NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997.
Constitutive Gene Expression
Advantages of retroviral vectors for studies involving constitutive gene expression include their ability to efficiently transduce a wide variety of cells types from different species and to induce high levels of protein expression. The most widely used and most easily accommodated vector insert is an intronless cDNA copy of an mRNA encoding a given protein. As discussed above (see above Principles of Retroviral Vector Design, Replication-defective Vectors), many strategies have been developed for expression of cDNAs in retroviral vectors. Large cDNAs such as the insulinlike growth factor I receptor (4.1-kb coding region) have been transmitted efficiently and expressed at high levels (>106 molecules per cell) in transduced cells (Kaleko et al. 1990). The presence of repetitive internal sequences, such as those found in a collagen cDNA, appear to be transmitted faithfully (Stacey et al. 1987), although the methods employed may not have detected subtle rearrangements. Investigators interested in such experiments should be aware that template switching during reverse transcription can cause expansion or contraction of such repetitive sequences.
Regulated Gene Expression
Retroviral vectors designed to provide regulated gene expression are quite useful in the context of gene therapy, where it may be important to limit expression to specific cell types (e.g., red cells for globin), to regulate the overall level of expression (e.g., for clotting factors), or to regulate expression in response to endogenous stimuli (e.g., to regulate insulin production in response to blood sugar levels). A number of experimental applications also benefit from the ability to regulate expression of vector-introduced genes. Retroviral vectors have been used to study the regulation of particular genes in cells; they can infect a wide range of cell types and stably insert single unrearranged copies of the gene of interest.
Regulated expression has been obtained by using retroviral vectors containing intronless “minigenes,” which are normal genes from which the introns have been removed (Miller et al. 1984; Bender et al. 1988). Minigenes contain the normal promoter, upstream enhancers, and transcription termination signals present in the parental gene and are potentially responsive to transcription factors that normally control gene activity. The introns are usually removed to reduce the size of the insert; in addition, any introns let in the vector are removed during viral replication (Shimotohno and Temin 1982; Sorge and Hughes 1982; Cepko et al. 1984; Kriegler et al. 1984; McIvor 1990). In some cases, these inserts contained the normal polyadenylation signal for the gene, and one might expect that inserting the genes into the vectors in the forward orientation would interfere with the viral life cycle. However, several retroviral vectors containing minigenes inserted in the forward orientation have been used to transfer their minigenes faithfully without a significant decrease in vector titer (less than fivefold) (Miller et al. 1984; Bender et al. 1988), suggesting that for at least some polyadenylation sites, polyadenylation is inefficient enough to allow accumulation of full-length viral RNA.
Entire genes containing introns have also been used in retroviral vectors (see Fig. 2E) (Bandyopadhyay and Temin 1984; Cone et al. 1987a,b; Karlsson et al. 1987; Stoeckert et al. 1987; Bender et al. 1988, 1989; Dzierzak et al. 1988; Miller et al. 1988). To avoid the removal of the introns, the gene must be inserted in the vector in the reverse orientation. This strategy is particularly important for genes containing regulatory elements within their introns, such as the immunoglobulin enhancer (Cone et al. 1987a) or elements that map to the second intron of the β-globin gene which are required for high-level gene expression (Miller et al. 1988). Reverse orientation gene inserts can have deleterious effects on vector titer or stability (Miller et al. 1988). Problems can arise because, even in the reverse orientation, sequences can be recognized as polyadenylation signals or introns. It is possible to mitigate such effects, for example, by removing sequences that serve as polyadenylation signals. Reverse-orientation gene inserts containing forward-orientation introns (see Fig. 2) can only be expressed if splicing occurs during replication. Such vectors can be useful probes for viral replication or retrotransposition (see Chapter 8.
