Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Glycerol Phenylbutyrate (Ravicti) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017 Apr.
Manufacturer’s Model Structure
The natural history for urea cycle disorders (UCDs) was modelled by the manufacturer using the structure in the subsequent figure.
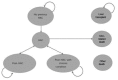
Figure 1
Manufacturer’s Model Structure. HAC = hyperammonemic crisis. Source: Manufacturer’s pharmacoeconomic submission.
A discussion of the model structure is provided in the main body of the CADTH Common Drug Review (CDR) pharmacoeconomic report. Treatment was incorporated into this natural history by modifying the costs of the “No previous HAC” (hyperammonemic crisis) and “HAC” states, and by modifying the probability of HAC. In this way, the changes to the model induced by treatment seek to account for how treatment modifies the natural history of the disease.
Table 10Data Sources
| Data Input | Description of Data Source | Comment |
|---|---|---|
| Efficacy | Evidence was claimed based on analysis of one week’s and two weeks’ trial data on short-term ammonia control, within the common technical document provided.2 These trials include one double-blind crossover trial (HPN-100-006), and three open-label fixed-sequence switchover trials (HPN-100-005, -012, and UP 1204-003). Evidence is presented relating blood ammonia levels to risk of HAC.4 | See note. |
| Natural history | Liver-transplantation probabilities based on reanalysis of a table within a single paper. One scenario analysis does consider the possibility of chronic conditions as a result of HAC, although no data were provided. | There is limited natural history in the model, although the model does include liver transplantation and HACs. There are issues with the way that these states are incorporated, as detailed subsequently. |
| Utilities | Clinical opinion was provided to obtain utility inputs for UCD patients (0.55), plus decrements for HAC (0.50). | All utilities were based upon clinical opinion. |
| Resource use | See “costs;” these were based on assumptions. | |
| AEs | Not considered in the model, and stated to be with the support of Canadian clinical experts5 | |
| Costs | ||
| Drug | GPB based on manufacturer’s price, Pheburane on Quebec list prices | |
| AEs | Not considered in the model, and stated to be with the support of Canadian clinical experts5 | Assumed to be comparable and minor |
| Health state | Costs for HAC states were estimated using detailed assumptions, and considered both drug treatment and hospital stays. Costs of follow-up treatments following HACs were again based on detailed assumptions, considering specialists, MRIs, dietitians, and nurse practitioners. A one-off liver-transplant cost was set at $127,700 based on data from Alberta.2 | Given that the disease is rare, some degree of assumption is expected. However, there is no broad comparison against other international figures to give context. |
| End of life | End-of-life costs were assumed from a Canadian source at around $68,000 per death.6 | The use of this cost may be controversial. |
AE = adverse events; GPB = glycerol phenylbutyrate; HAC = hyperammonemic crisis; MRI = magnetic resonance imaging; UCD = urea cycle disorder.
Note: (1) The primary outcomes reported from the clinical trials of GPB and sodium phenylbutyrate were ammonia levels. The manufacturer cites an estimated relationship between ammonia levels and HACs and uses linear interpolation between these two estimated points to further estimate HAC risks for dietary control well beyond the range defined by these points (a difference of 91.7 μmol/L, versus points at 10 μmol/L and 25 μmol/L). The estimated relative risk of 8.42 for dietary control is discussed further in Appendix IV.
