEYNOTE-087 study, there were 43 (62.3%), 54 (66.7%), and 36 (60.0%) events in cohorts 1, 2, and 3, respectively. In the KEYNOTE-204 study, the proportion of patients experiencing an event was similar between the pembrolizumab (53.6%) and BV (57.5%) arms. In the KEYNOTE-051 study, the median PFS was reported to be 11.1 months (95% confidence interval [CI], 2.6 to not reported). In the KEYNOTE-087 study, median survival was reported to be 16.4 months (95% CI, 11.3 to 27.6), 11.1 months (95% CI, 7.3 to 13.5), and 19.4 (95% CI, 8.4 to 22.1) months in cohorts 1, 2, and 3, respectively. In the KEYNOTE-204 study, the median PFS was higher in the pembrolizumab arm (13.2 months; 95% CI, 10.9 to 19.4) than the BV arm (8.3 months; 95% CI, 5.7 to 8.8). In the KEYNOTE-051 study, the PFS rate at 12 months was 27.8% (no 95% CI reported). In the KEYNOTE-087 study, the PFS rate at 12 months was 61.3%, 43.0%, and 53.9% in cohorts 1, 2, and 3, respectively (no 95% CI reported). In the KEYNOTE-204 study, the 12-month PFS rate was higher in the pembrolizumab arm (53.9%; 95% CI, 45.0 to 61.9) than the BV arm (35.6%; 95% CI, 26.9 to 44.4). In the KEYNOTE-087 study, the 24-month PFS rate was 41.6%, 21.9%, and 34.0% in cohorts 1, 2, and 3, respectively (no 95% CI reported). In the KEYNOTE-204 study, the 24-month PFS rate was 35.4% (95% CI, 26.2 to 44.6) in the pembrolizumab arm and 25.4% (95% CI, 17.1 to 34.5) in the BV arm. The hazard ratio for time to progression was 0.65 (95% CI, 0.48 to 0.88), which was statistically significant (P = 0.0027).
Overall Survival
In the KEYNOTE-051 study, minimal information regarding OS was provided. In the KEYNOTE-087 study, 15.9%, 16.0%, and 15.0% of patients in cohorts 1, 2, and 3, respectively, died. In the KEYNOTE-204 study, a smaller proportion of patients receiving pembrolizumab died relative to patients receiving BV (10.6% versus 19.6%). Median survival was not reported in the KEYNOTE-051 study and not reached in the KEYNOTE-087 or KEYNOTE-204 studies. In the KEYNOTE-051 study, 100% of patients were alive at 12 months. In the KEYNOTE-087 study, OS at 12 months was 95.7%, 96.,2% and 96.6% in cohorts 1, 2, and 3, respectively (95% CI not reported). |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| At 24 months in the KEYNOTE-087 study, 92.6%, 91.0%, and 89.4% of patients were alive in cohorts 1, 2, and 3, respectively (95% CI not reported). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Objective Response Rate
In the KEYNOTE-051 study, 42.9% (95% CI, 9.9 to 81.6) of patients experienced a partial or complete response. In the KEYNOTE-087 study, 78.3% (95% CI, 66.7 to 87.3), 64.2% (95% CI, 52.8 to 74.6), and 71.7% (95% CI, 58.6 to 82.5) of patients experienced a partial or complete response in cohorts 1, 2, and 3, respectively. In the KEYNOTE-204 study, more partial or completes responses were observed in the pembrolizumab arm relative to the BV arm (65.6%; 95% CI, 57.4 to 73.1 versus 54.2; 95% CI, 46.0 to 62.3), which was associated with a statistically insignificant 11.3% (95% CI, 0.2 to 22.1) difference in favour of pembrolizumab.
Complete Response Rate
In the KEYNOTE-051 study, 28.6% of patients (95% CI, 3.7 to 71.0) experienced a complete response. In the KEYNOTE-087 study, 26.1% (95% CI, 16.3 to 38.1), 25.9 (95% CI, 16.8 to 36.9), and 31.7% (95% CI, 20.3 to 45.0) of patients in cohorts 1, 2, and 3, respectively, experienced a complete response. In the KEYNOTE-204 study, the complete response rate was comparable between the pembrolizumab (24.5%; 95% CI, 17.9 to 32.2) and BV arms (24.2; 95% CI, 17.6 to 31.8).
Duration of Response
In the KEYNOTE-051 study, median DOR was not reached. In the KEYNOTE-087 study, the median DOR in cohorts 1, 2, and 3 were 25.0 months (range = 0 to 36.1), 11.1 months (range = 0 to 35.9), and 16.8 months (range = 0 to 39.1), respectively. In the KEYNOTE-204 study, the median DOR was higher among patients in the pembrolizumab arm (20.7 months; range = 0 to 33.2) than in patients in the BV arm (13.8 months; range = 0 to 33.9).
Time to Response
Median time to response in the KEYNOTE-051 study was 2.6 months (range = 2.1 to 2.8). The median time to response in cohort 1, cohort 2, and cohort 3 of the KEYNOTE-087 study were 2.7 months (range = 2.1 to 12.9), 2.8 months (range = 2.2 to 11.0), and 2.8 months (range = 2.6 to 16.5), respectively. Finally, the median time to response in the pembrolizumab arm of the KEYNOTE-204 study was 2.8 months (range = 1.0 to 31.2) and also 2.8 months (range = 1.3 to 7.3) in the BV arm.
Health-Related Quality of Life
HRQoL data were only measured in the KEYNOTE-087 and KEYNOTE-204 studies. In the KEYNOTE-087 study, the least squares mean change in the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) global health status between week 24 and baseline was 11.8, 13.9, and 6.6 in cohorts 1, 2, and 3, respectively. No CIs were reported in the KEYNOTE-087 study. In the KEYNOTE-204 study, the least squares mean change in EORTC QLQ-C30 global health status between baseline and week 24 was 8.60 points (95% CI, 3.89 to 13.31) higher in the pembrolizumab arm versus the BV arm. Consistent results were reported for the EORTC QLQ-C30 physical functioning scale (6.24; 95% CI, 1.87 to 10.62), EuroQol 5-Dimensions 3-Levels questionnaire (EQ-5D-3L) utility score (0.09; 95% CI, 0.04 to 0.14), and EQ-5D-3L visual analogue scale (6.12; 95% CI, 1.91 to 10.34).
Table 2
Summary of Key Efficacy Results From Pivotal and Protocol-Selected Studies.
Harms Results
In the KEYNOTE-051 study, 85.7% of patients experienced at least 1 AE. In the KEYNOTE-087 study, 98.6%, 98.8%, and 95.0% of patients experienced at least 1 AE in cohort 1, cohort 2, and cohort 3, respectively. In the KEYNOTE-204 study, 98.0% of patients in the pembrolizumab arm and 94.1% of those in the BV arm experienced an AE. The most common AEs were pyrexia, vomiting, headache, abdominal pain, anemia, cough, fatigue, diarrhea, and upper respiratory tract infections. In the KEYNOTE-204 study, pembrolizumab patients were more likely than BV patients to experience endocrine disorders (20.3% versus 3.9%); infections (66.2% versus 45.4%); musculoskeletal and connective tissue disorders (37.8% versus 31.6%); neoplasms (7.4% versus 1.3%); renal or urinary disorders (14.9% versus 4.6%); respiratory, thoracic, or mediastinal disorders (45.3% versus 26.3%); and skin and subcutaneous tissue disorders (43.9% versus 36.8%), but less likely to experience blood or lymphatic system disorders (18.2% versus 25.7%), gastrointestinal disorders (43.9% versus 52.0%), and nervous system disorders (26.4% versus 50.7%).
In the KEYNOTE-051 study, 28.6% of patients experienced at least 1 serious AE. In the KEYNOTE-087 study, 21.7%, 22.2,% and 25.0% of patients experienced a serious AE in cohort 1, cohort 2, and cohort 3, respectively. In the KEYNOTE-204 study, 29.7% of pembrolizumab and 21.1% of BV-treated patients experienced a serious AE. The most common serious AEs in the KEYNOTE-051 study were diaphragmatic hernia and pneumonia. The most common serious AEs in cohort 1 of KEYNOTE-087 were pneumonia and pericarditis. The most common serious AE in cohort 2 of the KEYNOTE-087 study was herpes zoster and the most common serious AEs in cohort 3 of the KEYNOTE-087 study were pyrexia and pneumonitis, There were no notable differences in frequency of serious AEs between the pembrolizumab and BV arms in the KEYNOTE-204 study. The most common serious AEs in the pembrolizumab arm of the KEYNOTE-204 study were infections or infestations; respiratory, thoracic, or mediastinal disorders; neoplasms; general disorders or administration site conditions; and hepatobiliary disorders. The most common serious AEs in the BV arm of the KEYNOTE-204 study were infections or infestations; respiratory, thoracic, or mediastinal disorders; nervous system disorders; gastrointestinal disorders; and general disorders or administration site conditions.
No patients in the KEYNOTE-051 study discontinued treatment due to an AE, while 11.6%, 6.2%, and 8.3% of patients in cohort 1, cohort 2, and cohort 3, respectively, of the KEYNOTE-087 study discontinued treatment due to an AE. In the KEYNOTE-204 study, 13.5% and 17.8% of patients receiving pembrolizumab and BV discontinued treatment due to an AE, respectively.
In the KEYNOTE-051 study, 28.6% of patients experienced at least 1 immune-mediated AE. In cohort 1, 2, and 3 of the KEYNOTE-087 study, 31.9%, 32.1%, and 38.3% of patients, respectively, experienced at least 1 immune-mediated AE. In the KEYNOTE-204 study, more patients in the pembrolizumab arm (35.8%) than the BV arm (13.8%) experienced an immune-mediated AE. No patients in the KEYNOTE-051 study experienced a serious immune-mediated AE. In the KEYNOTE-087 study, 4.3%, 2.5%, and 5.0% of patients in cohort 1, cohort 2, and cohort 3, respectively, experienced a serious immune-mediated AE. In the KEYNOTE-204 study, more pembrolizumab- than BV-treated patients experienced a serious immune-mediated AE (8.8% versus 3.3%).
Table 3
Summary of Key Harms Results From Pivotal and Protocol-Selected Studies.
Critical Appraisal
The KEYNOTE-051 and KEYNOTE-087 studies were single-arm, open-label trials, while the KEYNOTE-204 study was an open-label, randomized controlled trial. The single-arm trials will be unable to provide definitive evidence of a medication’s superiority over the standard of care while the open-label design of all trials puts them at risk of bias in either direction. However, some bias from the open-label design would be attenuated by the fact that tumour progression was assessed by an independent and blinded assessor in all 3 trials. Further, the randomized nature of the KEYNOTE-204 study will balance prognostic factors at the beginning of the study. The KEYNOTE-204 study permitted patients to be treated with a subsequent anticancer medication following discontinuation of the trial medication (pembrolizumab or BV) which may obscure the trial medication’s true impact on OS. Patients originally randomized to pembrolizumab were permitted to be subsequently treated with BV and vice versa. Almost all patients randomized to BV (97.4%) received a subsequent anticancer therapy while 70.2% of pembrolizumab-treated patients did so. Those randomized to BV were more likely to cross over and subsequently receive pembrolizumab (17.8% subsequently received pembrolizumab versus 1.4% of patients originally randomized to pembrolizumab retreated with BV). Those originally randomized to BV were also more likely to receive nivolumab (19.7%) relative to those randomized to pembrolizumab (3.4%). Finally, 25.0% of patients originally randomized to pembrolizumab received BV while 4.6% of patients originally randomized to BV were retreated with BV. The KEYNOTE-051 study identified 7 pediatric patients with refractory or relapsed cHL which is insufficient to be representative of the true treatment effect in children with this condition. Moreover, it is unclear if these patients failed or were ineligible for salvage chemotherapy and ASCT which is the population of interest in this review. Due to the methodological limitations of the KEYNOTE-051 study, the evidence base is limited to the KEYNOTE-087 and KEYNOTE-204 studies. While the KEYNOTE-204 study is methodologically superior to the KEYNOTE-087 study due to the randomized active control design of the KEYNOTE-204 study, only 1 active control (BV) was tested. The KEYNOTE-087 and KEYNOTE-204 studies excluded individuals with a ECOG status of 2 or greater which could limit its generalizability. Similarly, the KEYNOTE-204 study only compared pembrolizumab to BV. Notably, CADTH reviewed the use of BV in adults with HL after failure of at least 2 multi-agent chemotherapy regimens who are not candidates for ASCT and did not recommend reimbursement.35 However, the clinical experts consulted by CADTH confirmed that in jurisdictions where it is funded, BV is still standard of care due to the lack of superior alternatives. This is in part supported by more recent evidence suggesting the efficacy of BV as third-line therapy in patients who have not received a stem cell transplant.36
Conclusions
The body of evidence included in this review suggests that, when compared to BV, pembrolizumab provides statistically and clinically significant improvement in PFS as well as clinically significant improvements in || ORR, DOR, and HRQoL. |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||| Patients who received BV were generally less likely to experience AEs, serious AEs, or immune-mediated AEs but more likely to discontinue therapy due to an AE. A definitive explanation of this phenomenon cannot be derived from this evidence alone. However, 1 explanation could be that BV-treated patients expected or observed worse health outcomes and thus were less willing to tolerate AEs, even if the rates were lower than in the pembrolizumab arm. Discontinuation would be a viable alternative for these patients as receiving another anticancer medication, including pembrolizumab, was an option. Conversely, pembrolizumab patients may have been willing to tolerate more AEs as the expected benefits were commensurately higher. The body of evidence primarily evaluated pembrolizumab administered 200 mg every 3 weeks in adults but due to the nature of the disease, CADTH’s clinical experts believe that the benefits observed in adults would also be applicable to pediatric patients. However, because of insufficient evidence on the use of pembrolizumab in pediatric patients, it is uncertain what dose should be used to ascertain the benefits observed in adults. No other comparators to pembrolizumab aside from BV were evaluated in the included studies; thus, the comparative effect of pembrolizumab to other relevant treatments in the population under review, beyond BV, remains uncertain. Also, the KEYNOTE-087 and KEYNOTE-204 studies only recruited patients with an ECOG score of 0 or 1 but the CADTH clinical experts did not recommend limiting the use of pembrolizumab only to patients with low ECOG scores. In totality, the evidence suggests that pediatric and adult patients with relapsed or refractory cHL who failed ASCT or are ineligible for multi-agent salvage chemotherapy and ASCT are more likely to benefit from pembrolizumab than from BV; however, the dose required to ascertain these benefits in pediatrics is uncertain.
Introduction
Disease Background
The purpose of this report is to summarize the evidence regarding the use of pembrolizumab monotherapy in adult and pediatric patients with refractory or relapsed cHL who have failed ASCT or who are not candidates for salvage chemotherapy and ASCT. Pembrolizumab is an immune checkpoint inhibitor dosed at 200 mg every 3 weeks in adults and 2 mg/kg every 3 weeks in pediatrics.
The term HL refers to a group of lymphoid proliferations that share clinical and morphological features that distinguish them from other types of lymphoma. It is estimated that in 2020, 1,000 Canadians were diagnosed with cHL and 100 died from the disease.1 Clinically, HL presents most commonly with enlarged cervical lymph nodes, and spread is generally between contiguous nodal areas. Mediastinal masses and B symptoms (fever, weight loss, and night sweats) are common. A bimodal age distribution is appreciated for HL, with most patients diagnosed between the ages of 15 years to 39 years. A second peak is seen in individuals older than 70 years.2 HL is diagnosed by biopsy of an affected tissue or organ. On histopathology large, atypical, and malignant cells, termed Reed-Sternberg or Hodgkin cells, are observed in a heterogeneous background consisting of non-neoplastic inflammatory cells.3 Subclassification of HL into cHL (nodular sclerosis, mixed cellularity, lymphocyte rich, and lymphocyte depleted) and nodular lymphocyte-predominant HL is based on the degree of atypia of the malignant cells, their immunophenotype, and the features of the inflammatory background.
