Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Glycerol Phenylbutyrate (Ravicti) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017 Apr.
The manufacturer’s calculated relative risk for hyperammonemic crisis (HAC) appears to be a potential key driver of results, especially within the model in Subgroup 2 and Subgroup 4, in which dietary control is considered as the comparator for glycerol phenylbutyrate (GPB). As outlined in the preceding sections, the estimates for the relative risk of HAC are based on an extrapolation of already estimated points. Any linear interpolation of this type is inherently risky, as the relationships that underlie data within a given range do not necessarily determine the data over other ranges. In particular, the studies appear to compare the incidence of HACs in periods when patients receive GPB and periods when they receive sodium phenylbutyrate (NaPBA).
To illustrate the difficulty with using linear interpolation in this way, we present three diagrams. Figure 2 displays the reported relative risks for differences in ammonia levels versus GPB outcomes. These data are presented in the manufacturer’s submission Table 3, with error bars displaying the confidence intervals for each data point. The manufacturer proposes using a linear interpolation between the data points at a difference of 10 and a difference of 25. When plotted, it is noticeable that this relationship does not explain the points at differences of one and five very well at all. (There is an additional point at zero; trivially, if there is no difference, we would expect a relative risk of HACs of one.)
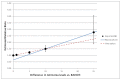
Figure 2
Manufacturer’s Reported Relative Risks and Alternative. RR = relative risk.
The second fitted curve shows the estimated relationship if there is a linear interpolation between a difference of zero and a difference of 10. This line passes within the confidence intervals for every one of the data points, and provides an arguably more robust fit.
Figure 3 shows these curves extended to allow them to explain points outside the range of the data. At the difference in ammonia levels claimed by the manufacturer for dietary control of 91.7 μmol/L, the difference is between the relative risk of 8.42 claimed by the manufacturer and relative risk of 5.62 in the alternative extrapolation. This establishes that, if using these points, there may be significant uncertainty about which point to use.
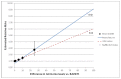
Figure 3
Manufacturer’s Reported Relative Risks and Alternative, Extrapolated. NaPBA = sodium phenylbutyrate; RR = relative risk.
Although the manufacturer uses linear interpolation, this is not consistent with the analysis that underlies the data presented (including the error bars, etc.). That is, the analysis determines the relationship between the two variables, and defines how this should be approached in any interpretation of that analysis.
In this case, the analysis appears to fit a linear relationship between log-relative risk and difference in ammonia levels. Since this estimated relationship can be extracted, the estimates based on this analysis are available and shown in Figure 4.

Figure 4
Relative Risk Inferred Using Prior Analysis, Versus Prior Estimates.
However, this leads to a relative risk of 42.18. As the GPB analysis uses an average of 0.27 HACs per patient, per year, this suggests 11.4 HACs per year under dietary control, so that those on dietary control are assumed to have HACs nearly every month if using this analysis.
Revised Results Based on Reconstructed Model (CDR Baseline)
A safer approach may be to use the data available. The manufacturer uses an observational study of patients receiving Pheburane,3 which reports data for 25 patients who had been unable to tolerate Buphenyl. That is, in the previous six months they either were unable to use Buphenyl (so used dietary control only) or used it but had extreme difficulty tolerating it. (The degree to which this really represents a “dietary control” group is unclear.) The manufacturer uses ammonia levels measured in this study, although the more relevant data are likely to be the historic number of HACs in the six months before the study. In the 25 patients enrolled, there were a total of 22 HACs. As there were 25 patients over six months, there were a total of 150 patient-months. HACs occurred in 22 of these patient-months, and did not occur in the remaining 128 months. Expressed as a probability, the parameter representing the monthly risk of HACs under dietary control can be conservatively modelled as coming from the distribution beta (22, 128), since some patients were still receiving NaPBA (poorly tolerated Buphenyl).
Likewise, Lee et al. 20154 provides data for the GPB and Pheburane groups. For the former, there were 27 HACs over 12 years among 100 patients (1,200 patient-months), yielding 0.27 HACs per year. For the latter, it was reported that there had been 54 HACs in the year before GPB treatment (on Pheburate), for a frequency of 0.54 HACs per year. The probabilities of HACs occurring under GPB and NaPBA can be computed in a similar fashion as with dietary control, leading to distributions beta (27, 1173) and beta (54, 1146), respectively.
Using this data directly allows us to avoid using the estimated relationship between ammonia levels and HAC rates. The short-term GPB trials are not used but, instead, the three longer-term studies and/or extensions are used (HPN-100-007, HPN-100-005 safety extension, HPN-100-012 safety extension). However, the methodological quality of this data is poor as these studies are uncontrolled (in the case of the 60 patients in HPN-100-007) or open-label (in the case of the 40 patients across the two safety extension studies).
- CLINICAL DATA USED IN REANALYSIS - Glycerol Phenylbutyrate (Ravicti)CLINICAL DATA USED IN REANALYSIS - Glycerol Phenylbutyrate (Ravicti)
- ISSUES FOR CONSIDERATION - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)ISSUES FOR CONSIDERATION - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)
- SUMMARY OF MANUFACTURER’S SENSITIVITY ANALYSES - Ustekinumab (Stelara)SUMMARY OF MANUFACTURER’S SENSITIVITY ANALYSES - Ustekinumab (Stelara)
- OBJECTIVES AND METHODS - Ustekinumab (Stelara)OBJECTIVES AND METHODS - Ustekinumab (Stelara)
- Clinical Review Report - Ustekinumab (Stelara)Clinical Review Report - Ustekinumab (Stelara)
Your browsing activity is empty.
Activity recording is turned off.
See more...
