Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Pembrolizumab (Keytruda): CADTH Reimbursement Review: Therapeutic area: Classical Hodgkin lymphoma [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021 Dec.

Pembrolizumab (Keytruda): CADTH Reimbursement Review: Therapeutic area: Classical Hodgkin lymphoma [Internet].
Show detailsExecutive Summary
The executive summary comprises 2 tables (Table 1 and Table 2) and a conclusion.
Table 1
Submitted for Review.
Table 2
Summary of Economic Evaluation.
Conclusions
Evidence suggests pembrolizumab provides statistically and clinically significant improvements in progression-free survival (PFS) |||||||||||||||||||||||||||||||||||| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||. Across all studies, brentuximab vedotin (BV) patients were generally less likely to experience adverse events but more likely to discontinue therapy due to an adverse event. No comparators beyond BV were evaluated. Therefore, the comparative evidence of pembrolizumab to chemotherapy in an autologous stem cell transplant (ASCT)-ineligible subpopulation is unknown, due to a lack of direct or indirect evidence for PFS and overall survival (OS).
CADTH addressed several key limitations of the sponsor’s model by selecting appropriate comparators for the Canadian context; using PFS data that censored upon stem cell transplant (SCT) events; considering a treatment effect waning; changing treatment-specific utilities to disease-specific; and incorporating drug wastage. According to CADTH’s base case for ASCT-eligible adult patients, pembrolizumab is associated with an incremental cost-effectiveness ratio (ICER) of $733,624 per quality-adjusted life-year (QALY) gained when compared with BV, and in an ASCT-eligible pediatric population pembrolizumab is dominant (i.e., cost saving and associated with more QALYs). In adults, a price reduction of approximately 13% is required for pembrolizumab to be cost-effective at a willingness-to-pay (WTP) threshold of $50,000 per QALY. This result is contingent on pembrolizumab generating approximately $43,000 of cost savings due to patients who receive pembrolizumab first line not receiving immunotherapies such as nivolumab and pembrolizumab second line. There is a significant amount of outstanding uncertainty regarding the degree of these cost savings. A scenario analysis that altered the subsequent therapy distribution based on data from the KEYNOTE-204 trial found the ICER in adults increased to $2,071,825 per QALY. At this level, a 29% price reduction is needed to achieve a $50,000 per QALY threshold.
The conclusions of CADTH’s analysis are specific to an ASCT-eligible population. CADTH was unable to assess the cost-effectiveness of pembrolizumab in an ASCT-ineligible population due to an absence of comparative evidence between pembrolizumab and chemotherapy. In Canada, individuals who are ineligible for ASCT rarely receive BV; therefore, chemotherapy is the most relevant comparator. Drug costs for pembrolizumab are substantially higher relative to chemotherapy, as such information on this comparison is needed to assess the value of pembrolizumab in this subgroup. Finally, results for the pediatric population are entirely extrapolated from the adult data, so any uncertainty with that extrapolation will impact the robustness of the pediatric results.
Stakeholder Input Relevant to the Economic Review
This section is a summary of the feedback received from the patient groups, registered clinicians, and drug plans that participated in the CADTH review process.
One patient group, Lymphoma Canada, conducted 2 online surveys of patients with Hodgkin lymphoma. The first was conducted in 2017 from June 5 to 30, 2017, and 91 participants responded, while the second collected responses from November 6, 2020, to January 13, 2021, and 37 responded. Of the participants who provided demographic information, 55% resided in Canada. Of the 9 participants with pembrolizumab experience, 7 resided in Canada. All 9 participants had at least 2 prior lines of conventional chemotherapy before initiating pembrolizumab. Those previous chemotherapy treatments included 8 reports of ABVD (Adriamycin-bleomycin-vinblastine-dacarbazine), 6 reports of GDP (gemcitabine-dexamethasone-cisplatin), 2 reports of COPP (cyclophosphamide-Oncovin-procarbazine-prednisone), and 1 report for either DHAP (dexamethasone-cytarabine-cisplatin), bendamustine, or Revlimid. The reason for starting pembrolizumab included no other treatment options available (n = 2), progression after ASCT and a desire not to risk toxicity of an allogeneic SCT (n = 4), hoping for remission to proceed to allogeneic transplant (n = 1), and lack of response to 3 previous chemotherapy lines and no desire to undergo ASCT (n = 2). Eight of the pembrolizumab patients tolerated the therapy well, while the remaining patient stopped treatment due to toxicity and side effects (i.e., neuropathy and inflammatory arthritis).
Clinician input was received from Ontario Health (Cancer Care Ontario) Hematology Disease Site Drug Advisory Committee, Lymphoma Canada Scientific Advisory Board, and the Pediatric Oncology Group of Ontario. For adult patients, the current pathway of care was divided into whether patients were eligible or ineligible for ASCT. In those eligible and who failed ASCT, BV is the standard of care, while in those not eligible there is no clear gold standard, and a variety of approaches can be used including combination chemotherapy, radiation, clinical trials, or occasionally novel agents such as BV or anti-PD1 antibodies (pembrolizumab or nivolumab). The introduction of pembrolizumab would replace BV as monotherapy in those who failed ASCT, and in those ineligible for ASCT it provides a therapy to patients with limited treatment options. For pediatric patients, the vast majority are eligible for ASCT. In pediatric patients who fail ASCT, pembrolizumab is expected to serve as an additional line of therapy with the aim to provide a curative therapeutic option.
Feedback from drug plans indicated that, in the subpopulation of adult patients with classic Hodgkin lymphoma (cHL) who are ASCT ineligible, the comparator is palliative care and not BV (due to its limited funding). In the pediatric population, they noted that there does not appear to be a standard of care, but most patients were ASCT eligible. The plans also noted drug wastage could be minimized by vial sharing, but it likely is not feasible in small outpatient cancer centres.
The following stakeholder input was addressed in the sponsor’s models:
- a comparison of pembrolizumab to BV in the ASCT-eligible population.
