Copyright © 2023 - Canadian Agency for Drugs and Technologies in Health. Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International licence (CC BY-NC-ND).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Amivantamab (Rybrevant): CADTH Reimbursement Review: Therapeutic area: Non–small cell lung cancer [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2023 May.

Amivantamab (Rybrevant): CADTH Reimbursement Review: Therapeutic area: Non–small cell lung cancer [Internet].
Show detailsExecutive Summary
The executive summary comprises 2 tables (Table 1 and Table 2) and a conclusion.
Table 1
Submitted for Review.
Table 2
Summary of Economic Evaluation.
Conclusions
The CADTH Clinical Review highlighted the high degree of uncertainty associated with the results of CHRYSALIS. The single-arm, open-label, nonrandomized, phase I/Ib design of the CHRYSALIS trial makes interpreting the efficacy and safety events attributable to amivantamab challenging, because all patients received the same treatment. While objective response rate (ORR) may be directly attributable to the drug’s antitumour activity, interpreting progression-free survival (PFS) and overall survival (OS) events is significantly limited. The extent to which the observed survival is due to the natural history of the tumour or the intervention remains unclear. The CADTH assessment of the sponsor-submitted adjusted treatment comparison identified several key limitations including small sample sizes, heterogeneity across study designs and pooled populations, and the inability to adjust for all potential confounders and prognostic variables, all of which significantly limited the ability to interpret the relative treatments effects observed between amivantamab and other treatments. Overall, it was noted that the phase I nature of the CHRYSALIS trial limits the ability to make firm conclusions on comparative efficacy given the short duration of follow-up, which results in immature data, as well as the small sample size of the CHRYSALIS trial and comparator cohorts.
Due to the high degree of unresolved uncertainty with the clinical data, CADTH was unable to derive an economic base case.
CADTH performed an exploratory analysis in which a Weibull parametric extrapolation was used for the OS data for amivantamab. Based on CADTH sequential reanalysis, amivantamab is associated with an incremental cost-effectiveness ratio (ICER) of $253,131 per quality-adjusted life-year (QALY) compared to non–platinum-based chemotherapy (NPBC), with a 0% probability of being cost-effective at a willingness-to-pay (WTP) threshold of $50,000 per QALY. This estimate is based on the sponsor’s clinical assumptions, which predict an additional 0.79 life-years (LYs) and 0.60 QALYs for those receiving amivantamab compared with NPBC. A price reduction of at least 77% would be required for amivantamab to be considered cost-effective compared to NPBC at this threshold.
If the comparative clinical data — particularly the PFS and OS data — are considered sufficiently robust, then the exploratory analysis performed by CADTH may be informative. However, in light of the available clinical information, CADTH’s results are based on assumptions (e.g., survival extrapolations, which result in OS benefits for amivantamab both preprogression and postprogression) that cannot be validated at this time and hence are associated with a large degree of uncertainty. As such, additional price reductions may be required to ensure the cost-effectiveness of amivantamab.
Due to the limitations with the clinical evidence and substantial uncertainty associated with the comparative clinical effects of amivantamab with relevant comparators, CADTH performed a scenario cost comparison analysis in which only drug acquisition costs were included. Results of this analysis suggest that amivantamab would require a price reduction of 86.4% to achieve cost parity with the least costly comparator, docetaxel.
Stakeholder Input Relevant to the Economic Review
This section is a summary of the feedback received from the patient groups, registered clinicians, and drug plans that participated in the CADTH review process.
Patient input was received from 2 groups, the Lung Health Foundation and Lung Cancer Canada. Lung Cancer Canada conducted 4 telephone interviews with patients living in Ontario, Canada, who were part of the amivantamab clinical trial. All 4 of the interviewed patients were epidermal growth factor receptor (EGFR) positive and 2 had EGFR exon 20 insertion mutations. The Lung Health Foundation conducted 3 phone interviews with Canadian lung cancer patients and received 2 responses to an online survey (demographic data not collected). Patients interviewed by the Lung Health Foundation reported previous treatment with surgery, radiation, chemotherapy, targeted therapy, and immunotherapy. The input indicated that most patients struggled with lingering side effects, including fatigue, nausea, weight loss, and hair loss, and that side effects from chemotherapy were specifically noted to affect patients’ quality of life, ability to work, and daily activities. The patient input noted a specific unmet need for targeted therapy to the exon 20 insertion mutation, which current chemotherapy does not address. The patients interviewed by Lung Cancer Canada were enrolled in the amivantamab clinical trial and had experience with the drug under review. Some patients experienced stability in tumours and metastases on amivantamab, while others experienced shrinkage. The most common adverse events (AEs) reported by patients were skin-related, including inflammation of the nail bed, rashes, acne, and dry and sensitive skin. All patients noted that side effects are manageable and that the potential benefits outweigh the negatives.
Clinician input was received from 2 groups, the Ontario Health – Cancer Care Ontario Lung Cancer Drug Advisory Committee and the Lung Cancer Canada Medical Advisory Committee. Clinicians indicated that current standard of care for patients in first-line therapy is platinum-based doublet chemotherapy, followed by docetaxel in second-line therapy, though response rates are poor. EGFR tyrosine kinase inhibitors (TKIs) may also be used for patients with EGFR mutations but patients with EGFR exon 20 insertion mutations are resistant to EGFR TKIs and have a low response rate to immunotherapy. Feedback from Ontario Health suggested that amivantamab would be used after all standard therapies acceptable to the patient have failed, suggested to be platinum-based doublet chemotherapy with maintenance pemetrexed and, potentially, docetaxel as well. The input from Lung Cancer Canada highlighted that targeted therapies against driver mutations should ideally be offered in a first-line setting, and that clinical trials in this setting are ongoing.
Drug plan input received for this review noted that additional resource use would be required with amivantamab due to its split first dose and escalating infusion-rate schedule. Additional labour will be required for drug preparation, and premedications are required to prevent infusion-related reactions (IRRs). The plans noted the presence of confidential prices for nivolumab, pembrolizumab, and atezolizumab.