Standards of Therapy
The treatment of cHL is guided by careful assessment of stage- and disease-specific risk factors.19 F-fluorodeoxyglucose PET is considered the gold standard for staging of HL and the Cotswold modification of the Ann Arbor staging system is applied to determine the numerical stage. In 1998 Hasenclever et al. published a prognostic score for adult patients with advanced HL consisting of 7 clinical (sex, age, and stage) and laboratory (anemia, leukocytosis, reduced serum albumin, and lymphopenia) factors. This score predicts freedom from progression (84% for patients with no risk factors to 42% for those with 5 or more factors) and OS (89% for patients with no risk factors to 56% for those with 5 or more factors) at 5 years.4 Treatment is risk-adapted, with low-risk patients (stage I to II, few risk factors) receiving a limited number of cycles of ABVD (doxorubicin-bleomycin-vinblastine-dacarbazine) chemotherapy, often with low-dose involved field radiotherapy5,6 while those with higher-risk disease receiving more extensive chemotherapy. Intensive chemotherapy, in the form of escalated BEACOPP (bleomycin-etoposide-doxorubicin-cyclophosphamide-vincristine-procarbazine-prednisone), is reserved for patients with the highest risk of adverse outcome.7 Response-adapted therapy with the use of interim PET restaging is used to de-escalate treatment for patients who are likely cured8,9 and to escalate treatment of patients who are not responding as expected.8,10 Risk- and response-adapted combined modality therapies are similarly used in pediatric protocols with some differences in chemotherapy backbones.11 Recognizing the shared clinic-pathological features of HL in the most affected age groups, North American adult and pediatric study groups have amalgamated efforts to study new agents using a common chemotherapy backbone.12 The outcome of HL has improved significantly over time and today more than 80% of patients with cHL are cured with initial therapy.2,13
Most patients who do not respond to or who relapse after first-line treatment for HL are treated with high-dose chemotherapy and autologous cell transplantation.14 This approach is supported by 2 randomized trials,15,16 several phase II17-20 and registry21 studies, and results in PFS in 50% to 60% of patients and OS in 60% to 80% of patients. Careful patient selection is required for successful ASCT as the presence of multiple or severe comorbidities may make the treatment-related mortality of high-dose therapy prohibitive. Patients may also not undergo ASCT if they fail to mobilize sufficient numbers of hematopoietic stem cells to support the use of high-dose chemotherapy, if they fail to respond to salvage chemotherapy, or for reasons of conscience as in the case of Jehovah’s Witnesses. Overall, approximately 85% of patients with relapsed or refractory cHL undergo ASCT.
There is currently no standard of care for patients with cHL who relapse after ASCT or who are ineligible for ASCT for 1 of the reasons noted above. Options to treat these patients include chemotherapy, radiotherapy (for those with localized recurrences), targeted therapy with BV, and immune checkpoint inhibitors. Although responses to standard-dose chemotherapy occur frequently in later lines of treatment, the use of conventional-dose salvage chemotherapy is unlikely to lead to cure in these patients.22 Good palliation can be achieved with oral single-agent or combination chemotherapy regimens.23,24 BV is an antibody-drug conjugate that targets CD30-positive cells, delivering the antimitotic agent MMAE into the cytoplasm of these cells by endocytosis.25 The utility of BV in relapsed or refractory cHL was demonstrated in a pivotal phase II study in 102 patients who failed ASCT. The overall response rate was 75% with complete responses seen in 34% of patients and toxicity was manageable, although peripheral neuropathy was frequently dose limiting.26 After 5 years of follow-up, OS in this cohort of 102 patients was 41% and PFS was 22%: Median OS and PFS were higher among those patients who achieved complete remission to BV.27 Real-world experience with BV in transplant-ineligible relapsed or refractory cHL was provided in a phase II study of 136 patients with a median age of 70 years at diagnosis. The most common reasons for transplant ineligibility in this cohort were comorbidities and age. A median of 8 cycles was given and overall and complete responses were observed in 74.3% and 34.6% of patients, respectively, similar to the results seen in patients who had previously undergone ASCT. Median progression-free and OS were 15.1 and 17.8 months, respectively.28
Immune checkpoint inhibitors affect the PD1/PD-L1 axis and lead to increased immune reactivity against cancers that have exploited this mechanism to escape immune control. Nivolumab is a human IgG monoclonal antibody that targets PD1. Nivolumab is licensed for treatment of patients with advanced hepatocellular carcinoma, non–small cell lung cancer, advanced renal cell carcinoma, and certain cases of colorectal carcinoma or malignant melanoma. It is also approved for treatment of patients with cHL who have progressed after ASCT and BV or 3 or more lines of systemic therapy including hematopoietic cell transplantation.29 The indication in cHL is based on the results of the CHECKMATE-205 and CHECKMATE-039 studies, which enrolled a total of 95 patients, demonstrating an overall response rate of 66% and complete and partial remission of 6% and 60%, respectively. Median DOR was 13.1 months. Toxicity was manageable, although immune-mediated toxicity was observed.30,31 A second immune checkpoint inhibitor, pembrolizumab, is the subject of this CADTH review.
Drug
The medication under review is pembrolizumab 200 mg in adults or 2 mg/kg in pediatrics, as monotherapy, administered intravenously every 3 weeks for patients with cHL who have failed ASCT or are ineligible for multi-agent salvage chemotherapy and ASCT. This indication is consistent with the Notice of Compliance provided by Health Canada. This medication has not been assessed by CADTH for this indication in the past.
Table 4
Key Characteristics of Pembrolizumab and Comparators.
Stakeholder Perspectives
Patient Group Input
This section was prepared by CADTH staff based on the input provided by patient groups.
One patient group, Lymphoma Canada, provided input for the review of pembrolizumab for adult and pediatric patients with refractory or relapsed cHL. The indication under consideration is pembrolizumab as monotherapy, for those who have failed ASCT, or who are not candidates for salvage chemotherapy and ASCT. Lymphoma Canada is a national charity that collaborates with patients, caregivers, health care professionals, and other stakeholders to empower the lymphoma community.
Lymphoma Canada conducted 2 anonymous, online surveys of patients with HL. One survey was conducted from June 5 to 30, 2017, to which 91 patients responded. No caregivers responded. The other survey was conducted from November 6, 2020, to January 13, 2021, to which 37 patients responded. The survey link was provided via email to patients registered with Lymphoma Canada, and made available via social media platforms, HL-specific forums, and social media groups, Canadian and American Cancer Society message boards, and with physicians at Canadian clinical trial sites. The survey included multiple-choice questions, rating questions, and open-ended questions. Three patients with HL in Canada, who had direct experience with pembrolizumab, were interviewed over the phone.
Not all respondents provided demographic information (103 out of 128 provided this information for country, and 94 out of 128 provided age and gender information). Of those that did provide demographic information, the majority (55%) were from Canada. Of the 9 patients with pembrolizumab experience, 7 reside in Canada, and 2 reside in the US. Table 5 details the country of survey respondents. Of those that provided demographic information, most respondents are male (54%). Most patients are between the ages of 20 to 59 years of age (78%). Table 6 details the gender and age of the survey respondents.
Table 5
Country of Survey Respondents (128 Respondents).
Table 6
Gender and Age of Survey Respondents.
Disease Experience
Experience at Diagnosis
Most of the survey respondents were between the ages of 13 years to 39 years when they were diagnosed (63%; 80 of 128 respondents). When asked about their experience receiving their diagnosis, 11% of respondents did not have all their questions answered, and 27% did not know what questions to ask their doctor who did not explain the disease to them (81 respondents). Some patients commented:
- “I was a teenager and was told I had Hodgkins disease – I didn’t know what that was and no one told me.”
- “Who can think of questions to ask when you receive this kind of news, especially when you’re 23…”
At diagnosis, the HL symptoms that most affected respondents’ quality of life were (based on the responses of 97 patients):
- fatigue or lack of energy (77%)
- enlarged lymph nodes (66%)
- drenching night sweats (44%)
- itching (40%)
- persistent cough (40%)
- unexplained weight loss (35%).
Other symptoms affecting the quality of life for greater than 10% of respondents included loss of appetite, trouble breathing, fever and chills, and chest pain.
When asked about the negative mental and emotional impacts of their disease and treatment, which affected their quality of life at diagnosis, most patients had 1 or more negative impacts (based on the responses of 97 patients):
- anxiety/worry (74%)
- stress of diagnosis (64%)
- difficulty sleeping (52%)
- problems concentrating (44%)
- loss of sexual desire (36%)
- depression (28%)
- memory loss (12%).
Current Experience
Thirty-five patients responded to questions about their current symptoms and quality of life. Current symptom experience, for those that responded, included the following:
- fatigue/lack of energy (51%)
- no symptoms (43%)
- trouble breathing (17%)
- fever/chills (14%)
- loss of appetite (14%)
- itching (14%).
In all, 113 patients provided information on current social and psychological impacts. These include the following:
- anxiety/worry (53%)
- problems concentrating (42%)
- loss of sexual desire (33%)
- memory loss (30%).
Table 7 describes the negative impact of HL on the quality of life of patients.
Table 7
Effect of HL on Day-to-Day Life of Patients (109 Respondents).
Three patients describe their experience with HL this way:
- “In remission since 2019 but dealing with long-term effects like ‘chemo brain,’ some PTSD and anxiety about recurrence. Plus, because of COVID and increased risk of complications if I catch it, I’m more nervous than most about gathering (when permitted) with even small groups of people.”
- “I immediately lost my job, as I worked in an environment not safe for someone with a compromised immune system. I had to give up my study at university, and both devastated me. I was very fit, but now if I try to exercise at the same level, I become exhausted very easily. It’s very hard.”
- “I experience more fatigue than I used to and although I’m able to work, I'm exhausted at the end of the day. Exercise is difficult to do on a weekday.”
Experiences With Currently Available Treatments
Of the 85 patients who provided information on previous treatments, all had received treatment or are currently undergoing treatment. Most (94%) had received at least 1 line of conventional chemotherapy, and 24% had received 3 or more lines of therapy. Regarding chemotherapy, the most common regimen received was ABVD (85%). GDP (gemcitabine, dexamethasone, cisplatin) was the next most common (11%), followed by BEACOPP (7%), and least common was BV (6%).
Regarding other treatments, 83 patients provided information, of which 49% received radiation therapy, 20% had an ASCT, and 25% had surgery.
In all, 101 patients indicated their current treatment phase. Following their most recent line of therapy, 85% of respondents are in remission, of which 32% have been in remission for longer than 5 years.
Many respondents are concerned with toxicity and side effects of previous treatments. Table 8 details the most common side effects experienced by respondents during their treatments for HL.
Table 8
Side Effects of HL Treatments (90 Respondents).
When asked which side effects patients found most difficult to tolerate, respondents reported nausea/vomiting (43%), fatigue (41%), hair loss (13%), mouth sores (10%), and bowel obstruction (4%) (68 respondents). Long-lasting side effects of treatments, reported lasting longer than 2 years or appearing 2 years or later after treatment, included fatigue (66%), “chemo brain” (60%), peripheral neuropathy (41%), loss of menstrual periods (18%) and sterility (18%), chest pain or infection (15%), and thyroid problems (20%) (80 respondents).
Table 9 details the impact of treatment on respondents’ quality of life. The question was a scale from 1 to 5, where 1 equals “no impact” and 5 equals “significant negative impact.”
Table 9
Impact of Treatment on Quality of Life (90 Respondents).
Table 10 details the impact of treatment on respondents’ daily living. The same scale as the question detailed in Table 9 was used.
Table 10
Impact of Treatment on Daily Living (90 Respondents).
When discussing treatments and side effects, 3 respondents reported their experience in this way:
- “Treatments were very difficult, and it took everything in me to complete my 6-month protocol. In fact, the last 2 [months] I almost begged not to have [treatment]. I have a lot of fear of recurrence because I feel I could not go through that experience again especially now that my body has changed so drastically since my initial experience with chemo. I felt young and fit prior to treatment and 3 years later I feel physically like an old woman which I was not mentally prepared for.”
- “The chemotherapy I received before and with my bone marrow transplant put me into premature menopause (I’m in my 20s) and that has negatively affected my intimate relations.”
- “I was unable to finish the first semester of nursing school at the time. I was unable to help coach basketball because of low self esteem from hair loss and fatigue. Did not really want to go places and visit friends because of hair loss.”
Access to Treatment
Many patients (83% of the 90 individuals who responded) were able to access treatment in their own communities. For the 15 patients who were unable to access treatment in their own communities, 73% lived in a community without a cancer care centre and for 26%, the treatment was not available at the local cancer care centre.
Table 11 details the financial impact of treatment.
Table 11
Financial Implications of Treatment for Patients With HL (86 Respondents).
Improved Outcomes
Patients seek individualized treatment options that provide disease control and remission, with fewer side effects than current treatment options available. When asked about important factors of new drugs or treatments for HL, “longer survival” and “longer remission” were the most important outcomes. Table 12 details the responses from the 24 patients who answered this question. The scale for the question ranged from 1 (“not important”) to 5 (“extremely important”).
Table 12
Patient Treatment Preferences (24 Respondents).
Of the 89 respondents who were asked if they would be willing to tolerate short-term side effects of a new treatment, 55% would be willing to tolerate potential short-term side effects, while 31% were not; the remaining 14% were unsure. Respondents were also asked if they would choose a treatment with known and potentially serious side effects if their doctor recommended it was the best option for them. Of the 100 patients who answered this question, 53% selected “Yes,” while only 3% selected “No”; the remaining 44% were unsure.
The survey asked respondents how important it is for patients and physicians to have a choice of therapy (a scale of 1 to 10, with 1 being “not important,” and 10 being “very important”). Most participants (79%) rated this a 7, 8, 9, or 10 (weighted average was 8.2).
A scale of 1 (“least important”) to 10 (“most important”) was used to ask survey participants which HL symptoms would be most important for a new treatment to control. One hundred respondents answered this question. Patients rated the most important symptoms for new treatments to control include difficulty breathing (8.1), drenching night sweats (7.2), chest pain (7.6), fatigue/lack of energy (7.4), and enlarged spleen or abdominal discomfort (7.0).
Experience With Drug Under Review
Nine patients had experience with pembrolizumab; 3 of these patients were interviewed for the patient input submission. The reasons for starting treatment with pembrolizumab include: no other treatment options were available (2 patients); HL progressed after autologous transplant and did not want to risk the potential toxicity of an allogeneic transplant (4 patients), hoping for remission to proceed to allogenic transplant (1 patient); did not respond to 3 previous lines of chemotherapy, and did not want to undergo an autologous transplant (2 patients).
Table 13 details the patients with pembrolizumab experience.
Table 13
Patients With HL With Pembrolizumab Experience.
All 9 patients had at least 2 prior lines of conventional chemotherapy, and 3 of these patients had received 6 or more lines of therapy. Previous chemotherapy treatments included ABVD (8), GDP (6), GVD (2), COPP (1), DHAP (1), bendamustine (1), lenalidomide (1), and unknown (1). Regarding other therapies, 7 patients had undergone an ASCT, 1 had undergone an allogeneic stem cell transplant and 4 had received treatment with BV before beginning treatment with pembrolizumab. When asked which symptoms pembrolizumab managed, 7 of the 9 patients responded that pembrolizumab managed all their HL symptoms, including fatigue, enlarged lymph nodes, frequent infections, weight loss, night sweats, shortness of breath, and pain. Two patients reported that pembrolizumab did not manage their fatigue.
Regarding the side effects of pembrolizumab, 8 patients tolerated this therapy well. However, 1 patient had to stop treatment with pembrolizumab because of toxicity and side effects, including peripheral neuropathy and inflammatory arthritis for which medication was taken. Table 14 outlines the side effects of pembrolizumab.
Table 14
Side Effects Experienced With Pembrolizumab (9 Respondents).
Knowing the potential side effects, all 9 patients responded that they would take this drug again if their doctor thought it was the best choice. The patient who had to stop treatment due to toxicity stated, “PFS was worth the side effects.”
When asked how the side effects of pembrolizumab compared to other treatments, 3 patients provided comments:
- “It’s night and day, compared to chemo. It should be the first treatment offered to patients – it is so much better than chemo, no awful side effects, only a 30-minute infusion.”
- “No side effects at all! This is the best drug ever given to me!”
- “Due to this drug, I’m able to go back to work as a nurse part-time. I don't have to take any other meds to manage side effects, which cost a lot when I was taking chemotherapy.”