Perhaps the best system now available for experimental control of gene expression from a retroviral vector is the recently developed tetracycline-controlled trans-activator-responsive promoter (Tet) system (Gossen and Bujard 1992). The system consists of two components: regulatory elements from the tetracycline resistance operon embedded in a minimal cytomegalovirus promoter and a hybrid activator protein (tTA) composed of the tetracycline repressor fused to the herpes simplex virus trans-activator protein. In the absence of tetracycline, tTA binds the promoter and activates transcription of linked DNA sequences. Gene expression is inhibited by tetracycline, which binds tTA and causes the trans-activator to dissociate from the promoter. Both the tTA-responsive promoter with a cloning site for cDNA insertion and the gene encoding tTA have been incorporated in a single retroviral vector (Paulus et al. 1996). With this system, tetracycline-dependent gene regulation ranged from 20-fold to more than 300-fold induction. This is a much greater induction than is typically achieved with other inducible promoters such as the steroid-responsive mouse mammary tumor virus (MMTV) and the metal-ion-responsive metallothionein promoters.
Cell Lineage Analysis
A determination of cell lineage in larger organisms, where cell fates cannot be directly observed, requires a technique that marks all of the progeny of a given cell. Retroviral vectors provide useful tools for cell lineage analysis because they integrate into the genome of the transduced cell and remain stable during cell division and differentiation. Retroviral vectors have been used to transduce neuronal cells in vivo in studies designed to follow the fates of the marked cells (Price et al. 1987; Turner and Cepko 1987; Galileo et al. 1990). Similar techniques have been applied to the developing chick embryo (Reddy et al. 1991a; Fekete and Cepko 1993a). β-galactosidase and alkaline phosphatase are convenient histochemical markers that have been used to mark transduced cells and their progeny. In addition to the usual problems with contaminating helper virus, which must be vigorously excluded, a potential problem with this technique is that the expression of the marker carried by the provirus can be modulated in different cell types due either to the specific integration site or to the effects of transcription factors on expression. The real worry is that there are cells that carry a marker provirus but do not express the marker gene product. Difficulties can also arise from the inability to define sibling relationships or clonal boundaries because all cells are marked with one or at most a few markers.
Some of the limitations of histochemical techniques for lineage analysis can be overcome by the incorporation of short random sequences in the vector. This technique can be used to generate a library of vectors for cell transduction (Golden et al. 1995). Each transducing vector is then unique, so that sibling relationships and boundaries between different clones can be precisely determined by polymerase chain reaction (PCR) amplification of the unique sequences carried by the vector, followed by sequencing or restriction enzyme analysis (Cepko et al. 1995; Golden et al. 1995).
cDNA Library Construction
Retroviral vectors offer important advantages for screening of cDNA libraries. The vectors can be used to transfer and express cDNAs in a wide range of cell types, including primary cells, providing an efficient means to transfer and express the cDNAs. In addition, the target cells usually contain a single copy of the retroviral vector, which carries a single unrearranged copy of the cDNA. This simplifies the analysis of the cDNAs, compared to transfection, which often causes rearrangements and where inserts that contain multiple tandem cDNAs are often present. The use of retroviral vectors is limited by the requirement that the cloned cDNA be able to go through the retroviral life cycle. For example, cDNAs that are very long may exceed the size limit for efficient vector packaging. Alternatively, cDNAs that destabilize mRNA will not be efficiently transferred (Lynch et al. 1993), and the presence of polyadenylation signals in the cDNAs also poses a potential problem.
Retroviral cDNA libraries are made by insertion of cDNAs into a retroviral vector in plasmid, followed by transfection of this plasmid library into retroviral packaging cells to generate virus carrying the inserted cDNAs. Target cells are then transduced with the resulting vector stock and screened for expression of the desired protein. The development of packaging cell lines that yield very high viral titers after transient transfection (Pear et al. 1993) can facilitate this approach (Kitamura et al. 1995), since the complexity of the library is a function of the number of infectious particles. Alternatively, the virus produced transiently from one packaging cell line can be used to infect a packaging cell line in a different interference group (Rayner and Gonda 1994; Zannettino et al. 1996). It should be pointed out that this type of amplification, which can be used to increase the titer of the viral stock, does not increase the complexity of the vector library.
