NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Show detailsThis PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of bile duct cancers. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
General Information About Bile Duct Cancer
Cancer of the bile duct (also called cholangiocarcinoma) is extremely rare. The true incidence of bile duct cancer is unknown because establishing an accurate diagnosis is difficult.
Traditionally, bile duct tumors located within the liver were classified with hepatocellular carcinoma as primary liver tumors.[1] In contrast, bile duct tumors located outside of the liver were classified with gallbladder cancer as extrahepatic biliary tract tumors.[1] The classification of bile duct tumors has changed to include intrahepatic tumors of the bile ducts and extrahepatic tumors (perihilar and distal) of the bile ducts.
Approximately 50% of cholangiocarcinomas arise in the bile ducts of the perihilar region, 40% in the distal region, and 10% in the intrahepatic region.
Many bile duct cancers are multifocal. In most patients, the tumor cannot be completely removed by surgery and is incurable. Palliative measures such as resection, radiation therapy (e.g., brachytherapy or external-beam radiation therapy), or stenting procedures may maintain adequate biliary drainage and allow for improved quality of life.
Anatomy
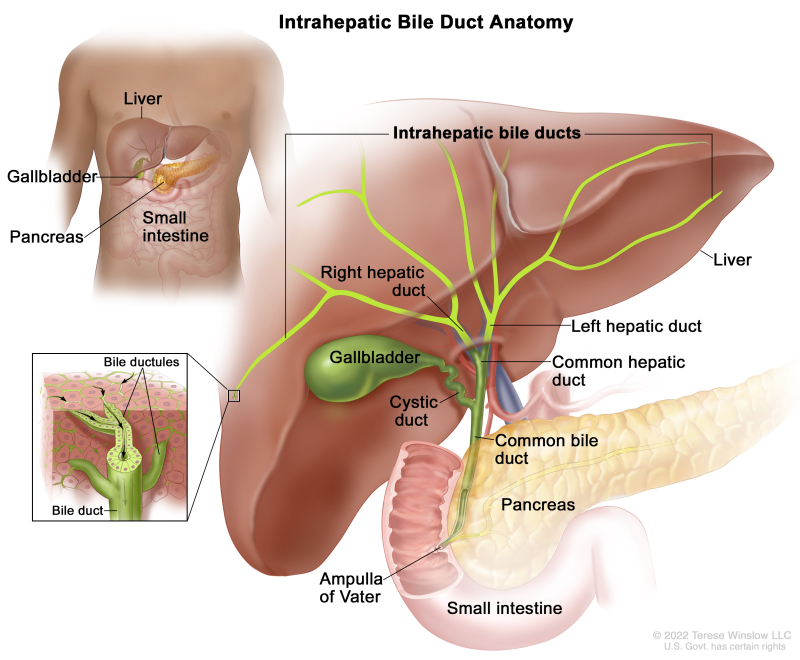
The biliary system consists of a network of ducts that carry bile from the liver to the small bowel and is classified by its anatomical location (Figure 1). Bile is produced by the liver and is important for fat digestion.
Intrahepatic bile duct
The bile ducts located within the liver are called intrahepatic bile ducts. Tumors of the intrahepatic bile ducts originate in small intrahepatic ductules or large intrahepatic ducts that are proximal to the bifurcation of the right and left hepatic ducts. These tumors are also known as intrahepatic cholangiocarcinomas.
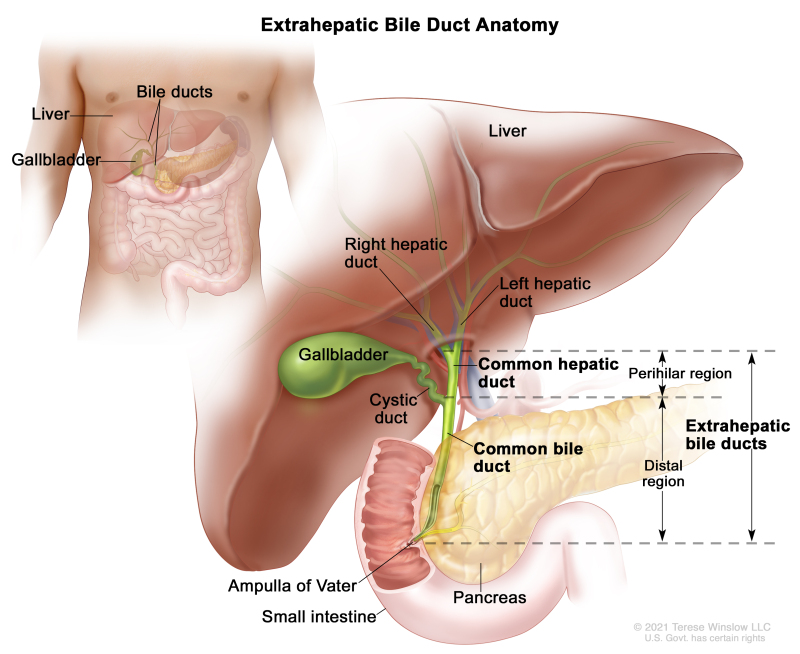
Extrahepatic bile duct
The bile ducts located outside of the liver are called extrahepatic bile ducts. They include part of the right and left hepatic ducts that are outside of the liver, the common hepatic duct, and the common bile duct. The extrahepatic bile ducts can be further divided into the perihilar (hilum) region and distal region.
- Perihilar (hilum) region. The hilum is the region where the right and left hepatic ducts exit the liver and join to form the common hepatic duct that is proximal to the origin of the cystic duct. Tumors of this region are also known as perihilar cholangiocarcinomas or Klatskin tumors.
- Distal region. This region includes the common bile duct and inserts into the small intestine. Tumors of this region are also known as extrahepatic cholangiocarcinomas (Figure 2).
Risk Factors
Bile duct cancer may occur more frequently in patients with a history of primary sclerosing cholangitis, chronic ulcerative colitis, choledochal cysts, or infections with the liver fluke Clonorchis sinensis.[2]
Clinical Features
Distal and perihilar bile duct cancers frequently cause biliary tract obstruction, leading to the following symptoms:
- Jaundice.
- Weight loss.
- Abdominal pain.
- Fever.
- Pruritus.
Intrahepatic bile duct cancer may be relatively indolent and difficult to differentiate clinically from metastatic adenocarcinoma deposits in the liver.
Diagnostic and Staging Evaluation
Clinical evaluation is dependent on laboratory and radiographic imaging tests that include the following:
- Liver function tests and other laboratory studies.
- Abdominal ultrasonography.
- Computed tomography.
- Magnetic resonance imaging.
- Magnetic resonance cholangiopancreatography.
These tests demonstrate the extent of the primary tumor and help determine the presence or absence of distant metastases.
If a patient is medically fit for surgery and the tumor is amenable to surgical resection, surgical exploration is performed. Pathological examination of the resected specimen is done to establish definitive pathological staging.
Prognosis
Prognosis depends in part on the tumor’s anatomical location, which affects resectability. Because of proximity to major blood vessels and diffuse extension within the liver, a bile duct tumor can be difficult to resect. Total resection is possible in 25% to 30% of lesions that originate in the distal bile duct; the resectability rate is lower for lesions that occur in more proximal sites.[3]
Complete resection with negative surgical margins offers the only chance of cure for bile duct cancer. For localized, resectable extrahepatic and intrahepatic tumors, the presence of involved lymph nodes and perineural invasion are significant adverse prognostic factors.[4-6]
Additionally, among patients with intrahepatic cholangiocarcinomas, worse outcomes have been associated with the following:[7-9]
- A personal history of primary sclerosing cholangitis.
- Elevated cancer antigen 19-9 level.
- Periductal infiltrating tumor growth pattern.
- Presence of hepatic venous invasion.