Regarding quality of life and impact on daily activities, 7 of the 9 patients reported that they did not experience any negative impact on work or school, family obligations, friendships, intimate relations, activities, or travel. One patient reported lasting fatigue that was thought to have been due to the drug, and the fatigue limited aspects of their life. The patient with lasting side effects of peripheral neuropathy and inflammatory arthritis had this negatively impact their family life and personal image.
Overall Experience With Pembrolizumab
All 9 patients said they had a good to excellent experience with pembrolizumab, and all would take this treatment if offered to them again. Based on their own experience, all 9 patients would recommend this therapy to other patients with HL.
When reflecting on their overall health and well-being, 3 patients had this to say:
- “I felt like I was back to normal for the first time since I was diagnosed. I was able to do everything again and not think about my cancer. I could work again and have a normal social life.”
- “I finally feel well enough to start looking forward in life. I still can't work because of side effects from previous treatments, but I’m able to enjoy life again.”
- “Everybody should be able to take this drug instead of going through chemo. It has been so much better for me.”
Companion Diagnostic Test
There is no companion diagnostic testing for pembrolizumab.
Clinician Input
Input From Clinical Experts Consulted by CADTH
All CADTH review teams include at least 1 clinical specialist with expertise regarding the diagnosis and management of the condition for which the drug is indicated. Clinical experts are a critical part of the review team and are involved in all phases of the review process (e.g., providing guidance on the development of the review protocol, assisting in the critical appraisal of clinical evidence, interpreting the clinical relevance of the results, and providing guidance on the potential place in therapy). The following input was provided by 3 clinical specialists with expertise in the diagnosis and management of pediatric and adult cHL.
Unmet Needs
For pediatric patients, the clinical expert highlighted that the current treatment options are aggressive cytotoxic chemotherapy, which can have serious side effects such as infections and organ toxicities. If patients do not respond to these treatments, there are limited remaining treatment options. The goal for pediatrics is cure of disease whenever possible, as well as minimizing AEs and treatment-related morbidities, improving HRQoL, and delaying disease progression.
The experts who treat adult patients stated several unmet needs in this patient population, mainly that none of the currently available treatments are curative. Current treatments are also limited by their means of administration (e.g., IV administration, which requires a hospital visit) and toxicity associated with treatment. There is a need for better tolerated treatments, formulations to improve convenience, and treatments for patients who do not respond or become refractory to current treatments.
Place in Therapy
The pediatric clinical expert highlighted that studies evaluating the addition of pembrolizumab to upfront and first salvage therapies is highly anticipated. The successful application of pembrolizumab in the relapsed and refractory cHL setting could offer a potential cure, prolonged disease control, and improved quality of life for these patients that are highly pre-treated with other drug therapies.
The adult clinical expert stated that pembrolizumab has the potential to be used earlier in the current treatment paradigm. They highlighted findings that support the use of pembrolizumab after failed ASCT, or as a second-line therapy for patients who are not eligible for salvage chemotherapy or ASCT. Pembrolizumab, while used as a single therapy currently, could potentially be combined with other therapies such as chemotherapy for patients who relapse.
Patient Population
For pediatric patients, the clinical expert suggests that patients meeting criteria, and without comorbidities that would make them ineligible, should be considered for treatment with pembrolizumab. For the adult population, patients with relapsed cHL would be identified by their hematologist or oncologist (their disease state may be apparent by patient symptoms, clinical examination, or medical imaging). Relapsed disease would be confirmed by a biopsy.
It was noted that patients with relapsed and refractory cHL are closely monitored for disease progression. If patients relapse, they are generally offered treatment regardless of whether or not their lymphoma is causing them symptoms; otherwise, their disease will continue to progress. None of the experts were aware of biomarkers that can determine which patients will respond to treatment; in early studies for checkpoint inhibitors, evidence of PD1 or PDL1 expression on biopsy samples was required, but this incidence is almost 100% in patients with cHL and no longer a requirement.
Patients least suited to pembrolizumab, as noted by the pediatric clinical expert, are those patients on therapy or recently on therapy for autoimmune disease, patients with poor performance status, or patients with organ failure. The adult experts highlighted that some patients with localized disease may be better suited for radiation therapy and that patients at high risk for autoimmune side effects with pembrolizumab would be least suited to this treatment.
Assessing Response to Treatment
The pediatric expert commented that a clinically meaningful response to treatment would be resolution of disease symptoms and radiologic evidence of disease response. This response would lead to an allogenic stem cell transplant, if eligible, or to ASCT if previously ineligible, along with improved HRQoL outcomes, and improved ability to perform activities of daily living. Treatment response should be assessed after the first 2 to 4 cycles of therapy (corresponding to 6 to 12 weeks), and then every 12 weeks thereafter.
For adult patients, the clinical experts highlighted improvement in symptoms, radiological evidence of disease response, and reduction in the size of lymph nodes and other disease sites. Goals of treatment would be response (either partial or complete); improved survival; improved quality of life, including ability to perform the activities of daily living and a return to work; and improved symptoms, but ideally complete resolution of symptoms. Patients receiving pembrolizumab are assessed clinically at every visit (every 3 weeks) and radiologically every 3 to 4 cycles.
Discontinuing Treatment
Treatment should be discontinued if there is disease progression, severe immune-related AEs, or severe infusion or hypersensitivity reactions. The pediatric expert noted that checkpoint inhibitors, including pembrolizumab, may be associated with a phenomenon called pseudoprogression, an inflammatory response which does not represent disease progression. Disease response should be carefully considered.
Prescribing Conditions
Pediatric oncologists or adult hematologists or oncologists are required to diagnose, treat, and monitor patients with relapsed or refractory cHL who are receiving pembrolizumab. If a patient experiences an immune-related AE, they may be referred to another specialist. Pembrolizumab can be given in the outpatient setting.
Clinician Group Input
This section was prepared by CADTH staff based on the input provided by patient groups.
Three registered clinician groups provided input for this review. One submission was by the Ontario Health Hematology Disease Site Drug Advisory Committee (Cancer Care Ontario; OH-CCO DAC), which included 6 physicians. OH-CCO DAC provides evidence-based clinical and health system guidance for the Provincial Drug Reimbursement Programs and Systemic Treatment Program. The Lymphoma Canada Scientific Advisory Board provided a separate submission; the group consisted of 5 clinicians. Lymphoma Canada is a not-for-profit organization for Canadian patients with lymphoma and chronic lymphocytic leukemia and more information on this organization can be found at www.lymphoma.ca. POGO also completed a submission; this submission was coordinated by 1 physician, with the input from POGO’s Therapeutic and Technology Advisory Committee. Ontario’s 5 specialized childhood cancer centres, and an official advisor to the Ontario Ministry of Health and Long-Term Care, comprise POGO. More information about POGO can be found at www.pogo.ca.
Unmet Needs
Both Lymphoma Canada and OH-CCO DAC identified highest unmet need in patients who have failed ASCT or are ineligible for ASCT. Lymphoma Canada noted that across the country there are “access gaps” for novel therapies (e.g., BV and anti-PD1 antibodies), due to lack of funding. OH-CCO DAC also echoed that BV is not covered for patients who are transplant ineligible. For patients that are not able to receive ASCT because of a lack of disease response, these patients need an effective therapy to position them to receive ASCT. Lymphoma Canada also stated that standard therapies (e.g., salvage chemotherapy) typically are more toxic and less effective than novel therapies; they also are not associated with favourable PFS or OS, or meaningful long-term disease control. They noted that novel therapies with a favourable efficacy to toxicity ratio are needed.
In the submission from POGO, they identified the greatest unmet need to be patients who have relapsed or refractory cHL and who have had previous exposure to BV. Even in the relapsed or refractory cHL child and adolescent population, the goal of therapy is cure and disease response.
Place in Therapy
In the submission from Lymphoma Canada, pembrolizumab, as supported by the KEYNOTE-204 clinical trial, would be used for patients with relapsed or refractory cHL after primary therapy if they are ineligible for ASCT, and in other patients who have received at least 2 prior lines of therapy, or have relapsed after ASCT. This is in-line with using pembrolizumab in second-line or beyond. It is anticipated that clinical practice would change based on the data from the KEYNOTE-204 study; for example, pembrolizumab would be used instead of BV in the post-ASCT population, or for patients who are not eligible for ASCT and have received prior therapy. It is thought that pembrolizumab might replace BV regarding place in therapy, for patients with relapsed or refractory cHL (funding for BV is limited).
The submission from OH-CCO DAC identified that younger patients who failed first-line therapy and do not respond to salvage chemotherapy (i.e., are ineligible for ASCT), would likely receive pembrolizumab. Older patients who fail first-line therapy and are not eligible for ASCT due to comorbidities or age would likely receive pembrolizumab instead of salvage chemotherapy.
For the pediatric population, where many patients have had past exposure to BV, pembrolizumab would be used after BV. Pembrolizumab would be appropriate for patients who have already received an ASCT and BV, or who fail to respond to BV or experience toxicity.
Patient Population
Both OH-CCO DAC and Lymphoma Canada identified patients who met the criteria outlined by the KEYNOTE-204 clinical trial as experiencing the greatest unmet need for patients with relapsed or refractory cHL, and most suited to receive pembrolizumab. Lymphoma Canada specifically noted adult patients who experienced failure of primary treatment, or who have not responded to second-line treatment. They also identified children and adolescents with relapsed or refractory cHL who have experienced failure after ASCT. Lymphoma Canada stated that most patients with relapsed or refractory cHL would be eligible for pembrolizumab. POGO identified patients who have progressive or relapsed disease after BV, or who are unable to tolerate it, but have acceptable performance status (ECOG 0 or 1 or Lansky Performance Scale score > 60) as most in need of and best likely to tolerate pembrolizumab.
Assessing Response to Treatment
To assess treatment response, Lymphoma Canada identified that patients typically undergo serial imaging (i.e., fluorodeoxyglucose PET) to monitor disease progression, regardless of ASCT eligibility. POGO also identified cross-sectional imaging and PET scans as the means for determining disease progression and treatment response. OH-CCO DAC stated that disease response is determined by standard response criteria, including imaging. Frequency of imaging varies across the country, but typically for the ASCT-eligible population it has typically been done at 3 months, and 1 year after ASCT. For the ASCT-ineligible population, imaging could be based on patient symptoms or after treatment. A clinically meaningful response to treatment would include improvement in disease related symptoms, tumour response, and disease control (i.e., PFS or OS). POGO and OH-CCO DAC suggested disease status should be assessed every 12 weeks, at minimum.
Discontinuing Treatment
When deciding to discontinue treatment, Lymphoma Canada and POGO indicated that disease progression and significant toxicities (i.e., AEs, particularly grade 3 or 4 events, and immune-related events) would be important considerations.
Prescribing Conditions
The submission from Lymphoma Canada stated that there is evidence to support the use of anti-PD1 antibodies in a variety of malignancies, with administration of treatment in the community setting, hospitals, and tertiary cancer centres. OH-CCO DAC stated that outpatient clinics would be suitable to administer pembrolizumab. For the pediatric population, POGO suggested that pembrolizumab be administered in specialized pediatric cancer programs only.
Drug Program Input
The drug programs provide input on each drug being reviewed through CADTH’s reimbursement review processes by identifying issues that may impact their ability to implement a recommendation. The implementation questions and corresponding responses from the clinical experts consulted by CADTH are summarized in Table 15.
Table 15
Summary of Drug Plan Input and Clinical Expert Response.
Clinical Evidence
The clinical evidence included in the review of pembrolizumab is presented as a systematic review which includes pivotal studies provided in the sponsor’s submission to CADTH and Health Canada, as well as those studies that were selected according to an a priori protocol.
Systematic Review (Pivotal and Protocol-Selected Studies)
Objectives
To perform a systematic review of the efficacy and safety of pembrolizumab, as monotherapy, in adult and pediatric patients with refractory or relapsed cHL, who have failed ASCT or who are not candidates for salvage chemotherapy and ASCT.
Methods
Studies selected for inclusion in the systematic review included pivotal studies provided in the sponsor’s submission to CADTH and Health Canada, as well as those meeting the selection criteria presented in Table 16.
Outcomes included in the CADTH review protocol reflect outcomes considered to be important to patients, clinicians, and drug plans.
Table 16
Inclusion Criteria for the Systematic Review.
The literature search for clinical studies was performed by an information specialist using a peer-reviewed search strategy according to the PRESS Peer Review of Electronic Search Strategies checklist.41 Published literature was identified by searching the following bibliographic databases: MEDLINE All (1946‒) via Ovid and Embase (1974‒) via Ovid. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were Keytruda (pembrolizumab) and classical HL. Clinical trials registries were searched: the US National Institutes of Health’s clinicaltrials.gov, WHO’s International Clinical Trials Registry Platform search portal, Health Canada’s Clinical Trials Database, and the European Union Clinical Trials Register. No filters were applied to limit the retrieval by study type. Retrieval was not limited by publication date or by language. Conference abstracts were excluded from the search results. See Appendix 1 for the detailed search strategies. The initial search was completed on March 17, 2021. Regular alerts updated the search until the meeting of the CADTH pan-Canadian Oncology Drug Review Expert Committee meeting on July 15, 2021.
Grey literature (literature that is not commercially published) was identified by searching relevant websites from the Grey Matters: A Practical Tool For Searching Health-Related Grey Literature checklist.42 Included in this search were the websites of regulatory agencies (FDA and European Medicines Agency). Google was used to search for additional internet-based materials. See Appendix 1 for more information on the grey literature search strategy. In addition, the manufacturer of the drug was contacted for information regarding unpublished studies.
Two CADTH clinical reviewers independently selected studies for inclusion in the review based on titles and abstracts, according to the predetermined protocol. Full-text articles of all citations considered potentially relevant by at least 1 reviewer were acquired. Reviewers independently made the final selection of studies to be included in the review, and differences were resolved through discussion.
A focused literature search for network meta-analyses dealing with HL was run in MEDLINE All (1946–) on March 17, 2021. No limits were applied to the search.
Findings From the Literature
A total of 359 studies were identified from the literature for inclusion in the systematic review (Figure 1). The included studies are summarized in Table 17. Six reports based on 3 unique studies were identified and were all provided by the sponsor; no additional unique studies were identified from the literature search. No network meta-analyses were identified.
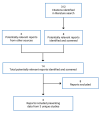
Figure 1
Flow Diagram for Inclusion and Exclusion of Studies.
Table 17
Details of Included Studies.
Description of Studies
KEYNOTE-051
The KEYNOTE-05132 study was a nonrandomized, open-label, single-arm trial of pembrolizumab 2 mg/kg administered every 3 weeks in 7 pediatric patients with cHL aged 3 years to 18 years. A 28-day screening period was performed before patient enrolment to collect necessary laboratory, diagnostic, and demographic information and assess study eligibility. The KEYNOTE-051 study evaluated safety and efficacy including ORR, DOR, PFS, and OS for 35 cycles of treatment or until discontinuation due to disease progression or AE. Post-treatment follow-up assessments occurred every 12 weeks. The study was funded by the sponsor and had a data cut-off date of January 2020.
KEYNOTE-087
The KEYNOTE-08733 study was a nonrandomized, single-arm study of pembrolizumab 200 mg administered every 3 weeks in adult patients with cHL. A 28-day screening period was performed before patient enrolment to collect necessary laboratory, diagnostic, and demographic information and assess study eligibility. The study evaluated ORR, PFS, DOR, HRQoL, and OS with a treatment duration up to 2 years, or until discontinuation of treatment due to disease progression, or occurrence of AE. Post-treatment follow-up assessments occurred every 12 weeks. The study was funded by the sponsor with a data cut-off date of March 2019. The study consisted of 3 cohorts:
- Cohort 1: Patients who failed to respond to or progressed after ASCT and also relapsed after or failed to respond to treatment with BV after ASCT (N = 69)
- Cohort 2: Patients who were ineligible for ASCT and relapsed after or failed to respond to BV (N = 81)
- Cohort 3: Patients who failed to respond to or progressed after ASCT and had not yet received BV (N = 60)
KEYNOTE-204
The KEYNOTE-20434 study was a phase III, randomized (1:1 ratio), active controlled, open-label clinical trial comparing pembrolizumab 200 mg administered intravenously every 3 weeks (N = 151) with BV 1.8 mg/kg (maximum dose of 180 mg) administered intravenously every 3 weeks (N = 153) in adult patients with cHL. A 28-day screening period was performed before patient enrolment to collect necessary laboratory, diagnostic, and demographic information and assess study eligibility. The study evaluated PFS, OS, ORR, DOR, time to response, HRQoL, and safety for 35 cycles of treatment or until early discontinuation due to disease progression, unacceptable AEs, or other reasons to withdraw therapy. Post-treatment follow-up assessments occurred every 12 weeks. The study was funded by the sponsor with data cut-off date of February 2020. A diagram of the KEYNOTE-204 study design is provided in Figure 2.
