Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Glycerol Phenylbutyrate (Ravicti) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2017 Apr.
3.1. Findings from the Literature
A total of one study was identified from the literature for inclusion in the systematic review (Figure 2). The included studies are summarized in Table 4 and described in Section 3.2. A list of excluded studies is presented in APPENDIX 3.
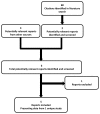
Figure 2
Flow Diagram for Inclusion and Exclusion of Studies.
Table 4
Details of Included Studies.
3.2. Included Studies
3.2.1. Description of Studies
One phase III randomized, double-blind (DB), double-dummy, active-controlled crossover study24 (HPN-100-006) met the inclusion criteria for this systematic review.
This study assessed the noninferiority of GPB to NaPBA by evaluating blood ammonia levels in adult patients with UCDs who had been on a stable dose of NaPBA (the mean baseline NaPBA dose was 14.54 ± 6.808 g per day [mean ± standard deviation (SD)]) for at least one week before study day 1. Eligible patients were randomly assigned at a 1:1 ratio to one of two treatment arms, using a computer-generated central randomization schedule. All investigators and study personnel, including the site pharmacist, were blinded to the study drug assignment. In the case of a medical emergency, when knowledge of the treatment assignment was essential to the well-being of the patient, the investigator or designee may have requested unblinding of the patient’s treatment assignment. In Arm A, patients received NaPBA plus GPB placebo for two weeks followed by GPB plus NaPBA placebo for two weeks; in Arm B, patients received GPB plus NaPBA placebo for two weeks followed by NaPBA plus GPB placebo for two weeks. There were no washout periods between the two treatments because of safety reasons.
No interim analysis was performed. The Data and Safety Monitoring Board (DSMB) convened on May 17, 2010, as planned, after approximately 50% of patients had received treatment, and reviewed available data on 19 of the 23 enrolled patients. No safety concerns were noted, and the DSMB recommended continuation of patient enrolment in the study. The final study visit was planned on day 29. Patients who completed the study and met study entry criteria were offered the opportunity to enrol in an open-label, long-term safety study of GPB (HPN-100-007).
3.2.2. Populations
a. Inclusion and Exclusion Criteria
To be eligible, patients were required to be at least 18 years of age and to have a confirmed diagnosis of a UCD. Deficiencies of CPS1, OTC, or ASS were included. Patients should have been on a stable dose of NaPBA for at least one week before day 1. For patients who were NaPBA-naive at the initial screening visit but had the potential to benefit from treatment, they could have started receiving NaPBA during the screening period and been enrolled in the study as long as they were on a stable dose of NaPBA for at least one week before day 1. Patients were excluded if their baseline ammonia level was greater than or equal to 100 μmol/L or if they had signs and symptoms suggesting hyperammonemia two weeks before screening; active infection or any other intercurrent condition that may have increased ammonia levels; any clinical or laboratory abnormality or medical condition that may put them at increased risk by participating in the study; investigational drug intake within 30 days of the study; or liver or hepatocellular transplant.
b. Baseline Characteristics
The mean age of patients was 32.73 years in HPN-100-006. In general, most patients were female (68.9%), white (77.8%), and had OTC deficiency (88.9%). Most patients had childhood (44.4%) or adult onset of a UCD (33.3%). Approximately 20% of the study participants had experienced at least one hyperammonemic crisis (HAC) within one year before the study. The mean ammonia level at the time of disease diagnosis was 165 μmol/L. The mean duration of previous treatment with NaPBA was 129 months. Details of patients’ baseline characteristics are presented in Table 5.
Table 5
Summary of Baseline Characteristics in Study HPN-100-006 (Safety Population).
3.2.3. Interventions
In study HPN-100-006, patients randomized to Arm A received NaPBA plus GPB placebo for two weeks followed by GPB plus NaPBA placebo for two weeks. In Arm B, patients received GPB plus NaPBA placebo for two weeks followed by NaPBA plus GPB placebo for two weeks. There was no washout period between the two treatment periods.
Each millilitre of Ravicti equals 1.1 g GPB and delivers 1.02 g PBA. Dose of GPB was calculated from the NaPBA dose determined by the investigator for each patient, such that each patient received the same amount of PBA during treatment with both drugs. The formula was: NaPBA dose (grams) × 0.95/1.1 = total daily GPB dose (millilitres). No adjustment to the dose or schedule of GPB was allowed during the study. The maximum allowed GPB dose was 17.4 mL per day, which was equivalent to 20 g per day of NaPBA. GPB and GPB placebo were supplied as liquid to be administered undiluted orally (by mouth or through gastrostomy, or nasogastric tube.