The feasibility of using retroviral cDNA libraries to isolate known cDNAs has been demonstrated in model systems. cDNAs encoding rat thymidine kinase (Murphy and Efstratiadis 1987; Murphy and Schimke 1991), the cytokines interleukin-3 (IL-3) and granulocyte macrophage–colony-stimulating factor (GM-CSF) (Rayner and Gonda 1994; Wong et al. 1994), and the cell-surface proteins CD2 and the IL-3 receptor O chain (Kitamura et al. 1995) have been isolated. Retroviral vectors have been used to identify cDNAs with transforming activity. Seven novel cDNAs with transforming activity, three known oncogenes, and nine previously cloned genes not known to have transforming activity have been isolated (Whitehead et al. 1995). A retroviral library was used to identify cDNAs encoding cell surface antigens following transduction of FDC-P1 hematopoietic cells (Zannettino et al. 1996). Retroviral vectors have also been used to express libraries of cDNA fragments that encode short peptides to screen for suppressors of drug resistance or other phenotypes in cultured cells (Gudkov et al. 1993, 1994). Taken together, these results show that retroviral cDNA libraries are useful for the isolation of additional cDNAs with defined phenotypes.
Immunoglobulin Rearrangement
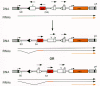
An innovative approach to the study of immunoglobulin gene rearrangement has been devised by constructing retroviral vectors that contain sequences from the immunoglobulin locus and studying the rearrangement of these sequences after the vector has been introduced into appropriate cells (Lewis et al. 1984; Landau et al. 1987; Desiderio and Wolff 1988). Although the same studies can be done by simply transfecting the immunoglobulin sequences, the advantage of using retroviral vectors is that the viral sequences are inserted in a precise, predictable manner into recipient cells and that the integration of a single copy per cell can be easily achieved. The vectors were constructed on the basis of the hypothesis that immunoglobulin rearrangement involves a reciprocal recombination between V and J elements which have opposite transcriptional orientations in the genomic DNA. Vectors were designed so that proper rearrangement of these sequences within the vector would result in expression of a drug resistance gene (gpt), which would permit selection for an appropriate rearrangement (Fig. 6). In the starting vector, the gpt-coding region is in the reverse orientation with regard to transcription and is not expressed; after rearrangement, the gpt gene is in the proper orientation for transcription. Splice acceptor and donor sequences are incorporated to promote the removal of sequences upstream of gpt, which, in the original configuration, prevented the proper translation of gpt. The vector also contains the neo selectable marker for an initial selection of cells containing the unrearranged vector. Cloning and sequencing of the rearranged immunoglobulin genes have permitted analysis of the recombination joints. This system can also be used to clone genes involved in immunoglobulin rearrangement and to study the effects of specific genes on rearrangement.
Insertional Mutagenesis for Gene Identification
Because retroviral vector integration does not generally lead to gross rearrangements of the genome of the infected cell, and because a helper-virus-free vector does not mobilize after the initial infection, retroviral vectors have been used as insertional mutagens. After mutagenesis, the presence of the vector at the site of the mutation provides a tag that marks the mutated gene. Provided the mutation is associated with a selectable phenotype, the mutated gene responsible for the phenotype can then be isolated by virtue of its association with vector sequences. This approach has been shown to work in cell culture in a model system involving mutations in the hprt gene (King et al. 1985). However, the direct application of this procedure to isolate new genes is limited to haploid genes or genes where mutation of one of two copies of the gene results in a selectable phenotype.
More recently, an elegant modification of this technique has been developed that allows functional knockout of the allelic locus as well as the mutated locus such that genes with recessive phenotypes can be identified (Li and Cohen 1996). The approach involves transduction of cells with a vector containing a β-geo-coding region (encoding a β-galactosidase/neomycin phosphotransferase fusion protein) located downstream from a splice acceptor, such that expression of β-geo is dependent on an upstream promoter and splice donor. The vector also contains an inducible reverse-orientation promoter that upon induction generates an antisense RNA that includes sequences from the mutated gene and that will block protein production from both the mutated and normal alleles. This approach was used by the authors to isolate a novel tumor suppressor gene by screening for mutant cells with a transformed phenotype.