The following concern was addressed in the sponsor’s model:
- the sponsor-included AEs, including IRRs.
CADTH was unable to address the following concerns raised from the stakeholder input:
- CADTH was unable to incorporate the presence of confidential, negotiated prices for immuno-oncology (IO) drugs.
- The cost of preinfusion medications was not considered by the sponsor.
- The model only considers the cost-effectiveness of amivantamab in a platinum-treated population.
Economic Review
The current review is for amivantamab (Rybrevant) for adult patients with locally advanced or metastatic non–small cell lung cancer (NSCLC) with activating EGFR exon 20 insertion mutations whose disease has progressed on, or after, platinum-based chemotherapy.
Economic Evaluation
Summary of Sponsor’s Economic Evaluation
Overview
The sponsor submitted a cost-utility analysis of amivantamab compared to IO drugs, TKIs, and NPBC. The modelled population is consistent with the Health Canada indication and the reimbursement request, and these populations are aligned with the sponsor’s CHRYSALIS trial population.1-3
Amivantamab is supplied in single-use vials containing 350 mg amivantamab in 7 mL solution (50 mg/mL).2 The recommended dose of amivantamab is 1,050 mg (3 vials) for patients weighing less than 80 kg and 1,400 mg (4 vials) for patients weighing greater than or equal to 80 kg.2 The recommended dosing schedule for amivantamab is once weekly for the first 4 weeks (first dose split on days 1 and 2) and every 2 weeks starting at week 5.2 Amivantamab should be reconstituted and administered by a health care professional with appropriate medical support to manage IRRs if they occur. Premedications consisting of antihistamines, antipyretics, and glucocorticoids should be administered to reduce the risk of IRRs. Amivantamab should be administered until disease progression or unacceptable toxicity.2 The cost for amivantamab is $1,676.00 per vial, leading to costs per dose of $5,028 and $6,704 for patients weighing less than 80 kg or greater than or equal to 80 kg, respectively.3 The cost in the first cycle is $20,112 for patients weighing less than 80 kg and $26,816 for patients weighing greater than or equal to 80 kg; the cost in subsequent cycles is $10,056 and $13,408, respectively.3
The sponsor selected 3 therapy classes as comparators: IO drugs consisting of atezolizumab (33.3%), nivolumab (33.3%), and pembrolizumab (33.3%); EGFR TKIs consisting of gefitinib (50%), afatinib (25%), and osimertinib (25%); and NPBC consisting of docetaxel (100%). Costs and effects for these comparators were weighted by these proportions, which were derived based on Canadian market share estimates and clinician input.3 The costs per cycle calculated by the sponsor were $10,052 for IO drugs, $3,471 for TKIs, and $2,775 for NPBC.3
The clinical outcomes of interest were QALYs and LYs over a lifetime horizon (10 years). Discounting (1.5% per annum) was applied to both costs and outcomes and a cycle length of 4 weeks was used along with a half-cycle correction. The base-case perspective was that of the Canadian publicly funded health care payer.
Model Structure
The sponsor submitted a partitioned survival model (PSM) consisting of 3 mutually exclusive health states: preprogression, postprogression, and death. All patients entered the model in the preprogression state and received amivantamab or a comparator. The allocation of patients into health states is based on treatment-specific PFS and OS functions. The proportion of patients in the preprogression health state followed the PFS curve for amivantamab from the CHRYSALIS trial, and the PFS curve generated from Kaplan-Meier (KM) data from a sponsor-commissioned real-world evidence (RWE) study for comparators.3 In the CHRYSALIS trial, PFS was derived based on investigator assessment and independent review committee assessment; in the RWE study, PFS was defined as human abstraction of physician evaluation of tumour progression.3 The proportion of patients in the postprogression health state was equal to the difference between the OS and the PFS curves. Patients in the postprogression state can receive subsequent-line therapy. Patients transitioning into the death state remained there until the end of the model time horizon. A figure of the sponsor’s model structure is available in Figure 1, Appendix 3.
Model Inputs
The target population of the economic evaluation was based on cohort D of the phase I/Ib single-arm CHRYSALIS trial, which included NSCLC patients with activating EGFR exon 20 insertion mutations whose disease had progressed on or after platinum-based chemotherapy. The mean age of the population was 62.3 years, mean weight was 67.5 kg, and 41% were male.1 The key clinical inputs (i.e., PFS, OS, and treatment discontinuation) for amivantamab were obtained from the primary efficacy population (n = 81), while safety data were informed from the expanded safety population (n = 153) of the trial. Data were based on the March 30, 2021, data cut-off date.4
In the absence of direct comparative evidence, comparative effectiveness data were derived from a sponsor-commissioned adjusted treatment comparison of CHRYSALIS versus an RWE cohort (n = 349). The sponsor-commissioned RWE study cohort was created using various RWE sources from the US (Flatiron Health Spotlight, ConcertAI, COTA) and Europe (Public Health England, 2 from Germany, and 1 from France). The adjusted treatment comparison used inverse probability of treatment weights derived from a propensity score model to account for variables including, but not limited to, age, sex, race, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, and time from initial diagnosis to advanced diagnosis.3 It was assumed that unobserved confounders did not have an impact on the comparator treatment-effect estimate. The covariates used to estimate the weights in the analysis of the European plus US cohort included age, gender, presence of brain metastases, and number of previous lines of therapy in the metastatic disease setting.3 For PFS, OS, and time to next treatment, time-to-event analyses were performed using weighted Cox proportional hazard models. KM curves were generated and used to estimate median time-to-event estimates for each treatment group.5
Extrapolations of the PFS, OS, and time to treatment discontinuation data were performed for amivantamab and comparators. For amivantamab, the PFS, OS, and time to treatment discontinuation data were fit with a lognormal, gamma, and exponential parametric fit, respectively. Full details of the parametric extrapolations are available in Table 10, Appendix 3.3