CADTH was able to address the following concerns raised from the stakeholder input:
- drug wastage was incorporated into the CADTH base case
- BV was removed as the comparator to pembrolizumab in the subpopulation who were ASCT ineligible, and chemotherapy was determined to be the relevant comparator.
CADTH was unable to address the following concerns raised from stakeholder input:
- a comparison of pembrolizumab to standard care rather than BV in the ASCT-ineligible population.
Economic Review
The current review is for pembrolizumab (Keytruda) for adult and pediatric patients with refractory or relapsed cHL who have failed ASCT or who are not candidates for multi-agent salvage chemotherapy and ASCT.
Economic Evaluation
Summary of Sponsor’s Economic Evaluation
Overview
The sponsor submitted a cost-utility analysis assessing pembrolizumab compared to BV in 2 distinct populations of adults or pediatric patients, with refractory or relapsed cHL who have failed ASCT or who are not candidates for multi-agent salvage chemotherapy and ASCT.1 The modelled population aligned with the Health Canada indication and the sponsor’s reimbursement request.
Pembrolizumab is a monotherapy available as a solution (100 mg/4 mL [25 mg/mL]) for IV infusion. The recommended dose of pembrolizumab in adult patients is 200 mg and 2 mg/kg (up to a maximum of 200 mg) in pediatric patients. Pembrolizumab is administered as an IV infusion for 30 minutes every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.2 The cost for pembrolizumab is $4,400 per 100 mg vial, equating to a cost per 21-day cycle of $8,800.00 in adults and $5,148.00 in a pediatric population (assuming no wastage). Pembrolizumab was compared to BV, which has a cost per 21-day cycle of $13,320.64 in adults and $10,193.04 in a pediatric population.
The clinical outcomes were QALYs and life-years, which were modelled over a 35-year time horizon. The base-case analysis was conducted from the Canadian publicly funded health care payer perspective, and a 1.5% discount rate was applied to both costs and outcomes.
Model Structure
The sponsor submitted a 3-state partitioned survival model with 3 mutually exclusive states consisting of “progression free,” “progressed disease,” and “death.” Membership of each state at any point in time is based on direct modelling of OS and PFS curves, which the sponsor extrapolated over the time horizon of the analysis. A piecewise approach was used to extrapolate Kaplan-Meier data, which consists of using Kaplan-Meier data until a set cut-off point (i.e., 52 weeks for PFS and time on treatment, and 135 weeks for OS), then fitting a parametric curves to the remaining data to extrapolate to the time horizon. During the model, the patient may discontinue treatment, at which point the cost of treatment is no longer incurred.
The cycle length is 1 week, with no correction made for events occurring within a cycle (i.e., half-cycle correction). The sponsor provided a graphical representation of the model which has been reproduced in Appendix 3, Figure 1.
Model Inputs
For the adult population, baseline characteristics of the target populations aligned with the KEYNOTE-204 trial, while the KEYNOTE-051 trial was used for the pediatric population.3,4 The mean ages for the adult and pediatric populations were 41.35 years and 14.90 years, respectively. Acquisition costs for weight-dependent or surface area–dependent therapies were calculated using the mean weight and body surface area of both the adult (weight: 76.45 kg; total body surface area: 1.90 m2) and pediatric populations (weight: 58.5 kg; total body surface area: 1.60 m2).
The KEYNOTE-204 trial was the primary source of efficacy data for the model.3 Treatment efficacy was modelled in terms of delaying time to progression and extending OS. PFS for both the adult and pediatric population were extrapolated from the KEYNOTE-204 trial data for both pembrolizumab and BV. The sponsor indicated PFS data from KEYNOTE-051 was not used for the pediatric population due to its small sample size of 7 patients with relapsed or refractory Hodgkin lymphoma.4 The other treatment efficacy outcome, OS, was not pulled from either of the previously mentioned KEYNOTE studies due to a lack of OS end point data. Therefore, it was derived from a phase II trial of BV in patients with relapsed or refractory Hodgkin lymphoma after failed ASCT.5 By using this data, the sponsor assumed no difference in OS between BV and pembrolizumab, and that the OS of those who failed ASCT was equivalent to the population ineligible for multi-agent salvage chemotherapy and ASCT. In addition to PFS and OS, time on treatment was extrapolated from the KEYNOTE-204 and KEYNOTE-051 trials for the adult and pediatric populations, respectively.3,4
For each treatment, Kaplan-Meier data were used until a user defined cut-off point (52 weeks for PFS and time on treatment and 135 weeks for OS), after which parametric survival curves were fitted to and extrapolated up to the time horizon. Seven different parametric approaches were considered, reflecting exponential, Weibull, log-normal, log-logistic, Gompertz, gamma, and generalized gamma distributions. The sponsor determined the “best-fitting” parametric distributions using statistical tests based on the Akaike Information Criterion and the Bayesian information criterion, combined with visual inspection. The sponsor also reported that clinical appropriateness was considered in selecting final distribution functions for the model. Based on these criteria, the sponsor adopted log-normal extrapolations for PFS, time on treatment, and OS in the adult and pediatric populations. The model allowed for consideration of “treatment effect waning,” but this was not considered by the sponsor in their base-case analysis.
In the sponsor’s base case, chemotherapy was not included as a comparator. The sponsor stated there was a lack of evidence to complete an analysis against pembrolizumab (in both the population who failed ASCT and who were ineligible for multi-agent salvage chemotherapy and ASCT), due to either insufficient outcome reporting, unrepresentative target population, or insufficient sample size. However, chemotherapy was included in a scenario analysis. In that scenario, parameters for time on treatment and PFS were assumed equal to BV, and the sponsor assumed a median OS of 36 months and extrapolated an OS curve using an exponential equation.
The dosing used in the model is consistent with that described in the Overview, where the recommended dose of pembrolizumab in adult patients is 200 mg and 2 mg/kg (up to a maximum of 200 mg) in pediatric patients. The reference case analysis assumed no drug wastage in the adult and pediatric populations using single-use vials of pembrolizumab.