Inclusion and Exclusion Criteria
The KEYNOTE-051 study recruited children and adolescents aged 3 years to 18 years with cHL who were either refractory to front-line therapy, high-risk and relapsed from front-line therapy, or relapsed or refractory to second-line therapy. The KEYNOTE-051 study did not explicitly recruit patients who failed ASCT or were ineligible for salvage chemotherapy and ASCT. The KEYNOTE-051 study recruited those with a Lansky Play-Performance Scale score of 50 or greater for children 16 years and younger or a Karnofsky score of 50 or greater in children 16 years and older. The KEYNOTE-051 study also excluded those who received prior systemic anticancer therapy including investigational agents within 2 weeks of the study’s start date or patients who had not recovered from AEs due to a previously administered agent. The KEYNOTE-087 and KEYNOTE-204 studies recruited adults 18 years or older with an ECOG score of 0 or 1. The KEYNOTE-087 study divided patients into 3 cohorts as follows.
- Cohort 1: Failed to respond to or progressed after ASCT and also relapsed after or failed to respond to treatment with BV after ASCT
- Cohort 2: Ineligible for ASCT and also relapsed after or failed to respond to BV
- Cohort 3: Failed to respond or progressed after ASCT and had not yet received BV
Finally, patients recruited into the KEYNOTE-204 study had relapsed or refractory cHL and met 1 of the following criteria.
- Failed to achieve a response or progressed after ASCT and had not previously been treated with BV
- Were not ASCT candidates due to chemo-resistant disease, advanced age (≥ 65 years), or a condition likely to have a negative impact on the tolerability to ASCT; these patients must have received at least 2 prior multi-agent chemotherapy regimens that did not include BV
Both the KEYNOTE-087 and KEYNOTE-204 studies excluded those who received a prior monoclonal antibody within 4 weeks before the study’s start date or who had not recovered from AEs due to agents administered more than 4 weeks earlier. Both studies also excluded those who had prior chemotherapy, targeted small molecule therapy, or radiation therapy within 2 weeks before the study’s start date or who had not recovered from AEs due to a previously administered agent.
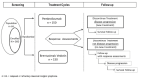
Figure 2
Study Design of KEYNOTE-204.
Baseline Characteristics
Patients in the KEYNOTE-051 study had a median age of 15 years while the median age in the KEYNOTE-087 and KEYNOTE-204 studies ranged from 32.0 years to 40.0 years. The proportion of female patients ranged from 41.2% among BV patients in the KEYNOTE-204 study to 47.8% among cohort 1 of the KEYNOTE-087 study. The proportion of patients with an ECOG score of 0 ranged from 42.0% in cohort 1 of KEYNOTE-087 to 65.4% among BV patients from the KEYNOTE-204 study. The proportion of patients with an ECOG score of 0 was 54.3% and 48.3% in cohorts 2 and 3, respectively, of KEYNOTE-087, and 57.0% in the pembrolizumab arm of the KEYNOTE-204 study. Cohorts 1 and 3 of the KEYNOTE-087 study had higher rates of prior radiation use (46.4% and 40.0%, respectively) relative to either arm in the KEYNOTE-204 study (pembrolizumab: 38.4% and BV: 39.9%), while those in cohort 2 had lower rates (25.9%). Patients in either arm of the KEYNOTE-204 study had more bulky disease (pembrolizumab: 23.2% and BV: 16.3%) relative to any cohort in the KEYNOTE-087 study (cohort 1: 2.9%, cohort 2: 6.2%, and cohort 3: 1.7%). Baseline B symptoms were present in 30.4%, 33.3%, and 31.7% of patients in cohort 1, cohort 2, and cohort 3 of the KEYNOTE-087 study, respectively. Baseline B symptoms were also present in 28.5% and 23.5% of pembrolizumab and BV patients, respectively, in the KEYNOTE-204 study. The 2 arms within the KEYNOTE-204 study seem relatively balanced except that pembrolizumab patients had higher rates of bulky disease (23.2% versus 16.3%). A complete summary of baseline characteristics is provided in Table 18. Patients in the KEYNOTE-204 study were permitted to be treated with a subsequent anticancer medication after pembrolizumab or BV was discontinued. Almost all patients randomized to BV (97.4%) received a subsequent anticancer therapy while 70.2% of pembrolizumab-treated patients did so. Those randomized to BV were more likely to cross over and subsequently receive pembrolizumab (17.8% versus 1.4% of patients originally randomized to pembrolizumab and retreated with pembrolizumab). Those originally randomized to BV were also more likely to receive nivolumab (19.7%) relative to those randomized to pembrolizumab (3.4%). Finally, 25.0% of patients originally randomized to pembrolizumab received BV while 4.6% of patients originally randomized to BV were retreated with BV (Table 19 and Appendix 2).
Table 18
Summary of Baseline Characteristics (Intention-to-Treat Analysis).
Interventions
KEYNOTE-051
KEYNOTE-051 studied pembrolizumab 2 mg/kg intravenously every 3 weeks until 35 cycles were administered, disease progression, unacceptable AEs, intercurrent illness preventing further administration, investigator decision to withdraw therapy, patient withdrawal, pregnancy, noncompliance, or administrative reasons. Patients were prohibited from concurrently using granulocyte-macrophage colony-stimulating factor, immunotherapy (other than pembrolizumab), chemotherapy, biologic therapy, investigational agents other than pembrolizumab, radiation, live vaccines, and glucocorticoids for any reason other than to treat an AE. Otherwise, patients were permitted to concurrently receive any medication necessary for the patient’s welfare so long as they adhered to standards of medical care and medication use was documented.
KEYNOTE-087
In each of the 3 cohorts of the KEYNOTE-087 study, pembrolizumab 200 mg was administered intravenously every 3 weeks for 2 years or until documented and confirmed disease progression, intolerable toxicity, or patient or investigator decision to withdraw. Patients were prohibited from concurrently using granulocyte-macrophage colony-stimulating factor, immunotherapy (other than pembrolizumab), chemotherapy, biologic therapy, investigational agents other than pembrolizumab, radiation, live vaccines, and glucocorticoids for any reason other than to treat an AE. Otherwise, patients were permitted to concurrently receive any medication necessary for the patient’s welfare so long as they adhered to standards of medical care and medication use was documented.
KEYNOTE-204
Pembrolizumab 200 mg or BV 1.8 mg/kg (maximum dose of 180 mg) was administered intravenously every 3 weeks for 35 cycles or until disease progression, unacceptable AEs, intercurrent illness preventing further administration, investigator decision to withdraw therapy, patient withdrawal, pregnancy, or administrative reasons. Patients in either arm were permitted to receive subsequent anticancer therapy after discontinuing pembrolizumab or BV. Further, those originally randomized to pembrolizumab were able to then receive BV while those originally randomized to BV were permitted to receive pembrolizumab. Patients were prohibited from concurrently using granulocyte-macrophage colony-stimulating factor, immunotherapy (other than pembrolizumab), chemotherapy (other than BV), biologic therapy, investigational agents other than pembrolizumab and BV, radiation, live vaccines, glucocorticoids for any reason other than to treat an AE, and in those receiving BV, potent CYP3A4 inhibitors or inducers or P-glycoprotein inhibitors. Otherwise, patients were permitted to concurrently receive any medication necessary for the patient’s welfare so long as they adhered to standards of medical care and medication use was documented.
In all 3 trials, if a patient experienced an immune-mediated AE, they could be treated with corticosteroids, anti-inflammatory agents if symptoms did not improve following corticosteroid treatment, insulin, non-selective beta blockers, thyroid hormone replacement therapy, antihistamines, nonsteroidal anti-inflammatory drugs, acetaminophen, opioids, vasopressors, and epinephrine. Supportive care was permitted as deemed necessary by the treating physician.
Table 19
Summary of Subsequent Use of Anticancer Medication Utilization in KEYNOTE-204; Greater Than 5% Utilization in Either Arm.
Outcomes
A list of efficacy end points identified in the CADTH review protocol that were assessed in the clinical trials included in this review is provided in Table 20. These end points are further summarized below. A detailed discussion and critical appraisal of the outcome measures are provided in Appendix 3.
Table 20
Summary of Outcomes of Interest Identified in the CADTH Review Protocol.
Progression-Free Survival
In the KEYNOTE-051 and KEYNOTE-087 studies, PFS was defined as time from first dosing date to the first documented progressive disease, death due to any cause, or start of new anticancer medication, whichever came first by blinded independent central radiology assessment and using International Working Group (IWG) response criteria. The IWG guidelines utilize diagnostic imaging, immunohistochemistry, and flow cytometry to define response to treatment in non-Hodgkin and HL.44
In the KEYNOTE-204 study, PFS was assessed by blinded independent central review according to the IWG response criteria including clinical and imaging data following autologous or allogenic stem cell transplant. PFS was defined as the time from randomization to the first documentation of progression or death from any cause. Patients were censored at the last disease assessment if they received an ASCT or another anticancer therapy. These analyses considered ASCT or initiation of another anticancer treatment as a censoring event. A sensitivity analysis of PFS considered the use of another anticancer medication as a progression event but otherwise had the same definition of PFS.
Overall Survival
In the KEYNOTE-051 and KEYNOTE-087 studies, OS was defined as time from first dose to the date of death. In KEYNOTE-204, OS was defined as the time from randomization to death from any cause. In the KEYNOTE-204 study, patients without death were censored at the date of the last assessment.
Objective Response Rate
In the KEYNOTE-051, KEYNOTE-087, and KEYNOTE-204 studies, ORR was defined as the proportion of patients who had a complete or partial response. All studies assessed response by blinded independent central review and used the IWG criteria.
Complete Remission Rate
Like ORR, complete remission rate was assessed by blinded independent central review using the IWG criteria in the KEYNOTE-051, KEYNOTE-087, and KEYNOTE-204 studies.
Duration of Response
In the KEYNOTE-051 and KEYNOTE-087 studies, DOR was defined as the time from first response to documented progressive disease or death from any cause in patients who achieved a partial response or better using IWG response criteria and by blinded independent central review. Those without a response were excluded from this analysis. DOR in the KEYNOTE-204 study was also assessed by blinded independent central review using IWG criteria but a clear definition of DOR was not provided.
Time to Response
No information was provided regarding time to response in the KEYNOTE-051, KEYNOTE-087, or KEYNOTE-204 studies.
Health-Related Quality of Life
HRQoL was not measured in the KEYNOTE-051 study. In the KEYNOTE-087 and KEYNOTE-204 studies, HRQoL was measured by the EORTC QLQ-C30 and EQ-5D-3L. The EORTC QLQ-30 is a widely used, cancer-specific HRQoL instrument consisting of 30 items measuring 5 functional dimensions (physical, role, cognitive, emotional, and social), 3 symptoms dimensions (fatigue, nausea/vomiting, and pain), 6 additional items (dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial impact), and a global HRQoL measure. The minimal important difference for adult cancer patients on the EORTC QLQ-C30 scale is 5 but no minimal important difference was identified specifically for patients with cHL.45 EQ-5D-3L is another standard instrument to measure health outcomes and is particularly useful to develop economic models. EQ-5D-3L measures mobility, self-care, usual activities, pain/discomfort, and anxiety/depression using a 3-point ordinal scale. These measurements can be pooled into a single utility score. Further, the EQ-5D-3L contains a visual analogue scale ranging from 0 to 100 so that participants may rate their general health state. Each of the HRQoL questionnaires were conducted at baseline and at 24 weeks so that the change could be calculated and compared between groups. The minimal important difference for adult cancer patients on the EQ-5D-3L visual analogue scale is 6 to 10 and the minimal important difference for American patients with cancer on the EQ-5D-3L utility scale is 0.05 to 0.08.46 No minimal important difference on the visual analogue scale or utility portion of the EQ-5D-3L scale for patients with cHL was identified.
Harms
All 3 studies assessed AEs, serious AEs, and immune-mediated AEs. An AE was defined as any untoward medical occurrence in a patient which does not necessarily have a causal relationship with the treatment. Serious AEs were defined as those which result in death, are life threatening, result in persistent or significant disability/incapacity, result in or prolong an existing inpatient hospitalization, result in a congenital anomaly/birth defect, is another important medical event, results in the development of a new cancer (different from the cancer under investigation), or is associated with an overdose. Immune-mediated AEs were defined as AEs of unknown etiology associated with drug exposure and consistent with an immune phenomenon. The following are examples: pneumonitis, diarrhea/colitis, elevated aspartate aminotransferase, elevated alanine aminotransferase, elevated bilirubin, type 1 diabetes mellitus, hypophysitis, hyperthyroidism, hypothyroidism, nephritis, renal dysfunction, and myocarditis.
Statistical Analysis
Progression-Free Survival
In KEYNOTE-087, PFS was estimated using the Kaplan-Meier method. In the KEYNOTE-204 study, PFS was analyzed using a stratified log rank test and the Kaplan-Meier method and the hazard ratio was estimated using a stratified Cox regression model using the Efron method to handle ties. The analysis was stratified by prior ASCT and cHL status after front-line therapy (primary refractory, relapsed disease < 12 months after completion of first-line therapy, or relapse 12 months or longer after completing first-line therapy). In the KEYNOTE-204 study, the analysis considered ASCT or initiation of another anticancer treatment as a censoring event. A sensitivity analysis of PFS considered the use of another anticancer medication as a progression event but otherwise had the same definition of PFS.
Overall Survival
In the KEYNOTE-087 study, OS was estimated using the Kaplan-Meier method. In the KEYNOTE-204 study, OS was analyzed in the same manner as the PFS analysis. The study protocol stated that if required to adjust for patients receiving subsequent anticancer therapies (following pembrolizumab or BV) in the OS analysis, the rank-preserving structural failure time and 2-stage analysis methods would be used. Rank-preserving structural failure time assumes all patients would receive equal benefit from identical interventions. This method compares time on and off treatment to estimate survival times without treatment and a treatment effect adjusted for subsequent utilization of anticancer therapy. The 2-stage adjustment assumes subsequent utilization of anticancer therapy only occurs following disease progression and uses this point to establish a “secondary” baseline. Within the control group, the treatment effect is estimated between those who do and do not subsequently use anticancer therapy that adjusts for “secondary” baseline characteristics. The incremental treatment effect between these groups is then used to discount the treatment effect observed in those who subsequently use additional anticancer therapies, which are then compared with the experimental group to estimate the treatment effect adjusted for subsequent use of anticancer therapies.
Objective Response Rate
In the KEYNOTE-087 and KEYNOTE-204 studies, ORR was estimated by a point estimate and 95% 2-sided binomial exact CIs using the Clopper-Pearson method. In the KEYNOTE-204 study, the difference in ORR was analyzed using the Miettinen and Nurminen method, weighted by stratum. In this analysis, data were stratified by previous ASCT and cHL status following front-line therapy (primary refractory, relapsed disease < 12 months after completion of first-line therapy, or relapse 12 months or longer after completing first-line therapy). Patients with missing data were assumed to be non-responders.
Complete Response Rate
In the KEYNOTE-087 study, complete response rate analysis consisted of the point estimate and 95% 2-sided exact CI. In the KEYNOTE-204 study, complete response rate was analyzed as in the ORR analysis.
Duration of Response
In the KEYNOTE-087 study, DOR was estimated using the Kaplan-Meier method. Patients without progression were censored on the date of the most recent assessment. In the KEYNOTE-204 study, no explicit statistical analysis was outlined for DOR.
Time to Response
In the KEYNOTE-087 and 204 studies, no explicit statistical analysis was outlined for time to response.
Health-Related Quality of Life
In the KEYNOTE-087 study, HRQoL data were collected at baseline and 24 weeks later in all individuals. The difference in scores between baseline and week 24 was analyzed using a longitudinal data analysis model adjusting for time and ECOG status and then estimated using a least squares mean score and standard error. In the KEYNOTE-204 study, no explicit statistical analysis was outlined for HRQoL.