Retroviral vectors can also be used to mutagenize totipotent mouse embryonic stem (ES) cells. Mouse ES cells can be efficiently transduced with retroviral vectors, and cell lines carrying at least 15 proviruses can be generated by cocultivation of ES cells with virus-producing cells (Robertson et al. 1986). The cells can then be reimplanted into mouse embryos to generate mice carrying the vector insertions. Since ES cells can contribute to the germ line, strains of mice carrying many integrated proviruses can be generated (Shih et al. 1988). Such animals should provide a rich source of interesting mutations, which will be marked by the presence of the integrated provirus. Mutagenesis of ES cells by transduction with a retroviral vector has also been used to generate specific mutations in mice by first selecting the appropriate phenotype in ES cells after vector transduction and then generating mice from the mutated cells. For example, hprt-deficient mice were generated from ES cells transduced with a retroviral vector that inactivated the hprt locus (Kuehn et al. 1987).
It should be noted, for these mutagenesis techniques, that since packaging cell lines transmit endogenous retroviral elements in addition to the intended retroviral vector (Rodland et al. 1987; Hatzoglou et al. 1990; Scadden et al. 1990; Ronfort et al. 1995; Purcell et al. 1996), some of the insertional mutations will not be marked by the vector, complicating the analysis of the mutations.
Transgenic Animals
Endogenous proviruses arise by the infection of germ cells or their precursors; new endogenous proviruses have been generated experimentally in mice (Huszar et al. 1985; Jahner et al. 1985; van der Putten et al. 1985; Soriano and Jaenisch 1986; Soriano et al. 1986; Stewart et al. 1987; Federspiel et al. 1996), chickens (Salter et al. 1987; Bosselman et al. 1989), and cattle (Kim et al. 1993; Haskell and Bowen 1995). These techniques are not likely to have much impact in mice, where transgenic techniques involving direct injection of DNA are well developed, but they may be important in other species where direct DNA transfer is difficult. For example, the reproductive physiology of chickens has made the direct injection of DNA into fertilized eggs quite difficult. In addition, the transgenic rate in cattle obtained with a GALV pseudotype retroviral vector was 7%, whereas the rate obtained by injection of DNA was only 0.1% (Haskell and Bowen 1995). This improvement in transgenic rates is important because it reduces the high cost of generating transgenic cattle to a more reasonable level. Limitations on the use of retroviral vectors for embryo gene transfer include all of the standard constraints on sequences that can be incorporated into retroviral vectors, such as the maximum size of the inserted DNA (see above Principles of Retroviral Vector Design, Replication-defective Vectors).
Vector Insertion as a Marker for Gene Activity during Development
Retroviruses can be designed such that expression of the integrated provirus is dependent on upstream cellular promoters and/or enhancers (von Melchner et al. 1989; Friedrich and Soriano 1991, 1993; Reddy et al. 1991b; Sablitzky et al. 1993). Typically, promoter-trap vectors are designed with the cDNA for a marker gene inserted into the U3 region of the vector so that expression of the marker is dependent on the insertion of the virus downstream from a promoter. In an enhancer-trap vector, a promoter is linked to the marker cDNA, but the enhancers are deleted so that expression is dependent on activation of the promoter by enhancers present near the site of integration. Such vectors can be used in the study of tissue-specific gene expression. The vector is introduced into ES cells and mice are generated, some of which show developmentally regulated expression of the marker gene on the vector. β-galactosidase is a good marker for such studies; histochemical staining can be used to monitor tissue-specific expression in the resultant mice (Friedrich and Soriano 1991).
Transfer of Genes That Regulate Development in Animals
Retroviral vectors have been used to transfer and analyze genes that regulate cell differentiation. Good examples include the transfer of genes regulating myogenesis, including MyoD, myogenin, Myf-5, and MRF4 (Weintraub et al. 1989; Weintraub 1993). Retroviral vectors are useful in this capacity because the high rates of gene transfer and the high levels of protein expression result in phenotypes that are more obvious than those seen with other gene transfer techniques.
In an extension of this work, a retroviral vector encoding MyoD has been used to develop a clinical screening test for Duchenne's muscular dystrophy (Sancho et al. 1993). The disease is characterized by a deficiency of dystrophin in muscle cells. Although muscle cells cannot be easily isolated from fetuses, other cell types, including amniocytes and chorionic villus cells, which are easy to obtain, can be induced to display a muscle phenotype by transduction of the MyoD gene. Immunohistochemical staining can then be used to determine the level of dystrophin expression that can be used for diagnosis. Again, retroviral vectors make this technique feasible because they can achieve a high level of efficient gene transduction into primary human cells.