References
- Siegel R, Ma J, Zou Z, et al.: Cancer statistics, 2014. CA Cancer J Clin 64 (1): 9-29, 2014 Jan-Feb. [PubMed: 24399786]
- de Groen PC, Gores GJ, LaRusso NF, et al.: Biliary tract cancers. N Engl J Med 341 (18): 1368-78, 1999. [PubMed: 10536130]
- Stain SC, Baer HU, Dennison AR, et al.: Current management of hilar cholangiocarcinoma. Surg Gynecol Obstet 175 (6): 579-88, 1992. [PubMed: 1280374]
- Wakai T, Shirai Y, Moroda T, et al.: Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer 103 (6): 1210-6, 2005. [PubMed: 15685618]
- Klempnauer J, Ridder GJ, von Wasielewski R, et al.: Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol 15 (3): 947-54, 1997. [PubMed: 9060532]
- Bhuiya MR, Nimura Y, Kamiya J, et al.: Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg 215 (4): 344-9, 1992. [PMC free article: PMC1242450] [PubMed: 1558415]
- Rosen CB, Nagorney DM, Wiesner RH, et al.: Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg 213 (1): 21-5, 1991. [PMC free article: PMC1358305] [PubMed: 1845927]
- Shirabe K, Mano Y, Taketomi A, et al.: Clinicopathological prognostic factors after hepatectomy for patients with mass-forming type intrahepatic cholangiocarcinoma: relevance of the lymphatic invasion index. Ann Surg Oncol 17 (7): 1816-22, 2010. [PubMed: 20135355]
- Isa T, Kusano T, Shimoji H, et al.: Predictive factors for long-term survival in patients with intrahepatic cholangiocarcinoma. Am J Surg 181 (6): 507-11, 2001. [PubMed: 11513774]
Cellular Classification of Bile Duct Cancer
Intrahepatic Bile Duct Cancer
The most common histopathological types of intrahepatic bile duct tumor include the following:[1]
- Intrahepatic cholangiocarcinoma.
- Biliary intraepithelial neoplasia, grade 3 (high-grade dysplasia).
- Combined hepatocellular-cholangiocarcinoma.
- Carcinosarcoma.
- Intraductal papillary neoplasm with an associated invasive carcinoma.
- Mucinous cystic neoplasm with an associated invasive carcinoma.
- Neuroendocrine carcinoma.
- Large cell neuroendocrine carcinoma.
- Small cell neuroendocrine carcinoma.
- Intraductal papillary neoplasm with high-grade dysplasia.
Perihilar Bile Duct Cancer
Adenocarcinomas are the most common type of perihilar bile duct tumor. The histological types of perihilar bile duct cancer include the following:[2]
- Carcinoma in situ.
- Biliary intraepithelial neoplasia, high grade.
- Intraductal papillary neoplasm with high-grade dysplasia.
- Mucinous cystic neoplasm with high-grade intraepithelial neoplasia.
- Adenocarcinoma.
- Adenocarcinoma, biliary type.
- Adenocarcinoma, gastric foveolar type.
- Adenocarcinoma, intestinal type.
- Clear cell adenocarcinoma.
- Mucinous carcinoma.
- Signet-ring cell carcinoma.
- Squamous cell carcinoma.
- Adenosquamous carcinoma.
- Undifferentiated carcinoma.
- High-grade neuroendocrine carcinoma.
- Small cell neuroendocrine carcinoma.
- Intraductal papillary neoplasm with an associated invasive carcinoma.
- Mucinous cystic neoplasm with an associated invasive carcinoma.
Distal Bile Duct Cancer
Adenocarcinomas are the most common type of distal bile duct tumor. The histological types of distal bile duct cancer include the following:[3]
- Carcinoma in situ.
- Biliary intraepithelial neoplasia, high grade.
- Intraductal papillary neoplasm with high-grade intraepithelial neoplasia.
- Mucinous cystic neoplasm with high-grade intraepithelial neoplasia.
- Adenocarcinoma.
- Adenocarcinoma, biliary type.
- Adenocarcinoma, intestinal type.
- Adenocarcinoma, gastric foveolar type.
- Mucinous adenocarcinoma.
- Clear cell adenocarcinoma.
- Signet-ring cell carcinoma.
- Squamous cell carcinoma.
- Adenosquamous carcinoma.
- Undifferentiated carcinoma.
- High-grade neuroendocrine carcinoma.
- Small cell neuroendocrine carcinoma.
- Mixed adenoneuroendocrine carcinoma.
- Intraductal papillary neoplasm with an associated invasive carcinoma.
- Mucinous cystic neoplasm with an associated invasive carcinoma.
References
- Intrahepatic Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 295–302.
- Perihilar bile ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 311–16.
- Distal bile duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 317–25.
Stage Information for Bile Duct Cancer
Staging for Bile Duct Cancer
Bile duct cancer is classified as resectable (localized) or unresectable, with obvious prognostic importance. The TNM (tumor, node, metastasis) staging system is used for staging bile duct cancer, commonly after surgery and pathological examination of the resected specimen. Evaluation of the extent of disease at laparotomy is an important component of staging.
AJCC Staging System for Bile Duct Cancer
AJCC staging system for intrahepatic bile duct cancer
The AJCC has designated staging by TNM classification to define intrahepatic bile duct cancer.[1]
Tables 1, 2, 3, 4, and 5 pertain to the intrahepatic bile duct cancer stages.
Table 1. Definitions of TNM Stage 0 Intrahepatic Bile Duct Cancera
| Stage | TNM | Description |
|---|---|---|
| 0 | Tis, N0, M0 | Tis = Carcinoma in situ (intraductal tumor). |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Intrahepatic Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 295–302.
Table 2. Definitions of TNM Stage I Intrahepatic Bile Duct Cancera
| Stage | TNM | Description | |
|---|---|---|---|
| I | IA | T1a, N0, M0 | T1a = Solitary tumor ≤5 cm without vascular invasion. |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| IB | T1b, N0, M0 | T1b = Solitary tumor >5 cm without vascular invasion. | |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Intrahepatic Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 295–302.
Table 3. Definitions of TNM Stage II Intrahepatic Bile Duct Cancera
| Stage | TNM | Description |
|---|---|---|
| II | T2, N0, M0 | T2 = Solitary tumor with intrahepatic vascular invasion or multiple tumors, with or without vascular invasion. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Intrahepatic Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 295–302.
Table 4. Definitions of TNM Stage III Intrahepatic Bile Duct Cancera
| Stage | TNM | Description | |
|---|---|---|---|
| III | IIIA | T3, N0, M0 | T3 =Tumor perforating the visceral peritoneum. |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| IIIB | T4, N0, M0 | T4 = Tumor involving local extrahepatic structures by direct invasion. | |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| Any T, N1, M0 | TX = Primary tumor cannot be assessed. | ||
| T0 = No evidence of primary tumor. | |||
| Tis = Carcinoma in situ (intraductal tumor). | |||
| T1 = Solitary tumor without vascular invasion, ≤5 cm or >5 cm. | |||
| –T1a = Solitary tumor ≤5 cm without vascular invasion. | |||
| –T1b = Solitary tumor >5 cm without vascular invasion. | |||
| T2 = Solitary tumor with intrahepatic vascular invasion or multiple tumors, with or without vascular invasion. | |||
| T3 = Tumor perforating the visceral peritoneum. | |||
| T4 = Tumor involving local extrahepatic structures by direct invasion. | |||
| N1 = Regional lymph node metastasis present. | |||
| M0 = No distant metastasis. | |||
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Intrahepatic Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 295–302.
Table 5. Definitions of TNM Stage IV Intrahepatic Bile Duct Cancera
| Stage | TNM | Description |
|---|---|---|
| IV | Any T, Any N, M1 | TX = Primary tumor cannot be assessed. |
| T0 = No evidence of primary tumor. | ||
| Tis = Carcinoma in situ (intraductal tumor). | ||
| T1 = Solitary tumor without vascular invasion, ≤5 cm or >5 cm. | ||
| –T1a = Solitary tumor ≤5 cm without vascular invasion. | ||
| –T1b = Solitary tumor >5 cm without vascular invasion. | ||
| T2 = Solitary tumor with intrahepatic vascular invasion or multiple tumors, with or without vascular invasion. | ||
| T3 = Tumor perforating the visceral peritoneum. | ||
| T4 = Tumor involving local extrahepatic structures by direct invasion. | ||
| NX = Regional lymph nodes cannot be assessed. | ||
| N0 = No regional lymph node metastasis. | ||
| N1 = Regional lymph node metastasis present. | ||
| M1 = Distant metastasis present. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Intrahepatic Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 295–302.
AJCC staging system for perihilar bile duct cancer
The AJCC has designated staging by TNM classification to define perihilar bile duct cancer.[2]
Tables 6, 7, 8, 9, and 10 pertain to the perihilar bile duct cancer stages.
Table 6. Definitions of TNM Stage 0 Perihilar Bile Duct Cancera
| Stage | TNM | Description |
|---|---|---|
| 0 | Tis, N0, M0 | Tis = Carcinoma in situ/high-grade dysplasia. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Perihilar Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 311–6.