Regarding the safety of amivantamab, the model included grade 3 or 4 AEs occurring in 5% or more of patients in cohort D of CHRYSALIS, as well as grade 1 to 2 IRRs.4 Product monographs for individual treatments were used to inform safety data, with AE incidence rates for selected index drugs used to represent the corresponding treatment class.3
Utility values were derived from Labbé et al., who performed a longitudinal cohort study of metastatic lung cancer; the values obtained were from the EGFR-mutated NSCLC population.6 The publication reported a utility value of 0.81 for progression-free disease and 0.70 for postprogression disease, which were employed in the sponsor’s model. Disutilities due to AEs were derived from various sources and were also applied in the model as a one-time disutility decrement.3
The economic model included costs related to drugs (acquisition, administration), AEs, monitoring and disease management, subsequent treatment, and terminal care. Dosing for amivantamab was as previously described, with weighted acquisition costs for the initial and subsequent cycles of $21,850 and $10,925, respectively, based on data from CHRYSALIS, which found that 74.1% of patients weighed less than 80 kg at baseline. Drug acquisition costs for comparators are as previously described. An administration cost of $201 per hour of chair time was assumed based on a published study;7 amivantamab administrations were assumed to require 2 to 6 hours of chair time, while comparators required 1 hour. Monitoring and disease management costs consisted of costs for outpatient visits, chest radiography, CT scans, nurse visits, and routine blood work, which were obtained from the Ontario Schedule of Benefits for Physician and Laboratory Services.8,9 Resource utilization frequency was assumed to be the same across all treatments, but differed for patients in the preprogression and postprogression health states. Costs for most AEs were derived from the Ontario Case Costing Initiative, while the cost of IRRs and febrile neutropenia came from published sources.7,10,11 Half of modelled patients were assumed to receive subsequent therapy upon progression, which consisted of IO drugs, TKIs, and NPBC, though patients were assumed not to receive the same therapy twice. Duration of subsequent therapy ranged from 2.8 months for NPBC to 4.2 months for IO drugs and amivantamab.12 Finally, a one-off terminal care cost of $53,008 was applied based on a published study.13
Summary of Sponsor’s Economic Evaluation Results
All analyses were run probabilistically (1,000 iterations for the base case and scenario analyses). The deterministic and probabilistic results were similar. The probabilistic findings are presented in the following sections.
Base-Case Results
In the sponsor’s base case, amivantamab was associated with an estimated cost of $233,445 and 1.81 QALYs over a lifetime horizon. In sequential analysis, amivantamab was associated with an ICER of $210,591 compared to NPBC (incremental cost: $142,316, incremental QALYs: 0.68). IO drugs were dominated by NPBC in the sponsor’s base case, resulting in fewer QALYs and higher cost. In the sponsor’s sequential analysis, amivantamab had a 0% probability of being cost-effective at a WTP threshold of $50,000 per QALY. Results of the base case suggest that 0.34 incremental QALYs were accrued after the maximum follow-up in CHRYSALIS of 30 months — that is, approximately 51% of the incremental benefit was obtained after the trial period. Less than 1% of patients on amivantamab remained alive after 10 years. Additional results from the sponsor’s submitted economic evaluation base case are available in Appendix 3.
Table 3
Summary of the Sponsor’s Economic Evaluation Results.
Sensitivity and Scenario Analysis Results
The sponsor conducted several scenario analyses involving an expanded efficacy population from CHRYSALIS (n = 114), definition of progression (based on independent review committee or investigator assessed), amivantamab PFS and OS extrapolations, treatment discontinuation, 5-year time horizon, and vial sharing. Results were generally similar among the scenarios tested, though sequential analysis was not performed on the scenarios. The ICER for amivantamab versus NPBC was most influenced by the choice of OS extrapolation for amivantamab, with ICERs ranging from $144,147 to $237,700 per QALY depending on the extrapolation chosen. The analysis with a 5-year time horizon resulted in an ICER of $238,659.
CADTH Appraisal of the Sponsor’s Economic Evaluation
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the economic analysis:
- The clinical data for amivantamab is based on a single-arm study with evidence for clinical response. The clinical efficacy of amivantamab was assessed in a single-arm, open-label, nonrandomized, phase I/Ib trial. As noted in the CADTH Clinical Review, the design of the trial makes interpreting the efficacy and safety events attributable to amivantamab challenging, because all patients received the same treatment. The lack of a control arm increases the risk of bias in the estimation of treatment effect due to the potential for confounding related to fluctuations in health status and other unidentified prognostic factors that could affect subjectively assessed outcomes. Patient selection and lack of randomization may have also introduced bias. Although the sponsor attempted to minimize the risk of bias by using an independent review committee assessment for key study outcomes, the open-label, single-arm design can increase the risk of bias in reporting of outcomes that are subjective in measurement and in interpretation, such as response and AEs. Additionally, the primary and secondary outcomes for cohort D were not controlled for multiple testing, and must be considered with respect to type I error and should be viewed as supportive evidence for the overall effect of amivantamab. While ORR may be directly attributable to the drug’s antitumour activity, interpreting other efficacy outcomes that rely on tumour biology, disease prognosis, and patients’ performance status — including PFS, OS, and duration of response — is significantly limited. The extent to which the observed survival is due to the natural history of the tumour or the intervention remains unclear. Finally, the median duration of follow-up was 14.5 months for the primary efficacy population, which was considered appropriate for the primary end point of ORR but was immature for survival outcomes such as OS. As such, the true benefit of amivantamab on survival outcomes is uncertain.
- CADTH was not able to address the limitations associated with the submitted clinical data. The clinical uncertainty directly affects the confidence that can be drawn from the results of the economic model.