The NaPBA formulation used in study HPN-100-006 was marketed in the US as Buphenyl. Each gram of NaPBA contained 0.88 g of PBA. Dose of NaPBA was determined by the investigator at the screening visit and was based on a variety of factors including severity of the patient’s enzyme deficiency and diet. No changes in the patient’s dosage regimen were required for entry and no changes were permitted during the study. The maximum dose levels were 600 mg/kg per day in patients weighing less than 20 kg, and 13 g/m2 per day in patients weighing 20 kg or more. NaPBA and NaPBA placebo were supplied as a tablet for oral administration or as a powder for oral, nasogastric, or gastrostomy tube administration.
In this study, GPB and GPB placebo were identical in appearance, as were NaPBA and NaPBA placebo.
Rescue medication (such as intravenous sodium phenylacetate/sodium benzoate), with or without hemodialysis, was allowed during HACs. Each patient was required to follow a low-protein diet and amino acid supplements throughout the study, as assessed by the investigator and/or dietician.
3.2.4. Outcomes
a. Mortality
This outcome was reported in the safety analysis in study HPN-100-006.
Hyperammonemic crises
The number and severity of symptomatic HACs were reported. HAC was defined as clinical symptoms associated with ammonia levels greater than or equal to 100 μmol/L. Clinical symptoms included vomiting, protein intolerance (becoming physically ill after high protein intake on multiple occasions leading to a self-imposed low-protein diet), lethargy, psychosis, abnormal neurological examination (hypotonia, spasticity, hyper-reflexia, and/or clonus), brain edema (evidence on magnetic resonance imaging or computed tomography scan), and headaches.
Cognitive development
These outcomes were not evaluated in the included study.
Anthropometric measurements
These outcomes were not evaluated in the included study.
Plasma ammonia levels
Ammonia levels were measured as: (1) 24-hour area under the curve (AUC0–24) for blood ammonia on days 14 and 28 (this was the primary outcome measure in study HPN-100-006); (2) maximum blood ammonia values observed on NaPBA versus GPB; and (3) percentage of blood ammonia values above the upper limit of normal (ULN) on NaPBA versus GPB at all time points of sample collection. On days 14 and 28, blood samples were collected at multiple time points for ammonia assessments (before first dose and after first dose at two, four, eight, 12, 16, 20, and 24 hours), and were processed by the laboratory at the investigator site per the facility standard operating procedures. AUC0–24 and 24-hour Cmax for blood ammonia levels were also assessed by age of UCD onset (two years old or younger, older than two years) on NaPBA versus GPB in post-hoc evaluation.
b. Glutamine levels
Blood samples for the glutamine levels were collected before first dose on day 1, day 14, and day 28. Glutamine levels on NaPBA versus GPB were assessed in post-hoc evaluation.
c. Health-related quality of life
These outcomes were not evaluated during the four-week treatment period.
d. Safety
Adverse events (AEs), serious adverse events (SAEs), as well as withdrawals due to adverse events were evaluated in the included study. An AE was defined as any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product; the occurrence did not necessarily need to have a causal relationship with this treatment. An SAE was defined as any AE that resulted in death, was immediately life-threatening, resulted in persistent or significant disability/incapacity, required or prolonged patient hospitalization, resulted in a congenital anomaly/birth defect, or was deemed serious for any other reason based on appropriate medical judgment. An independent DSMB was chartered to oversee the safety of study participants.
3.2.5. Statistical Analysis
In study HPN-100-006, a sample size of 44 patients was planned in order to provide 90% power at a one-sided significance level of 0.025 to demonstrate noninferiority of GPB to NaPBA, assuming an SD of the within-patient differences (natural log scale) of 0.225 and an expected ratio of the group means of 1.