Utility values were modelled based on EuroQol 5-Dimensions 3-Levels questionnaire data collected in the KEYNOTE-204 trial, mapped to the US tariff.3 Mean utility values for pembrolizumab and BV are 0.883 and 0.822 for “progression free,” and 0.861 and 0.766 for “progressed disease,” respectively. In scenario analyses, a mean disutility decrement of 0.075 is applied for grade 3 to 5 treatment-related adverse events identified in the KEYNOTE-204 trial.
The economic model included costs for drug acquisition, drug administration, subsequent treatment lines, SCTs, disease management by health state, terminal care (i.e., last 3 months before death), and adverse events. Drug acquisition costs were pulled from previous CADTH pan-Canadian Oncology Drug Review (pCODR) reports,6,7 while costs for SCTs were sourced from a cost-effectiveness analysis on chronic myeloid leukemia and data from the Canadian Institute for Health Information.8 Disease management costs were sourced from an economic evaluation on BV,9 and adverse events were derived from either the Canadian Institute for Health Information Patient Cost Estimator, Ontario Case Costing Initiative, or sponsor assumptions.
Summary of Sponsor’s Economic Evaluation Results
The results of the sponsor’s base case are presented below. The base case used a probabilistic analysis with 5,000 iterations, and scenario analyses were conducted in the adult and pediatric populations which disaggregated results into those eligible and ineligible for ASCT. Additional details pertaining to the sponsor’s submission are available in Appendix 3.
Base-Case Results
In the sponsor’s base-case analysis for the adult population, pembrolizumab was associated with an expected cost savings of $24,230 and a gain of 0.8408 QALYs over a 35-year time horizon (Table 3). As treatment with BV was more costly and produced fewer QALYs, the ICER of pembrolizumab compared to BV indicated pembrolizumab was dominant (less costly and more effective) in the target population (adult patients with refractory or relapsed cHL who have failed ASCT or who are not candidates for multi-agent salvage chemotherapy and ASCT). The sponsor reported that at a WTP threshold of $50,000 per QALY, the probability of pembrolizumab being cost-effective was 67% (note: errors were found in the cost-effectiveness acceptability curve resulting in the curve not being updated for pairwise comparisons; therefore, the sponsor-reported probabilities are uncertain).
Most of the total QALYs for each strategy were generated after the trial period (80%), with the remaining QALYs generated within the trial period (20%). Key savings in the total average costs for pembrolizumab included progressed disease costs (–$58,289) and subsequent treatment costs (–$47,243); while higher costs were primarily found for progression-free costs ($28,254) and acquisition costs ($52,976).
Table 3
Summary of the Sponsor’s Economic Evaluation Results (Adult Population).
In the sponsor’s base-case analysis for the pediatric population, pembrolizumab was associated with an expected cost savings of $47,937 and a gain of 0.8437 QALYs over a 35-year time horizon (Table 4). Similar to the results in the adult population, pembrolizumab was dominant over BV. At a WTP threshold of $50,000 per QALY, the probability of pembrolizumab being cost-effective was 67%.
Table 4
Summary of the Sponsor’s Economic Evaluation Results (Pediatric Population).
Sensitivity and Scenario Analysis Results
The sponsor conducted a number of sensitivity and scenario analyses. Key scenario analyses included modelling specific adult and pediatric subpopulations that were ASCT eligible and ASCT ineligible. Furthermore, in the scenario analysis for the ASCT-ineligible subpopulations, both BV and chemotherapy were chosen as pairwise comparators to pembrolizumab. Several of these scenario analyses did not result in pembrolizumab being dominant. In both the adult and pediatric population for ASCT-ineligible subpopulations, the ICER for pembrolizumab versus chemotherapy was $57,508 per QALY gained and $53,014 per QALY gained, respectively, while the ICER for an adult subpopulation who were ASCT eligible was $19,951 per QALY gained.
CADTH Appraisal of the Sponsor’s Economic Evaluation
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the economic analysis.
- In the Canadian setting, BV is not a relevant comparator for those ASCT ineligible: The sponsor’s base case evaluated pembrolizumab against BV in a mixed population consisting of individuals who were either ASCT eligible or ineligible. Across Canada, BV has limited utilization in an ASCT-ineligible subpopulation, as recommended by the CADTH pan-Canadian Oncology Drug Review which “did not recommend funding BV in patients with Hodgkin lymphoma who are not candidates for ASCT and who have relapsed disease following at least 2 prior multi-agent chemotherapies.”10
- Given that BV is not routinely funded for ASCT-ineligible patients, BV alone cannot be used to inform the cost-effectiveness of pembrolizumab in ASCT-ineligible patients. The conclusions CADTH draws from the cost-effectiveness of pembrolizumab versus BV relate solely to an ASCT-eligible population.
- In the ASCT-ineligible population, there is insufficient data to model chemotherapy as a comparator: As stated in the sponsor’s pharmacoeconomic evaluation, chemotherapy was not considered in their base case “due to a lack of evidence to complete an analysis against pembrolizumab.”1 Following a systematic literature review by the sponsor, no study including chemotherapy was deemed suitable for comparison in the ASCT-ineligible population “due to either insufficient outcome reporting, a population unrepresentative of the target population, insufficient sample size, or a combination of the 3, all of which could lead to important potential bias.”1 The sponsor provided an analysis that predicts OS and PFS for chemotherapy, but this is built entirely from assumptions. The main challenge is predicting the OS and PFS differences in a group receiving chemotherapy versus pembrolizumab, as even the OS and PFS from the trial is in a population that combines both ASCT-eligible and ineligible patients. Even if one could model the OS and PFS of chemotherapy, the pooled trial data for pembrolizumab is in a different patient population so uncertainty remains as to how health outcomes differ for pembrolizumab versus chemotherapy.
- Given chemotherapy is the most relevant comparator in the ASCT-ineligible population, CADTH was unable to conduct a base case for ASCT-ineligible adult and pediatric patients, and as such, the cost-effectiveness is unknown.