- The comparative clinical efficacy of amivantamab with relevant comparators is highly uncertain. Due to the lack of head-to-head evidence comparing amivantamab to relevant comparators in a randomized controlled trial, the sponsor submitted an adjusted treatment comparison that derived comparator information from an RWE cohort to inform the pharmacoeconomic model (PFS, OS, time to next treatment). In general, comparing trial evidence to RWE will result in greater uncertainty given that clinical trials and RWE measure different components; clinical trials measure treatment effects under controlled circumstances, while RWE aligns better with how treatments are used and assessed in clinical practice. As outlined in the CADTH Clinical Review, there were numerous limitations with the adjusted treatment comparison, which add considerable uncertainty to the analysis. The RWE data were analyzed retrospectively from electronic medical records and databases; these sources are more prone to biases that cannot be fully controlled for (e.g., selection bias, confounding, limited data availability) compared with those collected from prospective, interventional studies that cannot be fully controlled for. Inclusion and exclusion criteria from CHRYSALIS were applied to real-world data sources to select the appropriate population; however, any criteria that could not be applied to patients due to missing data were omitted from the list of criteria applied to that data source, which may have resulted in unaccounted-for differences in patient populations. No imputation method was applied to account for missing data, and if a substantial amount of data was missing, covariates were not included, which may affect the results of the comparisons. The sponsor performed propensity score matching without accounting for some important factors including smoking status, ECOG, and race; therefore, the comparisons were not balanced for confounders and were not mutually randomizable populations. It was assumed that unobserved confounders did not have an impact on the comparator treatment effect estimate, which is highly uncertain.Furthermore, the model results for NPBC do not align with published literature, further increasing the uncertainty with the sponsor’s analysis. The sponsor’s model predicts a median survival of 13 months for patients treated with NPBC. This is substantially higher than historical data for previously treated platinum chemotherapy patients with NSCLC who then receive docetaxel (median survival 7.0 months).14 The clinical experts consulted by CADTH acknowledged that advances in technology over the past 2 decades could be expected to improve OS in these patients, and suggested that a median survival of between 8 and 10 months would be reasonable for patients receiving docetaxel. This estimate is lower than the sponsor’s model predictions.
- CADTH was unable to address the limitations associated with the sponsor’s adjusted treatment comparison and notes that considerable uncertainty remains in the analysis that could not be resolved. CADTH tested alternate OS extrapolations for NPBC and noted that results varied little, with none resulting in median survival estimates that aligned with clinical expert opinion.
- The OS extrapolation for amivantamab is uncertain. The sponsor extrapolated the OS data from CHRYSALIS for amivantamab from a median follow-up of 14.5 months out to the 10-year model time horizon.1 As stated in the CADTH Clinical Review, these survival results were immature. The sponsor’s model predicted a median survival of 23 months for amivantamab, which, while aligned with the KM data from CHRYSALIS, is substantially higher than survival results for the comparators, even despite the overestimates for comparators described above. As shown in Figure 2, Appendix 3, patients in CHRYSALIS experienced a median PFS of approximately 8 months — that is, amivantamab is predicted to delay disease progression by 8 months. If that result is combined with clinical experts’ maximum predicted survival for this population (10 months), the theoretical maximum survival for patients on amivantamab is 18 months, which is lower than the 23 months predicted by the sponsor’s model. Ultimately, given the aforementioned limitations regarding the single-arm trial design and adjusted treatment comparison, OS results for amivantamab are substantially higher than that expected from current therapy with no robust comparative data to support these results.
- As part of an exploratory analysis, CADTH chose a Weibull extrapolation for the OS of amivantamab, which was deemed reasonable by clinical experts, to explore the impact of an alternate survival assumption.
- The sponsor’s model structure is not appropriate. Health Canada gave amivantamab, for patients with NSCLC with EGFR exon 20 insertion mutations, a Notice of Compliance with conditions in March 2022, pending the final study results of CHRYSALIS.15 Furthermore, the product monograph for amivantamab stated that the “clinical effectiveness of Rybrevant is based on ORR and duration of response from a single-arm trial in patients with activating EGFR exon 20 insertion mutations.”2 Given that PFS and OS for amivantamab have not been established in this patient population and that there is no robust evidence to confirm that response measures are a prognostic marker of PFS or OS, the sponsor’s PSM (which incorporated PFS and OS data based on the progression-free and postprogression health states) was not appropriate or supported by the available evidence. A model based on response rates may have been more appropriate based on the available data, though the output of such a model would still be constrained by the quality of the data used to inform it, which may have similar limitations to those described in the appraisal of the clinical evidence.
- CADTH could not address this limitation due to the submitted model structure.
- Safety outcomes were based on a naive comparison. The sponsor included grade 3 or 4 AEs occurring in 5% of more of patients in cohort D of CHRYSALIS along with grade 1 to 2 IRRs.4 However, because safety was not captured in the RWE study, product monographs for individual treatments were used to inform safety data, which were compared directly to AE rates in CHRYSALIS. This represents an uncontrolled, naive comparison of safety, because rates of AEs were not included in the adjusted treatment comparison. It is uncertain whether the metric used to define AEs (e.g., treatment-emergent or treatment-related) would be similar in all product monographs or whether additional experience gained with these treatments since publication of the product monograph might result in some mitigation of AE rates observed at the time of product approval.
- As part of a scenario analysis, CADTH set rates of AEs for all comparators equal to amivantamab.
One additional limitation was identified but was not considered to be a key limitation. The sponsor did not include the cost of preinfusion medications in its analysis despite the stipulation in the product monograph that these medications must be administered between 15 and 60 minutes before amivantamab.2 These medications, which consist of diphenhydramine, acetaminophen/paracetamol, and dexamethasone/methylprednisolone, would represent additional drug acquisition costs that are only incurred for patients on amivantamab; however, the cost of these drugs is minimal and unlikely to affect the base case.16
Additionally, the following key assumptions were made by the sponsor and have been appraised by CADTH (refer to Table 4).
Table 4
Key Assumptions of the Submitted Economic Evaluation (Not Noted as Limitations to the Submission).
CADTH Reanalyses of the Economic Evaluation
Base-Case Results
Due to the myriad of limitations outlined previously involving both the direct and indirect clinical evidence, CADTH was unable to derive a base case.
CADTH undertook a correction to the sponsor’s model to address a dosing error, and an exploratory analysis involving 1 change to the sponsor’s OS assumptions for amivantamab (refer to Table 5).