Many of the classical studies in embryology involved avian embryos, primarily because they are easy to observe and manipulate. Retroviral vectors can be used to modify the normal patterns of expression of genes in the developing chick embryos; both the tools and the techniques used in such experiments are described in a recent review (Morgan and Fekete 1996). This system has been used to study genes that are important in the developing limb bud (Morgan et al. 1992; Laufer et al 1994). In most exeriments, a replication-competent retroviral vector is used. Careful placement of the virus in the embryo results in localized expression; however, with time, the virus will spread. A replication-competent viral vector (and the gene it expresses) can be confined to a particular part of a chick embryo if a chimeric embryo is constructed by transplanting target tissue from a susceptible embryo onto a resistant embryo (Fekete and Cepko 1993b).
Chromosome Tagging
Retroviral vectors carrying selectable genes can also be used to mark individual chromosomes. By using high multiplicities of infection, it is possible to generate multiple integration events at independent sites in each of the cells in a large cell population. Single chromosomes can be isolated from these cells and introduced into cells of a different species by microcell fusion techniques. Cells that have successfully acquired the chromosomes are obtained by selection for the marker carried by the retroviral vector (Nelson et al. 1984; Lugo et al. 1987; Shih et al. 1988, Cuthbert et al. 1995). Furthermore, any location on a given chromosome can be marked, providing a method that can be used to select for small regions of a chromosome (Weis et al. 1984). This technique has been successfully applied to cell types, including lymphoblastoid cells, that are difficult to mark by other techniques (Warburton et al. 1990). The ability to control the number of vector integrants in an infected cell gives this technique wide flexibility; in addition, it is important that the retroviral vector does not induce adventitious damage to, or rearrangement in, the targeted chromosomes.
Shuttle Vectors
A variety of sequences have been included in retroviral vectors to allow shuttling of the vectors among animal cells, bacteria, and bacteriophage. Origins of replication and drug resistance genes from bacterial plasmids (amp and neo) have been included to facilitate the cloning of vector and flanking sequences from cells containing integrated vectors (Cepko et al. 1984; Berger and Bernstein 1985; Jhappan et al. 1986). Similarly, tRNA suppressor genes can be included in retroviral vectors to facilitate the cloning of proviral and flanking cellular sequences in bacteriophage libraries (Lobel et al. 1985; Reik et al. 1985). The inclusion of a tRNA suppressor in a retrovirus was instrumental in the first demonstration of integration in vitro, a critical step in the elucidation of the process (Brown et al. 1987) (see Chapter 5. Inclusion of the SV40 origin of replication in a vector allows a specific amplification of vector and flanking sequences when the infected cells are fused to COS cells. COS cells constitutively express SV40 T antigen, which acts at the SV40 origin in the vector to induce multiple rounds of DNA replication from the SV40 origin. Circular replicons are produced that are formed by recombination between vector sequences and/or flanking cellular sequences. Low-molecular-weight DNA obtained by Hirt extraction from such fused cells is a highly enriched source of vector DNA and of sequences flanking the retroviral integration site(s). These techniques provide simple methods for rescuing and recloning recombinant proviruses and flanking cellular sequences.