Table 11Manufacturer’s Key Assumptions
| Assumption | Comment |
|---|---|
| Mortality post–liver transplant based on three-year survival figures (93% survival) or background population mortality, whichever is larger | Mortality following a liver transplant is not necessarily constant over time. However, the impact is expected to be minor. |
| No uncertainty in liver transplant probability in patients with late-onset UCD | This appears inappropriate, as uncertainty was assumed for patients with early-onset UCD, as well as a relationship between the relative risks of both groups. |
| No separate state for initial and subsequent years following a liver transplant | The model applies the cost of liver transplantation only to new transplants but does not appear to apply an ongoing cost following transplantation, or to apply a different utility to the year of transplant than to following years. |
| Probabilistic sensitivity analysis run on 1,000 model runs. | This may be insufficient when parameters such as relative risks are modelled using lognormal distributions, as significant uncertainty exists. |
| Linear interpolation used to estimate HAC rates for Pheburane. | See Appendix 4 |
| All patients receiving sodium phenylbutyrate use Pheburane. | This is not necessarily accurate but is a conservative assumption and, given the information provided (higher patient acceptability, cheaper price), seems reasonable. |
| No increase in utility following liver transplant | This was considered beyond the scope of the analysis by the manufacturers. |
| Probabilistic sensitivity analyses use normal distribution to model uncertainty in cost parameters. | This is not good practice, since cost parameters are expected to be skewed. A lognormal or gamma distribution is more appropriate. |
| Probabilistic sensitivity analyses assume that standard errors are 20% of mean values when data are not available. | This is relevant for liver-transplantation probabilities. It is unclear how much of an impact this has, and for some parameters this would be a very small uncertainty to assume. In probabilities, this may not be as problematic. |
| Liver transplantation in neonatal patients based on a simulated cohort | A single paper was used4 to identify age of liver transplantation in a cohort diagnosed as neonates. However, most of these patients are living, and so the manufacturer’s analysis that assumes that all patients survive to 100 years of age, is inappropriate. See note (1). |
| Adherence is not considered in the model. | The manufacturer identifies that adherence with sodium phenylbutyrate is difficult for many patients, but the model does not fully reflect this alternative. See note (2). |
HAC = hyperammonemic crisis; UCD = urea cycle disorders.
Note:
- 1
In order to estimate the probability of receiving a transplant among patients with early onset UCD, the manufacturer uses a paper reporting on a birth cohort of patients with a UCD. This paper reports when patients received transplants across five age bands (to 6 years, from 6 years to 12 years, from 12 years to 24 years, from 24 years to 48 years, and 48 years and over) but does not report the current age of patients. In order to obtain probabilities, the manufacturer assumes that patients survive within each of the five age bands provided, i.e., that all patients in that birth cohort have either survived to 100 years of age, or have received a liver transplant at an earlier point in time. For example, when computing a probability of liver transplantation for patients between 12 and 24 years of age, the manufacturer assumes that all patients who do not receive a liver transplant have been observed through 24 years of age. A probability of not receiving a transplant is computed by dividing the numbers of patients who have not received transplants by the age 24 by the number who had not received transplants at age 12, and further dividing this figure by 12 years to obtain a “rate” (which is itself inappropriate). As we do not know that all patients survive, and as many patients will be within that range at the time of observation, this approach is likely to underestimate the true probability of liver transplantation. The same issues are likely to be the case for all of the probabilities for liver transplantation, and especially for those aged 48 years or older; the manufacturer assumes that all of these patients somehow survived until 100 years of age, despite the higher mortality rates assumed owing to urea cycle disorder. The manufacturer assumes that the relative risk of transplantation in patients with late-onset UCD versus those with early-onset UCD is 0.2, based on clinical opinion.
- 2
The impact of this is likely to unrealistically increase the cost-effectiveness of GPB versus sodium phenylbutyrate, since (1) this does not consider the possibility that GPB is not tolerated and (2) the alternative treatment (dietary control) appears to be more cost-effective generally than either GPB or sodium phenylbutyrate.
Manufacturer’s Results
Table 3 provides the manufacturer’s results for the probabilistic sensitivity analysis (PSA) for glycerol phenylbutyrate (GPB) in the four subgroups considered. In each of the subgroups, GPB becomes cost-effective only at incremental cost-utility ratios (ICURs) well above an indicative cost per quality-adjusted life-year (QALY) threshold of $50,000. In three of four subgroups, this figure exceeds $1 million per QALY, with the two subgroups comparing GPB with sodium phenylbutyrate (NaPBA) having ICURs exceeding $5 million per QALY.
These probabilistic results used selected parameters and produced results very similar to the deterministic analysis. This PSA was formed using 1,000 model runs, with the manufacturer’s model (as provided) able to accept up to 10,000 model runs. Parameters included in the PSA are summarized in Table 12.
Table 12
Parameters Included/Not Included in PSA.
Alongside the PSA, the manufacturer provided tornado diagrams displaying one-way sensitivity analyses for those variables that were varied in the PSA.
An additional set of scenario analyses was also conducted to assess other assumptions in the model.