Table 5
CADTH Revisions to the Submitted Economic Evaluation.
The results of the CADTH exploratory analysis suggested that amivantamab is associated with higher costs and QALYs than NPBC, the next comparator on the frontier (Table 6). The ICER for amivantamab compared to NPBC was $253,131, indicating that amivantamab is not cost-effective at a $50,000 WTP threshold, and had a 0% probability of being cost-effective at this threshold. Results are driven by the high drug acquisition and administration cost of amivantamab. Full results are available in Appendix 4.
Table 6
Summary of the CADTH Exploratory Analysis.
Scenario Analysis Results
CADTH undertook price reduction analyses based on the sponsor’s results and the CADTH exploratory analysis. The CADTH exploratory analysis suggested that a price reduction of 77% would be required to achieve cost-effectiveness of amivantamab relative to NPBC at a $50,000 per QALY threshold (Table 7).
Table 7
CADTH Price Reduction Analyses.
CADTH undertook 1 scenario analysis involving setting adverse event rates equal to amivantamab for all comparators. This scenario, detailed in Table 15, Appendix 4, resulted in similar results as the CADTH exploratory analysis. In addition, in light of the uncertainty about the magnitude of clinical benefit, CADTH performed a cost comparison analysis in which drug acquisition costs only were considered. Drug costs from the CADTH cost table were multiplied by the median PFS from the CHRYSALIS trial, 8.3 months, and compared to the least costly option within a given drug class.1 The sponsor’s base case assumptions about the proportion of patients weighing less than 80 kg were not modified. Results of this analysis are available in Table 15, Appendix 4. This analysis suggests that a price reduction of 86.4% would be required to achieve cost parity with docetaxel, the least costly comparator in this disease space.
Issues for Consideration
- CHRYSALIS was a phase I/Ib study that included a dose-escalation component in which amivantamab was trialled at doses ranging from 350 mg to 1,750 mg.1 The experts noted that, based on the findings of the trial, once patients received 700 mg or greater, the efficacy and safety results tended to plateau, suggesting that 700 mg may be sufficient for disease management. This has implications for the cost of amivantamab, which is priced per 350 mg vial — that is, the cost may be lower if patients receive a lower dose than recommended.
- Drug plan input highlighted the additional resource use required with amivantamab due to its split first dose and escalating infusion-rate schedule. Additional labour will also be required for drug preparation, and premedications are required to prevent IRRs. This may put a strain on resources (e.g., staff, pharmacists, overhead) in some settings.
Overall Conclusions
The CADTH Clinical Review highlighted the high degree of uncertainty associated with the results of CHRYSALIS. The single-arm, open-label, nonrandomized, phase I/Ib design of the CHRYSALIS trial makes interpreting the efficacy and safety events attributable to amivantamab challenging, because all patients received the same treatment. While ORR may be directly attributable to the drug’s antitumour activity, the ability to interpret PFS and OS events is significantly limited. The extent to which the observed survival is due to the natural history of the tumour or the intervention remains unclear. The CADTH assessment of the sponsor-submitted adjusted treatment comparison identified several key limitations, including small sample sizes, heterogeneity across study designs and pooled populations, and the inability to adjust for all potential confounders and prognostic variables, all of which significantly limited the ability to interpret the relative treatments effects observed between amivantamab and other treatments. Overall, it was noted that the phase I nature of the CHRYSALIS trial limits the ability to make firm conclusions about comparative efficacy given the short duration of follow-up, which resulted in immature data, and the small sample size of the CHRYSALIS trial and comparator cohorts.
Therefore, there exists a high degree of unresolved uncertainty with the clinical data, preventing CADTH from deriving an economic base case. In addition to the clinical uncertainty, the sponsor’s PSM model structure was not appropriate given that PFS and OS have not been established in this patient population, and there is no robust evidence to confirm that response measures (such as ORR) are a prognostic marker of PFS or OS.
CADTH performed an exploratory analysis in which a Weibull parametric extrapolation was used for the OS data for amivantamab. Based on CADTH sequential reanalysis, amivantamab is associated with an ICER of $253,131 per QALY compared to NPBC, with a 0% probability of being cost-effective at a WTP threshold of $50,000 per QALY. This estimate is based on the sponsor’s clinical assumptions, which predict an additional 0.79 LYs and 0.60 QALYs for those receiving amivantamab, compared to NPBC. A price reduction of at least 77% would be required for amivantamab to be considered cost-effective compared to NPBC at this threshold.
If the comparative clinical data — particularly the PFS and OS data — are considered sufficiently robust, then the exploratory analysis performed by CADTH may be informative. However, in light of the available clinical information, CADTH’s results are based on assumptions — for example, survival extrapolations that result in OS benefits for amivantamab in both the preprogression and postprogression states [(79 LYs gained), which cannot be validated at this time — and hence are associated with a large amount of uncertainty. As such, additional price reductions may be required to ensure the cost-effectiveness of amivantamab.
In light of the uncertainty with the available evidence, CADTH performed a scenario cost comparison analysis in which only drug acquisition costs were included, based on costs from the CADTH cost table and a median duration of treatment of 8.3 months. This analysis assumes equal efficacy and duration of treatment for all comparators. Results of this analysis suggest that amivantamab would require a price reduction of 86.4% to achieve cost parity with the least costly comparator, docetaxel. If amivantamab is deemed to be no more efficacious than other currently reimbursed alternatives, this price reduction will be required to ensure that costs to the health care system do not increase.
Abbreviations
- AE
adverse event
- EGFR
epidermal growth factor receptor
- ICER
incremental cost-effectiveness ratio
- IO
immunotherapy
- IRR
infusion-related reaction
- KM
Kaplan-Meier
- LY
life-year
- NPBC
non–platinum-based chemotherapy
- NSCLC
non–small cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PFS
progression-free survival
- PSM
partitioned survival model
- QALY
quality-adjusted life-year
- RWE
real-world evidence
- TKI
tyrosine kinase inhibitor
- WTP
willingness to pay
Appendix 1. Cost Comparison Table
Note that this appendix has not been copy-edited.