Harms
In the KEYNOTE-087 study, only descriptive statistics were provided. In the KEYNOTE-204 study, descriptive statistics including point estimates and 95% CIs were estimated and the unstratified Miettinen and Nurminen method was used to assess between-treatment differences.
Interim Analyses
The KEYNOTE-204 study results presented in this report constitute the second of 4 planned interim analyses. The study protocol planned for 1 interim analysis of PFS and 2 interim analyses of OS. An interim PFS analysis was to be conducted 3 months after all patients had been enrolled and once 110 PFS events had occurred. A final PFS analysis was planned once 221 events had occurred. If the PFS hypothesis was not rejected at the interim, then the first interim OS analysis would occur with the final PFS analysis. This assumes that 91 OS events would have been observed at this point. If the PFS hypothesis was rejected at the interim analysis then the interim OS analysis would occur 1 year from the interim PFS analysis or when 91 OS events occurred, whichever came first. If the OS hypothesis was not rejected at that point, a second interim OS analysis would occur at 119 events and if the OS hypothesis was not rejected at that point, a final OS analysis was planned to occur at 146 events. No interim analysis was planned for ORR. The final ORR analysis will occur with the final PFS analysis (when 221 PFS events have occurred).
Multiplicity
In the KEYNOTE-087 study, no multiplicity adjustments were required as each cohort was evaluated independently. All tests of significance were controlled at a 1-sided alpha of 0.025. In the KEYNOTE-204 study, the Mauer and Bretz method was used to allocate and re-allocate type I error between hypotheses and group sequential methods to allocate alpha between interim and final analyses. If the null hypothesis was rejected, the type I error allocated to that hypothesis would be redistributed to other hypotheses. For example, multiplicity could be controlled at 2.5% (1-sided) with 1.25% originally allocated to PFS and OS and none allocated to the ORR hypothesis. If the PFS null hypothesis was rejected, then 0.625% would be allocated to both the OS and ORR test. If the ORR null hypothesis was rejected, then 0.625% would be allocated to the OS analysis. OS testing was planned to occur at the 1.25% level if the PFS null hypothesis was not rejected, at 1.875% if the PFS but not ORR null hypotheses were rejected, or 2.5% if both the PFS and ORR null hypotheses were rejected.
Power Calculations
In the KEYNOTE-087 study, for cohorts 1 and 3, there is 93% power, at a 1-sided 2.5% alpha, to detect a 35% or higher ORR between pembrolizumab and a fixed control rate of 15% using the exact binomial test. This would require at least 16 responses if 60 patients are recruited. In cohort 2, there is 93% power, at a 1-sided 2.5% alpha, to detect a 20% or higher overall response rate in the pembrolizumab arm versus the fixed control rate of 5% using exact binomial test. This would require at least 8 responses out of 60 patients.
On the basis of 194 PFS events, the study had an 85% power to detect a hazard ratio of 0.622 (pembrolizumab versus BV) at and alpha of 1.2% (1-sided), assuming PFS would follow an exponential distribution with a median of 5·6 months in the control.
Table 21
Statistical Analysis of Efficacy End Points.
Analysis Populations
In the KEYNOTE-051 and KEYNOTE-087 studies, all patients who received at least 1 dose of study medication were analyzed in the efficacy and safety analyses. In the KEYNOTE-204 study, the intention-to-treat population was used in the efficacy analysis and the safety analysis consisted of all patients who received at least 1 dose of a study medication.
Results
Patient Disposition
No patient in the KEYNOTE-051 study discontinued the trial. Of the 7 patients in the KEYNOTE-051 study, 2 are still receiving treatment while 5 others had discontinued therapy at the time of data cut-off. In the KEYNOTE-087 study, 72.5%, 84.0%, and 76.7% of patients discontinued treatment in cohorts 1, 2, and 3, respectively, at the time of data cut-off. In the KEYNOTE-204 study, 338 patients were screened, 151 patients were randomized to pembrolizumab, and 153 were randomized to BV. Fewer patients in the pembrolizumab arm (13.2%) discontinued the trial compared to those in the BV arm (28.1%). A notable difference in trial discontinuation was due to deaths (10.6% in the pembrolizumab arm and 17.6% in the BV arm) and withdrawals (2.0% in the pembrolizumab arm and 8.5% in the BV arm). Similarly, treatment discontinuations were lower in the pembrolizumab arm (74.3%) than in the BV arm (96.1%). Notable differences in the reasons for treatment discontinuations were due to AEs (pembrolizumab: 13.5% versus BV: 19.1%) and progressive disease (pembrolizumab: 39.2% versus BV: 49.3%). Full details regarding patient disposition are available in Table 22.
Table 22
Patient Disposition.
Exposure to Study Treatments
In the KEYNOTE-051 study the median exposure to pembrolizumab was 344 days. Those in the KEYNOTE-087 study were exposed to pembrolizumab for a median of 506, 254, and 399.5 days in cohorts 1, 2, and 3, respectively. In the KEYNOTE-204 study, the median duration of exposure to pembrolizumab was 305 days and the median duration of exposure to BV was 146.5 days.
Efficacy
Only the efficacy outcomes that were identified in the review protocol are summarized below and in Table 23.
Progression-Free Survival
In the KEYNOTE-051 study, 3 patients (42.9%) experienced an event (disease progression or death). In the KEYNOTE-087 study, there were 43 (62.3%), 54 (66.7%), and 36 (60.0%) events in cohorts 1, 2, and 3, respectively. In the KEYNOTE-204 study, the proportion of patients experiencing an event were similar between the pembrolizumab (53.6%) and BV (57.5%) arms. In the KEYNOTE-051 study, the median PFS was reported to be 11.1 months (95% CI, 2.6 to not reported). In the KEYNOTE-087 study, median survival was reported to be 16.4 months (95% CI, 11.3 to 27.6), 11.1 months (95% CI, 7.3 to 13.5), and 19.4 (95% CI, 8.4 to 22.1) months in cohorts 1, 2, and 3, respectively. In the KEYNOTE-204 study, the median PFS was higher in the pembrolizumab arm (13.2 months; 95% CI, 10.9 to 19.4) than the BV arm (8.3 months; 95% CI, 5.7 to 8.8). In the KEYNOTE-051 study, the PFS rate at 12 months was 27.8% (no 95% CI reported). In the KEYNOTE-087 study, the PFS rate at 12 months was 61.3%, 43.0%, and 53.9% in cohorts 1, 2, and 3, respectively (no 95% CI reported). In the KEYNOTE-204 study, the 12-month PFS rate was higher in the pembrolizumab arm (53.9%; 95% CI, 45.0 to 61.9) than the BV arm (35.6%; 95% CI, 26.9 to 44.4). In the KEYNOTE-087 study, the 24-month PFS rate was 41.6%, 21.9%, and 34.0% in cohorts 1, 2, and 3, respectively (no 95% CI reported). In the KEYNOTE-204 study, the 24-month PFS rate was 35.4% (95% CI, 26.2 to 44.6) in the pembrolizumab arm and 25.4% (95% CI, 17.1 to 34.5) in the BV arm. The hazard ratio for time to progression was 0.65 (95% CI, 0.48 to 0.88), which was statistically significant (P = 0.0027).
In the KEYNOTE-204 study, the primary PFS analysis considered initiation of subsequent anticancer therapy or ASCT as a censoring event. A sensitivity analysis was conducted which treated these events as a progression event instead. In this analysis, 103 (68.2%) and 119 (77.8%) events were observed in the pembrolizumab and BV arms, respectively. The median PFS was higher in the pembrolizumab arm (9.5 months; 95% CI, 8.2 to 12.7) than the BV arm (5.7 months; 95% CI, 5.6 to 8.3). The hazard ratio for time to progression was 0.62 (95% CI, 0.48 to 0.82).
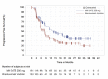
Figure 3
Kaplan-Meier Estimates of PFS in KEYNOTE-204.
Overall Survival
In the KEYNOTE-051 study, minimal information regarding OS was provided. In the KEYNOTE-087 study, 15.9%, 16.0%, and 15.0% of patients in cohorts 1, 2, and 3, respectively, died. |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||| Median survival was not reported in the KEYNOTE-051 study and not reached in the KEYNOTE-087 or KEYNOTE-204 studies. In the KEYNOTE-051 study, 100% of patients were alive at 12 months. In the KEYNOTE-087 study, OS at 12 months was 95.7%, 96.2% and 96.6% in cohorts 1, 2, and 3, respectively (95% CI not reported). |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| At 24 months in the KEYNOTE-087 study, 92.6%, 91.0%, and 89.4% of patients were alive in cohorts 1, 2, and 3, respectively (95% CI not reported). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Figure 4
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| .
Objective Response Rate
In the KEYNOTE-051 study, 42.9% (95% CI, 9.9 to 81.6) of patients experienced a partial or complete response. In the KEYNOTE-087 study, 78.3% (95% CI, 66.7 to 87.3), 64.2% (95% CI, 52.8 to 74.6), and 71.7% (95% CI, 58.6 to 82.5) of patients experienced a partial or complete response in cohorts 1, 2, and 3, respectively. In the KEYNOTE-204 study, more partial or completes responses were observed in the pembrolizumab arm relative to the BV arm (65.6%; 95% CI, 57.4 to 73.1 versus 54.2; 95% CI, 46.0 to 62.3), which was associated with a statistically insignificant 11.3% (95% CI, 0.2 to 22.1) difference in favour of pembrolizumab.
Complete Response Rate
In the KEYNOTE-051 study, 28.6% of patients (95% CI, 3.7 to 71.0) experienced a complete response. In the KEYNOTE-087 study, 26.1% (95% CI, 16.3 to 38.1), 25.9 (95% CI, 16.8 to 36.9), and 31.7% (95% CI, 20.3 to 45.0) of patients in cohorts 1, 2, and 3, respectively, experienced a complete response. In the KEYNOTE-204 study, the complete response rate was comparable between the pembrolizumab (24.5%; 95% CI, 17.9 to 32.2) and BV arms (24.2; 95% CI, 17.6 to 31.8).
Duration of Response
In the KEYNOTE-051 study, median DOR was not reached. In the KEYNOTE-087 study, the median DOR in cohorts 1, 2, and 3 were 25.0 months (range = 0 to 36.1), 11.1 months (range = 0 to 35.9), and 16.8 months (range = 0 to 39.1), respectively. In the KEYNOTE-204 study, the median DOR was higher among patients in the pembrolizumab arm (20.7 months; range = 0 to 33.2) than in patients in the BV arm (13.8 months; range = 0 to 33.9).
Time to Response
Median time to response in the KEYNOTE-051 study was 2.6 months (range = 2.1 to 2.8). The median time to response in cohort 1, cohort 2, and cohort 3 of the KEYNOTE-087 study were 2.7 months (range = 2.1 to 12.9), 2.8 months (range = 2.2 to 11.0), and 2.8 months (range = 2.6 to 16.5), respectively. Finally, the median time to response in the pembrolizumab arm of the KEYNOTE-204 study was 2.8 months (range = 1.0 to 31.2) and also 2.8 months (range = 1.3 to 7.3) in the BV arm.
Health-Related Quality of Life
HRQoL data were only measured in the KEYNOTE-087 and KEYNOTE-204 studies. In the KEYNOTE-087 study, the least squares mean square change in EORTC QLQ-C30 global health status between week 24 and baseline was 11.8, 13.9 and 6.6, in cohorts 1, 2, and 3, respectively. No CIs were reported in the KEYNOTE-087 study. In the KEYNOTE-204 study, the least squares mean change in EORTC QLQ-C30 global health status between baseline and week 24 was 8.60 points (95% CI, 3.89 to 13.31) higher in the pembrolizumab arm versus the BV arm. Consistent results were reported for the EORTC QLQ-C30 physical functioning scale (6.24; 95% CI, 1.87 to 10.62), EQ-5D-3L utility score (0.09; 95% CI, 0.04 to 0.14), and EQ-5D-3L visual analogue scale (6.12; 95% CI, 1.91 to 10.34).
Table 23
Key Efficacy Results in KEYNOTE-051, KEYNOTE-087, and KEYNOTE-204 (Intention-to-Treat Analysis).
Harms
The safety outcomes (harms) identified in the review protocol are reported below. See Table 24 for detailed harms data.
Adverse Events
In the KEYNOTE-051 study, 85.7% of patients experienced at least 1 AE. In the KEYNOTE-087 study, 98.6%, 98.8%, and 95.0% of patients experienced at least 1 AE in cohort 1, cohort 2, and cohort 3, respectively. In the KEYNOTE-204 study, 98.0% of patients in the pembrolizumab arm and 94.1% of those in the BV arm experienced an AE. The most common AEs were pyrexia, vomiting, headache, abdominal pain, anemia, cough, fatigue, diarrhea, and upper respiratory tract infections. In the KEYNOTE-204 study, pembrolizumab patients were more likely than BV patients to experience endocrine disorders (20.3% versus 3.9%); infections (66.2% versus 45.4%); musculoskeletal and connective tissue disorders (37.8% versus 31.6%); neoplasms (7.4% versus 1.3%), renal or urinary disorders (14.9% versus 4.6%); respiratory, thoracic, or mediastinal disorders (45.3% versus 26.3%); and skin and subcutaneous tissue disorders (43.9% versus 36.8%), but less likely to experience blood or lymphatic system disorders (18.2% versus 25.7%), gastrointestinal disorders (43.9% versus 52.0%), and nervous system disorders (26.4% versus 50.7%).
Serious Adverse Events
In the KEYNOTE-051 study, 28.6% of patients experienced at least 1 serious AE. In the KEYNOTE-087 study, 21.7%, 22.2%, and 25.0% of patients experienced a serious AE in cohort 1, cohort 2, and cohort 3, respectively. In the KEYNOTE-204 study, 29.7% of pembrolizumab and 21.1% of BV-treated patients experienced a serious AE. The most common serious AEs in the KEYNOTE-051 study were diaphragmatic hernia and pneumonia. The most common serious AEs in cohort 1 of the KEYNOTE-087 study were pneumonia and pericarditis. The most common serious AE in cohort 2 of the KEYNOTE-087 study was herpes zoster and the most common serious AEs in cohort 3 of the KEYNOTE-087 study were pyrexia and pneumonitis, There were no notable differences in frequency of serious AEs between the pembrolizumab and BV arms in the KEYNOTE-204 study. The most common serious AEs in the pembrolizumab arm of the KEYNOTE-204 study were infections or infestations; respiratory, thoracic, or mediastinal disorders; neoplasms; general disorders or administration site conditions; and hepatobiliary disorders. The most common serious AEs in the BV arm of the KEYNOTE-204 study were infections or infestations; respiratory, thoracic, or mediastinal disorders; nervous system disorders; gastrointestinal disorders; and general disorders or administration site conditions.
Treatment Discontinuation Due to AEs
No patients in KEYNOTE-051 discontinued treatment due to an AE while 11.6%, 6.2%, and 8.3% of patients in cohort 1, cohort 2, and cohort 3 of KEYNOTE-087, respectively, discontinued treatment due to an AE. In the KEYNOTE-204 study, 13.5% and 17.8% of patients receiving pembrolizumab and BV, respectively, discontinued treatment due to an AE.
Mortality
No patients in the KEYNOTE-051 study or cohort 1 of the KEYNOTE-087 study died. In the KEYNOTE-087 study, 2.5% and 1.7% of patients in cohort 2 and cohort 3, respectively, died. In the KEYNOTE-204 study, 2.0% and 1.3% of patients receiving pembrolizumab or BV, respectively, died.
Immune-Mediated AEs
In the KEYNOTE-051 study, 28.6% of patients experienced at least 1 immune-mediated AE. In cohort 1, 2, and 3 of the KEYNOTE-087 study, 31.9%, 32.1%, and 38.3% of patients, respectively, experienced at least 1 immune-mediated AE. In the KEYNOTE-204 study, more patients in the pembrolizumab arm (35.8%) than the BV arm (13.8%) experienced an immune-mediated AE.