An analysis of variance (ANOVA) model for the natural log-transformed blood ammonia AUC0–24 (primary efficacy end point) was constructed with factors for treatment, sequence, patient nested in sequence (as a random effect), and period in the intention-to-treat (ITT) population. The 95% confidence intervals (CIs) for the difference between GPB and NaPBA (GPB minus NaPBA) on the natural log scale were constructed using the least squares means from the ANOVA model. The difference and related CIs were exponentiated to express the results as geometric means, ratio of geometric means, and corresponding CI on the original scale. A one-sided alpha of 0.025 and 95% CIs were employed in assessing the noninferiority of GPB to NaPBA. Noninferiority of GPB to NaPBA was concluded when the upper bound of the 95% CI of ratio of the geometric means of blood ammonia AUC0–24 between GPB and NaPBA did not exceed 1.25. The margin of 1.25 was selected based on standard bioequivalence rules (i.e., the 95% CI for the ratio of mean AUCs being within 0.80 and 1.25); in addition, US Food and Drug Administration recommended that the manufacturer use 1.25 as the upper confidence limit (ratio of 24-hour AUC values for blood ammonia levels).20 All statistical comparisons for inequality between treatment groups were performed using two-sided alpha of 0.05 and 95% CIs.
The two-sample t-test and Wilcoxon rank sum test were used to analyze the maximum blood ammonia levels observed during the study, the number and percentage of ammonia values above the ULN, and the change from baseline in blood ammonia levels. The number of patients with at least one HAC was compared by treatment group using Fisher’s exact test.
Hochberg’s procedure was used to control for overall type I error. No adjustment for covariates was performed. Missing data were assumed to be missing at random. For patients who had incalculable blood ammonia AUC0–24 for both GPB and NaPBA treatment periods (completely missing data), their ammonia data were not imputed and were excluded from the analysis; for other patients who did not have a calculable blood ammonia AUC0–24 value for one but not both treatment periods, the missing ammonia data were handled using various methods, such as the last-observation-carried-forward approach. Sensitivity analyses were conducted to determine the effect of missing data on blood ammonia AUC0–24.
e. Analysis Populations
In HPN-100-006, the analysis set was defined as:
ITT population: including all patients who received any amount of either study treatment (NaPBA or GPB). The ITT population was used for the analysis of efficacy and pharmacokinetic parameters. Patients were included based on randomization assignment.
Per-protocol (PP) population: including all patients from the ITT population who received both study treatments (NaPBA and GPB) and 1) had a calculable blood ammonia AUC for both treatment periods; 2) had at least four blood ammonia samples, one of which was at either the eight-hour or 12-hour time point; 3) had the time zero blood ammonia sample drawn not more than 60 minutes after drug dosage and breakfast and the 24-hour blood ammonia sample drawn not more than 60 minutes after drug dosage and breakfast; 4) were compliant with study medication greater than 80% on day 14 and day 28; and 5) had not used sodium benzoate on either day 14 or day 28.
Safety population: including all patients who received any amount of study treatment. This was the primary population for all safety analyses. Patients were included based on study treatment received.
3.6. Patient Disposition
In total, 46 patients were randomized (Figure 3 and Table 6). One patient randomized to Arm B (GPB followed by NaPBA) withdrew before receiving any study treatment; therefore, 45 patients received at least one dose of study treatment (22 NaPBA followed by GPB, 23 GPB followed by NaPBA). One patient randomized to Arm A (NaPBA followed by GPB) withdrew on day 1 because of AEs (non-compliance with diet, including high blood ammonia levels [123 μmol/L], and headache). Another patient did not have a calculable AUC0–24 because they withdrew from the study after receiving one dose of NaPBA. Therefore, 44 patients in the ITT population completed the study and had evaluable data for the primary efficacy analysis. No patients in the safety population withdrew during the GPB treatment. No patients discontinued NaPBA or GPB treatment because of an HAC.
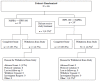
Figure 3
Patient Disposition in Study HPN-100-006. HPN-100 = glycerol phenylbutyrate; NaPBA = sodium phenylbutyrate. Source: Clinical Study Report of Study HPN-100-006.
Table 6
Patient Disposition.
3.7. Exposure to Study Treatments
In study HPN-100-006, treatment compliance was assessed based on the study drug compliance diaries collected from the patients at each visit, as well as visual inspection of the returned study drug.