The comparators presented in the following table have been deemed to be appropriate based on feedback from clinical experts. Comparators may be recommended (appropriate) practice or actual practice. Existing Product Listing Agreements are not reflected in the table and as such, the table may not represent the actual costs to public drug plans.
Table 8
CADTH Cost Comparison Table for Locally Advanced or Metastatic NSCLC.
Appendix 2. Submission Quality
Note that this appendix has not been copy-edited.
Table 9
Submission Quality.
Appendix 3. Additional Information on the Submitted Economic Evaluation
Note that this appendix has not been copy-edited.
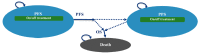
Figure 1
Model Structure.

Figure 2
PFS, OS, and TTD Kaplan-Meier Curves for Amivantamab (CHRYSALIS; n = 81).
Table 10
Predicted Survival and Reference Case Parametric Fittings for Amivantamab and Comparators.
Appendix 4. Additional Details on the CADTH Reanalyses and Sensitivity Analyses of the Economic Evaluation
Note that this appendix has not been copy-edited.
Detailed Results of CADTH Exploratory Analysis
Table 13
Disaggregated Summary of the CADTH Exploratory Analysis.
Table 14
Probabilistic Cost-Effectiveness Sequential Analysis From CADTH Exploratory Analysis.
Scenario Analyses
Table 15
Summary of Scenario Analyses Conducted on CADTH Exploratory Analysis.
Appendix 5. Submitted BIA and CADTH Appraisal
Note that this appendix has not been copy-edited.
Table 16
Summary of Key Takeaways.
Summary of Sponsor’s BIA
The submitted budget impact analysis (BIA) assessed the introduction of amivantamab for the treatment of post-platinum patients with EGFR Exon20ins mutated NSCLC. The analysis was taken from the perspective of the Canadian public drug plans using an epidemiology-based approach, with drug acquisition and administration costs included in the base case. A 3-year time horizon was used, from 2024 to 2026, with 2023 as a base year. The population size was estimated starting with incident lung cancer cases followed by a series of attritions. Key inputs to the BIA are documented in Table 17.
The reference case scenario included EGFR TKIs, IO drugs, and NPBC. The market share estimates were derived from Canadian chart review and a published conference proceeding.33 The new drug scenario included the same comparators along with amivantamab.
Table 17
Summary of Key Model Parameters.
Summary of the Sponsor’s BIA Results
The estimated budget impact of funding amivantamab for the treatment of post-platinum patients with EGFR Exon 20ins mutated NSCLC was $1,757,694 in year 1, $2,118,425 in year 2, and $1,963,051 in year 3, for a 3-year total budget impact of $5,839,170.
CADTH Appraisal of the Sponsor’s BIA
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the results of the BIA:
- The market share of amivantamab was underestimated in later years. The sponsor assumed market shares for amivantamab of 45%, 55%, and 50% in years 1, 2, and 3 of the BIA, respectively. These market share estimates do not meet face validity as they assume that the market share of amivantamab decreases in year 3. This implies that market share for amivantamab is being captured by an already existing product, which is unreasonable. Furthermore, the market shares themselves were felt to be underestimated by clinical experts, who expected the shift toward amivantamab to be more dramatic given the toxicities associated with TKIs and standard chemotherapy.
- As part of the base case, CADTH assumed market shares for amivantamab of 45%, 65%, and 70% in years 1, 2, and 3, respectively. The extra market share was assumed to come from TKIs, whose share was noted by the experts noted to be higher than expected in both the reference and new drug scenarios.
- Uncertainty regarding the final population size. The sponsor derived the population for the BIA using an epidemiology-based approach with a series of attritions, eventually resulting in about ██ incident patients per year that would potentially be eligible for amivantamab. This number was considered low by the clinical experts consulted by CADTH, and also considerably lower than the estimated annual incidence of 200 to 1000 patients provided by the clinician input from the Lung Cancer Canada Medical Advisory Committee. The experts considered an annual incidence of 100 patients to be more appropriate.
- As part of a scenario analysis, CADTH increased the population size to 100 incident cases per year.
- Subsequent therapy was not considered. The sponsor did not consider subsequent therapy in the BIA, despite including assumptions regarding subsequent therapy in their pharmacoeconomic model. This was an issue according to the clinical experts, who noted that patients would likely receive either a TKI, IO drug, or NPBC after progressing on amivantamab. The experts specifically stated that rates of chemotherapy usage would not be expected to change following administration of amivantamab. That is, amivantamab is expected to be used in addition to all currently approved therapies, meaning all costs associated with the drug would represent additional, incremental costs to the health care system.
- As part of a scenario analysis, CADTH considered only the drug and administration costs for amivantamab.
CADTH Reanalyses of the BIA
Based on the identified limitations, CADTH’s base case included 1 correction to docetaxel dosing and 1 change to the market shares of amivantamab in the new drug scenario.
Table 18
CADTH Revisions to the Submitted BIA.
The results of the CADTH step-wise reanalysis are presented in summary format in Table 19 and a more detailed breakdown is presented in Table 20. Based on the CADTH base case, the estimated budget impact of the reimbursement of amivantamab for the treatment of post-platinum patients with EGFR Exon20ins mutated NSCLC is expected to be $1,762,759 in year 1, $2,521,485 in year 2, and $2,782,311 in year 3, for a 3-year total of $7,066,555.
Scenario analyses conducted by CADTH indicate that the BIA results are sensitive to the population size, with the scenario assuming 100 incident patients per year resulting in a 3-year budget impact of $15,919,831. Results of the analysis in which only amivantamab costs are considered resulted in a 3-year budget impact of $8,403,136. Thus, the sponsor’s base case results may have underestimated the true budget impact.
Table 19
Summary of the CADTH Reanalyses of the BIA.
Table 20
Detailed Breakdown of the CADTH Reanalyses of the BIA.
References
- 1.
- Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol. 2021;39(30):3391-3402. [PMC free article: PMC8791812] [PubMed: 34339292]
- 2.
