NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997.
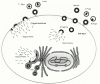
The polyprotein precursors used to initiate the process of assembly are encoded by the gag, pro, pol, and env genes common to all replication-competent retroviruses. The Gag protein contains the sequences of MA, CA, NC, and a few unnamed peptides. Gag is translated from the unspliced viral RNA on free ribosomes in the cytoplasm (Fig. 2). This polyprotein has the central role in assembly (for reviews, see Wills and Craven 1991; Hunter 1994; Kräusslich and Welker 1996; see also Chapter 2. In particular, Gag has the ability to direct the budding of virus-like particles from the cell, even when expressed in the absence of all the other virus-encoded components. Because of these properties, the Gag protein has been described as a “particle-making machine” (Dickson et al. 1984). The Gag protein is also involved in packaging most of the other components of the virion, including the two copies of genomic RNA.
The products of the pro and pol genes—PR, RT, and IN—are also synthesized from the unspliced viral RNA, but never as parts of an independent polyprotein. Rather, they are initially contained within the Gag-Pro or Gag-Pro-Pol fusion protein, the product of translational readthrough among the gag-, pro-, and pol-coding sequences. The Gag portion of the molecule serves to direct the Pro and Pol portions to the site of assembly and to mediate their assembly into particles, although the spumaviruses may use an alternative strategy for packaging a distinct Pro-Pol precursor.
The Env polyprotein contains the sequences for SU and TM. The route it travels to the plasma membrane is entirely distinct from the cytosolic pathway taken by the precursors to the internal proteins (Fig. 2). Env is synthesized from a spliced transcript and glycosylated as it is translocated through the membrane of the RER. A hydrophobic region located near the carboxyl terminus anchors it in the membrane. Subsequent vesicular transport carries the viral glycoproteins through the stacks of the Golgi apparatus and then to the plasma membrane, where they are exposed on the outside of the cell. Lateral movement brings them to the site of budding where they are assembled onto particles containing Gag, Gag-Pro-Pol, and viral RNA as they emerge and bud off the cell surface.
Variations of the Morphogenetic Pathways of Retroviruses
Electron microscopy has revealed a wealth of information about how retroviruses assemble. Although there are many subtle variations, two major patterns have been observed (Teich 1984; Gelderblom 1990). In the first type, the Gag-containing polyproteins assemble within the cytoplasm to form an obvious and stable structure, often called an intracytoplasmic A-type particle (ICAP) or A particle. ICAPs are subsequently transported to the plasma membrane where the envelope is acquired during budding (Figs. 3 and 4). The best characterized examples of viruses using this pathway are mouse mammary tumor virus (MMTV) and Mason-Pfizer monkey virus (M-PMV), the prototypes for the type-B and type-D viruses, respectively (see Chapter 2, as well as spumaviruses. Retroviruses using the other pathway do not appear to assemble their Gag and Gag-Pro-Pol proteins into ICAPs, at least on the basis of electron microscopy. Instead, macromolecular aggregates of these molecules are first evident as discrete, electrondense patches intimately associated with the cytoplasmic face of the plasma membrane (Figs. 2, 3, and 4). As budding proceeds at these sites, dome-shaped structures arise with the concurrent formation of the immature viral core and the acquistion of the envelope. For the viruses such as avian sarcoma/leukosis virus (ASLV) and murine leukemia virus (MLV), this pattern of assembly has been designated type C, and it is the pattern most often seen for retroviruses, including human immunodeficiency virus type 1 (HIV-1) and the other lentiviruses.
Why some retroviruses exhibit the type-C pattern of morphogenesis and others exhibit the type-B/D pattern is not known, but it is clear that the determinants for assembly are inherent in their Gag polyproteins. When expressed in the absence of the other viral components, Gag proteins from each type of virus continue to follow the appropriate morphogenetic pathway. Moreover, when the viral protease is expressed with Gag, the internal structures of the virus-like particles that emerge are remarkably similar in morphology to mature infectious particles (see, e.g., Delchambre et al. 1989; Gheysen et al. 1989; Rhee et al. 1990; Smith et al. 1990; Weldon et al. 1990). The central importance of the Gag protein as the key determinant of virion assembly is perhaps best illustrated by the demonstration that M-PMV can be converted from a D-type to a C-type assembly pattern by changing a single amino acid in its MA sequence (Rhee and Hunter 1990a). This suggests that the events mediated by the Gag protein during budding via these two different pathways are more similar among the different types of retroviruses than previously believed.