- Treatment-specific utility values are overestimated: The sponsor used treatment-specific utility values from the KEYNOTE-204 trial for the progression-free and progressed disease health states. These utility values have large incremental differences between pembrolizumab and BV, despite both treatments having similar adverse event profiles and routes of administration. This similar adverse event profile is outlined in the clinical evidence review where it found the proportion of patients experiencing at least 1 serious adverse event was similar among KEYNOTE trials, but highest among the pembrolizumab arm of the KEYNOTE-204 trial (29.7%). The use of treatment-specific utility values was also deemed inappropriate as the utility benefit for pembrolizumab during the trial was applied across the 35-year time horizon, thus assuming that even post-treatment discontinuation, pembrolizumab provides an indefinite utility benefit. The ability to measure utility difference within the trial period was also limited by poor compliance to EuroQol 5-Dimensions 3-Levels questionnaires. By week 24, pembrolizumab had 82.2% compliance and BV had 56.7% compliance, which dropped significantly by week 48 to 58.2% and 23.3% compliance, respectively. Finally, treatment-specific utilities are discouraged in the current CADTH economic guidelines, where it states health preferences (i.e., utilities) should reflect the health state in the model, not the treatment-specific state.11
- In the CADTH reanalysis, equal disease-specific utility values were assigned for each treatment, in the progression-free and progressed disease health states. This results in incremental QALY gain between treatments being attributable to time in the progression-free state, relative to time in the progressed disease state.
- Treatment effect waning not applied: The sponsor’s base case assumed the observed PFS benefits for pembrolizumab in the trial continued indefinitely past the trial duration. Although the sponsor submitted a revised economic model that allowed for the possibility of PFS treatment effect waning, the sponsor did not apply this in the submitted base-case analyses. CADTH’s clinical experts recommended treatment waning be implemented, with the expectation that PFS between treatments could be equal after 10 years.
- The sponsor’s provided analysis to implement treatment waning was unstable and resulted in some implausible extrapolations as shown in Appendix 3 (Figure 3). CADTH therefore opted to choose a parametric fit for the PFS curve for pembrolizumab so that PFS curves were equal at approximately 10 years.
- The inclusion of SCT events inflates PFS benefits: The sponsor used data which did not censor individuals who received SCT after primary treatment initiation, thus capturing the benefit of SCT on PFS. This approach was deemed inappropriate given insignificant but differential SCT rates between treatments, and the fact its inclusion artificially inflates PFS extrapolation across the model time horizon. Furthermore, the model includes SCT costs when SCT events are not censored in PFS, but the costs used are specific to an intention-to-treat population that is given either an autologous or allogeneic SCT. As only ASCT-eligible patients (who just failed treatment with ASCT) are analyzed in the CADTH base case, these costs do not reflect the target subpopulation. If differential rates of SCT are predicted, then a partition survival model would not be appropriate, as then modelling should have separate health states with differential probabilities, costs, and utilities.
- CADTH used PFS data that censored patients at the time of an SCT procedure.
- Uncertainty regarding subsequent therapies received: In the KEYNOTE-204 trial, approximately 21% of patients who started on BV received nivolumab as a subsequent therapy and 19% received pembrolizumab. In the model, patients can only receive subsequent therapies if they progress. Based on this assumption, in the model after 2 years, approximately 33% of patients who start on BV have received nivolumab and 33% have received pembrolizumab. Likewise, in the KEYNOTE trial, a few patients in the pembrolizumab arm received pembrolizumab or nivolumab as a subsequent anticancer therapy. The disconnect between subsequent therapies used in the model versus the trial is problematic as the PFS is likely informed by what subsequent therapies patients ended up receiving. Likewise, the trial showed that subsequent therapy use occurred in patients who had not progressed which is not an option within the model. CADTH noted that the sponsor’s model only allows for BV, pembrolizumab, gemcitabine, or no treatment to be considered as subsequent therapy options. However, clinical experts confirmed these would represent most treatment options in Canada.
- As the sponsor’s model only allowed consideration of subsequent therapy in the progressed state, it was not possible for CADTH to align the trial data with the model. Therefore, as a scenario analysis, CADTH assumed that subsequent therapy breakdown from the trial matched what was seen in the progressed state. This analysis is limited in that the subsequent therapy breakdown will still not match the trial, but this is not possible with the way in which the sponsor’s model is built. This analysis is used to demonstrate the influence of subsequent therapy breakdown on the model’s conclusions.
- Failure to include drug wastage in a single-use product: Drug wastage was not incorporated in the sponsor’s base case; however, pembrolizumab’s product monograph states it contains a single-use vial, and weight-based dosing is used in the pediatric population.2
- 5% drug wastage was included in the CADTH reanalysis.
- Incorrect gemcitabine cost: The sponsor sourced drug cost data for gemcitabine from a phase III clinical trial published in 2003.12 This cost was deemed inappropriate as it was significantly lower than gemcitabine list prices available in Canada.
- The price of gemcitabine was revised to the Canadian list price.
- Excessively complex model: The sponsor’s submitted Excel model was excessively complex and lacked transparency. The extent of Visual Basic code is in excess of what is necessary for a model of this type and served to prohibit rigorous validation of the model. Additionally, the sponsor used numerous IFERROR statements in their model. IFERROR statements lead to situations in which the parameter value is overwritten with an alternative value without alerting the user to the automatized overwriting. The systematic use of IFERROR statements makes thorough auditing of the sponsor’s model impossible, as it remains unclear whether the model is running inappropriately by overriding errors. Best programming practices are such that any errors alert the user to a specific error. Finally, data used to inform the model were repeated in numerous sheets making it unclear how to make required changes to the model. This made solving errors in the modelling challenging, such as 1 error where the cost-effectiveness acceptability curve was not updated following pairwise comparisons, and another where the model predicted substantial OS gains for 1 treatment despite the assumption that OS was equal across treatments.
- Given the complexity of the submitted Excel model, CADTH was unable to rigorously validate the model.
Additional limitations were identified but were not considered to be key limitations.
- There is a large degree of heterogeneity when pooling PFS data for the ASCT-eligible and ineligible populations. Given a CADTH base case was only established for ASCT-eligible patients, and clinical expert opinion indicated ASCT-ineligible patients are typically older and in poorer health, PFS may be underestimated in an ASCT-eligible subpopulation.