- RybrevantÒ (amivantamab for injection): 50 mg/mL concentrate for solution for infusion [product monograph]. Toronto (ON): Janssen Inc.; 2022 Mar 30.
- 3.
- Pharmacoeconomic evaluation [internal sponsor's report]. In: Drug Reimbursement Review sponsor submission: RYBREVANT® (amivantamab) 50 mg/mL concentrate for solution for infusion. Toronto (ON): Janssen Inc.; 2022 Apr 21.
- 4.
- Interim Clinical Study Report for Subjects With EGFR Exon 20 Insertion Mutations Treated With Amivantamab Monotherapy (30 March 2021Cutoff): 61186372EDI1001. A Phase 1, First-in-Human, Open-Label, Dose Escalation Study of JNJ-61186372, a Human Bispecific EGFR and cMet Antibody, in Subjects with Advanced Non-Small Cell Lung Cancer [internal sponsor's report]. Raritan (NJ): Janssen Research & Development, LLC; 2021 Oct 21.
- 5.
- Amivantamab for the treatment of adult patients with advanced non-small cell lung cancer with activating epidermal growth factor exon 20 insertion mutations, after failure of platinum-based therapy: adjusted treatment comparison versus standard of care from real-world evidence sources [internal sponsor's report]. In: Drug Reimbursement Review sponsor submission: RYBREVANT® (amivantamab) 50 mg/mL concentrate for solution for infusion. Toronto (ON): Janssen Inc.; 2022 Apr 21.
- 6.
- Labbé C, Leung Y, Lemes J, et al. Real-world EQ5D Health utility scores for patients with metastatic lung cancer by molecular alteration and response to therapy. Clin Lung Cancer. 2016;18(4):388-395. [PubMed: 28111120]
- 7.
- Tam VC, Ko YJ, Mittmann N, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20(2):e90-e106. [PMC free article: PMC3615875] [PubMed: 23559890]
- 8.
- Schedule of benefits for physician services under the Health Insurance Act: effective October 1, 2021. Toronto (ON): Ontario Ministry of Health; 2022: https://www
.health.gov .on.ca/en/pro/programs /ohip/sob/physserv/sob_master.pdf. Accessed 2022 Jun 10. - 9.
- Schedule of benefits for laboratory services: effective July 1, 2020. Toronto (ON): Ontario Ministry of Health; 2020: https://www
.health.gov .on.ca/en/pro/programs /ohip/sob/lab/lab_mn2020.pdf. Accessed 2022 Jun 10. - 10.
- Lathia N, Mittmann N, DeAngelis C, et al. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116(3):742-748. [PubMed: 20029970]
- 11.
- Ontario Case Costing Initiative (OCCI). Toronto (ON): Ontario Health and Long-Term Care; 2017: https://data
.ontario .ca/dataset/ontario-case-costing-initiative-occi. Accessed 2022 Jun 10. - 12.
- Park K, Bennouna J, Boyer M, et al. Sequencing of therapy following first-line afatinib in patients with EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2019;132:126-131. [PubMed: 31097085]
- 13.
- De Oliveira C, Pataky R, Bremner KE, et al. Estimating the Cost of Cancer Care in British Columbia and Ontario: A Canadian Inter-Provincial Comparison. Healthc Policy. 2017;12(3):95-108. [PMC free article: PMC5344366] [PubMed: 28277207]
- 14.
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095-2103. [PubMed: 10811675]
- 15.
- Health Canada. Notice of compliance (NOC) for Rybrevant. 2022; https:
//health-products .canada.ca/noc-ac/index-eng.jsp. Accessed 2022 Jun 15. - 16.
- Ontario Ministry of Health, Ontario Ministry of Long-Term Care. Ontario drug benefit formulary/comparative drug index. 2022; https://www
.formulary .health.gov.on.ca/formulary/. Accessed 2022 Jun 10. - 17.
- U.S. Food & Drug Administration (FDA). FDA grants accelerated approval to mobocertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations. 2021; https://www
.fda.gov/drugs /resources-information-approved-drugs /fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20. Accessed 2022 Jun 23. - 18.
- Spectrum Pharmaceuticals. Press release: Spectrum Pharmaceuticals announces acceptance of new drug application filing for poziotinib. 2022; https://investor
.sppirx .com/node/23976/pdf. Accessed 2022 Jun 23. - 19.
- DeltaPA. Ottawa (ON): IQVIA; 2022: https://www
.iqvia.com/. Accessed 2022 Jun 10. - 20.
- OPDIVO® (nivolumab for injection): 10 mg nivolumab /mL, 40 mg and 100 mg single-use vials for intravenous infusion [product monograph]. Montreal (QC): Bristol-Myers Squibb Canada Co.; 2022 Feb 28: https://pdf
.hres.ca/dpd_pm/00065021.PDF. Accessed 2022 Jun 10. - 21.
- JAMP Gefitinib (gefitinib): 250 mg oral tablet [product monograph]. Boucherville (QC): JAMP Pharma Corporation; 2022 Mar 11: https://pdf
.hres.ca/dpd_pm/00065057.PDF. Accessed 2022 Jun 10. - 22.
- Apo-erlotinib (erlotinib hydrochloride): 25 mg, 100 mg, 150 mg tablets [product monograph]. Toronto (ON): Apotex Inc.; 2018 Dec 5: https://pdf
.hres.ca/dpd_pm/00048641.PDF. Accessed 2022 Jun 10. - 23.
- TAGRISSO® (osimertinib as osimertinib mesylate): 40 mg and 80 mg oral tablets [product monograph]. Mississauga (ON): AstraZeneca Canada inc.; 2022 Mar 01: https://pdf
.hres.ca/dpd_pm/00064905.PDF. Accessed 2022 Jun 10. - 24.
- Taro-Docetaxel (docetaxel for injection): 20 mg / mL, 80 mg / 4 mL, 160 mg / 8 mL single vial formulation, concentrated solution for intravenous infusion [product monograph]. Brampton (ON): Taro Pharmaceuticals Inc.; 2021 Aug 10: https://pdf
.hres.ca/dpd_pm/00063672.PDF. Accessed 2022 Jun 10. - 25.