Table 7. Definitions of TNM Stage I Perihilar Bile Duct Cancera
| Stage | TNM | Description |
|---|---|---|
| I | T1, N0, M0 | T1 = Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Perihilar Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 311–6.
Table 8. Definitions of TNM Stage II Perihilar Bile Duct Cancera
| Stage | TNM | Description |
|---|---|---|
| II | T2a–b, N0, M0 | T2 = Tumor invades beyond the wall of the bile duct to surrounding adipose tissue, or tumor invades adjacent hepatic parenchyma. |
| –T2a = Tumor invades beyond the wall of the bile duct to surrounding adipose tissue. | ||
| –T2b = Tumor invades adjacent hepatic parenchyma. | ||
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Perihilar Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 311–6.
Table 9. Definitions of TNM Stage III Perihilar Bile Duct Cancera
| Stage | TNM | Description | |
|---|---|---|---|
| III | IIIA | T3, N0, M0 | T3 = Tumor invades unilateral branches of the portal vein or hepatic artery. |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| IIIB | T4, N0, M0 | T4 = Tumor invades the main portal vein or its branches bilaterally, or the common hepatic artery; or unilateral second-order biliary radicals with contralateral portal vein or hepatic artery involvement. | |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| IIIC | Any T, N1, M0 | TX = Primary tumor cannot be assessed. | |
| T0 = No evidence of primary tumor. | |||
| Tis = Carcinoma in situ/high-grade dysplasia. | |||
| T1 = Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue. | |||
| T2 = Tumor invades beyond the wall of the bile duct to surrounding adipose tissue, or tumor invades adjacent hepatic parenchyma. | |||
| –T2a = Tumor invades beyond the wall of the bile duct to surrounding adipose tissue. | |||
| –T2b = Tumor invades adjacent hepatic parenchyma. | |||
| T3 = Tumor invades unilateral branches of the portal vein or hepatic artery. | |||
| T4 = Tumor invades the main portal vein or its branches bilaterally, or the common hepatic artery; or unilateral second-order biliary radicals with contralateral portal vein or hepatic artery involvement. | |||
| N1 = One to three positive lymph nodes typically involving the hilar, cystic duct, common bile duct, hepatic artery, posterior pancreatoduodenal, and portal vein lymph nodes. | |||
| M0 = No distant metastasis. | |||
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Perihilar Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 311–6.
Table 10. Definitions of TNM Stage IV Perihilar Bile Duct Cancera
| Stage | TNM | Description | |
|---|---|---|---|
| IV | IVA | Any T, N2, M0 | TX = Primary tumor cannot be assessed. |
| T0 = No evidence of primary tumor. | |||
| Tis = Carcinoma in situ/high-grade dysplasia. | |||
| T1 = Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue. | |||
| T2 = Tumor invades beyond the wall of the bile duct to surrounding adipose tissue, or tumor invades adjacent hepatic parenchyma. | |||
| –T2a = Tumor invades beyond the wall of the bile duct to surround adipose tissue. | |||
| –T2b = Tumor invades adjacent hepatic parenchyma. | |||
| T3 = Tumor invades unilateral branches of the portal vein or hepatic artery. | |||
| T4 = Tumor invades the main portal vein or its branches bilaterally, or the common hepatic artery; or unilateral second-order biliary radicals with contralateral portal vein or hepatic artery involvement. | |||
| N2 = Four or more positive lymph nodes from the sites described for N1. | |||
| M0 = No distant metastasis. | |||
| IVB | Any T, Any N, M1 | TX = Primary tumor cannot be assessed. | |
| T0 = No evidence of primary tumor. | |||
| Tis = Carcinoma in situ/high-grade dysplasia. | |||
| T1 = Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue. | |||
| T2 = Tumor invades beyond the wall of the bile duct to surrounding adipose tissue, or tumor invades adjacent hepatic parenchyma. | |||
| –T2a = Tumor invades beyond the wall of the bile duct to surround adipose tissue. | |||
| –T2b = Tumor invades adjacent hepatic parenchyma. | |||
| T3 = Tumor invades unilateral branches of the portal vein or hepatic artery. | |||
| T4 = Tumor invades the main portal vein or its branches bilaterally, or the common hepatic artery; or unilateral second-order biliary radicals with contralateral portal vein or hepatic artery involvement. | |||
| NX = Regional lymph nodes cannot be assessed. | |||
| N0 = No regional lymph node metastasis. | |||
| N1 = One to three positive lymph nodes typically involving the hilar, cystic duct, common bile duct, hepatic artery, posterior pancreatoduodenal, and portal vein lymph nodes. | |||
| N2 = Four or more positive lymph nodes from the sites described for N1. | |||
| M1 = Distant metastasis. | |||
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Perihilar Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 311–6.
AJCC staging system for distal bile duct cancer
The AJCC has designated staging by TNM classification to define distal bile duct cancer.[3] Stages defined by TNM classification apply to all primary carcinomas arising in the distal bile duct or in the cystic duct; these stages do not apply to perihilar or intrahepatic cholangiocarcinomas, sarcomas, or carcinoid tumors.
Tables 11, 12, 13, 14, and 15 pertain to the distal bile duct cancer stages.
Table 11. Definitions of TNM Stage 0 Distal Bile Duct Cancera
| Stage | TNM | Definition |
|---|---|---|
| 0 | Tis, N0, M0 | Tis = Carcinoma in situ/high-grade dysplasia. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Distal Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 317–325.
Table 12. Definitions of TNM Stage I Distal Bile Duct Cancera
| Stage | TNM | Definition |
|---|---|---|
| I | T1, N0, M0 | T1 = Tumor invades the bile duct wall with a depth <5 mm. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Distal Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 317–325.
Table 13. Definitions of TNM II Distal Bile Duct Cancera
| Stage | TNM | Definition | |
|---|---|---|---|
| II | IIA | T1, N1, M0 | T1 = Tumor invades the bile duct wall with a depth <5 mm. |
| N1 = Metastasis in one to three regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T2, N0, M0 | Tumor invades the bile duct wall with a depth of 5–12 mm. | ||
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| IIB | T2, N1, M0 | T2 = Tumor invades the bile duct wall with a depth of 5–12 mm. | |
| N1 = Metastasis in one to three regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T3, N0, M0 | T3 = Tumor invades the bile duct wall with a depth >12 mm. | ||
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| T3, N1, M0 | T3 = Tumor invades the bile duct wall with a depth >12 mm. | ||
| N1 = Metastasis in one to three regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Distal Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 317–325.
Table 14. Definitions of TNM Stage III Distal Bile Duct Cancer
| Stage | TNM | Definition | |
|---|---|---|---|
| III | IIIA | T1, N2, M0 | T1 = Tumor invades the bile duct wall with a depth <5 mm. |
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T2, N2, M0 | T2 = Tumor invades the bile duct wall with a depth of 5–12 mm. | ||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T3, N2, M0 | T3 = Tumor invades the bile duct wall with a depth >12 mm. | ||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| IIIB | T4, N0, M0 | T4 = Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery. | |
| N0 = No regional lymph node metastasis. | |||
| M0 = No distant metastasis. | |||
| T4, N1, M0 | T4 = Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery. | ||
| N1 = Metastasis in one to three regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
| T4, N2, M0 | T4 = Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery. | ||
| N2 = Metastasis in four or more regional lymph nodes. | |||
| M0 = No distant metastasis. | |||
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Distal Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 317–325.
Table 15. Definitions of TNM Stage IV Distal Bile Duct Cancera
| Stage | TNM | Definition |
|---|---|---|
| IV | Any T, Any N, M1 | TX = Primary tumor cannot be assessed. |
| TIS = Carcinoma in situ/high-grade dysplasia. | ||
| T1 = Tumor invades the bile duct wall with a depth <5 mm. | ||
| T2 = Tumor invades the bile duct wall with a depth of 5–12 mm. | ||
| T3 = Tumor invades the bile duct wall with a depth >12 mm. | ||
| T4 = Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery. | ||
| NX = Regional lymph nodes cannot be assessed. | ||
| N0 = No regional lymph node metastasis. | ||
| N1 = Metastasis in one to three regional lymph nodes. | ||
| N2 = Metastasis in four or more regional lymph nodes. | ||
| M1 = Distant metastasis. |
T = primary tumor; N = regional lymph node; M = distant metastasis.
aReprinted with permission from AJCC: Distal Bile Duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 317–325.