No patients in the KEYNOTE-051 study experienced a serious immune-mediated AE. In the KEYNOTE-087 study, 4.3%, 2.5% and 5.0% of patients in cohort 1, cohort 2, and cohort 3, respectively, experienced a serious immune-mediated AE. In the KEYNOTE-204 study, more pembrolizumab- than BV-treated patients experienced a serious immune-mediated AE (8.8% versus 3.3%).
Table 24
Summary of Harms.
Table 25
Most Frequent Adverse Events in KEYNOTE-051.
Table 26
Most Frequent Adverse Events in KEYNOTE-087; Incidence of 10% or Greater in 1 or More Groups.
Table 27
Most Frequent Adverse Events in KEYNOTE-204; Incidence of 5% or Greater in 1 or More Groups.
Table 28
Most Frequent Serious Adverse Events in KEYNOTE-051.
Table 29
Most Frequent Serious Adverse Events in KEYNOTE-087; Incidence of 1% or Greater.
Table 30
Most Frequent Serious Adverse Events in KEYNOTE-204; Incidence of 1% or Greater.
Table 31
Most Frequent Immune-Mediated Adverse Events in KEYNOTE-051.
Table 32
Most Frequent Immune-Mediated Adverse Events in KEYNOTE-087.
Table 33
Most Frequent Immune-Mediated Adverse Events in KEYNOTE-204.
Critical Appraisal
Internal Validity of KEYNOTE-051
The KEYNOTE-051 study originally did not aim to specifically recruit patients with relapsed or refractory cHL. A study protocol amendment was made to identify patients who clearly had relapsed or refractory cHL which then identified 7 patients. Only 3 response events were observed, based on these 7 patients. Due to the low event rate and sample size, it is uncertain if these results alone are representative of the potential benefits and harms of pembrolizumab. The KEYNOTE-051 study was also an open-label trial without a comparator, thus any incremental benefit over standard of care is unknown and fails to mitigate the impact of confounding variables, but the trial assessed response to therapy using an independent and blinded assessor which reduces any bias introduced by the open-label design on outcomes such as PFS, ORR, complete response rate, DOR, and time to response.
External Validity of KEYNOTE-051
The KEYNOTE-051 study did not explicitly recruit patients who failed ASCT or were ineligible for ASCT and salvage chemotherapy. Thus, generalizability of the KEYNOTE-051 study to the requested patient population is uncertain. These results may also only be applicable to those with a Lansky Play-Performance Scale score of 50 or greater for children 16 and younger or a Karnofsky score of 50 or greater in children aged 16 years and older.
Internal Validity of KEYNOTE-087
The KEYNOTE-087 study is a single-arm, open-label trial and thus provides limited insight on any additional benefit over the current standard of care. The open-label nature of this trial may have biased the HRQoL assessment, but the trial did assess response to therapy using an independent and blinded assessor which reduces the bias introduced by the open-label design on outcomes such as PFS, ORR, complete response rate, DOR, and time to response.
External Validity of KEYNOTE-087
The results from the KEYNOTE-087 study are generalizable to most adult patients who have failed an ASCT or are ineligible for ASCT and salvage chemotherapy. The KEYNOTE-087 study excluded those with an ECOG performance score of 2 or greater; hence, its results may not be generalizable to this population. Notably, cohort 1 and 2 included patients who had received and failed on BV in addition to having a history of disease progression after ASCT (cohort 1) or being ineligible for ASCT (cohort 2), and thus do not completely align with the population of interest in this review.
Internal Validity of KEYNOTE-204
The KEYNOTE-204 study was an open-label trial which randomized patients centrally using an interactive voice response system and integrated web response system. Randomization was stratified based on prior ASCT (yes or no) and cHL status after first-line therapy (primary refractory, relapsed disease < 12 months after completion of first-line therapy, or relapse 12 months or longer after completing first-line therapy). Randomization helps to ensure prognostic balance at the start of the study and baseline characteristics were generally balanced between arms suggesting randomization was successful. There were slightly higher rates of bulky disease, baseline B symptoms, and baseline bone marrow involvement in the pembrolizumab arm; however, the clinical experts do not believe this imbalance detracts from the results favouring pembrolizumab as the patients in the pembrolizumab arm had more adverse baseline characteristics than those in the BV arm.
The KEYNOTE-204 study allowed patients to receive subsequent anticancer therapies once a trial medication (pembrolizumab or BV) was discontinued but did not allow concurrent use of other anticancer treatments with trial medications. Almost all patients randomized to BV (97.4%) received a subsequent anticancer therapy while 70.2% of pembrolizumab-treated patients did so. Those randomized to BV were more likely to cross over and subsequently receive pembrolizumab (17.8% versus 1.4% of patients who were originally randomized to pembrolizumab are retreated with pembrolizumab). Those originally randomized to BV were also more likely to receive nivolumab (19.7%) relative to those randomized to pembrolizumab (3.4%). Finally, 25.0% of patients originally randomized to pembrolizumab received BV while 4.6% of patients originally randomized to BV were retreated with BV (Table 19 and Appendix 2). Because these therapies were not used concurrently with a trial medication and were only used following a PFS event and after the trial medication was discontinued, it should not substantially impact the PFS analysis. However, subsequent utilization of anticancer therapies was considered a censoring event in the primary PFS analysis which may be a questionable assumption. In a pre-specified sensitivity analysis, initiation of subsequent anticancer therapies was considered a progression event and the resulting hazard ratio and 95% CI did not change substantially from the main analysis, suggesting the impact of subsequent anticancer therapy utilization on PFS might be limited. Subsequent utilization of anticancer therapies can impact the OS analysis; however, the use of subsequent anticancer medications was not evaluated by rank-preserving structural failure time or 2-stage analysis as suggested in the study protocol; thus, it is unclear what effect, if any, utilization of subsequent anticancer therapies would have on the OS results. |||||||||||||||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| While the sponsor confirmed the proportional hazards assumption likely was not violated in the PFS analysis, it is unclear if the proportional hazards assumption was met in the model of OS as the assumption was not tested.
Treatment discontinuation rates were higher in the BV (96.1%) arm than in the pembrolizumab arm (74.3%) mostly due to higher discontinuations due to AEs and progressive disease. Discontinuation from the trial was also higher in the BV group (28.1% versus 13.2%) due to higher rates of death and withdrawal. These discontinuations may not be cause for methodological concern as they may reflect the superiority of pembrolizumab over BV. Alternatively, a propensity to discontinue therapy or the trial early could be influenced by the open-label nature of the study and a patient’s view of the medication they were randomized to. Similarly, the open-label nature of the study may have impacted subjective outcomes measures such as HRQoL. HRQoL was measured at baseline and week 24 but is unclear if at this point patients had already discontinued the trial medication and began utilization of a subsequent anticancer medication. If this is the case, their HRQoL scores may have been influenced by the subsequent anticancer medication and not the trial medication alone.
External Validity of KEYNOTE-204
The KEYNOTE-204 study results are generalizable to patients with cHL who failed ASCT or are ineligible for ASCT and multi-agent salvage chemotherapy. The KEYNOTE-204 study did not recruit individuals with an ECOG score of 2 or greater; therefore, results may not be applicable to this group. This study only compared pembrolizumab to BV, but not to any other comparators of interest listed in the study protocol. Notably, CADTH reviewed the use of BV in adults with HL after failure of at least 2 multi-agent chemotherapy regimens who are not candidates for ASCT and did not recommend reimbursement.35 However, the clinical experts consulted in the current review confirmed that in jurisdictions where it is funded, BV is still standard of care due to the lack of superior alternatives. This is in part supported by more recent evidence suggesting the efficacy of BV as third-line therapy in patients who have not received a stem cell transplant.36 Of note, BV is not universally funded across Canada at this time. The KEYNOTE-204 study did not conduct a subgroup analysis to analyze any differential impact of pembrolizumab on patients who failed ASCT and those who were ineligible for ASCT and salvage chemotherapy, thus the review team was unable to determine if there was a differential effect in each group. Approximately 11% of patients in each arm discontinued their study medication and subsequently received a stem cell transplant who were then censored in the PFS analysis at the time of transplant.
Table 34
Assessment of Generalizability of Evidence for Pembrolizumab.
Discussion
Summary of Available Evidence
One study, the KEYNOTE-051 study, assessed the efficacy and safety of 2 mg/kg of pembrolizumab administered every 3 weeks in 7 pediatric patients with relapsing or refractory cHL while 2 studies, the KEYNOTE-087 (N = 210) and KEYNOTE-204 (N = 304) studies, did so in the adults at a dose of 200 mg every 3 weeks. The KEYNOTE-087 study was a single-arm trial which divided patients in to 3 cohorts as shown below. Notably, cohorts 1 and 2 are similar but not identical to the population of interest as they have received and then failed or relapsed on BV. Patients in cohort 3 are similar to the population of interest as they had failed or relapsed after ASCT but were BV naive.
- Cohort 1: Patients who failed to respond to or progressed after ASCT and also relapsed after or failed to respond to treatment with BV after ASCT (N = 69)
- Cohort 2: Patients who were ineligible for ASCT and relapsed after or failed to respond to BV (N = 81)
- Cohort 3: Patients who failed to respond to or progressed after ASCT and had not yet received BV (N = 60)
Finally, the KEYNOTE-204 study was an open-label, randomized trial comparing pembrolizumab (N = 151) with BV (N = 153) in patients with relapsed or refractory cHL who failed ASCT or were ineligible for ASCT but had trialled at least 2 multi-agent chemotherapy regimens. Although the KEYNOTE-204 study was an actively controlled trial, it is uncertain if the results observed could be extrapolated to other comparators.
Interpretation of Results
Efficacy
The KEYNOTE-051 study only recruited 7 pediatric patients; a sample size which is insufficient to be truly representative of pembrolizumab’s benefits and harms. The KEYNOTE-051 study was also an open-label, single-arm trial which limits the ability to estimate the incremental benefit of pembrolizumab over standard of care or mitigate the risk of confounding variables. Further, these patients had relapsed or refractory cHL but the study did not require patients to have a history of failure after ASCT, nor did it require patients to be ineligible for ASCT and multi-agent salvage therapy. Therefore, the KEYNOTE-051 study’s eligibility criteria do not completely align with the population of interest for this review. Due to the methodological limitations of the KEYNOTE-051 study, the conclusions made by the review team are mainly derived from the results reported in the KEYNOTE-087 and KEYNOTE-204 studies which only recruited adult patients. Among the cohorts of the KEYNOTE-087 study, cohort 3 most closely resembles the eligibility criteria for this review as these patients have failed ASCT and are BV naive while those in cohort 1 and 2 had trialled and failed BV.
Studies identified in this review do not provide a sufficient evidence base for a stand-alone reimbursement recommendation for the pediatric population. However, the clinical experts consulted by the review team confirmed that cHL is a disease which does not conform to the traditional and arguably artificial delineations of disease by age. Instead, cHL is often viewed as a disease with a similar biology and treatment approach, regardless of age. As such, while the median age of patients from the KEYNOTE-087 and KEYNOTE-204 studies ranges from 32.0 years to 40.0 years, the clinical experts believed these results were applicable to patients with cHL younger than 18 years of age. The clinical experts agreed that it might be biologically plausible to extrapolate the results of the KEYNOTE-087 and KEYNOTE-204 studies to pediatric patients and assume pembrolizumab may also benefit pediatric patients; however, evidence from studies with rigorous methodological quality are needed to confirm pembrolizumab’s benefits in the pediatric population. Further, it is uncertain if the pembrolizumab dosing regimen used in the KEYNOTE-087 and KEYNOTE-204 studies could be extrapolated to pediatric patients in whom the pharmacokinetic profile of pembrolizumab may differ. The KEYNOTE-051 study recruited 155 additional patients with other types of cancer, and in addition to past literature, pembrolizumab’s safety at this dose may be easier to establish but KEYNOTE-051 was a phase I/II study and thus it is unclear if 2 mg/kg is the most efficacious dose in pediatric patients. Such concerns would have been mitigated if the quality of evidence derived from the KEYNOTE-051 study was sufficiently robust to confirm that the effect of pembrolizumab administered at 2 mg/kg in pediatric patients was similar to the effect of pembrolizumab administered at 200 mg in adult patients. In the absence of this evidence, only clinical judgment and expertise can provide guidance on this issue.
Based on the KEYNOTE-087 and KEYNOTE-204 studies, pembrolizumab achieved clinically and statistically significant improvements over BV on PFS and clinically significant improvements on || ORR, DOR, and HRQoL. Patients treated with BV in the KEYNOTE-204 study had a median PFS of 8.3 months (95% CI, 5.7 to 8.8) while those treated with pembrolizumab had a median PFS of 13.2 months (95% CI, 10.9 to 19.4). The median PFS in cohort 3 of the KEYNOTE-087 study was the highest observed in all studies (19.4 months; 95% CI, 8.4 to 22.1) which supports the robust PFS evidence from the KEYNOTE-204 study. “Longer survival” was the most highly desired treatment outcome identified by patients. |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| PFS is a commonly used proxy indicator for OS in many oncology trials but CADTH staff were unable to identify any literature that quantified PFS power to predict OS in this patient population; thus, this report’s ability to translate pembrolizumab’s impact on PFS to OS will rely on clinical expertise.
ORR data were also encouraging as 11.3% (95% CI, 0.2 to 22.1) more patients in the pembrolizumab arm (65.6%; 95% CI, 57.4 to 73.1) of the KEYNOTE-204 study achieved a response relative to the BV arm (54.2%; 95% CI, 46.0 to 62.3). The proportion of patients in cohort 3 of the KEYNOTE-087 study achieving a response was even higher (71.7%; 95% CI, 58.6 to 82.5). However, the proportion of patients achieving a complete response was similar in the KEYNOTE-204 study regardless of treatment. CADTH’s clinical experts noted that while complete response rates are important, ORR is a more clinically relevant metric and patients may still have a relatively strong quality of life with a partial response or with disease stabilization. Patients also indicated that “longer remission” was a crucial treatment outcome. Relative to BV-treated patients, the DOR was higher in pembrolizumab-treated patients from the KEYNOTE-204 study and cohort 3 of the KEYNOTE-087 study.
Between baseline and week 24 in the KEYNOTE-204 study, HRQoL as measured by EORTC QLQ-C30 global health status improved by 8.60 (95% CI, 3.89 to 13.31) more points in the pembrolizumab arm relative to the BV arm. This is above the minimal important difference of 5 for cancer patients.45 Compared to the pembrolizumab arm of the KEYNOTE-204 study, EORTC QLQ-C30 global health status scores increased by a similar magnitude in cohort 3 of the KEYNOTE-087 study. Between baseline and week 24, HRQoL in pembrolizumab patients in the KEYNOTE-204 study improved more than BV patients when measured by the EQ-5D-3L utility scores (0.09; 95% CI, 0.04 to 0.14) and EQ-5D-3L visual analogue scores (6.12; 95% CI, 1.91 to 10.34) which exceeded the respective minimal important differences for patients with cancer of 0.05 and 6.47 While encouraging, HRQoL is a subjective measure which could have been influenced by the open-label nature of the trial and should be interpreted with caution. Finally, while minimal important differences were identified for patients with cancer, the minimal important differences were not specific to patients with cHL.
Harms
While BV patients in the KEYNOTE-204 study were least likely to experience an AE, serious AE, or immune-mediated AE, they were most likely to discontinue therapy due to an AE. There were no clear AEs that disproportionately affected the BV arm to an extent that would explain this phenomenon; hence, this observation cannot be clarified based on this data alone. One hypothesis is that BV-treated patients expected or observed fewer benefits and thus were less likely to tolerate the associated AEs and more likely to discontinue therapy. In this example, discontinuation is influenced by the fact that these patients were not blinded to their therapy and knew switching to pembrolizumab or another medication was an option. If they viewed BV as an inferior option, tolerance to AEs is decreased and propensity to discontinue BV in favour of another medication is increased. Conversely, pembrolizumab patients may be willing to tolerate more AEs and/or more serious AEs before discontinuing therapy if they felt pembrolizumab’s benefits, and hence remaining on pembrolizumab, was superior to other alternatives. Based on the feedback received from patients, 9 patients had previously been treated with pembrolizumab and generally spoke positively about pembrolizumab’s safety profile. Anecdotally, 7 of the 9 did not experience any negative impact on work or school and all reported a good to excellent experience with pembrolizumab, which is critical as “better quality of life,” and was the third most important treatment outcome to patients. The fourth most important outcome was fewer side effects and although BV generally had lower rates of AEs, 1 patient who trialled pembrolizumab and eventually discontinued it due to toxicity still stated the “PFS was worth the side effects.” Thus, from a patient’s perspective, pembrolizumab’s benefits may outweigh the harms while BV’s harms outweighed the benefits despite BV actually having fewer AEs.