- Dose intensity is the proportion of the dose actually received of the prescribed dose, and it was deemed to be a quality metric and not applicable to costing single-use pembrolizumab vials. Dose intensity’s exclusion would marginally increase total cost, but likely have minimal impact on incremental costs.
- Due to immature survival data, the sponsor used OS data from a single-arm BV trial that was specific to a population with relapsed or refractory Hodgkin lymphoma who were eligible for ASCT and failed treatment. This data does not include the ASCT-ineligible subpopulation, who are on average, older with poorer health outcomes. The use of these data in an ASCT-ineligible subpopulation could overestimate total QALYs gained, and further increase the uncertainty of estimating an ICER.
- In the pharmacoeconomic report, there is a lack of information describing the parameters used in the probabilistic sensitivity analysis, specifically the standard errors used alongside each distribution. Although Appendix B, Section 9 lists the distributions used, there is no clearly reported list of parameters and standard errors used in the probabilistic sensitivity analysis.
- The pharmacoeconomic report contains numerous errors. Specifically, an explanation for chemotherapy OS curve extrapolation was listed to be in Appendix B, Section 9.3; however, this section does not exist in the report. Further, sources for gemcitabine costs differed by report location, either referencing previous CADTH reviews that did not evaluate gemcitabine or referencing the published literature.
Additionally, the following key assumptions were made by the sponsor and have been appraised by CADTH (Table 5).
Table 5
Key Assumptions of the Submitted Economic Evaluation (Not Noted as Limitations to the Submission).
CADTH Reanalyses of the Economic Evaluation
Base-Case Results
Several limitations of the sponsor’s submission could not be adequately addressed, resulting in CADTH not being able to conduct a base case for ASCT-ineligible patients treated with pembrolizumab compared to chemotherapy. Therefore, the cost-effectiveness of pembrolizumab in ASCT-ineligible patients could not be determined and is unknown. CADTH was able to conduct a base case for adult and pediatric patients who were ASCT eligible and treated with pembrolizumab compared to BV.
To address limitations identified within the economic model, the CADTH base case was derived by making changes in model parameter values and assumptions, in consultation with clinical experts. Table 6 details each change made to derive the CADTH reanalysis, which was conducted in a step-wise approach to the sponsor’s base case to highlight the impact of each change. For treatment waning, alternate parametric distributions were fitted to PFS, as consulted clinical experts estimated PFS would be equal between pembrolizumab and BV after 10 years (Appendix 4, Figure 4 and Figure 5). For changes in utilities values, CADTH derived utilities from the same source as the sponsor’s base-case values, except the utilities were specific to the pooled analysis instead of being treatment-specific.13
Table 6
CADTH Revisions to the Submitted Economic Evaluation.
The summary results of the CADTH reanalyses for the adult and pediatric populations are presented in Table 7 and 8, respectively. CADTH undertook a stepped analysis, incorporating each change proposed in Table 6 to the sponsor’s base case to highlight the impact of each change.
In CADTH’s base case for an adult population, pembrolizumab had higher mean costs (incremental: $16,863) and QALYs gained (incremental: 0.0230) than BV. The ICER for pembrolizumab versus BV was $733,624 per QALY gained, and pembrolizumab had a 27.1% probability of being cost-effective at a WTP threshold of $50,000 per QALY gained. A detailed breakdown of the disaggregate results is available in Appendix 4, Table 15. Approximately 20% of the total QALYs gained in the model were generated within the trial period (1.53 QALYs), while the remaining 80% were generated over the extrapolated post-trial period (5.91 QALYs).
Table 7
Summary of the Stepped Analysis of the CADTH Adult Reanalysis Results.
In CADTH’s base case for the pediatric population, pembrolizumab had lower mean costs (incremental: –$16,685) and higher QALYs gained (incremental: 0.0393) than BV. The ICER indicated pembrolizumab was dominant (i.e., cost savings and more QALYs gained) versus BV, and pembrolizumab had a 46.7% probability of being cost-effective at a WTP threshold of $50,000 per QALY gained. A detailed breakdown of the disaggregate results is available in Appendix 4, Table 14. Similar to the adult population, 20% of the total QALYs gained in the model were generated within the trial period (1.53 QALYs), while the remaining 80% were generated over the extrapolated post-trial period (5.99 QALYs). This analysis is reliant on assuming that data from the adult population can be used for pediatrics.
Table 8
Summary of the Stepped Analysis of the CADTH Pediatric Reanalysis Results.
Scenario Analysis Results
CADTH conducted a scenario analysis with alternate subsequent therapy breakdowns for those patients who progress. In this analysis pembrolizumab was less cost-effective with an ICER of $2,071,825 relative to BV in the adult population and $532,115 in the pediatric population.
A price reduction analysis was performed based on the sponsor’s and CADTH’s reanalysis. Based on the CADTH base case, a price reduction of approximately 13% is required to make pembrolizumab cost-effective at a WTP threshold of $50,000 per QALY gained in an adult ASCT-eligible population (Table 9). Despite the high ICER, there is a relatively small price reduction as pembrolizumab is achieves cost savings across various cost categories (e.g., progressed disease, subsequent treatment, and adverse events) with the exception of drug acquisition costs. Furthermore, small incremental QALY gains of 0.023 result in changes in incremental costs, drastically altering the ICER. A price reduction was conducted in the pediatric population given the ICER was dominant (cost savings and more QALYs gained).
A price reduction analysis was performed based on CADTH’s scenario analysis that used a different breakdown of subsequent therapy. In this analysis, the cost savings from subsequent therapy use are substantially lower. Therefore, a higher price reduction of approximately 29% is required to ensure cost-effectiveness at a $50,000 per QALY threshold.
Table 9
CADTH Price Reduction Analysis for Adults (Deterministic).
Issues for Consideration
- pCODR made a 2019 recommendation to not fund BV in patients with Hodgkin lymphoma who are not candidates for ASCT, thereby eliminating it as a relevant comparator in this subpopulation.