References
- Intrahepatic Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 295–302.
- Perihilar Bile Ducts. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 311–6.
- Distal bile duct. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 317–25.
Treatment Option Overview for Bile Duct Cancer
The treatment of bile duct cancer depends primarily on whether the cancer can be completely removed by surgery.
Resectable (Localized) Bile Duct Cancer
Localized intrahepatic and extrahepatic bile duct cancer may be completely removed by surgery. These tumors represent a very small number of cases that are usually in the distal common bile duct. Among patients treated with surgical resection, long-term prognosis varies depending on primary tumor extent, margin status, lymph node involvement, and additional pathological features.[1,2]
Extended resections of hepatic duct bifurcation tumors (Klatskin tumors, also known as hilar tumors) to include adjacent liver, either by lobectomy or removal of portions of segments 4 and 5 of the liver, may be performed. If major hepatic resection is necessary to achieve a complete resection, postoperative hepatic reserve should be evaluated. For patients with underlying cirrhosis, the Child-Pugh class and the Model for End-Stage Liver Disease score are determined.
Unresectable (Including Metastatic and Recurrent) Bile Duct Cancer
Most cases of intrahepatic, distal, and perihilar bile duct cancer are unresectable and cannot be completely removed. Often the cancer invades directly into the portal vein, the adjacent liver, along the common bile duct, and to adjacent lymph nodes. Portal hypertension may result from invasion of the portal vein. Spread to distant parts of the body is uncommon, but intra-abdominal metastases, particularly peritoneal metastases, do occur. Transperitoneal and hematogenous hepatic metastases also occur with bile duct cancer of all sites. Moreover, most patients who undergo resection will develop recurrent disease within the hepatobiliary system or, less frequently, at distant sites.
In locally advanced disease, phase II trials have evaluated chemoradiotherapy with the goal of improved local control and potential downstaging for surgical resection.[3,4] These approaches have not been compared with standard therapy, and the curative potential is unknown.
For patients with unresectable bile duct cancer, management is directed at palliation.
Treatment options for bile duct cancer are described in Table 16.
Table 16. Treatment Options for Bile Duct Cancer
| Staging Criteria | Treatment Options |
|---|---|
| Resectable (Localized) Bile Duct Cancer | Surgery |
| Adjuvant therapy | |
| Unresectable (Including Metastatic and Recurrent) Bile Duct Cancer | Palliative therapy |
| Chemotherapy | |
| Immunotherapy | |
| Targeted therapy |
Capecitabine and Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[5,6] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[5-7] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[8-10] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[11] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[12]References
- Nagorney DM, Donohue JH, Farnell MB, et al.: Outcomes after curative resections of cholangiocarcinoma. Arch Surg 128 (8): 871-7; discussion 877-9, 1993. [PubMed: 8393652]
- Washburn WK, Lewis WD, Jenkins RL: Aggressive surgical resection for cholangiocarcinoma. Arch Surg 130 (3): 270-6, 1995. [PubMed: 7534059]
- Edeline J, Touchefeu Y, Guiu B, et al.: Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 6 (1): 51-59, 2020. [PMC free article: PMC6824230] [PubMed: 31670746]
- Cercek A, Boerner T, Tan BR, et al.: Assessment of Hepatic Arterial Infusion of Floxuridine in Combination With Systemic Gemcitabine and Oxaliplatin in Patients With Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 6 (1): 60-67, 2020. [PMC free article: PMC6824231] [PubMed: 31670750]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PMC free article: PMC8649021] [PubMed: 34506675]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PubMed: 27569869]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PubMed: 33410339]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PMC free article: PMC5760397] [PubMed: 29152729]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PubMed: 30348537]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PMC free article: PMC8742388] [PubMed: 34996412]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PMC free article: PMC10496767] [PubMed: 35668003]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PMC free article: PMC10414691] [PubMed: 36821823]
Treatment of Resectable (Localized) Bile Duct Cancer
Treatment Options for Resectable (Localized) Bile Duct Cancer
Treatment options for resectable (localized) bile duct cancer include the following:
Surgery
Intrahepatic bile duct cancer
For intrahepatic bile duct cancers, hepatic resection to achieve negative margins is potentially curative. If a major liver resection is necessary to achieve negative surgical margins, preoperative portal vein embolization may be considered to optimize the volume of the remnant liver.
Partial liver resection or partial hepatectomy to achieve negative margins is a procedure with curative intent for patients with intrahepatic cholangiocarcinoma.[1] The extent of liver resection necessary depends on the extent of hepatic parenchymal involvement and the proximity of the tumor to major blood vessels in this region.
The role of routine portal lymphadenectomy has not been well established because of the risk of common bile duct devascularization.
Perihilar bile duct cancer
For perihilar cholangiocarcinomas (Klatskin tumors), bile duct resection alone leads to high local recurrence rates resulting from the early confluence of the hepatic ducts and the caudate lobe. The addition of partial hepatectomy that includes the caudate lobe has improved long-term outcomes, but it may be associated with increased postoperative complications.[2] With this aggressive surgical approach, 5-year survival rates of 20% to 50% have been reported.[3] An understanding of both the normal and varied vascular and ductal anatomy of the porta hepatis has increased the number of hepatic duct bifurcation tumors that can be resected.
The primary site of relapse after surgical resection is local; however, distant recurrence is also frequently reported.[4]
The optimal surgical procedure for carcinoma of the perihilar bile duct varies according to the location of the tumor along the biliary tree, the extent of hepatic parenchymal involvement, and the proximity of the tumor to major blood vessels in this region. The state of the regional lymph nodes is assessed at the time of surgery because of their prognostic significance. Operations for bile duct cancer are usually extensive. A historical cohort reported an operative mortality rate of approximately 10%, along with a roughly 40% risk of disease recurrence.[5]
In jaundiced patients, the role of percutaneous transhepatic catheter drainage or endoscopic placement of a stent for relief of biliary obstruction is controversial because of inconsistent findings of significant clinical benefit and concerns of increased risk of postoperative complications.[6] However, percutaneous transhepatic catheter drainage or endoscopic placement of a stent for relief of biliary obstruction may be considered before surgery, particularly if jaundice is severe or an element of azotemia is present.[7,8]
Distal bile duct cancer
Complete surgical resection with negative surgical margins offers the only chance of cure for distal bile duct cancers. Bile duct tumors can be difficult to resect because of their proximity to major blood vessels and diffuse infiltration of adjacent bile ducts. Total resection is possible in 25% to 30% of lesions that originate in the distal bile duct; the resectability rate is lower for lesions that occur in more proximal sites.[9]
The optimum surgical procedure for carcinoma of the distal bile duct will vary according to the location of the tumor along the biliary tree, the extent of hepatic parenchymal involvement, and the proximity of the tumor to major blood vessels in this region. The regional lymph nodes are assessed at the time of surgery because they have prognostic significance. Patients with cancer of the lower end of the duct and regional lymph node involvement may warrant an extensive resection (Whipple procedure). The 5-year survival outcomes range between 20% and 50%.[10,11] Bypass operations or endoluminal stents are alternatives if intraoperatively the tumor is found to be unresectable.[10,11]
In jaundiced patients, the role of percutaneous transhepatic catheter drainage or endoscopic placement of a stent for relief of biliary obstruction is controversial, but these options may be considered before surgery, particularly if jaundice is severe or an element of azotemia is present.[7,8]
Adjuvant therapy
Chemotherapy
Numerous retrospective series have suggested that adjuvant chemotherapy after complete surgical resection may be beneficial.[12,13][Level of evidence C2] However, prospective randomized trials have failed to consistently show a significant benefit in overall survival (OS).
Evidence (chemotherapy):
- A multicenter phase III study in the United Kingdom (BILCAP) included 447 patients with cholangiocarcinoma or muscle-invasive gallbladder cancer who underwent a macroscopically complete resection with curative intent. Patients were randomly assigned to receive eight cycles of capecitabine (1,250 mg/m2 twice a day on days 1−14 of a 21-day cycle) or observation.[13][Level of evidence B1] At a median follow-up of 106 months, the following results were observed:
- There was no statistically significant difference in OS in the intention-to-treat analysis (median OS, 49.6 months in the capecitabine group vs. 36.1 months in the observation group; adjusted hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.67−1.06; P > .05).