Conclusions
The body of evidence included in this review suggests that, when compared to BV, pembrolizumab provides statistically and clinically significant improvement in PFS, as well as clinically significant improvements in || ORR, DOR, and HRQoL. |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||| Patients who received BV were generally less likely to experience AEs, serious AEs, or immune-mediated AEs, but more likely to discontinue therapy due to an AE. A definitive explanation of this phenomenon cannot be derived from this evidence alone. However, 1 explanation could be that BV-treated patients expected or observed worse health outcomes and thus were less willing to tolerate AEs, even if the rates were lower than in the pembrolizumab arm. Discontinuation would be a viable alternative for these patients as receiving another anticancer medication, including pembrolizumab, was an option. Conversely, pembrolizumab patients may have been willing to tolerate more AE as the expected benefits were commensurately higher. The body of evidence primarily evaluated pembrolizumab administered 200 mg every 3 weeks in adults but due to the nature of the disease, CADTH’s clinical experts believe that the benefits observed in adults would also be applicable to pediatric patients. However, because of insufficient evidence on the use of pembrolizumab in pediatric patients, it is uncertain what dose should be used to ascertain the benefits observed in adults. No other comparators to pembrolizumab aside from BV were evaluated in the included studies; thus, the comparative effect of pembrolizumab to other relevant treatments in the population under review, beyond BV, remains uncertain. Also, the KEYNOTE-087 and KEYNOTE-204 studies only recruited patients with an ECOG score of 0 or 1 but the CADTH clinical experts did not recommend limiting the use of pembrolizumab only to patients with low ECOG scores. In totality, the evidence suggests that pediatric and adult patients with relapsed or refractory cHL who failed ASCT or are ineligible for multi-agent salvage chemotherapy and ASCT are more likely to benefit from pembrolizumab than from BV; however, the dose required to ascertain these benefits in pediatrics is uncertain.
Abbreviations
Abbreviations
- AE
adverse event
- ASCT
autologous stem cell transplant
- BV
brentuximab vedotin
- cHL
classical Hodgkin lymphoma
- CI
confidence interval
- DOR
duration of response
- ECOG
Eastern Cooperative Oncology Group
- EORTC QLQ-C30
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30
- EQ-5D-3L
EuroQol 5-Dimensions 3-Levels questionnaire
- ES
effect size
- HL
Hodgkin lymphoma
- HRQoL
health-related quality of life
- IWG
International Working Group
- OH-CCO DAC
Ontario Health Hematology Disease Site Drug Advisory Committee (Cancer Care Ontario)
- ORR
objective response rate
- OS
overall survival
- PET
positron emission tomography
- PFS
progression-free survival
- POGO
Pediatric Oncology Group of Ontario
Appendix 1. Clinical Literature Search Strategy
Note that this appendix has not been copy-edited.
Overview
Interface: Ovid
Databases:
- MEDLINE All (1946–present)
- Embase (1974–present)
- Note: Subject headings and search fields have been customized for each database. Duplicates between databases were removed in Ovid.
Date of search: March 17, 2021
Alerts: Bi-weekly search updates until project completion
Study types: No filters were applied to limit the retrieval by study type.
Limits:
- No publication date limits
- No language limits
- Conference abstracts: excluded
Table 35
Syntax Guide.
Multi-Database Strategy
Search strategy:
- (Keytruda* or pembrolizumab* or lambrolizumab* or MK 3475 or MK3475 or Merck 3475 or HSDB 8257 or HSDB8257 or Sch 900475 or Sch900475 or DPT0O3T46P).ti,ab,ot,kf,hw,rn,nm.
- Hodgkin Disease/
- (Hodgkin* or reed sternberg*).ti,ab,kf,ot,hw.
- ((lymphoma* or lymphogranuloma* or granuloma*) adj5 malign*).ti,ab,kf,ot,hw.
- (classic* HL or classic* HD).ti,ab,kf,ot,hw.
- or/2-5
- 1 and 6
- 7 use medall
- *pembrolizumab/
- (Keytruda* or pembrolizumab* or lambrolizumab* or MK 3475 or MK3475 or Merck 3475 or HSDB 8257 or HSDB8257 or Sch 900475 or Sch900475).ti,ab,kw,dq.
- 9 or 10
- exp Hodgkin Disease/
- (Hodgkin* or reed Sternberg*).ti,ab,kw,dq.
- ((lymphoma* or lymphogranuloma* or granuloma*) adj5 malign*).ti,ab,kw,dq.
- (classic* HL or classic* HD).ti,ab,kw,dq.
- or/12-15
- 11 and 16
- 17 use oemezd
- 18 not (conference abstract or conference review).pt.
- 8 or 19
- remove duplicates from 20
Clinical Trials Registries
ClinicalTrials.gov
Produced by the US National Library of Medicine. Targeted search used to capture registered clinical trials.
Search -- Studies with results on Keytruda (pembrolizumab) AND (Hodgkin disease OR Hodgkin lymphoma)
WHO ICTRP
International Clinical Trials Registry Platform, produced by the World Health Organization. Targeted search used to capture registered clinical trials.
Search terms – Keytruda (pembrolizumab) AND (Hodgkin disease OR Hodgkin lymphoma)
Health Canada’s Clinical Trials Database
Produced by Health Canada. Targeted search used to capture registered clinical trials.
Search terms – Keytruda (pembrolizumab) AND (Hodgkin disease OR Hodgkin lymphoma)
EU Clinical Trials Register
European Union Clinical Trials Register, produced by the European Union. Targeted search used to capture registered clinical trials.
Search terms – Keytruda (pembrolizumab) AND (Hodgkin disease OR Hodgkin lymphoma)
Grey Literature
Search dates: March 12, 2021 – March 16, 2021
Keywords: Keytruda OR pembrolizumab OR MK-3475) AND (Hodgkin disease OR Hodgkin lymphoma)
Limits: No publication date limits
Updated: Search updated prior to the completion of stakeholder feedback period
Relevant websites from the following sections of the CADTH grey literature checklist Grey Matters: A Practical Tool For Searching Health-Related Grey Literature were searched:
- Health Technology Assessment Agencies
- Health Economics
- Clinical Practice Guidelines
- Drug and Device Regulatory Approvals
- Advisories and Warnings
- Drug Class Reviews
- Clinical Trials Registries
- Databases (free)
- Health Statistics
- Internet Search
- Open Access Journals
Appendix 2. Subsequent Anticancer Medication Utilization in KEYNOTE-204
Note that this appendix has not been copy-edited.
Table 36
Subsequent Anticancer Therapy in KEYNOTE-204.
Appendix 3. Description and Appraisal of Outcome Measures
Note that this appendix has not been copy-edited.
Aim
To describe the following outcome measures and review their measurement properties (validity, reliability, responsiveness to change, and minimal important difference (MID)):
- European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire – core 30 items (EORTC QLQ-C30)
- European Quality of Life Scale – 5 Dimensions – 3 Levels (EQ-5D-3L)
Findings
Table 37
Summary of Outcome Measures and Their Measurement Properties.
EORTC QLQ-C30
Description
The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30, or EORTC QLQ-C30, is 1 of the most commonly used patient-reported outcome measures in oncology clinical trials.48 It is a multi-dimensional, cancer-specific, evaluative measure of HRQoL. It was designed specifically for the purpose of assessing changes in participants’ HRQoL in clinical trials in response to treatment.49 The core questionnaire of the EORTC QLQ-C30 consists of 30 questions that are scored to create 5 multi-item functional scales, 3 multi-item symptom scales, 6 single-item symptom scales, and a 2-item quality of life (QoL) scale, as outlined in Table 25. The first 2 versions of the questionnaire have been previously validated in patients with cancer.50 Version 3.0 of the questionnaire is the most current version and has been in use since December of 1997.51 It is available in 90 languages and is intended for use in adult populations only. The global QoL scale is also known as the Global Health Status (GHS).52
Table 38
EORTC QLQ-C30 Scales.
Scoring
The EORTC QLQ-C30 uses a 1-week recall period to assess function and symptoms.51 Most questions have 4 response options (“not at all,” “a little,” “quite a bit,” “very much”), with scores on these items ranging from 1 to 4. For the 2 items that form the global QoL scale, the response format is a 7-point Likert-type scale with anchors at 1 = “very poor” and 7 = “excellent.”
Raw scores for each scale are computed as the average of the items that contribute to a particular scale.51 This scaling approach is based on the assumption that it is appropriate to provide equal weighting to each item that comprises a scale. There is also an assumption that, for each item, the interval between response options is equal (for example, the difference in score between “not at all” and “a little” is the same as “a little” and “quite a bit,” at a value of 1 unit). Each raw scale score is converted to a standardized score that ranges from 0 to 100 using a linear transformation, with a higher score reflecting better function on the function scales, higher symptoms on the symptom scales, and better HRQoL (i.e., higher scores simply reflect higher levels of response on that scale). Thus, a decline in score on the symptom scale would reflect an improvement, whereas an increase in score on the function and QoL scale would reflect an improvement. According to the EORTC QLQ-C30 scoring algorithm, if there are missing items for a scale (i.e., the participant did not provide a response), the score for the scale can still be computed if there are responses for at least one-half of the items. In calculating the scale score, the missing items are simply ignored — an approach that assumes that the missing items have values equal to the average of those items for what the respondent completed.
Validity
One cross-sectional study aimed to validate the EORTC QLQ-C30 in a convenience sample of cancer patients in Singapore.53 Most patients had breast and colorectal cancers, but leukemia, lung cancer, lymphoma, germ cell tumour, and other cancers were also reported. Construct validity was assessed by cross-sectional correlational evidence and discriminative evidence. First, convergent validity was assessed using Spearman’s correlations between QLQ-C30 and Short Form-36 (SF-36) scales, hypothesizing moderate to strong correlation (defined as correlation coefficient of 0.35 to 0.5, and > 0.5, respectively) between scales of these 2 instruments measuring similar dimensions of HRQoL. Results showed moderate to strong correlations between QLQ-C30 and SF-36 scales, ranging from 0.35 to 0.67 across the assessed scales. Next, known-groups approach was used to compare 6 QLQ-C30 scale scores between patients reporting mild and severe symptoms, as well as by stage of disease and presence of comorbid conditions. Except for emotional functioning, the remaining 5 scales showed better scores in patients with mild symptoms than those with severe symptoms (P < 0.05 for all other comparisons). Patients in early stages of cancer (or with no comorbid conditions) generally had better QLQ-C30 scores than those in advanced disease stages (or with comorbid conditions); however, none of these differences were statistically significant.
A recent cross-sectional study in Kenya was conducted to evaluate the psychometric properties of the EORTC QLQ-C30, using the English or Kiswahili version in 100 patients with cancer.52 Most patients had breast cancer, followed by prostate, Kaposi sarcoma, lung, and other cancers. Construct validity was assessed by examining the inter-scale correlations among the subscales of EORTC QLQ-C30. The inter-scale correlations were weak to strong with an absolute magnitude ranging from 0.07 to 0.73. Notably, apart from cognitive functioning, emotional functioning, nausea and vomiting, dyspnea, appetite loss, constipation, and diarrhea, the GHS correlated moderately with the remaining subscales (r ≥ 0.30). Cross-cultural validity was evaluated but not reported here.
Reliability
The Singaporean cross-sectional study above also assessed internal consistency reliability by calculating Cronbach alpha for all QLQ-C30 scales.53 Cronbach alpha was ≥ 0.70 for 6 of the 9 assessed QLQ-C30 scales; cognitive functioning, physical functioning, and nausea and vomiting had a Cronbach alpha ranging from 0.19 to 0.68. The Kenyan study described above assessed the internal consistency of each scale of the questionnaire using Cronbach alpha coefficients.52 With the exception of the cognitive function scale, all of the scales had a Cronbach alpha ≥ 0.70.
No studies evaluating the responsiveness of the instrument were found.
Minimum Important Difference
For use in clinical trials, scores on the EORTC QLQ-C30 can be compared between different groups of patients or within a group of patients over time. One study from 1998 conducted in patients with breast cancer and small cell lung cancer estimated a clinically relevant change in score on any scale of the EORTC QLQ-C30 to be 10 points.45 The estimate was based on a study that used an anchor-based approach to estimate the minimum important difference in which patients who reported “a little” change (for better or worse) on the subjective significance questionnaire had corresponding changes on a function or symptom scale of the EORTC QLQ-C30 of approximately 5 to 10 points. Participants who reported a “moderate” change had corresponding changes in the EORTC QLQ-C30 of about 10 to 20 points, and those who reported being “very much” changed had corresponding changes of more than 20 points.
More recently in 2015, a Canadian study estimated the MIDs of EORTC QLQ-C30 scales using data from 193 patients newly diagnosed with breast and colorectal cancers.54 The Supportive Care Needs Survey-Short Form-34 (SCNS-SF34) was used as an anchor; mean changes in EORTC QLQ-C30 scales associated with improvement, worsening, and no-change in supportive care based on the SCNS-SF34 was then calculated. MIDs were assessed for the following scales: physical function, role function, emotional function, global health/QoL (i.e., GHS), pain, and fatigue. For improvement, MIDs associated with a statistically significant improvement in supportive care needs ranged from 10 to 32 points. For worsening, MIDs associated with a statistically significant worsening of supportive care needs ranged from 9 to 21 points. The range for unchanged supportive care needs was from 1-point worsening to 16-point improvement in EORTC QLQ-C30 score. Based on this, the authors suggested a 10-point change in EORTC QLQ-C30 score represented changes in supportive care needs, and therefore, should be considered for clinical use.
In 2014, another Canadian study estimated the MID for EORTC QLQ-C30 in 369 patients with advanced cancer who completed the questionnaire at baseline and 1 month after radiation.55 The most common cancer type was breast cancer, followed by lung, prostate, gastrointestinal, renal cell, and other cancers. MID was estimated using both anchor- and distribution-based methods for improvement and deterioration. Two anchors of overall health and overall QoL were used, both taken directly from the EORTC QLQ-C30 (questions 29 and 30) where patients rated their overall health and QoL themselves. Improvement and deterioration were categorized as an increase or decrease by 2 units to account for the natural fluctuation of patient scoring. With these 2 anchors, the estimated MIDs across all EORTC QLQ-C30 scales ranged from 9.1 units to 23.5 units for improvement, and from 7.2 units to 13.5 units for deterioration. Distribution-based estimates were closest to 0.5 SD.
EQ-5D-3L
The European Quality of Life Scale (EQ-5D) is a generic HRQoL instrument that may be applied to a wide range of health conditions and treatments.56,57 The first of 2 parts of the EQ-5D is a descriptive system that classifies respondents (aged ≥12 years) based on the following 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ-5D-3L has 3 possible levels (1, 2, or 3) for each domain representing ‘no problems,’ ‘some problems,’ and ‘extreme problems,’ respectively. Respondents are asked to choose the level that reflects their health state for each of the 5 dimensions, corresponding with 243 different health states. A scoring function can be used to assign a value (EQ-5D-3L index score) to self-reported health states from a set of population-based preference weights.56,57 The second part is a 20 cm visual analogue scale (EQ-VAS) that has end points labelled 0 and 100, with respective anchors of ‘worst imaginable health state’ and ‘best imaginable health state.’ Respondents are asked to rate their health by drawing a line from an anchor box to the point on the EQ-VAS which best represents their health on that day. Hence, the EQ-5D produces 3 types of data for each respondent:
- A profile indicating the extent of problems on each of the 5 dimensions represented by a 5-digit descriptor, such as 11121, 33211, and so forth,
- A population preference-weighted health index score based on the descriptive system,
- A self-reported assessment of health status based on the EQ-VAS.