Regardless of the pathway utilized, the internal appearance of all immature retroviral particles is fundamentally the same and characterized by spherical structures with an electron-lucent center (Figs. 2, 3, and 4; see also Chapter 2. These are similar to the ICAPs of the type-B/D viruses, except that they are surrounded by a lipid bilayer. The Gag (and Gag-Pro-Pol) proteins contained in such particles are intact, but during the late stages of budding, or immediately thereafter, they are rapidly cleaved by the viral PR to generate the smaller species characteristic of the mature virion (Fig. 2). Mutants with inactive PR invariably release particles of immature morphology. Processing of the Gag and Gag-Pro-Pol molecules causes the internal structure of the particle to condense into an electrondense core, the shape and location of which are characteristic of the retroviral type. Spumaviruses differ from the standard retroviral pattern in that processing of Gag proteins into the separate domains does not occur and extracellular virions retain an “immature” morphology.
A third pattern of morphogenesis has been found among certain defective endogenous retroviruses, which bud exclusively from the ER membrane to produce intracisternal A-type particles (IAPs). These membrane-enclosed particles contain uncleaved Gag polyproteins and exhibit an immature morphology (Teich 1984; Kuff and Lueders 1988). Several IAP genomes have been sequenced (Ono et al. 1985; Mietz et al. 1987; Anderson et al. 1990; Dorner et al. 1991), and most contain multiple stop codons in their putative env genes, consistent with the lack of an extracellular phase. IAPs are thought to be targeted to the ER (rather than the plasma membrane) by virtue of unique targeting information near the beginning of their Gag sequences (Kuff and Lueders 1988). Hence, they have the appearance of type-C viruses that have been directed to the “wrong” site for budding.
Significance of Polyproteins
Although the central theme in retroviral assembly is the coordinated use of large polyproteins (Gag, Gag-Pro-Pol, and Env), it is not self-evident that such precursors would be required. Indeed, spumaviruses use a strategy of independent expression of the pol gene (Yu et al. 1996a). Furthermore, many other enveloped viruses (e.g., rhabdoviruses) efficiently assemble all of their internal components at the membrane without the need for covalent linkages between them. Likewise, in the more closely related hepatitis B virus, the RT is not expressed as a fusion protein with the core protein; rather, it is packaged by interacting directly with the RNA genome (Hirsch et al. 1990; Bartenschlager and Schaller 1992). Some retroviruses express small accessory proteins (e.g., Vpr) that are packaged into the virion with high efficiency through noncovalent interactions with the Gag protein and do not require proteolytic cleavage.
The polyprotein strategy is, however, an elegant design for viral assembly from many points of view. First, it minimizes the number of components that need to be targeted individually to the site of assembly. Second, proteolysis provides a means for regulating functions needed at different stages of the replication cycle. For example, the assembly functions of Gag proteins (needed to drive the budding process) are inactivated by proteolysis; this alteration prevents the molecules from reentering the budding pathway when released into the cytoplasm upon fusion with a newly infected cell. Conversely, proteolysis liberates and activates the various proteins and enzymes that are needed for the synthesis and integration of the DNA form of the viral genome (see, e.g., Craven et al. 1991; Stewart and Vogt 1991). Third, the highly conserved order of the MA, CA, NC, PR, RT, and IN sequences within the Gag and Gag-Pro-Pol proteins corresponds to their relative location in the virion, starting from the outside and working inward (see Figs. 1 and 5 and Chapter 2. Thus, the interactions that take place among the polyproteins during particle assembly serve to align the various functional domains, as envisioned by an early model of the retroviral virion (Bolognesi et al. 1978).
- Overview of Retroviral Assembly - RetrovirusesOverview of Retroviral Assembly - Retroviruses
- Retrotransposons, Endogenous Retroviruses, and the Evolution of Retroelements - ...Retrotransposons, Endogenous Retroviruses, and the Evolution of Retroelements - Retroviruses
- References - RetrovirusesReferences - Retroviruses
- Synthesis and Organization of Env Glycoproteins - RetrovirusesSynthesis and Organization of Env Glycoproteins - Retroviruses
- Membrane Fusion and Viral Entry - RetrovirusesMembrane Fusion and Viral Entry - Retroviruses
Your browsing activity is empty.
Activity recording is turned off.
See more...