- In the intention-to-treat analysis, median recurrence-free survival (RFS) was 24.3 months (95% CI, 18.6–34.6) in the capecitabine group and 17.4 months (95% CI, 11.8–23) in the observation group. An adjusted Cox proportional hazards model suggested potential improvement in RFS in the first 24 months from randomization (HR, 0.74; 95% CI, 0.57–0.96), but with no significant difference in the period after 24 months (HR, 1.57; 95% CI, 0.90–2.74).
- The open-label, randomized, phase II STAMP study (NCT03079427), presented in abstract form, included 101 patients with perihilar or distal bile duct cancer, at least one regional lymph node metastasis (N1 or greater), and complete macroscopic (R0 or R1) resection within 12 weeks. Patients were assigned to receive either eight cycles of capecitabine (1,250 mg/m2) twice a day on days 1–14 of a 21-day cycle (based on the BILCAP trial) or eight cycles of cisplatin (25 mg/m2) and gemcitabine (1,000 mg/m2) on days 1 and 8 of a 21-day cycle.[14][Level of evidence B1] At a median follow-up of 28.7 months, the following results were observed:
- The median disease-free survival (DFS), the primary end point, was 14.3 months (1-sided 90% CI, 10.7–16.5) in the cisplatin-and-gemcitabine group and 11.1 months in the capecitabine group (1-sided 90% CI, 8.4–12.7).
- The median OS was 35.7 months (1-sided 90% CI, 29.5–not estimated) in the cisplatin-and-gemcitabine group and 35.7 months (1-sided 90% CI, 30.9–not estimated) in the capecitabine group.
- The gemcitabine-and-cisplatin group had increased rates of toxicity. Grade 3 to 4 adverse events occurred in 84% of patients who received gemcitabine and cisplatin (most commonly neutropenia) and in 16% of patients who received capecitabine (most commonly hand-foot syndrome).
- Given the lack of significant difference in DFS and OS and the higher toxicity rate in the cisplatin-and-gemcitabine group, capecitabine remains the current reference standard for adjuvant therapy.
- A French multicenter phase III study (PRODIGE 12-ACCORD 18-UNICANCER GI) randomly assigned 196 patients with R0 or R1 resection of localized biliary tract cancer to 12 cycles of adjuvant gemcitabine plus oxaliplatin (GEMOX) or surveillance.[15][Level of evidence B1] After a median follow-up of 46.5 months the following results were observed:
- There was no statistically significant difference in the primary outcome of RFS (median, 30.4 months with GEMOX vs. 18.5 months with observation; HR, 0.88; 95% CI, 0.62–1.25, P = .48).
- There was also no statistically significant difference in the secondary outcome of OS (75.8 months with GEMOX vs. 50.8 months with observation; HR, 1.08; 95% CI, 0.7–1.66; P = .74).
- The Bile Duct Cancer Adjuvant Trial (BCAT), a Japanese multicenter phase III study, randomly assigned 225 patients with resected bile duct cancer to six cycles of adjuvant gemcitabine or observation.[16][Level of evidence B1]
- There was no significant difference in the primary outcome of OS (median, 62.3 months with gemcitabine vs. 63.8 months with observation; HR, 1.01; 95% CI, 0.7–1.45; P = .964).
- No OS differences were observed, even in subgroups stratified by lymph node status and surgical margin status.
- There was also no significant difference in the secondary outcome of RFS (median, 36 months with gemcitabine vs. 39.9 months with observation; HR, 0.93; P = .693).
- The European Study Group for Pancreatic Cancer (ESPAC-3 trial [NCT00058201]) randomly assigned 428 patients with periampullary cancer, which included 96 patients with bile duct cancers, to observation, 6 months of fluorouracil (5-FU)/leucovorin, or 6 months of gemcitabine.[17][Level of evidence B1]
- Among all patients, adjuvant chemotherapy, compared with observation, was not associated with significant OS benefit. However, after adjusting for prognostic variables by multivariable analysis, a statistically significant OS benefit was associated with adjuvant chemotherapy (HR, 0.75; 95% CI, 0.57–0.98; P = .03).
- In a preplanned subgroup analysis of the 96 patients with bile duct cancer, no benefit was seen among patients treated with chemotherapy. Limitations of this subgroup analysis include limited statistical power and difficulty in differentiating ampullary versus distal common bile duct tumors as the pathological site of origin.
- The median survival was 27 months for the observation-alone group, 18 months for the 5-FU/leucovorin group, and 20 months for the gemcitabine-alone group.[17]
- A multi-institutional Japanese study compared surgery alone with mitomycin and infusional 5-FU followed by 5-FU until disease progression.[18][Level of evidence B1]
- Among the subset of patients with bile duct cancer (n = 139), no survival benefit was seen.
For a list of chemotherapy regimens with potential activity, see the Treatment of Unresectable (Including Metastatic and Recurrent) Bile Duct Cancer section.
External-beam radiation therapy (EBRT)
Numerous retrospective studies have suggested that adding EBRT after complete surgical resection may be beneficial.[19,20][Level of evidence A1] However, no prospective randomized trials have demonstrated an OS benefit.
Evidence (EBRT):
- One small randomized trial of 207 patients with pancreatic and periampullary cancers demonstrated no survival benefit of adding chemoradiation therapy after surgery. This study is limited, however, because only a few patients had a diagnosis of bile duct cancer, and 20% of the patients randomly assigned to receive chemoradiation therapy did not receive treatment.[21][Level of evidence C3]
- A phase II cooperative group trial, SWOG S0809 (NCT00789958), evaluated adjuvant capecitabine and gemcitabine followed by chemoradiation therapy for resected extrahepatic cholangiocarcinoma and gallbladder cancer. In total, 79 eligible patients with pT2 to pT4 disease, node-positive disease, or positive-margin resection were enrolled (extrahepatic bile duct cancer, n = 54; gallbladder cancer, n = 25).[22][Level of evidence C2]
- The 2-year survival rate of 65% was significantly higher than expected, based on historical controls.[22][Level of evidence C2]
- Grade 3 toxicity was observed in 52% of patients, and grade 4 toxicity was observed in 11% of patients.
- On the basis of these results, this regimen was observed to be well tolerated, but it needs to be tested in a randomized controlled trial.
All patients are encouraged to enroll in clinical trials for adjuvant therapies. Information about ongoing clinical trials is available from the NCI website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Dodson RM, Weiss MJ, Cosgrove D, et al.: Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg 217 (4): 736-750.e4, 2013. [PubMed: 23890842]
- Burke EC, Jarnagin WR, Hochwald SN, et al.: Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 228 (3): 385-94, 1998. [PMC free article: PMC1191497] [PubMed: 9742921]
- Nakeeb A, Tran KQ, Black MJ, et al.: Improved survival in resected biliary malignancies. Surgery 132 (4): 555-63; discussion 563-4, 2002. [PubMed: 12407338]
- Hasegawa S, Ikai I, Fujii H, et al.: Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 31 (6): 1256-63, 2007. [PubMed: 17453285]
- Loehrer AP, House MG, Nakeeb A, et al.: Cholangiocarcinoma: are North American surgical outcomes optimal? J Am Coll Surg 216 (2): 192-200, 2013. [PubMed: 23266423]
- Liu F, Li Y, Wei Y, et al.: Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci 56 (3): 663-72, 2011. [PubMed: 20635143]
- Nimura Y: Preoperative biliary drainage before resection for cholangiocarcinoma (Pro). HPB (Oxford) 10 (2): 130-3, 2008. [PMC free article: PMC2504393] [PubMed: 18773090]
- Laurent A, Tayar C, Cherqui D: Cholangiocarcinoma: preoperative biliary drainage (Con). HPB (Oxford) 10 (2): 126-9, 2008. [PMC free article: PMC2504392] [PubMed: 18773089]
- Stain SC, Baer HU, Dennison AR, et al.: Current management of hilar cholangiocarcinoma. Surg Gynecol Obstet 175 (6): 579-88, 1992. [PubMed: 1280374]
- Fong Y, Blumgart LH, Lin E, et al.: Outcome of treatment for distal bile duct cancer. Br J Surg 83 (12): 1712-5, 1996. [PubMed: 9038548]
- Bortolasi L, Burgart LJ, Tsiotos GG, et al.: Adenocarcinoma of the distal bile duct. A clinicopathologic outcome analysis after curative resection. Dig Surg 17 (1): 36-41, 2000. [PubMed: 10720830]
- Murakami Y, Uemura K, Sudo T, et al.: Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg 250 (6): 950-6, 2009. [PubMed: 19953713]
- Bridgewater J, Fletcher P, Palmer DH, et al.: Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J Clin Oncol 40 (18): 2048-2057, 2022. [PubMed: 35316080]
- Yoo C, Jeong H, Kim K, et al.: Adjuvant gemcitabine plus cisplatin (GemCis) versus capecitabine (CAP) in patients (pts) with resected lymph node (LN)-positive extrahepatic cholangiocarcinoma (CCA): A multicenter, open-label, randomized, phase 2 study (STAMP). [Abstract] J Clin Oncol 40 (Suppl 16): A-4019, 2022.