- Giotrif (afatinib as afatinib dimaleate): 20, 30 and 40 mg tablets [product monograph]. Burlington (ON): Boehringer Ingelheim (Canada) Ltd.; 2019 Jun 6: https://www
.boehringer-ingelheim .ca/sites /ca/files/documents/giotrifpmen.pdf. Accessed 2022 Jun 10. - 26.
- Pemetrexed for injection (pemetrexed as pemetrexed disodium): 100 mg or 500 mg per vial, lyophilized powder [product monograph]. Mississauga (ON): Dr. Reddy’s Laboratories Canada Inc.; 2022 May 03: https://pdf
.hres.ca/dpd_pm/00065681.PDF. Accessed 2022 Jun 10. - 27.
- TECENTRIQ® (atezolizumab): 60 mg/mL, 1200 mg/20 mL and 840 mg/14 mL singal use vials, concentrate for solution for infusion [product monograph]. Mississauga (ON): Hoffman-La Roche Ltd.; 2022 Mar 29: https://pdf
.hres.ca/dpd_pm/00065218.PDF. Accessed 2022 Jun 10. - 28.
- KEYTRUDA® (pembrolizumab): 100 mg/4 mL vial, solution for infusion [product monograph]. Kirkland (QC): Merck Canada Inc.; 2022 May 04: https://pdf
.hres.ca/dpd_pm/00065684.PDF. Accessed 2022 Jun 10. - 29.
- Gemcitabine injection (as gemcitabine hydrochloride): 40 mg gemcitabine per mL, 200 mg/5 mL, 1 g/25 mL, 2 g/50 mL concentrate sterile solution for injection [product monograph]. Boucherville (QC): Sandoz Canada Inc.; 2014 Aug 14: https://pdf
.hres.ca/dpd_pm/00026822.PDF. Accessed 2022 Jun 10. - 30.
- VIZIMPRO™ (dacomitinib as dacomitinib monohydrate): 15mg, 30 mg, and 45 mg, oral tablets [product monograph]. Kirkland (QC): Pfizer Canada ULC; 2021 Jul 29: https://pdf
.hres.ca/dpd_pm/00062308.PDF. Accessed 2022 Jun 10. - 31.
- Drug Reimbursement Review clinical guidance and pharmacoeconomic reports: nivolumab (Opdivo) for esophageal or gastroesophageal junction cancer. Can J Health Technol. 2022;2(3). https://www
.cadth.ca /sites/default/files /DRR/2022/PC0253-Opdivo_combined.pdf. Accessed 2022 Jun 10. - 32.
- Drug Reimbursement Review clinical guidance and pharmacoeconomic reports: pembrolizumab (Keytruda) for metastatic microsatellite instability-high/mismatch repairdeficient colorectal cancer. Can J Health Technol. 2021;1(9). https://www
.cadth.ca /sites/default/files /DRR/2021/PC0235-Keytruda-Combined.pdf. Accessed 2022 Jun 10. - 33.
- Boyne DJ, Jarada TN, Yussuf A, et al. 51P: Testing patterns and outcomes of different egfr-positive metastatic non-small cell lung cancer patients in a Canadian real-world setting. Poster presented at: 2022 European Lung Cancer Congress (ELCC). Prague (CZ); 2022 Mar 30 - Apr 02.
- 34.
- Table: 17-10-0057-01 Projected population, by projection scenario, age and sex, as of July 1 (x 1,000). Ottawa (ON): Statistics Canada; 2022: https://www150
.statcan .gc.ca/t1/tbl1/en/tv .action?pid=1710005701. Accessed 2022 Mar 07. - 35.
- Brenner DR, Weir HK, Demers AA, et al. Appendix 4. In: Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192(9):E199-E205. [PMC free article: PMC7055947] [PubMed: 32122974]
- 36.
- Canadian Cancer Statistics Advisory Committee. Canadian cancer statistics: a 2020 special report on lung cancer. Toronto (ON): Canadian Cancer Society; 2020: www
.cancer.ca/Canadian-Cancer-Statistics-2020-EN. Accessed 2022 Jun 10. - 37.
- Bauml JM, Viteri S, Minchom A, et al. FP07.12 Underdiagnosis of EGFR Exon 20 Insertion Mutation Variants: Estimates from NGS-based Real-World Datasets. J Thorac Oncol. 2021;16(3 Suppl):S208-S209.
- Executive Summary
- Stakeholder Input Relevant to the Economic Review
- Economic Review
- Abbreviations
- Cost Comparison Table
- Submission Quality
- Additional Information on the Submitted Economic Evaluation
- Additional Details on the CADTH Reanalyses and Sensitivity Analyses of the Economic Evaluation
- Submitted BIA and CADTH Appraisal
- References
- Pharmacoeconomic Review - Amivantamab (Rybrevant)Pharmacoeconomic Review - Amivantamab (Rybrevant)
- Bacillus sonorensis strain N11B3I contig_0000018, whole genome shotgun sequenceBacillus sonorensis strain N11B3I contig_0000018, whole genome shotgun sequencegi|2410320279|ref|NZ_JALAMX01000001 gnl|WGS:NZ_JALAMX01|contig_0000018Nucleotide
- Excluded Studies - Clinical Review Report: Halobetasol Propionate and Tazarotene...Excluded Studies - Clinical Review Report: Halobetasol Propionate and Tazarotene (Duobrii)
- Submitted Budget Impact Analysis and CADTH Appraisal - Clinical and Economic Rev...Submitted Budget Impact Analysis and CADTH Appraisal - Clinical and Economic Review Report: Vedolizumab (ENTYVIO SC)
- Detailed Outcome Data - Clinical Review Report: Slexipag (Uptravi)Detailed Outcome Data - Clinical Review Report: Slexipag (Uptravi)
Your browsing activity is empty.
Activity recording is turned off.
See more...