- pCODR made a recommendation in January 2018 to fund pembrolizumab as a monotherapy in adult patients with refractory or relapsed cHL who have either failed ASCT and BV or who are not candidates for ASCT and have failed BV.
- CADTH notes that the patent for BV is expected to expire in 2023.
Overall Conclusions
Evidence suggests pembrolizumab provides statistically and clinically significant improvements in PFS |||||||||||||||||||||||||||||||||||| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||. Across all studies, BV patients were generally less likely to experience adverse events but more likely to discontinue therapy due to an adverse event. No comparators beyond BV were evaluated. Therefore, the comparative evidence of pembrolizumab to chemotherapy in an ASCT-ineligible subpopulation is unknown, due to a lack of direct or indirect evidence for PFS and OS.
CADTH addressed several key limitations of the sponsor’s model by selecting appropriate comparators for the Canadian context; evaluating an ASCT-eligible subpopulation with comparative evidence; using PFS data that censored upon SCT events; considering a treatment effect waning; changing treatment-specific utilities to disease-specific; and incorporating drug wastage. According to CADTH’s reanalyses, in adult patients, pembrolizumab is associated with an ICER of $733,624 per QALY gained when compared to BV, and in a pediatric population pembrolizumab is dominant (i.e., cost saving and associated with more QALYs gained). In adults, a price reduction of approximately 13% is required for pembrolizumab to be cost-effective at a WTP threshold of $50,000 per QALY. This result is contingent on pembrolizumab generating approximately $43,000 of cost savings due to patients who receive pembrolizumab first line and not receiving immunotherapies such as nivolumab and pembrolizumab second line. There is a significant amount of outstanding uncertainty regarding the degree of these cost savings. A scenario analysis that more closely matched subsequent therapy data from the KEYNOTE-204 trial found the ICER in adults increased to $2,071,825 per QALY. At this level, a 29% price reduction is needed to achieve a $50,000 per QALY threshold.
The probability that pembrolizumab is cost-effective at a WTP threshold of $50,000 per QALY was low in both the adult (27.1%) and pediatric (46.7%) populations. The low probability of pembrolizumab being cost-effective in a pediatric population differs from the mean ICER, which indicated pembrolizumab was dominant (less costly and more effective) versus BV. This finding highlights the uncertainty across probabilistic iterations, and the extreme ICERs generated due to marginal differences in costs and QALYs between pembrolizumab and BV.
The conclusions of CADTH’s analysis are specific to an ASCT-eligible population. CADTH was unable to assess the cost-effectiveness of pembrolizumab in an ASCT-ineligible population due to an absence of comparative evidence between pembrolizumab and chemotherapy. In Canada, individuals who are ineligible for ASCT rarely receive BV, therefore chemotherapy is the most relevant comparator. Drug costs for pembrolizumab are substantially higher relative to chemotherapy, as such information on this comparison is needed to assess the value of pembrolizumab in this subgroup. Finally, results for the pediatric population are almost entirely extrapolated from the adult data as well, so any uncertainty with that extrapolation will impact the robustness of the pediatric results.
Abbreviations
Abbreviations
- ASCT
autologous stem cell transplantation
- BIA
budget impact analysis
- BV
brentuximab vedotin
- cHL
classic Hodgkin lymphoma
- ICER
incremental cost-effectiveness ratio
- OS
overall survival
- pCODR
CADTH pan-Canadian Oncology Drug Review
- PFS
progression-free survival
- QALY
quality-adjusted life-year
- SCT
stem cell transplant
- WTP
willingness to pay
Appendix 1. Cost Comparison Table
Note that this appendix has not been copy-edited.
The comparators presented in the Table 10 have been deemed to be appropriate based on feedback from clinical experts. Comparators may be recommended (appropriate) practice or actual practice. Existing Product Listing Agreements are not reflected in the table and as such, the table may not represent the actual costs to public drug plans.
Table 10
CADTH Cost Comparison Table for Relapsed or Refractory Classical Hodgkin Lymphoma.
Appendix 2. Submission Quality
Note that this appendix has not been copy-edited.
Table 11
Submission Quality.
Appendix 3. Additional Information on the Submitted Economic Evaluation
Note that this appendix has not been copy-edited.
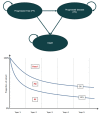
Figure 1
Model Structure.
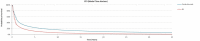
Figure 2
PFS Extrapolations for Pembrolizumab Versus BV (52-Week Cut-Off Point).
Table 12
Disaggregated Results of Adult Population With ITT Base Case.
Table 13
Disaggregated Results of Pediatric Population With ITT Base Case.
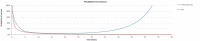
Figure 3
Errors in Treatment Waning for PFS Extrapolations for Pembrolizumab Versus BV (52-Week Cut-Off Point).
Appendix 4. Additional Details on the CADTH Reanalyses and Sensitivity Analyses of the Economic Evaluation
Note that this appendix has not been copy-edited.

Figure 4
PFS Extrapolations — Adult ASCT-Eligible Population.

Figure 5
PFS Extrapolations — Pediatric ASCT-Eligible Population.
Table 14
Disaggregated Summary of CADTH’s Economic Evaluation Results — Pediatric ASCT-Eligible Population.
Table 15
Disaggregated Summary of CADTH’s Economic Evaluation Results — Adult ASCT-Eligible Population.
Appendix 5. Submitted BIA and CADTH Appraisal
Note that this appendix has not been copy-edited.
Table 16
Summary of Key Take-Aways.
Summary of Sponsor’s BIA
The submitted budget impact analysis (BIA) assessed the introduction of pembrolizumab, as monotherapy, for adults and pediatric patients with relapsed/refractory classical Hodgkin lymphoma (cHL), for third-line treatment in those who relapsed post-ASCT or as second-line treatment in those ineligible for ASCT. The analysis was taken from the perspective of the Canadian public drug plans using an epidemiologic-based approach, with only drug acquisition costs considered. A 3-year time horizon was used, from 2022 to 2024, with 2021 as a base year.