- Edeline J, Benabdelghani M, Bertaut A, et al.: Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol 37 (8): 658-667, 2019. [PubMed: 30707660]
- Ebata T, Hirano S, Konishi M, et al.: Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 105 (3): 192-202, 2018. [PubMed: 29405274]
- Neoptolemos JP, Moore MJ, Cox TF, et al.: Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 308 (2): 147-56, 2012. [PubMed: 22782416]
- Takada T, Amano H, Yasuda H, et al.: Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95 (8): 1685-95, 2002. [PubMed: 12365016]
- Kim TH, Han SS, Park SJ, et al.: Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys 81 (5): e853-9, 2011. [PubMed: 21497455]
- Hughes MA, Frassica DA, Yeo CJ, et al.: Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys 68 (1): 178-82, 2007. [PubMed: 17276614]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al.: Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230 (6): 776-82; discussion 782-4, 1999. [PMC free article: PMC1420941] [PubMed: 10615932]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al.: SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol 33 (24): 2617-22, 2015. [PMC free article: PMC4534524] [PubMed: 25964250]
Treatment of Unresectable (Including Metastatic and Recurrent) Bile Duct Cancer
Treatment Options for Unresectable (Including Metastatic and Recurrent) Bile Duct Cancer
Treatment options for unresectable (including metastatic and recurrent) bile duct cancer include the following:
Palliative therapy
Relief of biliary obstruction is warranted when symptoms such as pruritus and hepatic dysfunction outweigh other symptoms of the cancer. When possible, such palliation can be achieved with the placement of bile duct stents by operative, endoscopic, or percutaneous techniques.[1,2]
Palliative radiation therapy may be beneficial, and patients may be candidates for stereotactic body radiation therapy [3] and intra-arterial embolization.[4]
Chemotherapy
Systemic chemotherapy is appropriate for selected patients with adequate performance status and intact organ function. The following agents have been reported to produce transient partial remissions in a minority of patients:
- Fluoropyrimidines (gemcitabine).
- Platinum agents.
- Docetaxel.
Evidence (chemotherapy):
- A phase III study (NCT00262769) randomly assigned 410 patients with unresectable, recurrent, or metastatic biliary tract carcinoma to receive cisplatin followed by gemcitabine or to receive gemcitabine alone for up to 6 months. The following results were observed:[5][Level of evidence A1]
- Median overall survival (OS) improved among patients treated with cisplatin and gemcitabine therapy (11.7 months) versus patients treated with gemcitabine alone (8.1 months) (hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.52−0.80; P < .001).[5]
- A similar median OS benefit was demonstrated in all subgroups, including 73 patients with extrahepatic bile duct cancer and 57 patients with hilar tumors.
- Grades 3 and 4 toxicities occurred with similar frequency in both study arms, with the exception of increased hematologic toxicity in patients randomly assigned to the gemcitabine/cisplatin arm and increased hepatic toxicity in patients randomly assigned to the single-agent gemcitabine arm.
- A phase III noninferiority study (NCT01470443) enrolled 114 patients with metastatic biliary tract cancers, including 30 (26%) with primary gallbladder cancer. Patients were randomly assigned to receive capecitabine plus oxaliplatin (XELOX) or gemcitabine plus oxaliplatin (GEMOX).[6][Level of evidence B1]
- OS was not significantly different, with 10.4 months (95% CI, 8.0−12.6) in the GEMOX group, compared with 10.6 months (95% CI, 7.3−15.5) in the XELOX group (P = .131).
- The primary end point of 6-month progression-free survival (PFS) was 44.6% for the GEMOX group and 46.7% for the XELOX group (95% CI of difference in 6-month PFS rate, -12% to 16%, meeting criteria for noninferiority).
- A predefined subgroup analysis based on primary site of disease did not reveal a difference in objective response rate between the two arms in patients with gallbladder cancer (P = .598).
Pending further clinical trials, cisplatin plus gemcitabine is considered the reference standard first-line chemotherapy backbone for patients with unresectable, metastatic, or recurrent bile duct cancer. Following the results of the TOPAZ-1 and KEYNOTE-966 trials, addition of a checkpoint inhibitor (either durvalumab or pembrolizumab) to front-line therapy has become the standard of care (for more information, see the Immunotherapy section). Potential alternatives include gemcitabine plus capecitabine, GEMOX, and XELOX. Clinical trials should be considered for all patients.
There is limited high-quality evidence to guide selection of a second-line regimen in refractory disease:
- A multicenter phase III trial in the United Kingdom (ABC-06 [NCT01926236]) randomly assigned 162 patients with locally advanced or metastatic biliary tract cancer and documented radiological disease progression on first-line cisplatin and gemcitabine to receive either FOLFOX (folinic acid, fluorouracil [5-FU], and oxaliplatin) with active symptom control (ASC) or ASC alone. The following was observed after a median follow-up of 21.7 months:[7][Level of evidence A1]
- The median OS was significantly longer in the FOLFOX group (6.2 months) than in the group who received ASC alone (5.3 months) (adjusted HR, 0.69; 95% CI, 0.50−0.97; P = .031). In the FOLFOX group, the OS rate was 50.6% at 6 months and 25.9% at 12 months, compared with 35.5% at 6 months and 11.4% at 12 months in the group who received ASC alone.
- Grade 3 to 5 adverse events were reported in 56 (69%) patients in the FOLFOX group, compared with 42 (52%) in the ASC alone group. The most frequently reported grade 3 to 5 FOLFOX-related adverse events were neutropenia (12%), fatigue/lethargy (11%), and infection (10%). There were three chemotherapy-related deaths, one each due to infection, acute kidney injury, and febrile neutropenia.
- A multicenter phase IIb trial in South Korea (NIFTY [NCT03524508]) randomly assigned 174 patients with metastatic biliary tract cancer that had progressed on first-line cisplatin and gemcitabine to receive 5-FU and leucovorin with or without liposomal irinotecan. The following was observed after a median follow-up of 6.1 months:[8][Level of evidence A1]
- The primary end point of median PFS was significantly longer in the group who received liposomal irinotecan (3.9 months) compared with the group who received 5-FU plus leucovorin alone (1.6 months) (HR, 0.38; 95% CI, not reported; P = .0001). A secondary end point of median OS was also significantly longer in the group who received liposomal irinotecan (8.6 months) compared with the group who received 5-FU plus leucovorin alone (5.3 months) (HR, 0.68; 95% CI, not reported; P = .024).
Immunotherapy
Based on results from the TOPAZ-1 and KEYNOTE-966 trials, all patients with unresectable, metastatic, or recurrent disease should consider treatment with a checkpoint inhibitor (either durvalumab or pembrolizumab) with cisplatin and gemcitabine (the previous standard-of-care doublet) in the first-line setting.[9,10]
Evidence (immunotherapy):
- An international, multicenter, phase III study (TOPAZ-1 [NCT03875235]) included 685 patients with locally advanced, recurrent, or metastatic biliary tract cancer that was unresectable and previously untreated. Patients were randomly assigned to receive either durvalumab or placebo with cisplatin plus gemcitabine for up to eight cycles, followed by durvalumab or placebo maintenance until disease progression or unacceptable toxicity occurred. After a median follow-up of 13.7 months for patients in the durvalumab arm, the following was observed:[10][Level of evidence A1]
- The primary end point of median OS was significantly improved in the durvalumab group (12.8 months) compared with the placebo group (11.5 months) (HR, 0.80; 95% CI, 0.66–0.97; P = .021). In the durvalumab group, the OS rate was 35.1% at 18 months and 24.9% at 24 months, compared with 25.5% at 18 months and 10.4% at 24 months in the placebo group.
- There was no significant difference between groups in the number of grade 3 or 4 treatment-related adverse events or the number of events leading to discontinuation of a study medication.