Table 13Parameters Modified in Sensitivity Analysis
| Parameter modified | Original value | Revised value(s) |
|---|---|---|
| Cost of alternative treatment, using Buphenyl as a sodium phenylbutyrate treatment, as opposed to Pheburane | $48.75/g | $19.20/g |
| Mortality rate from liver transplantation | 0.2% | 0% |
| Relative risk of UCD patients vs. general population | 3 | 2, 4 |
| Relative risk of HAC for DC patients | 8.42 | 6.5, 10.5 |
| Utility decrement associated with HAC | 0.5 | 0.3, 0.4 |
| Utility associated with UCD patients | 0.55 | 0.45, 0.65, 0.75 |
| Relative risk of liver transplant for patients with late onset compared with neonatal onset UCD | 0.02 | 0, 0.05, 1 |
| Length of HAC | 7 | 3.5, 10.5 |
| Time horizon | To 100 | 1, 5, 10 years |
| Discounting | 5% | 0%, 3% |
DC = dietary control plus supplementation only; HAC = hyperammonemic crisis; UCD = urea cycle disorder.
Source: Adapted from the manufacturer’s pharmacoeconomic submission.2
One-way sensitivity analyses
While the manufacturer’s tornado diagrams state that they display “net benefits,” it does not appear that this is the case, and instead ICURs are displayed. Overall, the changes considered in the one-way sensitivity analysis do not make the ICUR for GPB approach the indicative cost-effectiveness threshold values. For Subgroup 1 and Subgroup 3, the ICUR does not fall below $2,500,000 per QALY, whereas, for Subgroup 2 and Subgroup 4, the ICURs remain more than $700,000 and $500,000 per QALY, respectively.
Scenario analyses
Within the 88 different scenario analyses provided by the manufacturer, there are only three scenarios in which GPB would be considered cost-effective at a threshold of $500,000 per QALY (i.e., at 10 times an indicative $50,000 per QALY).
Table 14 displays the two scenarios in which GPB dominates alternative treatments, when NaPBA is provided using the more expensive Buphenyl option. In the base-case analysis, it is assumed that only Pheburane is used; if this is not the case, then this scenario suggests that there may be an impact on estimates of cost-effectiveness. (Note that the manufacturer submission’s Table 28, which suggests that GPB is dominated, is incorrect.)
Table 14
Selected Scenario Analyses.
The third scenario deals with Subgroup 4. The relative risk of HAC is assumed to be 10.5 times for those receiving dietary control versus GPB. In this case, the ICUR is $480,733, with details in Table 14.
In a sensitivity analysis, a separate “Post-HAC with chronic condition” state was added to the model, assuming that 50% of those who enter the model would have previously experienced a HAC and would begin in this state. In the model, an individual beginning a period in a post-HAC no chronic condition could transit to HAC, and then have a 50% chance (if surviving and not receiving a transplant) of being in either post-HAC (no chronic condition) or “Post-HAC with chronic condition.” However, the way this is coded also means that same individual could, after having a HAC, have a 50% chance (if surviving and not receiving a transplant) of returning to “Post-HAC no chronic condition.” This appears inappropriate, as a HAC may cure a chronic condition. While this is a potentially important issue in terms of the disease progression, the manufacturer’s model cannot address this without more data and substantial changes to the submitted model.
CADTH Common Drug Review Reanalyses
Several concerns were raised surrounding the manufacturer’s submission. These were modified in the ways identified within the main body of this report. In order to implement the threshold analysis, the final model was run with a 0%, 30%, 40%, and 50% price discount for GPB. Based on these results, the ICUR values were interpolated to identify likely price discounts at which each subgroup would likely “reach” a cost per QALY of $200,000 per QALY.
The CDR intermediate reanalysis provides findings relatively similar to the original manufacturer’s analysis, although the ICURs for GPB are generally slightly higher than those reported by the manufacturer.
Table 15CDR Intermediate Reanalysis – Results for Patients with Urea Cycle Disorder
| Costs | QALYs | Incremental Costs | Incremental QALYs | ICUR ($/QALYs) | |
|---|---|---|---|---|---|
| SUBGROUP 1 | |||||
| NaPBA | $2,580,167 | 9.81 | |||
| GPB | $3,893,845 | 10.02 | $1,313,678 | 0.21 | $6,214,030 |
| SUBGROUP 2 | |||||
| DC | $2,180,226 | 7.79 | |||
| GPB | $4,441,678 | 9.59 | $2,261,453 | 1.80 | $1,257,935 |
| SUBGROUP 3 | |||||
| NaPBA | $1,910,683 | 9.50 | |||
| GPB | $2,811,783 | 9.65 | $901,101 | 0.15 | $5,841,454 |
| SUBGROUP 4 | |||||
| DC | $2,063,474 | 7.98 | |||
| GPB | $3,333,151 | 9.60 | $1,269,677 | 1.63 | $780,570 |
CDR = CADTH Common Drug Review; DC = dietary control plus supplementation only; GPB = glycerol phenylbutyrate; ICUR = incremental cost-utility ratio; NaPBA = sodium phenylbutyrate; QALY = quality-adjusted life-year.