Forty-five of the 46 randomized patients received at least one dose of NaPBA study treatment. One patient withdrew before receiving any study treatment, and another patient, randomized to Arm A (NaPBA followed by GPB), withdrew from the study on day 1 and received only NaPBA. Therefore, 44 patients received at least one dose of GPB. Overall treatment compliance was high in the study, with 97.7% and 100% of patients being at least 80% compliant with the NaPBA and GPB treatments, respectively. The total mean dose of each study treatment was similar between the NaPBA and GPB treatment period (Table 7).
Table 7
Extent of Exposure to Study Drugs in Study HPN-100-006 (Safety Population).
3.8. Critical Appraisal
3.8.1. Internal Validity
HPN-100-006 was a phase III, DB, double-dummy, crossover randomized controlled trial (RCT) evaluating the noninferiority of GPB to NaPBA in blood ammonia AUC0–24 in patients with UCDs. NaPBA is an appropriate comparator in the study population. Treatment allocation was carried out using a computer-generated central randomization schedule. The method of blinding was questionable because NaPBA has an unfavourable taste and odour, while GPB and GPB placebo supplied in study HPN-100-006 was odourless and almost tasteless. This may have allowed some patients (and/or investigators) to surmise that they were randomized to receive a certain treatment. However, the primary outcome, change in blood ammonia levels, is an objective outcome measure. Therefore, it is unlikely that the method of blinding had an important impact on the study results for the primary analysis. In the case of a medical emergency, unblinding of the patient’s treatment assignment could be requested by the investigator for the well-being of the patient; however, no cases of unblinding occurred during the study. Forty-four out of 46 randomized patients completed the study; the overall loss to follow-up was low and treatment compliance was high. Because of the ethical consideration, there was no washout period between the two treatments. The potential carry-over effect may complicate the interpretation of the study findings, such as the comparison of drug-related AEs between treatment groups. A previous study demonstrated that, in healthy volunteers, after NaPBA administration, the mean plasma half-life of PBA was 0.7 ± 0.1 hours; after GPB administration, the mean plasma half-life of PBA was 1.9 ± 1.7 hours.25 Given the short half-lives of GPB and NaPBA, the carry-over effect would not be considered significant.
The primary outcome of this study was AUC0–24 for blood ammonia levels. Blood samples were drawn and processed by the laboratory at the investigator site rather than a central laboratory. Although the facility standard operating procedures were adopted for the process, there may still be discrepancies among the various sites, which may affect the accuracies of the results. Furthermore, important clinical outcomes such as cognitive development, anthropometric measurements, and health-related quality of life (HRQoL) were not measured, probably because of the short duration (four weeks) of HPN-100-006. The relationship between ammonia levels and clinical outcomes among patients with UCDs was not well established and there was conflicting evidence in previous research. While general trends suggest that higher levels of blood ammonia are associated with higher risks of HAC, identifying quantitative levels for targets and minimal clinically important differences remains elusive.26,27
In terms of the methods of statistical analysis, the method for the sample size calculation was described. The planned sample size was considered to provide 90% power to demonstrate noninferiority of GPB to the comparator. There was no rationale provided for the use of 0.225 as an SD. This is key information to be able to assess whether the sample size was determined in a proper manner. Due to the small sample size, analyses of some important subgroups (such as UCD subtypes and age) predefined in the research protocol were not feasible. The clinical expert consulted for this review noted that the current sample size is acceptable, given that a UCD is a rare disease. Missing data were assumed to be missing at random; however, this may not be an appropriate assumption because patient dropout may be due to differences in the treatment effects between the two groups. Sensitivity analyses were conducted by excluding patients with incalculable blood ammonia AUC0–24 to determine the effect of missing data on the primary study end point, and the results were consistent with the primary analysis, which was conducted in a PP or ITT population.
In HPN-100-006, efficacy and safety of GPB and NaPBA were assessed up to two weeks after the randomization. There was a lack of longer-term comparative efficacy and safety data available for GPB in the study population. Open-label extension studies were conducted to explore the treatment effect of GPB in adult and pediatric populations up to one year without comparing with a currently available active treatment (APPENDIX 7).
3.8.2. External Validity
The study participants were recruited from one Canadian centre and 21 US centres. The NaPBA formulation used in study HPN-100-006 was marketed in the US as Buphenyl. This was a taste-unmasked formulation that is not the same formulation as the taste-masked formulation (Pheburane) available in Canada. The active component in both formulations is NaPBA; the dose of Buphenyl was the same as the HC-approved dosage for Pheburane. It is uncertain whether this would limit the generalizability of the study results to current Canadian clinical practice.