Vectors Designed to Study Retroviral Recombination
Retroviral vectors have been quite useful in studying viral replication, mutation, and recombination. Most of these experiments are described in other chapters. However, it is appropriate to describe here how vectors can be specifically designed to measure, for example, steps in the process by which retroviruses acquire nonhomologous sequences. Although such events are rare, retroviruses can acquire oncogenes from cellular genes, and recombination could in theory occur at the level of DNA or RNA (see Chapters 4 and 10). Retroviral vectors can be used to create proviral elements with defined structure that can be used as substrates to study recombination. In one study, Zhang and Temin (1993) inserted a murine retroviral vector that expressed hygromycin phosphotransferase but lacked a 3′LTR in the reverse orientation in a self-inactivating spleen necrosis virus vector (see Fig. 4). The spleen necrosis virus-derived portion of the vector was inactivated after cell transduction, but the hygromycin phosphotransferase was expressed from the MLV LTR. Retroviral packaging cells were infected with this vector and with a complete murine vector that expressed neomycin phosphotransferase. Virus capable of transducing hygromycin resistance could be obtained from this cell line and was found to contain a variety of recombination events that repaired the 3′LTR of the vector carrying hygromycin phosphotransferase. In this case, where the two RNAs involved in the recombination could be efficiently packaged into virions, recombination probably occurred via strand switching during reverse transcription. Other studies have shown that recombination events can arise by readthrough transcription into sequences downstream from an integrated retrovirus, followed by further rearrangements to yield transmissible virus (Swain and Coffin 1992; see also Chapter 4.
Intron Removal
Retroviral vectors have been used to generate cDNAs from genomic copies of genes (Shimotohno and Temin 1982; Sorge and Hughes 1982; Kriegler et al 1984; Brown et al. 1986; Jat et al. 1986; Noda et al. 1986; Auffray et al. 1987; Feldman et al. 1987; Kaplan et al. 1987; Morgan et al. 1988; Joly and Oldstone 1991; Bates et al. 1993). The gene to be converted into a cDNA is inserted into a retroviral vector in a forward orientation. A bacterial origin of replication and a selectable marker that functions in bacteria can be included in the vector to allow facile cloning of the recombinant retrovirus (Cepko et al. 1984). The introns are removed during the RNA phase of the viral life cycle: A cDNA copy can be conveniently obtained from either integrated or unintegrated viral DNA. This approach has been used to obtain cDNAs that arise from differential gene splicing, such as the SV40 large T and small T antigens (Kriegler et al. 1984).
Cellular Immortalization
Many types of primary somatic cells, especially those from humans, grow only for relatively short periods of time in culture before the cells senesce and die. Many of the methods for generating immortal cell lines from these somatic cells, for example, infection of human fibroblasts with SV40, are relatively inefficient. Retroviral vectors expressing the SV40 T antigen are more efficient (Reilly 1990; Bartek et al. 1991; Paul et al. 1991; Pfeifer et al. 1993). Recently, a vector carrying the E6 and E7 genes of human papillomavirus type 16 has been shown to cause efficient immortalization of several primary cell types, including human keratinocytes (Halbert et al. 1991) and human smooth muscle cells (Perez-Reyes et al. 1992). This technique will probably be useful for generating cell lines from other tissues and will be especially useful when relatively small numbers of target cells are available.
Antisense RNA and Ribozyme Production
Retroviral vectors have been used to inhibit the expression of specific genes by the inclusion of transcriptional units intended to induce the synthesis of antisense RNA or specific ribozymes. Antisense RNAs have been used to inhibit infection of cells by retroviruses containing the sense-strand RNA (To et al. 1986; Han et al. 1991) and to inhibit expression of proteins from sense-strand RNAs (Trevor et al. 1987). These techniques are being explored for use against HIV (Yu et al. 1993; Gilboa and Smith 1994). However, it must be said at this point, despite the fact that a considerable effort has been made to use retroviral vectors as vehicles to express a variety of antisense RNAs, that there have been relatively few successes, and it would appear that this technique has limited utility. These data describing the production of specific ribozymes using retroviral vectors are much more limited, and a determination of the general usefulness of this technique awaits additional experimentation.
- Constitutive Gene Expression
- Regulated Gene Expression
- Cell Lineage Analysis
- cDNA Library Construction
- Immunoglobulin Rearrangement
- Insertional Mutagenesis for Gene Identification
- Transgenic Animals
- Vector Insertion as a Marker for Gene Activity during Development
- Transfer of Genes That Regulate Development in Animals
- Chromosome Tagging
- Shuttle Vectors
- Vectors Designed to Study Retroviral Recombination
- Intron Removal
- Cellular Immortalization
- Antisense RNA and Ribozyme Production
- Experimental Applications - RetrovirusesExperimental Applications - Retroviruses
Your browsing activity is empty.
Activity recording is turned off.
See more...