It is noticeable, however, that the revised analysis differs from the “intermediate” one. As predicted, the cost-effectiveness of GPB relative to NaPBA has increased (although it does not fall below $2,000,000 per QALY), but the cost-effectiveness of GPB relative to dietary control has decreased.
Table 16CDR Revised Analysis – Results for Patients with Urea Cycle Disorder (0% Price Discount)
| Costs | QALYs | Incremental Costs | Incremental QALYs | ICUR ($/QALYs) | |
|---|---|---|---|---|---|
| SUBGROUP 1 | |||||
| NaPBA | $2,719,926 | 9.56 | |||
| GPB | $3,895,776 | 10.02 | $1,175,850 | 0.46 | $2,559,450 |
| SUBGROUP 2 | |||||
| DC | $1,991,358 | 7.98 | |||
| GPB | $4,443,465 | 9.58 | $2,452,107 | 1.60 | $1,532,491 |
| SUBGROUP 3 | |||||
| NaPBA | $2,031,362 | 9.30 | |||
| GPB | $2,812,972 | 9.65 | $781,610 | 0.34 | $2,288,790 |
| SUBGROUP 4 | |||||
| DC | $1,874,190 | 8.16 | |||
| GPB | $3,333,535 | 9.60 | $1,459,345 | 1.44 | $1,012,665 |
CDR = CADTH Common Drug Review; DC = dietary control plus supplementation only; GPB = glycerol phenylbutyrate; ICUR = incremental cost-utility ratio; NaPBA = sodium phenylbutyrate; QALY = quality-adjusted life-year.
Table 17CDR Revised Analysis – Results for Patients with Urea Cycle Disorder (30% Price Discount)
| Costs | QALYs | Incremental Costs | Incremental QALYs | ICUR ($/QALYs) | |
|---|---|---|---|---|---|
| SUBGROUP 1 | |||||
| NaPBA | $2,720,038 | 9.56 | |||
| GPB | $2,867,735 | 10.02 | $147,697 | 0.45 | $324,737 |
| SUBGROUP 2 | |||||
| DC | $1,999,730 | 7.99 | |||
| GPB | $3,239,014 | 9.58 | $1,239,283 | 1.59 | $779,241 |
| SUBGROUP 3 | |||||
| NaPBA | $2,030,343 | 9.31 | |||
| GPB | $2,083,521 | 9.65 | $53,177 | 0.34 | $157,546 |
| SUBGROUP 4 | |||||
| DC | $1,876,453 | 8.17 | |||
| GPB | $2,456,356 | 9.60 | $579,903 | 1.43 | $405,100 |
CDR = CADTH Common Drug Review; DC = dietary control plus supplementation only; GPB = glycerol phenylbutyrate; ICUR = incremental cost-utility ratio; NaPBA = sodium phenylbutyrate; QALY = quality-adjusted life-year.
Table 18CDR Revised Analysis – Results for Patients with Urea Cycle Disorder (40% Price Discount)
| Costs | QALYs | Incremental Costs | Incremental QALYs | ICUR ($/QALYs) | |
|---|---|---|---|---|---|
| SUBGROUP 1 | |||||
| NaPBA | $2,716,249 | 9.56 | |||
| GPB | $2,523,318 | 10.02 | −$192,931 | 0.46 | DOMINATES |
| SUBGROUP 2 | |||||
| DC | $1,992,783 | 7.98 | |||
| GPB | $2,833,968 | 9.58 | $841,185 | 1.60 | $524,197 |
| SUBGROUP 3 | |||||
| NaPBA | $2,028,919 | 9.31 | |||
| GPB | $1,840,787 | 9.65 | −$188,132 | 0.34 | DOMINATES |
| SUBGROUP 4 | |||||
| DC | $1,877,778 | 8.15 | |||
| GPB | $2,164,177 | 9.60 | $286,399 | 1.45 | $198,052 |
CDR = CADTH Common Drug Review; DC = dietary control plus supplementation only; GPB = glycerol phenylbutyrate; ICUR = incremental cost-utility ratio; NaPBA = sodium phenylbutyrate; QALY = quality-adjusted life-year.