- An international, multicenter, phase III study (KEYNOTE-966 [NCT04003636]) enrolled 1,069 patients with previously untreated, unresectable, locally advanced or metastatic biliary tract cancer. Patients were randomly assigned to receive either pembrolizumab or placebo for up to 35 cycles. This was combined with gemcitabine (with no maximum duration) and cisplatin for up to 8 cycles. After a median follow-up of 25.6 months, the following results were observed:[9][Level of evidence A1]
- The median OS was 12.7 months in the pembrolizumab group and 10.9 months in the placebo group (HR, 0.83; 95% CI, 0.72–0.95; one-sided P = .0034).
- There was no difference in the total frequency of treatment-related adverse events between treatment groups, including grade 3 or grade 4 events. Death due to treatment-related adverse events was seen in a total of eight patients (2%) in the pembrolizumab arm and three patients (1%) in the placebo arm.
All patients with unresectable, metastatic, or recurrent disease who have not already received a checkpoint inhibitor should have molecular testing for deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H) tumors. Extrapolating from a subgroup of patients with gastrointestinal and hepatopancreatobiliary tumors in the I-PREDICT (NCT02534675) and KEYNOTE-158 (NCT02628067) studies, patients with either dMMR or MSI-H tumors can consider pembrolizumab treatment.[11,12][Level of evidence C3]
Targeted therapy
Clinical trials of investigational therapies should be considered for patients with targetable mutations. Currently, targeted therapies have only been approved for patients whose disease has progressed or who are ineligible for first-line therapies.
IDH1 inhibitors
Up to 15% of bile duct cancers express a mutation in the IDH1 gene.
Evidence (IDH1 inhibitors):
- The phase III ClarIDHy trial (NCT02989857) included 187 patients with IDH1-mutated cholangiocarcinoma that had progressed during previous systemic therapy. Patients were randomly assigned to receive either the IDH1 inhibitor ivosidenib or placebo. The following results were observed:[13,14][Level of evidence B1]
- The primary end point of median PFS was improved among patients treated with ivosidenib (2.7 months) compared with placebo (1.4 months) (HR, 0.37; 95% CI, 0.25−0.54; P < .001). PFS rates at 6 months and 12 months were 32% and 21.9%, respectively, in the ivosidenib arm. No patients in the placebo group were progression free at 6 months.
- In the intention-to-treat analysis, median OS was 10.3 months in the ivosidenib group compared with 7.5 months for the placebo group (HR, 0.79; one-sided P = .09), despite crossover of 57% of placebo patients to ivosidenib. When adjusted for crossover, median OS for the placebo group was 5.1 months.
- Grades 3 and 4 toxicities occurred in 46% of patients in the ivosidenib group compared with 36% in the placebo group.
Fibroblast growth factor receptor 2 (FGFR) inhibitors
FGFR2 fusions are present in approximately 15% of intrahepatic cholangiocarcinomas. Multiple phase II trials, some reported in abstract form, have suggested activity of FGFR inhibitors in patients with cholangiocarcinoma and FGFR2 fusions whose disease progressed after or who were ineligible for first-line chemotherapy.[15-17]
Evidence (FGFR inhibitors):
- The multicenter, open-label, single-arm phase II FIGHT-202 trial (NCT02924376) enrolled 147 patients with disease progression on or after at least one previous therapy. A total of 108 patients harbored FGFR2 rearrangements/fusions. All patients received pemigatinib 13.5 mg orally once daily for 14 consecutive days, followed by 7 days off therapy. At a median follow-up of 30.4 months, the following results were observed:[16][Level of evidence C3]
- The overall response rate in the cohort of patients with FGFR2 rearrangements/fusions was 37% (95% CI, 27.9%−46.9%), including four complete responses. Among the 40 patients who achieved an objective response, the median duration of response was 8.1 months.
- The median PFS in patients with FGFR2 rearrangements/fusions was 7.0 months (95% CI, 6.1–10.5), and the median OS was 17.5 months (95% CI, 14.4–22.9). Given the single-arm study design, the relative effect of pemigatinib on PFS and OS has not yet been established. However, in the cohort of study patients whose tumors did not harbor FGFR rearrangements, the median PFS was only 1.5 months and the median OS was only 4.0 months.
- The most common adverse effect was hyperphosphatemia, occurring in 58.5% of patients, although no adverse effect was grade 3 or higher. Adverse events led to treatment discontinuation in 10.2% of patients, dose reduction in 13.64% of patients, and dose interruptions in 42.2% of patients.
In April 2020, the FDA granted accelerated approval of pemigatinib for the treatment of adults with previously treated unresectable or metastatic cholangiocarcinoma with FGFR2 fusion or other rearrangement. - Futibatinib is an irreversible noncompetitive inhibitor of FGFR1–4. Preclinical in vitro studies showed that futibatinib was less susceptible to on-target resistance mutations than pemigatinib. However, there are no head-to-head clinical trial data comparing outcomes for the various FGFR inhibitors. The multinational, open-label, single-group, phase II FOENIX-CCA2 trial (NCT02052778) evaluated futibatinib in patients with previously treated intrahepatic cholangiocarcinoma and an FGFR2 fusion or rearrangement. The study enrolled 103 patients with disease progression after at least one previous line of systemic therapy. All patients received futibatinib at a continuous dose of 20 mg once daily.[18][Level of evidence C3]
- The overall response rate was 42% (95% CI, 31.1%–50.4%), including one complete response. Of the patients who had a response, the median duration of response was 9.7 months.
- The median PFS was 9 months (95% CI, 6.9–13.1) and the median OS was 21.7 months (95% CI 14.5–NR). Given the single-arm study design, the relative effect of futibatinib on PFS and OS has not yet been established.
- The most common adverse effect of any grade was hyperphosphatemia, which occurred in 85% of patients and was grade 3 in 30% of patients. Other common adverse effects included alopecia (33%), dry mouth (30%), dry skin (27%), and fatigue (25%). Other notable grade 3 toxicities included aspartate aminotransferase elevation (7%) and stomatitis (6%). Treatment-related adverse events led to dose interruptions in 50% of patients, dose reductions in 54% of patients, and permanent drug discontinuation in 2% of patients.
Patients with FGFR2 fusion−positive disease should be encouraged to enroll in a clinical trial.
HER2-targeted therapy
- The international, multicenter, single-arm, phase IIb HERIZON-BTC-01 trial (NCT04466891) enrolled 87 patients with HER2-amplified (by fluorescence in situ hybridization), unresectable, locally advanced or metastatic biliary tract cancer whose disease progressed on prior gemcitabine-based therapy. Cohort 1 included 80 patients with HER2 2+ or 3+ expression by immunohistochemistry (IHC), while cohort 2 included seven patients with HER2 0+ or 1+ expression by IHC. All patients received zanidatamab, a bispecific antibody targeting two distinct HER2 epitopes, at a dose of 20 mg/kg intravenously every 2 weeks. At a median follow-up of 12.4 months, the following results were observed:[19][Level of evidence C3]
- In cohort 1, the objective response rate was 41.3% (95% CI, 30.4%–52.8%). The median duration of response was 12.9 months (95% CI, 6.0–not reached).
- The median PFS was 5.5 months in cohort 1 (95% CI, 3.7–7.2) and 1.9 months (95% CI, 1.2–not estimable) in cohort 2.
- Serious treatment-related adverse events occurred in 8% of patients. Zanidatamab was discontinued in two patients: one due to reduced ejection fraction and one due to pneumonitis. Diarrhea (37%) and infusion reactions with the first cycle (33%) were relatively common, but mostly low-grade.
- The FDA has granted breakthrough therapy designation for zanidatamab in this setting, but it is not yet FDA approved.
Although not yet FDA approved in biliary tract cancer, a growing body of evidence demonstrated activity of the antibody-drug conjugate trastuzumab deruxtecan in patients with HER2-expressing solid tumors.
- The DESTINY-PanTumor02 trial (NCT04482309), reported in abstract form, was tumor-agnostic (enrolled patients with HER2-positive tumors from any site) but included a subset of 41 patients with biliary tract cancer.[20][Level of evidence C3]
- The overall response rate was 22% in this subset of patients.