A summary of the sponsor’s derivation of the eligible population size is presented in Figure 6, and key inputs to the BIA are documented in Table 17. The sponsor estimated the current population using an epidemiologic approach, derived from non-Canadian and Canadian publications, health technology assessment recommendations, and clinical expert opinions. The incidence of Hodgkin lymphoma patients in 2022 was estimated at 761 patients, of which 95% would have the classical subtype. Clinician input indicated that all cHL patients receive first-line ABVD (Adriamycin-bleomycin-vinblastine-dacarbazine) treatment because of its high cure rate. Of that treated population, Canadian data indicated the 5-year risk of relapse was 18.1%, which was used to calculate the relapse/refractory cHL population. Based on that same data in relapse/refractory cHL patients, 78% would undergo ASCT while the remaining was assumed to represent the ASCT-ineligible subpopulation. Each subpopulation was further stratified into adult and pediatric patients based on Statistics Canada data on Hodgkin lymphoma (to allow for differential drug dosing and costing), where approximately 12% of all patients were considered pediatric.
The sponsor’s submission had a reference scenario in which patients were initially treated with either BV or chemotherapy, and a new drug scenario in which pembrolizumab was reimbursed. The sponsor also conducted various sensitivity analyses where wastage, subsequent SCT costs, and administration costs were included.
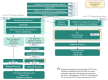
Figure 6
Sponsor’s Estimation of the Size of the Eligible Population.
Table 17
Summary of Key Model Parameters.
Summary of the Sponsor’s BIA Results
From the perspective of Canadian public drug plans, the estimated budget of reimbursing pembrolizumab for the treatment of relapsed/refractory cHL patients for both third-line treatment in those who relapsed post-ASCT and as second-line treatment in those ineligible for ASCT, is expected to be $277,229 in Year 1, $2,038,724 in Year 2, and $3,538,473 in Year 3, with a 3-year budget impact of $5,854,426. Note, these costs include both the initial and subsequent treatments.
CADTH Appraisal of the Sponsor’s BIA
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the results of the BIA:
- The sponsor assumed the market share for BV as a second-line treatment for ASCT-ineligible patients is currently 32%. However, across Canada BV has limited utilization in an ASCT-ineligible subpopulation, as recommended by the CADTH pan-Canadian Oncology Drug Review (pCODR) which “did not recommend funding BV in patients with Hodgkin lymphoma who are not candidates for ASCT and who have relapsed disease following at least 2 prior multi-agent chemotherapies.”10
- Given the local context and expert feedback, the market share of BV was reduced to 15% in a scenario analysis.
- Clinical trials are an inappropriate comparator: The sponsor included clinical trials as a relevant comparator in their base case and assumed those in clinical trials would incur no costs.
- Clinical trials were removed as an intervention, and its uptake was redistributed according to the prior market share distribution.
- Initial pembrolizumab uptake rate is low: After consultation with clinical experts, they indicated that based on KEYNOTE-204 results, the year 1 uptake rate for pembrolizumab would be higher than the current estimate of 45%.
- The growth of the market share of pembrolizumab was changed from following a linear regression to a logarithmic regression, thus increases the first-year uptake rate.
- Failure to include drug wastage in a single-use product: Drug wastage was not incorporated in the sponsor’s base case, however pembrolizumab’s product monograph states it contains a single-use vial, and weight-based dosing is used in the pediatric population.2
- 5% drug wastage was included in the CADTH reanalysis.
- Incorrect gemcitabine cost: The sponsor sourced drug cost data for gemcitabine from a phase III clinical trial published in 2003.12 This cost was deemed inappropriate as it was significantly lower than gemcitabine list prices available in Canada.
- The price of gemcitabine was revised to the Canadian list price.
Additional limitations were identified but were not considered to be key limitations. These limitations include:
- The BIA assumed 12% of all patients estimated using an epidemiological approach are pediatric. This input was derived from a Statistics Canada age breakdown of Hodgkin lymphoma patients, not relapsed/refractory classical Hodgkin lymphoma patients.
- The sponsor’s base case included subsequent treatments following initial therapy, which may have the effect of averaging out costs and reducing the budget impact of introducing pembrolizumab as third-line treatment in those who relapsed post-ASCT and as second-line treatment in those ineligible for ASCT. This approach is problematic in the case where people are initially treated with chemotherapy as the model assumes 100% move on to a clinical trial, which was determined to be an inappropriate comparator.
CADTH Reanalyses of the BIA
Based on the limitations identified, CADTH’s base case included: the removal of clinical trials as an intervention, an increased pembrolizumab uptake rate in year 1, accounting for drug wastage, and correcting gemcitabine’s price to the Canadian list price. A scenario analysis explored the reduction in BV market share (Table 20).
Table 18
CADTH Revisions to the Submitted Budget Impact Analysis.
The results of the CADTH step-wise reanalysis are presented in summary format in Table 1 and a more detailed breakdown is presented in Table 2. Based on the CADTH base case, the expected budget impact for funding pembrolizumab is $305,213 in year 1, $2,070,116 in year 2, and $3,035,408 in year 3, for a total 3-year budget impact of $5,410,737. Within the total 3-year budget impact, $1,503,571 was from the third-line ASCT-eligible subpopulation and $3,907,166 was from the second-line ASCT-ineligible subpopulation.
CATH conducted a scenario analysis where the BV market share was reduced to 15%. In this analysis the expected budget impact for funding pembrolizumab is $518,795 in year 1, $2,551,263 in year 2, and $3,587,068 in year 3, for a total 3-year budget impact of $6,657,127.
Table 19
Summary of the CADTH Reanalyses of the BIA.
Table 20
Detailed Breakdown of the CADTH Reanalyses of the BIA.
References
- 1.
- Pharmacoeconomic evaluation [internal sponsor's report]. In: Drug Reimbursement Review sponsor submission: keytruda (pembrolizumab), 200 mg intravenously (IV) Q3W (for adult patients); 2 mg/kg IV Q3W, up to a maximum of 200 mg (for pediatric patients). Kirkland (QC): Merck Canada Inc.; 2021 Jan 29.
- 2.