The baseline patient characteristics were somewhat different from a typical Canadian population with UCDs. According to the clinical expert consulted for this review, due to the restricted inclusion criteria and extensive exclusion criteria, patients in the included study had milder disease (such as lower baseline ammonia levels and less comorbidity) compared with the patients who are usually seen in clinical practice. This can limit the generalizability of the study results to a broader UCD population. The clinical expert noted that, through clinical experience, the results would be translated to the more severe UCD population. On the other hand, not all subtypes of UCDs were included in the study.
The study enrolled adult patients only; therefore, the clinical benefits and harms in pediatric patients cannot be examined. In addition, only patients with CPS1, OTC, and ASS subtypes were enrolled; thus the treatment effect of study medication on other UCD subtypes was uncertain. Generalizability of the study results would also be limited due to lack of evidence on some hard clinical outcomes, short study duration, and uncertain sustained effect of the study medication.
3.9. Efficacy
Only those efficacy outcomes identified in the review protocol are reported below (Section 2.2, Table 3). See APPENDIX 4 or detailed efficacy data.
3.9.1. Mortality
No deaths occurred in the study.
3.9.2. Number of Hyperammonemic Crises
No patients had an HAC during GPB treatment.
One patient had elevated blood ammonia levels that met the definition of an HAC while on NaPBA treatment; the elevated levels were due to noncompliance with the study treatment. This was also considered an SAE.
3.9.3. Cognitive Development
This was not assessed as an outcome in HPN-100-006.
3.9.4. Anthropometric Measurements
This was not assessed as an outcome in HPN-100-006.
3.9.5. Plasma Ammonia Levels
AUC0–24 for Blood Ammonia on Day 14 and Day 28
In the PP population, the mean AUC0–24 values for blood ammonia were 12% lower with GPB treatment compared with NaPBA (868.29 ± 668.145 μmol·h/L versus 985.47 ± 873.578 μmol·h/L, respectively). Consistent results were observed in the ITT population, with mean AUC0–24 values for blood ammonia 11% lower with GPB treatment compared with NaPBA (865.85 ± 660.529 μmol·h/L versus 976.63 ± 865.352 μmol·h/L, respectively). None of the differences between the GPB and NaPBA treatments with respect to blood ammonia assessed as AUC0–24 were statistically significant.
In the PP population, GPB achieved noninferiority to NaPBA. The upper bound of the 95% CI of ratio of the geometric means of blood ammonia AUC0–24 between GPB and NaPBA was 1.030, which was below the predefined noninferiority margin of 1.25. A consistent treatment effect was seen in the ITT population, in which GPB was shown to be noninferior to NaPBA in controlling blood ammonia (upper bound of the 95% CI of 1.034) (Table 8).
Table 8
Plasma Ammonia Levels in Study HPN-100-006 (AUC0–24, μmol·h/L).
Post-hoc analysis in subgroups suggested that mean blood ammonia AUC0–24 with GPB treatment was 17% lower compared with NaPBA treatment in patients who were diagnosed with a UCD during infancy (UCD onset at two years old or younger) and 10% lower in patients who were diagnosed with a UCD after infancy (UCD onset older than two years). The between-group differences in the subgroup analysis were not statistically significant APPENDIX 4.
a. Maximum Blood Ammonia Values
Twenty-four–hour Cmax values for blood ammonia were numerically but not statistically significantly lower with GPB treatment compared with NaPBA treatment in the patient populations. In the PP population, mean Cmax values for blood ammonia were 14% lower with GPB treatment compared with NaPBA (61.33 ± 46.686 μmol/L versus 71.47 ± 67.355 μmol/L, respectively), and it was 14% lower with GPB treatment compared with NaPBA treatment in the ITT population (60.94 ± 46.213 μmol/L versus 70.83 ± 66.71 μmol/L, respectively) (Table 9).
Table 9
Plasma Ammonia Levels in Study HPN-100-006 (24-Hour CMAX, μmol/L).