- The phase II HERB trial (NCT04482309), reported in abstract form, enrolled 32 patients (24 with HER2-positive disease, 8 with HER2-low disease) with biliary tract cancers refractory to, or intolerant of, a gemcitabine-containing regimen, all of whom were treated with trastuzumab deruxtecan.[21][Level of evidence C3]
- Among the HER2-positive patients, the overall response rate was 36.4%. The median PFS was 4.4 months and the median OS was 7.1 months.[21]
Similarly, the combination of tucatinib and trastuzumab—which the FDA has not approved for the treatment of biliary tract cancer but has approved for breast and colorectal cancer indications—was shown to have potential activity in previously treated patients.
- The tumor-agnostic phase II SGNTUC-019 study (NCT04579380) evaluated the combination of tucatinib and trastuzumab. The trial included a cohort of 30 patients with previously treated HER2-overexpressing or HER2-amplified biliary tract cancer.[22][Level of evidence C3]
- At a median follow-up of 10.8 months, the objective response rate was 46.7% (90% CI, 30.8%–63.0%), with a disease control rate of 76.7% (90% CI, 60.6%–88.5%).
- The median PFS was 5.5 months (90% CI, 3.9–8.2).
- The most common treatment-related adverse events were pyrexia (43.3%) and diarrhea (40%). Adverse events caused no deaths but led one patient to discontinue treatment.
Patients with HER2-amplified disease are candidates for clinical trials.
All patients are encouraged to enroll in clinical trials for adjuvant therapies. Information about ongoing clinical trials is available from the NCI website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Nordback IH, Pitt HA, Coleman J, et al.: Unresectable hilar cholangiocarcinoma: percutaneous versus operative palliation. Surgery 115 (5): 597-603, 1994. [PubMed: 7513906]
- Levy MJ, Baron TH, Gostout CJ, et al.: Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: An evidence-based approach. Clin Gastroenterol Hepatol 2 (4): 273-85, 2004. [PubMed: 15067620]
- Barney BM, Olivier KR, Miller RC, et al.: Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol 7: 67, 2012. [PMC free article: PMC3464963] [PubMed: 22553982]
- Hyder O, Marsh JW, Salem R, et al.: Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol 20 (12): 3779-86, 2013. [PubMed: 23846786]
- Valle J, Wasan H, Palmer DH, et al.: Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362 (14): 1273-81, 2010. [PubMed: 20375404]
- Kim ST, Kang JH, Lee J, et al.: Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: a multicenter, open-label, randomized, phase III, noninferiority trial. Ann Oncol 30 (5): 788-795, 2019. [PubMed: 30785198]
- Lamarca A, Palmer DH, Wasan HS, et al.: Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 22 (5): 690-701, 2021. [PMC free article: PMC8082275] [PubMed: 33798493]
- Yoo C, Kim KP, Kim I, et al.: Final results from the NIFTY trial, a phase IIb, randomized, open-label study of liposomal Irinotecan (nal-IRI) plus fluorouracil (5-FU)/leucovorin (LV) in patients (pts) with previously treated metastatic biliary tract cancer (BTC). Ann Oncol 33 (Suppl 7): S565, 2022.
- Kelley RK, Ueno M, Yoo C, et al.: Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401 (10391): 1853-1865, 2023. [PubMed: 37075781]
- Oh DY, He AR, Qin S, et al.: Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann Oncol 33 (Suppl 7): S565-S566, 2022.
- Sicklick JK, Kato S, Okamura R, et al.: Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med 25 (5): 744-750, 2019. [PMC free article: PMC6553618] [PubMed: 31011206]
- Marabelle A, Le DT, Ascierto PA, et al.: Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 38 (1): 1-10, 2020. [PMC free article: PMC8184060] [PubMed: 31682550]
- Abou-Alfa GK, Macarulla T, Javle MM, et al.: Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 21 (6): 796-807, 2020. [PMC free article: PMC7523268] [PubMed: 32416072]
- Zhu AX, Macarulla T, Javle MM, et al.: Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol 7 (11): 1669-1677, 2021. [PMC free article: PMC8461552] [PubMed: 34554208]
- Mazzaferro V, El-Rayes BF, Droz Dit Busset M, et al.: Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer 120 (2): 165-171, 2019. [PMC free article: PMC6342954] [PubMed: 30420614]
- Abou-Alfa GK, Sahai V, Hollebecque A, et al.: Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 21 (5): 671-684, 2020. [PMC free article: PMC8461541] [PubMed: 32203698]
- Droz Dit Busset M, Braun S, El-Rayes B, et al.: Efficacy of derazantinib (DZB) in patients (pts) with intrahepatic cholangiocarcinoma (ICCA) expressing FGFR2-fusion or FGFR2 mutations/amplifications. [Abstract] Ann Oncol 30 (Suppl 5): A-721P, 2019.
- Goyal L, Meric-Bernstam F, Hollebecque A, et al.: Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N Engl J Med 388 (3): 228-239, 2023. [PubMed: 36652354]
- Harding JJ, Fan J, Oh DY, et al.: Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol 24 (7): 772-782, 2023. [PubMed: 37276871]
- Meric-Bernstam F, Makker V, Oaknin A, et al.: Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results. [Abstract] J Clin Oncol 41 (Suppl 17): A-LBA3000, 2023. [PMC free article: PMC10730032] [PubMed: 37870536]
- Ohba A, Morizane C, Kawamoto Y, et al.: Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). [Abstract] J Clin Oncol 40 (Suppl 16): A-4006, 2022.
- Nakamura Y, Mizuno N, Sunakawa Y, et al.: Tucatinib and Trastuzumab for Previously Treated Human Epidermal Growth Factor Receptor 2-Positive Metastatic Biliary Tract Cancer (SGNTUC-019): A Phase II Basket Study. J Clin Oncol 41 (36): 5569-5578, 2023. [PMC free article: PMC10730072] [PubMed: 37751561]
Latest Updates to This Summary (03/28/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Unresectable (Including Metastatic and Recurrent) Bile Duct Cancer
Added text to state that the combination of tucatinib and trastuzumab—which the U.S. Food and Drug Administration has not approved for the treatment of biliary tract cancer but has approved for breast and colorectal cancer indications—was shown to have potential activity in previously treated patients.
Added text about the results of a tumor-agnostic phase II study that evaluated the combination of tucatinib and trastuzumab in a cohort of 30 patients with previously treated HER2-overexpressing or HER2-amplified biliary tract cancer (cited Nakamura et al. as reference 22 and level of evidence C3).
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of bile duct cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Bile Duct Cancer (Cholangiocarcinoma) Treatment are:
- Amit Chowdhry, MD, PhD (University of Rochester Medical Center)
- Valerie Lee, MD (Johns Hopkins University)
- Leon Pappas, MD, PhD (Dana-Farber Cancer Institute)
- Ari Seifter, MD (University of Illinois at Chicago)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Bile Duct Cancer (Cholangiocarcinoma) Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/liver/hp/bile-duct-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389308]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
- General Information About Bile Duct Cancer
- Cellular Classification of Bile Duct Cancer
- Stage Information for Bile Duct Cancer
- Treatment Option Overview for Bile Duct Cancer
- Treatment of Resectable (Localized) Bile Duct Cancer
- Treatment of Unresectable (Including Metastatic and Recurrent) Bile Duct Cancer
- Latest Updates to This Summary (03/28/2024)
- About This PDQ Summary
- Review Prostate Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Prostate Cancer Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Bladder Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Bladder Cancer Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Gallbladder Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Gallbladder Cancer Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Skin Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Skin Cancer Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Vaginal Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Vaginal Cancer Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Bile Duct Cancer (Cholangiocarcinoma) Treatment (PDQ®) - PDQ Cancer Information ...Bile Duct Cancer (Cholangiocarcinoma) Treatment (PDQ®) - PDQ Cancer Information Summaries
- Symptom Clusters in Cancer (PDQ®) - PDQ Cancer Information SummariesSymptom Clusters in Cancer (PDQ®) - PDQ Cancer Information Summaries
- yv03c09.r1 Soares fetal liver spleen 1NFLS Homo sapiens cDNA clone IMAGE:241648 ...yv03c09.r1 Soares fetal liver spleen 1NFLS Homo sapiens cDNA clone IMAGE:241648 5', mRNA sequencegi|1087192|gnl|dbEST|394055|gb|H916Nucleotide
- Homo sapiens transcription factor mRNA, 5' endHomo sapiens transcription factor mRNA, 5' endgi|487835|gb|L32162.1|HUMTRFANucleotide
- condensin-2 complex subunit D3 isoform 1 [Homo sapiens]condensin-2 complex subunit D3 isoform 1 [Homo sapiens]gi|45356151|ref|NP_056076.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...