The EQ-5D index score is generated by applying a multi-attribute utility function to the descriptive system. Different utility functions are available that reflect the preferences of specific populations (e.g., US or UK). The lowest possible overall score for the 3L version (corresponding to severe problems on all 5 attributes) varies depending on the utility function that is applied to the descriptive system (e.g., −0.59 for the UK algorithm and −0.109 for the US algorithm). Scores less than 0 represent health states that are valued by society as being worse than dead, while scores of 0 and 1.00 are assigned to the health states ‘dead’ and ‘perfect health,’ respectively. Reported MIDs for the 3L version of the scale have ranged from 0.033 to 0.074.46
Teckle et al. conducted a study of patients (n=184) who had either breast (36%), colorectal (31%), or lung (33%) cancer at the Vancouver Cancer Clinic to investigate if disease severity could be distinguished by cancer-specific and generic preference-based instruments.58 Internal consistency was calculated using Cronbach alpha and all 5 functioning scales along with GHS showed acceptable consistency (α > 0.7) with values ranging from 0.77 to 0.82. Validity was assessed using Pearson’s correlation coefficient (r) where r between 0 and 0.3 demonstrated weak correlation, between 0.3 and 0.49 was moderate, and greater than 0.5 was considered strong. Teckle et al. found the following, between the EORTC QLQ-C30, and EQ-5D, r = 0.43; comparing the EORTC QLQ-C30 and EQ-VAS, r = 0.73; and between EQ-5D and EQ-VAS, r = 0.43. External validity was estimated between cancer severity (self-reported health status, Eastern Cooperative Oncology Group-Performance Status [ECOG-PS], and cancer stage). An effect size (ES) between 0.2 and 0.5 was considered small, between 0.5 and 0.8 was medium, and greater than 0.8 was large. The EQ-5D was able to discriminate populations based on self-reported health status (excellent/good versus fair/very poor; ES = 0.90), and somewhat based on ECOG-PS (0 versus 1 to 3; ES = 0.31), but not for stage of cancer (stages 1 and 2 versus stages 3 and 4; ES = 0.06). The EORTC QLQ-C30 performed better in all 3 areas: self-reported health status (ES = 1.39), ECOG-PS (ES = 0.65), and stage of cancer (ES = 0.49). It is worth noting that the EQ-5D was based on a non-Canadian population and the comparison with EORTC QLQ-C30 was based solely on the 2 questions asking about overall health and HRQoL rather than the questionnaire as a whole. This study was a mixed population of 3 types of cancer and the results may not exactly reflect what would be observed in patients with relapsed or refractory cHL. Furthermore, there was no information on what type of treatment the patients were receiving when completing the questionnaires.
Pickard et al. conducted a retrospective analysis of 534 patients with 11 types of cancer (including colon/rectal cancer) to estimate the MID using distribution-based (SEM, 1/2 SD, and 1/3 SD) and anchor-based (ECOG) methods.47 After stratifying by ECOG status, the mean weighted index score MID for all cancer patients was estimated to be between 0.07 and 0.11 for UK-index scores and between 0.05 and 0.08 for US-index scores. The VAS MID was estimated to range from 6 to 11 points for all patients. No MID information was identified in patients with relapsed or refractory classical Hodgkin’s Lymphoma.
Appendix 4. PFS Survival Curves for KEYNOTE-051 and KEYNOTE-087 and OS Survival Curves for KEYNOTE-087
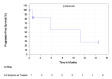
Figure 5
Kaplan-Meier Estimates of PFS in KEYNOTE-051.
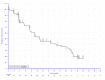
Figure 6
Kaplan-Meier Estimates of PFS in KEYNOTE-087, Cohort 1.
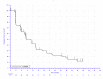
Figure 7
Kaplan-Meier Estimates of PFS in KEYNOTE-087, Cohort 2.
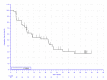
Figure 8
Kaplan-Meier Estimates of PFS in KEYNOTE-087, Cohort 3.
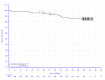
Figure 9
Kaplan-Meier Estimates of Overall Survival in KEYNOTE-087, Cohort 1.
Figure 10
Kaplan-Meier Estimates of Overall Survival in KEYNOTE-087, Cohort 2.
Figure 11
Kaplan-Meier Estimates of Overall Survival in KEYNOTE-087, Cohort 3.
References
- 1.
- Canadian Cancer Society. Hodgkin lymphoma statistics. 2021; https://www
.cancer.ca /en/cancer-information /cancer-type/hodgkin-lymphoma /statistics/?region=on. Accessed 2021 May 17. - 2.
- Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence patterns and outcomes for Hodgkin lymphoma patients in the United States. Adv Hematol. 2011;2011:725219. [PMC free article: PMC3010617] [PubMed: 21197477]
- 3.
- Piris MA, Medeiros LJ, Chang K-C. Hodgkin lymphoma: a review of pathological features and recent advances in pathogenesis. Pathology. 2020;52(1):154-165. [PubMed: 31699300]
- 4.
- Hasenclever D, Diehl V, Armitage JO, et al. A prognostic score for advanced Hodgkin's disease. N Engl J Med. 1998;339(21):1506-1514. [PubMed: 9819449]
- 5.
- Straus DJ, Jung S-H, Pitcher B, et al. CALGB 50604: risk-adapted treatment of nonbulky early-stage Hodgkin lymphoma based on interim PET. Blood. 2018;132(10):1013-1021. [PMC free article: PMC6128083] [PubMed: 30049811]
- 6.
- Sasse S, Bröckelmann PJ, Goergen H, et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: updated analyses of the German Hodgkin study group HD7, HD8, HD10, and HD11 trials. J Clin Oncol. 2017;35(18):1999-2007. [PubMed: 28418763]
- 7.
- Skoetz N, Will A, Monsef I, Brillant C, Engert A, von Tresckow B. Comparison of first‐line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2017(5). [PMC free article: PMC6481581] [PubMed: 28541603]
- 8.
- Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419-2429. [PMC free article: PMC4961236] [PubMed: 27332902]
- 9.
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598-1607. [PubMed: 25901426]
- 10.
- Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin's lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. The Lancet. 2017;390(10114):2790-2802. [PubMed: 29061295]
- 11.
- Mauz-Körholz C, Metzger ML, Kelly KM, et al. Pediatric Hodgkin lymphoma. J Clin Oncol. 2015;33(27):2975-2985. [PubMed: 26304892]
- 12.
- Castellino SM, LeBlanc ML, Herrera AF, et al. An intergroup collaboration for advanced stage classical Hodgkin lymphoma (cHL) in adolescents and young adults (AYA): SWOG S1826. J Clin Oncol. 2020;38(15_suppl):TPS8067-TPS8067.
- 13.
- Provencio M, Millán I, España P, et al. Analysis of competing risks of causes of death and their variation over different time periods in Hodgkin's disease. Clin Cancer Res. 2008;14(16):5300-5305. [PubMed: 18698050]
- 14.
- Broccoli A, Zinzani PL. The role of transplantation in Hodgkin lymphoma. Br J Haematol. 2019;184(1):93-104. [PubMed: 30407612]
- 15.
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. The Lancet. 2002;359(9323):2065-2071. [PubMed: 12086759]
- 16.
- Linch DC, Goldstone AH, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. The Lancet. 1993;341(8852):1051-1054. [PubMed: 8096958]
- 17.
- Fermé C, Mounier N, Diviné M, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Groupe d’Études des Lymphomes de l’Adulte H89 trial. J Clin Oncol. 2002;20(2):467-475. [PubMed: 11786576]
- 18.
- Stiff PJ, Unger JM, Forman SJ, et al. The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory hodgkin disease: A southwest oncology group phase II trial. Biol Blood Marrow Transplant. 2003;9(8):529-539. [PubMed: 12931122]
- 19.
- Tarella C, Cuttica A, Vitolo U, et al. High-dose sequential chemotherapy and peripheral blood progenitor cell autografting in patients with refractory and/or recurrent Hodgkin lymphoma. Cancer. 2003;97(11):2748-2759. [PubMed: 12767087]
- 20.
- Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin's disease. Blood. 1997;89(3):814-822. [PubMed: 9028312]
- 21.
- Lavoie JC, Connors JM, Phillips GL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106(4):1473-1478. [PubMed: 15870180]
- 22.
- Longo DL, Duffey PL, Young RC, et al. Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin's disease after combination chemotherapy: the low probability for cure. J Clin Oncol. 1992;10(2):210-218. [PubMed: 1732422]
- 23.
- Mead G, Harker, G., Kushlan P and Rosenberg SA. Single agent palliative chemotherapy for end-stage hodgkin's disease. Cancer. 1982;50:829-835. [PubMed: 6178495]
- 24.
- Proctor SJ, Lennard A.L., Jackson G.H, McKay P, Culligan D and Lucraft H.H. The role of an all-oral chemotherapy containing lomustine (CCNU) in advanced, progressive Hodgkin lymphoma: A patient-friendly palliative option which can result in long-term disease control. Ann Oncol. 2010;21(2):426-428. [PubMed: 19901016]
- 25.
- Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30(7):631-637. [PubMed: 22781692]
- 26.
- Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183-2189. [PMC free article: PMC3646316] [PubMed: 22454421]
- 27.
- Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562-1566. [PMC free article: PMC5034737] [PubMed: 27432875]
- 28.
- Bröckelmann PJ, Zagadailov EA, Corman SL, et al. Brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma who are Ineligible for autologous stem cell transplant: A Germany and United Kingdom retrospective study. Eur J Haematol. 2017;99(6):553-558. [PubMed: 28949403]
- 29.
- Opdivo (nivolumab for injection): intravenous infusion, 10 mg nivolumab /mL 40 mg and 100 mg single-use vials [product monograph]. Montreal (QC): Bristol-Myers Squibb Canada Co.; 2021 May 21:1-2.
- 30.
- Timmerman JM, Engert A, Younes A, et al. Checkmate 205 update with minimum 12-month follow up: a phase 2 study of nivolumab in patients with relapsed/refractory classical Hodgkin lymphoma. Blood. 2016;128(22):1110-1110.
- 31.
- Ansell S, Gutierrez ME, Shipp MA, et al. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039). Blood. 2016;128(22):183-183.
- 32.
- Clinical study report: P204V02MK3475. A phase I/II study of pembrolizumab (MK-3475) in children with advanced melanoma or a PD-L1 positive advanced, relapsed or refractory solid tumor or lymphoma (KEYNOTE-051). [internal sponsor's report]. Kirkland (QC): Merck Canada Inc.; 2020 Apr 24.
- 33.
- Clinical study report: P087V02MK3475. A phase II clinical trial of MK-3475 (pembrolizumab) in subjects with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL). [internal sponsor's report]. Kenilworth (NJ): Merck Sharp & Dohme Corp.; 2020 Apr 9.
- 34.
- Clinical study report: P204V01MK3475. A phase III, randomized, open-label, clinical trial to compare pembrolizumab with brentuximab vedotin in subjects with relapsed or refractory classical Hodgkin lymphoma. [internal sponsor's report]. Kirkland (QC): Merck Canada Inc.; 2020 Apr 10.
- 35.
- CADTH pCODR Expert Review Committee (pERC) final recommendation: brentuximab vedotin (Adcetris). Ottawa (ON): CADTH; 2019 Mar 7: https://cadth
.ca/sites /default/files/pcodr /Reviews2019/10145BrentuximabHL _FnRec_approvedbyChair _Post_07Mar2019_final.pdf. Accessed 2021 May 17. - 36.
- Brockelmann PJ, Engert A. Checkpoint inhibition in Hodgkin lymphoma - a review. Oncol Res Treat. 2017;40(11):654-660. [PubMed: 29065424]
- 37.
- Keytruda (pembrolizumab): powder for solution for infusion 50 mg solution for infusion 100 mg/4 mL vial antineoplastic agent, monoclonal antibody [product monograph]. Kirkland (QC): Merck Canada Inc.; 2021 Feb 5.
- 38.
- Adcetris (brentuximab vedotin for injection): lyophilized powder for reconstitution with 10.5 mL of sterile Water for Injection, USP 50 mg; Antineoplastic agent [product monograph]. Bothell (WA): Seattle Genetics, Inc.; 2013 Feb 1.
- 39.
- Fedorova LV, Lepik KV, Mikhailova NB, et al. Nivolumab discontinuation and retreatment in patients with relapsed or refractory Hodgkin lymphoma. Ann Hematol. 2021;100(3):691-698. [PubMed: 33528609]
- 40.
- Shah GL, Moskowitz CH. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood. 2018;131(15):1689-1697. [PMC free article: PMC5897866] [PubMed: 29500170]
- 41.
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. [PubMed: 27005575]
- 42.
- Grey matters: a practical tool for searching health-related grey literature. Ottawa (ON): CADTH; 2019: https://www
.cadth.ca/grey-matters. Accessed 2021 Feb 3. - 43.
- Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144-1153. [PMC free article: PMC6776792] [PubMed: 31409671]
- 44.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [PubMed: 17242396]
- 45.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139-144. [PubMed: 9440735]
- 46.
- Sinnott PL, Joyce VR, Barnett PG. Guidebook: preference measurement in economic analysis. Menlo Park (CA): Health Economics Research Center; 2007: https://www
.herc.research .va.gov/files/BOOK_419.pdf. Accessed 2021 May 17. - 47.
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. [PMC free article: PMC2248572] [PubMed: 18154669]
- 48.
- Giesinger JM, Kieffer JM, Fayers PM, et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79-88. [PubMed: 26327487]
- 49.
- EORTC Quality of life: FAQs. Brussels (Belgium): EORTC Data Center: https://qol
.eortc.org/faq/. Accessed 2021 Mar 8. - 50.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. [PubMed: 8433390]
- 51.
- EORTC QLC-C30 scoring manual. Brussels (Belgium): EORTC Data Center; 2001: https://www
.eortc.org /app/uploads/sites/2/2018/02/SCmanual .pdf. Accessed 2021 Mar 8. - 52.
- Davda J, Kibet H, Achieng E, Atundo L, Komen T. Assessing the acceptability, reliability, and validity of the EORTC Quality of Life Questionnaire (QLQ-C30) in Kenyan cancer patients: a cross-sectional study. Journal of Patient reported Outcomes. 2021;5(1):4. [PMC free article: PMC7790948] [PubMed: 33415528]
- 53.
- Luo N, Fones CS, Lim SE, Xie F, Thumboo J, Li SC. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-c30): validation of English version in Singapore. Qual Life Res. 2005;14(4):1181-1186. [PubMed: 16041912]
- 54.
- Snyder CF, Blackford AL, Sussman J, et al. Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients' supportive care needs. Qual Life Res. 2015;24(5):1207-1216. [PMC free article: PMC4405431] [PubMed: 25398495]
- 55.
- Bedard G, Zeng L, Zhang L, et al. Minimal important differences in the EORTC QLQ-C30 in patients with advanced cancer. Asia Pac J Clin Oncol. 2014;10(2):109-117. [PubMed: 23551530]
- 56.
- Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53-72. [PubMed: 10158943]
- 57.
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. [PubMed: 10109801]
- 58.
- Teckle P, Peacock S, McTaggart-Cowan H, et al. The ability of cancer-specific and generic preference-based instruments to discriminate across clinical and self-reported measures of cancer severities. Health Qual Life Outcomes. 2011;9:106. [PMC free article: PMC3236471] [PubMed: 22123196]
- Executive Summary
- Introduction
- Stakeholder Perspectives
- Clinical Evidence
- Discussion
- Conclusions
- Abbreviations
- Clinical Literature Search Strategy
- Subsequent Anticancer Medication Utilization in KEYNOTE-204
- Description and Appraisal of Outcome Measures
- PFS Survival Curves for KEYNOTE-051 and KEYNOTE-087 and OS Survival Curves for KEYNOTE-087
- References
- Clinical Review - Pembrolizumab (Keytruda)Clinical Review - Pembrolizumab (Keytruda)
- CDEC Final Recommendation - Ingenol Mebutate (Picato)CDEC Final Recommendation - Ingenol Mebutate (Picato)
- EXECUTIVE SUMMARY - Edoxaban (Lixiana)EXECUTIVE SUMMARY - Edoxaban (Lixiana)
- DETAILED OUTCOME DATA - Edoxaban (Lixiana)DETAILED OUTCOME DATA - Edoxaban (Lixiana)
- OBJECTIVES AND METHODS - Omalizumab (Xolair)OBJECTIVES AND METHODS - Omalizumab (Xolair)
Your browsing activity is empty.
Activity recording is turned off.
See more...