The mean blood ammonia Cmax levels were 14% lower with GPB treatment versus NaPBA treatment in patients who were diagnosed with a UCD during infancy (UCD onset at two years or younger) and in patients who were diagnosed with a UCD after infancy (UCD onset older than two years). The between-group differences in the subgroup analysis were not statistically significant APPENDIX 4.
b. Percentage of Blood Ammonia Values Above the Upper Limit of Normal on NaPBA Versus GPB
The number of ammonia samples above the ULN was similar with GPB and NaPBA treatments in the PP populations (35.4% and 36.8%, respectively; P > 0.05). The number of ammonia samples above the ULN was also similar with GPB and NaPBA treatments in the ITT population (35.6% and 36.2% of samples, respectively; P > 0.05) (Table 10).
Table 10
Ammonia Values Above thE Upper Limit of Normal in Study HPN-100-006.
c. Glutamine Levels
Glutamine levels were assessed in a post-hoc evaluation. Results of this outcome are presented in APPENDIX 4.
3.9.6. Health-Related Quality of Life
This was not assessed during the four-week treatment period in HPN-100-006.
3.9.7. Other Efficacy Outcomes
a. Hospitalization
Not assessed.
b. Patient Adherence
Not assessed.
c. Patient/Caregiver Satisfaction
Not assessed.
3.10. Harms
Only those harms identified in the review protocol are reported below (see 2.2.1, Protocol).
3.10.1. Adverse Events
At least one treatment-emergent AE was reported in 27 (61.4%) and 23 (51.1%) patients on GPB and NaPBA treatment, respectively (Table 11). Most treatment-emergent AEs were considered by the investigator to be mild, although one was a grade 3 hyperammonemia. Symptoms of lower GI tract disorders (diarrhea and flatulence) were reported more frequently on GPB treatment, whereas symptoms of upper GI disorders (abdominal discomfort, dyspepsia, nausea, and oral discomfort) were more likely to be reported during the NaPBA treatment. These events were generally mild. Dizziness was reported by more patients treated with NaPBA than GPB (8.9% versus 0).
Table 11
Harms.
3.10.2. Serious Adverse Events
Two patients reported treatment-emergent SAEs: one patient reported acute gastroenteritis on GPB treatment, and one patient reported a grade 3 hyperammonemia on NaPBA treatment.
3.10.3. Withdrawals Due to Adverse Events
No patients discontinued from GPB treatment, whereas one patient discontinued from NaPBA treatment because of high ammonia levels on day 1.
3.10.4. Mortality
No deaths occurred during the four-week treatment periods.
3.10.5. Notable Harms
During the baseline assessments, patients were queried regarding common symptoms associated with the use of NaPBA (decreased appetite/food aversion, increased appetite, body odour, burning sensation in mouth or throat, abdominal pain/distress, nausea, vomiting, heartburn, headache, amenorrhea/menstrual dysfunction, dizziness, or fatigue) and asked to record the frequency of their occurrence. The same set of inquiries was given to patients on day 14 and day 28 after treatment with NaPBA and GPB. If such symptoms were detected at baseline, they were to be also recorded as medical history before enrolling in the study. The symptoms were not reported as AEs if they existed at baseline. If these symptoms were first detected after the baseline visit, they were recorded as AEs and attributed to the last treatment received, either NaPBA or GPB.
In total, 33 patients reported “yes” to at least one of the UCD symptoms at baseline, while on day 14 and day 28, 21 of them did not report a UCD symptom after treatment with GPB. With the exception of amenorrhea/menstrual dysfunction, which showed no change in the reporting frequency, there was an overall numerical reduction in the number of patients still reporting “yes” to any of the UCD symptoms after treatment with GPB. Frequencies of these symptoms were less than 5%; therefore, they are are not presented in Table 11. In addition, mean changes from baseline in selected liver-function parameters were similar with NaPBA treatment and GPB treatment (data not presented). There were no clinically significant changes in liver-function values and potassium level during the study with either treatment.
- RESULTS - Glycerol Phenylbutyrate (Ravicti)RESULTS - Glycerol Phenylbutyrate (Ravicti)
- PATIENT INPUT - Ustekinumab (Stelara)PATIENT INPUT - Ustekinumab (Stelara)
- INTRODUCTION - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)INTRODUCTION - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)
- OBJECTIVES AND METHODS - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)OBJECTIVES AND METHODS - Somatropin (Genotropin) (0.15 mg/day to 0.3 mg/day)
- INTRODUCTION - Ustekinumab (Stelara)INTRODUCTION - Ustekinumab (Stelara)
Your browsing activity is empty.
Activity recording is turned off.
See more...