Table 19CDR Revised Analysis – Results for Patients with Urea Cycle Disorder (50% Price Discount)
| Costs | QALYs | Incremental Costs | Incremental QALYs | ICUR ($/QALYs) | |
|---|---|---|---|---|---|
| SUBGROUP 1 | |||||
| NaPBA | $2,718,236 | 9.57 | |||
| GPB | $2,179,612 | 10.02 | −$538,625 | 0.46 | DOMINATES |
| SUBGROUP 2 | |||||
| DC | $1,993,102 | 7.99 | |||
| GPB | $2,431,138 | 9.59 | $438,036 | 1.60 | $274,142 |
| SUBGROUP 3 | |||||
| NaPBA | $2,029,828 | 9.31 | |||
| GPB | $1,596,349 | 9.65 | −$433,478 | 0.34 | DOMINATES |
| SUBGROUP 4 | |||||
| DC | $1,876,895 | 8.16 | |||
| GPB | $1,869,395 | 9.60 | −$7,500 | 1.44 | DOMINATES |
CDR = CADTH Common Drug Review; DC = dietary control plus supplementation only; GPB = glycerol phenylbutyrate; ICUR = incremental cost-utility ratio; NaPBA = sodium phenylbutyrate; QALY = quality-adjusted life-year.
In the base-case analysis, the ICURs for NaPBA exceed $2 million per QALY, which is 40 times an indicative threshold of $50,000 per QALY. For those currently prescribed NaPBA, the price of GPB would need to be discounted by around 32% (late onset) and 30% (early onset) to obtain even an ICUR of $200,000 per QALY. To obtain an ICUR at an indicative threshold of $50,000 per QALY, these discounts would need to increase to 34% and 32%, respectively.
Higher discounts are required to obtain cost-effectiveness for those who do not tolerate NaPBA. This is because the cost of the alternative treatment (dietary control) is lower, and because dietary control represents a more cost-effective use of resources than NaPBA. In the case of those on dietary control, a discount of around 59% is necessary to obtain an ICUR below $50,000 per QALY in patients with late-onset UCD, and 47% in those with early-onset UCD. Even at a higher $200,000 per QALY figure, the discounts necessary are still 53% and 40%, respectively.
Table 20CDR Revised Analysis – Threshold Analysis for Discounts
| Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 | |
|---|---|---|---|---|
| GPB vs. NaPBA ICUR ($/QALY) | GPB vs. DC ICUR ($/QALY) | GPB vs. NaPBA ICUR ($/QALY) | GPB vs. DC ICUR ($/QALY) | |
| No discount | $2,559,450 | $1,532,491 | $2,288,790 | $1,012,665 |
| 30% discount | $324,737 | $779,241 | $157,546 | $405,100 |
| 32% discount | $173,471 | $722,734 | $13,665 | $358,819 |
| 34% discount | $24,578 | $682,118 | DOMINATES | $325,418 |
| 40% discount | DOMINATES | $524,197 | DOMINATES | $198,052 |
| 47% discount | DOMINATES | $347,151 | DOMINATES | $53,470 |
| 50% discount | DOMINATES | $274,142 | DOMINATES | DOMINATES |
| 53% discount | DOMINATES | $196,783 | DOMINATES | DOMINATES |
| 59% discount | DOMINATES | $43,860 | DOMINATES | DOMINATES |
CDR = CADTH Common Drug Review; DC = dietary control plus supplementation only; GPB = glycerol phenylbutyrate; ICUR = incremental cost-utility ratio; NaPBA = sodium phenylbutyrate; QALY = quality-adjusted life-year; vs. = versus.
- REVIEWER WORKSHEETS - Glycerol Phenylbutyrate (Ravicti)REVIEWER WORKSHEETS - Glycerol Phenylbutyrate (Ravicti)
- Executive Summary of the Pharmacoeconomic Submission - Pharmacoeconomic Review R...Executive Summary of the Pharmacoeconomic Submission - Pharmacoeconomic Review Report: dolutegravir (Tivicay)
- EXECUTIVE SUMMARY - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)EXECUTIVE SUMMARY - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)
- Pharmacoeconomic Review Report - Glycerol Phenylbutyrate (Ravicti)Pharmacoeconomic Review Report - Glycerol Phenylbutyrate (Ravicti)
Your browsing activity is empty.
Activity recording is turned off.
See more...