- Keytruda (pembrolizumab): powder for solution for infusion 50 mg, solution for infusion 100 mg/4 mL vial; Antineoplastic agent, monoclonal antibody [product monograph]. Kirkland (QC): Merck Canada Inc.; 2021 Jun 4.
- 3.
- Kuruvilla J, Ramchandren R, Santoro A, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(4):512-524. [PubMed: 33721562]
- 4.
- Geoerger B, Kang HJ, Yalon-Oren M, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020;21(1):121-133. [PubMed: 31812554]
- 5.
- Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562-1566. [PMC free article: PMC5034737] [PubMed: 27432875]
- 6.
- CADTH pCODR Expert Review Committee final economic guidance report: pembrolizumab (Keytruda) for renal cell carcinoma. 2020 Apr 2. https://cadth
.ca/sites /default/files/pcodr /Reviews2020/10185PembrolizumabRCC _fnEGR _NOREDACT-ABBREV_Post02Apr2020_final .pdf. Accessed May 17, 2021. - 7.
- CADTH Drug Reimbursement Review pharmacoeconomic report: brentuximab vedotin (Adcetris) for the treatment of previously untreated patients with stage IV Hodgkin lymphoma, in combination with doxorubicin, vinblastine, and dacarbazine. Ottawa (ON): CADTH; 2020 Dec 3: https://www
.cadth.ca /sites/default/files /pcodr/Reviews2020/10214BrentuximabVedotinHLAVD _fnEGR_NOREDACT-ABBREV _03Dec2020_final.pdf. Accessed 2021 May 11. - 8.
- Hirt C, Iannazzo S, Chiroli S, et al. Cost effectiveness of the third-generation tyrosine kinase inhibitor (TKI) ponatinib, vs. second-generation TKIs or stem cell transplant, as third-line treatment for chronic-phase chronic myeloid leukemia. Appl Health Econ Health Policy. 2019;17(4):555-567. [PubMed: 31168745]
- 9.
- Drug Reimbursement Review sponsor submission: Keytruda (pembrolizumab), 200 mg intravenously (IV) Q3W (for adult patients); 2 mg/kg IV Q3W, up to a maximum of 200 mg (for pediatric patients) [internal sponsor's package]. Kirkland (QC): Merck Canada Inc.; 2021 Jan 29.
- 10.
- CADTH pCODR Expert Review Committee final recomendation: brentuximab vedotin (Adcetris). 2019 Mar 7. https://cadth
.ca/sites /default/files/pcodr /Reviews2019/10145BrentuximabHL _FnRec_approvedbyChair _Post_07Mar2019_final.pdf. Accessed 2021 May 7. - 11.
- Guidelines for the economic evaluation of health technologies: Canada. CADTH methods and guidelines. 4th ed. Ottawa (ON): CADTH; 2017: https://www
.cadth.ca /sites/default/files /pdf/guidelines_for_the _economic_evaluation _of_health_technologies _canada_4th_ed.pdf. Accessed 2021 May 11. - 12.
- Gridelli C, Gallo C, Shepherd FA, et al. Gemcitabine plus vinorelbine compared with cisplatin plus vinorelbine or cisplatin plus gemcitabine for advanced non-small-cell lung cancer: a phase III trial of the Italian GEMVIN Investigators and the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21(16):3025-3034. [PubMed: 12837810]
- 13.
- Clinical study report: P204V01MK3475. A phase III, randomized, open-label, clinical trial to compare pembrolizumab with brentuximab vedotin in subjects with relapsed or refractory classical Hodgkin lymphoma. [internal sponsor's report]. Kenilworth (NJ): Merck Sharp & Dohme Corp; 2020 Apr 10.
- 14.
- DeltaPA. [Ottawa (ON)]: IQVIA; 2021: https://www
.iqvia.com/. Accessed 2021 May 11. - 15.
- Exceptional Access Program (EAP). Toronto (ON): Ontario Ministry of Health; Ontario Ministry of Long-Term Care; 2020: https://www
.health.gov .on.ca/en/pro/programs /drugs/odbf/odbf_except_access.aspx. Accessed 2021 May 11. - 16.
- Ontario drug benefit formulary/comparative drug index. Toronto (ON): Ontario Ministry of Health, Ontario Ministry of Long-Term Care; 2020: https://www
.formulary .health.gov.on.ca/formulary/. Accessed 2021 May 11. - 17.
- Budget impact analysis [internal sponsor's report]. In: Drug Reimbursement Review sponsor submission: keytruda (pembrolizumab), 200 mg intravenously (IV) Q3W (for adult patients); 2 mg/kg IV Q3W, up to a maximum of 200 mg (for pediatric patients). Kirkland (QC): Merck Canada Inc.; 2021 Jan 29.
- Executive Summary
- Stakeholder Input Relevant to the Economic Review
- Economic Review
- Abbreviations
- Cost Comparison Table
- Submission Quality
- Additional Information on the Submitted Economic Evaluation
- Additional Details on the CADTH Reanalyses and Sensitivity Analyses of the Economic Evaluation
- Submitted BIA and CADTH Appraisal
- References
- Pharmacoeconomic Review - Pembrolizumab (Keytruda)Pharmacoeconomic Review - Pembrolizumab (Keytruda)
- PREDICTED: Leopardus geoffroyi solute carrier family 25 member 14 (SLC25A14), tr...PREDICTED: Leopardus geoffroyi solute carrier family 25 member 14 (SLC25A14), transcript variant X2, mRNAgi|2166945362|ref|XM_045471601.1|Nucleotide
- REFERENCES - Edoxaban (Lixiana)REFERENCES - Edoxaban (Lixiana)
- Conjugative relaxosome accessory transposon protein (plasmid) [Salmonella enteri...Conjugative relaxosome accessory transposon protein (plasmid) [Salmonella enterica subsp. enterica serovar Typhimurium]gi|1905442734|emb|CAD5311246.1|Protein
- Protein TraQ (plasmid) [Salmonella enterica subsp. enterica serovar Typhimurium]Protein TraQ (plasmid) [Salmonella enterica subsp. enterica serovar Typhimurium]gi|1905442736|emb|CAD5311248.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...